- 1State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
- 2College of Medicine, Zhejiang University, Hangzhou, China
- 3Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, Hangzhou, China
- 4Shulan International Medical College, Zhejiang Shuren University, Hangzhou, China
Purpose: The mortality of invasive pulmonary aspergillosis (IPA) in patients with liver failure was high. However, the prophylactic treatment in those patients with a high-risk factor in IPA has not been researched.
Patients and methods: A multicenter, retrospective study was conducted in patients with liver failure. The study cohort of liver failure was randomly split into a training set for model development and the other served as the testing set for model verification. Multivariate analysis was performed to identify the risk factors of IPA. A weighted risk score for IPA was established. Anti-fungal treatment was prophylactically used in patients with medium and high IPA risk to evaluate the effect.
Results: In total, 1,722 patients with liver failure were enrolled. Fifty-seven patients who received prophylactic treatment were excluded from the risk factor system study. About 1,665 patients were randomly split at a ratio of 2:1 into two datasets. Diabetes, glucocorticoids, plasma exchange, and hepatorenal syndrome (HRS) were risk factors in IPA in patients with liver failure, with weighted risk scores of 4, 7, 2, and 3, respectively. In the validation set and test set, the patients with risk scores of ≤ 3 presented low incidences of IPA at 4 and 2.7%. Patients with risk scores of 4–5 had an IPA incidence of 7.6% and 10.1%, and could be considered as a medium-risk group (p < 0.01 vs. the group with scores of ≤ 3), whereas those with risk scores of >5 manifested a significantly higher IPA incidence of 21.2 and 12.7%, who were considered a high-risk group (p < 0.01 vs. the groups with scores of 4–5 and >5, respectively). The IPA risk scores in the training set and the testing set were also analyzed by the ROC with an area under the ROC of 0.7152 and 0.6912. In this study, 57 patients received antifungal prophylaxis; the incidence of IPA was 1.8%, which was significantly lower after prophylactic antifungal therapy (p < 0.001).
Conclusions: A weighted risk score for patients with liver failure, complicated with IPA, was established and confirmed in the testing cohort. Voriconazole prophylactic treatment to patients with liver failure with medium and high IPA risk can effectively prevent Aspergillus infection.
Introduction
Invasive pulmonary aspergillosis (IPA) is a devastating infectious disease in patients with liver failure; the mortality rate exceeds 80% (1, 2). Early diagnosis and treatment of IPA are particularly important. However, clinical diagnosis of IPA in liver failure is a huge challenge. The traditional G test and the GM test can diagnose IPA early in patients with allogeneic hematopoietic stem cell transplantation, but they lack sensitivity in patients with liver failure (3). Invasive diagnostic procedures are often not feasible due to prolonged prothrombin time and thrombocytopenia. So, at present, the clinical diagnosis of liver failure complicated with IPA infection depends more on symptom-triggered pulmonary CT screening. However, due to the lack of specificity in clinical symptoms and imaging of liver failure complicated with IPA infection, the study found that the positive predictive value of this method was 61% and the negative predictive value was 92% (4). Moreover, previous studies have shown that patients with IPA were diagnosed when the CLIF-SOFA lung score is >1 and had the worst prognosis, poor antifungal treatment effect, and the highest mortality (5). These studies demonstrated that early diagnosis and early treatment are the key factors to improve the prognosis of patients with liver failure, complicated with IPA infection, but it was difficult to achieve.
In view of the disastrous consequences of IPA in patients with liver failure, the risk factor in IPA has been researched extensively, such as hemodialysis and prior antibiotics use (3), prolonged and high-dose corticosteroid therapy (6, 7), and recent history of neutropenia (8, 9). The prophylactic antifungal treatment, which was based on the risk factors, had been evaluated in multiple clinical trials in different diseases. Rijnders et al. showed that prophylactic inhalation of liposomal amphotericin B significantly reduced the incidence of IPA in patients during prolonged neutropenia (10). The China Assessment of Antifungal Therapy in Hematological Diseases (CAESAR) study showed antifungal prophylaxis was beneficial in patients with hematological malignancies with an intermediate and high risk of invasive fungal disease (11). A clinical trial at Mayo Clinic (12) researched lung transplant recipients who receive prolonged and mostly lifelong azole antifungal prophylaxis; none of the patients developed disseminated invasive aspergillosis. However, the prophylactic treatment in patients with liver failure with a risk factor for IPA has not been researched.
In this study, we established a weighted risk score for IPA that accurately discriminated a cohort of patients with liver failure with low, intermediate, and high risks of IPA. Then, the efficacy of antifungal prophylaxis with voriconazole or caspofungin was evaluated in patients with different IPA risks in order to reduce mortality in this population.
Materials and Methods
Study Design
A multicenter, retrospective observational study was carried out in patients with liver failure admitted to two tertiary hospitals in Zhejiang province from December 2008 to July 2021: one is Shulan Hangzhou Hospital; the other is the First Affiliated Hospital, School of Medicine, Zhejiang University. The collected data included baseline characteristics, type of liver failure, clinical features, underlying disease, complication, antifungal treatment, treatment-related potential risk factors of IPA, and the prognosis. Because the study was retrospective and the data were analyzed anonymously, the need for consent was waived.
Enrollment Criteria
Liver failure was defined according to the Diagnosis and Treatment Guideline for Liver Failure in China (2018) (13). The definition of IPA was used by the European Organization for Research and Treatment of Cancer (EORTC) consensus (14), which was diagnosed if the following two criteria were fulfilled: (1) positive culture of Aspergillus spp. from sputum; (2) presence of one of the following three signs on computed tomography: dense, well-circumscribed lesions with or without a halo sign, air-crescent sign, or cavity.
Statistical Analysis
The study cohort of liver failure was randomly split at a ratio of 2:1 into two sets, of which one served as the training set for model development and the other served as the testing set for model verification. The characteristics of patients were compared between two datasets using Student's t-tests or Kruskal-Wallis tests for continuous variables where appropriate and χ2 or Fisher exact tests for categorical variables. We first, by using univariate analysis with p < 0.10, identified the risk factors that were individually complicated with probable IPA. The factors that demonstrated an individual association were demonstrated the multivariate logistic regression with the stepwise criteria of 0.05. Points were assigned for the variables and were weighted approximately by the corresponding regression β-coefficients. Receiver operator curves (ROC) were calculated to assess the discrimination capacity of the risk score. Once the model was determined, it was tested in the test set to confirm its performance in predicting the IPA incidence. All statistical analyses were performed using SPSS version 18.0 (SPSS, Chicago, IL, USA).
Results
Study Populations and Incidence of IPA
In this study, 1,722 patients with liver failure were enrolled. Fifty-seven patients who received voriconazole or caspofungin prophylactic treatment were excluded from the risk factor system study. Of the remaining 1,665 patients, the patients with IPA were 111, the overall incidence of IPA was 6.7%, and the mortality of IPA was 85.6%. Acute (subacute) liver failure, acute-on-chronic (subacute-on-chronic) liver failure, and chronic liver failure were 198, 902, and 565 cases, and the IPA incidences were 7.1, 7.1, and 5.9%, respectively. There was no significant difference between any two groups for IPA incidence.
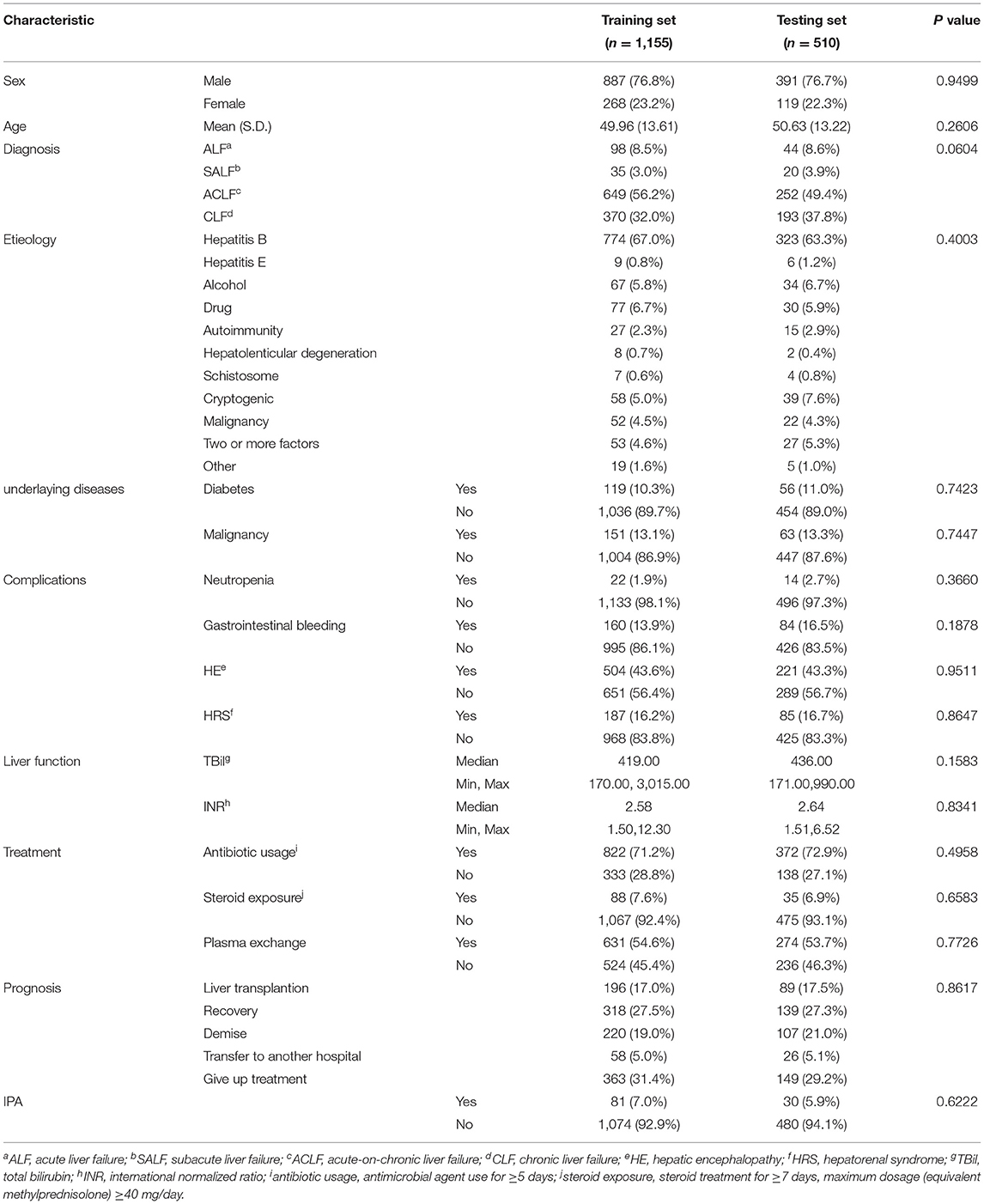
About 1,665 liver failure patients were randomly split at a ratio of 2:1 into two datasets, 1,155 patients were enrolled in the training set, and 510 patients were in the testing set.
The characteristics of patients in training and testing datasets were similar in all aspects, as shown in Table 1.
Risk Scores Associated With IPA in the Training Dataset
The variables of the training set that was associated with IPA incidence, including diabetes, corticosteroid, plasma exchange treatment, and INR, were significant in the multivariate logistic regression, and weighted points were assigned (Table 2).
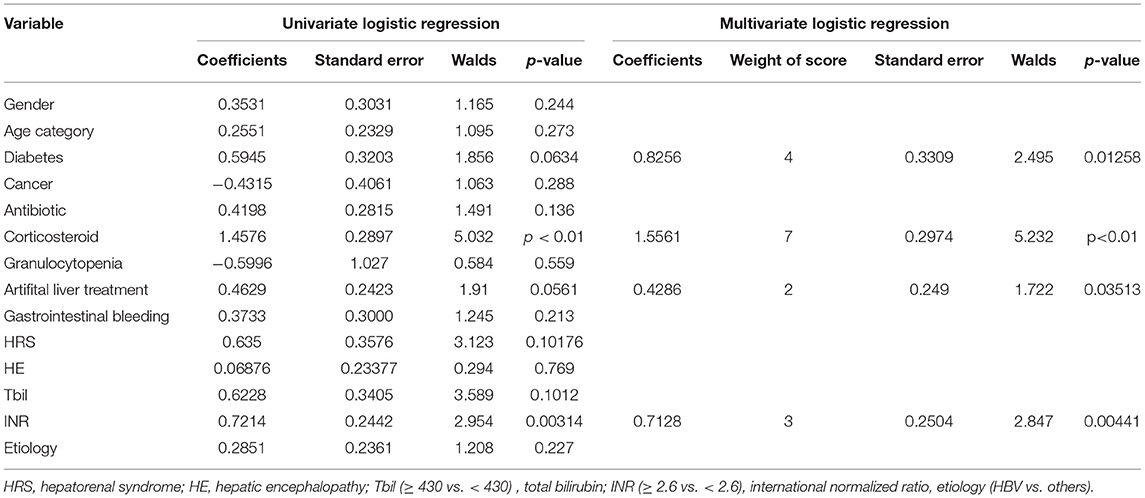
Table 2. Multivariate logistic regression analysis of risk factors associated with invasive pulmonary aspergillosis (IPA) development in the training dataset.
Based on the multivariate logistic regression analysis, IPA risk scores ranged from 0 to 16 (Table 3) and were calculated in the training set. The distribution of the risk scores and the cumulative incidence of IPA in liver failure patients were shown in Table 4. The patients with risk scores of ≤ 3 presented low incidences of IPA at 4%. Patients with risk scores of 4–5 had an IPA incidence of 7% and could be considered as a medium-risk group (p < 0.01 vs. the group with scores of ≤ 3), whereas those with risk scores of >5 manifested a significantly higher IPA incidence of 21.2%, who were considered as a high-risk group (p < 0.01 vs. the groups with scores of 4–5 and >5, respectively).
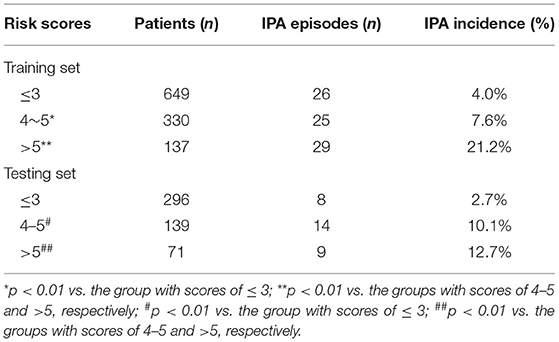
Table 4. Distribution of risk scores vs. the cumulative incidence of IPA in the training and testing datasets.
The ROC curve was used to identify the ability of the IPA risk scores, and the area under the curve was 0.6912, which is shown in Figure 1.
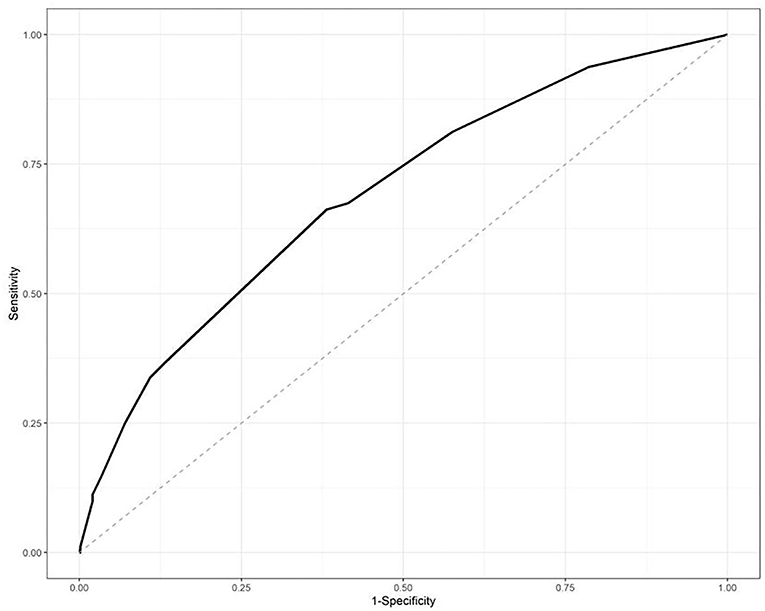
Figure 1. Receiver-operator curve (ROC) analysis of the risk scores in the training datasets. The area under the curve was 0.6912.
Risk Scores Associated With IPA in the Testing Dataset
The IPA risk score was used to assess the patients with liver failure in the testing dataset. The incidence of IPA was 2.7% when the risk scores of IPA were less than or equal to 3. As the risk scores reached 4–5, the IPA incidence increased to 7% (p < 0.01 vs. the group with scores of ≤ 3), whereas the IPA incidence was 12.7% in the patients with risk scores of >5 (p < 0.01 vs. the groups with scores of 4–5 and >5, respectively).
The IPA risk scores in the testing set were also analyzed by the ROC with an area under the ROC of 0.7152, as shown in Figure 2.
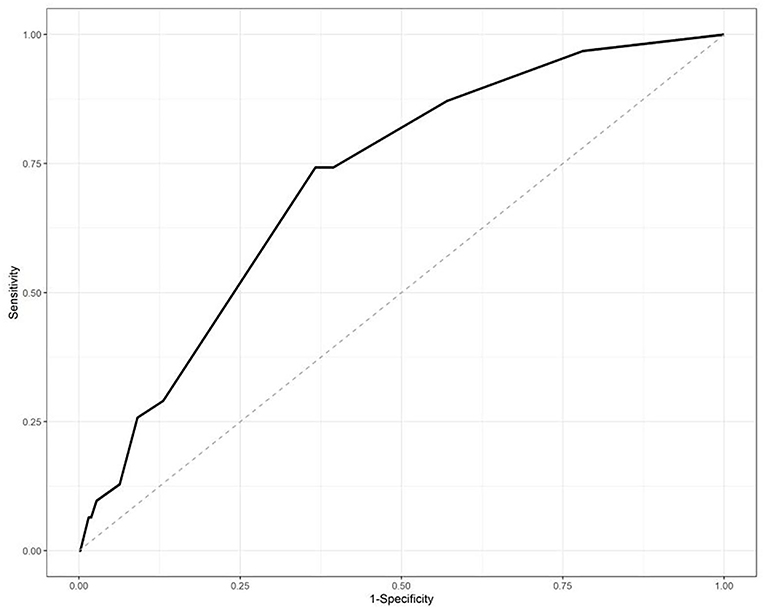
Figure 2. Receiver-operator curve (ROC) analysis of the risk scores in the testing datasets. The area under the curve was 0.7152.
Impact of Antifungal Prophylaxis in Patients With Different Risk Scores
In this study, 57 patients received antifungal prophylaxis, who had significantly more seriously impaired liver function than patients without prophylaxis (p < 0.001). Meanwhile, the risk scores were significantly higher in the population with antifungal prophylaxis (p < 0.001) (Table 5). However, the incidence of IPA was 1.8%, which was significantly lower after prophylactic antifungal therapy (p < 0.001).
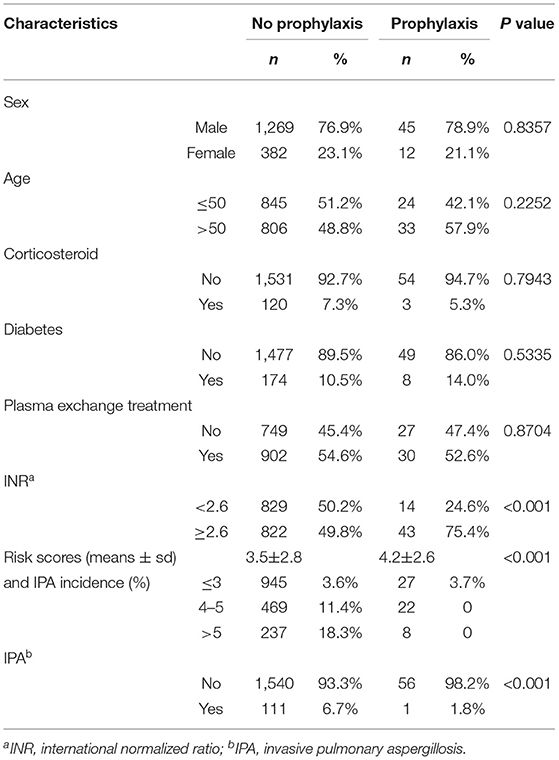
Table 5. The characteristics of patients with or without antifungal prophylaxis and impact of antifungal prophylaxis in patients with different risk scores.
Discussion
Patients with end-stage liver diseases are now considered additional risk factors of IPA, along with allogeneic bone marrow transplantation (15). The prevalence of IPA in HBV-related ACLF patients has been reported to be 5–8.3%. The short-term mortality observed in these patients ranged from 73.5 to 100% (1, 5, 7). In our study, the incidence of IPA was 6.7%, and the mortality was 85.6%, which was consistent with previous reports. It is not difficult to find that the IPA rate in patients with liver failure is not high, but, once it occurs, the mortality is very high. Hence, numerous studies have reported lots of IPA risk factors of liver failure (1, 2, 7, 16, 17) in order to provide a way for clinical early diagnosis and early treatment.
The previous study showed antibiotic use was an independent factor associated with the occurrence of IPA in patients with liver failure (2), and the patients were prone to many types of infections caused by opportunistic pathogens, including Aspergillus spp. after antibiotics use. However, our results showed there was no significant difference in antibiotic use between the patients with IPA and those without. Instead, diabetes, INR, plasma exchange, and steroid use were proved to be significant risk factors for IPA. Diabetes and steroid use indirectly reflect the immune status, which was easier to understand that they are the risk factors of IPA in patients with liver failure. But, in this study, we, for the first time, confirmed that the plasma exchange therapy was the risk factor of IPA in liver failure; this risk score is 2, which belongs to the low-level risk factor. This means that patients with severe liver failure treated with plasma exchange received steroid treatment or had diabetes at the same time, which will increase the risk of IPA.
Over the past three decades, plasma exchange has been employed to treat liver failure. Due to the lack of randomized controlled studies, the effect of plasma exchange on liver failure has always been controversial (18). Until Larsen et al. (19) published the first randomized control trial of plasma exchange in patients with acute liver failure in 2016, then, plasmapheresis was added to the European guidelines (20) as Level I, Grade 1 recommendation in management of acute liver failure. Its proposed mechanism is the removal of plasma cytokines and drivers of systemic inflammatory cascade by plasma exchange. But why does plasma exchange increase the risk of Aspergillus infection? We speculate whether plasma exchange removes some cytokines at the same time, which mediate the inflow of macrophage and might limit the degree of local tissue destruction of Aspergillus infection (21).
Despite so many studies on risk factors, there is no study that has considered an early prophylactic treatment for high-risk patients. There may be two reasons that hinder the measures to be taken for clinical prophylactic treatment. First, the risk factors found in different studies are inconsistent, there is no further verification of these risk factors, and there is no distinction between low-risk, medium-risk, and high-risk factors. So, it is difficult for clinical doctors to judge all of these risk factors and take steps. In fact, almost all liver failure will have 1–2 risk factors above mentioned, but it is not impossible to give prophylactic antifungal therapy to all the patients with liver failure; second, it is difficult to determine which prophylactic method has to be taken. If voriconazole prophylactic treatment is taken in line with HSCT (11), how much dosage of voriconazole needs to be used in patients with liver failure, because of the hepatotoxicity of voriconazole, whether its prophylactic use will be harmful to the liver of patients with liver failure. Different from creatinine clearance, the pharmacokinetics of drugs metabolized from the liver with different disease severities is not a simple linear relationship, and there are too many concerns about voriconazole metabolism and human CYP2C19 gene polymorphism at the same time (22, 23).
In this study, we conducted a multicenter-based study to build up a risk score system for IPA in patients with liver failure. We confirmed the discriminative performance of the IPA risk score system in both the training and testing sets. The effective concentration range of voriconazole is between 1 and 5 ug/ml. As long as the concentration is monitored, the risk can be avoided. Prevention failed in one patient among 27 low-risk patients, who were prevented with caspofungin. However, what we need to further study is the difference between caspofungin and voriconazole in the prevention of Aspergillus infection.
There are several limitations to our study. First, we can only, according to the results of sputum culture and pulmonary CT, diagnose IPA in patients with liver failure due to their coagulation function and platelet status, which will not lead to a precise diagnosis; second, this study is a retrospective analysis, which could lead to the unbalanced distribution of confounding factors when we evaluate the efficacy of the prophylactic treatment. Some factors may affect the results, such as the duration of the disease, the severity of the disease, or the dosage of the prophylactic antifungal drug. Third, we included liver failure caused by different causes, and they have different pathogenesis, which will affect the incidence rate of IPA. Finally, the sample size was insufficient to compare different prophylactic treatment effects in different subgroups.
Conclusion
To the best of our knowledge, this is the first report for developing an IPA risk score system based on a large patient population with liver failure. We established a weighted risk score for IPA that could reliably discriminate the incidence of IPA. The precise risk assessment of IPA may provide a chance for risk based antifungal treatment in patients with liver failure. For the first time, we tried to give voriconazole prophylactic treatment to patients with liver failure with medium and high IPA risk and found that it can effectively prevent Aspergillus infection. Furthermore, we need to expand the sample size and design a multicenter prospective study to further verify the effect of preventive antifungal therapy in high-risk groups and explore the best dose of preventive therapy in patients with liver failure.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Shulan Hangzhou Hospital. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
HG designed and conceptualized the study. XZ, SS, and XD designed the table to collect and analyze the data. YB, YW, YS, and JZ provided clinical data. XZ, SS, and HG wrote the manuscript. SS helped to revise the manuscript. All the authors have read and approved the final manuscript.
Funding
This work was supported by the Zhejiang Basic Public Welfare Research Program (No. LGF21H030012) and the Zhejiang Non-public Medical Specialty Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Jie Wu was involved in the development of the methodology and participated in the statistical analysis.
References
1. Chen J, Yang Q, Huang J, Li L. Risk factors for invasive pulmonary aspergillosis and hospital mortality in acute-on-chronic liver failure patients: a retrospective-cohort study. Int J Med Sci. (2013) 10:1625–31. doi: 10.7150/ijms.6824
2. Zhang X, Yang M, Hu J, Zhao H, Li L. Epidemiology of invasive pulmonary aspergillosis in patients with liver failure: clinical presentation, risk factors, and outcomes. J Int Med Res. (2018) 46:819–27. doi: 10.1177/0300060517729907
3. Verma N, Singh S, Taneja S, Duseja A, Singh V, Dhiman RK, et al. Invasive fungal infections amongst patients with acute-on-chronic liver failure at high risk for fungal infections. Liver Int. (2019) 39:503–13. doi: 10.1111/liv.13981
4. Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. (2012) 186:56–64. doi: 10.1164/rccm.201111-1978OC
5. Gao J, Zhang Q, Wu Y, Li Y, Qi T, Zhu C, et al. Improving survival of acute-on-chronic liver failure patients complicated with invasive pulmonary aspergillosis. Sci Rep. (2018) 8:876. doi: 10.1038/s41598-018-19320-2
6. Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. (2003) 362:1828–38. doi: 10.1016/S0140-6736:14904-5
7. Wu Z, Ling Z, Shao F, Sheng J, Li L. Invasive pulmonary aspergillosis in patients with acute-on-chronic liver failure. J Int Med Res. (2012) 40:1958–65. doi: 10.1177/030006051204000537
8. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the european organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. (2020) 71:1367–76. doi: 10.1093/cid/ciz1008
9. Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. (2011) 20:156–74. doi: 10.1183/09059180.00001011
10. Rijnders BJ, Cornelissen JJ, Slobbe L, Becker MJ, Doorduijn JK, Hop WC, et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clin Infect Dis. (2008) 46:1401–8. doi: 10.1086/586739
11. Wang L, Wang Y, Hu J, Sun Y, Huang H, Chen J, et al. Clinical risk score for invasive fungal diseases in patients with hematological malignancies undergoing chemotherapy: China Assessment of Antifungal Therapy in Hematological Diseases (CAESAR) study. Front Med. (2019) 13:365–77. doi: 10.1007/s11684-018-0641-0
12. Chong PP, Kennedy CC, Hathcock MA, Kremers WK, Razonable RR. Epidemiology of invasive fungal infections in lung transplant recipients on long-term azole antifungal prophylaxis. Clin Transplant. (2015) 29:311–8. doi: 10.1111/ctr.12516
13. Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology. Chinese Medical Association. Diagnositic and treatment guidelines for liver failure. Chinese Journal of Infectious Diseases. (2019) 37:1–9. doi: 10.3760/cma.j.issn.1000-6680.2019.01.001
14. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) Consensus Group. Clin Infect Dis. (2008) 46:1813–21. doi: 10.1086/588660
15. Koulenti D, Garnacho-Montero J, Blot S. Approach to invasive pulmonary aspergillosis in critically ill patients. Curr Opin Infect Dis. (2014) 27:174–83. doi: 10.1097/QCO.0000000000000043
16. Wang W, Zhao CY, Zhou JY, Wang YD, Shen C, Zhou DF, et al. Invasive pulmonary aspergillosis in patients with HBV-related liver failure. Eur J Clin Microbiol Infect Dis. (2011) 30:661–7. doi: 10.1007/s10096-010-1137-2
17. Lahmer T, Brandl A, Rasch S, Baires GB, Schmid RM, Huber W, et al. Prevalence and outcome of invasive pulmonary aspergillosis in critically ill patients with liver cirrhosis: an observational study. Sci Rep. (2019) 9:11919. doi: 10.1038/s41598-019-48183-4
18. Tan EX, Wang MX, Pang J, Lee GH. Plasma exchange in patients with acute and acute-on-chronic liver failure: a systematic review. World J Gastroenterol. (2020) 26:219–45. doi: 10.3748/wjg.v26.i2.219
19. Larsen FS, Schmidt LE, Bernsmeier C, Rasmussen A, Isoniemi H, Patel VC, et al. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol. (2016) 64:69–78. doi: 10.1016/j.jhep.2015.08.018
20. Wendon J, Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. (2017) 66:1047–81. doi: 10.1016/j.jhep.2016.12.003
21. Caglar K, Kalkanci A, Fidan I, Aydogan S, Hizel K, Dizbay M, et al. Investigation of interleukin-10, tumor necrosis factor-alpha and interferon-gamma expression in experimental model of pulmonary aspergillosis. Mikrobiyol Bul. (2011) 45:344–52.
22. Lin XB Li ZW, Yan M, Zhang BK, Liang W, Wang F, et al. Population pharmacokinetics of voriconazole and CYP2C19 polymorphisms for optimizing dosing regimens in renal transplant recipients. Br J Clin Pharmacol. (2018) 84:1587–97. doi: 10.1111/bcp.13595
Keywords: liver failure, invasive pulmonary aspergillosis, prophylaxis, voriconazole, risk score
Citation: Zhang X, Shen S, Dai X, Bi Y, Zhang J, Wu Y, Shi Y, Wei R and Gao H (2021) Clinical Risk Score for Invasive Pulmonary Aspergillosis in Patients With Liver Failure: A Retrospective Study in Zhejiang. Front. Med. 8:762504. doi: 10.3389/fmed.2021.762504
Received: 22 August 2021; Accepted: 11 October 2021;
Published: 22 November 2021.
Edited by:
Yu-Chen Fan, Shandong University, ChinaReviewed by:
Daxian Wu, First Affiliated Hospital of Nanchang University, ChinaYufeng Gao, First Affiliated Hospital of Anhui Medical University, China
Copyright © 2021 Zhang, Shen, Dai, Bi, Zhang, Wu, Shi, Wei and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hainv Gao, Z2FvaGFpbnZAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xuan Zhang1†
Xuan Zhang1† Sijia Shen
Sijia Shen Xiahong Dai
Xiahong Dai Hainv Gao
Hainv Gao