- Department of Dermatology, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang Province, China
Alopecia areata (AA) is a common non-scarring hair loss condition whose specific pathogenesis is not yet fully understood. In children, AA often co-occurs with atopic dermatitis (AD), complicating treatment. Here, we report the case of a child with myasthenia gravis who had severe AA and moderate AD. The child had previously been treated with local injections of corticosteroids and developed total hair loss and AD after discontinuing corticosteroid use. After approximately one year of treatment with baricitinib, 4 mg once daily, combined with twice-daily application of a corticosteroid ointment, a significant improvement in the child's condition was observed, with the Severity of Alopecia Tool score dropping from 100 to 24.4 and Eczema Area Severity Index score to 0. New vellus hairs were clearly observable under trichoscopy, which contrasted significantly with the pre-treatment state. Throughout the treatment process, the patient's clinical symptoms, blood cell counts, liver and kidney function, and coagulation functions were essentially normal, with no significant adverse reactions observed except for folliculitis on the scalp. We discuss common targets in the pathogenesis of AA and AD as well as the safety and prospects of Janus kinase inhibitors for the treatment of pediatric patients with these conditions.
1 Introduction
Alopecia areata (AA) is a chronic, relapsing autoimmune disease characterized by non-scarring, round or oval patches of hair loss, with alopecia universalis being the complete loss of all hair and body hair. AA often co-occurs with atopic dermatitis (AD). Both conditions are difficult to cure completely, with severe effects on the quality of life and mental health of patients (1), especially in children. Systemic treatments for moderate-tosevere AD in children over 12 years old include hormones, cyclosporine, antibodies targeting the type 2 inflammatory pathway (dupilumab), and Janus kinase (JAK) inhibitors (baricitinib, abrocitinib, upadacitinib), with only baricitinib being approved by the Food and Drug Administration (FDA), European Medicines Agency (EMA), and China Food and Drug Administration (CFDA) for the treatment of moderatetosevere AA in adults. There have been numerous reports in the literature on the efficacy and safety of baricitinib in children with severe AA in recent years. Here, we report the case of a 14-year-old child with severe AA and moderate AD who was successfully treated with baricitinib.
Informed consent was obtained from the patient and his grandmother for participation in the study and publication of the article, including publication of clinical photographs and laboratory test results.
2 Case description
We report the case of a 14-year-old male, born in southeast China, with a maternal history of allergic rhinitis. The patient had had eczema since the age of 2 years and myasthenia gravis since the age of 5 years. He had undergone high-dose corticosteroid pulse therapy for approximately 2 years at a local hospital. After his myasthenia gravis improved and the medication was discontinued, he began to develop exacerbated eczematous skin lesions all over his body, with the scalp being the most severely affected. The patient found the itching intolerable and repeatedly scratched his scalp. Since the age of 8 years, he had experienced patchy hair loss on both sides of his scalp, which was treated with local injections of corticosteroids. After treatment, his hair regrew, but after discontinuing the medication, all of his hair fell out and parts of his eyebrows and eyelashes were also affected. When the patient came to our department, erythema and scratch marks were visible on his head, face, trunk, and both upper limbs. His skin was dry and itching was significant, with an Eczema Area Severity Index (EASI) score of 18, indicating moderate AD. His hair and eyebrows had almost completely fallen out, his eyelashes were very sparse, and the Severity of Alopecia Tool (SALT) score was 100 (Figure 1), with no nail abnormalities observed, leading to a diagnosis of alopecia universalis. The patient was also quite introverted, avoiding direct eye contact, and wore a mask and hat throughout the visit.
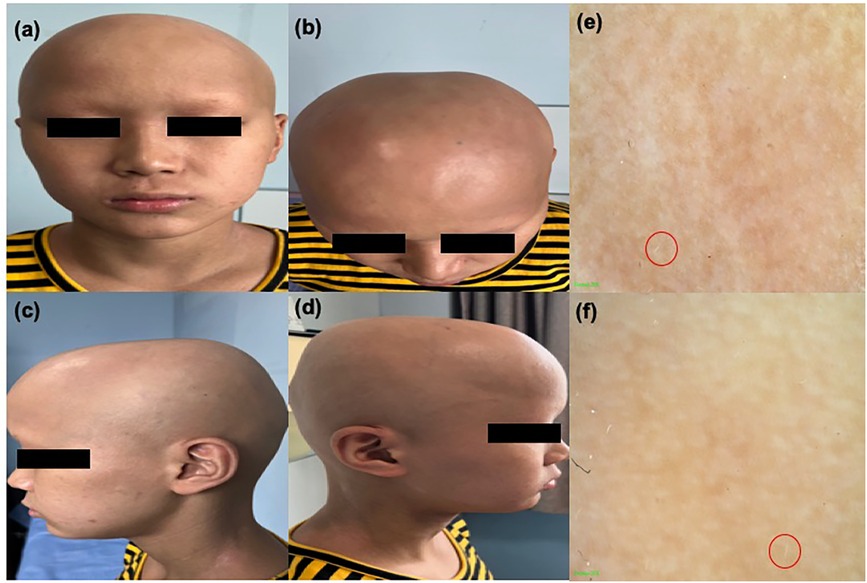
Figure 1. (a)–(d) Clinical image of the patient showing alopecia areata. (e) and (f) Trichoscopy image of alopecia areata; a small number of white vellus hairs can be observed within the red circle.
In addition to AA, we considered the possibility of other conditions such as trichotillomania, tinea capitis, scarring alopecia, and telogen effluvium. However, the diagnosis of AA can usually be confirmed based on the characteristic appearance of the bald patches, and the patient's medical history largely ruled out trichotillomania and telogen effluvium. Although the clinical symptoms of scarring alopecia are very similar to those of AA, in this condition, hair follicle openings are not visible upon clinical examination and tinea capitis can be excluded through fungal tests and hair microscopy.
After communicating with the patient and his family (who refused treatment with corticosteroid injections again) and considering the patient's medical history, we decided to treat the AA and AD with baricitinib 4 mg once daily combined with twice-daily application of dinideotide ointment. Baricitinib is currently the only drug approved in China for the treatment of adult AA. It has been approved by the FDA for adult rheumatoid arthritis, AA, and COVID-19, and by the EMA for the treatment of AD in children and adults over 2 years old, adult AA, and juvenile idiopathic arthritis in children over 2 years old. Before treatment, we performed a complete blood cell count, liver and kidney function tests, viral hepatitis markers, and tuberculosis screening. During treatment, complete blood cell counts, tests of liver and kidney function, and coagulation function tests were conducted regularly.
In the first 12 weeks of treatment, only a small number of new white vellus hairs could be seen by hair microscopy, and the patient reported being able to feel the new growth. At around 24 weeks of treatment, multiple clusters of short, dark new hairs were visible to the naked eye and the eczematous skin lesions on the patient's trunk and upper limbs had receded. The patient occasionally experienced itching and scaling on the face, which could be relieved with emollients. After 59 weeks of treatment, we observed significant clinical improvements in both the patient's AD and AA (Figure 2), with the SALT score dropping from 100 to 24.4 and the EASI score to 0. Hair microscopy indicated regrowth of hair on the scalp, eyebrows, and eyelashes. The patient's eczematous skin lesions had completely resolved. The patient reported occasional skin itching when exposed to heat, which could be alleviated with antihistamine medication.
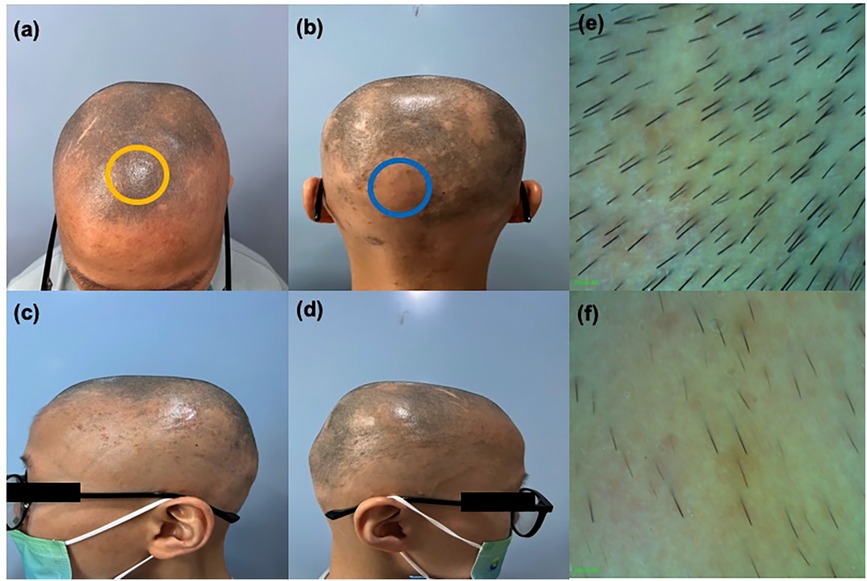
Figure 2. (a)–(d) Clinical image of the patient showing alopecia areata after 59 weeks of treatment. (e) Enlarged trichoscopy image of the yellow circle (f) enlarged trichoscopy image of the blue circle.
Throughout the treatment process, the blood cell counts, liver and kidney function, and coagulation function tests showed no significant abnormalities. Scattered episodes of folliculitis on the scalp occurred during the treatment, which increased noticeably after intense physical activity and sweating; these could be resolved with the application of fusidic acid ointment. At week 52 (during the hot summer months), a large number of folliculitis lesions appeared on the scalp that had not completely resolved by week 59. The patient's scalp folliculitis may have been related to his habit of wearing a hat, as no significant acne or folliculitis were observed on his face, neck, or trunk. The patient's family reported that because of his severe AA, the patient wore a hat every day when going out, including on the way to school, during meals and medical visits, and even during physical activities. The patient was also quite introverted, communicating with us through either nodding or shaking his head, during the early stages of treatment, but began to actively communicate with the doctor at week 24. By week 52, he could independently describe his condition, although he continued to wear a hat consistently outside of treatment sessions. The patient and family are very satisfied with the current treatment effects and are willing to continue the current treatment and follow up regularly.
Currently, the patient is still under follow-up. Our limitations include the lack of histopathological data and long-term follow-up information, especially regarding the potential impact on growth and development in children.
3 Discussion
Alopecia areata (AA) is a complex disease resulting from the interplay of multiple factors, and its pathogenesis involves multiple biological targets. The prevailing view is that AA compromises the immune privilege of hair follicles (2); however, the structure of the follicles is not destroyed the damage is mainly to the hair growth cycle (3). In skin biopsies of patients with AA, anagen hair follicles were found to be affected by the infiltration of inflammatory cells—primarily CD8+ T cells and CD4+ T cells, as well as antigen-presenting cells (APC), mast cells, NK cells, and eosinophils—around and within the follicle (4). Studies have shown that the levels of Th2-related markers (IL-13, CCL13, CCL17, CCL22, and CCL26) in the serum of patients with AA are significantly elevated, and the degree of Th2 activation increases with the severity of hair loss (5). Among them, IL-13 and IL-4 both bind to the so-called type II receptor, composed of the IL-4Rα chain and the IL-13Rα1 chain, thereby activating JAK1 and tyrosine kinase 2 (TYK2), which in turn leads to the activation of STAT6. There is literature indicating that dupilumab, which can block the IL-13 and IL-4 pathways exerts therapeutic effects in patients with AA (6).
During the pathogenesis of AD, Th2 cells expressing chemokine receptorhomologous molecules infiltrate the skin and produce IL-13, IL-31, and IL-4 (7). IL-4 and IL-13 significantly reduce the expression of key structural proteins of the skin barrier (such as filaggrin) by activating STAT6 (Figure 3), leading to an increase in transepidermal water loss that is typically measured, disrupting the integrity of the stratum corneum and exacerbating the severity of AD (8).
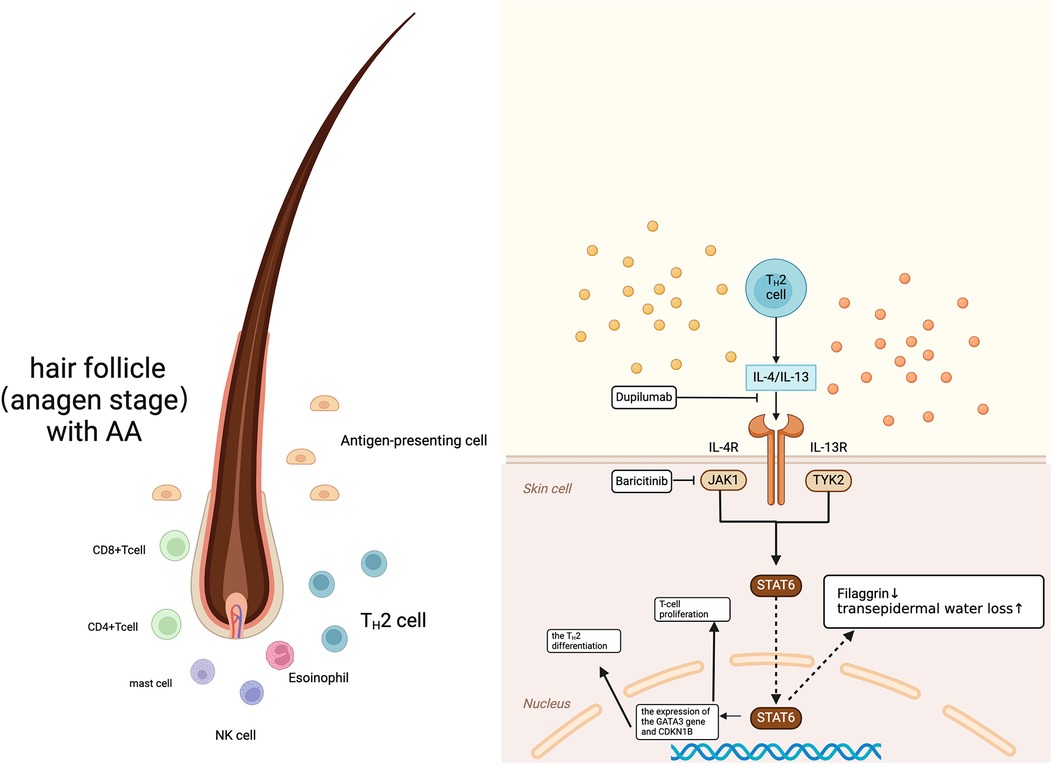
Figure 3. Common immunopathogenesis of alopecia areata AA and AD. Created in BioRender. Wang, S. (2024) https://BioRender.com/g04h745.
Other studies have found that filaggrin (FLG) gene mutations are an important risk factor for AD and are associated with the severity of AA (9). Palmer et al.'s research indicates that FLG loss-of-function mutations are closely related to AD (10). Regina C Betz et al.'s findings show that FLG mutations can drive the severity of comorbid AA in patients with AD. In the absence of FLG mutations, the comorbidity of atopic diseases has little or no impact on the severity of AA (11).
Drugs targeting the JAK pathway, such as baricitinib, can achieve therapeutic effectiveness in patients with AA and AD. In this case, owing to severe AA, the patient had been wearing a hat for a long time, which exacerbated his folliculitis. Furthermore, the patient had a relatively introverted personality, which is not uncommon (12). Children with AA and AD tend to have a much higher probability of depression than healthy children. A study by Peła et al. shows that the possibility of AA and AD appearing in patients with depression and in their families is higher. This may be because stress induces the HPA axis to release cortisol, adrenocorticotropic hormone, and beta-endorphins. Acute high levels of stress hormones have an immunosuppressive effect on Th1 cells while mediating the differentiation of T helper cells into Th2 cells. Th2 cells continue to induce the immunoglobulin class switch from IgM to IgE. IgE antibodies bind to mast cells, inducing the release of lipid mediators, chemical and protein mediators, and pro-inflammatory cytokines (such as TNF-α, TGF-β, IL-4, and IL-13), leading to eczematous skin lesions (13).
Currently, several JAK inhibitors have been proven effective in treating AA, including tofacitinib, baricitinib, abrocitinib, and upadacitinib (14–17). The discovery and clinical application of these drugs provide new treatment options for adolescent patients with comorbid AA and AD. Existing studies show that the success rate of systemic JAK inhibitors in children is comparable to that in adults, with many patients showing significant responses; furthermore, only few non-responders and minimal side effects have been reported, including mild infections, diarrhea, and transient laboratory abnormalities. Broader research in fields such as rheumatology and oncology has been conducted on JAK inhibitors in pediatric patients, and phase 1 studies of JAK inhibitors for the treatment of pediatric malignant tumors and inflammatory diseases support the potential safety of these drugs and provide dosage guidance (18). However, for a better understanding of their therapeutic efficacy in skin diseases, more data from randomized controlled trials remain necessary.
Baricitinib has been approved in several countries for the treatment of pediatric atopic dermatitis and adult alopecia areata. In phase III clinical trials of baricitinib for adult severe alopecia areata, it was observed that some patients with extensive hair loss had a delayed response, and some patients with a history of more than 4 years did not respond to a treatment dose of 2 mg daily (19, 20). The dose needed to be increased to 4 mg daily to observe clinical effects. In the clinical study, there were no cases of venous thromboembolic events, arterial thrombotic events, major adverse cardiovascular events, gastrointestinal perforations, malignant tumors, tuberculosis, or confirmed opportunistic infections. Growth assessments indicated that the patient's growth rate was consistent with their baseline height, weight, or body mass index (BMI) percentiles. A similar situation was observed in this pediatric case. In our current clinical use of baricitinib and other JAK inhibitors, and even corticosteroids, in adult patients, some have experienced hair loss again after discontinuing the medication. This means that current drugs cannot completely control the follicular inflammation in such patients, and further basic research is needed to substantiate this.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
SW: Writing – original draft. ZX: Writing – review & editing. XZ: Writing – review & editing. XF: Writing – review & editing. YY: Writing – review & editing. BL: Resources, Writing – review & editing. SX: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of the article. This work was supported by the Key Medical Discipline of Ningbo City, Rheumatology and Autoimmunology (Grant No. 2022-F08).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1497285/full#supplementary-material
References
1. Kridin K, Renert-Yuval Y, Guttman-Yassky E, Cohen AD. Alopecia areata is associated with atopic diathesis: results from a population-based study of 51,561 patients. J Allergy Clin Immunol Pract. (2020) 8(4):13231328.e1. doi: 10.1016/j.jaip.2020.01.052
2. Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. (2022) 13:955035. doi: 10.3389/fimmu.2022.955035
3. Paus R, Bulfone-Paus S, Bertolini M. Hair follicle immune privilege revisited: the key to alopecia areata management. J Investig Dermatol Symp Proc. (2018) 19(1):S12. doi: 10.1016/j.jisp.2017.10.014
4. Bertolini M, McElwee K, Gilhar A, Bulfone-Paus S, Paus R. Hair follicle immune privilege and its collapse in alopecia areata. Exp Dermatol. (2020) 29(8):703–25. doi: 10.1111/exd.14155
5. Song T, Pavel AB, Wen HC, Malik K, Estrada Y, Gonzalez J, et al. An integrated model of alopecia areata biomarkers highlights both TH1 and TH2 upregulation. J Allergy Clin Immunol. (2018) 142(5):16311634.e13. doi: 10.1016/j.jaci.2018.06.029
6. Tavoletti G, Valtellini L, Mattioli MA, Chiei-Gallo A, Barbareschi M, Marzano AV, et al. Effectiveness of dupilumab in the treatment of alopecia areata in patients with concurrent atopic dermatitis: a real-life retrospective study. Int J Dermatol. (2024) 63(12):e434–6. doi: 10.1111/ijd.17288
7. Mashiko S, Mehta H, Bissonnette R, Sarfati M. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J Dermatol Sci. (2017) 88(2):16774. doi: 10.1016/j.jdermsci.2017.07.003
8. Berdyshev E, Goleva E, Bronova I, Dyjack N, Rios C, Jung J, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight. (2018) 3(4):e98006. doi: 10.1172/jci.insight.98006
9. Suárez-Fariñas M, Ungar B, Noda S, Shroff A, Mansouri Y, Fuentes-Duculan J, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. (2015) 136(5):1277–87. doi: 10.1016/j.jaci.2015.06.032
10. Moosbrugger-Martinz V, Leprince C, Méchin MC, Simon M, Blunder S, Gruber R, et al. Revisiting the roles of filaggrin in atopic dermatitis. Int J Mol Sci. (2022) 23(10):5318. doi: 10.3390/ijms23105318
11. Betz RC, Pforr J, Flaquer A, Redler S, Hanneken S, Eigelshoven S, et al. Loss-of-function mutations in the filaggrin gene and alopecia areata: strong risk factor for a severe course of disease in patients comorbid for atopic disease. J Invest Dermatol. (2007) 127(11):2539–43. doi: 10.1038/sj.jid.5700915
12. Peła Z, Gałecka M, Murgrabia A, Kondratowicz A, Gałecki P. Depressive disorder and dermatological autoimmune diseases. J Clin Med. (2024) 13(11):3224. doi: 10.3390/jcm13113224
13. Mar K, Rivers JK. The mind body connection in dermatologic conditions: a literature review. J Cutan Med Surg. (2023) 27(6):628–40. doi: 10.1177/12034754231204295
14. Kołcz K, Żychowska M, Sawińska E, Reich A. Alopecia universalis in an adolescent successfully treated with upadacitinib-a case report and review of the literature on the use of JAK inhibitors in pediatric alopecia areata. Dermatol Ther (Heidelb). (2023) 13(3):843–56. doi: 10.1007/s13555-023-00889-0
15. Liu X, Song B, Jin H. Abrocitinib improved dupilumab-resistant severe atopic dermatitis with comorbid mild alopecia areata in a 12-year-old boy: a case report with 1-year follow-up. J Asthma Allergy. (2024) 17:305–11. doi: 10.2147/JAA.S458684
16. Zhao M, Wei Y, Cai L, Zhuo J, Zhang Z, Lin M. Baricitinib therapy for paediatric patients with severe alopecia areata. J Eur Acad Dermatol Venereol. (2025) 39(1):e82–3. doi: 10.1111/jdv.20108
17. Behrangi E, Barough MS, Khoramdad M, Hejazi P, Koltapeh MP, Goodarzi A. Efficacy and safety of tofacitinib for treatment of alopecia areata in children: a systematic review and meta-analysis. J Cosmet Dermatol. (2022) 21(12):6644–52. doi: 10.1111/jocd.15425
18. Hamilton CE, Craiglow BG. JAK Inhibitors for the treatment of pediatric alopecia areata. J Investig Dermatol Symp Proc. (2020) 20(1):S31. doi: 10.1016/j.jisp.2020.04.005
19. Ko JM, Mayo TT, Bergfeld WF, Dutronc Y, Yu G, Ball SG, et al. Clinical outcomes for uptitration of baricitinib therapy in patients with severe alopecia areata. JAMA Dermatol. (2023) 159(9):970–6. doi: 10.1001/jamadermatol.2023.2581
20. Torrelo A, Rewerska B, Galimberti M, Paller A, Yang CY, Prakash A, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in paediatric patients with moderate-to-severe atopic dermatitis with an inadequate response to topical corticosteroids: results from a phase III, randomized, double-blind, placebo-controlled study (BREEZE-AD PEDS). Br J Dermatol. (2023) 189(1):23–32. doi: 10.1093/bjd/ljad096
Keywords: alopecia areata, atopic dermatitis, JAK inhibitors, baricitinib, pediatric
Citation: Wang S, Xu Z, Zhu X, Fan X, Yu Y, Lin B and Xu S (2025) Case Report: Baricitinib improved alopecia areata in a pediatric patient with atopic dermatitis. Front. Pediatr. 12:1497285. doi: 10.3389/fped.2024.1497285
Received: 16 September 2024; Accepted: 27 December 2024;
Published: 10 January 2025.
Edited by:
Christian David Sadik, University of Lübeck, GermanyReviewed by:
Jing Guo, Hospital of Chengdu University of Traditional Chinese Medicine, ChinaDeming Liu, Chongqing Hospital of Traditional Chinese Medicine, China
Copyright: © 2025 Wang, Xu, Zhu, Fan, Yu, Lin and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingjiang Lin, linbingj@163.com; Suling Xu, xusuling@nbu.edu.cn
 Sihan Wang
Sihan Wang