- Department of Infectious Diseases, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China
Background: The development of metabolic dysfunction associated steatotic liver disease (MASLD) has been associated with lipid accumulation, oxidative stress, endoplasmic reticulum stress, and lipotoxicity. The Composite Dietary Antioxidant Index (CDAI) is a comprehensive score representing an individual intake of various dietary antioxidants, including vitamin A, vitamin C, vitamin E, selenium, zinc, and carotenoids. This study investigated the association between CDAI and MASLD.
Materials and methods: Clinical and demographic data, as well as ultrasound transient elastography measurements at baseline, were collected from the National Health and Nutrition Examination Survey 2017–2020 (NHANES 2017–2020). The controlled attenuation parameter was utilized to diagnose the presence of hepatic steatosis and to categorize individuals into those with and without MASLD. Liver stiffness was measured by ultrasound transient elastography, and subjects were classified as those with and without advanced liver fibrosis.
Results: This study included 5,884 adults, of whom 3,433 were diagnosed with MASLD, resulting in a weighted prevalence of 57.3%. After adjusting for covariates, the odds ratios for MASLD were 0.96 (95% CI: 0.82, 1.12) in the second quartile, 0.80 (95% CI: 0.68, 0.95) in the third quartile and 0.60 (95% CI: 0.49, 0.73) in the fourth quartile, respectively. CDAI, however, was not significantly associated with advanced liver fibrosis.
Conclusion: These findings suggested that scores on the CDAI were linearly and negatively associated with the prevalence of MASLD in the United States adults.
1 Introduction
In June 2023, non-alcoholic fatty liver disease (NAFLD) and metabolic associated fatty liver disease (MAFLD) were renamed metabolic dysfunction associated steatotic liver disease (MASLD) to emphasize the role of metabolic dysfunction in its development (1). MASLD has been estimated to affect 30% of the worldwide adult population. Metabolic dysfunction associated steatohepatitis (MASH), a step within the broad spectrum of lesions included in MAFLD, is histologically defined by the presence of lobular inflammation and hepatocellular ballooning, and associated with an increased risk of progression to fibrosis (2). Recently, the Food and Drug Administration (FDA) in the United States approved the drug Resmetirom for the treatment of MASH in adult patients, the first medication approved by the FDA for MASH in 40 years. Lifestyle adjustments are also important in the treatment of MASH (3, 4).
Currently, lifestyle interventions to achieve weight loss remain the cornerstone of MASLD management (5–13). Oxidative stress has been shown to play a significant role in the development of MASLD (14). A case–control study in Iran, enrolling 158 NAFLD patients and 357 healthy controls, revealed that the prevalence of NAFLD was significantly lower in subjects within the highest tertile than in subjects within the lowest tertile of dietary total antioxidant capacity (DTAC), after adjusting for potential confounding factors (odds ratio [OR] 0.19; 95% confidence interval [CI] 0.9, 0.34; p for trend <0.001) (15). DTAC, however, can itself be influenced by various factors, including seasonality, geographic location, storage conditions, and access to water and sunlight. Additionally, food preparation and cooking methods can also impact DTAC. These considerations were addressed by the formulation of the Composite Dietary Antioxidant Index (CDAI), a reliable and valid measurement of the overall antioxidant composition of an individual’s diet. The CDAI is a comprehensive score of multiple dietary antioxidants, including vitamins A, C, and E, selenium, zinc, and carotenoids, representing an individual’s overall dietary intake of antioxidants (16). A number of studies have demonstrated a negative association between CDAI and the prevalence of diabetes, hypertension, chronic kidney disease, and depression (17–20), as well as all-cause and cardiovascular mortality in the general United States population (21).
Although dietary antioxidants have been shown effective in the treatment of adverse health effects, including oxidative stress (22–26), the specific relationship between the CDAI and MASLD remains unclear. Based on the hypothesis that the CDAI is negatively associated with MASLD, the present study investigated the potential correlation between CDAI and MASLD using data from the National Health and Nutrition Examination Survey (NHANES) 2017–2020.
2 Methods
2.1 Study population and design
The study population consisted of subjects enrolled in the NHANES 2017–2020, due to the availability of liver ultrasound transient elastography data in this population. Using the Fibroscan to estimate the severity of liver steatosis and fibrosis (27), hepatic steatosis was defined as a controlled attenuation parameter (CAP) ≥ 248 dB/m, with a sensitivity of 68.8% and a specificity of 82.2% (28). Advanced liver fibrosis was defined as a liver stiffness measure (LSM) value ≥8.0 kPa (29). The study ultimately enrolled 5,884 subjects from an initial population of 15,560. Subjects aged <20 years (n = 6,328), those with missing CDAI data (n = 2,592), and those with missing Fibroscan measurements (n = 756) were excluded. According to the latest consensus diagnostic criteria from the EASL-AASLD-ALEH consensus statement in 2023 (1), 3,433 people were diagnosed with MASLD, while the others were included in the health control group (n = 2,451). The details are presented in Figure 1. The study protocol was approved and documented by the Research Ethics Review Committee of the National Center for Health Statistics of the United States. Before participants were enrolled in the study, they had to provide written informed consent.
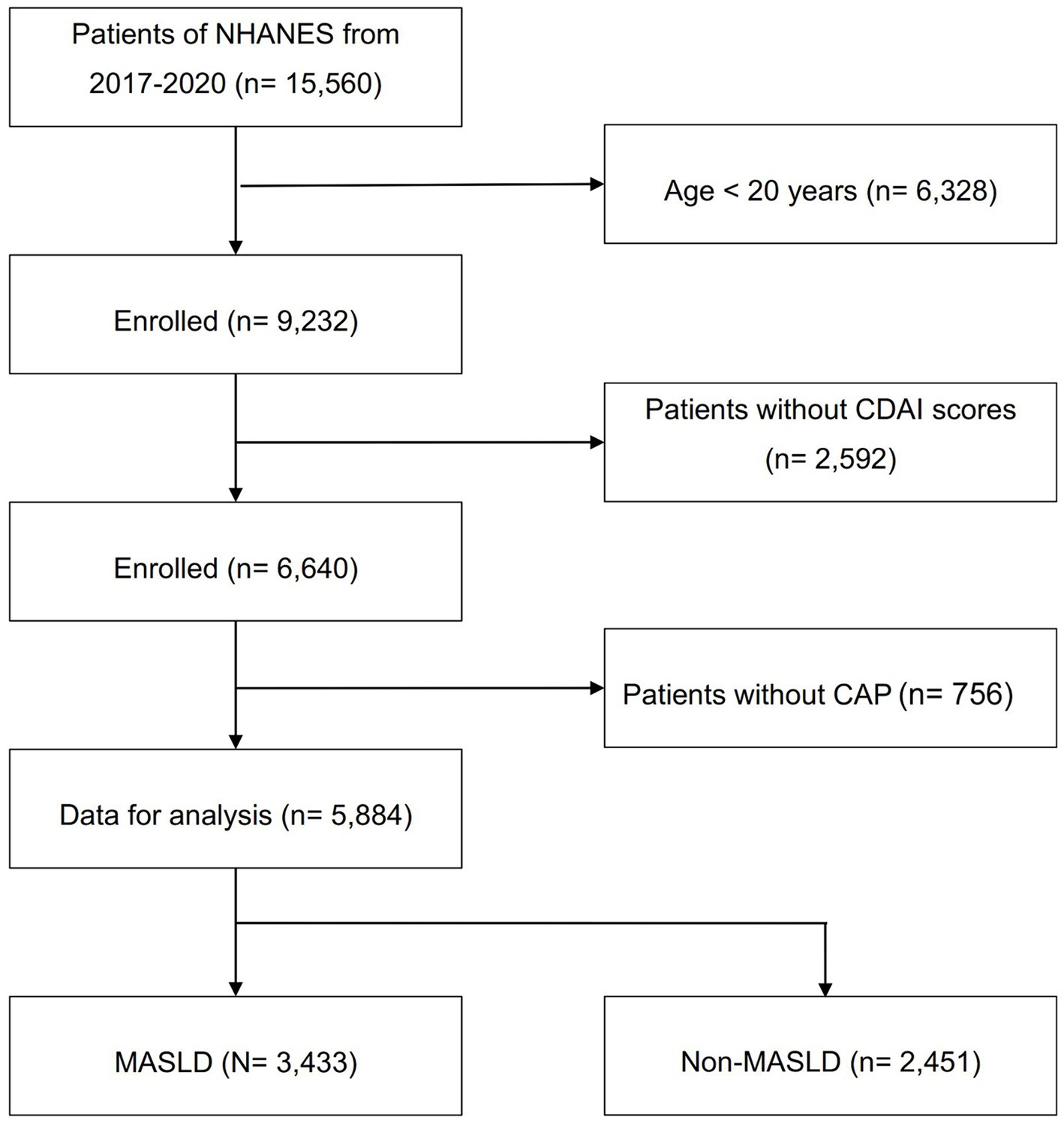
Figure 1. Flowchart of participant selection. NHANES, National Health and Nutrition Examination Survey; CAP, controlled attenuation parameter; CDAI, composite dietary antioxidant index; MASLD, metabolic dysfunction associated steatotic liver disease.
2.2 Definition of CDAI
Date were obtained from participants in the NHANES database, through two 24-h dietary recalls conducted at separate time points (30). The first recall was taken in-person at a mobile testing center, while the second recall was performed 3–10 days later via a telephone consultation. The CDAI of each participant was calculated based on the dietary recall of intake of six dietary antioxidants, vitamin A, vitamin C, vitamin E, zinc, selenium, and carotenoids (31).
2.3 Assessment of covariates
Referring to a previous study (32), the covariates included age, sex, race, education level, marital status, income status, physical activity, smoking status, alcohol consumption, and energy intake. Because other variables, such as blood glucose concentration, blood pressure, lipid profile, and body mass index were considered in the diagnosis of MASLD, these variables were not included in the regression model. Marital status was divided into married and unmarried; and education level into less than high school, high school, and more than high school. Income status was assessed by calculating the household income to poverty income ratio (PIR), with subjects divided into those with low (< 1.0), moderate (1.0–3.0), and high (≥ 3.0) PIR. Smoking status was categorized as never, former or current; physical activity was categorized as never, moderate and vigorous exercise (33); heavy drinking was defined as more than 14 drinks/week for men or more than 7 drinks/week for women (34). Detailed explanations of the methods used to calculate these variables are available on the NHANES website.1
2.4 Statistical analysis
All analyses considered the weights of each variable in the NHANES database. Continuous variables were described as weighted mean (standard error) and categorical variables were described as weighted n (weighted percentage). The CDAI was converted into quartiles, the relationship between CDAI and MASLD/advanced liver fibrosis was examined by three multivariate logistic regression models, all of which using the first CDAI quartile group as reference. Model 1 was a crude model without any adjustment for covariates; Model 2 included adjustments for age, sex, and race; and Model 3 included the adjustments in Model 2 as well as adjustments for education level, marital status, PIR, smoking, alcohol consumption, physical activity, and energy intake. To further eliminate relevant confounding factors, stratified analyses were performed based on factors such as age, sex, race, PIR, energy intake, and ethnicity. All analyses were performed using R software (version 4.3.2, Vienna, Austria: R Foundation for Statistical Computing, 2016) and Stata/SE software, v.16 (StataCorp, College Station, TX, USA), with bilateral p values <0.05 defined as statistically significant.
3 Results
3.1 Baseline characteristics of the enrolled population
The study included a total of 5,884 eligible participants, out of which 3,433 were diagnosed with MASLD, resulting in a weighted prevalence of 57.3% (92,050,707/160,526,094). Compared to the non-MASLD participants, MASLD participants had higher age and energy intake as 51.5 ± 0.4 years vs. 44.4 ± 0.5 years and 2,112 ± 20.0 kcal/day vs. 2,039 ± 23.9 kcal/day, respectively (p < 0.001). The proportion of male (51.4% vs. 43.8%, p < 0.001) and of married individuals (67.6% vs. 56.6%, p < 0.001) was significantly higher in MASLD. However, the MASLD group had a significantly lower score on CDAI than the non-MASLD group (0.6 ± 0.1 vs. 1.1 ± 0.1, p < 0.001). Race, educational level and smoking status also differed significantly in these two groups (all p < 0.05) (Table 1).
3.2 Association of CDAI with MASLD
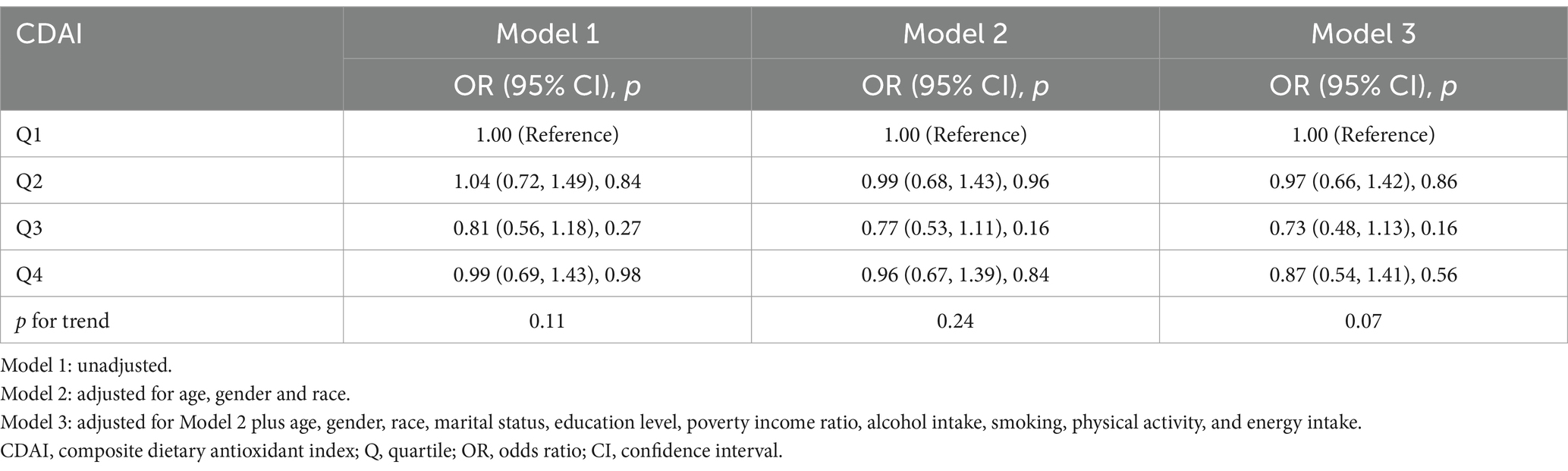
Weighted multivariate logistic regression analyses were performed to further access the relationship between CDAI and MASLD based on the quartile of CDAI. Univariate logistic regression analysis showed that the unadjusted OR (95% CI) of MASLD was significantly lower in the fourth quartile (Q4) (OR 0.83; 95% CI: 0.72, 0.96, p = 0.013) than in the first quartile (Q1) (used as reference). After adjusting for potential confounding factors, the risk of MASLD in Model 3 remained significantly lower in Q4 than in Q1 (OR 0.60; 95% CI: 0.49, 0.73, p < 0.001). In addition, there was a significant negative correlation between the CDAI index and the occurrence of MASLD for all three models (Table 2).
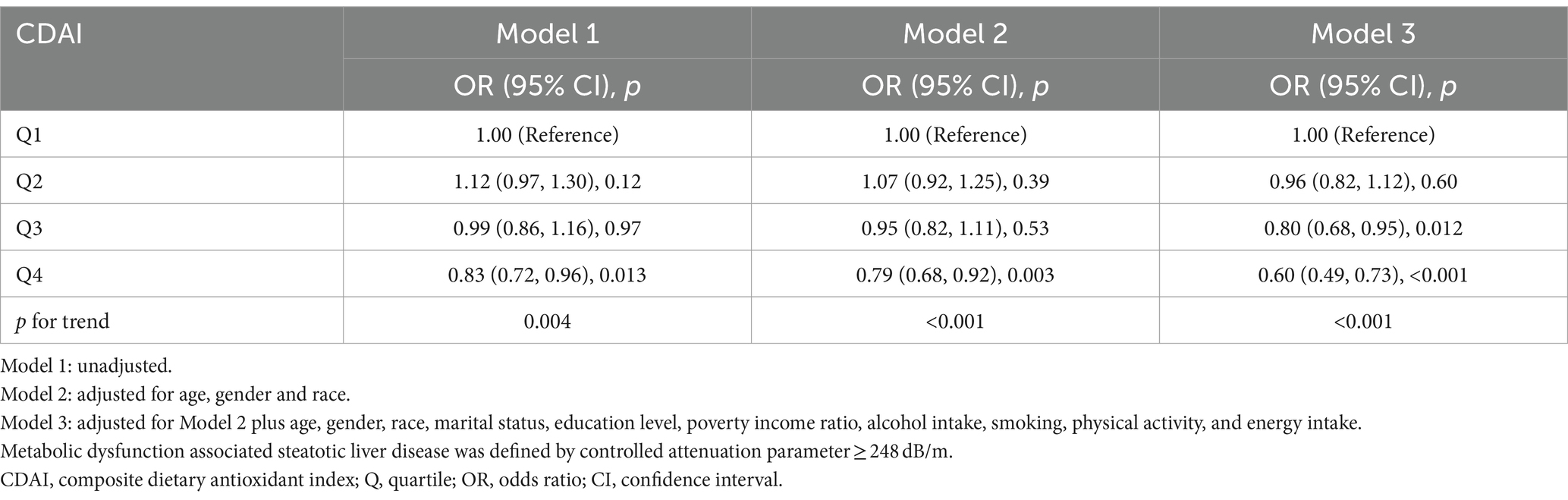
Table 2. Association of composite dietary antioxidant index and metabolic dysfunction associated steatotic liver disease.
We also analyzed separately the impact of each component of CDAI upon MASLD risk. These analyses found that vitamin C, vitamin E, and carotenoids may play an important role in the negative correlation between CDAI and MASLD (all p for trend <0.05) (Figure 2). Subsequently, restricted cubic spline (RCS) analysis to investigate the dose–response relationship between CDAI and MASLD risk showed that higher levels of CDAI were associated with a reduced likelihood of MASLD, with a non-linear p-value of 0.873 (Figure 3).
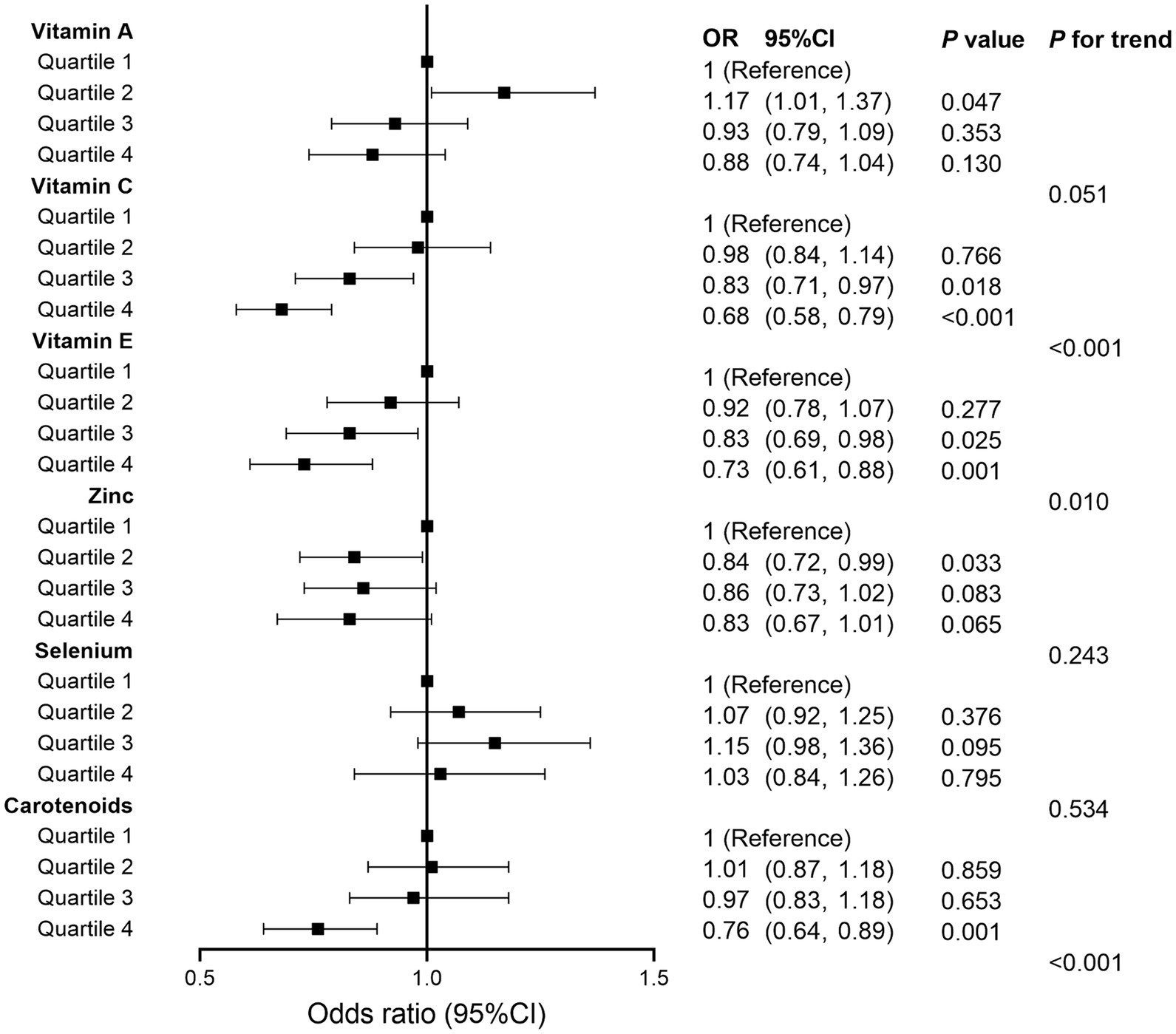
Figure 2. Forest plot for analyzing the relationship between each component of composite dietary antioxidant index and metabolic dysfunction associated steatotic liver disease. OR, odds ratio; CI, confidence interval.
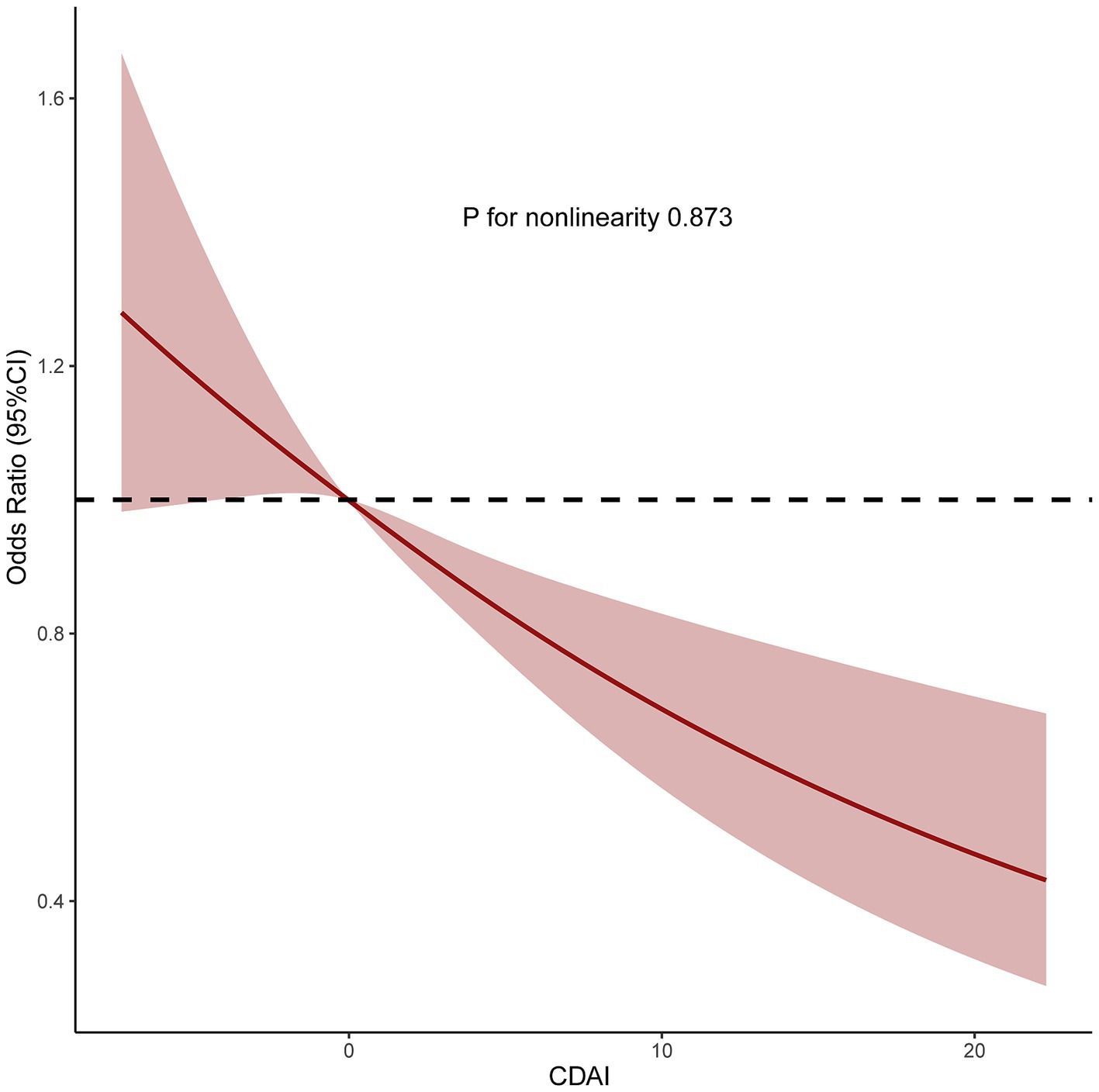
Figure 3. Restricted cubic spine model of the odds ratio of metabolic dysfunction associated steatotic liver disease with composite dietary antioxidant index. All were adjusted for age, sex, race, marital status, education level, poverty income ratio, alcohol intake, smoking, physical activity, and energy intake. CI, confidence interval.
Sensitivity analyses were used to assess the stability of the relationship between CDAI and MASLD. The fatty liver index (FLI) has been shown relatively effective in detecting MASLD in the United States population (35). The use of an FLI ≥ 60 to diagnose MASLD (36, 37) showed a significant negative correlation between CDAI and MASLD in all three models (Table 3). Even after excluding subjects with extremely high (> 8,000 kcal/day for men, > 5,000 kcal/day for women) and low (< 500 kcal/day for both sexes) daily energy consumption, a significant negative correlation between CDAI and the prevalence of MASLD persisted in all three models (Table 4). To rule out the possibility that higher CDAI may result from the intake of multivitamin and multimineral supplements, vitamin B6, vitamin D, vitamin K, calcium, phosphorus, and magnesium intake were included as covariates. However, CDAI and the prevalence of MASLD still showed a significant negative correlation in all three models (Table 5). Stratified analyses to explore the association between CDAI and MASLD by age, sex, race, PIR, and energy intake (converted to quartiles) showed that none of these variables significantly modified the relationship between CDAI and MASLD (p for interaction ≥0.05; Figure 4).
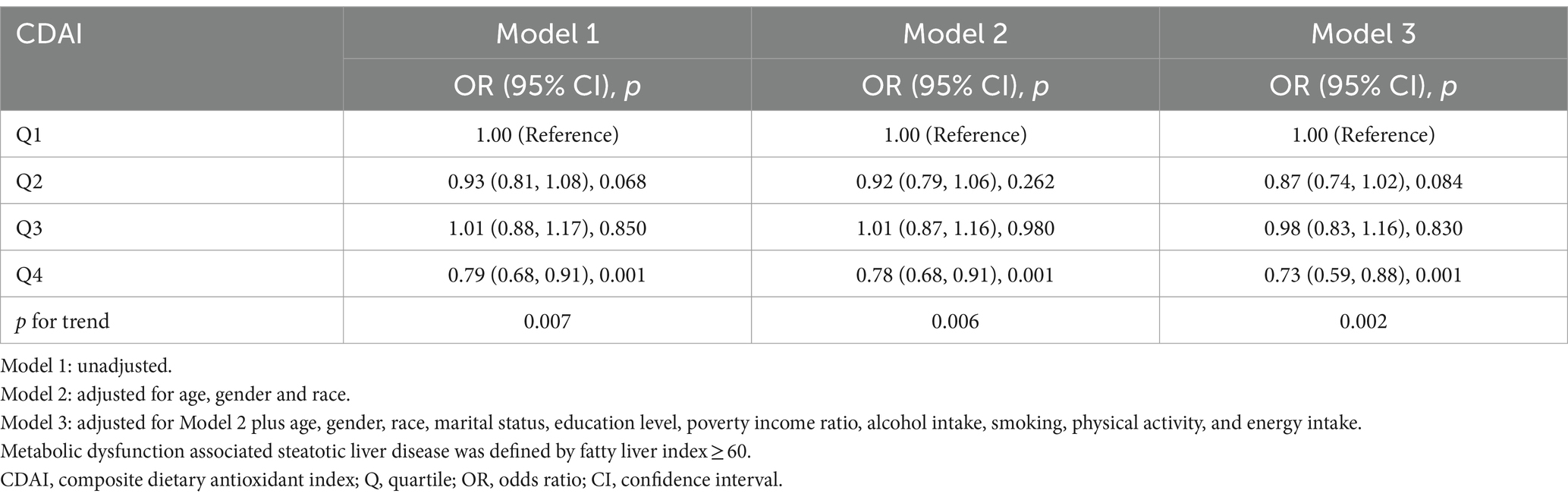
Table 3. Association of composite dietary antioxidant index and metabolic dysfunction associated steatotic liver disease defined by fatty liver index.
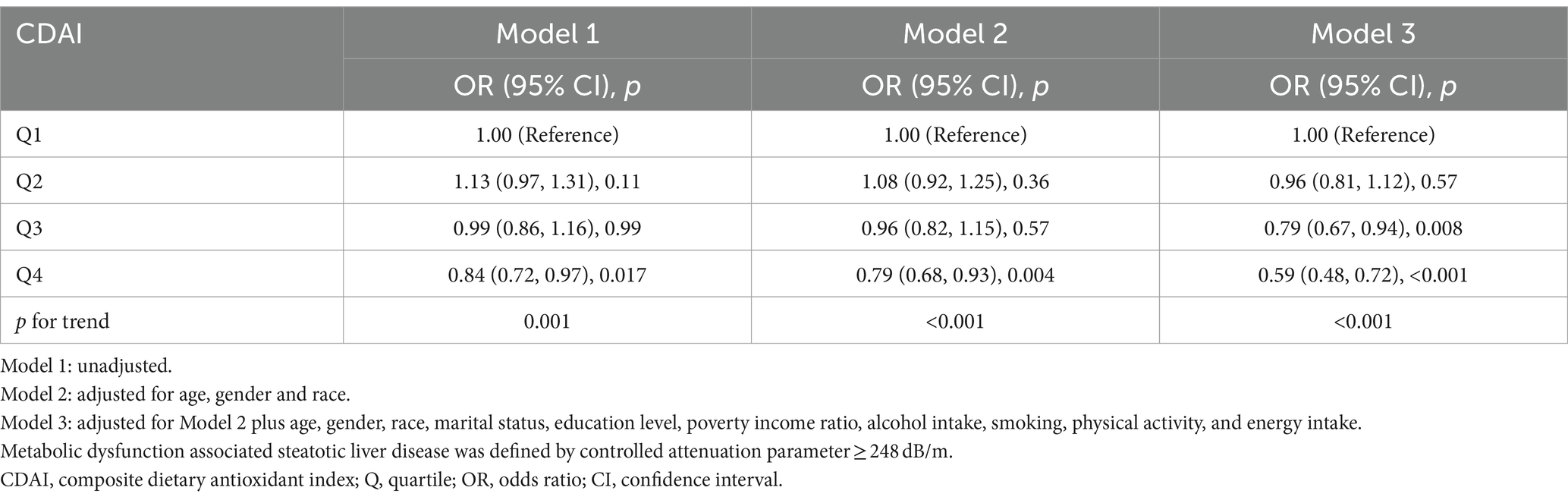
Table 4. Association of composite dietary antioxidant index and metabolic dysfunction associated steatotic liver disease excluding individuals with extreme energy intake.
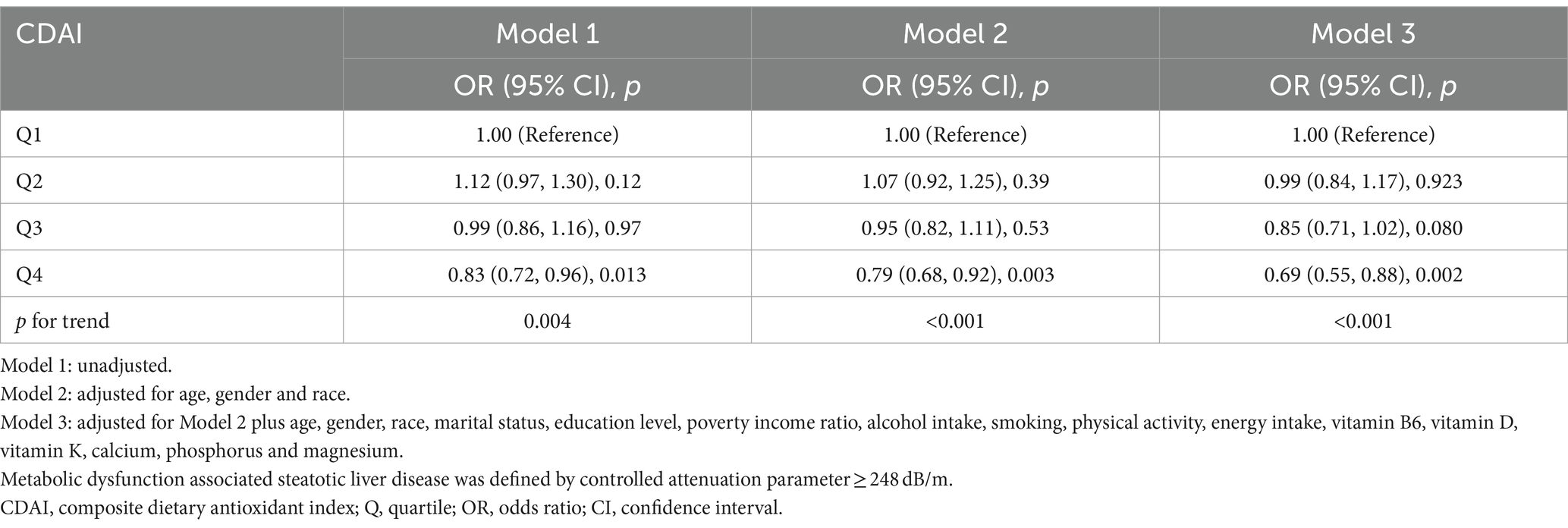
Table 5. Association of composite dietary antioxidant index and metabolic dysfunction associated steatotic liver disease after multivitamin and multimineral adjustment.
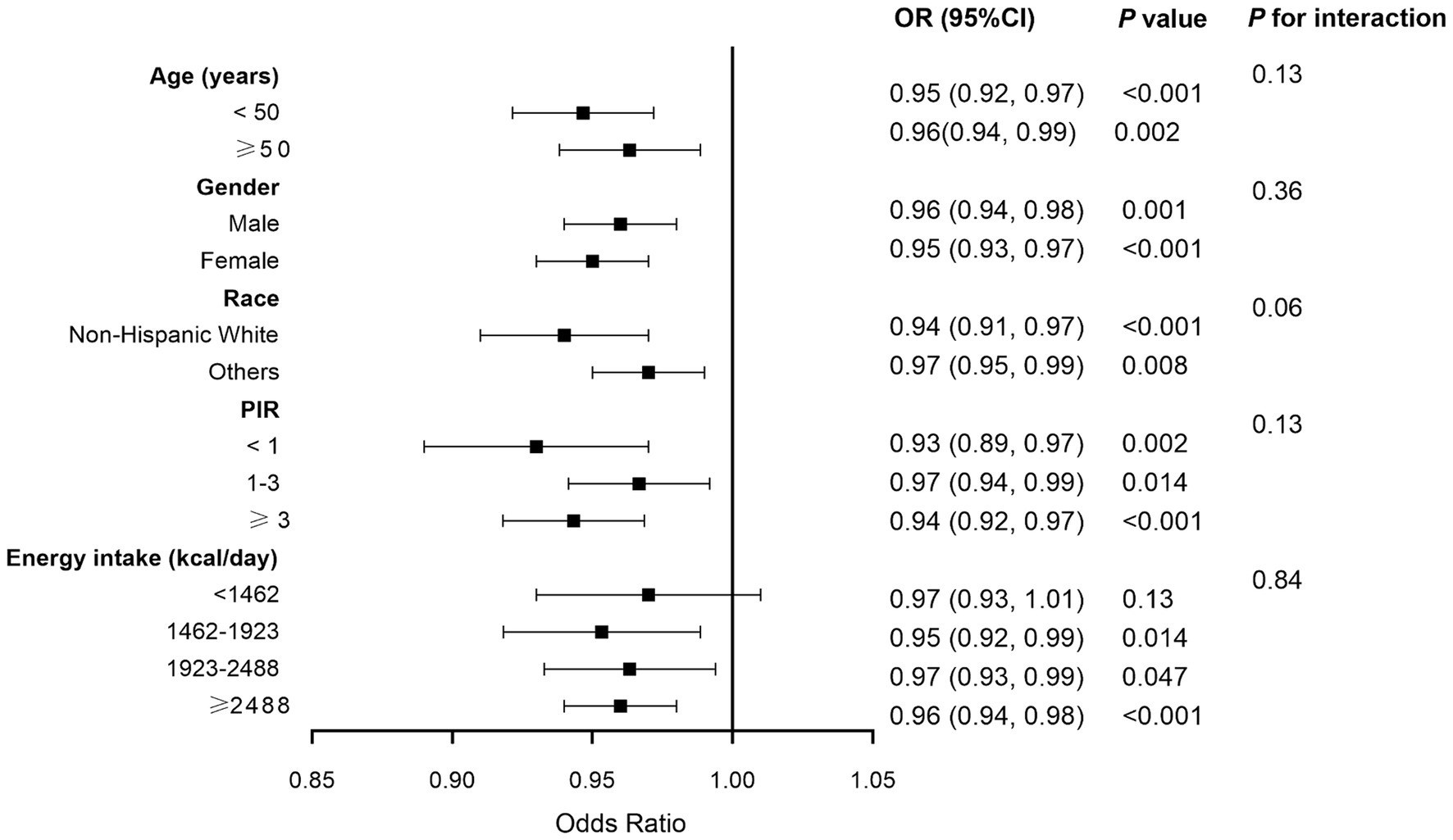
Figure 4. Forest plot of stratified analyses of the relationship between composite dietary antioxidant index and metabolic dysfunction associated steatotic liver disease. Analyses were adjusted for covariates age, gender, race, marital status, education level, poverty income ratio, alcohol intake, smoking, physical activity, and energy intake, when they were not the strata variables. OR, odds ratio; CI, confidence interval.
3.3 Association of CDAI with advanced liver fibrosis
Weighted multivariate logistic regression analysis showed that, without any adjustment, the prevalence of advanced liver fibrosis was lower in CDAI Q4 (OR 0.99; 95% CI: 0.69, 1.43, p = 0.98) than in Q1 (as reference), but this difference was not statistically significant. After adjusting for confounding factors, similarly no statistically significant difference was found between Q4 and Q1 in Model 3 (OR 0.87; 95% CI: 0.54, 1.41, p = 0.56). These findings are presented in Table 6.
4 Discussion
Increases in the prevalence of obesity and metabolic diseases have been associated with increases in the incidence of MASLD. Lifestyle modifications such as exercise and dietary interventions have been found effective in treating MASLD, but their underlying mechanisms remain unclear. To our knowledge, the present study is the first to explore the relationship between CDAI and MASLD in a large sample population. This study showed a significant negative linear association between CDAI scores and the risk of developing MASLD, even after adjusting for covariates.
MASLD is a complex multi-factorial disease, associated with various genetic, epigenetic, and environmental factors, with its pathogenesis being incompletely understood (38–40). The “multiple hit” hypothesis involving many potentially concurrent factors may provide a suitable explanation of MASLD. Oxidative stress, one of the factors contributing to these hits (39, 41), is a reflection of an imbalance between the generation of reactive species and the clearing capacity of the antioxidant system, favoring the former (42). At high concentrations, reactive species can induce oxidative modifications in cellular macromolecules (DNA, lipids, proteins, etc.), leading to the accumulation of damaged macromolecules and triggering liver injury (43, 44). Oxidative stress assessment involves (1) direct measurement of reactive species levels, (2) measurement of oxidative damage to biomolecules, and/or (3) assessment of antioxidant status. Many antioxidant biomarkers have been applied to evaluate the redox status of MASLD (44).
Pro-inflammatory diets can amplify the inflammatory responses within the body by increasing oxidative stress and immune dysregulation. Unhealthy dietary habits are often associated with higher levels of inflammatory cytokines, which can promote the development of atherosclerosis (45). The CDAI, which measures the dietary intake of antioxidants such as vitamins A, C, and E, selenium, zinc, and carotenoids, has been used to measure dietary antioxidant intake, population norms, and the overall impact of antioxidants on health outcomes (16, 46). Although individual antioxidants may play a role in the pathogenesis of MASLD, biological interactions among dietary antioxidants should also be considered (47–50). In our study, CDAI was measured to estimate combined exposure to six dietary antioxidants, resulting in a potential dose–response association between combined antioxidant intake and MASLD risk. That is, increased CDAI was associated with a lower risk of MASLD.
Several previous studies have assessed the relationship between intake of a specific vitamin with MASLD (51–54). These studies found that vitamin C, vitamin E, and carotenoids were negatively associated with the risk of MASLD, while vitamin A was positively associated with the risk of MASLD (51–54). In our study, a negative correlation between vitamin C, vitamin E, and carotenoids and the risk of developing MASLD was also found. Nevertheless, the results of our study did not indicate a significant correlation between vitamin A and MASLD (although a strong tendency was found p = 0.051). Furthermore, the present study found that the CDAI, a widely validated comprehensive antioxidant index, correlated negatively with the risk of developing MASLD.
To our knowledge, our study was the first to assess the relationship between CDAI and MASLD in a large national sample, finding a significant negative correlation between the two. Although the present study did not directly investigate the underlying mechanisms, several possibilities should be considered. Oxidative stress is a key factor in the pathogenesis of MASLD (9, 10, 39, 55). Antioxidant intake can reduce the overall level of oxidative stress (56–58). Using the CDAI index as a comprehensive assessment of dietary antioxidants may be a tool able to more comprehensively assess the relationship between the redox state and the pathogenesis of MASLD.
The present study had several limitations. Its cross-sectional design and lack of information on time of diagnosis prevented the determination of a causal relationship between CDAI and MASLD. Secondly, although the models controlled for covariates, all relevant covariates may not have been considered. Thirdly, due to the nature of the NHANES database, information on dietary intake was self-reported, which may have introduced recall bias. Large-scale prospective studies are therefore needed to better understand the relationship between the CDAI and MASLD.
5 Conclusion
This study demonstrated a significant negative association between CDAI scores and MASLD in United States adults. Moreover, these results suggested that diet rich in antioxidants may reduce the risk of MASLD, but additional studies in clinical cohorts are needed to confirm these findings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: the survey data are publicly available on the internet for data users and researchers throughout the world (www.cdc.gov/nchs/nhanes).
Ethics statement
The studies involving humans were approved by the parts of this study that involved human participants, human materials, or human data were conducted in compliance with the Declaration of Helsinki and were approved by the National Center for Health Statistics (NCHS) Ethics Review Board. The patients/participants provided written informed consent to participate in this study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZZ: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. HW: Visualization, Writing – original draft, Writing – review & editing. YC: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported Basic Research General Project of Shenzhen Science and Technology Plan Project (No. JCYJ20190809180211430), Key Disease Project of the Hospital: Viral Hepatitis Special Disease.
Acknowledgments
We appreciate the people who contributed to the NHANES data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CAP, Controlled attenuation parameter; CDAI, Composite Dietary Antioxidant Index; CI, Confidence interval; DTAC, Dietary total antioxidant capacity; FDA, Food and Drug Administration; FLI, Fatty liver index; LSM, Liver stiffness measurement; MAFLD, Metabolic associated fatty liver disease; MASH, Metabolic dysfunction associated steatohepatitis; MASLD, Metabolic dysfunction associated steatotic liver disease; NAFLD, Non-alcoholic fatty liver disease; NHANES, National Health and Nutrition Examination Survey; ORs, Odds ratios; PIR, Poverty income ratio; Q, Quartile; RCS, Restricted cubic spline.
Footnotes
References
1. Rinella, ME, Neuschwander-Tetri, BA, Siddiqui, MS, Abdelmalek, MF, Caldwell, S, Barb, D, et al. Aasld practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. (2023) 77:1797–835. doi: 10.1097/hep.0000000000000323
2. Chalasani, N, Younossi, Z, Lavine, JE, Charlton, M, Cusi, K, Rinella, M, et al. The diagnosis and Management of Nonalcoholic Fatty Liver Disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. (2018) 67:328–57. doi: 10.1002/hep.29367
3. Harrison, SA, Bedossa, P, Guy, CD, Schattenberg, JM, Loomba, R, Taub, R, et al. A phase 3, randomized, controlled trial of Resmetirom in Nash with liver fibrosis. N Engl J Med. (2024) 390:497–509. doi: 10.1056/NEJMoa2309000
4. Karim, G, and Bansal, MB. Resmetirom: an orally administered, Smallmolecule, liver-directed, Β-selective Thr agonist for the treatment of non-alcoholic fatty liver disease and non-alcoholic Steatohepatitis. touchREV Endocrinol. (2023) 19:60–70. doi: 10.17925/ee.2023.19.1.60
5. Long, MT, Noureddin, M, and Lim, JK. Aga clinical practice update: diagnosis and management of nonalcoholic fatty liver disease in lean individuals: expert review. Gastroenterology. (2022) 163:764–774.e1. doi: 10.1053/j.gastro.2022.06.023
6. Nádasdi, Á, Somogyi, A, Igaz, P, and Firneisz, G. Non-alcoholic fatty liver disease - a summary and update based on the Easl-Easd-Easo clinical practice guidelines of 2016. Orv Hetil. (2018) 159:1815–30. doi: 10.1556/650.2018.31231
7. Bischoff, SC, Bernal, W, Dasarathy, S, Merli, M, Plank, LD, Schütz, T, et al. Espen practical guideline: clinical nutrition in liver disease. Nutr Hosp. (2022) 39:434–72. doi: 10.20960/nh.03856
8. Rinella, ME, Tacke, F, Sanyal, AJ, and Anstee, QM. Report on the Aasld/Easl joint workshop on clinical trial endpoints in Nafld. J Hepatol. (2019) 71:823–33. doi: 10.1016/j.jhep.2019.04.019
9. Gonçalves, IO, Passos, E, Rocha-Rodrigues, S, Torrella, JR, Rizo, D, Santos-Alves, E, et al. Physical exercise antagonizes clinical and anatomical features characterizing Lieber-Decarli diet-induced obesity and related metabolic disorders. Clin Nutr. (2015) 34:241–7. doi: 10.1016/j.clnu.2014.03.010
10. Passos, E, Pereira, CD, Gonçalves, IO, Rocha-Rodrigues, S, Silva, N, Guimarães, JT, et al. Role of physical exercise on hepatic insulin, glucocorticoid and inflammatory signaling pathways in an animal model of non-alcoholic Steatohepatitis. Life Sci. (2015) 123:51–60. doi: 10.1016/j.lfs.2014.12.013
11. Ascensão, A, Martins, MJ, Santos-Alves, E, Gonçalves, IO, Portincasa, P, Oliveira, PJ, et al. Modulation of hepatic redox status and mitochondrial metabolism by exercise: therapeutic strategy for liver diseases. Mitochondrion. (2013) 13:862–70. doi: 10.1016/j.mito.2013.07.002
12. Semmler, G, Datz, C, Reiberger, T, and Trauner, M. Diet and exercise in Nafld/Nash: beyond the obvious. Liver Int. (2021) 41:2249–68. doi: 10.1111/liv.15024
13. Zhang, S, Huo, Z, Borné, Y, Meng, G, Zhang, Q, Liu, L, et al. Adherence to a healthy lifestyle including sleep and sedentary behaviors and risk of metabolic dysfunction-associated Steatotic liver disease in Chinese adults. Prev Med. (2024) 184:107971. doi: 10.1016/j.ypmed.2024.107971
14. Chen, Z, Tian, R, She, Z, Cai, J, and Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. (2020) 152:116–41. doi: 10.1016/j.freeradbiomed.2020.02.025
15. Sohouli, MH, Fatahi, S, Sayyari, A, Olang, B, and Shidfar, F. Associations between dietary total antioxidant capacity and odds of non-alcoholic fatty liver disease (Nafld) in adults: a case-control study. J Nutr Sci. (2020) 9:e48. doi: 10.1017/jns.2020.39
16. Wright, ME, Mayne, ST, Stolzenberg-Solomon, RZ, Li, Z, Pietinen, P, Taylor, PR, et al. Development of a comprehensive dietary antioxidant index and application to lung Cancer risk in a cohort of male smokers. Am J Epidemiol. (2004) 160:68–76. doi: 10.1093/aje/kwh173
17. Chen, X, Lu, H, Chen, Y, Sang, H, Tang, Y, and Zhao, Y. Composite dietary antioxidant index was negatively associated with the prevalence of diabetes independent of cardiovascular diseases. Diabetol Metab Syndr. (2023) 15:183. doi: 10.1186/s13098-023-01150-6
18. Wu, M, Si, J, Liu, Y, Kang, L, and Xu, B. Association between composite dietary antioxidant index and hypertension: insights from Nhanes. Clin Exp Hypertens. (2023) 45:2233712. doi: 10.1080/10641963.2023.2233712
19. Wang, M, Huang, ZH, Zhu, YH, He, P, and Fan, QL. Association between the composite dietary antioxidant index and chronic kidney disease: evidence from Nhanes 2011-2018. Food Funct. (2023) 14:9279–86. doi: 10.1039/d3fo01157g
20. Zhao, L, Sun, Y, Cao, R, Wu, X, Huang, T, and Peng, W. Non-linear association between composite dietary antioxidant index and depression. Front Public Health. (2022) 10:988727. doi: 10.3389/fpubh.2022.988727
21. Wang, L, and Yi, Z. Association of the composite dietary antioxidant index with all-cause and cardiovascular mortality: a prospective cohort study. Front Cardiovasc Med. (2022) 9:993930. doi: 10.3389/fcvm.2022.993930
22. Kaushik, AS, Strath, LJ, and Sorge, RE. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. (2020) 9:487–98. doi: 10.1007/s40122-020-00200-5
23. Zha, P, Wei, L, Liu, W, Chen, Y, and Zhou, Y. Effects of dietary supplementation with Chlorogenic acid on growth performance, antioxidant capacity, and hepatic inflammation in broiler chickens subjected to Diquat-induced oxidative stress. Poult Sci. (2023) 102:102479. doi: 10.1016/j.psj.2023.102479
24. Kashino, I, Mizoue, T, Serafini, M, Akter, S, Sawada, N, Ishihara, J, et al. Higher dietary non-enzymatic antioxidant capacity is associated with decreased risk of all-cause and cardiovascular disease mortality in Japanese adults. J Nutr. (2019) 9:nxz145. doi: 10.1093/jn/nxz145
25. Yamagata, K, and Yamori, Y. Inhibition of endothelial dysfunction by dietary flavonoids and preventive effects against cardiovascular disease. J Cardiovasc Pharmacol. (2020) 75:1–9. doi: 10.1097/fjc.0000000000000757
26. Sahu, P, Chhabra, P, and Mehendale, AM. A comprehensive review on non-alcoholic fatty liver disease. Cureus. (2023) 15:e50159. doi: 10.7759/cureus.50159
27. Eddowes, PJ, Sasso, M, Allison, M, Tsochatzis, E, Anstee, QM, Sheridan, D, et al. Accuracy of Fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1717–30. doi: 10.1053/j.gastro.2019.01.042
28. Karlas, T, Petroff, D, Sasso, M, Fan, JG, Mi, YQ, de Lédinghen, V, et al. Individual patient data Meta-analysis of controlled attenuation parameter (cap) Technology for Assessing Steatosis. J Hepatol. (2017) 66:1022–30. doi: 10.1016/j.jhep.2016.12.022
29. Kim, D, Cholankeril, G, Loomba, R, and Ahmed, A. Prevalence of fatty liver disease and fibrosis detected by transient Elastography in adults in the United States, 2017-2018. Clin Gastroenterol Hepatol. (2021) 19:1499–501.e2. doi: 10.1016/j.cgh.2020.08.017
30. Wang, L, Martínez Steele, E, Du, M, Pomeranz, JL, O'Connor, LE, Herrick, KA, et al. Trends in consumption of Ultraprocessed foods among us youths aged 2-19 years, 1999-2018. JAMA. (2021) 326:519–30. doi: 10.1001/jama.2021.10238
31. Xu, Q, Qian, X, Sun, F, Liu, H, Dou, Z, and Zhang, J. Independent and joint associations of dietary antioxidant intake with risk of post-stroke depression and all-cause mortality. J Affect Disord. (2023) 322:84–90. doi: 10.1016/j.jad.2022.11.013
32. Tian, T, Zhang, J, Xie, W, Ni, Y, Fang, X, Liu, M, et al. Dietary quality and relationships with metabolic dysfunction-associated fatty liver disease (Mafld) among United States adults, results from Nhanes 2017-2018. Nutrients. (2022) 14:4505. doi: 10.3390/nu14214505
33. Rehkopf, DH, Needham, BL, Lin, J, Blackburn, EH, Zota, AR, Wojcicki, JM, et al. Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: a cross-sectional study of us adults. PLoS Med. (2016) 13:e1002188. doi: 10.1371/journal.pmed.1002188
34. Raza, SA, Sokale, IO, and Thrift, AP. Burden of high-risk phenotype of heavy alcohol consumption among obese U.S. population: Results from National Health and nutrition examination survey, 1999-2020. Lancet Reg Health Am. (2023) 23:100525. doi: 10.1016/j.lana.2023.100525
35. Chen, J, Mao, X, Deng, M, and Luo, G. Validation of nonalcoholic fatty liver disease (Nafld) related steatosis indices in metabolic associated fatty liver disease (Mafld) and comparison of the diagnostic accuracy between Nafld and Mafld. Eur J Gastroenterol Hepatol. (2023) 35:394–401. doi: 10.1097/meg.0000000000002497
36. Koehler, EM, Schouten, JN, Hansen, BE, Hofman, A, Stricker, BH, and Janssen, HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. (2013) 11:1201–4. doi: 10.1016/j.cgh.2012.12.031
37. Meffert, PJ, Baumeister, SE, Lerch, MM, Mayerle, J, Kratzer, W, and Völzke, H. Development, external validation, and comparative assessment of a new diagnostic score for hepatic steatosis. Am J Gastroenterol. (2014) 109:1404–14. doi: 10.1038/ajg.2014.155
38. Chen, Z, Yu, Y, Cai, J, and Li, H. Emerging molecular targets for treatment of nonalcoholic fatty liver disease. Trends Endocrinol Metab. (2019) 30:903–14. doi: 10.1016/j.tem.2019.08.006
39. Passos, E, Ascensão, A, Martins, MJ, and Magalhães, J. Endoplasmic reticulum stress response in non-alcoholic Steatohepatitis: the possible role of physical exercise. Metabolism. (2015) 64:780–92. doi: 10.1016/j.metabol.2015.02.003
40. Li, Y, Yang, P, Ye, J, Xu, Q, Wu, J, and Wang, Y. Updated mechanisms of Masld pathogenesis. Lipids Health Dis. (2024) 23:117. doi: 10.1186/s12944-024-02108-x
41. Li, J, Cordero, P, Nguyen, V, and Oben, JA. The role of vitamins in the pathogenesis of non-alcoholic fatty liver disease. Integr Med Insights. (2016) 11:19–25. doi: 10.4137/imi.S31451
42. Takaki, A, Kawai, D, and Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic Steatohepatitis (Nash). Int J Mol Sci. (2013) 14:20704–28. doi: 10.3390/ijms141020704
43. Rolo, AP, Teodoro, JS, and Palmeira, CM. Role of oxidative stress in the pathogenesis of nonalcoholic Steatohepatitis. Free Radic Biol Med. (2012) 52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003
44. Serviddio, G, Bellanti, F, and Vendemiale, G. Free radical biology for medicine: learning from nonalcoholic fatty liver disease. Free Radic Biol Med. (2013) 65:952–68. doi: 10.1016/j.freeradbiomed.2013.08.174
45. Ignarro, LJ, Balestrieri, ML, and Napoli, C. Nutrition, physical activity, and cardiovascular disease: an update. Cardiovasc Res. (2007) 73:326–40. doi: 10.1016/j.cardiores.2006.06.030
46. Maugeri, A, Hruskova, J, Jakubik, J, Kunzova, S, Sochor, O, Barchitta, M, et al. Dietary antioxidant intake decreases carotid intima media thickness in women but not in men: a cross-sectional assessment in the Kardiovize study. Free Radic Biol Med. (2019) 131:274–81. doi: 10.1016/j.freeradbiomed.2018.12.018
47. Zou, Z, Xi, W, Hu, Y, Nie, C, and Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. (2016) 196:885–96. doi: 10.1016/j.foodchem.2015.09.072
48. Inoue, T, Komoda, H, Uchida, T, and Node, K. Tropical fruit Camu-Camu (Myrciaria Dubia) has anti-oxidative and anti-inflammatory properties. J Cardiol. (2008) 52:127–32. doi: 10.1016/j.jjcc.2008.06.004
49. Henning, T, and Weber, D. Redox biomarkers in dietary interventions and nutritional observation studies - from new insights to old problems. Redox Biol. (2021) 41:101922. doi: 10.1016/j.redox.2021.101922
50. Oyeyinka, BO, and Afolayan, AJ. Potentials of Musa species fruits against oxidative stress-induced and diet-linked chronic diseases: in vitro and in vivo implications of micronutritional factors and dietary secondary metabolite compounds. Molecules. (2020) 25:5036. doi: 10.3390/molecules25215036
51. Xie, ZQ, Li, HX, Tan, WL, Yang, L, Ma, XW, Li, WX, et al. Association of Serum Vitamin C with Nafld and Mafld among adults in the United States. Front Nutr. (2021) 8:795391. doi: 10.3389/fnut.2021.795391
52. Wegermann, K, Howe, C, Henao, R, Wang, Y, Guy, CD, Abdelmalek, MF, et al. Serum bile acid, vitamin E, and serotonin metabolites are associated with future liver-related events in nonalcoholic fatty liver disease. Hepatol Commun. (2021) 5:608–17. doi: 10.1002/hep4.1665
53. Xiao, ML, Chen, GD, Zeng, FF, Qiu, R, Shi, WQ, Lin, JS, et al. Higher serum carotenoids associated with improvement of non-alcoholic fatty liver disease in adults: a prospective study. Eur J Nutr. (2019) 58:721–30. doi: 10.1007/s00394-018-1678-1
54. Xiao, ML, Zhong, HL, Lin, HR, Liu, CY, Yan, Y, Ke, YB, et al. Higher serum vitamin a is associated with a worsened progression of non-alcoholic fatty liver disease in adults: a prospective study. Food Funct. (2022) 13:970–7. doi: 10.1039/d1fo03119h
55. García-Ruiz, C, and Fernández-Checa, JC. Mitochondrial oxidative stress and antioxidants balance in fatty liver disease. Hepatol Commun. (2018) 2:1425–39. doi: 10.1002/hep4.1271
56. Jimenez-Gutierrez, GE, Martínez-Gómez, LE, Martínez-Armenta, C, Pineda, C, Martínez-Nava, GA, and Lopez-Reyes, A. Molecular mechanisms of inflammation in sarcopenia: diagnosis and therapeutic update. Cells. (2022) 11:2359. doi: 10.3390/cells11152359
57. Oteiza, PI, Fraga, CG, and Galleano, M. Linking biomarkers of oxidative stress and disease with flavonoid consumption: from experimental models to humans. Redox Biol. (2021) 42:101914. doi: 10.1016/j.redox.2021.101914
58. Aranha, LN, Silva, MG, Uehara, SK, Luiz, RR, Nogueira Neto, JF, Rosa, G, et al. Effects of a Hypoenergetic diet associated with Açaí (Euterpe Oleracea Mart.) pulp consumption on antioxidant status, oxidative stress and inflammatory biomarkers in overweight, Dyslipidemic individuals. Clin Nutr. (2020) 39:1464–9. doi: 10.1016/j.clnu.2019.06.008
Keywords: metabolic dysfunction associated steatotic liver disease, composite dietary antioxidant index, oxidative stress, liver stiffness, restricted cubic spline
Citation: Zhang Z, Wang H and Chen Y (2024) Association between composite dietary antioxidant index and metabolic dysfunction associated steatotic liver disease: result from NHANES, 2017-2020. Front. Nutr. 11:1412516. doi: 10.3389/fnut.2024.1412516
Edited by:
Maria João Martins, University of Porto, PortugalReviewed by:
Alejandro Santos, University of Porto, PortugalCidália Pereira, Politechnic of Leiria, Portugal
Copyright © 2024 Zhang, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youpeng Chen, Y2hlbnlvdXBlbmdAc3lzdXNoLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zhaofu Zhang
Zhaofu Zhang Hao Wang†
Hao Wang† Youpeng Chen
Youpeng Chen