- Department of Bioscience and Technology for Food, Agriculture and Environment, University of Teramo, Teramo, Italy
Fermented foods have regained popularity in Western diets for their health-promoting potential, mainly related to the role of lactic acid bacteria (LAB) during the fermentation process. Nowadays, there is an increasing demand for vegetable-based fermented foods, representing an environmentally sustainable options to overcome the limitations of lactose intolerance, vegetarian, or cholesterol-restricted diets. Among them, table olives and their co-products (i.e., olive pomace) represent important plant-origin matrices, whose exploitation is still limited. Olives are an important source of fiber and bioactive molecules such as phenolic compounds with recognized health-promoting effects. Based on that, this minireview offer a brief overview about the potential beneficial role of fermented table olives/olive pomace, with a particular focus on the role of LAB to obtain healthy and/or probiotic-enriched fermented foods.
1 Introduction
Fermented foods have been a part of human diets for centuries, with the knowledge and practices being passed down through generations (1) and still today, fermentation represents one of the most used biotechnological processes for the biotransformation of raw materials into nutritious, palatable and organoleptically satisfying products (2). Recently, fermented foods have been scientifically defined as “those foods or beverages made through controlled microbial growth and enzymatic conversions of major and minor food components” (3). Almost all populations have applied the fermentation process to vegetal (such as fruits, seeds, tubers, and other materials) and animal matrices (such as eggs, fish, meat, and milk) (4). Fermentation is most often accomplished with selected microorganisms, mainly lactic acid bacteria (LAB), that when added to the substrate as starter cultures produce lactic acid and, eventually, other organic acids such as acetic acid, besides ethanol and carbon dioxide, lowering the pH of food matrix which, in its turn, contributes to the safety and shelf-life extension of the final product (5). Specifically, LAB improve food stability via physical and biochemical changes in fermented foods, contributing also to their distinctive taste, flavor and texture (6, 7).
In addition, LAB metabolic activities are associated with production of many beneficial compounds such as polyols, exopolysaccharides and antimicrobial compounds (i.e., bacteriocins) (8). Indeed, many LAB strains have been studied for their health-promoting properties and recognized as potential probiotics based on well-established criteria (9, 10). LAB fermentation can improve the overall functionality of the food itself by the production of secondary bioactive metabolites (11), which can increase the nutritional value of the food and confer health benefits, showing to be able to modify gut barrier function and intestinal microbiota in a positive way to prevent and/or treat various metabolic and inflammatory diseases (12, 13). Moreover, during fermentation LAB can act synergistically with dietary fibers and/or non-fiber substances, such as polyphenols, in conferring also prebiotic effects, namely providing beneficial effect through a microbiota-mediated mechanism. Indeed, the current scientific definition developed by ISAPP in 2016 of a prebiotic as a “substrate that is selectively utilized by host microorganisms conferring a health benefit,” clarify the need to selectively elicit a limited group of microorganisms in the host rather than the entire microbial ecosystem (14). Based on that, it has been suggested that fermented foods should be included as part of national dietary recommendations for their outstanding role in human nutrition (3). Moreover, the wide variety of raw material-microbe combinations results in a multitude of different fermented foods and beverages and the expanding range of vegetable-based matrices has spurred new research into plant products as matrices for the production of fermented and probiotic products (5). In addition, plant-based fermented products can also be consumed by people with lactose intolerance and milk allergies (15).
Furthermore, there is a growing attention among the population for a more sustainable diet (i.e., vegetarianism, veganism, and flexitarianism) and for consuming low-cholesterol foods (16), and at the same time in line with the Mediterranean diet, associated with a reduced risk of developing chronic diseases and a longer life expectancy. Among the most popular plant-derived fermented products, table olives are one of the oldest fermented vegetable foods in the Mediterranean area, with a millenary tradition and a significant economic importance due to their high appreciation by consumers. Besides that, fermented table olives also represent an important fermented food included in the Mediterranean diet (17), whose guidelines suggest the daily consumption of 1–2 portions of olives, seeds and nuts as healthy snacks (18). The high nutritional value of table olives is mainly due to the presence of several compounds with biological and functional value (2, 19, 20), and the nutritional composition is mainly influenced by several factors, including agronomical factors, type of cultivar and the ripening stage of the drupes as well as the fermentation and the storage processes (17, 21, 22). In general, the main constituents of olive pulp are water (60–75%) and lipids (10–25%). More in details, lipids are the most important fraction in contributing to the nutritional value of table olives, and most representative are triglycerides, combined with a small amount of sterols, fatty and triterpenic alcohols (23). In particular, table olives’ lipids are characterized by a high level of unsaturated fatty acids, as monounsaturated fatty acids (MUFA) (i.e., oleic acid), polyunsaturated fatty acids (PUFA) as linoleic acid and saturated fats (i.e., palmitic acid, steric acid) at lower level (23).
Soluble reducing and non-reducing sugars, such as glucose, fructose, galactose, mannitol and sucrose, are present in the raw olive pulp (3–6%), but they result almost absent in fermented table olives, due to their solubilization during washing step and their transformation during fermentation and storage in brine. The overall high nutrition value of table olives is also due to the presence of all essential amino acids, even though total proteins are very low (21).
Moreover, table olives can be considered a reservoir of dietary fiber (2.5–5%), mainly pectin, hemicelluloses, cellulose and lignin, and in small amount table olives provide also vitamins such as α-tocopherol, β-carotene, vitamin B complex (i.e., thiamin, niacin, pantothenic acid, vitamin B6), minerals and microelements (24).
Besides nutrients table olives contain high amount of phenolic compounds, with demonstrated biological and functional value. They include flavonols (i.e., quercetin-3-rutinoside); flavones (luteolin and apigenin glucosides), anthocyanins (cyanidin-3-O-glucoside), phenolic acids (5-O-caffeoylquinic acid), phenolic alcohols (i.e., tyrosol, hydroxytyrosol), secoiridoids (i.e., oleuropein, demethyloleuropein) and a hydroxycinnamic acid derivative (verbascoside). Although their composition depends on the olive variety, during fermentation processes the phenolic composition of table olives changed. Depending on the method, phenols can diffuse in the brine, or they were hydrolyzed. In particular, hydroxytyrosol and tyrosol result the major phenols in the fermented product with a significant reduction of secoridoids (oleuropein, demethyloleuropein) and their aglycon derivatives (3,4-DHPEA-EDA) in the olive pulp mainly due to microbial enzymatic activities while the decrease of verbascoside, is related to its release in the brine after the process (22, 24, 25). Oleuropein, hydroxytyrosol and tyrosol are considered the most natural and powerful antioxidants and their biological role, related on the protective role against oxidative stress, lipoprotein metabolism, inflammation and blood pressure has been widely assessed (17, 24).
A recent pilot study based on the administration of 12 green table olives (cv. Nocellara del Belice) per day for 30 days in healthy adult subjects demonstrated an anti-inflammatory and antioxidant effects related to the content of polyphenols and monounsaturated oleic acid, suggesting a clear nutraceutical potential of this food (26).
In addition, the Mediterranean Diet promotes the centrality of the consumption of extra-virgin olive oil, whose production generates high amount of olive pomace as a main co-product, still very rich in monounsaturated fatty acids, and polyphenols and their derivatives (i.e., hydroxytyrosol, tyrosol, oleuropein, hydroxytyrosol glucoside, etc.) (27–29), whose role in disease prevention has been recently recognized with a claim by the European Food Safety Authority (EFSA) for their potential health benefits, mainly in reducing oxidative stress and inflammation (30), boosting the research interest on investigating their potential health benefits (27–29, 31–34).
Although the huge health potential of these food matrices, their application as vegetable-based functional foods is still limited. Although olive oil and its nutritional and non-nutritional components have been extensively investigated for their effects on human health, regarding fermented table olives there is a lack in in vitro studies (17) and only few studies have investigated the beneficial effects of table olives and olive pomace as fermented foods in vivo (31, 35) in either animal or human trials. With that in mind, this mini-review provides a brief overview about the potential beneficial role of fermented table olives/olive pomace as functional foods, with a particular focus on the role of LAB to obtain healthy and/or probiotic-enriched fermented foods.
2 Table olives and olive pomace as vehicle for probiotics
Diet may represent a vehicle of exogenous microorganisms with positive effects on human health, and probiotics and prebiotics are the most used strategies for this purpose. Generally, probiotics are bacteria isolated from human sources, mostly from the gastro-intestinal tract, and are defined as “live microorganisms which, when administered in adequate amounts, as part of a food or a supplement, confer a health benefit on the host” (2).
Several studies have showed how native strains isolated from table olives, mainly belonging to Lpb. plantarum and Lacticaseibacillus casei taxonomic groups also possess specific probiotic traits and showed adhesion and co-aggregation capabilities to form biofilms on the surface of the fruit (36, 37). This could be considered beneficial because their presence appeared to effectively inhibit the adhesion of undesirable microorganisms during storage (38).
Scientific evidence has been reported on the genetic features of LAB strain adhesion on olive surfaces.
Different studies revealed that genes enoA1, gpi and obaC were necessary in Lpb. pentosus to form an organized biofilm on the olive skin during fermentation (39), as well as the presence of four moonlighting proteins over-produced in Lpb. pentosus strains isolated from green table olives (38).
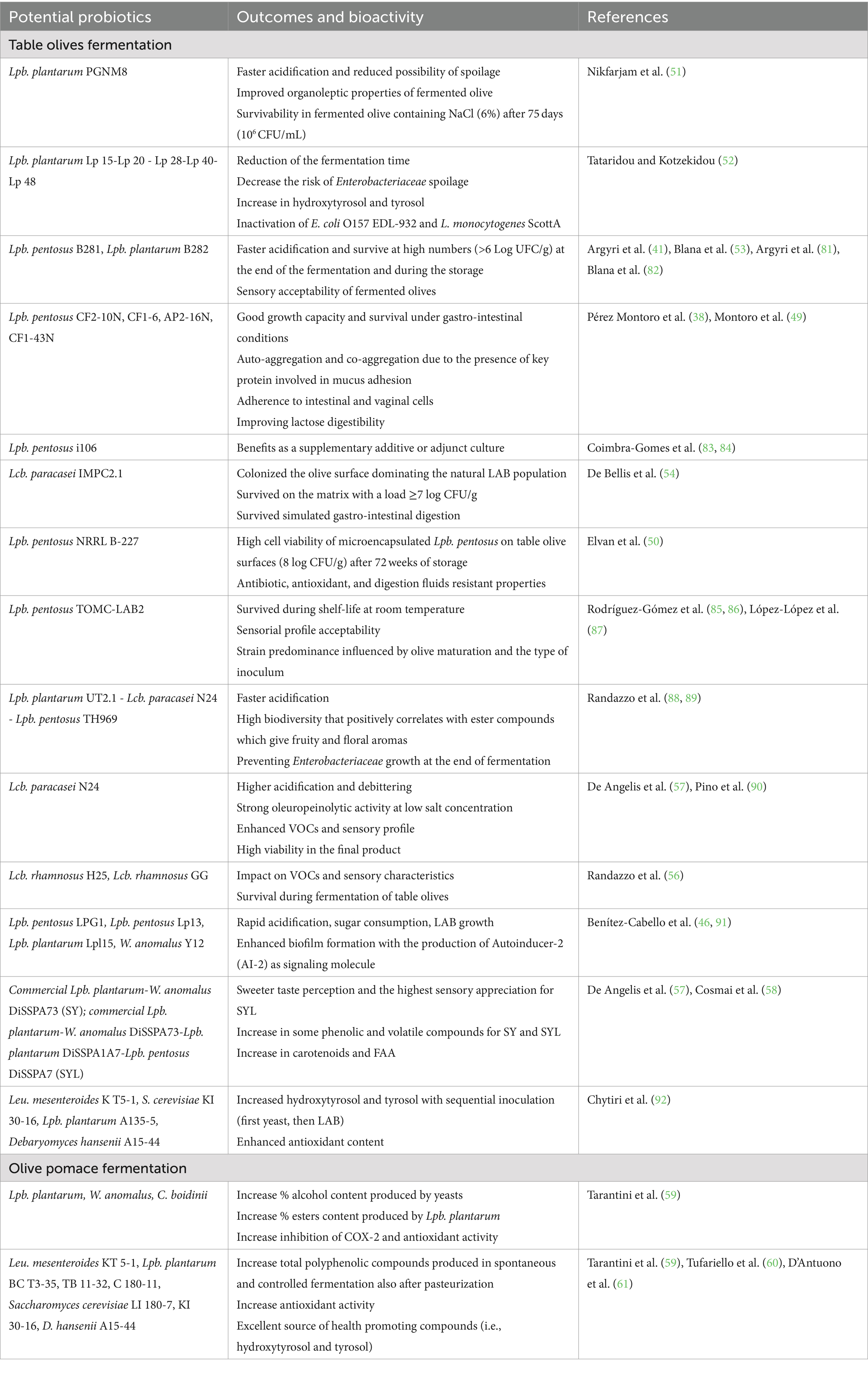
Therefore, the above mentioned properties turn table olives into valid carriers of potential probiotics (2, 38, 40–48). In fact, over the last 10 years, an increasingly growing number of experimental studies have emerged to evaluate the suitability of LAB probiotic strains to be inoculated during the fermentation (Table 1). Montoro et al. (49) have investigated the probiotic potential of 31 different Lpb. pentosus strains, which exhibited good growth capacity and survival under gastro-intestinal conditions, adherence to intestinal and vaginal cells, auto-aggregation and co-aggregation due to the presence of key protein involved in mucus adhesion. In addition, these microorganisms showed the capacity to improve lactose digestibility (38, 49). Other study revealed high cell viability on table olive surfaces of microencapsulated Lpb. pentosus strain that also exhibits antioxidative ability, antibiotic resistance and survivability after simulated digestion (50). Nikfarjam et al. (51) have demonstrated that the using of Lpb. plantarum PGNM8 in fermented olive as a starter with probiotic potential improved remarkably organoleptic properties of the product as well as had suitable survival in fermented olive containing NaCl (6%) after 75 days (106 CFU/mL) (51).
In their study, Tataridou and Kotzekidou (52) have used oleuropeinolytic strains of the Lpb. plantarum group as both the debittering agent of black and green olives. The results show the ability of these strains to increase biophenols, especially hydroxytyrosol and tyrosol, and inactivate the pathogens Escherichia coli O157 EDL-932 and Listeria monocytogenes ScottA (52).
Other researchers evaluated the performance of Lpb. plantarum and Lpb. pentosus strains co-inoculated in olive fermentations. Specifically, the probiotic strains, Lpb. pentosus B281 and Lpb. plantarum B282, could be considered also promising starter cultures to produce a high added value final product with improved sensory profile and probiotic potential. Among the two strains, Lpb. pentosus B281 was able to survive better in the different salt levels employed in fermentation and to colonize the olive surface at higher concentration compared to Lpb. plantarum B282, dominating even in the case of mixed inoculum (41, 53).
Among others, Lacticaseibacillus paracasei strains are assessed for their potential probiotic role in olive fermentations, also because this species has a close taxonomical relationship with Lcb. casei, involved in the natural fermentation of table olives (54). Indeed, De Bellis et al. (54) have investigated the dynamics of microbial populations adhering on the surface of debittered green olives cultivar cv. Bella di Cerignola combined with the probiotic strain Lcb. paracasei IMPC2.1 during the fermentation. The results illustrated a successfully colonization of the olive surface dominating the natural LAB population, establishing an accelerate fermentation process and reducing the survival period of potential spoilage microorganisms.
Moreover, related works concern the addition of Lcp. paracasei N24 strain in Sicilian table olives and its persistence after fermentation. This strain shows high acidification and debittering rate, strong oleuropeinolytic activity at low salt concentration and increased volatile organic compounds (VOCs), contributing to more pleasant flavors (54, 55).
Among the different species that predominate in table olives, Lcb. rhamnosus is not commonly found, but in their study Randazzo et al. (56) demonstrated that two probiotic Lcb. rhamnosus strains were able to survive during fermentation, even if their main ecological niche is dairy products. The presence of Lcb. rhamnosus at the end of the process is therefore attributed to the artificial addition of probiotics (56).
Recently, it has also been suggested the use of yeasts in combination with LAB to enhance the organoleptic quality and shelf-life of table olives (57, 58).
In their experimental study, De Angelis et al. (57) used different combination of potential probiotic LAB and yeasts with positive effects on the taste perception and sensory profile of table olives and olive pomace. The results highlight an increase in some volatile organic compounds, in carotenoids and free amino acids (FAA) (57, 58). Other studies applied a combined inoculum of LAB and yeasts to ferment table olives with beneficial impact on health-promoting compounds, antioxidant activity and total polyphenolic content (59–61).
Concerning by-products of olive oil process, Foti et al. (62) fermented the pâté olive cake with different microbial strains (Lpb. plantarum, Wickerhamomyces anomalus and Candida boidinii) obtaining an improvement of the VOCs profiles and the biological activity, as the antioxidant and anti-inflammatory potential. In particular, Lpb. plantarum positively affect the ester concentration whereas the yeasts mostly affect the alcohols (62).
Thus, all this experimental evidence suggests that the application of microorganisms characterized by probiotic properties could significantly improve the functional characteristics of table olives and their derivatives, conferring greater health benefits, opening a novel scenario on the development of functional/probiotic table olives and related matrices as healthy vegetable-based fermented foods.
3 Olive’s health benefits and LAB
The use of plant-based matrices and LAB in the production of fermented foods is an ancient tradition that is now being supported by scientific understanding and technological advancement (63).
Some plant-based matrices present undesirable properties that LAB species have proven to ameliorate by their fermenting action. In particular, olives and their co-products (i.e., olive pomace) contain high amount of bitter secoiridoids that must be hydrolyzed before consumption in order to reduce and/or remove the olives bitterness and, in turn achieve acceptable palatability. Following IOC standard procedures (64) olive debittering can be carried out through chemical hydrolysis by adding alkalin solution (2.5–3% w/v) followed by spontaneous fermentation (3–7 months) driven by LAB (Spanish-style method) (65, 66) or in the Greek-style method, the bitterness is removed by the enzymatic activities of the olives microbiota combined with the diffusion of polyphenols into a saline brine (6–10% w/v) in which olives are directly soaked, during a long spontaneous fermentation (8–12 months) driven by LAB and yeast (65).
More recently, the biotransformation using selected LAB as starter cultures with oleuropenolytic activity to drive olives and olive pomace fermentation has emerged as a valid and sustainable process, being highly recommended for table olives fermentation (2). The careful selection of microbial strains with inherent desirable characteristics has a critical impact on a predictable and reproducible improvement of the different quality and nutritional attributes of fermented products (27, 28). In fact, an appropriate inoculum (usually 6–7 log CFU/ml) reduces the phenomena of spoilage by deteriorating microorganisms, inhibits the growth of pathogenic microbes and contributes to obtain a controlled and standardized process, improving the sensorial and hygienic quality of the final product and significantly reducing fermentation times (2). LAB starter cultures are usually represented by a single strain or by a mixture of strains in a limited number selected based on their technological aptitude and their suitability to be cultivated, and subsequently used as an inoculum to accelerate and improve the fermentation process (17). The use of selected starter cultures, significantly improve the fermentation process, allowing to overcome unexpected outcomes due to the variability of spontaneous fermentation that occurs in both Spanish-style and Greek-style method (2, 22, 65).
Among LAB, Lactiplantibacillus (Lpb.) pentosus and Lpb. plantarum are the most characterized and used species as starter cultures, as a single inoculum or in combination with other bacterial or yeast species to drive the biological debittering process of olives (2). The use of these species is mainly due to their enzymatic (β-glucosidase and esterase) activities (67), able to degrade oleouropein and, in turn, release elenolic acid and hydroxytyrosol (65), recognized as beneficial compound against oxidative stress and inflammation (30), to their high survival capacity in the fermentation environment (low pH and high concentrations of salts), which allows, thanks to a greater rapidity of growth, to quickly dominate the endogenous microbiota present in the fermentation brines. Furthermore, these species are equipped with additional specific enzymatic activities to produce volatile compounds which contribute to the development of the sensorial characteristics of the product (68). Moreover, the LAB-mediated fermentation process can enhance the nutritional and functional value of the raw foods, by the production of a high portion of other beneficial substances such as phenolic acids, bioactive peptides and short-chain fatty acids (SCFAs), among others (69).
Several studies reported that the beneficial properties (i.e., antioxidant and anti-inflammatory activity) in plant-based matrices can be increased by using Lpb. plantarum, alone or in combination with other LAB, in the fermentation process (32–35, 63, 65, 67, 69, 70).
Moreover, the application of bacteria characterized by probiotic properties could significantly improve the functional characteristics of table olives and their derivatives, conferring greater health benefits.
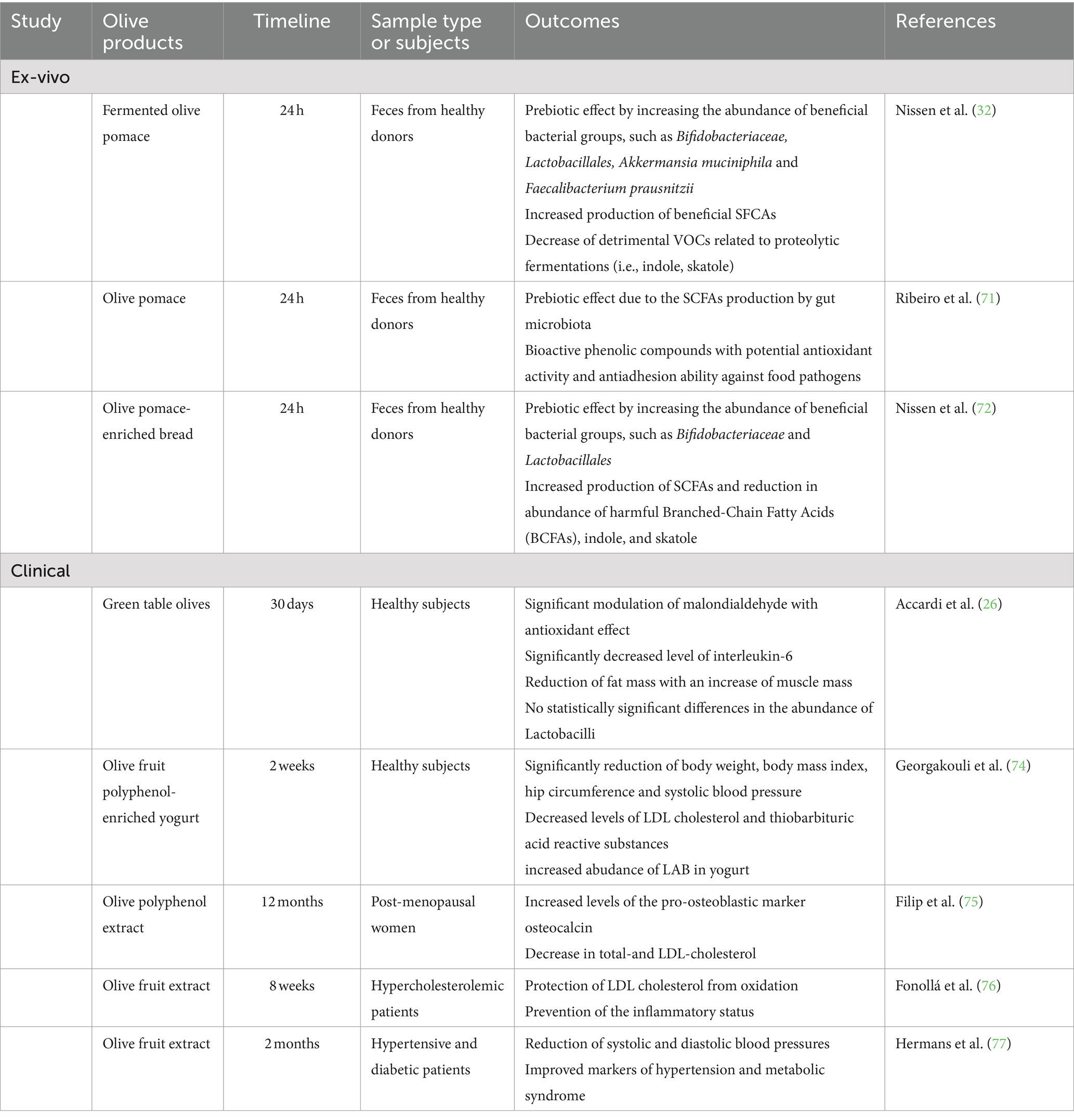
In our latest research, we have shown that the combined oral administration of a diet enriched with biologically debittered olive pomace and a specific probiotic strain (Lpb. plantarum IMC513) has a synergistic impact with anti-inflammatory and antifibrotic effects in a DSS-induced chronic colitis mice model (31). In addition, biologically debittered olive patè enriched with Lpb. plantarum with probiotics features showed to positively modulate intestinal human microbiota with a clear prebiotic effect (by increasing the abudance of Lactobacillales, Bifidobacteriaceae, Akkermansia municiphila and Faecalibacterium prausnitzii) and a positive correlation with the increased production of beneficial metabolites (i.e., SCFAs) in an ex-vivo gut model, confirming olive patè to be a good matrix for deliver beneficial microbes and, in turn, for the development of innovative fermented functional foods (32).
A similar ex-vivo fecal fermentation model has been also used by Ribeiro et al. (71) showing gastro-intestinal health benefits and prebiotic effects of olive pomace powders related to the stimulation of SCFAs production by gut microbiota as well as to asses the prebiotc role of olive pomace-enriched bread by Nissen et al. (72).
Similar prebiotic effect has been confirmed also in vivo in spontaneously hypertensive rats, after 7 weeks of daily intake of Arbequina table olives subjected to natural fermentation (73). The consumption of table olives promoted selectively the growth of intestinal beneficial microbes, such as Lactobacillus spp., Bifidobacterium spp. and Akkermansia muciniphila among others, with a related reduction in plasmatic concentrations of malondialdehyde and angiotensin II, demonstrating a clear antihypertensive activity (73), mainly due to the high amount of bioactive compounds, such as polyphenols delivered by table olives.
Olive polyphenols have been used to enrich yogurt, demonstrating a clear impact on body weight, body mass index, blood pressure, low density lipoprotein (LDL) cholesterol as well as a prebiotic effect by selectively increasing LAB population after 2 weeks of daily intake in a randomized, double-blind, placebo-controlled, crossover trial (74). This study indicates that 50 mg/day of olive polyphenols can help in decreasing LDL cholesterol and lipid peroxidation in healthy subjects.
Previously, a long term (12 months) administration of an olive polyphenol extract (Bonolive®) showed to improve lipid profile by decreasing total and LDL cholesterol in a double blind, randomized trial involving postmenopausal women (75). Hydroxytyrosol from olives fruit has been investigated for its potential cardioprotective effects in hypercholesterolemic (76) and hypertensive patients (77) in two pilot studies, confirming the positive effects of olives bioactive compounds in reducing LDL and preventing oxidation and inflammation, suggesting a promising use in the clinical management of hypercholesterolemic, hypertensive and metabolic disease.
More recently, the potential therapeutic role of these bioactive compounds, due to their antioxidant and anti-inflammatory properties, is emerging also as a potential strategy in the management of irritable bowel syndrome (IBD), for which successful and resolutive therapies are still missing (78). Up to now, experimental evidence are limited to intestinal cells and IBD-animal models that have indicated promising beneficial effects of olive polyphenols, (i.e., hydroxytyrosl, oleuropein) in decreasing pro-inflammatory cytokines (i.e., IL-1β, IL-6, IL-8, IL-17, TNF-α) and triggering NF-κB signaling and p38 MAPK pathway (68, 79, 80).
Based on that, the combination of bioactive compounds and beneficial microbes delivered by table olives and olive pomace can provide to the olive industry a new image of table olives and olive cream as health-promoting fermented foods with high nutraceutical value, with promising therapeutical application even though clinical evidence remain still limited (Table 2), and more well-designed randomized clinical trials are needed to confirm these beneficial effects.
4 Conclusion/future perspective
Consumers are increasingly interested in a healthy diet, mainly by decreasing their dietary intake of high-fat and animal-based food products, due to health, sustainability and ethical concerns. Plant-based matrices are in line with those consumers’ needs, and among them, olives represent suitable matrices to produce healthy innovative and environmentally sustainable fermented foods. Table olives represent an important fermented food included in the Mediterranean diet as a source of fiber and bioactive molecules such as phenolic compounds with recognized health-promoting effects.
The transformation of these products through fermentation and the exploitation of the potential combined effects of olive bioactive compounds and olive-associated LAB would lead (1) to overcome limitations on their consumption, (2) to open new market trends by meeting the needs of lactose free, vegan-vegetarian or low-cholesterol diets, (3) to improve adherence to the Mediterranean Diet, despite the constraints of modern society, and (4) to suggest a potential therapeutical application in dietary intervention (i.e., hypercholesterolemia, IBD).
Author contributions
FM: Investigation, Visualization, Writing – original draft, Writing – review & editing. FD: Investigation, Visualization, Writing – original draft. RP: Conceptualization, Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AC: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the European Union—Next Generation EU. Project Code: ECS00000041; Project CUP: C43C22000380007; Project Title: Innovation, digitalization and sustainability for the diffused economy in Central Italy—VITALITY.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Melini, F, Melini, V, Luziatelli, F, Ficca, AG, and Ruzzi, M. Health-promoting components in fermented foods: an up-to-date systematic review. Nutrients. (2019) 11:1189. doi: 10.3390/nu11051189
2. Perpetuini, G, Prete, R, Garcia-Gonzalez, N, Khairul Alam, M, and Corsetti, A. Table olives more than a fermented food. Food Secur. (2020) 9:178. doi: 10.3390/foods9020178
3. Marco, ML, Sanders, ME, Gänzle, M, Arrieta, MC, Cotter, PD, De Vuyst, L, et al. The international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat Rev Gastroenterol Hepatol. (2021) 18:196–208. doi: 10.1038/s41575-020-00390-5
4. Shah, AM, Tarfeen, N, Mohamed, H, and Song, Y. Fermented foods: their health-promoting components and potential effects on gut microbiota. Fermentation. (2023) 9:118. doi: 10.3390/fermentation9020118
5. Cichońska, P, and Ziarno, M. Legumes and legume-based beverages fermented with lactic acid bacteria as a potential carrier of probiotics and prebiotics. Microorganisms. (2022) 10:91. doi: 10.3390/microorganisms10010091
6. Frías, J, Martinez-Villaluenga, C, and Peñas, E. Fermented foods in health and disease prevention. Amsterdam, The Netherlands: Academic Press, Elsevier (2016).
7. Sharma, R, Garg, P, Kumar, P, Bhatia, SK, and Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation. (2020) 6:106. doi: 10.3390/fermentation6040106
8. Bintsis, T . Lactic acid bacteria as starter cultures: an update in their metabolism and genetics. AIMS Microbiol. (2018) 4:665–84. doi: 10.3934/microbiol.2018.4.665
9. Hill, C, Guarner, F, Reid, G, Gibson, GR, Merenstein, DJ, Pot, B, et al. Expert consensus document. The international scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66
10. De Filippis, F, Pasolli, E, and Ercolini, D. The food-gut axis: lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol Rev. (2020) 44:454–89. doi: 10.1093/femsre/fuaa015
11. Salminen, S, Collado, MC, Endo, A, Hill, C, Lebeer, S, Quigley, EMM, et al. The international scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. (2021) 18:649–67. doi: 10.1038/s41575-021-00440-6
12. Gille, D, Schmid, A, Walther, B, and Vergères, G. Fermented food and non-communicable chronic diseases: a review. Nutrients. (2018) 10:448. doi: 10.3390/nu10040448
13. Dimidi, E, Cox, SR, Rossi, M, and Whelan, K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. (2019) 11:1806. doi: 10.3390/nu11081806
14. Gibson, GR, Hutkins, R, Sanders, ME, Prescott, SL, Reimer, RA, Salminen, SJ, et al. Expert consensus document: the international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. (2017) 14:491–502. doi: 10.1038/nrgastro.2017.75
15. Sethi, S, Tyagi, SK, and Anurag, RK. Plant-based milk alternatives an emerging segment of functional beverages: a review. J Food Sci Technol. (2016) 53:3408–23. doi: 10.1007/s13197-016-2328-3
16. Hidalgo-Fuentes, B, De Jesús-José, E, Cabrera-Hidalgo, AJ, Sandoval-Castilla, O, Espinosa-Solares, T, González-Reza, RM, et al. Plant-based fermented beverages: nutritional composition, sensory properties, and health benefits. Food Secur. (2024) 13:844. doi: 10.3390/foods13060844
17. Rocha, J, Borges, N, and Pinho, O. Table olives and health: a review. J Nutr Sci. (2020) 9:e57. doi: 10.1017/jns.2020.50
18. Bach-Faig, A, Wickramasinghe, KK, Panadero, N, Fàbregues, S, Rippin, H, Halloran, A, et al. Dietary patterns for health and sustainability’ (DPHS). Project expert, dietary patterns for health and sustainability dietary patterns for health and sustainability: From experts’ opinions to action for the WHO European region. SSRN Prepr Lancet. (2021). doi: 10.2139/ssrn.3805860
19. Bonatsou, S, Tassou, CC, Panagou, EZ, and Nychas, GE. Table olive fermentation using starter cultures with multifunctional potential. Microorganisms. (2017) 5:30. doi: 10.3390/microorganisms5020030
20. De Bellis, P, Sisto, A, and Lavermicocca, P. Probiotic bacteria and plant-based matrices: an association with improved health-promoting features. J Funct Foods. (2021) 87:104821. doi: 10.1016/j.jff.2021.104821
21. López-López, A, Montaño, A, and Garrido-Fernández, A. Nutrient profiles of commercial table olives: fatty acids, sterols, and fatty alcohols In: VR Preedy and RR Watson, editors. Olives and olive oil in health and disease prevention. San Diego: Academic Press (2010). 715–24.
22. Perpetuini, A, Caruso, G, Urbani, S, Schirone, M, Esposto, S, Ciarrocchi, A, et al. Changes in polyphenolic concentrations of table olives (cv. Itrana) produced under different irrigation regimes during spontaneous or inoculated fermentation. Front Microbiol. (2018) 9:1287. doi: 10.3389/fmicb.2018.01287
23. Lanza, B . Nutritional and sensory quality of table olives, olive germplasm – The olive cultivation, table olive and olive oil industry in Italy. Intech Open: Innocenzo Muzzalupo (2005).
24. Boskou, D . Table olives: a vehicle for the delivery of bioactive compounds. J Exp Food Chem. (2017) 03:123. doi: 10.4172/2472-0542.1000123
25. Servili, M, Minnocci, A, Veneziani, G, Taticchi, A, Urbani, S, Esposto, S, et al. Compositional and tissue modifications induced by the natural fermentation process in table olives. J Agric Food Chem. (2008) 56:6389–96. doi: 10.1021/jf8007019
26. Accardi, G, Aiello, A, Gargano, V, Gambino, CM, Caracappa, S, Marineo, S, et al. Nutraceutical effects of table green olives: a pilot study with Nocellara del Belice olives. Immun Ageing. (2016) 13:11. doi: 10.1186/s12979-016-0067-y
27. Cavallo, P, Dini, I, Sepe, I, Galasso, G, Fedele, FL, Sicari, A, et al. An innovative olive pâté with nutraceutical properties. Antioxidants (Basel). (2020) 9:581. doi: 10.3390/antiox9070581
28. Ribeiro, TB, Oliveira, A, Campos, D, Nunes, J, Vicente, AA, and Pintado, M. Simulated digestion of an olive pomace water-soluble ingredient: relationship between the bioaccessibility of compounds and their potential health benefits. Food Funct. (2020) 11:2238–54. doi: 10.1039/C9FO03000J
29. Di Nunzio, M, Picone, G, Pasini, F, Chiarello, E, Caboni, MF, Capozzi, F, et al. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res Int. (2020) 131:108940. doi: 10.1016/j.foodres.2019.108940
30. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) . Scientific opinion on thesubstantiation of a health claim related to polyphenols in olive and maintenance of normal blood HDL-cholesterolconcentrations (ID 1639, further assessment) pursuant to article 13(1) of regulation (EC) no 1924/2006. EFSA J. (2012) 10:2848. doi: 10.2903/j.efsa.2012.2848
31. Vetuschi, A, Battista, N, Pompili, S, Cappariello, A, Prete, R, Taticchi, A, et al. The antiinflammatory and antifibrotic effect of olive phenols and Lactiplantibacillus plantarum IMC513 in dextran sodium sulfate-induced chronic colitis. Nutrition. (2022) 94:111511. doi: 10.1016/j.nut.2021.111511
32. Nissen, L, Prete, R, Casciano, F, Corsetti, A, Battista, N, Veneziani, G, et al. Modulation of human colon microbiota by naturally debittered olive patè enriched with Lactiplantibacillus plantarum in an in vitro intestinal model. LWT. (2024) 198:116014. doi: 10.1016/j.lwt.2024.116014
33. Tufariello, M, Durante, M, Veneziani, G, Taticchi, A, Servili, M, Bleve, G, et al. Patè olive cake: possible exploitation of a by-product for food applications. Front Nutr. (2019) 6:3. doi: 10.3389/fnut.2019.00003
34. Durante, M, Bleve, G, Selvaggini, R, Veneziani, G, Servili, M, and Mita, G. Bioactive compounds and stability of a typical italian bakery products “taralli” enriched with fermented olive paste. Molecules. (2019) 24:3258. doi: 10.3390/molecules24183258
35. Valero-Cases, E, Cerdá-Bernad, D, Pastor, JJ, and Frutos, MJ. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients. (2020) 12:1666. doi: 10.3390/nu12061666
36. Marco, ML, Heeney, D, Binda, S, Cifelli, CJ, Cotter, PD, Foligné, B, et al. Health benefits of fermented foods: microbiota and beyond. Curr Opin Biotechnol. (2017) 44:94–102. doi: 10.1016/j.copbio.2016.11.010
37. Garcia-Gonzalez, N, Battista, N, Prete, R, and Corsetti, A. Health-promoting role of Lactiplantibacillus plantarum isolated from fermented foods. Microorganisms. (2021) 9:349. doi: 10.3390/microorganisms9020349
38. Pérez Montoro, B, Benomar, N, Caballero Gómez, N, Ennahar, S, Horvatovich, P, Knapp, CW, et al. Proteomic analysis of Lactobacillus pentosus for the identification of potential markers of adhesion and other probiotic features. Int Food Res. (2018) 111:58–66. doi: 10.1016/j.foodres.2018.04.072
39. Perpetuini, G, Pham-Hoang, BN, Scornec, H, Tofalo, R, Schirone, M, Suzzi, G, et al. In Lactobacillus pentosus, the olive brine adaptation genes are required for biofilm formation. Int J Food Microbiol. (2016) 216:104–9. doi: 10.1016/j.ijfoodmicro.2015.10.002
40. Bautista-Gallego, J, Moreno-Baquero, JM, Garrido-Fernández, A, and López-López, A. Development of a novel Zn fortified table olive product. LWT Food Sci Technol. (2013) 50:264–71. doi: 10.1016/j.lwt.2012.05.017
41. Argyri, AA, Nisiotou, AA, Mallouchos, A, Panagou, EZ, and Tassou, CC. Performance of two potential probiotic Lactobacillus strains from the olive microbiota as starters in the fermentation of heat shocked green olives. Int J Food Microbiol. (2014) 171:68–76. doi: 10.1016/j.ijfoodmicro.2013.11.003
42. Botta, C, Langerholc, T, Cencič, A, and Cocolin, L. In vitro selection and characterization of new probiotic candidates from table olive microbiota. PLoS One. (2014) 9:94457. doi: 10.1371/journal.pone.0094457
43. Prete, R, Tofalo, R, Federici, E, Ciarrocchi, A, Cenci, G, and Corsetti, A. Food-associated Lactobacillus plantarum and yeasts inhibit the genotoxic effect of 4-nitroquinoline-1-oxide. Front Microbiol. (2017) 8:2349. doi: 10.3389/fmicb.2017.02349
44. Garcia-Gonzalez, N, Prete, R, Battista, N, and Corsetti, A. Adhesion properties of food-associated Lactobacillus plantarum strains on human intestinal epithelial cells and modulation of IL-8 release. Front Microbiol. (2018) 9:2392. doi: 10.3389/fmicb.2018.02392
45. Guantario, B, Zinno, P, Schifano, E, Roselli, M, Perozzi, G, Palleschi, C, et al. In vitro and in vivo selection of potentially probiotic lactobacilli from Nocellara del Belice table olives. Front Microbiol. (2018) 9:9. doi: 10.3389/fmicb.2018.00595
46. Benítez-Cabello, A, Calero-Delgado, B, Rodríguez-Gómez, F, Garrido-Fernández, A, Jiménez-Díaz, R, and Arroyo-López, FN. Biodiversity and multifunctional features of lactic acid bacteria isolated from table olive biofilms. Front Microbiol. (2019) 10:836. doi: 10.3389/fmicb.2019.00836
47. Prete, R, Long, SL, Joyce, SA, and Corsetti, A. Genotypic and phenotypic characterization of food-associated Lactobacillus plantarum isolates for potential probiotic activities. FEMS Microbiol Lett. (2020) 367:fnaa076. doi: 10.1093/femsle/fnaa076
48. Cufaro, MC, Prete, R, Di Marco, F, Sabatini, G, Corsetti, A, Gonzalez, NG, et al. A proteomic insight reveals the role of food-associated Lactiplantibacillus plantarum C9O4 in reverting intestinal inflammation. iScience. (2023) 26:108481. doi: 10.1016/j.isci.2023.108481
49. Montoro, BP, Benomar, N, Lavilla Lerma, L, Castillo Gutiérrez, S, Gálvez, A, and Abriouel, H. Fermented Aloreña table olives as a source of potential probiotic Lactobacillus pentosus strains. Front Microbiol. (2016) 7:7. doi: 10.3389/fmicb.2016.01583
50. Elvan, M, Baysal, AH, and Harsa, S. Microencapsulation of a potential probiotic Lactiplantibacillus pentosus and its impregnation onto table olives. LWT. (2022) 156:112975. doi: 10.1016/j.lwt.2021.112975
51. Nikfarjam, AK, Mahdian, E, Ghavidel, RA, and Karazhyan, R. Probiotic potential of Lactobacillus strains isolated from Iranian traditional pickled garlic and using them in fermented olive. J BioSci Biotechnol. (2021) 10:41–51.
52. Tataridou, M, and Kotzekidou, P. Fermentation of table olives by oleuropeinolytic starter culture in reduced salt brines and inactivation of Escherichia coli O157:H7 and Listeria monocytogenes. Int J Food Microbiol. (2015) 208:122–30. doi: 10.1016/j.ijfoodmicro.2015.06.001
53. Blana, VA, Grounta, A, Tassou, CC, Nychas, GJE, and Panagou, EZ. Inoculated fermentation of green olives with potential probiotic Lactobacillus pentosus and Lactobacillus plantarum starter cultures isolated from industrially fermented olives. Food Microbiol. (2014) 38:208–18. doi: 10.1016/j.fm.2013.09.007
54. De Bellis, P, Valerio, F, Sisto, A, Lonigro, SL, and Lavermicocca, P. Probiotic table olives: microbial populations adhering on olive surface in fermentation sets inoculated with the probiotic strain Lactobacillus paracasei IMPC2.1 in an industrial plant. Int J Food Microbiol. (2010) 140:6–13. doi: 10.1016/j.ijfoodmicro.2010.02.024
55. Pino, A, De Angelis, M, Todaro, A, Van Hoorde, K, Randazzo, CL, and Caggia, C. Fermentation of Nocellara Etnea table olives by functional starter cultures at different low salt concentrations. Front Microbiol. (2018) 9:9. doi: 10.3389/fmicb.2018.01125
56. Randazzo, CL, Todaro, A, Pino, A, Pitino, I, Corona, O, Mazzaglia, A, et al. Giarraffa and Grossa di Spagna naturally fermented table olives: effect of starter and probiotic cultures on chemical, microbiological and sensory traits. Int Food Res. (2014) 62:1154–64. doi: 10.1016/j.foodres.2014.05.056
57. De Angelis, M, Campanella, D, Cosmai, L, Summo, C, Rizzello, CG, and Caponio, F. Microbiota and metabolome of un-started and started Greek-type fermentation of Bella di Cerignola table olives. Food Microbiol. (2015) 52:18–30. doi: 10.1016/j.fm.2015.06.002
58. Cosmai, L, Campanella, D, De Angelis, M, Summo, C, Paradiso, VM, Pasqualone, A, et al. Use of starter cultures for table olives fermentation as possibility to improve the quality of thermally stabilized olive-based paste. LWT. (2018) 90:381–8. doi: 10.1016/j.lwt.2017.12.061
59. Tarantini, A, Crupi, P, Ramires, FA, D’Amico, L, Romano, G, Blando, F, et al. Study of the effects of pasteurization and selected microbial starters on functional traits of fermented table olives. Food Microbiol. (2024) 122:104537. doi: 10.1016/j.fm.2024.104537
60. Tufariello, M, Durante, M, Ramires, FA, Grieco, F, Tommasi, L, Perbellini, E, et al. New process for production of fermented black table olives using selected autochthonous microbial resources. Front Microbiol. (2015) 6:1007. doi: 10.3389/fmicb.2015.01007
61. D’Antuono, I, Bruno, A, Linsalata, V, Minervini, F, Garbetta, A, Tufariello, M, et al. Fermented Apulian table olives: effect of selected microbial starters on polyphenols composition, antioxidant activities and bioaccessibility. Food Chem. (2018) 248:137–45. doi: 10.1016/j.foodchem.2017.12.032
62. Foti, P, Randazzo, CL, Russo, M, Di Sanzo, R, Romeo, FV, Scilimati, A, et al. Effect of microbial fermentation on functional traits and volatiloma profile of pâté olive cake. Food Res Int. (2023) 174:113510. doi: 10.1016/j.foodres.2023.113510
63. Wuyts, S, Van Beeck, W, Allonsius, CN, van den Broek, MF, and Lebeer, S. Applications of plant-based fermented foods and their microbes. COBIOT. (2020) 61:45–52. doi: 10.1016/j.copbio.2019.09.023
64. International Olive Council . Trade standard applying to table olives. (2004). Available at: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/.
65. Corsetti, A, Perpetuini, G, Schirone, M, Tofalo, R, and Suzzi, G. Application of starter cultures to table olive fermentation: an overview on the experimental studies. Front Microbiol. (2012) 3:248. doi: 10.3389/fmicb.2012.00248
66. Arroyo-Lopez, FN, Garcıa-Garcıa, P, Rodrıguez-Gomez, F, and Garrido-Fernandez, A. Olives: types and consumption In: B Caballero, P Finglas, and F Toldrá, editors. Encyclopedia of food and health. Amsterdam, The Netherlands: Academic Press, Elsevier (2016). 167–70.
67. Campus, M, Değirmencioğlu, N, and Comunian, R. Technologies and trends to improve table olive quality and safety. Front Microbiol. (2018) 9:617. doi: 10.3389/fmicb.2018.00617
68. Muto, E, Dell’Agli, M, Sangiovanni, E, Mitro, N, Fumagalli, M, Crestani, M, et al. Olive oil phenolic extract regulates interleukin-8 expression by transcriptional and posttranscriptional mechanisms in Caco-2 cells. Mol Nutr Food Res. (2015) 59:1217–21. doi: 10.1002/mnfr.201400800
69. Bernal-Castro, C, Espinosa-Poveda, E, Gutiérrez-Cortés, C, and Díaz-Moreno, C. Vegetable substrates as an alternative for the inclusion of lactic acid bacteria with probiotic potential in food matrices. J Food Sci Technol. (2024) 61:833–46. doi: 10.1007/s13197-023-05779-z
70. Lorusso, A, Coda, R, Montemurro, M, and Rizzello, CG. Use of selected lactic acid bacteria and quinoa flour for manufacturing novel yogurt-like beverages. Food Secur. (2018) 7:51. doi: 10.3390/foods7040051
71. Ribeiro, TB, Costa, CM, Bonifácio-Lopes, T, Silva, S, Veiga, M, Monforte, AR, et al. Prebiotic effects of olive pomace powders in the gut: in vitro evaluation of the inhibition of adhesion of pathogens, prebiotic and antioxidant effects. Food Hydrocoll. (2021) 112:106312. doi: 10.1016/j.foodhyd.2020.106312
72. Nissen, L, Casciano, F, Chiarello, E, Di Nunzio, M, Bordoni, A, and Gianotti, A. Colonic in vitro model assessment of the prebiotic potential of bread fortified with polyphenols rich olive fiber. Nutrients. (2021) 13:787. doi: 10.3390/nu13030787
73. Gómez-Contreras, A, Franco-Ávila, T, Miró, L, Juan, ME, Moretó, M, and Planas, JM. Dietary intake of table olives exerts antihypertensive effects in association with changes in gut microbiota in spontaneously hypertensive rats. Food Funct. (2023) 14:2793–806. doi: 10.1039/D2FO02928F
74. Georgakouli, K, Mpesios, A, Kouretas, D, Petrotos, K, Mitsagga, C, Giavasis, I, et al. The effects of an olive fruit polyphenol-enriched yogurt on body composition, blood redox status, physiological and metabolic parameters and yogurt microflora. Nutrients. (2016) 8:344. doi: 10.3390/nu8060344
75. Filip, R, Possemiers, S, Heyerick, A, Pinheiro, I, Raszewski, G, Davicco, MJ, et al. Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J Nutr Health Aging. (2015) 19:77–86. doi: 10.1007/s12603-014-0480-x
76. Fonollá, J, Maldonado-Lobón, JA, Luque, R, Rodríguez, C, Bañuelos, Ó, López-Larramendi, JL, et al. Effects of a combination of extracts from olive fruit and almonds skin on oxidative and inflammation markers in hypercholesterolemic subjects: a randomized controlled trial. J Med Food. (2021) 24:479–86. doi: 10.1089/jmf.2020.0088
77. Hermans, MP, Lempereur, P, Salembier, JP, Maes, N, Albert, A, Jansen, O, et al. Supplementation effect of a combination of olive (Olea europea L.) leaf and fruit extracts in the clinical management of hypertension and metabolic syndrome. Antioxidants (Basel). (2020) 9:872. doi: 10.3390/antiox9090872
78. Larussa, T, Imeneo, M, and Luzza, F. Olive tree biophenols in inflammatory bowel disease: when bitter is better. Int J Mol Sci. (2019) 20:1390. doi: 10.3390/ijms20061390
79. Giner, E, Recio, MC, Ríos, JL, and Giner, RM. Oleuropein protects against dextran sodium sulfate-induced chronic colitis in mice. J Nat Prod. (2013) 76:1113–20. doi: 10.1021/np400175b
80. Voltes, A, Bermúdez, A, Rodríguez-Gutiérrez, G, Reyes, ML, Olano, C, Fernández-Bolaños, J, et al. Anti-inflammatory local effect of hydroxytyrosol combined with pectin-alginate and olive oil on trinitrobenzene sulfonic acid-induced colitis in wistar rats. J Investig Surg. (2020) 33:8–14. doi: 10.1080/08941939.2018.1469697
81. Argyri, AA, Nisiotou, AA, Pramateftaki, P, Doulgeraki, AI, Panagou, EZ, and Tassou, CC. Preservation of green table olives fermented with lactic acid bacteria with probiotic potential under modified atmosphere packaging. LWT Food Sci Technol. (2015) 62:783–90. doi: 10.1016/j.lwt.2014.11.046
82. Blana, VA, Polymeneas, N, Tassou, CC, and Panagou, EZ. Survival of potential probiotic lactic acid bacteria on fermented green table olives during packaging in polyethylene pouches at 4 and 20 °C. Food Microbiol. (2016) 53:71–5. doi: 10.1016/j.fm.2015.09.004
83. Coimbra-Gomes, J, Reis, PJM, Tavares, TG, Malcata, FX, and Macedo, AC. Study of lactic acid bacteria biodiversity in fermented Cobrançosa table olives to determine their probiotic potential. Food Secur. (2022) 11:3050. doi: 10.3390/foods11193050
84. Coimbra-Gomes, J, Reis, PJM, Tavares, TG, Faria, MA, Malcata, FX, and Macedo, AC. Evaluating the probiotic potential of lactic acid bacteria implicated in natural fermentation of table olives, cv. Cobrançosa. Molecules. (2023) 28:3285. doi: 10.3390/molecules28083285
85. Rodríguez-Gómez, F, Romero-Gil, V, García-García, P, Garrido-Fernández, A, and Arroyo-López, FN. Fortification of table olive packing with the potential probiotic bacteria Lactobacillus pentosus TOMC-LAB2. Front Microbiol. (2014) 5:5. doi: 10.3389/fmicb.2014.00467
86. Rodríguez-Gómez, F, Romero-Gil, V, Arroyo-López, FN, Roldán-Reyes, JC, Torres-Gallardo, R, Bautista-Gallego, J, et al. Assessing the challenges in the application of potential probiotic lactic acid bacteria in the large-scale fermentation of spanish-style table olives. Front Microbiol. (2017) 8:8. doi: 10.3389/fmicb.2017.00915
87. López-López, A, Moreno-Baquero, JM, Rodríguez-Gómez, F, García-García, P, and Garrido-Fernández, A. Sensory assessment by consumers of traditional and potentially probiotic green Spanish-style table olives. Front Nutr. (2018) 5:5. doi: 10.3389/fnut.2018.00053
88. Randazzo, CL, Todaro, A, Pino, A, Pitino, I, Corona, O, and Caggia, C. Microbiota and metabolome during controlled and spontaneous fermentation of Nocellara Etnea table olives. Food Microbiol. (2017) 65:136–48. doi: 10.1016/j.fm.2017.01.022
89. Randazzo, CL, Russo, N, Pino, A, Mazzaglia, A, Ferrante, M, Conti, GO, et al. Effects of selected bacterial cultures on safety and sensory traits of Nocellara Etnea olives produced at large factory scale. FCT. (2018) 115:491–8. doi: 10.1016/j.fct.2018.03.045
90. Pino, A, Vaccalluzzo, A, Solieri, L, Romeo, FV, Todaro, A, Caggia, C, et al. Effect of sequential inoculum of beta-glucosidase positive and probiotic strains on brine fermentation to obtain low salt Sicilian table olives. Front Microbiol. (2019) 10:10. doi: 10.3389/fmicb.2019.00174
91. Benítez-Cabello, A, Calero-Delgado, B, Rodríguez-Gómez, F, Bautista-Gallego, J, Garrido-Fernández, A, Jiménez-Díaz, R, et al. The use of multifunctional yeast-lactobacilli starter cultures improves fermentation performance of Spanish-style green table olives. Food Microbiol. (2020) 91:103497. doi: 10.1016/j.fm.2020.103497
92. Chytiri, A, Tasioula-Margari, M, Bleve, G, Kontogianni, VG, Kallimanis, A, and Kontominas, MG. Effect of different inoculation strategies of selected yeast and LAB cultures on Conservolea and Kalamàta table olives considering phenol content, texture, and sensory attributes. JSFAAE. (2020) 100:926–35. doi: 10.1002/jsfa.10019
Keywords: lactic acid bacteria, fermented foods, table olives, olive pomace, polyphenols, prebiotic effects, health benefits
Citation: Montagano F, Dell’Orco F, Prete R and Corsetti A (2024) Health benefits of fermented olives, olive pomace and their polyphenols: a focus on the role of lactic acid bacteria. Front. Nutr. 11:1467724. doi: 10.3389/fnut.2024.1467724
Edited by:
Zhuqing Dai, Jiangsu Academy of Agricultural Sciences (JAAS), ChinaReviewed by:
Elena Franciosi, Fondazione Edmund Mach, ItalyCopyright © 2024 Montagano, Dell’Orco, Prete and Corsetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberta Prete, cnByZXRlQHVuaXRlLml0
 Federica Montagano
Federica Montagano Francesca Dell’Orco
Francesca Dell’Orco Roberta Prete
Roberta Prete Aldo Corsetti
Aldo Corsetti