
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 22 November 2022
Sec. Marine Pollution
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1023996
This article is part of the Research TopicNovel Insights into the Circulation, Biogeochemistry, and Ecosystem Function of the Gulf of Mexico: A Perspective from Mexican Waters in Support of Oil Spill PreparednessView all 17 articles
Diversity of free-living marine nematode assemblages in the Southwestern Gulf of Mexico (SW GoM) is scarcely studied. Here, we aimed (1) to analyze the influence of a water depth gradient on the species richness, feeding type and taxonomic composition of assemblages; and (2) to document the regional diversity of free-living nematodes in the SW GoM. We sampled 63 sites along a water depth gradient from 186 to 3774 m during four oceanographic cruises. We found clear variations along the depth gradient of bottom water (temperature, salinity, and dissolved oxygen) and sediment variables (grain size and organic content). We identified 1881 nematodes belonging to 108 genera, 33 families, ten orders, and two classes. The abundance and species richness decreased with water depth. However, the expected number of species for a same level of abundance did not change along the depth gradient likely because the scarcity of food was compensated by reduced environmental stress (e.g., higher oxygen content and physical stability). Microbial sucker was the most abundant feeding type indicating the important role of sediment bacteria in the nematode’s diet. Species composition varied along the depth gradient with dominance in the upper slope sites of species of Comesomatidae tolerant to reduced sediments (e.g., Dorylaimopsis sp., Sabatieria spp., and Setosabatieria hilarula). Many congeneric species typical of deep sea were restricted to the deepest sites such as Acantholaimus spp., Ledovitia spp., Desmoscolex spp., and Halalaimus spp. The nematode regional diversity of SW GoM was 154 species, but the Chao 1 estimator indicated a richness of about 194 species and a highest limit of 246 species. The accumulation curves of richness were non-asymptotic suggesting a substantial fraction of undiscovered richness. Our study increased the free-living nematode fauna of GoM in 144 species (76%) respect to Hope’s list (2009). The large diversity of nematodes stands out the necessity of further studies to unravel the environmental drivers of α- and β-diversities and highlights the potential of this taxon for monitoring the deep sea of the Gulf of Mexico.
Nematoda is the 5th most diverse phylum of Animalia (Zhang, 2011) with about 28 537 described species (Hodda, 2022a). There is a large uncertainty about the total number of nematode species on the Earth, although Appeltans et al. (2012) estimated that only 19% of the total is known. Nematodes occur in all the habitats on the Earth, from the deep sea to the ice caps, and can be parasitic or free-living. The free-living nematodes are usually the most abundant metazoans in soils and sediments.
In the deep sea, free-living nematodes are particularly important in terms of abundance and diversity (Zeppilli et al., 2018). Nematodes have a body size in the order of 1 mm and therefore are part formally of the meiofauna, which is operationally defined as metazoans passing through 500 µm sieve and retained in 45/38 µm sieves. However, nematodes also occur in the macrofauna, i.e., the metazoans retained in 500 µm sieves. Many studies have been published on the ecology and diversity of deep-sea nematodes in the last 20 years resulting in an enormous body of knowledge. From an ecological point of view, four factors are mainly responsible of the meiofauna community structure in the deep sea (Rosli et al., 2018a): food availability, substrate structure, physico-chemical characteristics, and physical disturbance. These factors and associated processes are also main determinants of the nematode assemblage structure.
The studies on diversity of deep-sea nematode assemblages pose significant taxonomic challenges because usually each genus/species is represented by few individuals (Danovaro and Gambi, 2022). In addition, collections contain many undescribed species particularly of important families such as Xyalidae and Oxystominidae (Miljutin et al., 2010). Therefore, the identification and naming at species level is not always possible, although discrimination of congeneric species is usually possible.
The species richness of deep-sea nematodes is usually high at local scale (i.e., α-diversity). However, the magnitude of regional richness (i.e., γ-diversity) and its causes are still debatable. Lambshead and Boucher (2004) concluded that regional richness is rather modest because similar patches in a similar habitat are repeated for considerable distances. However, more recent evidence suggests that nematode genus/species turnover is very high in the deep sea and depends on processes acting at different scales (Pusceddu et al., 2009; Danovaro et al., 2013; Rosli et al., 2018b). In addition, water depth constitutes an important driver of nematode abundance (Trebukhova et al., 2013; dos Santos et al., 2020) and it is related fundamentally to trophic conditions and sediment properties (Udalov et al., 2005). These features explain the high rareness and endemism at regional scale reported by Danovaro and Gambi (2022) in a recent study over 246 sites of the deep sea.
The free-living nematodes in the deep waters of the Gulf of Mexico (GoM) have been the subject of few ecological/diversity studies. In a pioneer study, Sharma et al. (2011) detected a decreasing body size of macrobenthic nematodes along depth in the northern GoM. Sharma et al. (2012) studied 16 sites along two transects in the north-central GoM reporting that organic loads from Mississippi River drove the nematode assemblages. Jones et al. (2016) studied the nematode assemblages in 16 sites in the shelf and slope of northern GoM and concluded that west-east gradient in sediment characteristics and habitat type were the main drivers of assemblage structure. Soto et al. (2017) reported significant changes in the large-sized nematode assemblage structure in the northwestern GoM that they suggest could be due to the Deepwater Horizon oil spill. In the most recent study, Cisterna-Céliz et al. (2019) sampled 27 deep-sea sites over the southern GoM, and identified nematodes from seven sites, reporting that assemblage structure fitted into a “species-sorting” model that included environmental characteristics, niche differentiation, and limited dispersal. All these studies identified nematodes at genus level.
Studies of deep-sea nematodes at species level in GoM have been mostly devoted to taxonomy and the last revision on the diversity of the group was made by Hope (2009) about 12 years ago. He reported 190 species of nematodes, of which 126 (66%) were endemic and 64 (34%) were not (Hope, 2009).
Two massive accidents have occurred in the GoM related with oil industry. In 1979, a blowout in the exploratory oil well Ixtoc I in the Bay of Campeche released ca. 3.4 × 106 barrels of crude oil into the tropical waters of Campeche Sound during nine months (Soto et al., 2014). In 2010, a blowout of the well Macondo in the northcentral GoM released ca. 4.9 × 106 barrels of oil during 87 days (Lubchenco et al., 2012). In this scenario, our study was carried within the Gulf of Mexico Research Consortium (CIGoM) advocated to develop a baseline of environmental variables for evaluating potential large oil spill impact in the deep waters of GoM. A huge body of knowledge was generated during five years in this project, which was summarized in a set of synoptic atlases (https://atlascigom.cicese.mx) . The data gathered by this initiative go beyond the atlases and can give insights about patterns and processes in the deep waters of GoM. We gathered thousands of observations and hundreds of water and sediment samples derived from four oceanographic cruises (SOGOM 1–4) in a broad region of the southwestern GoM.
In this contribution, we analyzed the diversity patterns of nematode assemblages in the southwestern GoM with two objectives: (1) to assess the effects of a depth gradient on the species richness, taxonomic composition, and feeding type composition; and (2) to document the regional diversity of free-living nematodes.
We studied the seabed in the southwestern GoM from the upper continental slope to the abyssal plain. The oceanographic conditions in the southwestern GoM are typical of an oligotrophic regime and are influenced by the loop current. The sediments in the southwestern part of GoM are a mixture of siliciclastic and carbonate sediments that varies from west to east (Brooks et al., 2020). According to Brooks et al. (2020), the sediments in the westernmost part have a higher fraction of terrigenous origin related with the Grijalva-Usumacinta River System. Meanwhile in the easternmost part, sediments are mostly of carbonate nature due to pelagic deposition and downslope transport from the extensive shelf of Campeche Bank. The southwestern GoM is characterized by the presence of numerous oil seeps distributed along the continental shelf and deep waters (Godoy-Lozano et al., 2018). Also, there are many oil fields in the continental shelf of the SW GoM that largely support Mexican oil production (Gracia, 2010).
We designed a grid of 63 sampling sites encompassing a depth range from 186 to 3749 m and covering most of the southwestern GoM (Figure 1 and Table 1). We made four oceanographic cruises (SOGOM 1 to 4) onboard of the R/V Justo Sierra of the Universidad Nacional Autónoma de México during successive years: 2015 (June, 3–27), 2016 (August, 31-September, 20), 2017 (April, 21-May, 15), and 2018 (August, 29-September, 20), so each site was sampled four times.
We measured three bottom water variables (average 217 m over the seabed) in situ with a CTD probe Seabird SBE 9 plus: temperature (°C), salinity (PSU), and dissolved oxygen (mg L-1). The water depth (m) was measured with the vessel’s echosounder.
We collected seabed sediments with a Reineck-type box corer of 0.16 m2 of area. We made a single deployment of the box corer per cruise and site. For the study of meiofauna, we retrieved two smaller cores of each box core inserting a plastic syringe (12 cm2 of area) 10 cm into the sediment. We washed the sediments with filtered seawater on sieves of 500 and 45 µm of mesh aperture. We stored the sediment retained in the 45 µm sieve in a jar, added 10% formalin for preservation, and added also 5–10 drops of Bengal Rose for staining the organisms.
We collected 1000 cm3 of sediment from the box corer for determinations of organic matter content and grain size of sediments. We preserved these samples frozen until analysis in the laboratory.
We measured the organic matter content in sediment by oxidation with K2Cr2O7 (Jackson, 1958). Namely, 1 g of dried sediment reacted with 10 mL of K2Cr2O7, 10 mL of H2SO4, 10 mL of H3PO4, and 100 mL of distilled H2O. One milliliter of diphenylamine was added, and then organic matter was estimated by titration with 0.5 N of FeSO4. Sediment granulometry was measured with a Beckman Coulter LS230 laser diffraction analyzer and the particle size distribution expressed as percentage of sand, silt, and clay.
We observed the sediment meiofauna samples under a stereomicroscope Carl Zeiss Stemi 508 (maximum magnification 50×). We picked up all the nematodes in the samples with a handling needle and stored them in vials with 70% ethanol. The nematode processing followed the protocol described in Vincx (1996) with some modifications. Briefly, we transferred the nematodes in the vials into a staining cavity block, added 10 mL of pure glycerol and left the cavity for 24 hours at 35°C in an oven. This allowed for a slow evaporation of the ethanol and penetration of the glycerol that make the nematodes transparent. After, we placed 5–8 nematodes in a glycerol drop rounded by a paraffin ring on a glass microscope slide. We put a cover slide on top and the whole mounting slowly heated over a heating plate until the melting of paraffin ring and the nematodes were sealed inside the glycerol.
We observed the montages under a Carl Zeiss Primo Star microscope (maximum magnification 1000×) and identified the nematodes at the lowest taxonomic level, usually species level. We named many species as “sp.” because they did not match with any described species, being likely new. We used “species affinis” (aff.) for those species that resembles to known species, but differ slightly in some morphological characters (e.g., number of precloacal supplements, body size range). We used the book by Schmidt-Rhaesa (2014) and taxonomic articles for the identification. For systematics and nomenclature, we combined the revision by Hodda (2022a) and the World Register of Marine Species (https://marinespecies.org). We also classified each species into a feeding type using the recent scheme proposed by Hodda (2022b): microbial feeder (five subgroups: sucker, processor, piercer, crusher, and scraper) and predator (two subgroups: chewer and piercer).
We used the whole dataset of abiotic variables (bottom water and sediments) to describe changes along the depth gradient. We averaged the values of the four cruises (years) over the sites to obtain robust spatial trends. We represented the trends of abiotic factors in scatterplots along the depth gradient and added the best fit curves using LOESS method.
For the analysis of diversity along the water depth gradient, first we used the Spearman rank coefficient of correlation (rS) to calculate the association between pair of variables, namely abundance, number of species, and depth. After, we applied rarefaction for a fair comparison between sites using the expected number of species for n number of individuals (ES(n)) with the software EstimateS 9.0 (Colwell, 2013). Rarefaction to a same value of abundance reduces the positive influence of abundance on species richness (Gotelli and Colwell, 2011). We analyzed the species composition using a non-metric multidimensional scaling (NMDS) numerical ordination with the Sorensen index as measure of resemblance. We selected this presence/absence index to reduce the influence of abundance on the ordination. We used the software PRIMER 7.0.21 for the multivariate analyses (Clarke et al., 2014).
For the analysis of regional diversity, we built a matrix of species × samples for the analysis of the richness. We calculated accumulation curves of species versus individuals to analyze the completeness of the sampling and the maximum values of regional richness. We used two metrics of richness with 0.95 confidence intervals (CI) generated by permutations: the observed richness (SR) and the non-parametric estimator Chao 1, this last provides a minimum bound of diversity that included the unseen species in the assemblage (Gotelli and Colwell, 2011).
Bottom water and sediment variables had clear variation along the depth gradient. Temperature dropped in the shallow sites (< 1000 m depth) from 18.3 to 7.8°C, and in the deeper sites (> 1000 m) was very stable around 4.3°C (Figure 2A). Salinity had a similar pattern, with > 36 PSU in the four shallowest sites, a sharp decline of salinity in intermediate depths (300–600 m), and a very stable value around 35 PSU in all sites deeper than 600 m (Figure 2B). Dissolved oxygen showed an opposite pattern, the shallow sites (< 1000 m) had the lowest values around 2.4 mg L-1, increased in intermediate depths (1000–1500 m) around 4 mg L-1, and thereafter was stable for most of the sites deeper than 1500 m (Figure 2C). The silt/clay ratio increased from 180 to 1500 m depth indicating some relative increase of silt over clay fraction; and deeper than 1500 m occurred a steady increase of the relative content of clay in the sediments (Figure 2D). The organic matter content decreased steadily along the depth gradient from the shallow to deep sites. The nine sites shallower than 1000 m had large variability of organic content ranging from 1.7% to 3.0%; meanwhile, sites deeper than 1000 m had a clear decreasing trend from about 2% to 1% (Figure 2E).
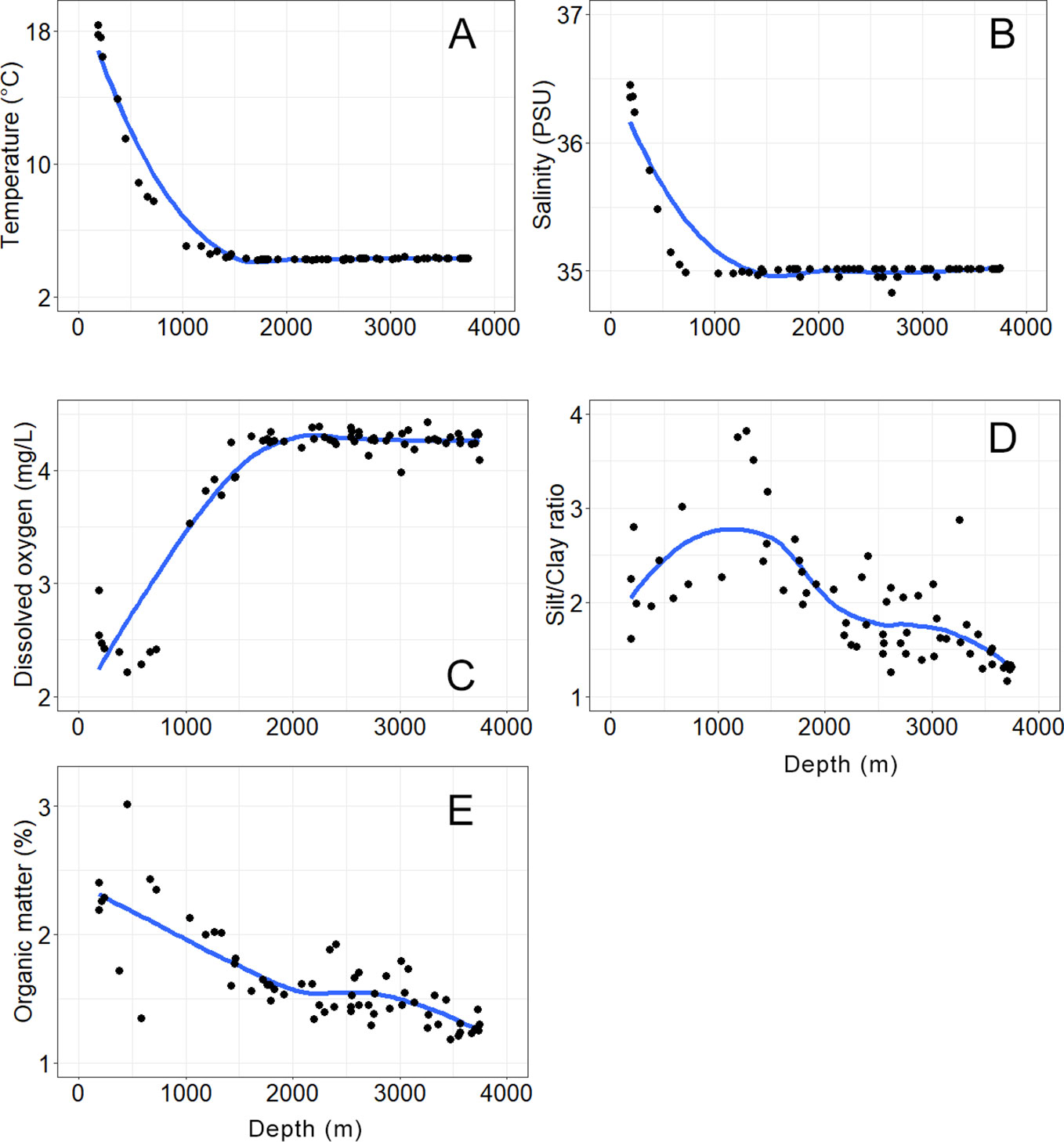
Figure 2 Abiotic variables in water column and sediments along a water depth gradient in 63 sites in the Southwestern Gulf of Mexico. (A) Temperature. (B) Salinity. (C) Dissolved oxygen. (D) Silt/clay ratio. (E) Organic matter content. Points represent averages over the four cruises. The best fit curves in blue were calculated by LOESS method.
As expected, water depth was negatively correlated with the nematode abundance (rS = -0.75, p < 0.001, n = 63); in turn, abundance was positively correlated with species richness (rS = 0.98, p < 0.001, n = 63). Therefore, we applied the technique of rarefaction for a value of 25 individuals. This number was a compromise between the number of sites to be removed (i.e., those with < 25 individuals) and the minimum number of individuals necessary for a comparison of richness along depth. We removed 44 sites with less than 25 individuals of abundance; so, we had a matrix of 143 species × 19 sites distributed along a depth gradient from 186 to 3049 m (average depth: 1118 m). The matrix of nematodes occurrence during the four cruises × 63 sites is given in the Supplementary Material S1.
The ES(25) did not change significantly along the depth gradient as indicated by a Spearman rank correlation (rs = 0.21, p = 0.39, n = 19). A scatterplot of ES(25) versus depth supported this lack of relationship, as indicated by the best-fit line almost horizontal (Figure 3A).
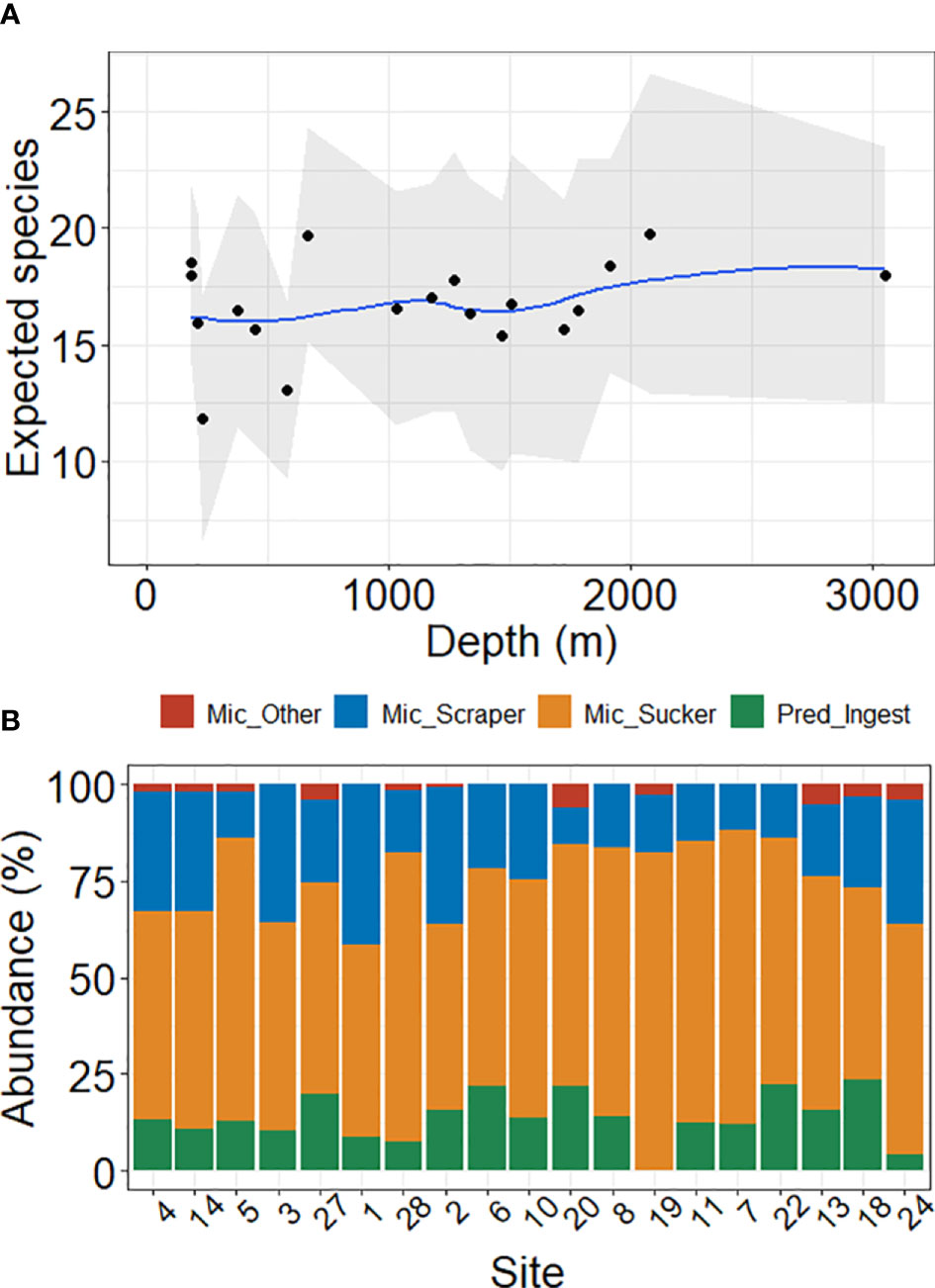
Figure 3 Species richness and feeding types of nematodes along a water depth gradient in 19 sites in the Southwestern Gulf of Mexico. (A) Expected number of nematode species [ES(25)]. Shaded area indicated 0.95 confidence intervals generated by permutations. The best fit curve in blue was calculated by LOESS method. (B) Relative abundance of the feeding types. Sites were ordered by depth (shallowest to the left). Mic, microbial feeders; Pred, predators; Ingest, ingester.
We calculated the relative abundance of the feeding types for each site. Crusher and Processor microbial feeders were less important (< 6% at any site), therefore we summed them and represented as “other” microbial feeder. The relative abundance of feeding types did not show any trend along the depth gradient (Figure 3B). Microbial sucker feeders were the most abundant nematodes (mean ± standard deviation: 62% ± 10%), followed by microbial scraper feeders (22% ± 9%) and predator ingester feeders (14% ± 6%).
The configuration of the sites in the NMDS plot suggested a gradual change of species composition related to water depth (Figure 4). Shallow sites (e.g., 1, 2, 4, 5, 14, 27) had more similar species composition than deeper sites (e.g., 13, 18, 20, 22, 24). Vectors of abiotic variables superimposed on the ordination, indicated the overarching effect of depth on the composition, but also on the other abiotic variables. Depth was positively correlated with dissolved oxygen and silt/clay ratio; meanwhile depth was negatively correlated with temperature, salinity, and organic matter content.
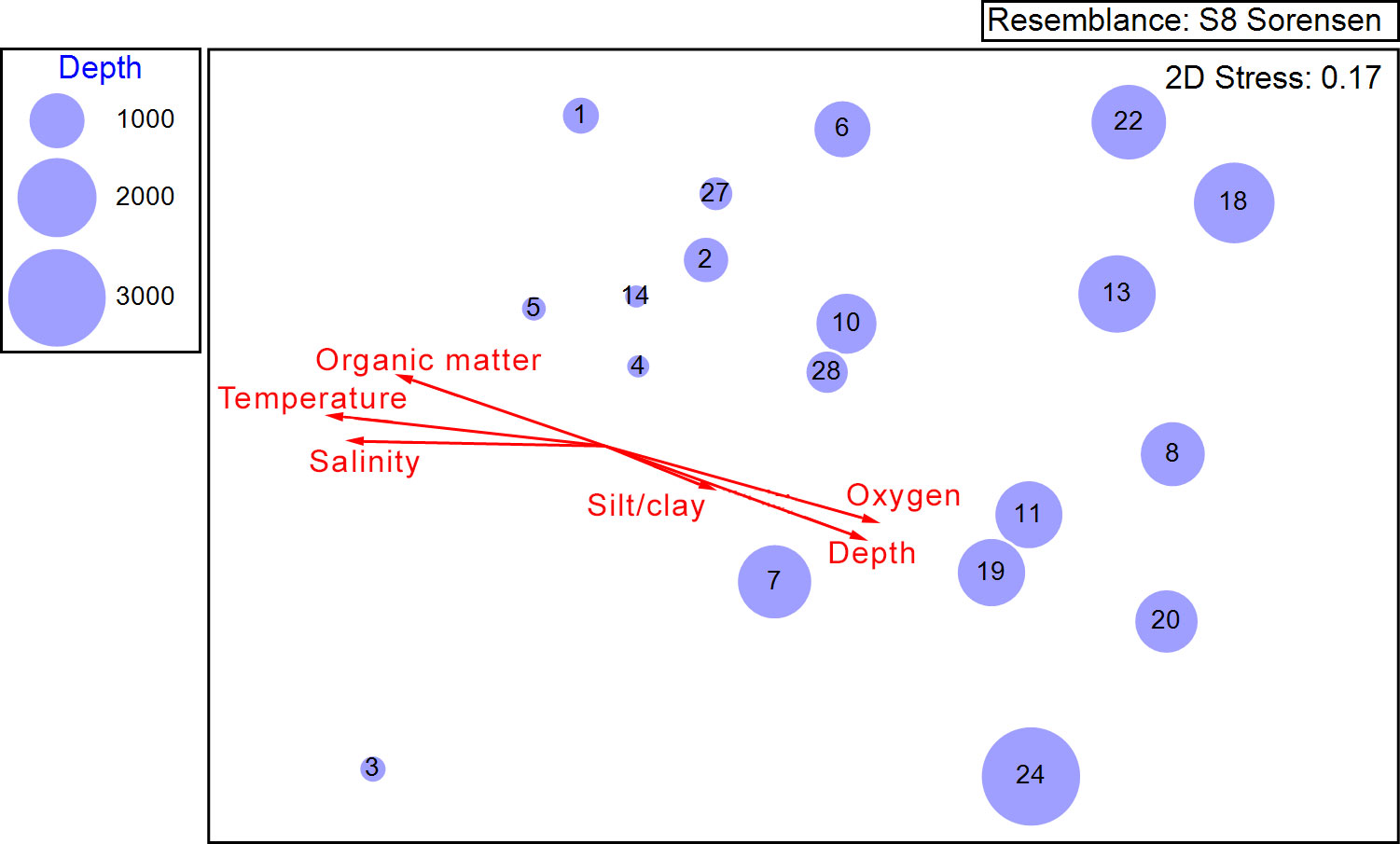
Figure 4 Nematode assemblage structure represented by a NMDS numerical ordination of 19 sites in the Southwestern Gulf of Mexico based on the presence/absence of species. Bubble size is proportional to the water depth at each site. Vectors represent the Pearson correlation between abiotic variables and the ordination axes.
We selected a subset of 17 species that contributed at least 8% to the total abundance in any site. Further, we represented the relative abundance of those species across the 19 sites ordered by depth in a heat map (Figure 5). There were species broadly distributed along the depth gradient such as Sabatieria aff. stekhoveni, S. aff. ancudiana, and S. conicauda. Other species were restricted to the shallower sites such as Cervonema sp. 2, Setosabatieria hilarula, Dorylaimopsis sp., and S. ornata. And another group of species was typical of deeper sites such as Acantholaimus spp., Ledovitia sp. 1, Viscosia aff. longissima, and Anguinoides stylosum.
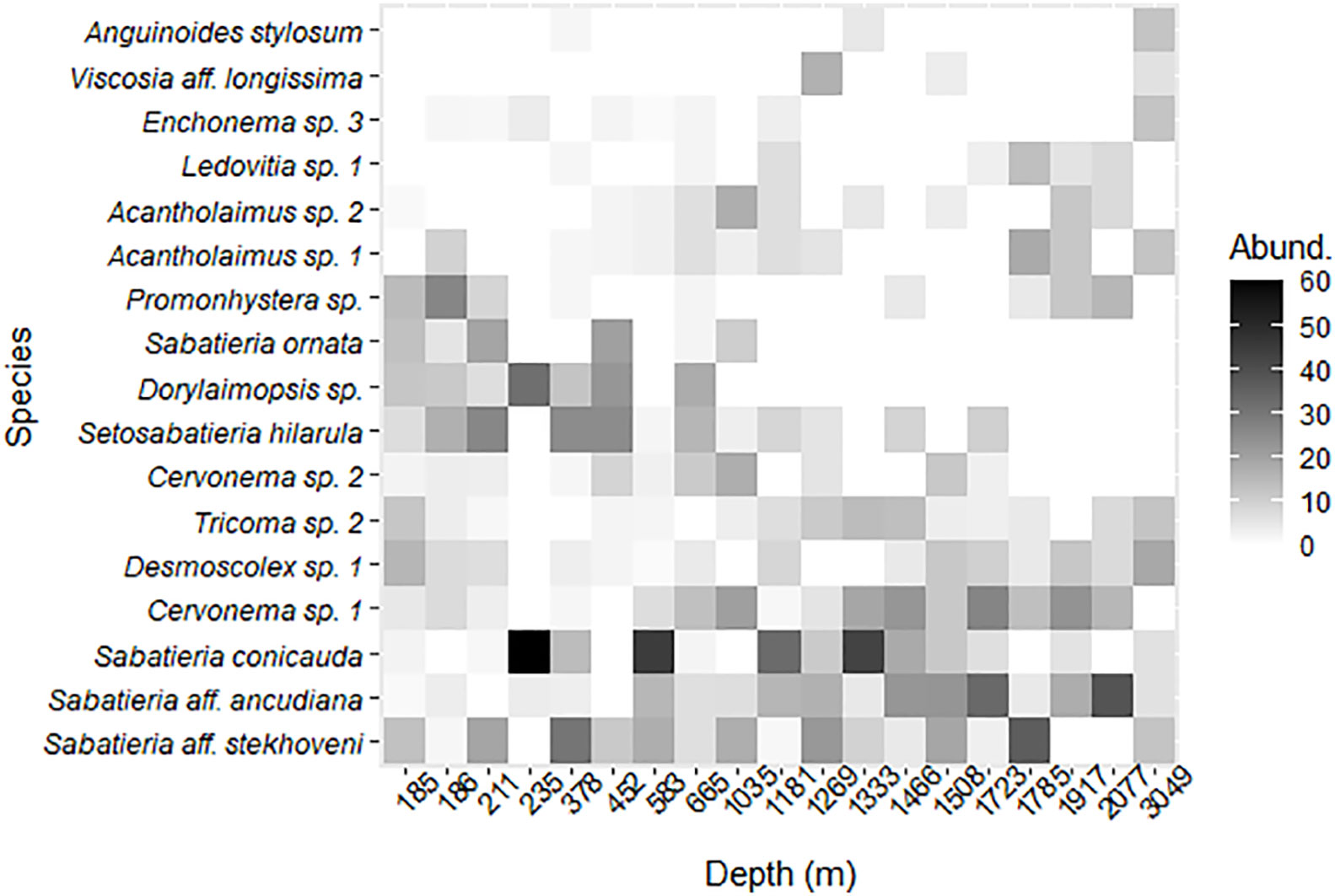
Figure 5 Relative abundance (%) heat map of nematode assemblage composition of the 17 most abundant species along a water depth gradient of 19 sites in the Southwestern Gulf of Mexico.
For the analysis of the regional diversity, we used the data from the 63 sampled sites. We identified 1881 nematodes belonging to 154 species, 108 genera, 33 families, ten orders, and two classes (Supplementary Material S2). The accumulation curves of observed richness and Chao 1 did not reach an asymptote (Figure 6). The observed richness had 0.95 CI between 142 and 166 species. Meanwhile, Chao 1 estimator suggested a mean value of richness of 194 species and a 0.95 CI between 171 and 246 species.
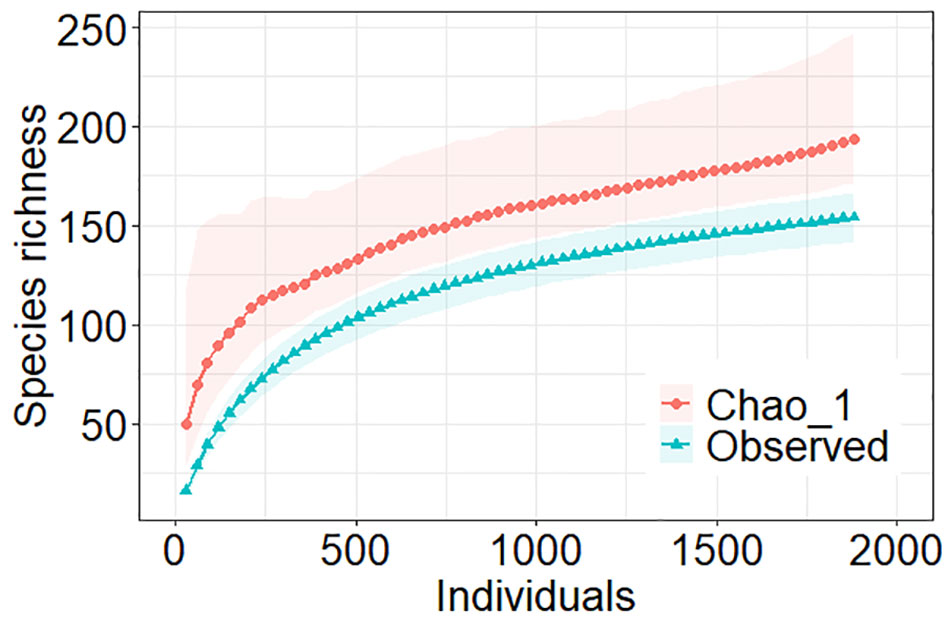
Figure 6 Accumulation curves of nematode species richness versus individuals of 63 sites in the Southwestern Gulf of Mexico. Two metrics of richness were given: Observed and non-parametric estimator Chao 1. Shaded areas indicated 0.95 confidence intervals generated by permutations.
The species composition indicated a moderate dominance with 15 species accounting for the 50% of the total abundance. The most abundant species (in parenthesis the % of abundance) were Sabatieria conicauda (6%), S. aff. stekhoveni (5%), Setosabatieria hilarula (4%), Desmoscolex sp. 1 (4%), S. aff. ancudiana (4%), Tricoma sp. 2 (4%), Cervonema sp. 1 (4%), and Acantholaimus sp. 1 (4%). The rareness of species was notable, as 27% (35 species) were represented by single individual and 9% (14 species) were represented by two individuals. These rare species were mostly restricted to the deepest sites such as Desmoscolex sp., Enchonema spp., Ledovitia spp., and Halalaimus spp.
The integration of our data provides a synoptic description of the abiotic environment and nematode assemblage structure in the southwestern Gulf of Mexico. For this region, this is the first time that deep-sea nematode assemblages are studied at species level. Unfortunately, our dataset underestimates the true nematode abundance due to methodological drawbacks during the processing of samples. Therefore, we are not able to make quantitative assessment of abundance, neither calculate abundance-based indexes such as trophic diversity or maturity index.
As expected, there were clear oceanographic gradients from the upper continental slope to the abyssal plane relative to bottom water (temperature, salinity, and dissolved oxygen) and sediment variables (granulometry and organic matter content). At sites shallower than 1000 m, temperature and salinity had larger variability according to the mix of water masses from North Atlantic and the Caribbean Sea (Cervantes-Díaz et al., 2022). At these sites, low oxygen in bottom waters (< 3 mg L-1) and high organic matter in sediments (> 2%) likely reflected a larger input of organic carbon derived of primary productivity in surface and influence of coastal river discharge. At sites deeper than 1000 m, temperature, salinity and dissolved oxygen were very stable and reflected the influence of the North Atlantic Deep Water mass (NADW) (Hamilton et al., 2018; Portela et al., 2018).
In general, the finest fraction of sediment (i.e., clay) became more important with increasing water depth, suggesting less hydrodynamic activity at higher depths. The detected variability in the silt/clay ratio in our study likely indicated the influence of topographic features such as canyons, knolls, and valleys which are commons in the seabed of southern GoM (Sahling et al., 2016). The steady decline of the sedimentary organic content along the depth gradient reflected the progressive remineralization of the organic particles sinking in the water column causing less carbon to reach the seabed (Stukel et al., 2022).
Nematode abundance in our study decreased significantly with water depth likely indicating a gradient in food availability, which is a common process in deep-sea sediments (e.g., Danovaro et al., 2008; Trebukhova et al., 2013; Gambi et al., 2014; dos Santos et al., 2020). Food availability in sediment has been related to water depth and nematode body size as well (Udalov et al., 2005). As abundance is closely related with species richness (Gotelli and Colwell, 2011), it is not surprising that species richness in our study decreased with increasing water depth. However, when rarefied to a same level of abundance, the richness did not change substantially along a water depth gradient (185–3049 m). The relationship between water depth and standardized richness of nematodes (for instance, expected species, ES) is not universal. According to some studies, ES increased with depth (dos Santos et al., 2020) and after other studies, ES decreased with depth (Danovaro et al., 2008; Trebukhova et al., 2013). Some other studies show that ES does not change with increasing depth (our study, Lambshead et al., 2000). The regional features of the seabed (e.g., canyons, oil seeps, surface primary productivity) seem decisive to yield a particular pattern.
In the Southwestern Gulf of Mexico, we hypothesize that a balance between food availability and environmental disturbance may explain the similar species richness along depth gradient. The Dynamic Equilibrium Model (DEM, Huston, 1994) proposes that resources availability and physical disturbance acting on the regional species pool determine the species richness. Moens et al. (2013) proposed this model as an explanation specifically for nematode diversity patterns. Also, DEM successfully explained the nematode richness patterns across nine habitat types that included deep-sea sediments (Armenteros et al., 2019). In the current study, the sites in the upper slope had higher food availability but also oxygen stress for nematodes. Sites from the lower slope to the abyssal plain showed lower food availability related to depth, but this could be counteracted by higher oxygen content and physical stability (indicated by higher content of clay in sediments). This hypothesis should be further tested with a larger sample size (i.e., more individuals) along a depth gradient that includes more sites deeper than 2000 m.
The dominance of microbial feeders (84% total abundance) and less contribution of predators (14%) broadly agrees with other studies (e.g., Ingels et al., 2010). In particular, microbial sucker feeders were the most abundant group indicating the important role of sediment bacteria in the nematodes’ diet (Mordukhovich et al., 2018). Microbial feeder nematodes were mostly represented by comesomatids belonging to six species: Sabatieria conicauda, S. aff. stekhoveni, Setosabatieria hilarula, S. aff. ancudiana, Cervonema sp. 1, and Dorylaimopsis sp.
There was a change in the nematode species composition along depth gradient. Sites in the upper slope were dominated by genera of the family Comesomatidae (Sabatieria, Dorylaimopsis, and Setosabatieria); which are recognized as tolerant to reduced conditions in sediments (Muthumbi et al., 2004; Soto et al., 2017). We hypothesize that the broad distribution of other Sabatieria spp. (S. conicauda, S. aff. ancudiana, and S. aff. stekhoveni) in the Southern GoM can be related to reduced sediments associated to chemosynthetic communities. Such communities are fueled by natural hydrocarbons seeps which are a common feature of the Southern GoM (Sahling et al., 2016, and references therein). However, further research of nematode assemblage composition in well-identified hydrocarbon seeps is needed for testing this hypothesis.
In the abyssal sites, occurred several genera typical of deep waters such as Acantholaimus, Desmoscolex, Ledovitia, and Halalaimus (Tietjen, 1989; Netto et al., 2005; Macheriotou et al., 2021). These genera were represented by very few individuals (typically one or two), but in a first taxonomic approach seems to contain different congeneric species. Unfortunately, the number of nematodes retrieved per site was low and we could not make a local-scale analysis of α- and β-diversities.
The γ-diversity of free-living nematodes was underestimated as indicated by the non-asymptotic shape of the accumulation curves. The Chao 1 estimator of richness indicated 194 species, which is the lower bound of diversity that could be expected considering the number of unseen species (Gotelli and Colwell, 2011). Table 2 shows a comparison of estimates of α- and γ-diversities, at genus level, among several areas of the GoM. The compilation suggest that α-diversity moves around 20 genera, but can be as high as 71 genera. Meanwhile γ-diversity is about 100 genera, ranging between 70 and 128 genera depending of the number of samples and area covered by the study. It is necessary to identify more specimens in order to improve the estimates of regional diversity for the southern Gulf of Mexico.
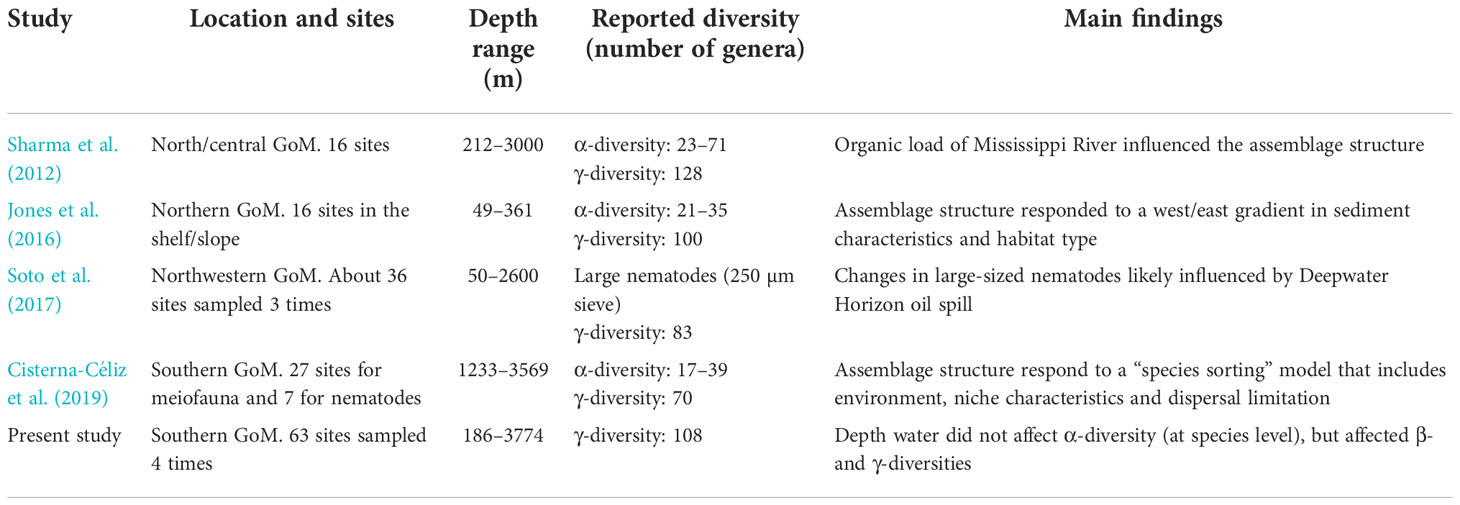
Table 2 Studies (in chronological order) of free-living nematode diversity in continental and deep waters of the Gulf of Mexico (GoM).
On the other hand, most of the species we found in our study did not match with described species and in consequence were named as “sp.”. We think that they are potentially new species, and they should be formally described. For many species identified in our study, organisms had slight morphological differences (e.g., body size, number of precloacal supplements) when compared with type specimens. Further taxonomic analyses using molecular markers and/or detailed morphometry are needed to unravel if the causes of these subtle differences between organisms are due to the environment (e.g., food availability) or to lineage sorting.
Hope (2009) compiled 112 genera and 190 species of free-living nematodes in the Gulf of Mexico. Here we report 70 genera and 144 species not previously registered by Hope’s compilation which means an increase of 76% of the number of species for the GoM. Only six species were shared by Hope’s and our list. These results suggest that the deep ocean could be a huge reservoir of endemic nematode species (Danovaro and Gambi, 2022).
Our study makes a significant contribution to the knowledge of the free-living nematode diversity in the deep-sea waters of the Gulf of Mexico. In particular, the substantial diversity of free-living nematodes suggests the necessity of further studies to unravel the environmental drivers of α- and β-diversities and highlights the potential of this taxon for monitoring the deep sea of the Gulf of Mexico.
This is the first study on nematode communities developed in the Southwestern GoM. We identified 108 genera and 154 species of free-living nematodes, which increases in a 76% the number of species reported for the Gulf of Mexico. Nonetheless, the non-asymptotic accumulation richness curves suggest that there could be a substantial number of species to be discovered. We found that the diversity of nematode assemblages was notably influenced by a depth gradient causing in turn changes in water (temperature, salinity, and dissolved oxygen) and sediment variables (grain size and organic content). The abundance and species richness decreased with water depth. Also, we identified that microbial sucker was the most abundant feeding type which indicates the important role of sediment bacteria in the nematodes’ diet.
The raw data supporting the conclusions of this article are given in the Supplementary Material.
AG and MA conceived and designed the study. AG coordinated the oceanographic cruises, the quantification of abiotic variables and acquired the funding. OQ-R collected the samples in the field. OQ-R and MA processed the samples. MA identified the nematodes and made the analyses of data. All authors contributed to the article and approved the submitted version.
This study was funded by the Mexican National Council for Science and Technology - Mexican Ministry of Energy - Hydrocarbon Fund, project 201441 as part of the Gulf of Mexico Research Consortium (CIGoM) due to PEMEX’s specific request to the Hydrocarbon Fund to address the environmental effects of oil spills in the Gulf of Mexico.
We acknowledge the officers and crew of R/V Justo Sierra for their support during the oceanographic cruises. We thank to H. M. Alexander, L. P. Ortega and B. Suárez for laboratory analyses. We also thank to A. J. Mercado and F. F. Velasco for sorting sediment samples. The collaboration of students and participants in the field job is also greatly appreciated. We are also grateful to the Posgrado en Ciencias Biológicas, UNAM for all the support granted to OQ-R. The comments of three reviewers that improved the manuscript are greatly appreciated.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1023996/full#supplementary-material
Appeltans W., Ahyong S. T., Anderson G., Angel M. V., Artois T., Bailly N., et al. (2012). The magnitude of global marine species diversity. Curr. Biol. 22, 1–14. doi: 10.1016/j.cub.2012.09.036
Armenteros M., Pérez-García J. A., Marzo-Pérez D., Rodríguez-García P. (2019). The influential role of the habitat on the diversity patterns of free-living aquatic nematode assemblages in the Cuban archipelago. Diversity 11, 166. doi: 10.3390/d11090166
Brooks G. R., Larson R. A., Schwing P. T., Diercks A. R., Armenteros M., Diaz-Asencio M., et al. (2020). “Gulf of Mexico (GoM) bottom sediments and depositional processes: A baseline for future oil spills, in scenarios and responses to future deep oil spills,” in Fighting the next war. Eds. Murawski S. A., Ainsworth C. H., Gilbert S., Hollander D. J., Paris C. B., Schluter M., et al (Switzerland: Springer), 75–95.
Cervantes-Díaz G. Y., Hernández-Ayón J. M., Zirino A., Herzka S. Z., Camacho-Ibar V., Norzagaray O., et al. (2022). Understanding upper water mass dynamics in the gulf of Mexico by linking physical and biogeochemical features. J. Mar. Syst. 225, 103647. doi: 10.1016/j.jmarsys.2021.103647
Cisterna-Céliz J. A., Marcelino-Barrios M., Herguera J. C., Rocha-Olivares A. (2019). Metacommunity analysis of meiobenthos of deep-sea sediments from the gulf of Mexico. Mar. Biodivers. 49, 1217–1231. doi: 10.1007/s12526-018-0899-0
Clarke K. R., Gorley R. N., Somerfield P. J., Warwick R. M. (2014). Change in marine communities: an approach to statistical analysis and interpretation. 3rd ed (Plymouth: Plymouth: PRIMER-E).
Colwell R. K. (2013) EstimateS: Statistical estimation of species richness and shared species from samples. Available at: http://purl.oclc.org/estimates.
Danovaro R., Carugati L., Corinaldesi C., Gambi C., Guilini K., Pusceddu A., et al. (2013). Multiple spatial scale analyses provide new clues on patterns and drivers of deep-sea nematode diversity. Deep. Res. II 92, 97–106. doi: 10.1016/j.dsr2.2013.03.035
Danovaro R., Gambi C. (2022). Cosmopolitism, rareness and endemism in deep-sea marine nematodes. Eur. Zool. J. 89, 653–665. doi: 10.1080/24750263.2022.2040621
Danovaro R., Gambi C., Lampadariou N., Tselepides A. (2008). Deep-sea nematode biodiversity in the Mediterranean basin: testing for longitudinal, bathymetric and energetic gradients. Ecography 31, 231–244. doi: 10.1111/j.2007.0906-7590.05484.x
dos Santos G. A. P., Silva A. C., Esteves A. M., Ribeiro-Ferreira V. P., Neres P. F., Valdes Y., et al. (2020). Testing bathymetric and regional patterns in the southwest Atlantic deep sea using infaunal diversity, structure, and function. Diversity 12, 485. doi: 10.3390/d12120485
Gambi C., Pusceddu A., Benedetti-Cecchi L., Danovaro R. (2014). Species richness, species turnover and functional diversity in nematodes of the deep Mediterranean Sea: searching for drivers at different spatial scales. Glob. Ecol. Biogeogr. 23, 24–39. doi: 10.1111/geb.12094
Godoy-Lozano E. E., Escobar-Zepeda A., Raggi L., Merino E., Gutiérrez-Ríos R. M., Juárez K., et al. (2018). Bacterial diversity and the geochemical landscape in the southwestern gulf of Mexico. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.02528
Gotelli N. J., Colwell R. K. (2011). “Estimating species richness,” in Biological diversity. frontiers in measurement and assessment. Eds. Magurran A. E., McGill B. J. (Oxford, UK: University Press), 39–54.
Gracia A. (2010). “Campaña oceanográfica (SGM 2010). informe final,” in Gerencia de seguridad industrial, protección ambiental y calidad, región Marina noreste, PEMEX-exploración y, vol. 1173. (Producción, México: Instituto de Ciencias del Mar y Limnología, UNAM).
Hamilton P., Leben R., Bower A., Furey H., Pérez-Brunius P. (2018). Hydrography of the gulf of Mexico using autonomous floats. J. Phys. Oceanogr. 48, 773–794. doi: 10.1175/JPO-D-17-0205.1
Hodda M. (2022a). Phylum Nematoda: a classification, catalogue and index of valid genera, with a census of valid species. Zootaxa 5114, 1–289. doi: 10.11646/zootaxa.5114.1.1
Hodda M. (2022b). Phylum Nematoda: feeding habits for all valid genera using a new, universal scheme encompassing the entire phylum, with descriptions of morphological characteristics of the stoma, a key, and discussion of the evidence for trophic relationships. Zootaxa 5114, 318–451. doi: 10.11646/zootaxa.5114.1.3
Hope D. W. (2009). “Free-living marine Nematoda of the gulf of Mexico,” in Gulf of Mexico–origins, waters, and biota. biodiversity. Eds. Felder D., Camp D. (Texas: Texas A&M Press), 1111–1123.
Huston M. (1994). Biological diversity: The coexistence of species on changing landscapes (Cambridge: Cambridge University Press).
Ingels J., Billett D. S. M., Van Gaever S., Vanreusel A. (2010). An insight into the feeding ecology of deep-sea canyon nematodes — results from field observations and the first in-situ 13C feeding experiment in the nazaré canyon. J. Exp. Mar. Bio. Ecol. 396, 185–193. doi: 10.1016/j.jembe.2010.10.018
Jones C. M., Sharma J., Miller J. M., Steward P. M., Landers S. C. (2016). Nematode assemblages of the northern gulf of Mexico continental shelf. Proc. Biol. Soc Wash. 129, 24–37. doi: 10.2988/0006-324X-129.Q1.24
Lambshead P. J. D., Boucher G. (2004). Marine nematode deep-sea biodiversity – hyperdiverse or hype? J. Biogeogr. 30, 475–485. doi: 10.1046/j.1365-2699.2003.00843.x
Lambshead P. J. D., Tietjen J. H., Ferrero T. J., Jensen P. (2000). Latitudinal diversity gradients in the deep sea with special reference to north Atlantic nematodes. Mar. Ecol. Prog. Ser. 194, 159–167. doi: 10.3354/meps194159
Lubchenco J., McNutt M. K., Dreyfus G., Murawski S. A., Kennedy D. M., Anastas P. T., et al. (2012). Science in support of the deepwater horizon response. Proc. Nat. Acad. Sci. U.S.A. 109, 20212–20221. doi: 10.1073/pnas.1204729109
Macheriotou L., Rigaux A., Olu K., Zeppilli D., Derycke S., Vanreusel A. (2021). Deep-sea nematodes of the Mozambique channel: Evidence of random community assembly dynamics in seep sediments. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.549834
Miljutin D. M., Gad G., Miljutina M. M., Mokievsky V. O., Fonseca-Genevois V. G., Esteves A. M. (2010). The state of knowledge on deep-sea nematode taxonomy: how many valid species are known down there? Mar. Biodivers. 40, 143–159. doi: 10.1007/s12526-010-0041-4
Moens T., Braeckman U., Derycke S., Fonseca G., Gallucci F., Gingold R., et al. (2013). “Ecology of free-living marine nematodes,” in Hand. zool, vol. 2 . Ed. Schmidt-Rhaesa (Berlin: De Gruyter) 109–152.
Mordukhovich V. V., Kiyashko S. I., Kharlamenko V. I., Fadeeva N. P. (2018). Determination of food sources for nematodes in the kuril basin and eastern slope of the kuril islands by stable isotope and fatty acid analyses. Deep. Res. Part II 154, 365–373. doi: 10.1016/j.dsr2.2018.01.003
Muthumbi A., Vanreusel A., Duineveld G., Soetaert K., Vincx M. (2004). Nematode community structure along the continental slope off the Kenyan coast, Western Indian ocean. Int. Rev. der gesamten Hydrobiol. und Hydrogr. 89, 188–205. doi: 10.1002/iroh.200310689
Netto S. A., Gallucci F., Fonseca G. (2005). Meiofauna communities of continental slope and deep-sea sites off SE Brazil. Deep. Res. I 52, 845–859. doi: 10.1016/j.dsr.2004.11.009
Portela E., Tenreiro M., Pallàs-Sanz E., Meunier T., Ruiz-Angulo A., Sosa-Gutiérrez R., et al. (2018). Hydrography of the central and Western gulf of Mexico. J. Geophys. Res. Ocean. 123, 5134–5149. doi: 10.1029/2018JC013813
Pusceddu A., Gambi C., Zeppilli D., Bianchelli S., Danovaro R. (2009). Organic matter composition, metazoan meiofauna and nematode biodiversity in Mediterranean deep-sea sediments. Deep. Res. II 56, 755–762. doi: 10.1016/j.dsr2.2008.10.012
Rosli N., Leduc D., Rowden A. A., Probert P. K. (2018a). Review of recent trends in ecological studies of deep-sea meiofauna, with focus on patterns and processes at small to regional spatial scales. Mar. Biodivers. 48, 13–34. doi: 10.1007/s12526-017-0801-5
Rosli N., Leduc D., Rowden A. A., Probert P. K., Clark M. R. (2018b). Regional and sediment depth differences in nematode community structure greater than between habitats on the new Zealand margin: Implications for vulnerability to anthropogenic disturbance. Prog. Oceanogr. 160, 26–52. doi: 10.1016/j.pocean.2017.11.006
Sahling H., Borowski C., Escobar-Briones E., Gaytán-Caballero A., Hsu C. W., Loher M., et al. (2016). Massive asphalt deposits, oil seepage, and gas venting support abundant chemosynthetic communities at the campeche knolls, southern gulf of Mexico. Biogeosciences 13, 4491–4512. doi: 10.5194/bg-13-4491-2016
Schmidt-Rhaesa A. (2014). “Nematoda. handbook of zoology,” in Gastrotricha, cycloneuralia and gnathifera. Ed. Schmidt-Rhaesa A. (Berlin: De Gruyter), 777. doi: 10.1515/9783110274257
Sharma J., Baguley J., Bluhm B. A., Rowe G. T. (2011). Do meio- and macrobenthic nematodes differ in community composition and body weight trends with depth? PLoS One 6, e14491. doi: 10.1371/journal.pone.0014491
Sharma J., Baguley J. G., Montagna P., Rowe G. T. (2012). Assessment of longitudinal gradients in nematode communities in the deep northern gulf of Mexico and concordance with benthic taxa. Int. J. Oceanogr. 2012, 903018. doi: 10.1155/2012/903018
Soto L. A., Botello A. V., Licea-Durán S., Lizárraga-Partida M. L., Yáñez-Arancibia A. (2014). The environmental legacy of the ixtoc-I oil spill in campeche sound, southwestern gulf of Mexico. Front. Mar. Sci. 1. doi: 10.3389/fmars.2014.00057
Soto L. A., Salcedo D. L., Arvizu K., Botello A. V. (2017). Interannual patterns of the large free-living nematode assemblages in the Mexican exclusive economic zone, NW gulf of Mexico after the deepwater horizon oil spill. Ecol. Ind. 79, 371–381. doi: 10.1016/j.ecolind.2017.03.058
Stukel M. R., Kelly T. B., Landry M. R., Selph K. E. (2022). Sinking carbon, nitrogen, and pigment flux within and beneath the euphotic zone in the oligotrophic, open-ocean gulf of Mexico. J. Plankton Res. 44, 711–727. doi: 10.1093/plankt/fbab001
Tietjen J. H. (1989). Ecology of deep-sea nematodes from the Puerto Rico trench area and hatteras abyssal plain. Deep. Res. 36, 1579–1594. doi: 10.1016/0198-0149(89)90059-9
Trebukhova J. A., Miljutin D. M., Pavlyuk O. N., Mar’yash A. A., Brenke N. (2013). Changes in deep-sea metazoan meiobenthic communities and nematode assemblages along a depth gradient (North-western Sea of Japan, pacific). Deep. Res. II 86–87, 56–65. doi: 10.1016/j.dsr2.2012.08.015
Udalov A. A., Azovsky A. I., Mokievsky V. O. (2005). Depth-related pattern in nematode size: What does the depth itself really mean? Prog. Oceanogr. 67, 1–23. doi: 10.1016/j.pocean.2005.02.020
Vincx M. (1996). “Meiofauna in marine and freshwater sediments,” in Methods for the examination of organismal diversity in soils and sediments. Ed. Hall G. S. (Wallinford: CAB International), 187–195.
Zeppilli D., Leduc D., Fontanier C., Fontaneto D., Fuchs S., Gooday A. J., et al. (2018). Characteristics of meiofauna in extreme marine ecosystems: a review. Mar. Biodivers. 48, 35–71. doi: 10.1007/s12526-017-0815-z
Keywords: nematodes, deep sea, richness, γ-diversity, Gulf of Mexico
Citation: Armenteros M, Quintanar-Retama O and Gracia A (2022) Depth-related patterns and regional diversity of free-living nematodes in the deep-sea Southwestern Gulf of Mexico. Front. Mar. Sci. 9:1023996. doi: 10.3389/fmars.2022.1023996
Received: 20 August 2022; Accepted: 08 November 2022;
Published: 22 November 2022.
Edited by:
M. Leopoldina Aguirre-Macedo, Center for Research and Advanced Studies - Mérida Unit, MexicoReviewed by:
Thadickal V. Joydas, King Fahd University of Petroleum and Minerals, Saudi ArabiaCopyright © 2022 Armenteros, Quintanar-Retama and Gracia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maickel Armenteros, maickel.armenteros@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.