
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Food. Sci. Technol., 29 January 2024
Sec. Food Biotechnology
Volume 3 - 2023 | https://doi.org/10.3389/frfst.2023.1270392
This article is part of the Research TopicBioactive Peptides in Functional Foods: Production, Characterization, and Health BenefitsView all articles
Marine collagen hydrolysates and purified peptides can be sourced from a variety of species. Application of collagen peptides to animal models of diabetes and obesity is contributing to the goal of elucidating a mode of action and their broad spectrum application includes wound healing and bone fracture, both of which are significant co-morbidities of diabetes and obesity related illnesses.
Obesity has reached epidemic proportions in the world and is increasing rapidly as traditional diets are replaced by more readily available processed and high fat content foods. Obesity is a complex condition with several drivers which can influence a person’s weight, this results in a number of co-morbidities including cardiovascular disease and type 2 diabetes. In Australia, it is estimated that over 66.9% of adults are overweight or obese and this is increased further in regional and remote communities (Obesity Evidence Hub). According to the World Health Organization 2016 report, 1.9 billion adults were overweight with an estimated 650 million being obese (WHO). In Australia, it is estimated that 1.3 million people have type 2 diabetes, and WHO estimates that around 422 million people have diabetes (WHO).
Metabolic syndrome is attributed to a collection of disorders and behaviors including obesity, lack of physical exercise which contributes to insulin resistance and development of diabetes and increased risk of cardiac and renal dysfunction.
Increasing studies have focused on nutritional and nutraceutical approaches to alleviate dysregulation associated with obesity and diabetes and this focused review will highlight advances in the effects of marine collagen in vitro and in vivo studies.
Collagen peptides are generated from a variety of sources, from marine creatures to land-based mammals including cows, pigs, and sheep (Table 1). A readily available source of collagen is waste products from the fish industry including fish skins and skeletons. Structurally, collagen as a triple helical molecule is conserved across species, however variation in amino acid composition may confer subtle differences particularly in relation to denaturation temperatures and bioactivity properties. Studies have shown differences in denaturation temperature between different sources of marine collagen from warm and cold waters. Figure 1 illustrates the recent exploration of therapeutic applications of marine collagen peptides in obesity related illnesses.
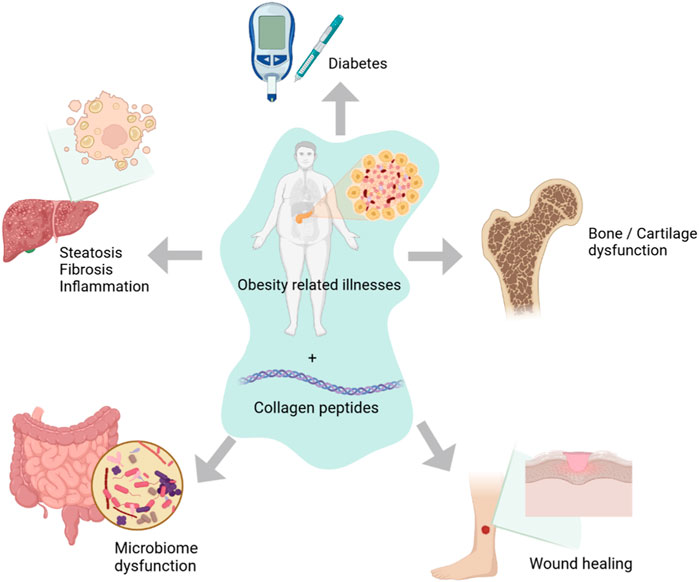
FIGURE 1. Target sites for applications of marine collagen peptides in obesity and diabetes related illnesses.
An original paper by Lee et al (2017) described the effects of tuna skin collagen hydrolysates on the differentiation of the preadipocyte 3T3 cell line and also in high fat diet (HFD) fed mice. Treatment of 3T3 cells with 0.5–1.0 mg/mL of hydrolysate resulted in a reduced intracellular accumulation of oil droplets, and reduced gene expression of adipogenic markers such as PPAR-gamma. In a parallel in vivo study, mice which were previously fed a high fat diet and received 300 mg/kg/day of hydrolysate and gavaged three time per week showed a reduction in body weight compared to control animals. Treatment of animals with marine collagen peptide also showed a reduction in the mRNA expression of key transcription factors involved in regulation of adipogenesis, namely, C/EBR-alpha and PPAR-γ. Histological assessment of HFD mice fed with peptide also showed a reduction in adipocyte size correlating with a reduction in body weight.
Commercially available sea fish collagen peptides, “Naticol” were also administered to mice fed a HFD, at a much higher dose of 4 g/kg/day in drinking water over a 20 week period resulted in a reduction in total body weight of the treated animals by week 12 (Astre et al., 2018). An amino acid analysis of Naticol showed a typical collagen profile of Glycine (approximately 20%) and proline, glutamic acid and Hydroxyproline). Other parameters affected by peptide treatment included some reduction in inflammatory cytokines (IL-6 and IL-1β) concentration in isolated adipocytes whilst no obvious changes in glucose tolerance and insulin sensitivity were observed.
A limited number of studies describe the effects of marine collagen peptides in human clinical trials. In one study by Zhu et al. (2010), a cohort of type 2 diabetic patients who received 13 g of peptide daily for upto 3 months showed changes in glucose and lipid metabolism markers with increased levels of insulin sensitivity reported together with reduced levels of fasting glucose, triglyceride, and free fatty acids. Improvements in kidney function were also seen. A similar study was performed in a group of type 2 diabetic patients with hypertension compared with to a non-hypertensive group who received 13.5 g of peptide per day. A therapeutic effect was more apparent in the non-hypertensive group (Zhu et al., 2010).
The effects of collagen peptide administration in animal models of obesity was recently described by Kalmikova et al (2023) using low molecular weight Antarctic fish collagen fragments generated by pepsin digestion followed by ultrafiltration. In rats fed a HFD together with collagen peptides (1 g/kg over a 6 weeks in period), a reduction in body mass and inflammation were observed, which may promote a decrease in adipose tissue content. This study follows on from an earlier investigation by (Raksha et al., 2018) where collagen peptides were associated with lower blood glucose, glycated hemoglobin and serum insulin levels.
Obesity and related illnesses, particularly diabetes, can have profound effects on the microbial community that inhabit the gut. This can have a variety of effects including; decreased production of short chain fatty acids (SCFAs), increased localized inflammation, disruption of the gut barrier and a higher abundance in pathogenic bacteria. A number of studies have investigated the effects of collagen peptides on gut microbiome. A recent study by Baek et al (2023) highlighted a change in the ratio of Firmicutes/Bacteriodetes in an obese mouse model using a fish (Tilapia) collagen peptide co-administered with a HFD compared with soybean and yeast. Whilst there was no change in the alpha index (microbial community richness) of treated animals a number of bacterial taxa were increased which have previously been associated with anti-obesogenic effects such as Faecalibaculum, however there were no changes in Akkermansia muciniphilia.
In a novel modification of collagen peptides through ferrous chelation, Jiang et al (2022) showed changes in the microbial community in a rat model of iron deficiency which reversed gut dysbiosis. The collagen peptide-iron complex increased the relative abundance of short chain fatty acid-producing bacteria, such as Blautia, Ruminococcus and Roseburia that can restore the pH of intestinal lumen, promote repair of intestinal tissue and inhibit inflammation. The collagen peptide-iron complex also enhanced the abundance of bacterial flora such as Subdoligranulum and Christensenellaceae_R-7_ group which have been linked to beneficial effects related to obesity and type II diabetes (Chen et al., 2021). In this study by Chen et al. (2021), the microbiota profile of patients with diabetic nephropathy was compared to patients with diabetes alone and a healthy control group. A number of distinguishing characteristics were found, including a lower diversity of gut microbiota in the group with an advanced stage of diabetic nephropathy. The presence of urinary protein following a 24 h collection correlated with certain species, including Alistipes and Subdoligranulum. A reduced estimated glomerular filtration rate (eGFR) was associated with the Ruminococcus (torques group).
Diabetic nephropathy is a major microvascular complication of chronic diabetes compromising the structural integrity of the nephron with increased extracellular matrix deposition, basement membrane thickening and tubular fibrosis. The effect of marine collagen peptides in the streptozotocin induced diabetes rat model was explored by Lin et al (2021) where tilapia skin peptides used at 3 g/kg/day over an 8 week period showed a reduction in the kidney hypertrophy index, and other biochemical parameters such as blood urea nitrogen (BUN), creatine and cholesterol. Histological examination also showed a reduction in kidney injury and the protective mechanism of the peptides was proposed to involve increased Bnip/nix signaling and mitophagy. Table 2 presents a summary of recent studies on the modulatory effects of marine derived collagen peptides in metabolic diseases.
Obesity is also linked to chronic kidney disease collagen peptides derived from monkfish meat which had the highest 2,2-diphenyl-1-picrylhydrazyl (DPPH) clearance rate, were administered to mice on HFD at 100 or 200 mg/kg for 8 weeks. Treatment showed a reduction in biochemical parameters (creatinine, uric acid, and BUN) in conjunction with a reduction in thickness around renal tubules (Ren et al., 2022). The peptide treated group also showed changes in gut microbial communities with an improvement in the Firmicutes/Bacteroidetes ratio. In a similar murine study of HFD induced kidney damage by Miao et al., 2022, the effect of monkfish peptides (at 100 or 200 mg/kg which showed the highest DPPH clearance rate), were associated with a reduction in biochemical parameters (creatinine, BUN, uric acid). Histopathological staining also showed reduced glomerular surface area and mesangial area compared to HFD controls. HFD fed mice exhibited a characteristic liver steatosis and monkfish collagen peptide fed mice showed a more regular hepatocyte morphology (Miao et al., 2022) Table 3.
An increasing number of studies have highlighted the protective effects of Monkfish peptides in non-alcoholic fatty liver disease (NAFLD) induced by HFD. Ye et al. (2022) applied monkfish peptides to a mouse model of NAFLD, specifically low molecular weight peptides of < 1 KDa and sequencing revealed a range of peptide sizes with octa and nona peptides being predominant. Monkfish peptides were administered at a dose range of 50–200 mg/kg/day for 8 weeks and NAFLD mice treated with peptides showed a decrease in body weight of up to 28%, lower triglyceride levels and elevated antioxidant enzymes. In this study, liver function (ALT and AST) was improved at the high dose together with a reduction in cholesterol and triglycerides. Liver pathology was also improved, including a reduction in the number of lipid droplets. The mechanism was proposed to be via enhancement of pathways that increased lipid beta oxidation and therefore reduced fatty acid synthesis.
Other non-fillet parts from the monkfish, including the swim bladder has been shown as a rich source of bioactive peptides. An extensive analysis of papain digests of the swim bladder (Shenkoohi et al., 2023) revealed a number of peptides with increased DPPH scavenging activity and protection of HepG2 cells for H2O2 induced oxidative stress. A comparison of enzyme treatments by Tian et al (2020) found that neutrase, alcalase and papain digestion of monkfish meat yielded peptides with greater DPPH clearance and ultrafiltration to acquire peptides of <1 kDa also contributed to greater antioxidant activity and upregulation of antioxidant enzymes in RAW 264.7 cells. An interesting approach in generating hydrolysates from monkfish muscle by Hu et al (2017), involved a gastrointestinal digestion mix of pepsin and trypsin and appropriate conditions with peptides showing increased DPPH and hydroxyl ion scavenging activity and protection of HepG2 cells from H2O2 oxidative stress.
Underutilized fish may also provide an alternative to generation of bioactive products for value adding. One example is sprat, a small oily fish that is widely used as a food source in Eastern Europe but otherwise underutilized. In a recent study sprat protein hydrolysate (Shekoohi et al., 2023) was characterized for its antioxidant activity and for its ability to stimulate muscle protein synthesis in the C2C12 myotube cell line. Ageing is associated with both a growing risk of diabetes and increased muscle loss and the features of type 2 diabetes, insulin resistance and inflammation have a negative impact on muscle mass. Sprat protein hydrolysate showed active antioxidant and free radical scavenging ability, promoting muscle protein synthesis and increased myotube thickness compared to controls. A similar study, although not involving purified collagen peptides, evaluated muscle hypertrophy in rats fed Alaska pollock protein (Uchida et al., 2022). This study demonstrated increased gastrocnemius muscle mass attributed to larger muscle fiber size in rats fed fish protein which was incorporated into a normal and HFD. The authors noted that the hypertrophy maybe associated with a suppression of pathways involved in protein degradation. In a related study, Ayabe et al. (2015) applied a novel peptide purified from tryptic digestion of Alaska pollock protein and when applied to a mouse model of type 2 diabetes, resulted in glucose lowering. A specific C-terminal peptide was used at a lower concentration of 1 mg/kg also lowered blood glucose and enhanced glucose uptake in a mouse skeletal muscle cell line.
The interplay between collagen peptides and muscle paracrine factors involved in wound healing was highlighted in a study by Li et al (2021) where squid cartilage type II collagen was shown to enhance tibial fracture healing in mice via upregulation of IGF-1 and Irisin.
Obesity and type 2 diabetes can both be associated with dysfunctional wound healing, in particular chronic ulcer development, caused by poor capillary flow. Peripheral artery disease is also prevalent in diabetic patients which can also contribute to delayed wound healing. There is growing interest in the application of marine collagen to improving wound healing and this has recently been reviewed by Cruz et al (2021) and Geachan et al (2022). Application of Tilapia skin collagen hydrolysates prepared by protease and papain digestion promoted in vitro wound healing at 50 μg/mL in the commonly used HaCat cell scratch assay (Hu et al., 2017). The same study also applied peptides to a rabbit model of scalding and wound healing was promoted by day 11 post scald. In an interesting combination of marine based bioactives, Ouyang et al (2018) developed a hydrogel composed of chitosan and tilapia peptides, which enhanced cell migration and proliferation of L929 cells in vitro. It also promoted wound healing as observed in a rabbit burn assay with increased epithelialization. Collagen peptides derived from marine sponges are attractive due to their relative ease of extraction from raw material. Pozzolini et al (2018) showed HPLC purified marine sponge collagen peptides exhibited antioxidant and oxygen free radical scavenging ability which also promoted cell proliferation in a variety of cells (L929, Raw 264.7 and HaCat). The sponge collagen also enhanced in vitro wound healing in the scratch assay.
Jellyfish species have also been investigated as a source of collagen peptides which may have wound healing capabilities. Felician et al (2019) isolated collagen peptides from Rhopilema esculentum using pepsin digestion and demonstrated in vitro proliferation in an endothelial cell line (HUVECs). The jellyfish derived collagen peptides when administered orally up to 0.9 g/kg for 6 days, improved wound closure in a mouse model.
Vascular endothelial growth factor (VEGF) is a major contributor to effective wound healing. An approach to elucidate the mode of action of marine collagen peptides, Yang et al (2019) tested peptides isolated from the skin of the giant croaker Nibea japonica. The study demonstrated that marine collagen peptides modulated the expression of VEGF together with other growth factors including fibroblast growth factor (FGF) and epidermal growth factor (EGF) via the NF-κ B signaling pathway.
Protein hydrolysates derived from salmon and mackerel skeletons were formulated as a nutritional supplement and fed to mice which had received a punch biopsy wound. The mode of action of collagen peptides in wound healing was highlighted by increased expression of Ccl3 and Cx3cl-1 chemokines which are associated with enhanced migration of inflammatory cells that can release pro-angiogenic growth factors such as VEGF (Lapi et al., 2021).
A further insight into the mechanism of collagen peptides in a wound healing setting is provided by Mei et al (2020), who applied marine collagen peptides (Salmo salar and Tilapia nilotica) via intragastric delivery (2 g/kg) to rats which received an incision wound for up to 12 days. Both peptides improved wound healing compared to controls and this was associated with decreased expression of pro-inflammatory cytokines (TNF-α, IL-6) and upregulated expression of VEGF. Interestingly, molecules associated with pathogen pattern recognition were upregulated and a wound healing bacterial community was promoted (Enterococcus and Bacillus).
Full-thickness wounds can be associated with an increased risk of bacterial infection, necrosis, and life-threatening complications. To overcome these challenges, a novel biomaterial which incorporate marine collagen may also accelerate wound healing. Feng et al (2020) described a hybrid hydrogel composed of aminated fish collagen, oxidized sodium alginate, and antimicrobials (polymyxin and bacitracin) applied to a full-thickness wound in a rat model. The hydrogel with marine peptides appeared to improve wound healing compared to controls, in terms of epithelialization and collagen deposition. In vitro cell proliferation and angiogenesis assays suggested that the addition of the antimicrobials also had an enhancing effect.
Wound healing complications can be associated with cesarean section and further compounded by co-morbidities such as obesity and diabetes. In a study by Peng et al (2020), marine collagen peptides (4.4 mg/kg) were delivered intragastrically to rats which has received a cesarean section. Higher dosages of peptides appeared to improve skin wound tensile strength and uterine bursting pressure. The use of marine peptides (Tilapia) in a composite biomaterial containing hydroxyapatite also showed wound healing potential via inhibition of inflammatory cytokine expression in a rabbit scald burn model (Ouyang et al., 2021). Incorporation of marine collagen peptide (Sipunculus nudus) into an ointment has been applied to a full-thickness excision wound in mice (Lin et al., 2021), with improved wound closure at day 10 compared to controls. Peptide treatment was again associated with a reduction in inflammatory markers and improved collagen deposition.
Obesity can affect overall bone health and function through a variety of mechanisms including increased weight and fat volume, dysregulation of bone formation, resorption and expression of pro-inflammatory cytokine. Adipokines, such as leptin can exert direct anabolic effects on osteoblasts. In diabetes, a complex interplay of hyperglycemia, insulin resistance and dysregulation of insulin-like growth factors maybe associated with increased risk of fracture.
Whilst collagen-based biomaterials have been extensively applied to models of fracture repair, recent studies have highlighted a potential role for marine collagen peptides to improve bone health directly or indirectly. An early study by Xu et al (2010) demonstrated that marine collagen based salmon hydrolysates administered to rats could increase serum osteocalcin levels which may enhance osteoblast activity and reduce bone resorption. The application of collagen scaffolds derived from jellyfish has shown promise in promoting bone formation via inflammatory macrophage recruitment. Flaig et al., 2020), implanted jelly fish 3D scaffolds into a rat model and demonstrated increased de novo bone formation after 60 days.
Squid derived collagen II has been shown to improve tibia fracture repair in a mouse model, where new bone formation was accelerated via upregulation of muscle paracrine factors, IGF-1 and Irisin (Li et al., 2021). Furthermore, Cruz et al (2020) generated a collagen scaffold for application in a rat cranial critical bone defect which in combination with photobiomodulation resulted in an increased amount of connective tissue and newly formed bone.
RW: Writing–original draft, Writing–review and editing. ZZ: Writing–review and editing. PS: Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Astre, G., Deleruyelle, S., Dortignac, A., Bonnet, C., Valet, P., and Dray, C. (2018). Diet-induced obesity and associated disorders are prevented by natural bioactive type 1 fish collagen peptides (Naticol®) treatment. J. Physiol. Biochem. 74 (4), 647–654. doi:10.1007/s13105-018-0650-0
Ayabe, T., Mizushige, T., Ota, W., Kawabata, F., Hayamizu, K., Han, Li, et al. (2015). A novel Alaska pollack-derived peptide, which increases glucose uptake in skeletal muscle cells, lowers the blood glucose level in diabetic mice. Food Funct. 6 (8), 2749–2757. doi:10.1039/c5fo00401b
Baek, Gh, Yoo, K. M., Kim, S. Y., Lee, D. H., Chung, H., Jung, S. C., et al. (2023). Collagen peptide exerts an anti-obesity effect by influencing the firmicutes/bacteroidetes ratio in the gut. Nutrients 15 (11), 2610. doi:10.3390/nu15112610
Chen, W., Zhang, M., Guo, Y., Zhen Liu, W. Q., Yan, R., Wang, Y., et al. (2021). The profile and function of gut microbiota in diabetic nephropathy. Diabetes Metab. Syndr. Obes. 14, 4283–4296. doi:10.2147/DMSO.S320169
CruzAraujo, M. A. T. A., Avanzi, I. R., Parisi, J. R., Martins de Andrade, A. L., and Muniz Rennó, A. C. (2021). Collagen from marine sources and skin wound healing in animal experimental studies: a systematic review. Mar. Biotechnol. (NY) 23 (1), 1–11. doi:10.1007/s10126-020-10011-6
CruzFernandesParisiValeJuniorFreitasSalesFortulanPeitl, M. A. K. R. J. RGCASRAF R. A. F. S. C. A. O., ZanottoGranito, E. R. N., and RibeiroRenno, A. M. A. C. M. (2020). Marine collagen scaffolds and photobiomodulation on bone healing process in a model of calvaria defects. Bone Min. Metab. 38 (5), 639–647. doi:10.1007/s00774-020-01102-4
Felician, F. F., Yu, R.-H., Li, M.-Z., Li, C.-J., Chen, H.-Q., Jiang, Y., et al. (2019). The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 22 (1), 12–20. Epub 2019 Feb 8. doi:10.1016/j.cjtee.2018.10.004
Feng, X., Zhang, X., Li, S., ZhengShi, Y. X., Li, F., Guo, S., et al. (2020). Preparation of aminated fish scale collagen and oxidized sodium alginate hybrid hydrogel for enhanced full-thickness wound healing. Int. J. Biol. Macromol. 164, 626–637. Epub 2020 Jul 12. doi:10.1016/j.ijbiomac.2020.07.058
Flaig, I., Radenković, M., Najman, S., Pröhl, A., Jung, O., and Barbeck, M. (2020). In vivo analysis of the biocompatibility and immune response of jellyfish collagen scaffolds and its suitability for bone regeneration. Int. J. Mol. Sci. 21 (12), 4518. doi:10.3390/ijms21124518
Gao, Q., and ShangZhouDengPeng, YWSC (2022). Marine collagen peptides: a novel biomaterial for the healing of oral mucosal ulcers. Dent. Mater J. 41 (6), 850–859. doi:10.4012/dmj.2021-323
Geahchan, A., Baharlouei, P., and Rahmam, A. (2022). Marine collagen: a promising biomaterial for wound healing, skin anti-aging, and bone regeneration. Mar. Drugs 20 (1), 61. doi:10.3390/md20010061
Hu, Z., Yang, P., Zhou, C., Li, S., and Hong, P. (2017). Marine collagen peptides from the skin of nile Tilapia (Oreochromis niloticus): characterization and wound healing evaluation. Mar. Drugs 15 (4), 102. doi:10.3390/md15040102
Jiang, S., Dong, W., Zhang, Z., Xu, J., Zhang, J., Dai, L., et al. (2022). A new iron supplement: the chelate of pig skin collagen peptide and Fe2+ can treat iron-deficiency anemia by modulating intestinal flora. Front. Nutr. 9, 1055725. doi:10.3389/fnut.2022.1055725
Jin, L., Zheng, D., Yang, G., Li, W., Yang, H., Jiang, Q., et al. (2020). Tilapia skin peptides ameliorate diabetic nephropathy in STZ-induced diabetic rats and HG-induced GMCs by improving mitochondrial dysfunction. Mar. Drugs 18 (7), 363. PMID: 32679664; PMCID: PMC7401261. doi:10.3390/md18070363
Kalmukova, O., Raksha, N., Vovk, T., Halenova, T., Dzerzhynsky, M., Mitrecic, D., et al. (2023). Low-molecular-mass fragments of collagen improve parameters related to mass and inflammation of the adipose tissue in the obese rat. Food Technol. Biotechnol. 61 (1), 51–63. doi:10.17113/ftb.61.01.23.7926
Lapi, I., Kolliniati, O., Aspevik, T., Deiktakis, E. E., Axarlis, K., Daskalaki, M. G., et al. (2021). Collagen-containing fish sidestream-derived protein hydrolysates support skin repair via chemokine induction. Mar. Drugs 19 (7), 396. doi:10.3390/md19070396
Lee, E. J., Hur, J., Ham, S. A., Jo, Y., Lee, S. Y., Choi, M.-J., et al. (2017). Fish collagen peptide inhibits the adipogenic differentiation of preadipocytes and ameliorates obesity in high fat diet-fed mice. Int. J. Biol. Macromol. 104, 281–286. Pt A. doi:10.1016/j.ijbiomac.2017.05.151
Li, Z., Tian, Y., Zhang, L., Zhang, T., Wang, P., and Wang, J. (2021). Type II collagen from squid cartilage mediated myogenic IGF-I and irisin to activate the Ihh/PThrp and Wnt/β-catenin pathways to promote fracture healing in mice. Food Funct. 12 (14), 6502–6512. doi:10.1039/d0fo03069d
Lin, H., Zheng, Z., Yuan, J., ZhangCao, C. W., and Qin, X. (2021). Collagen peptides derived from sipunculus nudus accelerate wound healing. Molecules 26 (5), 1385. doi:10.3390/molecules26051385
Mei, F., Liu, J., Wu, J., Duan, Z., Chen, M., Meng, K., et al. (2020). Collagen peptides isolated from Salmo salar and Tilapia nilotica skin accelerate wound healing by altering cutaneous microbiome colonization via upregulated NOD2 and BD14. J. Agric. Food Chem. 68 (6), 1621–1633. doi:10.1021/acs.jafc.9b08002
Miao, B., Zheng, J., Zheng, G., Tian, X., Zhang, W., Yuan, F., et al. (2022). Using collagen peptides from the skin of monkfish (Lophius litulon) to ameliorate kidney damage in high-fat diet fed mice by regulating the Nrf2 pathway and NLRP3 signaling. Front. Nutr. 9, 798708. doi:10.3389/fnut.2022.798708
Obesity evidence hub (2023). Find key evidence on obesity trends, impacts, prevention & treatment in Australia. Available at: https://www.obesityevidencehub.org.au/.
Ouyang, Q. Q., HuLinQuanDengLi, Z. Z. W.-Y. Y. F. D.-S., and LiChen, P. W. Y. (2018). Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int. J. Biol. Macromol. 112, 1191–1198. doi:10.1016/j.ijbiomac.2018.01.217
Ouyang, Q. Q., Kong, S., Huang, Y., Ju, X., Li, S., Li, P., et al. (2021). Preparation of nano-hydroxyapatite/chitosan/tilapia skin peptides hydrogels and its burn wound treatment. Int. J. Biol. Macromol. 181, 369–377. doi:10.1016/j.ijbiomac.2021.03.085
Peng, X., Xu, J., Tian, Y., Liu, W., and Peng, B. (2020). Marine fish peptides (collagen peptides) compound intake promotes wound healing in rats after cesarean section. Food Nutr. Res. 64, 64. doi:10.29219/fnr.v64.4247
Pozzolini, M., Millo, E., Oliveri, C., Mirata, S., Salis, A., Damonte, G., et al. (2018). Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge chondrosia reniformis. Mar. Drugs 16 (12), 465. doi:10.3390/md16120465
Raksha, N., Potalitsyn, P., Yurchenko, A., Halenova, T., Savchuk, O., and Ostapchenko, L. (2018). Prevention of diet-induced obesity in rats by oral application of collagen fragments. Archives Biol. Sci. 70 (1), 77–86. doi:10.2298/abs170401027r
Ren, X., Miao, B., Cao, H., Tian, X., Shen, L., Yang, Z., et al. (2022). Monkfish (Lophius litulon) peptides ameliorate high-fat-diet-induced nephrotoxicity by reducing oxidative stress and inflammation via regulation of intestinal flora. Molecules 28 (1), 245. doi:10.3390/molecules28010245
Salvatore, L., Gallo, N., Natali, M. L., Campa, L., Lunetti, P., Madaghiele, M., et al. (2020). Marine collagen and its derivatives: versatile and sustainable bio-resources for healthcare. Mater Sci. Eng. C Mater Biol. Appl. 113, 110963. doi:10.1016/j.msec.2020.110963
Shekoohi, N., Naik, A., Amigo-Benavent, M., Harnedy-Rothwell, P., Carson, B. P., and FitzGerald, R. J. (2023). Physicochemical, technofunctional, in vitro antioxidant, and in situ muscle protein synthesis properties of a sprat (Sprattus sprattus) protein hydrolysate. Front. Nutr. 10, 1197274. doi:10.3389/fnut.2023.1197274
Tian, X., Zheng, J., Xu, B., Ye, J., Yang, Z., and Yuan, F. (2020). Optimization of extraction of bioactive peptides from monkfish (Lophius litulon) and characterization of their role in H2O2-induced lesion. Mar. Drugs 18 (9), 468. doi:10.3390/md18090468
Uchida, K., Fujitani, M., Mizushige, T., Kawabata, F., Hayamizu, K., Uozumi, K., et al. (2022). Dietary Alaska pollack protein induces acute and sustainable skeletal muscle hypertrophy in rats. Nutrients 14 (3), 547. doi:10.3390/nu14030547
WHO (2023). WHO Obesity factsheet. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
Xu, Y., Han, X., and Li, Y. (2010). Effect of marine collagen peptides on long bone development in growing rats. J. Sci. Food Agric. . 90 (9), 1485–1491. doi:10.1002/jsfa.3972
Yang, F., Jin, S., and Tang, Y. (2019). Marine collagen peptides promote cell proliferation of NIH-3T3 fibroblasts via NF-κB signaling pathway. Molecules 24 (22), 4201. doi:10.3390/molecules24224201
Ye, J., Tian, X., Wang, Q., Zheng, J., Yang, Y., Xu, B., et al. (2022). Monkfish peptides mitigate high fat diet-induced hepatic steatosis in mice. Mar. Drugs 20 (5), 312. doi:10.3390/md20050312
Zheng, J., Tian, X., Xu, B., Yuan, F., Gong, J., and Yang, Z. (2020). Collagen peptides from swim bladders of giant croaker (Nibea japonica) and their protective effects against H2O2-induced oxidative damage toward human umbilical vein endothelial cells. Mar. Drugs 18 (8), 430. doi:10.3390/md18080430
Zhu, C., Zhang, W., Mu, B., Zhang, F., Lai, N., Zhou, J., et al. (2017). Effects of marine collagen peptides on glucose metabolism and insulin resistance in type 2 diabetic rats. J. Food Sci. Technol. 54 (8), 2260–2269. Epub 2017 Jun 14. PMID: 28740282; PMCID: PMC5502017. doi:10.1007/s13197-017-2663-z
Keywords: marine collagen, obesity, diabetes, wound healing, microbiome, bone
Citation: Wong RPM, Zhou ZK and Strappe PM (2024) The anti-obesogenic and anti-diabetic properties of marine collagen peptides. Front. Food. Sci. Technol. 3:1270392. doi: 10.3389/frfst.2023.1270392
Received: 31 July 2023; Accepted: 15 December 2023;
Published: 29 January 2024.
Edited by:
Pradyuman Kumar, Sant Longowal Institute of Engineering and Technology, IndiaReviewed by:
Naohisa Shobako, Kyoto University, JapanCopyright © 2024 Wong, Zhou and Strappe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Padraig M. Strappe, UGFkcmFpZy5zdHJhcHBlQGN1cnRpbi5lZHUuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.