- 1Department of Cardiology, Shaanxi Provincial People’s Hospital, Xi’an, China
- 2Department of Internal Medicine, Xi’an Jiaotong University Hospital, Xi’an, China
- 3Department of Cardiology, Pucheng County Hospital, Weinan, China
- 4Department of Interventional Radiography, Shanxi Provincial People’s Hospital, Xi’an, China
Background: Increased levels of serum Klotho have been associated with a reduced risk of several cardiovascular diseases (CVD). However, limited studies exist on the association between serum Klotho and mortality in patients with CVD.
Methods: We collected data from CVD patients in the National Health and Nutrition Examination Survey (NHANES) spanning 2007 to 2016. We linked NHANES data with the National Death Index to determine the survival status of participants. Univariate and multivariable Cox regression models were used to investigate the relationship between serum Klotho levels and mortality in CVD patients. The relationship between serum Klotho quartiles and mortality in CVD patients was visualized using Kaplan-Meier (KM) curves and restricted cubic spine. Finally, subgroup analyses were used to examine the association between serum Klotho and all-cause mortality in different populations.
Results: 1905 patients with CVD were finally enrolled in our study with a mean follow-up of 7.1 years. The average age of the participants was 63.4 years, with 58.40% being male. KM showed that lower Klotho levels were associated with lower survival rates. After adjusting for potential confounders, patients with higher serum Klotho levels had lower all-cause mortality (Q1: 1.00, Q2: 0.58 (0.42–0.80), Q3: 0.69 (0.47–1.01), and Q4:0.64 (0.45–0.92). However, the relationship between serum Klotho levels and cardiovascular mortality was not statistically significant. Dose-response analysis shows a U-shaped relationship between serum Klotho levels and all-cause mortality in patients with CVD (P nonlinear=0.002). Subgroup analysis indicated that participants with a history of hypertension had a higher risk of all-cause mortality in serum Klotho Q4 compared to Q1 (P trend <0.05).
Conclusion: The relationship between serum Klotho levels and all-cause mortality in CVD patients exhibits a U-shaped association. The underlying mechanisms of this association need further investigation.
Introduction
Cardiovascular disease (CVD) is a major cause of global disease burden and a key factor contributing to premature death and escalating healthcare costs (1, 2). CVD encompasses a range of conditions including heart failure, coronary heart disease, angina, heart attacks, and strokes. Identifying factors that predict mortality in patients with CVD is critical to promoting early prevention of the disease.
The Klotho protein is encoded by the Klotho gene, initially described for its anti-aging abilities. Mice lacking Klotho exhibit a range of syndromes resembling human aging, including shortened lifespan, infertility, vascular calcification, arteriosclerosis, skin atrophy, osteoporosis, and emphysema (3). Conversely, overexpression of Klotho can act as an anti-aging hormone (4). Two different types of Klotho proteins can be detected in humans: one located in the cytoplasmic membrane as a transmembrane protein, and the other as soluble Klotho, including soluble and secreted Klotho circulating in the blood (5). Numerous studies have shown that increased serum Klotho levels are closely associated with decreased cardiovascular risk. In middle-aged and elderly Americans, serum Klotho levels are negatively correlated with the presence of atrial fibrillation (6). Studies in diabetic mice have found that serum Klotho levels can improve diabetic cardiomyopathy and myocardial fibrosis (7, 8). In a large and diverse cohort, an independent inverse relationship was observed between serum Klotho levels and arterial stiffness indicator, pulse pressure (9). Serum Klotho, as a cardiac protector, can prevent the effects of aging on the heart and reduce the burden of CVD (10), but there is currently no research analyzing the relationship between serum Klotho levels and all-cause mortality and cardiovascular mortality in CVD patients.
By examining data from the National Health and Nutrition Examination Survey (NHANES), this study took into account potential confounding factors and investigated the relationship between serum Klotho levels and mortality in patients with CVD. This has important implications for the management of the health of patients with CVD.
Method
Study population
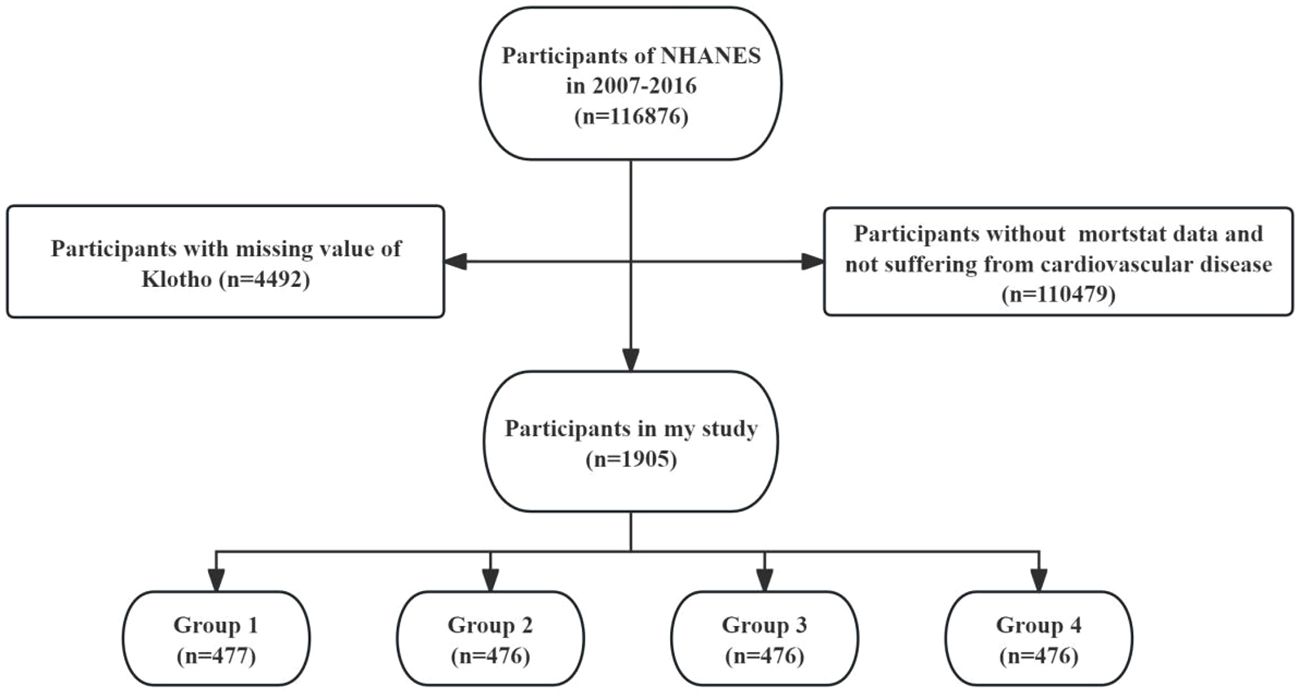
The data used in this study were obtained from the NHANES database (https://www.cdc.gov/nchs/nhanes/index.htm). NHANES is a vital national health and nutrition survey program conducted by the U.S. government. NHANES collects a vast amount of medical, nutritional, and health-related data through population surveys and physical examinations, providing a rich source of information for research in the field of public health. In this study, we used data from 5 cycles, involving a total of 116,876 participants from 2007 to 2016. After excluding individuals with missing mortality data and without cardiovascular disease (n = 110,479), and those with missing serum Klotho data (n = 4,492), a total of 1,905 participants with CVD were included in the final analysis. The selection process of this study is illustrated in Figure 1.
Outcome variable
CVD was defined as individuals who answered “yes” to the question: “Have you been told by a doctor or other health professional that you have congestive heart failure/CHD/myocardial infarction/stroke?”. All-cause mortality and cardiovascular mortality were obtained from the National Death Index (NDI) database as of December 31, 2019.
Exposure variable
Participants’ blood samples were collected in NHANES (National Health and Nutrition Examination Survey) and stored at -80°C until analyzed. Klotho concentrations in serum were measured using a commercially available ELISA kit (product offered by IBL International, Japan). The lower limit of detection for Klotho in this kit is typically set at 6 pg/mL prior to the assay, frozen serum samples were thawed and the manufacturer’s protocol was followed. Each sample is usually assayed twice to ensure accuracy and reproducibility of results. Two concentration levels of quality control samples (low and high Klotho concentrations) were included in the assay. If a sample’s duplicate assay value varies by more than 10%, that sample is subject to re-assay. If the value of the quality control sample is outside the range of two standard deviations from the known value, the result of the entire assay panel will be considered invalid and will need to be re-assayed. The final determination is expressed as the average of two measurements (11). For a detailed description of the Klotho detection method, please visit the NHANES website.
Covariates
We performed covariate inclusion through previous literature. Demographic, laboratory, physical examination, and questionnaire data were collected from the NHANES database. Age, poverty income ratio (PIR), and body mass index (BMI) were considered continuous variables (12, 13). Sex, race, smoking status, drinking status, education level, marital status, hypertension, and type 2 diabetes mellitus (DM) were considered categorical variables (14–16). Race was categorized as Mexican American, non-Hispanic Black, non-Hispanic White, other Hispanic, and other Race. Smoking status was categorized as former smoker, never smoker, and current smoker based on whether the participant had smoked 100 cigarettes in the past year. Drinking status was defined as non-drinker, low-to-moderate drinker, and heavy drinker. Education level was categorized as below high school, high school, and above high school. Marital status was classified as separated, married, and never married. Type 2 DM was defined according to the following criteria: (1) participants reporting a diagnosis of diabetes by a healthcare professional, (2) glycated hemoglobin testing 6.5% or higher, or (3) fasting blood glucose 126 mg/dL or higher. Hypertension was defined as self-reported hypertension, systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medication.
Statistical analysis
Continuous variables were expressed as mean ± standard error and differences between groups were compared using weighted t-tests or one-way ANOVA. Categorical variables were expressed as frequencies and percentages, and differences between groups were compared using Chi-square tests. Univariate and multivariate Cox proportional hazards regression models were used to examine the association between serum Klotho levels and all-cause mortality in patients with CVD. In this study, we used three models: Crude model (unadjusted), Model 1 (adjusted for age, gender, and race), and Model 2 (further adjusted on the basis of model 2 for PIR, education level, BMI, hypertension, type 2 DM, drinking status, and smoking status). Kaplan-Meier (KM) curves were used to show the association between serum Klotho levels and all-cause mortality in patients with CVD when stratified by serum Klotho quartiles. In addition, restricted cubic spline curves (RCS) adjusted for confounders were utilized to show the dose-response relationship between serum Klotho levels and mortality in CVD patients. Finally, we also performed subgroup analyses and interaction tests to examine the relationship between serum Klotho levels and all-cause mortality in CVD populations with different characteristics.
All results of this study were based on clustering, stratification, and weighting calculations from the NHANES database and were analyzed using R software (version: 4.1.3) and the survey package (version: 4.4–1). A two-tailed p value of less than 0.05 was considered statistically significant.
Result
Baseline characteristics
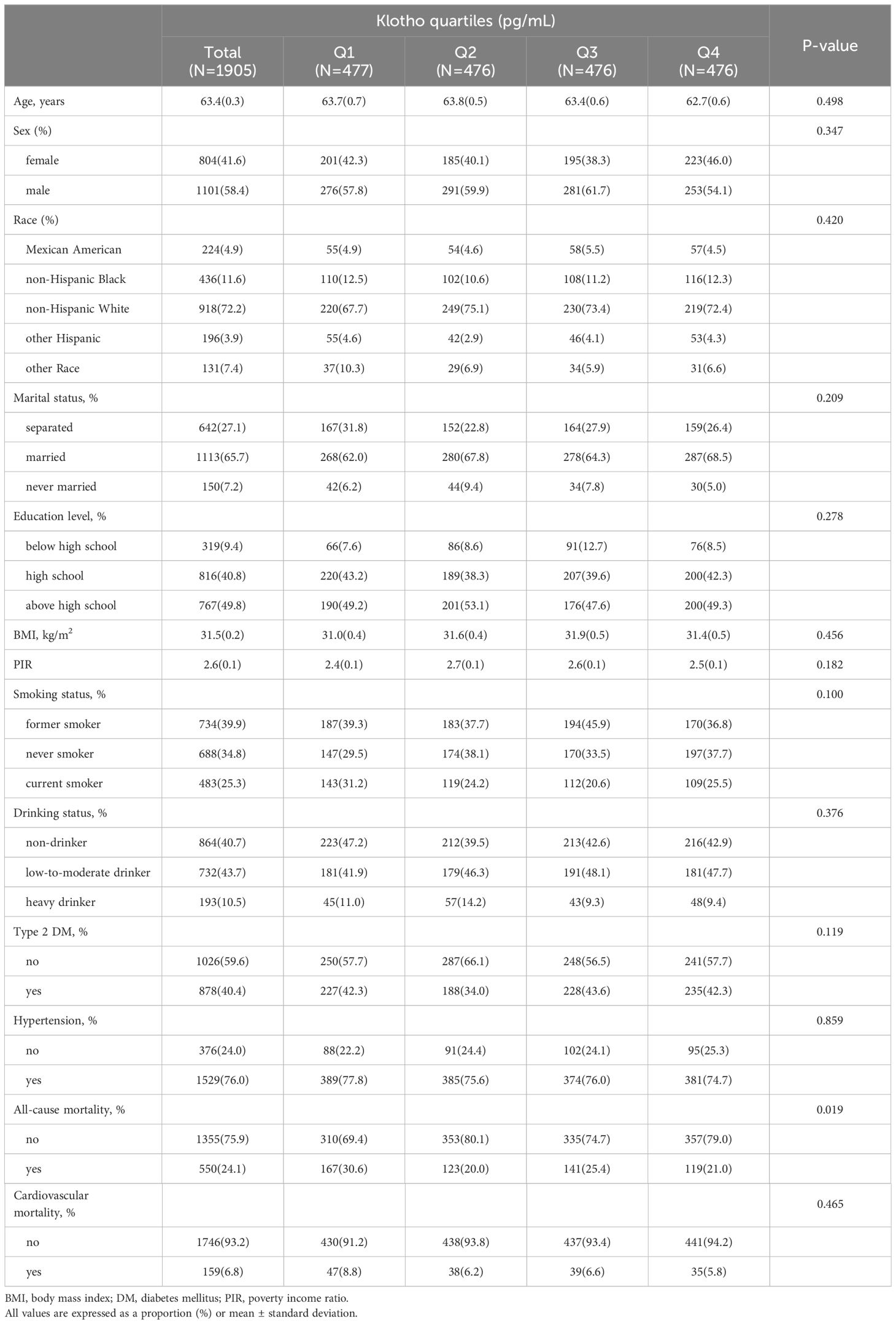
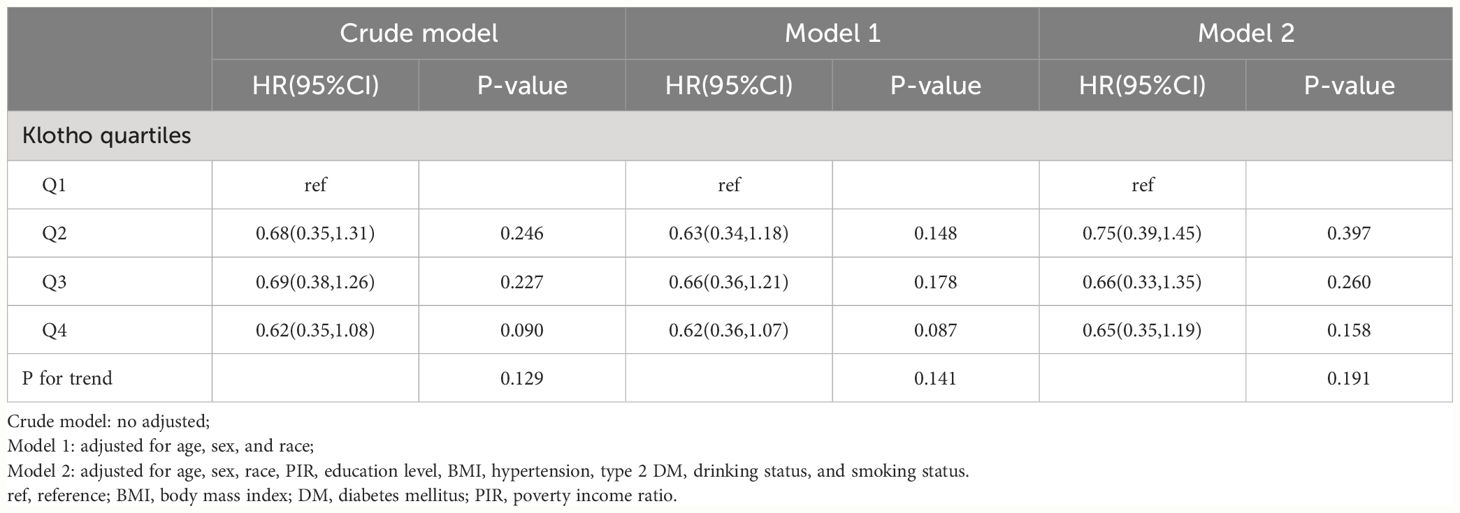
A total of 1905 subjects were enrolled in this study, with an average age of 63.4 years, 58.40% were male, and a mean follow-up time of 7.1 years (range 0.1 to 13.2 years) (Table 1). Serum Klotho levels were categorized into four groups based on quartiles (Q1 ≤ 614.1, 614.1 < Q2 ≤ 763.4, 763.4 < Q3 ≤ 946.2, 946.2 < Q4, pg/mL). There were no significant differences among the four groups of participants in terms of age, sex, race, marital status, education level, BMI, PIR, smoking status, drinking status, type 2 DM, hypertension, or cardiovascular mortality (P>0.05). Participants in higher quartiles of serum Klotho exhibited a lower all-cause mortality(Q1: 30.63%, Q2:19.95%, Q3:25.35%, Q4: 20.98%, P <0.05).
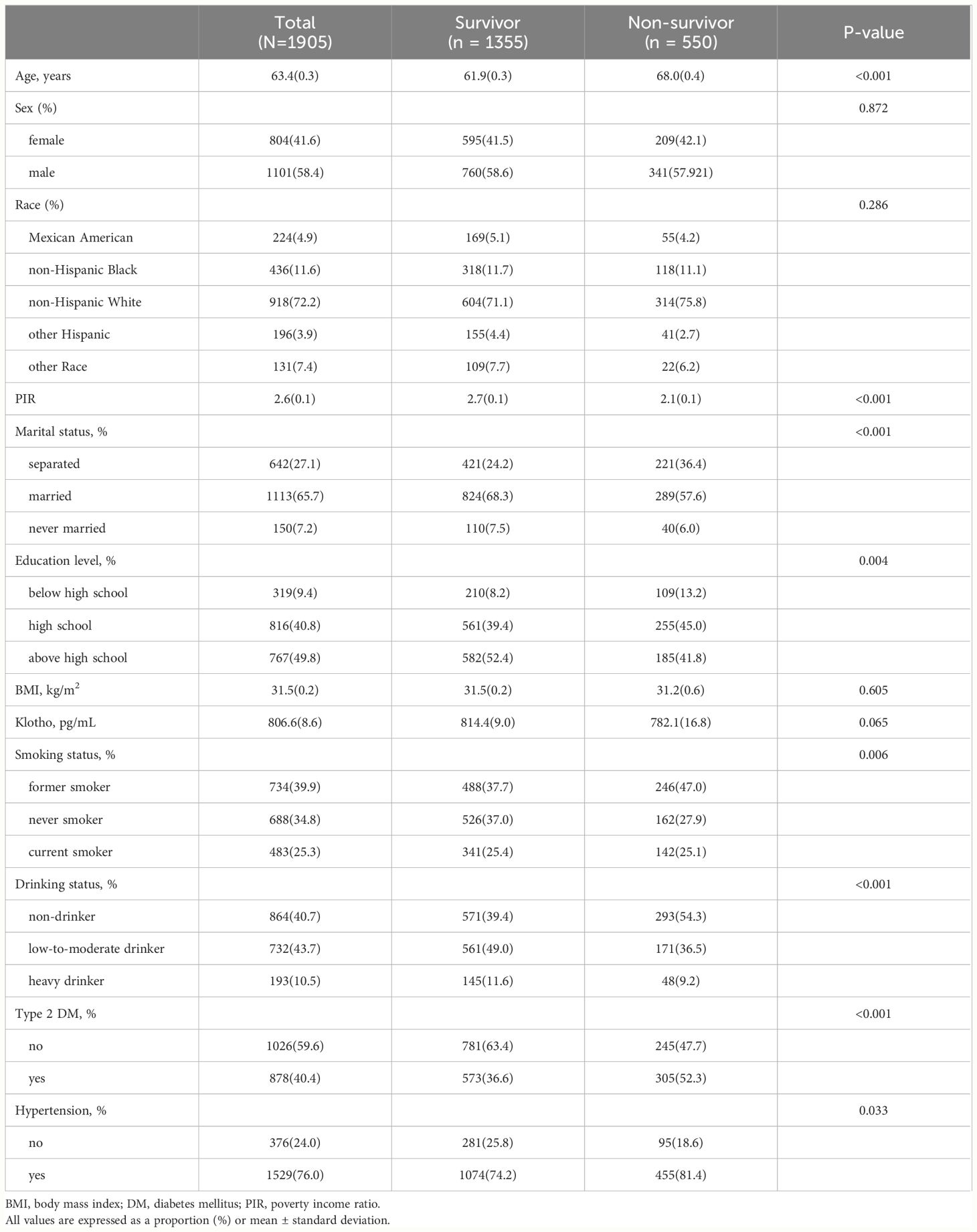
Table 2 stratified the participants into two groups based on their survival status, with 1355 individuals in the survival group and 55 individuals in the non-survival group. The two groups differed significantly in terms of age, marital status, education level, PIR, smoking status, drinking status, type 2 DM, hypertension, and cardiovascular mortality (P<0.05). Compared with the survivor group, the non-survivor group had a higher percentage of patients who were older, had lower PIR and education level, smoked, non-drinking, type 2 diabetes, and hypertension. In addition, cardiovascular mortality was higher in the non-survivor group.
The relationship between serum Klotho and mortality in CVD patients
According to the KM curve stratified by quartiles based on serum Klotho levels, the all-cause mortality in the lowest quartile (Q1) was significantly higher than in the other three groups (P = 0.014), but there was no difference in cardiovascular mortality (P >0.05) (Figure 2).
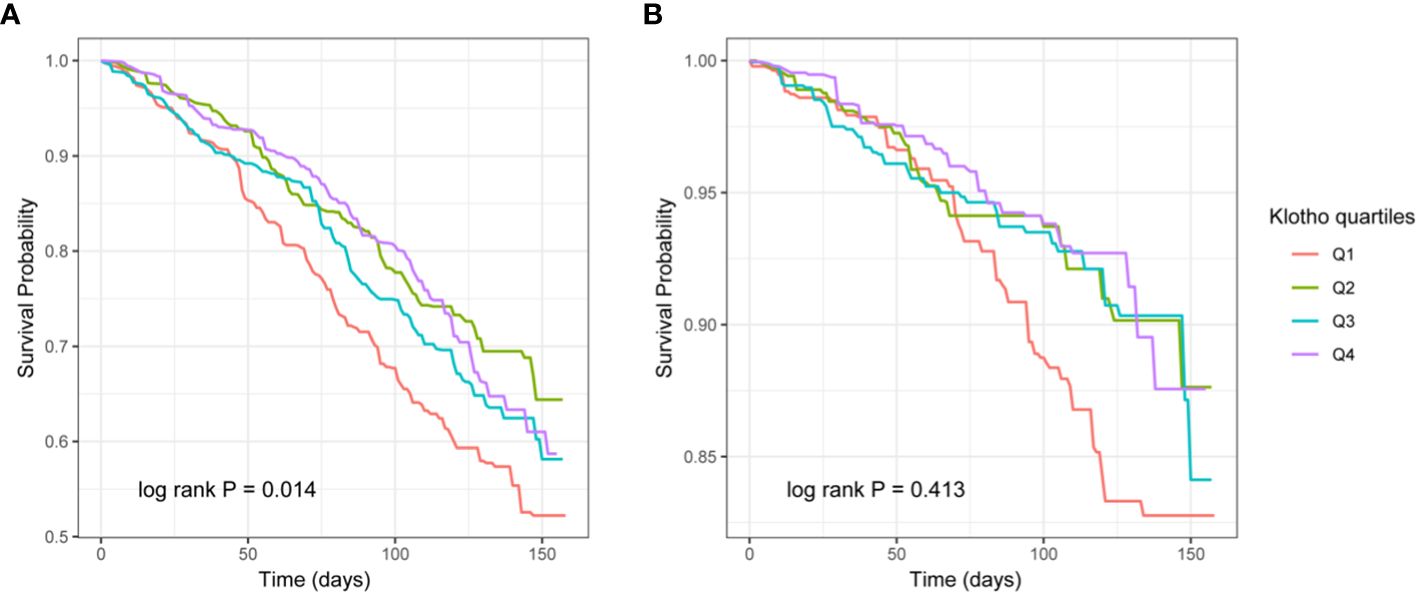
Figure 2 The Kaplan–Meier (KM) survival curves classified by Klotho quartiles (A: all-cause mortality, B: cardiovascular mortality).
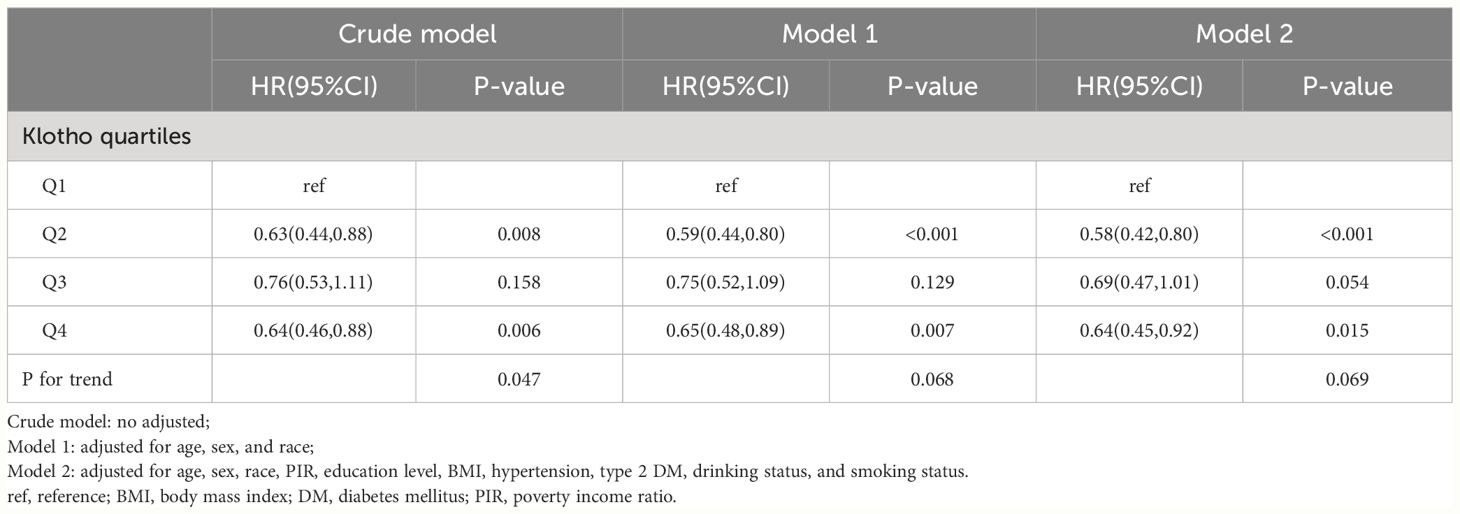
Further multivariate COX regression was used to analyze the independent association between serum Klotho levels and mortality (Table 3). In CVD patients, an inverse association was observed between serum Klotho levels and all-cause mortality across quartiles (Table 3). Compared with Q1, all-cause mortality was significantly lower in those with higher serum Klotho levels in fully adjusted Model 2 (Q2: 0.58 (0.42–0.80), Q3: 0.69 (0.47–1.01), and Q4: 0.64 (0.45–0.92), and the test for trend was no difference in the quartiles. However, in the study of cardiovascular mortality, no significant association was found between quartiles in the fully adjusted Model 2 (P>0.05) (Table 4).
Dose-response relationship between serum Klotho and mortality
RCS was used to portray a dose-response association between serum Klotho levels and all-cause and cardiovascular mortality in patients with CVD (Figure 3). There was a significant nonlinear association between serum Klotho and all-cause mortality and an insignificant association with cardiovascular mortality (all-cause mortality: nonlinear P = 0.002, cardiovascular mortality: overall P>0.05). When serum Klotho levels were below 763.4 pg/mL, all-cause mortality decreased with increasing serum Klotho levels, and when Klotho was above 763.4 pg/mL, all-cause mortality increased with increasing serum Klotho levels.
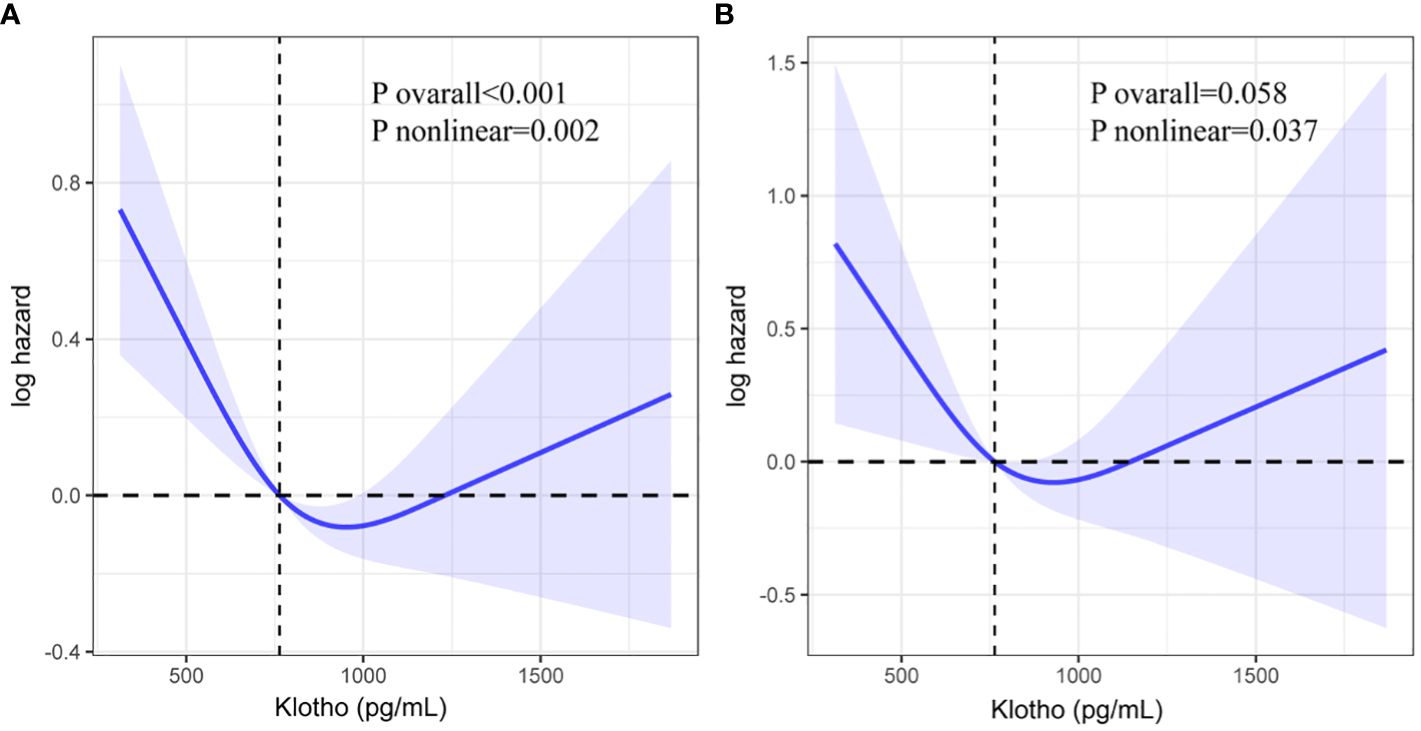
Figure 3 Restricted cubic spline (RCS) regression analysis of Klotho with all-cause mortality (A: all-cause mortality, B: cardiovascular mortality).
Subgroup analysis
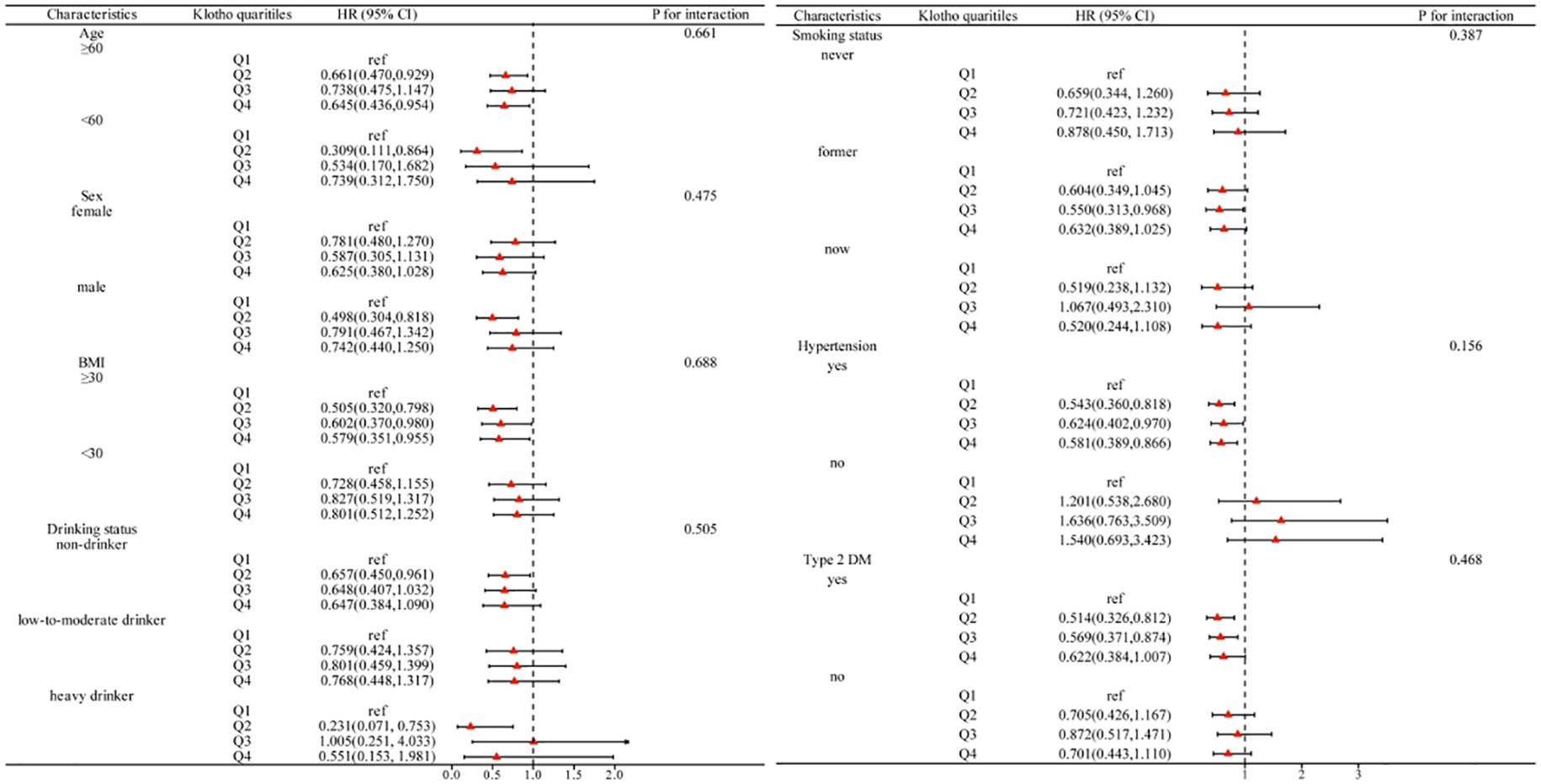
As depicted in Figure 4, a subgroup analysis was performed considering age, sex, BMI, drinking status, smoking status, hypertension, and type 2 DM. The inverse association between serum Klotho and all-cause mortality is maintained in most subgroups, and the interaction did not show differences between subgroups (P for interaction >0.05).
Discussion
Our study represents the first investigation into the relationship between Klotho levels and mortality among CVD patients within a large population. This extensive prospective cohort study analyzed data from 1905 CVD patients included in NHANES spanning from 2007 to 2016. Upon adjusting for confounding variables, we observed that higher all-cause mortality in CVD patients was linked to lower serum Klotho levels, a finding reinforced by KM curves. Notably, serum Klotho levels did not exhibit a statistically significant association with cardiovascular mortality in CVD patients. RCS curves revealed a U-shaped correlation between serum Klotho levels and all-cause mortality in CVD patients.
Serum Klotho, an aging-related protein, is characterized by a gradual decline in levels with advancing age (17). Studies have identified its protective effects against various degenerative diseases. Notably, Serum Klotho has exhibited kidney-protective properties in animal models of acute kidney injury and chronic kidney disease (18). Nephritis mice overexpressing Klotho transgene showed improved survival rates and kidney function, along with decreased morphological alterations in renal tubules and glomeruli (19). Additionally, the kidney-protective role of serum Klotho was evident in mice with renal fibrosis, where treatment with serum Klotho protein hindered the fibrotic process (20). Furthermore, Serum Klotho has demonstrated significant involvement in degenerative lung diseases, with serum Klotho shielding the lungs from oxidative damage and cell apoptosis by enhancing the endogenous antioxidant capacity of pulmonary epithelial cells (21, 22). In the realm of cancer research, investigations have revealed an inverse relationship between serum Klotho levels and cancer risk (23). Additionally, serum Klotho has been suggested to function as a tumor suppressor gene by modulating cell metabolism and various carcinogenic pathways (24).
Experimental studies conducted on laboratory animals have highlighted the direct protective impact of serum Klotho on the cardiovascular system. Notably, Klotho-deficient mice have demonstrated arterial wall calcification (3), a risk that can be mitigated by serum Klotho supplementation resulting in decreased blood phosphate levels (25). Additionally, cardiac dysfunction, as evidenced by hypertrophy and fibrosis in Klotho-deficient mice, can be ameliorated by supplementing serum Klotho (26, 27). Clinical investigations involving patients undergoing non-emergent coronary angiography revealed an association between lower levels of serum Klotho and the presence as well as the severity of coronary artery disease (28). Furthermore, studies involving hypertensive patients showed that lower serum Klotho levels were linked to an increased risk of all-cause mortality (29).
Our findings suggest that serum Klotho levels are associated with all-cause mortality but not cardiovascular mortality in a cardiovascular disease population. Similar results were obtained in a large-scale study involving hypertensive patients, where serum Klotho levels were linked to all-cause mortality in this population but not to cardiovascular mortality (29). While two independent studies identified serum Klotho as a predictive factor for cardiovascular mortality and all-cause mortality in chronic hemodialysis patients (30, 31), it is important to note that the cardiovascular death risk in this patient population is significantly elevated compared to the general population (32).
The observed negative association between serum Klotho levels and all-cause mortality in CVD patients can be elucidated through the mechanism of oxidative stress. The Klotho protein exhibits both antioxidant and anti-apoptotic properties, thereby exerting inhibitory effects on insulin, transforming growth factor-beta 1 signaling pathways, and the release of pro-inflammatory cytokine interleukin 6. These actions serve to inhibit oxidative stress, mitigate inflammation, and prevent fibrotic effects, ultimately contributing to a reduction in the risk of all-cause mortality (20, 33–37).
Our findings reveal a U-shaped relationship between serum Klotho levels and all-cause mortality in patients with CVD, a trend that aligns with previous research. Studies involving middle-aged and older adults have similarly reported an elevated risk of death for individuals at the extremes of Klotho levels (38). Furthermore, investigations in populations with type 2 DM and rheumatoid arthritis have also identified a U-shaped association between serum Klotho levels and the risk of mortality (39, 40). Given serum Klotho’s crucial role in mineral regulation and inflammation prevention within the body (41), it is hypothesized that individuals with extreme serum Klotho levels may experience disruption in their physiological balance, subsequently increasing the risk of death.
While our research utilizing a nationally representative population provided valuable insights into the association between serum Klotho levels and the risk of death in CVD patients, it is important to acknowledge several limitations when interpreting the results. Firstly, serum Klotho levels were measured only once, and there may be other factors interfering with the accuracy of the measurement results. Secondly, the use of stored residual serum for measuring serum Klotho may introduce measurement bias stemming from sample quality issues. Thirdly, despite adjusting for confounding factors like vital signs, lifestyle habits, and comorbidities, the presence of residual confounders could potentially impact our outcomes. As a result, prospective studies are necessary to validate and confirm the findings presented in our research.
Conclusion
Our study conducted on a nationally representative sample of American CVD patients revealed a U-shaped relationship between serum Klotho levels and all-cause mortality in this specific population. These findings offer valuable insights for prognostic assessment and treatment strategies tailored to individuals with CVD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SL: Writing – original draft. ZZ: Writing – original draft. KY: Writing – original draft. WZ: Writing – original draft. JP: Writing – original draft. YL: Writing – original draft. ZT: Writing – original draft. FL: Writing – original draft. YS: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Qin Chuangyuan Traditional Chinese Medicine Innovation Research and Development Transformation Project (No.2022-QCYZH-022), the Key Basic Natural Science Foundation of Shaanxi Province (No. 2022JZ-47), and the Natural Science Foundation of Shaanxi Province (No.2023-YBSF-653).
Acknowledgments
We acknowledge the staff at the National Center for Health Statistics at the CDC, who design, collect, and administer the NHANES data and release the data available for public use. We are thankful to all study participants for their cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: A compass for future health. J Am Coll Cardiol. (2022) 80:2361–71. doi: 10.1016/j.jacc.2022.11.005
2. Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
3. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. (1997) 390:45–51. doi: 10.1038/36285
4. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science. (2005) 309:1829–33. doi: 10.1126/science.1112766
5. Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. (2010) 24:3438–50. doi: 10.1096/fj.10-154765
6. Cai J, Zhang L, Chen C, Ge J, Li M, Zhang Y, et al. Association between serum Klotho concentration and heart failure in adults, a cross-sectional study from NHANES 2007–2016. Int J Cardiol. (2023) 370:236–43. doi: 10.1016/j.ijcard.2022.11.010
7. Li X, Li Z, Li B, Zhu X, Lai X. Klotho improves diabetic cardiomyopathy by suppressing the NLRP3 inflammasome pathway. Life Sci. (2019) 234:116773. doi: 10.1016/j.lfs.2019.116773
8. Alfonso-Munoz EA, Burggraaf-Sanchez de Las Matas R, Mataix Boronat J, Molina Martin JC, Desco C. Role of oral antioxidant supplementation in the current management of diabetic retinopathy. Int J Mol Sci. (2021) 22(8):4020. doi: 10.3390/ijms22084020
9. Alkalbani M, Prabhu G, Lagbo J, Qayyum R. Serum Klotho and pulse pressure; insight from NHANES. Int J Cardiol. (2022) 355:54–8. doi: 10.1016/j.ijcard.2022.02.021
10. Xie J, Cha SK, An SW, Kuro OM, Birnbaumer L, Huang CL. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun. (2012) 3:1238. doi: 10.1038/ncomms2240
11. Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. (2010) 398:513–8. doi: 10.1016/j.bbrc.2010.06.110
12. North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. (2012) 110:1097–108. doi: 10.1161/CIRCRESAHA.111.246876
13. Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. (2020) 395:795–808. doi: 10.1016/S0140-6736(19)32008-2
14. Headen AC, Siaw-ASamoah A, Julien HM. Race and modifiable factors influencing cardiovascular disease. Med Clin North Am. (2022) 106:401–9. doi: 10.1016/j.mcna.2021.11.008
15. Shufelt CL, Pacheco C, Tweet MS, Miller VM. Sex-specific physiology and cardiovascular disease. Adv Exp Med Biol. (2018) 1065:433–54. doi: 10.1007/978-3-319-77932-4
16. Dhindsa DS, Khambhati J, Schultz WM, Tahhan AS, Quyyumi AA. Marital status and outcomes in patients with cardiovascular disease. Trends Cardiovasc Med. (2020) 30:215–20. doi: 10.1016/j.tcm.2019.05.012
17. Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. (2011) 66:794–800. doi: 10.1093/gerona/glr058
18. Kuro OM. The Klotho proteins in health and disease. Nat Rev Nephrol. (2019) 15:27–44. doi: 10.1038/s41581-018-0078-3
19. Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U.S.A. (2007) 104:2331–6. doi: 10.1073/pnas.0611079104
20. Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. (2011) 286:8655–65. doi: 10.1074/jbc.M110.174037
21. Ravikumar P, Ye J, Zhang J, Pinch SN, Hu MC, Kuro-o M, et al. alpha-Klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol. (2014) 307:L566–75. doi: 10.1152/ajplung.00306.2013
22. Ravikumar P, Li L, Ye J, Shi M, Taniguchi M, Zhang J, et al. alphaKlotho deficiency in acute kidney injury contributes to lung damage. J Appl Physiol. (1985) 120:723–32. doi: 10.1152/japplphysiol.00792.2015
23. Qiao Y, Liu F, Peng Y, Wang P, Ma B, Li L, et al. Association of serum Klotho levels with cancer and cancer mortality: Evidence from National Health and Nutrition Examination Survey. Cancer Med. (2023) 12:1922–34. doi: 10.1002/cam4.5027
24. Sachdeva A, Gouge J, Kontovounisios C, Nikolaou S, Ashworth A, Lim K, et al. Klotho and the treatment of human Malignancies. Cancers (Basel). (2020) 12(6):1665. doi: 10.3390/cancers12061665
25. Smith RC, O’Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, et al. Circulating alphaKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest. (2012) 122:4710–5. doi: 10.1172/JCI64986
26. Xie J, Yoon J, An SW, Kuro-o M, Huang CL. Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol. (2015) 26:1150–60. doi: 10.1681/ASN.2014040325
27. Chen J, Fan J, Wang S, Sun Z. Secreted klotho attenuates inflammation-associated aortic valve fibrosis in senescence-accelerated mice P1. Hypertension. (2018) 71:877–85. doi: 10.1161/HYPERTENSIONAHA.117.10560
28. Navarro-Gonzalez JF, Donate-Correa J, Muros de Fuentes M, Perez-Hernandez H, Martinez-Sanz R, Mora-Fernandez C. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. (2014) 100:34–40. doi: 10.1136/heartjnl-2013-304746
29. Yan Y, Chen J. Association between serum Klotho concentration and all-cause and cardiovascular mortality among American individuals with hypertension. Front Cardiovasc Med. (2022) 9:1013747. doi: 10.3389/fcvm.2022.1013747
30. Marcais C, Maucort-Boulch D, Drai J, Dantony E, Carlier MC, Blond E, et al. Circulating klotho associates with cardiovascular morbidity and mortality during hemodialysis. J Clin Endocrinol Metab. (2017) 102:3154–61. doi: 10.1210/jc.2017-00104
31. Memmos E, Sarafidis P, Pateinakis P, Tsiantoulas A, Faitatzidou D, Giamalis P, et al. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol. (2019) 20:217. doi: 10.1186/s12882-019-1391-1
32. Sarafidis PA, Loutradis C, Karpetas A, Tzanis G, Piperidou A, Koutroumpas G, et al. Ambulatory pulse wave velocity is a stronger predictor of cardiovascular events and all-cause mortality than office and ambulatory blood pressure in hemodialysis patients. Hypertension. (2017) 70:148–57. doi: 10.1161/HYPERTENSIONAHA.117.09023
33. Ding J, Tang Q, Luo B, Zhang L, Lin L, Han L, et al. Klotho inhibits angiotensin II-induced cardiac hypertrophy, fibrosis, and dysfunction in mice through suppression of transforming growth factor-beta1 signaling pathway. Eur J Pharmacol. (2019) 859:172549. doi: 10.1016/j.ejphar.2019.172549
34. Xia W, Zhang A, Jia Z, Gu J, Chen H. Klotho contributes to pravastatin effect on suppressing IL-6 production in endothelial cells. Mediators Inflammation. (2016) 2016:2193210. doi: 10.1155/2016/2193210
35. Richter B, Haller J, Haffner D, Leifheit-Nestler M. Klotho modulates FGF23-mediated NO synthesis and oxidative stress in human coronary artery endothelial cells. Pflugers Arch. (2016) 468:1621–35. doi: 10.1007/s00424-016-1858-x
36. Lanzani C, Citterio L, Vezzoli G. Klotho: a link between cardiovascular and non-cardiovascular mortality. Clin Kidney J. (2020) 13:926–32. doi: 10.1093/ckj/sfaa100
37. Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. (2008) 389:233–41. doi: 10.1515/BC.2008.028
38. Chuang MH, Wang HW, Huang YT, Jiang MY. Association between soluble alpha-klotho and mortality risk in middle-aged and older adults. Front Endocrinol (Lausanne). (2023) 14:1246590. doi: 10.3389/fendo.2023.1246590
39. Chen L, Yin X, Zhao Y, Chen H, Tan T, Yao P, et al. Biological ageing and the risks of all-cause and cause-specific mortality among people with diabetes: a prospective cohort study. J Epidemiol Community Health. (2022) 76:771–8. doi: 10.1136/jech-2022-219142
40. Che QC, Jia Q, Zhang XY, Sun SN, Zhang XJ, Shu Q. A prospective study of the association between serum klotho and mortality among adults with rheumatoid arthritis in the USA. Arthritis Res Ther. (2023) 25:149. doi: 10.1186/s13075-023-03137-0
Keywords: cardiovascular disease, serum Klotho, NHANES, all-cause mortality, National Health and Nutrition Examination Survey
Citation: Liu S, Zhu Z, Yu K, Zhang W, Pu J, Lv Y, Tang Z, Liu F and Sun Y (2024) U-shaped association between serum Klotho and all-cause mortality in US cardiovascular patients: a prospective cohort study. Front. Endocrinol. 15:1405665. doi: 10.3389/fendo.2024.1405665
Received: 23 March 2024; Accepted: 31 May 2024;
Published: 13 June 2024.
Edited by:
Eduardo Hertel Ribeiro, Escola Superior de Ciências da Santa Casa de Misericórdia de Vitória (EMESCAM), BrazilReviewed by:
Catherine Bulka, University of South Florida, United StatesClaire Joanne Stocker, Aston University, United Kingdom
Copyright © 2024 Liu, Zhu, Yu, Zhang, Pu, Lv, Tang, Liu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongqiang Sun, cWlhbmcxOTg1MDQxOEAxNjMuY29t
 Shasha Liu1
Shasha Liu1 Kai Yu
Kai Yu Fuqiang Liu
Fuqiang Liu