
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 19 March 2025
Sec. Translational Pharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1567544
This article is part of the Research Topic Emerging Horizons of Metformin: Exploring Recent Advances and Addressing Challenges in Research and Clinical Utilization View all 7 articles
Background: Osteoarthritis (OA) and impaired glucose tolerance (IGT) frequently coexist, leading to compounded clinical and metabolic challenges. This study investigates the effects of metformin in improving both clinical outcomes (pain, stiffness, physical function) and metabolic parameters (inflammatory markers, lipid profile, BMI) in patients with knee OA and IGT.
Methods: The study included 60 patients diagnosed with knee OA and IGT. Participants were divided into two groups: 26 patients received standard OA treatment without metformin (Without Metf), while 34 received metformin (500 mg twice daily) for 3 months, in addition to standard treatment (With Metf). Clinical assessments (WOMAC, Lequesne Algofunctional Index, KOOS, VAS) and metabolic markers (CRP, NLR, SOD, lipid profile, BMI) were measured before treatment, after 1 month, and after 3 months.
Results: The With Metf group showed significantly greater improvements in pain, stiffness, physical function, and quality of life compared to the Without Metf group. Metformin also led to significant reductions in inflammatory markers and improvements in lipid profiles and metabolic health indicators. The With Metf group demonstrated enhanced BMI, waist-to-hip ratio, and waist-to-height ratio. Furthermore, the need for increased NSAID doses was predicted by factors such as pain severity and inflammatory markers.
Conclusion: Metformin effectively alleviates osteoarthritis symptoms and improves metabolic health in patients with both OA and IGT. Further research is needed to explore its long-term effects on joint health, inflammatory markers, and its potential role in OA management in patients without IGT.
The investigation of pleiotropic drugs in osteoarthritis (OA) is focused on identifying agents that simultaneously reduce symptoms and target underlying pathogenic mechanisms, potentially slowing disease progression (Fazio et al., 2024; Coppola et al., 2024; Halabitska et al., 2024a). Metformin, a first-line pharmacological agent widely used for managing type 2 diabetes mellitus (T2DM), has garnered considerable attention for its effects beyond glycemic control (Baker et al., 2021; Bailey, 2024). As an insulin-sensitizing agent, metformin is also frequently prescribed for individuals with impaired glucose tolerance (IGT), a prediabetic condition characterized by disrupted glucose homeostasis, systemic inflammation, and metabolic dysfunction (Ping et al., 2024; Hostalek et al., 2015; Rojas and Gomes, 2013). While its primary therapeutic role is in improving insulin sensitivity and reducing hepatic gluconeogenesis, growing evidence suggests that metformin exerts pleiotropic effects, including anti-inflammatory, antioxidant, and metabolic regulatory properties (Dutta et al., 2023; Apostolova et al., 2020; Drzewoski and Hanefeld, 2021). These attributes position metformin as a promising candidate for addressing a range of metabolic and degenerative disorders (Rotermund et al., 2018; Isop et al., 2023; Petrie, 2024).
OA, a chronic and progressive degenerative joint disease, has traditionally been associated with mechanical factors such as joint overload and injury (He et al., 2020; Felson, 2013; Hall et al., 2016; Halabitska and Babinets, 2021). OA is one of the most prevalent musculoskeletal disorders worldwide (Allen et al., 2022; Global, 2023). IGT affects a significant portion of the population, particularly in older adults (Kalyani and Egan, 2013; Fang et al., 2019; Hermans et al., 2005). Their comorbidity is common and poses challenges due to overlapping inflammatory and metabolic pathways (Aziz et al., 2024; Berenbaum and Walker, 2020). However, emerging evidence highlights the critical role of metabolic and inflammatory mechanisms in its pathogenesis, particularly in individuals with metabolic comorbidities such as obesity, T2DM, and IGT (Chandrasekaran and Weiskirchen, 2024; Rohm et al., 2022; Ruze et al., 2023; Redkva et al., 2021; Zemlyak et al., 2023). The comorbidity of osteoarthritis and obesity highlights a complex interplay of systemic inflammation and metabolic disturbances, exacerbating the progression of both conditions (Halabitska et al., 2021; Nedunchezhiyan et al., 2022; Halabitska et al., 2024b). These comorbidities create a vicious cycle (Li B. et al., 2024; Swain et al., 2022; Repchuk et al., 2021). This interaction not only accelerates joint degeneration but also contributes to chronic low-grade inflammation, insulin resistance, and impaired overall metabolic homeostasis (Halabitska et al., 2024c; Vinuesa et al., 2021; Roberts et al., 2013). OA is characterized by cartilage degradation, subchondral bone remodeling, and synovial inflammation, processes that are exacerbated by systemic metabolic dysfunction (He et al., 2020; De Roover et al., 2023; Halabitska et al., 2024d). In this context, the metabolic and anti-inflammatory actions of metformin may have a dual benefit: addressing systemic metabolic derangements and modulating local joint pathology (Foretz et al., 2023; Domingo et al., 2024; He, 2020).
Preliminary studies have demonstrated that metformin can mitigate key mechanisms underlying OA progression (Xu et al., 2024; Anis et al., 2012; Li et al., 2020; Lambova, 2023). These include reductions in systemic inflammation and oxidative stress, improvements in lipid metabolism, and direct modulation of chondrocyte function (Adam et al., 2024; Horváth et al., 2023; Su et al., 2022). Moreover, metformin has been shown to enhance the synthesis of extracellular matrix components, promoting cartilage repair and potentially slowing the degenerative processes associated with OA (Feng et al., 2020; Yao et al., 2023; Zheng et al., 2021; Song et al., 2022). Despite these promising findings, the clinical application of metformin in OA remains underexplored, and robust evidence from longitudinal studies and clinical trials is necessary to validate its therapeutic potential.
This article aims to explore the potential role of metformin in managing OA in patients with IGT. By addressing both systemic and local pathological mechanisms, metformin may offer a novel therapeutic approach for patients with these comorbid conditions. Further elucidation of its disease-modifying properties could pave the way for integrating metformin into broader treatment paradigms for OA, particularly in the context of metabolic health optimization.
The study included 60 patients diagnosed with knee OA and IGT. Inclusion criteria required participants to have a confirmed diagnosis of OA based on the American College of Rheumatology (ACR), EULAR, and National Institute for Health clinical and radiographic criteria (Peat et al., 2006; Wang et al., 2024), which include the presence of pain, stiffness, or functional limitation in at least one joint, along with radiographic evidence of joint space narrowing, osteophytes, and subchondral sclerosis. Additionally, participants had to meet the criteria for IGT, defined by a fasting blood glucose level between 5.6 and 6.9 mmol/L (100–125 mg/dL) or a 2-h postprandial glucose level between 7.8 and 11.0 mmol/L (140–199 mg/dL), in accordance with the World Health Organization (WHO) criteria (Bergman et al., 2024).
Exclusion criteria included the presence of other metabolic or systemic conditions that could confound the results, such as uncontrolled diabetes mellitus, cardiovascular disease, or inflammatory rheumatic diseases. Patients with a history of joint surgery, joint replacement, or other significant comorbidities such as malignancies were also excluded from the study.
The study was conducted in accordance with the core principles outlined in the Council of Europe’s Convention on Human Rights and Biomedicine, as well as the ethical guidelines set forth in the World Medical Association’s Declaration of Helsinki on medical research involving human subjects, including its subsequent revisions (World Medical Association Declaration of Helsinki, 2014). Additionally, the research adhered to the regulations specified in Ministry of Health of Ukraine Order No. 690, dated 23 September 2009. All participants provided written informed consent before their participation. Ethical approval for the study was granted by the Bioethics Committee of I. Horbachevsky Ternopil National Medical University, Ministry of Health of Ukraine (Protocol No. 78, 18 August 2024).
The study cohort was divided into two groups: 26 patients in the Without Metf group and 34 patients in the With Metf group, with matched characteristics in terms of age, gender, severity, and disease progression of osteoarthritis (Table 1). The Without Metf group received OA treatment according to the established protocol, along with recommendations for improving glucose tolerance, including dietary modifications (e.g., reducing carbohydrate intake, increasing dietary fiber, and promoting a balanced nutritional regimen), regular physical exercise, and other lifestyle interventions. The With Metf group received OA treatment in accordance with the protocol, in addition to receiving metformin at a dose of 500 mg twice daily for a period of 3 months. All clinical parameters were assessed before treatment, after 1 month, and after 3 months.
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) was employed to evaluate pain, stiffness, physical function, and overall health status in patients with OA. It consists of three subscales: Pain (P), Stiffness (S), and Function (F). An overall score (OS) is calculated based on the combined results from these subscales, offering a holistic assessment of the patient’s condition (Ebrahimzadeh et al., 2014).
The Lequesne Algofunctional Index (LI) was used to assess pain, functional impairment, and overall health status in patients with OA. It consists of two primary components: Pain Assessment (PA) and Functional Impairment Assessment (FIA). An Overall Score (OS) is derived from the combined results of both components, providing a comprehensive evaluation of the patient’s condition (Faucher et al., 2002).
The Knee injury and Osteoarthritis Outcome Score (KOOS) scale was utilized to evaluate various aspects of knee health in patients with osteoarthritis. It includes five subscales: Pain (P), Other Symptoms (OS), Function in Daily Living (FDL), Function in Sport/Recreation (FS/R), and Knee-Related Quality of Life (KRQL). These subscales collectively provide a comprehensive assessment of pain, functionality, and quality of life related to knee osteoarthritis (Roos and Lohmander, 2003).
The Visual Analog Scale (VAS) was employed to assess pain (VAS-P) in patients with osteoarthritis. Additionally, functional limitations (VAS-FL), stiffness (VAS-S), and physical activity and mobility (VAS-PAM) were evaluated to gain a more comprehensive understanding of the impact of osteoarthritis on daily functioning and quality of life (Delgado et al., 2018).
The Timed Up and Go (TUG) test and the 6-Minute Walk Test (6MWT) assessed functional mobility and endurance in patients with OA. The TUG measures the time to rise from a chair, walk a set distance, turn, return, and sit, while the 6MWT evaluates the distance walked in 6 minutes, indicating physical endurance and functional capacity (Montgomery et al., 2020; Buisseret et al., 2020).
The SF-36 Health Survey was used to assess health-related quality of life in patients at three time points: before treatment, after 1 month, and after 3 months. The survey included the following scales: Physical Functioning (SF-36-PF), Role Limitations due to Physical Health (SF-36-RP), Bodily Pain (SF-36-BP), General Health (SF-36-GH), Vitality (SF-36-VT), Social Functioning (SF-36-SF), Role Limitations due to Emotional Health (SF-36-RE), and Mental Health (SF-36-MH) (Ware and Sherbourne, 1992; Ware, 2000).
Anthropometric measurements were taken to assess patients’ metabolic health, including Body Mass Index (BMI), calculated as weight in kilograms divided by height in meters squared (kg/m2); Waist-to-Hip Ratio (WHR), determined by dividing waist circumference by hip circumference; and Waist-to-Height Ratio (WHtR), calculated as the ratio of waist circumference to height.
Fasting plasma glucose (FPG) was measured in mmol/L using an enzymatic method on the Cobas c311 analyzer (Roche Diagnostics, Germany); sensitivity: 0.11 mmol/L; measurement range: 0.11–41.7 mmol/L; intra-assay CV <2%; analyzed in duplicate. Glycated hemoglobin (HbA1c) was determined as a percentage (%) by high-performance liquid chromatography (HPLC) using the Tosoh G8 HPLC Analyzer (Tosoh Corporation, Japan); sensitivity: 0.1%; measurement range: 3.0%–18.0%; intra-assay CV <2%; analyzed in duplicate. The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was calculated using the formula: HOMA-IR = (FPG × fasting insulin)/22.5. C-peptide levels were measured in ng/mL using an immunoassay method on the Architect i2000SR analyzer (Abbott, USA); sensitivity: 0.02 ng/mL; measurement range: 0.02–20 ng/mL; intra-assay CV <5%; analyzed in duplicate.
The Neutrophil-to-Lymphocyte Ratio (NLR) was calculated by dividing the neutrophil count by the lymphocyte count, both measured in cells/µL both measured in cells/µL using the Sysmex XN-1000 hematology analyzer (Sysmex Corporation, Japan); sensitivity: 0.01 × 109/L; measurement range: neutrophils 0.01–100 × 109/L, lymphocytes 0.01–50 × 109/L; intra-assay CV <3%; analyzed in duplicate. C-Reactive Protein (CRP) was quantified in mg/L using an immunoturbidimetric assay using an immunoturbidimetric assay on the Cobas c501 analyzer (Roche Diagnostics, Germany); sensitivity: 0.3 mg/L; measurement range: 0.3–350 mg/L; intra-assay CV <3%; analyzed in duplicate. Hydroxyproline (HP) levels were determined in mg/L by colorimetric analysis using the Shimadzu UV-1800 spectrophotometer (Shimadzu, Japan); sensitivity: 0.5 mg/L; measurement range: 0.5–100 mg/L; intra-assay CV <5%; analyzed in triplicate. Malondialdehyde (MA) was measured in µmol/L using a thiobarbituric acid reactive substances assay on the Agilent Cary 60 UV-Vis spectrophotometer (Agilent Technologies, United States); sensitivity: 0.1 μmol/L; measurement range: 0.1–25 μmol/L; intra-assay CV <5%; analyzed in triplicate. Superoxide Dismutase (SOD) activity was assessed in U/mL using a spectrophotometric method on the Beckman Coulter DU 730 analyzer (Beckman Coulter, United States); sensitivity: 0.05 U/mL; measurement range: 0.05–25 U/mL; intra-assay CV <5%; analyzed in triplicate. α1-Antitrypsin (α1-AT) concentrations were measured in g/L by nephelometry on the BN ProSpec analyzer (Siemens Healthineers, Germany); sensitivity: 0.1 g/L; measurement range: 0.1–4.5 g/L; intra-assay CV <3%; analyzed in duplicate.
Total Cholesterol (TC) was measured in mmol/L using a colorimetric enzymatic method on the Abbott Architect c8000 analyzer (Abbott, United States); sensitivity: 0.1 mmol/L; measurement range: 0.1–15.0 mmol/L; intra-assay CV <3%; analyzed in duplicate. Low-Density Lipoprotein (LDL) cholesterol was quantified in mmol/L using the direct measurement method on the Abbott Architect c8000 analyzer (Abbott, United States); sensitivity: 0.2 mmol/L; measurement range: 0.2–10.0 mmol/L; intra-assay CV <3%; analyzed in duplicate. High-Density Lipoprotein (HDL) cholesterol levels were determined in mmol/L by a homogeneous enzymatic assay on the Abbott Architect c8000 analyzer (Abbott, United States); sensitivity: 0.1 mmol/L; measurement range: 0.1–4.0 mmol/L; intra-assay CV <3%; analyzed in duplicate. Triglycerides (TG) were assessed in mmol/L using an enzymatic colorimetric method on the Abbott Architect c8000 analyzer (Abbott, United States); sensitivity: 0.1 mmol/L; measurement range: 0.1–11.3 mmol/L; intra-assay CV <3%; analyzed in duplicate.
The OA exacerbation characteristics were assessed as follows: duration of exacerbation (≤7 days or >7 days) (OA exc. dur.), frequency of exacerbations in the last 3 months (≤2 times or >2 times) (OA exc. freq.), need for additional medical interventions during exacerbation (e.g., injections, physiotherapy) (OA exc. med. int.), requirement for work leave or exemption due to exacerbation (OA exc. work leave), occurrence of accompanying symptoms (e.g., swelling, redness) (OA exc. acc. sym.), need for increasing the dose of NSAIDs (NSAIDs dose), and need for increasing the duration of NSAID use (NSAID dur.).
Quantitative variables were first tested for normality using the Shapiro-Wilk test. Variables were described using median (Me) and lower and upper quartiles (Q1 – Q3). Categorical data were described with absolute and relative frequencies. The Mann-Whitney U-test was used to compare two groups on a quantitative variable. The comparison of frequencies in the analysis of 2 by 2 contingency tables was performed using Fisher’s exact test. As a measure of the effect size when comparing groups regarding binary variables, the odds ratio (OR) with a 95% confidence interval (95% CI) was calculated. The Friedman test was used, along with the Conover-Iman test with Holm correction as a post hoc method. A prognostic model for the probability of a specific outcome was constructed using logistic regression. The coefficient of determination, indicating the portion of variance explained by the logistic regression, was evaluated using Nagelkerke’s R2. For assessing the discriminatory ability of quantitative variables in predicting a specific outcome, ROC curve analysis was performed. The cut-off point for the quantitative variable was determined by the highest value of the Youden index. Differences were considered statistically significant at p < 0.05.
Statistical analyses were conducted using commercially available software packages, including IBM SPSS Statistics (version 25), R (version 4.0.3), and GraphPad Prism (version 9.3). These programs were used for data management, statistical testing, and generating visual representations of the results.
In both studied groups, statistically significant differences were observed. The analysis of WOMAC index dynamics revealed significant improvements in the WOMAC-P, WOMAC-S, WOMAC-F, and WOMAC-OS, with notable changes observed both before treatment versus after 3 months and after 1 month versus after 3 months across all scales. However, for the WOMAC-F, a significantly greater difference in the dynamics of the indicators was found in the With Metf group compared to the Without Metf group (Table 2).
In both studied groups, significant changes were observed in the Lequesne Algofunctional Index. In the Without Metf group, the analysis demonstrated significant changes in the LI-PA, LI-FIA, and LI-OS scales. Significant differences in the LI-FIA scale were observed between before treatment and after 1 month. Additionally, notable changes were identified in the LI-FIA and LI-OS scales between after 1 month and after 3 months (Table 3).
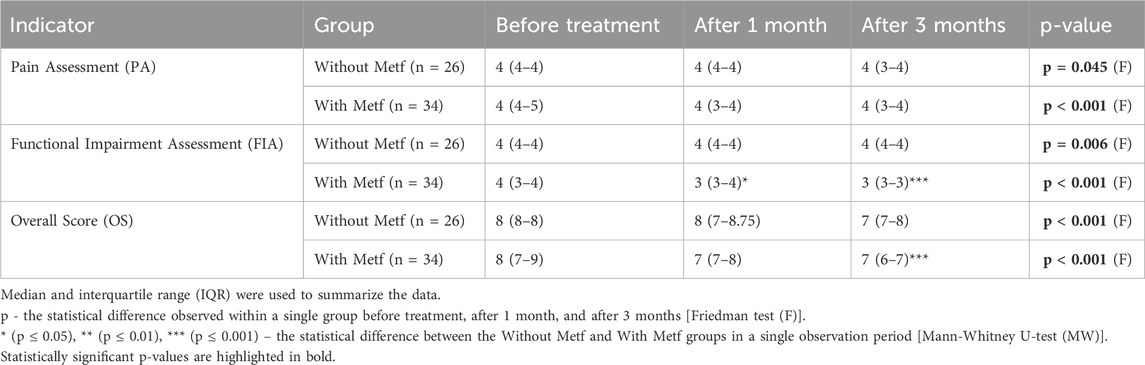
Table 3. Lequesne algofunctional index dynamics in the with and without Metf groups across treatment periods.
In the With Metf group, significant changes were observed across all Lequesne Algofunctional Index scales during treatment. Notable differences were found in the LI-PA and LI-OS scales between before treatment and after 1 month, before treatment and after 3 months, and after 1 month and after 3 months. Significant changes were also observed in the LI-FIA scale between before treatment and after 3 months, and between after 1 month and after 3 months (Table 3).
A significantly greater difference in the dynamics of the LI-PA and LI-FIA scales was found in the With Metf group compared to the Without Metf group (Table 3).
Significant statistical differences between the groups were observed in the Lequesne Algofunctional Index scales, particularly in the LI-FIA and LI-OS, after 3 months (Table 3).
In the Without Metf group, the KOOS scales analysis revealed significant changes throughout treatment, particularly in the KOOS-FS/R and KOOS-KRQL scales. Significant changes were observed in the KOOS-FS/R scale and the KOOS-KRQL scale between before treatment and after 3 months, and after 1 month and after 3 months (Table 4).
In the With Metf group, significant changes were observed across the KOOS scales – KOOS-P, KOOS-OS, KOOS-FDL, KOOS-FS/R, and KOOS- KRQL – during treatment. Notable changes were found between before treatment and after 1 month in the KOOS-OS and KOOS-FDL scales, and between before treatment and after 3 months in the KOOS-OS, KOOS-FDL, KOOS-FS/R, and KOOS- KRQL scales. Additionally, significant changes in the KOOS-P, KOOS-OS, and KOOS-FDL scales were observed between after 1 month and after 3 months (Table 4).
Additionally, a higher statistical significance of changes in the indicators was observed in the With Metf group for the KOOS-P, KOOS-OS, KOOS-FDL, and KOOS-FS/R scales, compared to the Without Metf group (Table 4).
In the Without Metf group, significant changes were noted in the VAS-P, VAS-FL, VAS-S, VAS-PA, and VAS-M scales throughout treatment. Notable differences were observed in the VAS-FL, VAS-S, and VAS-PA, and VAS-M scales between after 1 month and after 3 months, as well as in the VAS-PA and VAS-M scales between between before treatment and after 1 month (Table 5).
In the With Metf group, significant changes were observed throughout treatment in the VAS-P, VAS-FL, VAS-S, VAS-PA, and VAS-M scales. Statistically significant differences were found between before treatment and after 1 month, before treatment and after 3 months, as well as between after 1 month and after 3 months for all these scales (Table 5).
Furthermore, a greater statistical significance in the changes of the indicators was found in the With Metf group for your VAS-P, VAS-FL, and VAS-S scales, compared to the Without Metf group (Table 5).
Statistical differences between the groups were observed in the VAS-P and VAS-FL scales after 1 and 3 months. Furthermore, a significant difference was noted in the VAS-S scale between the groups after 3 months (Table 5).
In the Without Metf group, significant changes were observed in the TUG and 6MWT scales throughout the treatment period. Statistically significant differences were found between before treatment and after 3 months, as well as between after 1 month and after 3 months for both scales. Additionally, changes in the TUG scale were significant between before treatment and after 1 month (Table 6).
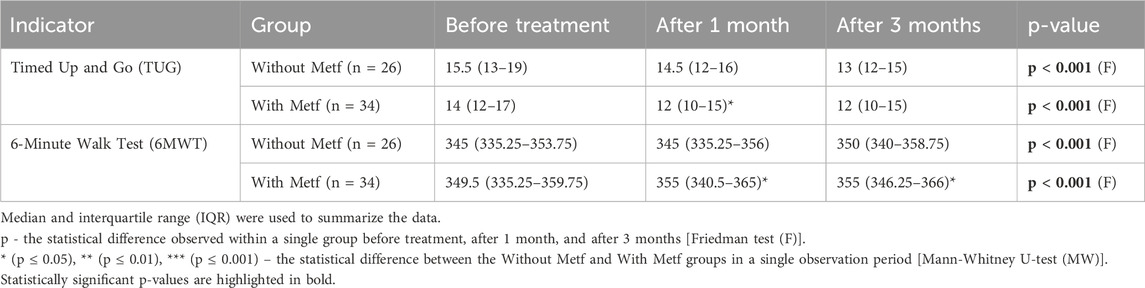
Table 6. Changes in TUG and 6MWT scales across treatment periods in the with and without Metf groups.
In the With Metf group, significant changes were observed in the TUG and 6MWT scales throughout the treatment period. Statistically significant differences were found in all indicators of both scales between before treatment and after 1 month, before treatment and after 3 months, as well as between after 1 month and after 3 months (Table 6).
Between the groups, statistical differences were observed for the TUG and 6MWT scales after 1 month, with a further significant difference noted in the 6MWT scale after 3 months (Table 6).
In the Without Metf group, statistically significant changes were observed across the OKS questionnaire scales during treatment, including OKS-P (p = 0.005) (Friedman test), OKS-S (p = 0.002) (Friedman test), and OKS-F (p = 0.001) (Friedman test). Additionally, significant differences were found in the OKS-F scale before treatment and after 1 month (p < 0.001) (Conover-Iman test with Holm correction), in the OKS-S scale before treatment and after 3 months, and in the OKS-F scale between after 1 month and after 3 months (p = 0.008) (Conover-Iman test with Holm correction) (Figure 1).
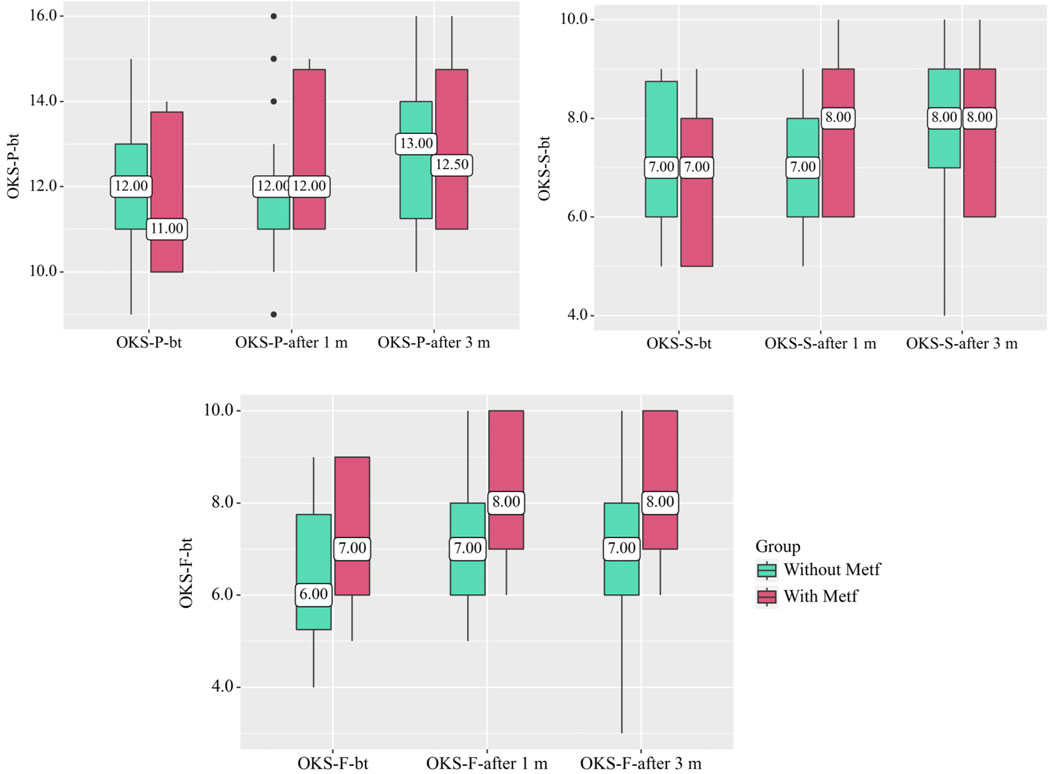
Figure 1. Changes in OKS Questionnaire Scales in the With and Without Metf Groups Across Treatment Periods. Statistical analysis was performed using the Friedman test for differences within groups over time, with post hoc comparisons conducted using the Conover-Iman test with Holm correction. Differences between groups at each time point were assessed using the Mann-Whitney U-test.
In the With Metf group, statistically significant changes were observed across all OKS questionnaire scales throughout treatment (p < 0.001) (Friedman test). Significant differences were also found between before treatment and after 1 month for all scales of the questionnaire (p < 0.001) (Conover-Iman test with Holm correction). Additionally, statistically significant changes were noted between after 1 month and after 3 months for all OKS scales (p < 0.001) (Conover-Iman test with Holm correction) (Figure 1).
Statistically significant differences between the groups were also observed in the OKS-F scale after 1 month (p = 0.043) (Mann-Whitney U-test) and after 3 months (p = 0.032) (Mann-Whitney U-test) (Figure 1).
In the Without Metf group, statistically significant changes were observed across several SF-36 scales throughout treatment. Specifically, SF-36-RP (p = 0.004) (Friedman test) [before treatment vs. after 1 month: p = 0.035, before treatment vs. after 3 months: p < 0.001, after 1 month vs. after 3 months: p = 0.035 (Conover-Iman test with Holm correction)]. SF-36-GH (p < 0.001) (Friedman test) [before treatment vs. after 3 months: p < 0.001, after 1 month vs. after 3 months: p < 0.001 (Conover-Iman test with Holm correction)], SF-36-VT (p < 0.001) (Friedman test) [before treatment vs. after 1 month: p < 0.001, before treatment vs. after 3 months: p < 0.001, after 1 month vs. after 3 months: p = 0.014 (Conover-Iman test with Holm correction)], SF-36-SF (p = 0.015) (Friedman test) [before treatment vs. after 3 months: p = 0.032, after 1 month vs. after 3 months: p = 0.046 (Conover-Iman test with Holm correction)], SF-36-RE showed (p < 0.001) (Friedman test) [before treatment vs. after 3 months: p < 0.001, after 1 month vs. after 3 months: p = 0.001 (Conover-Iman test with Holm correction)], and SF-36-MH (p < 0.001) (Friedman test) [before treatment vs. after 3 months: p < 0.001, after 1 month vs. after 3 months: p < 0.001 (Conover-Iman test with Holm correction)] (Figure 2).
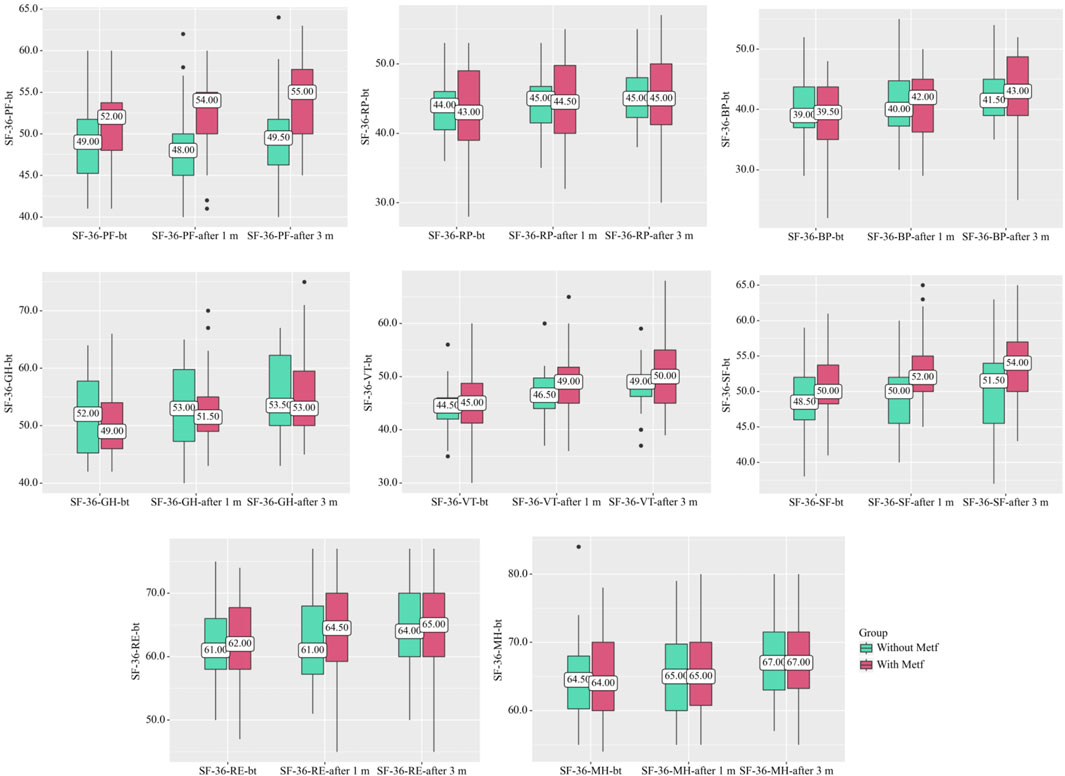
Figure 2. SF-36 Scale Dynamics in the With and Without Metf Groups Across Treatment Periods. Statistical analysis was performed using the Friedman test for differences within groups over time, with post hoc comparisons conducted using the Conover-Iman test with Holm correction. Differences between groups at each time point were assessed using the Mann-Whitney U-test.
In the With Metf group, statistically significant changes were observed across several SF-36 scales throughout treatment. Specifically, SF-36-PF (p < 0.001) (Friedman test) [before treatment vs. after 1 month: p = 0.004, before treatment vs. after 3 months: p < 0.001, after 1 month vs. after 3 months: p = 0.039 (Conover-Iman test with Holm correction)], SF-36-BP (p < 0.001) (Friedman test) [before treatment vs. after 1 month: p < 0.001, before treatment vs. after 3 months: p < 0.001, after 1 month vs. after 3 months: p = 0.004 (Conover-Iman test with Holm correction)], SF-36-GH (p < 0.001) (Friedman test) [before treatment vs. after 1 month: p < 0.001, before treatment vs. after 3 months: p < 0.001, after 1 month vs. after 3 months: p = 0.007 (Conover-Iman test with Holm correction)], SF-36-VT (p < 0.001) (Friedman test) [before treatment vs. after 1 month: p < 0.001, before treatment vs. after 3 months: p < 0.001, after 1 month vs. after 3 months: p = 0.012 (Conover-Iman test with Holm correction)], SF-36-RE (p < 0.001) (Friedman test) [before treatment vs. after 1 month: p = 0.004, before treatment vs. after 3 months: p < 0.001, after 1 month vs. after 3 months: p = 0.045 (Conover-Iman test with Holm correction)], and SF-36-MH (p < 0.001) (Friedman test) [before treatment vs. after 1 month: p = 0.003, before treatment vs. after 3 months: p < 0.001, after 1 month vs. after 3 months: p = 0.023 (Conover-Iman test with Holm correction)] (Figure 2).
A statistically significant difference was also found in the indicators between the groups on the scales SF-36-PF after 1 month (р = 0.002) (Mann-Whitney U-test) and SF-36-SF after 1 month (р = 0.005) (Mann-Whitney U-test), after 3 months (р = 0.020) (Mann-Whitney U-test) (Figure 2).
In the Without Metf group, significant changes in BMI and WHR were observed over the course of treatment. Furthermore, statistically significant differences in BMI and WHR were identified between before treatment and after 1 month of treatment. Similarly, significant changes in BMI and WHR were observed between after 1 month and after 3 months of treatment (Table 7).

Table 7. Changes in BMI, WHR, and WHtR across treatment periods in the with and without Metf groups.
In the With Metf group, significant changes were observed in BMI, WHR, and WHtR values throughout the course of treatment. Furthermore, statistically significant differences were identified for all indicators between before treatment and after 1 month, before treatment and after 3 months, as well as between after 1 month and after 3 months within this group (Table 7).
Moreover, a more significant statistical difference in the changes of the indicators was observed in the With Metf group for the BMI and WHtR scales, compared to the Without Metf group (Table 7).
Between the groups, significant statistical differences were observed after 1 month for BMI and WHtR, and after 3 months for BMI, WHR, and WHtR (Table 7).
In the Without Metf group, significant statistical changes were observed in FPG level throughout the course of treatment. Furthermore, notable differences in FPG were found between the measurements taken at 1 month and 3 months (Table 8).
In the With Metf group, statistically significant changes were observed throughout the treatment in the levels of FPG, HbA1c, HOMA-IR, and C-peptide. Additionally, significant changes were found across all indicators when comparing measurements before treatment to after 1 month, before treatment to after 3 months, and between after 1 month and after 3 months (Table 8).
Additionally, the With Metf group showed a more pronounced statistical difference in the changes of the FPG, HbA1c, HOMA-IR, and C-peptide indicators compared to the Without Metf group (Table 8).
Statistically significant differences between the groups were observed after 1 month for all indicators, with the exception of C-peptide. After 3 months, significant differences were found across all studied parameters (Table 8).
In the Without Metf group, statistically significant changes were observed throughout the treatment in the levels of NLR, CRP, MA, and SOD. Additionally, significant changes in NLR and SOD were found between before treatment and after 1 month. Statistically significant changes in NLR and CRP were observed between before treatment and after 3 months. Furthermore, significant differences were noted in NLR, CRP, MA, and SOD between after 1 month and after 3 months (Table 9).
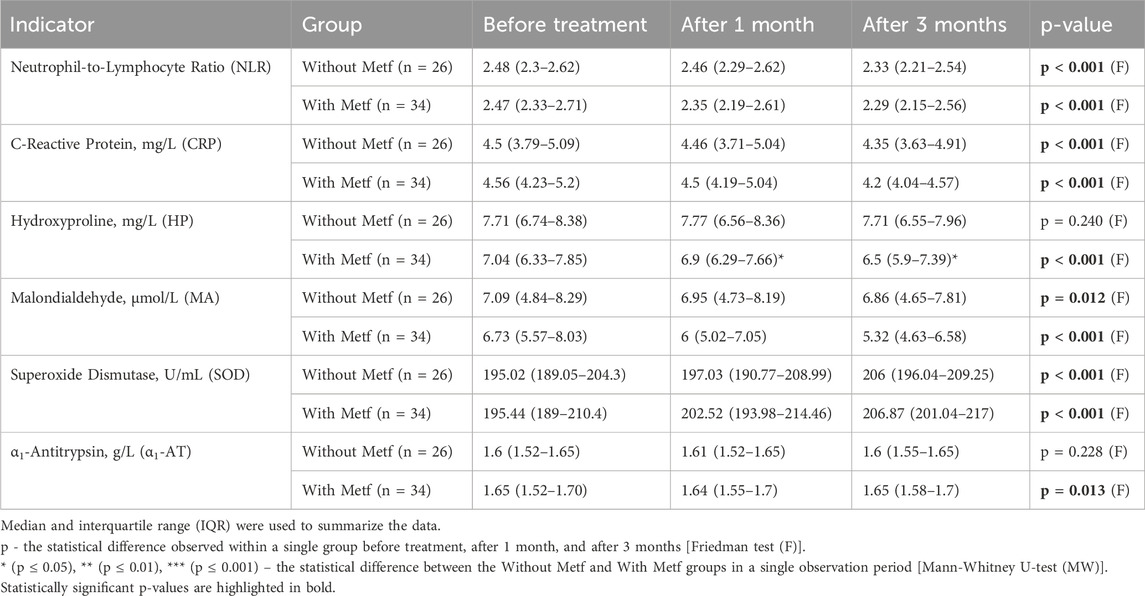
Table 9. Changes in inflammatory markers and antioxidant Enzyme levels throughout the treatment period.
In the With Metf group, statistically significant changes were observed during the treatment period in the levels of NLR, CRP, HP, MA, SOD, and α1-AT. Significant changes in NLR, CRP, MA, and SOD were found between before treatment and after 1 month. Additionally, statistically significant changes in NLR, CRP, HP, MA, and SOD were observed between before treatment and after 3 months. Furthermore, significant differences in NLR, CRP, HP, MA, SOD, and α1-AT were noted between after 1 month and after 3 months (Table 9).
Furthermore, a more significant statistical difference in the changes of the HP, MA, and α1-AT indicators was observed in the With Metf group compared to the Without Metf group (Table 9).
Statistically significant differences between the groups were observed in HP levels after 1 month and after 3 months (Table 9).
In the Without Metf group, statistically significant changes were observed in LDL levels throughout the treatment period. Additionally, significant changes in LDL levels were found between before treatment and after 3 months, as well as between after 1 month and after 3 months (Table 10).
In the Metf group, statistically significant changes were observed throughout the treatment period in TC, LDL, HDL, and TG levels. Additionally, significant changes were found in TC and LDL levels between before treatment and after 1 month. TC and HDL levels exhibited statistically significant changes between before treatment and after 3 months. Furthermore, TC, LDL, HDL, and TG levels showed significant changes between after 1 month and after 3 months (Table 10).
In addition, a more notable statistical difference in the changes of the TC, LDL, HDL, and TG indicators was found in the With Metf group compared to the Without Metf group (Table 10).
Statistically significant differences between the groups were found in TC and LDL levels after 1 and 3 months (Table 10).
Over a 3-month period, various aspects of osteoarthritis (OA) exacerbations were investigated, including the duration of flare-ups (OA exc. dur., ≤7 days or >7 days), their frequency (OA exc. freq., ≤2 times or >2 times), and the need for additional medical interventions such as injections or physiotherapy (OA exc. med. int.). Other factors assessed included the requirement for work leave or exemption due to exacerbations (OA exc. work leave), the occurrence of accompanying symptoms (OA exc. acc. sym., e.g., swelling, redness), and the necessity to adjust NSAID therapy by increasing either the dose (NSAIDs dose) or duration of use (NSAID dur.). These parameters provided insight into the severity and impact of OA exacerbations on patient management (Table 11).
No statistically significant difference was found between these indicators in the studied groups during the treatment period. Additionally, an analysis of the interrelationships among the investigated indicators of Osteoarthritis Exacerbation Characteristics was conducted, and a prognostic model was developed to predict the need for increased NSAID doses in patients with osteoarthritis and impaired glucose tolerance, based on factors such as VAS-P-after 3 months, OKS-F-after 3 months, HbA1c-after 3 months, C-peptide-after 3 months, CRP-after 3 months, HP-after 3 months, and α1-AT-after 3 months using binary logistic regression. The model was constructed using 60 observations, and the relationship between these variables is described by the following equation:
where P represents the probability estimate for “yes,” z denotes the value of the logistic function, XVAS-P-after 3 m refers to VAS-P-after 3 m, XOKS-F-after 3 m refers to OKS-F-after 3 m, Xhba1c-after 3 m refers to HbA1c-after 3 m, XC-peptide-after 3 m refers to C-peptide-after 3 m, XCRP-after 3 m refers to CRP-after 3 m, XHP-after 3 m refers to HP-after 3 m, and Xα1-at-after 3 m refers to α1-AT-after 3 months.
The resulting regression model, in terms of the alignment between the predicted and observed values upon the inclusion of predictors compared to the model without predictors, is statistically significant (p < 0.001). The Nagelkerke pseudo-R2 was 67.9% (Table 12).
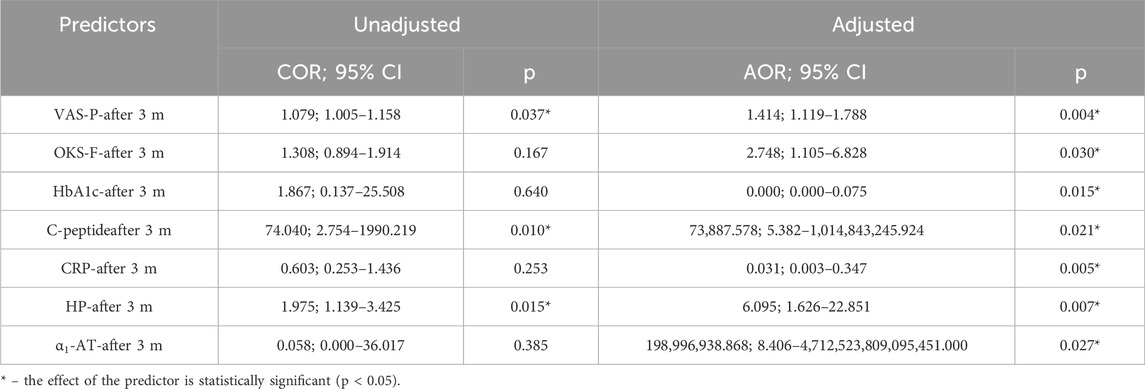
Table 12. Characteristics of the association between predictors of the model and the odds of the need for increased NSAID doses.
An increase of 1 in VAS-P-after 3 months increased the odds of the need for increased NSAID doses by a factor of 1.414. An increase of 1 in OKS-F-after 3 months increased the odds of the need for increased NSAID doses by a factor of 2.748. An increase of 1 in HbA1c-after 3 months decreased the odds of the need for increased NSAID doses by a factor of 488,307.202. An increase of 1 in C-peptide-after 3 months increased the odds of the need for increased NSAID doses by a factor of 73,887.578. An increase of 1 in CRP-after 3 months decreased the odds of the need for increased NSAID doses by a factor of 32.756. An increase of 1 in HP-after 3 months increased the odds of the need for increased NSAID doses by a factor of 6.095. An increase of 1 in α1-AT-after 3 months increased the odds of the need for increased NSAID doses by a factor of 198,996,938.868 (Figure 3).
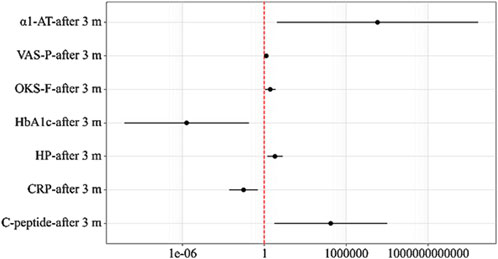
Figure 3. Odds ratios with 95% CI estimates for the studied predictors of the Need for Increased NSAID Doses.
The following curve was obtained when assessing the discriminatory ability of the regression model using ROC analysis (Figures 4, 5).
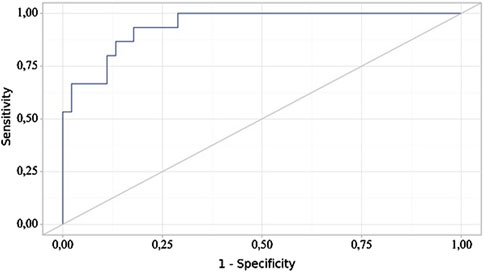
Figure 4. ROC curve illustrating the discriminatory ability of the regression model in predicting NSAIDs dose.
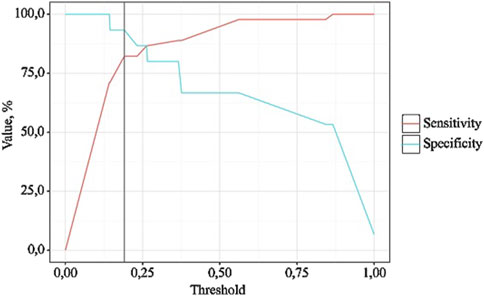
Figure 5. Analysis of model sensitivity and specificity depending on the threshold values of NSAIDs dose probability estimates.
The probability estimate P is a statistically significant predictor of the Need for Increased NSAID Doses (AUC = 0.942; 95% CI: 0.858–1.000, p < 0.001). The threshold value of the probability estimate P at the cut-off point, corresponding to the highest Youden’s index, was 0.191. A “yes” was predicted when the probability estimate P was greater than or equal to this value. The sensitivity and specificity of the resulting predictive model were 93.3% and 82.2%, respectively.
A statistical analysis was conducted to evaluate the relationship between BMI and the duration of osteoarthritis exacerbations (OA exc. dur.) (Table 13).
According to the presented table, when analyzing BMI depending on OA exc. dur., statistically significant differences were found (p = 0.015) (Figure 6).
The discriminatory capacity of >7 days from BMI was evaluated using ROC analysis, which yielded the following curve (Figures 7, 8).
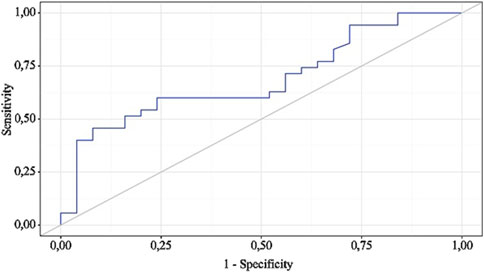
Figure 7. ROC curve characterizing the discriminatory ability of BMI-after 1 m in predicting OA exc. dur.
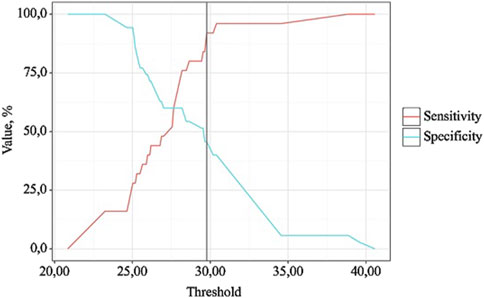
Figure 8. Analysis of the sensitivity and specificity of the model depending on the threshold values of the probability assessments for OA exc. dur.
BMI is a statistically significant predictor of OA exc. dur (AUC = 0.686; 95% CI: 0.553–0.820, p = 0.015). The threshold value of BMI at the cut-off point, corresponding to the highest Youden index, was 29.770. >7 days was predicted when BMI was equal to or greater than this value. The sensitivity and specificity of the obtained predictive model were 45.7% and 92.0%, respectively.
The application of metformin in OA has garnered attention for its metabolic and anti-inflammatory properties, with additional implications for oxidative stress and lipid profile modulation (Song et al., 2022; Lai et al., 2022; Chen et al., 2022; Ragab et al., 2024). Previous studies have demonstrated that metformin exerts a beneficial effect on oxidative stress, an important factor in OA pathogenesis (Alimoradi et al., 2025; Zuliani et al., 2020; Arinno et al., 2023). Oxidative stress plays a significant role in the degeneration of cartilage and the progression of OA by promoting inflammation and joint tissue damage (Ansari et al., 2020; Liu et al., 2022). Our findings support this, as metformin treatment resulted in reduced levels of markers such as superoxide dismutase, which is associated with oxidative stress. This is consistent with previous research indicating that metformin can reduce oxidative stress and protect against joint degeneration in OA patients, potentially contributing to a slower progression of the disease (Xu et al., 2024; Ruan et al., 2022; Barnett et al., 2017; Hyun et al., 2013). Metformin exerts its therapeutic effects in OA through a combination of systemic and local mechanisms that address both metabolic and inflammatory pathways contributing to disease progression (Yao et al., 2023; Song et al., 2021; Wang et al., 2019). At the systemic level, metformin significantly reduces insulin resistance and hyperglycemia, which are strongly linked to the chronic low-grade inflammation characteristic of metabolic disorders, including IGT (Tsalamandris et al., 2019; Tizazu et al., 2019). By activating AMP-activated protein kinase (AMPK), metformin plays a pivotal role in inhibiting the mechanistic target of rapamycin (mTOR) signaling pathway (Amin et al., 2019; Nair et al., 2014; Putilin et al., 2020). This inhibition is critical, as mTOR activation is associated with chondrocyte hypertrophy, extracellular matrix breakdown, and cartilage degeneration, all of which are hallmark features of OA pathology (Fazio et al., 2024; Chawla et al., 2022; Dong and Jin, 2025).
Locally, metformin demonstrates potent anti-inflammatory properties by downregulating the production of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) (Jia et al., 2024; Scafidi et al., 2024; Tsuji et al., 2020). These cytokines are major drivers of synovial inflammation, joint swelling, and cartilage erosion (Lubberts and van den Berg, 2003; Miller et al., 2014; Lubberts et al., 2001; Sokolove and Lepus, 2013).
Metformin has also demonstrated antiviral properties, particularly in inhibiting the replication of several viruses, including COVID-19 (Halabitska et al., 2024e; Petakh et al., 2022; Buchynskyi et al., 2023). Its potential to modulate viral infections, coupled with its anti-inflammatory effects, suggests that metformin may offer therapeutic benefits beyond metabolic conditions (Petakh et al., 2023a; Martin et al., 2023; Amengual-Cladera et al., 2024). Metformin exhibits antimicrobial properties, influencing pathogens and gut microbiota (Nosulenko et al., 2014; Jauvain et al., 2021; Bilyi et al., 2015; Garg and Mohajeri, 2024). Metformin affects stress responses, hormonal balance, and gut microbiota, potentially reducing inflammation and improving metabolic resilience (Topol and Kamyshny, 2013; Bilous et al., 2021; Petakh et al., 2023b).
Additionally, metformin’s effects on lipid profile are a notable area of interest (Gillani et al., 2021; Machado et al., 2012; Garimella et al., 2016). OA patients often present with metabolic disturbances, including dyslipidemia, which can exacerbate joint inflammation and cartilage degradation (Adam et al., 2024; Wei et al., 2023; Zhu et al., 2022). In our study, metformin led to significant improvements in lipid markers, including total cholesterol, LDL, HDL, and triglycerides. This is in line with other studies that have shown metformin’s ability to improve lipid profiles, suggesting that it may not only mitigate inflammation and oxidative stress but also correct underlying metabolic dysfunctions that worsen OA symptoms (Xing et al., 2022; Pradas et al., 2019; Zou et al., 2024). In fact, metformin’s ability to improve lipid metabolism may offer an additional mechanism for its positive effects in OA, as dyslipidemia is associated with increased risk of systemic inflammation and accelerated joint damage (Chen et al., 2022; Sobieh et al., 2023; Gkretsi et al., 2010; Mocanu et al., 2024).
When comparing our findings to other studies, the reduction in inflammatory markers such as C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio with metformin use is also well-documented in the literature (Cameron et al., 2016; Hambly et al., 2023; Pitsavos et al., 2007; Rahnavard et al., 2022). These results highlight metformin’s dual role in both controlling blood glucose and exerting anti-inflammatory effects, which have been linked to improved clinical outcomes in OA patients (Veronese et al., 2019; Kim et al., 2022; Lin et al., 2023). While our study demonstrates significant improvements in pain, stiffness, and functional limitations, differences in patient populations, dosages, or treatment durations align with the variability observed in the broader literature (Magni et al., 2021; Ferreira et al., 2024; Nahin et al., 2016). These discrepancies emphasize the need for further research to determine optimal dosing regimens and long-term efficacy of metformin in OA management.
Furthermore, the potential impact of metformin on neuropathic aspects of OA should not be overlooked (Zhang et al., 2024; Cao et al., 2024; Pușcașu et al., 2024). As OA can be associated with peripheral neuropathy, particularly in patients with comorbid diabetes or metabolic dysfunction, the ability of metformin to influence glucose metabolism may offer additional therapeutic benefits (Song et al., 2022; Chen et al., 2022; Kaur et al., 2023; Li S. et al., 2024). Previous research has indicated that metformin may reduce nerve damage and improve pain perception in OA patients with diabetes, providing a rationale for its broader application in OA management (Alenazi et al., 2023; Alimoradi et al., 2023; Aiad et al., 2024). Genetic determination plays a crucial role in individual responses to medications (Sydorchuk et al., 2020; Mroziewicz and Tyndale, 2010; Chen et al., 2024). Genetic factors influence the expression and effectiveness of pleiotropic drug effects, including those of metformin, which are being actively studied by various researchers (Buchynskyi et al., 2024a; Pawlyk et al., 2014; Froldi, 2024; Buchynskyi et al., 2024b; Lyubomirskaya et al., 2020).
Finally, while metformin has shown promise in improving metabolic disturbances and reducing inflammation in OA, its efficacy may be limited in certain patient groups, particularly the elderly or those with renal impairment (Kulkarni et al., 2020; Ala and Ala, 2021; Kloppenburg et al., 2025). Our study highlights the need for careful patient selection and monitoring to avoid potential risks, such as lactic acidosis, in vulnerable populations. Further research is needed to investigate the long-term effects of metformin on joint health, its impact on oxidative stress, lipid metabolism, and inflammatory markers, as well as its potential for combination therapy with other disease-modifying agents for OA.
This study has several limitations that should be considered when interpreting the results. First, the relatively small sample size limits the generalizability of the findings to a broader population of patients with osteoarthritis (OA) and impaired glucose tolerance (IGT). Larger, multicenter studies are required to validate these results and confirm their applicability to different populations. Additionally, the study’s observational nature and the lack of randomization may introduce selection bias, and further randomized controlled trials are needed to better assess the causal effects of metformin on OA symptoms and metabolic outcomes.
Moreover, the study only focused on a specific cohort of patients with both OA and IGT, without considering those with OA and normal glucose tolerance, which limits our understanding of metformin’s potential effects across different metabolic states. The absence of long-term follow-up data also prevents us from fully assessing the sustained impact of metformin on joint health, pain, and inflammation over time.
Another limitation lies in the lack of detailed mechanistic data on how metformin influences the molecular pathways underlying both OA and metabolic dysfunction. Future studies should focus on elucidating these pathways and assessing the long-term effectiveness of metformin in modifying OA progression.
Despite these limitations, the findings provide valuable insights into the potential benefits of metformin for managing OA symptoms and metabolic dysfunction, and future research is needed to explore its broader application and long-term impact.
Patients receiving metformin showed significantly greater improvements in both clinical and metabolic outcomes compared to those not receiving metformin. The metformin group demonstrated reductions in pain, stiffness, and improved physical function, as measured by the WOMAC, Lequesne Algofunctional Index, KOOS, and VAS scales, with notable improvements in quality of life and mobility. In contrast, the non-metformin group showed less significant changes. Metformin also led to reduced inflammatory markers, including C-reactive protein, neutrophil-to-lymphocyte ratio, and superoxide dismutase, suggesting decreased systemic inflammation. Additionally, improvements in lipid profiles, such as reductions in total cholesterol, LDL, HDL, and triglycerides, were observed, highlighting metformin’s metabolic benefits. Patients on metformin also showed significant improvements in BMI, waist-to-hip ratio, and waist-to-height ratio, indicating enhanced metabolic health. The need for increased NSAID doses in patients with osteoarthritis and impaired glucose tolerance can be predicted by factors such as pain severity, functional limitations, and inflammatory markers. Further studies are needed to confirm these findings and assess the long-term effects of dose adjustments. BMI has been identified as a potential predictor of OA exacerbation duration, with a threshold of 29.77. These findings suggest that metformin is effective in alleviating osteoarthritis symptoms and improving metabolic health in patients with osteoarthritis and impaired glucose tolerance. However, further research is needed to explore its long-term effects on joint health and inflammatory markers, as well as its potential role in managing osteoarthritis in patients without impaired glucose tolerance.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Local Ethics Committee of the I. Horbachevsky Ternopil National Medical University as protocol N78, dated 18 August 2024. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
IH: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing–original draft. PP: Writing–review and editing. OK: Supervision, Visualization, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adam, M. S, Zhuang, H, Ren, X, Zhang, Y, and Zhou, P (2024). The metabolic characteristics and changes of chondrocytes in vivo and in vitro in osteoarthritis. Frontiers in endocrinology 15, 1393550. doi:10.3389/fendo.2024.1393550
Aiad, A. A. E, El-Haggar, S. M, El-Barbary, A. M, and El-Afify, D. R (2024). Metformin as adjuvant therapy in obese knee osteoarthritis patients. Inflammopharmacology 32 (4), 2349–2359. doi:10.1007/s10787-024-01495-y
Ala, M, and Ala, M (2021). Metformin for cardiovascular protection, inflammatory Bowel disease, osteoporosis, periodontitis, polycystic ovarian syndrome, neurodegeneration, cancer, inflammation and senescence: what is next? ACS pharmacology and translational science 4 (6), 1747–1770. doi:10.1021/acsptsci.1c00167
Alenazi, A. M, Alhowimel, A. S, Alshehri, M. M, Alqahtani, B. A, Alhwoaimel, N. A, Segal, N. A, et al. (2023). Osteoarthritis and diabetes: where are we and where should we Go? Diagnostics 13 (8), 1386. doi:10.3390/diagnostics13081386
Alimoradi, N, Ramezani, A, Tahami, M, and Firouzabadi, N (2025). Metformin exhibits anti-inflammatory effects by regulating microRNA-451/CXCL16 and B cell leukemia/lymphoma 2 in patients with osteoarthritis. ACR open rheumatology. 7 (1), e11755. doi:10.1002/acr2.11755
Alimoradi, N, Tahami, M, Firouzabadi, N, Haem, E, and Ramezani, A (2023). Metformin attenuates symptoms of osteoarthritis: role of genetic diversity of Bcl2 and CXCL16 in OA. Arthritis research and therapy. 25, 35. doi:10.1186/s13075-023-03025-7
Allen, K. D, Thoma, L. M, and Golightly, Y. M (2022). Epidemiology of osteoarthritis. Osteoarthritis and cartilage 30 (2), 184–195. doi:10.1016/j.joca.2021.04.020
Amengual-Cladera, E, Morla-Barcelo, P. M, Morán-Costoya, A, Sastre-Serra, J, Pons, D. G, Valle, A, et al. (2024). Metformin: from diabetes to cancer—unveiling molecular mechanisms and therapeutic strategies. Biology 13 (5), 302. doi:10.3390/biology13050302
Amin, S, Lux, A, and O'Callaghan, F (2019). The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth. British journal of clinical pharmacology 85 (1), 37–46. doi:10.1111/bcp.13780
Anis, M. W, Iqbal, A, Younus, M. I, Aamir, A, and Khalid, W (2012)2024). Metformin: pioneering a path forward in knee osteoarthritis care? Annals of medicine and surgery 86 (8), 4333–4335. doi:10.1097/MS9.0000000000002318
Ansari, M. Y, Ahmad, N, and Haqqi, T. M (2020). Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomedicine and pharmacotherapy = Biomedecine and pharmacotherapie 129, 110452. doi:10.1016/j.biopha.2020.110452
Apostolova, N, Iannantuoni, F, Gruevska, A, Muntane, J, Rocha, M, and Victor, V. M (2020). Mechanisms of action of metformin in type 2 diabetes: effects on mitochondria and leukocyte-endothelium interactions. Redox biology 34, 101517. doi:10.1016/j.redox.2020.101517
Arinno, A, Maneechote, C, Khuanjing, T, Prathumsap, N, Chunchai, T, Arunsak, B, et al. (2023). Melatonin and metformin ameliorated trastuzumab-induced cardiotoxicity through the modulation of mitochondrial function and dynamics without reducing its anticancer efficacy. Biochimica et biophysica acta Molecular basis of disease 1869 (2), 166618. doi:10.1016/j.bbadis.2022.166618
Aziz, A, Ganesan, N. K, Kamarul, T, Mobasheri, A, and Sharifi, A (2024). The interplay between dysregulated metabolites and signaling pathway alterations involved in osteoarthritis: a systematic review. Therapeutic advances in musculoskeletal disease 16, 1759720x241299535. doi:10.1177/1759720X241299535
Bailey, C. J (2024). Metformin: therapeutic profile in the treatment of type 2 diabetes. Diabetes, obesity & metabolism 26 (Suppl. 3), 3–19. doi:10.1111/dom.15663
Baker, C, Retzik-Stahr, C, Singh, V, Plomondon, R, Anderson, V, and Rasouli, N (2021). Should metformin remain the first-line therapy for treatment of type 2 diabetes? Therapeutic advances in endocrinology and metabolism 12, 2042018820980225. doi:10.1177/2042018820980225
Barnett, L, Jordan, K, Edwards, J, and van der Windt, D (2017). Does metformin protect against osteoarthritis? An electronic health record cohort study. Primary Health Care Research and Development 18, 623–628. doi:10.1017/S1463423617000287
Berenbaum, F, and Walker, C (2020). Osteoarthritis and inflammation: a serious disease with overlapping phenotypic patterns. Postgraduate Medicine 132, 377–384. doi:10.1080/00325481.2020.1730669
Bergman, M, Manco, M, Satman, I, Chan, J, Schmidt, M. I, Sesti, G, et al. (2024). International Diabetes Federation Position Statement on the 1-hour post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes. Diabetes research and clinical practice 209, 111589. doi:10.1016/j.diabres.2024.111589
Bilous, I. I, Pavlovych, L. L, and Kamyshnyi, A. M (2021). Primary hypothyroidism and autoimmune thyroiditis alter the transcriptional activity of genes regulating neurogenesis in the blood of patients. Endocrine regulations 55 (1), 5–15. doi:10.2478/enr-2021-0002
Bilyi, A. K, Antypenko, L. M, Ivchuk, V. V, Kamyshnyi, O. M, Polishchuk, N. M, and Kovalenko, S. I (2015). 2-Heteroaryl-[1,2,4]triazolo[1,5-c]quinazoline-5(6 H)-thiones and their S-substituted derivatives: synthesis, spectroscopic data, and biological activity. ChemPlusChem 80 (6), 980–989. doi:10.1002/cplu.201500051
Buchynskyi, M, Kamyshna, I, Lyubomirskaya, K, Moshynets, O, Kobyliak, N, Oksenych, V, et al. (2023). Efficacy of interferon alpha for the treatment of hospitalized patients with COVID-19: a meta-analysis. Frontiers in immunology 14, 1069894. doi:10.3389/fimmu.2023.1069894
Buchynskyi, M, Oksenych, V, Kamyshna, I, Budarna, O, Halabitska, I, Petakh, P, et al. (2024a). Genomic insight into COVID-19 severity in MAFLD patients: a single-center prospective cohort study. Frontiers in genetics 15, 1460318. doi:10.3389/fgene.2024.1460318
Buchynskyi, M, Oksenych, V, Kamyshna, I, Vorobets, I, Halabitska, I, and Kamyshnyi, O (2024b). Modulatory roles of AHR, FFAR2, FXR, and TGR5 gene expression in metabolic-associated fatty liver disease and COVID-19 outcomes. Viruses 16 (6), 985. doi:10.3390/v16060985
Buisseret, F, Catinus, L, Grenard, R, Jojczyk, L, Fievez, D, Barvaux, V, et al. (2020). Timed up and Go and six-minute walking tests with wearable inertial sensor: one step further for the prediction of the risk of fall in elderly nursing home people. Sensors (Basel, Switzerland) 20 (11), 3207. doi:10.3390/s20113207
Cameron, A. R, Morrison, V. L, Levin, D, Mohan, M, Forteath, C, Beall, C, et al. (2016). Anti-inflammatory effects of metformin irrespective of diabetes status. Circulation research 119 (5), 652–665. doi:10.1161/CIRCRESAHA.116.308445
Cao, B, Xu, Q, Shi, Y, Zhao, R, Li, H, Zheng, J, et al. (2024). Pathology of pain and its implications for therapeutic interventions. Signal transduction and targeted therapy 9 (1), 155. doi:10.1038/s41392-024-01845-w
Chandrasekaran, P, and Weiskirchen, R (2024). Effects of probiotics on gut microbiota: an overview. International journal of molecular sciences 25 (3), 6022. doi:10.3390/ijms25116022
Chawla, S, Mainardi, A, Majumder, N, Dönges, L, Kumar, B, Occhetta, P, et al. (2022). Chondrocyte hypertrophy in osteoarthritis: mechanistic studies and models for the identification of new therapeutic strategies. Cells 11 (24), 4034. doi:10.3390/cells11244034
Chen, S, Gan, D, Lin, S, Zhong, Y, Chen, M, Zou, X, et al. (2022). Metformin in aging and aging-related diseases: clinical applications and relevant mechanisms. Theranostics 12 (6), 2722–2740. doi:10.7150/thno.71360
Chen, Y. C, Wang, Y. C, Lee, M. C, Chen, Y. H, Su, W, Ko, P. S, et al. (2024). Decisive gene strategy on osteoarthritis: a comprehensive whole-literature based approach for conclusive gene targets. Aging 16 (17), 12346–12378. doi:10.18632/aging.206094
Coppola, C, Greco, M, Munir, A, Musarò, D, Quarta, S, Massaro, M, et al. (2024). Osteoarthritis: insights into diagnosis, pathophysiology, therapeutic avenues, and the potential of natural extracts. Current Issues in Molecular Biology 46 (5), 4063–4105. doi:10.3390/cimb46050251
Delgado, D. A, Lambert, B. S, Boutris, N, McCulloch, P. C, Robbins, A. B, Moreno, M. R, et al. (2018). Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. Journal of the American Academy of Orthopaedic Surgeons Global research and reviews 2 (3), e088. doi:10.5435/JAAOSGlobal-D-17-00088
De Roover, A, Escribano-Núñez, A, Monteagudo, S, and Lories, R (2023). Fundamentals of osteoarthritis: inflammatory mediators in osteoarthritis. Osteoarthritis Cartilage 31 (10), 1303–1311. doi:10.1016/j.joca.2023.06.005
Domingo, E, Marques, P, Francisco, V, Piqueras, L, and Sanz, M. J (2024). Targeting systemic inflammation in metabolic disorders. A therapeutic candidate for the prevention of cardiovascular diseases? Pharmacological research 200, 107058. doi:10.1016/j.phrs.2024.107058
Dong, D.-L, and Jin, G.-Z (2025). Targeting chondrocyte hypertrophy as strategies for the treatment of osteoarthritis. Bioengineering 12 (1), 77. doi:10.3390/bioengineering12010077
Drzewoski, J, and Hanefeld, M (2021). The current and potential therapeutic use of metformin-the good old drug. Pharmaceuticals (Basel, Switzerland) 14 (2), 122. doi:10.3390/ph14020122
Dutta, S, Shah, R. B, Singhal, S, Dutta, S. B, Bansal, S, Sinha, S, et al. (2023). Metformin: a review of potential mechanism and therapeutic utility beyond diabetes. Drug design, development and therapy 17, 1907–1932. doi:10.2147/DDDT.S409373
Ebrahimzadeh, M. H, Makhmalbaf, H, Birjandinejad, A, Keshtan, F. G, Hoseini, H. A, and Mazloumi, S. M (2014). The western Ontario and McMaster Universities osteoarthritis index (WOMAC) in Persian speaking patients with knee osteoarthritis. The archives of bone and joint surgery 2 (1), 57–62.
Fang, F, Wang, N, Yan, S, Wang, L, Lu, Y, Li, J, et al. (2019). Impaired glucose tolerance predicts all-cause mortality among older men at high risk for cardiovascular disease in China. Primary care diabetes 13 (6), 495–504. doi:10.1016/j.pcd.2019.01.004
Faucher, M, Poiraudeau, S, Lefevre-Colau, M. M, Rannou, F, Fermanian, J, and Revel, M (2002). Algo-functional assessment of knee osteoarthritis: comparison of the test-retest reliability and construct validity of the WOMAC and Lequesne indexes. Osteoarthritis Cartilage 10 (8), 602–610. doi:10.1053/joca.2002.0533
Fazio, A, Di Martino, A, Brunello, M, Traina, F, Marvi, M. V, Mazzotti, A, et al. (2024). The involvement of signaling pathways in the pathogenesis of osteoarthritis: an update. Journal of orthopaedic translation 47, 116–124. doi:10.1016/j.jot.2024.06.002
Felson, D. T (2013). Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage 21 (1), 10–15. doi:10.1016/j.joca.2012.09.012
Feng, X, Pan, J, Li, J, Zeng, C, Qi, W, Shao, Y, et al. (2020). Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR. Aging 12 (2), 1087–1103. doi:10.18632/aging.102635
Ferreira, R. M, Martins, P. N, and Gonçalves, R. S (2024). Non-pharmacological and non-surgical interventions to manage patients with knee osteoarthritis: an umbrella review 5-year update. Osteoarthritis and cartilage open 6 (3), 100497. doi:10.1016/j.ocarto.2024.100497
Foretz, M, Guigas, B, and Viollet, B (2023). Metformin: update on mechanisms of action and repurposing potential. Nature reviews Endocrinology 19 (8), 460–476. doi:10.1038/s41574-023-00833-4
Froldi, G (2024). View on metformin: antidiabetic and pleiotropic effects, pharmacokinetics, side effects, and sex-related differences. Pharmaceuticals 17 (4), 478. doi:10.3390/ph17040478
Garg, K, and Mohajeri, M. H (2024). Potential effects of the most prescribed drugs on the microbiota-gut-brain-axis: a review. Brain research bulletin 207, 110883. doi:10.1016/j.brainresbull.2024.110883
Garimella, S, Seshayamma, V, Rao, H, Kumar, S, Kumar, U, and Saheb, S (2016). Effect of metformin on lipid profile of type II diabetes. International Journal of Integrative Medical Sciences 3, 449–453. doi:10.16965/ijims.2016.155
Gillani, S. W, Ghayedi, N, Roosta, P, Seddigh, P, and Nasiri, O (2021). Effect of metformin on lipid profiles of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Journal of pharmacy and bioallied sciences 13 (1), 76–82. doi:10.4103/jpbs.JPBS_370_20
Gkretsi, V, Simopoulou, T, and Tsezou, A (2010). Lipid metabolism and osteoarthritis: lessons from atherosclerosis. Progress in lipid research 50, 133–140. doi:10.1016/j.plipres.2010.11.001
Global, regional (2023). Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet Rheumatology 5 (9), e508–e522. doi:10.1016/S2665-9913(23)00163-7
Halabitska, I, and Babinets, L (2021). Different consequences of the treatment of osteoarthritis in gastrointestinal comorbidity with exocrine pancreatic insufficiency. Family Medicine and Primary Care Review 23 (4), 422–428. doi:10.5114/fmpcr.2021.108207
Halabitska, I, Babinets, L, and Kotsaba, Y (2021). Pathogenetic features of comorbidity of primary osteoarthritis and diseases with exocrine pancreatic insufficiency. Georgian medical news (321), 57–62.
Halabitska, I, Babinets, L, Oksenych, V, and Kamyshnyi, O (2024d). Diabetes and osteoarthritis: exploring the interactions and therapeutic implications of insulin, metformin, and GLP-1-based interventions. Biomedicines 12 (8), 1630. doi:10.3390/biomedicines12081630
Halabitska, I, Oksenych, V, and Kamyshnyi, O (2024a). Exploring the efficacy of alpha-lipoic acid in comorbid osteoarthritis and type 2 diabetes mellitus. Nutrients 16 (19), 3349. doi:10.3390/nu16193349
Halabitska, I, Petakh, P, Kamyshna, I, Oksenych, V, Kainov, D. E, and Kamyshnyi, O (2024c). The interplay of gut microbiota, obesity, and depression: insights and interventions. Cellular and molecular life sciences CMLS 81 (1), 443. doi:10.1007/s00018-024-05476-w
Halabitska, I, Petakh, P, Lushchak, O, Kamyshna, I, Oksenych, V, and Kamyshnyi, O (2024e). Metformin in antiviral therapy: evidence and perspectives. Viruses 16 (12), 1938. doi:10.3390/v16121938
Halabitska, I, Petakh, P, Oksenych, V, and Kamyshnyi, O (2024b). Predictive analysis of osteoarthritis and chronic pancreatitis comorbidity: complications and risk factors. Frontiers in endocrinology 15, 1492741. doi:10.3389/fendo.2024.1492741
Hall, M, Bryant, A. L, Wrigley, T. V, Pratt, C, Crossley, K. M, Whitehead, T. S, et al. (2016). Does meniscal pathology alter gait knee biomechanics and strength post-ACL reconstruction? Knee surgery, sports traumatology, arthroscopy official journal of the ESSKA 24 (5), 1501–1509. doi:10.1007/s00167-015-3908-x
Hambly, R, Kearney, N, Hughes, R, Fletcher, J. M, and Kirby, B (2023). Metformin treatment of hidradenitis suppurativa: effect on metabolic parameters, inflammation, cardiovascular risk biomarkers, and immune mediators. International journal of molecular sciences 24 (8), 6969. doi:10.3390/ijms24086969
He, L (2020). Metformin and systemic metabolism. Trends in pharmacological sciences 41 (11), 868–881. doi:10.1016/j.tips.2020.09.001
He, Y, Li, Z, Alexander, P. G, Ocasio-Nieves, B. D, Yocum, L, Lin, H, et al. (2020). Pathogenesis of osteoarthritis: risk factors, regulatory pathways in chondrocytes, and experimental models. Biology 9 (8), 194. doi:10.3390/biology9080194
Hermans, M. P, Pepersack, T. M, Godeaux, L. H, Beyer, I, and Turc, A. P (2005). Prevalence and determinants of impaired glucose metabolism in frail elderly patients: the Belgian Elderly Diabetes Survey (BEDS). The journals of gerontology Series A, Biological sciences and medical sciences 60 (2), 241–247. doi:10.1093/gerona/60.2.241
Horváth, E, Sólyom, Á, Székely, J, Nagy, E. E, and Popoviciu, H (2023). Inflammatory and metabolic signaling interfaces of the hypertrophic and senescent chondrocyte phenotypes associated with osteoarthritis. International journal of molecular sciences 24 (22), 16468. doi:10.3390/ijms242216468
Hostalek, U, Gwilt, M, and Hildemann, S (2015). Therapeutic use of metformin in prediabetes and diabetes prevention. Drugs 75 (10), 1071–1094. doi:10.1007/s40265-015-0416-8
Hyun, B, Shin, S, Lee, A, Lee, S, Song, Y, Ha, N. J, et al. (2013). Metformin down-regulates TNF-α secretion via suppression of scavenger receptors in macrophages. Immune network 13 (4), 123–132. doi:10.4110/in.2013.13.4.123
Isop, L. M, Neculau, A. E, Necula, R. D, Kakucs, C, Moga, M. A, and Dima, L (2023). Metformin: the winding path from understanding its molecular mechanisms to proving therapeutic benefits in neurodegenerative disorders. Pharmaceuticals (Basel, Switzerland) 16 (12), 1714. doi:10.3390/ph16121714
Jauvain, M, Courtois, S, Lehours, P, and Bessède, E (2021). Metformin modifies the gut microbiota of mice infected with Helicobacter pylori. Pharmaceuticals (Basel, Switzerland) 14 (4), 329. doi:10.3390/ph14040329
Jia, R, Ma, H, Hao, H, Wang, F, and Yang, H (2024). Metformin inhibits activation of NLRP3 inflammasome and inflammatory response in preeclamptic rats. Gene 919, 148509. doi:10.1016/j.gene.2024.148509
Kalyani, R. R, and Egan, J. M (2013). Diabetes and altered glucose metabolism with aging. Endocrinology and metabolism clinics of N. Am. 42 (2), 333–347. doi:10.1016/j.ecl.2013.02.010
Kaur, M, Misra, S, Swarnkar, P, Patel, P, Das Kurmi, B, Das Gupta, G, et al. (2023). Understanding the role of hyperglycemia and the molecular mechanism associated with diabetic neuropathy and possible therapeutic strategies. Biochemical pharmacology 215, 115723. doi:10.1016/j.bcp.2023.115723
Kim, J. W, Choe, J. Y, and Park, S. H (2022). Metformin and its therapeutic applications in autoimmune inflammatory rheumatic disease. The Korean journal of internal medicine 37 (1), 13–26. doi:10.3904/kjim.2021.363
Kloppenburg, M, Namane, M, and Cicuttini, F (2025). Osteoarthritis. Lancet (London, England) 405 (10472), 71–85. doi:10.1016/S0140-6736(24)02322-5
Kulkarni, A. S, Gubbi, S, and Barzilai, N (2020). Benefits of metformin in attenuating the hallmarks of aging. Cell metabolism 32 (1), 15–30. doi:10.1016/j.cmet.2020.04.001
Lai, F. T. T, Yip, B. H. K, Hunter, D. J, Rabago, D. P, Mallen, C. D, Yeoh, E. K, et al. (2022). Metformin use and the risk of total knee replacement among diabetic patients: a propensity-score-matched retrospective cohort study. Scientific reports 12 (1), 11571. doi:10.1038/s41598-022-15871-7
Lambova, S. N (2023). Pleiotropic effects of metformin in osteoarthritis. Life 13 (2), 437. doi:10.3390/life13020437
Li, B, Yang, Z, Li, Y, Zhang, J, Li, C, and Lv, N (2024a). Exploration beyond osteoarthritis: the association and mechanism of its related comorbidities. Frontiers in endocrinology 15, 1352671. doi:10.3389/fendo.2024.1352671
Li, J, Zhang, B, Liu, W. X, Lu, K, Pan, H, Wang, T, et al. (2020). Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Annals of the rheumatic diseases 79 (5), 635–645. doi:10.1136/annrheumdis-2019-216713
Li, S, Yang, D, Zhou, X, Chen, L, Liu, L, Lin, R, et al. (2024b). Neurological and metabolic related pathophysiologies and treatment of comorbid diabetes with depression. CNS neuroscience and therapeutics 30 (4), e14497. doi:10.1111/cns.14497
Lin, H, Ao, H, Guo, G, and Liu, M (2023). The role and mechanism of metformin in inflammatory diseases. Journal of inflammation research 16, 5545–5564. doi:10.2147/JIR.S436147
Liu, L, Luo, P, Yang, M, Wang, J, Hou, W, and Xu, P (2022). The role of oxidative stress in the development of knee osteoarthritis: a comprehensive research review. Frontiers in molecular biosciences 9, 1001212. doi:10.3389/fmolb.2022.1001212
Lubberts, E, Joosten, L. A, Oppers, B, van den Bersselaar, L, Coenen-de Roo, C. J, Kolls, J. K, et al. (2001). IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. Journal of immunology (Baltimore, Md 1950) 167 (2), 1004–1013. doi:10.4049/jimmunol.167.2.1004
Lubberts, E, and van den Berg, W. B (2003). Cytokines in the pathogenesis of rheumatoid arthritis and collagen-induced arthritis. Advances in experimental medicine and biology 520, 194–202. doi:10.1007/978-1-4615-0171-8_11
Lyubomirskaya, E. S, Kamyshnyi, A. M, Krut, Y. Y, Smiianov, V. A, Fedoniuk, L. Y, Romanyuk, L. B, et al. (2020). SNPs and transcriptional activity of genes of innate and adaptive immunity at the maternal-fetal interface in woman with preterm labour, associated with preterm premature rupture of membranes. Wiadomosci lekarskie (Warsaw, Poland 1960) 73 (1), 25–30. doi:10.36740/wlek202001104
Machado, H. A, Vieira, M, Cunha, M. R, Correia, M. R, Fukui, R. T, Santos, R. F, et al. (2012). Metformin, but not glimepiride, improves carotid artery diameter and blood flow in patients with type 2 diabetes mellitus. Clinics (Sao Paulo, Brazil) 67 (7), 711–717. doi:10.6061/clinics/2012(07)03
Magni, A, Agostoni, P, Bonezzi, C, Massazza, G, Menè, P, Savarino, V, et al. (2021). Management of osteoarthritis: expert opinion on NSAIDs. Pain and therapy 10 (2), 783–808. doi:10.1007/s40122-021-00260-1
Martin, D. E, Cadar, A. N, and Bartley, J. M (2023). Old drug, new tricks: the utility of metformin in infection and vaccination responses to influenza and SARS-CoV-2 in older adults. Frontiers in aging. 4, 1272336. doi:10.3389/fragi.2023.1272336
Miller, R. E, Miller, R. J, and Malfait, A. M (2014). Osteoarthritis joint pain: the cytokine connection. Cytokine 70 (2), 185–193. doi:10.1016/j.cyto.2014.06.019
Mocanu, V, Timofte, D. V, Zară-Dănceanu, C.-M, and Labusca, L (2024). Obesity, metabolic syndrome, and osteoarthritis require integrative understanding and management. Biomedicines 12 (6), 1262. doi:10.3390/biomedicines12061262
Montgomery, G, McPhee, J, Pääsuke, M, Sipilä, S, Maier, A. B, Hogrel, J. Y, et al. (2020). Determinants of performance in the timed up-and-go and six-minute walk tests in young and old healthy adults. Journal of clinical medicine 9 (5), 1561. doi:10.3390/jcm9051561
Mroziewicz, M, and Tyndale, R. F (2010). Pharmacogenetics: a tool for identifying genetic factors in drug dependence and response to treatment. Addiction science and clinical practice 5 (2), 17–29.
Nahin, R. L, Boineau, R, Khalsa, P. S, Stussman, B. J, and Weber, W. J (2016). Evidence-based evaluation of complementary health approaches for pain management in the United States. Mayo Clinic proceedings 91 (9), 1292–1306. doi:10.1016/j.mayocp.2016.06.007
Nair, V, Sreevalsan, S, Basha, R, Abdelrahim, M, Abudayyeh, A, Rodrigues, H. A, et al. (2014). Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. The Journal of biological chemistry 289 (40), 27692–27701. doi:10.1074/jbc.M114.592576
Nedunchezhiyan, U, Varughese, I, Sun, A. R, Wu, X, Crawford, R, and Prasadam, I (2022). Obesity, inflammation, and immune system in osteoarthritis. Frontiers in immunology 13, 907750. doi:10.3389/fimmu.2022.907750
Nosulenko, I. S, Voskoboynik, O. Y, Berest, G. G, Safronyuk, S. L, Kovalenko, S. I, Kamyshnyi, O. M, et al. (2014). Synthesis and antimicrobial activity of 6-Thioxo-6,7-dihydro-2H-[1,2,4]triazino[2,3-c]-quinazolin-2-one derivatives. Scientia pharmaceutica 82 (3), 483–500. doi:10.3797/scipharm.1402-10
Pawlyk, A. C, Giacomini, K. M, McKeon, C, Shuldiner, A. R, and Florez, J. C (2014). Metformin pharmacogenomics: current status and future directions. Diabetes 63 (8), 2590–2599. doi:10.2337/db13-1367
Peat, G, Thomas, E, Duncan, R, Wood, L, Hay, E, and Croft, P (2006). Clinical classification criteria for knee osteoarthritis: performance in the general population and primary care. Annals of the rheumatic diseases 65 (10), 1363–1367. doi:10.1136/ard.2006.051482
Petakh, P, Griga, V, Mohammed, I. B, Loshak, K, Poliak, I, and Kamyshnyiy, A (2022). Effects of metformin, insulin on hematological parameters of COVID-19 patients with type 2 diabetes. Medical archives (Sarajevo, Bosnia and Herzegovina) 76 (5), 329–332. doi:10.5455/medarh.2022.76.329-332
Petakh, P, Kamyshna, I, Oksenych, V, Kainov, D, and Kamyshnyi, A (2023a). Metformin therapy changes gut microbiota alpha-diversity in COVID-19 patients with type 2 diabetes: the role of SARS-CoV-2 variants and antibiotic treatment. Pharmaceuticals (Basel, Switzerland) 16 (6), 904. doi:10.3390/ph16060904
Petakh, P, Kobyliak, N, and Kamyshnyi, A (2023b). Gut microbiota in patients with COVID-19 and type 2 diabetes: a culture-based method. Frontiers in cellular and infection microbiology 13, 1142578. doi:10.3389/fcimb.2023.1142578
Petrie, J. R (2024). Metformin beyond type 2 diabetes: emerging and potential new indications. Diabetes, obesity and metabolism 26 (Suppl. 3), 31–41. doi:10.1111/dom.15756
Ping, W. X, Hu, S, Su, J. Q, and Ouyang, S. Y (2024). Metabolic disorders in prediabetes: from mechanisms to therapeutic management. World journal of diabetes 15 (3), 361–377. doi:10.4239/wjd.v15.i3.361
Pitsavos, C, Tampourlou, M, Panagiotakos, D, Skoumas, Y, Chrysohoou, C, Nomikos, T, et al. (2007). Association between low-grade systemic inflammation and type 2 diabetes mellitus among men and women from the ATTICA study. The review of diabetic studies RDS 4, 98–104. doi:10.1900/RDS.2007.4.98
Pradas, I, Rovira-Llopis, S, Naudí, A, Bañuls, C, Rocha, M, Hernandez-Mijares, A, et al. (2019). Metformin induces lipid changes on sphingolipid species and oxidized lipids in polycystic ovary syndrome women. Scientific reports 9, 16033. doi:10.1038/s41598-019-52263-w
Pușcașu, C, Negreș, S, Zbârcea, C. E, Ungurianu, A, Ștefănescu, E, Blebea, N. M, et al. (2024). Evaluating the antihyperalgesic potential of sildenafil–metformin combination and its impact on biochemical markers in alloxan-induced diabetic neuropathy in rats. Pharmaceuticals 17 (6), 783. doi:10.3390/ph17060783
Putilin, D. A, Evchenko, S. Y, Fedoniuk, L. Y, Tokarskyy, O. S, Kamyshny, O. M, Migenko, L. M, et al. (2020). The influence of metformin to the transcriptional activity of the mTOR and FOX3 genes in parapancreatic adipose tissue of streptozotocin-induced diabetic rats. Journal of medicine and life 13 (1), 50–55. doi:10.25122/jml-2020-0029
Ragab, E. A, Abd El-Wahab, M. F, Doghish, A. S, Salama, R. M, Eissa, N, and Darwish, S. F (2024). The journey of boswellic acids from synthesis to pharmacological activities. Naunyn-Schmiedeberg's archives of pharmacology 397 (3), 1477–1504. doi:10.1007/s00210-023-02725-w
Rahnavard, A, Mann, B, Giri, A, Chatterjee, R, and Crandall, K. A (2022). Metabolite, protein, and tissue dysfunction associated with COVID-19 disease severity. Scientific reports 12 (1), 12204. doi:10.1038/s41598-022-16396-9
Redkva, O. V, Babinets, L. S, and Halabitska, I. M (2021). Evaluation of parameters of actual typical pathogenetic syndromes in comorbidity of type 2 diabetes mellitus and chronic pancreatitis. Wiadomosci lekarskie (Warsaw, Poland 1960) 74 (10 cz 2), 2557–2559. doi:10.36740/wlek202110204
Repchuk, Y, Sydorchuk, L. P, Sydorchuk, A. R, Fedonyuk, L. Y, Kamyshnyi, O, Korovenkova, O, et al. (2021). Linkage of blood pressure, obesity and diabetes mellitus with angiotensinogen gene (AGT 704T>C/rs699) polymorphism in hypertensive patients. Bratislavske lekarske listy 122 (10), 715–720. doi:10.4149/BLL_2021_114
Roberts, C. K, Hevener, A. L, and Barnard, R. J (2013). Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Comprehensive Physiology 3 (1), 1–58. doi:10.1002/cphy.c110062
Rohm, T. V, Meier, D. T, Olefsky, J. M, and Donath, M. Y (2022). Inflammation in obesity, diabetes, and related disorders. Immunity 55 (1), 31–55. doi:10.1016/j.immuni.2021.12.013
Rojas, L. B, and Gomes, M. B (2013). Metformin: an old but still the best treatment for type 2 diabetes. Diabetology and metabolic syndrome 5 (1), 6. doi:10.1186/1758-5996-5-6
Roos, E. M, and Lohmander, L. S (2003). The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health and quality of life outcomes 1, 64. doi:10.1186/1477-7525-1-64
Rotermund, C, Machetanz, G, and Fitzgerald, J. C (2018). The therapeutic potential of metformin in neurodegenerative diseases. Frontiers in endocrinology 9, 400. doi:10.3389/fendo.2018.00400
Ruan, G, Yuan, S, Lou, A, Mo, Y, Qu, Y, Guo, D, et al. (2022). Can metformin relieve tibiofemoral cartilage volume loss and knee symptoms in overweight knee osteoarthritis patients? Study protocol for a randomized, double-blind, and placebo-controlled trial. BMC musculoskeletal disorders 23 (1), 486. doi:10.1186/s12891-022-05434-2
Ruze, R, Liu, T, Zou, X, Song, J, Chen, Y, Xu, R, et al. (2023). Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Frontiers in endocrinology 14, 1161521. doi:10.3389/fendo.2023.1161521
Scafidi, A, Mogensen, F, Campus, E, Pailas, A, Neumann, K, Legrave, N, et al. (2024). Metformin impacts the differentiation of mouse bone marrow cells into macrophages affecting tumour immunity. Heliyon 10, e37792. doi:10.1016/j.heliyon.2024.e37792
Sobieh, B. H, El-Mesallamy, H. O, and Kassem, D. H (2023). Beyond mechanical loading: the metabolic contribution of obesity in osteoarthritis unveils novel therapeutic targets. Heliyon 9 (5), e15700. doi:10.1016/j.heliyon.2023.e15700
Sokolove, J, and Lepus, C. M (2013). Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Therapeutic advances in musculoskeletal disease 5 (2), 77–94. doi:10.1177/1759720X12467868
Song, P, Hwang, J. S, Park, H. C, Kim, K. K, Son, H. J, Kim, Y. J, et al. (2021). Therapeutic applications of type 2 diabetes mellitus drug metformin in patients with osteoarthritis. Pharmaceuticals (Basel, Switzerland) 14 (2), 152. doi:10.3390/ph14020152
Song, Y, Wu, Z, and Zhao, P (2022). The effects of metformin in the treatment of osteoarthritis: current perspectives. Frontiers in pharmacology 13, 952560. doi:10.3389/fphar.2022.952560
Su, Z, Zong, Z, Deng, J, Huang, J, Liu, G, Wei, B, et al. (2022). Lipid metabolism in cartilage development, degeneration, and regeneration. Nutrients 14 (19), 3984. doi:10.3390/nu14193984
Swain, S, Kamps, A, Runhaar, J, Dell'Isola, A, Turkiewicz, A, Robinson, D, et al. (2022). Comorbidities in osteoarthritis (ComOA): a combined cross-sectional, case-control and cohort study using large electronic health records in four European countries. BMJ open 12 (4), e052816. doi:10.1136/bmjopen-2021-052816
Sydorchuk, L, Dzhuryak, V, Sydorchuk, A, Levytska, S, Petrynych, V, Knut, R, et al. (2020). The cytochrome 11B2 aldosterone synthase gene rs1799998 single nucleotide polymorphism determines elevated aldosterone, higher blood pressure, and reduced glomerular filtration, especially in diabetic female patients. Endocrine regulations 54 (3), 217–226. doi:10.2478/enr-2020-0024
Tizazu, A. M, Nyunt, M. S. Z, Cexus, O, Suku, K, Mok, E, Xian, C. H, et al. (2019). Metformin monotherapy downregulates diabetes-associated inflammatory status and impacts on mortality. Frontiers in physiology 10, 572. doi:10.3389/fphys.2019.00572
Topol, I, and Kamyshny, A (2013). Study of expression of TLR2, TLR4 and transcription factor NF-kB structures of galt of rats in the conditions of the chronic social stress and modulation of structure of intestinal microflora. Georgian medical news (225), 115–122.
Tsalamandris, S, Antonopoulos, A. S, Oikonomou, E, Papamikroulis, G. A, Vogiatzi, G, Papaioannou, S, et al. (2019). The role of inflammation in diabetes: current Concepts and future perspectives. European cardiology 14 (1), 50–59. doi:10.15420/ecr.2018.33.1
Tsuji, G, Hashimoto-Hachiya, A, Vu, Y, Takemura, M, Yumine, A, Furue, K, et al. (2020). Metformin inhibits IL-1β secretion via impairment of NLRP3 inflammasome in keratinocytes: implications for preventing the development of psoriasis. Cell Death Discovery 6, 11. doi:10.1038/s41420-020-0245-8
Veronese, N, Cooper, C, Reginster, J. Y, Hochberg, M, Branco, J, Bruyère, O, et al. (2019). Type 2 diabetes mellitus and osteoarthritis. Seminars in arthritis and rheumatism 49 (1), 9–19. doi:10.1016/j.semarthrit.2019.01.005
Vinuesa, A, Pomilio, C, Gregosa, A, Bentivegna, M, Presa, J, Bellotto, M, et al. (2021). Inflammation and insulin resistance as risk factors and potential therapeutic targets for Alzheimer's disease. Frontiers in neuroscience 15, 653651. doi:10.3389/fnins.2021.653651
Wang, Q, Runhaar, J, Kloppenburg, M, Boers, M, Bijlsma, J. W. J, Bierma-Zeinstra, S. M. A, et al. (2024). Evaluation of the diagnostic performance of American College of Rheumatology, EULAR, and national Institute for health and clinical excellence criteria against clinically relevant knee osteoarthritis: data from the CHECK cohort. Arthritis care and research 76 (4), 511–516. doi:10.1002/acr.25270
Wang, Y, Hussain, S. M, Wluka, A. E, Lim, Y. Z, Abram, F, Pelletier, J. P, et al. (2019). Association between metformin use and disease progression in obese people with knee osteoarthritis: data from the Osteoarthritis Initiative-a prospective cohort study. Arthritis Res Ther 21 (1), 127. doi:10.1186/s13075-019-1915-x
Ware, J. E (2000). SF-36 health survey update. Spine 25 (24), 3130–3139. doi:10.1097/00007632-200012150-00008
Ware, J. E, and Sherbourne, C. D (1992). The MOS 36-lem short-form health survey (SF-36): I. Conceptual framework and item selection. Medical care 30 (6), 473–483. doi:10.1097/00005650-199206000-00002
Wei, G, Lu, K, Umar, M, Zhu, Z, Lu, W. W, Speakman, J. R, et al. (2023). Risk of metabolic abnormalities in osteoarthritis: a new perspective to understand its pathological mechanisms. Bone research 11 (1), 63. doi:10.1038/s41413-023-00301-9
World Medical Association Declaration of Helsinki (2014). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. The Journal of the American College of Dentists 81 (3), 14–18.
Xing, H, Liang, C, Wang, C, Xu, X, Hu, Y, and Qiu, B (2022). Metformin mitigates cholesterol accumulation via the AMPK/SIRT1 pathway to protect osteoarthritis chondrocytes. Biochemical and biophysical research communications 632, 113–121. doi:10.1016/j.bbrc.2022.09.074
Xu, T, Liu, K, Fan, J, Jia, X, Guo, X, Zhao, X, et al. (2024). Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy. Open life sciences 19 (1), 20220922. doi:10.1515/biol-2022-0922
Yao, Q, Wu, X, Tao, C, Gong, W, Chen, M, Qu, M, et al. (2023). Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal transduction and targeted therapy 8 (1), 56. doi:10.1038/s41392-023-01330-w
Zemlyak, O. S, Babinets, L. S, and Halabitska, I. M (2023). The role of endotoxicosis and inflammation in deepening the pancreatic functional insufficiency in chronic pancreatitis in combination with type 2 diabetes. Polski merkuriusz lekarski organ Polskiego Towarzystwa Lekarskiego 51 (3), 207–215. doi:10.36740/Merkur202303104
Zhang, S, Zou, W, Leng, Y, Mu, Z, and Zhan, L (2024). Neuroprotective effects of metformin on cerebral ischemia-reperfusion injury: modulation of JNK and p38 MAP kinase signaling pathways. Cell biochemistry and biophysics 82 (3), 2597–2606. doi:10.1007/s12013-024-01373-y
Zheng, L, Zhang, Z, Sheng, P, and Mobasheri, A (2021). The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing research reviews 66, 101249. doi:10.1016/j.arr.2020.101249
Zhu, Z, Huang, J. Y, Ruan, G, Cao, P, Chen, S, Zhang, Y, et al. (2022). Metformin use and associated risk of total joint replacement in patients with type 2 diabetes: a population-based matched cohort study. CMAJ Canadian Medical Association journal = journal de l'Association medicale canadienne 194 (49), E1672–E1684. doi:10.1503/cmaj.220952
Zou, Z, Hu, W, Kang, F, Xu, Z, Li, Y, Zhang, J, et al. (2024). Interplay between lipid dysregulation and ferroptosis in chondrocytes and the targeted therapy effect of metformin on osteoarthritis. Journal of advanced research 69, 515–529. doi:10.1016/j.jare.2024.04.012
Zuliani, I, Urbinati, C, Valenti, D, Quattrini, M. C, Medici, V, Cosentino, L, et al. (2020). The anti-diabetic drug metformin rescues aberrant mitochondrial activity and restrains oxidative stress in a female mouse model of rett syndrome. Journal of clinical medicine 9 (6), 1669. doi:10.3390/jcm9061669
Keywords: osteoarthritis, impaired glucose tolerance, metformin, inflammatory markers, disease-modifying therapy
Citation: Halabitska I, Petakh P and Kamyshnyi O (2025) Metformin as a disease-modifying therapy in osteoarthritis: bridging metabolism and joint health. Front. Pharmacol. 16:1567544. doi: 10.3389/fphar.2025.1567544
Received: 27 January 2025; Accepted: 27 February 2025;
Published: 19 March 2025.
Edited by:
Agnieszka Śliwińska, Medical University of Lodz, PolandReviewed by:
Joseph S. Marino, University of North Carolina at Charlotte, United StatesCopyright © 2025 Halabitska, Petakh and Kamyshnyi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pavlo Petakh, cGF2bG8ucGV0YWtoQHV6aG51LmVkdS51YQ==; Oleksandr Kamyshnyi, a2FteXNobnlpX29tQHRkbXUuZWR1LnVh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.