- 1Infection Department, Ningbo Yinzhou No. 2 Hospital, Ningbo, China
- 2The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 3The First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 4Intensive Care Unit, Hospital of Zhejiang People's Armed Police, Hangzhou, Zhejiang, China
- 5Emergency Medical Center, Ningbo Yinzhou No. 2 Hospital, Ningbo, China
Objective: This meta-analysis explores the impact of vitamin D supplementation on antibiotic utilization.
Methods: We systematically searched for relevant randomized controlled trials (RCTs) in PubMed, Web of Science, EMBASE, and Science Direct from inception to April 2024. These trials compared antibiotic use rates between groups receiving vitamin D supplements and placebo.
Results: We included seven RCTs involving 35,160 participants. There was no significant difference in antibiotic use between the two groups in the general population (Odds Ratio [OR] = 0.98, p = 0.232), including elderly participants (OR = 0.98, p = 0.295). However, antibiotic use was lower in the intervention group compared to the placebo group among participants under 70 years of age (OR = 0.95, p = 0.015), those with relative vitamin D deficiency [25(OH)D < 75 nmol/L, OR = 0.95, p = 0.024; 25(OH)D < 50 nmol/L, OR = 0.96, p = 0.026], and those with respiratory tract infections (RTIs) (OR = 0.51, 95% CI: 0.24–1.08, p = 0.080), although these differences were not statistically significant for RTIs.
Conclusion: Vitamin D supplementation does not affect antibiotic use in the general population. However, it does reduce antibiotic utilization in individuals with RTIs, relative vitamin D deficiency, or aged below 70 years.
Systematic review registration: This meta-analysis adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and is registered with the International Prospective Register of Systematic Reviews (PROSPERO), registration number CRD42024543246.
Introduction
A spatial modeling study published in 2021 reported that global antibiotic consumption increased by 46% from 2000 to 2018, peaking at 40.1 defined daily doses (DDDs) in 2018 (1). Antibiotics, pivotal in reducing the morbidity and mortality associated with many infectious diseases, are considered life-saving drugs. However, their widespread availability and perceived cost-effectiveness have led to increased irrational and misuse (2). This is compounded by a lack of adequate awareness among both the public and medical professionals. Such overuse has accelerated the development of drug-resistant bacteria, posing a significant threat to global health due to the ensuing antibiotic resistance (3).
In response to the critical issue of antibiotic resistance, no effective alternatives have been developed, which necessitates the continuous development of new antibiotics (4). Recent studies suggest that combining antibiotics with non-antibiotic compounds could improve treatment outcomes against multi-resistant bacteria by possibly aiding in antibacterial action or repairing metabolic defects (5). For instance, it has been demonstrated that the addition of substrates like glucose or alanine can enhance the tricarboxylic acid cycle, thereby increasing bacterial uptake of antibiotics and improving their efficacy (6). Additionally, existing studies have suggested that vitamin D deficiency also played an important impact on extra-skeletal diseases, especially on respiratory tract infections (RTIs) such as bacterial pneumonia and acute respiratory infections (ARIs) (7). Notably, vitamin D has been recognized for its substantial immunomodulatory effects, such as activating immune cell chemotaxis, enhancing phagocytic capabilities of macrophages (8), and inducing the production of antimicrobial peptides (9). These properties suggest that vitamin D could serve as a supportive antimicrobial agent. A prior meta-analysis involving 25 randomized controlled trials (RCTs) indicated that vitamin D supplementation could lower the incidence of ARIs (10). Moreover, a prospective observational study in Sweden showed that vitamin D supplementation was associated with a reduction in antibiotic usage days (11). There was, in addition, a cohort study shown that low serum vitamin D levels were an independent predictor of adverse outcomes of COVID-19 and might result in higher levels of inflammation and more serious tissue damage in patients with severe or non-severe cases (12). Despite these findings, there were no meta-analyses that explore the effect of vitamin D supplementation on antibiotic use. In light of these considerations, this study aims to review published RCTs to perform a meta-analysis assessing the relationship between vitamin D supplementation and antibiotic usage frequency in adults.
Methods
Search strategy
This meta-analysis adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13), and is registered with the International Prospective Register of Systematic Reviews (PROSPERO), registration number CRD42024543246. We conducted a systematic search for RCTs examining the effects of vitamin D supplementation on antibiotic use from inception to April 2024. Searches were performed using PubMed, Web of Science, EMBASE, and Science Direct, with keywords including (vitamin D) AND (antibiosis OR antibiotic OR antibiotics OR anti-infection OR infection). A secondary search was conducted through the references of all identified studies to ensure the comprehensiveness of our search.
Selection and exclusion criteria
The inclusion and exclusion of studies were guided by the PICOS (participants, intervention, comparison, outcomes, and study design) framework (14). The inclusion criteria were as follows: (1) participants: adults aged 16 years or older, or those at high risk of antibiotic use due to certain diseases (excluding tuberculosis); (2) intervention: oral administration of vitamin D in the intervention group; (3) comparison: placebo given to the control group; (4) outcomes: measures related to antibiotic use; (5) study Design: only RCTs were considered.
Exclusion criteria included: (1) studies where relevant data could not be extracted or were unsuitable for statistical analysis; (2) studies where the full text was unavailable; (3) studies not published in English; (4) studies with outdated or superseded publications, where articles with the most recent and comprehensive data were given preference.
Data extraction and quality assessment
Data extraction was performed independently by two investigators using a predefined form. This form captured essential information including the authors, year of publication, country, clinical trial number, participant characteristics (age, number, recruitment year, physical condition, and vitamin D supplementation regimen), and antibiotic-related outcomes.
Risk of bias was assessed according to the guidelines provided by the Cochrane Collaboration Network (15). The assessment covered several domains: (1) random sequence generation (to address selection bias), (2) allocation concealment (to address selection bias), (3) blinding of participants and personnel (to mitigate performance bias), (4) blinding of outcome assessment (to mitigate detection bias), (5) completeness of outcome data (to address attrition bias), (6) selective reporting (to address reporting bias), and (7) other potential biases. Each domain was rated as ‘high risk,’ ‘low risk,’ or ‘unclear risk’. Disagreements between investigators were resolved through discussion to reach a consensus.
Statistical analysis
Statistical analyses were conducted using Stata Software version 12.0 (Stata Corporation LLC, College Station, United States). The impact of vitamin D supplementation on antibiotic use was evaluated using odds ratios (ORs) and 95% confidence intervals (CIs). Heterogeneity among the studies was assessed with a chi-square test and quantified using the I2 statistic. I2 values over 50% were considered indicative of significant heterogeneity, values between 25 and 50% indicated moderate heterogeneity, and values below 25% indicated low heterogeneity (16).
Due to potential variations among study participants and differences in study protocols, analyses were performed using a random-effects model to enhance the reliability of the results. Subgroup analyses were conducted to explore the sources of heterogeneity further. Publication bias was assessed using Begg’s test, and sensitivity analyses were performed to verify the stability of the findings. All statistical tests were two-sided, with a significance threshold set at p < 0.05.
Results
Study selection
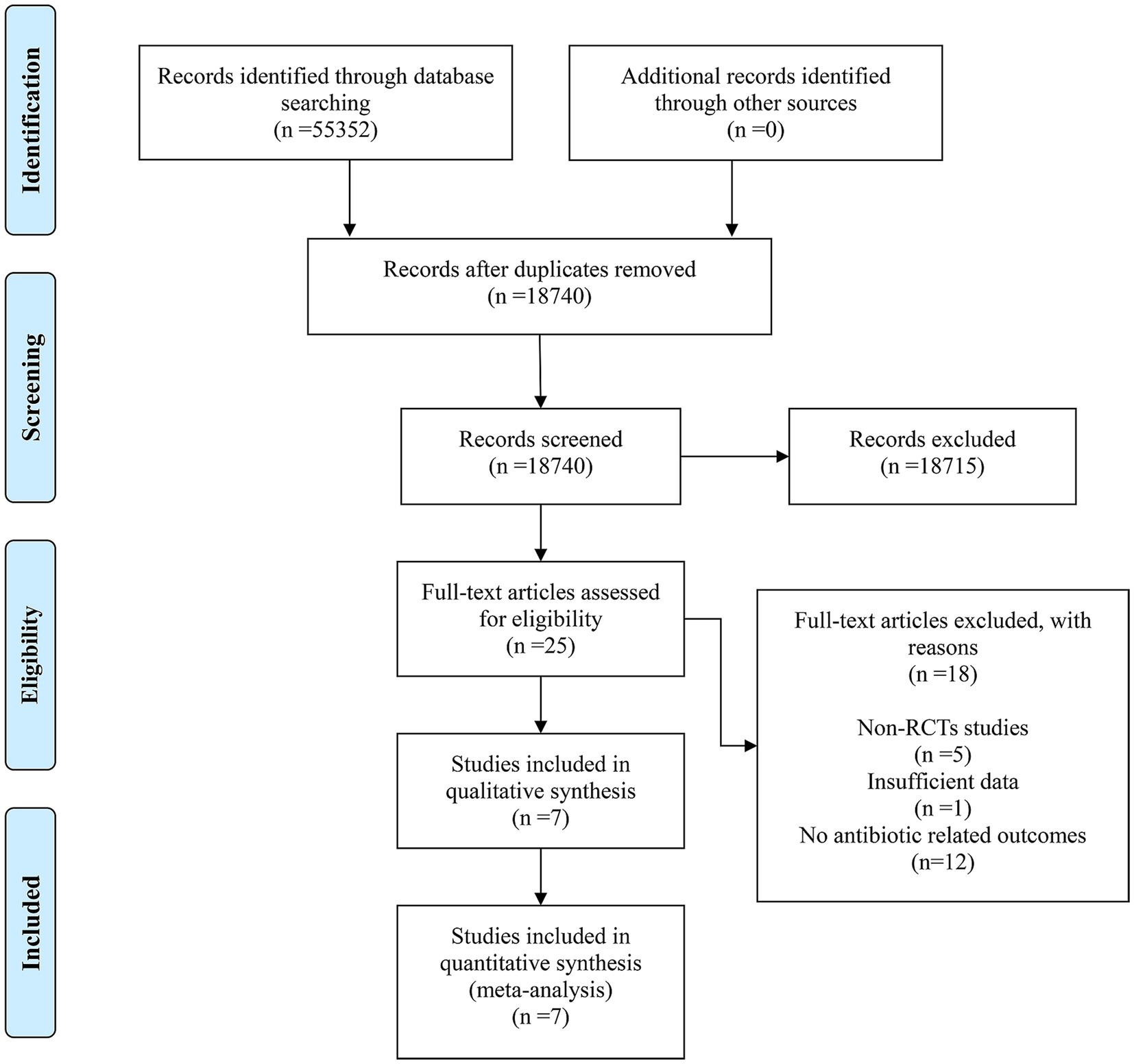
From four electronic databases, a search identified 55,352 records under the specified research strategy. No additional records were identified through other sources. After removing duplicates, 18,740 records remained. Screening of titles and abstracts led to the exclusion of 18,715 records due to low relevance, leaving 25 full-text articles for detailed evaluation. Out of these, 18 articles were excluded for the following reasons: 5 were non-RCTs, 1 had insufficient data, and 12 did not report antibiotic-related outcomes. Ultimately, 7 studies met the inclusion criteria and were included in the meta-analysis. The detailed retrieval process is illustrated in Figure 1.
Characteristics and quality assessment of included studies
The 7 RCTs (17–23), spanning from 2007 to 2022 and cited as references, investigated the relationship between vitamin D supplementation and antibiotic use, involving 35,160 participants from five countries (Sweden, United Kingdom, Australia, Netherlands, New Zealand). The intervention groups in these studies received oral vitamin D supplementation, while control groups were administered a placebo. Six (17, 18, 20–23) of the studies involved oral cholecalciferol and one (19) involved oral D-Peals capsules produced in Denmark. Duration of intervention varied: three studies (17, 20, 23) had durations exceeding 1 year, and the remaining four (18, 19, 21, 22) were conducted for 1 year or less.
Participants’ physical conditions varied across studies: one (18) involved participants with antibody deficiencies or frequent RTIs; one (19) included individuals with 25(OH)D levels below 75 nmol/L; one (21) focused on patients with a history of chronic obstructive pulmonary disease (COPD) exacerbation within the last 12 months and 25(OH)D levels below 50 nmol/L; one (17) targeted patients with low trauma and osteoporotic fractures; and three (20, 22, 23) included elderly individuals from the general community. Further characteristics and details of the included studies are presented in Table 1 and Supplementary Table S1.
The risk of bias was assessed for each study using the Cochrane Collaboration’s tool, as depicted in Supplementary Figures S1, S2. One study (19) was deemed to have a high risk of selection and performance bias due to inadequate concealment of treatment allocation and lack of stratified randomization. Another study (21) was identified as having a high risk of bias due to not achieving the designed sample size. Three studies (18, 22, 23) presented challenges in determining other biases. Overall, the studies were considered to be of high quality (Table 2).
Analysis of the primary result
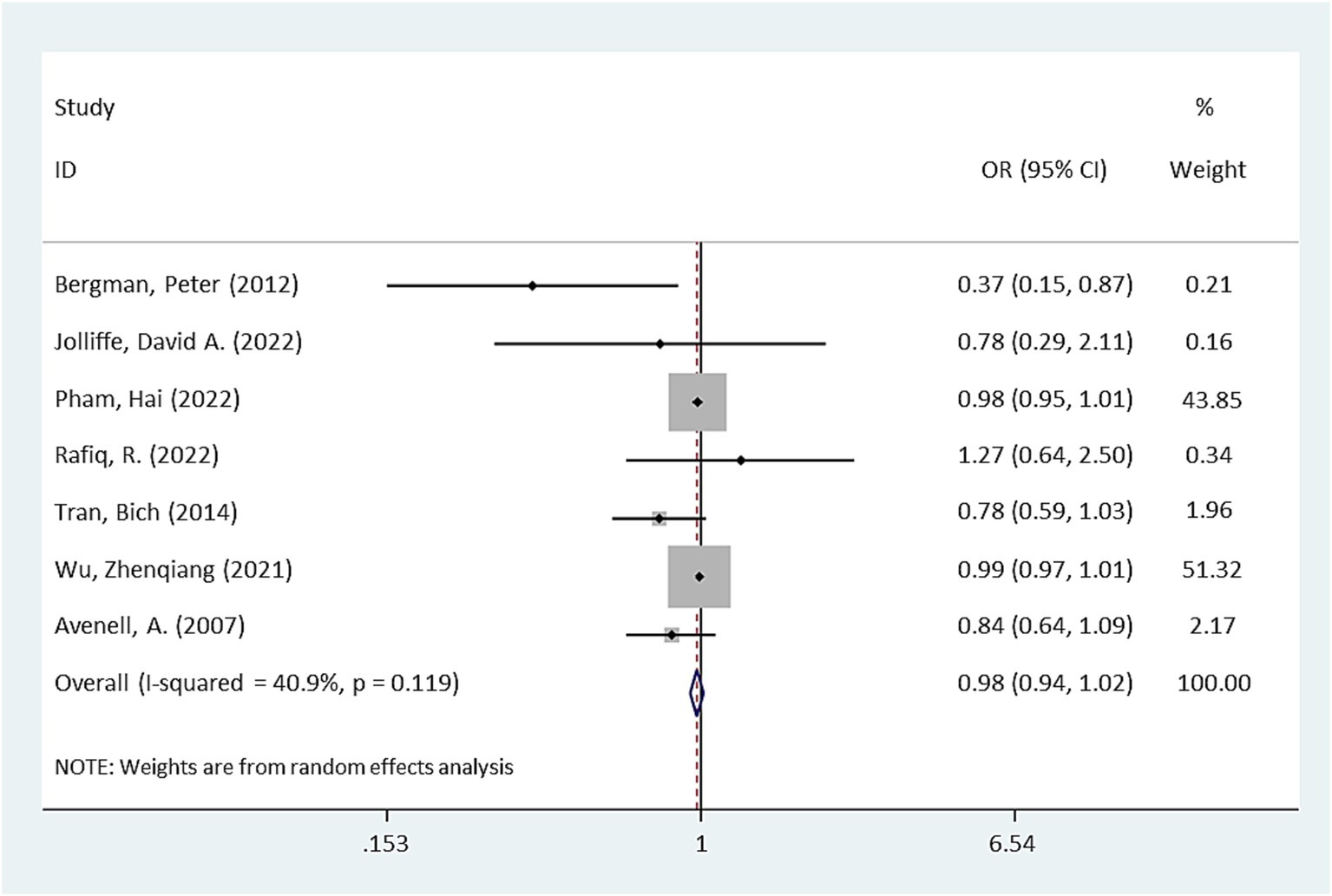
Pooling the results from seven RCTs (17–23), no significant difference in antibiotic use was observed between the intervention group receiving vitamin D supplementation and the placebo group (OR = 0.98, 95% CI: 0.94–1.02, p = 0.232, Figure 2). However, there was moderate heterogeneity among the studies (I2 = 40.9%).
Subgroup analysis
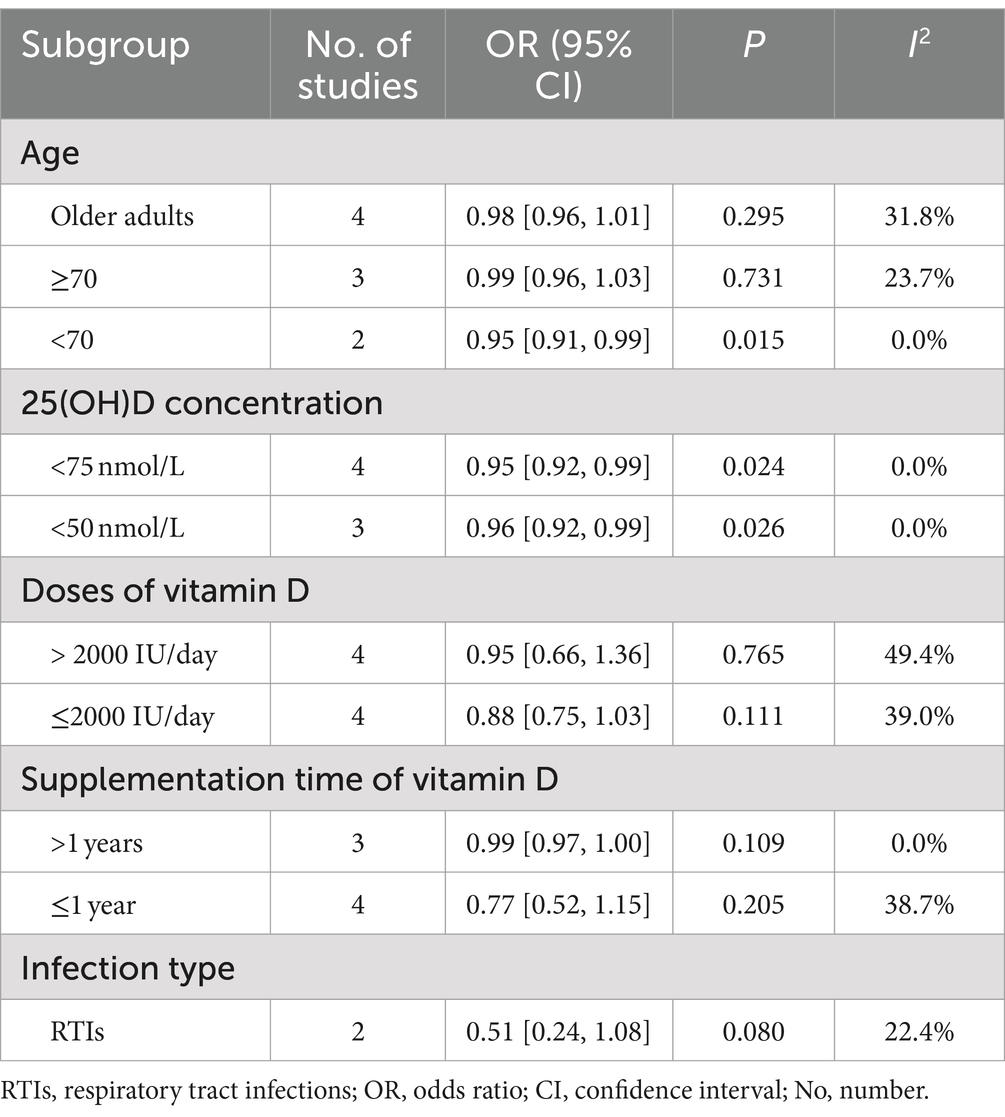
Subgroup analyses were conducted based on participant age thresholds. For participants aged ≥70 years, no statistical difference in antibiotic use was observed between the intervention and control groups (OR = 0.99, p = 0.731). Conversely, among participants aged <70 years, the intervention group exhibited a reduced use of antibiotics compared to the control group (OR = 0.95, p = 0.015). Additional subgroup analysis among older adults similarly showed no significant differences in antibiotic use between the groups (OR = 0.98, p = 0.295).
Vitamin D concentration levels of less than 75 nmol/L or 50 nmol/L were considered relatively inadequate (24). Among participants with 25(OH)D levels <75 nmol/L, four RCTs (19–21, 23) indicated reduced antibiotic use in the intervention group compared to the control group (OR = 0.95, p = 0.024). For participants with 25(OH)D levels <50 nmol/L, results from three RCTs (17, 18, 22) demonstrated that the vitamin D-receiving group used fewer antibiotics than the placebo group (OR = 0.96, p = 0.026), with no significant heterogeneity (I2 = 0%).
Regarding vitamin D dosage, participants were categorized based on daily intake exceeding 2000 IU (high-dose supplementation group) or not (low-dose supplementation group). Neither the high-dose group (OR = 0.95, p = 0.765) nor the low-dose group (OR = 0.88, p = 0.111) showed significant differences in antibiotic use. Similarly, based on the duration of supplementation, no significant differences were found either for durations greater than 1 year (OR = 0.99, p = 0.109) or less than or equal to 1 year (OR = 0.77, p = 0.205).
For participants suffering from RTIs in two RCTs (18, 19) the intervention group exhibited a lower rate of antibiotic utilization compared to the control group, though the difference was not statistically significant (OR = 0.51, 95% CI: 0.24–1.08, p = 0.080).
Publication bias and sensitivity analysis
Publication bias was assessed using Begg’s test, which indicated no significant bias in the results related to the effect of vitamin D on antibiotic use (p = 0.230, Supplementary Figure S3). Sensitivity analysis was conducted by sequentially excluding each study, confirming that the results remained stable (Supplementary Figure S4).
Discussion
In this meta-analysis of seven RCTs (17–23), no significant association was observed between vitamin D supplementation and the risk of antibiotic use, encompassing elderly participants and various subgroup analyses concerning dosage and duration of vitamin D supplementation. However, among participants under 70 years of age, those with relative vitamin D deficiency, or those suffering from RTIs, vitamin D supplementation appears to reduce antibiotic usage.
Vitamin D, recognized as a multifunctional health-promoting molecule (25), is absorbed into the bloodstream and converted in the kidneys to its active form, 1,25(OH)2D3, via the catalytic action of 1α-hydroxylase (26). It primarily exerts its effects through interaction with the vitamin D receptor (VDR), facilitating the receptor complex’s migration to the nucleus and modulating the expression of numerous genes related to immune regulation and infection control (27). The immunomodulatory mechanisms of vitamin D include enhancing phagocytosis and chemotaxis of innate immune cells such as macrophages and monocytes, thereby improving pathogen clearance (28); inducing dendritic cell tolerance through the expression of CYP27B1, which enhances localized concentrations of active vitamin D at infection sites (29); and integrating with pattern recognition receptors (PRRs) to detect microbial signals and activate downstream infection-fighting pathways (30). Additionally, vitamin D helps regulate inflammatory responses by inhibiting pro-inflammatory cytokines like interleukin-2 (IL-2) and promoting the production of anti-inflammatory cytokines such as IL-10 (31).
Beyond immune regulation, vitamin D also plays a significant role in anti-infection processes: it promotes the production of host defense peptides, including cathelicidin antimicrobial peptide (CAMP) and human β2-defensins (Defb2) (32), with vitamin D response elements directly influencing their gene expression (33). It mediates the synthesis of nitric oxide (NO), which enhances antimicrobial activity (34) and may reduce the viability of Streptococcus pneumoniae and the emergence of antibiotic resistance (35). Furthermore, vitamin D reduces the activity of mammalian target of rapamycin (mTOR), supports the recruitment of ATG16L1 by NOD2 to induce autophagy, and aids in the elimination of intracellular bacteria (36). The antioxidant properties of vitamin D help eliminate harmful reactive oxygen species (ROS), moderating inflammation and maintaining mitochondrial function, which is crucial in reducing TNF-α-induced lung epithelial inflammation and mitochondrial autophagy (37, 38). In conclusion, while vitamin D’s role in reducing antibiotic use was not uniformly observed across all study participants, its various immunomodulatory and antibacterial properties theoretically support the reduction of antibiotic usage, particularly in certain subpopulations.
Although the mechanisms discussed were not substantiated across all participants in our study, several factors could explain these findings. Firstly, certain disease states and environmental factors might suppress antimicrobial peptide levels, thus diminishing the anti-infective efficacy of vitamin D. Studies have demonstrated that diabetes can down-regulate the expression of antimicrobial peptides (39), and prolonged exposure to polluted air reduces these peptide levels in mice (40). Secondly, genetic variations in the VDR genes affect individual responsiveness to vitamin D. A meta-analysis has shown that genotypes with the TaqI polymorphism and the FF variant in the FokI gene are more responsive to vitamin D supplementation (41). Thirdly, in individuals who are not deficient in vitamin D, additional supplementation may lead to inefficient binding of the vitamin to its receptors, as excess vitamin D is converted to 1,24,25(OH)2D3, which has minimal affinity for VDR (42). This hypothesis is supported by subgroup analysis indicating that high-dose vitamin D supplementation does not significantly reduce the risk of antibiotic use. This is consistent with a single-center RCT finding that additional vitamin D supplementation did not decrease hospital-acquired infection rates among sepsis patients (43), suggesting that vitamin D supplementation may not universally contribute to reduced antibiotic use.
Moreover, while one study suggested that high doses of vitamin D could decrease inflammation levels and enhance anti-infection capabilities (44), our subgroup analysis found no significant benefits from either low or high doses of vitamin D supplementation in reducing antibiotic use. Possible explanations include: (1) active vitamin D maintains a dynamic equilibrium in the body, with excess being converted to an inactive form that cannot be effectively utilized (42); (2) variability in the effectiveness of different vitamin D supplementation regimens; and (3) the prevalence of adequate serum vitamin D levels among our study participants, which could obscure any potential benefits for those with insufficient vitamin D levels. Previous studies have demonstrated that vitamin D supplementation had a more phenomenal impact on participants with vitamin D deficiency (45).
Emerging evidence underscores the association between low serum vitamin D levels and increased infection risk (46–48). The Third National Health and Nutrition Examination Survey demonstrated an inverse relationship between serum vitamin D levels and recent upper respiratory tract infections in the American population (49). Furthermore, a meta-analysis confirmed that vitamin D deficiency heightens susceptibility to serious infections (50). Our subgroup analysis for participants with low serum vitamin D levels (25(OH)D < 50 nmol/L or <75 nmol/L) corroborates these findings, suggesting that vitamin D deficiency may compromise neutralizing antibody production and immune cell function (51). Therefore, vitamin D supplementation could potentially enhance immune responses and infection resistance.
Specifically, studies indicate that antibiotic usage is more prevalent among the elderly, women (52), and individuals in poorer health (53). Despite the theoretical benefits of vitamin D in boosting immunity among the elderly to combat infections, our findings did not support this hypothesis for participants aged ≥70 years. This discrepancy may be attributable to several factors: (1) age-related decline in organ function associated with calcium metabolism may lead to decreased expression of VDR, resulting in inefficient utilization of vitamin D (54); (2) the prevalence of chronic kidney disease in older adults impairs the kidneys’ ability to activate vitamin D (55); (3) parathyroid hormone, known to enhance the synthesis of 1α-hydroxylase (56)—which converts vitamin D to its active form—is often diminished in older women, as evidenced by higher rates of hypoparathyroidism in this group (57); (4) comorbid conditions such as diabetes can negatively affect antimicrobial peptide production (39). A meta-analysis involving 41,552 elderly patients revealed that vitamin D supplementation did not significantly reduce the incidence of ARIs or lower respiratory infections (58), further supporting our observations.
Conversely, vitamin D supplementation was found to be beneficial in participants under 70 years of age. Possible explanations include: (1) younger individuals often engage in higher levels of physical activity, which may enhance vitamin D metabolism in adipose tissue (59); (2) higher physiological requirements and lower dietary intake of vitamin D in younger populations may lead to more pronounced deficiencies (60, 61), which supplementation can effectively address.
As for the type of infection, many studies available now have confirmed that vitamin D can relieve the symptoms of infection or reduce the onset of RTIs. This was consistent with our findings. The reasons may be as follows: (1) vitamin D can promote the repair of epithelial cells and inhibit the apoptosis of epithelial cells, thereby improving lung function (62); (2) vitamin D can enhance mucosal immunity including respiratory mucosa (63); (3) existing study showed that daily supplementation of vitamin D could increase the antibacterial activity of airway surface fluids (37); (4) RTIs activated T and B lymphocytes and significantly up-regulated the expression of VDR (64, 65). Thus, vitamin D supplementation can conduce to the promotion of the ability to fight infection and reduce the risk of antibiotic use in people suffering from RTIs.
For all we know, this was the first meta-analysis to conduct a comprehensive and systematic exploration of the relationship between the antibiotic use and the supplementation of vitamin D. It could provide a reference value for the field of antibiotic use. Importantly, the meta-analysis was based on the RCTs with high quality. Nevertheless, there were some limitations in the present meta-analysis. Firstly, the number of studies was limited. Besides, on account of the limited data, we could not perform further subgroup analysis including the sex or the body mass index. Moreover, certain heterogeneity was produced due to the different physical conditions of the subjects and various programs of vitamin D supplementation.
Conclusion
This meta-analysis revealed that vitamin D supplementation does not significantly impact antibiotic usage in the general population, including elderly individuals. The regimen of vitamin D supplementation also showed no effect on antibiotic use. However, vitamin D supplementation may be beneficial in reducing antibiotic use among individuals under 70 years of age, those with relative vitamin D deficiency, or those suffering from RTIs. To substantiate these findings, more multicenter RCTs on a larger scale are necessary.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MW: Writing – original draft, Conceptualization. YW: Writing – original draft, Methodology, Investigation, Data curation. ZX: Writing – original draft, Investigation. YZ: Writing – original draft, Formal analysis. TH: Writing – review & editing. BC: Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1502835/full#supplementary-material
Abbreviations
DDDs, Defined Daily Doses; RTIs, Respiratory Tract Infections; ARIs, Acute Respiratory Infections; RCTs, Randomized Controlled Trials; OR, Odds Ratio; CI: Confidence Interval; COPD, Chronic Obstructive Pulmonary Disease; VDR, Vitamin D Receptor; PRRs, Pattern Recognition Receptors; IL, Interleukin; CAMP, Cathelicidin Antimicrobial Peptide; Defb2, Human β2-Defensins; NO, Nitric Oxide; mTOR, Mammalian Target of Rapamycin; ROS, Reactive Oxygen Species; No, Number.
References
1. Browne, AJ, Chipeta, MG, Haines-Woodhouse, G, Kumaran, EPA, Hamadani, BHK, Zaraa, S, et al. Global antibiotic consumption and usage in humans, 2000-18: a spatial modelling study. Lancet Planet Health. (2021) 5:e893–904. doi: 10.1016/S2542-5196(21)00280-1
2. Machowska, A, and Stålsby Lundborg, C. Drivers of irrational use of antibiotics in Europe. Int J Environ Res Public Health. (2018) 16:27. doi: 10.3390/ijerph16010027
3. Sabtu, N, Enoch, DA, and Brown, NM. Antibiotic resistance: what, why, where, when and how? Br Med Bull. (2015) 116:105–13. doi: 10.1093/bmb/ldv041
4. Chen, L, Kumar, S, and Wu, H. A review of current antibiotic resistance and promising antibiotics with novel modes of action to combat antibiotic resistance. Arch Microbiol. (2023) 205:356. doi: 10.1007/s00203-023-03699-2
5. Xiao, G, Li, J, and Sun, Z. The combination of antibiotic and non-antibiotic compounds improves antibiotic efficacy against multidrug-resistant Bacteria. Int J Mol Sci. (2023) 24:5493. doi: 10.3390/ijms242015493
6. Peng, B, Su, YB, Li, H, Han, Y, Guo, C, Tian, YM, et al. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab. (2015) 21:249–62. doi: 10.1016/j.cmet.2015.01.008
7. Giustina, A, Bilezikian, JP, Adler, RA, Banfi, G, Bikle, DD, Binkley, NC, et al. Consensus statement on vitamin D status assessment and supplementation: whys, whens, and hows. Endocr Rev. (2024) 45:625–54. doi: 10.1210/endrev/bnae009
8. Golpour, A, Bereswill, S, and Heimesaat, MM. Antimicrobial and immune-modulatory effects of vitamin D provide promising antibiotics-independent approaches to tackle bacterial infections – lessons learnt from a literature survey. Eur J Microbiol Immunol. (2019) 9:80–7. doi: 10.1556/1886.2019.00014
9. Gombart, AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. (2009) 4:1151–65. doi: 10.2217/fmb.09.87
10. Martineau, AR, Jolliffe, DA, Hooper, RL, Greenberg, L, Aloia, JF, Bergman, P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. (2017) 356:i6583. doi: 10.1136/bmj.i6583
11. Norlin, AC, Hansen, S, Wahren-Borgström, E, Granert, C, Björkhem-Bergman, L, and Bergman, P. Vitamin D3 supplementation and antibiotic consumption – results from a prospective, observational study at an immune-deficiency unit in Sweden. PLoS One. (2016) 11:e0163451. doi: 10.1371/journal.pone.0163451
12. di Filippo, L, Uygur, M, Locatelli, M, Nannipieri, F, Frara, S, and Giustina, A. Low vitamin D levels predict outcomes of COVID-19 in patients with both severe and non-severe disease at hospitalization. Endocrine. (2023) 80:669–83. doi: 10.1007/s12020-023-03331-9
13. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
14. Rudwaleit, M, Landewe, R, van der Heijde, D, Listing, J, Brandt, J, Braun, J, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. (2009) 68:770–6. doi: 10.1136/ard.2009.108217
15. Higgins, JP, Altman, DG, Gotzsche, PC, Juni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
16. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
17. Avenell, A, Cook, JA, MacLennan, GS, and MacPherson, GC. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing. (2007) 36:574–7. doi: 10.1093/ageing/afm091
18. Bergman, P, Norlin, AC, Hansen, S, Rekha, RS, Agerberth, B, Björkhem-Bergman, L, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. (2012) 2:e001663. doi: 10.1136/bmjopen-2012-001663
19. Jolliffe, DA, Holt, H, Greenig, M, Talaei, M, Perdek, N, Pfeffer, P, et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT). BMJ. (2022) 378:e071230. doi: 10.1136/bmj-2022-071230
20. Pham, H, Waterhouse, M, Baxter, C, Duarte Romero, B, McLeod, DSA, Armstrong, BK, et al. Vitamin D supplementation and antibiotic use in older Australian adults: an analysis of data from the D-health trial. J Infect Dis. (2022) 226:949–57. doi: 10.1093/infdis/jiac279
21. Rafiq, R, Aleva, FE, Schrumpf, JA, Daniels, JM, Bet, PM, Boersma, WG, et al. Vitamin D supplementation in chronic obstructive pulmonary disease patients with low serum vitamin D: a randomized controlled trial. Am J Clin Nutr. (2022) 116:491–9. doi: 10.1093/ajcn/nqac083
22. Tran, B, Armstrong, BK, Ebeling, PR, English, DR, Kimlin, MG, van der Pols, JC, et al. Effect of vitamin D supplementation on antibiotic use: a randomized controlled trial. Am J Clin Nutr. (2014) 99:156–61. doi: 10.3945/ajcn.113.063271
23. Wu, Z, Camargo, CA Jr, Sluyter, J, Waayer, D, Toop, L, and Scragg, R. Effect of monthly vitamin D supplementation on antibiotic prescribing in older adults: a post hoc analysis of a randomized controlled trial. Am J Clin Nutr. (2021) 114:314–21. doi: 10.1093/ajcn/nqab015
24. Vieth, R. Why the minimum desirable serum 25-hydroxyvitamin D level should be 75 nmol/L (30 ng/ml). Best Pract Res Clin Endocrinol Metab. (2011) 25:681–91. doi: 10.1016/j.beem.2011.06.009
25. Paul, S, Kaushik, R, Chawla, P, Upadhyay, S, Rawat, D, and Akhtar, A. Vitamin-D as a multifunctional molecule for overall well-being: an integrative review. Clin Nutr ESPEN. (2024) 62:10–21. doi: 10.1016/j.clnesp.2024.04.016
26. Kumar, R, Tebben, PJ, and Thompson, JR. Vitamin D and the kidney. Arch Biochem Biophys. (2012) 523:77–86. doi: 10.1016/j.abb.2012.03.003
27. Sassi, F, Tamone, C, and D'Amelio, P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. (2018) 10:1656. doi: 10.3390/nu10111656
28. Baeke, F, Takiishi, T, Korf, H, Gysemans, C, and Mathieu, C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. (2010) 10:482–96. doi: 10.1016/j.coph.2010.04.001
29. Ismailova, A, and White, JH. Vitamin D, infections and immunity. Rev Endocr Metab Disord. (2022) 23:265–77. doi: 10.1007/s11154-021-09679-5
30. Van Belle, TL, Gysemans, C, and Mathieu, C. Vitamin D in autoimmune, infectious and allergic diseases: a vital player? Best Pract Res Clin Endocrinol Metab. (2011) 25:617–32. doi: 10.1016/j.beem.2011.04.009
31. Boonstra, A, Barrat, FJ, Crain, C, Heath, VL, Savelkoul, HFJ, and O’Garra, A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. (2001) 167:4974–80. doi: 10.4049/jimmunol.167.9.4974
32. Amado Diago, CA, García-Unzueta, MT, Fariñas Mdel, C, and Amado, JA. Calcitriol-modulated human antibiotics: new pathophysiological aspects of vitamin D. Endocrinol Nutr. (2016) 63:87–94. doi: 10.1016/j.endonu.2015.09.005
33. Wang, TT, Nestel, FP, Bourdeau, V́, Nagai, Y, Wang, Q, Liao, J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. (2004) 173:2909–12. doi: 10.4049/jimmunol.173.5.2909
34. Guo, C, and Gombart, AF. The antibiotic effects of vitamin D. Endocr Metab Immune Disord Drug Targets. (2014) 14:255–66. doi: 10.2174/1871530314666140709085159
35. Allan, RN, Morgan, S, Brito-Mutunayagam, S, Skipp, P, Feelisch, M, Hayes, SM, et al. Low concentrations of nitric oxide modulate Streptococcus pneumoniae biofilm metabolism and antibiotic tolerance. Antimicrob Agents Chemother. (2016) 60:2456–66. doi: 10.1128/AAC.02432-15
36. Dimitrov, V, Barbier, C, Ismailova, A, Wang, Y, Dmowski, K, Salehi-Tabar, R, et al. Vitamin D-regulated gene expression profiles: species-specificity and cell-specific effects on metabolism and immunity. Endocrinology. (2021) 162:bqaa218. doi: 10.1210/endocr/bqaa218
37. Chen, YC, Sung, HC, Chuang, TY, Lai, TC, Lee, TL, Lee, CW, et al. Vitamin D(3) decreases TNF-α-induced inflammation in lung epithelial cells through a reduction in mitochondrial fission and mitophagy. Cell Biol Toxicol. (2022) 38:427–50. doi: 10.1007/s10565-021-09629-6
38. Cojic, M, Kocic, R, Klisic, A, and Kocic, G. The effects of vitamin D supplementation on metabolic and oxidative stress markers in patients with type 2 diabetes: a 6-month follow up randomized controlled study. Front Endocrinol. (2021) 12:610893. doi: 10.3389/fendo.2021.610893
39. Mohanty, S, Kamolvit, W, Scheffschick, A, Björklund, A, Tovi, J, Espinosa, A, et al. Diabetes downregulates the antimicrobial peptide psoriasin and increases E. coli burden in the urinary bladder. Nat Commun. (2022) 13:4983. doi: 10.1038/s41467-022-32636-y
40. Almaraz-de-Santiago, J, Solis-Torres, N, Quintana-Belmares, R, Rodríguez-Carlos, A, Rivas-Santiago, B, Huerta-García, J, et al. Long-term exposure to particulate matter from air pollution alters airway β-defensin-3 and -4 and cathelicidin host defense peptides production in a murine model. Peptides. (2021) 142:170581. doi: 10.1016/j.peptides.2021.170581
41. Usategui-Martín, R, de Luis-Román, DA, Fernández-Gómez, JM, Ruiz-Mambrilla, M, and Pérez-Castrillón, JL. Vitamin D receptor (VDR) gene polymorphisms modify the response to vitamin D supplementation: a systematic review and Meta-analysis. Nutrients. (2022) 14:360. doi: 10.3390/nu14020360
42. Tebben, PJ, Milliner, DS, Horst, RL, Harris, PC, Singh, RJ, Wu, Y, et al. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. J Clin Endocrinol Metab. (2012) 97:E423–7. doi: 10.1210/jc.2011-1935
43. Thampi, SJ, Basheer, A, and Thomas, K. Calcitriol in Sepsis-a single-Centre randomised control trial. J Clin Med. (2024) 13:3823. doi: 10.3390/jcm13133823
44. Tabatabaeizadeh, SA, Avan, A, Bahrami, A, Khodashenas, E, Esmaeili, H, Ferns, GA, et al. High dose supplementation of vitamin D affects measures of systemic inflammation: reductions in high sensitivity C-reactive protein level and neutrophil to lymphocyte ratio (NLR) distribution. J Cell Biochem. (2017) 118:4317–22. doi: 10.1002/jcb.26084
45. di Filippo, L, and Giustina, A. Vitamin D deficiency and type 2 diabetes: the dangerous link between two modern pandemics. J Clin Endocrinol Metab. (2024) 13:dgae390. doi: 10.1210/clinem/dgae390
46. Ahmad, S, Arora, S, Khan, S, Mohsin, M, Mohan, A, Manda, K, et al. Vitamin D and its therapeutic relevance in pulmonary diseases. J Nutr Biochem. (2021) 90:108571. doi: 10.1016/j.jnutbio.2020.108571
47. Gomes, TL, Fernandes, RC, Vieira, LL, Schincaglia, RM, Mota, JF, Nóbrega, MS, et al. Low vitamin D at ICU admission is associated with cancer, infections, acute respiratory insufficiency, and liver failure. Nutrition. (2019) 60:235–40. doi: 10.1016/j.nut.2018.10.018
48. Kalluri, HV, Sacha, LM, Ingemi, AI, Shullo, MA, Johnson, HJ, Sood, P, et al. Low vitamin D exposure is associated with higher risk of infection in renal transplant recipients. Clin Transpl. (2017) 31:e12955. doi: 10.1111/ctr.12955
49. Ginde, AA, Mansbach, JM, and Camargo, CA. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third National Health and nutrition examination survey. Arch Intern Med. (2009) 169:384–90. doi: 10.1001/archinternmed.2008.560
50. de Haan, K, Groeneveld, ABJ, de Geus, HRH, Egal, M, and Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit Care. (2014) 18:660. doi: 10.1186/s13054-014-0660-4
51. Deluca, HF, and Cantorna, MT. Vitamin D: its role and uses in immunology. FASEB J. (2001) 15:2579–85. doi: 10.1096/fj.01-0433rev
52. Chen, Y, Kirk, MD, Stuart, R, Cheng, AC, Pearson, SA, Hayen, A, et al. Socio-demographic and health service factors associated with antibiotic dispensing in older Australian adults. PLoS One. (2019) 14:e0221480. doi: 10.1371/journal.pone.0221480
53. Olesen, SW, Barnett, ML, MacFadden, DR, Brownstein, JS, Hernández-Díaz, S, Lipsitch, M, et al. The distribution of antibiotic use and its association with antibiotic resistance. eLife. (2018) 7:7. doi: 10.7554/eLife.39435
54. de Jongh, RT, van Schoor, NM, and Lips, P. Changes in vitamin D endocrinology during aging in adults. Mol Cell Endocrinol. (2017) 453:144–50. doi: 10.1016/j.mce.2017.06.005
55. Khan, SS, Petkovich, M, Holden, RM, and Adams, MA. Megalin and vitamin D metabolism-implications in non-renal tissues and kidney disease. Nutrients. (2022) 14:3690. doi: 10.3390/nu14183690
56. Goltzman, D, Mannstadt, M, and Marcocci, C. Physiology of the calcium-parathyroid hormone-vitamin D Axis. Front Horm Res. (2018) 50:1–13. doi: 10.1159/000486060
57. Palermo, A, Jacques, R, Gossiel, F, Reid, DM, Roux, C, Felsenberg, D, et al. Normocalcaemic hypoparathyroidism: prevalence and effect on bone status in older women. The OPUS study. Clin Endocrinol. (2015) 82:816–23. doi: 10.1111/cen.12732
58. Jia, H, Sheng, F, Yan, Y, Liu, X, and Zeng, B. Vitamin D supplementation for prevention of acute respiratory infections in older adults: a systematic review and meta-analysis. PLoS One. (2024) 19:e0303495. doi: 10.1371/journal.pone.0303495
59. Hengist, A, Perkin, O, Gonzalez, JT, Betts, JA, Hewison, M, Manolopoulos, KN, et al. Mobilising vitamin D from adipose tissue: the potential impact of exercise. Nutr Bull. (2019) 44:25–35. doi: 10.1111/nbu.12369
60. Borecka, O, Farrar, MD, Osman, JE, Rhodes, LE, and Webb, AR. Older adults who spend more time outdoors in summer and have higher dietary vitamin D than younger adults can present at least as high vitamin D status: a pilot study. Int J Environ Res Public Health. (2021) 18:3364. doi: 10.3390/ijerph18073364
61. Płudowski, P, Ducki, C, Konstantynowicz, J, and Jaworski, M. Vitamin D status in Poland. Pol Arch Med Wewn. (2016) 126:530–9. doi: 10.20452/pamw.3479
62. Zheng, S, Yang, JX, Hu, X, Li, M, Wang, Q, Dancer, RCA, et al. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition. Biochem Pharmacol. (2020) 177:113955. doi: 10.1016/j.bcp.2020.113955
63. Sun, J. Vitamin D and mucosal immune function. Curr Opin Gastroenterol. (2010) 26:591–5. doi: 10.1097/MOG.0b013e32833d4b9f
64. Chen, S, Sims, GP, Chen, XX, Gu, YY, Chen, S, and Lipsky, PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. (2007) 179:1634–47. doi: 10.4049/jimmunol.179.3.1634
Keywords: vitamin D supplementation, antibiotic use, infection, respiratory tract infections, vitamin D deficiency, meta-analysis
Citation: Wang M, Wu Y, Xiang Z, Zhang Y, Huang T and Chen B (2024) The effect of vitamin D supplementation on antibiotic use: a meta-analysis based on randomized controlled trials. Front. Nutr. 11:1502835. doi: 10.3389/fnut.2024.1502835
Edited by:
Luigi Di Filippo, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Sara Menotti, Vita-Salute San Raffaele University, ItalyUmberto Terenzi, Vita-Salute San Raffaele University, Italy
Copyright © 2024 Wang, Wu, Xiang, Zhang, Huang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bangsheng Chen, Y2JzMTEzQDEyNi5jb20=
 Mian Wang1
Mian Wang1 Bangsheng Chen
Bangsheng Chen