
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 09 June 2022
Sec. Family Medicine and Primary Care
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.875682
 Moritz Mirna1*
Moritz Mirna1* Lukas Schmutzler1
Lukas Schmutzler1 Albert Topf1
Albert Topf1 Brigitte Sipos1
Brigitte Sipos1 Lukas Hehenwarter2
Lukas Hehenwarter2 Uta C. Hoppe1
Uta C. Hoppe1 Michael Lichtenauer1
Michael Lichtenauer1Background: Acute myocarditis and acute coronary syndrome (ACS) are important differential diagnoses in patients with new-onset chest pain. To date, no clinical score exists to support the differentiation between these two diseases. The aim of this study was to develop such a score to aid the physician in scenarios where discrimination between myocarditis and ACS appears difficult.
Materials and Methods: Patients with ACS (n = 233) and acute myocarditis (n = 123) were retrospectively enrolled. Least absolute shrinkage and selection operator (LASSO) regression was conducted to identify parameters associated with the highest or least probability for acute myocarditis. Logistic regression was conducted using the identified parameters and score points for each level of the predictors were calculated. Cutoffs for the prediction of myocarditis were calculated. Validation was conducted in a separate cohort of 90 patients.
Results: A score for prediction of acute myocarditis was calculated using six parameters [age, previous infection, hyperlipidemia, hypertension, C-reactive protein (CRP), and leukocyte count]. Logistic regression analysis showed a significant association between total score points and the presence of myocarditis (B = 0.9078, p < 0.0001). Cutoff #1 for the prediction of myocarditis was calculated at ≥ 4 (Sens.: 90.3%, Spec.: 93.1%; 46.3% predicted probability for acute myocarditis), cutoff #2 was calculated at ≥ 7 (Sens.: 73.1%, Spec.: > 99.9%; 92.9% pred. prob.). Validation showed good discrimination [area under the curve (AUC) = 0.935] and calibration of the score.
Conclusion: Our clinical score showed good discrimination and calibration for differentiating patients with acute myocarditis and ACS. Thus, it could support the differential diagnosis between these two disease entities and could facilitate clinical decisions in affected patients.
Patients with acute myocarditis and acute coronary syndrome (ACS) often resemble each other in terms of their clinical presentation and diagnostic findings. Because of the possible similarity of both diseases, the European Society of Cardiology (ESC) recommends timely coronary angiography in patients with suspected myocarditis in order to rule out ACS (1, 2). However, a cardiac catheterization laboratory with a physician on-call is, especially in rural areas or developing countries, frequently not available, necessitating inter-hospital transfer accompanied by an emergency care physician (3, 4). Coronary angiography further constitutes an invasive procedure, which is associated with a risk of 1–2% for severe complications, such as allergic reactions to the contrast agent, contrast-induced nephropathy, vascular complications, arrhythmias, peri-procedural myocardial infarction, or cerebrovascular events (5, 6).
Although the risk profiles and comorbidities of patients with acute myocarditis and ACS differ substantially (i.e., age of the patients, presence of classical cardiovascular risk factors, and previous infection), there is, to the best of our knowledge, currently no validated clinical score available to support the differentiation between acute myocarditis and ACS. In compliance with the current recommendations of the ESC, coronary angiography is thus frequently conducted in patients with clinically suspected myocarditis, albeit with a low pretest probability for coronary artery disease. This is especially problematic considering the associated risk of complications, socioeconomic implications, and the significant exposure to radiation during coronary angiography, which is equivalent to 300 conventional chest X-rays (7, 8).
The aim of the current study was to design a score to support the differential diagnosis between myocarditis and ACS, especially in clinical scenarios where differentiation appears difficult, e.g., when patients are neither old nor very young. By strengthening the tentative diagnosis of acute myocarditis, a score could reduce risks and costs associated with potentially avoidable coronary angiography procedures, thus offering benefits in the management of affected patients.
The study was reviewed by the ethical review board of the state of Salzburg, Austria (EK Nr: 1181/2020) prior to enrollment and was conducted in compliance with the Declaration of Helsinki and the principles of Good Clinical Practice. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Eligible patients were identified through a database search on all patients admitted to the University Hospital of Salzburg, Austria, in the time period of 2009–2019. The search was performed for discharge diagnoses, which were classified according to the International Classification of Diseases, Tenth Revision (ICD-10) diagnostic codes (myocarditis: I40.0, I40.1, I40.8, I40.9, and I51.4; ACS: I21.0, I21.1, I21.2, I21.3, I21.4, I21.9, and I24.9). In total, 224 patients with a discharge diagnosis of myocarditis (time period 2009–2019) and 668 patients with a discharge diagnosis of ACS (the year 2019) were identified by database search.
The presence of acute myocarditis or ACS was defined according to the current recommendations of the ESC (1, 9, 10) and was confirmed by revision of all clinical records of identified patients. Patients with myocarditis were included in the subsequent analysis if they fulfilled the criteria for clinically suspected myocarditis by the ESC (1) and had evidence of acute myocarditis on endomyocardial biopsy (EMB) or cardiac magnetic resonance imaging (MRI). Patients with ACS were included if they fulfilled the criteria for ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation myocardial infarction (NSTEMI) by the ESC (9, 10). Patients with chronic or recurrent myocarditis, chronic infections (e.g., hepatitis, HIV etc.), or patients admitted for elective procedures were excluded from the study. Thus, 123 patients with acute myocarditis and 233 randomly selected patients with ACS were included in this study.
Demographical data, clinical data, and comorbidities were extracted from the initial hospital record at the presentation. Laboratory data were acquired from the first complete set of laboratory results, i.e., from the emergency department or the intensive care unit.
Statistical analyses were performed with R [version 4.0.2., R Core Team (11), R Foundation for Statistical Computing, Vienna, Austria1 ] using the packages “ggplot2,” “pastecs,” “Hmisc,” “ggm,” “polycor,” “QuantPsyc,” “glmnet,” “ResourceSelection,” and “rms,” as well as SPSS (Version 23.0, IBM, Armonk, NY, United States). Skew and kurtosis of continuous data were assessed visually, and data distribution was assessed by performing a Shapiro–Wilk test. Data were depicted as median ± interquartile range (IQR) and compared using a Mann-Whitney U test since most were not normally distributed. Categorical data were analyzed using Fisher’s exact test.
The cohort was split for the calculation of the score: three-fourths of the data (n = 266) were used for score calculation and one-fourth (n = 90) was used for validation. The least absolute shrinkage and selection operator (LASSO) regression analysis was conducted to identify parameters associated with the highest or least probability of myocarditis. Logistic regression for the presence of myocarditis was conducted using the identified predictors, Akaike information criterion (AIC) and R2 were calculated. Average leverage and standardized residuals were calculated to detect relevant outliers, absence of multicollinearity was checked by calculating variance inflation factors (see Table 3). Then, a locally estimated scatterplot smoothing (LOESS) function was performed to identify cut points for numeric variables. Logistic regression analysis was conducted for the newly generated ordinal variables. The total score was calculated for each patient, and binomial logistic regression for the prediction of myocarditis using total score values was performed in the three-fourths cohort. Receiver operating characteristic (ROC) curve and area under the curve (AUC) measurements for the prediction of myocarditis were performed, and cutoff points were defined in the three-fourths of cohort. Cutoff #1 was calculated by means of the Youden index (12), and cutoff #2 was chosen to depict maximum specificity for myocarditis. Validation was performed in the one-fourth validation cohort. A p-value of < 0.05 was considered statistically significant.
A total of 356 patients were enrolled in this study. Of these, 34.6% (n = 123) were in the myocarditis subgroup and 65.4% (n = 233) in the ACS subgroup. Patients with myocarditis were significantly younger than patients with ACS {median 34 years [interquartile range (IQR) 24 − 44] vs. median 62 years (IQR 55 − 74), p < 0.0001}. In both subgroups, the majority of patients was male (myocarditis: 80.5% vs. ACS: 70.8%, p = 0.056). Among patients with ACS, 131 patients (56.2%) had STEMI and 102 patients had (43.8%) NSTEMI.
While an infection within the last 4 weeks was significantly more prevalent in patients with myocarditis (66.1 vs. 8.6%, p < 0.0001), classical cardiovascular risk factors, such as diabetes mellitus, hyperlipidemia, obesity [body mass index (BMI) > 30 kg/m2], hypertension, and history of smoking occurred more frequently in the ACS subgroup. While coronary artery disease, cerebral artery disease, and peripheral artery disease were more prevalent in patients with ACS, there were no statistically significant differences in the frequencies of active malignancies, autoimmune disorders, or anti-inflammatory/immunosuppressive treatments between both groups (see Table 1).
During hospital stay, coronary angiography, and percutaneous coronary intervention (PCI) were conducted significantly more often in patients with ACS [coronary angiography: 93.6% (n = 218) vs. 46.3% (n = 57), p < 0.0001; PCI: 85.4% (n = 187) vs. 0% (n = 0), p < 0.0001]. In contrast, EMB and MRI were performed more frequently in patients with myocarditis [EMB: 9.9% (n = 12) vs. 0% (n = 0), p < 0.0001; MRI: 98.4% (n = 121) vs. 1.7% (n = 4), p < 0.0001].
Serum concentrations of C-reactive protein (CRP) were significantly higher in patients with myocarditis [median 40.0 mg/l (IQR 6.0–84.0) vs. median 3.0 mg/l (IQR 1.0–10.0), p < 0.0001], whereas peripheral leukocyte counts were higher in patients presented with ACS [11.03 G/l (IQR 8.98–14.00) vs. median 8.50 G/l (IQR 6.76–11.92)]. Furthermore, serum levels of serum creatinine, lactate dehydrogenase (LDH), prothrombin time, and thrombocyte count were significantly higher in patients of the ACS group (see Table 2). Of note, there were no significant differences in the concentrations of high sensitivity troponin (hsTnT), creatinine kinase, or pro-brain natriuretic peptide (pBNP) between patients of both investigated groups.
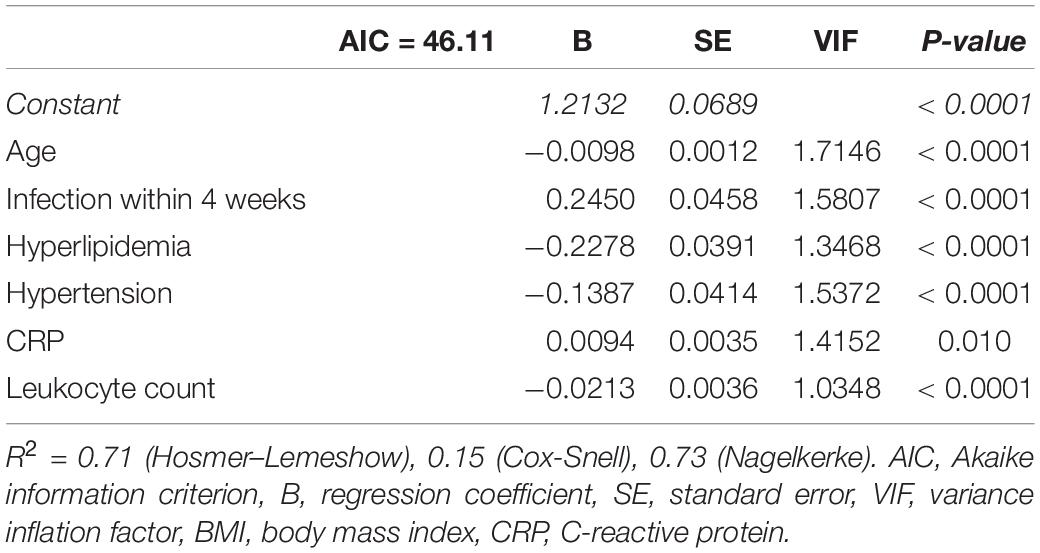
Table 3. Logistic regression analysis for the presence of myocarditis using the 6 predictors identified by LASSO regression.
Of a total of 26 parameters, 14 with significant differences between both subgroups were included in the following analysis (age, previous infection, diabetes mellitus, hyperlipidemia, obesity, hypertension, history of smoking, coronary artery disease, peripheral artery disease, cerebral artery disease, serum creatinine, CRP, leukocyte count, and thrombocyte count). LASSO regression analysis identified six of these at λ1SE as associated with the highest or least probability of myocarditis (age, previous infection, hyperlipidemia, hypertension, CRP, and leukocyte count; see Supplementary Figure 1). Logistic regression using the six identified predictors is depicted in Table 3.
Locally estimated scatterplot smoothing function was performed to identify cut points for numeric data (age, leukocyte count, and CRP) in order to generate ordinal variables (see Supplementary Figure 2). Then, logistic regression analysis was conducted to identify regression coefficients for dichotomous and ordinal variables, and coefficients were rounded to the nearest integer to create score points for each level of parameters (see Figure 1). The total score was calculated for each patient. Patients in the myocarditis subgroup had significantly higher score values than patients with ACS [median 8 (IQR 6–9) vs. median − 1 (IQR − 2 to 1), p < 0.0001]. Predicted probabilities for myocarditis for each sum of score points are depicted in Table 4, a plot of predicted probabilities vs. observed values in the three-fourths cohort is depicted in Figure 2.
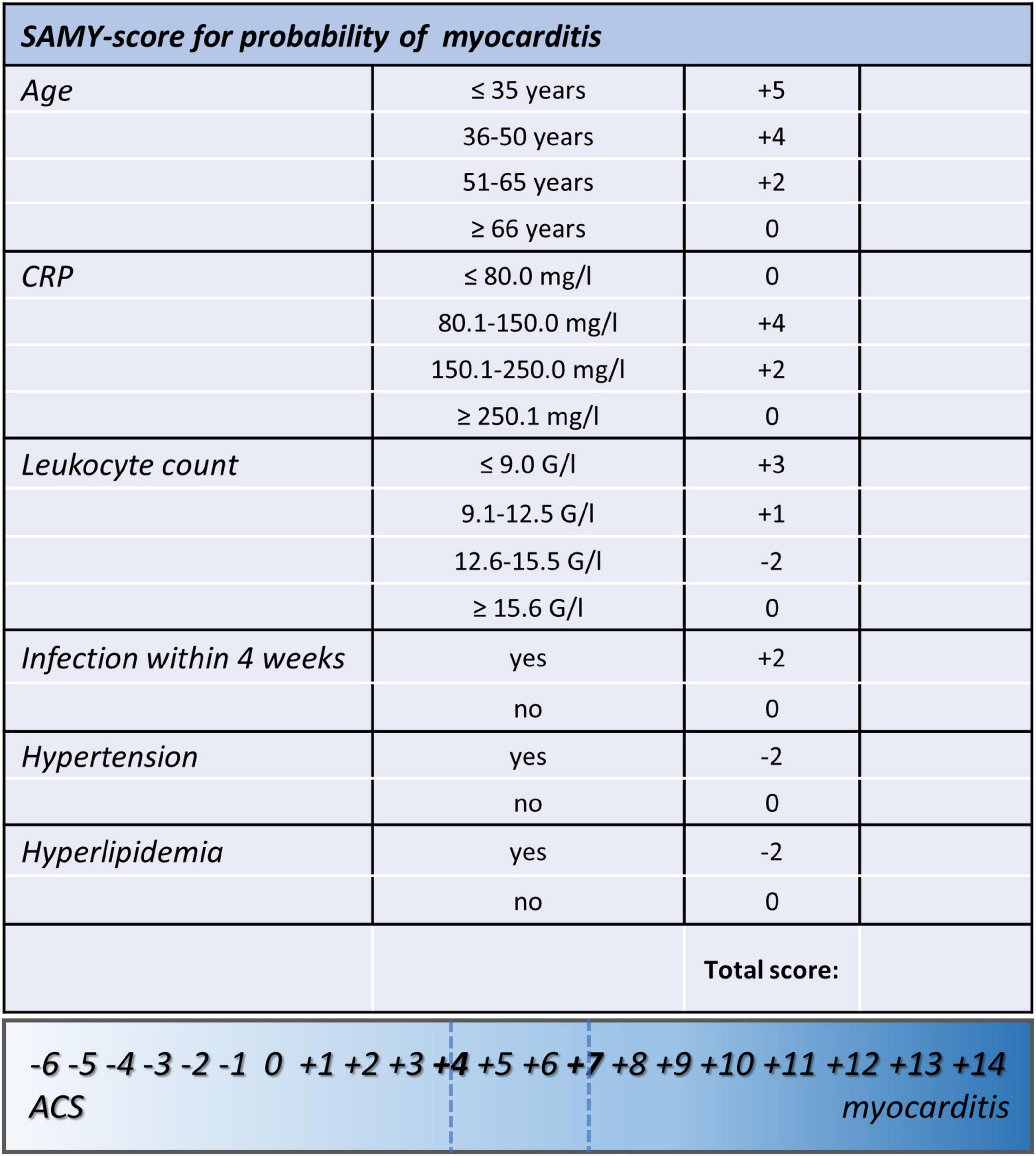
Figure 1. Score sheet of the proposed clinical score for the prediction of myocarditis that includes the two calculated cutoffs (#1: ≥ 4, Sens.: 90.3%, Spec.: 93.1%, PPV: 87.5%, NPV 94.7%; 46.3% predicted probability for myocarditis; #2: ≥ 7, Sens.: 73.1%, Spec.: > 99.9%, PPV: > 99.9%, NPV 87.4%; 92.9% predicted probability for myocarditis). SAMY, SAlzburg MYocarditis score; ACS, acute coronary syndrome; BMI, body mass index; CRP, C-reactive protein.
Univariate logistic regression analysis in the three-fourths cohort showed a significant association with the presence of myocarditis [B = 0.9078 (SE = 0.1159), p < 0.0001; R2 = 0.71 (Hosmer–Lemeshow), 0.60 (Cox-Snell), 0.83 (Nagelkerke); AIC = 104.83] and adequate goodness-of-fit (X2 = 5.20, p = 0.736). AUC of the score was calculated at 0.975, ROC curve is depicted in Figure 3A. Cutoff #1 was calculated by means of the Youden index (12) (#1: ≥ 4, Sens.: 90.3%, Spec.: 93.1%, positive predictive value (PPV): 87.5%, negative predictive value (NPV) 94.7%; 46.3% predicted probability for myocarditis) and cutoff #2 was calculated to depict optimal specificity for myocarditis (#2: ≥ 7, Sens.: 73.1%, Spec.: > 99.9%, PPV: > 99.9%, NPV 87.4%; 92.9% predicted probability for myocarditis).
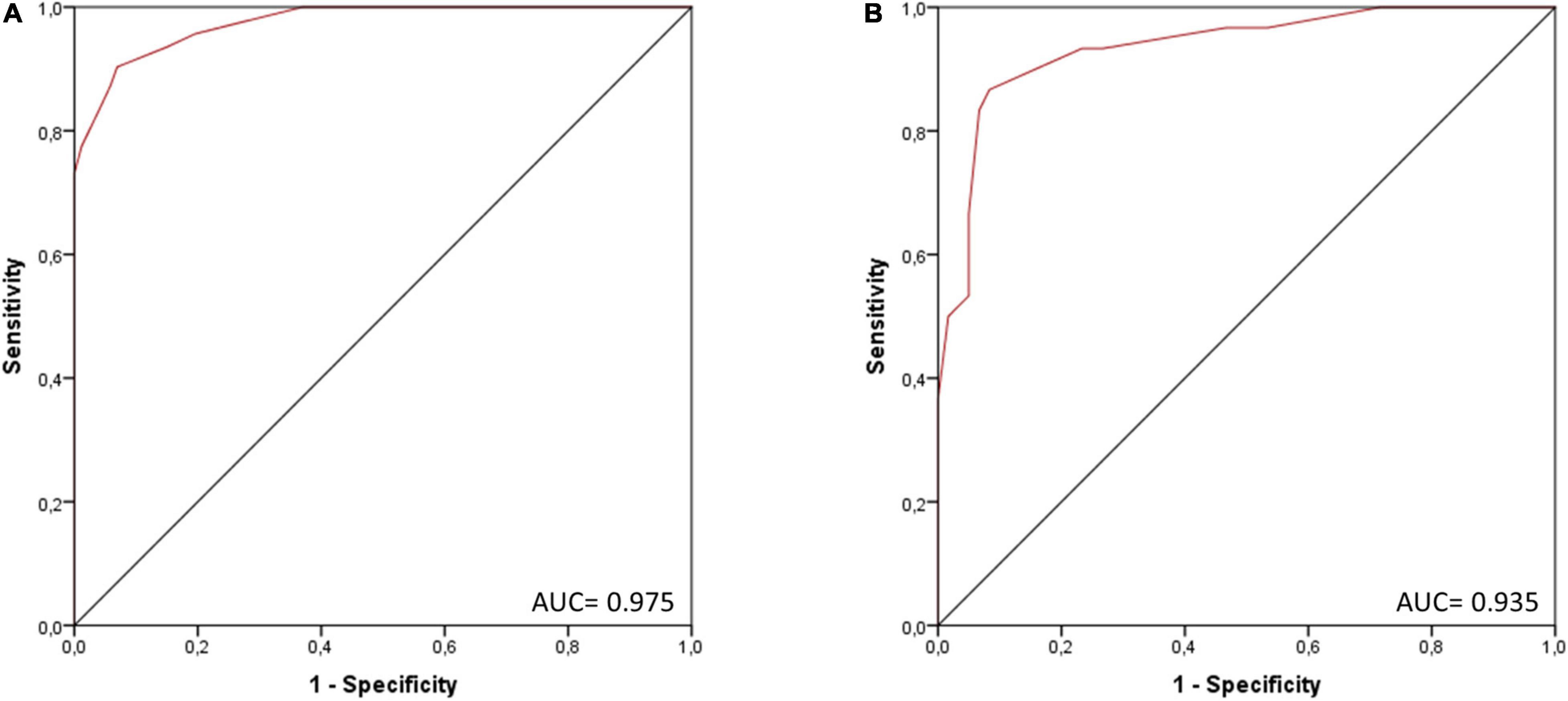
Figure 3. ROC curve of the score for the presence of myocarditis in (A) the three-fourths cohort (n = 266) and (B) the validation cohort (one-fourth of the total cohort, n = 90). AUC, area under the curve.
Validation of the score in one-fourth cohort (n = 90) showed good discrimination (AUC = 0.935; see Figure 3B) and calibration (see Supplementary Figure 3).
New-onset chest pain constitutes a challenging symptom, which requires timely and targeted diagnostic workup (13, 14). In the past, several scoring systems have been implemented in clinical routine to support diagnostic and therapeutic decisions in patients presenting with chest pain. For example, the Wells score and the Geneva score have been developed to facilitate the diagnosis of suspected pulmonary embolism (15, 16), whereas the Aortic Dissection Detection Risk Score (ADD-RS) has been validated to estimate the pretest probability of aortic dissection (17). Similarly, InterTAK Diagnostic Score was developed to support the differentiation between ACS and Takotsubo syndrome (TTS), which frequently resemble each other in terms of clinical presentation, laboratory findings, and abnormalities on electrocardiogram (ECG) and transthoracic echocardiography (TTE) (18).
Similar to TTS, acute myocarditis can mimic ACS. Therefore, current guidelines of the ESC advocate coronary angiography with a class IC recommendation in patients with suspected myocarditis in order to rule out ACS (1, 2). However, in highlight of the risk of complications, the associated exposure to radiation, as well as socioeconomic implications, coronary angiography should only be conducted after strict evaluation of its indication and all risks and benefits for the patient. In addition, patients are often admitted to an intensive care unit for heart rhythm monitoring until ACS is securely ruled out, which is problematic especially in highlight of limited resources during the COVID-19 pandemic (19). In this regard, the absence of a clinical scoring system to support the differential diagnosis between ACS and acute myocarditis becomes apparent, which is why we aimed to develop such a score in this study.
The novel SAlzburg MYocarditis (SAMY) score comprises six clinical parameters, which were selected by LASSO regression due to their high or low probability of myocarditis from a total of 26 possible variables. Interestingly, all of the selected parameters have been associated with the presence of acute myocarditis or ACS in previous studies. As such, acute myocarditis was identified to predominately affect patients of young age and to be associated with a preceding viral infection (1, 20, 21). In contrast, classical cardiovascular risk factors, such as hyperlipidemia or arterial hypertension, are commonly found in patients with ACS (9, 22, 23).
All of the six included parameters can easily be obtained in the emergency department by assessing the patient’s medical history and performing a standard laboratory analysis. Together, they provide a novel clinical score, which estimates the probability of acute myocarditis and shows good discrimination and calibration in our study. Interestingly, of all included predictors, young age was associated with the highest regression coefficients for acute myocarditis, which is depicted by the number of score points per level and is a direct result of the diverse distribution of age between the two investigated groups. Furthermore, patients in both investigated groups showed an increment of CRP and leukocyte count, however, leukocytosis was more pronounced in patients with ACS. Consequently, attribution of score points for CRP and leukocyte count does not follow a linear relationship, but is different for each level of the predictor variable depending on its individual probability for myocarditis (also see Supplementary Figure 2), in order to confer with the well-known and often transient increase of inflammatory biomarkers in patients presented with ACS (24–26).
The novel SAMY score can easily be calculated in the emergency department by using six clinical variables, hereby supporting the differential diagnosis between ACS and acute myocarditis in clinical scenarios where the differentiation between both disease entities appears difficult for the attending physician, e.g., when patients with chest pain are neither old nor very young. However, our score only provides a probability estimate for myocarditis and is not diagnostic per se. As such, a low score does not exclude acute myocarditis and a high score is not definitely diagnostic for myocarditis. Nevertheless, it could support the attending physicians in their clinical decisions that may help to allocate resources correctly and to weigh the risks and benefits of coronary angiography for the individual patient.
Using six clinical parameters, the novel SAMY score provides an estimate of the probability of acute myocarditis in order to support the differentiation between myocarditis and ACS in the acute setting. Hereby, it could help to avoid unnecessary coronary angiography procedures and could support the clinical decisions of the attending physician, especially in clinical scenarios where resources are limited or coronary angiography is not rapidly available.
Because of the low prevalence of myocarditis, we chose a retrospective study design for this study. However, a retrospective design is inferior to a prospective design in terms of the achieved level of evidence. Furthermore, the exclusion of patients without evidence of myocarditis on MRI or EMB greatly reduced the number of enrolled patients, which could have affected statistical analyses, especially regarding the validation of the score in the comparatively small validation cohort (n = 90). Therefore, our findings need to be validated in a larger prospective and, preferably multicentric, a study in the future. Moreover, the aim of this study was to create a score to help clinicians differentiate between ACS and acute myocarditis; whether it can be applied to other clinical scenarios or aim in the diagnosis of chronic myocarditis remains to be elucidated in further studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Review Board of the state of Salzburg, Austria (EK Nr: 1181/2020). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MM and ML concepted and designed the study. LS performed the data collection. MM conducted the statistical analyses. MM, AT, BS, and LS wrote the manuscript. UH, LH, and ML reviewed the manuscript and provided substantial improvements prior to submission. All authors read the final version of the manuscript and agreed to its contents.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to express their gratitude to all patients involved in this scientific project. Furthermore, our warmest thanks go out to our colleagues, all medical personnel, and nursing staff, who unrelentingly provide loving care for our patients, even in these troubled times of a global pandemic.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.875682/full#supplementary-material
ACS, acute coronary syndrome; ADD-RS, Aortic Dissection Detection Risk Score; AIC, Akaike information criterion; AUC, area under the curve; BMI, body mass index; CRP, C-reactive protein; ECG, electrocardiogram; EMB, endomyocardial biopsy; ESC, European Society of Cardiology; HIV, human immunodeficiency virus; hsTnT, high sensitivity troponin; IQR, interquartile range; LASSO, least absolute shrinkage and selection operator; LDH, lactate dehydrogenase; LOES, locally estimated scatterplot smoothing; MRI, magnetic resonance imaging; NSAID, non-steroidal anti-inflammatory drug; NSTEMI, non-ST-segment elevation myocardial infarction; pBNP, pro brain natriuretic peptide; PCI, percutaneous coronary intervention; ROC, receiver operating characteristic; SAMY, SAlzburg MYocarditis score; SE, standard error; STEMI, ST-segment elevation myocardial infarction; TTE, transthoracic echocardiography; TTS, Takotsubo syndrome; VIF, variance inflation factor.
1. Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. (2013) 34:2636–48. doi: 10.1093/eurheartj/eht210
2. Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases. Eur Heart J. (2015) 36:2921–64. doi: 10.1093/eurheartj/ehv318
3. Thompson SC, Nedkoff L, Katzenellenbogen J, Hussain MA, Sanfilippo F. Challenges in managing acute cardiovascular diseases and follow up care in rural areas: a narrative review. Int J Environ Res Public Health. (2019) 16:5126. doi: 10.3390/ijerph16245126
4. Medagama A, Bandara R, De Silva C, Galgomuwa MP. Management of acute coronary syndromes in a developing country; time for a paradigm shift? An observational study. BMC Cardiovasc Disord. (2015) 15:133. doi: 10.1186/s12872-015-0125-y
5. Tavakol M, Ashraf S, Brener SJ. Risks and complications of coronary angiography: a comprehensive review. Glob J Health Sci. (2012) 4:65–93. doi: 10.5539/gjhs.v4n1p65
6. Manda YR, Baradhi KM. Cardiac Catheterization, Risks and Complications. Treasure Island, FL: StatPearls Publishing (2019).
7. Mohammadi M, Danaee L, Alizadeh E. Reduction of radiation risk to interventional cardiologists and patients during angiography and coronary angioplasty. J Tehran Univ Hear Cent. (2017) 12:101–6.
8. Tarighatnia A, Mohammadalian A, Ghojazade M, Pourafkari L, Farajollahi A. Beam projections and radiation exposure in transradial and transfemoral approaches during coronary angiography. Anatol J Cardiol. (2017) 18:298–303. doi: 10.14744/AnatolJCardiol.2017.7724
9. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 Esc guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
10. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent St-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European society of cardiology (ESC). Eur Heart J. (2016) 37:267–315. doi: 10.1093/eurheartj/ehv320
11. R Core Team (2013) R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org (accessed July 23, 2021).
12. Martínez-Camblor P, Pardo-Fernández JC. The Youden index in the generalized receiver operating characteristic curve context. Int J Biostat. (2019) 15. doi: 10.1515/ijb-2018-0060
13. Fruergaard P, Launbjerg J, Hesse B, Jorgensen F, Petri A, Eiken P, et al. The diagnoses of patients admitted with acute chest pain but without myocardial infarction. Eur Heart J. (1996) 17:1028–34. doi: 10.1093/oxfordjournals.eurheartj.a014998
14. Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American heart association. Circulation. (2010) 122:1756–76. doi: 10.1161/CIR.0b013e3181ec61df
15. Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. (2001) 135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010
16. Shen JH, Chen HL, Chen JR, Xing JL, Gu P, Zhu BF. Comparison of the wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. (2016) 41:482–92. doi: 10.1007/s11239-015-1250-2
17. Nazerian P, Mueller C, De Matos Soeiro A, Leidel BA, Salvadeo SAT, Giachino F, et al. Diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes the ADvISED prospective multicenter study. Circulation. (2018) 137:250–8. doi: 10.1161/CIRCULATIONAHA.117.029457
18. Ghadri JR, Cammann VL, Jurisic S, Seifert B, Napp LC, Diekmann J, et al. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: results from the International Takotsubo Registry. Eur J Heart Fail. (2017) 19:1036–42. doi: 10.1002/ejhf.683
19. Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. (2020) 8:506–17. doi: 10.1016/S2213-2600(20)30161-2
21. Krejci J, Mlejnek D, Sochorova D, Nemec P. Inflammatory cardiomyopathy: a current view on the pathophysiology, diagnosis, and treatment. Biomed Res Int. (2016) 2016:4087632. doi: 10.1155/2016/4087632
22. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa575
23. D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. (2008) 117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579
24. Seropian IM, Sonnino C, Van Tassell BW, Biasucci LM, Abbate A. Inflammatory markers in STelevation acute myocardial infarction. Eur Hear J Acute Cardiovasc Care. (2016) 5:382–95. doi: 10.1177/2048872615568965
25. Grzybowski M, Welch RD, Parsons L, Ndumele CE, Chen E, Zalenski R, et al. The association between white blood cell count and acute myocardial infarction in-hospital mortality: findings from the national registry of myocardial infarction. Acad Emerg Med. (2004) 11:1049–60. doi: 10.1197/j.aem.2004.06.005
Keywords: cardiology, myocarditis, acute coronary syndrome, clinical score, inflammatory heart disease, score, ACS
Citation: Mirna M, Schmutzler L, Topf A, Sipos B, Hehenwarter L, Hoppe UC and Lichtenauer M (2022) A Novel Clinical Score for Differential Diagnosis Between Acute Myocarditis and Acute Coronary Syndrome – The SAlzburg MYocarditis (SAMY) Score. Front. Med. 9:875682. doi: 10.3389/fmed.2022.875682
Received: 14 February 2022; Accepted: 16 May 2022;
Published: 09 June 2022.
Edited by:
Marcos Ferreira Minicucci, São Paulo State University, BrazilReviewed by:
Cristoforo Pomara, University of Catania, ItalyCopyright © 2022 Mirna, Schmutzler, Topf, Sipos, Hehenwarter, Hoppe and Lichtenauer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moritz Mirna, m.mirna@salk.at; orcid.org/0000-0001-5679-4872
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.