- Center for Mind/Brain Sciences, University of Trento, Rovereto, Italy
Various motion cues can lead to the perception of animacy, including (1) simple motion characteristics such as starting to move from rest, (2) motion patterns of interactions like chasing, or (3) the motion of point-lights representing the joints of a moving biological agent. Due to the relevance of dogs in comparative research and considering the large variability within the species, studying animacy perception in dogs can provide unique information about how selection for specific traits and individual-level (social) differences may shape social perception. Despite these advantages, only a few studies have investigated the phenomenon in dogs. In this mini-review, we discuss the current findings about how specific motion dynamics associated with animacy drive dogs' visual attention.
1 Introduction
Attention to animate agents can facilitate to learn about the differences between animate and inanimate objects from birth, and later on it can also help to quickly detect predators, preys or social partners, and predict their future behavior (Scholl and Tremoulet, 2000; Lorenzi and Vallortigara, 2021; Schultz and Frith, 2022). Cues directing attention to such agents can be fairly simple, for example, two blobs on top and one in the bottom in the arrangement of eyes and mouth (face perception, for a recent review, see Kobylkov and Vallortigara, 2024) or the ability to initiate motion without external force (e.g., Premack, 1990; Mascalzoni et al., 2010; Di Giorgio et al., 2017). Some cues are more complex, either involving multiple objects representing a social interaction (e.g., chasing perception, Dittrich and Lea, 1994; Gao and Scholl, 2011; Frankenhuis et al., 2013; Meyerhoff et al., 2014; Atsumi and Nagasaka, 2015; Abdai et al., 2022a) or depicting the motion of a biological agent by point-lights representing the major joints of the body (biological motion, Johansson, 1973) (Figure 1). The phenomenon has been found in various species, including invertebrate species [e.g., human (Di Giorgio et al., 2021); dog (Canis familiaris) (Abdai et al., 2017b), chick (Gallus gallus) (Mascalzoni et al., 2010), common toad (Bufo bufo) (Ewert and Burghagen, 1979), zebrafish (Danio rerio) (Nunes et al., 2020), jumping spiders (Menemerus semilimbatus) (De Agrò et al., 2021)]. However, there are still a number of open questions about the evolutionary background, whether and how social and ecological environment influences animacy perception, and regarding potential changes in the perception (or behavioral response) during development.
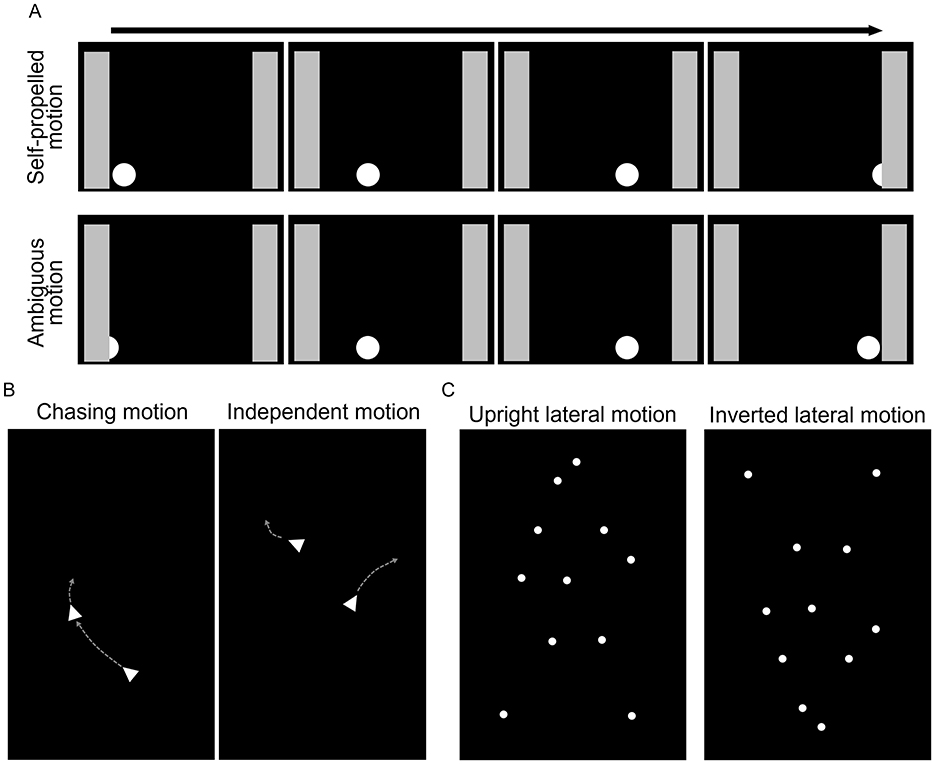
Figure 1. Examples of displays applied to investigate the perception of animate motion. (A) Schematic representation of the continuous motion of the dot. On the top, the onset of the dot's motion is visible and it moves out of view, disappearing behind the gray screen (self-propelled, animate), whereas on the right side the onset is ambiguous as the dot appears from behind the gray screen, and it stops when reaching the gray screen on the other side (ambiguous/inanimate motion). (B) On the left side, one dot is chasing the other, while on the right side they move independently from each other; gray arrows indicate the motion direction. (C) Point-light display of a laterally moving human figure, in an upright position on the left, and inverted position on the right side (figure based on Eatherington et al., 2019).
Investigating animacy perception in dogs is advantageous because there is large variability within the species (e.g., selection for specific behavior traits), broad interindividual differences (e.g., sociability, training for specific tasks), and differences in social/ecological environment (e.g., pet dogs vs. free-ranging dogs) allowing the investigation of the influence of wide range of factors. Dogs are considered as an important model species to understand human social behavior (Miklósi and Topál, 2013). The possibility to compare their behavior to that of wolves with which they share a recent common ancestor but has evolved in a different environment in the past ca. 16–32,000 years (e.g., Kubinyi et al., 2007), or with other pet species (e.g., cats or miniature pigs; e.g., Marino and Colvin, 2015; Pongrácz and Lugosi, 2024) whose evolutionary and ecological histories, as well as the domestication processes differ, further highlights the relevance of dogs in comparative research. From a methodological point of view, it is also important that a wide variety of approaches can be easily applied for the investigation, which can provide us with a more complex overview of the phenomenon.
Although there is a significant interest in animacy perception in humans (Torabian and Grossman, 2023), research in dogs is scarce despite the advantages mentioned above. In the following, we will review the current findings of animacy perception in dogs. As research about static cues of animacy, such as face detection, role of fur and having filled rather than hollow insides (for a review, see Lorenzi and Vallortigara, 2021) is limited in dogs, we focus on dynamic cues, including (1) simple dynamic stimuli, (2) chasing pattern, and (3) biological motion.
Although animacy and agency are strongly related concepts, researchers have hypothesized that they are processed differently and thus should be treated distinctly (e.g., Spelke, 2000; Leslie, 2010). Animacy refers to the presence of some “life-like” features of the object, such as self-propulsion, whereas agency includes the agent having (some level of) control over its action, for example, moving in a goal-directed manner. Thus, in the case of displays of simple motion cues we refer to the acting object as “object,” whereas in the case of chasing and biological motion perception as “object/agent” as it is unclear which aspect of the motion might influence dogs' perception.
2 Main methodological approaches
In dogs, the phenomenon has been investigated by either using the video displays of specific stimuli, or by the live demonstration of motion patterns performed by artificial agents unfamiliar to dogs (Unidentified Moving Object, UMO). Applying video displays not only allows assessing the spontaneous visual interest/preference of subjects with highly controlled and reproducible stimuli, but by measuring pupil size changes further information can be obtained (Völter and Huber, 2022). Pupillometry in humans has been suggested to be a reliable measurement of arousal, attention and cognitive load (for a review, see Mathôt, 2018). Studies show that dogs' pupil size also increases when presented with angry emotional expressions (arousal; Somppi et al., 2017; Karl et al., 2020), and in the case of expectancy violation (Völter et al., 2023), providing a promising basis for animacy perception research.
The above methods give an insight about how specific motion characteristics can drive the attention and trigger the perception of animacy, but they do not provide information about whether and how it influences the subsequent behavior of dogs in relation to the observed object/agent (cf. Don't-Get-Caught task or wolfpack-effect in humans, Gao et al., 2009, 2010). Using robots to present the stimuli facilitates maintaining high control, replicability and reproducibility, and subjects can engage in physical interaction with the performing objects. Applying UMOs, that is, artificial agents capable of self-propelled motion and having an embodiment not resembling any animal species, allows to separate the influence of physical characteristics and motion on subjects' behavior. Flexible changes in the embodiment and motion features of the robot further contributes to the presentation of a wide range of stimuli (Abdai et al., 2018).
3 Perceiving animacy based on motion
3.1 Simple dynamic stimuli
One of the simplest motion cues that triggers animacy perception is self-propelledness, that is, the ability of an object to carry out (changes in) motion without visible external force (e.g., Premack, 1990; Leslie, 2010; Vallortigara, 2012; Schultz and Frith, 2022). These simple stimuli can include different motion characteristics, such as, initiating motion from rest (e.g., Mascalzoni et al., 2010; Di Giorgio et al., 2017), changes in speed (e.g., Rosa-Salva et al., 2016; Di Giorgio et al., 2021), change in the direction of motion (e.g., Tremoulet and Feldman, 2000), aligning the main axis of the bilateral body toward the direction of motion (e.g., Ewert and Burghagen, 1979; Hernik et al., 2014; Rosa-Salva et al., 2018; but see Rosa-Salva et al., 2023), or moving against gravity (Szego and Rutherford, 2008; Bliss et al., 2023).
In Völter and Huber (2022), dogs observed videos of events showing (1) objects being dropped by a human (inanimate) vs. the same event in reverse order, that is, the object initiating its own motion (animate); and (2) variability in the speed of an object (animate) vs. moving with constant speed (inanimate). Although regarding the looking time toward the events, they only found a difference between the animate and inanimate conditions in one instance, in all of the cases dogs' pupil size changed significantly during the presentation of the animate, but not the inanimate motion. Völter and Huber (2022) suggested that changes in pupil dilation in their study reflected an orienting response, balancing between visual sensitivity (dilated pupil) and acuity (constricted pupil) (see also Mathôt, 2018). In another study by Völter and Huber (2021) focusing on contact causality (Michotte, 1963), they further found that dogs' pupil size changed more and was overall larger when there was no contact between the two objects, that is, the second (“launched”) object started to move without a visible external cause. However, dogs looked longer at the “launched” object in the contact, and the “launching” object in the no-contact scenario (i.e., not at the object showing self-propulsion). These findings indicate that although overall looking time may not indicate preference, pupillometry may reveal sensitivity to animate motion cues in dogs.
Applying artificial agents (UMOs) (Abdai et al., 2022b), dogs were presented with the animate motion of a UMO including start-from-rest, visible acceleration and deceleration, and sharp change in direction; and with inanimate (ambiguous) motion, having the same dynamics of motion, but the key elements (e.g., moment of speed change) being occluded from the dogs. Subjects showed more interest toward the UMO that displayed animate motion, regarding both their looking behavior and physical contact with the UMO. Thus, it seems that simple visual cues lead dogs' attention to an object having animate motion characteristics, and facilitating dogs to enter into an interaction with these objects/agents.
3.2 Chasing perception
Simple motion dynamics provide a foundation for detecting animacy, but using more complex patterns may offer additional insights into the perception of animate entities. Chasing is an ecologically relevant pattern for many species, either in the context of predation (for both the predator and prey) or in social interaction (e.g., play). Several characteristics of the motion pattern can elicit the perception of the objects as animate, and parameters of the pattern are easy to manipulate, allowing to investigate the influence of different characteristics on the perception (e.g., Nahin, 2007; Scholl and Gao, 2013).
In a series of studies, Abdai and colleagues investigated chasing perception in dogs, by assessing dogs' looking duration toward geometric shapes displaying chasing pattern (dependent motion) vs. moving independently from each other, presented simultaneously on two sides of a screen. Both when using dots (Abdai et al., 2017b) and isosceles triangles (aligning their main axis with their motion direction) (Abdai et al., 2021) as moving shapes, dogs turned their visual attention to the independently moving figures shortly after the presentation started. Similar results were found in adult humans (Rochat et al., 1997; Abdai et al., 2017b, 2021) and 5-month-old human infants (Rochat et al., 1997). Such looking preference was suggested to be the result of the rapid perception of the chasing motion, which quickly led observers' attention to the independent motion, that is, the “unrecognized” pattern (for similar explanations in animacy perception, see Rochat et al., 1997; Kovács et al., 2016).
One interesting aspect of studying dogs' behavior lies in the large variability within the species. Selection for specific traits resulted in marked differences between breeds, including social behavior (e.g., Gácsi et al., 2009) and vision (e.g., distribution of ganglion cells in the retina and the visual field including; Peichl, 1992; McGreevy et al., 2004). When comparing chasing perception in hunting dogs (selected for chasing vs. retrieving), no overall difference was found between the two groups of dogs regarding their looking preference (Abdai and Miklósi, 2022). Thus, the basic mechanisms of animacy perception seem to be independent of the changes introduced by artificial selection in dogs.
Dogs were also presented with the live demonstration of chasing and independent motion patterns using UMOs (Abdai et al., 2017a). Following the observation of the UMOs' motion, subjects approached sooner the UMO that participated in the chasing interaction, also touching and grabbing sooner a ball attached to these UMOs after the demonstration. Thus, it seems that dogs were more likely to consider the UMOs from the chasing as potential interactive partners.
Although the above results indicate that dogs discriminate between a chasing and an independent motion pattern, it is unclear whether they indeed recognize the motion as chasing or reacted to another aspect of the motion (e.g., predictability, Lemaire et al., 2022). Although our findings showed that selection for specific behavior traits did not influence dogs' perception of the chasing motion (Abdai and Miklósi, 2022), between species comparisons may reveal how evolutionary background of the species or their socio-ecological environment influences their perception of the chasing motion. For example, predator and prey species may react differently or their perception is influenced by different motion characteristics. Also, within predator species, solitary vs. group hunting may influence perception of the moving object. For example, when using the video display of the chasing vs. independent stimuli, we previously found that although cats (Felis catus) also discriminate between the two patterns, they react differently than dogs (Abdai et al., 2022a). However, more information would be needed to reveal whether different behavior in cats was driven by differences in the perception of the pattern or other aspect of the stimuli or the method influenced their visual preference.
3.3 Biological motion
Applying chasing pattern facilitates the investigation of perceiving the interaction of multiple objects (dependency in the motion dynamics of two or more objects), but in animacy research it is also important whether stylized depiction of an animal's body can lead to its perception as biological motion and what information can be obtained from it. Despite the interest in the perception of biological motion in humans (for a recent review, see Troje and Chang, 2023), to date only five studies have investigated the phenomenon in dogs. In these studies, researchers presented the point-light displays (Johansson, 1973) of human or dog figures, that is, their regular social partners.
Eatherington et al. (2019) found that dogs preferentially looked at the motion of an upright dog figure compared to its inverted display, regardless of whether the point-lights representing the joints moved coherently or were scrambled. However, dogs did not react when the figure was a laterally moving human. The results of Delanoeije et al. (2020) were similar when presenting lateral motion of the human point-light figure, but applying a frontal moving human vs. an inverted-and-scrambled or just scrambled version of it, dogs preferred to look at the upright, coherent human motion. Thus, it seems that not only moving in accordance with gravity, but the global form of the figure is also important. Dogs reacting to the frontally but not to the laterally moving human figure indicates that spatial arrangement of the motion may be important for the perception. However, it cannot be excluded that their looking preference is not influenced by the (lack of) perception of the human figure but rather lateral motion is irrelevant from the viewpoint of interaction, resulting in lower visual interest (see also Ishikawa et al., 2018; Delanoeije et al., 2020).
Indeed, the results of Ishikawa et al. (2018) show that social relevance of the moving figure might influence dogs' perception of biological motion, or at least the behavioral response to the display. Frontal motion of a socially relevant agent (dog or human in this case) can be perceived as an initiation of interaction which can be positive for a highly sociable dog whereas taken negatively by a less sociable one. On the other hand, lateral motion can be of less interest if one seeks for social encounters, but may provide safer observation for an individual that prefers to avoid social interactions (Ishikawa et al., 2018). Their results were in line with this assumption, that is, dogs that scored higher on sociability toward humans looked less at the laterally moving human figure than those scoring lower. Dogs rated as highly social with other dogs also preferred to look at the frontal compared to lateral upright display of a dog, whereas those scoring low on sociability toward dogs showed the opposite looking preference (Ishikawa et al., 2018).
Ishikawa et al. (2018) relied on the general sociability of the dogs to see how it influences their perception of, and reaction to biological motion. Kovács et al. (2016) applied a different approach, in which they intranasally administered oxytocin to dogs (or placebo) that has been shown to increase social behavior toward other dogs and humans (e.g., Romero et al., 2014; Oliva et al., 2015). Oxytocin was found to increase sensitivity to biological motion in adult humans (Kéri and Benedek, 2009), but the results of Kovács et al. (2016) showed that although after receiving placebo, dogs looked longer at the biological than at the non-biological (inverted-and-scrambled) motion, this preference disappeared when they received oxytocin. Authors proposed that increased oxytocin might indeed facilitate the recognition of the biological motion in their subjects, but instead of focusing on this display, they rather directed their visual attention to the “unrecognized” stimulus (for similar explanation in chasing perception, see Abdai et al., 2017b).
Humans can obtain many information from point-light displays of a human figure, such as the gender of the figure (Mather and Murdoch, 1994), the action it performs (Manera et al., 2010), or the emotional state (Parkinson et al., 2017). Although dogs can find a hidden reward based on the pointing gesture of a human displayed on a screen (Péter et al., 2013; Eatherington et al., 2021), they did not follow the pointing when it was performed by a silhouette or a point-light display of a human (Eatherington et al., 2021).
Eatherington et al. (2021) suggested that dogs may react to the biological motion itself and do not recognize the displays as representing a human (or a dog), and based on the current findings, biological motion perception is not analogous in dogs and humans. However, aspects of the stimuli presentation beside animate motion might influence dogs' looking behavior (see below).
4 Conclusion and future directions
Although investigating animacy perception relying on looking preferences is a common approach in dogs (and humans), several factors other than animacy perception per se may influence dogs' looking behavior, either leading to the lack of, or opposite as (generally) expected preference (Kovács et al., 2016; Abdai et al., 2017b; Ishikawa et al., 2018). Dogs may be less motivated to watch two-dimensional displays on a screen as it is an artificial context for them, and it is also difficult to take into account all aspects specific to dogs' vision (e.g., differences in the visual field). As the results of Abdai and Miklósi (2022) suggests, differences in looking preference of dogs and humans may be influenced not by animacy perception, but rather by basic differences in the visual characteristics of the two species (e.g., dogs having slower and bigger saccades, and longer fixations than humans; see also Park et al., 2020). Also, the specific stimuli may be irrelevant for dogs (e.g., Ishikawa et al., 2018), or interest is influenced by another feature of the display (e.g., unfamiliarity) (Kovács et al., 2016; Abdai et al., 2017b). These can result in drawing false conclusions about the perceptual abilities of dogs. Relying on measurements other than looking preference, such as, changes in pupil dilation (e.g., Völter and Huber, 2021, 2022) can provide important insight about perception in dogs. Further, showing actions that are relevant for dogs or potentially leading to an interaction may also facilitate research on the topic (Abdai et al., 2017a, 2022b; Eatherington et al., 2021).
Research indicates that (1) simple motion cues associated with animacy influences dogs' perception of these objects/agents, (2) they rely on similar kinematic characteristics as other species, and (3) perception of an object as animate may provide a basis for dogs to establish further interaction with the agent. Still, we know little about, for example, (1) which cues may elicit such rich, spontaneous social perception, (2) how different animate cues may influence dogs' behavior toward an object/agent, (3) whether sensitivity to specific cues changes during development, (4) whether perceiving an object as animate leads to further expectations about its behavior (e.g., goal-directed motion; Biro and Leslie, 2007), and (5) about the neural mechanisms. Recent brain imaging studies in dogs investigated face- and/or body-sensitive (Bunford et al., 2020; Boch et al., 2023) brain areas, and neural representation of animate stimuli (human, dog, and/or cat pictures) vs. inanimate stimuli (Boch et al., 2023; Farkas et al., 2024); however, there is no information about the neural mechanisms underlying animacy perception (e.g., chasing or biological motion perception) in dogs. Applying neuroimaging and electrophysiological measures could provide meaningful contribution to comparative research on perceptual animacy.
Current data suggest that cats show preference to a UMO previously moving in an animate manner (Abdai et al., 2022b), they discriminate between chasing and independently moving motion patterns (although react differently than dogs in the same context) (Abdai et al., 2022a), and they prefer biological over non-biological motion (Blake, 1993). However, it is unclear which motion characteristics influence their perception, and whether and how different evolutionary and ecological background of cats and dogs might contribute to differences in their visual preference (see Abdai et al., 2022a). Comparison of dogs with other species (e.g., wolves or cats), and taking the large variability within the species, dogs may become important in testing the effect of a wide range of factors on animacy perception, including for example, selection for specific behavior traits (Abdai and Miklósi, 2022), individual differences (e.g., Ishikawa et al., 2018), or anatomy (see e.g., McGreevy et al., 2004; Bognár et al., 2018). Testing dogs also provide a unique opportunity to study how training for specific behaviors (e.g., hunting or herding), or different environment (e.g., pet vs. free-ranging dogs) may influence the perception. Future research in dogs may provide further insight about the evolutionary background and potential influence of environment on the perception of animacy or its influence on behavior.
Author contributions
JA: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. JA was supported by the MSCA-PF Seal-of-Excellence by University of Trento (DM 737 AZIONE B).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdai, J., Baño Terencio, C., and Miklósi, Á. (2017a). Novel approach to study the perception of animacy in dogs. PLoS ONE 12:e0177010. doi: 10.1371/journal.pone.0177010
Abdai, J., Ferdinandy, B., Baño Terencio, C., Pogány, Á., and Miklósi, Á. (2017b). Perception of animacy in dogs and humans. Biol. Lett. 13:20170156. doi: 10.1098/rsbl.2017.0156
Abdai, J., Ferdinandy, B., Lengyel, A., and Miklósi, Á. (2021). Animacy perception in dogs (Canis familiaris) and humans (Homo sapiens): comparison may be perturbed by inherent differences in looking patterns. J. Comp. Psychol. 135, 82–88. doi: 10.1037/com0000250
Abdai, J., Korcsok, B., Korondi, P., and Miklósi, Á. (2018). Methodological challenges of the use of robots in ethological research. Anim. Behav. Cogn. 5, 326–340. doi: 10.26451/abc.05.04.02.2018
Abdai, J., and Miklósi, Á. (2022). Selection for specific behavioural traits does not influence preference of chasing motion and visual strategy in dogs. Sci. Rep. 12:2370. doi: 10.1038/s41598-022-06382-6
Abdai, J., Uccheddu, S., Gácsi, M., and Miklósi, Á. (2022a). Chasing perception in domestic cats and dogs. Anim. Cogn. 25, 1589–1597. doi: 10.1007/s10071-022-01643-3
Abdai, J., Uccheddu, S., Gácsi, M., and Miklósi, Á. (2022b). Exploring the advantages of using artificial agents to investigate animacy perception in cats and dogs. Bioinspir. Biomim. 17:065009. doi: 10.1088/1748-3190/ac93d9
Atsumi, T., and Nagasaka, Y. (2015). Perception of chasing in squirrel monkeys (Saimiri sciureus). Anim. Cogn. 18, 1243–1253. doi: 10.1007/s10071-015-0893-x
Biro, S., and Leslie, A. M. (2007). Infants' perception of goal-directed actions: development through cue-based bootstrapgpin. Dev. Sci. 10, 379–398. doi: 10.1111/j.1467-7687.2006.00544.x
Blake, R. (1993). Cats perceive biological motion. Psychol. Sci. 4, 54–57. doi: 10.1111/j.1467-9280.1993.tb00557.x
Bliss, L., Vasas, V., Freeland, L., Roach, R., Ferrè, E. R., and Versace, E. (2023). A spontaneous gravity prior: newborn chicks prefer stimuli that move against gravity. Biol. Lett. 19:20220502. doi: 10.1098/rsbl.2022.0502
Boch, M., Wagner, I. C., Karl, S., Huber, L., and Lamm, C. (2023). Functionally analogous body- and animacy-responsive areas are present in the dog (Canis familiaris) and human occipito-temporal lobe. Commun. Biol. 6:645. doi: 10.1038/s42003-023-05014-7
Bognár, Z., Iotchev, I. B., and Kubinyi, E. (2018). Sex, skull length, breed, and age predict how dogs look at faces of humans and conspecifics. Anim. Cogn. 21, 447–666. doi: 10.1007/s10071-018-1180-4
Bunford, N., Hernández-Pérez, R., Farkas, E. B., Cuaya, L. V., Szabó, D., Szabó, Á. G., et al. (2020). Comparative brain imaging reveals analogous and divergent patterns of species and face sensitivity in humans and dogs. J. Neurosci. 40, 8396–8408. doi: 10.1523/JNEUROSCI.2800-19.2020
De Agrò, M., Rößler, D. C., Kim, K., and Shamble, P. S. (2021). Perception of biological motion by jumping spiders. PLoS Biol. 19:e3001172. doi: 10.1371/journal.pbio.3001172
Delanoeije, J., Gerencsér, L., and Miklósi, Á. (2020). Do dogs mind the dots? Investigating domestic dogs' (Canis familiaris) preferential looking at human-shaped point-light figures. Ethology 126, 637–650. doi: 10.1111/eth.13016
Di Giorgio, E., Lunghi, M., Simion, F., and Vallortigara, G. (2017). Visual cues of motion that trigger animacy perception at birth: the case of self-propulsion. Dev. Sci. 20, 1–12. doi: 10.1111/desc.12394
Di Giorgio, E., Lunghi, M., Vallortigara, G., and Simion, F. (2021). Newborns' sensitivity to speed changes as a building block for animacy perception. Sci. Rep. 11:542. doi: 10.1038/s41598-020-79451-3
Dittrich, W. H., and Lea, S. E. (1994). Visual perception of intentional motion. Perception 23, 253–268. doi: 10.1068/p230253
Eatherington, C. J., Marinelli, L., Lõoke, M., Battaglini, L., and Mongillo, P. (2019). Local dot motion, not global configuration, determines dogs' preference for point-light displays. Animals 9:661. doi: 10.3390/ani9090661
Eatherington, C. J., Mongillo, P., Lõoke, M., and Marinelli, L. (2021). Dogs fail to recognize a human pointing gesture in two-dimensional depictions of motion cues. Behav. Processes 189:104425. doi: 10.1016/j.beproc.2021.104425
Ewert, J. P., and Burghagen, H. (1979). Configurational prey selection by Bufo, Alytes, Bombina and Hyla. Brain. Behav. Evol. 16, 157–175. doi: 10.1159/000121834
Farkas, E. B., Hernández-Pérez, R., Cuaya, L. V., Rojas-Hortelano, E., Gácsi, M., and Andics, A. (2024). Comparative fMRI reveals differences in the functional organization of the visual cortex for animacy perception in dogs and humans. bioRxiv. doi: 10.1101/2024.11.12.623268
Frankenhuis, W. E., House, B., Barrett, H. C., and Johnson, S. P. (2013). Infants' perception of chasing. Cognition 126, 224–233. doi: 10.1016/j.cognition.2012.10.001
Gácsi, M., McGreevy, P., Kara, E., and Miklósi, Á. (2009). Effects of selection for cooperation and attention in dogs. Behav. Brain Funct. 5:31. doi: 10.1186/1744-9081-5-31
Gao, T., McCarthy, G., and Scholl, B. J. (2010). The wolfpack effect: perception of animacy irresistibly influences interactive behavior. Psychol. Sci. 21, 1845–1853. doi: 10.1177/0956797610388814
Gao, T., Newman, G. E., and Scholl, B. J. (2009). The psychophysics of chasing: a case study in the perception of animacy. Cogn. Psychol. 59, 154–179. doi: 10.1016/j.cogpsych.2009.03.001
Gao, T., and Scholl, B. J. (2011). Chasing vs. stalking: interrupting the perception of animacy. J. Exp. Psychol. Hum. Percept. Perform. 37, 669–684. doi: 10.1037/a0020735
Hernik, M., Fearon, P., and Csibra, G. (2014). Action anticipation in human infants reveals assumptions about anteroposterior body-structure and action. Proc. R. Soc. B: Biol. Sci. 281:20133205. doi: 10.1098/rspb.2013.3205
Ishikawa, Y., Mills, D., Willmott, A., Mullineaux, D., and Guo, K. (2018). Sociability modifies dogs' sensitivity to biological motion of different social relevance. Anim. Cogn. 21, 245–252. doi: 10.1007/s10071-018-1160-8
Johansson, G. (1973). Visual perception of biological motion and a model for its analysis. Percept. Psychophys. 14, 201–211. doi: 10.3758/BF03212378
Karl, S., Boch, M., Zamansky, A., van der Linden, D., Wagner, I. C., Völter, C. J., et al. (2020). Exploring the dog–human relationship by combining fMRI, eye-tracking and behavioural measures. Sci. Rep. 10:22273. doi: 10.1038/s41598-020-79247-5
Kéri, S., and Benedek, G. (2009). Oxytocin enhances the perception of biological motion in humans. Cogn. Affect. Behav. Neurosci. 9, 237–241. doi: 10.3758/CABN.9.3.237
Kobylkov, D., and Vallortigara, G. (2024). Face detection mechanisms: nature vs. nurture. Front. Neurosci. 18:1404174. doi: 10.3389/fnins.2024.1404174
Kovács, K., Kis, A., Kanizsár, O., Hernádi, A., Gácsi, M., and Topál, J. (2016). The effect of oxytocin on biological motion perception in dogs (Canis familiaris). Anim. Cogn. 19, 513–522. doi: 10.1007/s10071-015-0951-4
Kubinyi, E., Virányi, Z., and Miklósi, Á. (2007). Comparative social cognition: from wolf and dog to humans. Comp. Cogn. Behav. Rev. 2, 26–46. doi: 10.3819/ccbr.2008.20002
Lemaire, B. S., Rosa-Salva, O., Fraja, M., Lorenzi, E., and Vallortigara, G. (2022). Spontaneous preference for unpredictability in the temporal contingencies between agents' motion in naive domestic chicks. Proc. R. Soc. B: Biol. Sci. 289:20221622. doi: 10.1098/rspb.2022.1622
Leslie, A. M. (2010). “ToMM, ToBY, and agency: core architecture and domain specificity,” in Mapping the Mind: Domain Specificity in Cognition and Culture, eds. L. A. Hirschfeld and S. A. Gelman (Cambridge: Cambridge University Press), 119–148. doi: 10.1017/cbo9780511752902.006
Lorenzi, E., and Vallortigara, G. (2021). “Evolutionary and neural bases of the sense of animacy,” in The Cambridge Handbook of Animal Cognition, eds. A. B. Kaufman, J. Call, and J. C. Kaufman (Cambridge: Cambridge University Press), 295–321. doi: 10.1017/9781108564113.017
Manera, V., Schouten, B., Becchio, C., Bara, B. G., and Verfaillie, K. (2010). Inferring intentions from biological motion: a stimulus set of point-light communicative interactions. Behav. Res. Methods 42, 168–178. doi: 10.3758/BRM.42.1.168
Marino, L., and Colvin, C. M. (2015). Thinking pigs: A comparative review of cognition, emotion, and personality in Sus domesticus. Int. J. Comp. Psychol. 28:23859. doi: 10.46867/ijcp.2015.28.00.04
Mascalzoni, E., Regolin, L., and Vallortigara, G. (2010). Innate sensitivity for self-propelled causal agency in newly hatched chicks. Proc. Natl. Acad. Sci. 107, 4483–4485. doi: 10.1073/pnas.0908792107
Mather, G., and Murdoch, L. (1994). Gender discrimination in biological motion displays based on dynamic cues. Proc. R. Soc. B. Biol. Sci. 258, 273–279. doi: 10.1098/rspb.1994.0173
Mathôt, S. (2018). Pupillometry: psychology, physiology, and function. J. Cogn. 1:16. doi: 10.5334/joc.18
McGreevy, P., Grassi, T. D., and Harman, A. M. (2004). A strong correlation exists between the distribution of retinal ganglion cells and nose length in the dog. Brain. Behav. Evol. 63, 13–22. doi: 10.1159/000073756
Meyerhoff, H. S., Schwan, S., and Huff, M. (2014). Perceptual animacy: visual search for chasing objects among distractors. J. Exp. Psychol. Hum. Percept. Perform. 40, 702–717. doi: 10.1037/a0034846
Miklósi, Á., and Topál, J. (2013). What does it take to become “best friends”? Evolutionary changes in canine social competence. Trends Cogn. Sci. 17, 287–294. doi: 10.1016/j.tics.2013.04.005
Nahin, P. J. (2007). Chases and Escapes: The Mathematics of Pursuit and Evasion. Princeton, NJ: Princeton University Press.
Nunes, A. R., Carreira, L., Anbalagan, S., Blechman, J., Levkowitz, G., and Oliveira, R. F. (2020). Perceptual mechanisms of social affiliation in zebrafish. Sci. Rep. 10:3642. doi: 10.1038/s41598-020-60154-8
Oliva, J. L., Rault, J. L., Appleton, B., and Lill, A. (2015). Oxytocin enhances the appropriate use of human social cues by the domestic dog (Canis familiaris) in an object choice task. Anim. Cogn. 18, 767–775. doi: 10.1007/s10071-015-0843-7
Park, S. Y., Bacelar, C. E., and Holmqvist, K. (2020). Dog eye movements are slower than human eye movements. J. Eye Mov. Res. 12:4. doi: 10.16910/jemr.12.8.4
Parkinson, C., Walker, T. T., Memmi, S., and Wheatley, T. (2017). Emotions are understood from biological motion across remote cultures. Emotion 17, 459–477. doi: 10.1037/emo0000194.supp
Peichl, L. (1992). Topography of ganglion-cells in the dog and wolf retina. J. Comp. Neurol. 324, 603–620. doi: 10.1002/cne.903240412
Péter, A., Miklósi, Á., and Pongrácz, P. (2013). Domestic dogs' (Canis familiaris) understanding of projected video images of a human demonstrator in an object-choice task. Ethology 119, 898–906. doi: 10.1111/eth.12131
Pongrácz, P., and Lugosi, C. A. (2024). Predator for hire: the curious case of man's best independent friend, the cat. Appl. Anim. Behav. Sci. 271:106168. doi: 10.1016/j.applanim.2024.106168
Premack, D. (1990). The infant's theory of self-propelled objects. Cognition 36, 1–16. doi: 10.1016/0010-0277(90)90051-K
Rochat, P., Morgan, R., and Carpenter, M. (1997). Younf infants' sensitivity to movement information specifying social causality. Cogn Dev 12, 537–561. doi: 10.1016/S0885-2014(97)90022-8
Romero, T., Nagasawa, M., Mogi, K., Hasegawa, T., and Kikusui, T. (2014). Oxytocin promotes social bonding in dogs. Proc. Natl. Acad. Sci. USA 111, 9085–9090. doi: 10.1073/pnas.1322868111
Rosa-Salva, O., Grassi, M., Lorenzi, E., Regolin, L., and Vallortigara, G. (2016). Spontaneous preference for visual cues of animacy in naïve domestic chicks: the case of speed changes. Cognition 157, 49–60. doi: 10.1016/j.cognition.2016.08.014
Rosa-Salva, O., Hernik, M., Broseghini, A., and Vallortigara, G. (2018). Visually-naïve chicks prefer agents that move as if constrained by a bilateral body-plan. Cognition 173, 106–114. doi: 10.1016/j.cognition.2018.01.004
Rosa-Salva, O., Hernik, M., Fabbroni, M., Lorenzi, E., and Vallortigara, G. (2023). Naïve chicks do not prefer objects with stable body orientation, though they may prefer behavioural variability. Anim. Cogn. 26, 1177–1189. doi: 10.1007/s10071-023-01764-3
Scholl, B. J., and Gao, T. (2013). “Perceiving animacy and intentionality: Visual processing or higher-level judgment,” in Social Perception: Detection and Interpretation of Animacy, Agency, and Intention, eds. M. D. Rutherford and V. A. Kuhlmeier (Cambridge, MA: MIT Press), 197–229.
Scholl, B. J., and Tremoulet, P. D. (2000). Perceptual causality and animacy. Trends Cogn. Sci. 4, 299–309. doi: 10.1016/S1364-6613(00)01506-0
Schultz, J., and Frith, C. D. (2022). Animacy and the prediction of behaviour. Neurosci. Biobehav. Rev. 140:104766. doi: 10.1016/j.neubiorev.2022.104766
Somppi, S., Törnqvist, H., Topál, J., Koskela, A., Hänninen, L., Krause, C. M., et al. (2017). Nasal oxytocin treatment biases dogs' visual attention and emotional response toward positive human facial expressions. Front. Psychol. 8:1854. doi: 10.3389/fpsyg.2017.01854
Szego, P. A., and Rutherford, M. D. (2008). Dissociating the perception of speed and the perception of animacy: a functional approach. Evol. Hum. Behav. 29, 335–342. doi: 10.1016/j.evolhumbehav.2008.04.002
Torabian, S., and Grossman, E. D. (2023). When shapes are more than shapes: perceptual, developmental, and neurophysiological basis for attributions of animacy and theory of mind. Front. Psychol. 14:1168739. doi: 10.3389/fpsyg.2023.1168739
Tremoulet, P. D., and Feldman, J. (2000). Perception of animacy from the motion of a single object. Perception 29, 943–951. doi: 10.1068/p3101
Troje, N. F., and Chang, D. H. F. (2023). Life detection from biological motion. Curr. Dir. Psychol. Sci. 32, 26–32. doi: 10.1177/09637214221128252
Vallortigara, G. (2012). “Aristotle and the chicken: animacy and the origins of beliefs,” in The Theory of Evolution and its Impact, ed. A. Fasolo (New York, NY: Springer), 189–200. doi: 10.1007/978-88-470-1974-4
Völter, C. J., and Huber, L. (2021). Dogs' looking times and pupil dilation response reveal expectations about contact causality. Biol. Lett. 17:20210465. doi: 10.1098/rsbl.2021.0465
Völter, C. J., and Huber, L. (2022). Pupil size changes reveal dogs' sensitivity to motion cues. iScience 25:104801. doi: 10.1016/j.isci.2022.104801
Keywords: dog, animacy perception, biological motion, agency, animal-robot interaction, chasing
Citation: Abdai J (2025) Perception of animate motion in dogs. Front. Psychol. 15:1522489. doi: 10.3389/fpsyg.2024.1522489
Received: 04 November 2024; Accepted: 10 December 2024;
Published: 03 January 2025.
Edited by:
Marina A. Pavlova, University Hospital Tübingen, GermanyReviewed by:
Jan Van den Stock, KU Leuven, BelgiumCopyright © 2025 Abdai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Judit Abdai, anVkaXQuYWJkYWlAdW5pdG4uaXQ=
 Judit Abdai
Judit Abdai