
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 07 March 2025
Sec. Nutrition and Metabolism
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1560610
This article is part of the Research Topic Nutrition, Inflammation and Oxidative Stress in Obstetrics and Gynecology View all 12 articles
It is well acknowledged that metabolic disorder binds closely with preeclampsia, though some of the causal relationships are still ambiguous. This review systematically summarizes the metabolic characteristics of carbohydrates, lipids, amino acids, and glycans in preeclampsia, highlighting their roles in oxidative stress, trophoblast autophagy, inflammatory response, and vascular tone regulation. Key findings include upregulated glycolysis and impaired mitochondrial function contributing to ATP deficiency, dysregulated lipid metabolism exacerbating oxidative stress and vascular dysfunction, and amino acid imbalances disrupting immune responses and redox homeostasis. Emerging therapies, such as metformin and pravastatin, demonstrate potential in targeting these pathways for prevention and treatment. Here, we reviewed thoroughly the related literature with a view to delineating the potential association of nutrient metabolism with preeclampsia, so that we could explore a promising therapeutic approach.
• Metabolism of nutrients is identified as a field in the pathogenesis of preeclampsia.
• Preeclampsia undergoes metabolic reprogramming of nutrients.
• Metabolites interact between placental trophoblast and immune cells in preeclampsia.
• Metabolites can serve as potential prevention and treatment targets for preeclampsia.
• Validations on metabolites in prenatal diagnosis of preeclampsia are expected.
Preeclampsia, a serious but mysterious pregnancy disorder syndrome, is characterized by newly-onset hypertension which occurs at or after 20-week gestation and terminal organ dysfunction in the form of proteinuria, acute kidney injury, liver dysfunction, eclampsia, and even death (1, 2). Specific to pregnancy, preeclampsia has an incidence of approximately 3–5%, characterized by preterm birth of 15% and maternal death of 42% (3). The problem is that there is a lack of effective interventions, since the exact mechanism of preeclampsia is unknown; once preeclampsia occurs, only the termination of pregnancy can relieve the symptoms. Therefore, it is imperative that research be conducted on the mechanism of preeclampsia, which plays a significant role in its prevention and treatment, thereby significantly reducing perinatal adverse outcomes.
Increasingly recognized as a metabolic disease, preeclampsia can be explained by its increased susceptibility in pregnant women with higher body mass index (BMI) (4). Studies have shown that metabolic reprogramming in preeclampsia involves significant alterations in nutrient metabolism. For example, impaired placental mitochondrial function and upregulated glycolysis result in ATP deficiency and oxidative stress, contributing to disease progression (5). Additionally, dysregulated lipid metabolism, characterized by elevated fatty acid levels and lipid peroxidation, has been linked to vascular dysfunction and endothelial damage (6). Though the mechanism of preeclampsia is scarcely understood, a consensus has been reached that it is attributed to oxidative stress, trophoblast cell autophagy, systematic inflammation, platelet aggregation and increased vascular tone (7–9). Thus, the metabolic changes that are involved in the occurrence and development of preeclampsia are worthy of due attention in the clinic.
Metabolism is a general term for a series of chemical reactions that occur in living organisms which need to sustain life. Since metabolism is a relatively macro concept, we mainly focused on the metabolism of nutrients such as carbohydrates, lipids, amino acids and glycans, which have disparate metabolic pathways to be connected through certain intermediate metabolites, so as to generate energy and synthesize substances that maintain normal functions of cells. In the field of oncology, up to now, the studies on metabolism catch the spotlight of the public, where researchers have found that metabolic reprogramming in tumor cells can help their proliferation by despoiling nutrients from the microenvironment (10). In the case of preeclampsia, currently, the metabolic investigations center on placental energy and metabolic intermediates, the alterations of which are caused by metabolic reprogramming, ultimately leading to insufficient placental energy and perturbation of nutrient synthesis. Although much research has been performed in this field, a systematic summary has not been made on the overall metabolic changes in preeclampsia. In the current review, we outlined the metabolic characteristics of nutrients systematically and completely, based on which we proposed some metabolism-related predictors and therapies for preeclampsia (11).
A comprehensive literature search was conducted using PubMed, Web of Science, and Scopus databases. The search included articles published in English up to 2023, using keywords such as “preeclampsia,” “nutrient metabolism,” “carbohydrates,” “lipids,” “amino acids,” and “glycans.” Inclusion criteria were peer-reviewed articles that investigated metabolic mechanisms or therapeutic approaches related to preeclampsia. Exclusion criteria included studies unrelated to nutrient metabolism or lacking experimental/clinical data.
The nutrients that can regulate the development of preeclampsia include carbohydrates, lipids, amino acids and glycans, which can have a principal effect on placental energy supply, inflammatory response, vasoconstriction, oxidative stress, trophoblast autophagy and vascular tone. As indicated in Figure 1, a category has been made of different nutrients and their metabolites, which may contribute to or inhibit the progression of preeclampsia through certain pathways.

Figure 1. Pathogeneesis of preeclampsia regulated by nutrients metabolism. Several classic mechanisms of preeclampsia including energy deficiency, oxidative stress, increased vascular tone, inflammation, platelet aggregation and trophoblast autophagy can be regulated by metabolites.
Carbohydrates, as organic compounds composed of carbon, hydrogen and oxygen, exist mostly in nature, with a broad spectrum of chemical structure and biological function. The most vital metabolic pathway of carbohydrates is central carbon metabolism involving glycolysis, pentose phosphate and tricarboxylic acid cycle, as the main source of energy for organisms and also as precursor for other metabolisms in the human body (12). As early as 1987, researchers observed reduced adenosine triphosphate (ATP) production in preeclamptic placenta, which was caused by placental ischemia and hypoxia, thus impairing energy-dependent placental functions such as active transport, i.e., amino acids transfer, and protein synthesis; at this point, glycolysis was upregulated to maintain ATP synthesis (13, 14). This could be explained by the changes in metabolic enzyme activity, which is the researchers’ initial understanding of carbohydrate metabolic reprogramming. Recent research provided a deeper understanding of this notion, revealing that carbohydrate metabolism could adapt to the hypoxia condition in mild preeclampsia while a decompensation of carbohydrate metabolism reprogramming was discovered in severe preeclampsia (11) (Figure 2).
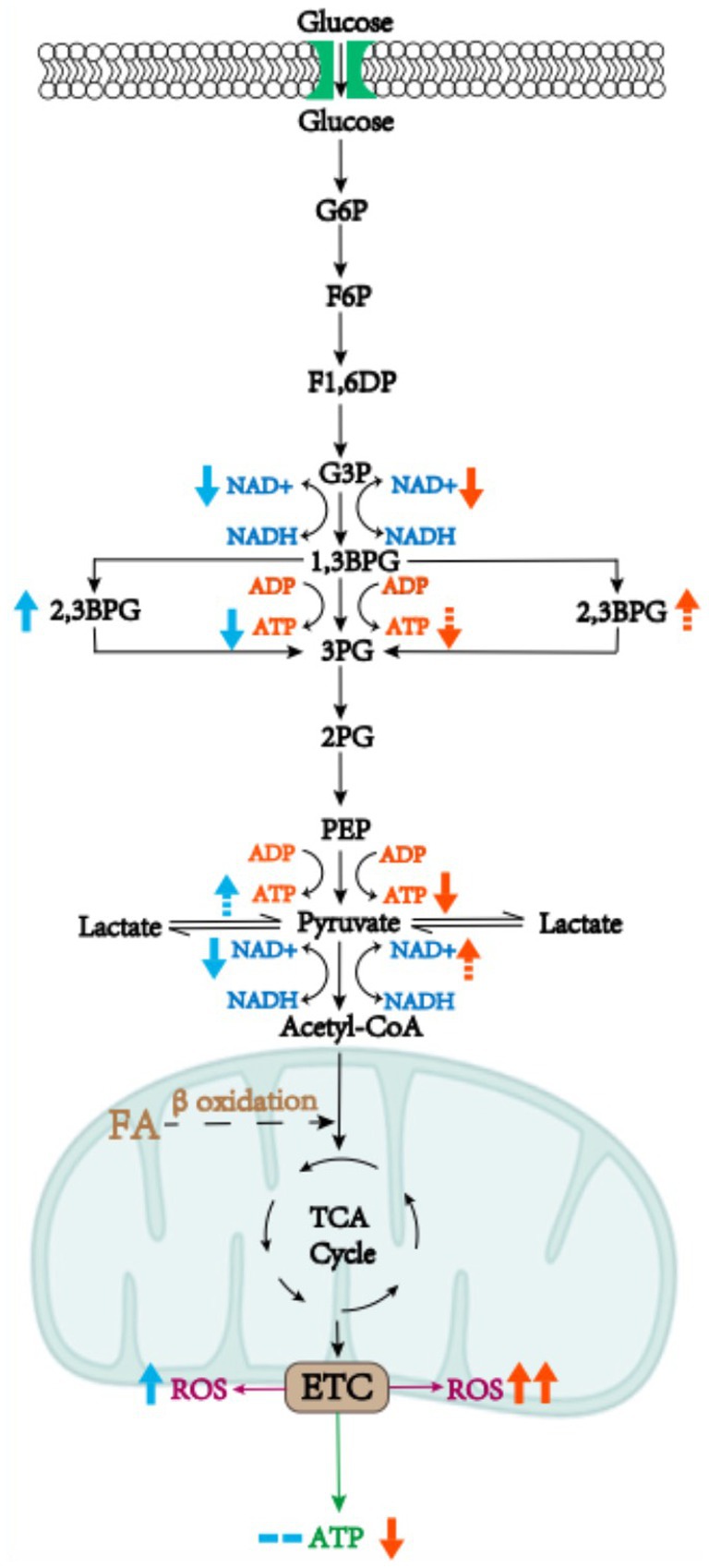
Figure 2. Metabolic reprogramming of carbohydrates in mild or severe preeclamptic placenta. In preeclampsia, the glycolytic pathway is activated and large amounts of NAD+ are consumed, leading to a blockage in the synthesis of vital substances in the placenta. Moreover, some of the ATP synthesis pathways are bypassed, ending up with less ATP production. Metabolic reprogramming of carbohydrates in mild preeclampsia compensates by increasing glycolytic intermediates, such as increasing oxidation of fatty acids to provide acetyl-coA, whereas in severe preeclampsia a decompensation of metabolic reprogramming of carbohydrates has been witnessed. The blue arrows represent the direction of metabolic reprogramming in mild preeclampsia and the red arrows represent decompensation of metabolic reprogramming in severe preeclampsia. Solid arrows represent changes that have been reported in the literature, dotted arrows represent presumed changes, and the number of arrows is proportional to the magnitude of the changes.
In cells, glycolysis produces a small amount of ATP, while most of energy is produced via oxidative phosphorylation in mitochondria. Mitochondrial dysfunction and oxidative stress, manifested as the increased production of reactive oxygen species (ROS), are known to occur in different subtypes of preeclampsia (15). In mild preeclampsia, mitochondria may compensate by enhancing oxidative phosphorylation and antioxidant activity, and the glycolytic pathway is also compensatively activated to replenish ATP (16). Moreover, it has been demonstrated in mouse models that the levels of 2,3-Bisphosphoglycerate (2,3-BPG) derived from 1,3-Bisphosphoglycerate (1,3-BPG) are significantly increased, which bypassed ATP-producing pathway where 1–3 BPG synthesizes 3-Bisphosphoglycerate (3-BPG) (5). Activation of glycolysis is known to lead to a lack of NAD+, resulting in a decrease in the conversion from pyruvate to acetyl-CoA where NAD+ act as a coenzyme of pyruvate dehydrogenase. Tricarboxylic acid (TCA) cycle, another energy-supplying process buttressed by NAD+ as coenzyme, cannot proceed smoothly. Acetyl-CoA also has a compensatory pathway, coming from fatty acid β oxidation in addition to glycolysis (17). β oxidation of fatty acids is likely to produce acetyl-CoA as compensation, which can be demonstrated by increased acylcarnitine levels (5). Therefore, we hypothesize that in energy production, a compensatory increase in ATP production from glycolysis combined with mitochondrial oxidative phosphorylation could maintain overall ATP levels in mild preeclampsia, and that in terms of substance synthesis, the substances that need to maintain the normal function of the placenta can be ensured, thanks to the compensatory production of acetyl-CoA.
In the case of severe preeclampsia, the bypass pathway from 1,3-BPG to 2,3-BPG could also be activated, as it brought about ATP deficiency (5), a major character of preeclampsia. Mitochondrial dysfunction could not be compensated by increasing oxidative phosphorylation, so that it was only compensated by activating the glycolytic pathway, which was inefficient at producing ATP (18). A loss of bioenergy and biosynthetic homeostasis was reported, which could be explained by significantly reduced concentrations of intermediate metabolites such as pyruvate, lactic acid, and pyruvate kinase (a key enzyme in glycolysis) in placenta (16). Thus, we propose a hypothesis that the former part of glycolysis is activated in severe preeclampsia, but bypass the ATP-producing pathway, while the latter part is inhibited, resulting in decreased ATP production in glycolysis along with overall ATP decrease; this process in turn could lead to the clinical manifestation of severe preeclampsia, which comprised premature birth and fetal growth restriction (11).
To meet the needs of fetal growth and development in normal pregnancy, the capacity of intestinal lipid absorption is enhanced (19–21); the elevated levels of blood lipid (such as cholesterol and triglycerides) lead to increased lipid peroxidation (22, 23). In contrast, women with preeclampsia are characterized by higher BMI, higher levels of blood lipid and lipid peroxides (24), which can be decomposed into more free radicals, thus damaging vascular endothelial cells, causing the decrease of prostacyclin (PGI2) by inhibiting PGI2 synthetase and activating thromboxin (TXA2) synthetase to produce TXA2 (25). PGI2/TXA2 ratio thereafter decreases, leading to a series of pathophysiological changes of vasospasm and contraction. Furthermore, free radicals could cause mitochondrial damages, followed by reduced energy production, and increased oxidative stress (6, 26). The free radicals induced by oxidative stress actively interact with polyunsaturated fatty acids (PUFAs) to produce lipid peroxides, forming a vicious cycle (27). Once confirmed in preeclampsia, the altered lipid metabolism is likely to be the result of genetic predisposition, which also acts as a risk factor for the development of cardiovascular disease in later life.
Although the effect of abnormal lipid metabolism on the classical mechanisms of preeclampsia has been well documented, the mechanisms of the various abnormalities in lipid metabolism have not been clearly elucidated, except for that of fatty acids, the important metabolites of fat which are increased in the second and third trimesters in normal pregnancy (28). Significantly elevated fatty acids is usually seen in preeclampsia, which can be observed prior to the onset of the disease (29), and attributed to decreased activity of corresponding metabolic enzymes (30). Gene mutation and polymorphism of short-chain acyl-coenzyme A dehydrogenase (SCAD, a type of enzyme in charge of fatty acid β oxidation), to name a few, are known to relate to the decreased activity of SCAD, thus raising the level of short-chain fatty acids, which acts as a risk factor in preeclampsia (30). In addition to alternation in genes, disturbance of intestinal flora can also leads to the reduction of fatty acid levels in the intestine and blood, which is related to the occurrence of preeclampsia because fewer fatty acid cracking products are found in the feces of pregnant women with preeclampsia (31). Therefore, the role of fatty acids in preeclampsia needs further study.
Additionally, the disorder of long chain fatty acid oxidation has been found in preeclampsia, which is characterized by the reduced level of mRNA and protein expression of long chain omega-3 hydroxy CoA dehydrogenase (LCHAD) in the placenta of preeclampsia, which leads to lipid deposition. The earlier the onset of preeclampsia, the more significant abnormal LCHAD expression and the more lipid deposition can be found (32). The altered activity of SCAD and LCHAD working together could lead to the overloaded titers of fatty acid in the serum, which could contribute to oxidative stress and increased vascular tone through the pathways aforementioned (33). The notion of SCAD deficiency has been validated based on a single clinical case; therefore, it is important that a large-sample-based analysis be conducted to search for the gene-induced fatty acid enzymes to induce irregularities in preeclampsia (30), and that future studies focus on the enzymes related to fatty acid metabolism and the gene expressions in the activity regulation of enzymes so as to prevent the occurrence and development of preeclampsia.
Beyond fatty acids, recent studies have highlighted the role of metabolic reprogramming in other major lipid classes, such as glycerophospholipids, sphingolipids, and long-chain polyunsaturated fatty acids (LCPUFAs), in the pathophysiology of preeclampsia (34). For instance, sphingolipids, particularly ceramides, have been implicated in trophoblast dysfunction and apoptosis, leading to impaired spiral artery remodeling, a hallmark of early-onset preeclampsia (35). Similarly, altered glycerophospholipid metabolism disrupts endothelial function and inflammatory signaling pathways, exacerbating vascular dysfunction (36). LCPUFAs, such as docosahexaenoic acid (DHA) and arachidonic acid (AA), are critical for maintaining vascular tone and regulating inflammation. Disruptions in the DHA/AA balance have been linked to increased oxidative stress and endothelial damage in preeclampsia (37).
The metabolism of the multiple varieties of amino acids, peptides and proteins have been newly proposed to correlate with the development of preeclampsia. As indicated in Figure 3, a focus has been made on glutathione (GSH), tryptophan (Trp), arginine (Arg) and homocysteine (Hcy) metabolism, which involve in the classical mechanism of preeclampsia, respectively.

Figure 3. Metabolic pathways of various animo acid and their contributions to preeclampsia. In normal placental blood vessels, a variety of amino acids and their metabolites can regulate oxidative stress, vasoconstriction, inflammation and so on. In preeclamptic placentas, changes in the activity of enzymes that metabolize certain amino acid can lead to changes in the level of corresponding amino acid, thus having an impact on the above pathways. T, T cells; CD4T, CD4T cells; TS, inhibitory T cells.
It has been experimentally verified that preeclampsia is responsible for oxidative stress, and that GSH is a prominent component of the non-enzymatic oxidative defense system (38). GSH is a major intracellular antioxidant compound, abundant in the cytoplasm, nucleus and mitochondria (39). Capable of bioconversion and elimination of biomass, GSH plays an antioxidant role when oxidized to glutathione oxidized (GSSG) through the catalysis of glutathione peroxidase (GPx) and catalase (CAT) (40). Moreover, GSH contributes to the conversion from H2O2 to H2O, thus alleviating oxidative stress where H2O2 acts as a vital type of ROS (41).
The higher level of GPx in the umbilical cord of preeclampsia has been observed, which may function as a compensatory mechanism against oxidative stress (21). This could explain the similar levels of H2O2 in preeclampsia in comparison with normal pregnancies (42, 43). Though the higher level of GPx has been found in preeclampsia, the changing activities of CAT remain controversial. It has been suggested that CAT is compensatively increased in preeclampsia (43), or that decreased CAT increases the risk of preeclampsia (44).
Additionally, the decreased expression of Glutathione S-transferase theta 2 (GSTT2) has been found in preeclampsia, which is a major type of enzyme in charge of combining ROS with GSH, thus reducing oxidative stress (45). In general, the decompensation of the aforementioned enzymes inhibits the scavenging of ROS, which is the root cause of redox imbalance in favor of pro-oxidants (43). In view of a considerable variety of enzymes involved in the metabolism of GSH, their changing activities in redox reaction and their potential roles in the prediction and therapeutic significance in preeclampsia can be of an intriguing discipline to pursue.
Trp, an essential amino acid in the human body, is known to have two breakdown pathways: the kynurenine (KYN) and the 5-HT pathway. It was previously reported that a small part of Trp produced 5-HT through tryptophan hydroxylase (TPH), whereas about 95% of Trp produced KYN through the catalysis of indolamine 2,3-dioxygenase (IDO) or tryptophan2,3-dioxygenase (TDO) (46).
By depleting Trp, IDO is known to inhibit the function of immune cells, including T lymphocyte proliferation, and to potentially downregulate the inflammatory response. IDO inhibits the function of immune cells through a specific L-Trp depletion pathway, where L-Trp depletion by IDO leads to its dissociation with L-Trp tRNA, thus activating general control non-derepressible 2 (GCN2) due to the presence of its allosteric regulatory site which senses free tRNAs (47, 48). Phosphorylated by activated GCN2, the activity of eukaryotic translation initiation factor-2α (eIF-2α) is weakened, thus inhibiting the transcription of various RNAs and the translation of proteins in T cells (27). Moreover, L-Trp deficiency can inhibit master amino acid-sensing kinase 1 (GLK1), and further inhibit m-TOR signaling molecules, triggering T cell impotence and autophagy (27). Additionally, KYN produced by IDO is an endogenous ligand of aromatic hydrocarbon receptor (AhR). KYN binding to AhR leads to differentiation of immature CD4+ T cells into inhibitory T cells; it can also induce IDO expression, further suppressing T cell immune response and inflammation (49).
In normal pregnancy, plasma KYN/Trp ratio increases with gestational age. In preeclampsia, however, a decrease in plasma KYN/Trp ratio has been discovered, which can be attributed to decreased placental IDO activity (50). IDO-caused failure of Trp depletion was reported to induce excessive inflammatory response in preeclampsia (50). On the other hand, a significant increase in TDO expression has been found in preeclampsia, but since inflammatory response is involved in the pathogenesis of preeclampsia, it can be hypothesized that upregulation of TDO cannot fully compensate for the decrease in IDO in this regard. It is important that future studies address the differential regulation of IDO and TDO in placenta to determine their mechanistic role in preeclampsia (51, 52).
As a metabolite of Trp, 5-HT has been described primarily as a potent vasoconstrictor in the placental circulation (52). 5-HT activates its receptors on vascular smooth muscle and platelets, thereby promoting vasoconstriction and platelet aggregation. Furthermore, 5-HT synergistically amplifies the effects of other vasoconstricting substances (53). Catalyzed by monoamine oxidase (MAO), 5-HT metabolizes into 5-hydroxyindole acetic acid (5-HIAA), which is excreted from the body in the form of urine (54).
Women with preeclampsia have less 5-HIAA excreted from the urine, the evidence that cannot be explained in terms of impaired renal function, which is one of the manifestations in preeclampsia (55). At the same time, the patients’ level of 5-HT is high (40). The increased serum concentration of 5-HT and the simultaneously decreased excretion of 5-HIAA can be attributed to the decreased activity of MAO (50, 54, 56). Increased 5-HT is known to exacerbate the effect of vasoconstriction and platelet aggregation. One piece of evidence has shown that the involvement of increased 5-HT in the pathogenesis of preeclampsia could be the therapeutic effect of ketanserin, a 5-HT2 antagonist (53). Ketanserin functions as antihypertensive and antithrombotic, improving hemodynamics (57). Experimental 5-HT infusion in the pregnant animal could cause the similar kidney and placental damages as those seen in preeclampsia, which can be prevented by ketanserin (50). Even though less is known about the reason behind the decreased expression of MAO, MAO activity can determine, partially at least, the maternal systemic 5-HT concentration.
Arg, a non-essential amino acid produced at a slow speed, plays an irreplaceable role in the human body as a basic component of various proteins. There exist three Arg metabolic pathways, of which the one producing NO under the catalysis of nitric oxide synthase (NOS) is of the most significance. NO is known to serve as a strong vasodilator, playing an effective role in maintaining the constancy of vascular tone and regulating the stability of blood pressure (58, 59). Studies have shown that NO affects cardiovascular system mainly by activating guanylate cyclase (GUC) to increase the concentration of guanosine cyclomonophosphate (cGMP) (60–65). As a second messenger, cGMP mediates the inhibition of calcium ion flow through receptor-mediated calcium channels (53). Activation of GUC mediated by NO completes various physiological functions such as vasodilation and inhibition of platelet aggregation (66).
In preeclamptic placentas, reduced L-Arg and NO formation contributes to the inactivation of GUC-related channel, which may be attributed to the increased level of asymmetric dimethylarginine (ADMA), down-regulated eNOS and dimethylarginine dimethylamine hydrolase (DDAH) expression respectively (2, 67). NO produced by eNOS is downregulated through ADMA-caused reversible competitive inhibition (68). Since ADMA competes with Arg for eNOS, the bioavailability of NO depends on the balance between Arg and ADMA, namely the Arg/ADMA ratio; in preeclampsia the lower Arg/ADMA ratio contributes to less production of NO (69), yet eNOS do not differ significantly between the early-onset and late-onset of preeclampsia (70). ADMA itself is metabolized to L-citrulline and dimethylamine by DDAH, whose reduced activity in preeclampsia can result in increased ADMA level, which competes with eNOS for NO and reduces NO production (58). The contributing factors aforementioned can cause the overall level of NO to decrease, thus inactivating GUC-related channel, hence the platelet aggregation and high blood pressure ultimately in preeclampsia (71).
According to other researches, nonetheless, women with preeclampsia tend to increase the major metabolite of NO in their serum and urine, mainly in the form of FeNO (72, 73). Moreover, placental eNOS activity is not significantly different between preeclampsia and normal pregnancies (74). Evidence has shown that the changes in placental NO metabolism are unlikely to be the main cause of placental lesions, that the higher level of circulating NO metabolites may be to compensate for the vasoconstrictor effect of preeclampsia, and that the vascular system of patients with preeclampsia may also have some degree of desensitization or resistance to the effects of NO (74). Therefore, the exact changes of NO in preeclampsia remain to be further investigated.
Hcy, a member of the methionine-homocysteine metabolism (MHM), is transformed into methionine (MET) (75) by catalyzing methyltransferase (MTR), which uses 5-methyltetrahydrofolate (5-MTHF) as a methyl donor (76). 5-methyltetrahydrofolate-homocysteine methyltransferase reductase (MTRR) is capable of regenerating functionally active MTR by reducing methylation. 5,10-methylenetetrahydrofolate reductase (MTHFR) provides methyl groups with tetrahydrogen folic acid (THF) into 5-MTHF so that MHM receives methyl groups. Using ATP as an adenosine donor, MET is transformed into S-adenosylhomocysteine (SAM), which gets rid of a methyl group to transform SAM, which goes through the process of deadenylation and changes into Hcy. Catalyzed by cystathionine β synthase (CBS), Hcy turns into cysteine (Cys) (77).
In the normal pregnant women, plasma concentration of Hcy is low during the first trimester, which reaches its lowest level during the second half of pregnancy (78). In those who were diagnosed with preeclampsia; however, the level is increased, with hyper-homocysteinemia arising from MHM to cause oxidative stress and imbalance of plasma NO/ET level. In preeclampsia, hyper-homocysteinemia could be ascribed to the alternations of MTHFR and CBS, which, from the perspective of gene, are likely to be induced by single nucleotide polymorphisms (75). Since MTHFR is a polymorphic enzyme (79), the preeclamptic women are broadly observed to be homozygous for MTHFR T/T, MTHFR C677T and A1298C (80), which thus can lead to an increase in Hcy level. When the lower mRNA expression of CBS is found in preeclamptic placenta, moreover, a failure may occur in the elimination of Hcy (81).
Even though the changes in single nucleotide polymorphisms of MHM enzymes can lead to increased Hcy, MHM can be compensatorily activated in preeclampsia. 2-methoxyestradiol (2-ME), a metabolite of 17-𝛽-estradiol synthesized by Catechol-O-Methyltransferase (COMT), induces the differentiation of the endovascular cytotrophoblast cells into its invasive phenotype under the condition of hypoxia (75). With COMT being responsible for methylating 2-Hydroxyestradiol (2-HE) into 2-ME, it has been demonstrated that low activity or expression of this enzyme could be involved in the pathogenesis of preeclampsia (82). MHM, the process responsible for supplying COMT with the methyl group necessary for 2-ME synthesis, could be compensatorily activated in preeclampsia to supply methyl groups enough to sustain adequate concentration of 2-ME (83).
The notion that MHM is activated as compensation can be justified by the post-transcriptional changes of the related enzymes in MHM. In the preeclamptic placentas, RNA expression of MTHFR and MTR is elevated, but this change is not reflected in protein content, which highlights a potential compensatory mechanism for MHM (75). This underlines a possible role of MHM as a compensation mechanism in the presence of low 2-ME levels (83).
In severe preeclampsia (sPE), oxidative stress, inflammatory responses, and endothelial dysfunction collectively contribute to significant alterations in various metabolic molecules. GSH, as an important endogenous antioxidant, is significantly depleted due to elevated oxidative stress, leading to impaired antioxidant defense systems (84, 85). Additionally, the level of Trp decreases as a result of increased activity of IDO, which metabolizes Trp into KYN, reflecting enhanced inflammation and immune activation (86). Arg levels are reduced in sPE, primarily due to endothelial dysfunction. Arg, as a substrate for NO synthesis, is metabolized by arginase into ornithine and urea, further reducing NO bioavailability (87, 88). Additionally, Hcy levels increase due to disrupted folate metabolism or deficiencies in vitamins B6/B12. The accumulation of Hcy exacerbates oxidative stress and endothelial dysfunction, promoting the progression of preeclampsia (89, 90). These amino acid alterations in sPE illustrate the intricate interplay between oxidative stress, inflammation, and endothelial dysfunction, providing valuable insights into the disease’s pathophysiology. Figure 4 summarizes the key changes and their associated mechanisms.
Glycan, a complex composed of monosaccharides, can covalently bind with proteins or lipids, forming such biomolecules as glycoproteins, proteoglycans and glycolipids. The complex is known to be involved in cell recognition, cell adhesion, cell differentiation, immune recognition, and even tumor metastasis. In the placenta, the expression of glycan is a dynamic reflection of placental developmental and pathophysiological state. In the case of preeclampsia, oxidative stress can lead to the excessive production of ROS, which can regulate the expression of glycan (26). The higher expression of mannosan, a subtype of glycan, can be found at the end of placental villi in the early-onset of severe preeclampsia, and it can be recognized by cytotoxic NK cell mannose-receptors, which activate NK cells to make systemic inflammatory responses (91). In addition to preeclampsia, glycation of trophoblastic cells is also associated with pregnancy-induced hypertension and fetal growth restriction (92).
As an important “interpreter” of glycan, galectin can bind with glycan to act as an “alarm protein-like” molecule signalizing tissue damages induced by oxidative stress (91). Since apoptosis results partially from oxidative stress, the galectin-glycan circuit acts indispensably in regulating pro-survival and pro-apoptosis pathways, maintaining homeostasis under microenvironmental damages (92). The changes in the glycan of placental villous, caused by excessive ROS, can lead to the decreased level of Gal-9, a type of galectins most expressed at the maternal fetal interface, whose increased levels indicate that the placental cells are susceptible to apoptosis (93). Autophagy exists in normal human placenta and is an important manifestation of placenta’s normal physiological function. Apoptosis is found in normal placental trophoblast, stromal and endothelial cells, but mainly in syncytiotrophoblast (94). It has been confirmed in animal models that the placenta of mice with autophagy deficiency shows typical pathological changes of preeclampsia, that is, superficial trophoblast invasion and vascular remodeling failure, affecting the normal pregnancy process (95). Given that apoptosis and autophagy are mutually inhibited, apoptosis facilitates Gal-9-mediated autophagy reduction, which results in the decrease of invasiveness in the trophoblastic cells, and the obstruction of spiral artery recasting, hence placental hypoperfusion (96). Therefore, the dynamic changes in the galectin-glycan network associated with oxidative stress may play an important role in the pathogenesis of preeclampsia. This local stress response can eventually spread throughout the body, resulting in the development of preeclampsia (92).
However, the effect of glycosylation on autophagy and reactive immune cell regulation in the trophoblast requires further investigation, as excessive oxidative stress has been found to reduce the expression of GnT-III, a key mannose glycosyl transferase, contrary to the previously stated hyper glycan expression in the terminal of the placental villus in preeclampsia (97). This may be due to different cell lines in different experiments. In conclusion, oxidative stress-induced glycosylation of the placental trophoblast cells can change their metabolic traits, exerting an impact on the development of preeclampsia.
Interaction exists between immune cells and trophoblast, as manifested by the evidence that CD8 + T cells induce trophoblast to express matrix metalloproteinase-2/9 (MMP-2/9) to promote trophoblast invasion and facilitate embryo implantation. As aforementioned, the high concentration of fatty acid is observed in the preeclamptic placenta, as a risk factor. Even though the role of fatty acid in the interaction between immune cells and trophoblast cells remains obscure, it can be referred to as the microenvironment of tumor metabolism, as tumor and trophoblast cells share the common character of metabolic reprogramming. In the microenvironment of tumor metabolism, the higher levels of fatty acid inhibit CD8 + T cell function and promote tumor growth by altering the metabolic pattern of tumor cells (98). Tumor and CD8 + T cells appear to reprogram fatty acid metabolism differently, for the tumor cells adapt themselves by increasing fatty acid utilization, whereas CD8 + T cells do not (98). The uptake of fatty acids is enhanced by tumor cells, which may contribute to the deficiency of fatty acids in the tumor microenvironment of CD8 + T cells, whose normal function is impaired (98). Thus, the higher levels of fatty acid exacerbate metabolic reprogramming, which can lead to the nutrient availability and immune dysfunction altered in the tumor microenvironment.
Therefore, we hypothesize that in the preeclamptic placenta the high fatty acid concentrations can be responsible for metabolic reprogramming as in the case of tumor cells, resulting in insufficient fatty acid availability for CD8 + T cells and inhibited CD8 + T cell function. Decidual CD8 + T cells could recognize human leukocyte antigen (HLA)-C expressed by extravillous trophoblast cells (99). Recognition of HLA-C by CD8 + T cells may be important to normal pregnancy, especially when those who lack killer cell activating receptors to interact with HLA-C are more likely to suffer from preeclampsia (100). Recognition by decidual CD8 + T cells of HLA-C expressed by trophoblasts tend to result in the generation of IL8, which has been reported to increase production of both MMP-2 and MMP-9 by endothelial cells (101). This can be responsible for the vascular remodeling required for the establishment of the placenta (98). In other words, CD8 + T cell function, when inhibited, can impair trophoblast invasion, which is acknowledged as pathogenesis of preeclampsia. In view of this, it is imperative that the metabolite-mediated immune cell function and its interaction with the trophoblast cells be further studied in the future. Apart from the role of CD8 + T cell, other types of immune cells are likely to interfere with metabolites, which is an intriguing aspect of further investigation.
Although definitive therapies are unavailable, some metabolism-related ones have recently been shown to be promising in the prevention and treatment of preeclampsia by interfering with the classic mechanisms (Figure 5).
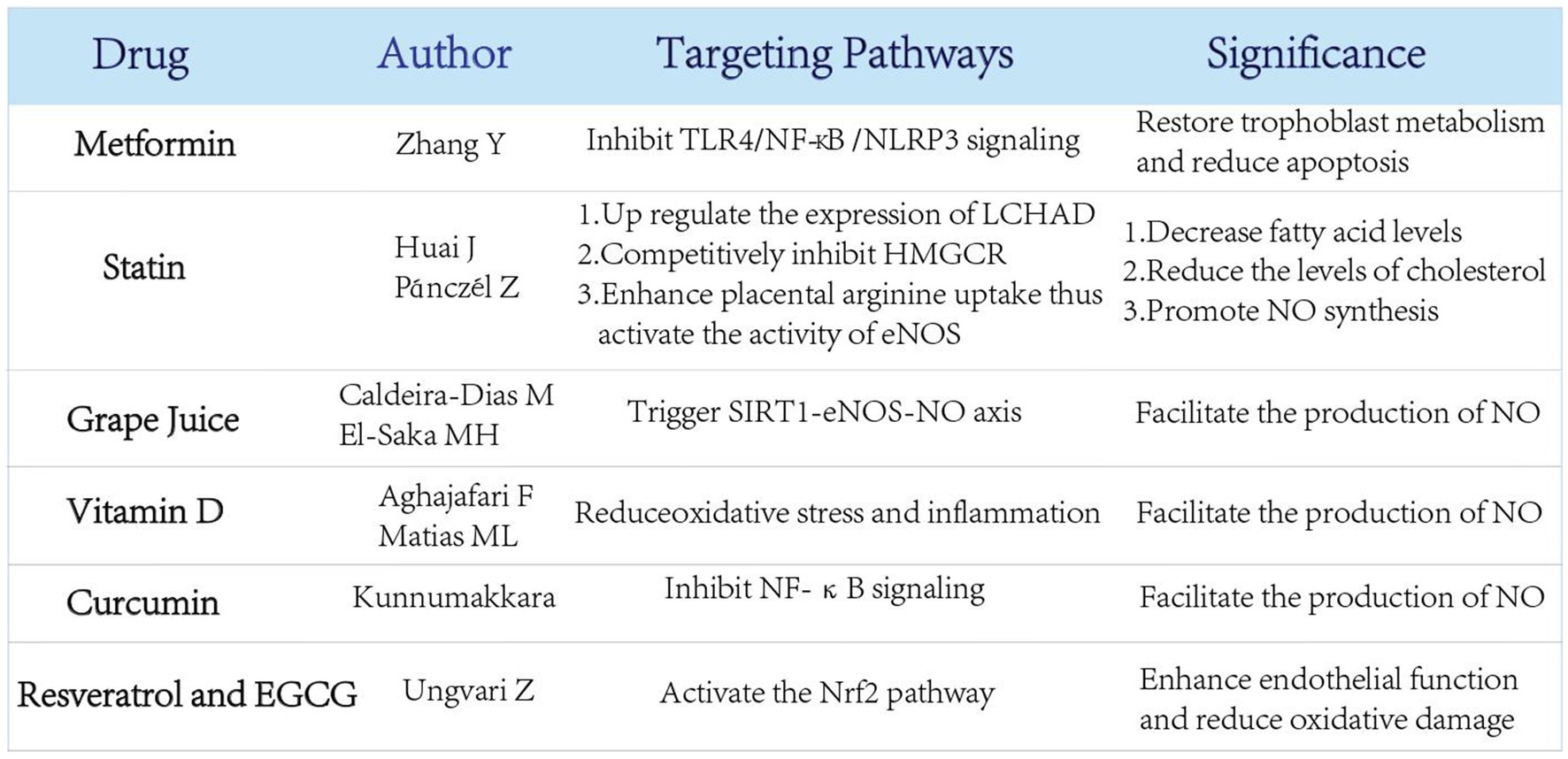
Figure 5. Several newly proposed therapies for preeclampsia related to nutrient metabolism and their potential effects. Several emerging treatments related to nutrient metabolism have been proposed to prevent the occurrence of preeclampsia through multiple regulatory pathways.
Preeclampsia is thought to be associated with abnormal apoptosis of trophoblast cells. As recent studies have suggested that preeclamptic trophoblasts are highly likely to undergo glycolytic reprogramming, the newly discovered TLR4/NF-κB/PFKFB3 pathway may function as a link between metabolic reprogramming and NLRP3 inflammasome induced trophoblast apoptosis (102). In this pathway, TLR4/NF-κB signaling causes mitochondrial destruction and dysfunction, thus reprogramming the glycometabolism to glycolysis with increased PFKFB3 expression, which induces NLRP3 inflammasome assisted apoptosis (103–105). Activation of TLR4/NF-κB/PFKFB3 pathway in preeclampsia causes trophoblast cells to preferentially use glycolysis over mitochondrial oxidative phosphorylation, ultimately resulting in trophoblast ATP deficiency and increased apoptosis (102).
Metformin (MET), the first-line drug for type II diabetes mellitus, has long been clinically administered to regulate glucose metabolism. MET can reduce NLRP3-induced apoptosis and restore trophoblast metabolism by effectively inhibiting TLR4/NF-κB signaling (102, 106, 107). Beyond that, MET is partially capable of suppressing apoptosis by blocking the binding of the transcription factor NF-κB to PFKFB3 promoter and reducing PFKFB3 transcription (102).
Metformin is widely used during pregnancy for conditions such as gestational diabetes mellitus and polycystic ovary syndrome, with studies showing no significant increase in adverse outcomes for mothers or neonates. A Phase II clinical trial involving 180 pregnant women suggests that metformin (3 g daily dose) can extend the gestational weeks of early-onset preeclampsia by about 1 week and reduce neonatal hospital stay (108).
However, the long-term physiological effects of metformin therapy in pregnancy women without diabetes remains unclear till now. Therefore, MET and its long-term effect can have promising potential to be explored in preeclampsia.
Pravastatin, as a natural compound of statin, is conventionally applied to the treatment of primary hypercholesterolemia, type IIa and type IIb hyperlipidemia. In the case of preeclampsia, the level of LCHAD is found to be decreased, which is explained by the decreased level of fatty acid oxidation in this disease (102). Pravastatin comes in to play when the expression of LCHAD is upregulated in the liver and placenta, thus significantly decreasing fatty acid levels (109). Acting as a competitive inhibitor of 3-hydroxy-3-methylglutarate monoacyl-CoA reductase (HMGCR), the rate-limiting enzyme of cholesterol synthesis, pravastatin can also reduce the levels of cholesterol, low-density lipoprotein, very low-density lipoprotein and triacylglycerol in the body in a direct or indirect way, so as to regulate blood lipid level (110). Additionally, the potency of pravastatin can even be administered to regulate blood lipid so that the clinical manifestations of preeclampsia can be alleviated (111).
Apart from the lipid-regulating properties, pravastatin is capable of significantly increasing NOS activity in the placenta, thereby promoting NO synthesis (112). Preeclamptic pregnancies are known to have low concentrations of Arg in serum, where pravastatin induces Arg uptake at low Arg levels, rapidly activating eNOS whose activity increases with the supply of substrate (113). Future studies are acquired to explore the effect of pravastatin on the unknown levels of Arg in preeclamptic placentas and in severity-categorized preeclamptic samples.
As aforementioned, NO acts as a vasodilator in pregnancy. Since grape juice is found to interfere with NO production, a recent proposal has been made to use it as original add-on therapy for preeclampsia (114). Upon an ingestion of grape juice, NO production is increased in the serum endothelial cells of preeclampsia patients (115), which is recognized to trigger SIRT1-eNOS-NO axis. However, grape juice’s ability to increase the production of NO in endothelial cells does not appear to rely solely on its major antioxidant named resveratrol. In an in vitro PE model, grape juice intake appears to have a different effect than resveratrol supplementation alone, suggesting that other bioactive molecules in grape juice combined with SIRT1-eNOS-NO have therapeutic potential in PE (116). This suggests that grape juice can be of a feasible therapy for preeclampsia, although further experiments are required to measure its appropriate dose and other effective substances apart from resveratrol to better understand its synergistic effect along with SIRT1-eNOS-NO axis.
Recent studies have highlighted the role of vitamin D and natural plant-derived compounds in reducing oxidative stress and inflammation, which are key contributors to preeclampsia pathogenesis. Vitamin D has been shown to regulate immune responses and improve endothelial function, with low maternal vitamin D levels being associated with an increased risk of preeclampsia (117). Supplementation with vitamin D has demonstrated potential in reducing preeclampsia risk in randomized controlled trials, although further large-scale studies are needed to confirm its efficacy (118).
Natural compounds of plant origin, including flavonoids, epigallocatechin gallate (EGCG), quercetin, resveratrol, and curcumin, have attracted attention for their antioxidant and anti-inflammatory properties. For example, curcumin has been reported to modulate oxidative stress pathways and inflammation through the inhibition of NF-κB signaling (119). Similarly, resveratrol and EGCG enhance endothelial function and reduce oxidative damage by activating the Nrf2 pathway (120). Quercetin, a potent flavonoid, has shown promise in preclinical studies for its ability to reduce vascular inflammation and improve placental function (121). These compounds may represent complementary therapeutic strategies, but more clinical studies are required to evaluate their safety and efficacy in pregnancy (122).
In the pathogenesis of preeclampsia, the alternations of carbohydrates, lipids, amino acids and glycans are involved in the multiple classic mechanisms, the influence of which is significantly extensive. The main reason for the changes in metabolites, which still remains unclear though, is focused on the changes of relevant enzymes. However, the post-translational modification of proteins or other regulatory effects of metabolites have not been fully elucidated. Some newly proposed therapies which target nutrient metabolism have been shown promising in the prevention and treatment of preeclampsia in either animal models or patients. These findings reveal that metabolic abnormalities may involve in the pathophysiological mechanism of preeclampsia, which suggests that in the future researches, more specific metabolic pathways need to be explored in preeclampsia based on animals and in vitro models, which is of great significance to the development of new metabolic drugs for the alleviation of preeclamptic symptoms. Furthermore, large-scale cohort studies are urgently needed to validate the role of specific metabolites in prenatal diagnosis. These studies would help identify potential pathways and biomarkers critical to improving early detection and therapeutic strategies for preeclampsia.
SL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. YZ: Software, Supervision, Validation, Visualization, Writing – review & editing. PA: Investigation, Methodology, Project administration, Resources, Writing – review & editing. HZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – review & editing. YX: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Research and Development Program (2021YFC2701600 and 2021YFC2701601); Clinical Research Plan of SHDC (SHDC2020CR6021); National Science Foundation of China (81741047); the National Science Fund of Shanghai, China (No. 22ZR1409000); and Medical Innovation Research Program of Shanghai, China (No. 21Y11908000).
The authors would like to thank Zhengliu Liang (College of Foreign Languages and Literature, Fudan University) for polishing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. von Versen-Hoeynck, FM, and Powers, RW. Maternal-fetal metabolism in normal pregnancy and preeclampsia. Front Biosci. (2007) 12:2457–70. doi: 10.2741/2247
2. Azizi, F, Omrani, MD, Amiri, V, Mirfakhraie, R, Dodangeh, F, Shahmirzadi, SA, et al. Altered methylation and expression patterns of genes regulating placental nitric oxide pathway in patients with severe preeclampsia. Hum Antibodies. (2019) 27:117–24. doi: 10.3233/hab-180356
3. Joffe, H, Petrillo, L, Viguera, A, Koukopoulos, A, Silver-Heilman, K, Farrell, A, et al. Eszopiclone improves insomnia and depressive and anxious symptoms in perimenopausal and postmenopausal women with hot flashes: a randomized, double-blinded, placebo-controlled crossover trial. Am J Obstet Gynecol. (2010) 202:171.e1–171.e11. doi: 10.1016/j.ajog.2009.10.868
4. Sun, Y, Yang, H, and Sun, WJ. Risk factors for pre-eclampsia in pregnant Chinese women with abnormal glucose metabolism. Int J Gynaecol Obstet. (2008) 101:74–6. doi: 10.1016/j.ijgo.2007.10.008
5. Sato, E, Tsunokuni, Y, Kaneko, M, Saigusa, D, Saito, R, Shimma, S, et al. Metabolomics of a mouse model of preeclampsia induced by overexpressing soluble fms-like tyrosine kinase 1. Biochem Biophys Res Commun. (2020) 527:1064–71. doi: 10.1016/j.bbrc.2020.04.079
6. Zhou, X, Han, TL, Chen, H, Baker, PN, Qi, H, and Zhang, H. Impaired mitochondrial fusion, autophagy, biogenesis and dysregulated lipid metabolism is associated with preeclampsia. Exp Cell Res. (2017) 359:195–204. doi: 10.1016/j.yexcr.2017.07.029
7. Aouache, R, Biquard, L, Vaiman, D, and Miralles, F. Oxidative stress in preeclampsia and placental diseases. Int J Mol Sci. (2018) 19:19. doi: 10.3390/ijms19051496
8. Cristofalo, R, Bannwart-Castro, CF, Magalhães, CG, Borges, VT, Peraçoli, JC, Witkin, SS, et al. Silibinin attenuates oxidative metabolism and cytokine production by monocytes from preeclamptic women. Free Radic Res. (2013) 47:268–75. doi: 10.3109/10715762.2013.765951
9. Redman, CW, and Sargent, IL. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J Reprod Immunol. (2007) 76:61–7. doi: 10.1016/j.jri.2007.03.008
10. Gao, P, Tchernyshyov, I, Chang, TC, Lee, YS, Kita, K, Ochi, T, et al. C-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. (2009) 458:762–5. doi: 10.1038/nature07823
11. Aye, I, Aiken, CE, Charnock-Jones, DS, and Smith, GCS. Placental energy metabolism in health and disease-significance of development and implications for preeclampsia. Am J Obstet Gynecol. (2022) 226:S928–s944. doi: 10.1016/j.ajog.2020.11.005
12. Xiao, Y, and Zhang, X. Association between maternal glucose/lipid metabolism parameters and abnormal newborn birth weight in gestational diabetes complicated by preeclampsia: a retrospective analysis of 248 cases. Diabetes Ther. (2020) 11:905–14. doi: 10.1007/s13300-020-00792-3
13. Bloxam, DL, Bullen, BE, Walters, BN, and Lao, TT. Placental glycolysis and energy metabolism in preeclampsia. Am J Obstet Gynecol. (1987) 157:97–101. doi: 10.1016/s0002-9378(87)80354-x
14. Soleymanlou, N, Jurisica, I, Nevo, O, Ietta, F, Zhang, X, Zamudio, S, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. (2005) 90:4299–308. doi: 10.1210/jc.2005-0078
15. Marín, R, Chiarello, DI, Abad, C, Rojas, D, Toledo, F, and Sobrevia, L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim Biophys Acta Mol basis Dis. (2020) 1866:165961. doi: 10.1016/j.bbadis.2020.165961
16. Holland, OJ, Cuffe, JSM, Dekker Nitert, M, Callaway, L, Kwan Cheung, KA, Radenkovic, F, et al. Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis. (2018) 9:1150. doi: 10.1038/s41419-018-1190-9
17. Lin, T, Yang, WQ, Luo, WW, Zhang, LL, Mai, YQ, Li, ZQ, et al. Disturbance of fatty acid metabolism promoted vascular endothelial cell senescence via acetyl-CoA-induced protein acetylation modification. Oxidative Med Cell Longev. (2022) 2022:1198607–24. doi: 10.1155/2022/1198607
18. Rigoulet, M, Bouchez, CL, Paumard, P, Ransac, S, Cuvellier, S, Duvezin-Caubet, S, et al. Cell energy metabolism: an update. Biochim Biophys Acta Bioenerg. (2020) 1861:148276. doi: 10.1016/j.bbabio.2020.148276
19. Chen, DQ, Zhao, HY, Fang, Q, He, J, and Chai, Y. Relationship between blood lipid levels of pregnant women with glucose metabolism disorders and perinatal outcomes. Zhonghua Fu Chan Ke Za Zhi. (2007) 42:366–9.
20. Melland-Smith, M, Ermini, L, Chauvin, S, Craig-Barnes, H, Tagliaferro, A, Todros, T, et al. Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy. (2015) 11:653–69. doi: 10.1080/15548627.2015.1034414
21. López-Tinoco, C, Roca, M, Fernández-Deudero, A, García-Valero, A, Bugatto, F, Aguilar-Diosdado, M, et al. Cytokine profile, metabolic syndrome and cardiovascular disease risk in women with late-onset gestational diabetes mellitus. Cytokine. (2012) 58:14–9. doi: 10.1016/j.cyto.2011.12.004
22. Yang, X, Xu, P, Zhang, F, Zhang, L, Zheng, Y, Hu, M, et al. AMPK hyper-activation alters fatty acids metabolism and impairs invasiveness of trophoblasts in preeclampsia. Cell Physiol Biochem. (2018) 49:578–94. doi: 10.1159/000492995
23. Ma, HZ, Chen, Y, Guo, HH, Wang, J, Xin, XL, Li, YC, et al. Effect of resveratrol in gestational diabetes mellitus and its complications. World J Diabetes. (2023) 14:808–19. doi: 10.4239/wjd.v14.i6.808
24. Zhang, L, Bi, S, Liang, Y, Huang, L, Li, Y, Huang, M, et al. Integrated Metabolomic and Lipidomic analysis in the placenta of preeclampsia. Front Physiol. (2022) 13:807583. doi: 10.3389/fphys.2022.807583
25. Cong, KJ, Wang, TT, and Liu, GR. Lipid metabolism and pregnancy induced hypertension. Zhonghua Fu Chan Ke Za Zhi. (1994) 29:697–58.
26. Bhat, PV, Vinod, V, Priyanka, AN, and Kamath, A. Maternal serum lipid levels, oxidative stress and antioxidant activity in pre-eclampsia patients from Southwest India. Pregnancy Hypertens. (2019) 15:130–3. doi: 10.1016/j.preghy.2018.12.010
27. Cabunac, P, Karadžov Orlić, N, Ardalić, D, Banjac, G, Ivanišević, J, Janać, J, et al. Unraveling the role of oxidative stress and lipid status parameters in the onset of preeclampsia. Hypertens Pregnancy. (2021) 40:162–70. doi: 10.1080/10641955.2021.1921790
28. Stadler, JT, Scharnagl, H, Wadsack, C, and Marsche, G. Preeclampsia affects lipid metabolism and HDL function in mothers and their offspring. Antioxidants. (2023) 12:12. doi: 10.3390/antiox12040795
29. Ray, JG, Diamond, P, Singh, G, and Bell, CM. Brief overview of maternal triglycerides as a risk factor for pre-eclampsia. BJOG. (2006) 113:379–86. doi: 10.1111/j.1471-0528.2006.00889.x
30. Iruretagoyena, JI, and Shah, D. A case of severe preeclampsia leading to the diagnosis of de novo abnormal fatty acid metabolism and ACE gene deletion. J Obstet Gynaecol Can. (2010) 32:695–7. doi: 10.1016/s1701-2163(16)34575-3
31. Mackay, CR, and Marques, FZ. Dysbiosis in preeclampsia and treatment with short chain fatty acids. Circ Res. (2022) 131:507–9. doi: 10.1161/circresaha.122.321701
32. Li, F, Yang, Z, Zhang, A, Sun, X, Wang, J, and Meng, R. The changes of LCHAD in preeclampsia with different clinical features and the correlation with NADPH P47-phox, p38MAPK-α, COX-2 and serum FFA and TG. Zhonghua Fu Chan Ke Za Zhi. (2015) 50:92–100.
33. Murai, JT, Muzykanskiy, E, and Taylor, RN. Maternal and fetal modulators of lipid metabolism correlate with the development of preeclampsia. Metabolism. (1997) 46:963–7. doi: 10.1016/s0026-0495(97)90088-3
34. Sánchez-Aranguren, LC, Prada, CE, Riaño-Medina, CE, and Lopez, M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol. (2014) 5:372. doi: 10.3389/fphys.2014.00372
35. Yung, HW, Atkinson, D, Campion-Smith, T, Olovsson, M, Charnock-Jones, DS, and Burton, GJ. Differential activation of placental unfolded protein response pathways implies heterogeneity in causation of early- and late-onset pre-eclampsia. J Pathol. (2014) 234:262–76. doi: 10.1002/path.4394
36. Gao, LM, Xie, CY, Zhang, TY, Wu, X, and Yin, YL. Maternal supplementation with calcium varying with feeding time daily during late pregnancy affects lipid metabolism and transport of placenta in pigs. Biochem Biophys Res Commun. (2018) 505:624–30. doi: 10.1016/j.bbrc.2018.09.143
37. Devarshi, PP, Grant, RW, Ikonte, CJ, and Hazels, MS. Maternal Omega-3 nutrition, placental transfer and fetal brain development in gestational diabetes and preeclampsia. Nutrients. (2019) 11:11. doi: 10.3390/nu11051107
38. Tanzilli, G, Arrivi, A, Placanica, A, Viceconte, N, Cammisotto, V, Nocella, C, et al. Glutathione infusion before and 3 days after primary angioplasty blunts ongoing NOX2-mediated inflammatory response. J Am Heart Assoc. (2021) 10:e020560. doi: 10.1161/jaha.120.020560
39. Kawasaki, K, Kondoh, E, Chigusa, Y, Kawamura, Y, Mogami, H, Takeda, S, et al. Metabolomic profiles of placenta in preeclampsia. Hypertension. (2019) 73:671–9. doi: 10.1161/hypertensionaha.118.12389
40. Samarghandian, S, Azimi-Nezhad, M, Farkhondeh, T, and Samini, F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother. (2017) 87:223–9. doi: 10.1016/j.biopha.2016.12.105
41. Burton, GJ, and Jauniaux, E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. (2011) 25:287–99. doi: 10.1016/j.bpobgyn.2010.10.016
42. Fragoso, MBT, Ferreira, RC, Tenório, M, Moura, FA, de Araújo, ORP, Bueno, NB, et al. Biomarkers of inflammation and redox imbalance in umbilical cord in pregnancies with and without preeclampsia and consequent perinatal outcomes. Oxidative Med Cell Longev. (2021) 2021:9970627. doi: 10.1155/2021/9970627
43. Ferreira, RC, Fragoso, MBT, Tenório, M, Martins, A, Borbely, AU, Moura, FA, et al. Biomarkers of placental redox imbalance in pregnancies with preeclampsia and consequent perinatal outcomes. Arch Biochem Biophys. (2020) 691:108464. doi: 10.1016/j.abb.2020.108464
44. Ahmad, IM, Zimmerman, MC, and Moore, TA. Oxidative stress in early pregnancy and the risk of preeclampsia. Pregnancy Hypertens. (2019) 18:99–102. doi: 10.1016/j.preghy.2019.09.014
45. Jin, X, Xu, Z, Cao, J, Shao, P, Zhou, M, Qin, Z, et al. Proteomics analysis of human placenta reveals glutathione metabolism dysfunction as the underlying pathogenesis for preeclampsia. Biochim Biophys Acta Proteins Proteom. (2017) 1865:1207–14. doi: 10.1016/j.bbapap.2017.07.003
46. Gál, EM, and Sherman, AD. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem Res. (1980) 5:223–39. doi: 10.1007/bf00964611
47. Li, F, Zhang, R, Li, S, and Liu, J. IDO1: an important immunotherapy target in cancer treatment. Int Immunopharmacol. (2017) 47:70–7. doi: 10.1016/j.intimp.2017.03.024
48. Zhai, L, Spranger, S, Binder, DC, Gritsina, G, Lauing, KL, Giles, FJ, et al. Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for Cancer immunotherapy. Clin Cancer Res. (2015) 21:5427–33. doi: 10.1158/1078-0432.Ccr-15-0420
49. Guo, Y, Liu, Y, Wu, W, Ling, D, Zhang, Q, Zhao, P, et al. Indoleamine 2,3-dioxygenase (Ido) inhibitors and their nanomedicines for cancer immunotherapy. Biomaterials. (2021) 276:121018. doi: 10.1016/j.biomaterials.2021.121018
50. Kudo, Y, Boyd, CA, Sargent, IL, and Redman, CW. Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia. Am J Obstet Gynecol. (2003) 188:719–26. doi: 10.1067/mob.2003.156
51. Keaton, SA, Heilman, P, Bryleva, EY, Madaj, Z, Krzyzanowski, S, Grit, J, et al. Altered tryptophan catabolism in placentas from women with pre-eclampsia. Int J Tryptophan Res. (2019) 12:1178646919840321. doi: 10.1177/1178646919840321
52. Broekhuizen, M, Klein, T, Hitzerd, E, de Rijke, YB, Schoenmakers, S, Sedlmayr, P, et al. L-tryptophan-induced vasodilation is enhanced in preeclampsia: studies on its uptake and metabolism in the human placenta. Hypertension. (2020) 76:184–94. doi: 10.1161/hypertensionaha.120.14970
53. Steyn, DW, and Odendaal, HJ. Randomised controlled trial of ketanserin and aspirin in prevention of pre-eclampsia. Lancet. (1997) 350:1267–71. doi: 10.1016/s0140-6736(97)06408-8
54. Carrasco, G, Cruz, MA, Gallardo, V, Miguel, P, Dominguez, A, and González, C. Transport and metabolism of serotonin in the human placenta from normal and severely pre-eclamptic pregnancies. Gynecol Obstet Investig. (2000) 49:150–5. doi: 10.1159/000010237
55. Carrasco, G, Cruz, MA, Gallardo, V, Miguel, P, Lagos, M, and González, C. Plasma and platelet concentration and platelet uptake of serotonin in normal and pre-eclamptic pregnancies. Life Sci. (1998) 62:1323–32. doi: 10.1016/s0024-3205(98)00066-6
56. Schäfer, CA, du Bois, A, Vach, W, Prömpeler, H, Bauknecht, T, and Breckwoldt, M. Changes in serotonin metabolism in pre-eclampsia. Geburtshilfe Frauenheilkd. (1996) 56:418–22. doi: 10.1055/s-2007-1023257
57. Brogden, RN, and Sorkin, EM. Ketanserin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in hypertension and peripheral vascular disease. Drugs. (1990) 40:903–49. doi: 10.2165/00003495-199040060-00010
58. Ehsanipoor, RM, Fortson, W, Fitzmaurice, LE, Liao, WX, Wing, DA, Chen, DB, et al. Nitric oxide and carbon monoxide production and metabolism in preeclampsia. Reprod Sci. (2013) 20:542–8. doi: 10.1177/1933719112459231
59. Cindrova-Davies, T. Gabor than award lecture 2008: pre-eclampsia – from placental oxidative stress to maternal endothelial dysfunction. Placenta. (2009) 30:55–65. doi: 10.1016/j.placenta.2008.11.020
60. Li, DY, Gao, SJ, Sun, J, Zhang, LQ, Wu, JY, Song, FH, et al. Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain. Neural Regen Res. (2023) 18:996–1003. doi: 10.4103/1673-5374.355748
61. Arnold, WP, Mittal, CK, Katsuki, S, and Murad, F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA. (1977) 74:3203–7. doi: 10.1073/pnas.74.8.3203
62. Denninger, JW, and Marletta, MA. Guanylate cyclase and the.NO/cGMP signaling pathway. Biochim Biophys Acta. (1999) 1411:334–50. doi: 10.1016/s0005-2728(99)00024-9
63. Hussain, MB, Hobbs, AJ, and MacAllister, RJ. Autoregulation of nitric oxide-soluble guanylate cyclase-cyclic GMP signalling in mouse thoracic aorta. Br J Pharmacol. (1999) 128:1082–8. doi: 10.1038/sj.bjp.0702874
64. Derbyshire, ER, and Marletta, MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem. (2012) 81:533–59. doi: 10.1146/annurev-biochem-050410-100030
65. Weisbrod, RM, Griswold, MC, Yaghoubi, M, Komalavilas, P, Lincoln, TM, and Cohen, RA. Evidence that additional mechanisms to cyclic GMP mediate the decrease in intracellular calcium and relaxation of rabbit aortic smooth muscle to nitric oxide. Br J Pharmacol. (1998) 125:1695–707. doi: 10.1038/sj.bjp.0702233
66. Engelberger, RP, Pittet, YK, Henry, H, Delodder, F, Hayoz, D, Chioléro, RL, et al. Acute endotoxemia inhibits microvascular nitric oxide-dependent vasodilation in humans. Shock. (2011) 35:28–34. doi: 10.1097/SHK.0b013e3181ec71ab
67. Tamás, P, Bódis, J, Sulyok, E, Kovács, GL, Hantosi, E, Molnár, G, et al. L-arginine metabolism in early-onset and late-onset pre-eclamptic pregnancies. Scand J Clin Lab Invest. (2013) 73:436–43. doi: 10.3109/00365513.2013.803230
68. Huang, S, Xu, Y, Peng, WF, Cheng, J, Li, HH, Shen, LS, et al. A correlational study between serum asymmetric dimethylarginine level and impaired glucose tolerance patients associated with obesity. J Cell Physiol. (2019) 234:10640–5. doi: 10.1002/jcp.27743
69. Jarquin Campos, A, Risch, L, Baumann, M, Purde, MT, Neuber, S, Renz, H, et al. Shrunken pore syndrome, preeclampsia, and markers of NO metabolism in pregnant women during the first trimester. Scand J Clin Lab Invest. (2019) 79:91–8. doi: 10.1080/00365513.2019.1568150
70. ElMonier, AA, El-Boghdady, NA, Abdelaziz, MA, and Shaheen, AA. Association between endoglin/transforming growth factor beta receptors 1, 2 gene polymorphisms and the level of soluble endoglin with preeclampsia in Egyptian women. Arch Biochem Biophys. (2019) 662:7–14. doi: 10.1016/j.abb.2018.11.022
71. Erlandsson, L, Ducat, A, Castille, J, Zia, I, Kalapotharakos, G, Hedström, E, et al. Alpha-1 microglobulin as a potential therapeutic candidate for treatment of hypertension and oxidative stress in the STOX1 preeclampsia mouse model. Sci Rep. (2019) 9:8561. doi: 10.1038/s41598-019-44639-9
72. Principe, P, Mukosera, GT, Gray-Hutto, N, Tugung, A, Gheorghe, CP, and Blood, AB. Nitric oxide affects Heme Oxygenase-1, Hepcidin, and transferrin receptor expression in the placenta. Int J Mol Sci. (2023) 24:24. doi: 10.3390/ijms24065887
73. Mukosera, GT, Principe, P, Mata-Greenwood, E, Liu, T, Schroeder, H, Parast, M, et al. Iron nitrosyl complexes are formed from nitrite in the human placenta. J Biol Chem. (2022) 298:102078. doi: 10.1016/j.jbc.2022.102078
74. Nishizawa, H, Pryor-Koishi, K, Suzuki, M, Kato, T, Sekiya, T, Tada, S, et al. Analysis of nitric oxide metabolism as a placental or maternal factor underlying the etiology of pre-eclampsia. Gynecol Obstet Investig. (2009) 68:239–47. doi: 10.1159/000238381
75. Pérez-Sepúlveda, A, España-Perrot, PP, Fernández, XB, Ahumada, V, Bustos, V, Arraztoa, JA, et al. Levels of key enzymes of methionine-homocysteine metabolism in preeclampsia. Biomed Res Int. (2013) 2013:731962. doi: 10.1155/2013/731962
76. Herrmann, W, Hübner, U, Koch, I, Obeid, R, Retzke, U, and Geisel, J. Alteration of homocysteine catabolism in pre-eclampsia, HELLP syndrome and placental insufficiency. Clin Chem Lab Med. (2004) 42:1109–16. doi: 10.1515/cclm.2004.228
77. Also-Rallo, E, Lopez-Quesada, E, Urreizti, R, Vilaseca, MA, Lailla, JM, Balcells, S, et al. Polymorphisms of genes involved in homocysteine metabolism in preeclampsia and in uncomplicated pregnancies. Eur J Obstet Gynecol Reprod Biol. (2005) 120:45–52. doi: 10.1016/j.ejogrb.2004.08.008
78. Kasture, VV, Sundrani, DP, and Joshi, SR. Maternal one carbon metabolism through increased oxidative stress and disturbed angiogenesis can influence placental apoptosis in preeclampsia. Life Sci. (2018) 206:61–9. doi: 10.1016/j.lfs.2018.05.029
79. Mislanova, C, Martsenyuk, O, Huppertz, B, and Obolenskaya, M. Placental markers of folate-related metabolism in preeclampsia. Reproduction. (2011) 142:467–76. doi: 10.1530/rep-10-0484
80. Wu, X, Yang, K, Tang, X, Sa, Y, Zhou, R, Liu, J, et al. Folate metabolism gene polymorphisms MTHFR C677T and A1298C and risk for preeclampsia: a meta-analysis. J Assist Reprod Genet. (2015) 32:797–805. doi: 10.1007/s10815-014-0408-8
81. Mohanraj, PS, Rahat, B, Mahajan, A, Bagga, R, and Kaur, J. Temporal expression of genes involved in folate metabolism and transport during placental development, preeclampsia and neural tube defects. Mol Biol Rep. (2019) 46:3193–201. doi: 10.1007/s11033-019-04776-w
82. Liang, S, Liu, X, Fan, P, Liu, R, Zhang, J, He, G, et al. Association between Val158Met functional polymorphism in the COMT gene and risk of preeclampsia in a Chinese population. Arch Med Res. (2012) 43:154–8. doi: 10.1016/j.arcmed.2012.03.002
83. Hertig, A, Liere, P, Chabbert-Buffet, N, Fort, J, Pianos, A, Eychenne, B, et al. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstet Gynecol. (2010) 203:477.e1–9. doi: 10.1016/j.ajog.2010.06.011
84. Xu, X, Ye, X, Zhu, M, Zhang, Q, Li, X, and Yan, J. FtMt reduces oxidative stress-induced trophoblast cell dysfunction via the HIF-1α/VEGF signaling pathway. BMC Pregnancy Childbirth. (2023) 23:131. doi: 10.1186/s12884-023-05448-1
85. Gupta, S, Agarwal, A, and Sharma, RK. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet Gynecol Surv. (2005) 60:807–16. doi: 10.1097/01.ogx.0000193879.79268.59
86. Austdal, M, Thomsen, LC, Tangerås, LH, Skei, B, Mathew, S, Bjørge, L, et al. Metabolic profiles of placenta in preeclampsia using HR-MAS MRS metabolomics. Placenta. (2015) 36:1455–62. doi: 10.1016/j.placenta.2015.10.019
87. Maas, R, Böger, RH, Schwedhelm, E, Casas, JP, López-Jaramillo, P, Serrano, N, et al. Plasma concentrations of asymmetric dimethylarginine (ADMA) in Colombian women with pre-eclampsia. JAMA. (2004) 291:823–4. doi: 10.1001/jama.291.7.823
88. Morris, SM Jr. Arginine: beyond protein. Am J Clin Nutr. (2006) 83:508s–12s. doi: 10.1093/ajcn/83.2.508S
89. Gurbuz, A, Karateke, A, and Mengulluoglu, M. Elevated plasma homocysteine levels in preeclampsia and eclampsia. Int J Gynaecol Obstet. (2004) 87:165–6. doi: 10.1016/j.ijgo.2004.06.024
90. Powers, RW, Evans, RW, Majors, AK, Ojimba, JI, Ness, RB, Crombleholme, WR, et al. Plasma homocysteine concentration is increased in preeclampsia and is associated with evidence of endothelial activation. Am J Obstet Gynecol. (1998) 179:1605–11. doi: 10.1016/s0002-9378(98)70033-x
91. Campuzano, M, Bueno-Sánchez, J, Agudelo-Jaramillo, B, Quintana-Castillo, JC, Chaouat, GC, and Maldonado-Estrada, JG. Glycan expression in chorionic villi from histocultures of women with early-onset preeclampsia: immunomodulatory effects on peripheral natural killer cells. J Reprod Immunol. (2020) 142:103212. doi: 10.1016/j.jri.2020.103212
92. Blois, SM, Prince, PD, Borowski, S, Galleano, M, and Barrientos, G. Placental glycoredox dysregulation associated with disease progression in an animal model of superimposed preeclampsia. Cells. (2021) 10:10. doi: 10.3390/cells10040800
93. Miko, E, Meggyes, M, Bogar, B, Schmitz, N, Barakonyi, A, Varnagy, A, et al. Involvement of Galectin-9/TIM-3 pathway in the systemic inflammatory response in early-onset preeclampsia. PLoS One. (2013) 8:e71811. doi: 10.1371/journal.pone.0071811
94. Ji, L, Chen, Z, Xu, Y, Xiong, G, Liu, R, Wu, C, et al. Systematic characterization of autophagy in gestational diabetes mellitus. Endocrinology. (2017) 158:2522–32. doi: 10.1210/en.2016-1922
95. Nakashima, A, Tsuda, S, Kusabiraki, T, Aoki, A, Ushijima, A, Shima, T, et al. Current understanding of autophagy in pregnancy. Int J Mol Sci. (2019) 20:20. doi: 10.3390/ijms20092342
96. Hariharan, N, Shoemaker, A, and Wagner, S. Pathophysiology of hypertension in preeclampsia. Microvasc Res. (2017) 109:34–7. doi: 10.1016/j.mvr.2016.10.002
97. Deng, Q, Yin, N, Chen, Y, Shan, N, Liu, X, and Qi, H. Downregulated N-acetylglucosaminyltransferase III is involved in attenuating trophoblast migration and invasion under hypoxia-reoxygenation condition. J Matern Fetal Neonatal Med. (2019) 32:2369–75. doi: 10.1080/14767058.2018.1438392
98. Ringel, AE, Drijvers, JM, Baker, GJ, Catozzi, A, García-Cañaveras, JC, Gassaway, BM, et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. (2020) 183:1848–1866.e26. doi: 10.1016/j.cell.2020.11.009
99. King, A, Burrows, TD, Hiby, SE, Bowen, JM, Joseph, S, Verma, S, et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta. (2000) 21:376–87. doi: 10.1053/plac.1999.0496
100. Vianna, P, Mondadori, AG, Bauer, ME, Dornfeld, D, and Chies, JA. HLA-G and CD8+ regulatory T cells in the inflammatory environment of pre-eclampsia. Reproduction. (2016) 152:741–51. doi: 10.1530/rep-15-0608
101. Chen, J, and Khalil, RA. Matrix metalloproteinases in Normal pregnancy and preeclampsia. Prog Mol Biol Transl Sci. (2017) 148:87–165. doi: 10.1016/bs.pmbts.2017.04.001
102. Zhang, Y, Liu, W, Zhong, Y, Li, Q, Wu, M, Yang, L, et al. Metformin corrects glucose metabolism reprogramming and NLRP3 Inflammasome-induced Pyroptosis via inhibiting the TLR4/NF-κB/PFKFB3 signaling in trophoblasts: implication for a potential therapy of preeclampsia. Oxidative Med Cell Longev. (2021) 2021:1806344. doi: 10.1155/2021/1806344
103. Matias, ML, Gomes, VJ, Romao-Veiga, M, Ribeiro, VR, Nunes, PR, Romagnoli, GG, et al. Silibinin downregulates the NF-κB pathway and NLRP1/NLRP3 Inflammasomes in monocytes from pregnant women with preeclampsia. Molecules. (2019) 24:24. doi: 10.3390/molecules24081548
104. Matias, ML, Romao-Veiga, M, Ribeiro, VR, Nunes, PR, Gomes, VJ, Devides, AC, et al. Progesterone and vitamin D downregulate the activation of the NLRP1/NLRP3 inflammasomes and TLR4-MyD88-NF-κB pathway in monocytes from pregnant women with preeclampsia. J Reprod Immunol. (2021) 144:103286. doi: 10.1016/j.jri.2021.103286
105. Mei, Z, Huang, B, Qian, X, Zhang, Y, and Teng, B. Gastrodin improves preeclampsia-induced cell apoptosis by regulation of TLR4/NF-κB in rats. Food Sci Nutr. (2020) 8:820–9. doi: 10.1002/fsn3.1342
106. Alzokaky, AA, Al-Karmalawy, AA, Saleh, MA, Abdo, W, Farage, AE, Belal, A, et al. Metformin ameliorates doxorubicin-induced cardiotoxicity targeting HMGB1/TLR4/NLRP3 signaling pathway in mice. Life Sci. (2023) 316:121390. doi: 10.1016/j.lfs.2023.121390
107. Zhou, Y, Ma, XY, Han, JY, Yang, M, Lv, C, Shao, Y, et al. Metformin regulates inflammation and fibrosis in diabetic kidney disease through TNC/TLR4/NF-κB/miR-155-5p inflammatory loop. World J Diabetes. (2021) 12:19–46. doi: 10.4239/wjd.v12.i1.19
108. Cluver, CA, Hiscock, R, Decloedt, EH, Hall, DR, Schell, S, Mol, BW, et al. Use of metformin to prolong gestation in preterm pre-eclampsia: randomised, double blind, placebo controlled trial. BMJ. (2021) 374:n2103. doi: 10.1136/bmj.n2103
109. Huai, J, Yang, Z, Yi, YH, and Wang, GJ. Role of mammalian target of rapamycin signaling pathway in regulation of fatty acid oxidation in a preeclampsia-like mouse model treated with pravastatin. Chin Med J. (2019) 132:671–9. doi: 10.1097/cm9.0000000000000129
110. Li, M, Jin, R, Qi, Y, Zhou, H, Zhu, T, Liu, L, et al. Cholesterol partially rescues the inhibition effect of pravastatin on keratinocytes proliferation by regulating cell cycle relative proteins through AKT and ERK pathway. Dermatol Ther. (2020) 33:e14305. doi: 10.1111/dth.14305
111. Huai, J, Yang, Z, Yi, YH, Wang, GJ, and Xiang, QQ. Regulation of pravastatin on long-chain fatty acid oxidative enzyme in pre-eclampsia-like mouse model. Zhonghua Fu Chan Ke Za Zhi. (2018) 53:183–9. doi: 10.3760/cma.j.issn.0529-567X.2018.03.008
112. Tong, S, Kaitu'u-Lino, TJ, Hastie, R, Brownfoot, F, Cluver, C, and Hannan, N. Pravastatin, proton-pump inhibitors, metformin, micronutrients, and biologics: new horizons for the prevention or treatment of preeclampsia. Am J Obstet Gynecol. (2022) 226:S1157–s1170. doi: 10.1016/j.ajog.2020.09.014
113. Pánczél, Z, Kukor, Z, Supák, D, Kovács, B, Kecskeméti, A, Czizel, R, et al. Pravastatin induces NO synthesis by enhancing microsomal arginine uptake in healthy and preeclamptic placentas. BMC Pregnancy Childbirth. (2019) 19:426. doi: 10.1186/s12884-019-2507-0
114. Caldeira-Dias, M, Viana-Mattioli, S, De Souza Rangel Machado, J, Carlström, M, De Carvalho Cavalli, R, and Sandrim, VC. Resveratrol and grape juice: effects on redox status and nitric oxide production of endothelial cells in in vitro preeclampsia model. Pregnancy Hypertens. (2021) 23:205–10. doi: 10.1016/j.preghy.2021.01.001
115. El-Saka, MH, Madi, NM, Ibrahim, RR, Alghazaly, GM, Elshwaikh, S, and El-Bermawy, M. The ameliorative effect of angiotensin 1-7 on experimentally induced-preeclampsia in rats: targeting the role of peroxisome proliferator-activated receptors gamma expression and asymmetric dimethylarginine. Arch Biochem Biophys. (2019) 671:123–9. doi: 10.1016/j.abb.2019.07.006
116. Viana-Mattioli, S, Cinegaglia, N, Bertozzi-Matheus, M, Bueno-Pereira, TO, Caldeira-Dias, M, Cavalli, RC, et al. SIRT1-dependent effects of resveratrol and grape juice in an in vitro model of preeclampsia. Biomed Pharmacother. (2020) 131:110659. doi: 10.1016/j.biopha.2020.110659
117. Aghajafari, F, Nagulesapillai, T, Ronksley, PE, Tough, SC, O'Beirne, M, and Rabi, DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. (2013) 346:f1169. doi: 10.1136/bmj.f1169
118. Bodnar, LM, Catov, JM, Simhan, HN, Holick, MF, Powers, RW, and Roberts, JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. (2007) 92:3517–22. doi: 10.1210/jc.2007-0718
119. Kunnumakkara, AB, Bordoloi, D, Padmavathi, G, Monisha, J, Roy, NK, Prasad, S, et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. (2017) 174:1325–48. doi: 10.1111/bph.13621
120. Ungvari, Z, Bagi, Z, Feher, A, Recchia, FA, Sonntag, WE, Pearson, K, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. (2010) 299:H18–24. doi: 10.1152/ajpheart.00260.2010
121. Boots, AW, Haenen, GR, and Bast, A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. (2008) 585:325–37. doi: 10.1016/j.ejphar.2008.03.008
122. Sibai, BM, and Stella, CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. (2009) 200:481.e1–7. doi: 10.1016/j.ajog.2008.07.048
BMI - body mass index
ATP - adenosine triphosphate
ROS - reactive oxygen species
2,3-BPG - 2,3-Bisphosphoglycerate
1,3-BPG - 1,3-Bisphosphoglycerate
3-BPG - 3-Bisphosphoglycerate
TCA - tricarboxylic acid
PGI2 - prostacyclin
TXA2 - thromboxin
PUFAs - polyunsaturated fatty acids
SCAD - short-chain acyl-coenzyme A dehydrogenase
LCPUFAs - long-chain polyunsaturated fatty acids
DHA - docosahexaenoic acid
AA - arachidonic acid
LCHAD - long chain omega-3 hydroxy CoA dehydrogenase
GSH - glutathione
Trp - tryptophan
Arg - arginine
Hcy - homocysteine
GSSG - glutathione oxidized
GPx - glutathione peroxidase
CAT - catalase
GSTT2 - Glutathione S-transferase theta 2
KYN - kynurenine
TPH - tryptophan hydroxylase
IDO - indolamine 2,3-dioxygenase
TDO - tryptophan2,3-dioxygenase
GCN2 - general control non-derepressible 2
eIF-2α - eukaryotic translation initiation factor-2α
GLK1 - master amino acid-sensing kinase 1
AhR - aromatic hydrocarbon receptor
MAO - monoamine oxidase
5-HIAA - 5-hydroxyindole acetic acid
NO - nitric oxide
NOS - nitric oxide synthase
GUC - guanylate cyclase
cGMP - guanosine cyclomonophosphate
ADMA - asymmetric dimethylarginine
DDAH - dimethylarginine dimethylamine hydrolase
MHM - methionine-homocysteine metabolism
MET - methionine
MTR - methyltransferase
5-MTHF - 5-methyltetrahydrofolate
MTRR - 5-methyltetrahydrofolate-homocysteine methyltransferase reductase
MTHFR - 5,10-methylenetetrahydrofolate reductase
THF - tetrahydrogen folic acid
SAM - S-adenosylhomocysteine
CBS - cystathionine β synthase
Cys - cysteine
2-ME - 2-methoxyestradiol
COMT - Catechol-O-methyltransferase
2-HE - 2-Hydroxyestradiol
sPE - severe preeclampsia
MMP-2/9 - matrix metalloproteinase-2/9
MET - metformin
HMGCR - 3-hydroxy-3-methylglutarate monoacyl-CoA reductase
EGCG - epigallocatechin gallate
Keywords: preeclampsia, nutrient metabolism, pathogenesis, carbohydrate, lipid, amino acids
Citation: Li S, Zhu J, Zhao Y, An P, Zhao H and Xiong Y (2025) Metabolic disorder of nutrients—an emerging field in the pathogenesis of preeclampsia. Front. Nutr. 12:1560610. doi: 10.3389/fnut.2025.1560610
Received: 14 January 2025; Accepted: 24 February 2025;
Published: 07 March 2025.
Edited by:
Dorota Formanowicz, Poznan University of Medical Sciences, PolandReviewed by:
Suniti Vaishya, Bharati Vidyapeeth Deemed University, IndiaCopyright © 2025 Li, Zhu, Zhao, An, Zhao and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huanqiang Zhao, emhhb2h1YW5xaWFuZ0AxNjMuY29t; Yu Xiong, eGlvbmd5dTE1MzVAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.