- 1College of Animal Science and Technology, Hunan Agricultural University, Changsha, China
- 2Laboratory of Animal Nutritional Physiology and Metabolic Process, Key Laboratory of Agro-Ecological Processes in Subtropical Region, National Engineering Laboratory for Pollution Control and Waste Utilization in Livestock and Poultry Production, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China
Dietary supplementation with aromatic amino acids (AAAs) has been demonstrated to alleviate intestinal inflammation induced by lipopolysaccharide (LPS) in the piglets. But the mechanism of AAA sensing and utilization under inflammatory conditions is not well-understood. The study was conducted with 32 weanling piglets using a 2 × 2 factorial arrangement (diet and LPS challenge) in a randomized complete block design. Piglets were fed as basal diet or the basal diet supplemented with 0.16% tryptophan (Trp), 0.41% phenylalanine (Phe), and 0.22% tyrosine (Tyr) for 21 days. The results showed that LPS treatment significantly reduced the concentrations of cholecystokinin (CCK) and total protein but increased leptin concentration, the activities of alanine transaminase, and aspartate aminotransferase in serum. Dietary supplementation with AAAs significantly increased the serum concentrations of CCK, peptide YY (PYY), and total protein but decreased the blood urea nitrogen. LPS challenge reduced the ileal threonine (Thr) digestibility, as well as serum isoleucine (Ile) and Trp concentrations, but increased the serum concentrations of Phe, Thr, histidine (His), alanine (Ala), cysteine (Cys), and serine (Ser) (P < 0.05). The serum-free amino acid concentrations of His, lysine (Lys), arginine (Arg), Trp, Tyr, Cys, and the digestibilities of His, Lys, Arg, and Cys were significantly increased by feeding AAA diets (P < 0.05). Dietary AAA supplementation significantly increased the serum concentrations of Trp in LPS-challenged piglets (P < 0.05). In the jejunal mucosa, LPS increased the contents of Ala and Cys, and the mRNA expressions of solute carrier (SLC) transporters (i.e., SLC7A11, SLC16A10, SLC38A2, and SLC3A2), but decreased Lys and glutamine (Gln) contents, and SLC1A1 mRNA expression (P < 0.05). In the ileal mucosa, LPS challenge induced increasing in SLC7A11 and SLC38A2 and decreasing in SLC38A9 and SLC36A1 mRNA expressions, AAAs supplementation significantly decreased mucosal amino acid (AA) concentrations of methionine (Met), Arg, Ala, and Tyr, etc. (P < 0.05). And the interaction between AAAs supplementation and LPS challenge significantly altered the expressions of SLC36A1 and SLC38A9 mRNA (P < 0.05). Together, these findings indicated that AAAs supplementation promoted the AAs absorption and utilization in the small intestine of piglets and increased the mRNA expressions of SLC transports to meet the high demands for specific AAs in response to inflammation and immune response.
Introduction
During the immunological stress, amino acids (AAs) are redistributed away from protein production toward tissues involved in inflammation and immune response (1–3). The metabolism reprogramming in the immune process could affect the animal's ability to sense and demand AAs because AAs are used as a substrate for the synthesis of inflammatory proteins and immunoglobulins (4). Therefore, the transportation and metabolism of AA are important for immune cells. Immunological stress and inflammation will lead to the increase of basal metabolic rate, which directly leads to metabolic changes (5). The increased synthesis of immune system metabolites such as acute phase proteins, immunoglobulin, and glutathione is accompanied by the increased demand for specific AA (6). For example, the dietary tyrosine (Tyr), phenylalanine (Phe), and tryptophan (Trp) requirements are increased to support the immune response under inflammation conditions in pigs (7). Circulating aromatic AAs (AAAs) as the crucial mediators in the communication between gut and brain participates in immune regulation (8).
The absorptions of dietary AAs by the small intestine play critical roles on extraintestinal tissues and the serum AA profiles are correlated with the mRNA expression levels for key AA transporters in the small intestine (9). Some AA transporters are transceptors with both transporting and sensing functions, which trigger the downstream signal transduction pathway such as the Target of Rapamycin Complex 1 (mTORC1) pathway and general control non-derepressible kinase pathway (10–13). The previous study has demonstrated that dietary supplementation with AAAs activated the Ca2+-sensing receptor (CaSR) signaling pathway and alleviated intestinal inflammation induced by LPS in piglets (14). CaSR is expressed in the enterocytes cells throughout the intestine and responds to a broad range of AAs, especially aromatic compounds (15). CaSR couples to the phosphatidylinositol pathway and has been linked to AAAs stimulation of CCK release and intracellular Ca2+ mobilization (16). It has been extensively documented that CaSR coordinated food digestion and nutrient absorption, promoted cell proliferation and differentiation, regulated energy metabolism and immune response, stimulated hormone secretion, mitigated secretory diarrhea, and enhanced intestinal barrier function (17–19). This would appear to provide a molecular explanation for AA absorption and utilization to support the inflammatory response in the intestine. The signaling pathways downstream of CaSR, phospholipase Cβ2, and NF-κB have been confirmed to be involved in the regulation of AAAs on intestinal inflammation (14). But it is not clear whether the CaSR activation is accompanied by the change of availability of AAs.
Therefore, the present study is conducted to investigate the effects of dietary supplementation with AAAs on the ileal apparent digestibility of AAs, serum and mucosal AAs profiles as well as AA transporters in the small intestine of LPS-challenged piglets.
Materials and Methods
The animal trial was approved by the Institutional Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (2013020).
Animal Experiment Design
The animal experimental design was based on the same experimental protocol that has been presented by Liu et al. (14). Briefly, a total of 32 cross-bred (Duroc × Landrace) weanling gilts and barrows (6.66 ± 0.31 kg body weight) were randomly assigned into four treatments (eight piglets /treatment) using a 2 × 2 factorial arrangement. The main factors were dietary treatment (piglets were fed the basal diet or the 0.16% Trp, 0.41% Phe, and 0.22% Tyr supplemented diet) and LPS challenge (piglets were challenged with LPS or treated with sterile saline). The diets preparation, feeding, and management of piglets were the same as the description in the previous study (14).
On the morning of day 21 after the initiation of the treatment, the piglets were intraperitoneally injected with either 100 μg/kg BW LPS (Escherichia coli strain O5:B55) or the same volume of 0.9% sterilized saline, respectively. Blood samples were collected from the jugular vein at 4 h after injection and serum samples were obtained by centrifugation at 2,000 g for 15 min and then stored at −80°C until further analysis. Jejunal and ileal mucosa were collected and immediately snap-frozen in liquid nitrogen and stored at −80°C for the analysis of free AA profiles and gene expression. In addition, digesta samples were collected from terminal ileum for the AAs digestibility analysis.
Analysis of Serum Metabolites and Hormones
Serum biochemical parameters, including total protein (TP), albumin (ALB), alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), glucose (GLU), and lactic dehydrogenase (LDH), were measured using Biochemical Analytical Instrument (Beckman CX4) and commercial kits (Sino-German Beijing Leadman Biotech Ltd, Beijing, China).
Serum samples were treated with sulfosalicylic acid, centrifuged, and filtered through a 0.45 μm filtration membrane. Then the amino acid concentrations were determined using an automatic amino acid analyzer (Model L-8900, Hitachi Ltd. Tokyo, Japan).
The serum concentrations of cholecystokinin (CCK), peptide YY (PYY), ghrelin, glucagon, and leptin were determined using the corresponding pig ELISA Kit (CUSABIO, Wuhan, China) in accordance with the manufacturer's instructions.
Determination of Free Amino Acids in the Intestinal Mucosa
About 0.5 g of jejunal and ileum mucosal tissues were weighed, and 5 ml of 0.1 M HCl homogenate was added, centrifuged for 10 min at 5,000 g. We took 0.5 ml of the supernatant, mixed it with the same volume of 8% sulfosalicylic acid, and left it resting at 4°C overnight. Centrifugation was performed at 12,000 g for 10 min. Then the supernatant was absorbed and centrifuged again at 12,000 g for 10 min, and then through filter membranes. Then the AA content was determined by a high-performance liquid chromatography (Agilent 1200, Agilent Technologies, USA). The test conditions were as following: wavelength: 254 nm; flow rate: 1 ml/min; column temperature: elution at 40°C; Acetonitrile: 0.02 mol/L; Ammonium formate = 30:70 (V:V).
Digestibility of Amino Acid Analysis
Feed samples and terminal ileal digesta samples (0.5 g) were accurately weighed and put into an ampere tube, 10 ml of 6 M hydrochloric acid was added. The tube was sealed with an alcohol torch, hydrolyzed at 110 ± 2°C for 24 h, and then transferred to a 100 ml volumetric flask after cooling. Took a constant volume of 1–25 ml from the above solution. Then filtered into the injection flask with a 0.22 μm membrane. The AAs content was determined by high-performance liquid chromatography (Agilent 1200, Agilent Technologies, USA).
The feed samples and ileal digesta after freeze-drying were weighed in parallel samples for analysis and determination. The AA profiles were detected by high-performance liquid chromatography (Agilent 1200, Agilent Technologies, USA). Lysine and threonine were detected after hydrolyzing with 6 mol/L HCl at 105°C for 24 h. Methionine was analyzed as methionine sulfone after cold performic acid oxidation overnight before hydrolysis. Tryptophan was determined after hydrolyzing with 4 mol/L LiOH at 110°C for 20 h. The apparent ileal digestibility (AID) of AAs was calculated using the following formula:
Where (Diet component/Chromium) d = ratio of diet component to Chromium in the diet and (Diet component/ Chromium) i = ratio of diet component to Chromium in the ileal digesta (20, 21).
Real-Time Quantitative RT-PCR
Total RNA was isolated from the liquid nitrogen-pulverized jejunal, and ileal mucosa samples, and cDNA were synthesized as previously described (9). The mRNA abundance of AA transporters including SLC1A1, SLC7A11, SLC1A5, SLC6A19, SLC6A20, SLC16A10, SLC36A1, SLC38A2, SLC38A9, SLC3A1, SLC6A14, SLC7A1, SLC7A2, SLC7A7, SLC7A9, and SLC3A2 were analyzed using quantitative real-time polymerase chain reaction analysis. The primer sequences for the tested genes are shown the previous study (9). Data are expressed as the relative values to those for piglets of the basal diet with saline injection treatment.
Statistical Analysis
All data were analyzed by ANOVA using the general linear model procedures of SPSS for a 2 × 2 factorial design (SPSS Inc., Chicago, IL, USA, 2001). The statistical model included the effects of challenge (saline or LPS), diet (basal or AAAs), and their interactions. When there was significant interaction. The differences among treatments were evaluated using the Duncan test. P < 0.05 was considered significant.
Results
Serum Concentrations of Gastrointestinal Hormones
Compared to the saline injected piglets, LPS administration remarkably decreased the serum CCK and increased leptin concentration. However, the supplementation of AAAs in the diet markedly increased the concentrations of CCK and PYY (P < 0.05). LPS challenge × diet had an interactive effect on serum leptin concentration (P < 0.05). There were no significant differences in serum concentrations of ghrelin and glucagon among all treatments (P > 0.05) (Table 1).
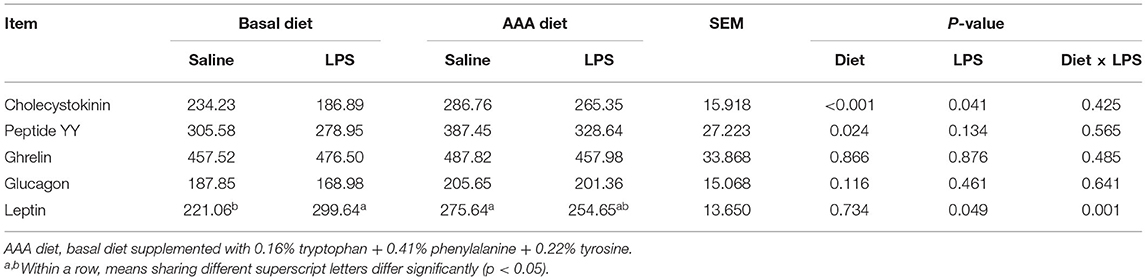
Table 1. Effects of dietary supplementation with AAA on serum concentrations of gastrointestinal hormones in piglets (pg/ml).
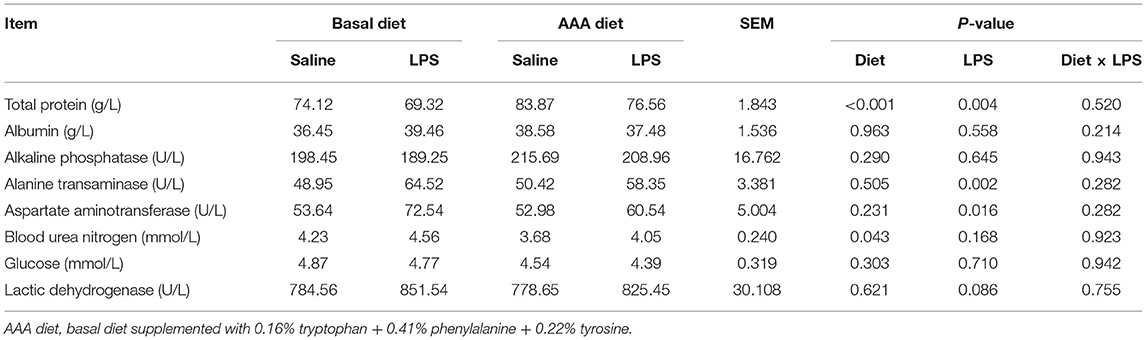
Serum Biochemical Parameters
As shown in Table 2, LPS treatment markedly increased the activity of alanine transaminase and aspartate aminotransferase but decreased the serum concentration of total protein (P < 0.05). Dietary AAAs supplementation increased the serum concentration of total protein and decreased the concentration of blood urea nitrogen (P < 0.05). There was no significant change of other determined serum biochemical parameters in response to LPS or AAA treatment (P > 0.05).
Serum Amino Acids Profiles
The results of serum AAs profiles were shown in Table 3. LPS treatment induced the increases in serum concentrations such as His, Phe, Thr, Ala, Cys, and Ser, also including total non-essential amino acids (NEAA), but the decreases of serum Ile and Trp concentrations (P < 0.05). Dietary supplementation of the AAAs improved the serum concentrations of His, Lys, Arg, Trp, Tyr, and Cys (P < 0.05). An interaction of LPS challenge × diet was observed for Trp content (P < 0.05).
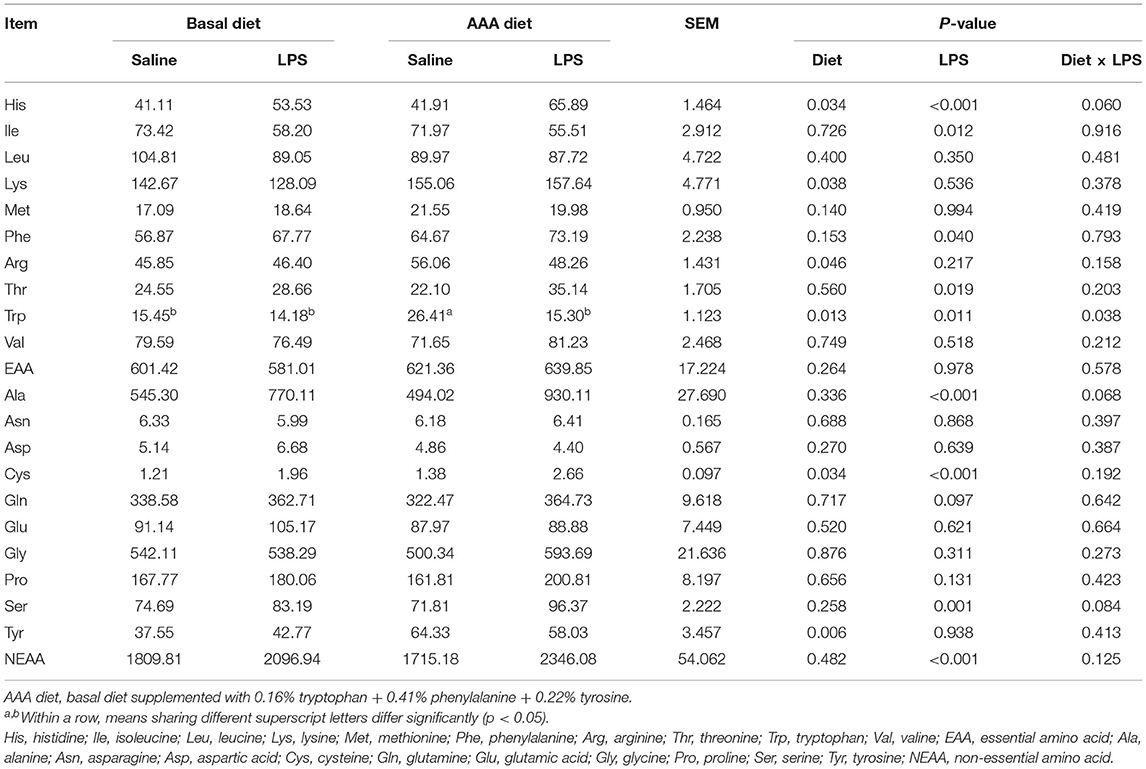
Table 3. Effects of dietary supplementation with AAA on serum concentration of amino acids in piglets (μmol/L).
The Jejunal and Ileal Mucosal Amino Acid Profiles and Apparent Ileal Digestibility of Amino Acid
In the jejunal mucosa, LPS significantly increased the contents of Ala and Cys but decreased the Lys and Gln contents (P < 0.05). Dietary supplementation with AAA had interactive effects with LPS (Diet × LPS, P < 0.05) on the Gly and Ser contents (Table 4).
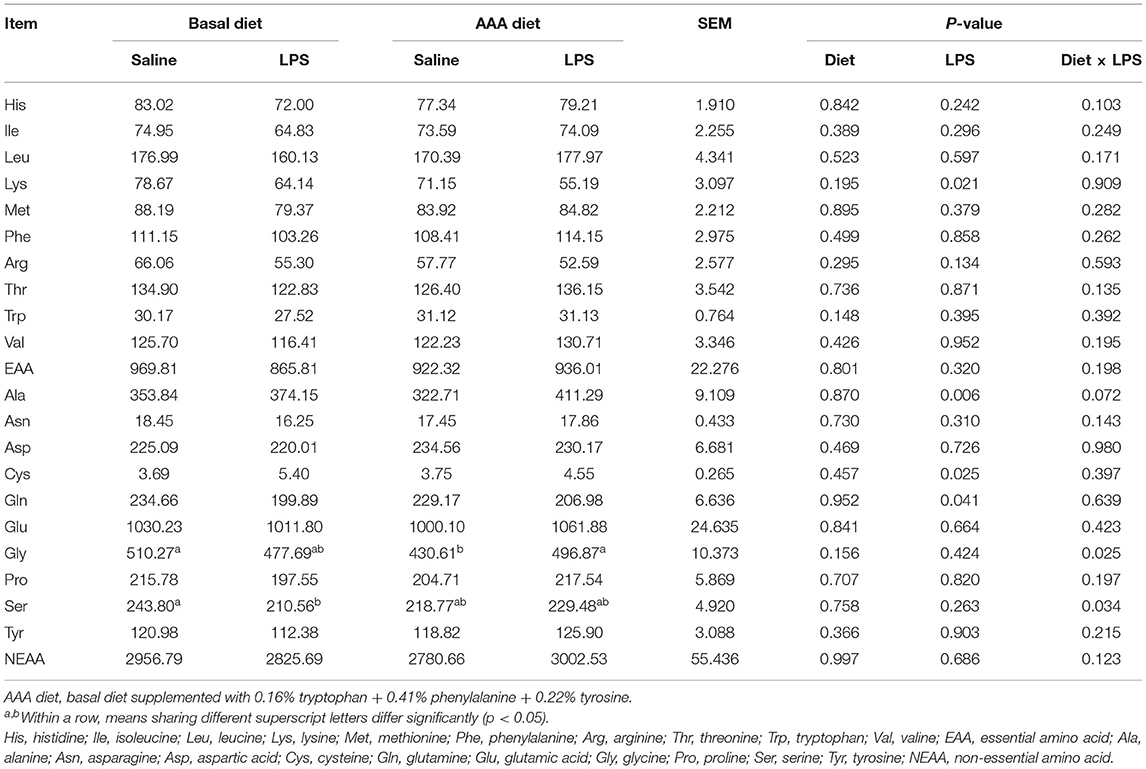
Table 4. Effects of dietary supplementation with AAA on the concentration of amino acid content in the jejunal mucosa (μg/g).
In the ileal mucosa, LPS injection had no effects on all determined AAs (P > 0.05). Dietary supplementation with AAA decreased the contents of Ile, Leu, Met, Phe, Thr, Val, Ala, Asn, Gly, Pro, Ser, and Tyr, as well as the total NEAA (P < 0.05). There was no interaction of LPS challenge × diet on all determined AAs contents (P > 0.05) (Table 5).
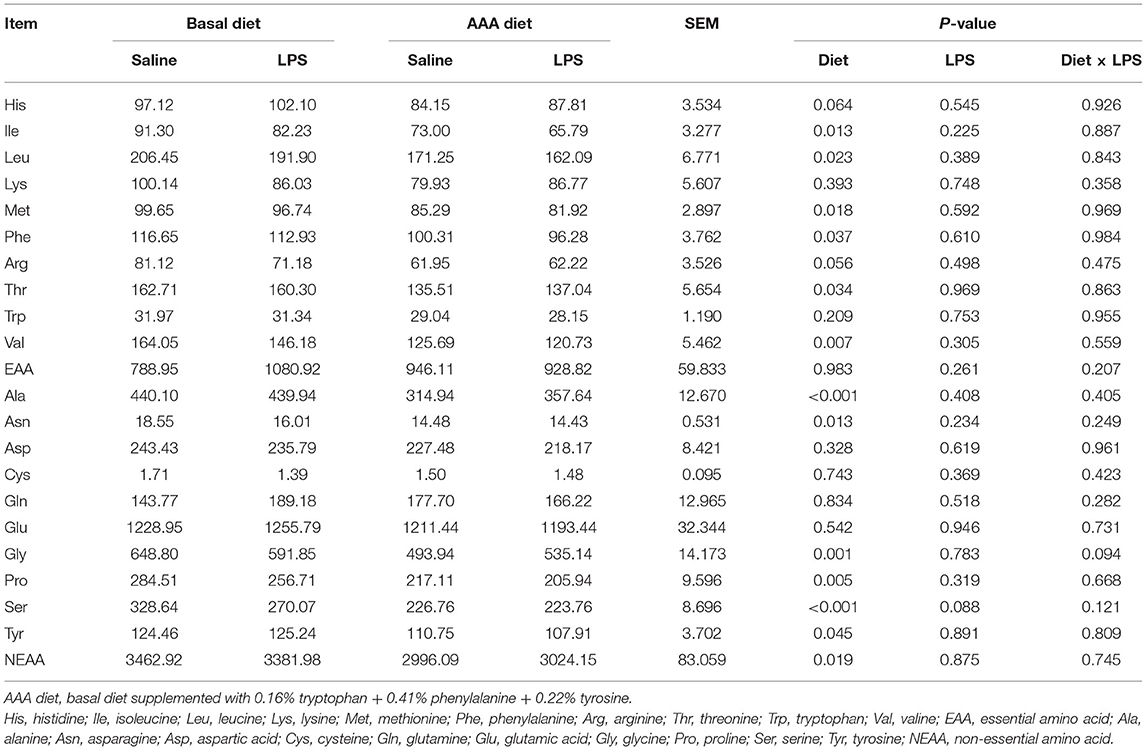
Table 5. Effects of dietary supplementation with AAA on the concentration of amino acid content in the ileal mucosa (μg/g).
The apparent ileal digestibility of AAs was shown in Table 6, LPS significantly increased the digestibility of His but decreased the digestibility of Thr. Dietary AAA supplementation enhanced the digestibility of His, Lys, Arg, and Cys (P < 0.05). There was no interaction of LPS challenge × diet on the apparent ileal digestibility of AAs (P > 0.05).
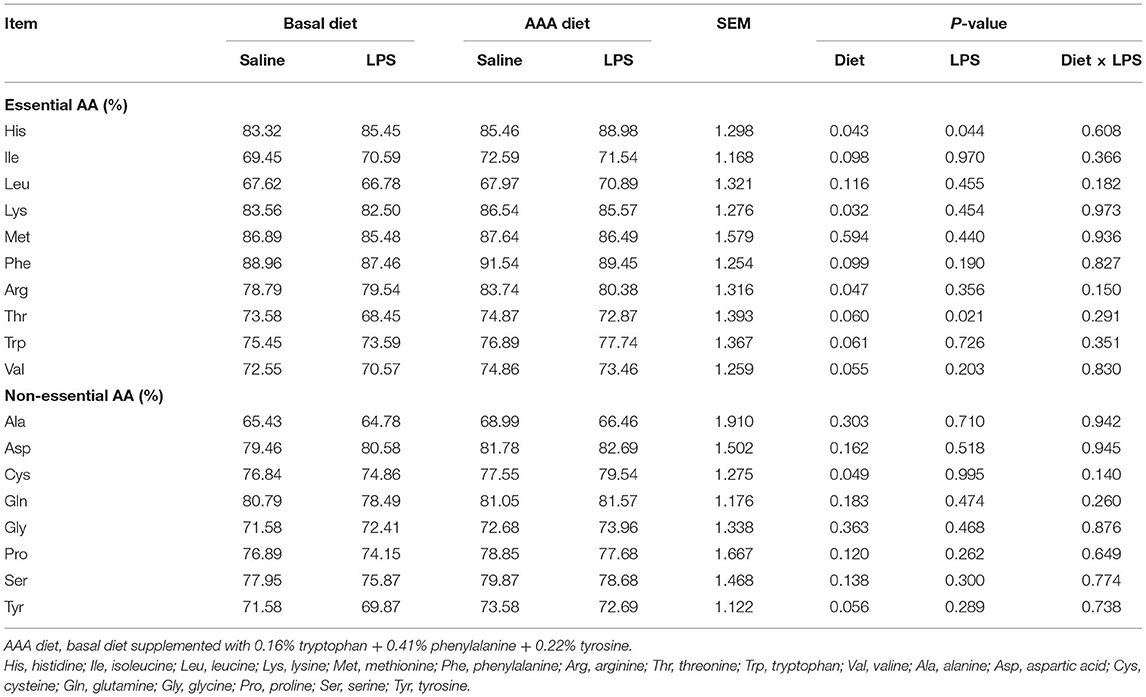
Table 6. Effects of dietary supplementation with AAA on apparent ileal digestibility of amino acid in piglets.
The MRNA Expression Level of Amino Acids Transporters in the Jejunal and Ileal Mucosa
In the jejunum, the relative mRNA expressions of SLC7A11, SLC16A10, SLC38A2, SLC6A14, SLC3A2, and SLC7A2 were markedly increased but the expression of SLC1A1 was decreased by LPS challenge (P < 0.05). AAAs supplementation only decreased the expression of SLC6A19 mRNA (P < 0.05). The interaction between AAAs supplementation and LPS challenge notably affected the expression of SLC1A1, SLC36A1, SLC3A1, SLC7A2, and SLC7A9 mRNA (P < 0.05) (Table 7).

Table 7. Effects of dietary supplementation with AAA on the mRNA expression level of amino acids transporters in the jejunum and ileum of piglets (P < 0.05).
In the ileum, the AAAs supplementation had significantly up-regulated the mRNA expressions of SLC16A10 and SLC38A9 (P < 0.05). The mRNA expression of SLC38A2 and SLC7A11 were increased, but the expression of SLC36A1 was decreased by the LPS challenge (P < 0.05). The interaction between AAAs supplementation and the LPS challenge significantly altered the expression of SLC36A1 and SLC38A9 (P < 0.05) (Table 7).
Discussion
The immune system stimulation alters the animals' physiology and metabolism via a complex system involving innate and adaptive immune response, several cytokines and acute-phase proteins, as well as the central nervous system (22). During the period of immune system stimulation, especially the metabolism and demand of Glu, Arg, Trp, Thr, and sulfur-containing AAs undergo certain changes (23). The present study showed the changes of AAs metabolism in piglets in response to LPS change including the AAs profiles in serum and intestinal mucosa, as well as apparent ileal digestibility of AA, etc. However, dietary supplementation with AAAs showed to improve the AAs sensing and utilization under inflammatory conditions.
Firstly, LPS significantly reduced the amount of CCK and increased the concentrations of leptin. But supplementation with AAAs counteracted the negative effect of LPS and stimulated CCK secretion. It has been well-documented that AAs stimulate cholecystokinin release through the Ca2+-sensing receptor (14). In non-calcified tissues, CaSR affected gastrointestinal nutrient sensing and intestinal endocrine hormone secretion (24). Additionally, it is allosterically sensed and associated with AAAs (15). As a metabolic substrate for Clostridium sporogenes, AAAs could be metabolized into 12 compounds, 9 of which could accumulate in the serum and affect systemic immunity (25). Trp and Phe were the most potent CaSR activators in mobilization assays (26). The supplementation of AAAs alleviated intestinal inflammation mediated by the CaSR signaling pathway (14). CaSR mediated the secretion of CCK induced by AAAs in the native intestinal I cell. And L-Phe stimulated CCK secretion enhancement in the presence of extracellular calcium levels (27). L-Phe increased serum glucagon and PYY levels but reduced the ghrelin levels in plasma (28).
Secondly, the addition of AAAs not only increased the total protein levels but also significantly reduced the blood urea nitrogen content, which partly indicated the decrease of N excretion and increase of AAs utilization in piglets. The increased N excretion, which occurs during the immune response, is a reflection of a relative imbalance in the profile of AAs released from peripheral tissues (29). Numerous compelling investigations have indicated that a metabolic alteration will occur in intestinal inflammation, resulting in the change of serum profile of AAs (30–33). LPS challenge reduced the ileal Thr digestibility, as well as serum total protein, Ile, and Trp concentrations, but increased the serum concentrations of Phe, Thr, His, Ala, Cys, and Ser in the present study. Reduced total protein means that AA is redistributes inflammation and immunity, not protein synthesis (5, 34). Therefore, it requires the increased provision of particular AAs from the diet in order to spare body protein stores (29). Some dispensable AAs become limiting because their de novo synthesis could be impaired. Several strands of evidence suggest that sulfur AAs, and AAs that are metabolically related to them, may be required in increased amounts (35). The demand for Cys increases under immune system stimulation and is used for the synthesis of glutathione (36) and acute phase proteins (22). AAAs demands are also increased to support the immune response under inflammation conditions in pigs (12). Therefore, dietary supplementation with AAAs showed the anti-inflammatory effects in LPS-challenged piglets (10), which could be explained by the increase in AID of His, Lys, Arg, and Cys, as well as serum concentrations of His, Arg, Trp, and Cys. The increased levels of AAs in serum may be due to the increase in hepatic catabolism and AA requirements for utilization. Interestingly, the levels of 12 kinds of AA were reduced in the jejunal mucosa, where after only the level of Trp was elevated significantly in serum. This is because the capture, absorption, transformation, and metabolism of intestinal epithelial cells and liver metabolism were jointly responsible for regulating the amount of AAs in peripheral blood (37, 38). It was also found in rat liver that His, Phe, Leu, Tyr, Gln, Pro, Trp, and Met inhibit intracellular proteolysis (39). And AA catabolism by the mucosal cells was quantitatively greater than AA incorporation into mucosal protein (40). The catabolism of AAs in the small intestine plays an important role in regulating the availability of dietary AAs to extraintestinal tissues (41).
It is well-known that AAs are absorbed through AA transporters, which may act as an initiator of nutritional signaling. Signaling pathways are intrinsically linked to amino acid transporter activity as well as to intracellular AAs metabolism (42). The SLC family mediates the transport of AAs on the plasma membrane (43). Both A2 and A9 of the SLC38 family are involved in the mTOR pathway. SLC38A9, which is a lysosomal Arg sensor machinery in the mTORC1 pathway (44). The Arg activates mTORC1 through the SLC38A9 sensor and binds it to other essential AAs in lysosomes as a lysosomal messenger, including Phe, Leu, Ile, Trp, Tyr, Val, Pro, Ser, and Met (45). The expression of SLC38A9 in the ileum and the content of Arg, Trp, and Tyr in serum were significantly increased by the addition of AAAs. This suggests that LPS-induced immunity activated the mTOR pathway and increased the Arg requirements in animals. SLC38A2 participates in the regulation of AA availability (46) and also as an AA sensor upstream of mTOR (47). SLC38A2 knockdown in rat myocytes and leads to a drop in intracellular concentrations of both Gln and Leu (48). Gln has been demonstrated to be a rate-limiting nutrient for mTOR activation (49). The content of Gln in the jejunum mucosa was significantly decreased by the LPS stimulation. Compared to the basal diet group, the relative expression of SLC7A11 was significantly increased in the LPS-challenged group, especially in the jejunal mucosa. It promoted Cys uptake and Glu biosynthesis, resulting in protection from oxidative stress (50). The Cys levels were markedly increased both in jejunal mucosa and serum. SLC7A11 imports extracellular Cys with intracellular Glu release at a ratio of 1:1 (51). According to reports, SLC6A19 as a major transporter for neutral AAs is the main agent of branched-chain AAs and Met absorption in the intestinal tract (52). However, the AAA diet significantly reduced its expression in the jejunum, but had no significant effect on the content of three branched-chain AAs and Met in the jejunal mucosa. AAA diet up-regulated significantly the expression level of SLC16A10. This proves the roles of SLC16A10 in mediating facilitated diffusion of AAAs across membranes and maintaining homeostasis by balancing AAAs concentrations between plasma and liver cells (53). SLC16A10 realizes the regulation of neutral AAs through the recovery of aromatic substrates (54).
In conclusion, the present results showed that the inflammation induced by LPS altered the AAs metabolism including the AAs profiles in serum and intestinal mucosa, as well as apparent ileal digestibility of AAs, etc. However, dietary supplementation with AAAs showed to improve the AAs sensing and utilization, which may meet the high demands for specific AAs in response to inflammation and immune response and then exert the anti-inflammatory effects. These findings may provide guidelines for the use of AAAs in animal and human nutrition.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (2013020).
Author Contributions
QDu: writing—original draft. BT: supervision, project administration, and funding acquisition. JW: writing—review and editing. BH: data curation and supervision. JL, MK, KH, QDe, and YY: supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (32072745), Earmarked Fund for China Agriculture Research System (CARS-35), and Innovation Province Project (2019RS3021).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AA, amino acid; AAA, aromatic amino acid; AID, apparent ileal digestibility; Ala, alanine; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; Arg, arginine; Asn, asparagine; Asp, aspartic acid; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CaSR, calcium-sensing receptor; CCK, cholecystokinin; Cys, cysteine; EAA, essential amino acid; Gln, glutamine; Glu, glutamic acid; GLU, glucose; Gly, glycine; His, histidine; Ile, isoleucine; LDH, lactic dehydrogenase; Leu, leucine; LPS, lipopolysaccharide; Lys, lysine; Met, methionine; mTOR, mammalian target of rapamycin; NEAA, non-essential amino acid; Phe, phenylalanine; Pro, proline; Ser, serine; SLC, solute carrier; Thr, threonine; Tp, total protein; Trp, tryptophan; Tyr, tyrosine; Val, valine.
References
1. Bruins MJ, Soeters PB, Deutz NE. Endotoxemia affects organ protein metabolism differently during prolonged feeding in pigs. J Nutr. (2000) 130:3003–13. doi: 10.1093/jn/130.12.3003
2. Bruins MJ, Soeters PB, Lamers WH, Deutz NE. L-arginine supplementation in pigs decreases liver protein turnover and increases hindquarter protein turnover both during and after endotoxemia. Am J Clin Nutr. (2002) 75:1031–44. doi: 10.1093/ajcn/75.6.1031
3. Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. (2002) 20:125–63. doi: 10.1146/annurev.immunol.20.082401.104914
4. Melchior D, Sève B, Le Floc'h N. Chronic lung inflammation affects plasma amino acid concentrations in pigs. J Anim Sci. (2004) 82:1091–9. doi: 10.2527/2004.8241091x
5. Klasing KC, Johnstone BJ. Monokines in growth and development. Poult Sci. (1991) 70:1781–9. doi: 10.3382/ps.0701781
6. Daly JM, Reynolds J, Sigal RK, Jian S, Liberman M. Effect of dietary protein and amino acids on immune function. Crit Care Med. (1990) 18:86–93. doi: 10.1097/00003246-199002003-00002
7. Gao K, Pi Y, Mu CL, Peng Y, Huang Z, Zhu WY. Antibiotics-induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J Neurochem. (2018) 146:219–34. doi: 10.1111/jnc.14333
8. Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. (2019) 18:379–401. doi: 10.1038/s41573-019-0016-5
9. Duan Y, Tan B, Li J, Liao P, Huang B, Li F, et al. Optimal branched-chain amino acid ratio improves cell proliferation and protein metabolism of porcine enterocytesin in vivo and in vitro. Nutrition. (2018) 54:173–81. doi: 10.1016/j.nut.2018.03.057
10. Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. (2013) 14:133–9. doi: 10.1038/nrm3522
11. Kim SG, Buel GR, Blenis J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol Cells. (2013) 35:463–73. doi: 10.1007/s10059-013-0138-2
12. Poncet N, Taylor PM. The role of amino acid transporters in nutrition. Curr Opin Clin Nutr Metab Care. (2013) 16:57–65. doi: 10.1097/MCO.0b013e32835a885c
13. Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. (2009) 20:436–43. doi: 10.1016/j.tem.2009.05.008
14. Liu H, Tan B, Huang B, Li J, Wang J, Liao P, et al. Involvement of calcium-sensing receptor activation in the alleviation of intestinal inflammation in a piglet model by dietary aromatic amino acid supplementation. Br J Nutr. (2018) 120:1321–31. doi: 10.1017/S0007114518002891
15. Conigrave AD, Franks AH, Brown EM, Quinn SJ. L-amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism? Eur J Clin Nutr. (2002) 56:1072–80. doi: 10.1038/sj.ejcn.1601463
16. Ling S, Shi P, Liu S, Meng X, Zhou Y, Sun W, et al. Structural mechanism of cooperative activation of the human calcium-sensing receptor by Ca(2+) ions and L-tryptophan. Cell Res. (2021) 31:383–94. doi: 10.1038/s41422-021-00474-0
17. Reimann F, Ward P, Gribble F. Signaling Mechanisms Underlying the Release of Glucagon-Like Peptide 1. Diabetes. (2006) 55:10. doi: 10.2337/db06-S010
18. Wang Y, Chandra R, Samsa LA, Gooch B, Fee BE, Cook JM, et al. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol. (2011) 300:G528–37. doi: 10.1152/ajpgi.00387.2010
19. Knerr I, Gröschl M, Rascher W, Rauh M. Endocrine effects of food intake: insulin, ghrelin, and leptin responses to a single bolus of essential amino acids in humans. Ann Nutr Metab. (2003) 47:312–8. doi: 10.1159/000072405
20. Li H, Yin J, He X, Li Z, Ma X. Enzyme-treated soybean meal replacing extruded full-fat soybean affects nitrogen digestibility, cecal fermentation characteristics and bacterial community of newly weaned piglets. Front Vet Sci. (2021) 8:e639039. doi: 10.3389/fvets.2021.639039
21. Neto M, Gallardo C, Perna F, Dadalt JC. Apparent total and ileal digestibility of rice bran with or without multicarbohydrase and phytase in weaned piglets. Livest Sci. (2021) 245:104423. doi: 10.1016/j.livsci.2021.104423
22. Reeds PJ, Jahoor F. The amino acid requirements of disease. Clin Nutr. (2001) 20:15–22. doi: 10.1054/clnu.2001.0402
23. Rakhshandeh A. Immune system stimulation in the pig: effect on performance and implications for amino acid nutrition. J Anim Sci. (2011) 97:735–44. doi: 10.1093/jas/sky468
24. Hannan FM, Kallay E, Chang W, Brandi ML, Thakker RV. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat Rev Endocrinol. (2018) 15:33–51. doi: 10.1038/s41574-018-0115-0
25. Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. (2017) 551:648–52. doi: 10.1038/nature24661
26. Conigrave AD, Mun HC, Delbridge L, Quinn SJ, Wilkinson M, Brown EM. L-amino acids regulate parathyroid hormone secretion. J Biol Chem. (2004) 279:38151–9. doi: 10.1074/jbc.M406373200
27. Liou AP, Sei Y, Zhao X, Feng J, Lu X, Thomas C, et al. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol. (2011) 300:G538–46. doi: 10.1152/ajpgi.00342.2010
28. Alamshah A, Spreckley E, Norton M, Kinsey-Jones JS, Amin A, Ramgulam A, et al. l-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. Int J Obes. (2017) 41:1693–701. doi: 10.1038/ijo.2017.164
29. Obled C. Amino acid requirements in inflammatory states. Can J Anim Sci. (2003) 83:365–73. doi: 10.4141/A03-021
30. McGilvray WD, Klein D, Wooten H, Dawson JA, Hewitt D, Rakhshandeh AR, et al. Immune system stimulation induced by Escherichia coli lipopolysaccharide alters plasma free amino acid flux and dietary nitrogen utilization in growing pigs. J Anim Sci. (2019) 97:315–26. doi: 10.1093/jas/sky401
31. Rakhshandeh A, Htoo JK, Karrow N, Miller SP, de Lange CF. Impact of immune system stimulation on the ileal nutrient digestibility and utilisation of methionine plus cysteine intake for whole-body protein deposition in growing pigs. Br J Nutr. (2014) 111:101–10. doi: 10.1017/S0007114513001955
32. McGaha TL, Huang L, Lemos H, Metz R, Mautino M, Prendergast GC, et al. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev. (2012) 249:135–57. doi: 10.1111/j.1600-065X.2012.01149.x
33. Kelly B, Pearce EL. Amino assets: how amino acids support immunity. Cell Metab. (2020) 32:154–75. doi: 10.1016/j.cmet.2020.06.010
34. Rakhshandeh A, Weber TE, Dekkers J, Tuggle CK, Gabler NK. Impact of systemic immune system stimulation on intestinal integrity and function in pigs. J Exp Bio. (2013) 34:1. doi: 10.1096/fasebj.27.1_supplement.867.2
35. Grimble RF, Grimble GK. Immunonutrition: role of sulfur amino acids, related amino acids, and polyamines. Nutrition. (1998) 14:605–10. doi: 10.1016/S0899-9007(98)80041-5
36. Malmezat T, Breuillé D, Capitan P, Mirand PP, Obled C. Glutathione turnover is increased during the acute phase of sepsis in rats. J Nutr. (2000) 130:1239–46. doi: 10.1093/jn/130.5.1239
37. Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. (2017) 27:R1147–51. doi: 10.1016/j.cub.2017.09.019
38. Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. (2009) 37:1–17. doi: 10.1007/s00726-009-0269-0
39. Pösö AR, Wert JJ Jr., Mortimore GE. Multifunctional control of amino acids of deprivation-induced proteolysis in liver. Role of leucine. J Biol Chem. (1982) 257:12114–20. doi: 10.1016/S0021-9258(18)33686-X
40. Stoll B, Henry J, Reeds PJ, Yu H, Jahoor F, Burrin DG. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J Nutr. (1998) 128:606–14. doi: 10.1093/jn/128.3.606
41. Wu G. Intestinal mucosal amino acid catabolism. J Nutr. (1998) 128:1249–52. doi: 10.1093/jn/128.8.1249
42. Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochemical J. (2003) 373:1–18. doi: 10.1042/bj20030405
43. Kandasamy P, Gyimesi G, Kanai Y, Hediger MA. Amino acid transporters revisited: New views in health and disease. Trends Biochem Sci. (2018) 43:752–89. doi: 10.1016/j.tibs.2018.05.003
44. Rebsamen M, Superti-Furga G. SLC38A9: A lysosomal amino acid transporter at the core of the amino acid-sensing machinery that controls MTORC1. Autophagy. (2016) 12:1061–2. doi: 10.1080/15548627.2015.1091143
45. Wyant GA, Abu-Remaileh M, Wolfson RL, Chen WW, Freinkman E, Danai LV, et al. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell. (2017) 171:642–54.e12. doi: 10.1016/j.cell.2017.09.046
46. Gazzola GC, Franchi R, Saibene V, Ronchi P, Guidotti GG. Regulation of amino acid transport in chick embryo heart cells. I. Adaptive system of mediation for neutral amino acids. Biochim Biophys Acta. (1972) 266:407–21. doi: 10.1016/0005-2736(72)90097-1
47. Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. (2009) 296:E603–13. doi: 10.1152/ajpendo.91002.2008
48. Evans K, Nasim Z, Brown J, Clapp E, Amin A, Yang B, et al. Inhibition of SNAT2 by metabolic acidosis enhances proteolysis in skeletal muscle. J Am Soc Nephrol. (2008) 19:2119–29. doi: 10.1681/ASN.2007101108
49. Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. (2009) 136:521–34. doi: 10.1016/j.cell.2008.11.044
50. Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. (2018) 38:12. doi: 10.1186/s40880-018-0288-x
51. Lin W, Wang C, Liu G, Bi C, Wang X, Zhou Q, et al. SLC7A11/xCT in cancer: biological functions and therapeutic implications. Am J Cancer Res. (2020) 10:3106–26.
52. Bröer S. The role of the neutral amino acid transporter B0AT1 (SLC6A19) in Hartnup disorder and protein nutrition. IUBMB Life. (2009) 61:591–9. doi: 10.1002/iub.210
53. Mariotta L, Ramadan T, Singer D, Guetg A, Herzog B, Stoeger C, et al. T-type amino acid transporter TAT1 (Slc16a10) is essential for extracellular aromatic amino acid homeostasis control. J Physiol. (2012) 590:6413–24. doi: 10.1113/jphysiol.2012.239574
Keywords: aromatic amino acid, amino acids sensing, transporters, sensors, piglets
Citation: Duanmu Q, Tan B, Wang J, Huang B, Li J, Kang M, Huang K, Deng Q and Yin Y (2022) The Amino Acids Sensing and Utilization in Response to Dietary Aromatic Amino Acid Supplementation in LPS-Induced Inflammation Piglet Model. Front. Nutr. 8:819835. doi: 10.3389/fnut.2021.819835
Received: 22 November 2021; Accepted: 16 December 2021;
Published: 17 January 2022.
Edited by:
Yongqing Hou, Wuhan Polytechnic University, ChinaReviewed by:
Zhigang Song, Shandong Agricultural University, ChinaXi Ma, China Agricultural University, China
Copyright © 2022 Duanmu, Tan, Wang, Huang, Li, Kang, Huang, Deng and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bie Tan, YmlldGFuQGh1bmF1LmVkdS5jbg==
 Qing Duanmu
Qing Duanmu Bie Tan
Bie Tan Jing Wang1
Jing Wang1