
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 13 November 2024
Sec. Nutrition and Metabolism
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1439738
This article is part of the Research Topic The Model of Ramadan Diurnal Intermittent Fasting: Unraveling the Health Implications - Volume 3 View all 14 articles
 Raoua Triki1,2
Raoua Triki1,2 Abderraouf Ben Abderrahman1,2
Abderraouf Ben Abderrahman1,2 Iyed Salhi1,2
Iyed Salhi1,2 Fatma Rhibi3
Fatma Rhibi3 Ayoub Saeidi4*‡
Ayoub Saeidi4*‡ Abdullah Almaqhawi5
Abdullah Almaqhawi5 Anthony C. Hackney6
Anthony C. Hackney6 Ismail Laher7
Ismail Laher7 Urs Granacher8*†‡
Urs Granacher8*†‡ Hassane Zouhal3,9*†‡
Hassane Zouhal3,9*†‡Objectives: We investigated the timing of resistance training (RT) during Ramadan fasting (RF) on muscle strength, hormonal adaptations, and sleep quality.
Methods: Forty healthy and physically active male Muslims (age = 25.7 ± 5.6 years, body mass = 85.1 ± 17.5 kg, height = 175 ± 9 cm, BMI = 28.3 ± 5.7 kg/m2) were enrolled in this study and 37 completed pre and post-tests. Subjects were randomly allocated into two experimental groups. Group 1 (FAST, n = 20) completed an 8-week whole-body RT in the late afternoon (between 16 h and 18 h) while fasting. Group 2 (FED, n = 20) completed the similar RT protocol compared with FAST at night (between 20 h and 22 h). The following parameters were analyzed at various time-points: 2 weeks before the start of RF (T0), on the 15th day of Ramadan (T1), on the 29th day of Ramadan (T2), and 21 days after the last day of RF (T3) where both groups were in a fed state. One-repetition maximum tests (1-RM) were conducted for the squats (1-RMSQ), the deadlift (1-RMDL) and the bench press (1-RMBP). Sleep quality was assessed using the full Pittsburgh Sleep Quality Index (PSQI). Blood samples were taken to determine cortisol, testosterone and IGF-1 levels. Additionally, acute hormonal responses were evaluated before (BF), immediately after (AF), and 30 min after a RT session (AF-30 min) at T0, T1, T2, and T3.
Results: Significant group-by-time interactions were identified for 1-RMSQ (p = 0.001; effect size [ES] = 0.43) and 1-RMDL (p = 0.001; ES = 0.36). Post-hoc tests indicated significant 1-RMSQ (p = 0.03; ES = 0.12) and 1-RMDL (p = 0.04; ES = 0.21) improvements from T0-T2 for FED. Additionally, significant group-by-time interactions were observed for the chronic effects on cortisol (p = 0.03; ES = 0.27) and testosterone levels (p = 0.01; ES = 0.32). Post-hoc tests indicated significant increases of cortisol levels among FAST at T1 and T2 compared to T0 (p = 0.05; ES = 0.41, p = 0.03; ES = 0.34) and a significant increase in cortisol levels in FED at T1 (p = 0.05; ES = 0.29) and T2 (p = 0.04; ES = 0.25). However, the observed increase was lower compared to FAST. Post-hoc tests also indicated significant increases of testosterone only among FED at T2 (p = 0.04; ES = 0.31). A significant group-by-time interaction was found for the acute effect of exercise on cortisol level (p = 0.04; ES = 0.34). The cortisol level immediately after RT was higher in FAST only at T1 (p = 0.03; ES = 0.39) and T2 (p = 0.05; ES = 0.22) compared with T0. No significant group-by-time interactions were identified for sleep quality (p = 0.07; ES = 0.43).
Conclusion: Muslims can safely practice RT during RF. However, training in a fed state during Ramadan might be more effective than during fasted state for the enhancement of maximal strength with better hormonal responses observed.
More than 1.9 billion healthy Muslims worldwide fast intermittently during the Ramadan month (RF) as one of the fundamental obligations of the Muslim faith according to the Pew Research Center (1). During this religious month, Muslims abstain from any type of food and fluid intake, engaging in sexual activities, and smoking from dawn (suhoor) to sunset (iftar) (2, 3). Thus, consuming food and drinks during RF is restricted to the hours of darkness (4). Additionally, the timing of meals and caloric restriction have previously been associated with changes in physiological, chronobiological, and social behaviors (5–8). Hormonal secretions (catecholamines, steroids, growth, and gut hormones) and sleep–wakefulness patterns are modified by circadian changes in food intake and social habits during RF (3, 9, 10).
The effect of engaging in sports during RF was underlined during the London Olympic Games 2012 and also the FIFA Soccer World Cup 2014, as they staged during the RF (11, 12). Some studies reported that training during RF is an effective strategy to improve physical fitness and overall health despite the dietary challenges (13, 14). Regular physical activity during fasting can improve cardiovascular health, reduce the risk of chronic diseases, and improve insulin sensitivity (15). Additionally, integrating sports and physical activity into the life style routine during RF helps to increase spiritual awareness and mindfulness. Many Muslims find that training during RF helpful to connect with their bodies and their faith (16).
Some researchers observed that low energy intake alters the availability and utilization of energetic substrates that can have adverse effects on metabolic, immune, and inflammatory responses and may thus lead to impaired physical fitness (17–20). In addition, insufficient sleep can affect physical fitness by increasing fatigue perception and decreasing mental alertness in response to the same exercise load during RF (8, 21).
It is therefore important to implement effective strategies such as a balanced diet during the non-fasting period, scheduling sports activities, and exercise outside fasting hours, and reducing the intensity and duration of exercise in order to maintain physical performance and adapt to the physiological changes associated with fasting (22).
The maintenance of physical exercise during RF is also challenging for athletes and physically active people who continue resistance training (RT). While there is a plethora of research on the effects of RT applied during RF on measures of body composition and muscular fitness such as muscle power, strength, and local muscular endurance (23, 24), there is limited data on the effects of RT during RF on sleep patterns and hormonal adaptations. Accordingly, we examined the timing of RT during RF when RT is performed either after breaking fast during a fed state to minimize fasting-induced performance degradation (25, 26), or during a fasting state to prevent sleep and hormonal perturbation after night training that could negatively affect muscle strength (27, 28). Additionally, researchers from previous studies also investigated the acute effects of exercise during fasting on hormonal secretion of growth hormones (GH), testosterone, and cortisol levels, which are higher after exercise and remain elevated 60 min after exercise during caloric restriction (29, 30).
Therefore, it is important to choose the right time of day for RT training during RF to minimize the effects of changes in mealtimes and the disruption of hormonal responses and sleep quality during this month, thus offering better physical performance and muscle maintenance. This study aimed to investigate the effects of performing RT in fasted state (FAST, i.e., training before breaking fast) or fed state (FED, i.e., training after breaking fast) on measures of muscle strength, sleep quality, and chronic hormonal responses in healthy and physically active young male adults. A secondary aim was to verify the effects of RT during FED or FAST on acute hormonal responses. With reference to the literature (23, 24), it was hypothesized that RT could be safely practiced during RF with both schedules; and that training after breaking fast may increase muscle strength while preserving normal hormonal responses.
This longitudinal study was conducted during the Ramadan of the lunar year 1442 Hijri (from March 12th to April 13th, 2021), with 15 ± 1 h of fasting every day. The study was conducted in Tunisia, where daytime temperatures during the study period were ~ 23 ± 4°C with a relative humidity of 65 ± 5%.
Data on maximal strength, sleep quality, and hormonal adaptations (cortisol, testosterone, and IGF-1) were collected at four different time points: 2 weeks before RF (T0), on the 15th day of (T1), on the 29th day of (T2), and 21 days after the end of Ramadan (T3). All tests were conducted in the same order and time of day during pre and post-tests (see Figure 1).
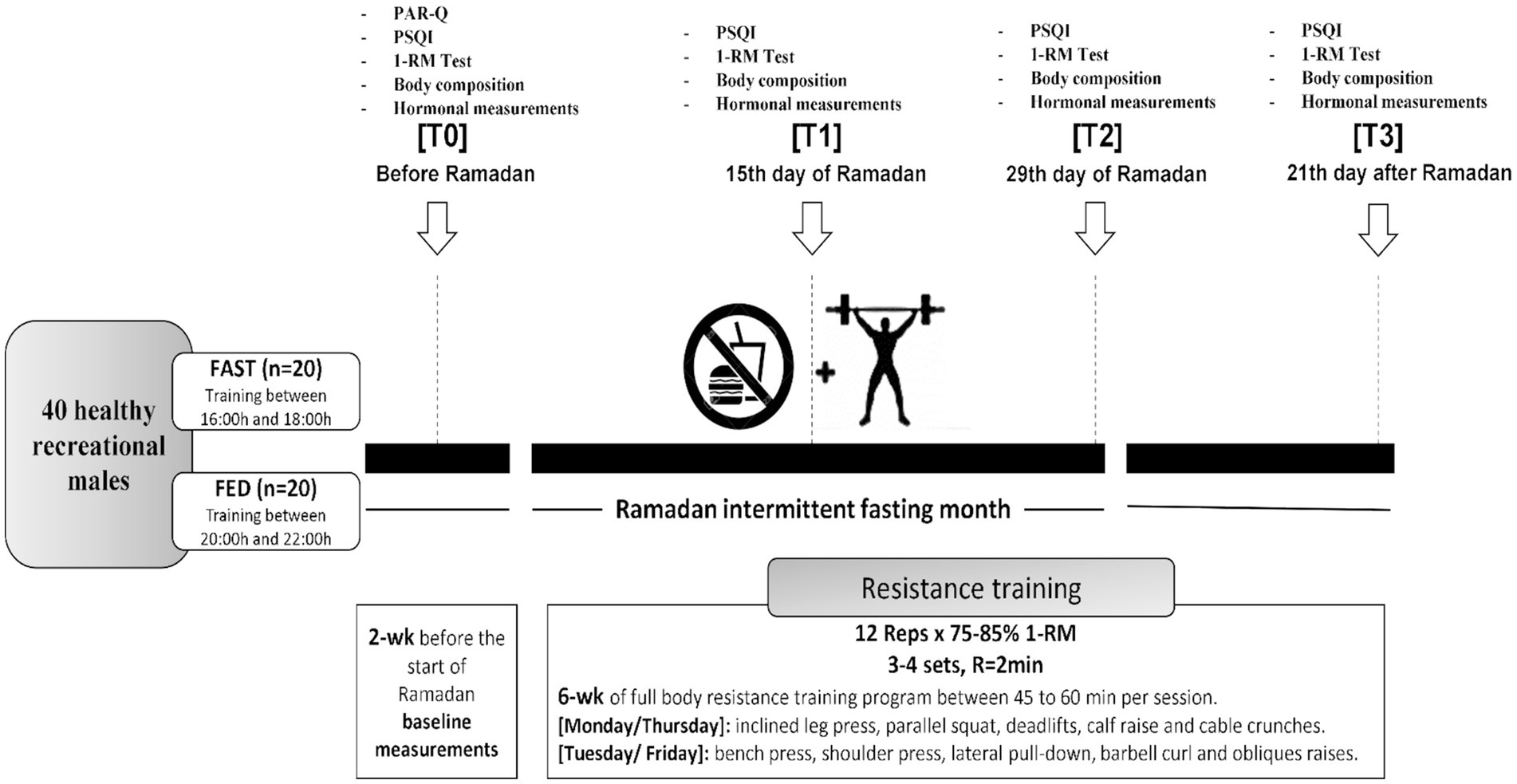
Figure 1. Study design. PSQI, Pittsburgh Sleep Quality Index; PAR-Q, physical activity readiness questionnaire; 1-RM, one repetition maximum; R, rest; wk, week; reps, repetitions.
The sample size of our study was determined using the power analysis program G*Power (version 3.1.9.3, University of Kiel, Kiel, Germany). The a priori power analysis was calculated using the F-test family (i.e., ANOVA repeated measures within-between interaction; power = 0.8, alpha = 0.05, effect size Cohen’s f = 0.3), and a related study that examined the effects of RF intermittent fasting on 1-RM performance (31). Outcomes from the power analysis indicated that a total sample size of 24 subjects to be sufficient (31). The sample size was increased to 40 to allow for potential dropouts of study participants (Figure 2).
Forty healthy and physically active males aged 25.7 ± 5.6 years (body mass = 85.1 ± 17.5 kg, height = 175 ± 9 cm, BMI = 28.3 ± 5.7 kg/m2) volunteered to participate in this study. Subjects were randomly assigned into two groups: FAST (n = 20) practiced RT in the afternoon before breaking fast (4–6 pm); FED (n = 20) practiced RT in the evening 1–2 h after breaking fast (8–10 pm) during RF. We added a control period of 21 days after the end of RF. During this time, both groups continued with the same training program at the same time of day under a normal nutritional regime (fed state). The study goal was to investigate whether the time-of-day training has an effect under the same nutritional conditions in either of the study groups.
Subjects were eligible for the study if they were healthy males aged between 18 and 35 years old, had at least 2 years of RT experience, lacked a history of cardiometabolic diseases or muscle injuries that could have precluded participation in the RT program, did not use medications, ergogenic aids, anabolic steroids or dietary supplements. Individuals had to refrain from other physical exercise during the study duration. All subjects had a medical examination and were fully informed about all experimental procedures, possible discomforts, risks, and benefits of the participation. Thereafter, they were kindly asked to sign the written informed consent form prior to the start of the study. The applied study procedures were in accordance with the principles outlined in the latest version of the Declaration of Helsinki. The study was approved by the local University Ethics Committee on Human Research of the University of Manouba, Tunisia (Ethics Code: Tn, UM2019-73).
Subjects visited the laboratory to become acquainted with all procedures before the start of data collection. The first day of testing was devoted to signing the informed consent form, answering the physical activity readiness questionnaire (PAR-Q), and the Pittsburgh Sleep Quality Index (PSQI), undergoing anthropometric measurements, and completing the one repetition maximum (1-RM) test. On the second test day, the 1-RM tests were repeated to determine the loads that were used during the RT programs and to assess test–retest reliability of the applied tests. During the third test day, blood samples were collected to analyze testosterone, cortisol, and the insulin-like growth factor-1 (IGF-1). The test sessions were separated by 48 h.
A whole-body RT program was performed on 4 days per week (Monday, Tuesday, Thursday, and Friday) for 8 weeks (1 week before Ramadan, 4 weeks during Ramadan, and 3 weeks after the end of Ramadan). Each workout included five exercises that were repeated twice. The applied exercises on Monday and Thursday involved the inclined leg press, the parallel squat exercise, deadlifts, calf raises, and cable crunches. On Tuesday and Friday, subjects performed the bench press, the shoulder press, the lateral pull-down, the barbell curl, and obliques raises.
Each exercise session lasted 45–60 min and started with a general warm-up (10 min) and included a cool-down period (5–10 min) of low-intensity aerobic and dynamic stretching exercises. Subjects performed 4 sets × 12 repetitions at 75–85% of the 1-RM for every exercise with 2 min of passive recovery between sets and exercises. Exercise intensities were gradually increased during the 8-week training period, from 75% of the 1-RM at the start of the program to 85% of the 1-RM at week 3, until the end of the intervention. Each Subject was individually supervised by an experienced strength and conditioning specialist (RT) who managed load progression to ensure that each exercise was performed with adequate exercise technique during the concentric and eccentric phases of movement. Time under tension amounted to 2 s during the concentric phase and 2 s during the eccentric phase. The person responsible for exercise supervision monitored compliance and individual workout data for each exercise session (e.g., number of repetitions and sets, rest, movement velocity, and intensity).
Body composition was measured in the morning (10:00 a.m.) at T0, T1, T2, and T3 following an overnight fast (10:00 h) and after more than 36 h following the last exercise bout.
Height was measured using a stadiometer. Body mass was assessed using an electronic scale with a resolution of 50 g (Medisana®, Neuss, Germany). The percentage of body fat was determined with a Harpenden caliper (Baty international. RH15 9LR. England) in four skinfolds (biceps, triceps, subscapular, and suprailiac) (32). Lean body mass (LBM) was calculated as body mass minus body fat mass. All measurements were performed by the same investigator following standardized procedures (33).
Sleep quality was assessed directly after taking the anthropometric and body composition measurements at T0, T1, T2, and T3 using the full Pittsburgh Sleep Quality Index (PSQI) validated for the Arabic language (34). The global PSQI score is composed of seven components: subjective sleep quality (individual’s sleep quality perception), sleep latency (time between lying in bed and starting sleeping), sleep duration (period sleeping reported by the individual), habitual sleep efficiency (ratio between the time sleeping and the time in bed), sleep disturbance (problems to sleep expressed by subjects), sleeping medication (use of medication to sleep), and daytime dysfunction (intensity of diurnal somnolence) (35). The seven domains were separately scored from 0 (no difficulty) to 3 (very difficult), with a total or global score ranging from 0 to 21 to reflect sleep quality and disturbances over a 1-month period. Better sleep quality is indicated through have a lower global PSQI score, while higher scores indicate poor sleep quality.
The 1-RM tests for the squat, bench press, and deadlift exercises were determined for each subject at T0, T1, T2, and T3 to evaluate the development of maximal strength during the study. Additionally, 1-RM tests were re-assessed every 2 weeks to maintain the targeted exercise intensity (75–85% 1-RM). The 1-RM testing and the start of the training sessions were separated by 10 min of rest to allow for an appropriate recovery.
Subjects were asked to perform 10 repetitions with the estimated 10-RM as a warm-up before the start of the test for each exercise using previously described procedures (36, 37). The 1-RM was determined with a maximum of six attempts, with a 5-min rest period between each attempt (37). In addition, a 3-min rest was allowed between the tests. Pilot data were obtained on 2 test days from 40 subjects to determine test–retest reliability. Our data revealed the following ICCs 1-RM parallel squat (0.929; 95%CI lower 0.929–upper 0.996), 1-RM bench press (0.779; 95%CI lower 0.581–upper 0.883), 1-RM deadlift (0.886; 95%CI lower 0.783–upper 0.940).
Four venous blood samples were taken at T0–T3. A heparinized catheter (Insyte-W, 1.1 mmo.d. × 30 mm, Biopol, Tunis, Tunisia) was inserted into an antecubital vein by using a 20-gauge needle and Vacutainer tubes before, at 0 min (immediately after), and 30 min after the training sessions. The blood was collected in test tubes containing EDTA and centrifuged at 1,500 × g for 10 min at 4°C, resultant serum was then removed and stored at −80°C until subsequently testosterone (nmol/L), cortisol (nmol/L), and IGF-1(ng/mL) were analyzed using radioimmunoassay commercial kits (Beckman Coulter and Diagnostic Systems Laboratories, France) according to the manufacturer’s procedures. The limits of sensitivity were 0.5 nmol/L for testosterone, 5.79 nmol/L for cortisol, and 3 ng/mL for IGF-1. The intra-assay coefficients of variation were between 0.9 and 4.6% for testosterone, between 2.24 and 6.01% for cortisol, and between 6.3 and 6.8% for IGF-1. All samples were assayed in duplicate and were decoded only after the analyses were completed (i.e., blinded analysis procedure). All samples were analyzed on the same assay for each analyte to eliminate inter-assay variance.
Participants were instructed to record the estimated quantities of all food and beverages consumed for at least 3 days per week (2 week-day and 1 day on the weekend), to offer a reliable estimate of food intake for the entire week. Total water intake was defined as the fluid volume of consumed beverages plus the water content of consumed foods.
A nutritional tracking application1 and the food composition tables of the National Institute of Statistics of Tunis (1978) were used to determine the distribution of macronutrients and the calories ingested. Each food item was individually entered into the application to provide data on total energy consumption, and the amounts of energy derived from proteins, fats, and carbohydrates for each period analyzed. The mean of nutrients consumed during the intervention was calculated using the method described by McCance and Widdowson (38).
Protein intake was supervised by a specialist to ensure that dietary protein needs were met. Subjects were instructed to consume dietary supplements on the training days containing 24 g of protein and 1 g of carbohydrate before sleeping (Iso100 Hydrolyzed Whey Protein Isolate; Dymatize Nutrition, Dallas, TX). There were no differences in the estimated nutrients consumed during the study between the experimental groups (Table 1).
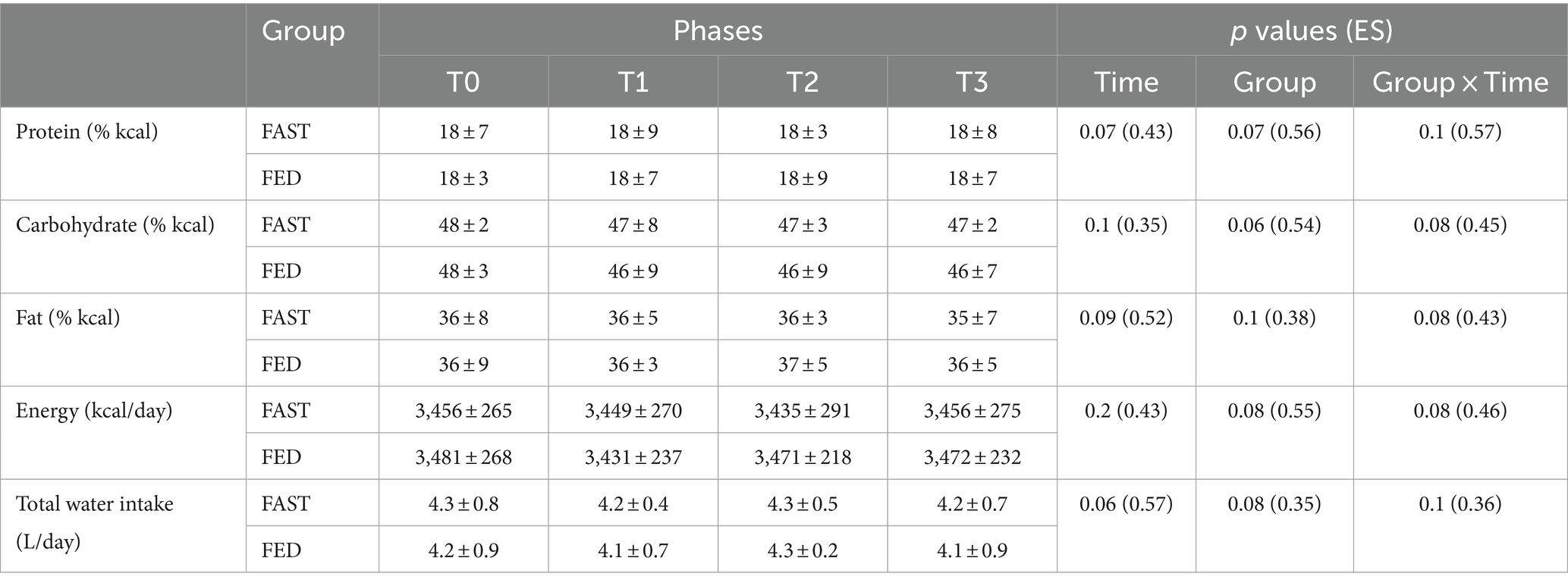
Table 1. Estimated daily dietary intake (mean ± SD) recorded at T0, T1, T2, and T3 in FAST and FED groups (N = 37).
Data were analyzed using SPSS software (v. 16.0, SPSS Inc., Chicago, IL, United States) and were expressed as means ± standard deviations (SD). The normality of data distribution was confirmed using the Shapiro–Wilk test. The effects of time of training during RF were evaluated using a 2 (groups: FAST, FED) × 4 (time: T0, T1, T2, T3) mixed model ANOVA. Additionally, hormonal data were assessed using a 2 (groups: FAST, FED) × 3 (time: BF, AF, AF-30 min) mixed model ANOVA. Where significant group-by-time interactions occurred, post hoc tests were computed using the Bonferroni adjustments to identify group-specific changes over time.
Effect sizes (ES) were calculated using ANOVA output by converting partial eta-squared ηp2 to Cohen’s d values. In addition, within-group ES were computed using the following equation: ES = (mean post—mean pre)/SD. ES were considered trivial (<0.2), small (0.2–0.6), moderate (0.6–1.2), large (1.2–2, 0), and very large (2.0–4.0) (39). The level of statistical significance was set at p < 0.05. Means, standard deviations, and 95% confidence intervals (CI) were presented for all data.
Three subjects (one from FED and two from FAST) were not included in the final analyses due to low compliance (<90%). The remaining subjects (N = 37) met the 100% adherence requirement for continued participation in the study. There were no between-group differences for any anthropometric measures, strength parameters, and diurnal variation of hormonal secretions at baseline between the two groups. ES magnitudes ranged from ignored, small to moderate for all measurements.
Means, standard deviations, and 95% CI of anthropometric parameters and body composition characteristics are presented in Table 2 for the study sample. No significant group × time interactions were found for all anthropometric measurements. However, a significant main time effect was reported for both FAST and FED for body mass (p = 0.001; ES = 0.37), BMI (p = 0.001; ES = 0.32), and body fat (p = 0.001; ES = 0.41) during Ramadan, with no significant main time effect for lean body mass (p = 0.57; ES = 0.42).
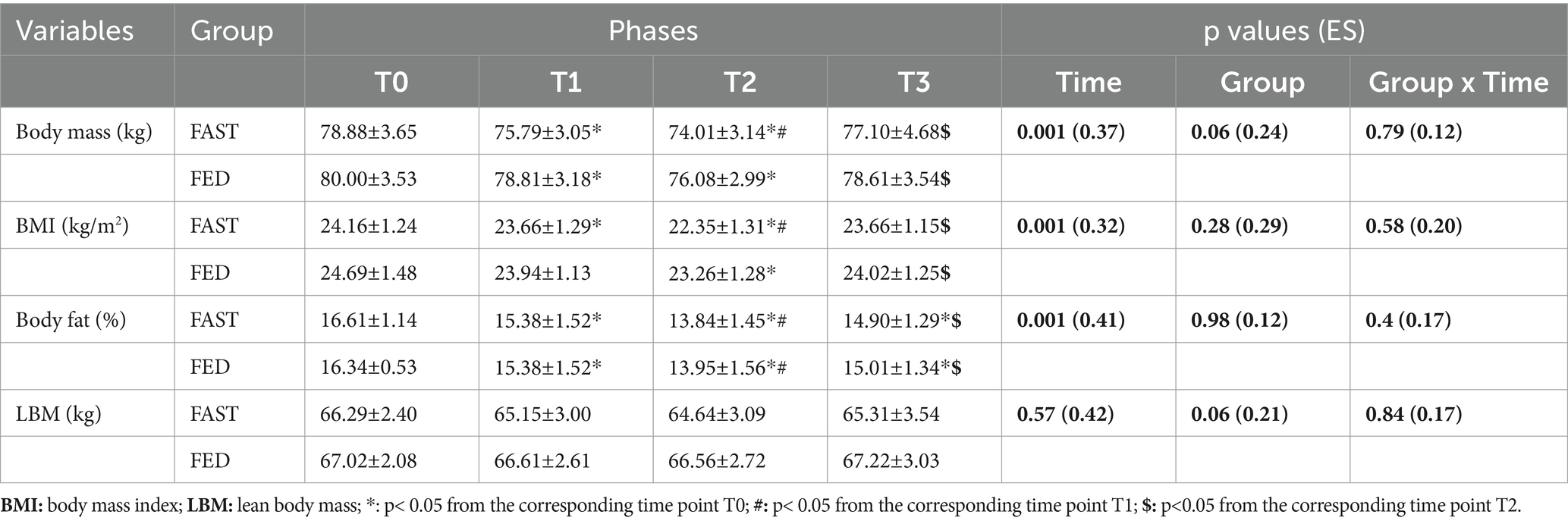
Table 2. Anthropometric and body composition characteristics (means ± SD) at T0, T1, T2 and T3 in FAST and FED groups (N=37).
Table 3 contains the primary findings with regards to maximal strength performance. There were significant group × time interactions for 1-RMSQ (p = 0.001; ES = 0.43) and 1-RMDL (p = 0.001; ES = 0.36). Post-hoc tests indicated significant increases for 1-RMSQ (p = 0.03; ES = 0.13) and 1-RMDL (p = 0.04; ES = 0.21) from T0-T2 among the FED group. No significant group × time effect was found for 1-RMBP (p = 0.68; ES = 0.21). At T3, significant improvements were observed for 1-RMSQ (p = 0.04; ES = 0.24), 1-RMBP (p = 0.04; ES = 0.20), and 1-RMDL (p = 0.02; ES = 0.18) in the FED group and for 1-RMSQ (p = 0.02; ES = 0.18), 1-RMBP (p = 0.02; ES = 0.23), and 1-RMDL (p = 0.04; ES = 0.19) in the FAST group in comparison with T0.
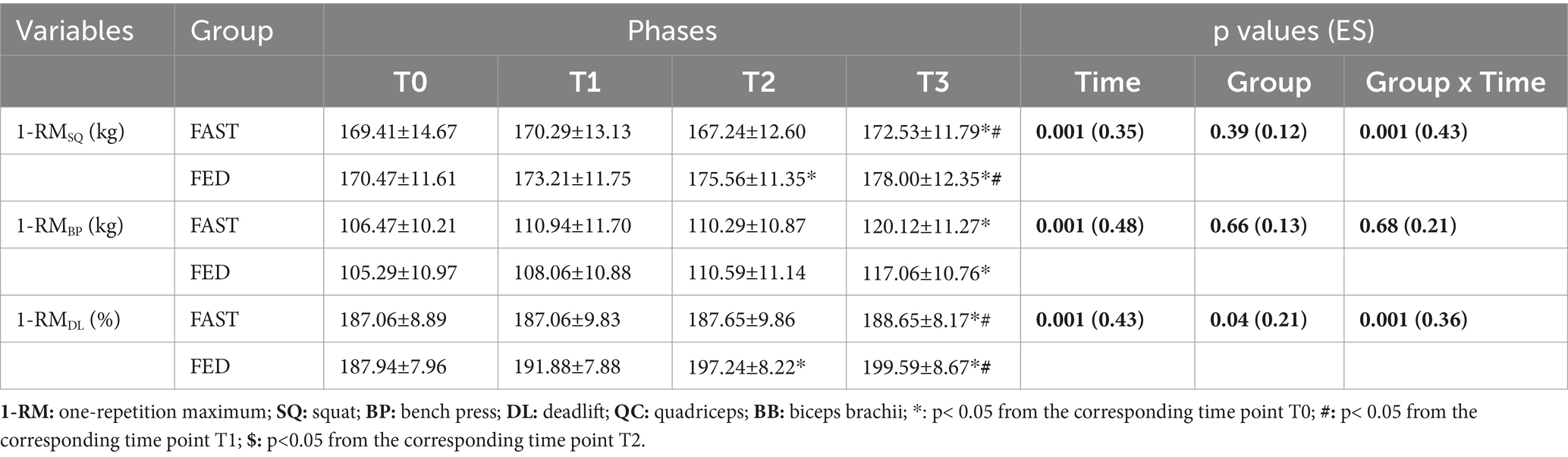
Table 3. One-repetition maximum (1-RM) changes (means ± SDs) at T0, T1, T2 and T3 in FAST and FED groups (N=37).
Means, standard deviations, and 95% confidence intervals (CI) of the Pittsburgh Sleep Quality Index at T0, T1, T2, and T3 among FAST or FED groups are reported in Table 4. No significant group × time interaction was found for the total score of the PSQI (p = 0.07; ES = 0.43). There was a main effect of time for the total score of PSQI. A higher global PSQI score was observed RF in both groups at T1 (p = 0.02; ES = 0.36 for FAST and p = 0.04; ES = 0.26 for FED) and T2 (p = 0.001; ES = 0.46 for FAST and p = 0.02; ES = 0.35 for FED) compared to T0. Total PSQI scores were lower 21 days after RF (T3) compared to T1 (p = 0.01; ES = 0.32 for FED and p = 0.01; ES = 0.27 for FAST) and T2 (p = 0.001; ES = 0.16 for FED and p = 0.03; ES = 0.18 for FAST).
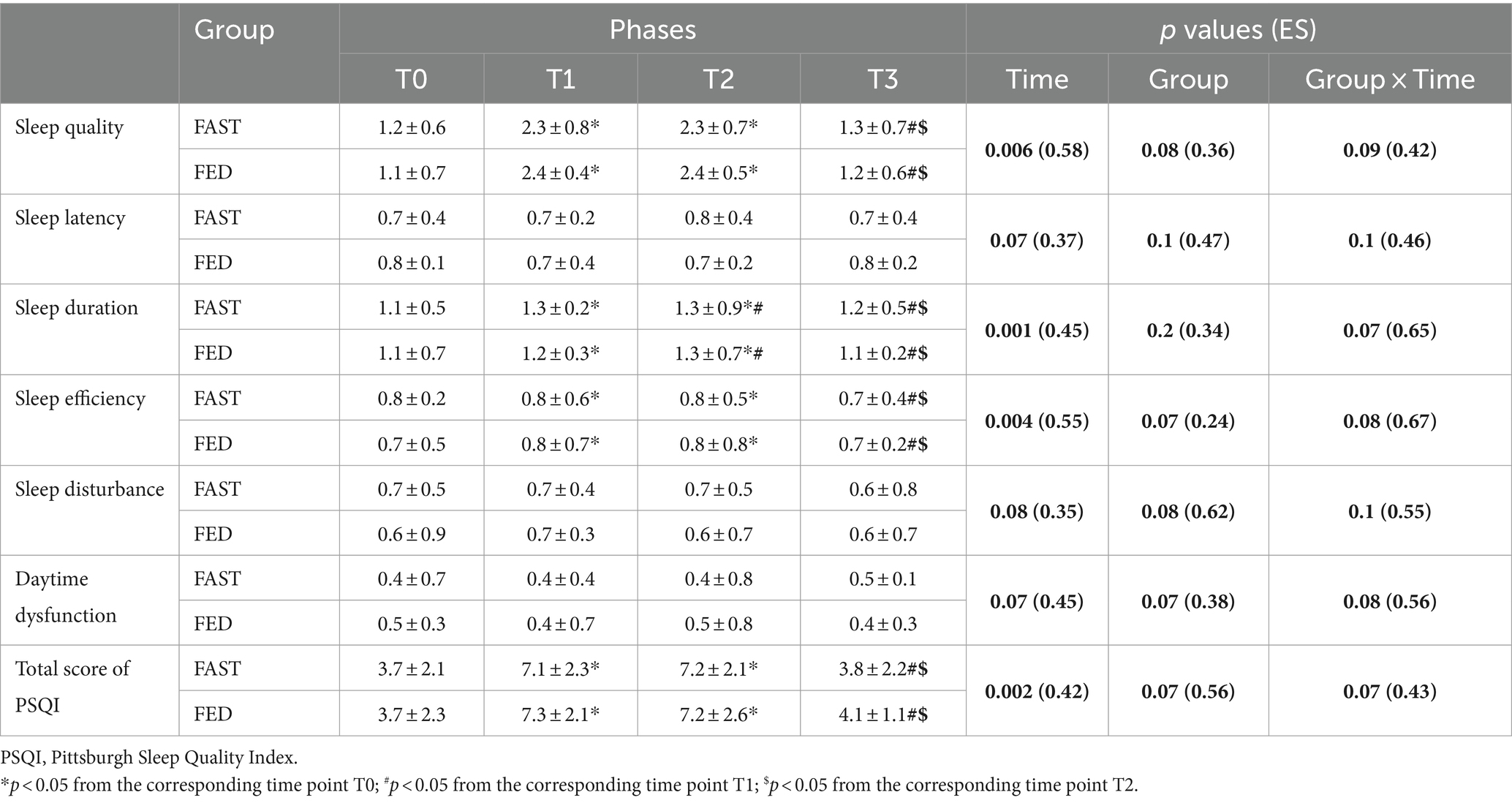
Table 4. Sleep quality (mean ± SD) recorded at T0, T1, T2, and T3 in the FAST and FED groups (N = 37).
Means, standard deviations, and 95% confidence intervals (CI) of the acute and chronic effects of exercise (FED, FAST) during RF are illustrated in Figure 3.
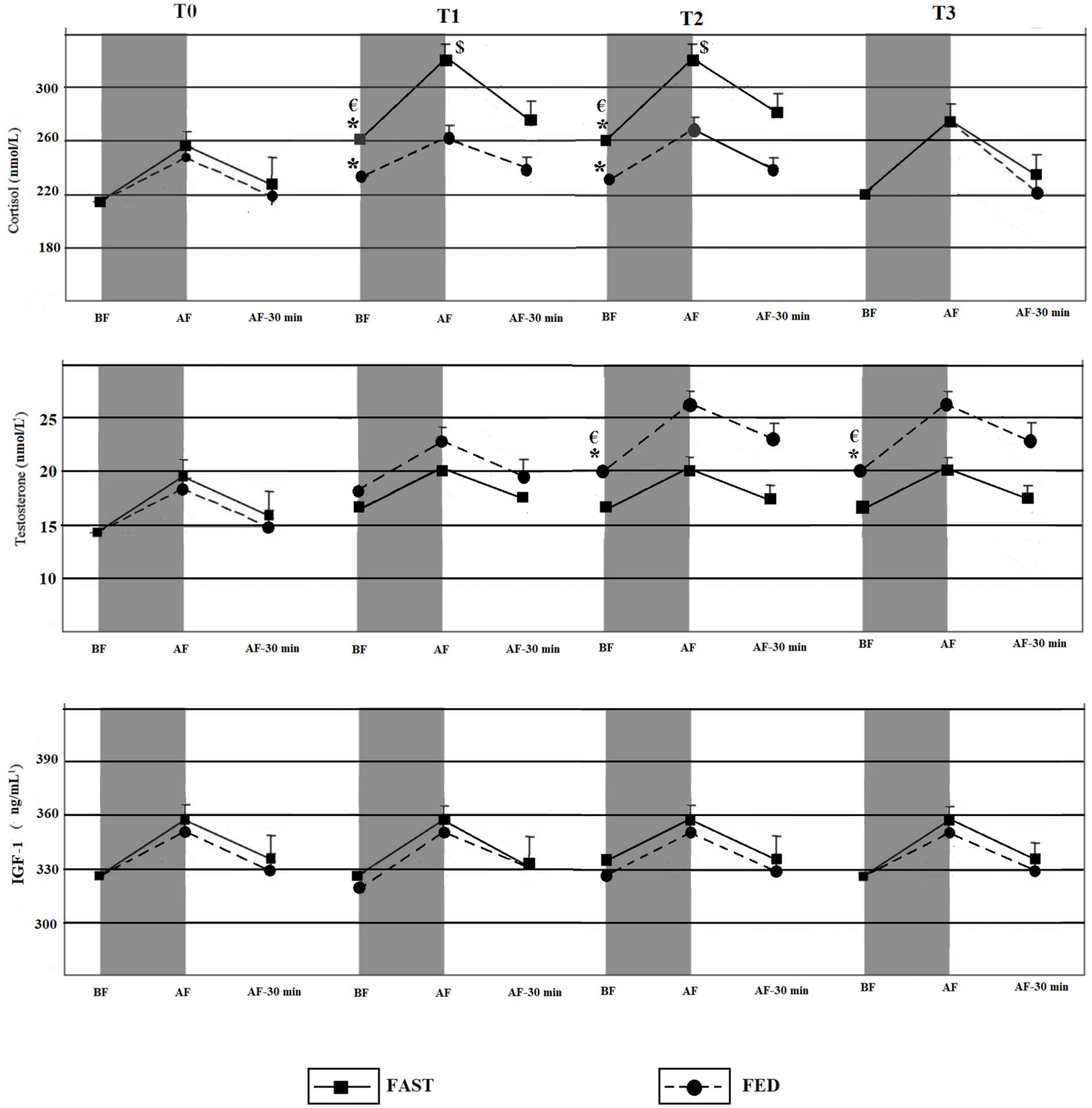
Figure 3. Acute and chronic effects of training during RF among FED and FAST groups. BF, Before exercise; AF, Immediately after exercise; AF-30 min, 30 min after exercise. *Significant time effect compared to T0 (p < 0.05); €Significant group × time chronic effect of training (p < 0.05); $significant group × time acute effect of exercise (p < 0.05).
Significant group × time interactions were identified for the chronic RT effects on measures of cortisol (p = 0.03; ES = 0.27). Post-hoc tests indicated significant increases in cortisol levels in the FAST group at T1 and T2 compared to T0 (p = 0.05; ES = 0.41 and p = 0.03; ES = 0.34). Significant increases in cortisol levels also occurred in the FED group at T1 (p = 0.05; ES = 0.29) and T2 (p = 0.04; ES = 0.25) compared to T0. However, the increases were significantly lower compared to the FAST group. Cortisol levels decreased after the Ramadan, and no significant differences were found for cortisol level at T3 compared with T0 among both groups (p > 0.05).
Significant group × time interactions occurred with regards to the chronic RT effects for testosterone levels (p = 0.01; ES = 0.32). Post-hoc tests indicated significant increases of testosterone among FED at T2 and T3 compared to T0 (p = 0.04; ES = 0.31 and p = 0.03; ES = 0.42). Additionally, testosterone levels remained significantly elevated after the end of RF at T3 vs. T0 (p = 0.05; ES = 0.28) and T3 vs. T1 (p = 0.03; ES = 0.25).
A significant group × time interaction occurred for cortisol levels (p = 0.04; ES = 0.34) for the acute effects of exercise. The level of cortisol immediately after RT was higher at T1 (p = 0.03; ES = 0.39) and T2 (p < 0.05; ES = 0.22) compared with T0 among the FAST group. However, there were no significant differences in the cortisol levels between T0 and T3 (i.e., for the acute effects of exercise) in the FAST group, immediately after RT.
No significant group × time interactions were detected for IGF-1 (p = 0.07; ES = 0.27).
This study was conducted with healthy young and RT experienced males. In this study, we aimed to evaluate the effects of RT applied during RF on muscular strength, sleep quality, and selected blood hormonal concentrations (chronic and acute effects) in a FED state vs. in a FAST state. To the best of our knowledge, this is the first study that investigates the importance of choosing the adequate day time RT training for a better muscle performance, taking into consideration the effects on sleep quality and hormonal secretions.
The key findings of our study include: (1) significant improvements for 1-RMDL and 1-RMSQ only in FED during RF (T1), however, while after the end of RF (T2) 1-RMDL, 1-RMSQ, and 1-RMBP improved in both groups (2) significant chronic effects on hormonal concentrations was detected in both groups, cortisol level increased among FAST at the second day of RF (T1) and lasted till its end (T2), while testosterone increased in FED at the last week of the RF (T1) and remained to increase even after the end of RF (T3); (3) significant increases in cortisol levels were reported only among FAST, increased cortisol levels immediately after RT sessions during RF (T1) were higher than before the start of RF, while they returned to the normal level after the end of RF (T2). No significant between group differences were observed for sleep quality during the experiment.
The observed significant deadlift and squat improvements were found in the FED group only and might be due to adequate pre-exercise energy and fluid intake levels. The RT sessions of FED occurred at 21:00 h under favorable conditions as the athletes were able to refuel and rehydrate for ∼90 min before the exercise sessions, while the FAST group trained after at least 13 h of fasting. Previous studies reported that the athletes’ pre-exercise blood glucose concentration levels and fluid balance were within the normal range during the evenings, while training at 18:00 h was the least favorable time of day for training as the athletes abstained from food and fluid for more than 10 h (40).
The effects of RT applied during RF on measures of muscle strength were also reported by others who obtained similar results (41, 42). Authors from previous studies indicated that the optimal time of day to perform high-intensity exercises during RF is in the evening, after breaking the fast. Training-induced adaptations might be diminished during the fasting state of RF (25, 40).
Hormonal changes were also reported in our study, and showed significant increases in cortisol levels in FAST likely due to a chronic effect of training during fasting. Increases in cortisol levels were also higher after breaking fast (FED) compared to before RF levels, but remained within ranges. The modification of the time difference between the food and water intakes and training sessions could underly increases in cortisol levels in FAST compared with pre-RF levels and to the FED state group. The higher level of cortisol concentration during fasting is a response to a catabolic process to restore glucose needs by stimulating gluconeogenesis, proteolytic activity and increasing skeletal protein degradation after 12 h of fasting (44). Increases in cortisol levels during RF were also reported previously (45–47). However, some studies observed decreases in cortisol levels in judo athletes after a higher training load during RF (12). One reason for this discrepancy could be the timing of blood sampling collection, which occurred between 8:00 h and 10:00 h in the later study (12), and in the afternoon as in our study.
The cortisol levels immediately after a single bout of RT in FAST were also higher compared to pre-RF levels. Differences in the magnitude of acute RT-induced cortisol responses may be due to the effects of a new physiological stress (fasting) that could lead to increased catabolic processes to mobilize fatty acids from fat reserves, and activation of protein breakdown from muscle tissue to raise blood glucose concentrations during food restriction during exercise in the FAST state (48). Similar study results were reported previously. The reported increases in cortisol levels after exercise in a fasting state (caloric restriction) were greater than in fed state (30, 49).
Significant increases of testosterone levels were only found in the FED state group during RF, which may favor physiological responses to modulate the balance between hormone-mediated anabolic and catabolic activities after the slight increase of cortisol concentrations during RF. Thus, the observed increase in muscle strength in FED can be explained by favoring anabolic functions of testosterone in skeletal muscle and neuronal tissue (50, 51). Similar findings were reported by Mira et al. (52) who observed significant increases in testosterone during RF in the evening hours and decreases in the morning. These findings were explained by the effects of RF on changes in the circadian rhythm and metabolic regulation during RF (52). The effects of fasting and training were also investigated in other studies, where testosterone levels were unchanged when measured before breaking the fast during RF (17, 53). To the best of our knowledge, no study investigated the effects of training times on testosterone secretion during RF.
Additionally, there were continued increases in testosterone levels only in the FED group even after the end of Ramadan (T3) in comparison with T0 and T1. Researchers from previous studies reported elevations in testosterone levels after long-term resistance training (8 weeks of training) in a non-fasting state while there were no increases in the FAST group (T3) undergoing only 3 weeks of training in a fed state (45, 46).
There were non-significant changes in IGF-1 levels in the two study groups. IGF-1 levels can be affected by food restriction and dehydration during RF or by changes in physical activity levels (54, 55). This was not the case in our study where there were no differences in total energy and water intake, or in total training volume between the two study groups. However, this non-significant change (tendency to decrease) may be due to increased cortisol levels which can decrease the skeletal IGF-I synthesis (44).
Sleep is an essential component of improving performance by promoting post-training recovery (56). Previous studies indicated that training at night causes sleep disturbances due to high pre-sleep arousal levels, especially if it occurs during 3 h before going to bed (57). Researchers from other studies reported that RF affects the circadian rhythm and produces modifications in the sleep quality with significant reductions in total sleep time (58, 59).
Our results showed a non-significant group × time effect for the factor day time training on sleep quality. However, significant main time effects were found for sleep quality during RF fasting. In fact, the Ramadan month affords substantial lifestyle adjustments and traditions involving longer nightly prayers, sleep disturbances due to the timing of suhur meal with poor sleep experiences.
This study was conducted during the COVID partial lock down period, and training sessions for the FED group occurred between 20:00 h and 22:00 h before gym closing times (as mandated by the Tunisian Government), making it important to study late-night RT and its effects on sleep quality and hormonal secretion.
Additionally, it could be useful to add a third day-time point to investigate the effects of RT after a short time after starting fasting. In fact, a morning training in a FAST state, after a short time of taking suhur meal could provide important insights.
Our study examined the effects of RT during RF on body composition, strength performance, and hormonal secretion. The results of our study suggest that RT during RF induces favorable changes in body composition (loss of body fat, maintaining lean tissue). Moreover, practicing RT after breaking fast (Fed state) improved muscle strength, probably due to a more favorable anabolic and hormonal status compared with the FAST. Thus, the results of this study can guide coaching strategies for strength and conditioning specialists. Choosing the appropriate time of day to practice RT training during RF is an important consideration for recreational weightlifters, and also for athletes from other (strength-dominated) sports to avoid physiological and psychological challenges that occur during this month of daily fasting.
The aim of this study was to investigate the appropriate time of day for recreational athletes who continue to train during RF to maintain and improve muscle performance in order to minimize the effects of meal timing changes on muscle performance. Our study demonstrated that RT applied after breaking fast in a FED state improved energy intake and testosterone secretion, thus enhancing muscle strength. Additionally, practicing RT in a FAST state had no adverse effects on measures of muscle strength when maintaining the same training volume and energy intake.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The applied study procedures were in accordance with the principles outlined in the latest version of the Declaration of Helsinki. The study was approved by the local University Ethics Committee on Human Research of the University of Manouba, Tunisia (Ethics Code: Tn, UM2019-73). The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
RT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AAb: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. IS: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – review & editing. FR: Data curation, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. AS: Data curation, Formal analysis, Validation, Visualization, Writing – review & editing. AAl: Data curation, Formal analysis, Funding acquisition, Resources, Software, Validation, Writing – review & editing. AH: Data curation, Formal analysis, Validation, Writing – review & editing. IL: Data curation, Formal analysis, Validation, Writing – review & editing. UG: Data curation, Formal analysis, Resources, Validation, Writing – review & editing. HZ: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors wish to express their sincere gratitude to all subjects, especially considering that our study coincided with the corona virus pandemic. The authors thank Oxygen Nutrition for supplying the whey protein used in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer KI declared a past co-authorship with the authors UG and HZ to the handling editor.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lugo, L, Cooperman, A, O’Connell, E, and Stencel, S. (2011). The future of the global Muslim population. USA: Pew Research Center, 1–209.
3. Faris, M’e A-IE, Jahrami, HA, Alhayki, FA, Alkhawaja, NA, Ali, AM, Aljeeb, SH, et al. Effect of diurnal fasting on sleep during Ramadan: a systematic review and meta-analysis. Sleep Breath. (2020) 24:771–82. doi: 10.1007/s11325-019-01986-1
4. Gharbi, M, Akrout, M, and Zouari, B. Food intake during and outside Ramadan. East Mediterran Health J. (2003) 9:2003–140. doi: 10.26719/2003.9.1-2.131
5. Alkandari, JR, Maughan, RJ, Roky, R, Aziz, AR, and Karli, U. The implications of Ramadan fasting for human health and well-being. J Sports Sci. (2012) 30:S9–S19. doi: 10.1080/02640414.2012.698298
6. Zouhal, H, Bagheri, R, Triki, R, Saeidi, A, Wong, A, Hackney, AC, et al. Effects of Ramadan intermittent fasting on gut hormones and body composition in males with obesity. Int J Environ Res Public Health. (2020) 17:5600. doi: 10.3390/ijerph17155600
7. Trabelsi, K, Masmoudi, L, Ammar, A, Boukhris, O, Khacharem, A, Jemal, M, et al. The effects of Ramadan intermittent fasting on sleep-wake behaviour and daytime sleepiness in team sport referees. J Sports Sci. (2021) 39:2411–7. doi: 10.1080/02640414.2021.1935672
8. Boukhris, O, Trabelsi, K, Shephard, RJ, Hsouna, H, Abdessalem, R, Chtourou, L, et al. Sleep patterns, alertness, dietary intake, muscle soreness, fatigue, and mental stress recorded before, during and after Ramadan observance. Sports. (2019) 7:118. doi: 10.3390/sports7050118
9. al-Rawi, N, Madkour, M, Jahrami, H, Salahat, D, Alhasan, F, BaHammam, A, et al. Effect of diurnal intermittent fasting during Ramadan on ghrelin, leptin, melatonin, and cortisol levels among overweight and obese subjects: a prospective observational study. PLoS One. (2020) 15:e0237922. doi: 10.1371/journal.pone.0237922
10. Bogdan, A, Bouchareb, B, and Touitou, Y. Ramadan fasting alters endocrine and neuroendocrine circadian patterns. Meal–time as a synchronizer in humans? Life Sci. (2001) 68:1607–15. doi: 10.1016/S0024-3205(01)00966-3
11. Chaouachi, A, Coutts, AJ, Chamari, K, Wong, DP, Chaouachi, M, Chtara, M, et al. Effect of Ramadan intermittent fasting on aerobic and anaerobic performance and perception of fatigue in male elite judo athletes. J Strength Cond Res. (2009) 23:2702–9. doi: 10.1519/JSC.0b013e3181bc17fc
12. Aloui, A, Chtourou, H, Hammouda, O, Souissi, H, Chaouachi, A, Chamari, K, et al. Effects of Ramadan on the diurnal variations of physical performance and perceived exertion in adolescent soccer players. Biol Rhythm Res. (2013) 44:869–75. doi: 10.1080/09291016.2013.780697
13. Wallis, GA, and Gonzalez, JT. Is exercise best served on an empty stomach? Proc Nutr Soc. (2019) 78:110–7. doi: 10.1017/S0029665118002574
14. Warburton, DE, Nicol, CW, and Bredin, SS. Health benefits of physical activity: the evidence. CMAJ. (2006) 174:801–9. doi: 10.1503/cmaj.051351
15. Zouhal, H, Saeidi, A, Salhi, A, Li, H, Essop, MF, Laher, I, et al. Exercise training and fasting: current insights. Open Access J Sports Med. (2020) 11:1–28. doi: 10.2147/OAJSM.S224919
16. El Ansari, W, and Stock, C. Relationship between attainment of recommended physical activity guidelines and academic achievement: undergraduate students in Egypt. Global J Health Sci. (2014) 6:274–83. doi: 10.5539/gjhs.v6n5p274
17. Chennaoui, M, Desgorces, F, Drogou, C, Boudjemaa, B, Tomaszewski, A, Depiesse, F, et al. Effects of Ramadan fasting on physical performance and metabolic, hormonal, and inflammatory parameters in middle-distance runners. Appl Physiol Nutr Metab. (2009) 34:587–94. doi: 10.1139/H09-014
18. Chaouachi, A, Coutts, AJ, Wong, DP, Roky, R, Mbazaa, A, Amri, M, et al. Haematological, inflammatory, and immunological responses in elite judo athletes maintaining high training loads during Ramadan. Appl Physiol Nutr Metab. (2009) 34:907–15. doi: 10.1139/H09-095
19. Abaïdia, A-E, Daab, W, and Bouzid, MA. Effects of Ramadan fasting on physical performance: a systematic review with meta-analysis. Sports Med. (2020) 50:1009–26. doi: 10.1007/s40279-020-01257-0
20. Maughan, R, Fallah, J, and Coyle, E. The effects of fasting on metabolism and performance. Br J Sports Med. (2010) 44:490–4. doi: 10.1136/bjsm.2010.072181
21. Trabelsi, K, Bragazzi, N, Zlitni, S, Khacharem, A, Boukhris, O, el-Abed, K, et al. Observing Ramadan and sleep-wake patterns in athletes: a systematic review, meta-analysis and meta-regression. Br J Sports Med. (2020) 54:674–80. doi: 10.1136/bjsports-2018-099898
22. Said, I. Sport during the Ramadan fasting period: health benefits and risks and recommendations for practicing. Int J Sport Stud Health. (2023) 6:1–4. doi: 10.61838/kman.intjssh.6.1.1
23. Keenan, S, Cooke, MB, and Belski, R. The effects of intermittent fasting combined with resistance training on lean body mass: a systematic review of human studies. Nutrients. (2020) 12:2349. doi: 10.3390/nu12082349
24. Tinsley, GM, Butler, NK, Forsse, JS, Bane, AA, Morgan, GB, Hwang, PS, et al. Intermittent fasting combined with resistance training: effects on body composition, muscular performance, and dietary intake. J Int Soc Sports Nutr. (2015) 12:P38. doi: 10.1186/1550-2783-12-S1-P38
25. Chtourou, H, Hammouda, O, Aloui, A, Souissi, N, and Chaouachi, A. The optimal time of day for training during Ramadan: a review study. J Nutr Fast Health. (2014) 2:46–52.
26. Hammouda, O, Chtourou, H, Aloui, A, Mejri, MA, Chahed, H, Miled, A, et al. Does Ramadan fasting affect the diurnal variations in metabolic responses and total antioxidant capacity during exercise in young soccer players? Sport Sci Health. (2014) 10:97–104. doi: 10.1007/s11332-014-0179-8
27. Ammar, A, Chtourou, H, Turki, M, Hammouda, O, Chaari, A, Boudaya, M, et al. Acute and delayed responses of steroidal hormones, blood lactate and biomarkers of muscle damage after a resistance training session: time-of-day effects. J Sports Med Phys Fitness. (2017) 58:980–9. doi: 10.23736/S0022-4707.17.07048-7
28. Issaoui, M, Zouhal, H, Yousfi, N, Salhi, A, Aloui, A, Bragazzi, N, et al. Effect of Ramadan fasting: association with time of day on time-motion, technical aspect and psychophysiological response to simulated karate competition in young amateur competitors. Int J Sports Sci Coach. (2020) 15:195–203. doi: 10.1177/1747954119900993
29. Abedelmalek, S, Chtourou, H, Souissi, N, and Tabka, Z. Caloric restriction effect on proinflammatory cytokines, growth hormone, and steroid hormone concentrations during exercise in judokas. Oxidative Med Cell Longev. (2015) 2015:1–8. doi: 10.1155/2015/809492
30. Nindl, BC, Rarick, KR, Castellani, JW, Tuckow, AP, Patton, JF, Young, AJ, et al. Altered secretion of growth hormone and luteinizing hormone after 84 h of sustained physical exertion superimposed on caloric and sleep restriction. J Appl Physiol. (2006) 100:120–8. doi: 10.1152/japplphysiol.01415.2004
31. Triki, R, Zouhal, H, Chtourou, H, Salhi, I, Jebabli, N, Saeidi, A, et al. Timing of resistance training during Ramadan fasting and its effects on muscle strength and hypertrophy. Int J Sports Physiol Perform. (2023) 18:579–89. doi: 10.1123/ijspp.2022-0268
32. Durnin, J, and Rahaman, MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. (1967) 21:681–9. doi: 10.1079/BJN19670070
33. De Onis, M, and Habicht, J-P. Anthropometric reference data for international use: recommendations from a World Health Organization expert committee. Am J Clin Nutr. (1996) 64:650–8. doi: 10.1093/ajcn/64.4.650
34. Suleiman, KH, Yates, BC, Berger, AM, Pozehl, B, and Meza, J. Translating the Pittsburgh sleep quality index into Arabic. West J Nurs Res. (2010) 32:250–68. doi: 10.1177/0193945909348230
35. Buysse, DJ, Reynolds, CF III, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
36. Lacerda, LT, Costa, CG, Lima, FV, Martins-Costa, HC, Diniz, RCR, Andrade, AGP, et al. Longer concentric action increases muscle activation and neuromuscular fatigue responses in protocols equalized by repetition duration. J Strength Cond Res. (2019) 33:1629–39. doi: 10.1519/JSC.0000000000002148
37. Lacerda, LT, Martins-Costa, HC, Diniz, RCR, Lima, FV, Andrade, AGP, Tourino, FD, et al. Variations in repetition duration and repetition numbers influence muscular activation and blood lactate response in protocols equalized by time under tension. J Strength Cond Res. (2016) 30:251–8. doi: 10.1519/JSC.0000000000001044
38. MacCance, R., and Widdowson, E. (2014). Food Standards Agency. Public Health England McCance and Widdowson’s the Composition of Foods.
39. Hopkins, WG, Marshall, SW, Batterham, AM, and Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. (2009) 41:3–12. doi: 10.1249/MSS.0b013e31818cb278
40. Aziz, AR, Chia, MYH, Low, CY, Slater, GJ, Png, W, and Teh, KC. Conducting an acute intense interval exercise session during the Ramadan fasting month: what is the optimal time of the day? Chronobiol Int. (2012) 29:1139–50. doi: 10.3109/07420528.2012.708375
41. Aziz, AR, Chia, M, Wahid, M, Png, W, Wong, J, and Teh, KC. Effect of Ramadan Fasting on Maximal Bench Press Strength Performance in Trained Athletes. Proceedings of the iii international conference of physical education and sports science 25 - 28 MAY M Chia, J Wang, G Balasekaran, and N Chatzisarantis Editors. (Singapore: National Institute of Education) (2010).
42. Rebaï, H, Chtourou, H, Zarrouk, N, Harzallah, A, Kanoun, I, Dogui, M, et al. Reducing resistance training volume during Ramadan improves muscle strength and power in football players. Int J Sports Med. (2014) 35:432–7. doi: 10.1055/s-0033-1353216
44. McCarthy, JJ, and Esser, KA. Anabolic and catabolic pathways regulating skeletal muscle mass. Curr Opin Clin Nutr Metab Care. (2010) 13:230–5. doi: 10.1097/MCO.0b013e32833781b5
45. Maughan, RJ, Leiper, JB, Bartagi, Z, Zrifi, R, Zerguini, Y, and Dvorak, J. Effect of Ramadan fasting on some biochemical and haematological parameters in Tunisian youth soccer players undertaking their usual training and competition schedule. J Sports Sci. (2008) 26:S39–46. doi: 10.1080/02640410802491368
46. Sharifi, F, Masoud, AK, Rezai, M, Ziaei, M, and Hedayati, M. Evaluation of serum levels of IL-1α, TGF-β, TNF-α, IFN-α, IFN-γ, cortisol and immunoglobulins in Islamic Ramadan fasting. Iran J Endocrinol Metabol. (2007) 9:201–4.
47. Bahijri, S, Borai, A, Ajabnoor, G, Abdul Khaliq, A, AlQassas, I, al-Shehri, D, et al. Relative metabolic stability, but disrupted circadian cortisol secretion during the fasting month of Ramadan. PLoS One. (2013) 8:e60917. doi: 10.1371/journal.pone.0060917
48. Schoenfeld, BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res. (2010) 24:2857–72. doi: 10.1519/JSC.0b013e3181e840f3
49. Degoutte, F, Jouanel, P, Bègue, RJ, Colombier, M, Lac, G, Pequignot, JM, et al. Food restriction, performance, biochemical, psychological, and endocrine changes in judo athletes. Int J Sports Med. (2006) 27:9–18. doi: 10.1055/s-2005-837505
50. Kraemer, WJ, Ratamess, NA, Hymer, WC, Nindl, BC, and Fragala, MS. Growth hormone (s), testosterone, insulin-like growth factors, and cortisol: roles and integration for cellular development and growth with exercise. Front Endocrinol. (2020) 11:33. doi: 10.3389/fendo.2020.00033
51. Kraemer, WJ, Ratamess, NA, and Nindl, BC. Recovery responses of testosterone, growth hormone, and IGF-1 after resistance exercise. J Appl Physiol. (2017) 122:549–58. doi: 10.1152/japplphysiol.00599.2016
52. Mira, S. Changes in testosterone levels during the fasting month of Ramadan. Qatar Med J. (2002) 2002:12. doi: 10.5339/qmj.2002.2.12
53. Brini, S, Marzouki, H, Ouerghi, N, Ouergui, I, Castagna, C, and Bouassida, A. Effects of Ramadan observance combined with two training programs on plasma lipids and testosterone/cortisol ratio in male senior basketball players. Med Sport. (2019) 72:47–58. doi: 10.23736/S0025-7826.18.03407-5
54. Bouhlel, E, Zaouali, M, Miled, A, Tabka, Z, Bigard, X, and Shephard, R. Ramadan fasting and the GH/IGF-1 axis of trained men during submaximal exercise. Ann Nutr Metab. (2008) 52:261–6. doi: 10.1159/000140517
55. Nemet, D, Connolly, PH, Pontello-Pescatello, AM, Rose-Gottron, C, Larson, JK, Galassetti, P, et al. Negative energy balance plays a major role in the IGF-I response to exercise training. J Appl Physiol. (2004) 96:276–82. doi: 10.1152/japplphysiol.00654.2003
56. Kellmann, M, Bertollo, M, Bosquet, L, Brink, M, Coutts, AJ, Duffield, R, et al. Recovery and performance in sport: consensus statement. Int J Sports Physiol Perform. (2018) 13:240–5. doi: 10.1123/ijspp.2017-0759
57. Sargent, C, Halson, S, and Roach, GD. Sleep or swim? Early-morning training severely restricts the amount of sleep obtained by elite swimmers. Eur J Sport Sci. (2014) 14:S310–5. doi: 10.1080/17461391.2012.696711
58. Herrera, CP. Total sleep time in Muslim football players is reduced during Ramadan: a pilot study on the standardized assessment of subjective sleep–wake patterns in athletes. J Sports Sci. (2012) 30:S85–91. doi: 10.1080/02640414.2012.676666
Keywords: fasting, muscle performance, training, hormonal adaptation, sleep quality
Citation: Triki R, Ben Abderrahman A, Salhi I, Rhibi F, Saeidi A, Almaqhawi A, Hackney AC, Laher I, Granacher U and Zouhal H (2024) Effects of time-of-day resistance training on muscle strength, hormonal adaptations, and sleep quality during Ramadan fasting. Front. Nutr. 11:1439738. doi: 10.3389/fnut.2024.1439738
Received: 28 May 2024; Accepted: 09 October 2024;
Published: 13 November 2024.
Edited by:
Philip Calder, University of Southampton, United KingdomReviewed by:
Piotr Zmijewski, Józef Piłsudski University of Physical Education in Warsaw, PolandCopyright © 2024 Triki, Ben Abderrahman, Salhi, Rhibi, Saeidi, Almaqhawi, Hackney, Laher, Granacher and Zouhal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayoub Saeidi, c2FlaWRpX2FzNjhAeWFob28uY29t; Hassane Zouhal, aGFzc2FuZS56b3VoYWxAdW5pdi1yZW5uZXMyLmZy; Urs Granacher, dXJzLmdyYW5hY2hlckBzcG9ydC51bmktZnJlaWJ1cmcuZGU=
‡ORCID: Ayoub Saeidi, https://orcid.org/0000-0003-2458-6256
Urs Granacher, https://orcid.org/0000-0002-7095-813X
Hassane Zouhal, https://orcid.org/0000-0001-6743-6464
†These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.