
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 23 May 2018
Sec. Waste Management in Agroecosystems
Volume 2 - 2018 | https://doi.org/10.3389/fsufs.2018.00017
This article is part of the Research Topic Treatment of organic waste in the framework of circular economy View all 5 articles
The anaerobic thermophilic co-digestion of mixtures of the organic fraction of municipal solid waste (OFMSW) and the liquid fraction from the hydrothermal carbonization (LFHTC) of dewatered secondary sludge was studied. Mixtures with a low OFMSW to LFHTC ratio (50, 75, and 100% LFHTC) exhibited accumulation of volatile fatty acids (VFA) as well as low degradation of organic matter and methane production. However, the mixture containing 25% LFHTC performed quite well in terms of methane production: (179 ± 3) mL CH4 STP g−1 CODadded, which was only slightly lower than the value obtained with 100% OFMSW. The experimental results fitted the modified Gompertz model reasonably well and the maximum methane production rate for the mixture containing 25% LFHTC (11.96 mL CH4 g−1 COD d−1) was 29.3% higher than that obtained with the substrate with 100% OFMSW. Therefore, centralized co-digestion of OFMSW in mixtures with 25% LFHTC seemingly provides an effective method for valorizing the latter substrate.
Thermochemical conversion processes are typically used to convert biomass into valuable products or biofuel. Specifically, hydrothermal carbonization (HTC) at 180–260°C under auto-generated pressure is a promising method for converting wet biomass (Tekin et al., 2014; Kambo and Dutta, 2015). Thus, HTC converts biomass into a valuable solid product called “hydrochar” in addition to a liquid fraction (LFHTC) and a gas stream. HTC is especially suitable for biomass waste with a high moisture content such as sewage sludge produced in wastewater treatment plants (WWTP). Because this hydrochar possesses a high heating value (ca. 22 MJ kg−1), hydrothermal conversion of sewage sludge can be an effective, inexpensive choice for its management. Also, the resulting liquid fraction (LFHTC) has a total chemical oxygen demand (COD) of nearly 100 g O2 L−1 (Villamil et al., 2018), which justifies its valorization to recover organic matter up to 15% of all initial carbon (Broch et al., 2014).
The liquid fraction obtained by HTC of organic waste is used as feedstock for chemical production or recycled in consecutive HTC runs to improve carbon yield (Xiao et al., 2012; Stemann et al., 2013); also, it can be subjected to wet air oxidation, aerobic degradation or anaerobic digestion (Eibisch et al., 2013; Becker et al., 2014; Smith and Ross, 2016). However, the presence of recalcitrant compounds formed during the thermal treatment (furfural, phenols, furans, pyrazines, and pyridines) detracts from methane yields in anaerobic digestion (Becker et al., 2014; Danso-Boateng et al., 2015; De la Rubia et al., 2018b; Villamil et al., 2018).
One of the advantages of co-digestion processes (viz., the simultaneous digestion of two or more substrates) is that it dilutes toxic compounds (Angeriz-Campoy et al., 2015). This makes co-digestion of LFHTC with another organic waste a suitable choice for LFHTC valorization. Anaerobic co-digestion is by now a well-established technology (De Clercq et al., 2017; Hagos et al., 2017; Komilis et al., 2017), and co-digestion of sewage sludge (SS) and the organic fraction of municipal solid waste (OFMSW) is most widely explored combination (Mata-Alvarez et al., 2014).
OFMSW anaerobic digestion is a widely used green method and an advantageous alternative to traditional management choices for organic solid wastes (e.g., landfill refuse) with reduced methane emission and energy production (Fernández-Rodríguez et al., 2015). However, the usual practice of performing OFMSW digestion under mesophilic conditions necessitates revision and improvement. In fact, processing OFMSW at a thermophilic temperature rather than at mesophilic levels affords high waste loads, increased biogas production, and effective destruction of pathogenic microorganisms, which leads to improved hygienization of solid waste material for use on land (Mata-Alvarez et al., 2014). Also, using a digestion co-substrate can increase biogas production or even methane yield in traditional anaerobic digestion processes for organic wastes (Martín-González et al., 2011). LFHTC can be an useful co-substrate for this purpose on account of its high organic matter content (Villamil et al., 2018). In addition, anaerobic co-digestion could aid the process by balancing the C:N ratio and increasing buffering capacity (Capson-Tojo et al., 2018). However, optimizing co-digestion requires using the best possible blend to exploit synergistic and complementary effects, as well as to maximize methane production while avoiding inhibition (Sensai et al., 2014).
In this work, we explored the batchwise anaerobic co-digestion under thermophilic conditions of mixtures of the liquid fraction from the hydrothermal carbonization of dehydrated sewage sludge and the organic fraction of municipal solid waste in variable ratios with a view to improving methane yields in relation to the processing of either substrate alone. Process performance was assessed in terms of various parameters including methane yield.
The starting anaerobic digestate was collected from a full-scale mesophilic reactor processing OFMSW from a municipal solid waste treatment plant (MSWTP) in the Spanish region of Madrid. The mixed anaerobic culture used as thermophilic inoculum was obtained by directly switching from mesophilic (35°C) to thermophilic conditions (55°C) according to De la Rubia et al. (2013). The main properties of the inoculum were as follows: pH 8.2 ± 0.1, total solids (97.9 ± 0.4) g TS kg−1; volatile solids (45.3 ± 0.5) g VS kg−1; and total chemical oxygen demand (36.6 ± 3.3) g O2 L−1.
OFMSW was collected from the waste reception area of the aforementioned MSWTP. Although the solid waste delivered at the treatment plant is source segregated at household level, it still contains considerable amounts of plastic, paper, cardboard, metal, and glass. An amount of ~100 kg of OFMSW was sorted by hand and its non-OFMSW portion removed prior to grinding in a mill. Finally, the shredded organic waste was sieved to a final particle size smaller than 0.02 m for use. This substrate contained (24.5 ± 2.9)% C, (1.6 ± 0.5)% H, (1.9 ± 0.2)% N and (0.1 ± 0.05)% S, and its main characteristics were as follows: (437 ± 9) g TS kg−1; (283 ± 3) g VS kg−1; and total COD (1157 ± 10) mg O2 g−1 TS.
LFHTC was obtained by hydrothermal carbonization of dehydrated sewage sludge, with an 85% moisture content, collected from a full-scale membrane bioreactor processing industrial wastewater from a cosmetics factory. The substrate was stored at −20°C until use. HTC was performed by using 1.5 kg of dewatered sludge in an electrically heated 4 L ZipperClave® pressure vessel at 208°C. The operating temperature was reached at a heating rate of 3°C min−1 and held for 1 h. The reaction was stopped by cooling with an internal heat exchanger using tap water. The slurry obtained (470 g of wet hydrochar and 530 g of LFHTC for each kg of wet material treated) was centrifuged at 3,500 rpm for 1 h, filtered (Albet FV-C, 0.45 μm) and stored at 4°C until anaerobic digestion. HTC allowed obtaining a dry basis hydrochar with 40.1% yield, 21.6 MJ kg−1 heating heat value and the following elemental composition: 43.1% C, 5.8% H, 0.2% S, and 4.6% N.
The composition and main properties of the LFHTC were as follows, each given as the average of three determinations ± standard deviation: pH 5.1 ± 0.1, (94.6 ± 2.0) g L−1 soluble COD (SCOD), (42.6 ± 1.7) g TOC L−1, (55.7 ± 0.5) g TS L−1, (46.2 ± 0.5) g VS L−1, and (8.7 ± 0.1) g L−1 total Kjeldahl nitrogen (TKN). This co-substrate was analyzed by GC/MS (De la Rubia et al., 2018a), which allowed the identification of nitrogen-containing species (pyrazines and aromatic amines) and oxygen-containing aromatic compounds (phenols and furans).
Anaerobic co-digestion runs were done batchwise in 120 mL glass serum vials that were kept under thermophilic conditions (55 ± 1°C) in a Julabo thermostatic water bath shaker operating at 80 rpm. The operational sequence used in each run comprised 9 fed reactors and 3 controls. The fed reactors were initially loaded with the required amounts of the two co-substrates, six being sacrificed and removed each day initially and then weekly until the end of the experiment, in order to assess changes in chemical parameters at different times during the anaerobic digestion process. The other three reactors were used for biogas analysis (volume and composition) only. Methane production through biomass decay, and residual substrate potentially present in the inoculum, as determined in the controls, were subtracted from the experimental values. All experiments were allowed to develop until no significant gas production was observed and biodegradation was thus essentially complete as in the controls with starch (ca. 350 mL CH4 g−1 CODadded), which took ~55 days.
Batch co-digestion runs used an inoculum concentration of 15 g VS L−1 and an ISR value of 2.0, on a COD basis, as widely recommended (Raposo et al., 2011b; Alzate et al., 2012; Pellera and Gidarakos, 2016; Villamil et al., 2018). The co-substrates were used in different ratios, namely: 0, 25, 50, 75, and 100% LFHTC, all on a COD basis. A basal medium of macro- and micronutrients was prepared according to Villamil et al. (2018). Also, a 10% (v/v) solution of 50 g NaHCO3 L−1 was used to obtain a TA value of 3.4 g CaCO3 L−1 at the beginning of the reaction (time zero). Finally, the reactors were filled up to 60 mL with distilled water, the reaction medium being flushed with N2 for 3 min in order to ensure anaerobic conditions and the vials sealed with rubber stoppers and metal crimps.
Dry matter, TS and VS were determined according to standard methods 2540B and 2540E (APHA, 1998), and total COD according to Raposo et al. (2008). pH was measured with a Crison 20 Basic pH meter. Partial alkalinity (PA) and total alkalinity (TA) were determined by titration to pH 5.75 and 4.3, respectively (Jenkins et al., 1983). Intermediate alkalinity (IA), defined as the difference between TA and PA, and SCOD were assessed by closed digestion and with standard colorimetric method 5220D, respectively (APHA, 1998). TKN was measured as described elsewhere (Villamil et al., 2018); total ammonia nitrogen (TAN) by distillation and titration according to standard method 4500E (APHA, 1998); and free ammonia nitrogen (FAN) according to Hansen et al. (1998). The elemental composition of the inoculum and co-substrates was determined with a LECO CHNS-932 CHNS analyzer. The concentrations of individual volatile fatty acids (VFA) from acetic to heptanoic, iso forms included, were determined on a Varian 430 gas chromatograph equipped with a flame ionization detector (FID) and a capillary column filled with Nukol (polyethylene glycol modified by nitroterephthalic acid) according to De la Rubia et al. (2018a). Biogas and methane production expressed at standard temperature (273 K) and pressure (1 bar) were measured once daily over the first 3 days and 12 more times through the incubation period. H2, CO2, and CH4 were determined on a Thermo Scientific Trace 1300 gas chromatograph equipped with a thermal conductivity detector (TCD) and a 8 ft × 1/8 in SS column packed with HayeSep Q 80/100 mesh (De la Rubia et al., 2018a).
The pH values at the end of the co-digestion process were 8.1–8.2, and hence very similar in all runs and typical for anaerobic digestion under thermophilic conditions (Sosnowski et al., 2008; De la Rubia et al., 2013). These values are suitable for growing methanogenic Archaea, which are extremely sensitive to pH changes (Wang et al., 2014).
Table 1 shows the TA, PA, and IA/TA ratios, as well as the final TAN and FAN value. As can be seen, TA ranged from 10.3 to 11.3 g CaCO3 L−1 and was thus high enough to ensure acceptable buffering capacity. In fact, an IA/TA ratio lower than 0.3 ensures efficient operation of anaerobic processes, so the buffering capacity of the medium was adequate. Hydrolysis reactions are favored by thermophilic conditions, which boost protein degradation and ammonia nitrogen production as a result (De la Rubia et al., 2013). Nitrogen is essential for protein synthesis and primarily a nutrient for microorganisms in anaerobic digestion; on the other hand, TAN has some buffering effect on anaerobic digestion systems. Nitrogen in the form of ammonium ion () can react with bicarbonate ion to form NH4HCO3. However, ammonia (NH3) has been suggested to be the active component inhibiting microbial methanogenesis (Fricke et al., 2007). The free ammonia level depends mainly on three parameters, namely: TAN concentration, temperature, and pH (Hansen et al., 1998). An increase in pH results in a marked concentration increase in free ammonia (e.g., 8 times for a pH rise from 7 to 8; Yangin-Gomec and Ozturk, 2013). As can be seen, TAN ranged from 2,044 to 2,212 mg L−1 and FAN from 709 to 791 mg L−1. According to Chen et al. (2008), TAN concentrations over the range 1,500–7,000 mg L−1, which is very broad and encompasses a variety of substrates and conditions, can inhibit methanogenesis. Our TAN values fell slightly above the lower limit of inhibition but were very far from the upper limit. As consequence, slight inhibition of methanogenic activity by ammonia nitrogen might have occurred. However, our FAN values were lower than the limit reported by Hansen et al. (1998) for inhibition in the anaerobic degradation of swine manure in batch cultures (1.1 g L−1). As can be seen from Table 1, FAN increased with decreasing OFMSW/LFHTC ratio, probably due to the intimate relationship between the pH of the medium, higher than 8 in all runs, and the proportion of ammonia/ammonium as a function of temperature.
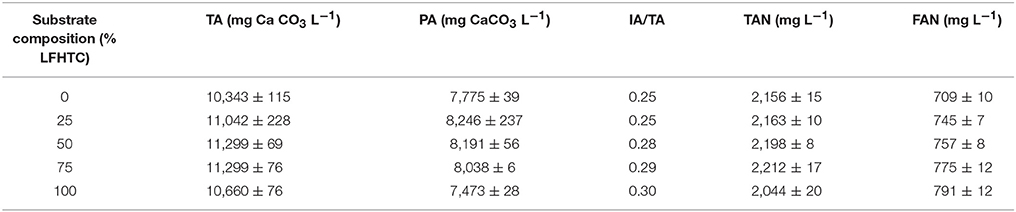
Table 1. Total alkalinity (TA), partial alkalinity (PA), intermediate to total alkalinity (IA/TA) ratio, total ammonia nitrogen (TAN), and free ammonia (FAN) at the end of the experiments.
VFA evolution is central to the development of anaerobic digestion. Figure 1 shows the VFA profile obtained at the end of the experiments with the different OFMSW/LFHTC ratios studied. As can be seen, accumulation of VFA such as propionic, isobutyric, isovaleric, and valeric increased with increasing proportion of LFHTC. The presence of these acids has a negative effect on methane production; in fact, the acids are difficult to degrade (particularly propionic), so their accumulation can lead to inhibition of methanogenic Archaea. De la Rubia et al. (2013) reported that thermophilic conditions tend to generate high concentrations of VFA in the medium, which coincides with the results obtained experimentally. As can be seen in Figure 1, using a proportion of LFHTC higher than 50% led to a propionic acid concentration exceeding 850 mg L−1. Wang et al. (2009) found that propionic acid concentration of 900 mg L−1 led to considerable anaerobic digestion inhibition. This propionic acid concentration was also found to have inhibitory effects by Qiao et al. (2013) in processing the supernatant of hydrothermally treated municipal sludge in an upflow anaerobic sludge blanket reactor. In fact, conditions with of LFHTC higher than 50% reached lower methane yields, as more forward will be checked.
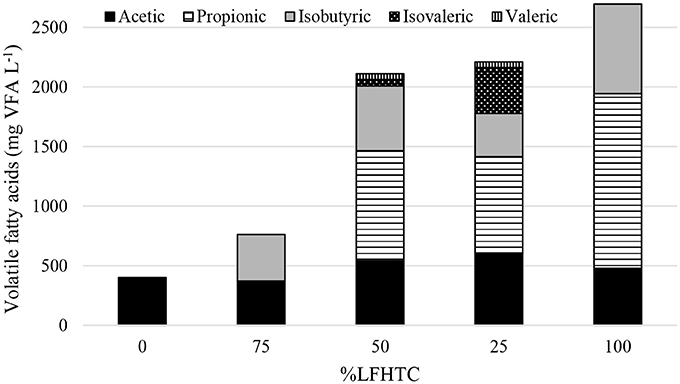
Figure 1. VFA profile (expressed as mg L−1) at the end of the experiments with substrates of variable composition.
Table 2 shows the SCOD values at the end of the experiments. As can be seen, there were two clear-cut trends. Thus, the mixtures with the highest proportions of OFMSW (100 and 75%) exhibited the lowest SCOD values. Since the initial COD concentration was identical in all trials, these runs were those providing the highest methane yields. On the other hand, the mixtures with high proportions of LFHTC (50, 75, and 100%) led to high SCOD levels (i.e., to less efficient degradation of organic matter and hence to decreased methane generation). As expected, the runs with high SCOD values also led to high VFA concentrations. VFA, in mg COD L−1, accounted for a substantial fraction of SCOD in the samples containing more than 50% LFHTC, probably as a result of its partially inhibiting the methanogenic population.
Figure 2 shows the time course of methane yield, in mL CH4 STP g−1 CODadded, in the anaerobic co-digestion runs. Methane production at the end of the runs ranged from (51 ± 1) to (209 ± 1) mL CH4 STP g−1 CODadded for the individual substrates (LFHTC and OFMSW), respectively, while methane yields relative to the theoretical value (350 mL CH4 g−1 COD) were 14.6 and 59.6%. The maximum theoretical methane production was never reached because some organic matter is usually inaccessible, some compounds are difficult to degradable and a fraction of the substrate is used for cell growth and maintenance (Raposo et al., 2011a). The highest yields were obtained in the runs involving the highest proportions of OFMSW (100 and 75%); on the other hand, methane production decreased with increasing LFHTC content in the mixture. The OFMSW substrate contained large amounts of organic matter of easy degradation by anaerobic microorganisms and thus had a high potential for biogas production. By contrast, the LFHTC substrate contained recalcitrant compounds (viz., nitrogen-containing species such as pyrazines and aromatic amines, and oxygen-containing aromatic compounds such as phenols and furans) and such compounds were difficult to degrade and led to lower methane yields (De la Rubia et al., 2018b).
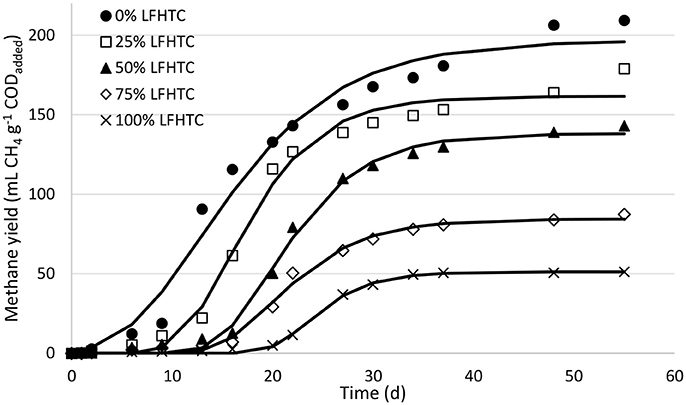
Figure 2. Time-course of methane production in the experiments. Symbols represent experimental values and the solid lines predicted values.
A comparison of the methane yields obtained in the runs with 100 and 75% OFMSW under thermophilic conditions [(209 ±1) and (179 ± 3) mL CH4 STP g−1 CODadded, respectively] with those obtained under mesophilic conditions [(194 ± 1) and (188 ± 1) mL CH4 STP g−1 CODadded, respectively] (De la Rubia et al., 2018a), revealed an increase by 7.7% with 100% OFMSW under thermophilic conditions, but similar yields with 75% OFMSW.
Cabbai et al. (2013) studied the anaerobic co-digestion of OFMSW and sewage sludge obtaining a similar trend in methane yield by reducing the proportion of OFMSW in the starting mixture. Also, our yields were lower than those reported by Qiao et al. (2011) for the anaerobic digestion of the liquid fraction from the HTC of mixed (primary and secondary) sewage sludge (257 mL CH4 g−1 COD). However, these authors performed HTC at 120–190°C, which is much lower than the temperature used here (208°C). In any case, Wirth et al. (2015) obtained similar methane yields (120–180 mL CH4 STP g−1 CODadded) by using the liquid fraction from HTC of digested sewage sludge as their sole substrate in the continuous feed mode.
Methane yields were fitted to the modified Gompertz model. The model uses a sigmoidal function similar to a time series where the growth rate is very low at the beginning and end (Amiri et al., 2017). This equation is probably one of the best functions for predicting biogas production in batchwise anaerobic digestion processes; also, it has been calibrated and tested with large amounts of experimental data by a number of researchers (Donoso-Bravo et al., 2010; Li et al., 2011; Amiri et al., 2017).
In the modified Gompertz model, cumulative methane production is related to digestion time by the following equation:
where B is the cumulative methane production at time t (mL CH4 g−1 CODadded), Bm the maximum methane production or methane yield potential (mL CH4 g−1 CODadded), Rm the maximum methane production rate (mL CH4 g−1 CODadded d), λ the lag time (d), t the digestion time (d) at which methane production is calculated and e denotes exp(1) = 2.7183. Parameters Bm, Rm, and λ for each run were calculated by using the non-linear regression routine in the software SigmaPlot 11.0.
Table 3 shows the values of the model parameters for each run as obtained with the modified Gompertz model. As can be seen, the model fitted the experimental data quite acceptably, with low standard errors of estimate (SEE). The differences between the experimental and calculated values of Bm were smaller than 5% in most cases. On the other hand, the determination coefficients exceeded 0.99 in almost all. A decrease in theoretical ultimate methane yield with increasing proportion of LFHTC was observed. The substrate mixture containing 75% OFMSW exhibited a smaller value than the sole OFMSW substrate. However, the ultimate methane yield was considerably lower with all other substrate compositions, (138, 84, and 51 mL CH4 STP g−1 CODadded, respectively). The highest methane production rate was that for the mixture 75% OFMSW−25% LFHTC: 11.96 mL CH4 g−1 COD d−1, which was 29.3% higher than that for the 100% OFMSW. Also, increasing the proportion of OFMSW decreased the maximum methane production rate from 11.96 to 5.33 mL CH4 g−1 COD d−1. In addition, the lag time increased considerably (from 4.9 to 19.7 days) as the proportion of LFHTC in the mixture was increased as a result of the presence in this substrate of complex organic acids and other inhibitory substances that are difficult to degrade anaerobically.
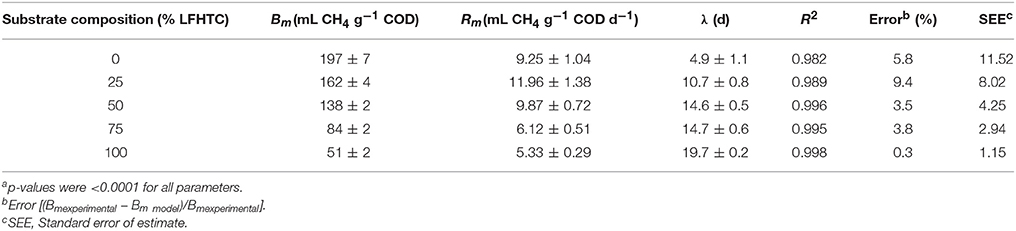
Table 3. Parametersa of the Modified Gompertz model for the individual substrates and the co-substrate mixtures.
Thermophilic anaerobic co-digestion of OFMSW and LFHTC mixtures failed to increase methane yield with respect to the anaerobic digestion of OFMSW alone; also, no synergistic effect was observed. However, a mixture containing 25% LFHTC provided methane yields very close to those obtained with 100% OFMSW. This substrate combination provides an alternative management method for this waste (LFHTC): co-digestion with OFMSW. Methane production decreased with increasing content of LFHTC in the mixtures because this substrate contains recalcitrant compounds that are difficult to degrade by methanogenic microorganisms. However, anaerobic tests with mixtures containing more than 25% LFHTC inhibited methanogenesis through increasing accumulation of VFA as the proportion of this substrate in the mixture was increased. The time course of methane production fitted the modified Gompertz model quite well.
JV and AR contributed conception and design of the study. JV organized the database. AR, AM, JR, and RB wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors wish to express their gratitude to Spain's MINECO (CTM2016-76564-R) and UAM-Santander (Project CEAL-AL/2015-29) for funding this work. AR acknowledges additional funding from the Spanish Ministry of Economy and Competitiveness (RYC-2013-12549). The valuable contribution of A. Eguiluz is also acknowledged.
Alzate, M. E., Muñoz, R., Rogalla, F., Fdz-Polanco, F., and Pérez-Elvira, S. I. (2012). Biochemical methane potential of microalgae: influence of substrate to inoculum ratio, biomass concentration and pretreatment. Bioresour. Technol. 123, 488–494. doi: 10.1016/j.biortech.2012.06.113
Amiri, L., Abdoli, M. A., Gitipour, S., and Madadian, E. (2017). The effects of co-substrate and thermal pretreatment on anaerobic digestion performance. Environ. Technol. 38, 2352–2361. doi: 10.1080/09593330.2016.1260643
Angeriz-Campoy, R., Álvarez-Gallego, C. J., and Romero-García, L. I. (2015). Thermophilic anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW) with food waste (FW): enhancement of bio-hydrogen production. Bioresour. Technol. 194, 291–296. doi: 10.1016/j.biortech.2015.07.011
APHA (1998). Standard Methods for the Examination of Water and Wastewater, 20th Edn. Washington, DC: American Public Health Association; American Water Works Association and Water Environment Federation.
Becker, R., Dorgerloh, U., Paulke, E., Mumme, J., and Nehls, I. (2014). Hydrothermal carbonization of biomass: major organic components of the aqueous phase. Chem. Eng. Technol. 37, 511–518. doi: 10.1002/ceat.201300401
Broch, A., Jena, U., Hoekman, S. K., and Langford, J. (2014). Analysis of solid and aqueous phase products from hydrothermal carbonization of whole and lipid-extracted algae. Energies 7, 62–79. doi: 10.3390/en7010062
Cabbai, V., Ballico, M., Aneggi, E., and Goi, D. (2013). BMP tests of source selected OFMSW to evaluate anaerobic codigestion with sewage sludge. Waste Manage. 33, 1626–1632. doi: 10.1016/j.wasman.2013.03.020
Capson-Tojo, G., Trably, E., Rouez, M., Crest, M., Bernet, N., Steyer, J. P., et al. (2018). Cardboard proportions and total solids contents as driving factors in dry co-fermentation of food waste. Bioresour. Technol. 248, 229–237. doi: 10.1016/j.biortech.2017.06.040
Chen, Y., Cheng, J. J., and Creamer, K. S. (2008). Inhibition of anaerobic digestion process: a review. Bioresour. Technol. 99, 4044–4064. doi: 10.1016/j.biortech.2007.01.057
Danso-Boateng, E., Shama, G., Wheatley, A. D., Martin, S. J., and Holdich, R. G. (2015). Hydrothermal carbonisation of sewage sludge: effect of process conditions on product characteristics and methane production. Bioresour. Technol. 177, 318–327. doi: 10.1016/j.biortech.2014.11.096
De Clercq, D., Wen, Z., Gottfried, O., Schmidt, F., and Fei, F. (2017). A review of global strategies promoting the conversion of food waste to bioenergy via anaerobic digestion. Renew. Sustain. Energy Rev. 79, 204–221. doi: 10.1016/j.rser.2017.05.047
De la Rubia, M. A., Riau, V., Raposo, F., and Borja, R. (2013). Thermophilic anaerobic digestion of sewage sludge: focus on the influence of the start-up. A review. Crit. Rev. Biotechnol. 33, 448–460. doi: 10.3109/07388551.2012.726962
De la Rubia, M. A., Villamil, J. A., Rodriguez, J. J., Borja, R., and Mohedano, A. F. (2018a). Mesophilic anaerobic co-digestion of the organic fraction of municipal solid waste with the liquid fraction from hydrothermal carbonization of sewage sludge. Waste Manage. doi: 10.1016/j.wasman.2018.02.046. [Epub ahead of print].
De la Rubia, M. A., Villamil, J. A., Rodríguez, J. J., and Mohedano, A. F. (2018b). Effect of inoculum source and initial concentration on the anaerobic digestion of the liquid fraction from hydrothermal carbonisation of sewage sludge. Renew. Energ. 127, 697–704. doi: 10.1016/j.renene.2018.05.002
Donoso-Bravo, A., Pérez-Elvira, S. I., and Fdz-Polanco, F. (2010). Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chem. Eng. J. 160, 607–614. doi: 10.1016/j.cej.2010.03.082
Eibisch, N., Helfrich, M., Don, A., Mikutta, R., Kruse, A., Ellerbrock, R., et al. (2013). Properties and degradability of hydrothermal carbonization products. J. Environ. Qual. 42, 1565–1573. doi: 10.2134/jeq2013.02.0045
Fernández-Rodríguez, J., Pérez, M., and Romero, L. I. (2015). Temperature-phased anaerobic digestion of Industrial Organic Fraction of Municipal Solid Waste: a batch study. Chem. Eng. J. 270, 597–604. doi: 10.1016/j.cej.2015.02.060
Fricke, K., Santen, H., Wallmann, R., Hüttner, A., and Dichtl, N. (2007). Operating problems in anaerobic digestion plants resulting from nitrogen in MSW. Waste Manage. 27, 30–43. doi: 10.1016/j.wasman.2006.03.003
Hagos, K., Zong, J., Li, D., Liu, C., and Lu, X. (2017). Anaerobic co-digestion process for biogas production: progress, challenges and perspectives. Renew. Sustain. Energy Rev. 76, 1485–1496. doi: 10.1016/j.rser.2016.11.184
Hansen, K. H., Angelidaki, I., and Ahring, B. K. (1998). Anaerobic digestion of swine manure: inhibition by ammonia. Water Res. 32, 5–12. doi: 10.1016/S0043-1354(97)00201-7
Jenkins, S. R., Morgan, J. M., and Sawyer, C. L. (1983). Measuring anaerobic sludge digestion and growth by a simple alkalimetric titration. J. Water Pollut. Control Fed. 55, 451–453.
Kambo, H. S., and Dutta, A. (2015). A comparative review of biochar and hydrochar in terms of production, physico–chemical properties and applications. Renew. Sustain. Energy Rev. 45, 359–378. doi: 10.1016/j.rser.2015.01.050
Komilis, D., Barrena, R., Grando, R. L., Vogiatzi, V., Sánchez, A., and Font, X. (2017). A state of the art literature review on anaerobic digestion of food waste: influential operating parameters on methane yield. Rev. Environ. Sci. Biotechnol. 16, 347–360. doi: 10.1007/s11157-017-9428-z
Li, M., Li, W., and Liu, S. (2011). Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr. Res. 346, 999–1004. doi: 10.1016/j.carres.2011.03.020
Martín-González, L., Castro, R., Pereira, M. A., Alves, M. M., Font, X., and Vicent, T. (2011). Thermophilic co-digestion of organic fraction of municipal solid wastes with FOG wastes from a sewage treatment plant: reactor performance and microbial community monitoring. Bioresour. Technol. 102, 4734–4741. doi: 10.1016/j.biortech.2011.01.060
Mata-Alvarez, J., Dosta, J., Romero-Güiza, M. S., Fonoll, X., Peces, M., and Astals, S. (2014). A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 36, 412–427. doi: 10.1016/j.rser.2014.04.039
Pellera, F.-M., and Gidarakos, E. (2016). Effect of substrate to inoculum ratio and inoculum type on the biochemical methane potential of solid agroindustrial waste. J. Environ. Chem. Eng. 4, 3217–3229. doi: 10.1016/j.jece.2016.05.026
Qiao, W., Peng, C., Wang, W., and Zhang, Z. (2011). Biogas production from supernatant of hydrothermally treated municipal sludge by upflow anaerobic sludge blanket reactor. Bioresour. Technol. 102, 9904–9911. doi: 10.1016/j.biortech.2011.08.037
Qiao, W., Takayanagi, K., Niu, Q., Shofie, M., and Li, Y. Y. (2013). Long-term stability of thermophilic co-digestion submerged anaerobic membrane reactor encountering high organic loading rate, persistent propionate and detectable hydrogen in biogas. Bioresour. Technol. 149, 92–102. doi: 10.1016/j.biortech.2013.09.023
Raposo, F., de la Rubia, M. A., Borja, R., and Alaiz, M. (2008). Assessment of a modified and optimised method for determining chemical oxygen demand of solid substrates and solutions with high suspended solid content. Talanta 76, 448–453. doi: 10.1016/j.talanta.2008.03.030
Raposo, F., De La Rubia, M. A., Fernández-Cegrí, V., and Borja, R. (2011a). Anaerobic digestion of solid organic substrates in batch mode: an overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 16, 861–877. doi: 10.1016/j.rser.2011.09.008
Raposo, F., Fernández-Cegrí, V., De la Rubia, M. A., Borja, R., Béline, F., Cavinato, C., et al. (2011b). Biochemical methane potential (BMP) of solid organic substrates: evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 86, 1088–1098. doi: 10.1002/jctb.2622
Sensai, P., Thangamani, A., and Visvanathan, C. (2014). Thermophilic co-digestion feasibility of distillers grains and swine manure: effect of C/N ratio and organic loading rate during high solid anaerobic digestion (HSAD). Environ. Technol. 35, 2569–2574. doi: 10.1080/09593330.2014.913688
Smith, A. M., and Ross, A. B. (2016). Production of bio-coal, bio-methane and fertilizer from seaweed via hydrothermal carbonisation. Algal Res. 16, 1–11. doi: 10.1016/j.algal.2016.02.026
Sosnowski, P., Klepacz-Smolka, A., Kaczorek, K., and Ledakowicz, S. (2008). Kinetic investigations of methane co-fermentation of sewage sludge and organic fraction of municipal solid wastes. Bioresour. Technol. 99, 5731–5737. doi: 10.1016/j.biortech.2007.10.019
Stemann, J., Putschew, A., and Ziegler, F. (2013). Hydrothermal carbonization: process water characterization and effects of water recirculation. Bioresour. Technol. 143, 139–146. doi: 10.1016/j.biortech.2013.05.098
Tekin, K., Karagöz, S., and Bektaş, S. (2014). A review of hydrothermal biomass processing. Renew. Sustain. Energy Rev. 40, 673–687. doi: 10.1016/j.rser.2014.07.216
Villamil, J. A., Mohedano, A. F., Rodriguez, J. J., and de la Rubia, M. A. (2018). Valorisation of the liquid fraction from hydrothermal carbonisation of sewage sludge by anaerobic digestion. J. Chem. Technol. Biotechnol. 93, 450–456. doi: 10.1002/jctb.5375
Wang, K., Yin, J., Shen, D., and Li, N. (2014). Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum : effect of pH. Bioresour. Technol. 161, 395–401. doi: 10.1016/j.biortech.2014.03.088
Wang, Y., Zhang, Y., Wang, J., and Meng, L. (2009). Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenerg. 33, 848–853. doi: 10.1016/j.biombioe.2009.01.007
Wirth, B., Reza, T., and Mumme, J. (2015). Influence of digestion temperature and organic loading rate on the continuous anaerobic treatment of process liquor from hydrothermal carbonization of sewage sludge. Bioresour. Technol. 198, 215–222. doi: 10.1016/j.biortech.2015.09.022
Xiao, L. P., Shi, Z. J., Xu, F., and Sun, R. C. (2012). Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 118, 619–623. doi: 10.1016/j.biortech.2012.05.060
Keywords: biochemical methane potential (BMP), co-digestion, dewatered sewage sludge, hydrothermal carbonization, organic fraction of municipal solid waste (OFMSW)
Citation: Villamil JA, Mohedano AF, Rodríguez JJ, Borja R and De la Rubia MA (2018) Anaerobic Co-digestion of the Organic Fraction of Municipal Solid Waste and the Liquid Fraction From the Hydrothermal Carbonization of Industrial Sewage Sludge Under Thermophilic Conditions. Front. Sustain. Food Syst. 2:17. doi: 10.3389/fsufs.2018.00017
Received: 22 February 2018; Accepted: 02 May 2018;
Published: 23 May 2018.
Edited by:
José Luis Garcia-Morales, University of Cádiz, SpainReviewed by:
Victor Riau, Institut de Recerca i Tecnologia Agroalimentàries (IRTA), SpainCopyright © 2018 Villamil, Mohedano, Rodríguez, Borja and De la Rubia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Angeles de la Rubia, YW5nZWxlcy5kZWxhcnViaWFAdWFtLmVz
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.