
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol., 01 September 2023
Sec. Obstetric and Pediatric Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1213734
This article is part of the Research TopicPatient Safety in Obstetric and Pediatric PharmacologyView all 6 articles
Objective: This study aims to determine the drug concentration of etomidate, remifentanil, and rocuronium bromide for general anesthesia in fetus as well as the placental transport rate between term and preterm delivery, twins, and singleton.
Study design: Sixty parturients with 72 fetuses undergoing cesarean section under general anesthesia were included. According to whether the fetus was a twin or premature, parturients were divided into Group I (term singleton), Group II (premature singleton), Group III (term twins), and Group IV (premature twins). The preoperative demographic characteristics and laboratory examination of parturients, hemodynamic indicators, the Apgar score of neonates at 1, 5, and 10 min after delivery and at specific assigned values, umbilical artery blood gas analysis results, neonatal weight, and resuscitative measures were recorded. Anesthetic drug concentrations in maternal arterial (MA), umbilical arterial (UA), and umbilical venous (UV) blood were detected by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry (UPLC-MS/MS).
Result: No significant differences were observed in the concentrations of etomidate, remifentanil, and rocuronium bromide in MA, UV, and UA blood, or in the UV/MA and UA/UV ratios between term and preterm infants, twins, and singletons. Moreover, there was no variation in the anesthetic drug concentration among each pair of twins. Additionally, no correlation was found between the neonatal weight and the plasma concentrations of anesthetic drugs in UV and UA blood, except for remifentanil in UA blood.
Conclusion: Preterm or twin deliveries do not affect the neonatal concentration of etomidate, remifentanil, and rocuronium bromide used in general anesthesia for cesarean sections.
Clinical Trial Registration: www.chictr.org.cn, identifier ChiCTR2100046547
Intraspinal anesthesia remains the preferred option for cesarean sections, surpassing general anesthesia in numerous aspects. Nonetheless, in certain critical cases, general anesthesia may be more suitable or had to be the sole alternative. Recent reports indicate that general anesthesia has accounted for 5.8% of total cesarean section anesthesia and 14.6% of emergency cesarean sections due to various reasons (Juang et al., 2017). In terms of general anesthesia, etomidate, remifentanil, and rocuronium bromide are widely used in practice.
Remifentanil belongs to μ opioid receptor agonists and is administered intravenously during anesthesia for cesarean section. It offers an optimal balance between reducing maternal stress response and minimizing neonatal respiratory depression (White et al., 2019). Remifentanil easily passes through the placental barrier due to its fat-soluble characteristics. However, it can be hydrolyzed in cord blood due to its rapid metabolism features and will rapidly be redistributed and can even enter the fetus. The distribution volume of remifentanil in newborns and infants is larger than that in adults or toddlers, and the clearance rate is faster (Kan et al., 1998). Our previous data demonstrate that there is no correlation between the plasma concentration of remifentanil in full-term newborns and induction to delivery (I-D) intervals time. This finding is consistent with another study (Hu et al., 2017) that reported that the mean concentrations of remifentanil in the MA, UA, and UV blood at delivery in the shorter I-D group were similar to those in the longer I-D group.
Etomidate has been used in cesarean sections under general anesthesia for approximately 50 years (Downing et al., 1979). In particular, etomidate offers several advantages for parturients with previous congenital heart disease (Clivatti et al., 2012; Ho et al., 2018). Remifentanil combined with etomidate and muscle relaxant (rocuronium bromide) are becoming much more popular for general anesthesia in cesarean sections; however, the concentration and placental transfer of these three drugs in twins and premature infants is poorly understood.
Due to advancements in assisted reproductive technology, the incidence of twin pregnancies has escalated significantly. It is noteworthy that twin pregnancies have a higher likelihood of premature birth compared to singleton pregnancies. Premature newborns and full-term newborns exhibit significant differences in many aspects of organ development, and the risk factors affecting respiratory depression also vary between them (Condo et al., 2017). Recent research (Louchet et al., 2021) indicates that cord concentrations of antiretroviral differed in nearly one-third of twins in the same pair, which may be due to inter-individual genetic variability of placental transporters or physiological differences between twins.
For general anesthesia drugs, concerns are arising regarding the side effects of these anesthetic agents for neonates due to the lack of well-established dosing regimens for these drugs in the specific population. It remains unclear whether there are variations in drug concentrations or placental transport rates between term and preterm newborns as well as between twins. The primary objectives of this study were to ascertain the drug concentrations in the fetus and the placental transport rates for twin pairs and preterms versus full-term deliveries during cesarean sections performed under general anesthesia. This research has potential implications in clinics for understanding how anesthesia drug administration during cesarean sections affects fetal exposure to medications, especially in the context of multiple pregnancies and premature births.
This study was implemented in accordance with the Declaration of Helsinki, was approved by the Research Ethics Committee of the Women and Children’s Hospital of Chongqing Medical University (Number 2021-011), and was registered with the Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org) (registration ID: ChiCTR2100046547). Informed consent was obtained from all participants. The inclusion criteria in this study are parturients with a preoperative history of lumbar disc herniation or lumbar surgery, thrombocytopenia or other medical conditions that contraindicated to intraspinal anesthesia. Sixty parturients with 72 fetuses undergoing cesarean section under intravenous–inhalation combined anesthesia were included. The exclusion criteria for this study were as follows: mental illness, cognitive impairment, high risk of difficult airway assessment, or full stomach needing awake tracheal intubation. According to whether the parturient was twin or premature, enrolled parturients were divided into four groups as follows: Group I (full-term singleton), Group II (premature singleton), Group III (full-term twins), and Group IV (premature twins) (see Figure 1).
All parturients were strictly fasted for at least 8 h and forbidden to drink for 2 h before surgery. After entering the operating room, all parturients lay on their left side at 30° to reduce the compression of the inferior vena cava and abdominal aorta by the uterus and to prevent the occurrence of supine hypotension syndrome. Firstly, a peripheral venous route in the forearm was established. Parturients were administered oxygen via a face mask and non-invasive arterial pressure (NIBP), heart rate (HR), and peripheral oxygen saturation (SpO2) were monitored as normal. Anesthesia was induced with target-controlled infusion (TCI) of remifentanil at 5 ng/mL combined with 5% sevoflurane inhalation (sevoflurane was administered for a brief duration and was discontinued if the parturients lost consciousness). In the meantime, etomidate (0.25 mg/kg) and rocuronium (0.6 mg/kg) were administered intravenously. Once the muscle relaxant rocuronium took effect, endotracheal intubation was inserted and connected with an anesthesia machine for mechanical ventilation. Anesthesia was maintained using remifentanil (TCI, 5 ng/mL) and propofol (TCI, 3.5 μg/mL) after umbilical cord ligation.
The recorded data for statistical comparisons include the preoperative demographic characteristics and laboratory examination of parturients, hemodynamic indicators, the Apgar score at 1, 5, and 10 min after delivery, umbilical artery blood gas analysis, neonatal weight, and resuscitative measures, including tactile stimulation and bag-mask ventilation or tracheal intubation of newborns. Maternal arterial (MA), umbilical arterial (UA), and umbilical venous (UV) blood samples were obtained after umbilical cord ligation for analysis of drug concentrations. The samples were centrifuged in sodium citrate–coated anticoagulant tubes at 3,000 rpm at 4°C for 10 min. The plasma was isolated and stored at −80°C for later analysis.
The following chemicals were used for the analysis procedure: methanol and acetonitrile were used for Ultra Performance Liquid Chromatography (UPLC) (Fisher Scientific, United States), remifentanil standard was purchased from Stanford Chemicals (FJTI-8736, Oregon, United States), etomidate (E933300) and rocuronium (R639500) standards were purchased from Toronto Research Chemicals (Toronto, Canada), internal standards (IS) sufentanil was purchased from Stanford Chemicals (SREP-5931, Oregon, United States), metomidate was purchased from MACKLIN (Shanghai, China), vecuronium (V102500) was purchased from Toronto Research Chemicals (Toronto, Canada), Milli-Q apparatuses (Millipore, Bedford, MA, United States) were used to purify the water during the study, and formic acid was purchased from Shanghai Aladdin Bio-Chem Technology Co., LTD. (Shanghai, China).
A tandem mass spectrometer (Waters, United States) equipped with an electrospray ionization source operated in the positive mode provided the analytical platform. A reverse-phase BEH C18 column (50 × 2.1 mm, 1.7 m, Waters Acquity) was used for separation. This was conducted at 45 °C with a 0.4 mL/min flow of milli-Q water containing 0.1% (v/v) formic acid (solution A) along with a 1:1 (v/v) mixture of methanol and acetonitrile containing 0.1% (v/v) formic acid (solution B). From 0 to 0.6 min, the gradient was 10% solution B; from 0.6 to 2.8 min, 10%–90% solution B; and from 2.8 to 3.6 min, 10% solution B.
Using the tandem mass spectrometer as an electrospray ionization source, eluted drugs were analyzed at 150°C; capillary voltage, 3.0 kV; desolvation temperature, 500°C; gas flow for desolvation, 800 L/h; and gas flow for cone, 150 L/h. An MRM event was recorded every time-scheduled minute. Data were processed and quantified using MassLynx mass spectrometry software (Waters, Massachusetts, United States). The three target drugs were identified using structurally similar drugs of protonated molecules and by comparing their retention times with those of IS. As part of the MRM mode, extracted ion chromatograms were created using the mass of the precursor ions that collided in the collision chamber to produce fragment ions. The most stable fragment ion with the highest intensity was used for quantification, from which the area under the peak was calculated.
Statistical analyses were performed using SPSS version 22.0 for Windows. Measurement data are expressed as the mean ± standard deviation (
There was no statistically significant difference among the four groups in preoperative general information, except for gestational weeks. For preoperative laboratory examination of parturients, the D-dimer of Group IV was nearly twice as high as that of Group I (p < 0.05). These differences were related to the grouping criteria. Table 1 shows the comparison of preoperative general information and laboratory examination of parturients among the four groups. The hemodynamic indicators, including HR, NIBP, and SpO2, are shown in Supplementary Table S1.
A total of 60 parturients and 72 newborns were included in this analysis. The weight of singleton premature newborns and twin newborns was significantly lower than that of the singleton term group (p < 0.05). The incidence of neonatal asphyxia in Group IV (35.71%) was significantly higher than that in full-term delivery groups (I, III) (p < 0.05). Although there was no statistically significant difference in 1-5-10 min Apgar scores among the groups, when the sub-items of the score were compared, the reflex and color scores of the 1-min Apgar scores in Group IV were worse than those in other groups (p < 0.05). Additionally, the color scores of the 5-min Apgar scores in Groups II and IV were also worse than those in Group I (p < 0.05). The proportion of bag-mask ventilation usage for newborns and the send to NICU rate in premature newborns (Groups II and IV) were higher than those in term newborns (Groups I and III). The proportion of tracheal intubation in Group IV was significantly higher than that in Group I (p < 0.05). The results of the blood gas analysis were consistent with the neonatal condition; the umbilical artery blood pH of Group IV was significantly lower than that of Group I (p < 0.05) (see Table 2).
Twin birth and premature delivery had no effect on the concentrations of general anesthetics at delivery. There was no significant difference in the plasma concentrations of the anesthetic drugs etomidate, remifentanil, and rocuronium bromide in MA, UV, and UA blood as well as the UV/MA ratio and UA/UV ratio among the four groups (p > 0.05) (see Tables 3–5). Among the 12 twin parturients, only 2 of them were monochorionic diamniotic, while the remaining 10 were dichorionic diamniotic. As shown in Supplementary Table S2, there were no statistically significant differences between monochorionic diamniotic and dichorionic diamniotic at the three anesthetics concentrations in MA, UV, and UA blood.
During a cesarean section of a twin pregnancy, the birth order has no impact on the blood concentration of anesthetic drugs (etomidate, remifentanil, and rocuronium bromide) in UV or UA blood and UV/MA ratio and UA/UV ratio (p > 0.05) (see Table 6).
Except for remifentanil in UA blood, neonatal weight showed no significant correlation with the plasma concentration of anesthetic drugs in UV or UA blood (see Figures 2–4). A positive correlation was found between remifentanil plasma concentrations and neonatal weight in UA blood (p < 0.05) (see Table 7).

FIGURE 2. Correlations of neonatal remifentanil concentrations and neonatal weight. Ther is no correlation between neonatal weight and neonatal remifentanil plasma concentration in UV blood. There is a positive correlation between neonatal weight and remifentanil plasma concentrations in UA blood (p < 0.05).
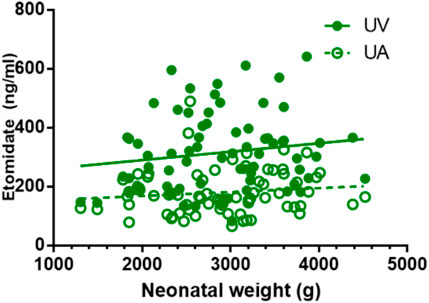
FIGURE 3. Correlations of neonatal etomidate concentrations and neonatal weight. There is no correlation between neonatal weight and etomidate plasma concentration in UV and UA blood (p > 0.05).
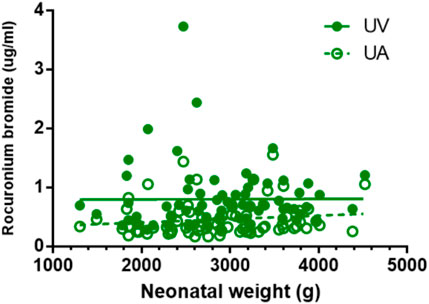
FIGURE 4. Correlations of neonatal rocuronium bromide concentrations and neonatal weight. There is no correlation between neonatal weight and rocuronium bromide plasma concentration in UV and UA blood (p > 0.05).
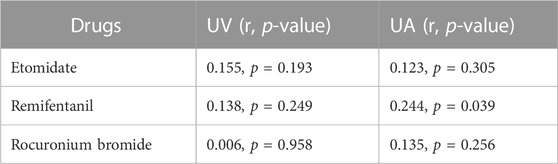
TABLE 7. Correlation coefficient of neonatal blood concentrations of etomidate, remifentanil, rocuronium bromide with neonatal weight.
The utilization of opioid induction for CS remains a controversial topic, with numerous theorized risks and benefits. The rising incidence of caesarean deliveries for premature babies under general anesthesia raises concerns about the assumption that neonate exposure to anesthetics will lead to respiratory depression after birth. Cord blood concentrations reflect the drug transfer across the placenta and pharmacokinetics in the fetus, both of which may differ within a twin pair or between preterm and term infants. In this study, the blood concentrations of various anesthetic drugs (etomidate, remifentanil, and rocuronium bromide) in MA, UV, and UA blood at delivery undergoing caesarean section between term and preterm infants, twins, and singletons were analyzed by UPLC-MS/MS. No differences were found for these anesthetic drugs concentrations in MA, UV, and UA blood or UV/MA and UA/UV ratios between term and preterm infants, twins, and singletons. Moreover, there was no variation in the anesthetic drug concentration among each pair of twins. Additionally, no correlation was found between the neonatal weight and the plasma concentrations of anesthetic drugs in UV and UA blood, except for remifentanil in UA blood.
Our data reveals that the incidence of neonatal asphyxia in twin premature delivery was up to 35.71% for caesarean section under general anesthesia. Immediately after birth, we observed that the reflex and color of newborns were predominantly affected, while respiration seemed less impacted. This suggests that the adverse effects on preterm twin infants due to general anesthesia may not be directly attributed to respiratory depression caused by the anesthesia drugs. In addition, the proportion of tracheal intubation in the premature twin group was significantly higher than that in the full-term singleton group, suggesting extra attention should be paid to the issue of asphyxia in newborns born through cesarean section under general anesthesia.
No significant differences were observed in etomidate, remifentanil, and rocuronium bromide concentrations between term and preterm infants; twins and singletons are the main findings of this study. Etomidate has been used in cesarean sections under general anesthesia for many years. A previous study (Gregory and Davidson, 1991) demonstrated that the umbilical/maternal vein ratio of etomidate was 0.5 (SD 0.18), with no relation to time in the range encountered, and that the uterine artery/uterine vein ratio of etomidate was 0.86 (SD 0.33), suggesting that etomidate uptake into the fetus is effectively complete. Another study (Esener et al., 1992) in 40 patients undergoing cesarean deliveries found that the mean plasma etomidate concentration decreased rapidly (1,242.0 ng/mL at 5 min to undetectable 2 h after injection) and that the umbilical:maternal vein ratio was 1:24. And etomidate levels in colostrum were 79.3 ng/mL after 30 min, which reduced to undetectable after 4 h, indicating that a single injection of etomidate is safe for parturients and lactation.
Remifentanil is a μ-opioid receptor agonist with weak affinity to δ- and κ-receptors. Blood-brain balance is rapidly reached in the human body in approximately 1 min and the drug is rapidly hydrolyzed in tissue and blood, resulting in a fast onset and short maintenance time, which is obviously different from other fentanyl analogues. Remifentanil is mainly metabolized by non-specific esterase hydrolysis in plasma and tissues. Approximately 95% of remifentanil is metabolized and excreted in urine, with the main metabolite activity being only 1/4,600. There is no change in metabolic rate and no accumulation in the body after long-term infusion or repeated injection, and the incidence of delayed respiratory depression is very low (Kan et al., 1998; Sridharan and Sivaramakrishnan, 2019). A previous study (Welzing et al., 2011) demonstrated that very preterm infants exhibited a high nonspecific esterase activity in umbilical cord blood compared with term infants, which contributed to the rapid drug metabolization in preterm infants. This rapid metabolic feature also explains the results in our study, wherein the plasma concentrations of remifentanil and etomidate are not different between the groups. As for the positive correlation found between remifentanil plasma concentrations and neonatal weight in UA blood, it is inferred that preterm infants exhibit a high nonspecific esterase activity in umbilical cord blood compared with term infants, affecting the metabolism and distribution of remifentanil in newborns.
Rocuronium provides the shortest onset of action of non-depolarizing blocking agents, with few adverse reactions. It is highly water-soluble and difficult to pass through the placental barrier. As early as 1994, it was used for general anesthesia in cesarean section (Abouleish et al., 1994). Due to its characteristic large molecular weight, most studies related to rocuronium (Abu-Halaweh et al., 2007; Blaha et al., 2020) focused on the onset time and muscle relaxant effects, while the exact placental transfer rate was absent. Our results show that the placental transfer rate of rocuronium in combination with etomidate and remifentanil was 16% in single term neonates.
Apgar scores are commonly used as surrogate measures to predict subsequent adverse neonatal outcomes (Cnattingius et al., 2020). Due to the grouping conditions, the Apgar scores between groups were different for neonates, with those from twin premature delivery being inferior in most indicators (e.g., weight, the reflex and color scores of 1 min and 5 min Apgar scores, the proportion of using bag-mask ventilation and tracheal intubation, and umbilical artery blood pH). In addition, D-dimer was different (higher in twins) between groups due to the grouping conditions, which is consistent with the changing model results of D-dimer in twins (Bar et al., 2000; Morikawa et al., 2011).
This study is the first to describe the concentrations and placental transfer rates of intravenous drugs in twins and premature infants born by cesarean section under general anesthesia. However, the study has several limitations. Firstly, the sample size of the study is small, resulting in a large data standard deviation and some differences (such as the positive correlation between remifentanil concentration in UA and neonatal weight) that cannot be clearly explained. It is necessary to collect a larger sample size to clarify this confusion. Secondly, cord blood concentrations were determined only at birth, which does not reflect dynamic changes in the features of neonates after birth. In addition, monochorionic and dichorionic diamniotic twins were pooled for analysis, since obtaining a sufficient number of cases for twins under general anesthesia is challenging.
In conclusion, preterm or twin deliveries do not affect the neonatal concentrations of etomidate, remifentanil, and rocuronium bromide used in general anesthesia for cesarean sections.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Medical Ethics Committee of Chongqing Health Center for Women and Children (reference number: 2021-011). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
HL laboratory tests, data curation, writing-original draft. J-KM methodology, supervision, investigation, writing–original draft. MC, LG, and H-QZ investigation, data curation, formal analysis. X-FL writing–review, resource, supervision. JY conceptualization, investigation, formal analysis, writing–review and editing. All authors contributed to the article and approved the submitted version.
This study was supported by Natural Science Foundation of Chongqing of China (No. cstc2021jcyj-msxmX0763), Institutional Research Grants of Chongqing Health Center for Women and Children (No. 2021YJMS03) and National Key Clinical Specialty Construction Project (Obstetrics and Gynecology).
We are grateful to Prof. Yongchun Su at Chongqing Youyoubaobei Women and Children’s Hospital for his manuscript preparation assistance. We thank all the participating families and the research assistants involved in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1213734/full#supplementary-material
Abouleish, E., Abboud, T., Lechevalier, T., Zhu, J., Chalian, A., and Alford, K. (1994). Rocuronium (Org 9426) for caesarean section. Br. J. Anaesth. 73, 336–341. doi:10.1093/bja/73.3.336
Abu-Halaweh, S. A., Massad, I. M., Abu-Ali, H. M., Badran, I. Z., Barazangi, B. A., and Ramsay, M. A. (2007). Rapid sequence induction and intubation with 1 mg/kg rocuronium bromide in cesarean section, comparison with suxamethonium. Saudi Med. J. 28, 1393–1396.
Bar, J., Blickstein, D., Hod, M., Bar-Hava, I., Ben-Rafael, Z., Rahmany-Babai, J., et al. (2000). Increased D-dimer levels in twin gestation. Thromb. Res. 98, 485–489. doi:10.1016/s0049-3848(00)00187-0
Blaha, J., Noskova, P., Hlinecka, K., Krakovska, V., Fundova, V., Bartosova, T., et al. (2020). Surgical conditions with rocuronium versus suxamethonium in cesarean section: A randomized trial. Int. J. Obstet. Anesth. 41, 14–21. doi:10.1016/j.ijoa.2019.08.005
Clivatti, J., Smith, R. L., Sermer, M., Silversides, C., and Carvalho, J. C. (2012). Cardiac output monitoring during Cesarean delivery in a patient with palliated tetralogy of Fallot. Can. J. Anaesth. 59, 1119–1124. doi:10.1007/s12630-012-9793-6
Cnattingius, S., Johansson, S., and Razaz, N. (2020). Apgar score and risk of neonatal death among preterm infants. N. Engl. J. Med. 383, 49–57. doi:10.1056/NEJMoa1915075
Condo, V., Cipriani, S., Colnaghi, M., Bellu, R., Zanini, R., Bulfoni, C., et al. (2017). Neonatal respiratory distress syndrome: are risk factors the same in preterm and term infants? J. Matern. Fetal Neonatal Med. 30, 1267–1272. doi:10.1080/14767058.2016.1210597
Downing, J. W., Buley, R. J., Brock-Utne, J. G., and Houlton, P. C. (1979). Etomidate for induction of anaesthesia at caesarean section: comparison with thiopentone. Br. J. Anaesth. 51, 135–140. doi:10.1093/bja/51.2.135
Esener, Z., Sarihasan, B., Guven, H., and Ustun, E. (1992). Thiopentone and etomidate concentrations in maternal and umbilical plasma, and in colostrum. Br. J. Anaesth. 69, 586–588. doi:10.1093/bja/69.6.586
Gregory, M. A., and Davidson, D. G. (1991). Plasma etomidate levels in mother and fetus. Anaesthesia 46, 716–718. doi:10.1111/j.1365-2044.1991.tb09762.x
Ho, Y. C., Boey, S. K., Varughese Mathews, A. M., See, H. G., and Hwang, N. C. (2018). An unusual case of a parturient with uncorrected pentalogy of fallot presenting for elective cesarean section delivery of twins. Anesth. Essays Res. 12, 267–270. doi:10.4103/aer.AER_126_17
Hu, L., Pan, J., Zhang, S., Yu, J., He, K., Shu, S., et al. (2017). Propofol in combination with remifentanil for cesarean section: placental transfer and effect on mothers and newborns at different induction to delivery intervals. Taiwan J. Obstet. Gynecol. 56, 521–526. doi:10.1016/j.tjog.2016.09.010
Juang, J., Gabriel, R. A., Dutton, R. P., Palanisamy, A., and Urman, R. D. (2017). Choice of anesthesia for cesarean delivery: an analysis of the national anesthesia clinical outcomes Registry. Anesth. analgesia 124, 1914–1917. doi:10.1213/ANE.0000000000001677
Kan, R. E., Hughes, S. C., Rosen, M. A., Kessin, C., Preston, P. G., and Lobo, E. P. (1998). Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology 88, 1467–1474. doi:10.1097/00000542-199806000-00008
Louchet, M., Peytavin, G., Didelot, H., Le, M., Bourgeois-Moine, A., Carbillon, L., et al. (2021). Frequency of differential placental transfer to twins of maternal antiretroviral medications. Eur. J. Obstet. Gynecol. Reprod. Biol. 256, 405–411. doi:10.1016/j.ejogrb.2020.11.004
Morikawa, M., Yamada, T., Yamada, T., Akaishi, R., Koyama, T., and Minakami, H. (2011). Changes in D-dimer levels after cesarean section in women with singleton and twin pregnancies. Thromb. Res. 128, e33–e38. doi:10.1016/j.thromres.2011.05.011
Sridharan, K., and Sivaramakrishnan, G. (2019). Comparison of fentanyl, remifentanil, sufentanil and alfentanil in combination with propofol for general anesthesia: A systematic review and meta-analysis of randomized controlled trials. Curr. Clin. Pharmacol. 14, 116–124. doi:10.2174/1567201816666190313160438
Welzing, L., Ebenfeld, S., Dlugay, V., Wiesen, M. H., Roth, B., and Mueller, C. (2011). Remifentanil degradation in umbilical cord blood of preterm infants. Anesthesiology 114, 570–577. doi:10.1097/ALN.0b013e318204e043
Keywords: cesarean section, general anesthesia, preterm, twins, drug concentration
Citation: Liu H, Miao J-K, Cai M, Gan L, Zhao H-Q, Lei X-F and Yu J (2023) Anesthetic drug concentrations and placental transfer rate in fetus between term and preterm infants, twins, and singletons. Front. Pharmacol. 14:1213734. doi: 10.3389/fphar.2023.1213734
Received: 28 April 2023; Accepted: 17 August 2023;
Published: 01 September 2023.
Edited by:
Geert W. 't Jong, University of Manitoba, CanadaReviewed by:
Yinyin Qu, Peking University Third Hospital, ChinaCopyright © 2023 Liu, Miao, Cai, Gan, Zhao, Lei and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Yu, dodoes@qq.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.