
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci. , 22 February 2022
Sec. Deep-Sea Environments and Ecology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.838274
This article is part of the Research Topic 16th Deep-Sea Biology Symposium View all 27 articles
This study reports the first attempt to quantitatively describe a Dendrophyllia ramea population on the Apollo bank (Ionian Sea), revealed in summer 2021 through a remotely operated vehicle (ROV) survey. The habitat description, bathymetric distribution, population density, and structure of the species were assessed by image analysis. A well-developed population of D. ramea, located on boulders on a sedimentary plateau at 70–80 m depth, was observed. The density ranged on average between 0.17 ± 0.04 and 0.8 ± 2.4 colonies m–2 with dense patches up to 8 colonies m–2. The population consisted primarily of many isolated single corallites and colonies of various sizes, some of which reached a maximum height of more than 40 cm. Deepwater fishing activities, primarily longline fishing, negatively affected this species. The newly collected data add knowledge about this vulnerable scleractinian coral. The documented negative effect of fishing activities on vulnerable marine ecosystem (VME) species further highlights the need for urgent conservation measures.
The orange tree coral Dendrophyllia ramea (Linnaeus, 1758) is an Atlantic-Mediterranean species belonging to the family Dendrophyllidae Gray, 1847. It is an arborescent scleractinian with white tentacles and pale orange polyps and can form large, branched colonies exceeding 100 cm in height (Salomidi et al., 2010; Bo and Fisheries and Aquaculture Resources Use and Conservation Division, 2017).
It is distributed in the eastern Atlantic Ocean, recorded in Azores (Braga-Henriques et al., 2013), Canary Islands (Arístegui et al., 1987; Bianchi et al., 2000; Reyes et al., 2000; Brito and Ocaña, 2004; Aguilar et al., 2010a), in the Gulf of Cadiz (Aguilar et al., 2010b), and further south along the African coast [Morocco, Cape Verde, Gulf of Guinea, Ghana, and Nigeria (Zibrowius, 1980)]. In the Mediterranean, this species has been reported mainly in the southern basin with a patchy distribution, and it is considered rare (Orejas et al., 2019a). Recently, Salvati et al. (2021) updated the D. ramea Mediterranean distribution with new records from northeastern Sicily. In this basin, D. ramea generally developed in the circalittoral zone on rocky substratum or bioconstruction characterized by moderate currents and turbidity, usually between 40 and 80 m depth, up to the maximum recorded depth of ∼240 m (Bonfitto et al., 1994; Jiménez et al., 2016; Orejas et al., 2017). In the Aegean Sea (Greece and Cyprus), this scleractinian has also been observed dwelling on the soft bottom at 125–170 m depth (Salomidi et al., 2010; Orejas et al., 2019a).
Knowledge about D. ramea is scarce. Some biological information comes from experiments carried out in aquaria (Orejas et al., 2019b; Reynaud and Ferrier-Pagès, 2019). It is known for its use for ornamental purposes in both the Mediterranean Sea and the Atlantic Ocean. Moreover, it is affected by bycatch and entanglement in lost fishing gear (Salvati et al., 2021). For these reasons, it is listed as Vulnerable in the Mediterranean Red List (Anthozoans) (Otero et al., 2017) of the International Union for the Conservation of Nature (IUCN) and as Data Deficient in the Italian IUCN Red List (Italian corals) (Salvati et al., 2014). Recently (December 2017), it has also been included in Annex II (List of endangered or threatened species) of the Barcelona Convention for all European States of the Mediterranean (EC No. 338/97, UNEP/MAP-SPA/RAC, 2018).
Assessing the species distribution, population structure, and conservation status is of paramount importance to obtain valuable information to establish adequate management and conservation plans, particularly for vulnerable species. Understanding the ecological interactions of vulnerable marine ecosystem (VME) with environmental and human constraints has important implications for biodiversity conservation.
Considering the limited knowledge on the ecology of this scleractinian, a population of D. ramea was investigated in the southern shelf off Syracuse (Sicily, Ionian Sea, Italy) to contribute to bridge this gap. The presence of this species in the area has been reported by technical divers and local fishermen, but, to the best of our knowledge, no information is available on its density and distribution in the Ionian Sea.
The aim of this study was to characterize the D. ramea population by investigating its distribution and density patterns as well as population structure, and associated megabenthic species. Moreover, the main threats affecting this vulnerable coral in the study area are also discussed.
The study area is in the southeastern part of the Sicilian continental margin (Ionian Sea, Central Mediterranean Sea) and encompasses the so-called Apollo bank. The insular shelf is narrow, up to 4-km wide, with the shelf break characterized by the presence of numerous canyon heads. The oceanography of the area is characterized by three water masses (Bonanno et al., 2006; Bergamasco and Malanotte-Rizzoli, 2010; Celentano et al., 2020): the Modified Atlantic Water (MAW) coming from the Strait of Sicily and flowing eastward in the upper layer (∼200 m); the Levantine Intermediate Water (LIW) flowing in the intermediate layer, westward toward the Strait of Sicily, and northward entering the Adriatic Sea; and water of Adriatic origin in the deepest layer. The Ionian Sea is also characterized by a bimodal phenomenon in the subbasin, i.e., Adriatic-Ionian Bimodal Oscillating System (BiOS, Gačić et al., 2010, 2011), a reversal of the circulation from cyclonic to anticyclonic at decadal timescale, having important implications for the surface transport of larvae and litter (Bergamasco and Malanotte-Rizzoli, 2010; Celentano et al., 2020).
No precise information is available in the literature about the Apollo bank, but the information collected by fishermen and recreational/technical divers reported the presence of D. ramea colonies, locally called “Apollo corals,” along the entire continental margin at the bathymetric range between 70 and 90 m depth. The area is of high importance for fisheries; several fleets operate targeting both pelagic and demersal species, by longlines, and at deeper depths by trawling. The explored sites are located approximately ≈1.5 NM offshore the coast between Plemmirio and Avola villages (Figure 1). They are in front of the Natural Reserve Cavagrande and of the mouth of the Cassibile river, one of the most important rivers of Sicily.
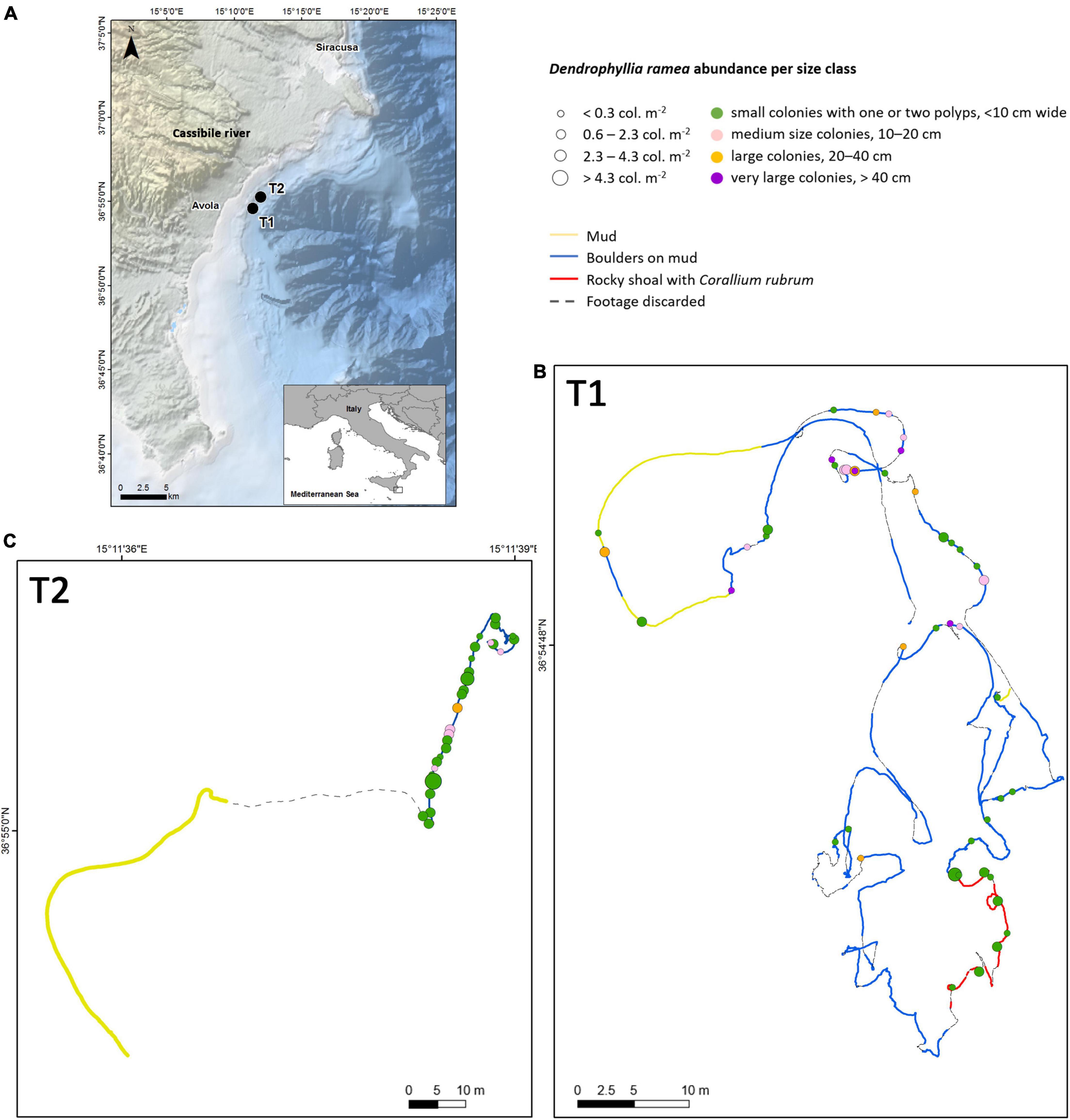
Figure 1. Investigated area: (A) Apollo Bank. Black dots represent the locations of the two remotely operated vehicle (ROV) tracks where Dendrophyllia ramea was found. Background bathymetry was obtained from EMODnet. (B,C) ROV track for each exploratory dive with the location of relative coral range abundance per size class and an indication of the type of substratum.
In summer 2021, a research campaign was carried out on board the R/V Astrea of Istituto Superiore per la Protezione e Ricerca Ambientale (ISPRA). Two exploratory dives, carried out in the depth range of 70–80 m using a remotely operated vehicle (ROV, Perseo, L3 Calzoni), revealed the presence of D. ramea in the study area (Figure 1 and Table 1). The ROV was equipped with a Kongsberg high-definition video camera (1,920 × 1,080 pixels), two lasers 16 cm apart used as a metric scale, and a manipulator arm to take samples. In addition, ROV position was acquired every 2 s using an ultrashort baseline (USBL) underwater compact positioning system (μPAP 200, Kongsberg) with up to 0.25° accuracy. Specific attention was paid to maintain a constant ROV cruising speed of approximately 0.5 knots and an altitude of approximately 1.5 m from the bottom.
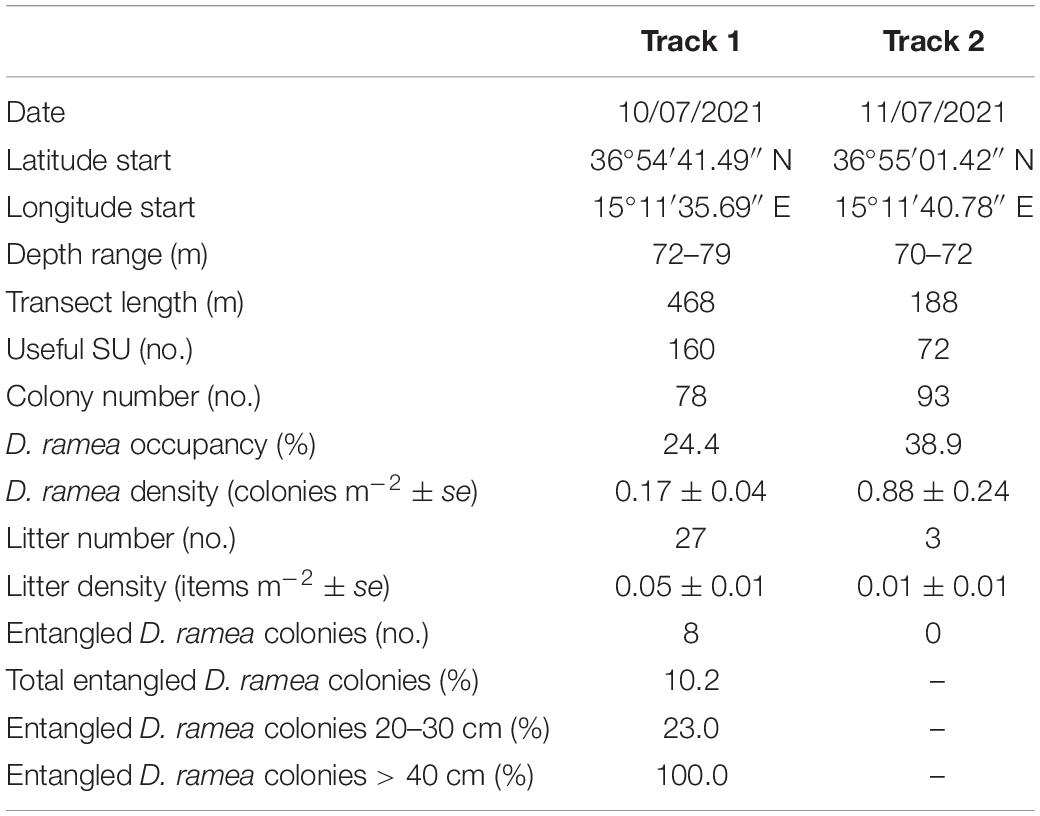
Table 1. Summary of remotely operated vehicle (ROV) tracks performed in the study area with an indication of geographical coordinates, depth, total length, useful sampling units (SUs), Dendrophyllia ramea colonies (no.), occupancy (%), density (colonies m–2), and percentage frequency (%) of entangled colonies by abandoned, lost, or otherwise discarded fishing gear (ALDFG), along with litter number (no.) and density (items m–2).
The smooth plot of the georeferenced ROV tracks was imported into the geographic information system (GIS) software ArcGIS v10.3.1, and each ROV video track was divided into 2-m-long sampling units (SUs; 2 m long × 1.5 m wide), according to the studies by Corbera et al. (2019) and Castellan et al. (2020). Overall, 1 h 30 min of ROV footage at the bottom was processed using the free Internet software VLC. The portions of the video (SUs) that is not relevant (i.e., ascent and descent ROV phases, sample collection, recording close-up images, and frames with poor visibility or out of focus) were not considered in the analyses.
The substratum type along the tracks was visually classified according to three categories, namely, mud, boulders on mud, and rocky shoal. The prevalent substratum type was assigned to each SU. A total of 328 SUs were extrapolated, and all megafaunal organisms visible along each SU were classified to the lowest taxonomic level. The population of D. ramea was estimated both by the occupancy (frequency of occurrence in the SUs, %) and by the density (no. of colonies m–2), calculated for each SU, and expressed as mean ± SE. Information on the habitat and megabenthic species associated with D. ramea was also collected.
Still images, captured from each video by DVDVideoSoft software, were used for the morphometric analysis of D. ramea colonies. Each frame was analyzed, and, when possible, colonies were measured using ImageJ/Fiji software and the laser beams as a scale. The estimated length values and/or the number of calyxes were used for a preliminary assessment of the population structure in terms of skewness and kurtosis. Each coral colony was assigned to one of the four size classes as defined by Orejas et al. (2019a).
The presence of benthic litter and abandoned, lost, or otherwise discarded fishing gear (ALDFG) along with their interaction with coral species was recorded following the method described by Angiolillo et al. (2021).
The explored sites were mainly characterized by soft bottom with scattered boulders. Overall, hard substratum covered up to 75.8% of the explored sea bottom (Figure 2A). The boulders were covered by calcareous algae and encrusting sponges and dwelled by bryozoans, serpulids, mollusks, and tunicates.

Figure 2. (A) Percentage composition of substratum type. (B) Size distribution of colony height of D. ramea colonies in the study area.
Turbidity was high, and a thin layer of sediment covered the encrusted boulders. Rocky shoal substratum was recorded only in the first dive (T1) and was characterized by the co-presence of D. ramea and several healthy colonies of Corallium rubrum (Figures 1, 3A). No other relevant structuring of Cnidarian species was observed, except for the scattered presence of the gorgonian Eunicella cavolini and some specimens of Alcyonium acaule and Cerianthus sp. Several echinoderms were observed, such as the starfish Echinaster sepositus, Chaetaster longipes, and Peltaster placenta and the echinoids Echinus melo, Cidaris cidaris, and Centrostephanus longispinus. The presence of the lobster Palinurus elephas is remarkable. Associated megafauna observed within the coral forest included 25 taxa. Three of the taxa found are listed in at least one of the main legal instruments for species conservation and management ongoing in the Mediterranean Sea (Relini and Tunesi, 2009).
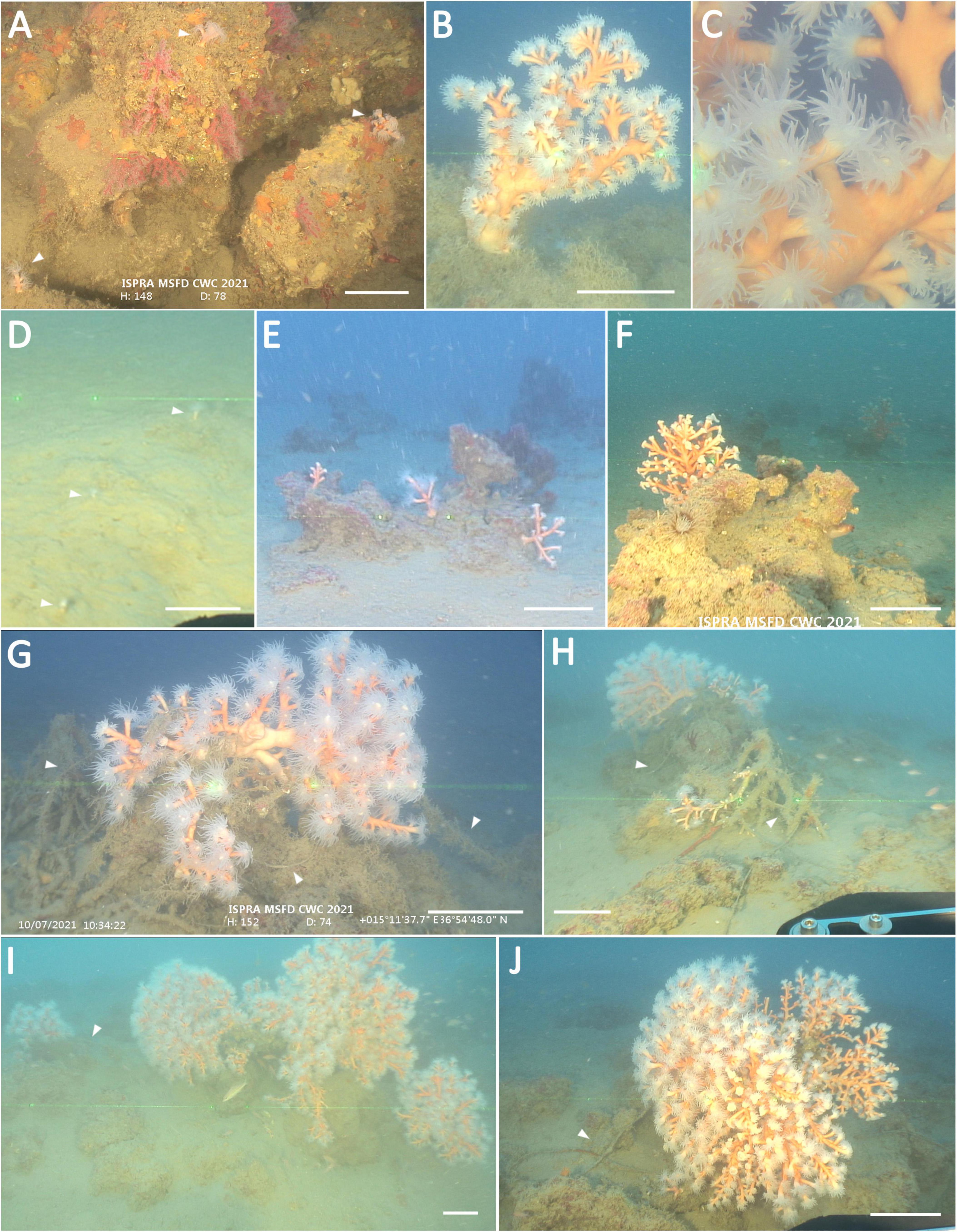
Figure 3. D. ramea and the anthropogenic impact in the study area. (A) D. ramea (white arrows) and healthy Corallium rubrum colonies dwelling on a rocky shoal. (B,C) Candelabra-shaped D. ramea colony with a close-up image. (D) Single corallites (white arrows) on the soft bottom. (E,F) Boulders with different sized branched colonies of D. ramea. (G,H) Larger colonies of D. ramea entangled and broken by old lines (white arrows). (I,J) Very large bushy-shaped colonies of D. ramea abraded by lines (white arrows). Approximated scale bar: 16 cm.
A total of 171 colonies of D. ramea (Figure 3) were counted along the two tracks over an area of approximately 350 m2. No colonies were observed on the soft substratum, except for a few single corallites (Figure 3D) recorded in the proximity of boulders (Figure 1). Overall, the colonies showed 24.4–38.9% occupancy (Table 1), up to 82.4% when considering the part of tracks characterized only by hard substratum. An average abundance of 0.6 ± 0.4 to 1.1 ± 2.2 colonies per SU was observed, with a density ranged between 0.17 ± 0.04 colonies m–2 and 0.88 ± 0.24 colonies m–2 (maximum density of 8 colonies m–2) (Table 1).
Size-frequency histograms for each site showed that 97.0% of the colonies were smaller than 40 cm (Figure 2B). About 50.2% of the overall colonies showed single corallites. The distributions were leptokurtic and highly skewed, with evident right tails represented by big adults up to 49.5 cm in height and 79.9 cm in wide. Particularly, the second track (T2) shows the dominance of small colonies and some medium-sized colonies (Figures 3D–F), whereas, in the track T1, very large colonies were more abundant (Figures 1, 3B,C,G–J). Along the two tracks, small and larger colonies were associated with each other, with a patchy distribution.
A total of 30 litter items were recorded. The dominant litter types (96.6%) were related to fishing activities: lost lines and bottom longlines made up the most significant portion of the litter (63.3%), followed by ropes (30%) and nets (3.3%). An unidentified waste was also found. All the three size classes of marine litter were observed; however, 60% of the items were assigned to the third class (>10 m2), mainly represented by ropes and longlines, which showed the highest occurrence. Average litter density ranged between 0.05 ± 0.01 and 0.01 ± 0.01 items m–2 (Table 1). Of the total litter items, 26.6% were observed to entangle sessile invertebrates (Figures 3G–J). Entanglement affected 44% of the largest colonies of D. ramea (Table 1), followed by a specimen of ascidian Microcosmus sp. Fresh rubble of scleractinian was observed on the seafloor near living colonies. The presence of epibionts was observed only on the larger colonies entangled in ALDFG. Unidentified bryozoans, sponges, and bivalves were settled on dead parts of the colonies.
The remaining litter was recorded lying on the bottom (73.3%). In these cases, it was not always possible to verify if litter produced injury to organisms. Fishing-related litter was almost completely covered by epibionts: hydroids were always recorded, followed by high occurrences of coverage by encrusting sponges, coralline algae, oysters, and bryozoans.
To the best of our knowledge, this is the first quantitative study of a Dendrophyllia ramea population on the Apollo bank, despite the presence of the species in this area is known so far.
In the study area, this species thrives on boulders on a sedimentary plateau at 70–80 m depth, as described by several authors in other Mediterranean areas (Zibrowius, 1980; Aguilar et al., 2006; Templado et al., 2009). Moreover, the bathymetric range is similar to that observed by Salvati et al. (2021) in northern Sicily. Some isolated D. ramea corallites were also observed on the soft substratum (Figure 3D) between boulders. However, colonies growing directly on the soft bottom, as described by Orejas et al. (2017), were not recorded. This species inhabits different geomorphologic settings, all characterized by highly dynamic systems (Salvati et al., 2021). The peculiar characteristic of the Ionian Sea in the dynamics and processes of water exchange, important in determining biological patterns (Bonanno et al., 2006; Bergamasco and Malanotte-Rizzoli, 2010; Celentano et al., 2020), could support the flourishing settlement of D. ramea in this area. More detailed studies of the environmental and hydrographical conditions should be performed to confirm this hypothesis.
The population of D. ramea studied showed a patchy distribution, with maximum densities up to 8 colonies m–2 and average densities of 0.17 ± 0.04 and 0.8 ± 2.4 colonies m–2 (Table 1). Smaller colonies formed patches at higher densities. These results are comparable with those recently obtained by Orejas et al. (2017), while they are higher than those reported by Salvati et al. (2021) for northern Sicily.
The size structure of the D. ramea population is asymmetrical, mainly dominated by small colonies and single corallites. There are also very large, lush colonies that show a bushy shape (Figure 3J). The presence of small- and medium-sized colonies together with large colonies suggests an active recruitment (Orejas et al., 2017). The high number of small-sized colonies may indicate young colonies, originated from sexual reproduction and low larvae dispersion or by the fragmentations of larger colonies (Wallace, 1985; Bruckner and Bruckner, 2001; Okubo et al., 2007). Fragmentation can be a consequence of both natural and anthropic stresses, as observed with other anthozoans (e.g., Wallace, 1985; Coppari et al., 2019). Relatively small colony sizes might also be due to the presence of fishing impacts.
The anthropogenic impact revealed by the ROV footage suggests that the remarkable D. ramea population described is far from being in a pristine state. All largest colonies are entangled by fishing gear (Table 1 and Figures 3I,J), and broken branches are observed together with fragments, suggesting a frequent disturbance to the coral population in the area. An entangled colony shows detached branches with the basal portions conspicuously turned up and exposed. Periodic disturbances could cause upturning of entire colonies and their possible fragmentation (Orejas et al., 2019c). No dead D. ramea colonies have been recorded, but the colonies at direct contact with fishing gear are often covered by epibionts, suggesting that the tissue abrasion may enhance infections as well as the susceptibility to epibiosis (Bo et al., 2014; Angiolillo, 2019).
Almost all litter items observed are related to fishing activities and are more abundant in the track with more rocky outcrops and more larger colonies (T1). The rocky nature and abrupt morphology of the sites that were explored meant that fishermen frequently damage bottom nets or snag their lines in rocks or corals. The Apollo bank is severely exploited by commercial fishing activities targeting valuable commercial species such as lobsters and large fishes. Local fishermen knew about the presence of the Dendrophyllia population at Apollo bank, which they call “Apollo coral,” because all along the southern coast of Syracuse at that bathymetric range of D. ramea is bycatch; nets can completely remove entire colonies, and longlines can bring up, damage, or cut off the branches of colonies. Moreover, in the proximity of canyon heads, the area is also intensively trawled (Global Fishing Watch, globalfishingwatch.org, and Marine Traffic, marinetraffic.com). Although it has not yet been quantified and characterized, sediment loads and turbidity may increase if trawling occurs nearby of the area and may cause mechanical damage, producing colony fragmentation and material accumulation.
The destructive effects of ALDFG on benthic VME and habitat-forming species are widely documented (Bo et al., 2014; Galgani et al., 2018; Angiolillo and Fortibuoni, 2020), and its use should be regulated or banned in areas characterized by coral forests (Chimienti et al., 2019), because it may severely threaten the survival of arborescent corals (Fabri et al., 2014; Angiolillo, 2019; Otero and Marin, 2019; Chimienti et al., 2020; Angeletti et al., 2021).
The co-presence of the vulnerable D. ramea with the precious C. rubrum and other protected species such as C. longispinus and P. elephas, all listed in different annexes of several legal instruments (i.e., SPA/BIO Protocol of Barcelona Convention, Berne Convention, and Habitat Directive 92/43/EEC), is remarkable for the area.
Understanding the species and community distribution, population structure, and state of conservation of vulnerable corals and their ecological interactions with environmental and human constraints has important implications for biodiversity conservation (de la Torriente et al., 2019; Moccia et al., 2019). This type of information is fundamental to identify and implement appropriate management and conservation plans to ensure biodiversity conservation/restoration and to shape specific management measures also within the Marine Strategy Framework Directive (EC, 2008).
These preliminary data on the D. ramea population at Apollo bank highlight the need for the conservation of this remarkable assemblage in the area. The collection of new data at the Mediterranean scale is required to enlarge the knowledge on the distribution of this vulnerable scleractinian and add information about the biodiversity of the mesophotic zone. Further exploratory studies are essential to deepen the biology and ecology of the species and to assess the ongoing and future responses of this coral to natural- and human-induced changes (e.g., increasing human pressures and climate change) affecting the Mediterranean Sea. Data acquired in this study, although spatially limited, together with those recently reported by Salvati et al. (2021) for the northeastern Sicilian waters, add new knowledge on D. ramea and its vulnerability to fishing activity and may be useful to bridge the gap in the data-deficient assessment in the Italian IUCN Red List.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
MA and MG conceived the idea, designed the methodology of the manuscript, and analyzed and interpreted the data. MA, MG, and LR collected the data. MA processed the video of the biological data and wrote the manuscript. LT acquired funding. All authors contributed critically to the drafts and gave final approval for publication.
This study was financially supported by the Italian Ministry of the Ecological Transition (MITE) within the framework of the “Marine Strategy Framework Monitoring Program” (PR ISPRA X0SM0001, 2018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the ship crew of the R/V Astrea of ISPRA and the colleagues Francesco Sande Rende, Alfredo Pazzini, and Alessia Izzi of ISPRA Marine Robotic Department (Sezione per lo sviluppo tecnologico e supporto del monitoraggio e della ricerca applicata all’ambiente marino profondo) for their professional collaboration and helpfulness in the collection of data. We would also like to thank two reviewers who provided useful comments that helped improve the manuscript.
Aguilar, R., Pastor, X., and de Pablo, M. J. (2006). Habitats en peligro. Propuesta de proteccion de OCEANA. Islas Canarias: Oceana-Fundacion Biodiersidad, 81.
Aguilar, R., Torriente, A., Penalver, J., Lopez, J., Greenberg, R., and Calzadilla, C. (2010a). Propuesta de Areas marinas de Importancia Ecológica. Islas Canarias: Oceana-Fundación Biodiversidad.
Aguilar, R., Pardo, E., Cornax, M. J., García, S., and Ubero, J. (2010b). Doñana Y El Golfo De Cádiz: Propuesta Para La Ampliación Del Área Marina Protegida. Islas Canarias: Oceana-Fundación Biodiversidad.
Angeletti, L., D’Onghia, G., Otero, M. M., Settanni, A., Spedicato, M. T., and Taviani, M. (2021). A Perspective for Best Governance of the Bari Canyon Deep-Sea Ecosystems. Water 13:1646. doi: 10.3390/w13121646
Angiolillo, M. (2019). “Debris in Deep Water,” in World Seas: An Environmental Evaluation, Vol III: Ecological Issues and Environmental Impacts, ed. C. Sheppard (Amsterdam: Elsevier Ltd), 251–268. doi: 10.1016/B978-0-12-805052-1.00015-2
Angiolillo, M., and Fortibuoni, T. (2020). Impacts of Marine Litter on Mediterranean Reef Systems: From Shallow to Deep Waters. Front. Mar. Sci. 7:581966. doi: 10.3389/fmars.2020.581966
Angiolillo, M., Gérigny, O., Valente, T., Fabri, M.-C., Tambutté, E., Rouanet, E., et al. (2021). Distribution of seafloor litter and its interaction with benthic organisms in deep waters of the Ligurian Sea (Northwestern Mediterranean). Sci. Total Environ. 788:147745. doi: 10.1016/j.scitotenv.2021.147745
Arístegui, J., Brito, A., Cruz, T., Bacallado, J. J., Barquin, J., Nunez, J., et al. (1987). El poblamiento de los fondos marinos de Dendrophyllia ramea (Anthozoa, Scleractinia) en las Islas Canarias. Cuadernos Marisqueros Publicaciones Técnicas 11, 163–181.
Bergamasco, A., and Malanotte-Rizzoli, P. (2010). The circulation of the Mediterranean Sea: a historical review of experimental investigations. Adv. Oceanogr. Limnol. 1, 11–28. doi: 10.1080/19475721.2010.491656
Bianchi, C. N., Haroun, R., Morri, C., and Wirtz, P. (2000). The subtidal epibenthic communities off Puerto del Carmen (Lanzarote, Canary Islands). Arquipélago. Life Mar. Sci. 2, 145–155.
Bo, M., Bava, S., Canese, S., Angiolillo, M., Cattaneo-Vietti, R., and Bavestrello, G. (2014). Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biol. Conserv. 171, 167–176. doi: 10.1016/j.biocon.2014.01.011
Bo, M., and Fisheries and Aquaculture Resources Use and Conservation Division. (2017). Poster: Deep-Sea Corals of the Mediterranean Sea. Rome: FAO.
Bonanno, A., Goncharov, S., Mazzola, S., Popov, S., Cuttitta, A., Patti, B., et al. (2006). Acoustic evaluation of anchovy larvae distribution in relation to oceanography in the Cape Passero area (Strait of Sicily). Chem. Ecol. 22, 265–273. doi: 10.1080/02757540600670307
Bonfitto, A., Bigazzi, M., Fellegara, I., Impiccini, R., Gofas, S., Oliverio, M., et al. (1994). Rapporto scientifico sulla crociera DP 91 (Margine orientale della Sardegna, Mar Mediterraneo). Bollettino Malacologico 30, 129–140.
Braga-Henriques, A., Porteiro, F. M., Ribeiro, P. A., Matos, V. D., Sampaio, Í, Ocaña, O., et al. (2013). Diversity, distribution and spatial structure of the cold-water coral fauna of the Azores (NE Atlantic). Biogeosci. 10, 4009–4036. doi: 10.5194/bg-10-4009-2013
Brito, A., and Ocaña, B. (2004). Corales de las Islas Canarias: Antozoos con Esqueleto de los Fondos Litorales y Profundos, ed. F. Lemus (La Laguna: San Cristóbal de la Laguna), 70.
Bruckner, A. W., and Bruckner, R. J. (2001). Condition of restored Acropora palmata fragments off Mona island, Puerto Rico, 2 years after the Fortuna reefer ship grounding. Coral. Reefs 20, 235–243. doi: 10.1007/s003380100164
Castellan, G., Angeletti, L., Correggiari, A., Foglini, F., Grande, V., and Taviani, M. (2020). “Visual methods for monitoring mesophotic-to-deep reefs and animal forests: finding a compromise between analytical effort and result quality,” in Perspectives on the Marine Animal Forests of the World, eds S. Rossi and L. Bramanti (Cham: Springer), 487–514. doi: 10.1007/978-3-030-57054-5_15
Celentano, P., Falco, P., and Zambianchi, E. (2020). Surface connection between the Ionian Sea and different areas of the Mediterranean derived from drifter data. Deep Sea Res. Part I Oceanogr. Res. Pap. 166:103431. doi: 10.1016/j.dsr.2020.103431
Chimienti, G., De Padova, D., Mossa, M., and Mastrototaro, F. (2020). A mesophotic black coral forest in the Adriatic Sea. Sci. Rep. 10:8504. doi: 10.1038/s41598-020-65266-9
Chimienti, G., Mastrototaro, F., and D’Onghia, G. (2019). “Chapter 6- Mesophotic and deep-sea vulnerable coral habitats of the Mediterranean sea: overview and conservation perspectives,” in Advances in the Studies of the Benthic Zone, ed. L. Soto (London: IntechOpen), 1–20. doi: 10.5772/intechopen.90024
Coppari, M., Mestice, F., Betti, F., Bavestrello, G., and Bo, M. (2019). Fragmentation, re-attachment ability and growth rate of the Mediterranean black coral Antipathella subpinnata. Coral Reefs 38, 1–14. doi: 10.1007/s00338-018-01764-7
Corbera, G., Lo Iacono, C., Gràcia, E., Griny, O. J., Pierdomenico, M., Huvenne, V. A. I et al. (2019). Ecological characterisation of a Mediterranean cold-water coral reef: Cabliers Coral Mound Province (Alboran Sea, western Mediterranean). Progr. Oceanogr. 175, 245–262. doi: 10.1016/j.pocean.2019.04.010
de la Torriente, A., Gonz’alez-Irusta, J. M., Aguilar, R., Fern’andez-Salas, L. M., Punz’on, A., and Serrano, A. (2019). Benthic habitat modelling and mapping as a conservation tool for marine protected areas: A seamount in the western Mediterranean. Aquatic Conserv. 29, 732–750. doi: 10.1002/aqc.3075
Fabri, M. C., Pedel, L., Beuck, L., Galgani, F., Hebbeln, D., and Freiwald, A. (2014). Megafauna of vulnerable marine ecosystems in French Mediterranean submarine canyons: Spatial distribution and anthropogenic impacts. Deep Sea Res. II 104, 184–207. doi: 10.1016/j.dsr2.2013.06.016
Gačić, M., Civitarese, G., Eusebi-Borzelli, G. L., Kovačević, V., Poulain, P.-M., Theocharis, A., et al. (2011). On the relationship between the decadal oscillations of the northern Ionian Sea and the salinity distributions in the eastern Mediterranean. J. Geophys. Res. 116:12002. doi: 10.1029/2011JC007280
Gačić, M., Eusebi-Borzelli, G. L., Civitarese, G., Cardin, V., and Yari, S. (2010). Can internal processes sustain reversals of the ocean upper circulation? Ionian Sea example. Geophys. Res. Lett. 37:L09608. doi: 10.1029/2010GL043216
Galgani, F., Pham, C. K., Claro, F., and Consoli, P. (2018). Marine animal forests as useful indicators of entanglement by marine litter. Mar. Pollut. Bull. 135, 735–738. doi: 10.1016/j.marpolbul.2018.08.004
Jiménez, C., Achilleos, K., Abu Alhaija, R., Gili, J. M., Orejas, C., et al. (2016). Living in close quarters: epibionts on Dendrophyllia ramea deep-water corals (Cyprus and Menorca channel). Rapp. Comm. Int. Mer. Médit. 41:466.
Moccia, D., Cannas, R., Cau, A., Alvito, A., Follesa, M. C., et al. (2019). New sites expanding the “Sardinian cold-water coral province” extension: A new potential cold - water coral network? Aquatic Conserv. 29, 153–160. doi: 10.1002/aqc.2975
Okubo, N., Motokawa, T., and Omori, M. (2007). When fragmented coral spawn? Effect of size and timing on survivorship and fecundity of fragmentation in Acropora Formosa. Mar. Biol. 151, 353–363. doi: 10.1007/s00227-006-0490-2
Orejas, C., Gori, A., Jimenez, C., Rivera, J., Iacono, C. L., Hadjioannou, L., et al. (2017). First in situ documentation of a population of the coral Dendrophyllia ramea off Cyprus (Levantine Sea) and evidence of human impacts. Galaxea. J. Coral Reef Studies 19, 15–16. doi: 10.3755/galaxea.19.1_15
Orejas, C., Gori, A., Jiménez, C., Rivera, J., Kamidis, N., Abu Alhaija, R., et al. (2019a). Occurrence and distribution of the coral Dendrophyllia ramea in Cyprus insular shelf: Environmental setting and anthropogenic impacts. Deep Sea Res. Part II 164, 190–205. doi: 10.1016/j.dsr2.2019.04.006
Orejas, C., Taviani, M., Ambroso, S., Andreou, V., Bilan, M., Bo, M., et al. (2019b). “38 - Cold-Water Coral in Aquaria: Advances and Challenges. A Focus on the Mediterranean,” in Mediterranean Cold-Water Corals: Past, Present and Future. Coral Reefs of the World, Vol. 9, eds C. Orejas and C. Jiménez (Berlin: Springer), 435–471. doi: 10.1007/978-3-319-91608-8_38
Orejas, C., Jiménez, C., Gori, A., Rivera, J., Iacono, C. L., Aurelle, D., et al. (2019c). “23 - Corals of Aphrodite: Dendrophyllia ramea populations of Cyprus,” in Mediterranean cold-water corals: Past, present and future. Coral Reefs of the World, Vol. 9, eds C. Orejas and C. Jimenez (Berlin: Springer), 257–260. doi: 10.1007/978-3-319-91608-8_23
Otero, M. M., and Marin, P. (2019). “Conservation of cold-water corals in the Mediterranean: Current status and future prospects for improvement,” in Mediterranean cold-Water corals: Past, present and future. Coral Reefs of the World, Vol. 9, eds C. Orejas and C. Jimenez (Berlin: Springer).
Otero, M. M., Numa, C., Bo, M., Orejas, C., Garrabou, J., Cerrano, C., et al. (2017). Overview of the conservation status of Mediterranean anthozoans. Malaga: IUCN, 73.
Relini, G., and Tunesi, L. (2009). Laminaria rodriguezii bornet. in:protected species according to the spa/bio protocol (barcelona convention) present in italy. identification sheets. Bio. Mar. Mediterr. 16, 433.
Reyes, J., Ocaña, O., Sanson, M., and Brito, A. (2000). Description de comunidades bentonicas infralitorales en la Reserva marina de La Graciosa e islotes del Norte de Lanzarote (Islas Canarias). Vieraea 28, 137–154.
Reynaud, S., and Ferrier-Pagès, C. (2019). “Biology and Ecophysiology of Mediterranean Cold–Water Corals,” in Mediterranean Cold-Water Corals: Past, Present And Future, eds C. Orejas and C. Jimenez (Berlin: Springer), 391–404. doi: 10.1007/978-3-319-91608-8_35
Salomidi, M., Zibrowius, H., Issaris, Y., and Milionis, K. (2010). Dendrophyllia in Greek waters, Mediterranean Sea, with the first record of D. ramea (Cnidaria, Scleractinia) from the area. Mediterr. Mar. Sci. 11, 189–194. doi: 10.12681/mms.102
Salvati, E., Bo, M., Rondinini, C., Battistoni, A., and Teofili, C. (2014). Lista Rossa IUCN Dei coralli Italiani. Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare. Roma: IUCN.
Salvati, E., Giusti, M., Canese, S., Esposito, V., Romeo, T., Andaloro, F., et al. (2021). New contribution on the distribution and ecology of Dendrophyllia ramea (Linnaeus, 1758): abundance hotspots off north-eastern Sicilian waters. Aquatic Conserv. 31, 1322–1333. doi: 10.1002/aqc.3533
Templado, J., Guallart, J., Capa, M., and Luque, A. (2009). 1170 arrecifes. In: V.V.A.A. (Ed.), Bases ecologicas preliminares para la conservacion de los tipos de habitat de interes comunitario en Espana, 142. Available online at: http://hdl.handle.net/10261/80300
Wallace, C. C. (1985). Reproduction, recruitment and fragmentation in nine sympatric species of the coral genus Acropora. Mar. Biol. 88, 217–233. doi: 10.1007/bf00392585
Keywords: scleractinian corals, Dendrophyllia ramea, fishing impact, Mediterranean Sea, ROV imaging, MPAs
Citation: Angiolillo M, Giusti M, Rossi L and Tunesi L (2022) A Dendrophyllia ramea Population in the Ionian Sea (Central Mediterranean Sea) Threatened by Anthropogenic Impacts. Front. Mar. Sci. 9:838274. doi: 10.3389/fmars.2022.838274
Received: 17 December 2021; Accepted: 25 January 2022;
Published: 22 February 2022.
Edited by:
Daniela Zeppilli, Institut Français de Recherche pour l’Exploitation de la Mer (IFREMER), FranceReviewed by:
Lorenzo Angeletti, Institute of Marine Science, National Research Council (CNR), ItalyCopyright © 2022 Angiolillo, Giusti, Rossi and Tunesi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michela Angiolillo, michela.angiolillo@isprambiente.it
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.