
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 10 November 2021
Sec. Gastroenterology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.763370
This article is part of the Research Topic Acute-On-Chronic Liver Failure: Natural History, Mechanism, And Treatment View all 20 articles
Background: The aim of this study was to investigate the impact of proton pump inhibitor (PPI) therapy on complications and prognosis in cirrhosis patients with and without acute-on-chronic liver failure (ACLF).
Materials and Methods: Cirrhosis patients with acute decompensation (AD) (n = 489) admitted in our center were enrolled in this prospective observational cohort study. According to treatment received, patients were identified as users or nonusers of PPI. Clinical and laboratory data, complications during hospitalization, and overall survival were recorded in all the patients.
Results: Of the 489 patients, 299 (61.1%) patients received PPI therapy. The logistic regression analysis showed that age, albumin, history of previous hepatic encephalopathy (HE), and the chronic liver failure-sequential organ failure assessment (CLIF-SOFA) score were independent risk factors for HE in patients with decompensated cirrhosis [odds ratio (OR) = 1.07, 95% CI: 1.03–1.12, p = 0.001; OR = 1.13, 95% CI: 1.04–1.24, p = 0.006; OR = 242.52, 95% CI: 40.17–1464.11, p < 0.001; and OR = 2.89, 95% CI: 2.11–3.96, p < 0.001, respectively]. Previous severe liver injury and previous bacterial infections were independent risk factors for spontaneous bacterial peritonitis (SBP) in patients with decompensated cirrhosis (OR = 3.43, 95% CI: 1.16–10.17, p = 0.026 and OR = 6.47, 95% CI: 2.29–18.29, p < 0.001, respectively). The multivariate Cox proportional hazards regression model showed that the type and dose of the PPI used were not related to 28-day and 90-day mortality in cirrhosis patients with AD or ACLF.
Conclusion: PPI use does not appear to increase mortality or the risk of HE and SBP in the hospitalized cirrhosis patients with and without ACLF.
Proton pump inhibitors (PPIs) are commonly used in cirrhosis patients to treat gastrointestinal disorders, especially gastrointestinal bleeding (1–3). Gastrointestinal hemorrhage may be due to bleeding varices or ulcers or portal hypertensive gastropathy and is one of the most serious complications seen in acute-on-chronic liver failure (ACLF) (4–6). While PPI is useful in these cases, the drug may alter the composition of the gut microbiota and increase the risk of spontaneous bacterial peritonitis (SBP), hepatic encephalopathy (HE), Clostridium difficile infection, and mortality (7–14). Although some recent studies have indicated that PPI therapy does not increase the risk of SBP or mortality in cirrhosis, these studies did not include patients with ACLF (15–18). The aim of this study was to assess whether PPI therapy increases the incidence of SBP and HE and decreases survival in cirrhosis patients with and without ACLF.
Patients diagnosed as cirrhosis with acute decompensation (AD) and hospitalized in the Department of Infectious Diseases at the First Affiliated Hospital, School of Medicine, Zhejiang University, Zhejiang, China, between February 2014 and March 2015 were eligible for inclusion in this study. Patients were excluded if they: (1) were <18 years old; (2) were pregnant; (3) had evidence of infection with human immunodeficiency virus, Epstein-Barr virus, or human cytomegalovirus; (4) had aplastic anemia, myelodysplastic syndrome, thrombocytopenia, hemophilia, or disseminated intravascular coagulation; (5) were currently using immunosuppressant medications or adrenocortical hormones; (6) had hepatocellular carcinoma or other malignant tumors; (7) had chronic renal disease or other serious comorbidity; or (8) had history of liver transplantation (19, 20). The diagnosis of liver cirrhosis was based on clinical evidence, endoscopic or radiologic examination, and liver biopsy (6, 21). Cirrhosis patients were diagnosed with AD if they had more than one of the following major complications: ascites, encephalopathy, gastrointestinal hemorrhage, severe liver injury, and infection (20). Severe liver injury was diagnosed if alanine aminotransferase (ALT) was ≥ five times the normal upper limit or more than twice the baseline value along with elevation of serum bilirubin to ≥ 85 μmol/l and international normalized ratio (INR) to ≥ 1.5 at any time during the preceding 1 month (21). The chronic liver failure-sequential organ failure assessment (CLIF-SOFA) score was used to diagnose organ failure (6). The diagnosis of ACLF was based on the criteria proposed by the Chinese Group on the Study of Severe Hepatitis B (COSSH) (22). The diagnosis of gastrointestinal bleeding and bacterial infections was made as previously described (1, 23, 24). PPI treatment was generally used in patients with gastrointestinal bleeding and gastric ulcer, but was extended in patients receiving endoscopic variceal ligation and those manifesting gastrointestinal disturbances such as hiccups, epigastric pain, nausea, or vomiting. For this study, “PPI use” was defined as intravenous administration of any PPI for at least 6 days with daily dose higher than that recommended by the WHO (13). Patients receiving lower doses were considered to be nonusers. PPI doses are classified according to the cumulative defined daily dose (cDDD) (25).
Informed consent for participation in this study was obtained from each participant or his or her legal surrogate. This study was approved by the Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University, Zhejiang, China and all the procedures were in accordance with the latest version of the Declaration of Helsinki.
Demographic data, clinical history, reason for admission, physical examination findings, laboratory results, cirrhosis complications, events of organ failure, and prognosis were recorded. Potential precipitating factors for SBP and HE were noted. Survival at 28 days and 90 days following enrollment was recorded. Survival information was obtained by telephonic interview.
The Statistical Package for the Social Sciences (SPSS) version 26.0 (IBM Corporation, Armonk, New York, USA) was used for the data analysis. Categorical variables were summarized as numbers and percentages and compared by using the chi-squared test. Continuous variables were summarized as means ± SDs or medians (and interquartile ranges) and compared by using the Student's t-test or the Mann–Whitney U test. Variables found to be associated with SBP and HE on the univariate analysis (p < 0.10) were entered into the multivariate logistic regression analysis to identify the independent risk factors for SBP or HE. Baseline characteristics were compared between patients receiving and not receiving PPI therapy before and after propensity score matching (PSM). We performed PSM to adjust differences in baseline characteristics of decompensated cirrhosis and ACLF including gender, age, leukocyte count, platelet count, hemoglobin, serum bilirubin, albumin, aspartate aminotransferase (AST), ALT, INR, creatinine, serum sodium, the CLIF-SOFA score, and the model for end-stage liver disease (MELD) score by matching non-PPI users with comparable patients with PPI users in a ratio of 1:1 with a caliper of 0.1 of the SD. A standardized difference < 0.1 indicated a good balance between PPI and non-PPI groups. The multivariate Cox proportional hazards regression model was used to analyze the risk factors for 28-day and 90-day mortality. For the multivariate analysis, the probability for stepwise entry was at p = 0.05 and for removal at p = 0.10; only variables with p < 0.05 were retained in the final model. Statistical significance was at p ≤ 0.05.
A total of 489 acute decompensated patients with liver cirrhosis were selected in this study (Figure 1). Among these 489 patients (409 patients without ACLF and 80 patients with ACLF), PPI therapy was used in 299 (61.1%) patients: 256/409 (62.6%) patients without ACLF and 43/80 (53.8%) patients with ACLF (Figure 1; Table 1). The PPIs included omeprazole (n = 98; daily dose 40–80 mg), pantoprazole (n = 132; daily dose 40–80 mg), and lansoprazole (n = 69; daily dose 30–60 mg).
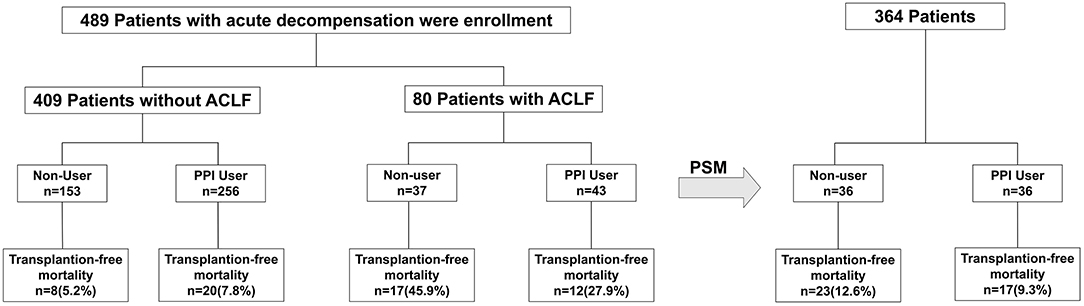
Figure 1. Flowchart showing patient selection for the study. A total of 489 acute decompensated patients with liver cirrhosis were selected and then propensity score matched with 1:1. PPI, proton pump inhibitor; ACLF, acute-on-chronic liver failure.
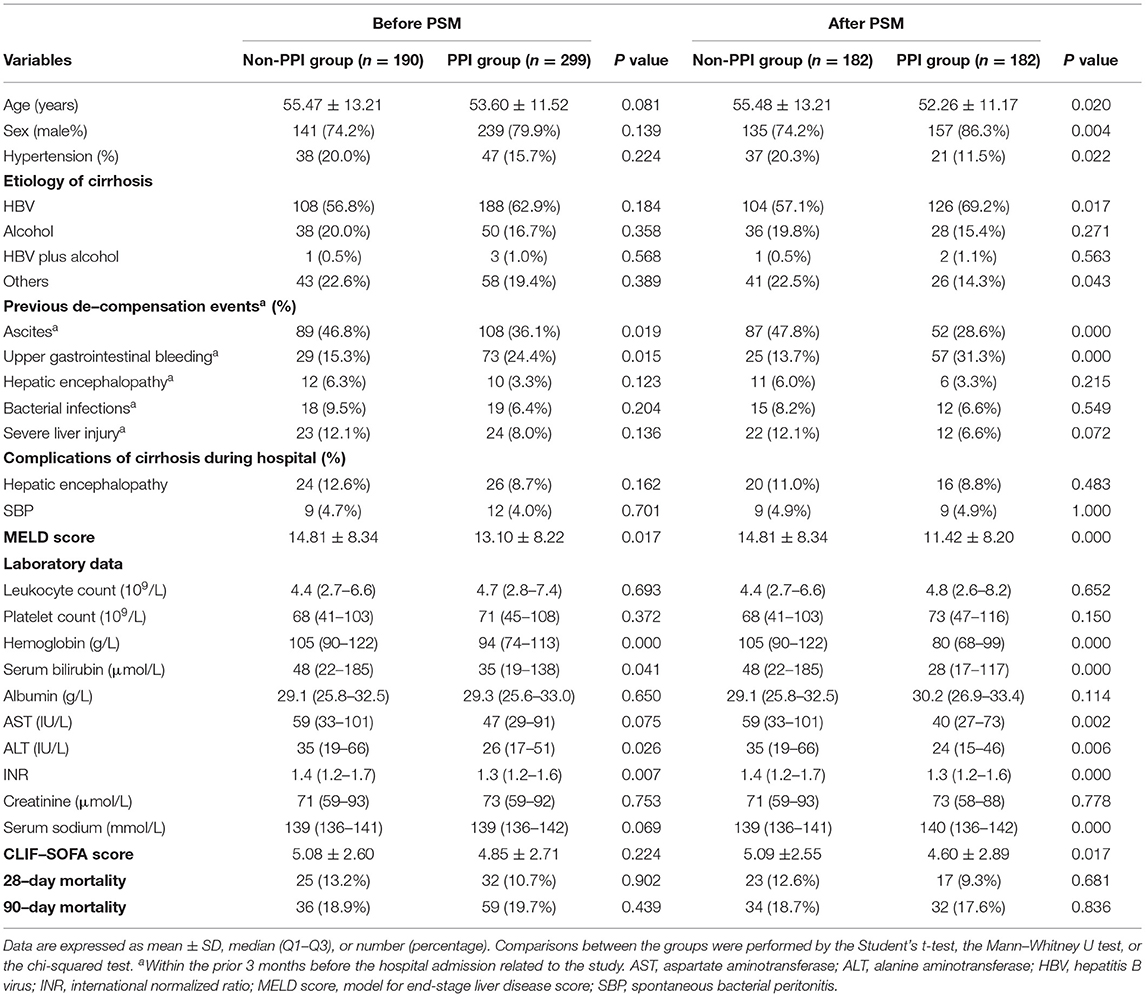
Table 1. Baseline characteristics of the enrolled patients receiving and not receiving proton pump inhibitor (PPI) therapy.
Proton pump inhibitor non-users (n = 190) and users (n = 299) did not differ significantly with respect to the baseline features (hepatitis B, 56.8 vs. 62.9%); (alcohol abuse, 20.0 vs. 16.7%); age (55.47 ± 13.21 vs. 53.60 ± 11.52 years); gender composition (male, 74.2 vs. 79.9%); prevalence of hypertension (20.0 vs. 15.7%); and history of HE (6.3 vs. 3.3%), bacterial infections (9.5 vs. 6.4%), or severe liver injury (12.1 vs. 8.0%) in the preceding 3 months. Gastrointestinal bleeding in the 3 months prior to admission was significantly more common in PPI users than in nonusers (24.4 vs. 15.3%, p = 0.015), while ascites in the 3 months prior to admission was significantly less common in PPI users (36.1 vs. 46.8%, p = 0.019). The incidence of HE (8.7 vs. 12.6%) and SBP (4.0 vs. 4.7%) during hospitalization was not differ significantly between PPI users and nonusers (Table 1). PPI users had significantly lower hemoglobin, serum bilirubin, ALT, INR, and the MELD score (p = 0, p = 0.041, p = 0.026, p = 0.007, and p = 0.017, respectively) (Table 1).
Then, we matched 489 patients with acute decompensated cirrhosis with 1:1. After PSM, there are 182 patients in PPI user group and non-user group, respectively (Table 1). Compared with the decompensated cirrhosis patients before PSM, some baseline characteristics have changed. PPI users were younger than nonusers (52.26 ± 11.17 vs. 55.48 ± 13.21 years, p = 0.020) included more male individuals (86.3 vs. 74.2%, p = 0.004) and fewer hypertension patients (11.5 vs. 20.3%, p = 0.022). Among PPI users, decompensated cirrhosis was more commonly caused by hepatitis B virus alone compared to PPI non-users (69.2 vs. 57.1%, p = 0.005) other than by other causes (14.3 vs. 22.5%, p = 0.043). In addition, PPI users had remarkably higher serum sodium level (p = 0.000), lower AST level, and the CLIF-SOFA score (p = 0.002 and p = 0.017). Other baseline characteristics did not change significantly.
We also analyzed 80 patients with ACLF in detail and matched them by the same method as above (Table 2). Among patients with ACLF, there were no significant differences between PPI users and non-users in mean age, gender composition, prevalence of hypertension, etiology of cirrhosis, incidence of previous decompensation, complications of cirrhosis during hospital stay, laboratory data, and scores of the MELD and the CLIF-SOFA. Similarly, PPI users and non-users had comparable 28-day mortality (27.8 vs. 45.9%, 0.482) and 90-day mortality rates (47.2 vs. 56.8%, 0.887). After PSM, other features did not change significantly, except for the lower prevalence of hypertension in PPI users (25.0 vs. 5.6%, p = 0.023).
The proportion of patients developing HE during hospitalization was comparable between PPI users and nonusers before and after PSM (8.7 vs. 12.6%, p = 0.162; 8.8 vs. 11.0%, p = 0.483) (Table 1). This was true even when patients with ACLF (14.0 vs. 16.2%, p = 0.779; 16.7 vs. 13.9%, p = 0.745) (Table 2). On the multivariate analysis, age [odds ratio (OR) = 1.07, 95% CI: 1.03–1.12, p = 0.001], albumin (OR = 1.13, 95% CI: 1.04–1.24, p = 0.006), history of previous HE (OR = 242.52, 95% CI: 40.17–1464.11, p < 0.001) and the CLIF-SOFA score (OR =2.89, 95% CI: 2.11–3.96, p < 0.001) were significantly associated with the risk of developing HE in decompensated cirrhosis patients during hospitalization (Table 3).
The proportion of patients developing SBP during hospitalization was comparable between PPI users and nonusers (4.0 vs. 4.7%, p = 0.701; 4.9 vs. 4.9%, p = 1) (Table 1). This was true even when patients with ACLF were considered separately (4.0 vs. 2.7%, p = 0.649; 5.6 vs. 2.8%, p = 0.558) (Table 2). On the multivariate analysis, history of previous severe liver injury (OR = 3.43, 95% CI: 1.16–10.17, p = 0.026) and previous bacterial infections (OR = 6.47, 95% CI: 2.29–18.29, p < 0.001) were independent risk factors for SBP in patients with decompensated cirrhosis (Table 3).
The cDDD of PPI in patients with decompensated cirrhosis and ACLF is summarized in Figure 2. The cDDDs of PPI in patients with decompensated cirrhosis and ACLF were divided into four groups: 1–7, 8–14, 15–30, and >30 patients. Among all the patients with decompensated cirrhosis, 25.15% were not treated with PPI. Especially, among all the patients with ACLF, 26.25% were not treated with PPI. Before and after PSM, the group of cDDD 15–30 patients accounted for the highest proportion of PPI users in both the decompensated cirrhosis group and ACLF group. But, the proportion of patients with cDDD > 30 patients was the smallest.
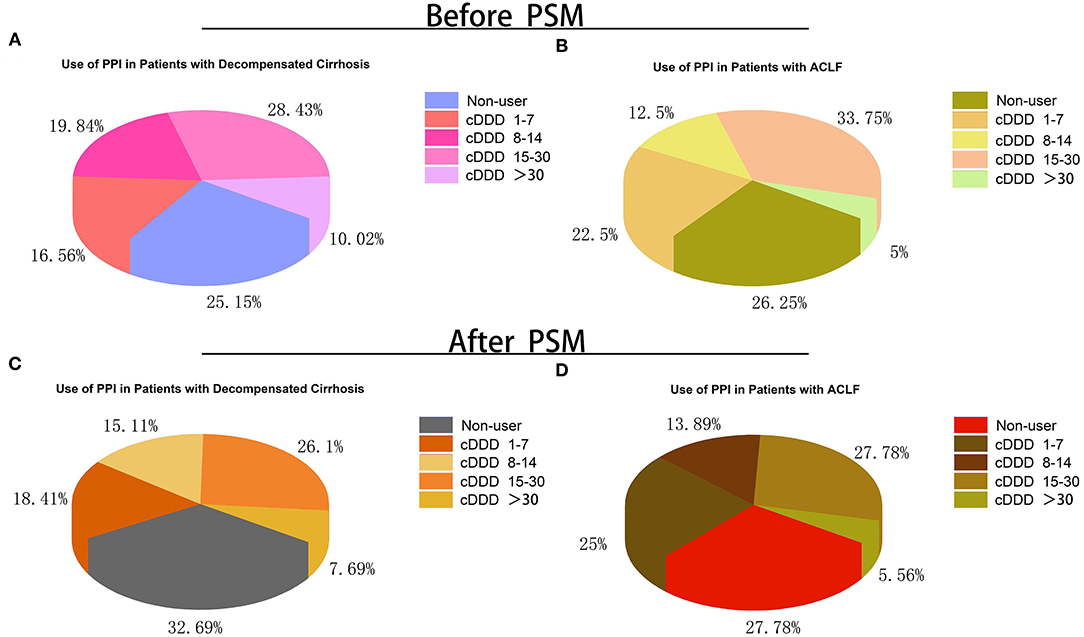
Figure 2. Proportion of patients with different doses and without PPI treatment during hospitalization. (A) Proportion of patients with different doses and without PPI treatment in acute decompensated cirrhosis before PSM; (B) Proportion of patients with different doses and without PPI treatment in ACLF before PSM; (C) Proportion of patients with different doses and without PPI treatment in acute decompensated cirrhosis after PSM; (D) Proportion of patients with different doses and without PPI treatment in ACLF after PSM. cDDD, cumulative defined daily dose; PPI, proton pump inhibitor; ACLF, acute-on-chronic liver failure; PSM, propensity score matching.
Then, we further discussed the relationship between the patients with HE and SBP during hospitalization and the cDDD of PPI (Figure 3). Before PSM, cDDD of 32% patients with HE and cDDD of 29% patients with SBP in decompensated cirrhosis were 15–30 patients, which had the highest proportion. The PPI non-user and cDDD 15–30 patients complicated with HE accounted for a high proportion of 33% in ACLF. After PSM of patients with decompensated cirrhosis, the PPI non-users and cDDD 15–30 patients complicated with HE accounted for a high proportion of 31%, while cDDD 1–7 patients and cDDD 15–30 patients with SBP accounted for a high proportion of 28%. ACLF patients with SBP had the same proportion in the three cDDD groups (1–7, 8–14, and 15–30 patients). Meanwhile, the type and dose of the PPI used were not related to the risk of developing HE or SBP (Table 3).
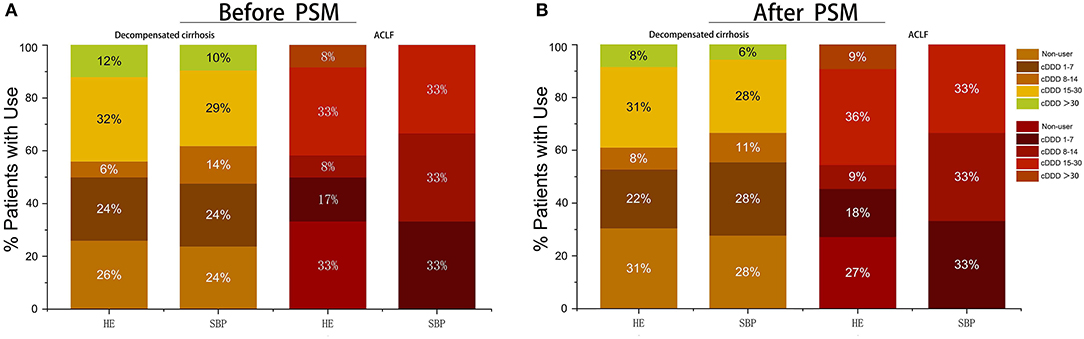
Figure 3. Proportion of patients with different doses and without PPI treatment who suffer from complications during hospitalization. (A) Proportion of patients with different doses and without PPI treatment before PSM; (B) Proportion of patients with different doses and without PPI treatment after PSM. cDDD, cumulative defined daily dose; PPI, proton pump inhibitor; ACLF, acute-on-chronic liver failure.
After median follow-up of 23 months (IQR 6–26 months), the 28-day and 90-day mortality rates were 11.7% (57/489) and 19.4% (95/489), respectively (Table 1; Figure 1).
The multivariate Cox proportional hazards regression model did not show significant association between the type and dose of the PPI and 28-day and 90-day mortality (Table 4; Figures 4, 5). The p-value of the cumulative incidence of mortality death between patients with decompensated liver cirrhosis and the dose of the PPI was 0.018 before PSM, but after PSM, there was no statistical significance. However, age, leukocyte count, and the high MELD score were independent predictors of 28-day and 90-day mortality in cirrhosis patients with AD (Table 4). In addition, previous severe liver injury was an independent predictor of 28-day mortality (Table 4).
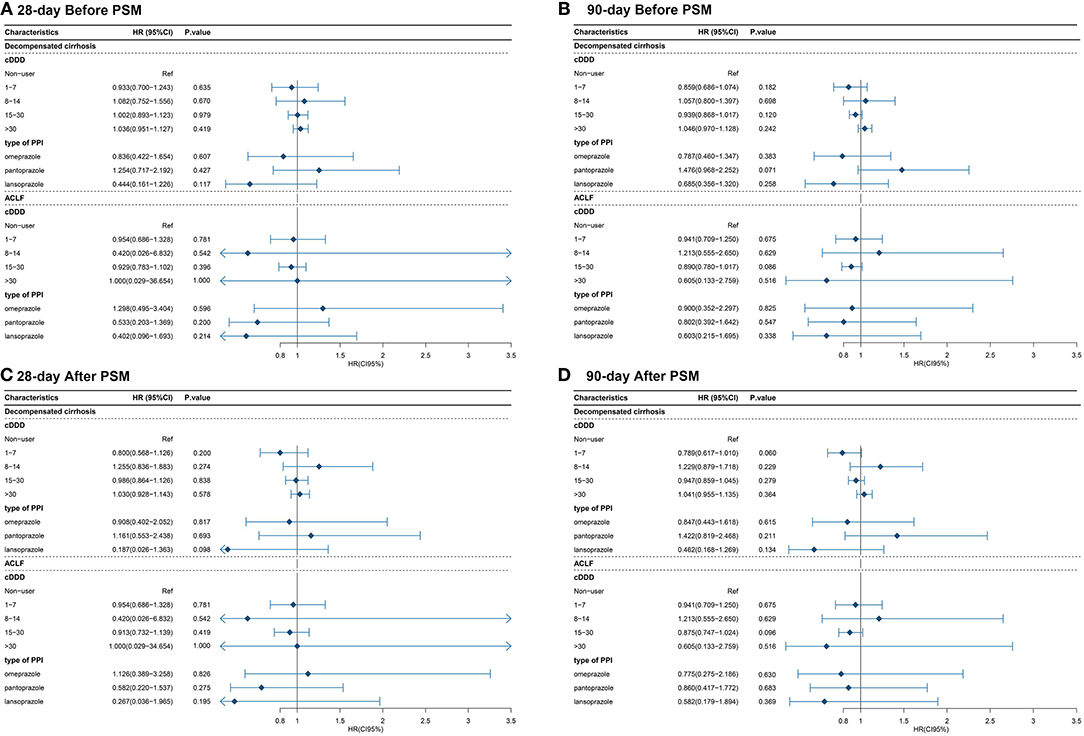
Figure 4. The relationship between the type and dose of the PPI therapy and 28-day and 90-day mortality (A) The relationship between the type and dose of the PPI therapy and 28-day mortality before PSM; (B) The relationship between the type and dose of the PPI therapy and 90-day mortality before PSM; (C) The relationship between the type and dose of the PPI therapy and 28-day mortality after PSM; (D) The relationship between the type and dose of the PPI therapy and 90-day mortality after PSM. cDDD, cumulative defined daily dose; PPI, proton pump inhibitor; ACLF, acute-on-chronic liver failure.
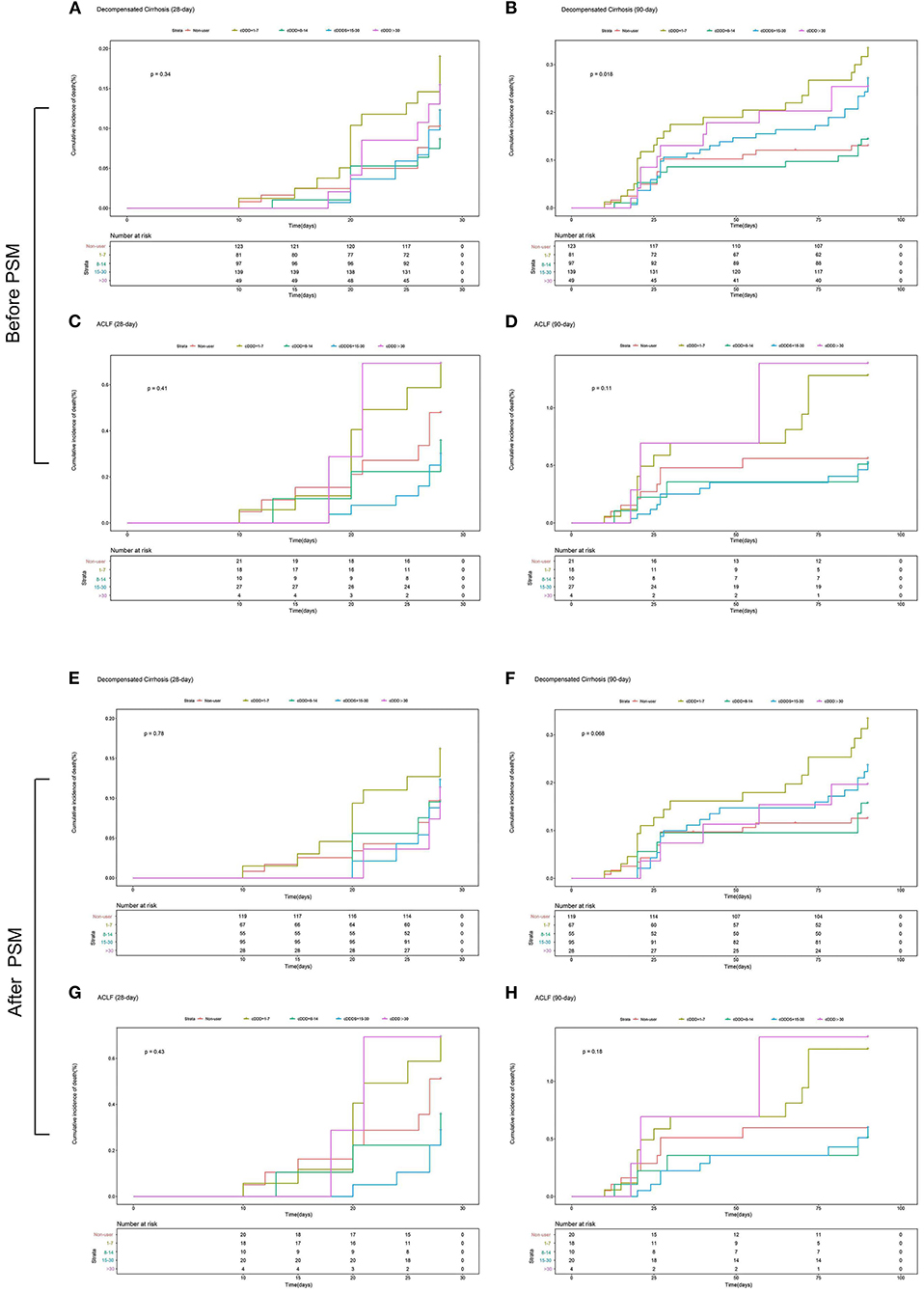
Figure 5. The 28-day or 90-day cumulative incidence of death of patients with and without ACLF with different doses or without PPI treatment during hospitalization. (A) The 28-day cumulative incidence of death of patients with decompensated cirrhosis with different doses or without PPI treatment before PSM; (B) The 90-day cumulative incidence of death of patients with decompensated cirrhosis with different doses or without PPI treatment before PSM; (C) The 28-day cumulative incidence of death of patients with ACLF with different doses or without PPI treatment before PSM; (D) The 90-day cumulative incidence of death of patients with ACLF with different doses or without PPI treatment before PSM; (E) The 28-day cumulative incidence of death of patients with decompensated cirrhosis with different doses or without PPI treatment after PSM; (F) The 90-day cumulative incidence of death of patients with decompensated cirrhosis with different doses or without PPI treatment after PSM; (G) The 28-day cumulative incidence of death of patients with ACLF with different doses or without PPI treatment after PSM; (H) The 90-day cumulative incidence of death of patients with ACLF with different doses or without PPI treatment after PSM. cDDD, cumulative defined daily dose; PPI, proton pump inhibitor; ACLF, acute-on-chronic liver failure.
This study aimed to investigate the impact of PPI therapy on complications and prognosis in cirrhosis patients with and without ACLF. PPI therapy is used in as much as 46–78% of cirrhosis patients (7). In this study, PPI was used in 61.1% of cirrhosis patients including 62.6% of patients without ACLF and 53.8% of patients with ACLF. Thus, consistent with previous studies, our results also demonstrate that PPI use is common in cirrhosis patients with and without ACLF (2). The proportions of patients with history of previous gastrointestinal bleeding and low hemoglobin were higher among PPI users than among non-users. This is not surprising because PPIs are commonly used in cirrhosis patients with gastrointestinal bleeding (2, 26).
The type of PPI and the dose were not associated with the risk of HE and SBP. Previous reports have indicated that treatment with acid-suppressing drugs increase the incidence of SBP and HE (7, 11, 14). The explanation offered was that PPIs might promote small intestinal bacterial overgrowth with subsequent bacterial translocation (7, 27). Another explanation was that PPIs may inhibit the functions of neutrophils and natural killer cells (28). However, the association between PPI and SBP has not been consistently demonstrated (15, 21, 29). In one prospective multicenter study of many cirrhosis patients with AD, PPI use did not increase the risk of SBP (17). Our result is in accordance with the conclusion of an earlier meta-analysis that did not support the association between PPI use and SBP or HE (18).
In this study, age, albumin, history of previous HE, and the CLIF-SOFA score were significantly associated with HE during hospitalization. In addition, previous severe liver injury and previous bacterial infections were shown to be independent risk factors for SBP in cirrhosis patients. A previous study has also shown that history of HE is associated with increased incidence of HE during hospitalization (30).
This study found that older age, high leukocyte count, and the high MELD score independently predicted the 28-day and 90-day mortality in cirrhosis patients with AD. Previous studies have also found that age, leukocyte count, and the MELD score have to be independently associated with the risk of death in cirrhosis (31, 32). In this study, PPI therapy was not associated with the 28-day and 90-day mortality in cirrhosis patients with AD or ACLF. Others have also reported that PPI use does not increase the mortality rate in cirrhosis patients with or without ACLF (16, 18).
In conclusion, PPI therapy does not appear to increase the risk of HE or SBP or to shorten survival in cirrhosis patients with or without ACLF. Further prospective multicenter studies with large samples are necessary to confirm our findings and to clarify how PPI therapy is related to complications and disease progression in cirrhosis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital, School of Medicine of Zhejiang University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JS and HZ conceived and designed the study and draw the manuscript. SS, WY, and RZ collected and analyzed the data. JH, XZ, and MY participated in the data interpretation. SS, HZ, and JS revised the manuscript. All authors contributed to the manuscript and approved the submitted version of the manuscript.
This study was supported by the National Natural Science Foundation of China (Grant No. 81971982) and the Special Anti-epidemic Project of Zhejiang Provincial Department of Education (Y202043368).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer QY declared a shared affiliation with the authors to the handling editor at time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the colleagues who provide enthusiastic help for the study in the First Affiliated Hospital, Zhejiang University, Zhejiang, China.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.763370/full#supplementary-material
1. Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. (2015) 47:a1–46. doi: 10.1055/s-0034-1393172
2. Lu Y, Adam V, Teich V, Barkun A. timing or dosing of intravenous proton pump inhibitors in acute upper gastrointestinal bleeding has low impact on costs. Am J Gastroenterol. (2016) 111:1389–98. doi: 10.1038/ajg.2016.157
3. Scarpignato C, Gatta L, Zullo A, Blandizzi C. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med. (2016) 14:179. doi: 10.1186/s12916-016-0718-z
4. Arroyo V, Moreau R, Jalan R, Gines P. Acute-on-chronic liver failure: a new syndrome that will re-classify cirrhosis. J Hepatol. (2015) 62:S131–43. doi: 10.1016/j.jhep.2014.11.045
5. Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. (2014) 61:1038–47. doi: 10.1016/j.jhep.2014.06.012
6. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. (2013) 144:1426–37. doi: 10.1053/j.gastro.2013.02.042
7. Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. (2016) 64:1265–72. doi: 10.1002/hep.28737
8. Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, et al. Proton pump inhibitors affect the gut microbiome. Gut. (2016) 65:740–8. doi: 10.1136/gutjnl-2015-310376
9. Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. (2016) 65:749–56. doi: 10.1136/gutjnl-2015-310861
10. Lin ZN, Zuo YQ, Hu P. Association of proton pump inhibitor therapy with hepatic encephalopathy in hepatitis b virus-related acute-on-chronic liver failure. Hepat Mon. (2014) 14:e16258. doi: 10.5812/hepatmon.16258
11. Min YW, Lim KS, Min BH, Gwak GY, Paik YH, Choi MS, et al. Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: a propensity score matched cohort study. Aliment Pharmacol Ther. (2014) 40:695–704. doi: 10.1111/apt.12875
12. Ratelle M, Perreault S, Villeneuve JP, Tremblay L. Association between proton pump inhibitor use and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Can J Gastroenterol Hepatol. (2014) 28:330–4. doi: 10.1155/2014/751921
13. Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. (2015) 64:1680–704. doi: 10.1136/gutjnl-2015-309262
14. Tsai CF, Chen MH, Wang YP, Chu CJ, Huang YH, Lin HC, et al. Proton pump inhibitors increase risk for hepatic encephalopathy in patients with cirrhosis in a population study. Gastroenterology. (2017) 152:134–41. doi: 10.1053/j.gastro.2016.09.007
15. Kim JH, Lim KS, Min YW, Lee H, Min BH, Rhee PL, et al. Proton pump inhibitors do not increase the risk for recurrent spontaneous bacterial peritonitis in patients with cirrhosis. J Gastroenterol Hepatol. (2017) 32:1064–70. doi: 10.1111/jgh.13637
16. Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Summereder C, et al. Proton pump inhibitor intake neither predisposes to spontaneous bacterial peritonitis or other infections nor increases mortality in patients with cirrhosis and ascites. PLoS ONE. (2014) 9:e110503. doi: 10.1371/journal.pone.0110503
17. Terg R, Casciato P, Garbe C, Cartier M, Stieben T, Mendizabal M, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol. (2015) 62:1056–60. doi: 10.1016/j.jhep.2014.11.036
18. Yu T, Tang Y, Jiang L, Zheng Y, Xiong W, Lin L. Proton pump inhibitor therapy and its association with spontaneous bacterial peritonitis incidence and mortality: a meta-analysis. Dig Liver Dis. (2016) 48:353–9. doi: 10.1016/j.dld.2015.12.009
19. Shi Y, Yan H, Zhou Z, Fang H, Li J, Ye H, et al. The clinical course of cirrhosis patients hospitalized for acute hepatic deterioration: a prospective bicentric study. Medicine. (2015) 94:e2031. doi: 10.1097/MD.0000000000002031
20. Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. (2015) 62:232–42. doi: 10.1002/hep.27795
21. Bajaj JS, O'Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. (2012) 56:2328–35. doi: 10.1002/hep.25947
22. Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. (2018) 67:2181–91. doi: 10.1136/gutjnl-2017-314641
23. Fernandez J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. (2018) 67:1870–80. doi: 10.1136/gutjnl-2017-314240
24. Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. (2012) 61:297–310. doi: 10.1136/gutjnl-2011-300779
25. Roza MADe, Kai L, Kam JW, Chan YH, Kwek A, Ang TL, et al. Proton pump inhibitor use increases mortality and hepatic decompensation in liver cirrhosis. World J Gastroenterol. (2019) 25:4933–44. doi: 10.3748/wjg.v25.i33.4933
26. Ray WA, Chung CP, Murray KT, Smalley WE, Daugherty JR, Dupont WD, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. (2016) 151:1105–12e10. doi: 10.1053/j.gastro.2016.08.054
27. Bauer TM, Steinbruckner B, Brinkmann FE, Ditzen AK, Schwacha H, Aponte JJ, et al. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol. (2001) 96:2962–7. doi: 10.1111/j.1572-0241.2001.04668.x
28. Zedtwitz-Liebenstein K, Wenisch C, Patruta S, Parschalk B, Daxbock F, Graninger W. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit Care Med. (2002) 30:1118–22. doi: 10.1097/00003246-200205000-00026
29. D Law, M. Pimentel. Proton pump inhibitor therapy does not affect hydrogen production on lactulose breath test in subjects with IBS. Dig Dis Sci. (2010) 55:2302–8. doi: 10.1007/s10620-009-1010-2
30. Patidar KR, Thacker LR, Wade JB, Sterling RK, Sanyal AJ, Siddiqui MS, et al. Covert hepatic encephalopathy is independently associated with poor survival and increased risk of hospitalization. Am J Gastroenterol. (2014) 109:1757–63. doi: 10.1038/ajg.2014.264
31. Ampuero J, Simon M, Montoliu C, Jover R, Serra MA, Cordoba J, et al. Minimal hepatic encephalopathy and critical flicker frequency are associated with survival of patients with cirrhosis. Gastroenterology. (2015) 149:1483–9. doi: 10.1053/j.gastro.2015.07.067
Keywords: proton pump inhibitor, acute decompensated cirrhosis, acute-on-chronic liver failure, prognosis, complications
Citation: Sun S, Ye W, Zhao R, Hu J, Zhang X, Yang M, Zhao H and Sheng J (2021) Proton Pump Inhibitor Therapy Does Not Affect Prognosis of Cirrhosis Patients With Acute Decompensation and Acute-on-Chronic Liver Failure: A Single-Center Prospective Study. Front. Med. 8:763370. doi: 10.3389/fmed.2021.763370
Received: 23 August 2021; Accepted: 07 October 2021;
Published: 10 November 2021.
Edited by:
Yu-Chen Fan, Shandong University, ChinaReviewed by:
Qiao Yang, Sir Run Run Shaw Hospital, ChinaCopyright © 2021 Sun, Ye, Zhao, Hu, Zhang, Yang, Zhao and Sheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Zhao, zjuzhaohong@zju.edu.cn; Jifang Sheng, jifang_sheng@zju.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.