- 1Department of Cardiothoracic Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Anesthesiology, Children's Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Health Management Center, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: Gut microbiota is reported to be related to the onset of insulin resistance (IR) and type 2 diabetes mellitus (T2DM). The dietary index for gut microbiota (DI-GM) is a novel index for reflecting gut microbiota diversity. We aimed to evaluate the association of DI-GM with T2DM and IR.
Methods: This cross-sectional research comprised 10,600 participants aged ≥20 from the National Health and Nutrition Examination Survey (NHANES) 2007–2018. We employed weighted multivariable linear and logistic regression models to examine the correlation of DI-GM with T2DM and IR. Linear or nonlinear relationships were examined by restricted cubic spline (RCS) regression. Additionally, subgroup and sensitivity analyses were performed to ensure the reliability of the results. Mediation analysis explored the roles of body mass index (BMI) and inflammatory factors in these associations.
Results: Higher DI-GM were inversely associated with T2DM (OR = 0.93, 95%CI: 0.89–0.98) and IR (OR = 0.95, 95%CI: 0.91–0.99) after adjusting for confounders. DI-GM ≥ 6 group showed significantly lower risks of T2DM (OR = 0.74, 95%CI: 0.60–0.91) and IR (OR = 0.77, 95%CI: 0.62–0.95). RCS demonstrated a linear relationship between DI-GM and T2DM, as well as IR. DI-GM was also inversely correlated with the risk markers of T2DM. Mediation analysis showed that BMI and the systemic inflammation response index partly mediated the association of DI-GM with T2DM and IR, while the systemic immune-inflammation index mediated only the association with T2DM.
Conclusion: DI-GM is inversely associated with T2DM and IR, with BMI and inflammatory markers partly mediating this association.
1 Introduction
The high incidence of diabetes has become a critical health problem worldwide. The global incidence is estimated at 9.3% of the adult population in 2019, with projections indicating a significant increase (1). Type 2 diabetes mellitus (T2DM) represents the predominant form of diabetes, the burden of T2DM extends beyond its health implications, imposing significant economic challenges on healthcare systems worldwide (2). Diabetes care accounts for one-quarter of healthcare expenditures, with 61% directly attributable to the disease in the U.S. (3). Additionally, insulin resistance (IR) is a pathological condition marked by reduced cellular responsiveness to insulin. IR is typically accompanied by hyperinsulinemia and is closely linked to the onset of T2DM (4). Therefore, early detection and timely management of T2DM and IR are imperative.
In recent years, studies of the gut microbiota have already become a hotspot, and its association with T2DM has been widely discussed. Multiple studies suggested that the gut microbiota was associated with the occurrence and progression of T2DM and could represent a promising therapeutic target (5, 6). Furthermore, several studies suggested that modulating gut microbiota through dietary interventions is a promising approach to improving host health (7). The dietary index for gut microbiota (DI-GM) is a new tool developed by Kase et al. (8) for reflecting the diversity of gut microbiota. They identified 14 dietary components, having a positive or a negative effect on gut microbiota by systematically reviewing the literature and using these components to calculate the DI-GM. Additionally, they demonstrated that DI-GM was significantly related to urinary enterolignans, highlighting an association with gut microbiota diversity. This tool may help to design effective dietary patterns to prevent or alleviate dysbiosis-related diseases. Currently, a few studies have explored the relationship between DI-GM and other medical conditions (9, 10). There is a lack of studies discussing the association of DI-GM with T2DM and IR. Therefore, it’s necessary to explore if healthy gut microbiota dietary pattern identified by DI-GM was associated with a reduced risk of T2DM and IR.
Obesity is a major T2DM risk factor, visceral fat accumulation leads to adipokines and pro-inflammatory cytokines secretion, which contributes to systemic inflammation and IR (11, 12). Chronic low-grade inflammation is usually regarded to play a crucial role in the progression of IR and T2DM (13). Studies indicated that gut microbiota disorders leading to increased intestinal permeability can trigger low-grade systemic inflammation, this is a crucial risk factor in the onset of IR and obesity, leading to T2DM development (14). Therefore, we hypothesized that following a promoting healthy gut microbiota dietary pattern identified by DI-GM could mitigate T2DM and IR risk by reducing obesity and systemic inflammation.
This study aimed to investigate the correlation of DI-GM with T2DM and IR by utilizing data from the National Health and Nutrition Examination Survey (NHANES) and to investigate the mediation of BMI and inflammatory markers in this relationship. This study may offer new perspectives for the prevention and control of T2DM.
2 Materials and methods
2.1 Data sources and study population
All data obtained in this research is from the NHANES, which employs a cross-sectional, multistage, stratified, and subgroup probability sampling design to evaluate the nutritional and health conditions of the U.S. population.
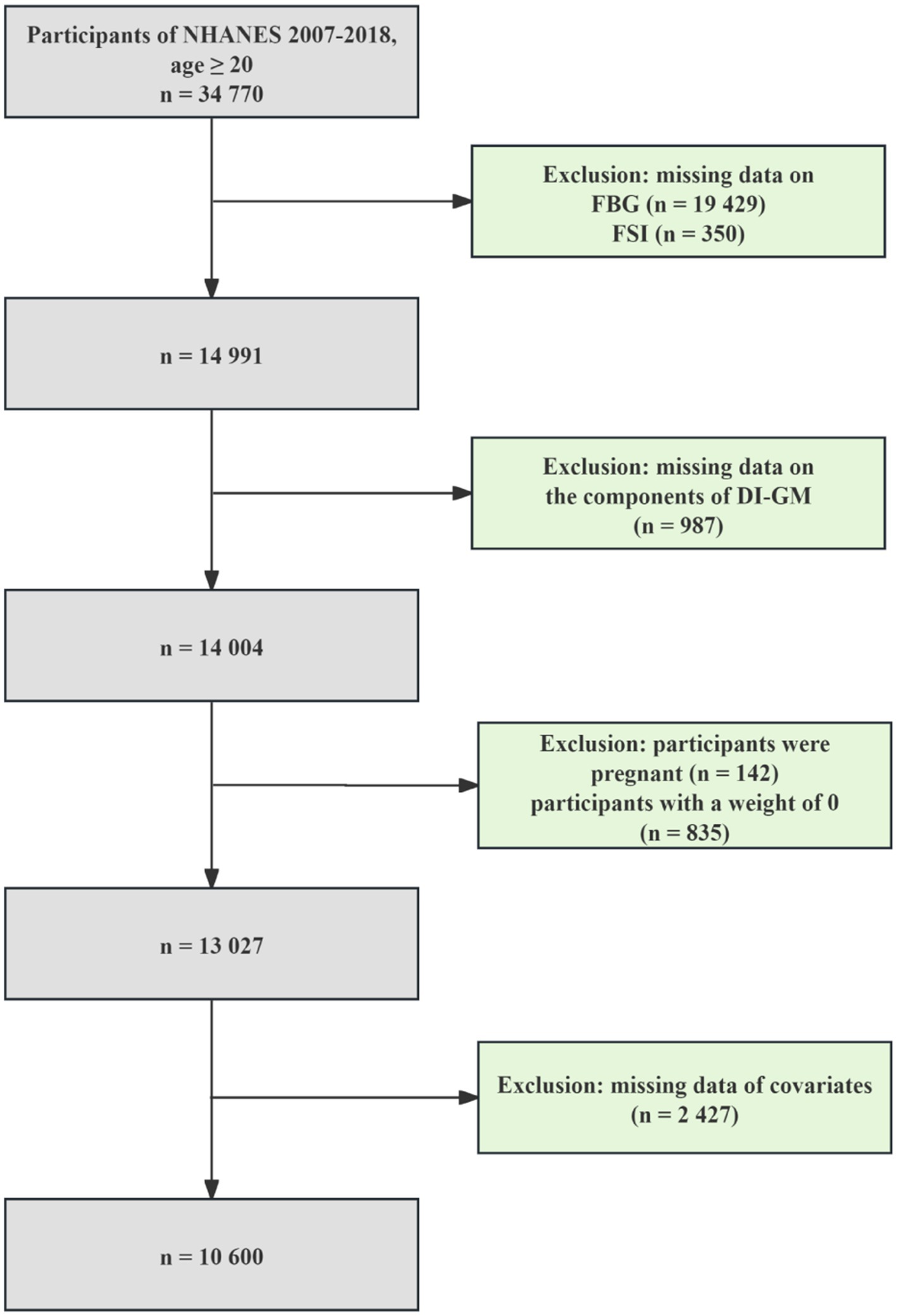
We pooled six NHANES cycles data from 2007 to 2018 for this study, involving 34,770 participants aged ≥20 years. The exclusion of participants was determined based on the following criteria: (1) missing data on the diagnosis of T2DM (n = 19,429) and IR (n = 350); (2) missing information on DI-GM (n = 987); (3) participants were pregnant (n = 142); (4) participants with a weight of 0 (n = 835); (5) missing data on covariates (n = 2,427). Finally, 10,600 eligible participants were enrolled. The specific processes are shown in Figure 1.
2.2 Evaluation of the DI-GM
The dietary data are jointly gathered by the National Center for Health Statistics (NCHS) and the U.S. Department of Agriculture (USDA) through the What We Eat in America (WWEIA) survey (15). Trained surveyors utilize the computer-assisted USDA Automated Multiple-Pass Method to conduct irregular dietary recalls, asking participants about their consumption of beverages, foods, and dietary supplements from the previous day (16). An additional dietary recall was administered by telephone approximately 3–10 days following the initial assessment. An average of the values from both days was calculated for each diet component.
The DI-GM was constituted of 14 food items or nutrients, following the evaluation standards outlined in the article by Kase et al., including coffee, green tea, fermented dairy, avocado, broccoli, chickpeas, cranberries, fiber, whole grains, and soybean as beneficial elements, while unfavorable elements included processed meat, red meat, a high-fat diet (≥ 40% energy from fat), and refined grains (8). Participants whose consumption was exceeding the sex-specific median for beneficial elements or below the sex-specific median for unfavorable elements were assigned a score of 1. Conversely, a score of 0 was given for consuming below the sex-specific median for beneficial elements or exceeding the sex-specific median for unfavorable elements. DI-GM is obtained by summing the scores for each element, yielding a range from 0 to 14. Specific details are given in the Supplementary Table S1. Based on previous research, DI-GM scores were divided into three group: 0–3, 4–5, and ≥ 6 points (9).
2.3 Outcomes
T2DM, IR, and associated risk markers, including fasting blood glucose (FBG, mmol/L), fasting serum insulin (FSI, μU/ml), and homeostasis model assessment of insulin resistance (HOMA-IR) (17, 18) were considered as outcome variables.
In our study, T2DM was diagnosed as any of the following criteria: (1) participants with a self-reported diabetes diagnosis; (2) FBG level ≥ 7.0 mmol/L; (3) 2-h oral glucose tolerance test (OGTT) plasma glucose ≥11.1 mmol/L; (4) a glycosylated hemoglobin A1c (HbA1c) level ≥ 6.5% (19). Although the NHANES database does not provide explicit information on the specific types of diabetes, this study likely enrolled a majority of T2DM subjects since the proportion of type 1 diabetes mellitus (T1DM) in adulthood is low.
IR was evaluated by HOMA-IR (HOMA-IR = FSI (μU/ml) * FBG (mmol/L) / 22.5). Based on previous studies, the values greater than or equal to the 75th percentile were diagnosed as IR (HOMA-IR > 4.53 in our study) (20).
2.4 Definitions of mediating variables
The determination of the anthropometric, complete blood count test and basic biochemical characteristics of the participants in NHANES have been reported in previous publications (21–24). Inflammatory markers included the Systemic Immune Inflammation Index (SII), the Systemic Inflammation Response Index (SIRI). SII and SIRI were widely used in research to quantify systemic inflammation, calculated by neutrophil count (NEUT), platelet count (PLA), lymphocyte count (LYM), and monocyte count (MONO). The calculation formulas are as follows: SII = (NEUT × PLA) / LYM, SIRI = (NEUT × MONO) / LYM (24–26).
BMI was calculated through the standard formula: BMI = weight/height2 (27).
2.5 Covariates
Covariates were selected based on some published studies (9, 17, 28). Therefore, we included the following covariables: age, race, gender, education level, poverty-to-income ratio (PIR), marital status, smoking status, drinking status, physical activity (PA), BMI, whether they have hypertension, hyperlipidemia, hyperuricemia and cardiovascular disease (CVD). The definition and measurement of the covariates are shown in the Supplementary Appendix.
2.6 Statistical analysis
All analyses considered the fasting subsample weights (WTSAF2YR) and were divided by six to obtain pooled weights across six survey cycles, as this constituted the smallest subsample of the study, according to NHANES analysis guidelines.
Continuous variables are presented as mean ± standard deviation (SD) or median (interquartile range, IQR), and other variables are reported as weighted percentages (%). Differences between participants grouped by DI-GM scores (0–3, 4–5, ≥6) were analyzed using a weighted t-test (for continuous variables) and a weighted chi-square test (for categorical variables). Pairwise comparisons (eg, 0–3 vs 4–5, 0–3 vs ≥ 6, and 4–5 vs ≥ 6) were considered to be statistically significant if the corresponding unadjusted pairwise p ≤ 0.017 (ie, < 0.05/3 for Bonferroni correction).
Multivariable weighted logistic and linear regression models were utilized to investigate the association of DI-GM with T2DM, IR, and the risk markers of T2DM (FBG, FSI, and HOMA-IR). The Odds ratios (ORs), Beta (β) values, and 95% confidence intervals were calculated, respectively. DI-GM was analyzed as a continuous variable and a grouped variable (0–3, 4–5, ≥6). Model 1 was unadjusted. Model 2 was adjusted by age, gender, and race. Model 3 was adjusted for PIR, marital status, education level, BMI, smoking status, drinking status, PA, hypertension, hyperlipidemia, hyperuricemia, and CVD based on Model 2.
Linear or non-linear associations of DI-GM with T2DM and IR were investigated by restricted cubic splines (RCS), adjusting for all confounding variables in model 3. Additionally, we performed interaction and subgroup analyses to investigate potential confounders affecting the association of DI-GM with T2DM and IR. Subgroup analyses were detected by stratifying by sex, age, race, BMI, PA, hypertension, hyperuricemia, hyperlipidemia, and CVD. Mediation analyses (1,000 bootstraps) were conducted to assess the mediation effect of BMI and inflammatory markers on the association of DI-GM with T2DM and IR. To explore the potential inflammatory pathways, we also conducted mediation analyses on white blood cells (WBC) and the inflammatory cells included in the two indicators (NEUT, LYM, and MONO).
Sensitivity analyses were conducted to evaluate the reliability of the results. First, we applied multiple imputation (MI) by chained equations to input the missing data on covariates (9, 28). Subsequently, the imputed dataset was transferred to weighted multifactor regression analysis. Second, unweighted regression analyses were conducted. Third, further adjustments were made for liver and kidney function (alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, alkaline phosphatase, serum creatinine, blood urea nitrogen) and total energy intake (kcal/day) to evaluate whether these factors influenced the association of DI-GM with T2DM and IR. Finally, given the close association between CVD, hyperuricemia and T2DM, we performed regression analyses again after excluding participants who had CVD or hyperuricemia at baseline.
All statistical analyses were completed with R software version 4.2.2 and Free Statistics software version 2.0. A two-sided p < 0 0.05 was considered as statistically significant.
3 Results
3.1 Participants characteristics
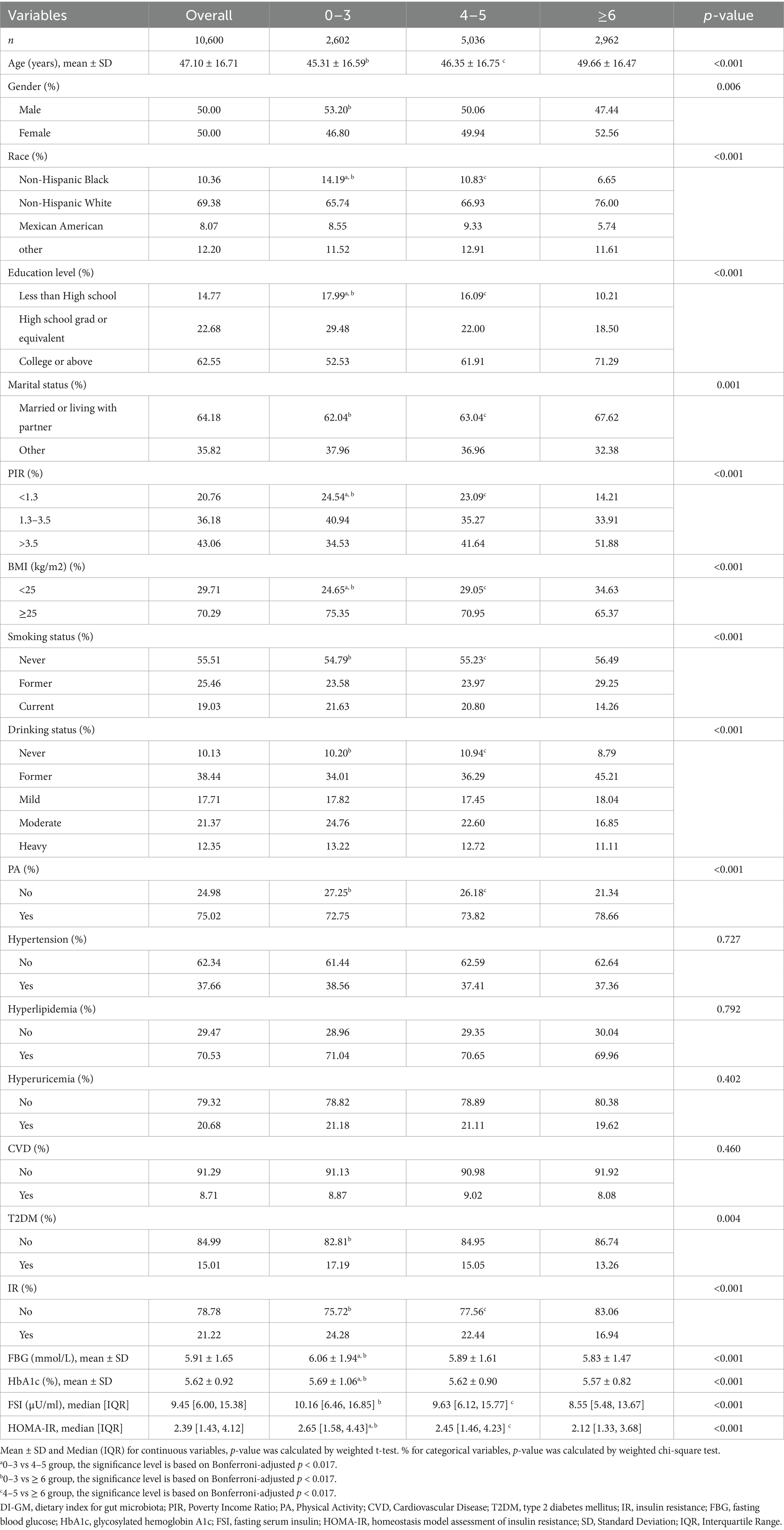
The baseline characteristics of participants stratified by the DI-GM scores are shown in Table 1. The mean age of the participants was 47.10 ± 16.71 years. The proportion of participants diagnosed with T2DM was 15.01%, while 21.22% were defined as having IR. Compared with the group with the lowest DI-GM level, participants in the highest DI-GM group were predominantly older, predominantly female, Non-Hispanic White, and more likely to be married or living with a partner. Additionally, this group exhibited higher levels of physical activity, education, and PIR. They also had a lower BMI, were less likely to smoke or engage in heavy drinking, and had a lower prevalence of T2DM and IR.
3.2 Associations of DI-GM with T2DM and IR
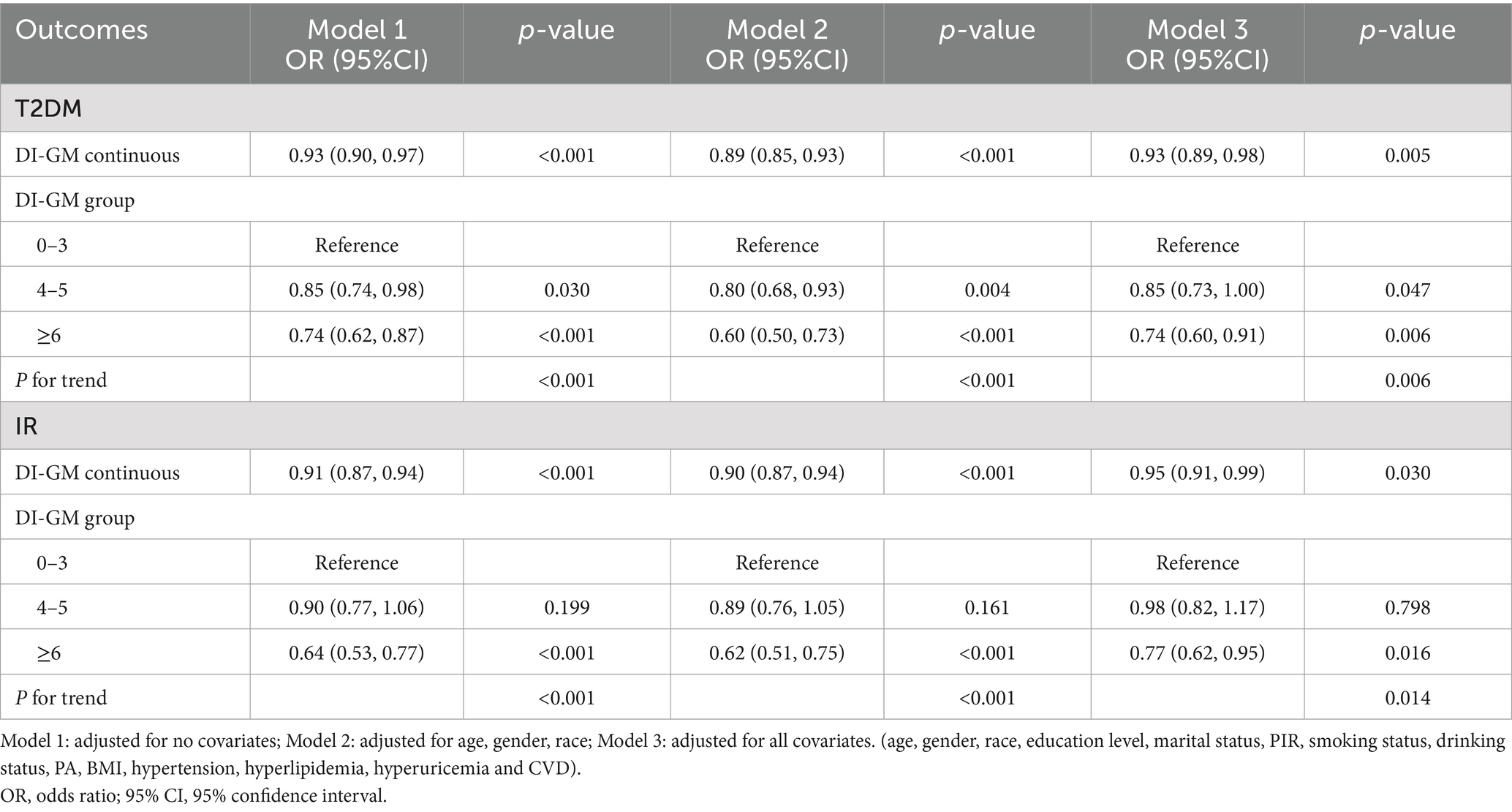
Table 2 illustrates the associations of DI-GM with T2DM and IR. The results of analysis when including DI-GM as a continuous variable have shown that DI-GM is inversely correlated with T2DM occurrence (OR = 0.93, 95% CI = 0.90, 0.97), the association remained robust after adjustment (OR = 0.93, 95% CI = 0.89, 0.98), and so was the association of DI-GM with IR (OR = 0.95, 95% CI = 0.91, 0.99). Moreover, after grouping DI-GM, DI-GM ≥ 6 group participants were inversely associated with the risk of T2DM, compared to those in the lowest DI-GM group in model 3 (OR = 0.74, 95% CI = 0.60, 0.91), and the trend test was significant (P for trend = 0.006). Meanwhile, the relationship between DI-GM and IR was also significantly negative (OR = 0.77, 95% CI = 0.62, 0.95) with the P for trend = 0.014 in model 3.
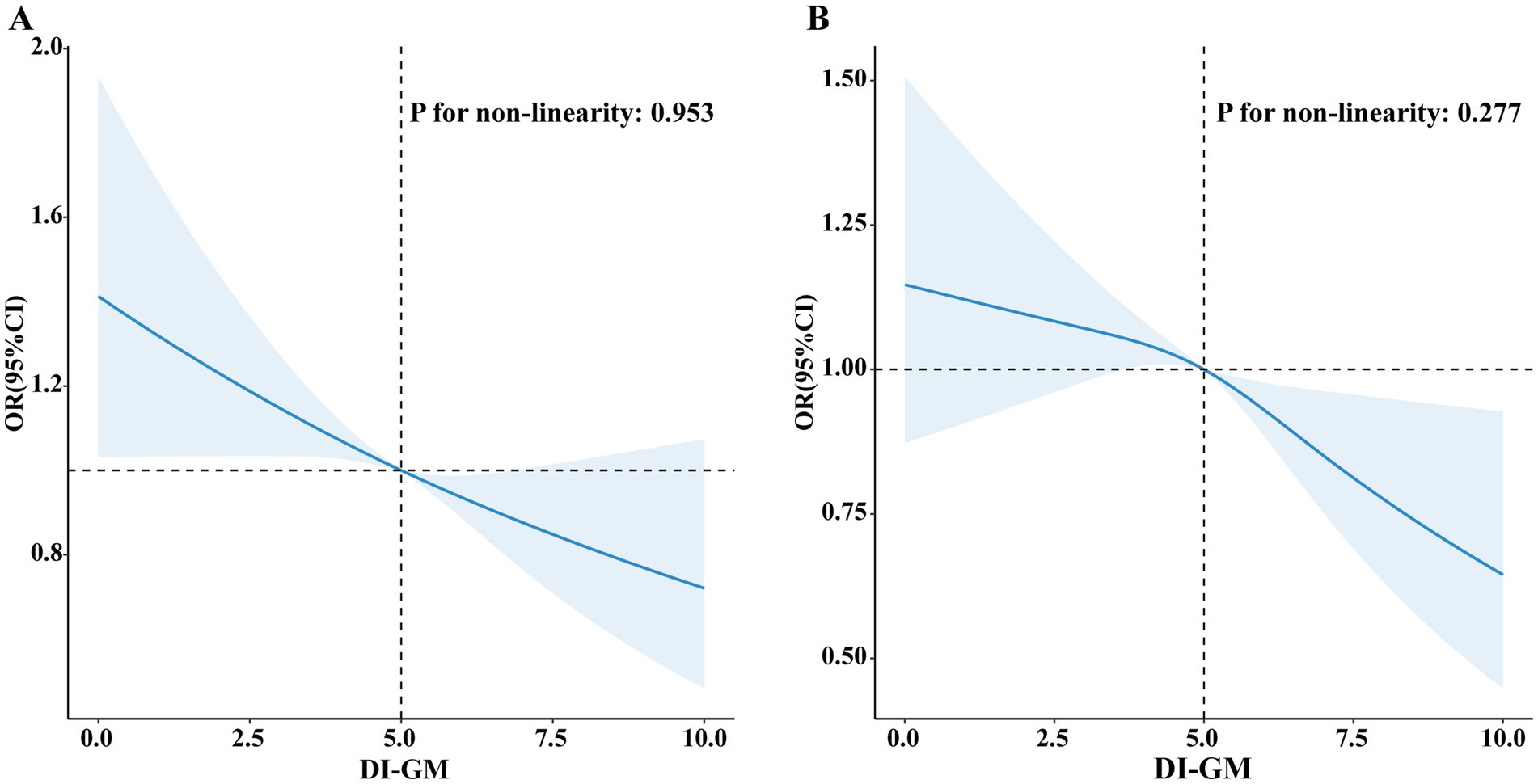
Figure 2 illustrates a visual representation of the relationship between DI-GM and T2DM as well as IR. The RCS revealed a linear association between DI-GM and T2DM (non-linearity: p = 0.953), as well as IR (non-linearity: p = 0.277) in the fully adjusted models.
3.3 Relationship between DI-GM and risk markers of T2DM
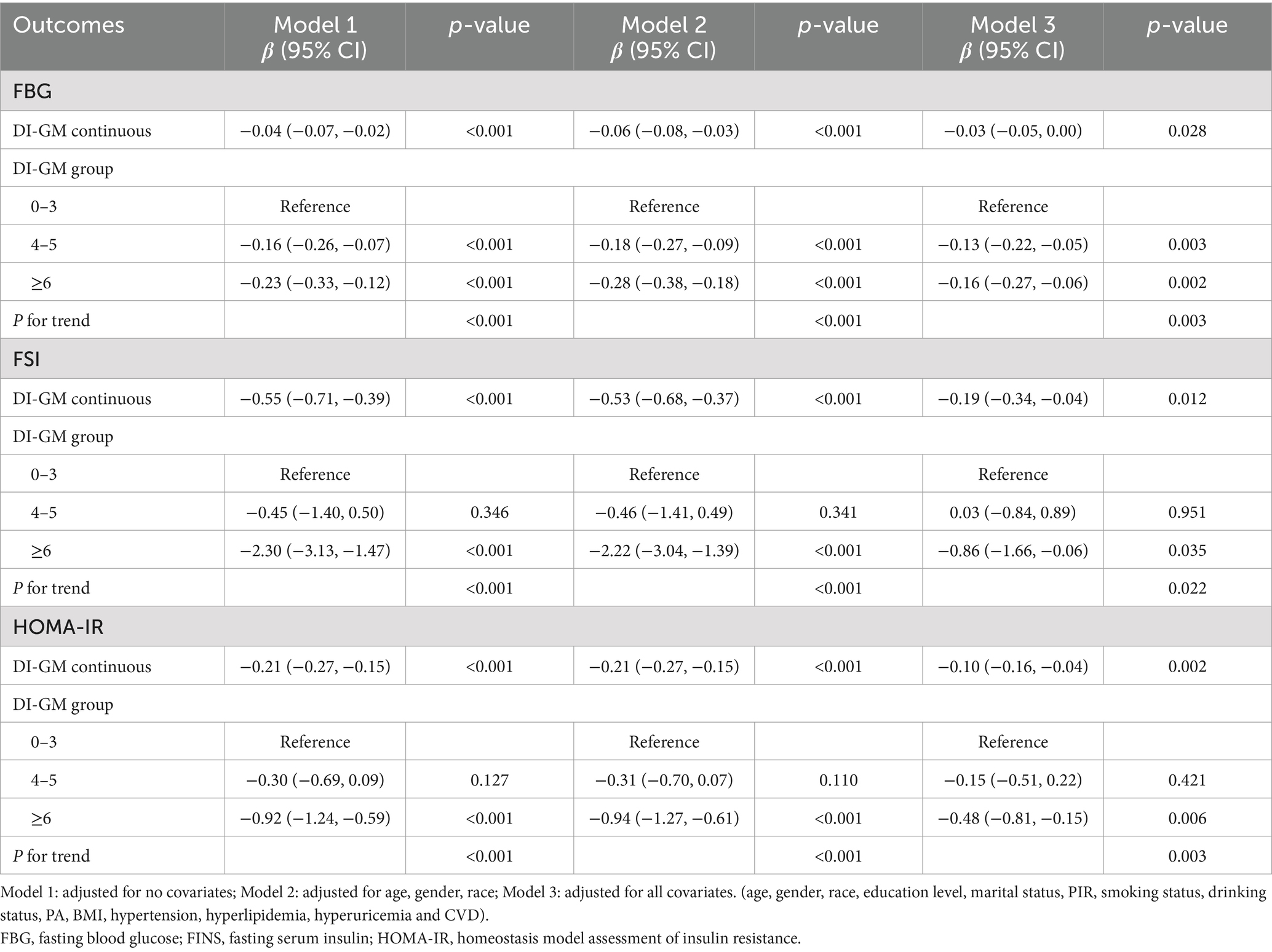
Table 3 presents the relationship between DI-GM and risk markers for T2DM. The DI-GM was inversely associated with FBG (β = −0.03, 95% CI = −0.05, 0.00), FSI (β = −0.19, 95% CI = −0.34, −0.04), and HOMA-IR (β = −0.10, 95% CI = −0.16, −0.04) as a continuous variable after adjusting for all confounders. Moreover, when used as a categorical variable, the lowest DI-GM group was used as a reference, the associations of DI-GM ≥ 6 with FBG (β = −0.16, 95% CI = −0.27, −0.06), FSI (β = −0.86, 95% CI = −1.66, −0.06), and HOMA-IR (β = −0.48, 95% CI = −0.81, −0.15) were also significant negative, with all P for trend <0.05 in the full adjusted model.
3.4 Subgroup analyses
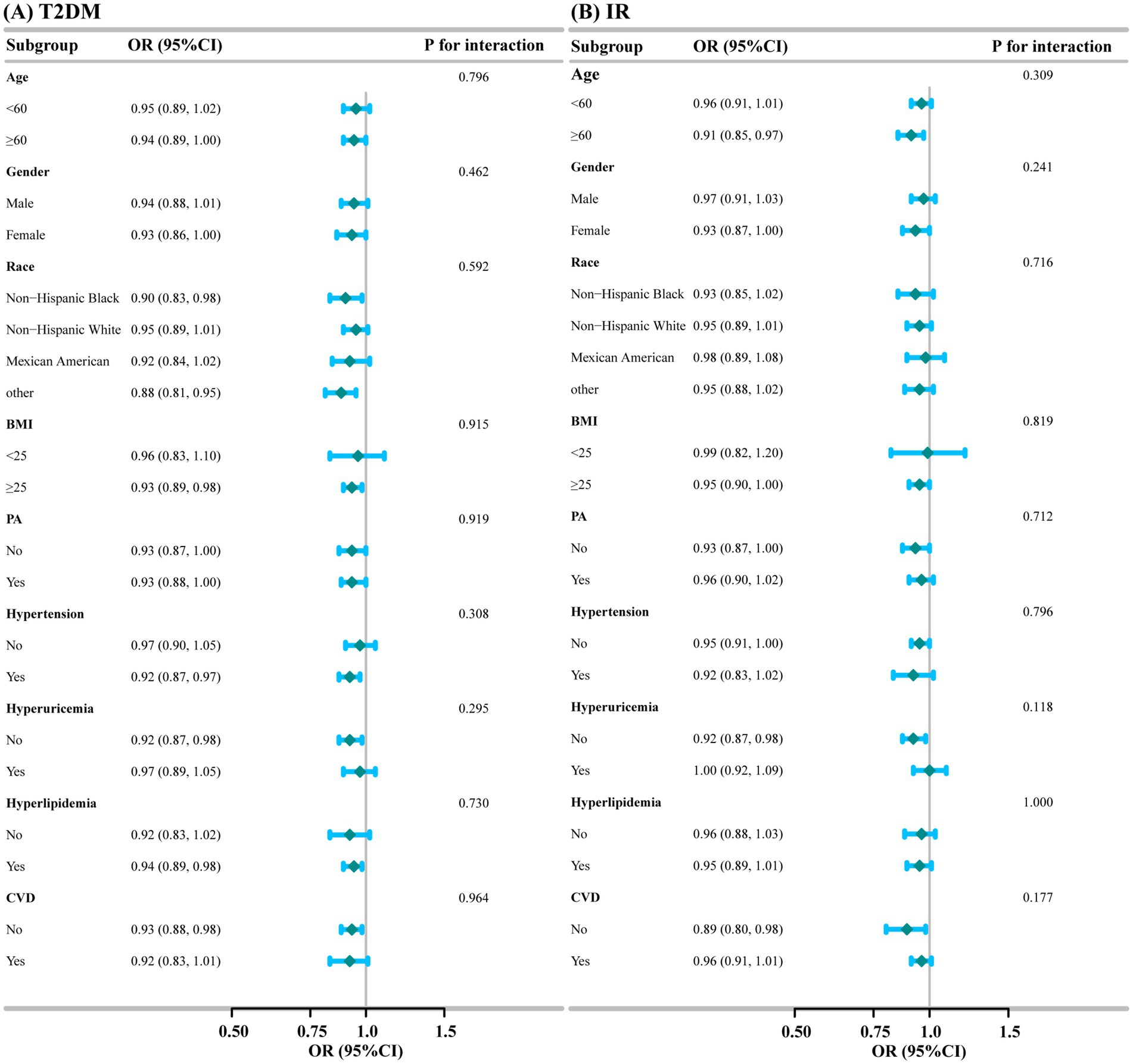
Subgroup and interaction analyses were conducted for the association of DI-GM with T2DM and IR based on age, gender, race, BMI, PA, Hypertension, Hyperuricemia, Hyperlipidemia, and CVD. As shown in Figure 3, subgroup analyses illustrated the stability of the results. We saw no significant interaction between DI-GM and T2DM (All P for interaction>0.05), nor between DI-GM and IR (All P for interaction >0.05).
3.5 Mediation analyses
We conducted the mediation analyses to explore the mediating effect of BMI and inflammatory markers. As shown in Figure 4, BMI significantly mediated the associations of DI-GM with T2DM and IR, explaining 9.08%, and 26.5%, respectively (p < 0.001). Meanwhile, two inflammatory markers had a significant mediation on the association between DI-GM and T2DM, SII and SIRI explained 2.07% (p = 0.026), and 2.16% (p = 0.008), respectively. In addition, SIRI partially mediated the relationship between DI-GM and IR, with a mediation ratio of 2.22% (p = 0.008), and SII had no significant mediating effect (p > 0.05). Further analysis revealed that NEUT mediated 6.31% (p < 0.001) of the association between DI-GM and T2DM and 8.07% (p < 0.001) of the association with IR. No significant mediation effects were observed for other inflammatory cells (all p > 0.05) as shown in Supplementary Figure S1.
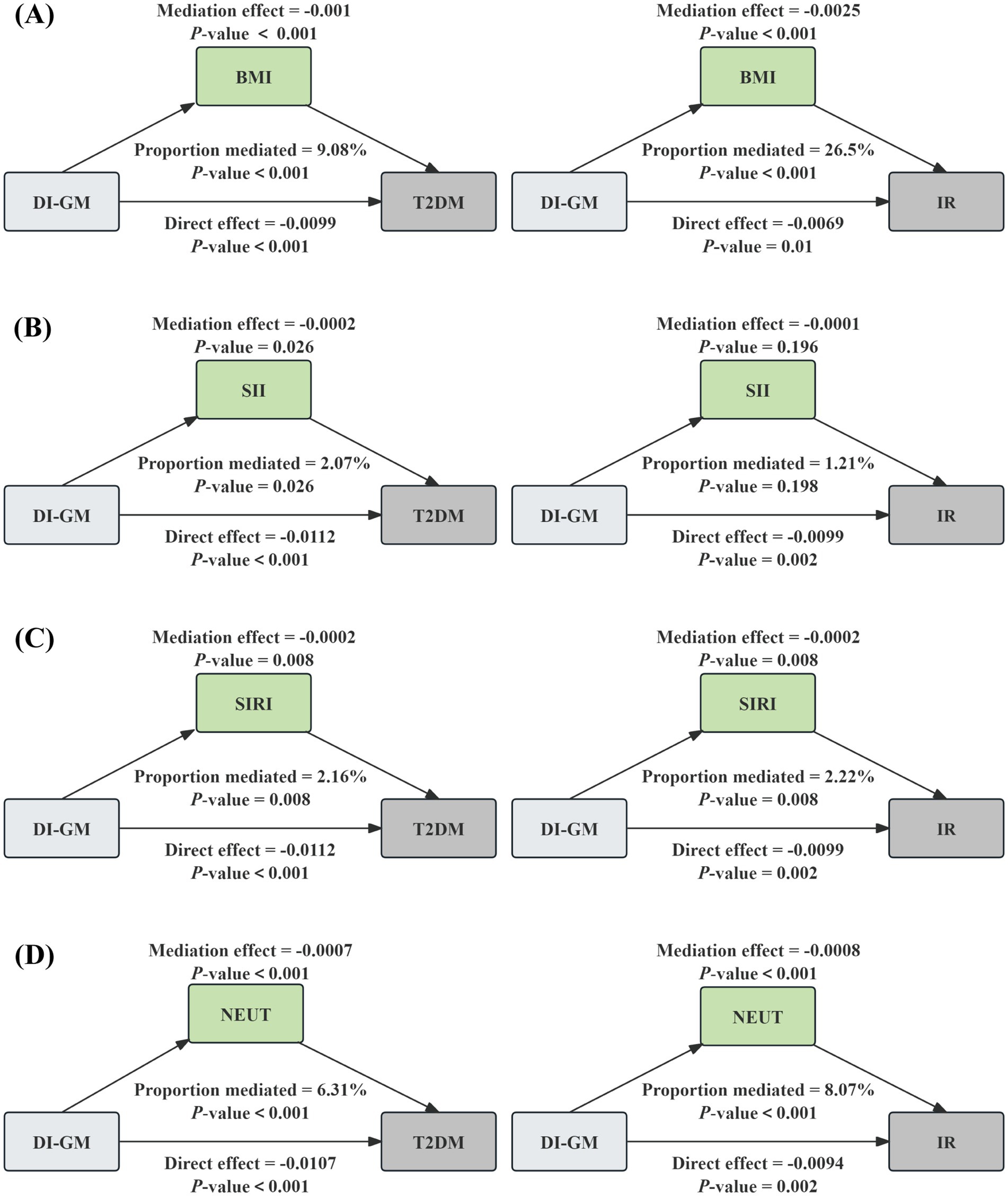
Figure 4. The mediation analysis of BMI, inflammatory biomarkers, and NEUT on the association of DI-GM with T2DM and IR. The graphs in (A–D) represented the mediating role of BMI, SII, SIRI, and NEUT, respectively.
3.6 Sensitivity analyses
In addition, sensitivity analyses were implemented. Firstly, we used multiple imputation for missing data on covariates, multivariable regression analyses were conducted subsequently. The results were shown in Supplementary Tables S2, S3, a significant inverse correlation between DI-GM and T2DM, IR, and risk markers of T2DM was found, which was consistent with the result of the main analysis. Secondly, the results of the unweighted multivariate regression analyses were similar to the weighted analyses, this further illustrates the robustness of the results, as shown in Supplementary Tables S4, S5. Thirdly, when further adjusting for the liver and kidney function indicators and total energy intake, respectively, the results remained unchanged significantly (Supplementary Tables S6, S7). Lastly, the results remained robust in participants without CVD (Supplementary Tables S8, S9) or hyperuricemia (Supplementary Tables S10, S11).
4 Discussion
The results of our study, which included 10,600 participants, demonstrated that DI-GM (continuous variable) and DI-GM ≥ 6 group were significantly inversely correlated with T2DM, IR, and the risk markers of T2DM (FBG, FSI, and HOMA-IR), the correlation remained significant after adjusting the covariates. RCS visualized a linear correlation of DI-GM with T2DM and IR. Moreover, subgroup and sensitivity analyses were conducted to further validate the reliability of the primary results. Mediation analysis showed that BMI and two inflammatory markers (SII and SIRI) partly mediated the associations of DI-GM with T2DM and IR.
The association between gut microbiota and T2DM has attracted much attention recently. Several researches have demonstrated that individuals with T2DM frequently exhibit a unique gut microbiota composition compared to healthy controls. For example, a study conducted on Indian individuals with T2DM identified alterations in eubacterial, archaeal, and eukaryotic components, highlighting pervasive dysbiosis in newly diagnosed and long-standing diabetic patients (29). Furthermore, metagenomic analyses have identified specific bacterial taxa associated with metabolic dysfunction in individuals with T2DM, such as the increased abundance of Firmicutes and diminished levels of butyrate-producing bacteria like the Ruminococcaceae (29). The animal experiment indicated significant alterations in gut microbial diversity and metabolite profiles, which were found to be closely associated with glucose metabolism and IR (30). Moreover, Mendelian randomization studies have further clarified the possible causality between gut microbiota and T2DM, identifying specific genera that may influence the risk of developing the disease (31, 32). These findings emphasize the potential for targeting gut microbiota as a treatment approach for managing T2DM and its associated complications.
DI-GM is a newly developed dietary index to capture dietary patterns that are beneficial or harmful to gut health. Our study preliminarily established a linear negative relationship between DI-GM and T2DM as well as IR. This provides some evidence that adhering to a dietary pattern promoting healthy gut microbiota identified by DI-GM may reduce the occurrence of T2DM and IR. The impact of diet on gut microbiota and its potential role in T2DM development is widely discussed. Recent researches have revealed that dietary patterns play important roles in affecting the gut microbiota composition and function, which can subsequently affect metabolic health and the development of conditions such as T2DM (33, 34). For instance, high-fiber diets were demonstrated to be related to improvements in gut microbiota diversity and the enrichment of beneficial bacteria, which can enhance glucose metabolism and reduce systemic inflammation in T2DM patients (35). Additionally, the Mediterranean diet, rich in polyphenols and healthy fats, has been shown to positively modulate gut microbiota composition, potentially lowering T2DM risk (36). Moreover, probiotic supplementation has been shown to significantly impact oxidative stress and inflammation biomarkers in diabetic patients (37). These findings suggested a promising new avenue for dietary interventions in managing T2DM.
Our findings revealed that BMI and inflammation markers (SII and SIRI) may mediated the relationship between DI-GM and T2DM. It is noteworthy that SII and SIRI mediated only approximately 2% of this association. However, we should not overlook this finding, as it provides theoretical support and direction for future longitudinal studies or interventional trials. Gut microbiota dysbiosis was reported to result in the excessive leakage of gram-negative bacterial products, such as lipopolysaccharides (LPS), which promote systemic low-grade inflammation and elevate the risk of metabolic disorders. This process leads to local endotoxemia in the small intestine and colon, particularly with an influx of gram-negative genera, including Bacteroides, Prevotella, and Escherichia. Such microbial changes represent a critical risk factor for obesity and IR, ultimately contributing to the development of T2DM (14, 38, 39). In addition, gut dysbiosis can lead to intestinal barrier disruption, permitting excessive leakage of LPS into the bloodstream. This triggers an inflammatory response, leading to IR and disrupted glucose homeostasis (14, 40, 41). These findings are consistent with our results. Our further analysis also revealed a significant mediating role of NEUT in the relationship between DI-GM and T2DM or IR. Previous studies also suggest that NEUT play an important role in the occurrence of IR, via secreted elastase (42). However, due to the cross-sectional study design, we need to interpret these findings with caution. Further studies are necessary to validate these findings and investigate the underlying mechanisms.
This study has several strengths. Firstly, DI-GM is a new novel dietary index, we are the first to investigate its correlation with T2DM and IR, as far as we know. Secondly, all data we used were from NHANES, a comprehensive nationally representative database, which utilizes a multistage sampling methodology to improve the reliability and robustness of the findings. Thirdly, potential confounders were adjusted to ensure consistent and reliable conclusions across various subgroups. Furthermore, multiple sensitivity analyses were conducted to evaluate the stability of the findings.
Several limitations exist in this study. Firstly, the cross-sectional design was unable to draw causal relationships. Secondly, although a variety of confounders were considered, unknown residual confounding factors cannot be excluded. Thirdly, dietary data were obtained through self-reported 24-h dietary recalls, which are prone to recall bias. However, potential errors were mitigated by averaging the results from two 24-h dietary recall interviews. Lastly, although our study did not include individuals under the age of 20, which would reduce the misclassification of T1DM, the possibility of residual inclusion of T1DM cases cannot be eliminated.
5 Conclusion
The newly proposed dietary index, i.e., DI-GM, was negatively correlated with the prevalence of T2DM, IR, and the risk markers of T2DM (FBG, FSI, HOMA-IR). In addition, we found the mediating role of BMI and inflammatory markers (SII and SIRI). Our results suggest that DI-GM is expected to identify the dietary patterns that are beneficial to gut health, thus, reducing the incidence of T2DM. Additional studies are required to verify and support our findings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://wwwn.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by NHANES was approved by the National Center for Health Statistics Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HQ: Conceptualization, Data curation, Formal analysis, Validation, Visualization, Writing – original draft. YY: Data curation, Formal analysis, Validation, Visualization, Writing – original draft. QX: Formal analysis, Writing – review & editing. LY: Conceptualization, Methodology, Project administration, Validation, Writing – original draft. YS: Conceptualization, Methodology, Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We extend our gratitude to all NHANES participants and staff for their contributions to the project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1557280/full#supplementary-material
References
1. Saeedi, P, Petersohn, I, Salpea, P, Malanda, B, Karuranga, S, Unwin, N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
2. Weisman, A, Fazli, GS, Johns, A, and Booth, GL. Evolving trends in the epidemiology, risk factors, and prevention of type 2 diabetes: a review. Can J Cardiol. (2018) 34:552–64. doi: 10.1016/j.cjca.2018.03.002
3. Parker, ED, Lin, J, Mahoney, T, Ume, N, Yang, G, Gabbay, RA, et al. Economic costs of diabetes in the U.S. in 2022. Diabetes Care. (2024) 47:26–43. doi: 10.2337/dci23-0085
4. Lu, X, Xie, Q, Pan, X, Zhang, R, Zhang, X, Peng, G, et al. Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy. Signal Transduct Target Ther. (2024) 9:262. doi: 10.1038/s41392-024-01951-9
5. Yang, G, Wei, J, Liu, P, Zhang, Q, Tian, Y, Hou, G, et al. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. (2021) 117:154712. doi: 10.1016/j.metabol.2021.154712
6. Scheithauer, T, Rampanelli, E, Nieuwdorp, M, Vallance, BA, Verchere, CB, van Raalte, DH, et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol. (2020) 11:571731. doi: 10.3389/fimmu.2020.571731
7. Wang, J, Dong, L, Hu, JQ, Wang, YY, Li, A, Peng, B, et al. Differential regulation and preventive mechanisms of green tea powder with different quality attributes on high-fat diet-induced obesity in mice. Front Nutr. (2022) 9:992815. doi: 10.3389/fnut.2022.992815
8. Kase, BE, Liese, AD, Zhang, J, Murphy, EA, Zhao, L, and Steck, SE. The development and evaluation of a literature-based dietary index for gut microbiota. Nutrients. (2024) 16:1045. doi: 10.3390/nu16071045
9. Zhang, X, Yang, Q, Huang, J, Lin, H, Luo, N, and Tang, H. Association of the newly proposed dietary index for gut microbiota and depression: the mediation effect of phenotypic age and body mass index. Eur Arch Psychiatry Clin Neurosci. [Epub ahead of print] (2024). doi: 10.1007/s00406-024-01912-x
10. An, S, Qin, J, Gong, X, Li, S, Ding, H, Zhao, X, et al. The mediating role of body mass index in the association between dietary index for gut microbiota and biological age: a study based on NHANES 2007-2018. Nutrients. (2024) 16:4164. doi: 10.3390/nu16234164
11. Ghemiș, L, Goriuc, A, Minea, B, Botnariu, GE, Mârțu, MA, Ențuc, M, et al. Myeloid-derived suppressor cells (MDSCs) and obesity-induced inflammation in type 2 diabetes. Diagnostics (Basel). (2024) 14:2453. doi: 10.3390/diagnostics14212453
12. Zatterale, F, Longo, M, Naderi, J, Raciti, GA, Desiderio, A, Miele, C, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. (2019) 10:1607. doi: 10.3389/fphys.2019.01607
13. Kubota, T, Inoue, M, Kubota, N, Takamoto, I, Mineyama, T, Iwayama, K, et al. Downregulation of macrophage Irs2 by hyperinsulinemia impairs IL-4-indeuced M2a-subtype macrophage activation in obesity. Nat Commun. (2018) 9:4863. doi: 10.1038/s41467-018-07358-9
14. Guraka, A, Sreedharan, S, Arasaradnam, R, Tripathi, G, and Kermanizadeh, A. The role of the gut microbiome in the development and progression of type 2 diabetes and liver disease. Nutr Rev. [Epub ahead of print] (2024). doi: 10.1093/nutrit/nuae172
15. O'Connor, LE, Wambogo, EA, Herrick, KA, Parsons, R, and Reedy, J. A standardized assessment of processed red meat and processed poultry intake in the US population aged ≥2 years using NHANES. J Nutr. (2022) 152:190–9. doi: 10.1093/jn/nxab316
16. Moshfegh, AJ, Rhodes, DG, Baer, DJ, Murayi, T, Clemens, JC, Rumpler, WV, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. (2008) 88:324–32. doi: 10.1093/ajcn/88.2.324
17. Yin, B, Wu, Z, Xia, Y, Xiao, S, Chen, L, and Li, Y. Non-linear association of atherogenic index of plasma with insulin resistance and type 2 diabetes: a cross-sectional study. Cardiovasc Diabetol. (2023) 22:157. doi: 10.1186/s12933-023-01886-5
18. Shu, Y, Wu, X, Wang, J, Ma, X, Li, H, and Xiang, Y. Associations of dietary inflammatory index with prediabetes and insulin resistance. Front Endocrinol (Lausanne). (2022) 13:820932. doi: 10.3389/fendo.2022.820932
19. Cheng, YJ, Kanaya, AM, Araneta, M, Saydah, SH, Kahn, HS, Gregg, EW, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011-2016. JAMA. (2019) 322:2389–98. doi: 10.1001/jama.2019.19365
20. Meigs, JB, Larson, MG, Fox, CS, Keaney, JJ, Vasan, RS, and Benjamin, EJ. Association of oxidative stress, insulin resistance, and diabetes risk phenotypes: the Framingham offspring study. Diabetes Care. (2007) 30:2529–35. doi: 10.2337/dc07-0817
21. Zhang, X, Zhao, L, Ducatman, A, Deng, C, von Stackelberg, KE, Danford, CJ, et al. Association of per- and polyfluoroalkyl substance exposure with fatty liver disease risk in US adults. JHEP Rep. (2023) 5:100694. doi: 10.1016/j.jhepr.2023.100694
22. You, Y, Chen, Y, Fang, W, Li, X, Wang, R, Liu, J, et al. The association between sedentary behavior, exercise, and sleep disturbance: a mediation analysis of inflammatory biomarkers. Front Immunol. (2022) 13:1080782. doi: 10.3389/fimmu.2022.1080782
23. Luo, R, Chen, M, Hao, S, Hun, M, Luo, S, Huang, F, et al. Associations of exposure to bisphenol-a or parabens with markers of liver injury/function among US adults in NHANES 2011-2016. J Expo Sci Environ Epidemiol. [Epub ahead of print] (2024). doi: 10.1038/s41370-024-00704-8
24. Luo, S, Liu, Z, Jiao, R, Li, W, Sun, J, Ma, S, et al. The associations of two novel inflammation indexes, systemic immune-inflammation index (SII) and system inflammation response index (SIRI), with periodontitis: evidence from NHANES 2009-2014. Clin Oral Investig. (2024) 28:129. doi: 10.1007/s00784-024-05529-1
25. Qi, Q, Zhuang, L, Shen, Y, Geng, Y, Yu, S, Chen, H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.30057
26. Tang, M, Chang, X, Zheng, H, Zeng, F, Zhang, G, He, M, et al. Association of dietary inflammatory index and systemic inflammatory markers with mortality risk in depressed adults: a mediation analysis of NHANES data. Front Nutr. (2024) 11:1472616. doi: 10.3389/fnut.2024.1472616
27. Zhang, Y, Wang, F, Tang, J, Shen, L, He, J, and Chen, Y. Association of triglyceride glucose-related parameters with all-cause mortality and cardiovascular disease in NAFLD patients: NHANES 1999-2018. Cardiovasc Diabetol. (2024) 23:262. doi: 10.1186/s12933-024-02354-4
28. Lv, C, and Huo, R. Association between visceral adiposity index, lipid accumulation product and type 2 diabetes mellitus in US adults with hypertension: a cross-sectional analysis of NHANES from 2005 to 2018. BMC Endocr Disord. (2024) 24:216. doi: 10.1186/s12902-024-01750-x
29. Bhute, SS, Suryavanshi, MV, Joshi, SM, Yajnik, CS, Shouche, YS, and Ghaskadbi, SS. Gut microbial diversity assessment of Indian Type-2-diabetics reveals alterations in eubacteria, Archaea, and eukaryotes. Front Microbiol. (2017) 8:214. doi: 10.3389/fmicb.2017.00214
30. Zhao, JD, Sun, M, Li, Y, Yu, CJ, Cheng, RD, Wang, SH, et al. Characterization of gut microbial and metabolite alterations in faeces of Goto Kakizaki rats using metagenomic and untargeted metabolomic approach. World J Diabetes. (2023) 14:255–70. doi: 10.4239/wjd.v14.i3.255
31. Zheng, X, Chen, M, Zhuang, Y, Zhao, L, Qian, Y, Xu, J, et al. Genetic associations between gut microbiota and type 2 diabetes mediated by plasma metabolites: a Mendelian randomization study. Front Endocrinol (Lausanne). (2024) 15:1430675. doi: 10.3389/fendo.2024.1430675
32. Zhang, H, Ma, L, Peng, W, Wang, B, and Sun, Y. Association between gut microbiota and onset of type 2 diabetes mellitus: a two-sample Mendelian randomization study. Front Cell Infect Microbiol. (2024) 14:1327032. doi: 10.3389/fcimb.2024.1327032
33. Rinninella, E, Mele, MC, Merendino, N, Cintoni, M, Anselmi, G, Caporossi, A, et al. The role of diet, micronutrients and the gut microbiota in age-related macular degeneration: new perspectives from the gut−Retina Axis. Nutrients. (2018) 10:10. doi: 10.3390/nu10111677
34. Attaye, I, Pinto-Sietsma, SJ, Herrema, H, and Nieuwdorp, M. A crucial role for diet in the relationship between gut microbiota and Cardiometabolic disease. Annu Rev Med. (2020) 71:149–61. doi: 10.1146/annurev-med-062218-023720
35. Chen, L, Liu, B, Ren, L, Du, H, Fei, C, Qian, C, et al. High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients. Front Cell Infect Microbiol. (2023) 13:1069954. doi: 10.3389/fcimb.2023.1069954
36. Sánchez-Quintero, MJ, Delgado, J, Medina-Vera, D, Becerra-Muñoz, VM, Queipo-Ortuño, MI, Estévez, M, et al. Beneficial effects of essential oils from the Mediterranean diet on gut microbiota and their metabolites in ischemic heart disease and Type-2 diabetes mellitus. Nutrients. (2022) 14:14. doi: 10.3390/nu14214650
37. Zheng, HJ, Guo, J, Jia, Q, Huang, YS, Huang, WJ, Zhang, W, et al. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2019) 142:303–13. doi: 10.1016/j.phrs.2019.02.016
38. Boulangé, CL, Neves, AL, Chilloux, J, Nicholson, JK, and Dumas, ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. (2016) 8:42. doi: 10.1186/s13073-016-0303-2
39. Sun, KY, Xu, DH, Xie, C, Plummer, S, Tang, J, Yang, XF, et al. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine. (2017) 92:1–11. doi: 10.1016/j.cyto.2017.01.003
40. Cani, PD, Amar, J, Iglesias, MA, Poggi, M, Knauf, C, Bastelica, D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. (2007) 56:1761–72. doi: 10.2337/db06-1491
41. Gomes, J, Costa, JA, and Alfenas, R. Metabolic endotoxemia and diabetes mellitus: a systematic review. Metabolism. (2017) 68:133–44. doi: 10.1016/j.metabol.2016.12.009
Keywords: gut microbiota, type 2 diabetes mellitus, insulin resistance, BMI, inflammatory marker, NHANES
Citation: Qu H, Yang Y, Xie Q, Ye L and Shao Y (2025) Linear association of the dietary index for gut microbiota with insulin resistance and type 2 diabetes mellitus in U.S. adults: the mediating role of body mass index and inflammatory markers. Front. Nutr. 12:1557280. doi: 10.3389/fnut.2025.1557280
Edited by:
Jasmina D. Debeljak Martacic, University of Belgrade, SerbiaReviewed by:
Azin Vakilpour, University of Pennsylvania, United StatesHaoxian Tang, First Affiliated Hospital of Shantou University Medical College, China
Copyright © 2025 Qu, Yang, Xie, Ye and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Shao, c2hhb3l1ZWNxQDE2My5jb20=; Liu Ye, bGl1eWVjcW11QDEyNi5jb20=
†These authors have contributed equally to this work
 Haoran Qu
Haoran Qu Yiyun Yang2†
Yiyun Yang2† Liu Ye
Liu Ye