
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 23 February 2022
Sec. Heart Valve Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.812692
This article is part of the Research Topic Insights in Heart Valve Disease: 2022 View all 10 articles
 Kangning Han1,2,3
Kangning Han1,2,3 Dongmei Shi1,2,3
Dongmei Shi1,2,3 Lixia Yang1,2,3
Lixia Yang1,2,3 Meng Xie4
Meng Xie4 Rongrong Zhong5
Rongrong Zhong5 Zhijian Wang1,2,3
Zhijian Wang1,2,3 Fei Gao1,2,3
Fei Gao1,2,3 Xiaoteng Ma1,2,3*
Xiaoteng Ma1,2,3* Yujie Zhou1,2,3*
Yujie Zhou1,2,3*Background: Mounting evidence indicates that rapid progression of aortic stenosis (AS) is significantly associated with poor prognosis. Whether diabetes accelerates the progression of AS remains controversial.
Objectives: The purpose of the present study was to investigate whether diabetes was associated with rapid progression of AS.
Methods: We retrospectively analyzed 276 AS patients who underwent transthoracic echocardiography at least twice with a maximum interval ≥ 180 days from January 2016 to June 2021. AS severity was defined by specific threshold values for peak aortic jet velocity (Vmax) and/or mean pressure gradient. An increase of Vmax ≥ 0.3 m/s/year was defined as rapid progression. The binary Logistic regression models were used to determine the association between diabetes and rapid progression of AS.
Results: At a median echocardiographic follow-up interval of 614 days, the annual increase of Vmax was 0.16 (0.00–0.41) m/s. Compared with those without rapid progression, patients with rapid progression were older and more likely to have diabetes (P = 0.040 and P = 0.010, respectively). In the univariate binary Logistic regression analysis, diabetes was associated with rapid progression of AS (OR = 2.02, P = 0.011). This association remained significant in the multivariate analysis based on model 2 and model 3 (OR = 1.93, P = 0.018; OR = 1.93, P = 0.022). After propensity score-matching according to Vmax, diabetes was also associated rapid progression of AS (OR = 2.57, P = 0.045).
Conclusions: Diabetes was strongly and independently associated with rapid progression of AS.
Aortic stenosis (AS) is one of the most common valvular heart diseases affecting up to nearly 10% of the senior population (1–3). AS is a progressive disease and its rapid progression has been shown to be associated with poor prognosis (4–8). Patients with rapid progression of AS may not be easily identified at the initial diagnosis; however, these patients should be considered intervention early rather than at the onset of symptoms (8). Indeed, one study showed that early intervention in asymptomatic severe AS patients with rapid progression of AS was associated with a significant reduction in mortality (9). Many studies defined rapid progression of AS as an increase of aortic jet velocity (Vmax) ≥ 0.3 m/s/year (7, 10). Both ACC/AHA and ESC guidelines consider an increase of Vmax ≥ 0.3 m/s/year as one of the indications for AS intervention (classes of recommendations: IIa, level of evidence: B-C) (11, 12). Although the pathogenesis of AS is similar to that of coronary artery disease (CAD), the determinants of its rapid progression remain unclear (13). Although many promising pharmacotherapeutic studies have shown the potential benefits in detaining the progression of AS, currently, there is no effective drug treatment for AS, and valve replacement represents the only treatment for end-stage AS (14). Identification of risk factors for rapid progression of AS may allow for its secondary prevention.
Two studies have shown that diabetes can accelerate the progression of AS (15, 16). However, one of the studies only included mild AS patients above the age of 60 and defined AS progression by the yearly increase of peak transvalvular gradient (15). The other study comprised more than 99% of male patients and defined AS progression according to the annual decrease of aortic valve area (16). Whether diabetes can significantly accelerate AS progression as shown by Vmax is unknown. Since the rapid increase in Vmax is currently an indication for AS intervention, therefore, the present study was to investigate the association of diabetes with the progression of AS according to Vmax.
Patients diagnosed with AS were retrospectively identified by the electronic medical system of Beijing Anzhen Hospital, Capital Medical University from January 2016 to June 2021. AS was diagnosed by transthoracic echocardiography according to Vmax and/or mean pressure gradient (MPG). All patients underwent comprehensive echocardiography by experienced sonographers and the following diagnostic criteria were used: (1) mild AS (Vmax 2.00–2.99 m/s and/or MPG 10.0–19.9 mmHg); (2) moderate AS (Vmax 3.00–3.99 m/s and/or MPG 20.0–39.9 mmHg); (3) severe AS (Vmax ≥ 4.00 m/s and/or MPG ≥ 40.0 mmHg). Then, a total of 3,780 adult patients who underwent transthoracic echocardiography at least twice were selected. If the patient had more than one echocardiography after the first echocardiography, the one with the highest Vmax was selected as the last echocardiography. The exclusion criteria were: (1) congenital heart disease other than bicuspid aortic valve (BAV); (2) history of aortic valve surgery or transcatheter aortic valve implantation; (3) lack of clinical history, echocardiographic or laboratory data; (4) maximal interval from the first echocardiography to the last echocardiography < 180 days; (5) left ventricular ejection fraction (LVEF) at the first or last echocardiography < 40% (Figure 1). Patients with a history of atrial fibrillation (AF) or concomitant significant valvular diseases were not excluded. For patients with AF, echocardiography was performed over at least five cardiac cycles, and the Vmax was averaged. The first (baseline) and the last echocardiography were used for analysis. Information about age, sex, smoking, drinking, hypertension, diabetes, dyslipidemia, CAD, heart failure, chronic kidney disease (CKD), BAV, and medications (such as statins, ACEI/ARBs, and β-blockers) during the observation period were obtained from the electronic medical system or telephone interviews. Rapid progression of AS was defined as the rate of Vmax progression ≥ 0.3 m/s/year.
Continuous variables with normal distribution were expressed as mean ± standard deviation, otherwise median and quartile. For continuous variables, the unpaired t-test and Mann-Whitney U-test were used as appropriate. Categorical variables were expressed as numbers and percentages, where the χ2 test or Fisher exact test was used accordingly. Three different Logistic regression models were used to determine the association of diabetes with rapid progression: (1) unadjusted; (2) adjusted for sex and age; (3) adjusted for sex, age, hypertension, CKD, dyslipidemia, and smoking. Paired patient data depending on the diagnosis of diabetes was acquired by propensity score-matching based on the baseline Vmax. All statistical analyses were performed by SPSS 26.0 (SPSS, Inc, Chicago, Illinois) and a 2-sided P-value < 0.05 was considered statistically significant.
A total of 276 AS patients constituted the study population, including 69 (25.0%) mild, 129 (46.7%) moderate, and 78 (28.3%) severe AS. The baseline characteristics of the population were summarized in Table 1. Among the study population, the mean age was 65 ± 11 years, 131 (47.5%) were female, 76 (27.5%) had diabetes, and 82 (29.7%) had BAV. Patients with rapid progression were older (P = 0.040) and more likely to have diabetes (P = 0.010). Compared with those without diabetes, patients with diabetes had lower baseline Vmax and MPG, but had higher rates of hypertension, dyslipidemia, CAD, and use of medications (Table 2). The progression of AS in diabetic patients was more pronounced than in non-diabetic patients (Table 2).
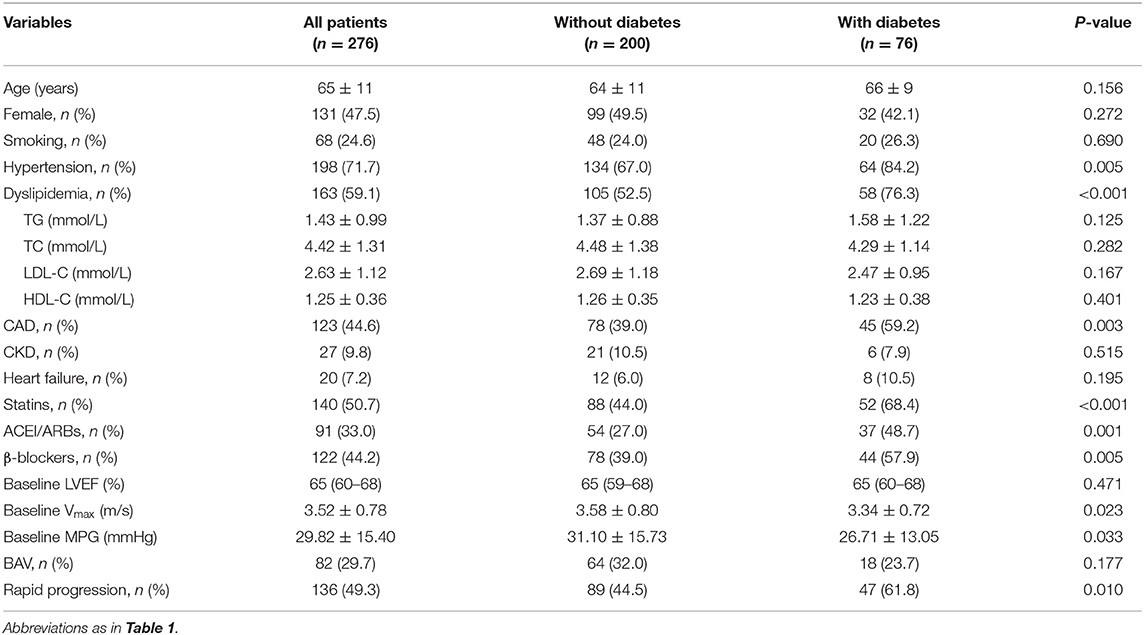
Table 2. Baseline characteristics of the study population according to the with and without diabetes.
The median time interval from the first echocardiography to the last echocardiography was 614 days (interquartile range: 350–932 days). During the period, the Vmax and MPG were increasing. The Vmax increased from 3.52 ± 0.78 m/s to 3.88 ± 0.87 m/s, with an annual increase of 0.16 (0.00–0.41) m/s. The MPG was increased from 30 ± 15 mmHg to 36 ± 18 mmHg, with an annual increase of 2.78 (0.00–6.86) mmHg. Compared with those without diabetes, patients with diabetes had a more significant Vmax progression [0.21 (0.09–0.41) m/s/year vs. 0.13 (−0.02 to 0.42) m/s/year, P = 0.024].
At follow-up, the proportion of patients with severe AS increased by 16.3%, while the proportions of patients with mild and moderate AS decreased by 8.7% and 7.6%, respectively. In the 76 diabetic patients, the proportion of moderate-to-severe AS increased by 17.1%, and particularly, severe AS increased by 19.8%. In the patients without diabetes, the proportion of moderate-to-severe AS increased by 3.5%, and severe AS increased by 15.0% (Figure 2).
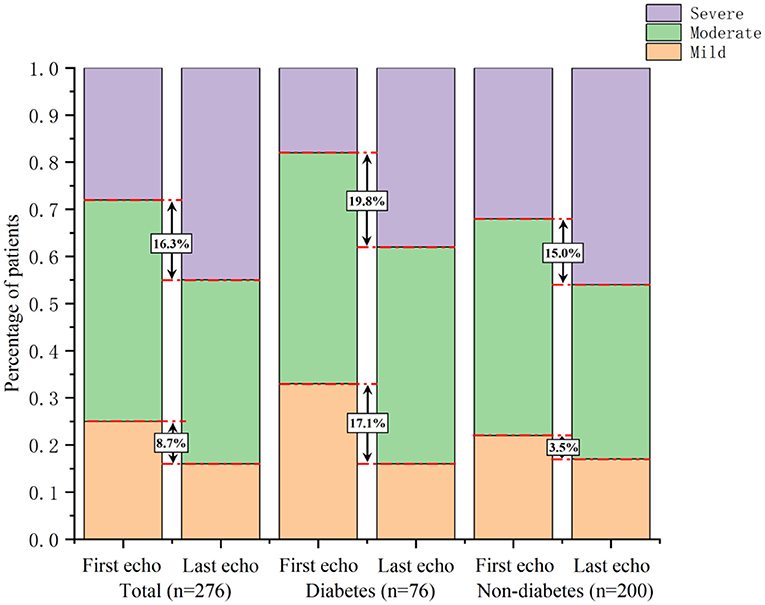
Figure 2. Comparison of AS severity changes between patients with and without diabetes. In diabetic patients, the proportion of moderate-to-severe AS increased by 17.1%, and severe AS increased by 19.8%. In non-diabetic patients, the proportion of moderate-to-severe AS increased by 3.5%, and severe AS increased by 15.0%.
In the univariate binary Logistic regression analysis (model 1), diabetes was associated with rapid progression of AS (unadjusted OR = 2.02, 95% CI: 1.18–3.47, P = 0.011). After adjustment for potential confounders in model 2 and model 3, this association remained significant (Table 3). After propensity score-matching, multivariate Logistic regression analysis (adjusted by sex, age, hypertension, CKD, dyslipidemia, and smoking) showed diabetes was associated with rapid progression of AS (OR = 2.57, 95% CI: 1.02–6.47, P = 0.045).
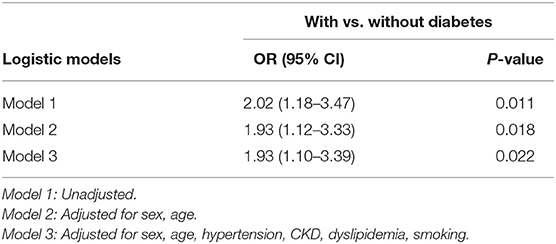
Table 3. Odds ratios and 95% confidence intervals for rapid progression according to with and without diabetes.
In this study, we demonstrated that diabetes was significantly and independently associated with the rapid progression of AS. Previous study of Deutscher et al. showed that diabetes was associated with the development of AS (17). Likewise, diabetes was significantly associated with an increased incidence of AS, according to a recent retrospective cohort study involving 78,805 patients with diabetes and 78,805 patients without diabetes (18). Another retrospective cohort study comprised 71,813 subjects showed that diabetes was associated with a 34% increased risk of AS (19). Diabetes leads to elevated valvular and plasma accumulation of glycation end products in AS patients. Additionally, there was a positive correlation between the amount of glycation end products and the severity of AS in diabetic patients (20).
Diabetes can lead to aortic valve calcification, which is a major cause of AS, especially in the elderly. The study of Katz et al. revealed that diabetes was associated with an increased risk of aortic valve calcification detected by computed tomography (21). Furthermore, diabetes has been shown to be associated with stenotic and non-stenotic aortic valve calcification (22). Tucureanu et al. found increased expression of cell adhesion molecules, extracellular matrix remodeling, and osteogenic markers in hyperlipidemia ApoE−/− diabetic mice (23). High glucose can induce osteogenic molecules and increase calcium deposits by increasing the expression of cytokines, cell adhesion molecules, and matrix metalloproteinases in valvular endothelial cells and interstitial cells (24). In the animal model, diabetes can lead to early aortic valve mineralization and calcific AS (25).
Diabetes can also cause inflammation and degeneration that can lead to AS. AS is associated with macrophages, mast cells, T cells and other immune cells infiltrations, emphasizing the role of local inflammation in AS (26–28). In severe AS patients before aortic valve replacement, diabetic individuals had higher levels of C-reactive protein in plasma and aortic valve (29). Diabetes can decrease the number and induce dysfunction of endothelial progenitor cells, impairing valve endothelial repair (30). Indeed, diabetes has been shown to be associated with aortic valve and bioprosthetic aortic valve degeneration (31, 32). At the molecular level, diabetes can lead to the upregulation of biglycan, which may contribute to aortic valve degeneration (33).
In AS patients, diabetes was associated with a worse prognosis. It has been reported that diabetes can impair coronary microvascular function in patients with asymptomatic AS (34). Some studies have shown that diabetes has an adverse effect on hypertrophic remodeling and is associated with reduced systolic function (35, 36). Moreover, diabetes was demonstrated to be associated with systemic inflammatory response syndrome after aortic valve replacement (37). A previous study by Lancellotti et al. showed that diabetes was associated with higher mortality in severe AS patients (38).
In this study, we found diabetes could accelerate the progression of AS, and particularly, lead to a rapid progression (Vmax ≥ 0.3 m/s/year). Several studies have demonstrated the association between diabetes and AS progression. The study of Aronow et al. first showed that diabetes was associated with the progression of AS. However, this study only included mild AS patients aged more than 60 years and defined AS progression according to annual decrease of peak transvalvular gradient (15). The study of Kamalesh et al. showed that diabetes significantly accelerated the progression of AS in patients with moderate AS. However, this study included more than 99% of male patients and defined AS progression according to annual decrease of aortic valve area (16). Diabetes was found to be a strong risk factor for the development of severe AS in a prospective cohort study which included 1.12 million individuals without valvular disease history (39). Moreover, diabetes has been shown to accelerate the progression of AS by enhancing the inflammatory response measured by C-reactive protein, where an increase in the inflammatory response was observed within the aortic valve of AS patients (29). Additionally, the density of immune cell infiltration within aortic valve was demonstrated to be correlated with the progression of AS (40). Of note, an increased inflammatory response has also been shown to be associated with the development and progression of diabetes, underlying a potential link between diabetes and the progression of AS (41).
Several limitations must be considered when interpreting the results of our study. First, this study was a single-center retrospective study, so the influence of unmeasured and residual confounding variables could not be excluded. Second, the data of this study were limited to the Chinese population, so the ethnic difference cannot be eradicated. Third, this study did not perform the external validation, which may hamper the general applicability of the current findings. Fourth, aortic valve area was not included in this study since it was not measured routinely by sonographers in our cardiovascular center. Fifth, to better reflect the impact of diabetes on the progression of AS in the real world, similar to many previous studies, we did not exclude patients with AF and concomitant significant valve diseases. Excluding patients with those conditions may determine the association between diabetes and AS progression more directly and accurately. Future studies on such patients are needed.
In this study, we demonstrated that diabetes was strongly and independently associated with the rapid progression of AS. Further prospective studies are needed to confirm our results.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Beijing Anzhen Hospital, Capital Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
KH and XM analyzed the data and drafted the manuscript. KH and MX extracted the echocardiography data from the electronic medical system. MX guided the echocardiography analysis. RZ analyzed the data and drew the figures. XM and YZ designed the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by National Key Research and Development Program of China (2017YFC0908800); China Postdoctoral Science Foundation (2021M692253); Beijing Postdoctoral Research Foundation (2021-ZZ-023); Beijing Municipal Administration of Hospitals Mission Plan (SML20180601).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Joseph J, Naqvi SY, Giri J, Goldberg S. Aortic stenosis: pathophysiology, diagnosis, and therapy. Am J Med. (2017) 130:253–63. doi: 10.1016/j.amjmed.2016.10.005
2. Carabello BA, Paulus WJ. Aortic stenosis. Lancet. (2009) 373:956–66. doi: 10.1016/S0140-6736(09)60211-7
3. Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromsø Study. Heart. (2013) 99:396–400. doi: 10.1136/heartjnl-2012-302265
4. Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by doppler echocardiography. J Am Coll Cardiol. (1989) 13:545–50. doi: 10.1016/0735-1097(89)90590-1
5. Roger VL, Tajik AJ, Bailey KR, Oh JK, Taylor CL, Seward JB. Progression of aortic stenosis in adults: newappraisal using doppler echocardiography. Am Heart J. (1990) 119:331–8. doi: 10.1016/S0002-8703(05)80024-9
6. Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. (2000) 343:611–7. doi: 10.1056/NEJM200008313430903
7. Nistri S, Faggiano P, Olivotto I, Papesso B, Bordonali T, Vescovo G, et al. Hemodynamic progression and outcome of asymptomatic aortic stenosis in primary care. Am J Cardiol. (2012) 109:718–23. doi: 10.1016/j.amjcard.2011.10.035
8. Seo JS, Kang DH, Kim DH, Song JM, Song JK. Predictors of echocardiographic progression in patients with mild aortic stenosis. Korean Circ J. (2011) 41:649–53. doi: 10.4070/kcj.2011.41.11.649
9. Head SJ, Gahl B, Çelik M, Head SJ, Vanoverschelde JL, Pibarot P, et al. Natural history of asymptomatic severe aortic stenosis and the association of early intervention with outcomes: a systematic review and meta-analysis. JAMA Cardiol. (2020) 5:1102–12. doi: 10.1001/jamacardio.2020.2497
10. Mateos N, Gómez M, Homar A, Garcia-Elias A, Yáñez L, Tajes M, et al. Plasmatic PCSK9 levels are associated with very fast progression of asymptomatic degenerative aortic stenosis. J Cardiovasc Transl Res. (2021). doi: 10.1007/s12265-021-10138-4. [Epub ahead of print].
11. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: Executive summary a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2021) 143:E35–71. doi: 10.1161/CIR.0000000000000932
12. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–86. doi: 10.1093/eurheartj/ehx391
13. Stefanini GG, Stortecky S, Meier B, Windecker S, Wenaweser P. Severe aortic stenosis and coronary artery disease. EuroIntervention. (2013) 9:S63–8. doi: 10.4244/EIJV9SSA12
14. Kraler S, Blaser MC, Aikawa E, Camici GG, Lüscher TF. Calcific aortic valve disease: from molecular and cellular mechanisms to medical therapy. Eur Heart J. (2021). doi: 10.1093/eurheartj/ehab757. [Epub ahead of print].
15. Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. (2001) 88:693–5. doi: 10.1016/S0002-9149(01)01821-5
16. Kamalesh M, Ng C, El Masry H, Eckert G, Sawada S. Does diabetes accelerate progression of calcific aortic stenosis? Eur J Echocardiogr. (2009) 10:723–5. doi: 10.1093/ejechocard/jep048
17. Deutscher S, Rockette HE, Krishnaswami V. Diabetes and hypercholesterolemia among patients with calcific aortic stenosis. J Chronic Dis. (1984) 37:407–15. doi: 10.1016/0021-9681(84)90108-5
18. Roderburg C, Loosen SH, Luedde T, Kostev K, Luedde M. Diabetes mellitus is associated with an increased incidence of aortic valve stenosis. Diabetes Vasc Dis Res. (2021) 18:147916412110338. doi: 10.1177/14791641211033819
19. Larsson SC, Wallin A, Håkansson N, Stackelberg O, Bäck M, Wolk A. Type 1 and type 2 diabetes mellitus and incidence of seven cardiovascular diseases. Int J Cardiol. (2018) 262:66–70. doi: 10.1016/j.ijcard.2018.03.099
20. Kopytek M, Za̧bczyk M, Mazur P, Undas A, Natorska J. Accumulation of advanced glycation end products (AGEs) is associated with the severity of aortic stenosis in patients with concomitant type 2 diabetes. Cardiovasc Diabetol. (2020) 19:92. doi: 10.1186/s12933-020-01068-7
21. Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, et al. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the multi-ethnic study of atherosclerosis. Circulation. (2006) 113:2113–9. doi: 10.1161/CIRCULATIONAHA.105.598086
22. Boon A, Cheriex E, Lodder J, Kessels F. Cardiac valve calcification: characteristics of patients with calcification of the mitral annulus or aortic valve. Heart. (1997) 78:472–4. doi: 10.1136/hrt.78.5.472
23. Tucureanu MM, Filippi A, Alexandru N, Ana Constantinescu C, Ciortan L, Macarie R, et al. Diabetes-induced early molecular and functional changes in aortic heart valves in a murine model of atherosclerosis. Diabetes Vasc Dis Res. (2019) 16:562–76. doi: 10.1177/1479164119874469
24. Ciortan L, Macarie RD, Cecoltan S, Vadana M, Tucureanu MM, Mihaila AC, et al. Chronic high glucose concentration induces inflammatory and remodeling changes in valvular endothelial cells and valvular interstitial cells in a gelatin methacrylate 3d model of the human aortic valve. Polymers. (2020) 12:1–15. doi: 10.3390/polym12122786
25. Quang K Le, Bouchareb R, Lachance D, Laplante MA, El Husseini D, Boulanger MC, et al. Early development of calcific aortic valve disease and left ventricular hypertrophy in a mouse model of combined dyslipidemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. (2014) 34:2283–91. doi: 10.1161/ATVBAHA.114.304205
26. Natorska J, Marek G, Hlawaty M, Sobczyk D, Sadowski J, Tracz W, et al. Evidence for tissue factor expression in aortic valves in patients with aortic stenosis. Pol Arch Med Wewn. (2009) 119:636–43. doi: 10.20452/pamw.791
27. Galante A, Pietroiusti A, Vellini M, Piccolo P, Possati G, De Bonis M, et al. C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J Am Coll Cardiol. (2001) 38:1078–82. doi: 10.1016/S0735-1097(01)01484-X
28. Mathieu P, Bouchareb R, Boulanger MC. Innate and adaptive immunity in calcific aortic valve disease. J Immunol Res. (2015) 2015. doi: 10.1155/2015/851945
29. Natorska J, Wypasek E, Grudzień G, Sobczyk D, Marek G, Filip G, et al. Does diabetes accelerate the progression of aortic stenosis through enhanced inflammatory response within aortic valves? Inflammation. (2012) 35:834–40. doi: 10.1007/s10753-011-9384-7
30. Filippi A, Constantin A, Alexandru N, Voicu G, Constantinescu CA, Rebleanu D, et al. Integrins α4β1 and αVβ3 are reduced in endothelial progenitor cells from diabetic dyslipidemic mice and may represent new targets for therapy in aortic valve disease. Cell Transplant. (2020) 29:1–8. doi: 10.1177/0963689720946277
31. Manduteanu I, Simionescu D, Simionescu A, Simionescu M. Aortic valve disease in diabetes: molecular mechanisms and novel therapies. J Cell Mol Med. (2021) 25:9483–95. doi: 10.1111/jcmm.16937
32. Selig JI, Ouwens DM, Raschke S, Thoresen GH, Fischer JW, Lichtenberg A, et al. Impact of hyperinsulinemia and hyperglycemia on valvular interstitial cells – A link between aortic heart valve degeneration and type 2 diabetes. Biochim Biophys Acta Mol Basis Dis. (2019) 1865:2526–37. doi: 10.1016/j.bbadis.2019.05.019
33. Barth M, Selig JI, Klose S, Schomakers A, Kiene LS, Raschke S, et al. Degenerative aortic valve disease and diabetes: implications for a link between proteoglycans and diabetic disorders in the aortic valve. Diabetes Vasc Dis Res. (2019) 16:254–69. doi: 10.1177/1479164118817922
34. Banovic M, Brkovic V, Nedeljkovic I, Nedeljkovic M, Popovic D, Djordjevic-Dikic A, et al. Diabetes mellitus and coronary microvascular function in asymptomatic patients with severe aortic stenosis and nonobstructed coronary arteries. Diabetes Vasc Dis Res. (2016) 13:220–7. doi: 10.1177/1479164115627107
35. Lindman BR, Arnold S V., Madrazo JA, Zajarias A, Johnson SN, Pérez JE, et al. The adverse impact of diabetes mellitus on left ventricular remodeling and function in patients with severe aortic stenosis. Circ Heart Fail. (2011) 4:286–92. doi: 10.1161/CIRCHEARTFAILURE.110.960039
36. Czestkowska E, Rozanowska A, Długosz D, Bolt K, Świerszcz J, Kruszelnicka O, et al. Depressed systemic arterial compliance and impaired left ventricular midwall performance in aortic stenosis with concomitant type 2 diabetes: a retrospective cross-sectional study. Cardiovasc Diabetol. (2019) 18:92. doi: 10.1186/S12933-019-0894-1
37. Lindman BR, Goldstein JS, Nassif ME, Zajarias A, Novak E, Tibrewala A, et al. Systemic inflammatory response syndrome after transcatheter or surgical aortic valve replacement. Heart. (2015) 101:537–45. doi: 10.1136/heartjnl-2014-307057
38. Lancellotti P, Magne J, Dulgheru R, Clavel MA, Donal E, Vannan MA, et al. Outcomes of patients with asymptomatic aortic stenosis followed up in heart valve clinics. JAMA Cardiol. (2018) 3:1060–8. doi: 10.1001/jamacardio.2018.3152
39. Yan AT, Koh M, Chan KK, Guo H, Alter DA, Austin PC, et al. Association between cardiovascular risk factors and aortic stenosis: The CANHEART aortic stenosis study. J Am Coll Cardiol. (2017) 69:1523–32. doi: 10.1016/j.jacc.2017.01.025
40. Coté N, Mahmut A, Bosse Y, Couture C, Pagé S, Trahan S, et al. Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation. (2013) 36:573–81. doi: 10.1007/s10753-012-9579-6
Keywords: diabetes, aortic stenosis, rapid progression, transthoracic echocardiography, valvular disease
Citation: Han K, Shi D, Yang L, Xie M, Zhong R, Wang Z, Gao F, Ma X and Zhou Y (2022) Diabetes Is Associated With Rapid Progression of Aortic Stenosis: A Single-Center Retrospective Cohort Study. Front. Cardiovasc. Med. 8:812692. doi: 10.3389/fcvm.2021.812692
Received: 11 November 2021; Accepted: 15 December 2021;
Published: 23 February 2022.
Edited by:
Ernesto Greco, Sapienza University of Rome, ItalyReviewed by:
Amiliana Mardiani Soesanto, Universitas Indonesia, IndonesiaCopyright © 2022 Han, Shi, Yang, Xie, Zhong, Wang, Gao, Ma and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoteng Ma, maxiaotengai@163.com; Yujie Zhou, azzyj12@163.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.