
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 02 April 2024
Sec. Endocrinology of Aging
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1370457
Introduction: Serum Klotho (S-Klotho) is a transmembrane protein holds pivotal roles in anti-aging. The Dietary Inflammation Index (DII), a meticulously dietary tool, quantifies the inflammatory potential of an individual's diet. The existing research strongly suggests that a low DII diet plays a significant role in delaying aging and reducing aging-related symptoms in males. Testosterone could potentially act as a mediating intermediary between DII and S-Klotho. However, this aspect remains unexplored. This study aims to investigate the potential causal link of testosterone between DII and S-Klotho in males.
Methods: We utilized data from National Health and Nutrition Examination Survey (NHANES) which focused on male participants from 2013-2016. Mediation analyses were used to investigate the effects of testosterone (TT), free testosterone (FT), and free androgen index (FAI) on the DII-S-Klotho relationship, using three modes adjusting for covariates.
Results: Mediation analysis unveiled a significant inverse correlation between DII and S-Klotho levels (model 1: c = -14.78, p = 0.046). The interaction between DII and S-Klotho was modulated by TT in model 1 (ab = -1.36; 95% CI: -5.59, -0.55; p = 0.008), but lost significance after adjustments (model 2: ab = -0.39; 95% CI: -4.15, 1.66; p = 0.378; model 3: ab = -0.59; 95% CI: -4.08, 2.15; p = 0.442). For FT, the mediating impact was not statistically significant (model 1: ab = 0.43; 95% CI: -0.51, 5.44; p = 0.188; model 2: ab = 0.72; 95% CI: -0.26, 5.91; p = 0.136; model 3: ab = 0.84; 95% CI: -0.02, 8.06; p = 0.056). Conversely, FAI consistently influenced the DII-S-Klotho relationship (model 1: ab = 2.39; 95% CI: 0.69, 9.42; p = 0.002), maintaining significance after adjustments (model 2: ab = 3.2; 95% CI: 0.98, 11.72; p = 0.004; model 3: ab = 3.15; 95% CI: 0.89, 14.51; p = 0.026).
Discussion: This study observed no mediating influence of TT or FT on the correlation between DII and S-Klotho after covariate control. Remarkably, FAI continued to significantly mediate the DII-S-Klotho connection even following covariate adjustment, although its significance in males warrants careful consideration.
Serum Klotho (S-Klotho) is a transmembrane protein that plays pivotal roles in anti-aging and is considered a longevity-associated protein (1). The Klotho protein exists in two forms: membrane-bound and secretory. The detached membrane-bound variant is referred to as S-Klotho and can be detected in blood, urine, and cerebrospinal fluid (2, 3). Intriguingly, the dearth of Klotho gene expression in mice leads to a syndrome mirroring human aging, encompassing shortened lifespan, infertility, arteriosclerosis, skin atrophy, osteoporosis, and emphysema (4, 5). Conversely, overexpression of this protein appears to extend lifespan by 20-30% (6). In humans, plasma Klotho concentrations have demonstrated an inverse correlation with aging-related symptoms and overall mortality (7, 8).
The Dietary Inflammation Index (DII), a meticulously dietary tool, quantifies the inflammatory potential of an individual’s diet, has been shown to be highly correlated with six major inflammatory biomarkers across diverse populations (9–12). Emerging evidence suggests a crucial link between the DII and various health outcomes, particularly in relation to aging-related symptoms including mortality, cancer, cardiovascular diseases, musculoskeletal disorders, and mental well-being (1–4). The existing research strongly suggests that a low DII diet plays a significant role in delaying aging and reducing aging-related symptoms (13).
Population-based studies have also indicated a negative correlation between DII and S-Klotho (14). However, stratified analysis by gender revealed that this negative correlation is evident only in males (14). Considering the pivotal roles of sex hormones in metabolic and aging differences between males and females, and in light of existing research data, we hypothesize that sex hormones, particularly testosterone, may play an intermediary role in the negative correlation between DII and S-Klotho in the male population.
Mechanistically, testosterone activates klotho gene promoters through androgen receptor-mediated pathways, thereby enhancing klotho mRNA expression (15). Plausible mechanisms linking pro-inflammatory diets to diminished testosterone levels might involve pro-inflammatory markers such as increased IL-1, IL-6, IL-17, and TNF. Experimental evidence suggests that these markers impede testosterone secretion by influencing both central (hypothalamic-pituitary) and peripheral (testicular) components of the gonadal axis (16–18). In addition to the aforementioned mechanistic research, clinical studies from the United States (19–21) and Spain (22) have unveiled a negative correlation between DII and testosterone, while testosterone showed a positive association with S-Klotho in males. The amalgamation of these findings suggests that testosterone could conceivably serve as a mediating intermediary between DII and S-Klotho in adult males.
Notably, despite these intriguing possibilities, there is an evident dearth of research addressing this mediating role. Consequently, this study endeavors to explore the conceivable mediating function of testosterone within the complex interrelation between dietary inflammation and S-Klotho levels in adult males.
In this cross-sectional study, we utilized data from NHANES (National Health and Nutrition Examination Survey), an ongoing nationwide survey conducted by the National Center for Health Statistics at the U.S. Centers for Disease Control and Prevention. NHANES aims to assess the nutritional and health status of the U.S. population and is conducted in two-year cycles with representative sample weights. The study protocol of NHANES has been approved by the institutional review board, and all participants provided written informed consent in accordance with the principles of the Declaration of Helsinki. The data used in our study are publicly available on the CDC (Centers for Disease Control and Prevention) website: https://www.cdc.gov/nchs/nhanes/.
We obtained the data from NHANES 2013-2016, which included comprehensive information on sex hormones, sex hormone-binding globulin (SHBG), dietary inflammatory index (DII) calculations, and serum Klotho (S-Klotho) levels. Our study focused on male participants who completed 24-hour dietary recall, underwent sex hormone and SHBG tests, as well as S-Klotho testing. If there was any absence of the information above, participants were excluded from our cohort. The detailed selection and exclusion procedure was as follows: (1) Exclude female participants. (2) Exclude male participants with kidney failure which is defined based on the question: ‘has he/she ever been told by a doctor or other health professional that had weak or failing kidneys’ in the questionnaire data or the information of kidney condition is unavailable. (3) Exclude participants with incomplete dietary data to calculate DII. (4) Exclude participants without S-Klotho serum levels. (5) Participants without testosterone test data or SHBG test data were excluded. This procedure is also presented in Figure 1.
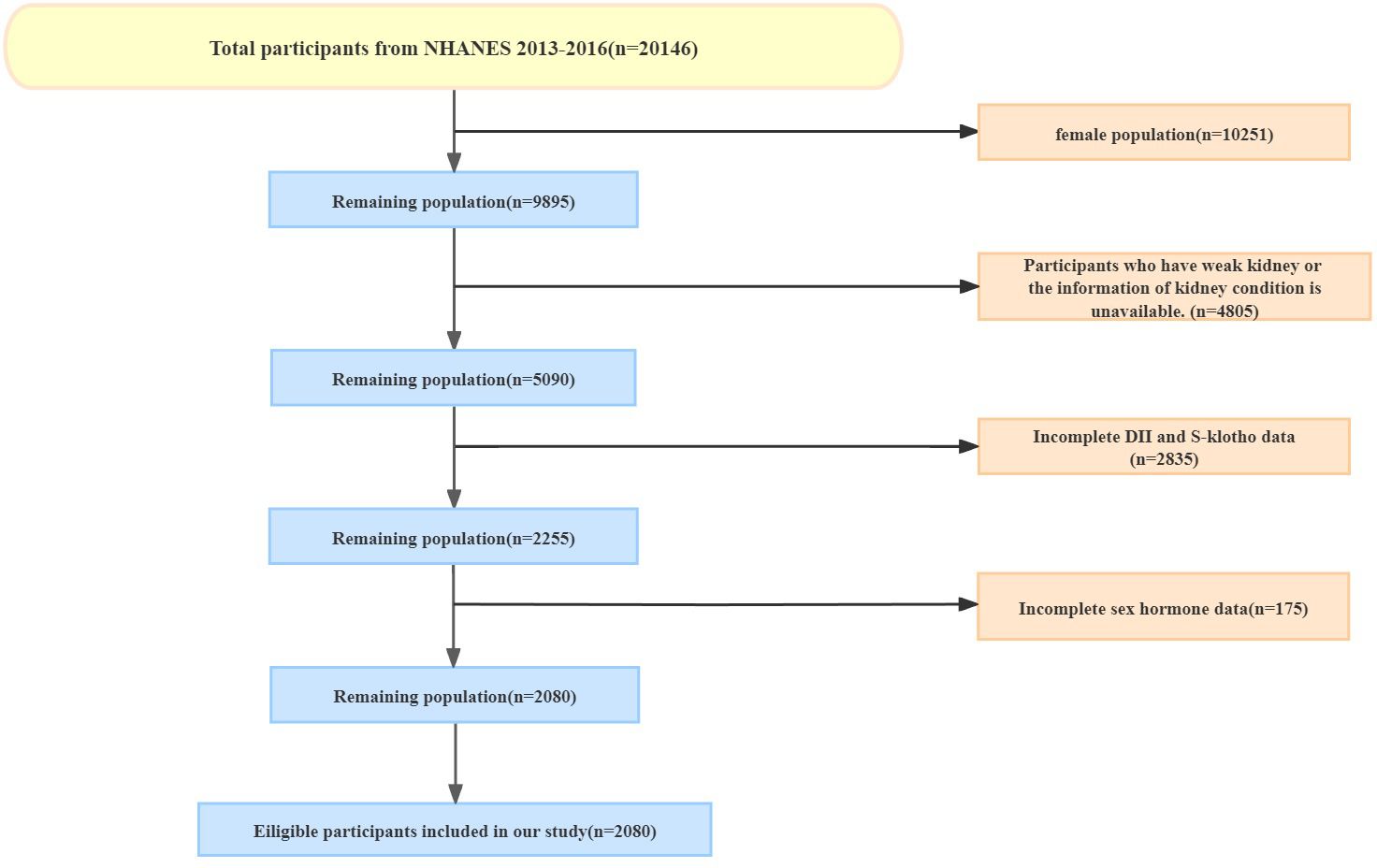
Figure 1 The participants enrollment. A total of 2080 people were included from NHANES (from 2013 to 2016).
The dietary data used for calculating the DII were obtained from NHANES. The validity of these dietary data has been established by the Nutrition Methodology Working Group (23). We calculated the DII following the protocols described by Shivappa et al. (24). A lower DII score indicates a more anti-inflammatory diet, while a higher DII score suggests a more proinflammatory diet. Our study included 27 out of 45 food parameters available in the NHANES database, including protein, fat, alcohol, carbohydrates, fiber, cholesterol, omega-3 and omega-6 polyunsaturated fatty acids, saturated, monounsaturated, and polyunsaturated fatty acids, niacin, vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin D, and vitamin E, iron, magnesium, zinc, selenium, folic acid, beta carotene, and caffeine. We treated the DII as a continuous variable and stratified it into four quarters for further statistical analysis.
Testosterone (TT) levels were measured using the isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) method, which is based on the reference method developed by the National Institute for Standards and Technology (NIST). The measurement of SHBG involved the reaction of SHBG with immuno-antibodies and chemoluminescence measurements of the reaction products, which occurred after two incubation periods and subjecting the samples to a magnetic field. The chemiluminescent reaction on the captured microparticles was measured using a photomultiplier tube. Detailed measurement protocols can be found at www.cdc.gov/nchs/nhanes/. Additionally, we calculated the free androgen index (FAI) by multiplying TT (ng/dL) by 100 and 0.288 and divided by SHBG (nmol/L), which provides an approximate estimation of the amount of circulating free testosterone (25). We also calculated the free testosterone (FT) based on the algorithm published by Vermeulen et al. (26).
Available pristine serum samples from 40-79 year old participants in NHANES were analyzed with the IBL ELISA method. The Northwest Lipid Metabolism and Diabetes Research Laboratories, Division of Metabolism, Endocrinology, and Nutrition, University of Washington, performed analyses on all but four fresh-frozen (pristine) samples received from the Centers for Disease Control and Prevention. All sample analyses were performed in duplicate according to the manufacturer’s protocol and all the results were checked to meet the laboratory’s standardized criteria for acceptability prior to being released for reporting.
Covariates in our study included age (in years), body mass index (BMI, in kg/m2), race/ethnicity (categorized as Mexican American, Other Hispanic, non-Hispanic White, non-Hispanic Black, or other race), ratio of family income to poverty (PIR), marital status (categorized as Married, Widowed, Divorced, Separated, Never married or Living with partner), smoking status, and alcohol intake. Smoking status was classified according to the NCHS classifications, where individuals who had smoked fewer than 100 cigarettes in their lifetime were considered never smokers, those who had smoked more than 100 cigarettes but were not currently smoking at the time of the survey were classified as former smokers, and those who had smoked more than 100 cigarettes in their lifetime and were currently smoking at the time of the survey were categorized as current smokers. Alcohol intake was categorized based on the question, “Have you had at least 12 alcohol drinks in the past year?” BMI was calculated using self-reported weight and height measurements and was categorized as underweight or healthy weight (<25 kg/m2), overweight (25-29.9 kg/m2), or obese (≥30 kg/m2) (19). PIR was categorized as low income (<1), middle income (1-4), or high income (≥4).
We conducted the statistical analysis using R (version 3.5.3) and EmpowerStats (www.empowerstats.com; X&Y Solutions Inc.). Categorical variables were presented as percentages, while continuous variables were reported as means ± standard deviation. To analyze differences among the DII quarters, we employed weighted chi-square tests for categorical variables and weighted linear regression for continuous variables if the data fit the normal distribution. Otherwise, the weighted kruskal-wallis test will be used.
Mediation was estimated using the indirect effect, which represents the changes in the effect of the independent variable on the outcome that can be attributed to the proposed mediator (27). The “a” path represents effect of the independent variable on the mediator variable while the “b” path represents effect of the mediator variable on the dependent variable. Indirect effects (a × b paths) with confidence intervals that did not include zero were considered statistically significant which could occur regardless of the significance of the total effect (i.e. c path, effect of the independent variable on the dependent variable) and the direct effect (i.e. c’ path, effect on the dependent variable when both the independent and the mediator variables are included as independent variables) (27). To explore the effects of TT, SHBG and FAI on the relationship between DII and S-Klotho levels respectively, we performed weighted mediation analyses. Three models were used to assess the impact of covariates on this association: Model 1 included no covariate adjustments, Model 2 adjusted for age, race, and BMI group, and Model 3 adjusted for age, race, BMI group, Ratio of family income to poverty which is used as a categorical variable (low income: <1, middle income: 1-4, high income: ≥4), smoking status, alcohol drinking, and marital status. To quantify the magnitude of the total effect explained by mediation analysis, we calculated the percentage of mediation ([indirect effect/total effect] × 100) (28).
A total of 2080 males were included in the current analysis (Figure 1). The baseline characteristics of the participants are shown in Table 1. Participants adhering to the most anti-inflammatory diets (DII quarter 1) exhibited higher levels of S-Klotho (824.26 pg/mL vs. 798.52 pg/mL, p<0.001), free testosterone (7.08 ng/mL vs. 6.44 ng/mL, p=0.005), and free androgen index (35.42 vs. 31.15, p=0.001). and were more likely to have higher incomes (59.55% vs. 31.53%, p<0.001) and be non-smokers (56.33% vs. 44.42%, p<0.001) compared with participants with the most proinflammatory diets (DII quarter 4). The participants’ mean age was similar across DII quarters, as were testosterone, estradiol, SHBG, blood pressure, the proportions of BMI group, alcohol drinking and marital status.
The results indicated an inverse correlation between DII and S-2Klotho plasma levels (model 1: c = -14.78; 95% CI: -43.82, -0.39; p = 0.046; model 2: c = -16.14; 95% CI: -47.9, -2.55; p = 0.020; model 3: c = -15.28; 95% CI: -45.83, -0.37; p = 0.048) (Figure 2). Mediation analyses were carried out to test whether the association of DII with S-Klotho levels in males could be mediated by TT, FT or FAI respectively (Tables 2, 3).
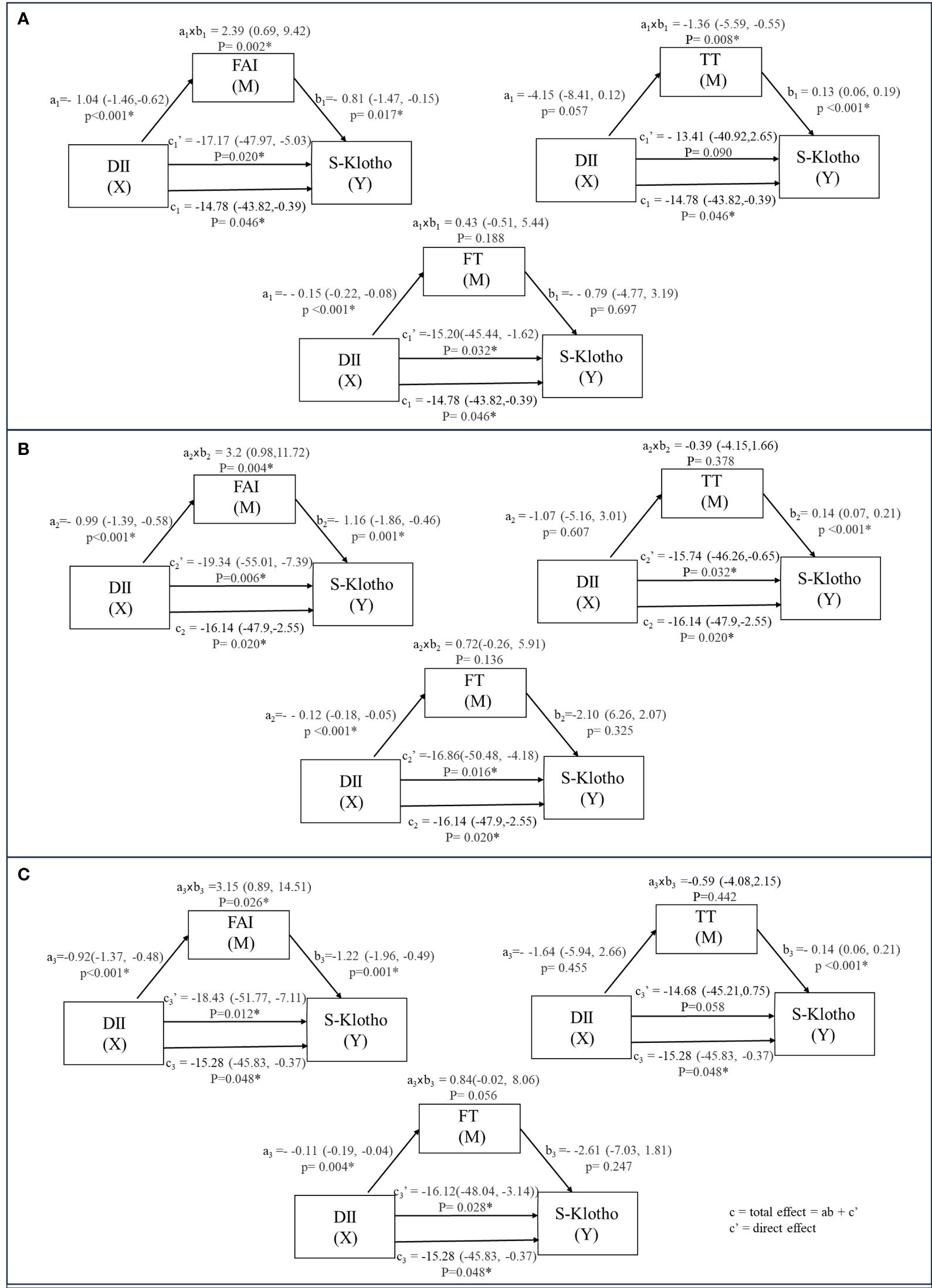
Figure 2 The relation between DII and S-Klotho through the indirect effect of TT, FT and FAI (weighted). a: Coefficients of the relationship between intermediary variable and independent variable. b: Coefficients of the relationship between implicit variable and intermediary variable. axb: Value of intermediary effect. (A) model 1 unadjusted. (B) model 2 adjusted for Age, Race, BMI group. (C) model 3 adjusted for Age; Race; BMI group; Ratio of family income to poverty; Smoking status; Alcohol drinking; Marital status.

Table 2 Mediation analysis of the estimated effect of Dietary Inflammatory Index on the S-Klotho concentrations through Testosterone, FAI and FT among males in NHANES 2013-2016 (weighted).
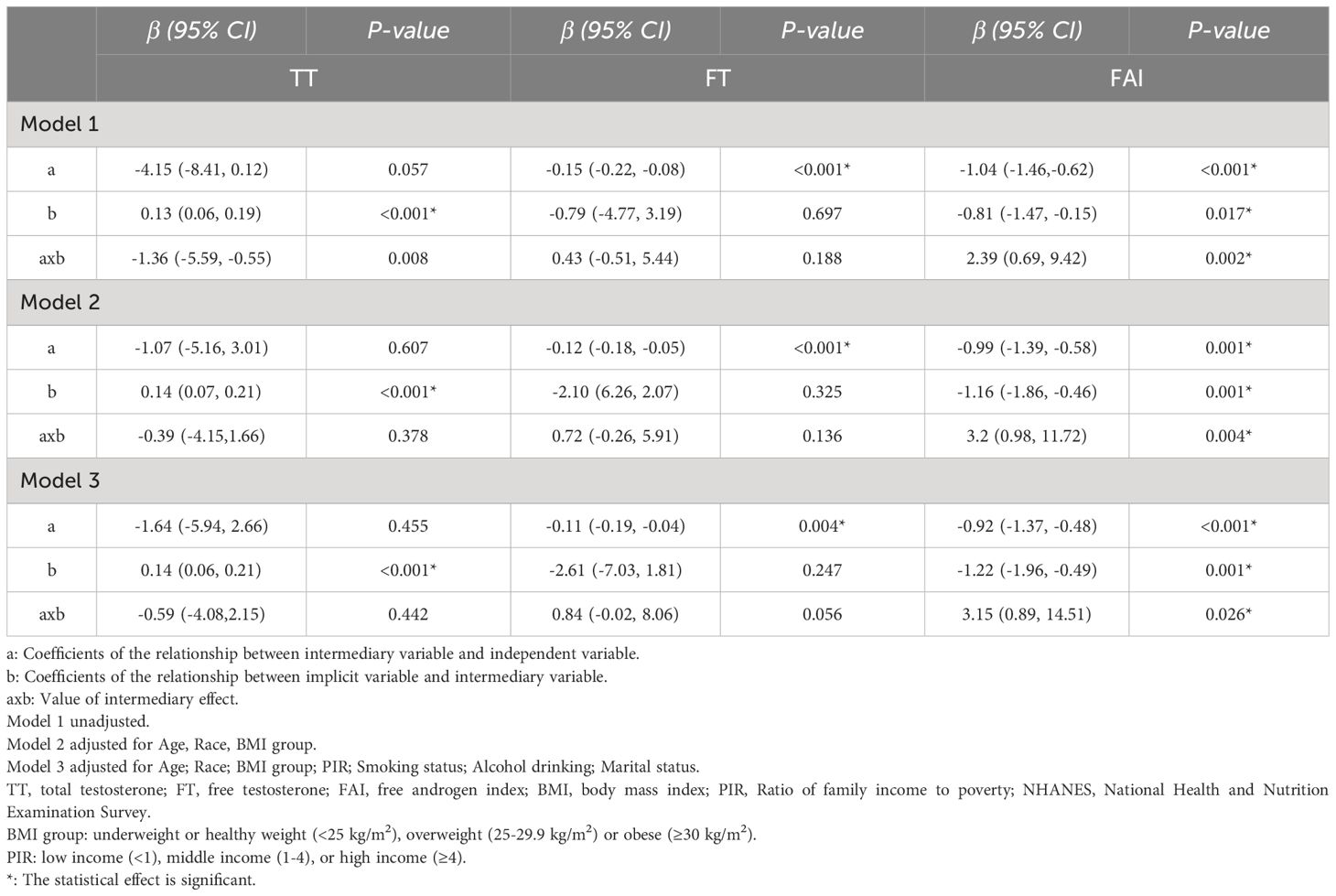
Table 3 Mediation analysis of the estimated effect of Dietary Inflammatory Index on the serum S-Klotho concentrations through Testosterone, FAI and FT among males in NHANES 2013-2016 (weighted).
Regarding TT, we found that the relation between DII and S-Klotho levels was significantly operated indirectly through TT (model 1: ab = -1.36; 95%Cl: -5.59, -0.55; p = 0.008), with a percentage of mediation of 9.23%. However, this mediating effect lost significance after adjustments (model 2: ab = -0.39; 95%Cl: -4.15,1.66; p = 0.378; model 3: ab = -0.59; 95%Cl: -4.08,2.15; p = 0.442). There was no significant association between TT and DII(model 1: a = -4.15; 95%Cl: -8.41, 0.12; p = 0.057; model 2: a = -1.07 95%Cl:-5.16, 3.01; p = 0.607; model 3: a = -1.64; 95%Cl: -5.94, 2.66; p = 0.455), but a positive direct association between TT and S-Klotho was observed (model 1: b = 0.13; 95%Cl: 0.06, 0.19; p <0.001; model 2: b = 0.14 95%Cl:0.07, 0.21; p <0.001; model 3: b = 0.14; 95%Cl: 0.06, 0.21; p<0.001).
For FT, the mediation analysis did not support a significant indirect influence of FT on the DII-S-Klotho relationship (model 1: ab = 0.43; 95%Cl: -0.51, 5.44; p = 0.188; model 2: ab = 0.72; 95%Cl: -0.26, 5.91; p = 0.136; model 3: ab = 0.84; 95%Cl: -0.02, 8.06; p = 0.056). There was a negative association between FT and DII(model 1: a = -0.15; 95%Cl: -0.22, -0.08; p <0.001; model 2: a = -0.12 95%Cl:-0.18, -0.05; p <0.001; model 3: a = -0.11; 95%Cl: -0.19, -0.04; p = 0.004), but no significant direct association was found between FT and S-Klotho levels (model 1: b =-0.79; 95%Cl: -4.77, 3.19; p = 0.697; model 2: b =-2.10 95%Cl:-6.26, 2.07; p =0.325; model 3: b = -2.61; 95%Cl: -7.03, 1.81; p= 0.247).
In the case of FAI, the mediation analysis established a significant indirect influence of FAI on the DII-S-Klotho relationship (model 1: ab = 2.39; 95%Cl: 0.69, 9.42; p = 0.002) and maintained stability after adjusted (model 2: ab = 3.2; 95%Cl: 0.98, 11.72; p = 0.004; model 3: ab = 3.15; 95%Cl: 0.89, 14.51; p = 0.026) with a percentage of mediation of -16.20%, -19.85% and -20.62% respectively. There was a negative association between FAI and DII(model 1: a = -1.04; 95%Cl: -1.46,-0.62; p <0.001; model 2: a = -0.99 95%Cl:-1.39, -0.58; p <0.001; model 3: a = -0.92; 95%Cl: -1.37, -0.48; p <0.001), as well as a negative direct association between FAI and S-Klotho levels (model 1: b =-0.81; 95%Cl: -1.47, -0.15; p = 0.017; model 2: b =-1.16; 95%Cl: -1.86, -0.46; p = 0.001; model 3: b = -1.22; 95%Cl: -1.96, -0.49; p= 0.001).
The study conducted mediation analyses to explore the potential impacts of testosterone (TT), free testosterone (FT), and free androgen index (FAI) on the relationship between the dietary inflammatory index (DII) and S-Klotho levels among males aged 40-79, using data from NHANES 2013-2014 and 2015-2016. Initially, a notable mediation effect was observed in the case of TT, indicating that the link between DII and S-Klotho levels was indirectly influenced by TT. This mediating influence accounted for a percentage of mediation. However, subsequent adjustments rendered this mediation effect statistically non-significant. While no direct significant correlation emerged between TT and DII, a positive direct association was detected between TT and S-Klotho levels. Conversely, the mediation analysis pertaining to FT failed to provide substantial support for an indirect impact of FT on the DII-S-Klotho relationship. Concurrently, a negative connection between FT and DII was established, though no significant direct association surfaced between FT and S-Klotho levels. On the other hand, the investigation into FAI yielded noteworthy outcomes, revealing a significant indirect influence of FAI on the DII-S-Klotho relationship through mediation. This mediating effect remained consistent even after adjustments were made and was quantified as a percentage of mediation. The alignment of our unadjusted findings with our hypothesis indicated that testosterone could potentially act as a causal mediator between DII and S-Klotho. Nevertheless, this effect weakened after accounting for covariates, possibly due to confounding factors such as Body Mass Index (BMI). Prior research, including systematic reviews and meta-analyses, has illuminated the strong connection between a pro-inflammatory diet and heightened annual weight gain, as well as a higher risk of developing overweight or obesity (29–31). Given the association between obesity and increased levels of various inflammatory markers, the presence of chronic low-grade inflammation in obesity can impact the production of testosterone and Sex Hormone-Binding Globulin (SHBG) through the hypothalamic-pituitary-gonadal axis (32–34). Studies on elderly males from the United States (35, 36) and Europe (37) have consistently demonstrated that obesity, as defined by parameters such as waist circumference, waist-to-hip ratio, or BMI, is correlated with declining levels of total and free testosterone.
The free hormone hypothesis emphasizes the biological activity of hormones based on free hormone concentrations in the plasma, rather than protein-bound hormones (38). Notably, observational studies within the EMAS cohort have underscored the importance of calculated Free Testosterone (FT) serum levels in understanding symptoms related to androgen deficiency in males (39). Contrary to expectations, our study showed that the mediating effect of FT on the DII-S-Klotho association did not reach statistical significance, but displayed a trend towards significance after controlling for confounding factors (p = 0.056). This intriguing finding suggests the potential for an independent mediating role of FT, warranting further investigation. The interplay between sex hormones, inflammatory markers, DII, and S-Klotho might involve complex interactions influenced by multifactorial conditions, necessitating comprehensive research for clarification.
Significantly, our study substantiated a robust mediating impact of the Free Androgen Index (FAI) on the DII-S-Klotho relationship, a result that persisted across model adjustments. FAI, albeit correlated with FT, remains distinct due to its dependence on measurements of TT and SHBG (40, 41). Despite its wide usage in clinical practice for assessing FT status in females, the applicability of FAI for estimating FT in males is limited due to assumptions inherent in its formula that are not applicable to males (42–44). As such, the statistical significance of FAI’s mediating effect should be interpreted with caution, as it might suggest an independent mediating role of FT in the DII-S-Klotho association.
The strengths of our study include the utilization of a nationally representative population and a relatively large sample size, enhancing the reliability and generalizability of our findings. Additionally, we employed liquid chromatography-tandem mass spectrometry (LC-MS/MS) for precise measurement of TT and the Vermeulen calculator for FT estimation, both of which improve accuracy and reliability. However, limitations must also be acknowledged. The cross-sectional nature of our study restricts causal inferences, necessitating prospective research. Furthermore, the reliance on 24-hour dietary recalls introduces recall bias, potentially affecting the DII and testosterone relationship. Additionally, measuring plasma testosterone at a single time point might not capture its dynamic fluctuations over time. The absence of crucial medical data, such as the usage of testosterone inhibitors, and a more precise assessment of potential confounding factors (e.g., alcohol intake assessment through the Alcohol Use Disorders Identification Test), could also introduce bias into the results.
In conclusion, the present study unveiled intricate connections among dietary inflammation, sex hormones, and S-Klotho levels, highlighting the mediating effects mediated by testosterone. Although the mediating effect of TT no longer had statistical significance after accounting for covariates, a positive association with S-Klotho levels remained evident. FT did not exhibit a significant mediating effect. Notably, the considerable mediating effect of FAI on the relationship between the DII and S-Klotho persisted even after adjusting for covariates. However, its significance warrants careful consideration due to its limited effect in males. These findings underscore the imperative for future investigations aimed at unraveling the intricate mechanisms that underlie the dynamic interplay between inflammation, hormonal factors, and the intricate processes related to aging.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The studies involving humans were approved by NHANES Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SD: Writing – original draft, Writing – review & editing. JZ: Writing – original draft, Writing – review & editing. XC: Writing – original draft, Writing – review & editing. JP: Data curation, Writing – original draft. QC: Writing – original draft, Writing – review & editing. YZ: Formal analysis, Writing – original draft, Writing – review & editing. AL: Conceptualization, Writing – original draft. XW: Funding acquisition, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Technology Innovation Research and Development Projects in Chengdu (No. 2019-YF05-00333-SN).
The authors have no acknowledgment to declare.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. (1997) 390:45–51. doi: 10.1038/36285
2. Cheikhi A, Barchowsky A, Sahu A, Shinde SN, Pius A, Clemens ZJ, et al. Klotho: an elephant in aging research. J Gerontol A Biol Sci Med Sci. (2019) 74:1031–42. doi: 10.1093/gerona/glz061
3. Kuro OM. The Klotho proteins in health and disease. Nat Rev Nephrol. (2019) 15:27–44. doi: 10.1038/s41581-018-0078-3
4. da Costa JP, Vitorino R, Silva GM, Vogel C, Duarte AC, Rocha-Santos T. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res Rev. (2016) 29:90–112. doi: 10.1016/j.arr.2016.06.005
5. Chalhoub D, Marques E, Meirelles O, Semba RD, Ferrucci L, Satterfield S, et al. Association of serum Klotho with loss of bone mineral density and fracture risk in older adults. J Am Geriatrics Society. (2016) 64:e304–e8. doi: 10.1111/jgs.14661
6. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Sci (New York NY). (2005) 309:1829–33. doi: 10.1126/science.1112766
7. Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. (2011) 66:794–800. doi: 10.1093/gerona/glr058
8. Kresovich JK, Bulka CM. Low serum Klotho associated with all-cause mortality among a nationally representative sample of American adults. Journals Gerontol Ser A Biol Sci Med Sci. (2022) 77:452–6. doi: 10.1093/gerona/glab308
9. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. (2006) 48:677–85. doi: 10.1016/j.jacc.2006.03.052
10. Kotemori A, Sawada N, Iwasaki M, Yamaji T, Shivappa N, Hebert JR, et al. Validating the dietary inflammatory index using inflammatory biomarkers in a Japanese population: A cross-sectional study of the JPHC-FFQ validation study. Nutr (Burbank Los Angeles County Calif). (2020) 69:110569. doi: 10.1016/j.nut.2019.110569
11. Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, et al. Development and validation of an empirical dietary inflammatory index. J Nutr. (2016) 146:1560–70. doi: 10.3945/jn.115.228718
12. Shivappa N, Hebert JR, Marcos A, Diaz LE, Gomez S, Nova E, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res. (2017) 61:2. doi: 10.1002/mnfr.201600707
13. Xie R, Ning Z, Xiao M, Li L, Liu M, Zhang Y. Dietary inflammatory potential and biological aging among US adults: a population-based study. Aging Clin Exp Res. (2023) 35:1273–81. doi: 10.1007/s40520-023-02410-1
14. Zhang C, Zhang Z, Li J, Deng L, Geng J, Jin K, et al. Association between Dietary Inflammatory Index and serum Klotho concentration among adults in the United States. BMC Geriatrics. (2022) 22:528. doi: 10.1186/s12877-022-03228-8
15. Hsu SC, Huang SM, Lin SH, Ka SM, Chen A, Shih MF, et al. Testosterone increases renal anti-aging klotho gene expression via the androgen receptor-mediated pathway. Biochem J. (2014) 464:221–9. doi: 10.1042/BJ20140739
16. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. (2004) 89:3313–8. doi: 10.1210/jc.2003-031069
17. Tremellen K, McPhee N, Pearce K, Benson S, Schedlowski M, Engler H. Endotoxin-initiated inflammation reduces testosterone production in men of reproductive age. Am J Physiol Endocrinol Metab. (2018) 314:E206–e13. doi: 10.1152/ajpendo.00279.2017
18. Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol. (2002) 57:3–18. doi: 10.1016/S0165-0378(02)00020-7
19. Zhang C, Bian H, Chen Z, Tian B, Wang H, Tu X, et al. The association between dietary inflammatory index and sex hormones among men in the United States. J Urol. (2021) 206:97–103. doi: 10.1097/JU.0000000000001703
20. Qin Z, Liu N, Liao R, Jiang L, Su B. The association between dietary inflammatory potential and sex hormones in male children and adolescents aged 6-19 years. Front Endocrinol. (2021) 12:722941. doi: 10.3389/fendo.2021.722941
21. Zhang Z, Qiu S, Huang X, Jin K, Zhou X, Lin T, et al. Association between testosterone and serum soluble α-klotho in U.S. males: a cross-sectional study. BMC Geriatrics. (2022) 22:570. doi: 10.1186/s12877-022-03265-3
22. Dote-Montero M, Amaro-Gahete FJ, De-la OA, Jurado-Fasoli L, Gutierrez A, Castillo MJ. Study of the association of DHEAS, testosterone and cortisol with S-Klotho plasma levels in healthy sedentary middle-aged adults. Exp Gerontol. (2019) 121:55–61. doi: 10.1016/j.exger.2019.03.010
23. Manning F, Jack A, Jacob F, Gail F, Peter H, Robert I, et al. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat Ser 1 Programs Collection Procedures. (1994) 32):1–407.
24. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
25. Cienfuegos S, Corapi S, Gabel K, Ezpeleta M, Kalam F, Lin S, et al. Effect of intermittent fasting on reproductive hormone levels in females and males: A review of human trials. Nutrients. (2022) 14:3. doi: 10.3390/nu14112343
26. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. (1999) 84:3666–72. doi: 10.1210/jcem.84.10.6079
27. Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monographs. (2009) 76:408–20. doi: 10.1080/03637750903310360
28. Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach Vol. xvii. . New York, NY, US: Guilford Press (2013) p. 507–xvii.
29. Yi Q, Li X, He Y, Xia W, Shao J, Ye Z, et al. Associations of dietary inflammatory index with metabolic syndrome and its components: a systematic review and meta-analysis. Public Health Nutr. (2021) 24:5463–70. doi: 10.1017/S1368980021000288
30. Farhangi MA, Vajdi M. The association between dietary inflammatory index and risk of central obesity in adults: An updated systematic review and meta-analysis. Int J Vitam Nutr Res. (2020) 90:535–52. doi: 10.1024/0300-9831/a000648
31. Ramallal R, Toledo E, Martínez JA, Shivappa N, Hébert JR, Martínez-González MA, et al. Inflammatory potential of diet, weight gain, and incidence of overweight/obesity: The SUN cohort. Obes (Silver Spring). (2017) 25:997–1005. doi: 10.1002/oby.21833
32. Khanna D, Khanna S, Khanna P, Kahar P, Patel BM. Obesity: A chronic low-grade inflammation and its markers. Cureus. (2022) 14:e22711. doi: 10.7759/cureus.22711
33. de Mello RN, de Gois BP, Kravchychyn ACP, Dâmaso AR, Horst MA, Lima GC, et al. Dietary inflammatory index and its relation to the pathophysiological aspects of obesity: a narrative review. Arch Endocrinol Metab. (2023) 67:e000631. doi: 10.20945/2359-3997000000631
34. Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab. (1994) 79:997–1000. doi: 10.1210/jcem.79.4.7962311
35. Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol. (2006) 65:125–31. doi: 10.1111/j.1365-2265.2006.02560.x
36. Cooper LA, Page ST, Amory JK, Anawalt BD, Matsumoto AM. The association of obesity with sex hormone-binding globulin is stronger than the association with ageing – implications for the interpretation of total testosterone measurements. Clin Endocrinol. (2015) 83:828–33. doi: 10.1111/cen.12768
37. Wu FCW, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European male aging study. J Clin Endocrinol Metab. (2008) 93:2737–45. doi: 10.1210/jc.2007-1972
38. Mendel CM. The free hormone hypothesis: A physiologically based mathematical model*. Endocr Rev. (1989) 10:232–74. doi: 10.1210/edrv-10-3-232
39. Antonio L, Wu FCW, O'Neill TW, Pye SR, Ahern TB, Laurent MR, et al. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab. (2016) 101:2647–57. doi: 10.1210/jc.2015-4106
41. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Utility, limitations, and pitfalls in measuring testosterone: an endocrine society position statement. J Clin Endocrinol Metab. (2007) 92:405–13. doi: 10.1210/jc.2006-1864
42. Kapoor P, Luttrell BM, Williams D. The free androgen index is not valid for adult males. J Steroid Biochem Mol Biol. (1993) 45:325–6. doi: 10.1016/0960-0760(93)90350-6
43. Vermeulen A. Reflections concerning biochemical parameters of androgenicity. Aging Male. (2004) 7:280–9. doi: 10.1080/13685530400016615
Keywords: klotho, testosterone, dietary inflammatory index, NHANES, male, mediating effect
Citation: Du S, Zhao J, Chou X, Peng J, Cao Q, Zeng Y, Ao L and Wang X (2024) Testosterone does not mediate the correlation between dietary inflammation and serum klotho levels among males: insights from NHANES database. Front. Endocrinol. 15:1370457. doi: 10.3389/fendo.2024.1370457
Received: 18 January 2024; Accepted: 18 March 2024;
Published: 02 April 2024.
Edited by:
Masashi Tanaka, Health Science University, JapanReviewed by:
Zhou-Xin Yang, Zhejiang Hospital, ChinaCopyright © 2024 Du, Zhao, Chou, Peng, Cao, Zeng, Ao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Cao, q.cao_6362@scu.edu.cn
†These authors have contributed equally to this work and share the first authorship
‡ORCID: Qi Cao, orcid.org/0000-0001-6970-8654
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.