- 1Medical and Translational Oncology Unit, AOU Renato Dulbecco, Catanzaro, Italy
- 2Department of Experimental and Clinical Medicine, Magna Græcia University, Catanzaro, Italy
- 3Radiology Unit, AOU Renato Dulbecco, Catanzaro, Italy
- 4Institute for Biomedical Research and Innovation (IRIB), National Research Council of Italy (CNR), Catanzaro, Italy
Background: The optimal strategy for the treatment of recurrent and/or advanced endometrial cancer is still undefined. Recently, despite the lack of any predictive biomarker, the combination of pembrolizumab with lenvatinib has improved survival outcomes. We here report the long-term management of lung toxicity in a patient with endometrial cancer, and we critically review the current therapeutic options for this disease.
Results: A patient with heavily pretreated endometrial cancer took pembrolizumab plus lenvatinib for 1 year, achieving a persistent partial response with a time to treatment failure of 18 months, despite relevant lung toxicity that did not affect the remarkable overall clinical benefit. A systematic review of this combination underlines the efficacy outcome despite toxicity. Interestingly, the literature review on lung toxicity suggested the role of anti-angiogenetic agents in the pathogenesis of lung cavitation, probably related to direct treatment activity, and disclosed a potential radiological sign predictive of the activity of anti-angiogenetic agents.
Conclusion: We underline the efficacy of pembrolizumab plus lenvatinib in the current treatment landscape of endometrial cancer, underscoring the relevance of a correct management of toxicity.
Introduction
Endometrial cancer (EC) is the sixth most common malignancy in women in the world (1).
At present, two major pathological conditions have been recognized: endometrioid carcinoma (type I), which represents 80% of all cases, and non-endometrioid carcinoma (type II), which includes the remaining 10%–20%, including serous carcinoma, clear cell carcinoma, and carcinosarcoma (2).
Recently, The Cancer Genome Atlas (TCGA) project analyzed about 400 different EC genomes using array- and sequencing-based technologies, which allowed the re-classification of EC into four different clusters according to genomics: POLE ultra-mutated, MSI hyper-mutated, copy number low, and copy number high (serous-like). This new classification is characterized by high biological heterogeneity able to impact the efficacy of adjuvant treatments, especially for women with aggressive and/or advanced-stage tumors, or predicting different outcomes (e.g., the POLE ultra-mutated type has the best prognosis whereas copy number high has the worst survival outcome) (3, 4).
Germline mutation or hereditary impairment of MMR genes, including MLH1, MSH2, MSH6, PMS2, and EPCAM, determined at least 5%–10% of Lynch syndrome, while epigenetic alterations, such as hypermethylation of the MLH1 promoter, epigenetic inactivation of MSH2, or downregulation of MMR genes by microRNAs, cause inhibition of transcription and interference with the expression of MMR genes and DNA repair function (5–7). Although several pathways are involved in the pathogenesis and proliferation of this tumor, particularly PI3K/AKT/mTOR alterations and mismatch repair pathway (8, 9), there is no gold standard second-line recurrent and/or advanced EC treatment after the failure of platinum-based regimens. Therefore, the current treatment of metastatic setting is still based on conventional treatment in which carboplatin plus paclitaxel is the standard (10, 11).
Moreover, hormone therapy could be considered for low-grade ER-positive cancer in adjuvant or front-line settings; targeted therapy, including trastuzumab, for tumors with HER2 overexpression; immunotherapy for dMMR/MSI-H patients; and antiangiogenetic therapy such as bevacizumab in monotherapy or in combination with carboplatin and paclitaxel, and lenvatinib with pembrolizumab for non-dMMR tumors/non-MSI high (11–19).
In Keynote-775, the lenvatinib plus pembrolizumab combination confirmed efficacy data from a previous study (Keynote-146), in terms of both PFS and OS irrespective of MRR status (20, 21).
Lenvatinib is a multitargeted tyrosine kinase inhibitor selectively acting on VEGFR 1–3, FGFR 1–4, PDGFRα, KIT, and RET, but, unlike other TKIs, it is also active in FGFR inhibition (22). Pembrolizumab is a monoclonal antibody targeting programmed death-1 (PD-1), an immune checkpoint receptor expressed on tumor-infiltrating T cells; it interacts with programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2)—expressed both on several human and on some tumor cells—and causes the inactivation of T cells, preventing T cell-mediated tumor cytolysis (23). Pembrolizumab was evaluated in gynecological cancer that progressed after standard therapy in two different studies, phase Ib Keynote-028 and phase II Keynote-158, obtaining 13.5% ORR and 48% ORR, respectively, and encouraging survival improvement (24, 25).
Drug combination emerged as a therapeutic strategy for advanced/recurrent EC in the phase Ib/II trial Keynote-146/Study 111 where lenvatinib plus pembrolizumab was associated with a 38.0% ORR irrespective of MMR status in patients with advanced previously treated (two or less prior therapies) EC (20, 26). Recent results of the multicenter, open-label, randomized phase III Keynote-775 confirmed these preliminary data in 827 advanced, recurrent EC patients after one previous platinum-based chemotherapy (no exposure to vascular endothelial growth factors or anti PD-1 therapy). Patients were randomly assigned to receive both lenvatinib 20 mg orally once daily plus pembrolizumab flat dose of 200 mg IV every 3 weeks or chemotherapy of the physician’s choice (doxorubicin 60 mg/m2 IV every 3 weeks or paclitaxel 80 mg/m2 IV weekly). They were stratified according to MMR status (130 in the dMMR population and 697 in the pMMR population) and among pMMR patients. Primary endpoints were PFS and OS, while secondary endpoints included ORR according to RECIST version 1.1, safety and side effects and health-related quality of life. All endpoints were assessed in the pMMR population and among all patients. This trial showed a significant benefit in terms of mPFS and mOS in both pMMR EC [mPFS: 6.6 vs. 3.8 months, HR 0.60 (95% CI, 0.50–0.72) p < 0.001; mOS: 17.4 vs. 12.0 months, HR 0.68 (95% CI, 0.56–0.84) p < 0.001] and overall population [mPFS: 7.2 vs. 3.8 months, HR 0.56 (95% CI, 0.47–0.66) p < 0.001; mOS: 18.3 vs. 11.4 months, HR 0.62 (95% CI, 0.51–0.75) p < 0.001]. ORR was 30.3% for lenvatinib plus pembrolizumab and 15.1% for chemotherapy for the pMMR population and 31.9% and 14.7%, respectively, in the overall population (20, 21).
We here report the use of lenvatinib plus pembrolizumab in a heavily pre-treated EC patient, not expressing actionable mutations at liquid biopsy, and who underwent immunotherapy and antiangiogenetic treatment with a long-lasting major response. We focus on the management of the lung toxicity in this patient.
Case presentation
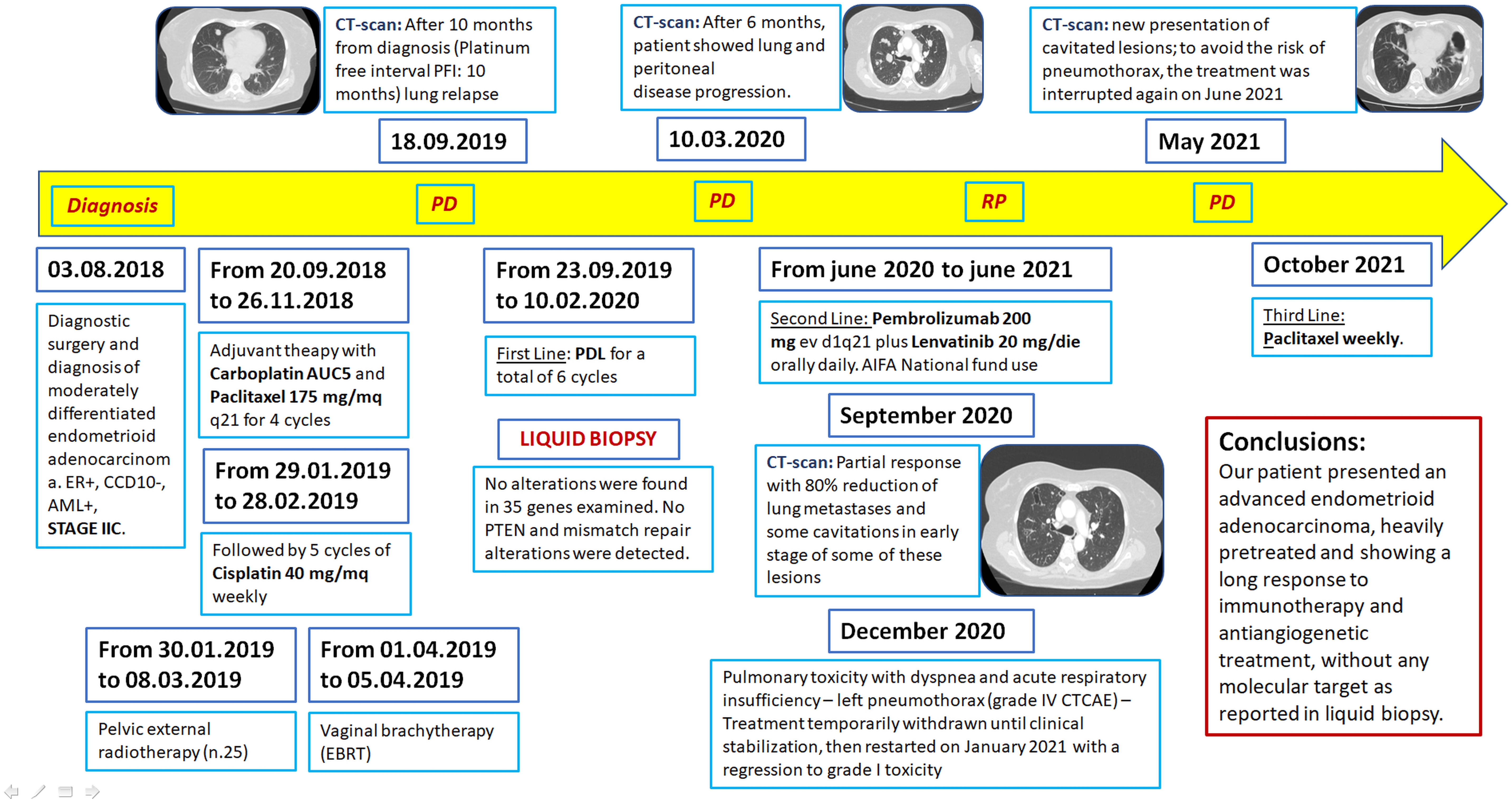
On August 2018, a 61-year-old woman with EC was taken cared of by our team, after diagnostic surgery and diagnosis of moderately differentiated endometrioid adenocarcinoma, ER +, CD10 -, AML +, IIC stage. According to the stage of disease, she received, from September to November 2018, adjuvant therapy with AUC5 carboplatin and 175 mg/m2 q21 paclitaxel for four cycles followed by 40 mg/m2 weekly cisplatin for five cycles, concomitant with pelvic external radiotherapy and vaginal brachytherapy. After 13 months from diagnosis [platinum-free interval (PFI): 10 months], in September 2019, the patient reported the first lung relapse and took carboplatin plus pegylated liposomal doxorubicine (PLD), for a total of six cycles, without improvement. To offer a personalized treatment, we performed a molecular profile with an FDA-approved NGS blood-based liquid biopsy (Foundation One® Liquid CDX). This in vitro diagnostic test was used for analyzing 311 genes including blood tumor mutational burden (TMB), microsatellite instability high (MSI-H), and tumor fraction values. In particular, it was performed for profiling a complete exonic sequence of 35 explored genes, introns of 7 genes involved in rearrangements, and select exons of an additional 35 genes. Unfortunately, no actionable mutations were found in this patient (27–29).
In particular, the absence of known biomarkers determined a condition of “not-addicted” EC, focusing on several factors: no PTEN and mismatch repair alterations were detected, BRCAwt, and no PI3k mutations. Moreover, the Dako test showed a lack of PD-L1 expression. After 6 months, the patient showed lung and peritoneal disease progression (PS ECOG 0) and was highly motivated to try new treatment options.
Taking into account the available data on pembrolizumab plus lenvatinib in “addicted-EC” and “not-addicted-EC” patients (Keynote-146), in June 2020, following our request, we were able to administer, through the AIFA national fund allowance, 200 mg ev d1q21 pembrolizumab plus 20 mg/day orally daily lenvatinib to this specific cancer patient (14, 30, 31).
The interesting preliminary Keynote-146 results encouraged this decision for a significant improvement in terms of ORR (37.2% in all populations, regardless of PD-L1 expression) as a potentially good approach compared to the less effective standard chemotherapy (32, 33).
The patient received this regimen for a total of 12 months (June 2020 to June 2021). Three months later (September 2020), a CT scan evaluation showed partial response with 80% reduction of lung metastases (Figures 1A–F) and appearance of some cavitations (Figures 2A, B) (34). At 6 months, CT scan showed a drastic change of lung imaging with evidence of cavitation in several lesions, previously described as a radiologic “air crescent sign” (35, 36). During treatment, we assessed routine blood test and tumor markers (CA 125, CA 15.3, and HE4) to follow tumor progression and treatment toxicity. At baseline, laboratory test and organ function results were as follows: hemoglobin 12.1 g/dl, platelet 311 x 103/μl, neutrophils 7.0 x 103/μl, and normal values of liver function and renal function. During treatment, no significant changes of these parameters were observed. At the time of progression, laboratory test results were as follows: hemoglobin 11.2 g/dl, platelet 570 x 103/μl, neutrophils 23 x 103/μl, and normal values of renal function and cholestasis index alterations (alkaline phosphatase 111 U/l and gamma glutamyl transferase 105 UI/l).
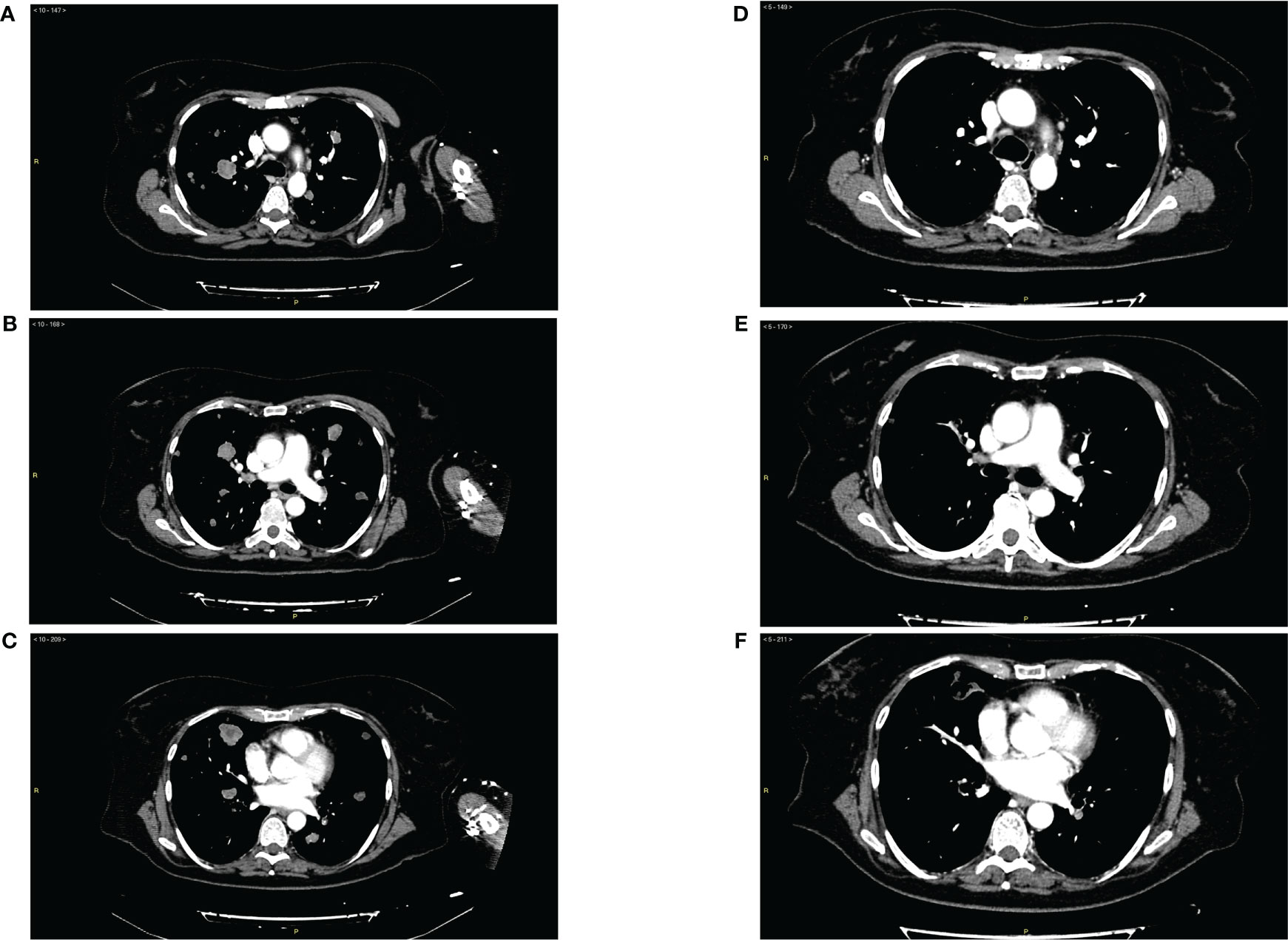
Figure 1 (A–C) Panel of radiological imaging concerning basal condition (June 2020) and presence of lung metastases. (D–F) Panel of radiological imaging concerning response to treatment (September 2020).
In particular, we focused on the trajectory of CA 125 and neutrophil-to-lymphocyte ratio (NLR) for a total evaluation of overall efficacy outcome and potential inflammation role. Indeed, NLR is a peculiar indicator of systemic inflammation and a potential parameter of immune response (37, 38).
Interestingly, the CA 125/NLR curve disclosed an increase of these values after treatment discontinuation (Figure 3) (39).
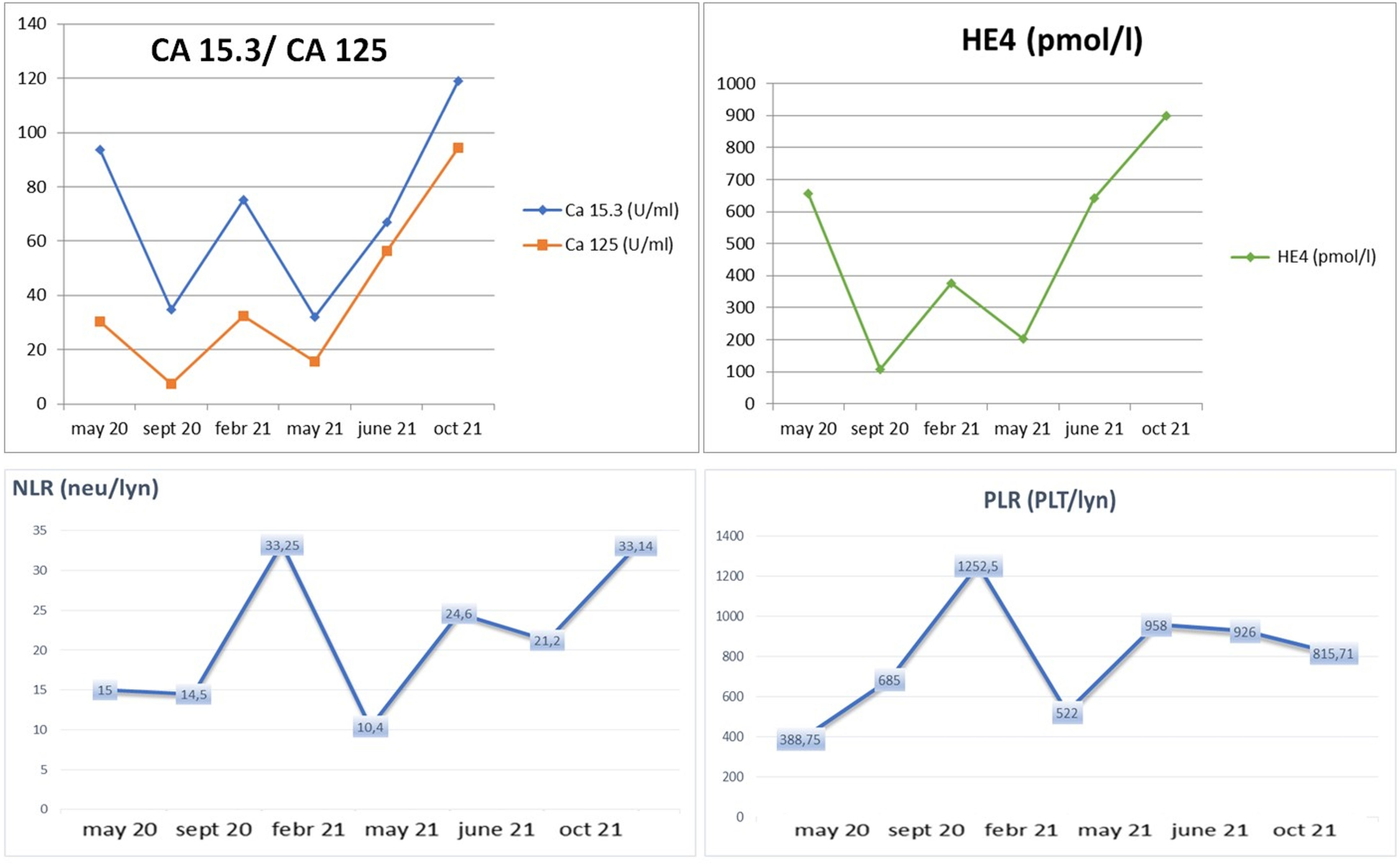
Figure 3 Clinical trend of biochemical markers (CA 125, CA 15.3, and HE4) and hematological inflammatory marker (NLR) according to response to treatment and “stop and go” strategy.
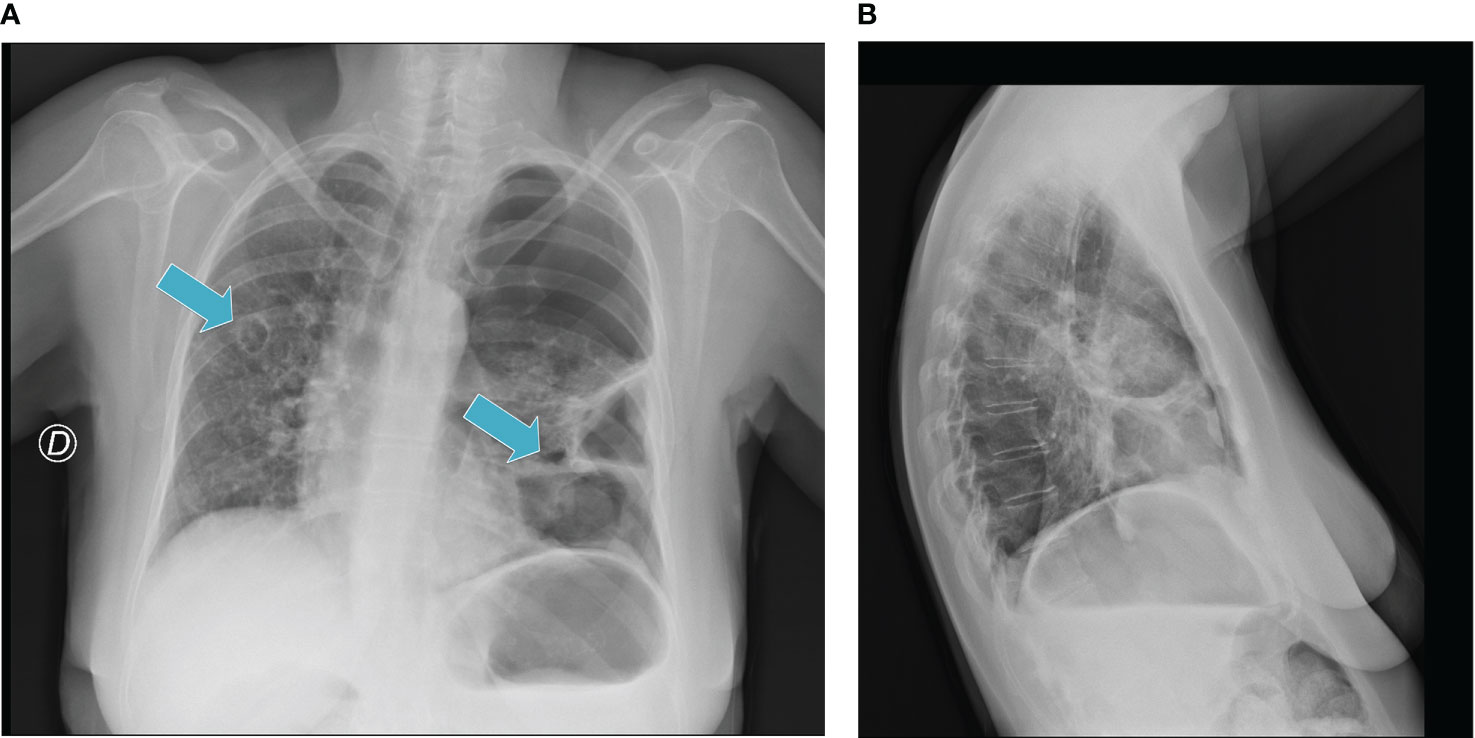
In December 2020, the pulmonary toxicity with dyspnea and acute respiratory failure required hospital admission for left pneumothorax (grade IV CTCAE) with initial contralateral mediastinal slip and the patient underwent thoracic drainage. The treatment was temporarily withdrawn and then restarted in January 2021 with regression to grade I toxicity. However, we observed an overall tumor control for the entire treatment period and more: this particular outcome seems to relate to a potential immunotherapy “carryover effect”. In May 2021, despite clinical benefit, CT scan showed new cavitated lesions (the major lesion was 48 x 72 mm); thus, in order to avoid the risk of pneumothorax, the treatment was interrupted again in June 2021, maintaining partial response for almost 4 months. Finally, in October 2021, because of the lack of clinical improvement and owing to disease progression, based on high patient’s motivation, she underwent new treatment with weekly paclitaxel until disease progression and then the PS progressively declined until death.
Finally, Figure 4 shows the timeline detailing case history by diagnosis to death with particular attention on clinical tests, lines of treatment, and disease progression.
Systematic review on drug toxicity
After case report description, we focus on the management of lung toxicity in this patient.
Methods
Case presentation was performed according to the CARE statement since the introduction of antiangiogenic agents in clinical practice, considering the improvement of the survival outcome in several solid tumors, the identification of new radiological response parameters has emerged (31). We performed a systematic review in order to select and describe all literature reports on this topic, according to the PRISMA statement.
Searching
As previously performed, we conducted the bibliographic research on PubMed by using the search terms “anti-PD1”, “anti-PDL1”, “immunotherapy”, “anti-VEGF”, “antiangiogenetic therapy”, “lung toxicity”, and “lung cavitations”. The time frame was 2015–2022. For this search, only papers in English language were considered.
Data extraction
The works were examined, independently, by investigators (M.C., G.R., and N.S.) to select homogeneous studies on lung toxicity reported as pembrolizumab toxicity, lenvatinib toxicity, or drug combination toxicity. The evaluation was extended to immunotherapy lung toxicity and antiangiogenetic drug-lung toxicity.
Results
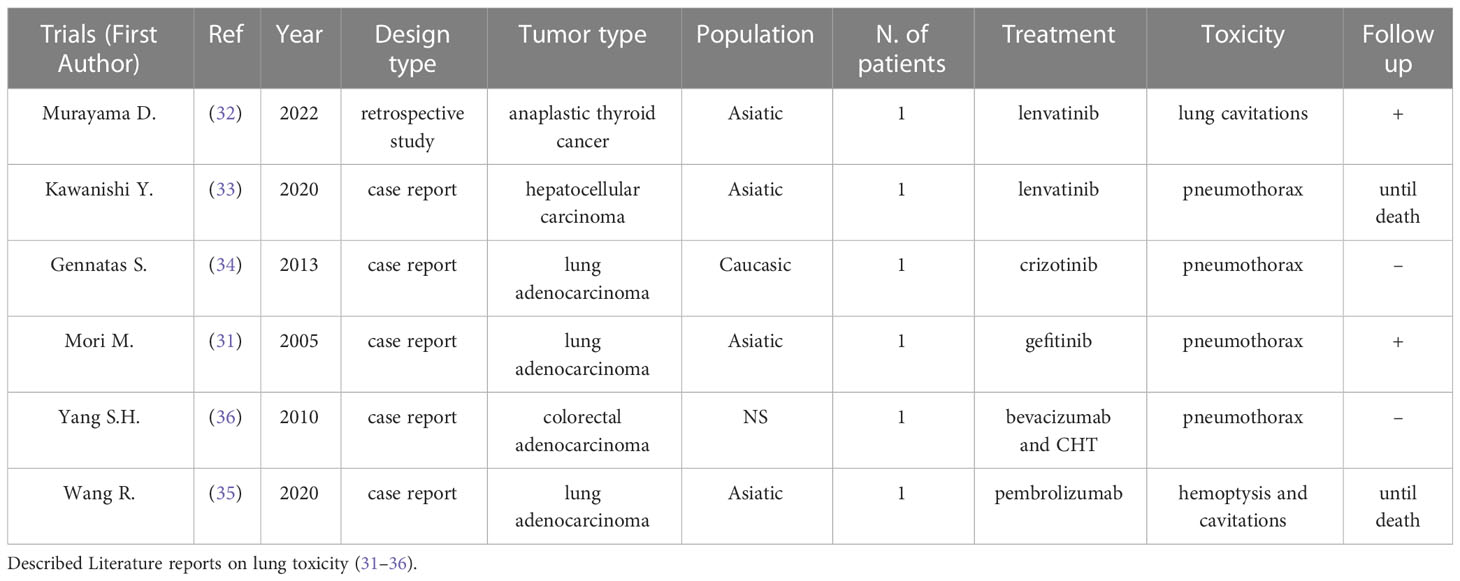
According to our selection, Table 1 describes literature reports on lung toxicity (40–45). Several data described similar experience in terms of pulmonary toxicity related to the use of these drugs in different types of tumors. Murayama et al., evaluating 26 patients with anaplastic thyroid cancer with lung metastasis, underlined the prognostic value of lung cavitations in patients treated with lenvatinib [OS 186 days vs. 89 days for the cavitation (+) group and the cavitation (–) group, respectively] (41). Similarly, Kawanishi et al. reported a case of cavitation and pneumothorax in a 71-year-old man suffering from hepatocarcinoma (HCC) with lung metastasis and treated with lenvatinib (42). Although CT scan showed tumor size stability, they considered this event suggestive of a response to the treatment. Differently, Gennatas et al. showed how pneumothorax during TKI treatment (in this case with crizotinib for lung adenocarcinoma) can occur spontaneously, in the absence of cavitations of the lung metastasis (43). Furthermore, spontaneous pneumothorax was described during an anti-VEGF therapy with bevacizumab plus FOLFOXIRI for a metastatic colorectal cancer with lung metastases (45). Finally, gefinitib was associated with simultaneous bilateral spontaneous pneumothorax in a patient treated for lung adenocarcinoma with multiple parenchymal metastases (40).
Conversely, no instances of pulmonary toxicity (cavitation or pneumothorax) and immunotherapy, such as pembrolizumab, were reported in the literature. Pneumonitis is the only pulmonary disease of immune checkpoint inhibitors (frequency <5%). Moreover, an interesting report on unusual pulmonary toxicity was described in Beijing, where a patient suffering from non-small cell lung cancer (NSCLC) and treated with pembrolizumab presented an atypical lung toxicity with hemoptysis and cavitation, in the absence of other possible causes (44).
Consistent with literature description, our experience underlined the potential prognostic value of pulmonary toxicity for different types of solid tumors with or without lung metastasis, suggesting that toxicity may represent a predictor of long-term disease control. Cavitation of metastatic lung lesions, determining pneumothorax, can be the clinical presentation of this toxicity, but pneumothorax can even occur in patients with no lung metastatic lesions. Of the lung cancer patients who took bevacizumab the 14%–24% presented cavitation. This radiologic sign is described as a density modification of tumor mass reported as “air-filled”. This event, whose mechanism and prognostic role remain unclear, seems to correlate to response to therapy.
Conversely, the absence of comparative data regarding the disease control rate of patients with cavitated lesions, compared to patients who did not present cavitations, prevents us from identifying it as a predictive response marker. Indeed, the fragility of the patient, with a greater predisposition to infections, could result in a worse prognosis. A new point of view of our work is focused on the maintenance of efficacy despite a “stop and go” treatment administration. What we observed in this case is how pembrolizumab plus lenvatinib can be discontinued because of toxicity and, despite this discontinuation, how antitumor effect can be maintained. We had to stop the therapy twice in order to treat toxicity, but our patient continued to show clinical and radiological improvement.
Discussion
The literature review showed several cases of lung toxicity related to systemic treatments, such as taxanes, tyrosine kinase inhibitors (TKIs), anti-VEGF agents, and immunotherapy, with a different clinical presentation: taxane-induced pneumonitis, TKI-associated interstitial lung disease, spontaneous pneumothorax by anti-VEGF drugs, and immunotherapy-related pneumonitis (46, 47).
In our experience, the metastatic EC patient treated with both immunotherapy and TKI in a second-line setting developed lung toxicity, characterized by cavitation and pneumothorax.
After 3 months of treatment, we observed cavitation of the metastatic lesions that caused left pneumothorax (shown in the chest radiograph in December 2020) with dyspnea and acute pulmonary failure. We treated the pneumothorax with thoracic drainage and the treatment was discontinued until clinical resolution (January 2021). The treatment was interrupted twice in May 2021 for a second episode of lung metastasis cavitation and in June 2021. In October 2021, treatment was withdrawn due to disease progression. Considering the important role of microenvironment regulation, several authors showed higher activity of anti-VEGF plus anti-PD-L1 combinatory approaches in vitro and in vivo (26). Based on this observation, we hypothesized the possible interaction between immunotherapy and antiangiogenetic therapy in this toxicity event. Antiangiogenetic therapy seems to reprogram the tumor immune microenvironment. Abnormal angiogenesis changes the function and expression of antitumor lymphocytes downregulating adhesion molecules, increasing interstitial fluid pressure and inducing hypoxia that upregulates some inhibitory signals for antitumor immune response (48). The ICI-ILD mechanism is still unclear. An increase of inflammatory cytokines could probably trigger the onset of irAEs. The inflammatory cytokine interleukin (IL)-6 induces the differentiation of naive CD4 T cells to Th17 cells, and it has been related to the incidence of irAEs (25). In particular, Th17 cells are involved in several autoimmune diseases (49, 50). Moreover, tumor necrosis factor-α (TNF-α) could be associated with irAEs. Conversely, a potential anti-angiogenic activity mediated by IFN-γ signaling that could condition the vascular endothelial cells’ function and promote T-cell infiltration was reported. Several works showed that lenvatinib reduced TAMs and increased CD8+ T cells (51). This condition could perhaps suggest negative feedback that is able to increase and regulate dendritic cell and macrophage function related to immunoinflammatory response.
Conclusions
Our patient history represents an interesting example of a persistent response to immunotherapy and antiangiogenetic combination, beyond the toxicity interruption. To the best of our knowledge, it is the only case of “stop and go” treatment administration that configures a significant maintenance of efficacy, showing a durable response to treatment. Indeed, in this case, we observed how pembrolizumab plus lenvatinib can be discontinued because of toxicity, and despite this, its antitumoral effect can be maintained. We had to stop the therapy twice in order to treat and prevent toxicity, but our patient continued to show clinical and radiologic improvement. Conversely, according to the prognostic role of NLR, recognized as an inflammatory biomarker, the increase of this parameter (NLR 33 vs. NLR 15) was related to disease progression and dramatically occurred together with rapid clinical decline.
Our interesting result is consistent with literature data, if we consider that PD-L1 did not represent a predictive biomarker in EC as reported in the above-described clinical trials. Moreover, MMR status is an immunotherapy predictive biomarker in advanced EC, and the confirmatory Keynote-775 study supports the evidence of significant OS benefit in both pMMR-status patients and dMMR-status patients. Indeed, our patient showed long-term clinical benefit and a significant PFS advantage regardless of PD-L1 and MSI condition (20).
In future studies, the use of novel pharmacogenomics platforms may help in the identification of predictive biomarkers, which, in turn, will help in the understanding of biological events and in improving the quality of life and survival of these patients (52–55).
Data availability statement
The datasets presented in this article are not readily available because Not available. Requests to access the datasets should be directed to not available.
Ethics statement
All authors confirm that the patient provided and signed the informed consent that is authorized and approved in our Centre (Mater Domini Teaching Hospital), approved by our local ethical committee. Written informed consent was obtained from the participant for the publication of this case report.
Author contributions
NS and PTg: conception and study design. NS, MC, GR, and FF: manuscript writing and sample collection. NS, DC, and MA: manuscript review and data analysis. AS and VF: manuscript review, teamwork had in charge of patients during treatment. PTg and PTs: supervision of manuscript review and in charge. All authors contributed to the article and approved the submitted version.
Funding
Publication costs are supported by Institutional funds of Department of Clinical and Experimental Medicine, Magna Græcia University of Catanzaro (Tagliaf18)
Acknowledgments
Publication costs are supported by Institutional funds of DMSC. The authors published this manuscript in memory of the patient and the family.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature (2013) 497(7447):67–73. doi: 10.1038/nature12325
3. Lu KH, Broaddus RR. Endometrial cancer. N Engl J Med (2020) 383(21):2053–64. doi: 10.1056/NEJMra1514010
4. Arend RC, Jones BA, Martinez A, Goodfellow P. Endometrial cancer: molecular markers and management of advanced stage disease. Gynecol Oncol (2018) 150(3):569–80. doi: 10.1016/j.ygyno.2018.05.015
5. Deshpande M, Romanski PA, Rosenwaks Z, Gerhardt J. Gynecological cancers caused by deficient mismatch repair and microsatellite instability. Cancers (Basel) (2020) 12(11):3319. doi: 10.3390/cancers12113319
6. Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res (1997) 57(5):808–11.
7. Ligtenberg MJ, Kuiper RP, Chan TL, Goossens M, Hebeda KM, Voorendt M, et al. Heritable somatic methylation and inactivation of MSH2 in families with lynch syndrome due to deletion of the 3' exons of TACSTD1. Nat Genet (2009) 41(1):112–7. doi: 10.1038/ng.283
8. Barra F, Evangelisti G, Ferro Desideri L, Di Domenico S, Ferraioli D, Vellone VG, et al. Investigational PI3K/AKT/mTOR inhibitors in development for endometrial cancer. Expert Opin Investig Drugs (2019) 28(2):131–42. doi: 10.1080/13543784.2018.1558202
9. Velasco A, Bussaglia E, Pallares J, Dolcet X, Llobet D, Encinas M, et al. PIK3CA gene mutations in endometrial carcinoma: correlation with PTEN and K-RAS alterations. Hum Pathol (2006) 37(11):1465–72. doi: 10.1016/j.humpath.2006.05.007
10. Connor EV, Rose PG. Management strategies for recurrent endometrial cancer. Expert Rev Anticancer Ther (2018) 18(9):873–85. doi: 10.1080/14737140.2018.1491311
11. Rose PG, Ali S, Moslemi-Kebria M, Simpkins F. Paclitaxel, carboplatin, and bevacizumab in advanced and recurrent endometrial carcinoma. Int J Gynecol Cancer (2017) 27(3):452–8. doi: 10.1097/IGC.0000000000000891
12. Kailasam A, Langstraat C. Contemporary use of hormonal therapy in endometrial cancer: a literature review. Curr Treat Options Oncol (2022) 23(12):1818–28. doi: 10.1007/s11864-022-01031-6
13. Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol (2012) 13(8):e353–61. doi: 10.1016/S1470-2045(12)70213-9
14. Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol (2020) 38(26):2981–92. doi: 10.1200/JCO.19.02627
15. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite Instability/Mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol (2020) 38(1):1–10. doi: 10.1200/JCO.19.02105
16. Oaknin A, Tinker AV, Gilbert L, Samouelian V, Mathews C, Brown J, et al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol (2020) 6(11):1766–72. doi: 10.1001/jamaoncol.2020.4515
17. Roncolato F, Lindemann K, Willson ML, Martyn J, Mileshkin L. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database Syst Rev (2019) 10(10):CD012160. doi: 10.1002/14651858.CD012160.pub2
18. Rubinstein MM, Dickinson S, Narayan P, Zhou Q, Iasonos A, Ma W, et al. Bevacizumab in advanced endometrial cancer. Gynecol Oncol (2021) 161(3):720–6. doi: 10.1016/j.ygyno.2021.04.016
19. Di Dio C, Bogani G, Di Donato V, Cuccu I, Muzii L, Musacchio L, et al. The role of immunotherapy in advanced and recurrent MMR deficient and proficient endometrial carcinoma. Gynecol Oncol (2022) 169:27–33. doi: 10.1016/j.ygyno.2022.11.031
20. Makker V, Colombo N, Herraez AC, Monk BJ, Mackay H, Santin AD, et al. Lenvatinib plus pembrolizumab in previously treated advanced endometrial cancer: updated efficacy and safety from the randomized phase III study 309/KEYNOTE-775. J Clin Oncol (2023) 386(5):437. doi: 10.1200/JCO.22.02152
21. Marth C, Tarnawski R, Tyulyandina A, Pignata S, Gilbert L, Kaen D, et al. Phase 3, randomized, open-label study of pembrolizumab plus lenvatinib versus chemotherapy for first-line treatment of advanced or recurrent endometrial cancer: ENGOT-en9/LEAP-001. Int J Gynecol Cancer (2022) 32(1):93–100. doi: 10.1136/ijgc-2021-003017
22. Fogli S, Gianfilippo G, Cucchiara F, Del Re M, Valerio L, Elisei R, et al. Clinical pharmacology and drug-drug interactions of lenvatinib in thyroid cancer. Crit Rev Oncol Hematol (2021) 163:103366. doi: 10.1016/j.critrevonc.2021.103366
23. Miquelotti LB, Sari MHM, Ferreira LM. Immunotherapy in cancer management: a literature review of clinical efficacy of pembrolizumab in the non-small cell lung cancer treatment. Adv Pharm Bull (2023) 13(1):88–95. doi: 10.34172/apb.2023.007
24. Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol (2019) 37(17):1470–8. doi: 10.1200/JCO.18.01265
25. Frenel JS, Le Tourneau C, O'Neil B, Ott PA, Piha-Paul SA, Gomez-Roca C, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase ib KEYNOTE-028 trial. J Clin Oncol (2017) 35(36):4035–41. doi: 10.1200/JCO.2017.74.5471
26. Eisinger C, Muluneh B. Combined use of pembrolizumab and lenvatinib: a review. J Oncol Pharm Pract (2023) 10781552231178461. doi: 10.1177/10781552231178461
27. Lin DI, Fine A, Danziger NA, Huang RSP, Mata DA, Decker B, et al. Molecular analysis of endometrial serous carcinoma reveals distinct clinicopathologic and genomic subgroups. Gynecol Oncol (2022) 164(3):558–65. doi: 10.1016/j.ygyno.2021.12.030
28. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol (2020) 21(10):1353–65. doi: 10.1016/S1470-2045(20)30445-9
29. Milbury CA, Creeden J, Yip WK, Smith DL, Pattani V, Maxwell K, et al. Clinical and analytical validation of FoundationOne(R)CDx, a comprehensive genomic profiling assay for solid tumors. PloS One (2022) 17(3):e0264138. doi: 10.1371/journal.pone.0264138
30. Makker V, Aghajanian C, Cohn AL, Romeo M, Bratos R, Brose MS, et al. A phase Ib/II study of lenvatinib and pembrolizumab in advanced endometrial carcinoma (Study 111/KEYNOTE-146): long-term efficacy and safety update. J Clin Oncol (2023) 41(5):974–9. doi: 10.1200/JCO.22.01021
31. Paulino E. Pembrolizumab plus lenvatinib for all advanced endometrial cancer? maybe not. Int J Gynecol Cancer (2022) 32(4):579. doi: 10.1136/ijgc-2022-003419
32. Humber CE, Tierney JF, Symonds RP, Collingwood M, Kirwan J, Williams C, et al. Chemotherapy for advanced, recurrent or metastatic endometrial cancer: a systematic review of cochrane collaboration. Ann Oncol (2007) 18(3):409–20. doi: 10.1093/annonc/mdl417
33. Restaino S, Dinoi G, La Fera E, Gui B, Cappuccio S, Campitelli M, et al. Recurrent endometrial cancer: which is the best treatment? systematic review of the literature. Cancers (Basel) (2022) 14(17):4176. doi: 10.3390/cancers14174176
34. Peng Y, Chen Y, Zhang X, Yang Y, Cao D, Bi F, et al. Tumoral cavitation in colorectal cancer patients with unresectable lung metastasis treated with bevacizumab and chemotherapy. J Cancer Res Clin Oncol (2018) 144(7):1339–46. doi: 10.1007/s00432-018-2656-y
35. Sevilha JB, Rodrigues RS, Barreto MM, Zanetti G, Hochhegger B, Marchiori E. Infectious and non-infectious diseases causing the air crescent sign: a state-of-the-Art review. Lung (2018) 196(1):1–10. doi: 10.1007/s00408-017-0069-3
36. Stechele M, Kunz WG. Air crescent sign in fast-responding lung metastases. Radiol Imaging Cancer (2022) 4(2):e220012. doi: 10.1148/rycan.220012
37. Muzykiewicz KP, Iwanska E, Janeczek M, Glanowska I, Karolewski K, Blecharz P. The analysis of the prognostic value of the neutrophil/ lymphocyte ratio and the platelet/lymphocyte ratio among advanced endometrial cancer patients. Ginekol Pol (2021) 92(1):16–23. doi: 10.5603/GP.a2020.0164
38. Njoku K, Ramchander NC, Wan YL, Barr CE, Crosbie EJ. Pre-treatment inflammatory parameters predict survival from endometrial cancer: a prospective database analysis. Gynecol Oncol (2022) 164(1):146–53. doi: 10.1016/j.ygyno.2021.11.009
39. Rocconi RP, Matthews KS, Kemper MK, Hoskins KE, Huh WK, Straughn JM Jr. The timing of normalization of CA-125 levels during primary chemotherapy is predictive of survival in patients with epithelial ovarian cancer. Gynecol Oncol (2009) 114(2):242–5. doi: 10.1016/j.ygyno.2009.04.019
40. Mori M, Nakagawa M, Fujikawa T, Iwasaki T, Kawamura T, Namba Y, et al. Simultaneous bilateral spontaneous pneumothorax observed during the administration of gefitinib for lung adenocarcinoma with multiple lung metastases. Intern Med (2005) 44(8):862–4. doi: 10.2169/internalmedicine.44.862
41. Murayama D, Yamamoto Y, Matsui A, Yasukawa M, Okamoto S, Toda S, et al. Lung cavitation in patients with anaplastic thyroid cancer treated with lenvatinib. Gland Surg (2022) 11(6):963–9. doi: 10.21037/gs-22-71
42. Kawanishi Y, Kuwahara M, Utsunomiya M, Ishida N, Ishikawa Y, Hiroi M, et al. Pneumothorax during lenvatinib treatment for hepatocellular carcinoma with lung metastasis. Clin J Gastroenterol (2021) 14(1):288–92. doi: 10.1007/s12328-020-01273-7
43. Gennatas S, Stanway SJ, Thomas R, Min T, Shah R, O'Brien ME, et al. Early pneumothorax as a feature of response to crizotinib therapy in a patient with ALK rearranged lung adenocarcinoma. BMC Cancer (2013) 13:207. doi: 10.1186/1471-2407-13-207
44. Wang R, Li K, Pi J, Meng L, Zhu M. Cavitation and fatal hemoptysis after immunotherapy for advanced lung adenocarcinoma: a case report. Thorac Cancer (2020) 11(9):2727–30. doi: 10.1111/1759-7714.13578
45. Yang SH, Lin JK, Chen WS, Lin TC, Yang SH, Jiang JK, et al. Pneumothorax after bevacizumab-containing chemotherapy: a case report. Jpn J Clin Oncol (2011) 41(2):269–71. doi: 10.1093/jjco/hyq195
46. Nigen B, Chene AL, Liberge R, Sagan C, Blanc FX. [Immune-related pneumonitis: a differential diagnosis of SARS-CoV-2 pneumonia]. Rev Mal Respir (2022) 39(7):626–32. doi: 10.1016/j.rmr.2022.07.001
47. Anoop TM, Joseph R, Unnikrishnan P, Thomas F, Venugopal M. Taxane-induced acute interstitial pneumonitis in patients with breast cancer and outcome of taxane rechallenge. Lung India (2022) 39(2):158–68. doi: 10.4103/lungindia.lungindia_126_21
48. Ren S, Xiong X, You H, Shen J, Zhou P. The combination of immune checkpoint blockade and angiogenesis inhibitors in the treatment of advanced non-small cell lung cancer. Front Immunol (2021) 12:689132. doi: 10.3389/fimmu.2021.689132
49. Niewada M, Macioch T, Konarska M, Mela A, Goszczynski A, Przekopinska B, et al. Immune checkpoint inhibitors combined with tyrosine kinase inhibitors or immunotherapy for treatment-naive metastatic clear-cell renal cell carcinoma-a network meta-analysis. focus on cabozantinib combined with nivolumab. Front Pharmacol (2022) 13:1063178. doi: 10.3389/fphar.2022.1063178
50. Bukamur H, Katz H, Alsharedi M, Alkrekshi A, Shweihat YR, Munn NJ. Immune checkpoint inhibitor-related pulmonary toxicity: focus on nivolumab. South Med J (2020) 113(11):600–5. doi: 10.14423/SMJ.0000000000001166
51. Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology (2021) 74(5):2544–60. doi: 10.1002/hep.31921
52. Arbitrio M, Di Martino MT, Scionti F, Barbieri V, Pensabene L, Tagliaferri P. Pharmacogenomic profiling of ADME gene variants: current challenges and validation perspectives. High Throughput (2018) 7(4):40. doi: 10.3390/ht7040040
53. Arbitrio M, Scionti F, Di Martino MT, Caracciolo D, Pensabene L, Tassone P, et al. Pharmacogenomics biomarker discovery and validation for translation in clinical practice. Clin Transl Sci (2021) 14(1):113–9. doi: 10.1111/cts.12869
54. Scionti F, Di Martino MT, Caracciolo D, Pensabene L, Tagliaferri P, Arbitrio M. Tools in pharmacogenomics biomarker identification for cancer patients. Methods Mol Biol (2022) 2401:1–12. doi: 10.1007/978-1-0716-1839-4_1
Keywords: advanced endometrial cancer, immunotherapy, targeted therapy, pembrolizumab, lenvatinib, lung toxicity
Citation: Staropoli N, Salvino A, Falcone F, Farenza V, Costa M, Rossini G, Manti F, Crispino A, Riillo C, Ciliberto D, Arbitrio M, Tassone P and Tagliaferri P (2023) Pembrolizumab plus lenvatinib in advanced endometrial cancer: case report and systematic review of lung toxicity. Front. Oncol. 13:1145986. doi: 10.3389/fonc.2023.1145986
Received: 15 March 2023; Accepted: 07 June 2023;
Published: 10 July 2023.
Edited by:
Chunhua Song, The Ohio State University, United StatesReviewed by:
Dongqin Yang, Fudan University, ChinaSoo Mun Ngoi, The Ohio State University, United States
Copyright © 2023 Staropoli, Salvino, Falcone, Farenza, Costa, Rossini, Manti, Crispino, Riillo, Ciliberto, Arbitrio, Tassone and Tagliaferri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicoletta Staropoli, nicolettastaropoli@gmail.com; Pierosandro Tagliaferri, tagliaferri@unicz.it
†These authors have contributed equally to this work
 Nicoletta Staropoli
Nicoletta Staropoli