
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 24 May 2017
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 4 - 2017 | https://doi.org/10.3389/fmars.2017.00157
This article is part of the Research Topic Challenges and Opportunities for The EU Common Fisheries Policy application in The Mediterranean and Black Sea View all 18 articles
In the Adriatic Sea, shifts in benthic community structure have been attributed to multiple stressors, from the effects of climate change to the impacts of commercial fishing. Some fishing practices, such as bottom trawling, have caused a widespread decline in exploited fish stocks. Bottom trawling is also expected to have negative impacts on benthic habitats, usually structured by and hosting a large array of invertebrate species, which provide important ecological services to fish and commercial invertebrate stocks. However, in contrast to commercial species for which long-term time series of the abundance exist, data on these habitat-forming invertebrates are scarce, as they are usually caught as bycatch and discarded. Therefore, there is great uncertainty about their long-term trends, and if these populations are stable or declining. Here we used interview surveys conducted with bottom-trawling fishers of the central Adriatic Sea to gather local ecological knowledge on megabenthos abundance occurring in their fishing domain, as an alternative source of information to conventional fisheries data. We interviewed 44 fishers, from the most important ports of the Marche region of Italy, to understand how megabenthic species have changed in abundance within the area since the 1980s. Specifically, we asked fishers to provide qualitative abundance scores for 18 invertebrate species in five phyla (Porifera, Cnidaria, Bryozoa, Mollusca, and Echinodermata) based on their recollection of these species' presence in bycatch. We stratified responses in homogeneous temporal periods and geographic sectors of the study area, and analyzed their response with mixed effect ordered logistic regression models in order to evaluate spatiotemporal changes in the perceived abundance of each species. Our analysis suggests that the abundance of the sponge Geodia cydonium, the molluscs Pecten jacobaeus, Atrina fragilis, Neopycnodonte cochlear, and the group of holothurians, have declined. From fishers' perceptions, only the bryozoan Amathia semiconvoluta has increased. Local ecological knowledge can provide important information on environmental change and can highlight species and ecosystems at risk when conventional scientific data are scarce or absent. This approach can be expanded to other regions of the Adriatic and broader Mediterranean Sea to reconstruct change of this heavily exploited marine region.
Marine ecosystems are subject to escalating pressure from the cumulative impact of multiple anthropogenic stressors (e.g., pollution, eutrophication, ocean acidification, and fishing), causing biodiversity loss, habitat degradation, and stock declines (Halpern et al., 2008, 2015; Coll et al., 2012; Micheli et al., 2013a). Fishing activities, in particular those employing non-selective gear such as bottom trawling and drift nets, are considered one of the most important anthropogenic sources of marine ecosystem decline, causing both direct (crushes and buries marine animals) and indirect (sediment removal, alteration of water-column fluxes, reduction of the original complexity of fishing grounds) impacts on marine populations and habitats (Watling and Norse, 1998; Jackson et al., 2001; Puig et al., 2012). These impacts are evident in the Mediterranean Sea, which combines a long history of exploitation with a high level of social, economic, and political complexity that present major challenges for effective marine management and conservation (Coll et al., 2012; Micheli et al., 2013a,b). To date, 85% of assessed Mediterranean stocks are overfished (Colloca et al., 2013), and current fisheries management is considered inadequate (Fouzai et al., 2012). The management strategies adopted in the Mediterranean basin largely take a single species approach instead of an ecosystem-base management approach (de Juan et al., 2012). Most regulations are aimed at reducing fishing effort and fishing capacity, and/or at implementing technical measures such as the regulation of mesh size, the establishment of a minimum landing size, and temporal, mostly seasonal fishing closures (de Juan et al., 2012; Fouzai et al., 2012; Colloca et al., 2013). However, scientific advice is rarely used to implement the spatial and temporal fisheries management strategies needed to achieve sustainable yields and to preserve the ecological role of the exploited species and their habitats (Colloca et al., 2013).
A major shift in management focus has occurred over the last 10 years through an increased awareness of the fundamental role played by habitat in fished stocks conservation and recovery, which has, in turn, led to the key concepts of Vulnerable Marine Ecosystems (VMEs) and Essential Fish Habitats (EFHs) (UNGA, 2006; FAO, 2009). VMEs and EFHs include both water column and sea bottom areas that support the productivity of commercial species and that are vulnerable to human activities, in particular to bottom trawling (Rosenberg et al., 2000; FAO, 2009). VMEs and EFHs include spawning, nursery and feeding grounds, together with foundation species (Dayton, 1972), i.e., “a single species that defines much of the structure of a community by creating locally stable conditions for other species, and by modulating and stabilizing fundamental ecosystem processes.” This is the role played for examples, by the animal forests, in particular anthozoans (Cerrano et al., 2010; Valisano et al., 2016) whose functional and structural role is receiving increasing attention (Rossi et al., 2017). Numerous initiatives have been developed in order to map the presence and distribution of VMEs and EFHs, and to provide useful tools to help managers and decision makers in the selection of priority areas and in the definition of management plans to ensure the long-term conservation and sustainable use of marine resources (Stecf, 2006; OSPAR Commission, 2010; Rogers and Gianni, 2010; Rengstorf et al., 2013).
Unfortunately, the lack of historical information limits our ability to reconstruct habitat distribution and trends, and assess the current status of VMEs and EFHs. Most of the studies of changes through time have focused on decline of exploited fish populations or top predators (Barausse et al., 2011; Ferretti et al., 2013; Mazzoldi et al., 2014). More limited historical information is available for non-target species, such as benthic invertebrates caught as bycatch. Thus, reconstructing past distribution and abundances of benthic habitats and species is challenging. Such baselines and trends, however, are critical for assessing the current status of EFHs and VMEs and establishing reference targets for their recovery (Engelhard et al., 2016). Over the last decades, “Local Ecological Knowledge” (LEK) has emerged as an alternative approach to collecting information on species presence or abundances when historical data are lacking (Huntington, 2000; Anadón et al., 2009). However, up to now, the use of LEK in the Mediterranean Sea has been limited to collecting information and describing trends in fish diversity and abundances (Azzurro et al., 2011), and discarding of commercially important fish species in the bottom trawl fishery (Damalas et al., 2015a,b). Here, we apply LEK to examine the temporal change of habitat-forming invertebrates in the Adriatic Sea.
The Adriatic Sea is one of the most productive regions of the Mediterranean Sea, hosting a variety of endemic species, and important nursery, spawning, and foraging grounds (Coll et al., 2010; de Juan and Lleonart, 2010; Colloca et al., 2015). Humans have exploited the Adriatic Sea since the prehistoric era (Lotze et al., 2011). This long history of human use, together with global environmental changes (Conversi et al., 2010; Zenetos et al., 2011; Giani et al., 2012) have greatly altered the Adriatic marine environment and ecosystems (Coll et al., 2007, 2009, 2010; Lotze et al., 2011), and ranked the basin as one of the most threatened regions of the Mediterranean Sea (Micheli et al., 2013b). The description and distribution of Adriatic benthic communities have been studied from ancient time both, on a larger scale (Vatova, 1949; Gamulin-Brida, 1974) and a local scale (Paolucci, 1923; Scaccini, 1967; Scaccini and Piccinetti, 1969; Fedra et al., 1976; Crema et al., 1991) with an exhaustive description of its biocoenosis and biodiversity of megabenthic species. Several studies, most of which conducted in the northern Adriatic Sea, have described negative trends and chronic effects of commercial species and benthic communities due to trawling activities (Hall-Spencer et al., 1999; Jukic-Peladic et al., 2001; Pranovi et al., 2001, 2005; Morello et al., 2005; Romanelli et al., 2009). More than 90% of Adriatic marine resources are depleted and the current management of fisheries is inadequate (Lotze et al., 2011; Fouzai et al., 2012). Mean discard rate in Adriatic bottom trawl fisheries ranges between 20 and 67% of total catches, higher than the Mediterranean average (Tsagarakis et al., 2013; FAO, 2016), with a rate that varies according to fishing intensity.
Little is known about temporal variation in the abundance of megabenthic species, foundation species, VMEs and EFHs in the Adriatic Sea. In the northern Adriatic, studies have revealed a shift from benthic communities characterized by the presence of filter-feeding epifaunal organisms forming complex 3D habitat (such as sponges, sea pens, ascidians, holothurians, and large bryozoans) to a community dominated by infaunal and scavengers species (Raicevich et al., 2004; Lotze et al., 2011). This information is not available for other Adriatic sectors. In this study, we used LEK to describe changes in the abundance of habitat-forming megabenthos, and highlight species and ecosystem at risk.
The study was conducted from January to April 2016, in the main fishing ports of the Marche region (Italy, central Adriatic Sea): Ancona, Civitanova Marche, and San Benedetto del Tronto (Figure 1A). The area is characterized by sandy-muddy bottoms (Brambati et al., 1983; Spagnoli et al., 2014) with depths that do not exceed 100 m, apart from the Pomo pit (Russo and Artegiani, 1996). Benthic assemblages on the western side and offshore are dominated by endofauna, where the main variety, richness, and biomass is represented by bivalve mollusks, and polychaetes (Vatova, 1949; Gamulin-Brida, 1974; McKinney, 2007). Epifauna biomass is higher in areas around 50–75 m depth, and the most representative organisms include sponges, ascidians, and anemones (Scaccini, 1967; Piccinetti, 1976; McKinney, 2007).
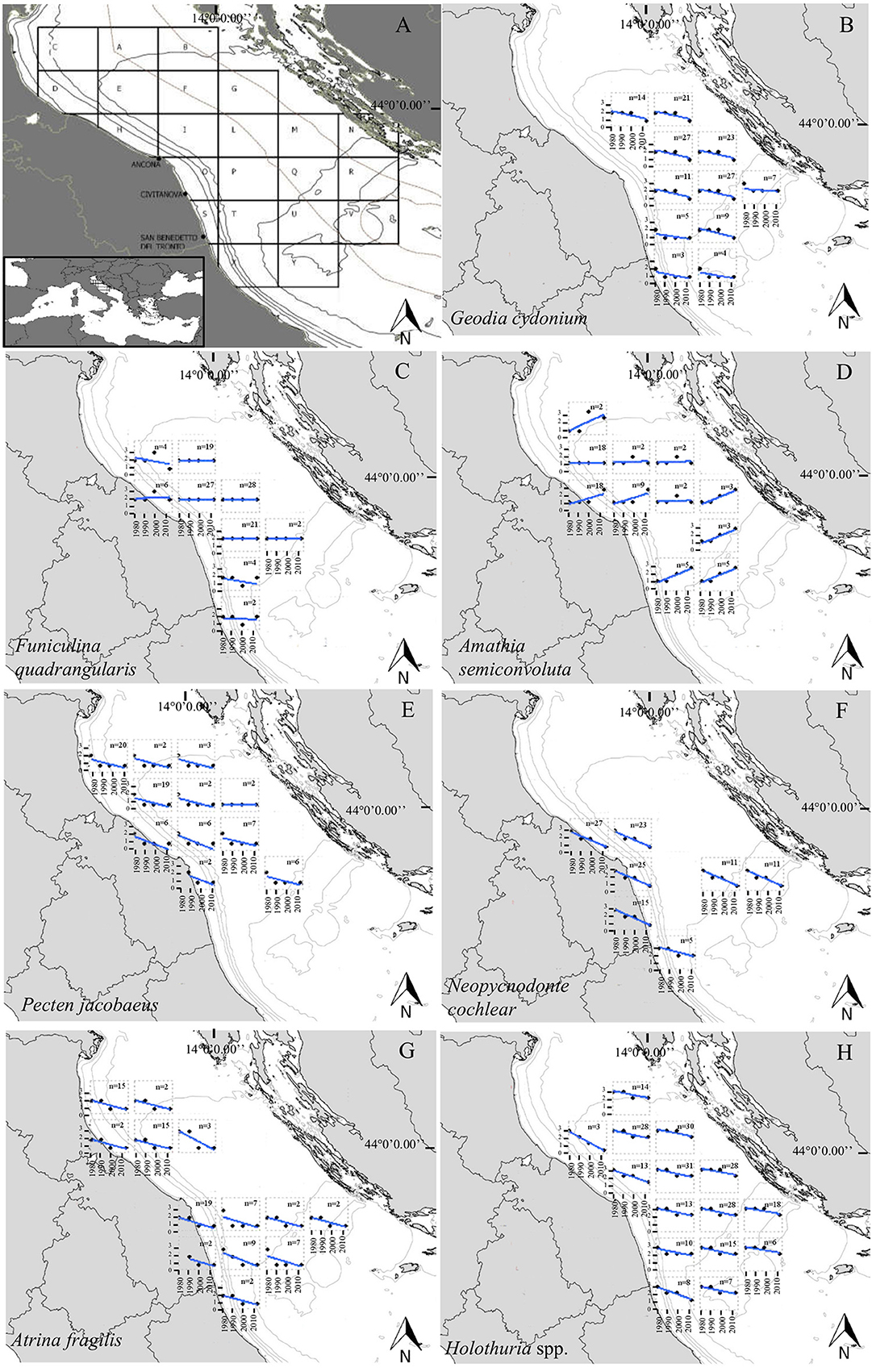
Figure 1. Maps showing the geographical location and ports where fishers were interviewed (Ancona; Civitanova Marche; San Benedetto del Tronto) and trends of the taxa or species analyzed in our study. (A) Grid used to stratify the focal area. Letters are sector codes used to collect information about fishing locations during interviews. Gray lines identify areas outside Italian and Croatian territorial seas; black lines show the bathymetry. (B) Trends in the abundance of the sponge Geodia cydonium; (C) Trends in the abundance of the sea-pen Funiculina quadrangularis; (D) Trends in the abundance of the bryozoan Amathia semiconvoluta; (E) Trends in the abundance of the scallop Pecten jacobaeus; (F) Trends in the abundance of the fan mussel Atrina fragilis; (G) Trends in the abundance of the deepsea oyster Neopycnodonte cochlear; (H) Trends in the abundance of Holothuria spp. Dots are the class predictions according to the ordinal regression models. The trend lines (blue lines) were included for visual purposes to aid the detection of overall temporal trends in the abundance classes. Even if some sector specific panel is falling on land, it is intended that the relative data has been collected in the portion of the square on the sea. n, number of fishers that gave information per species per sub-area.
Fishing is intense in the Adriatic region. The main Italian fisheries are small-scale fishing (around 49% of the total number of vessels), followed by dredges (around 26% of vessels), and bottom otter trawl (24% of vessels) (EU fleet register, 20171). In 2011, the Marche region was the third highest region for total volume landings in the Italian Adriatic region, with more than 7,000 tons of total landings in volume coming from bottom trawls. However, landings decreased by 28% between 2004 and 2011 (IREPA Onlus, 2011).
Information was gathered using a structured interview (Supplementary Materials). In each port, we interviewed only otter trawl fishers, identified through their main associations or cooperatives. These groups included the cooperative “Pescatori Motopescherecci” of Ancona, which includes 54 members (51 vessels are trawlers and 3 vessels are small fishing vessels); the association “Casa del Pescatore” of Civitanova Marche, formed by 34 bottom trawlers; and finally, the fishery located in San Benedetto del Tronto, which includes 35–38 vessels practicing bottom trawling. Fishers were selected on their availability to participate to our survey. An “Oral Consent Procedure” was followed: all potential interviewees were provided with the purpose of the study and with the usage of collected data before obtaining their consent. All involved fishers willingly agreed to participate in the survey. Interviews were kept anonymous and responses were coded with a numeric identifier making it impossible to disclose any personal sensitive data and track the individual fishers.
We selected 18 invertebrate species in five phyla (Porifera, Cnidaria, Bryozoa, Mollusca, and Echinodermata). Species were selected according to one or more of the following criteria: the species should be easily recognizable, common/abundant in the catches, a habitat-forming species, or play a fundamental ecological role (i.e., add tridimensionality to the substrate or acting as a nursery, providing refuge for eggs or small fishes and/or invertebrates; Table 1). Among the selected species, only the scallop (Pecten jacobaeus) is actively targeted by fishing, while the others are all discarded.
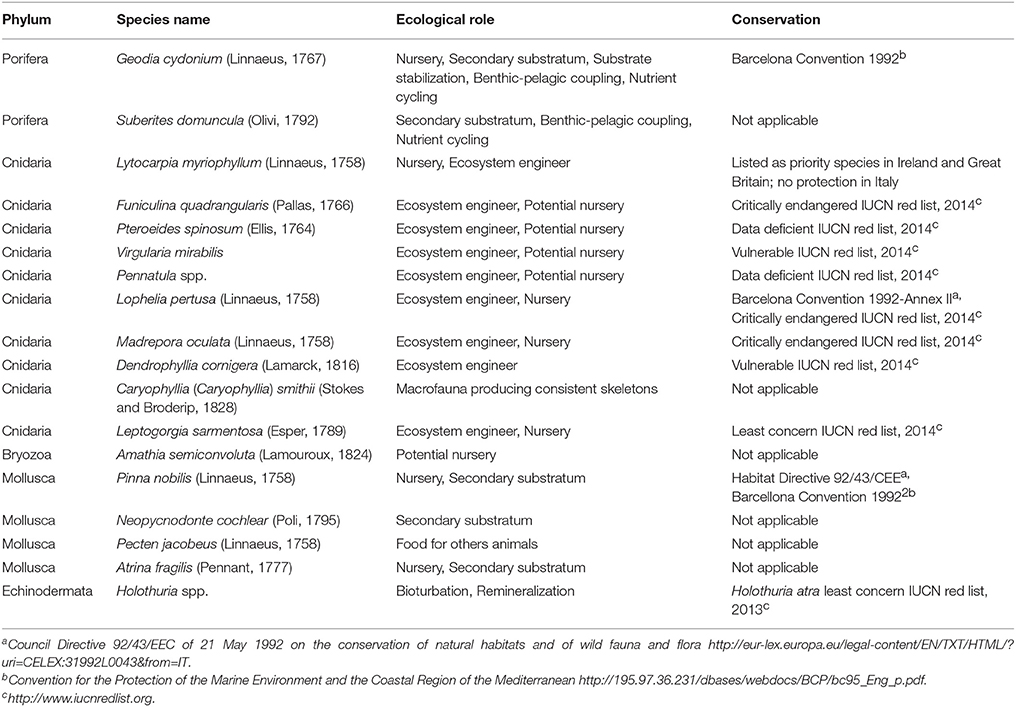
Table 1. List of the selected megabenthic species for which we asked fishers to provide qualitative abundances in the central Adriatic Sea, with the ecological, and functional role played by each species and their conservation status.
First, we asked questions helping us to characterize the profile of each fisher: age, year he started fishing, and the characteristics of fishing gear used (such as size of the horizontal opening, mesh size of the cod-end nets). Then we used a photographic guide to identify and match local and common species names with the scientific names of the animals for which we were asking questions.
We stratified responses in homogeneous temporal periods and geographic sectors of the study area (Figure 1A) to evaluate spatiotemporal changes in the perceived abundance of the focal species. We asked fishers to relate information to four periods: 1980–1989, 1990–1999, 2000–2010, and 2010 up to the present. Once the different species were identified as present in the bycatch for a given period, with the aid of a nautical map (1:750.000) of the Adriatic Sea, we asked the fishers to localize the areas where they usually found each species. The area of interest (minimum latitude and longitude: 42°40′N–12°30′E, maximum latitude and longitude: 44°40′N–15°30′E) was divided into 22 sub-areas. Each sub-area has a size of around 55 × 40 km and was identified by a letter to easily analyze the collected information (Figure 1A).
We defined four qualitative classes of reported species abundance, using different metrics (abundance vs. catch volume) for different species depending on the possibility to count single specimens. In particular, for colonial specimens such as cnidarians and bryozoan, we used catch volume metric. Thus, the used qualitative classes of abundance were: 0 = never observed; 1 = rare (1–10 specimens in the cod-end of the net, or for colonial specimens such as cnidarians and bryozoan, “rare” corresponds to an overall dimensions of <¼ of the net in volume); 2 = common (11–50 specimens; for cnidarians and bryozoan ¼–¾ of the cod-end of the net in volume); 3 = very abundant (more than 50 organisms; for cnidarians and bryozoan >¾ of the cod-end of the net in volume). A detected change in abundance class has to be interpreted in relative terms within the species being analyzed but cannot be compared across species. The fishers thus attributed a rank of abundance for each species (0–3), in each time period (1980–1989, 1990–1999, 2000–2010, and 2000–2016), depending on their experience, and fishing location. We asked fishers to identify the abundance of each species for each time period, for each sub-area present on the map. In this manner, the response of each fisher, and the resulting temporal change over time would apply to all single sub-area identified by the fisher.
Statistical analyses were performed using the open access software R (version 3.3.1). All the selected benthic species observed by the fishers as discards in different locations (i.e., identified sub-areas) and in the different time periods were reported in the respective class of abundance. In particular, each row of the final dataset reported the anonymous identifier of the fisher, the age, the port of origin, the species name, the taxonomic group (phylum) it belonged to, the class of abundance (from 0 to 3), the spatial location (latitude and longitude of the centroid of each sub-area), the distance of each sub-area from the coast, the mean depth of each sub-area, and the time period. When fishers could not determine whether a species was present in the catch, the location in the map or its abundance because they did not remember, NA was entered in the dataset. Only species observed more than twice, for each fishers-sub-areas combination (filter observation for n > 2), and for which we had more than half of fishers' answers, were included in the analyses (Table 2).
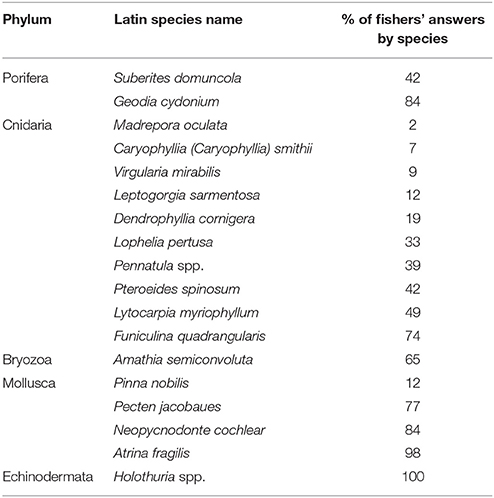
Table 2. Percentages of fishers that clearly remembered (including geographical localization) the selected megabenthic invertebrate species in their by catch and for which we collected clear answers to our questions.
We performed an ordered logistic regression, using the clmm2 function from the ordinal package, in order to assess the temporal changes of the different species. An ordered logistic regression model is a multinomial regression model where the dependent variable has more than two nominal ordered response categories. In particular, we fitted the cumulative link mixed model
where P(Yi ≤ j) is the cumulative probability of the ith observation falling in the jth category or below. Because perceptions about the abundances of the species in the bycatch are expected to vary across fishers, we included fishers as a random effect.
In our study, the response ordered categories were the classes of abundance (with four levels, each one representing a different qualitative class of abundance), while time, spatial location (latitude and longitude), depth and distance of each subarea from the coastline were the explanatory variables. Ordinal regression enabled us to determine which of our independent variables (if any) had a statistically significant effect on the cumulative probabilities of 4 abundance classes (Christensen, 2015). In particular, we tested the influence of time, of spatial location, of depth, and the relative distance from the coasts (i.e., where we hypothesized a higher fisheries impact on coastal benthic communities), on the abundances of Adriatic megabenthos groups. To avoid collinearity, we first tested the correlation among the available explanatory variables. Then, we calculate the variance inflation factor (VIF). In our case, depth was strongly related to longitude (correlation coefficient > 0.9), which in turn, was an important covariate to account for spatial correlation among observations. Therefore, in interpreting the results of the models, we took longitude as a proxy of depth. Also, latitude and longitude was strongly correlated (VIF ≥ 2), and to include these variables in the models, we followed a sequential regression method (Graham, 2003), which linearly regresses explanatory variables (latitude and longitude) against each other and uses the residuals to represent them. Finally, we also wanted to test whether the perceived temporal change of species abundance varied with distance from the coast. We predicted that as fishers operated farther from the coast, we would have expected a lower rate of change over time. This is because more distant sectors would have been exposed to a lower cumulative amount of effort than closer-to-coast areas. We tested this aspect by including an interaction between distance and time in our initial models. Thus, the final equation of our model was:
We fitted the mixed effects model by maximum likelihood estimation through Laplace approximation and the final model was selected following a backward stepwise selection procedure, and selecting the model with the lowest Akaike Information Criterion (AIC). The predicted probabilities for an average fisher's perceptions (u = 0) have been calculated by including the data used to fit the model.
Georeferenced plots were produced to visualize areas where temporal changes of the selected megabenthic species have occurred, according to fishers' perceptions. To easily and clearly communicate the temporal abundance trends of the analyzed species, a linear regression line, when the temporal effect was significant, was added to the plot. Some species were excluded from the analyses because of a small number of fishers' answers. In these cases we only mapped them to show their presence in the fishing grounds.
We conducted a total of 44 interviews (to 25 fishers from Ancona, 12 from San Benedetto, and 7 from Civitanova Marche). The age of interviewees ranged from 42 to 82 years, with 80% of them older than 50 (around 64% of fishermen were 50–60 years old; fishermen between 40–50 years and between 60–80 years were 18% of interviewees). Only 20% of fishers gave a detailed description of the otter trawl gears they use, the others only stated they use otter trawl gear.
The results of the ordinal regression mixed models indicate an overall reduction of the analyzed species over time (p-values for time ranges from <0.001–0.04; Figures 1B–G; Table 3). Of all the independent variables used, time was significant for all species (p-values <0.001–0.04; Table 3), longitude was significant in P. jacobaeus, Neopycnodonte cochlear, and Holothuria spp, (p-values <0.001–0.005; Table 3). The residuals of the regression between latitude and longitude was significant in Amathia semiconvoluta (p-values < 0.002; Table 3). Distance from the coast was significant in P. jacobaeus, A. semiconvoluta, and Holothuria spp. (p-values from <0.001 to 0.33; Table 3), while the interaction between time and distance from the coast was significant in A. semiconvoluta, P. jacobaeus, and Holothuria spp. (p-values <0.001–0.007; Table 3). Moreover, in each model, the random effect was significant (p-values always < 0.001) indicating that individual fishers added a non-negligible level of subjectivity in their perception of the changes in abundance of the selected megabenthic species.
Declining abundances were reported for the sponge Geodia cydonium (Figure 1B), while the abundances of the sea pen Funiculina quadrangularis remained relatively stable from the 1980s up to now, except for very few sub-areas where the species showed a slight decline (Figure 1C). The bryozoan A. semiconvoluta is the only species that, based on the fishers' perception, had an increasing trend in the last 40 years (Figure 1D). Species belonging to the phylum Mollusca, in particular the scallop P. jacobaeus, the fan mussel Atrina fragilis and the deepsea oyster N. cochlear, have declined from very abundant to rare in the study area from the 1980s to the present time (Figures 1E–G). The abundance of the holothurians also shows declining trends, even though Holothuria spp. are perceived by fishers as still common in most of the central Adriatic sea-bottoms (Figure 1H).
Our results did not reveal significant spatial patterns in the trends of species. These trends were similar throughout the study area and no significant differences were apparent between coastal and offshore areas.
Fishers recognized all the species listed in our survey allowing us to map several of them in the central Adriatic fishing grounds (Figure 1; Supplementary Figure 1). In particular, the sponge G. cydonium was recognized by 84% of interviewed fishers, the bryozoan A. semiconvoluta by 65%, the sea pen F. quadrangularis by 74%, the bivalves P. jacobaeus, A. fragilis, and N. cochlear by 77, 98, and 84% respectively, and Holothuria spp. were recognized by 100% of the interviewed fishers (Table 2; Figure 1). The sponge G. cydonium was reported mainly in offshore sub-areas (Figure 1B). The bryozoan A. semiconvoluta has been found in several sub-areas of the central Adriatic, usually as a rare occurrence, but with increasing abundances moving toward offshore sub-areas (Figure 1D). P. jacobaeus was reported mainly in the northern sectors and in sub-areas no deeper than 70 m, while A. fragilis and N. cochlear were collected also in deeper sub-areas (Figures 1E–G). Holothurians were reported in almost all of the analyzed sea bottoms of the central Adriatic Sea (Figure 1H). Although all fishers provided us with answers about the abundances and the geographical location of common invertebrates (such as molluscs bivalves or holothurians), only a fraction of them detected the presence in the bycatch of anthozoan species, which occurrences are less frequent and distribution more patchy in the Adriatic sea-bottoms (Table 2; Supplementary Figure 1).
The impact of towed gears on benthic communities has been extensively studied in many exploited demersal ecosystems of the world (Dayton et al., 1995; Collie et al., 1997; Jennings and Kaiser, 1998; Hall-Spencer et al., 1999; Thrush and Dayton, 2002). These destructive practices have contributed to the decline of habitat-forming species, VMEs and EFHs worldwide. This study reveals that using fishers' LEK can provide a useful tool to describe long-term trends of both target and non-target species. Other studies have recently demonstrated this methods is useful to detect patterns in exploited Mediterranean fish populations (Fortibuoni et al., 2010; Azzurro et al., 2011). Here we highlighted its utility also for a broader range of bycatch species across different marine taxa, including those important to structure benthic habitats. Species such as sponges, bivalves, and holothurians that historically were reported as common in the soft-bottom communities of the central Adriatic Sea (Scaccini, 1967; Scaccini and Piccinetti, 1969; Gamulin-Brida, 1974) were perceived to decline in the last 40 years, especially in the decade 1980–1990 (this study). In contrast, the bryozoan A. semiconvoluta increased its distribution and abundance in some areas during the surveyed period, revealing that fishers can easily detect the increase of megabenthic species, particularly those that may affect fishing activities. Fishing grounds where A. semiconvoluta is present in high abundance, in fact, are usually not trawled because colonies of this bryozoan can clog trawl nets' meshes (Grati et al., 2013; Salvalaggio et al., 2014).
Our study reveals that LEK may also provide a reliable and alternative source of information to study the spatial distribution of the benthic invertebrates. Clear spatial patterns in the distribution of the selected species in the Adriatic fishing grounds were apparent. In the Adriatic Sea, P. jacobaues lives in sandy bottoms shallower than 70 m (Piccinetti et al., 1986), and this aspect was confirmed in our interviews. Moreover, our analysis showed that P. jacobaeus is found by fishers also in southern areas respect those previously described, even if in the same bathymetric range were the bivalves used to live. Higher numbers and biomass of Holothuria tubulosa and Holothuria forskali were found between 20 and 100 m depth, unevenly distributed (Šimunović, 1997; Šimunović et al., 2000). Our analysis revealed that the presence of Holothuria spp. goes from 20 m depth down to the deeper sub-areas of the central Adriatic Sea. Thus, we can suppose that environmental factors, such as depth, may be considered directly related to the distribution of Adriatic benthic invertebrates. LEK was also useful to detect rare and spatial restricted species such as the cnidarians Madrepora oculata, and Lophelia pertusa, which were only previously recorded in death assemblages in the Pomo/Jabuka Pit (Angeletti et al., 2014; UNEP/MAP-RAC/SPA, 2015). Although a small number of fishers gave us answers in relation to these scleractinan species, probably because a small fraction of the interviewees trawled in the Pomo pit area, our maps overlap with the known species distributions (Supplementary Figure 1).
LEK can be an instrumental management tool to reconstruct historical information, such as changes in fish community structure following commercial exploitation and climatic change, or to detect rare species, and species invasions (Berkes et al., 2000; Drew, 2005; Azzurro et al., 2011). In addition, LEK can be used to describe changes in fishing methods and strategies (Damalas et al., 2015a,b), leading in some cases, to approaches of adaptive and qualitative management strategies of marine resources and ecosystems (Berkes et al., 2000). Here we aimed to demonstrate LEK's utility and potential applications as an information tool to characterize the structural changes and alteration of benthic invertebrate assemblages, often unmonitored in conventional fisheries management. Fishers' perceptions may represent in some cases the only option to reconstruct historical baselines for habitats status and to map potential VMEs. Thus, LEK may represent an additional tool to help driving actions needed to reach the ecological targets of “Good Environmental Status” (GES). In fact, the maintenance of benthic biodiversity, sea-floor integrity, and a good status of benthic ecosystems through the protection and restoration of benthic sensitive species and habitats are among the targets of the 11 descriptors of GES of the European Marine Strategy Framework Directive (MSFD-EU, 2008). Moreover, LEK may contribute to the Habitat Directive (92/43 CEE) through the identification of priority habitats present in the central Adriatic Sea, such as biogenic-carbonate reefs or oyster reefs, representing rich and fragile biotopes affected by the high pressure of destructive fishing (Conti et al., 2002; Beck et al., 2011; Taviani et al., 2015). Thus, LEK could provide important information for defining areas to be protected from trawling, providing maps of hotspots of biodiversity, priority habitats and areas with presence of VMEs and EFHs, promoting the development of an efficient and sustainable management of the Adriatic fishing as aimed by the Common Fisheries Policy (CFP) of the European Union (EU).
Despite the potential of LEK for describing temporal changes and spatial distribution of benthic invertebrate species, some limitations of this approach emerged from our analysis. In particular, for some of the selected species (see Table 2) information about their presence in the bycatch is limited. This could be related to the fact that fishers do not pay particular attention to species that are not commercially important, or that these species are not so abundant to be commonly observed, or do not affect fishing activities. The interviewed fishers also trawled different fishing grounds with different bottom characteristics and species associations. Thus, the description and identification of the selected megabenthic species, and the likelihood they are observed by fishers, could be related to the natural distribution of the benthic species and to the characteristics of the Adriatic fishing grounds. Moreover, the difference in the number of fishers' responses for the sub-areas identified in our study could be related to the port of origin. In particular, the northern and southern analyzed sectors might be trawled only by a subset of the interviewed fishers, depending on the geographic location of their port of origin. Thus, the number of observations for these sub-areas is smaller compared to the central sub-areas because of their greater distance from the different ports of the Marche region. It was not possible to control for these aspects in this study, but future analyses should address these issues. Finally, our models suggested that there was a significant variability in the response of the individual fishers (random intercept in our model). This aspect needs to be considered when analyzing results from interview surveys to obtain unbiased parameter estimates for other fixed effects. The variability among fisher may be due to a variable perception of abundance among individuals due to experience, recollection ability, and any other factor capable of biasing the index of abundance being modeled (Grant and Berkes, 2007). In the absence of specific information to control for these biasing factors, it is reasonable to assume that each fisher influenced the variability of the responses in a random fashion according to a normal distribution with mean 0 and standard deviation to be estimated from the data.
The widespread perceived decline of benthic species playing important ecological roles (Table 1) in the central Adriatic may have altered the Adriatic marine ecosystem functioning over the past decades. Changes in benthic invertebrates we described here are congruent with patterns of decline described by other authors in the northern Adriatic through use of standard sampling methodologies such as dredges and trawl surveys (Scardi et al., 1999; Raicevich et al., 2004). These studies reported a net reduction of the ratio discards/commercial species, with a decline or disappearance of large filter feeding organisms (e.g., the sponge Geodia) documented from 1980s to 2000s (Raicevich et al., 2004) together with a general decreasing trend of the diversity of macrobenthic assemblages (Scardi et al., 1999). In other ocean sectors, the declines in benthic invertebrates triggered entire regime shifts (Kaiser et al., 2000; Jackson, 2001) and we expect that similar consequences may have occurred also for the Adriatic Sea. Detecting the occurrence of these ecological changes is of paramount importance for future studies.
Several factors may have driven the declines of megabenthos species living on soft bottoms of the Adriatic basin. Declines of sponges and other benthic invertebrates, for example, has been associated with anoxic events (Fedra et al., 1976) in the northern Adriatic basin. Climate change, such as temperature anomalies, caused mass mortalities events in the central basin (Di Camillo et al., 2013; Di Camillo and Cerrano, 2015; Kružić and Popijač, 2015), and direct and indirect impacts of human activities, such as fishing, have reduced the biodiversity and the complexities of the Adriatic benthic communities (Raicevich et al., 2004; Pronzato and Manconi, 2008; Lotze et al., 2011). While we cannot exclude the influence of multiple factors in driving the decline of megabenthic species described here, our analysis supports the hypothesis that intense trawling in the Adriatic Sea over the past decades may have been a major factor determining the alteration of the Adriatic soft bottom communities. In 1980s, Italian Adriatic regions reached the maximum number of fishing vessels together with the complete development of highly damaging fisheries introduced in the 1960s (Froglia, 2000; AdriaMed, 2004; Romanelli et al., 2009). In the 2000s the total number of fishing vessels decreased (AdriaMed, 2004), however, new technologies such as GPS systems have been introduced, improving the exploitation of new fishing ground (Fortibuoni et al., 2017) and the total fishing pressure on Adriatic seabed bottoms is currently considered unsustainable. Because the LEK data we collected in our study to detect fishers' perceptions is mainly qualitative, our models did not detect clear patterns moving from coastal to offshore areas. However, distance from the coast is one of the most important variables affecting our regression models, for example for P. jacobaeus. In particular, our model suggests that at increasing distance from the coast, higher classes of abundance are more likely (Table 3). This relation with the proximity to the coastline is characteristic of a community being exploited, such as coastal communities that typically are exposed to a heavier and more prolonged history of exploitation than those offshore. Automatic Identification System and Vessel Monitoring System analysis clearly revealed that trawling fishing effort is higher in coastal areas with respect to offshore areas in the central Adriatic Sea (Santelli et al., 2017). However, chronic and intensive effects of bottom trawling fishing, with habitat degradation are well-known (Pusceddu et al., 2014), and the long-term exploitation of the Adriatic basin could have homogenized and simplified Adriatic soft bottoms habitats and species composition even in offshore areas. In particular, habitats formed by slow growing and long-lived specimens such as sea pens, hydroids, or corals, have a high vulnerability to fishing and even reduced fishing effort may cause considerable damage to these species, preventing their recovery (Troffe et al., 2005; Greathead et al., 2014). Moreover, the impacts of trawl fishing gears on the seabed differ depending on the sediment compositions and on bottom trawl target species (Pranovi et al., 2001, 2005; Eigaard et al., 2016). Gear characteristics (e.g., changes in number, the size of meshes in the cod end net, modification of the design of the doors, and other parts of the trawl net) also possibly affected the level and the type of damage by trawling gear on megabenthos. Our study did not consider different gear types, thus the pattern described by fishers is only relevant to a specific type of fishing gear. All the interviewed fishers were otter trawler and used a fishing gear that is generally standard across our focal area (that is an Italian otter trawl as specified in Fiorentini et al., 1999). However, because the interviewed fishers in most cases did not give us the specific characteristics of their fishing gears (e.g., detailed size of trawl net and numbers of used gears per haul), it was not possible to confirm and clearly relate the fishing effort and fishing gear characteristics with the observed megafauna trends. More detailed analysis and new interviews are needed to fill these gaps and to explore the most adequate restoration measures (Bastari et al., 2016) that need to be urgently adopted.
Historical studies are fundamental for understanding long-term changes in marine ecosystems. LEK surveys provide an opportunity to fill this knowledge gaps as we demonstrated here by focusing on historical changes of benthic invertebrates species in the exploited Adriatic Sea. These approaches provide an opportunity to reconstruct reference points for benthic communities and may help management in setting recovery target for ecosystem structure and even function at local and regional scale. Therefore, extending these studies on a broader geographic scale is a promising approach for drawing historical baselines and inform marine management.
Ethics approval was not required for this study as per institutional guidelines and Italian law and regulations. In compliance with the aforementioned guidelines, laws and regulations, oral informed consent was obtained from all research participants. All potential interviewees were provided of the purpose of the study and of the usage of collected data before obtaining their consent. Their answers were anonymized and it is not possible to link the statements back to individual subjects.
AB gave substantial contributions to the conception and design of the work, analysis of data, and drafting the work, and final approval of the version to be published. JB gave substantial contributions to the acquisition and analysis of data. FF gave substantial contributions to the work conception, analysis and interpretation of data, and revising the work critically for important intellectual content, and final approval of the version to be published. FM and CC conceive the work and gave substantial contributions to its design, revising the work critically for important intellectual content, and the writing of the article.
CC and AB were supported by a grant from the Polytechnic University of Marche (AMER project) and by European Union (EU) project MERCES (689518). FM and FF were supported by a grant from Stanford's Woods Institute for the Environment (Environmental Venture Projects).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank all fishers who dedicated time to our work, sharing their precious knowledge and allowing the description of long-term changes of the megabenthic Adriatic assemblages.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00157/full#supplementary-material
1. ^Available online at: http://ec.europa.eu/fisheries/fleet/index.cfm
AdriaMed (2004). AdriaMed Seminar on Fishing Capacity: Definition, Measurement and Assessment. AdriaMed Technical Documents, FAO-MiPAF Scientific Cooperation to Support Responsible Fisheries in the Adriatic Sea, Termoli.
Anadón, J. D., Giménez, A., Ballestar, R., and Pérez, I. (2009). Evaluation of local ecological knowledge as a method for collecting extensive data on animal abundance. Conserv. Biol. 23, 617–625. doi: 10.1111/j.1523-1739.2008.01145.x
Angeletti, L., Taviani, M., Canese, S., Foglini, F., Mastrototaro, F., Argnani, A., et al. (2014). New deep-water cnidarian sites in the southern Adriatic Sea. Mediterr. Mar. Sci. 15, 1–11. doi: 10.12681/mms.558
Azzurro, E., Moschella, P., and Maynou, F. (2011). Tracking signals of change in Mediterranean fish diversity based on local ecological knowledge. PLoS ONE 6:e24885. doi: 10.1371/journal.pone.0024885
Barausse, A., Michieli, A., Riginella, E., Palmeri, L., and Mazzoldi, C. (2011). Long-term changes in community composition and life-history traits in a highly exploited basin (northern Adriatic Sea): the role of environment and anthropogenic pressures. J. Fish Biol. 79, 1453–1486. doi: 10.1111/j.1095-8649.2011.03139.x
Bastari, A., Micheli, F., Ferretti, F., Pusceddu, A., and Cerrano, C. (2016). Large marine protected areas (LMPAs) in the Mediterranean Sea: the opportunity of the Adriatic Sea. Mar. Policy 68, 165–177. doi: 10.1016/j.marpol.2016.03.010
Beck, M. W., Brumbaugh, R. D., Airoldi, L., Carranza, A., Coen, L. D., Crawford, C., et al. (2011). Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 61, 107–116. doi: 10.1525/bio.2011.61.2.5
Berkes, F., Colding, J., and Folke, C. (2000). Rediscovery of traditional ecological knowledge as adaptive management. Ecol. Appl. 10, 1251–1262. doi: 10.1890/1051-0761(2000)010[1251:ROTEKA]2.0.CO;2
Brambati, A., Ciabatti, M., Fanzutti, G. P., Marabini, F., and Marocco, R. (1983). A new sedimentological textural map of the northern and central Adriatic Sea. Bull. Soc. Geol. Ital. 98, 293–326.
Cerrano, C., Danovaro, R., Gambi, C., Pusceddu, A., Riva, A., and Schiaparelli, S. (2010). Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodivers. Conserv. 19, 153–167. doi: 10.1007/s10531-009-9712-5
Christensen, R. H. B (2015). A Tutorial on fitting Cumulative Link Models with the Ordinal Package. Available online at: https://cran.r-project.org/web/packages/ordinal/vignettes/clm_tutorial.pdf
Coll, M., Piroddi, C., Albouy, C., Ben Rais Lasram, F., Cheung, W. W. L., Christensen, V., et al. (2012). The Mediterranean Sea under siege: spatial overlap between marine biodiversity, cumulative threats and marine reserves. Glob. Ecol. Biogeogr. 21, 465–480. doi: 10.1111/j.1466-8238.2011.00697.x
Coll, M., Piroddi, C., Steenbeek, J., Kaschner, K., Lasram, F. B. R., Aguzzi, J., et al. (2010). The biodiversity of the Mediterranean Sea: ESTIMATES, patterns, and threats. PLoS ONE 5:e11842. doi: 10.1371/journal.pone.0011842
Coll, M., Santojanni, A., Palomera, I., and Arneri, E. (2009). Food-web changes in the Adriatic Sea over the last three decades. Mar. Ecol. Prog. Ser. 381, 17–37. doi: 10.3354/meps07944
Coll, M., Santojanni, A., Palomera, I., Tudela, S., and Arneri, E. (2007). An ecological model of the Northern and Central Adriatic Sea: analysis of ecosystem structure and fishing impacts. J. Mar. Syst. 67, 119–154. doi: 10.1016/j.jmarsys.2006.10.002
Collie, J., Escanero, G., and Valentine, P. (1997). Effects of bottom fishing on the benthic megafauna of Georges Bank. Mar. Ecol. Prog. Ser. 155, 159–172. doi: 10.3354/meps155159
Colloca, F., Cardinale, M., Maynou, F., Giannoulaki, M., Scarcella, G., Jenko, K., et al. (2013). Rebuilding Mediterranean fisheries: a new paradigm for ecological sustainability. Fish Fish. 14, 89–109. doi: 10.1111/j.1467-2979.2011.00453.x
Colloca, F., Garofalo, G., Bitetto, I., Facchini, M. T., Grati, F., Martiradonna, A., et al. (2015). The seascape of demersal fish nursery areas in the north mediterranean sea, a first step towards the implementation of spatial planning for trawl fisheries. PLoS ONE 10:e0119590. doi: 10.1371/journal.pone.0119590
Conti, A., Stefanon, A., and Zuppi, G. M. (2002). Gas seeps and rock formation in the northern Adriatic Sea. Cont. Shelf Res. 22, 2333–2344. doi: 10.1016/S0278-4343(02)00059-6
Conversi, A., Fonda Umani, S., Peluso, T., Molinero, J. C., Santojanni, A., and Edwards, M. (2010). The Mediterranean Sea regime shift at the end of the 1980s, and intriguing parallelisms with other European Basins. PLoS ONE 5:e10633. doi: 10.1371/journal.pone.0010633
Crema, R., Castelli, A., and Prevedelli, D. (1991). Long term eutrophication effects on macrofaunal communities in northern Adriatic Sea. Mar. Pollut. Bull. 22, 503–508. doi: 10.1016/0025-326X(91)90405-H
Damalas, D., Maravelias, C. D., Osio, G. C., Maynou, F., Sbrana, M., and Sartor, P. (2015a). “Once upon a time in the Mediterranean” long term trends of Mediterranean fisheries resources based on fishers' traditional ecological knowledge. PLoS ONE 10:e0119330. doi: 10.1371/journal.pone.0119330
Damalas, D., Maravelias, C. D., Osio, G. C., Maynou, F., Sbrana, M., Sartor, P., et al. (2015b). Historical discarding in Mediterranean fisheries: a fishers' perception. ICES J. Mar. Sci. J. Cons. 72, 2600–2608. doi: 10.1093/icesjms/fsv141
Dayton, P. K. (1972). “Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica,” in Proceeding of Colloquium Conservation Antarctica (Kansas, MI: Allen Press; Lawrence), 81–95.
Dayton, P. K., Thrush, S. F., Agardy, M. T., and Hofman, R. J. (1995). Environmental effects of marine fishing. Aquat. Conserv. Mar. Freshw. Ecosyst. 5, 205–232. doi: 10.1002/aqc.3270050305
de Juan, S., and Lleonart, J. (2010). A conceptual framework for the protection of vulnerable habitats impacted by fishing activities in the Mediterranean high seas. Ocean Coast. Manag. 53, 717–723. doi: 10.1016/j.ocecoaman.2010.10.005
de Juan, S., Moranta, J., Hinz, H., Barberá, C., Ojeda-Martinez, C., Oro, D., et al. (2012). A regional network of sustainable managed areas as the way forward for the implementation of an Ecosystem-Based Fisheries Management in the Mediterranean. Ocean Coast. Manag. 65, 51–58. doi: 10.1016/j.ocecoaman.2012.04.024
Di Camillo, C. G., and Cerrano, C. (2015). Mass mortality events in the NW Adriatic Sea: phase shift from slow-to fast-growing organisms. PLoS ONE 10:e0126689. doi: 10.1371/journal.pone.0126689
Di Camillo, C. G., Bartolucci, I., Cerrano, C., and Bavestrello, G. (2013). Sponge disease in the Adriatic Sea. Mar. Ecol. 34, 62–71. doi: 10.1111/j.1439-0485.2012.00525.x
Drew, J. A. (2005). Use of traditional ecological knowledge in marine conservation. Conserv. Biol. 19, 1286–1293. doi: 10.1111/j.1523-1739.2005.00158.x
Eigaard, O. R., Bastardie, F., Breen, M., Dinesen, G. E., Hintzen, N. T., Laffargue, P., et al. (2016). Estimating seabed pressure from demersal trawls, seines, and dredges based on gear design and dimensions. ICES J. Mar. Sci. J. Cons. 73, i27–i43. doi: 10.1093/icesjms/fsw116
Engelhard, G. H., Thurstan, R. H., MacKenzie, B. R., Alleway, H. K., Bannister, R. C. A., Cardinale, M., et al. (2016). ICES meets marine historical ecology: placing the history of fish and fisheries in current policy context. ICES J. Mar. Sci. J. Cons. 73, 1386–1403. doi: 10.1093/icesjms/fsv219
FAO (2009). FAO 2010-2016. Fisheries and Aquaculture Activities. The FAO International Guidelines for the Management of Deep-Sea Fisheries in the High Seas. FAO Fisheries and Aquaculture Department, Rome. Available online at: ftp://ftp.fao.org/docrep/fao/011/i0816t/i0816t.pdf (Accessed October 24, 2016).
FAO (2016). The State of Mediterranean and Black Sea Fisheries. General Fisheries Commission for the Mediterranean, Rome.
Fedra, K., Ölscher, E. M., Scherübel, C., Stachowitsch, M., and Wurzian, R. S. (1976). On the ecology of a North Adriatic benthic community: distribution, standing crop and composition of the macrobenthos. Mar. Biol. 38, 129–145. doi: 10.1007/BF00390766
Ferretti, F., Osio, G. C., Jenkins, C. J., Rosenberg, A. A., and Lotze, H. K. (2013). Long-term change in a meso-predator community in response to prolonged and heterogeneous human impact. Sci. Rep. 3:1057. doi: 10.1038/srep01057
Fiorentini, L., Dremière, P. Y., Leonori, I., Sala, A., and Palumbo, V. (1999). Efficiency of the bottom trawl used for the Mediterranean international trawl survey (MEDITS). Aquat. Living Resour. 12, 187–205. doi: 10.1016/S0990-7440(00)88470-3
Fortibuoni, T., Giovanardi, O., Pranovi, F., Raicevich, S., Solidoro, C., and Libralato, S. (2017). Analysis of long-term changes in a Mediterranean marine ecosystem based on fishery landings. Front. Mar. Sci. 4:33. doi: 10.3389/fmars.2017.00033
Fortibuoni, T., Libralato, S., Raicevich, S., Giovanardi, O., and Solidoro, C. (2010). Coding early naturalists' accounts into long-term fish community changes in the Adriatic Sea (1800-2000). PLoS ONE 5:e15502. doi: 10.1371/journal.pone.0015502
Fouzai, N., Coll, M., Palomera, I., Santojanni, A., Arneri, E., and Christensen, V. (2012). Fishing management scenarios to rebuild exploited resources and ecosystems of the Northern-Central Adriatic (Mediterranean Sea). J. Mar. Syst. 102–104, 39–51. doi: 10.1016/j.jmarsys.2012.05.003
Froglia, C. (2000). Il contributo della ricerca scientifica alla gestione della pesca dei molluschi bivalvi con draghe idrauliche. Biol. Mar. Mediterr. 7, 71–82.
Gamulin-Brida, H. (1974). Biocoenoses benthiques de la mer Adriatique. Inst. Oceanogr. Ribar XV, 1–61.
Giani, M., Djakovac, T., Degobbis, D., Cozzi, S., Solidoro, C., and Umani, S. F. (2012). Recent changes in the marine ecosystems of the northern Adriatic Sea. Estuar. Coast. Shelf Sci. 115, 1–13. doi: 10.1016/j.ecss.2012.08.023
Graham, M. H. (2003). Confronting multicollinearity in ecological multiple regression. Ecology 84, 2809–2815. doi: 10.1890/02-3114
Grant, S., and Berkes, F. (2007). Fisher knowledge as expert system: a case from the longline fishery of Grenada, the Eastern Caribbean. Fish. Res. 84, 162–170. doi: 10.1016/j.fishres.2006.10.012
Grati, F., Scarcella, G., Polidori, P., Domenichetti, F., Bolognini, L., Gramolini, R., et al. (2013). Multi-annual investigation of the spatial distributions of juvenile and adult sole (Solea solea L.) in the Adriatic Sea (northern Mediterranean). J. Sea Res. 84, 122–132. doi: 10.1016/j.seares.2013.05.001
Greathead, C., González-Irusta, J. M., Clarke, J., Boulcott, P., Blackadder, L., Weetman, A., et al. (2014). Environmental requirements for three sea pen species: relevance to distribution and conservation. ICES. J. Cons. 72, 576–586. doi: 10.1093/icesjms/fsu129
Hall-Spencer, J. M., Froglia, C., Atkinson, R. J., and Moore, P. G. (1999). The impact of Rapido trawling for scallops, Pecten jacobaeus (L.), on the benthos of the Gulf of Venice. ICES J. Mar. Sci. 56, 111–124. doi: 10.1006/jmsc.1998.0424
Halpern, B. S., Frazier, M., Potapenko, J., Casey, K. S., Koenig, K., Longo, C., et al. (2015). Spatial and temporal changes in cumulative human impacts on the world's ocean. Nat. Commun. 6, 7615. doi: 10.1038/ncomms8615
Halpern, B. S., McLeod, K. L., Rosenberg, A. A., and Crowder, L. B. (2008). Managing for cumulative impacts in ecosystem-based management through ocean zoning. Ocean Coast. Manag. 51, 203–211. doi: 10.1016/j.ocecoaman.2007.08.002
Huntington, H. P. (2000). Using traditional ecological knowledge in science: methods and applications. Ecol. Appl. 10, 1270. doi: 10.2307/2641282
IREPA Onlus (2011). Osservatorio Economico Sulle Strutture Produttive Della Pesca Marittima in Italia 2011. Napoli: Edizioni Scientifiche Italiane, 252.
Jackson, J. B. (2001). What was natural in the coastal oceans?. Proc. Natl. Acad. Sci. U.S.A. 98, 5411–5418. doi: 10.1073/pnas.091092898
Jackson, J. B., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637. doi: 10.1126/science.1059199
Jennings, S., and Kaiser, M. J. (1998). The effects of fishing on marine ecosystems. Adv. Mar. Biol. 34, 201–352. doi: 10.1016/S0065-2881(08)60212-6
Jukic-Peladic, S., Vrgoc, N., Krstulovic-Sifner, S., Piccinetti, C., Piccinetti-Manfrin, G., Marano, G., et al. (2001). Long-term changes in demersal resources of the Adriatic Sea: comparison between trawl surveys carried out in 1948 and 1998. Fish. Res. 53, 95–104. doi: 10.1016/S0165-7836(00)00232-0
Kaiser, M. J., Ramsay, K., Richardson, C. A., Spence, F. E., and Brand, A. R. (2000). Chronic fishing disturbance has changed shelf sea benthic community structure. J. Anim. Ecol. 69, 494–503. doi: 10.1046/j.1365-2656.2000.00412.x
Kružić, P., and Popijač, A. (2015). Mass mortality events of the coral Balanophyllia europaea (Scleractinia, Dendrophylliidae) in the Mljet National Park (eastern Adriatic Sea) caused by sea temperature anomalies. Coral reefs 34, 109–118. doi: 10.1007/s00338-014-1231-5
Lotze, H. K., Coll, M., and Dunne, J. A. (2011). Historical changes in marine resources, food-web structure and ecosystem functioning in the Adriatic Sea, Mediterranean. Ecosystems 14, 198–222. doi: 10.1007/s10021-010-9404-8
Mazzoldi, C., Sambo, A., and Riginella, E. (2014). The Clodia database: a long time series of fishery data from the Adriatic Sea. Sci. Data 1, 140018. doi: 10.1038/sdata.2014.18
McKinney, F. K. (2007). The Northern Adriatic Ecosystem: Deep Time in a Shallow Sea. New York, NY: Columbia University Press.
Micheli, F., Halpern, B. S., Walbridge, S., Ciriaco, S., Ferretti, F., Fraschetti, S., et al. (2013a). Cumulative human impacts on Mediterranean and Black Sea marine ecosystems: Assessing current pressures and opportunities. PLoS ONE 8:e79889. doi: 10.1371/journal.pone.0079889
Micheli, F., Levin, N., Giakoumi, S., Katsanevakis, S., Abdulla, A., Coll, M., et al. (2013b). Setting priorities for regional conservation planning in the Mediterranean Sea. PLoS ONE 8:e59038. doi: 10.1371/journal.pone.0059038
Morello, E. B., Froglia, C., Atkinson, R. J., and Moore, P. G. (2005). Impacts of hydraulic dredging on a macrobenthic community of the Adriatic Sea, Italy. Can. J. Fish. Aquat. Sci. 62, 2076–2087. doi: 10.1139/f05-122
MSFD (2008). Directive 2008/56/EC of the European Parliament and the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). Available online at: http://ec.europa.eu/environment/water/marine/index_en.htm
OSPAR Commission (2010). Background Document for Seapen and Burrowing Megafauna Communities Biodiversity Series. OSPAR Commission.
Paolucci, C. (1923). Il primo esperimento governativo di pesca con battello a vapore nell'Adriatico. Rivist. Pesca Idrobiol. 33–60.
Piccinetti, C. (1976). Aspetti della fauna marina delle Marche. Plant Biosyst. 110, 427–436. doi: 10.1080/11263507609426406
Piccinetti, C., Simunovic, A., and Jukic, S. (1986). Distribution and Abundance of Chlamys opercularis (L.) and Pecten jacobaeus L. in the Adriatic Sea. FAO Fisheries Report (FAO).
Pranovi, F., Raicevich, S., Franceschini, G., Torricelli, P., and Giovanardi, O. (2001). Discard analysis and damage to non-target species in the “rapido” trawl fishery. Mar. Biol. 139, 863–875. doi: 10.1007/s002270100646
Pranovi, F., Raicevich, S., Libralato, S., Da Ponte, F., and Giovanardi, O. (2005). “Trawl fishing disturbance and medium-term microfaunal recolonization dynamics: a functional approach to the comparison between sand and mud habitats in the Adriatic Sea (Northern Mediterranean Sea),” in American Fisheries Society Symposium, Vol. 41, (Bethesda, MD: American Fisheries Society), 545.
Pronzato, R., and Manconi, R. (2008). Mediterranean commercial sponges: over 5000 years of natural history and cultural heritage. Mar. Ecol. 29, 146–166. doi: 10.1111/j.1439-0485.2008.00235.x
Puig, P., Canals, M., Company, J. B., Martín, J., Amblas, D., Lastras, G., et al. (2012). Ploughing the deep sea floor. Nature 489, 286–289. doi: 10.1038/nature11410
Pusceddu, A., Bianchelli, S., Martín, J., Puig, P., Palanques, A., Masqué, P., et al. (2014). Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning. Proc. Natl. Acad. Sci. U.S.A. 111, 8861–8866. doi: 10.1073/pnas.1405454111
Raicevich, S., Pranovi, F., Libralato, S., and Giovanardi, O. (2004). “The ecological role of potential scavengers in exploited ecosystems,” in Poster Presentato al Simposio Internazionale “Quantitative Ecosystem Indicators for Fishery Management” (ambito UNESCO, SCOR, PICES)”, Parigi.
Rengstorf, A. M., Yesson, C., Brown, C., and Grehan, A. J. (2013). High-resolution habitat suitability modeling can improve conservation of vulnerable marine ecosystems in the deep sea. J. Biogeogr. 40, 1702–1714. doi: 10.1111/jbi.12123
Rogers, A. D., and Gianni, M. (2010). The Implementation of UNGA Resolutions 61/105 and 64/72 in the Management of Deep-Sea Fisheries on the High Seas. Management, 97. Available online at: http://www.stateoftheocean.org/pdfs/61105-Implemention-finalreport.pdf.
Romanelli, M., Cordisco, C. A., and Giovanardi, O. (2009). The long-term decline of the Chamelea gallina L. (Bivalvia : Veneridae) clam fishery in the Adriatic Sea : is a synthesis possible ? Acta Adriat. 50, 171–204. Available online at: http://161.53.31.3/acta/pdf/50_2_pdf/50_2_6.pdf
Rosenberg, A., Bigford, T. E., Leathery, S., Hill, R. L., and Bickers, K. (2000). Ecosystem approaches to fishery management through essential fish habitat. Bull. Mar. Sci. 66, 535–542.
Rossi, S., Bramanti, L., Gori, A., and Orejas Saco del Valle, C. (eds.) (2017). Marine Animal Forests. The Ecology of Benthic Biodiversity Hotspots. Springer International Publishing.
Salvalaggio, V., Brunetti, B., Despalatović, M., Fabi, G., Grati, F., Polidori, P., et al. (2014). Distribuzione spaziale e persistenza del briozoo Amathia semiconvoluta in mar Adriatico centrale e settentrionale. Biol. Mar. Mediterr. 21, 249–250.
Santelli, A., Cvitković, I., Despalatović, M., Fabi, G., Grati, F., Marčeta, B., et al. (2017). Spatial persistence of megazoobenthic assemblages in the Adriatic Sea. Mar. Ecol. Prog. Ser. 566, 31–48. doi: 10.3354/meps12002
Scaccini, A. (1967). Dati preliminari sulle zoocenosi bentoniche e sulla biomassa in una zona dell'Alto e Medio Adriatico. Note del Lab. di Biol. Mar. Pesca Fano II, 3, 25–56.
Scaccini, A., and Piccinetti, C. (1969). Il Fondo del Mare da Falconara. Tortoreto: con annessa carta di pesca. C.N.R. Bologna.
Scardi, M., Crema, R., Di Dato, P., Fresi, E., and Orel, G. (1999). “Le comunità bentoniche dell'Alto Adriatico: un'analisi preliminare dei cambiamenti strutturali dagli anni'30 ad oggi,” in Proceedings of the Workshop “Impact of Trawl Fishing on Benthic communities”, (Rome), 95–108.
Šimunović, A., Piccinettti, C., Bartulović, M., and Grubelić, I. (2000). Distribution and abundance of the species Holothuria tubulosa Gmelin, 1788 and Holothuria forskali Delle Chiaje, 1823 (Holothuria, Echinodermata) in the Adriatic Sea. Acta Adriat. 41, 3–16.
Šimunović, A. (1997). Quantitative and qualitative investigations of benthic communities in the areas of mobile bottoms of the Adriatic Sea. Acta Adriat. 38, 77–194.
Spagnoli, F., Dinelli, E., Giordano, P., Marcaccio, M., Zaffagnini, F., and Frascari, F. (2014). Sedimentological, biogeochemical and mineralogical facies of Northern and Central Western Adriatic Sea. J. Mar. Syst. 139, 183–203. doi: 10.1016/j.jmarsys.2014.05.021
Taviani, M., Franchi, F., Angeletti, L., Correggiari, A., López-Correa, M., Maselli, V., et al. (2015). Biodetrital carbonates on the Adriatic continental shelf imprinted by oxidation of seeping hydrocarbons. Mar. Pet. Geol. 66, 511–531. doi: 10.1016/j.marpetgeo.2015.03.015
Thrush, F. S., and Dayton, K. P. (2002). Disturbance to marine benthic habitats by trawling and dredging: implications for marine biodiversity. Annu. Rev. Ecol. Syst. 33, 449–473. doi: 10.1146/annurev.ecolsys.33.010802.150515
Troffe, P. M., Levings, C. D., Piercey, G. B. E., and Keong, V. (2005). Fishing gear effects and ecology of the sea whip (Halipteris willemoesi (Cnidaria: Octocorallia: Pennatulacea)) in British Columbia, Canada: preliminary observations. Aquat. Conserv. Mar. Freshw. Ecosyst. 15, 523–533. doi: 10.1002/aqc.685
Tsagarakis, K., Palialexis, A., and Vassilopoulou, V. (2013). Mediterranean fishery discards: review of the existing knowledge. ICES J. Mar. Sci. J. Cons. 71, 1219–1234. doi: 10.1093/icesjms/fst074
UNEP/MAP-RAC/SPA. (2015). Adriatic Sea: Description of the Ecology and Identification of the Areas that May Deserve to be Protected. Tunis, 92.
UNGA (2006). United Nations General Assembly. A/RES/61/105. A/RES/61/105 - Sustainable fisheries, including through the 1995 Agreement for the Implementation of the Provisions of the United Nations Convention on the Law of the Sea of 10 December 1982 relating to the Conservation and Management of Straddling Fish Stocks and Highly Migratory Fish Stocks, and related instruments.
Valisano, L., Notari, F., Mori, M., and Cerrano, C. (2016). Temporal variability of sedimentation rates and mobile fauna inside and outside a gorgonian garden. Mar. Ecol. 37, 1303–1314. doi: 10.1111/maec.12328
Watling, L., and Norse, E. A. (1998). Disturbance of the seabed by mobile fishing gear: a comparison to forest clearcutting. Conserv. Biol. 12, 1180–1197. doi: 10.1046/j.1523-1739.1998.0120061178.x
Zenetos, A., Gofas, S., Verlaque, M., Inar, M. E., Garci, J. E., Bianchi, C. N., et al. (2011). Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union's Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution. Mediterr. Mar. Sci. 11, 509–514. doi: 10.12681/mms.87
Keywords: Adriatic Sea, local ecological knowledge, megabenthic species, historical trends, fishers perceptions
Citation: Bastari A, Beccacece J, Ferretti F, Micheli F and Cerrano C (2017) Local Ecological Knowledge Indicates Temporal Trends of Benthic Invertebrates Species of the Adriatic Sea. Front. Mar. Sci. 4:157. doi: 10.3389/fmars.2017.00157
Received: 31 January 2017; Accepted: 09 May 2017;
Published: 24 May 2017.
Edited by:
Simone Libralato, National Institute of Oceanography and Experimental Geophysics, ItalyReviewed by:
Konstantinos Tsagarakis, Hellenic Centre for Marine Research, GreeceCopyright © 2017 Bastari, Beccacece, Ferretti, Micheli and Cerrano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Azzurra Bastari, YXp6dWJhc3RAdW5pdnBtLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.