- 1College of Tropical Agriculture and Forestry, Hainan University, Haikou, China
- 2Donghua (Anhui) Ecological Planning Institute Co., Ltd., Hefei, China
- 3Hainan Academy of Forestry, Haikou, China
Research on understory plant diversity and its response to environmental factors helps in the sustainable development of plantation forests. We investigated the characteristics of understory plant diversity in Eucalyptus plantation forests located in Dongfang, Ding'an, Tunchang, and Lingao on Hainan Island by leveraging the plot survey method, and analyzing how the understory plant diversity in these Eucalyptus plantation forests responds to environmental factors. The results showed that a total of 124 plant species belonging to 62 families and 112 genera were recorded in the sampled plots of the Dongfang, Ding’an, Tunchang, and Lingao regional sites on Hainan Island, among which species of Fabaceae and Poaceae comprised the largest number of plants. The number of species and plant diversity indices of the shrub layer and herb layer in Eucalyptus plantation forests varied at different sites, The richest understory vegetation in Tunchang, located in the center of Hainan Island, and the highest α-diversity whether gauged by species or phylogenetically. The similarity of the understory plant community species was greatest between Ding’an and Tunchang, whereas the difference in composition was largest between Dongfang and the other three sites. Phylogenetically, the understory plant community at Ding’an had the most distant affinities among species, whereas that at Tunchang had the closest affinities among species. The results of the Mantel test and redundancy analysis revealed differing correlations between plant diversity in the shrub layer versus herb layer and various environmental factors. In particular, elevation and annual average temperature are the two main factors influencing plant diversity in the shrub layer, and soil available nitrogen and annual average sunshine duration are the two main factors influencing plant diversity in the herb layer. Variance decomposition showed that the combined effect of soil, climate, and topography factors is the main driver shaping plant diversity in the shrub layer of the understory in Eucalyptus plantation forests, while the combined effect of climate and soil factors is the main one determining plant diversity in their herb layer.
1 Introduction
Since the mid-20th century, planting of monoculture plantation forests has been prevalent worldwide because of the increasing demand for timber and industrial raw materials (Malkamäki et al., 2017). According to recent statistics, the world’s total forested area is 4.06×109 ha, of which plantation forests cover 2.94×108 ha or 7.24% (Food and Agriculture Organization of the United Nations, 2020). However, when meeting the increasing demand for timber and forest products, plantation forests have also caused many ecological problems such as reduction in soil or water conservation capacity and pronounced decline in biodiversity, and, consequently, have contributed to a fragile ecological environment (Xie et al., 2020). Yet some studies have shown that, under the premise of sustainable management, the biodiversity of plantation forests is not low, and it could also fulfill an important ecological service function (Coote et al., 2012). Therefore, identifying ways to implement the scientific management of plantation forests, maintain and improve their biodiversity, enhance their stability, and improve ecological service functions is of generally great significance to global biodiversity conservation and sustainable development.
Eucalyptus collectively refers to eucalypt tree species (Eucalyptus spp.) in the myrtle family (Myrtaceae). Eucalyptus has been widely used worldwide because of its high adaptability, fast growth rate, and ease of management (Zhang et al., 2015; Cook et al., 2016). Biodiversity decline is an ecological problem that is often criticized during the operation and management of Eucalyptus plantation forests. Forestation measures such as excessive application of herbicides, slash burning, and non-use of fertilizers, coupled with the physiological characteristics of Eucalyptus trees (high rate of water uptake and high soil nutrient depletion), could mainly be responsible for the loss of plant diversity in the understory of Eucalyptus plantation forests (Wen et al., 2010; Chu et al., 2014; He et al., 2014; Zhou et al., 2018). Eucalyptus plantations have always been an ecological issue of great concern to governments, the public, and research community. Hence, continuous and in-depth research on the ecology of Eucalyptus plantation forests can provide guidance for their scientific management and operation.
Given its substantial heterogeneity and complex stratification structure, understory vegetation is an indispensable component of forest communities and plays a central role in the dynamics of forest ecosystems. The multifunctionality of forest ecosystems and their provisioning of ecosystem services are mainly attributed to biodiversity, especially the diversity of understory plants (Evy et al., 2016). Accordingly, exploring and revealing the diversity of forest communities and the key influencing factors is an imperative task in both community ecology and conservation biology research, and this is a “hotspot” of current ecological research (Grierson et al., 2011; Ma, 2017). With progress and refinement of various analytical methods, empirical studies of understory vegetation diversity in relation to environmental factors in Eucalyptus plantation forests have gradually increased in frequency. Yet, most of these studies have focused on its relationship with soil factors in Eucalyptus plantation forests at the local scale (Yang et al., 2007; Ye et al., 2010; Li et al., 2014; Zhou et al., 2021), leaving relatively few studies addressing its relationship with climatic factors and topographic factors. Further, there is a lack of studies on the relationship between understory plant species and phylogenetic diversity and environmental factors in Eucalyptus plantation forests at the regional scale. Therefore, this study aimed to address these research questions: (1) Are there crucial differences in the diversity of understory vegetation species and phylogenetic in Eucalyptus plantation forests in different geographic regions of Hainan Island? (2) What are the relationships or species diversity or phylogenetic diversity of the understory vegetation in relation to environmental factors? (3) What are the main environmental factors driving understory plant diversity in Eucalyptus plantations on Hainan Island?
2 Materials and methods
2.1 Study site
Hainan Island is the second largest island in China, located in the northwestern part of the South China Sea (18°10′–20°10′ N, longitude 108°37′–111°03′ E; Figure 1). Situated at the northern edge of the tropics, there are small intra-annual differences in temperature, but dry and wet seasons are pronounced, which are part of a prevailing tropical oceanic monsoon climate. Here, the average annual temperature is 22–27°C, and there is abundant rainfall (annual precipitation of 1000–2600 mm) and abundant irradiance. The soil type of Hainan Island is mainly brick red soil (Ren et al., 2014). Wuzhishan Mountain and the Parrot RidgIn have the highest elevation in the middle of Hainan Island, which gradually descends to the surrounding mountains, hills, terraces, and plains, forming a ring-stratified landform of medium height around low. This unique topography has created a rich environmental gradient across Hainan Island, leading to a variety of vegetation types. Its natural vegetation types mainly include tropical rainforest, monsoon rainforest, alpine cloud forest, and tropical coniferous forest, in addition to plantation forests planted with Hevea brasiliensis, Areca catechu, Eucalyptus, and Acacia mangium (Yang et al., 2021).
Eucalyptus was introduced to Hainan Island at the start of the 20th century, serving as one of the most important afforestation species at this island for more than 100 years (Zhang et al., 2006). Thus far, Eucalyptus has been planted on at least 129 400 ha, with a storage capacity of 5 556 000 m3 (State Forestry and Grassland Administration, 2019).
Eucalyptus plantation forests are rich in terms of their understory vegetation, with the main dominant species in the shrub layer being Aporosa dioica, Clerodendrum cyrtophyllum, Mallotus apelta, and Mallotus philippensis, among others, and those in the herb layer being Oplismenus compositus, Praxelis clematidea, Chromolaena odorata, and Imperata cylindrica, among others.
2.2 Plot set-up and survey
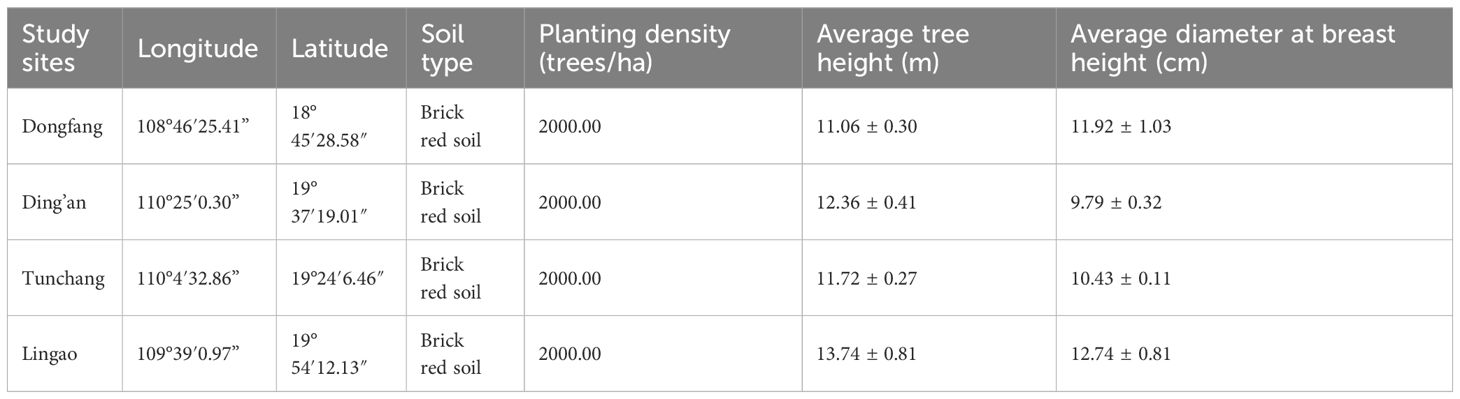
Based on the different rainfall distribution conditions and the distribution of Eucalyptus plantations on Hainan Island, four sites having a large plantation area of Eucalyptus in Dongfang, Ding’an, Tunchang, and Lingao were selected as the study sites (Figure 1). The Eucalyptus variety planted in these Eucalyptus plantations was Eucalyptus urophylla. The age of the trees, as well as their management methods, was relatively consistent across the sites (Table 1). In August 2020, we randomly established 12 shrub layer sampling plots (each 5 m × 5 m) at each study site, maintaining a distance of minimum 20 meters between plots to survey and record the species and number of woody plants. In the middle of each plot, a single herb layer quadrat (1 m × 1 m) was set up to survey and record the species and cover of herbaceous plants.
2.3 Soil sample collection and determination
In each herb layer quadrat, a ring knife (volume: 100 cm3) was used to collect three soil samples from the 0–20 cm surface layer. The drying method (LY/T 1215-1999) was used to determine their respective soil bulk weight, total soil porosity, and soil capillary porosity. From each 0–20 cm soil sample, 150 g was collected at the same time as the ring knife sampling and transported to the laboratory in a self-sealing bag for the determination of soil chemical properties. The potentiometric method (NY/T 1121.2-2006) was used to determine the soil pH (in a water-to-soil ratio of 2.5:1). The potassium dichromate-sulfuric acid digestion method (NY/T 1121.6-2006) was used to determine the soil organic matter content. The Kjeldahl nitrogen fixation method (HJ717-2014) was used to determine the soil total nitrogen content; the acid fusion-molybdenum-antimony colorimetric method was used to determine the total phosphorus content of the soil (LY/T 1232-1999); alkali fusion-flame photometry (LY/T 1234-2015) was used to determine the soil total potassium content. The hydrochloric acid-ammonium fluoride extraction-molybdenum-antimony colorimetric method was used (LY/T 1232-2015) to quantify the soil effective phosphorus content, while ammonium acetic acid leaching-flame photometry was used for the determination of soil quick-acting potassium content (LY/T 1234-2015), with the alkali lysis diffusion method (LY/T 1228-2015) applied to determine soil alkaline dissolved nitrogen content. Soil physical and chemical properties were measured by referring to international standards.
2.4 Environmental factor selection and calculation
A total of 18 factors were investigated, of which environmental factors included topography, climate, and soil. The elevation (ELE), slope degree (SD), and slope aspect (SA) data of each fixed sampling plot were recorded as topographic factors; the SA was converted from 0°~360° to 0~1 using the TRASP index (Roberts and Cooper, 1989). The annual average temperature (TEMP), annual average relative humidity (RH), annual average rainfall (PRCP), and annual average sunshine duration (DH) of the plots from 2009 to 2019 were obtained from the “Climate Research Unit 30′ × 30′ raster data set” as climatic factors of interest (CRU TS v4.04,<www.cru.uea.ac.uk/>, Harris et al., 2020). The experimentally measured soil bulk weight (SBD), soil total porosity (STP), soil capillary porosity (SCP), soil pH (PH), soil organic matter content (OM), soil total nitrogen (TN), soil total phosphorus (TP), soil total potassium (TK), soil effective phosphorus (OP), soil immediate potassium (AK), and soil alkaline nitrogen (AN) were the soil factors.
2.5 Selection and calculation of phylogenetic and species diversity indices
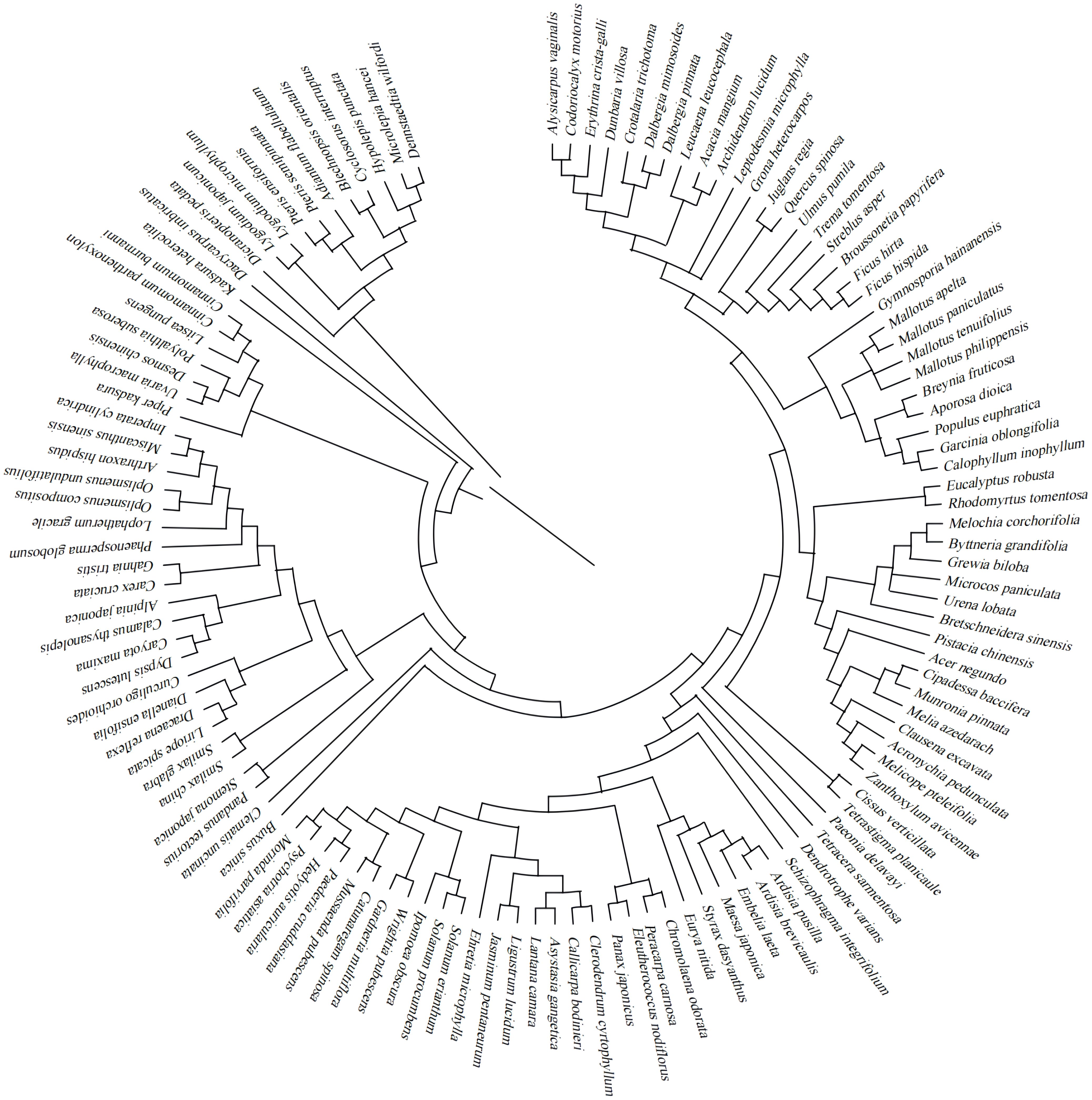
Based on the plant survey data of plantation forest samples from each study site, obtained using the TPL function of the “Taxonstand” package (Cayuela et al., 2012), the Plant List website (http://www.theplantlist.org) was visited to determine the information of 124 plant species found in the understory of Eucalyptus plantations. The “S3” method of the “phylo.maker” function in the “V.PhyloMaker2” package (Jin and Qian, 2019) was used to construct the species-level phylogenetic tree of understory plants in Eucalyptus plantations. The Faith phylogenetic diversity (PD) index was used to measure the phylogenetic α-diversity of plants in the sample data; the mean pairwise phylogenetic distance (MPD) and mean nearest taxonomic unit distance (MNTD) were used to quantify the phylogenetic β-diversity of plants in the sample data (Equations 1, 2; Faith, 1992; Webb, 2000).
where i or j denotes the object species during traversal calculations, na or nb represents the total number of species in sample plots a or b, respectively; and min(d) and denote the minimum and average branch lengths, respectively, between any species present in a given sample and all species in the other sample.
The Margalef, Shannon–Wiener, Simpson, and Pielou indices were used to measure the α-diversity of plant species in the understory of Eucalyptus plantation forests (Equations 3–6; Ma and Liu, 1994).
where S is the total number of species in the sample, N is the total number of individuals in the sample, and is the ratio of the total number of individuals of the ith species to the total number of individuals in the community.
The Jaccard index was used to measure the β-diversity of community species (Equation 7; Ma and Liu, 1995).
where ma denotes the number of species in community A, mb denotes the number of species in community B, and mj denotes the number of species shared by communities A and B.
2.6 Statistical analyses
One-way analysis of variance was used to compare the species and phylogenetic diversity indices among the four study sites and to test for significant differences. The “linkET” package (Guillot and Rousset, 2013) was used to analyze the species α-diversity, phylogenetic diversity, and environmental factors of the shrub and herb layers in Eucalyptus plantations, using the Mantel test. Canoco 5.0 software was used to implement the redundancy analysis (RDA) of species α-diversity, phylogenetic diversity, and environmental factors in shrub and herb layers of Eucalyptus plantations. The variance decomposition method in the “vegan” package (Stoffel et al., 2017) for R 4.3.1 was used to quantitatively analyze the explanatory contribution of the climatic, and topographic, and soil factors, and their interactions as environmental factors for the diversity of understory plants in Eucalyptus plantations.
3 Results
3.1 Species composition of understory plants
The species composition of the surveyed shrub and herb layers in the understory of Eucalyptus plantations in Hainan Island was rich. A total of 124 species of plants belonging to 62 families and 112 genera were recorded and investigated. The developmental composition of the species lineages is relatively discrete, with the ratio of species to genera being 1.11 (Figure 2). The dominant groups of Eucalyptus plantations in Hainan Island are mainly Fabaceae (12 spp.) and Poaceae (7 spp.). Although these two families account only for 3.22% of the total number of plant families encountered, they harbor 15.32% of the total number of species found, indicating that these two families have traits conferring significant fitness advantages in the Eucalyptus plantations on this island. The angiosperms were the largest in number, with 112 species accounting for 90.32% of the total, plus 11 species of ferns (8.87%), and gymnosperms represented by a single species. Differences were observed in the number of plant species in Eucalyptus plantations at different sites. Among them, Tunchang had the most differences, where 67 species belonging to 41 families and 63 genera were recorded, followed by Lingao, where 41 species belonging to 30 families and 41 genera were recorded, and then by Dongfang, where 31 species belonging to 24 families and 29 genera were recorded. The least number of species was found at Ding’an, where 21 species belonging to 17 families and 21 genera were recorded.
3.2 Alpha and beta diversity of understory plant species
The species α-diversity values of the shrub and herb layers in Eucalyptus plantations in different regions of Hainan Island are shown in Figure 3. For the shrub layer, the ranking of the Margalef index was Tunchang > Dongfang > Ding’an > Lingao, while all three Shannon–Wiener, Simpson, and Pielou indices were ranked as follows: Tunchang > Lingao > Ding’an > Dongfang. The Margalef index, Shannon–Wiener index, and Simpson index of Tunchang were significantly higher (P< 0.05) than those of Dongfang, Ding’an, and Lingao; conversely, the Simpson and Pielou indices of Dongfang were significantly lower (P< 0.05) than those of Tunchang, Ding’an, and Lingao. For the herb layer, the Margalef, Shannon–Wiener, and Simpson indices showed the following ranking: Tunchang > Lingao > Dongfang > Ding’an, while the Pielou index was ranked as Dongfang > Tunchang > Lingao > Ding’an. However, all four indices were similar (not significantly different) between Tunchang and Lingao; the Margalef, Shannon–Wiener and Simpson indices of Ding’an were significantly lower than those of Tunchang, Lingao, or Dongfang (P< 0.05). No significant difference was observed in the Pielou index between Dongfang and Tunchang, but it was significantly higher at Dongfang than at either Lingao or Ding’an (P< 0.05). The above results showed that the α diversity of understory plant species in the Eucalyptus plantation of Tunchang was the highest.

Figure 3 Species α-diversity of understory plants in Eucalyptus plantations in four regions of Hainan Island. Margalef index (A); Shannon–Wiener index (B); Simpson index (C); Pielou index (D). Lower-case letters (a–d) indicate significant differences (P< 0.05).
The Jaccard Index differences between the four sites are presented in Table 2. The disparity in the Jaccard index of the shrub layer and the herb layer for Ding’an in relation to Tunchang was greatest overall (0.150 and 0.194, respectively), while that of the shrub layer between Dongfang and Tunchang was smallest (0.059), and that of the herb layer between Dongfang and Lingao was smallest (0.056). The smaller the Jaccard index value was, the greater the difference in species composition was between different site regions.
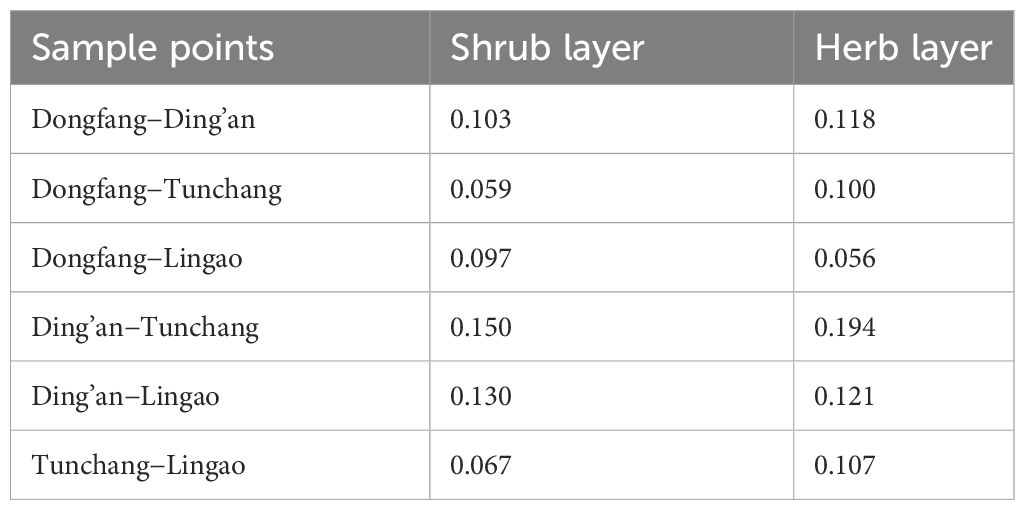
Table 2 Species β-diversity of understory plants in Eucalyptus plantations in four regions of Hainan Island.
3.3 Understory plant phylogenetic α- and β-diversity
The phylogenetic α-diversity of the shrub layer and the herb layer of Eucalyptus plantations at different sites is shown in Figure 4. For the shrub layer, its PD was ranked as Tunchang > Dongfang > Ding’an > Lingao. Tunchang showed a significantly higher PD than Dongfang, Ding’an, and Lingao (P< 0.05). Likewise, Dongfang demonstrated a significantly higher PD than both Ding’an and Lingao (P< 0.05). However, Ding’an and Lingao exhibited similar PD values. For the herb layer, its PD took this ranking: Tunchang > Lingao > Ding’an > Dongfang. Although similar, Tunchang and Lingao were each significantly higher than either Dongfang or Ding’an (P< 0.05), but no significant difference was observed between Dongfang and Ding’an. Altogether, these results showed that the phylogenetic α-diversity of understory plants in Eucalyptus plantations in Tunchang was highest.
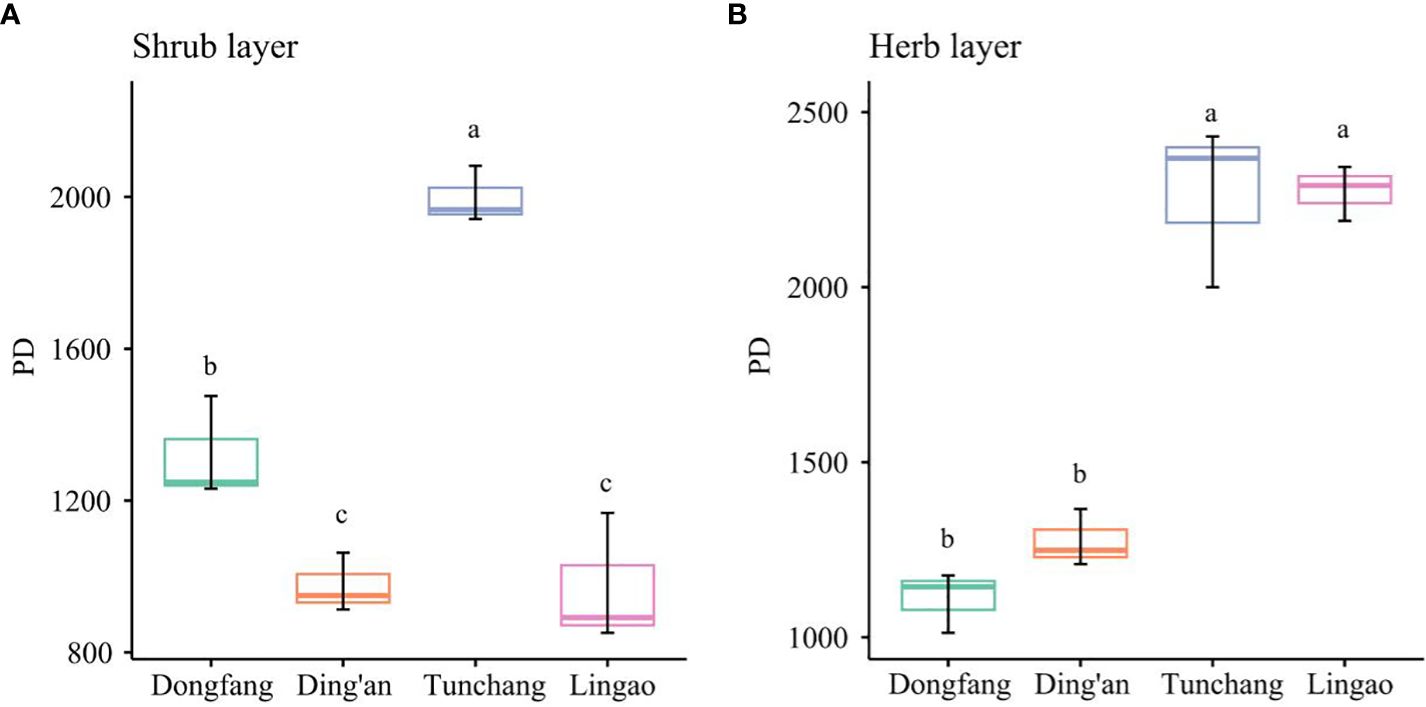
Figure 4 Phylogenetic α-diversity of understory plants in Eucalyptus plantations in four regions of Hainan Island. Shrub layer PD (A). Herb layer PD (B). Lower-case letters (a–d) indicate significant differences (P< 0.05).
The phylogenetic β-diversity of the shrub and herb layers under Eucalyptus plantations in different regions is shown in Figure 5. For the shrub layer, its MPD was ranked as Dongfang > Ding’an > Lingao > Tunchang, with no significant difference between Dongfang, Ding’an, and Lingao. However, Dongfang had a significantly larger MPD than Tunchang (P< 0.05), whereas the differences between Ding’an, Tunchang, and Lingao were not significant. For MNTD, its ranking was Ding’an > Lingao > Dongfang > Tunchang, with no significant difference between Ding’an and Lingao and between Dongfang and Tunchang. However, the MNTD values of Ding’an and Lingao significantly surpassed those of Dongfang and Tunchang (P< 0.05). For the herb layer, its MPD was ranked as Ding’an > Tunchang > Lingao > Dongfang, with significant differences among Ding’an, Tunchang, Lingao, and Dongfang (P< 0.05). For MNTD, its ranking was Ding’an > Dongfang > Lingao > Tunchang, with no significant difference between Ding’an and Dongfang; Ding’an and Dongfang each significantly higher than Lingao or Tunchang (P< 0.05); and Lingao was significantly higher than Tunchang (P< 0.05). To sum up, these results showed that the phylogenetic β-diversity of the shrub and herb layers at Ding’an was highest, while that in Tunchang was lowest.
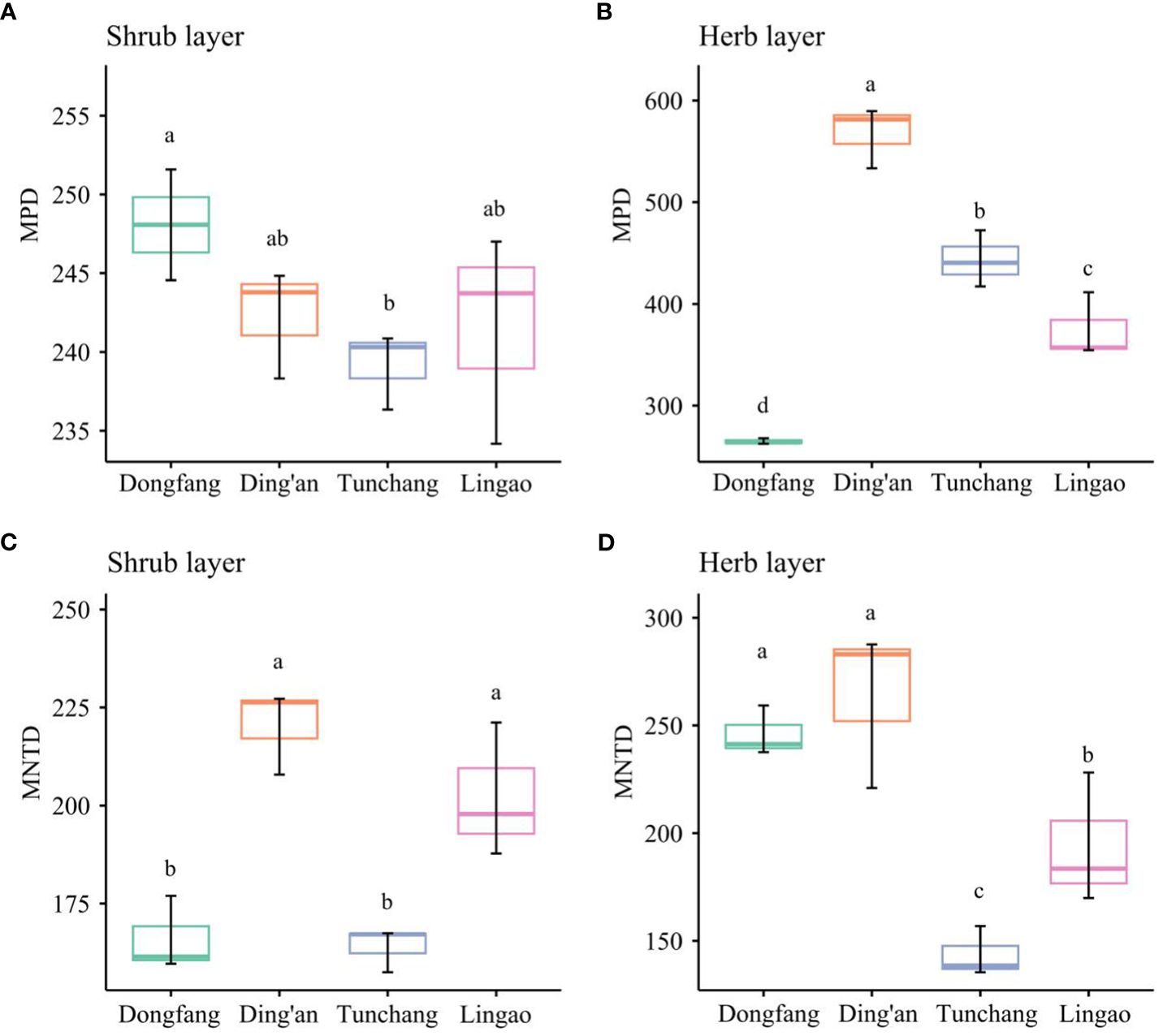
Figure 5 Phylogenetic β-diversity of understory plants in Eucalyptus plantations in four regions of Hainan Island. Shrub layer MPD (A); herb layer MPD (B); shrub layer MNTD (C); herb layer MNTD (D). Lower-case letters (a-d) indicate significant differences (P< 0.05).
3.4 Relationship between understory plant diversity and environmental factors
Figure 6 shows the results of the Mantel test analyses of plant diversity in the shrub and herb layers of Eucalyptus plantation forests in relation to their environmental factors. The plant diversity index in the shrub layer of the Eucalyptus plantation forest was significantly and positively correlated with OM and RH (P< 0.001), with SD and ELE (P< 0.01), and with TK and TEMP (P< 0.05) but did not significantly correlate with any other environmental factor. Plant diversity in the herb layer of the Eucalyptus plantation forest was significantly and positively correlated with SCP, DH, and TEMP (P< 0.001), with STP, AN, TP, and PRCP (P< 0.01), and with PH, OP, and AK (P< 0.05) but did not significantly correlate with other environmental factors.
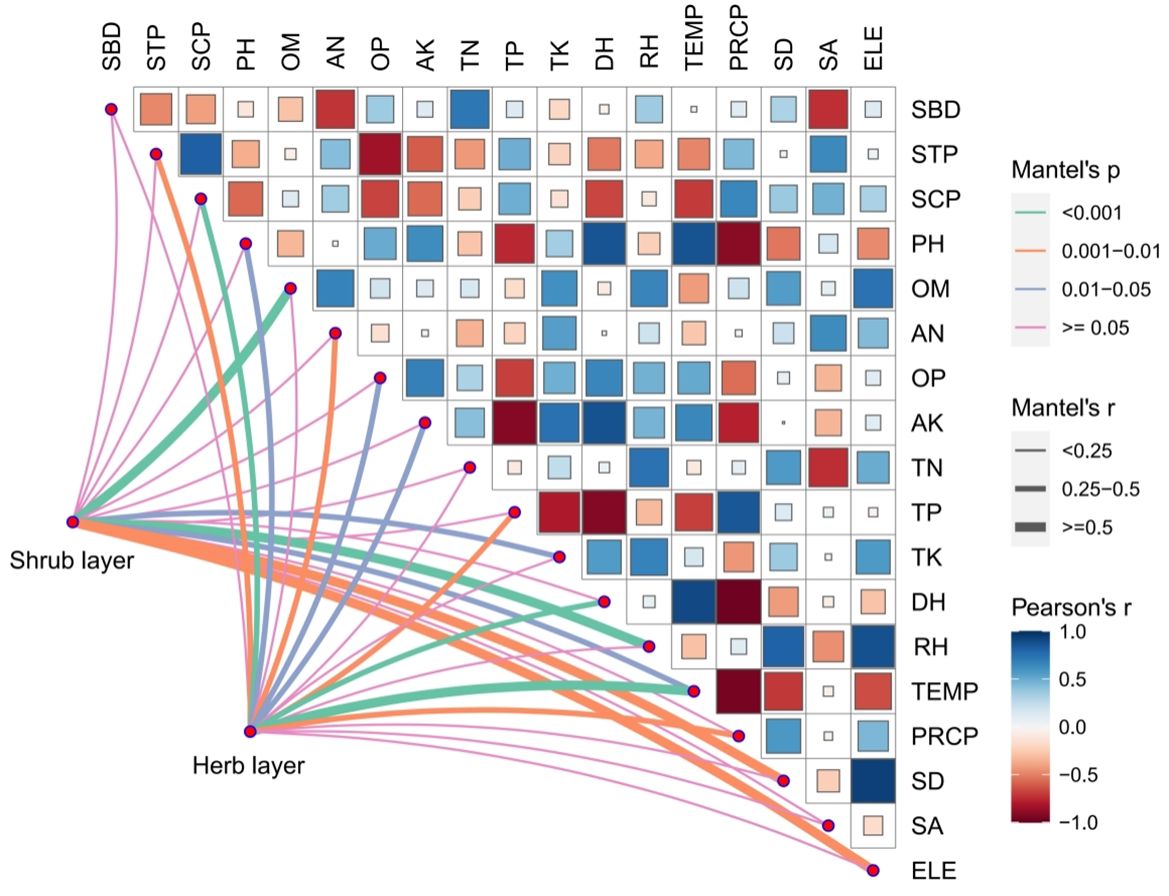
Figure 6 Mantel test of plant diversity index and environmental factors in the understory of Eucalyptus plantation forests. SBD, STP, SCP, PH, OM, AN, OP, AK, TN, TP, TK, DH, RH, TEMP, PRCP, SD, SA, and ELE respectively are soil bulk density, soil total porosity, soil capillary porosity, soil pH, soil organic matter, soil available nitrogen, soil available phosphorus, soil available potassium, soil total nitrogen, soil total phosphorus, soil total potassium, annual sunshine hours, annual air humidity, annual air temperature, annual rainfall, slope degree, slope aspect, and elevation.
The RDA ranking plot of understory plant diversity and environmental factors in Eucalyptus plantation forests is shown in Figure 7. Together, DH, RH, TEMP, ELE, PH, TP, OM, OP, AK, and TK—a total of 10 environmental factors—accounted for 99.99% of the total variance in plant diversity information for in the shrub layer of the Eucalyptus plantation forests, with the first and second ranked axes contributing to 66.35% and 23.83%, respectively, for a cumulative 90.18% of the overall variance explained. Particularly, ELE and TEMP contributed to 59.00% (p = 0.002) and 21.60% (p = 0.004) of the variance in the shrub layer plant diversity information, respectively. These two environmental factors mainly influenced the plant diversity of the shrub layer in the Eucalyptus plantation forest understory. Ten environmental factors, namely DH, RH, PRCP, TEMP, SA, AN, TK, PH, SCP, and AK, together explained 100% of the total variance in plant diversity information in the herb layer of Eucalyptus plantation forests. In this regard, the first and second sorting axes contributed to 66.28% and 27.15%, respectively, together explaining 93.43% of the variance information. Notably, explanatory contribution of soil AN and sunshine for the variance in plant diversity information in the herb layer was 55.60% (p = 0.002) and 25.10% (p = 0.006), respectively. These two environmental factors mainly influenced the diversity in the herb layer in the understory of Eucalyptus plantation forests on Hainan Island.
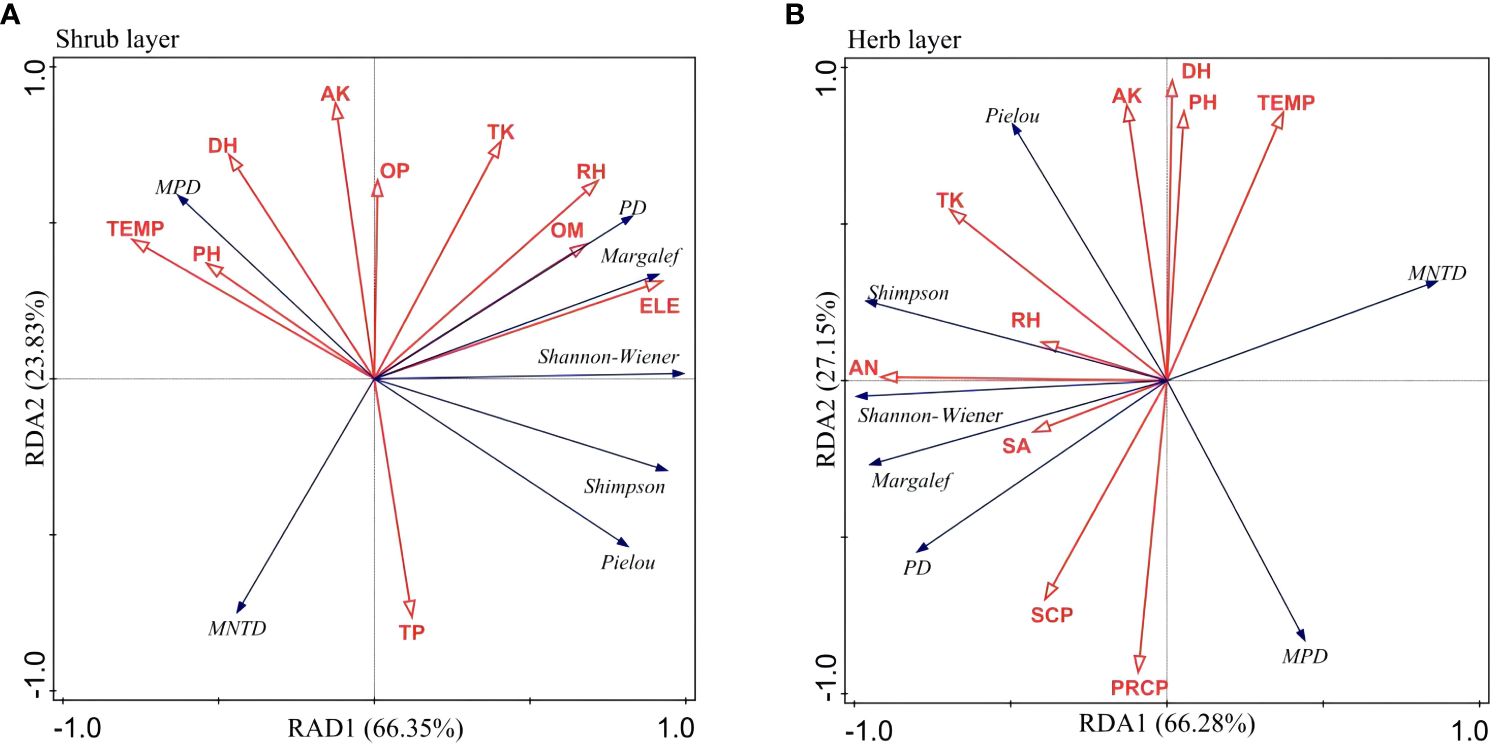
Figure 7 RDA ranking of understory plant diversity indices of Eucalyptus plantation forests with environmental factors. Shrub layer (A); Herb layer (B). SBD, STP, SCP, PH, OM, AN, OP, AK, TN, TP, TK, DH, RH, TEMP, PRCP, SD, SA, and ELE respectively are soil bulk density, soil total porosity, soil capillary porosity, soil pH, soil organic matter, soil available nitrogen, soil available phosphorus, soil available potassium, soil total nitrogen, soil total phosphorus, soil total potassium, annual sunshine hours, annual air humidity, annual air temperature, annual rainfall, slope degree, slope aspect, and elevation.
According to the above RDA results, the environmental factors influencing the shrub and herb layers could be divided and examined in terms of soil, climatic, and topographic factors. Next, the variance decomposition method was applied to quantitatively analyze the relative contribution of these environmental factors toward shaping the diversity of understory plants in Eucalyptus plantation forests. As shown in Figure 8, for the shrub layer, the independent contribution of climatic factors was the largest, at 6.61%, followed by soil factors, at 4.55%, while that of topographic factors was the smallest, at 3.22%. The coupling effect of climatic and soil factors contributed to 16.37%, whereas that of topographic and climatic factors, as well as that of topographic and soil factors, each contributed to<0.01%. In stark contrast, the contribution arising from coupling the effects of all three sets of factors was 73.18%. This indicated that the interaction of climatic factors, soil factors, and topographic factors plays a major role in the changing the shrub layer plant diversity in the sampled Eucalyptus plantations. For the herb layer, the independent contribution of climatic factors was the largest, amounting to 10.59%, followed by soil factors, at 3.17%, whereas that of topographic factors was the smallest, at 1.93%. The coupling effect of climatic and soil factors contributed to 66.94%, but that of topographic and climatic factors, as well as that of topographic and soil factors, was<0.01%. The contribution attained from the coupling effect of all three sets of factors was 21.78% and much lower than that for the shrub layer. This indicated that the combined effect of climatic and soil factors played a major role in altering plant diversity in the herb layer of Eucalyptus plantations on Hainan Island.

Figure 8 Variance decomposition of the effects of environmental factors upon understory plant diversity in Eucalyptus plantations. Shrub layer (A). Herb layer (B).
4 Discussion
Species diversity and phylogenetic diversity of communities can be understood better by analyzing their species composition (Aldana et al., 2017). The species composition of the understory shrub and herb layers of the Eucalyptus plantation forests on Hainan Island is evidently rich, with a total of 124 plant species, belonging to 62 families and 112 genera, recorded in our survey. Among them, the species of Fabaceae and Poaceae have the highest representation (number of spp.) in the composition of understory vegetation of Eucalyptus plantation forests, which may be attributed to the fact that members of these two families, such as Codariocalyx motorius, Acacia mangium, Archidendron lucidum, Oplismenus compositus, and Lophatherum gracile, among others, are well adapted to the environment and are widely distributed in different regions of Hainan Island (Chen, 1964). In addition, their dominance may be related to the insensitivity of Fabaceae and Poaceae to chemosensory substances of Eucalyptus (Chen et al., 2003) Therefore, we infer that Fabaceae and Poaceae play an important ecological role in the understory plant community of Eucalyptus plantation forests on Hainan Island.
By analyzing the differences in species composition, species diversity, and phylogenetic diversity of the understory vegetation of Eucalyptus plantation forests in different regions of Hainan Island, we showed that the environmental gradient has an important effect on plant species composition and diversity. Because of the topographic features of Hainan Island, whose elevation is high in the middle region and surrounded by mountainous terrain, hilly areas and plains, and tableland landscapes, which make the environmental factors such as climate, soil, and topography of its various regions have clear differences (Zhang et al., 2020). For example, mountain ranges act as barrier that can cause changes in the local climate, soil, and runoff, which could lead to differences in species composition and diversity in different regions (Rahbek et al., 2019). In the present study, the number of species and α-diversity indices of the understory shrub and herb layers of Eucalyptus plantation forests differed among the sampled sites. Notably, Tunchang, which is located in the central region of Hainan Island, harbored significantly more shrub species and greater diversity than the other three regional sites, which may be related to its higher rainfall and soil organic matter.
Species β-diversity describes the similarity of species composition between communities or the rate of replacement along an environmental gradient. The similarity of plant communities often shows a significant distance decay pattern with an increasing distance (Liu et al., 2015; Murphy et al., 2016). Here, the Jaccard index of the shrub and herb layers reached its largest disparity between Tunchang and Ding’an, but its disparity was smallest between Dongfang and Tunchang for the shrub layer and between Dongfang and Lingao for the herb layer. This shows that as the spatial distance increases, the similarity of species composition of understory plants in Eucalyptus plantations in different regions of Hainan Island gradually decreases. Environmental filtering can lead to phylogenetic clustering (Wiens and Graham, 2005), while competitive exclusion may lead to phylogenetic dispersion (Burns and Strauss, 2011). We found regional differences in both the MPD and MNTD of the shrub and herb layers in the Eucalyptus plantations; hence, we may infer their understory vegetation is shaped by both environmental filtering and competitive exclusion. The MNTD of the shrub layer and MPD and MNTD of the herb layer are highest overall at the Ding’an site, while MPD and MNTD index of the shrub layer and MNTD of the herb layer are the lowest at the Tunchang site. This pattern indicates a farther phylogenetic relationship between understory plant species in the Eucalyptus plantation at Ding’an, whereas a closer phylogenetic relationship characterizes Tunchang’s Eucalyptus plantation.
Previous studies have shown that non-biological determinism tends to increase with an increase in spatial scale, while biological determinism tends to reduce with a decrease in the spatial scale. Abiotic determinism is more important than interactions between species in the conservation of biodiversity at the regional scale (Cardillo, 2011; Villalobos et al., 2013; Yang et al., 2014). The RDA results show that the environmental factors’ contribution is high, in that they explain much of the diversity of the shrub and herb layers in the Eucalyptus plantations, which suggests that the variation in this plant diversity on Hainan Island basically depends on changes in the abiotic environment. This may be related to the short rotation period of Eucalyptus plantations, the short time span of understory vegetation formation, and the lack of long-term interaction between species.
In this study, differences emerged in the relationship between plant diversity and environmental factors in the herb versus shrub layers of Eucalyptus plantation forests. According to the Mantel test results, when compared with the herb layer, the shrub layer plant diversity is more closely related to the topographic factors ELE and SD. Furthermore, the RDA results show that ELE and TEMP are the two environmental factors mainly influencing the plant diversity of the shrub layer in the understory of Eucalyptus plantation forests, for which ELE explained 59.00% of the variance (P=0.002). This result suggests that ELE strongly shapes the shrub diversity of Eucalyptus plantation forests, an effect that is extremely important. It is widely accepted that species diversity declines with increasing elevation (Tang and Fang, 2004), but some studies have shown that plant diversity can exhibit different trends in response to increasing elevation (Xing et al., 2010; Zhong et al., 2022). In the present study, the shrub layer diversity of Eucalyptus plantations was positively correlated with ELE, which may be related to the distribution of vegetation types as well as the seed dispersal and spread of woody plant species on Hainan Island. The distribution of secondary and primary forests having higher species richness at higher elevation in the central region (Yang et al., 2021) should enable more woody plants to settle into the Eucalyptus plantation community through seed dispersal dynamics.
In this study, herb layer plant diversity was closely related to soil and climatic factors, but no significant correlation was found with topographic factors. This may be due to the direct impact of soil factors and climatic factors on the diversity of herbaceous plants, which weakens the indirect ecological effects of topographic factors (Wu et al., 2013; Xu et al., 2014). In the Mantel test analysis, soil AN, OP, and AK were significantly and positively correlated with herbaceous plant diversity, and the RDA showed that soil AN explained 55.60% (p = 0.002) of the variance in herbaceous plant diversity. This suggests that the fast-acting nutrient content of the soil plays an important role in maintaining and enhancing the plant diversity of the herb layer in the understory of Eucalyptus plantation forests.
There are often complex interactions between environmental factors affecting community species composition and diversity, which can lead to overlapping effects in the interpretation of community species composition and diversity by different environmental factors (Lin, 2021). Variance decomposition is often relied on to explain the contribution of different environmental factors, either individually or interactively, to shaping community composition and diversity (Zhao et al., 2020; Zhang et al., 2022). In this study, the variance decomposition results showed that the combined effect of soil, climatic, and topographic factors is what predominantly influences the plant diversity of the shrub layer in the understory of Eucalyptus plantation forests. For the herb layer, however, it is the combined effect of climatic and soil factors that chiefly influences its plant diversity in the understory of Eucalyptus plantation forests.
5 Conclusions
A total of 124 species of plants were recorded in the understory of Eucalyptus plantations sampled at four sites, among which those in the Fabaceae and Poaceae families were the most abundant. The species composition and diversity of understory plants in Eucalyptus plantations in different regions of Hainan Island are not alike. The species composition at Tunchang, located in the central part of Hainan, is the richest (highest number of spp.), and its plant diversity is generally the greatest overall. The species similarity of Eucalyptus plantations at different regions increases as the spatial distance is shortened. Environmental factors (climate, soil, and topography) are the main factors affecting the diversity of understory plants in Eucalyptus plantations. The responses of the shrub and herb layers of Eucalyptus plantations to environmental factors were, however, different. The shrub layer’s diversity is mainly affected by elevation (ELE) and annual average temperature (TEMP), which are the two main factors affecting its plant diversity. The herb layer’s diversity is chiefly affected by soil alkali-hydrolyzable nitrogen and annual average sunshine hours (DH), which are the two main factors affecting its plant diversity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HC: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. SD: Data curation, Investigation, Methodology, Writing – review & editing. HH: Investigation, Methodology, Writing – review & editing. LT: Methodology, Resources, Supervision, Writing – review & editing. HZ: Investigation, Methodology, Writing – review & editing. JW: Investigation, Methodology, Writing – review & editing. XY: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Science and Technology Department of Hainan Province (grant number ZDYF2023SHFZ174).
Acknowledgments
We would also like to thank The Charlesworth Author Services Team (https://www.cwauthors.com.cn) for them assistance with English language and grammatical editing of the manuscript.
Conflict of interest
Author SD was employed by the company Donghua Anhui Ecological Planning Institute Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aldana A. M., Carlucci M. B., Fine P. V., Stevenson P. R. (2017). Environmental filtering of eudicot lineages underlies phylogenetic clustering in tropical South American flooded forests. Oecologia 183, 327–335. doi: 10.1007/s00442-016-3734-y
Burns J. H., Strauss S. Y. (2011). More closely related species are more ecologically similar in an experimental test. Proc. Natl. Acad. Sci. U.S.A. 108, 5302–5307. doi: 10.1073/pnas.1013003108
Cardillo M. (2011). Phylogenetic structure of mammal assemblages at large geographical scales: linking phylogenetic community ecology with macroecology. Philos. Trans. R. Soc Lond. B Biol. Sci. 366, 2545–2553. doi: 10.1098/rstb.2011.0021
Cayuela L., Granzow-De La Cerda I. N. I., Albuquerque F. S., Golicher D. J. (2012). Taxonstand: an R package for species names standardisation in vegetation databases. Methods Ecol. Evol. 3, 1078–1083. doi: 10.1111/j.2041-210X.2012.00232.x
Chen Q. B., Wang Z. H., Lin W. F., Peng L. X., He L. M., Bai C. J. (2003). Allelopathic effects of eucalyptus 12ABL on four legume species. J. Trop. Crop 24, 67–72. doi: 10.3969/j.issn.1000-2561.2003.03.015
Chu C. J., Mortimer P. E., Wang H. C., Wang Y. F., Liu X. B., Yu S. X. (2014). Allelopathic effects of eucalyptus on native and introduced tree species. For. Ecol. Manage. 323, 79–84. doi: 10.1016/j.foreco.2014.03.004
Cook R. L., Binkley D., Stape J. L. (2016). Eucalyptus plantation effects on soil carbon after 20 years and three rotations in Brazil. For. Ecol. Manage. 359, 92–98. doi: 10.1016/j.foreco.2015.09.035
Coote L., French L. J., Moore K. M., Mitchell F., Kelly D. (2012). Can plantation forests support plant species and communities of semi-natural woodland. Forest. Ecol. Manage. 283, 86–95. doi: 10.1016/j.foreco.2012.07.013
Evy A., Federico S., Harald A., Lander B., Sigrid B., Elisa C., et al. (2016). Driving mechanisms of overstorey–understorey diversity relationships in European forests. Perspect. Plant Ecol. Evol. Syst. 19, 21–29. doi: 10.1016/j.ppees.2016.02.001
Faith D. P. (1992). Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10. doi: 10.1016/0006-3207(92)91201-3
Food and Agriculture Organization of the United Nations (2020). Global Forest Resources Assessment 2020 Main report (Rome: Food and Agriculture Organization of the United Nations).
Grierson C., Barnes S., Chase M. W., Clarke M., Grierson D., Edwards K., et al. (2011). One hundred important questions facing plant science research. New Phytol. 192, 6–12. doi: 10.1111/j.1469-8137.2011.03859.x
Guillot G., Rousset F. (2013). Dismantling the mantel tests. Methods Ecol. Evol. 4, 336–344. doi: 10.1111/2041-210x.12018
Harris I., Osborn T. J., Jones P., Lister D. (2020). Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 7, 109. doi: 10.1038/s41597-020-0453-3
He H., Song Q., Wang Y. F., Yu S. X. (2014). Phytotoxic effects of volatile organic compounds in soil water taken from a Eucalyptus urophylla plantation. Plant Soil 377, 203–215. doi: 10.1007/s11104-013-1989-1
Jin Y., Qian H. (2019). PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography 42, 1353–1359. doi: 10.1111/ecog.04434
Li W., Zhang C. P., Wei R. P. (2014). Relationship of under-story vegetation diversity with stand age and soil factor of eucalypt plantations in central-western Guangdong. Acta Ecol. Sin. 34, 4957–4965. doi: 10.5846/stxb201301060041
Lin H. (2021). Species composition and diversity of alluvial fan vegetation in Lhasa River basin and its environmental interpretation. Yangling, China: Northwest Agriculture and Forestry University. Master’s thesis.
Liu Y. M., Tang Z. Y., Fang J. Y., Ewald J. (2015). Contribution of environmental filtering and dispersal limitation to species turnover of temperate deciduous broad-leaved forests in China. Appl. Veg. Sci. 18, 34–42. doi: 10.1111/avsc.12101
Ma K. P. (2017). Frontiers in biodiversity science: insular biogeography, community assembly and application of big data. Biodivers. Sci. 25, 343–344. doi: 10.17520/biods.2017137
Ma K. P., Liu Y. M. (1994). Measuring methods of biological community diversity: Iα diversity measurement method (Part 2). Biodivers. Sci. 2, 231–239. doi: 10.17520/biods.1994038
Ma K. P., Liu Y. M. (1995). Methods for measuring the diversity of biological communities ii methods for measuring the diversity of β. Biodivers. Sci. 3, 38–43. doi: 10.17520/biods.1995007
Malkamäki A., D’amato D., Hogarth N. J., Kanninen M., Pirard R., Toppinen A., et al. (2017). The socioeconomic impacts of large-scale tree plantations on local communities: a systematic review protocol (Kota Hujan: Center for International Forestry Research).
Murphy S. J., Salpeter K., Comita L. S. (2016). Higher β-diversity observed for herbs over woody plants is driven by stronger habitat filtering in a tropical understory. Ecology 97, 2074–2084. doi: 10.1890/15-1801.1
Rahbek C., Borregaard M. K., Antonelli A., Colwell R. K., Holt B. G., Nogues-Bravo D., et al. (2019). Building mountain biodiversity: geological and evolutionary processes. Science 365, 1114–1119. doi: 10.1126/science.aax0151
Ren H., Li L. J., Liu Q., Wang X., Li Y., Hui D., et al. (2014). Spatial and temporal patterns of carbon storage in forest ecosystems on Hainan island, southern China. PloS One 9, e108163. doi: 10.1371/journal.pone.0108163
Roberts D. W., Cooper S. V. (1989). Concepts and Techniques of Vegetation Mapping (New York: USDA Forest Service).
State Forestry and Grassland Administration (2019). China Forest Resources News 2014-2018 (Beijing: China Forestry Publishing House).
Stoffel M. ,. A., Nakagawa S., Schielzeth H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. doi: 10.1111/2041-210X.12797
Tang Z. Y., Fang J. Y. (2004). A review on the elevational patterns of plant species diversity. Biodivers. Sci. 12, 20–28. doi: 10.17520/biods.2004004
Villalobos F., Rangel T. F., Diniz-Filho J. A. F. (2013). Phylogenetic fields of species: cross-species patterns of phylogenetic structure and geographical coexistence. Proc. R. Soc B-Biol. Sci. 280, 20122570. doi: 10.1098/rspb.2012.2570
Webb C. O. (2000). Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am. Nat. 156, 145–155. doi: 10.1086/303378
Wen Y. G., Ye D., Chen F., Liu S. R., Liang H. W. (2010). The changes of understory plant diversity in continuous cropping system of Eucalyptus plantations, South China. J. Forest. Res. 15, 252–258. doi: 10.1007/s10310-010-0179-8
Wiens J. J., Graham C. H. (2005). Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36, 519–539. doi: 10.1146/annurev.ecolsys.36.102803.095431
Wu H., Zhang M. X., Wang D. X. (2013). Diversity characteristics in different layers of Pinus tabuliformis-Quercus aliena var. accuteserrata mixed forest and environmental interpretation in the southern slope of Qinling mountains. Acta Bot. Sin. 33, 2086–2094. doi: 10.7606/j.issn.1000-4025.2013.10.2086
Xie H., Fawcett J. E., Wang G. G. (2020). Fuel dynamics and its implication to fire behavior in loblolly pine-dominated stands after southern pine beetle outbreak. Forest. Ecol. Manage. 466, 118130. doi: 10.1016/j.foreco.2020.118130
Xing S. H., Cui G. F., Lin D. Y., Yao Y. G., Li H. L., Yang S. Y. (2010). Vertical distribution patterns of plant species diversity in the mountains of Beijing. J. Beijing For. Univ. S1, 45–50. doi: 10.13332/j.1000-1522.2010.s1.004
Xu C. Y., Chen Z. C., Hao C. Y., Ding X. D. (2014). Research on the correlation between plant species diversity and its main environmental factors of Mt. Baiyunshan in the transitional region from warm temperate zone to subtropical zone. J. Ecol. Environ. 23, 371–376. doi: 10.3969/j.issn.1674-5906.2014.03.002
Yang X. B., Chen Z. Z., Li D. H. (2021). Classification and distribution of vegetation in Hainan, China. Sci. Sin. Vitae 51, 321–333. doi: 10.1360/SSV-2020-0286
Yang Z. H., Yu X. B., Yang X. B., Li Y. L., Wu Q. S. (2007). Analysis of the relationship between undergrowth diversity in Hainan eucalyptus plantation and its relative factors. Chin. J. Trop. Agr. 27, 54–57. doi: 10.3969/j.issn.1009-2196.2007.04.015
Yang J., Zhang G. C., Ci X. Q., Swenson N. G., Cao M., Sha L., et al. (2014). Functional and phylogenetic assembly in a Chinese tropical tree community across size classes, spatial scales and habitats. Funct. Ecol. 28, 520–529. doi: 10.1111/1365-2435.12176
Ye S. M., Wen Y. G., Yang M., Liang H. W. (2010). Correlation analysis on biodiversity and soil physical & chemical properties of eucalyptus spp. plantations under successive rotation. J. Soil Water Conserv. 24, 246–250. doi: 10.13870/j.cnki.stbcxb.2010.04.05
Zhang C. H., Dong L. J., Wu Y., Feng W., Guo D. Y., Wu H., et al. (2020). Influence of mountainous terrain on weather and climate in central Hainan Island. Prog. Meteorol. Sci. Technol. 10, 70–73. doi: 10.3969/j.issn.2095-1973.2020.04.019
Zhang Y. B., Qin H., Meng Q. X., Zhang F., Tang Z. Y. (2022). Spatial pattern of forest community species diversity and its influencing factors in Taihang Mountain. J. Appl. Environ. Biol. 28, 331–338. doi: 10.19675/j.cnki.1006-687x.2020.11014
Zhang X. H., Yu X. B., Huang J. C. (2006). Evaluation of ecosystem service function of eucalyptus plantation in Hainan Province. Trop. For. 34, 25–27. doi: 10.3969/j.issn.1672-0938.2006.03.007
Zhang K., Zheng H., Chen F. L., Ouyang Z. Y., Wang Y., Wu Y., et al. (2015). Changes in soil quality after converting pinus to eucalyptus plantations in southern China. Solid Earth 6, 115–123. doi: 10.5194/se-6-115-2015
Zhao Y., Li M., Wang X., Deng J., Zhang Z., Wang B. (2020). Influence of habitat on the phylogenetic structure of Robinia pseudoacacia forests in the eastern Loess Plateau, China. Glob. Ecol. Conserv. 24, e01199. doi: 10.1016/j.gecco.2020.e01199
Zhong Y. M., Guo X. S., Yao Z. Y. (2022). Study on community composition and species diversity of deciduous oak forest in South subtropical region of western Guangxi. Acta Bot. Sin. 42, 1945–1953. doi: 10.7606/j.issn.1000-4025.2022.11.1945
Zhou R. H., Tang Y. B., Wang M., Dong H. J., Yu F. Y., Chen C. L., et al. (2021). Species diversity and soil physicochemical properties of eucalyptus plantations at different ages in Weiyuan, China. J. Appl. Environ. Biol. 27, 742–748. doi: 10.19675/j.cnki.1006-687x.2020.03004
Zhou X. G., Zhu H. G., Wen G., Goodale U. M., Li X., You Y., et al. (2018). Effects of understory management on trade-offs and synergies between biomass carbon stock, plant diversity and timber production in Eucalyptus plantations. Forest. Ecol. Manage. 410, 164–173. doi: 10.1016/j.foreco.2017.11.015
Keywords: Eucalyptus plantation, undergrowth vegetation, species diversity, phylogenetic diversity, environmental factors
Citation: Chen H, Du S, Huang H, Tian L, Zhou H, Wu J and Yu X (2024) Plant diversity in the understory of Eucalyptus plantations on Hainan Island and its response to environmental factors. Front. Ecol. Evol. 12:1366094. doi: 10.3389/fevo.2024.1366094
Received: 01 March 2024; Accepted: 06 May 2024;
Published: 21 May 2024.
Edited by:
Yang Huai, International Center for Bamboo and Rattan, ChinaReviewed by:
Jianfeng Liu, Chinese Academy of Forestry, ChinaLin Peiqun, Chinese Academy of Tropical Agricultural Sciences, China
Copyright © 2024 Chen, Du, Huang, Tian, Zhou, Wu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuebiao Yu, eXV4dWViaWFvQDE2My5jb20=
 Haihui Chen
Haihui Chen Shan Du1,2
Shan Du1,2