
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 20 August 2021
Sec. Paleoecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.688768
This article is part of the Research Topic Early Human Colonization of Remote Indian Ocean Islands and its Ecological Impacts View all 11 articles
The Mascarenes are sadly famous worldwide for the massive extinction of their native vertebrates since recent human colonization. However, extinction patterns show astonishing disparities between the two main islands and between lineages of forest vertebrates. On Réunion (2,512 km2, 3,070 m) where about a third of native habitats remains, most large-bodied vertebrates, especially frugivores, collapsed by the first half of the 18th century, while several have survived longer and some still exist on Mauritius (1,865 km2, 828 m) where more than 95% of native habitats have been transformed. Considering lineages of forest vertebrates shared by both islands (23 genera, 53 species), we test the hypothesis that differing patterns of lowland suitable habitat destruction is the main cause behind this paradox. Before that, we assess the potential impact of other major drivers of extinctions since first contact with humans. Firstly, Mauritius shows earlier and more numerous introductions of mammal predators known for their devastating impact (except northern islets which have thus become important sanctuaries for several squamates). Secondly, settlers were inveterate hunters on both islands, but while Réunion was overhunted before Mauritius, the burst of human population in the latter in late 18th century has not led to the rapid extinction of all large native vertebrates. These two factors alone therefore cannot explain the observed paradox. Rather, the early destruction of lowland habitats (<400 m) on Réunion is concomitant with most extinctions of forest vertebrate, notably frugivores that rapidly lost most lowland habitats dominated by large fleshy-fruited plants. Moreover, landform-induced fragmentation has likely decreased the ability of adjacent habitats to act as effective refuges. Conversely, Mauritius retained suitable low-fragmented habitats until the late 19th which probably allowed, at least for a time, several native vertebrates to escape from multiple human-induced disturbances. Despite the almost total destruction of native habitats since then on Mauritius, conservation actions have saved several threatened vertebrate species that play a fundamental role in the functioning of native ecosystems. The fact that there are now more favorable habitats on Réunion than on Mauritius argues for the rewilding of Réunion with these extant large vertebrates.
The Dodo of Mauritius became one of the most emblematic species of human-induced extinction but this species is only a symbol among numerous other insular species that constitute the bulk of documented extinctions at the Holocene (Steadman, 1995; Alcover et al., 1998; Blackburn et al., 2004; Whittaker and Fernandez-Palacios, 2007; Kier et al., 2009). Islands show indeed a more pronounced extinction rate than mainland ecosystems, which often leads to the global extinction of species due to high endemism. The primary drivers of vertebrate extinction, all induced by human colonization, are the destruction and fragmentation of habitats, the introduction of predators and competing vertebrate species, as well as overhunting (Blackburn et al., 2004; Cheke and Hume, 2008; Triantis et al., 2010; Duncan et al., 2013; Heinen et al., 2017; Osuri et al., 2020). However, biogeographic factors such as high isolation, small area or high elevation, have also been shown to increase vertebrate extinction risk on islands (Blackburn et al., 2004; Duncan et al., 2013; Heinen et al., 2017).
Habitat destruction is a major cause of global diversity loss (Tilman et al., 1994; Triantis et al., 2010). It results in small and isolated populations, susceptible to stochastic factors such as erosion of genetic diversity or random fluctuation in demography, and can lead these populations to extinction in a few generations. These populations are, however, also vulnerable to other human-induced factors. For example, the introduction of invasive predators has been a major cause of vertebrate extinctions on oceanic islands worldwide, because most islands have few native predators (Blackburn et al., 2004; Doherty et al., 2016). Invasive mammals are particularly damaging, having contributed to considerable species decline and extinction. Introduced rodents and cats have for instance been listed as causal factors in 44% of modern species extinctions of bird, mammal, and reptile (Doherty et al., 2016). In addition, non-predator introduced vertebrates can also negatively impact native vertebrates by competing for resources and nest sites, or being reservoirs of exotic diseases (Jones, 1996; McClure et al., 2020).
Among biogeographic factors, island isolation increases the risk of vertebrate extinction (Heinen et al., 2017). Indeed, isolation may cause exaggerated ecological release, resulting in traits such as flightlessness, which make species more vulnerable to extinctions (MacArthur and Wilson, 1967). Smaller islands are also more prone to vertebrate extinctions because resources as well as refuges from anthropogenic overhunting or deforestation are more limited (Blackburn et al., 2004; Duncan et al., 2013; Heinen et al., 2017). Although montane areas were also expected to act as refuges from anthropogenic disturbances, greater extinction risks are nevertheless associated with island maximum elevation (Blackburn et al., 2004; Heinen et al., 2017). Heinen et al. (2017) suggested this might be explained by the great heterogeneity of habitat suitability along elevational gradients, making species more vulnerable to the destruction of lowland habitats. This “habitat suitability” hypothesis was first proposed by Cheke and Dahl (1981) who discussed a striking paradox in the Mascarenes. Despite the almost total destruction of native habitats, one of the two native species of fruit bats survived on Mauritius whereas they have long gone extinct on Réunion, where vast areas of native habitats remain. Thiollay and Probst (1999) made a comparable observation concerning the two extant Mascarene cuckoo-shrikes: the species confined to native remnants on Mauritius is doing better than the sister species on Réunion, where major conservation efforts are hardly succeeding in significantly increasing its population size in montane forest (see Salmona et al., 2012). In West Indies and Hawai’i, where similar patterns exist, it has been postulated that the loss of dry lowland habitats long before the arrival of Europeans might have caused the extinction of numerous vertebrates (Olson and James, 1989). Yet it has never been tested on oceanic islands, where historical contingency is generally poorly known.
Here, we use the Mascarene archipelago as a study system to test the “habitat suitability” hypothesis. It is probably one of the only archipelagos worldwide where natural history has been so well documented since first human settlement both in terms of historical accounts (Lougnon, 2005; Cheke and Hume, 2008) and paleontological works on extinct vertebrates (Cheke and Dahl, 1981; Hume, 2007, 2011, 2013, 2014, 2019; Arnold and Bour, 2008; Rijsdijk et al., 2009). With a very low filter effect (sensu Blackburn et al., 2004), we have a unique opportunity to study the main drivers of a paradoxical vertebrate extinction pattern: among the 23 forest vertebrate genera shared by Réunion and Mauritius, 12 genera (55.2% of species involved) went extinct on Réunion that still harbors 35% of native habitats (Strasberg et al., 2005), while five genera (31.4% of species involved) went extinct on Mauritius that retains less than 5% of native vegetation (Hammond et al., 2015; Figure 1 and Table 1). This contrast between the two islands is all the more puzzling since the extinction of vertebrates on Réunion has been staggering (Lougnon, 2005; Cheke and Hume, 2008). To decipher this paradox, we study the three main drivers of extinction, i.e., introduction of non-native vertebrates, overhunting and deforestation of suitable habitats, and compare the associated timeline on Réunion and Mauritius. To better characterize changes in habitat suitability since human colonization, we focus on an important guild of forest vertebrates, the frugivores of which spatial distribution is globally associated with the distribution of fleshy-fruited plants (Dehling et al., 2014; Ferger et al., 2014; Correa et al., 2015; Hazell, 2020).
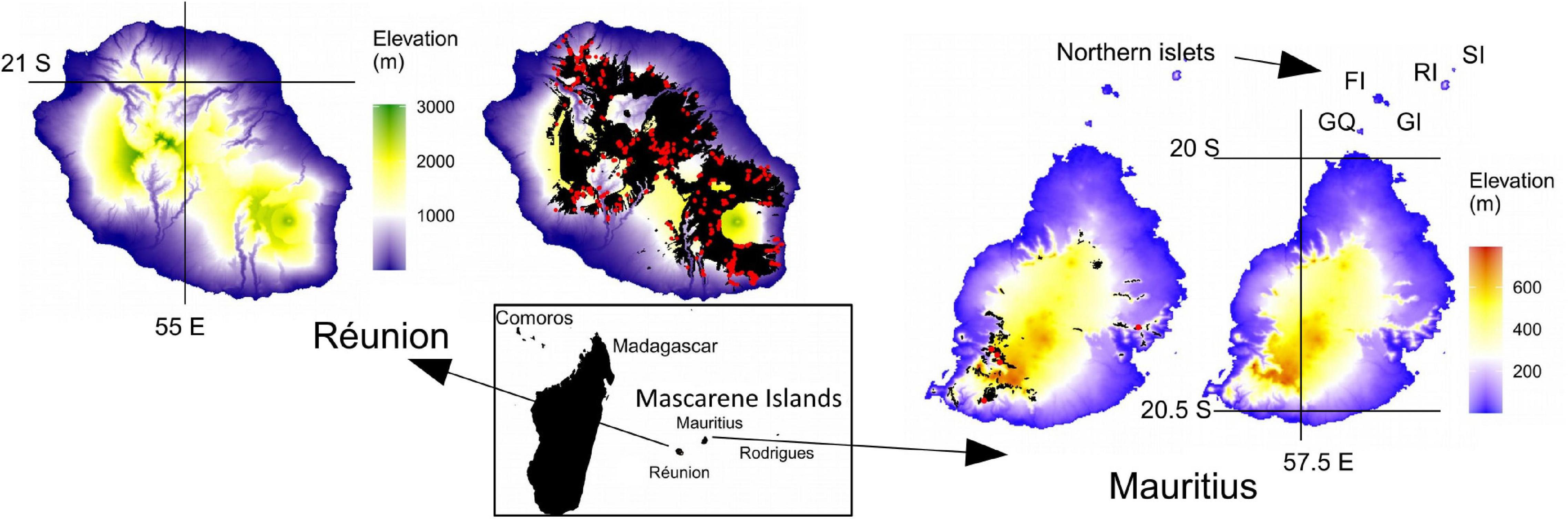
Figure 1. Topography and native forest remnants of the two main islands of the Mascarenes. Réunion is a relatively recent high-elevation island with only one tiny islet, while geologically older Mauritius shows a much less rugged topography and a large number of islets. The northern islets that have played an important role for the conservation of several endemic reptiles are indicated by letters: from South to North, “GQ” Gunner’s Quoin, “GI” Gabriel Island, “FI,” Flat Island,” “RI” Round Island and “SI” Serpent Island. The small Pigeon House Rock is not visible on the map. These six islets are located between 4 km (Gunner’s Quoin) and 24 km (Serpent Island) from the mainland. Extant native forests and shrublands are shown in black. On both islands, they are mainly located at the highest elevations, but two native forest corridors between sea level and montane habitats still persist in the South-East and North on Réunion. Red points display the location of historical surveys used for analyses of plant diversity (430 and 5 plots on Réunion and Mauritius, respectively).
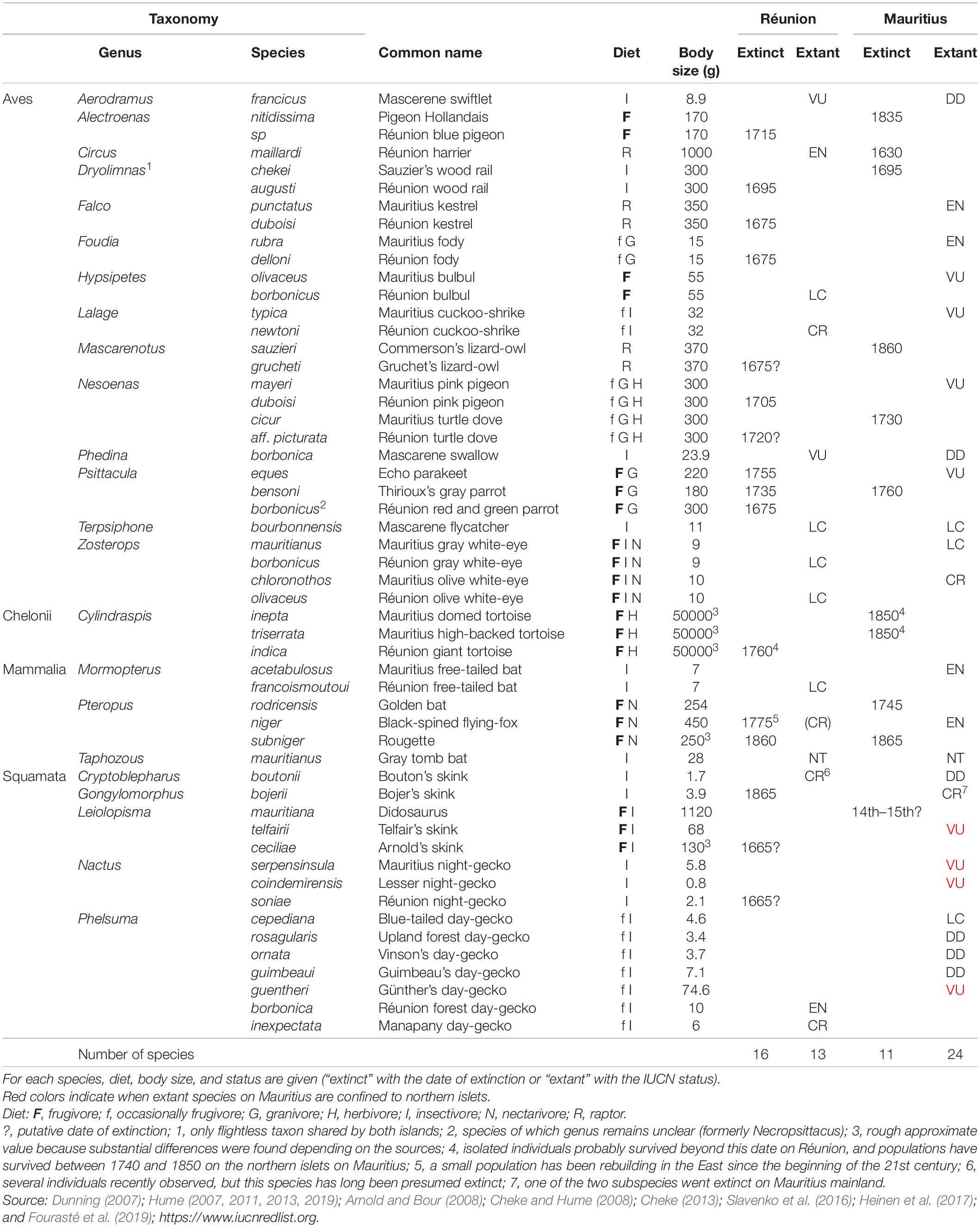
Table 1. Overview of forest vertebrate lineages shared by Réunion and Mauritius in the pristine Mascarenes.
Réunion (2,512 km2) and Mauritius (1,865 km2) are the two main islands of the Mascarenes (Figure 1). Rodrigues (109 km2), the smallest and easternmost island, was not included in this study. Indeed, the natural history of this island provides little information to disentangle the different drivers of extinctions due to the early and almost complete destruction of native habitats. Mauritius and Réunion have very different topographical characteristics. The first one is a shield volcano that peaks at 3,070 m asl with 50% of areas located above 770 m. The island is marked by a very rugged relief that induces strong geographical barriers, e.g., the Northwest/Southeast diagonal, the Cirques and other radially deep valleys (Figure 1). Conversely, 50% of areas are located under 161 m on Mauritius. Even if the island has some steep massifs that peak above 800 m asl, they are located in a rather lateral position, the center of Mauritius being occupied by a large, relatively flat plateau (Figure 1). Both islands experience a tropical oceanic climate, but the relief on Réunion induces far greater variations in terms of temperature and rainfall. Frost can appear above 1,500 m and is frequent above 2,000 m in winter, and the windward and leeward coasts, respectively, record the maximum (11,000 mm) and minimum (450 mm) annual rainfall in the archipelago (Réchou et al., 2019). The result is a zoning of habitats that has been the subject of various typological works (Cadet, 1977; Dupouey and Cadet, 1986; Strasberg et al., 2005). On Mauritius, an elevational zoning of habitats has also been described although the whole island is included in the megathermic domain (Vaughan and Wiehe, 1937; Page and D’Argent, 1997).
Until the 17th century, Réunion and Mauritius were almost totally wooded (except at high elevations on Réunion) and were home to a particularly abundant and relatively diverse fauna for oceanic islands. Here, we focus on vertebrates that are (were) closely dependent on forest habitats, so that we excluded seabirds and vertebrates dependent on wetlands. Both islands shared 23 genera of forest vertebrates (14 birds, one chelonian, three mammals, five squamates, Table 1). However, 15 genera of forest vertebrates were not found on the sister island. Réunion had other endemic bird species such as the Solitaire (Threskiornis), the Oiseau bleu (“Cyanornis”), the Mascarin parrot (Mascarinus), the Hoopoe starling (Fregilupus) and the Réunion stonechat (Saxicola), but also insectivorous bats, i.e., the Pale house bat (Scotophilus) and the Bory’s white bat (Boryptera). Mauritius sheltered endemic flightless birds, the Dodo (Raphus) and the Red hen (Aphanapterix), but also the Raven parrot (Lophopsittacus), the Wood pigeon (Columba) and the Mauritius starling (Cryptopsar) (Hume, 2013, 2014). More surprisingly, Mauritius also had several endemic snakes: the Keel-scaled boa (Casarea), the Burrowing boa (Bolyeria) and the Carié’s blind-snake (Typhlops). All these vertebrates went extinct since human colonization, except the Stonechat on Réunion and the Keel-scaled boa that survives on the northern islets of Mauritius (Cheke and Hume, 2008). Here, we studied the genera present both on Mauritius and Réunion, assuming that they shared intrinsic characteristics to allow a direct comparison of extinction factors.
The chronology of extinctions of forest vertebrates was documented on the basis of a literature review. Most of the data regarding the dates of extinction were drawn from the appendices of Cheke and Hume (2008) and relative updated publications (Table 1). We also compiled the body sizes of focal species and assessed changes in median body size of forest vertebrates since first contact with humans.
We established a chronological overview of terrestrial vertebrate naturalizations on Réunion and Mauritius since the beginning of human colonization. We characterized their introduction date on Réunion, Mauritius mainland or Mauritius islets, as well as their diet. The aim was not to provide a complete picture of vertebrate introductions on both islands, but to present all those that potentially negatively impacted the native fauna or have impacted the extant fauna. This impact may be directly mediated via predation or indirectly via competition, sometimes between related lineages. We also presented vertebrates that potentially contribute to alter the structure of native habitats. Most of the data regarding vertebrate introductions were drawn from the appendices of Cheke and Hume (2008) and relative updated publications (see Supplementary Appendix 1).
Overhunting is primarily related to human demography, and this was especially the case in the Mascarenes where the first settlers were described as inveterate hunters on both islands (Lougnon, 2005; Cheke and Hume, 2008). Using data available for Mauritius in Cheke and Hume (2008) and for Réunion in INSEE (2014), we compared the demography on Réunion and Mauritius since the beginning of human colonization. We also tried to document the extent to which marooning, i.e., slaves fleeing their captive terrible condition, might have contributed to overhunting on both islands.
We assessed the destruction of native forest habitats since the beginning of human settlement on Mauritius using digitized maps of native forest clearings in 1773, 1835, 1872, 1935 and 1997 available in Norder et al. (2017). For Réunion, we created digitized maps of native forest clearings based on Selhausen’s maps of 1793 and 1818. Earlier maps were not used because they were far too inaccurate regarding forest clearings (Germanaz, 2016). We also produced a map of native forest clearings in 1925 (J.-C. Notter, unpublished data), which marked the end of the Pelargonium cropping (Cheke and Hume, 2008), and used the recent work of Strasberg et al. (2005) as the most recent assessment of habitat transformation (Supplementary Appendix 2).
By cross-referencing the current habitat maps with maps of the clearing progress since the beginning of human colonization, we assessed the chronology of the destruction of native habitats. Habitat maps were based on common elevational zoning: 0–400 m, lowland; 400–850 m, mid-elevation; 850–1,200 m, submontane; 1,200–2,000 m, montane; 2,000–3,070, subalpine. This zoning generally follows elevational boundaries classically described for the two islands. The “lowland” area here is close to the definition of Dupouey and Cadet (1986) on Réunion and the elevational boundary between the so-called humid and subhumid forests was probably a bit below on Mauritius (Vaughan and Wiehe, 1937). The elevation of 850 m corresponds more or less to the upper limit of the megathermic area (Cadet, 1977) and to the top of Mauritius. Numerous lowland woody species occur up to 1,200 m in the “submontane” area on Réunion (Strasberg et al., 2005). The “montane” area then extends up to the average elevation of the trade-wind inversion (Cadet, 1977; Strasberg et al., 2005). The “subalpine” area also includes elevations above 2,700 m which are in an “alpine” environment.
Finally, given the importance of frugivores among forest vertebrates in the Mascarenes, we focused on the spatial distribution of fleshy-fruited plants that provide(d) crucial resources for these vertebrates. In particular, we studied the influence of elevation on the proportions of fleshy-fruited plants and large fleshy-fruited plants (i.e., mean fruit diameter >13 mm) within woody plant communities. On Réunion where sampling is substantial, we used spatial statistics for modeling (for more details, see Supplementary Appendix 3; Albert et al., 2018). Such modeling could not be performed on Mauritius because extant forests are too scarce and do not currently display sufficient environmental heterogeneity (Figure 1). Nevertheless, Florens (2008) surveyed native forest remnants, which enabled to roughly characterize the proportions of (large) fleshy-fruited plants on Mauritius. We then cross-referenced the spatial distribution of fleshy-fruited plants with land clearing maps at studied dates.
Human-induced extinctions within taxa of forest vertebrates shared by Réunion and Mauritius might have begun in the 14th century on Mauritius mainland after the arrival of Arab traders (Hume, 2013; Figure 2A and Table 1). Several reptile species may have survived only on the northern islets from that time. Most extinctions occurred from the beginning of permanent human colonization, i.e., 1638 on Mauritius and 1665 on Réunion. Shared lineages of forest vertebrates were dominated by frugivores in terms of body mass (Figure 2B). Frugivores went extinct more often than other vertebrates and this phenomenon was much more marked on Réunion which lost a large part of its large frugivores as early as 1750. Most large-bodied frugivores were present until the middle of the 19th century on Mauritius: giant tortoises went extinct on the northern islets in 1850 and the Pigeon Hollandais and the Rougette around 1870 on Mauritius mainland. The balance of extinctions, i.e., 16 and 11 species extinct on Réunion and Mauritius, respectively, remains unchanged since that time and Mauritius still has 68% of its native forest vertebrates (Figure 2A and Table 1). The largest surviving native frugivore on Réunion for more than 150 years is the Réunion bulbul (the only larger extant forest vertebrate is the Réunion harrier). The median body size of frugivores remains much larger on Mauritius compared to Réunion due to the extant Black-spined flying-fox, Echo parakeet and Telfair skink (on northern islets only for the latter; Figure 2B).
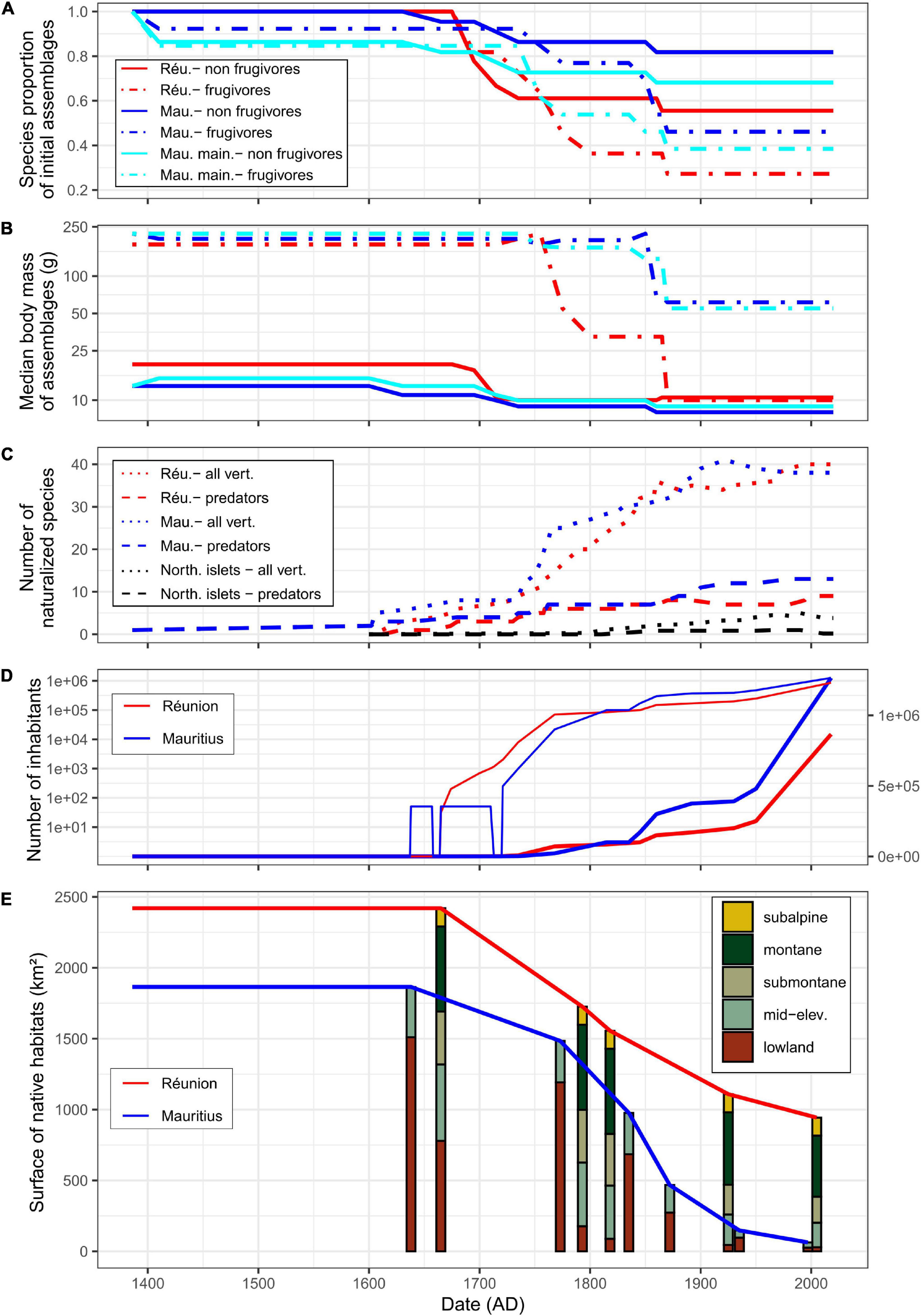
Figure 2. Chronology of extinctions of native forest vertebrates in lineages shared between Réunion and Mauritius (A,B) and of main extinction factors (C–E) since first human arrival. Islands are displayed by colors: Réunion in red, Mauritius in blue (i.e., considering mainland and the northern islets), Mauritius mainland in cyan [panels (A,B) only], northern islets in black [panel (C) only]. (A) Changes in species richness of native frugivores (dotdash lines) and non-frugivores (solid lines) due to extinctions as proportions of initial species assemblages. (B) Changes in median body mass of native assemblages of frugivores and non-frugivores [same legend as in panel (A)]. Note the log-scale for the y-axis. (C) Changes in the number of vertebrate naturalizations: all land vertebrates (dotted lines) and predators (dashed lines). For the northern islets, the mean number is given. Note the non-monotonicity of the curves which reflects extinctions (for more details, see Supplementary Appendix 1). (D) Human population size (bold lines: arithmetic scale on right y-axis, thin lines: log-scale on left y-axis). There are no accurate estimate of the number of Dutch settlers who occupied Mauritius more or less permanently during the 17th century. Similarly, after the abandonment of Mauritius by the Dutch, the human population officially fell to zero even though there is evidence of maroons persistence before the French took possession. (E) Changes in areas of native habitats; for all habitats (solid lines) and elevational habitats (colored bars: lowland, 0–400 m; mid-elevation, 400–850 m; submontane, 850–1,200 m; montane, 1,200–2,000 m; subalpine, 2,000–3,070 m). For Réunion, approximately 100 km2 of unfavorable areas (mostly bare lava flows in the caldera) have been excluded from analyses.
Réunion and Mauritius have experienced the naturalization of about 50 vertebrate species that have potentially impacted native terrestrial biota (Supplementary Appendix 1), excluding the two species of non-native tortoises voluntarily introduced on Round Island as taxon substitutes of native giant tortoises (Griffiths et al., 2011; Cole, 2012). Mauritius mainland showed earlier introductions of vertebrates than Réunion, especially predators such as the Ship rat introduced in the 14th century, the Crab-eating macaque and the Pig in the 16th century (Hume, 2013; Figure 2C). Potentially harmful birds and reptiles were introduced late except the Common myna in the mid-18th century (Supplementary Appendix 1). Hence, the main vertebrates involved in early extinctions were land mammals that were introduced before or at the beginning of permanent settlements on Réunion and Mauritius (Figure 2C).
The early introduction of predators such as the Ship rat, the Pig and especially the Cat, caused a carnage among flightless birds, e.g., Wood rails (Dryolimnas), but also among other ground nesting birds (Cheke, 2013) and giant tortoises, as reported many times by settlers and travelers (Lougnon, 2005; Cheke and Hume, 2008). Moreover, the absence of any mention in the historical literature of the world’s greatest skink on Mauritius is likely the direct result of the early introduction of the Ship rat (Hume, 2013). As stated by Blackburn et al. (2004), species most susceptible to introduced mammals probably went extinct rapidly on both islands, i.e., before 1750 (Figure 2C). However, several large vertebrates were able to survive on Mauritius until now despite the persistence of mammal predators, sometimes at high density, while the Pig went extinct in the meantime on Réunion (Table 1). Even taxa such as fruit pigeons (Alectroenas) that went extinct on both islands have lasted longer on Mauritius mainland than on Réunion (Table 1). This is all the more puzzling since several mammal species known for their severe impact, such as the Crab-eating macaque and the Indian mongoose, have been introduced on Mauritius, but never on Réunion (Supplementary Appendix 1). The northern islets experienced few introductions of vertebrates, and especially few and relatively late introductions of predators (Figure 2C), which is probably the main reason for the capacity of the Telfair skink and several endemic geckos to persist until now (Figure 2A and Table 1). Thus, although introduced vertebrates have had a major impact on the native fauna of the archipelago, apart from the northern islets which shelter several endemic reptiles, this factor cannot explain alone why all large forest vertebrates went extinct so quickly on Réunion compared to Mauritius.
The Dutch claimed Mauritius in 1598 and occupied the island between 1638 and 1710, with a pause between 1657 and 1664. During this period, the European population of Mauritius never numbered more than 50 according to Hume (2013). When the French actually took control of Mauritius in 1721, Réunion already had about 2,000 inhabitants (Figure 2D). On that date, flightless birds were extinct on both islands and many forest vertebrate populations were already strongly reduced or were collapsing, especially on Réunion where increasing demography led to a strong demand for meat. The local authorities actually early realized how quickly the populations of large vertebrates were collapsing, which particularly worried them because the French East India Company was using the native fauna to supply its ships free of charge (Lougnon, 2005). Large frugivores were particularly prized because they were, by necessity, large body-sized game (Figure 2B). Settlers were literally obsessed with the areas that were still home to abundant wildlife at the beginning of the 18th century, areas known as “the land of food” (Defos Du Rau, 1960). Attempts to legally regulate hunting on Réunion proved totally unsuccessful (Cheke and Hume, 2008). The arrival of the governor Mahé de La Bourdonnais in 1735 coincided with strong economic development based on sugar production and a sharp increase in the number of inhabitants on Mauritius (Figure 2D). In 1768, the number of inhabitants was still three times larger on Réunion than on Mauritius, but the balance was reversed in the last decades of the century (Figure 2D). The number of inhabitants on Mauritius has remained higher since that date. Settlers were known as active and efficient hunters on both islands (Lougnon, 2005; Cheke and Hume, 2008), deer hunting has for instance remained an institution on Mauritius since that time (Cheke and Hume, 2008). However, the sharp increase in inhabitants on Mauritius has surprisingly not led, as on Réunion, to the rapid extermination of all large native frugivores.
Marooning may have significantly contributed to vertebrates being overhunted in the archipelago. Indeed, maroons have early taken refuge in the remote forests of Réunion and Mauritius, and lived largely on the fauna of both islands between the 17th and 19th centuries (Lougnon, 2005; Cheke and Hume, 2008; Dijoux, 2013). We do not have accurate estimates of the number of maroons on both islands (Figure 2D). The phenomenon was quite intense in the first half of the 18th century on Réunion where small villages of maroons may have existed in the Cirques before the settlers launched man-hunts that gradually pushed the maroons back up to the highlands (Dijoux, 2013). Marooning was also prominent in the early phases of the colonization of Mauritius (Hume, 2013) and succeeded in posing severe difficulties to the first French settlers (Cheke and Hume, 2008). The phenomenon then seems to have been essentially confined to south-western Mauritius where large native forests have belatedly remained. On both islands, marooning probably contributed to increased pressure on wildlife in remote areas that could act as refuges. However, while overhunting has played an important role in the extinction of vertebrates on both islands, it fails to explain alone why the native fauna that had survived the arrival of introduced predators went rapidly extinct on Réunion compared to Mauritius.
Before permanent colonization, lowland forests (under 400 m asl) represented 31.8 % of total area on Réunion against 81% on Mauritius (Figure 2E). On Réunion, the belt of lowland habitats was rapidly cleared which generated large-scale fires on the leeward as reported by La Houssaye in 1698 (Lougnon, 2005). During the 18th century, deforestation eventually affected almost all lowlands that could be cultivated on the windward. Despite the intense exploitation of ebony trees on Mauritius by the Dutch and the early use of fires (Gosling et al., 2017), the island was still largely wooded when the French took control in 1721 (Figure 2E). The permanent colonization of Mauritius led to intense deforestation that has not stopped until the 20th century. Around 1800, 610 km2 of native forests have been cleared on Réunion and Mauritius, which represented ca 87% and 40% of lowland habitats, respectively (Figure 2E). Hence, Réunion lost its overall tropical lowland forest in less than 135 years of settlement, which probably had dramatic functional consequences for native forest vertebrates, notably for frugivores.
On Réunion, fleshy-fruited plants overwhelmingly dominated woody plant communities at sea level (estimated proportion >80%) and were dominant up to 1,400 m asl (Figure 3). The estimated proportion of large fleshy-fruited plants was ca 25% at sea level and sharply decreased with elevation (<10% in submontane habitats and <3% in montane habitats). The early destruction of the lowland belt has thus led to the rapid loss of highly favorable areas for frugivores (Figures 2E, 3). Moreover, due to belt structuring, favorable remnants were systematically adjacent (i) at lower elevations, to cleared areas (and subsequently subject to high hunting pressure), and (ii) at higher elevations, to (sub)montane habitats where proportions of (large) fleshy-fruited plants rapidly decreased with elevation (Supplementary Appendix 4). Thus, effective refuges were rare on Réunion, not only because the original area of favorable habitats was relatively small, but also because the relief accentuated the fragmentation of potentially suitable remnants (Supplementary Appendix 5).
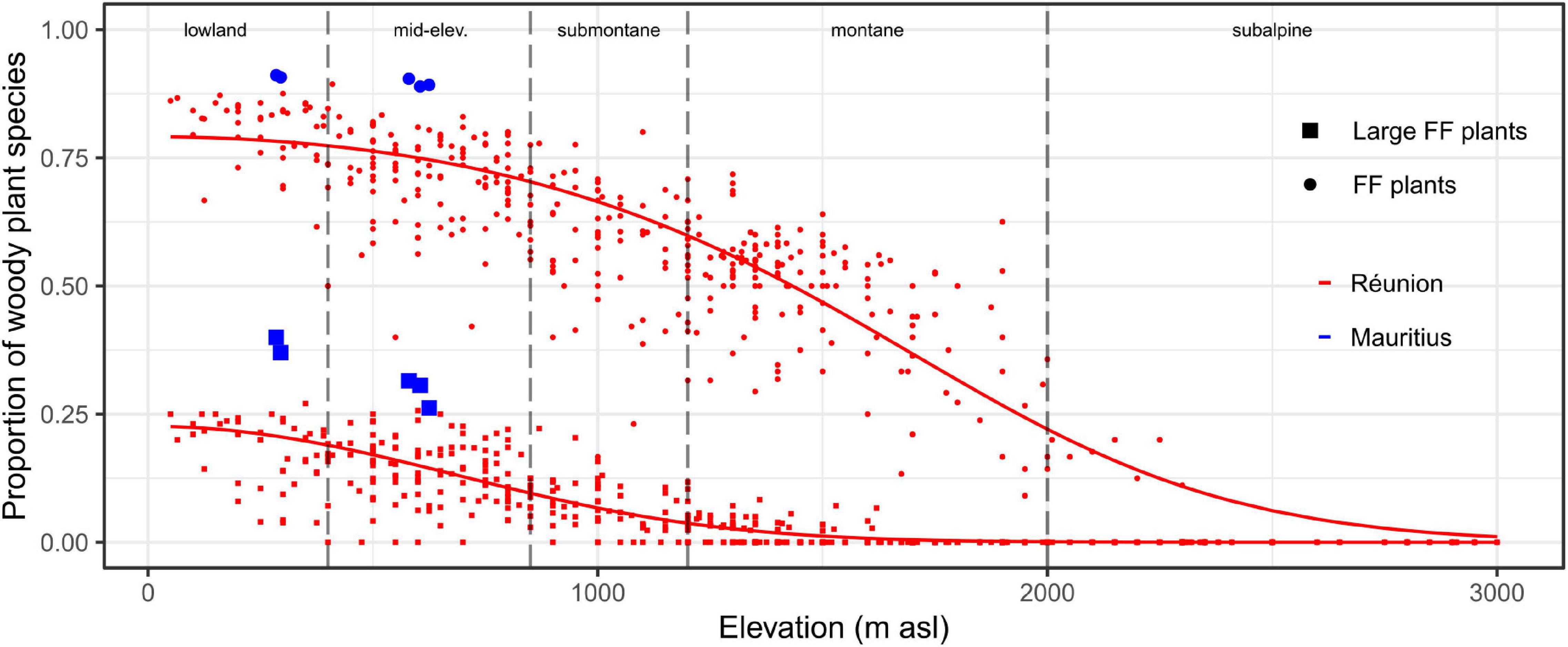
Figure 3. Proportion of (large) fleshy-fruited plants within woody plant communities depending on elevation on Réunion and Mauritius. The x-axis is divided according to the typology of elevational habitats (dashed vertical lines). Dots and squares symbolize the proportions of fleshy-fruited (FF) and large fleshy-fruited plants, respectively. Sampling plots on Réunion and Mauritius are displayed by red and blue colors, respectively. For Réunion, predicted probabilities are displayed by solid lines (spatial modeling is visible at Supplementary Appendix 4).
On Mauritius, fleshy-fruited plants dominated woody plant communities in the lowlands even more than on Réunion (Figure 3). This was mainly due to higher proportions of large-fruited plants with up to 40% of woody plants on Mauritius. Hence, native forest habitats are supposed to have been formerly highly favorable to native frugivores throughout the island. This high suitability implies that, with a comparable level of deforestation in 1800, native forests probably remained much more favorable to native frugivores on Mauritius than on Réunion (Figures 2E, 3). Finally, native areas have belatedly persisted on the central plateau of Mauritius as a compact habitat (Supplementary Appendix 5), with the possibility for native vertebrates to better escape, at least for a time, from the multiple human-induced disturbances.
The majority of native forest vertebrates in the Mascarenes has disappeared since human colonization, but this phenomenon nevertheless shows important disparities in the archipelago (Cheke and Dahl, 1981; Cheke and Hume, 2008; Hume, 2013). Considering vertebrates from lineages shared by Réunion and Mauritius, we sought to understand why the former island, which still hosts 35% of native habitats, has lost all of its large forest vertebrates (except the Réunion harrier, Circus) while the latter, whose habitats have been almost completely destroyed, has retained a greater diversity of squamates (Gongylomorphus, Leiolopisma, Nactus), birds (Falco, Foudi, Nesoenas, Psittacula) and mammals (Pteropus). With the exception of the northern islets where squamates avoided extinction thanks to low densities of introduced predators (Cheke and Hume, 2008; Cole, 2012), this paradox cannot be explained by differential pressures of mammal introduction and overhunting. On the contrary, Mauritius experienced a greater number of introductions of predators known for their negative impact on native vertebrates (Florens, 2013; Reinegger et al., 2021). Meanwhile, although overhunting likely impacted more Réunion during early human colonization, it has not spared Mauritius, where the number of inhabitants has been higher than on Réunion since the late 18th century. The introduction of invasive vertebrates and overhunting being put aside, the major explanatory difference between the two islands is the rate of deforestation of lowland habitats since human colonization. While on Mauritius, low-fragmented favorable forests have long persisted, lowland habitats on the leeward coast of Réunion had disappeared as soon as the early 18th century and were massively destroyed along the century course on the windward coast. The suitability of mid-elevation remnants on Réunion has probably also been reduced due to the fragmentation induced by landform configuration. Montane habitats, especially in steep places, may have acted as inhospitable refuges from overhunting, but not from introduced mammal predators (Thiollay and Probst, 1999; Pinet et al., 2009). In fact, there may be a critical size/configuration of suitable home-range for a given level of disturbance. This was demonstrated on Mauritius after the disappearance of Les Mares in 1975 with the sharp decline of the populations of the Pink pigeon, the Echo parakeet and the Mauritius olive white-eye which had resisted well until 1950 (Jones, 1987; Jones et al., 1998). Additional drivers, beyond the scope of this study, may also have played a role in the extinctions observed. For example, avian malaria has been mentioned as a possible cause of extinction of the Hoopoe starling on Réunion in the 19th (Cheke, 2013). However, vector-borne diseases seem unlikely to have decimated focal vertebrates on Réunion, but not on Mauritius, where introductions of potential host vertebrates have been more massive and where there is no elevational escape from Culex pipiens fatigans (see Peirce et al., 1977).
We show that large frugivores are more prone to extinction than other forest vertebrates in the Mascarenes, as already demonstrated elsewhere (Steadman, 1995). Their extinction has been especially rapid and massive on Réunion where the lowland belt rich in large fleshy-fruited plants was concomitantly destroyed. These plants probably provided crucial resources for frugivores and their spatial distributions may have coincided, as already demonstrated in the tropical Andes (Dehling et al., 2014; Correa et al., 2015), on Mount Kilimanjaro (Ferger et al., 2014) or in Papua New Guinea (Hazell, 2020). All forests on Mauritius were probably widely dominated by fleshy-fruited species with an even higher proportion of large fleshy-fruited plants than on Réunion. Therefore, the long-standing persistence of low-fragmented suitable forests probably played a key role in the survival of the Pigeon Hollandais until 1850 (see Morante-Filho et al., 2018) and in the maintenance of the Black-spined flying-fox and the Echo parakeet until today. Even introduced Coracopsis parrots from Madagascar that naturalized in the 1790s on Réunion failed to survive in lowland forest remnants, while the Ring-necked parakeet has naturalized in Mauritius native forests since its introduction in the 19th century (Cheke and Hume, 2008). Hence, in regards to the strong modulation by elevation of suitable surfaces observed in the Mascarenes, it is not surprising that high-elevation oceanic islands, all subject to strong environmental gradients (Irl et al., 2016), experience globally higher rates of frugivore extinctions (Heinen et al., 2017). In all these islands, the systematic destruction and fragmentation of lowland native forests by humans have led to the early destruction of the most suitable habitats for frugivores.
Because of the assumed overlap of spatial distributions of fleshy-fruited plants and frugivores in the Mascarenes, it is more convenient to study changes in habitat suitability for frugivores compared to vertebrates with different diets. However, the study of habitat suitability goes beyond food availability and encompasses a panel of biotic and abiotic conditions to maintain a population. Although belonging to radiated lowland lineages (Cheke and Hume, 2008; Kehlmaier et al., 2019), several extinct vertebrates were sometimes described as frequenting montane habitats on Réunion, which fueled the idea that these habitats may have acted as effective refuges. Yet, these vertebrates may have been unable to breed and/or to feed all year long there. The case of giant tortoises is emblematic, as they were observed in steep and cool montane slopes (Lougnon, 2005), but probably lacked suitable nesting localities without the possibility of returning to the coast (Hume et al., 2021). Likewise, vertical migrations were attributed to several bird species, of which passerines, pigeons and parrots (Lougnon, 2005). As suggested by fruit pigeons Alectroenas in Madagascar, vertical migrations of pigeons on the leeward were probably seasonal, descending in the lowlands during the wet season, and returning in winter to the mid-elevations, probably because of food availability (Hume, 2011). Hence, these forest vertebrates were capable of adapting to heterogeneous insular contexts, but they probably remained highly dependent on lowland habitats. In this respect, the South-West Indian Ocean islands offer interesting perspectives to understand the distribution of extinct vertebrates. For example, four sympatric pigeons and doves related to the extinct Mascarene columbids have persisted in the Comoros where forests still remain along elevational gradients (Hume, 2011). Recent deforestation in this archipelago thus provides a clinical case to better understand the capacity of these species to adapt to montane conditions that are presumed to be much less favorable.
Paleontology plays a central role in the knowledge of extinct fauna and extinction factors. In the Mascarenes, Réunion paleoecosystems remain the less well investigated (Hume, 2013). The main reasons are geomorphological as the island has few favorable sites (but see Mourer-Chauviré et al., 1999) compared to Mauritius and Rodrigues where caves, limestone deposits and Lagerstätte as La Mare aux Songes have yielded numerous subfossil bones (Griffiths and Florens, 2006; Cheke and Hume, 2008; Rijsdijk et al., 2009, 2015; Hume, 2013; Hume et al., 2021). Due to the lack of osteological material, several emblematic vertebrates have not yet been described and remain poorly known, such as the Réunion blue pigeon, the Réunion turtle dove or the Réunion red and green parrot (Hume, 2011, 2013). The existence of certain species remains putative, e.g., the Réunion wood pigeon (Columba) (Hume, 2011), and Mourer-Chauviré et al. (1999) stated that the near absence of flightless birds on Réunion might be a sampling bias. Moreover, osteological details allow us to better understand the ecology of extinct species of which we usually have very sketchy accounts. For example, the extinct Mauritius turtle dove differs from the Madagascar turtle dove in a number of osteological details, which shows that this subspecies was probably more terrestrial than the introduced Madagascar turtle dove (Hume, 2011). Finally, osteological material can also be used to better trace the chronology of native vertebrate extinction. For example, the first material evidence of contact between the Mascarenes and the Arab traders was obtained by dating a mandible of ship rat collected from La Mare aux Songes, which provided at the same time a credible explanation for the early extinction of several endemic animals on Mauritius mainland (Hume, 2013).
Lowland leeward habitats have been completely transformed and may have been the richest in large vertebrates such as giant tortoises or skinks in the Mascarenes (Cheke and Hume, 2008), as elsewhere (Olson and James, 1989). Our analyses, based on extrapolation of statistical estimators (Supplementary Appendix 6), suggest that the lowlands on Réunion leeward were rich in large fleshy-fruited plants and that ecosystems more complex than a latan/benjoin savanna were probably encountered there. Our work therefore supports the hypothesis that the structure and composition of the whole coastal dry forest have been early altered and the leeward lowlands early savannised [in Lougnon (2005), Houssaye reported large areas of recently burned land in 1689, while stating that the dry Pointe des Galets was forested]. Moreover, Mourer-Chauviré et al. (1999) excavated a great amount of large seeds belonging to Latania, Terminalia, Cassine, Foetidia, Pandanus, or Sideroxylon among giant tortoise bones in the swamp of l’Ermitage (D. Strasberg, personal communication). These findings suggest that plant community composition might have been relatively close to what was revealed by Rijsdijk et al. (2009) at La Mare aux Songes on Mauritius. Hence, we need a thorough paleoecological work on Réunion to better understand what may have been the likely main habitat of the largest forest vertebrates.
While the majority of native forest vertebrate species are extinct, the situation is highly contrasted regarding extant ones. There is little doubt that many of them would have gone extinct if the conservationists had not taken action on both islands to face the extinction debt. While clearings no longer seem to occur, conservationists have focused on improving the quality of native remnants that are often severely degraded by exotic plants, on combating poaching and introduced predators, while attempting to control the introduction of new harmful organisms (Cheke and Hume, 2008; Sanchez and Probst, 2016). At the same time, concerns about the impact of vertebrate loss on the functioning of tropical forests have globally grown and recent studies with dramatic results for the regeneration of Mascarene tropical forests have shown the urgency of restoring plant-vertebrate interactions.
Some vertebrates were able to adapt to disturbances induced by human colonization in the Mascarenes. For example, the Réunion gray white-eye is able to colonize all kinds of habitats (Barré et al., 2005; Cheke and Hume, 2008). Likewise, the Mascarene swiftlet or microbats such as the Réunion free tailed bat, hold up well as long as breeding and roosting sites are preserved, especially caves and lava tunnels. Bourbon gecko’s and Réunion passerines (except the cuckoo-shrike) are widely distributed where native forest still remain, even though the size of populations is sometimes much smaller than what it used to be, e.g., the Réunion bulbul (Cheke, 1987). However, most extant vertebrates are threatened and subject to considerable conservation effort, especially on Mauritius. The reintroduction of individuals of the Pink pigeon and the Echo parakeet reproduced in captivity has recently allowed to significantly increase their population size. Likewise, the Réunion harrier and the Mauritius kestrel have recolonized a large part of their initial range in their respective island. The rescue of the latter is regarded as a tremendous success story in conservation biology. Populations of native reptiles have reached satisfactory sizes on northern islets of Mauritius since the extirpation of all non-native mammals in the 1990s (Cheke and Hume, 2008). Some actions, however, are currently unable to enhance populations of threatened species, for example for the Mauritius olive white-eye, the Manapany gecko or the Réunion cuckoo-shrike. The latter, which previously had a wider range including the lowlands, is now confined to montane habitats to northern Réunion (Barré et al., 2005). The impressive efforts to control introduced predators into a specially created reserve have been unsuccessful to downlist the species from the “Critically Endangered” category. Hence, this species may be a lowland species which survives in sub-optimal habitat (Thiollay and Probst, 1999). In light of our results, the establishment of a second population at lower elevations should be reconsidered.
Among forest vertebrates, the guild of frugivores plays a crucial role in the functioning of tropical forests by ensuring seed dispersal of most woody plants. Building on works showing the negative impact of the extirpation of large frugivores in continental forests (Terborgh et al., 2008; Effiom et al., 2013; Harrison et al., 2013), recent studies have investigated the consequences of their massive defaunation on the regeneration of Mascarene forests. They showed that primary succession has been profoundly disturbed on Réunion for several centuries with a dramatic loss of native plant diversity on historical lava flows (Albert et al., 2020), but also that numerous tree species show a worrying lack of recruitment in old-growth forests, particularly on Réunion where all large frugivores went extinct (Albert et al., 2021). In contrast, the regeneration success for many small- and medium-seeded plants in Mauritian remnants is probably due to the extant Black-spined flying-fox and Echo parakeet (Florens et al., 2017). While experimental direct sowing on Réunion has already shown that the recruitment of large-seeded trees can be restored (Albert, 2020), the future should focus on rewilding ecosystems to restore processes across space and time. From this point of view, the situation is contrasted on Mauritius. On the one hand, the Black-spined flying-fox is facing a massive slaughter for demagogic reasons instead of being protected (Florens et al., 2017). On the other hand, the population of the Echo parakeet has reached a threshold which may coincide with the carrying capacity of native remnants (S. Henshaw, personal communication); rewilding with substitute giant tortoises has also been undertaken to restore seed dispersal and herbivory on northern islets and Ile aux Aigrettes (Griffiths et al., 2011; Cole, 2012), which are now formidable labs for ecological restoration. The most promising possibilities may be found on Réunion where there are now more suitable areas than on Mauritius and where the need to restore plant-animal interactions in native ecosystems should be a high-rank priority (Albert et al., 2021). The Echo parakeet being rescued on Mauritius, it is now possible to consider its reintroduction on Réunion, where populations could reach even higher levels than on Mauritius. French and Mauritian stakeholders are currently reflecting on the feasibility of this project (K. Leclerc, personal communication). Finally, the Black-spined flying-fox is back to Réunion after more than two centuries of absence (Fourasté et al., 2019). If the embryonic population is not hunted and/or persecuted, this species may be able to recolonize native forests where it has a crucial role to play as double mutualist vertebrate (Florens et al., 2017; Albert et al., 2021). Needless to say, this will depend on the ability of conservationists to learn from the socio-ecological context and create the conditions for consent.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
SA, OF, CA-P, and DS developed the ideas for the research. SA and DS collected the data. SA analyzed the data. All authors contributed critically to the drafts of the article and have given their approval for publication.
This research was supported by the University of Réunion Island, Région Réunion and FEDER financed through the Ph.D grant from the doctoral school EDI-STS (SA), Fédération OMNCG for the research project PALEOE (OF, CA-P, and DS), and FEDER DIVINES (OF, CA-P, and DS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Jean-Cyrille Notter at Réunion National Park who provided support for the production of digitized maps, Kalyan Leclerc at SEOR for constructive exchanges concerning the avifauna and two reviewers whose relevant comments significantly improved the manuscript. We would also like to thank Solène Irion for proofreading the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.688768/full#supplementary-material
Albert, S. (2020). Rupture des Interactions Mutualistes Plantes à Fruits Charnus-vertébrés Frugivores, et Conséquences Sur la régénération des Forêts Tropicales Dans les Mascareignes. Ph.D. Saint-Denis, Réunion: Université de la Réunion. Available Online at: https://tel.archives-ouvertes.fr/tel-03126708
Albert, S., Flores, O., Baider, C., Florens, F. B. V., and Strasberg, D. (2021). Differing severity of frugivore loss contrasts the fate of native forests on the land of the Dodo (Mascarene archipelago). Biol. Conserv. 257:109131. doi: 10.1016/j.biocon.2021.109131
Albert, S., Flores, O., Rouget, M., Wilding, N., and Strasberg, D. (2018). Why are woody plants fleshy-fruited at low elevations? Evidence from a high-elevation oceanic island. J. Veg. Sci. 29, 847–858. doi: 10.1111/jvs.12676
Albert, S., Flores, O., and Strasberg, D. (2020). Collapse of dispersal trait diversity across a long-term chronosequence reveals a strong negative impact of frugivore extinctions on forest resilience. J. Ecol. 108, 1386–1397. doi: 10.1111/1365-2745.13359
Alcover, J. A., Sans, A., and Palmer, M. (1998). The extent of extinctions of mammals on islands. J. Biogeogr. 25, 913–918. doi: 10.1046/j.1365-2699.1998.00246.x
Arnold, E. N., and Bour, R. (2008). A new Nactus gecko (Gekkonidae) and a new Leiolopisma skink (Scincidae) from La Réunion, Indian Ocean, based on recent fossil remains and ancient DNA sequence. Zootaxa 1705, 40–50. doi: 10.11646/zootaxa.1705.1.3
Barré, N., Barau, A., and Jouanin, C. (2005). Le grand livre des oiseaux de la Réunion. La Réunion. Paris: Orphie Éd. du Pacifique.
Blackburn, T. M., Cassey, P., Duncan, R. P., Evans, K. L., and Gaston, K. J. (2004). Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958. doi: 10.1126/science.1101617
Cadet, T. (1977). La végétation de l’île de la Réunion: étude Phytoécologique et Phytosociologique. Ph.D. Thesis. France: Université d’Aix Marseille
Cheke, A. S. (1987). “The ecology of the surviving native land-birds of Reunion,” in Studies of Mascarene Island Birds, ed. A. W. Diamond (Cambridge: Cambridge University Press), 301–358. doi: 10.1017/cbo9780511735769.008
Cheke, A. S. (2013). Extinct birds of the Mascarenes and Seychelles - a review of the causes of extinction in the light of an important new publication on extinct birds. Phelsuma 21, 4–19.
Cheke, A. S., and Dahl, J. F. (1981). The status of bats on western Indian Ocean islands, with special reference to Pteropus. Mammalia 45, 205–238. doi: 10.1515/mamm.1981.45.2.205
Cheke, A. S., and Hume, J. P. (2008). Lost Land of the Dodo: An Ecological History of Mauritius, Réunion & Rodrigues. New Haven, CT: Yale University Press.
Cole, N. (2012). Restoration of island ecosystems in Mauritius: the Mauritius reptile recovery programme annual report 2013. Durrell Wildlife Conservation Trust, Jersey.
Correa, D. F., Álvarez, E., and Stevenson, P. R. (2015). Plant dispersal systems in Neotropical forests: availability of dispersal agents or availability of resources for constructing zoochorous fruits? Glob. Ecol. Biogeogr. 24, 203–214. doi: 10.1111/geb.12248
Defos Du Rau, J. (1960). “Une île créole : le peuplement et l’occupation du sol,” in L’île de La Réunion: étude de Géographie Humaine. Bordeaux: Institut de géographie. 129–177.
Dehling, D. M., Töpfer, T., Schaefer, H. M., Jordano, P., Böhning-Gaese, K., and Schleuning, M. (2014). Functional relationships beyond species richness patterns: trait matching in plant-bird mutualisms across scales. Glob. Ecol. Biogeogr. 23, 1085–1093. doi: 10.1111/geb.12193
Dijoux, A.-L. (2013). La « vallée secrète » à La Réunion. Un refuge extrême pour les esclaves « marrons » caractérisé pour la première fois par l’archéologie. Archéopages 38, 20–23. doi: 10.4000/archeopages.491
Doherty, T. S., Glen, A. S., Nimmo, D. G., Ritchie, E. G., and Dickman, C. R. (2016). Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. U. S. A. 113, 11261–11265. doi: 10.1073/pnas.1602480113
Duncan, R. P., Boyer, A. G., and Blackburn, T. M. (2013). Magnitude and variation of prehistoric bird extinctions in the Pacific. Proc. Natl. Acad. Sci. U. S. A. 110, 6436–6441. doi: 10.1073/pnas.1216511110
Dupouey, J.-L., and Cadet, T. (1986). Subdivisions de la forêt de bois de couleur à l’île de la Réunion. Ann. Sci. For. 43, 103–114. doi: 10.1051/forest:19860108
Effiom, E. O., Nunez-Iturri, G., Smith, H. G., Ottosson, U., and Olsson, O. (2013). Bushmeat hunting changes regeneration of African rainforests. Proc. R. Soc. B Biol. Sci. 280:20130246. doi: 10.1098/rspb.2013.0246
Ferger, S. W., Schleuning, M., Hemp, A., Howell, K. M., and Böhning-Gaese, K. (2014). Food resources and vegetation structure mediate climatic effects on species richness of birds. Glob. Ecol. Biogeogr. 23, 541–549. doi: 10.1111/geb.12151
Florens, F. B. V. (2008). Ecologie Des Forêts Tropicales De L’ile Maurice Et Impact Des Espèces Introduites Envahissantes. Ph.D. Thesis. Saint-Denis, Reunion: Université de la Réunion.
Florens, F. B. V., Baider, C., Marday, V., Martin, G. M. N., Zmanay, Z., Oleksy, R., et al. (2017). Disproportionately large ecological role of a recently mass-culled flying fox in native forests of an oceanic island. J. Nat. Conserv. 40, 85–93. doi: 10.1016/j.jnc.2017.10.002
Florens, V. F. B. (2013). “Conservation in Mauritius and Rodrigues: challenges and achievements from two ecologically devastated oceanic islands,” in Conservation Biology: Voices From the tropics, eds N. Sodhi, L. Gibson, and P. H. Raven (Hoboken: John Wiley & Sons, Ltd), 40–50. doi: 10.1002/9781118679838.ch6
Fourasté, S., Prolhac, E., and Monnier, G. (2019). Ecologie alimentaire de la Roussette noire, interactions avec les cultures fruitières et implications pour la conservation de l’espèce sur l’île de La Réunion. Saint-Paul, Réunion: Groupe Chiroptères Océan Indien.
Germanaz, C. (2016). Un tour des cartes de Bourbon. Matériaux pour une histoire de la représentation cartographique de La Réunion. Bull. Acad. L’Ile La Réun. 32, 47–73.
Gosling, W. D., de Kruif, J., Norder, S. J., de Boer, E. J., Hooghiemstra, H., Rijsdijk, K. F., et al. (2017). Mauritius on fire: tracking historical human impacts on biodiversity loss. Biotropica 49, 778–783. doi: 10.1111/btp.12490
Griffiths, C. J., Hansen, D. M., Jones, C. G., Zuël, N., and Harris, S. (2011). Resurrecting extinct interactions with extant substitutes. Curr. Biol. 21, 762–765. doi: 10.1016/j.cub.2011.03.042
Griffiths, O. L., and Florens, V. F. (2006). A Field Guide to the Non-Marine Molluscs of the Mascarene Islands: (Mauritius, Rodrigues, and Réunion) and The Northern Dependencies of Mauritius. European Union: Bioculture Press.
Hammond, D. S., Gond, V., Baider, C., Florens, F. B. V., Persand, S., and Laurance, S. G. W. (2015). Threats to environmentally sensitive areas from peri-urban expansion in Mauritius. Environ. Conserv. 42, 256–267. doi: 10.1017/S0376892914000411
Harrison, R. D., Tan, S., Plotkin, J. B., Slik, F., Detto, M., Brenes, T., et al. (2013). Consequences of defaunation for a tropical tree community. Ecol. Lett. 16, 687–694. doi: 10.1111/ele.12102
Hazell, R. J. (2020). Functional Relationships Between Birds and Fruits on an Elevational Gradient in Papua New Guinea. Ph.D. thesis. United Kingdom: University of Sussex.
Heinen, J. H., van Loon, E. E., Hansen, D. M., and Kissling, W. D. (2017). Extinction-driven changes in frugivore communities on oceanic islands. Ecography 41, 1245–1255. doi: 10.1111/ecog.03462
Hume, J. P. (2007). Reappraisal of the parrots (Aves:Psittacidae) from the Mascarene Islands, with comments on their ecology, morphology, and affinities. Zootaxa 1513, 1–76. doi: 10.11646/zootaxa.1513.1.1
Hume, J. P. (2011). Systematics, morphology, and ecology of pigeons and doves (Aves: columbidae) of the Mascarene Islands, with three new species. Zootaxa 3124, 1–62. doi: 10.11646/zootaxa.3124.1.1
Hume, J. P. (2013). “A synopsis of the pre-human avifauna of the Mascarene Islands,” in Proceedings of the 8th International Meeting of the Society of Avian Paleontology and Evolution. Austria: Naturhistorisches Museum, Wien. 195–237.
Hume, J. P. (2014). Systematics, morphology, and ecological history of the Mascarene starlings (Aves: sturnidae) with the description of a new genus and species from Mauritius. Zootaxa 3849, 1–75. doi: 10.11646/zootaxa.3849.1.1
Hume, J. P. (2019). Systematics, morphology and ecology of rails (Aves: Rallidae) of the Mascarene Islands, with one new species. Zootaxa 4626, 1–107. doi: 10.11646/zootaxa.4626.1.1
Hume, J. P., Griffiths, O., Andre, A. A., Meunier, A., and Bour, R. (2021). Discovery of the first Mascarene giant tortoise nesting site on Rodrigues Island, Indian Ocean (Testudinidae: Cylindraspis). Herpetol. Notes 14, 103–116.
INSEE. (2014). Tableau économique de La Réunion - Edition 2014. Saint-Denis: Institut national de la statistique et des études économiques.
Irl, S. D. H., Anthelme, F., Harter, D. E. V., Jentsch, A., Lotter, E., Steinbauer, M. J., et al. (2016). Patterns of island treeline elevation - a global perspective. Ecography 39, 427–436. doi: 10.1111/ecog.01266
Jones, C. G. (1987). “The larger landbirds of Mauritius,” in Studies of Mascarene Island Birds, ed. A. W. Diamond (Cambridge: Cambridge University Press), 208–300. doi: 10.1017/cbo9780511735769.007
Jones, C. G. (1996). “Bird introductions to Mauritius: status and relationship with native birds,” in The Introduction & Naturalisation of Birds, eds J. S. Holmes and J. R. Simons (London: Stationery Office Publication Centre), 113–123.
Jones, C. G., Swinnerton, K., Thorsen, M., and Greenwood, A. (1998). “The biology and conservation of the echo parakeet Psittacula eques of Mauritius,” in Proceedings of IV International Parrot Convention 17-20 September 1998, ed. W. D. Kissling (Tenerife: Loro Parque), 110–123.
Kehlmaier, C., Graciá, E., Campbell, P. D., Hofmeyr, M. D., Schweiger, S., Martínez-Silvestre, A., et al. (2019). Ancient mitogenomics clarifies radiation of extinct Mascarene giant tortoises (Cylindraspis spp.). Sci. Rep. 9:17487. doi: 10.1038/s41598-019-54019-y
Kier, G., Kreft, H., Lee, T. M., Jetz, W., Ibisch, P. L., Nowicki, C., et al. (2009). A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. U. S. A. 106, 9322–9327. doi: 10.1073/pnas.0810306106
Lougnon, A. (2005). Sous le signe de la tortue: voyages anciens à l’Île Bourbon, 1611-1725. 5. éd. France: Orphie
MacArthur, R. H., and Wilson, E. O. (1967). The Theory of Island Biogeography. Princeton NJ: Princeton University Press.
McClure, K. M., Fleischer, R. C., and Kilpatrick, A. M. (2020). The role of native and introduced birds in transmission of avian malaria in Hawai’i. Ecology 101:e03038. doi: 10.1002/ecy.3038
Morante-Filho, J. C., Arroyo-Rodríguez, V., Pessoa, M., de, S., Cazetta, E., and Faria, D. (2018). Direct and cascading effects of landscape structure on tropical forest and non-forest frugivorous birds. Ecol. Appl. 28, 2024–2032. doi: 10.1002/eap.1791
Mourer-Chauviré, C., Bour, R., Ribes, S., and Moutou, F. (1999). The avifauna of Reunion Island (Mascarene Islands) at the time of the arrival of the first Europeans. Smithson. Contrib. Paleobiol. 89, 1–38.
Norder, S. J., Seijmonsbergen, A. C., Rughooputh, S. D. D. V., van Loon, E. E., Tatayah, V., Kamminga, A. T., et al. (2017). Assessing temporal couplings in social-ecological island systems: historical deforestation and soil loss on Mauritius (Indian Ocean). Ecol. Soc. 22:29. doi: 10.5751/ES-09073-220129
Olson, S. L., and James, H. F. (1989). “The role of Polynesians in the extinction of the avifauna of the Hawaiian Islands,” in Quaternary Extinctions: A Prehistoric Revolution, eds P. S. Martin and R. G. Klein (Tucson: University of Arizona Press), 768–781.
Osuri, A. M., Mendiratta, U., Naniwadekar, R., Varma, V., and Naeem, S. (2020). Hunting and forest modification have distinct defaunation impacts on tropical mammals and birds. Front. For. Glob. Change 2:87. doi: 10.3389/ffgc.2019.00087
Page, W., and D’Argent, G. (1997). A Vegetation Survey of Mauritius to Identify Priority Rain Forest Areas for Conservation Management. Port Louis: Mauritian Wildlife Foundation.
Peirce, M. A., Cheke, A. S., and Cheke, R. A. (1977). A survey of blood parasites of birds in the Mascarene Islands. IBIS 119, 451–461. doi: 10.1111/j.1474-919x.1977.tb02053.x
Pinet, P., Salamolard, M., Probst, J.-M., Russell, J. C., Jaquemet, S., and Corre, M. L. (2009). Barau’s Petrel Pterodroma baraui: history, biology and conservation of an endangered petrel. Mar. Ornithol. 37, 107–113.
Réchou, A., Flores, O., Jumeaux, G., Duflot, V., Bousquet, O., Pouppeville, C., et al. (2019). Spatio−temporal variability of rainfall in a high tropical island: patterns and large−scale drivers in Réunion Island. Q. J. R. Meteorol. Soc. 145, 893–909. doi: 10.1002/qj.3485
Reinegger, R. D., Oleksy, R. Z., Bissessur, P., Naujeer, H., and Jones, G. (2021). First come, first served: fruit availability to keystone bat species is potentially reduced by invasive macaques. J. Mammal. 102, 428–439. doi: 10.1093/jmammal/gyaa182
Rijsdijk, K. F., Hume, J. P., Bunnik, F., Florens, F. B. V., Baider, C., Shapiro, B., et al. (2009). Mid-Holocene vertebrate bone Concentration-Lagerstätte on oceanic island Mauritius provides a window into the ecosystem of the dodo (Raphus cucullatus). Quat. Sci. Rev. 28, 14–24. doi: 10.1016/j.quascirev.2008.09.018
Rijsdijk, K. F., Hume, J. P., Louw, P. G. B. D., Meijer, H. J. M., Janoo, A., De Boer, E. J., et al. (2015). A review of the dodo and its ecosystem: insights from a vertebrate concentration Lagerstätte in Mauritius. J. Vertebr. Paleontol. 35, 3–20. doi: 10.1080/02724634.2015.1113803
Salmona, J., Salamolard, M., Fouillot, D., Ghestemme, T., Larose, J., Centon, J.-F., et al. (2012). Signature of a pre-human population decline in the critically endangered Reunion Island endemic forest bird Coracina newtoni. PLoS One 7:e43524. doi: 10.1371/journal.pone.0043524
Sanchez, M., and Probst, J.-M. (2016). L’herpétofaune allochtone de l’île de La Réunion (Océan Indien) : état des connaissances en 2015. Bull. Soc. Herp. Fr. 160, 49–78.
Slavenko, A., Tallowin, O. J. S., Itescu, Y., Raia, P., and Meiri, S. (2016). Late Quaternary reptile extinctions: size matters, insularity dominates. Glob. Ecol. Biogeogr. 25, 1308–1320. doi: 10.1111/geb.12491
Steadman, D. W. (1995). Prehistoric extinctions of Pacific island birds: biodiversity meets zooarchaeology. Science 267, 1123–1131. doi: 10.1126/science.267.5201.1123
Strasberg, D., Rouget, M., Richardson, D. M., Baret, S., Dupont, J., and Cowling, R. M. (2005). An assessment of habitat diversity and transformation on La Réunion Island (Mascarene Islands, Indian Ocean) as a basis for identifying broad-scale conservation priorities. Biodivers. Conserv. 14, 3015–3032. doi: 10.1007/s10531-004-0258-2
Terborgh, J., Nuñez-Iturri, G., Pitman, N. C. A., Valverde, F. H. C., Alvarez, P., Swamy, V., et al. (2008). Tree recruitment in an empty forest. Ecology 89, 1757–1768. doi: 10.1890/07-0479.1
Thiollay, J.-M., and Probst, J.-M. (1999). Ecology and conservation of a small insular bird population, the Reunion cuckoo-shrike Coracina newtoni. Biol. Conserv. 87, 191–200. doi: 10.1016/s0006-3207(98)00062-7
Tilman, D., May, R. M., Lehman, C. L., and Nowak, M. A. (1994). Habitat destruction and the extinction debt. Nature 371, 65–66. doi: 10.1038/371065a0
Triantis, K. A., Borges, P. A. V., Ladle, R. J., Hortal, J., Cardoso, P., Gaspar, C., et al. (2010). Extinction debt on oceanic islands. Ecography 33, 285–294. doi: 10.1111/j.1600-0587.2010.06203.x
Vaughan, R. E., and Wiehe, P. O. (1937). Studies on the vegetation of Mauritius: I. A preliminary survey of the plant communities. J. Ecol. 25, 289–343. doi: 10.2307/2256197
Keywords: biodiversity loss, biological invasion, elevational gradients, habitat destruction and fragmentation, Mascarene archipelago, overhunting
Citation: Albert S, Flores O, Ah-Peng C and Strasberg D (2021) Forests Without Frugivores and Frugivores Without Forests –An Investigation Into the Causes of a Paradox in One of the Last Archipelagos Colonized by Humans. Front. Ecol. Evol. 9:688768. doi: 10.3389/fevo.2021.688768
Received: 31 March 2021; Accepted: 23 July 2021;
Published: 20 August 2021.
Edited by:
Simon Haberle, Australian National University, AustraliaReviewed by:
Julien Louys, Griffith University, AustraliaCopyright © 2021 Albert, Flores, Ah-Peng and Strasberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sébastien Albert, candidalbert@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.