- 1Department of Immunology, Institute for Cellular and Molecular Medicine, SAMRC Extramural Unit for Stem Cell Research and Therapy, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa
- 2Department of Oral and Maxillofacial Pathology, School of Dentistry, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa
- 3CNC—Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal
- 4CIBB- Center for Innovative Biomedicine and Biotechnology, University of Coimbra, Coimbra, Portugal
- 5III—Institute for Interdisciplinary Research, University of Coimbra, Coimbra, Portugal
- 6GeneT, Center for Excellence in Gene Therapy in Portugal, University of Coimbra, Coimbra, Portugal
Editorial on the Research Topic
Mesenchymal and induced-pluripotent stem cells as models to study biological processes
Mesenchymal stromal/stem cells (MSCs) are isolated directly from various body tissues and are known for their capacity to self-renew, multipotent lineage differentiation and the secretion of bioactive factors, making them suitable for potential use in tissue repairs, regenerative medicine and as cellular therapy (Oliveira Miranda et al., 2018; Barros et al., 2020; Jovic et al., 2022; Ghasemi et al., 2023; Li et al., 2024; Sitbon et al., 2024). Additionally, MSCs can be used as cellular models for understanding biological processes (Visweswaran et al., 2015; Ambele et al., 2016; Jiang et al., 2019; Liang et al., 2022). On the other hand, induced pluripotent stem cells (iPSCs), are adult somatic cells that have been genetically reprogrammed by inducing pluripotency similar to embryonic stem cells. iPSCs posses unlimited self–renewal capacity and can be induced to differentiate into all adult cell types, thereby making them a unique model for studying a variety of biological processes (Yin et al., 2024). Together, current research trend puts MSCs and iPSCs as promising tools at the forefront of groundbreaking research that seeks to explore cellular therapy as alternative treatment for various conditions or for use in mimicking the in vivo physiological environment to provide better understanding of the pathophysiological processes of disease.
Research over the years have extensively explored the potential of MSCs as cellular models to study biological processes such as adipogenesis (Ambele et al., 2016), osteogenesis (Jiang et al., 2019) and chondrogenesis (Liang et al., 2022), as well as cellular therapy in treating different conditions globally (Jovic et al., 2022) by paracrine mechanism. This Research Topic brings together current works that uses MSCs and iPSCs to provide new insights on 1) the development of biological systems, and 2) the understanding of molecular and cellular processes as illustrated in Figure 1.
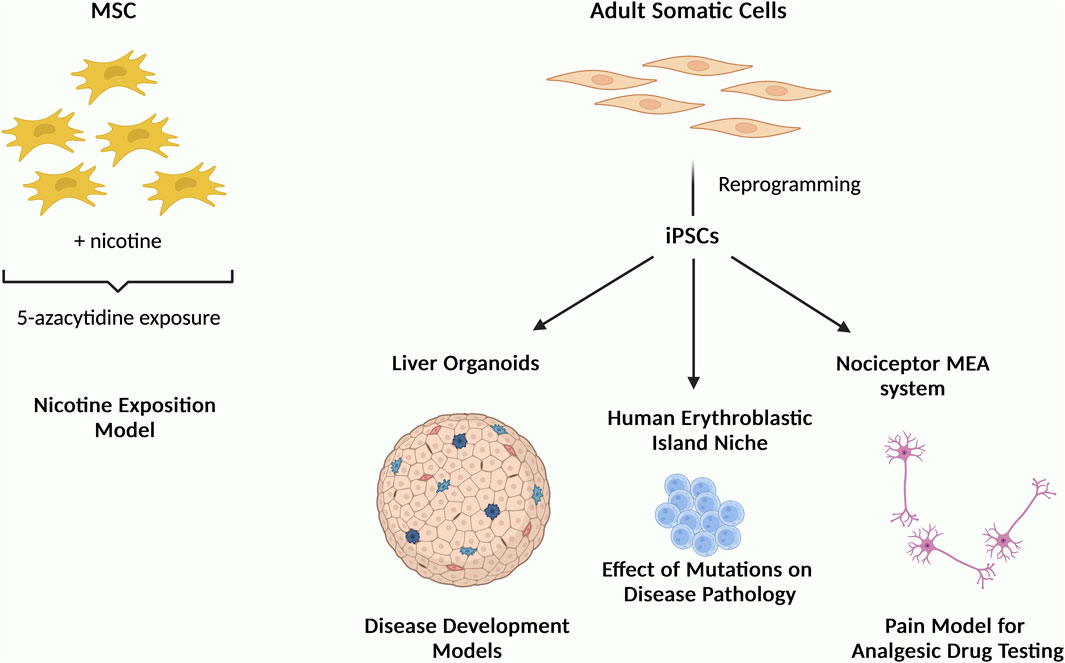
Figure 1. Schematic illustration summarizing the studies of this Research Topic “Mesenchymal and Induced-Pluripotent Stem Cells as Models to Study Biological Processes” showing the use of MSCs and iPSCs as cellular models to understand disease mechanisms and for drug screening.
In one of these studies, MSCs were exposed to nicotine and then treated with 5-azacytidine to mimic the risk of smoking on cells expressing the cardiac markers GATA4 and troponin (Figure 1). The authors reported nicotine reduced cells viability and the expression of these markers (Gheisari et al.). As has been previously reported in many other studies, this study highlights the potential use of MSC in understanding molecular process.
Regarding iPSC, a study showed that iPSCs-derived nociceptor culture system integrated with microelectrode arrays could be used for nociceptive analgesic drug testing. This is particularly important given the global health crisis in opioid abuse and addiction for chronic pain management and that this kind of studies are undesirable to be performed in animals and/or humans. This system offers alternative strategy to search for new effective analgesics and opioid substitutes (Nimbalkar et al.). Generally, one of the advantages of using iPSCs is the ability to produce cellular models that resemble human disorders as well as to generate organoids that allow cells to assembled in a 3D configuration resembling the tissue structure in vivo. For example, iPSC-derived liver organoids can serve as useful tool to study organogenesis, pathological modeling through cell-cell interactions, disease modeling and drug screening (Ouchi and Koike). One study used iPSCs to recapitulate the human erythroblastic island (EBI) niche in congenital dyserythropoietic anaemia (CDA) type IV to study the effect of KLF1 mutation Glu325Lys (E325K) on the disease pathology. The authors reported this mutation to negatively affect the production of erythroid cells. Also, upon expression of this mutated gene KLF1-E325K, a slight reduction of both RBC enucleation and macrophage maturation were observed. Furthermore, this provides an effective strategy to study the effects of other KLF1 mutations on EBI niche (May et al.).
Notwithstanding, the potential of iPSCs is enormous extending beyond disease modeling and drug screening to encompass cellular therapy and biomarkers discovery, although there is limited information regarding their safety for use as cellular therapies (Yin et al., 2024).
In summary, this Research Topic highlights the use of both MSCs and iPSCs as cellular models, providing new insights into different fields of research and evidence of their potential to study molecular and cellular processes.
Author contributions
MA: Conceptualization, Data curation, Funding acquisition, Writing–original draft, Writing–review and editing. CM: Conceptualization, Data curation, Funding acquisition, Writing–original draft, Writing–review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. MAA is funded by the NHLS Research Trust Pathology Research Award (GRANT004_94920), National Research Foundation grant no. 114044 and South African Medical Research Council (Grant No. A1A982). COM is funded by the ERDF through the Regional Operational Program Center 2020, Competitiveness Factors Operational Program (COMPETE 2020), and National Funds through FCT (Foundation for Science and Technology)—BrainHealth2020 projects (CENTRO-01-0145-FEDER-000008), UID/NEU/04539/2019, UIDB/04539/2020, UIDP/04539/2020, LA/P/0058/2020, 2022.06127.PTDC, ViraVector (CENTRO-01-0145- FEDER-022095), CortaCAGs (PTDC/NEU-NMC/0084/2014|POCI-01-0145-FEDER-016719), SpreadSilencing POCI-01-0145-FEDER-029716, Imagen POCI-01-0145-FEDER-016807, Interdisciplinary Scientific Research Seed Projects (University of Coimbra), 2022.06127.PTDC, CancelStem POCI-01-0145-FEDER-016390, POCI-01-0145-FEDER- 032309, ARDAT under the IMI2 JU Grant agreement No 945473 supported by EU and EFPIA; GeneT—Teaming Project 101059981 supported by the European Union’s Horizon Europe program; as well as SynSpread, ESMI and ModelPolyQ under the EU Joint Program—Neurodegenerative Disease Research (JPND), the last two co-funded by the European Union H2020 program, GA No.643417; by National Ataxia Foundation (USA), the American Portuguese Biomedical Research Fund (APBRF) and the Richard Chin and Lily Lock Machado-Joseph Disease Research Fund.
Acknowledgments
The authors would like to thank the contributing authors and reviewers to this Research Topic.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ambele, M. A., Dessels, C., Durandt, C., and Pepper, M. S. (2016). Genome-wide analysis of gene expression during adipogenesis in human adipose-derived stromal cells reveals novel patterns of gene expression during adipocyte differentiation. Stem Cell Res. 16, 725–734. doi:10.1016/j.scr.2016.04.011
Barros, I., Marcelo, A., Silva, T. P., Barata, J., Rufino-Ramos, D., Pereira de Almeida, L., et al. (2020). Mesenchymal stromal cells' therapy for polyglutamine disorders: where do we stand and where should we go? Front. Cell Neurosci. 14, 584277. doi:10.3389/fncel.2020.584277
Ghasemi, M., Roshandel, E., Mohammadian, M., Farhadihosseinabadi, B., Akbarzadehlaleh, P., and Shamsasenjan, K. (2023). Mesenchymal stromal cell-derived secretome-based therapy for neurodegenerative diseases: overview of clinical trials. Stem Cell Res. Ther. 14, 122. doi:10.1186/s13287-023-03264-0
Jiang, H., Hong, T., Wang, T., Wang, X., Cao, L., Xu, X., et al. (2019). Gene expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation. J. Cell Physiol. 234, 7070–7077. doi:10.1002/jcp.27461
Jovic, D., Yu, Y., Wang, D., Wang, K., Li, H., Xu, F., et al. (2022). A brief overview of global trends in MSC-based cell therapy. Stem Cell Rev. Rep. 18, 1525–1545. doi:10.1007/s12015-022-10369-1
Li, Y., Yue, G., Yu, S., Cheng, X., Cao, Y., and Wang, X. (2024). Evaluating the efficacy of mesenchymal stem cells for diabetic neuropathy: a systematic review and meta-analysis of preclinical studies. Front. Bioeng. Biotechnol. 12, 1349050. doi:10.3389/fbioe.2024.1349050
Liang, T., Li, P., Liang, A., Zhu, Y., Qiu, X., Qiu, J., et al. (2022). Identifying the key genes regulating mesenchymal stem cells chondrogenic differentiation: an in vitro study. BMC Musculoskelet. Disord. 23, 985. doi:10.1186/s12891-022-05958-7
Oliveira Miranda, C., Marcelo, A., Silva, T. P., Barata, J., Vasconcelos-Ferreira, A., Pereira, D., et al. (2018). Repeated mesenchymal stromal cell treatment sustainably alleviates machado-joseph disease. Mol. Ther. 26, 2131–2151. doi:10.1016/j.ymthe.2018.07.007
Sitbon, A., Delmotte, P. R., Pistorio, V., Halter, S., Gallet, J., Gautheron, J., et al. (2024). Mesenchymal stromal cell-derived extracellular vesicles therapy openings new translational challenges in immunomodulating acute liver inflammation. J. Transl. Med. 22, 480. doi:10.1186/s12967-024-05282-9
Visweswaran, M., Pohl, S., Arfuso, F., Newsholme, P., Dilley, R., Pervaiz, S., et al. (2015). Multi-lineage differentiation of mesenchymal stem cells - to Wnt, or not Wnt. Int. J. Biochem. Cell Biol. 68, 139–147. doi:10.1016/j.biocel.2015.09.008
Keywords: mesenchymal stromal/stem cells, induced-pluripotent stem cells, organoids, disease models, biological processes
Citation: Ambele MA and Miranda CO (2024) Editorial: Mesenchymal and induced-pluripotent stem cells as models to study biological processes. Front. Genet. 15:1439306. doi: 10.3389/fgene.2024.1439306
Received: 27 May 2024; Accepted: 10 July 2024;
Published: 25 July 2024.
Edited by:
Atsushi Asakura, University of Minnesota Twin Cities, United StatesReviewed by:
Alejandro Correa, Oswaldo Cruz Foundation, BrazilAhmed Lotfy, Medical University of South Carolina, United States
Copyright © 2024 Ambele and Miranda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melvin A. Ambele, melvin.ambele@up.ac.za; Catarina O. Miranda, csmiranda@cnc.uc.pt
 Melvin A. Ambele
Melvin A. Ambele