
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 16 December 2021
Sec. Coronary Artery Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.773200
This article is part of the Research TopicInsights in Coronary Artery Disease: 2021View all 15 articles
 Xiao-Teng Ma†
Xiao-Teng Ma† Qiao-Yu Shao†
Qiao-Yu Shao† Qiu-Xuan Li
Qiu-Xuan Li Zhi-Qiang Yang
Zhi-Qiang Yang Kang-Ning Han
Kang-Ning Han Jing Liang
Jing Liang Hua Shen
Hua Shen Xiao-Li Liu
Xiao-Li Liu Yu-Jie Zhou
Yu-Jie Zhou Zhi-Jian Wang*
Zhi-Jian Wang*Background: Malnutrition has been shown to be associated with adverse cardiovascular outcomes in many patient populations.
Aims: To investigate the prognostic significance of malnutrition as defined by nutritional risk index (NRI) in patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI) and whether NRI could improve the GRACE score based prognostic models.
Methods: This study applied NRI among 1,718 patients with ACS undergoing PCI. Patients were divided into three nutritional risk groups according to their baseline NRI: no nutritional risk (NRI ≥ 100), mild nutritional risk (97.5 ≤ NRI <100), and moderate-to-severe nutritional risk (NRI <97.5). The primary endpoint was the composite of major adverse cardiovascular events (MACE), including all-cause death, non-fatal stroke, non-fatal myocardial infarction, or unplanned repeat revascularization.
Results: During a median follow-up of 927 days, 354 patients developed MACE. In the overall population, compared with normal nutritional status, malnutrition was associated with increased risk for MACE [adjusted HR for mild and moderate-to-severe nutritional risk, respectively: 1.368 (95%CI 1.004–1.871) and 1.473 (95%CI 1.064–2.041)], and NRI significantly improved the predictive ability of the GRACE score for MACE (cNRI: 0.070, P = 0.010; IDI: 0.005, P < 0.001). In the diabetes subgroup, malnutrition was associated with nearly 2-fold high adjusted risk of MACE, and the GRACE score combined with NRI appeared to have better predictive ability than that in the overall population.
Conclusion: Malnutrition as defined by NRI was independently associated with MACE in ACS patients who underwent PCI, especially in individuals with diabetes, and improved the predictive ability of the GRACE score based prognostic models.
Patients with acute coronary syndrome (ACS) are still at an unacceptably high risk of cardiovascular (CV) death and thrombotic events, even after they have undergone percutaneous coronary intervention (PCI). Comprehensive and accurate risk assessment plays an important role in making appropriate treatment decisions for these patients. The GRACE (Global Registry of Acute Coronary Events) score was a strong predictor of 6-month mortality and reinfarction after ACS (1, 2). However, some important predictors associated with poor prognosis are not included in the scoring system. Malnutrition has been proved to be associated with the development of atherosclerosis and a higher rate of CV mortality in elderly patients (3). Alternative nutritional indicators such as body mass index (BMI), serum albumin (ALB), and serum total cholesterol (TC) are predictors of survival in patients with ACS (4–6). Recently, nutritional status has been demonstrated to be a promising prognostic factor (7), and it is considered a modifiable clinical characteristic which physicians may perform interventions on to reduce the risk of adverse CV events.
The nutritional risk index (NRI) was developed as a simplified screening tool to assess nutritional status and predict clinical outcomes based on weight, height, and ALB (8). It has been reported that the malnutrition as defined by NRI was associated with the poor prognosis among patients with advanced age (9, 10), myocardial infarction (MI) (11), heart failure (HF) (12), valvular heart disease (13), atrial fibrillation (14), or chronic kidney disease (CKD) (15). So far, few studies have added nutritional status to the GRACE score for risk stratification assessment, and little is known about whether the predictive value of NRI differs among different subgroups of ACS patients. The present study aimed to evaluate the prognostic significance of nutritional status measured by NRI and the incremental predictive value of adding NRI to the GRACE score in patients with ACS undergoing PCI.
The present study is derived from a single-center prospective observational cohort study (ChiCTR1800017417) which was described in detail elsewhere (16). A total of 1770 patients who underwent coronary angiography for ACS and were treated with primary or elective PCI in our CV center were consecutively and prospectively enrolled in the database from June 2016 to November 2017. The exclusion criteria of this study included patients with prior coronary artery bypass graft surgery, cardiogenic shock, left ventricular ejection fraction (LVEF) <30%, renal failure with creatinine clearance <15 ml/min, and known cancer history. Four patients were also excluded because of missing follow-up data despite at least four separate attempts to contact them. Ultimately, 1,718 patients were included in the final analysis (Figure 1). The study complied with the Declaration of the Helsinki with respect to investigation in humans, was approved by the institutional review committee of Beijing Anzhen Hospital, Capital Medical University, and conducted in accordance with the guidelines of the ethics committee at participating institutions. Written informed consent was obtained from all patients.
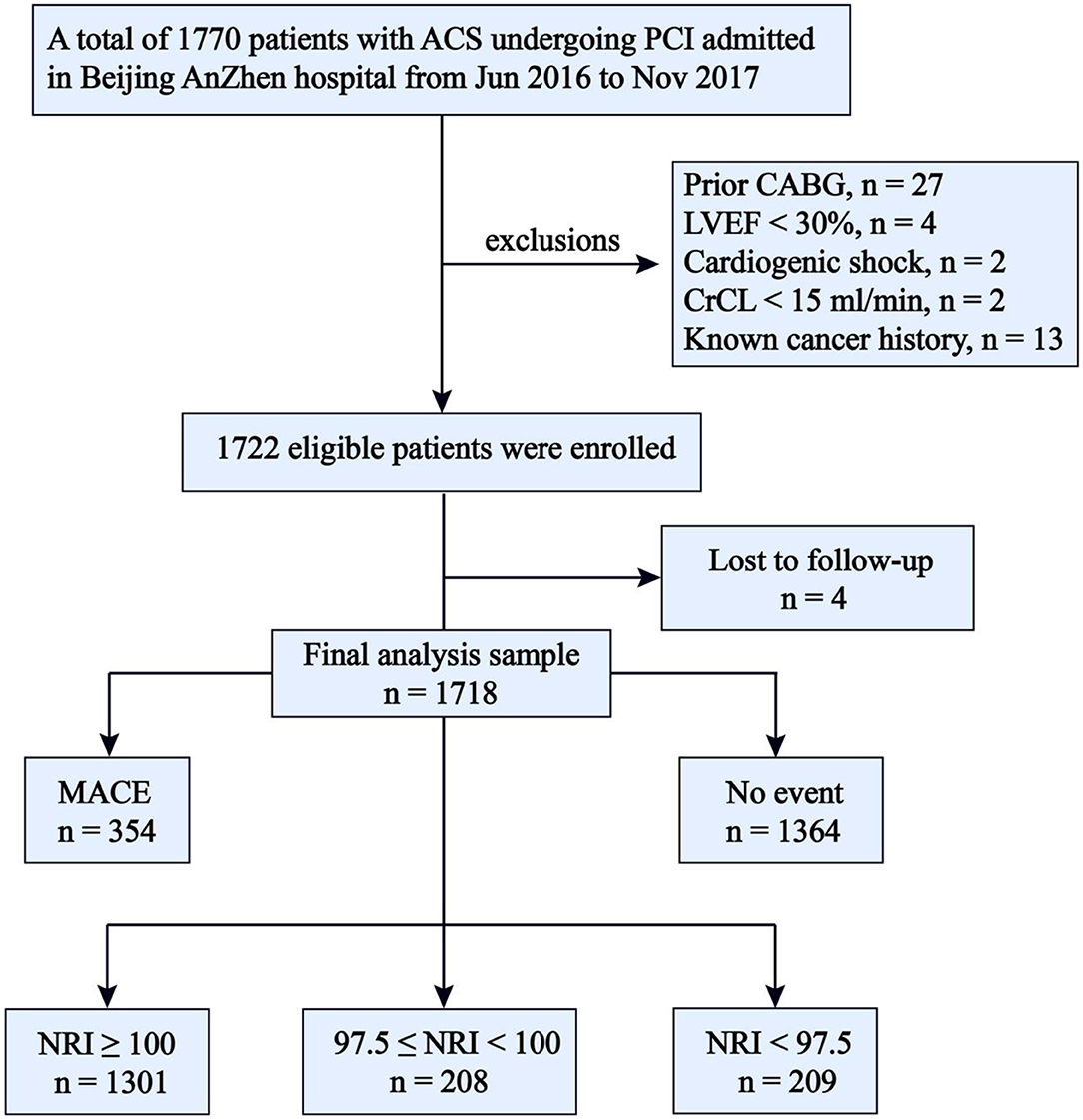
Figure 1. Flow chart. ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery; LVEF, left ventricular ejection fraction < 30%, CrCL, creatinine clearance < 15 ml/min; NRI, nutritional risk index; MACE, major adverse cardiovascular events; NRI, nutritional risk index.
All patients were followed up at 1, 6, 12, 18, 24, 30, 36 months after hospital discharge. The information regarding adverse events was collected from the medical records and telephone interviews by three trained personnel blinded to the baseline characteristics. The first participant was recruited in June 2016 and the follow-up ended in December 2019.
The primary endpoint was the composite of major adverse cardiovascular events (MACE), which included all-cause death, non-fatal stroke, non-fatal MI, or unplanned repeat revascularization. The stroke was defined as ischemic cerebral infarction with evidence of neurological dysfunction requiring hospitalization with clinically documented lesions on brain computed tomography or magnetic resonance imaging. MI was defined as an elevated level of cardiac troponin or creatine kinase greater than the upper limit of the normal range with either ischemic symptoms or electrocardiograph changes implicating ischemia. The presence of new pathological Q waves in ≥ 2 contiguous electrocardiogram leads was also diagnosed as MI. Within 1 week after PCI, only Q-wave MI was defined as MI. Unplanned repeat revascularization referred to any non-staged revascularization after the index PCI. Staged revascularization was defined as scheduled revascularization within 90 days after the index PCI, without a revascularization status of emergency or salvage or without treatment of a coronary artery territory which had been treated. The most severe endpoint event was selected for the primary endpoint analysis if > 1 event occurred during follow-up (death > stroke > MI > revascularization). If more than one stroke or MI or revascularization occurred, the first stroke or MI or revascularization was selected. Meanwhile, the first event was also selected for the primary endpoint analysis.
Data on demographics, medical history, and medication history were collected using a standard questionnaire. The blood pressure on admission was recorded. The ALB, lipid profiles, fasting plasma glucose (FPG), glycosylated hemoglobin, high-sensitivity C-reactive protein (hs-CRP), and creatinine levels in the first fasting blood samples during the stay in the hospital, which were obtained after 12 h of fasting, were determined at the central laboratory of Beijing Anzhen Hospital. The GRACE score was calculated on admission for predicting 6-month death or MI. The symptoms of diabetes and casual plasma glucose ≥ 11.1 mmol/L, FPG ≥ 7.0 mmol/L, or 2-h plasma glucose of 75 g oral glucose tolerance test ≥ 11.1 mmol/L, and/or antidiabetic drug use were the diagnostic criteria for diabetes. Hypertension was defined as at least two blood pressure recordings greater than 140/90 mmHg, and/or use of antihypertensive drugs. Fasting TC > 5.17 mmol/L, low-density lipoprotein-cholesterol (LDL-C) > 3.36 mmol/L, triglycerides (TG) > 1.69 mmol/L, high-density lipoprotein-cholesterol (HDL-C) <1.03 mmol/L, and/or chronic use of lipid-lowering drugs were considered criteria for dyslipidemia.
Baseline NRI was calculated from ALB and BMI obtained on admission as previously described: NRI = 14.89 × ALB (g/dl) + 41.7 × [measured body weight (kg)/ideal body weight (kg)] [8]. The ideal body weight was calculated as follows: body height (cm)−100–{[body height (cm)−150]/4} for males, body height (cm)−100–{[body height (cm)−150]/2.5} for females (17). In accordance with prior studies, we set current body weight/ideal body weight = 1 when current body weight exceeded ideal body weight [7]. In our study, all patients were classified into three nutritional risk groups according to their baseline NRI, as defined in previous studies: normal nutrition (NRI ≥ 100), mild nutritional risk (97.5 ≤ NRI <100), and moderate-to-severe nutritional risk (NRI <97.5) (7). Due to the limitation of the sample size in this study, we did not separate a severe group since there was no patient with severe nutritional risk (NRI <83.5). GRACE score was assessed on admission for predicting 6 months death or MI.
Continuous variables were presented as the mean ± standard deviation or the median and interquartile range (IQR) in the case of normal or non-normal distribution and differences between two groups were examined by independent-sample t-test or Manne-Whitney U test correspondingly. Categorical variables were expressed as counts (percentages). The Chi-squared test or Fisher's exact test was used to analyze differences in categorical variables between groups. ANOVA or the Kruskal–Wallis H test was applied to analyze differences in continuous variables between groups. Spearman analysis was used to analyze the correlation between two continuous variables. Kaplan–Meier methods were used to derive the event rates at follow-up and to plot time-to-event curves. The NRI was analyzed in two ways: (1) as a categorical variable; and (2) as a continuous variable. Multivariate Cox proportional hazards analysis was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) of NRI for MACE after adjustment for multiple confounders including other nutrition-related laboratory parameters, clinically relevant risk factors, and variables with statistical significance in the univariate analysis: lymphocyte count, neutrophil count, monocyte count, TC, hs-CRP, GRACE score, sex, BMI, current smoking, family history of CAD, hypertension, dyslipidemia, diabetes, past MI, past PCI, SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) score, complete revascularization, discharged with aspirin, angiotensin converting enzyme inhibitor/angiotensin II receptor blockers (ACEI/ARBs), β-blockers, insulins, and oral antidiabetic agents. The interaction effect was tested with a likelihood ratio test, and the proportional hazard assumption was tested by demonstrating no importance of variables multiplied by time as time-dependent variables. The C-statistic, continuous net reclassification improvement (cNRI), and integrated discrimination improvement (IDI) were calculated to assess the discrimination capacity of NRI to predict CV events. All P-values were two-sided, and values < 0.05 were considered significant. All statistical analyses were performed using IBM SPSS Statistics version 26.0 (IBM Corporation, Chicago, IL) and R version 4.0.2 software (Vienna, Austria).
The median follow-up duration was 927 days (IQR, 927 to 1,109 days), and during the follow-up period, a total of 354 patients had at least one primary endpoint event, including 239 patients from the normal nutrition group (n = 1301), 53 rom the mild nutritional risk group (n = 208), and 62 from the moderate-to-severe nutritional risk group (n = 209). There were 44 cases of death (37 CV deaths and seven non-CV deaths), 24 cases of non-fatal stroke, 49 cases of non-fatal MI, and 289 cases of unplanned repeat revascularization. Fifty-two patients suffered more than one primary endpoint event. The clinical outcomes according to NRI degree are shown in Table 1. The distribution of NRI and incidence rate curve of MACE across continuous NRI are shown in Figure 2. The lower the NRI, the significantly higher the incidence of MACE.
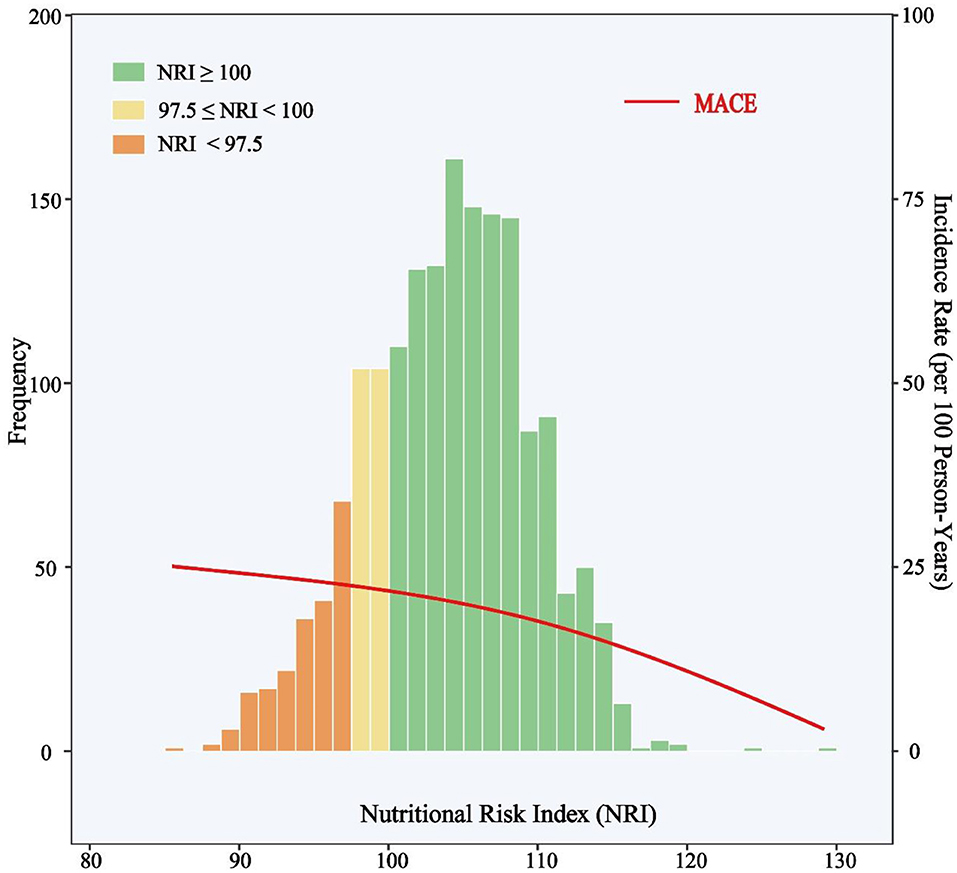
Figure 2. Distribution of NRI and Incidence Rate of MACE. The incidence rate curve of MACE is shown in the red line. Histograms show the population distribution of NRI. The left y-axis shows the frequency of subjects in each NRI intervals and the right y-axis shows the incidence rate (events per 100 person-years) of MACE. The x-axis shows the score of NRI by 1.5 intervals. MACE include all-cause death, non-fatal stroke, non-fatal myocardial infarction, or unplanned repeat revascularization. NRI, nutritional risk index; MACE, major adverse cardiovascular events.
At baseline, the majority of patients were male (76.7%), and the mean age was 60 years. The NRI-defined malnutrition rate was 24.3% in the total population, whereas 32.5% in the primary endpoint group, which was significantly higher than that in the event-free population. Patients with a primary endpoint event had higher heart rate, systolic blood pressure, and higher rates of family history of CAD, diabetes, previous MI and prior PCI. In terms of laboratory measurements, patients with a primary endpoint event had higher levels of creatinine, hs-CRP, neutrophil, monocyte, TC, TG, LDL-C, FPG, and glycosylated hemoglobin, but lower levels of ALB and HDL-C. As for the angiographic findings and procedural results, patients with an endpoint event had a higher SYNTAX score, a higher rate of left main or multi-vessel disease, and a lower rate of complete revascularization. Compared with those with normal nutritional status, patients with malnutrition had a higher GRACE score and higher rates of female, non-ST segment elevation MI (NSTEMI), ST segment elevation MI (STEMI), and proximal left anterior descending artery stenosis. Patients with malnutrition had higher levels of hs-CRP, neutrophil, and monocyte, but had a lower rate of complete revascularization. The baseline characteristics of the study population are shown in Table 2 and Supplementary Table 1. One-point decrease of NRI was positively correlated with hs-CRP (r = 0.231, P < 0.001), neutrophil count (r = 0.064, P = 0.008), monocyte count (r = 0.204, P < 0.001), but not significantly correlated with lymphocyte count (r = −0.024, P = 0.325).
NRI was introduced into multivariate COX regression analysis as a category variable, and after adjustment for multiple confounding factors, compared with those with normal nutritional status, patients with malnutrition had significantly higher adjusted risk of MACE in both mild and moderate-to-severe group [HR for mild and moderate-to-severe nutritional risk respectively: 1.368 (95%CI 1.004–1.871) and 1.473 (95%CI 1.064–2.041)] (Table 3). When NRI was used as a continuous variable in the multivariate Cox regression model, decreased NRI was associated with a higher risk of MACE [HR 1.026, (95%CI 1.004–1.049), P = 0.022] (Supplementary Table 2). Kaplan–Meier analysis revealed that patients with malnutrition showed higher incidence of the MACE (log-rank P < 0.001). This difference was mainly driven by the increase in death (log-rank P < 0.001) and stroke (log-rank P = 0.006), while the incidence of MI (log-rank P = 0.870) and repeat revascularization (log-rank P = 0.094) was similar between non-malnourished and malnourished patients during the follow-up. Kaplan–Meier curves of the incidence of the primary endpoint and each component of the primary endpoint for NRI are presented in Figures 3, 4.
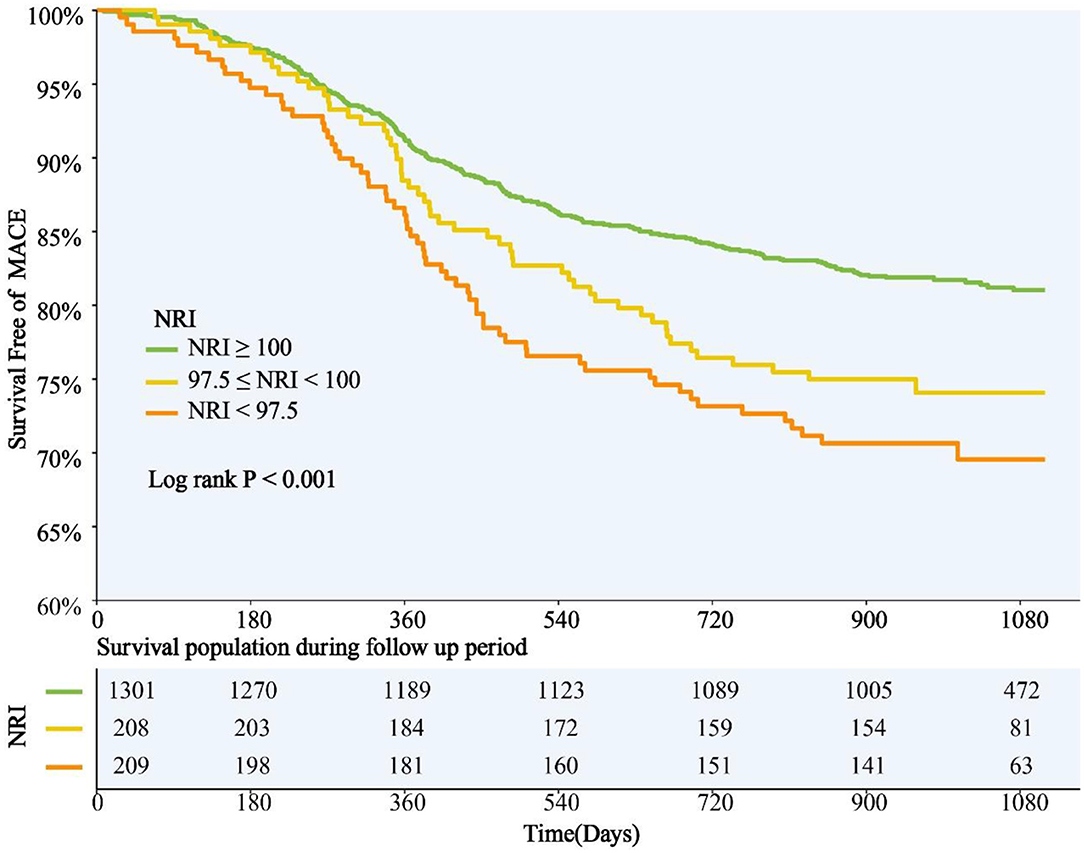
Figure 3. NRI Degree and Risk of MACE. Kaplan-Meier curves for MACE by the NRI degree. NRI, nutritional risk index; MACE, major adverse cardiovascular events.
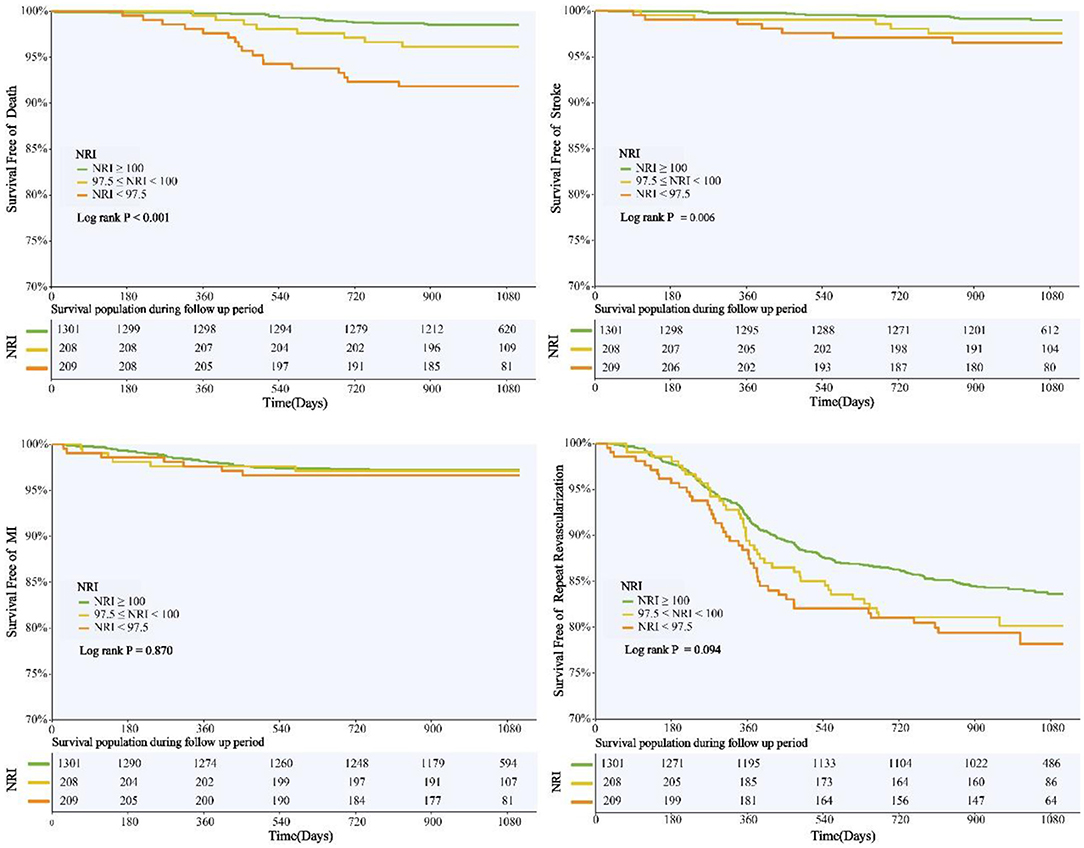
Figure 4. NRI Degree and Risk of Death, Stroke, MI, and Revascularization. Kaplan-Meier curves for all-cause death, non-fatal stroke, non-fatal myocardial infarction, and unplanned repeat revascularization by the NRI degree. NRI, nutritional risk index; MI, myocardial infarction.
Subgroup analyses were also conducted to investigate whether the predictive value of NRI was similar among patients with different demographic characteristics or comorbidities. We found a significant interaction effect between continuous NRI and diabetes subgroup (the predictive value of NRI seemed to be more prominent in patients with diabetes). However, NRI was a significant predictor of MACE regardless of age ≥ or <60 years, male or female, BMI ≥ or <25 kg/m2, current smoking or not, hypertension or not, STEMI or NSTE-ACS (unstable angina + NSTEMI) (all P for interaction > 0.05) (Figure 5).
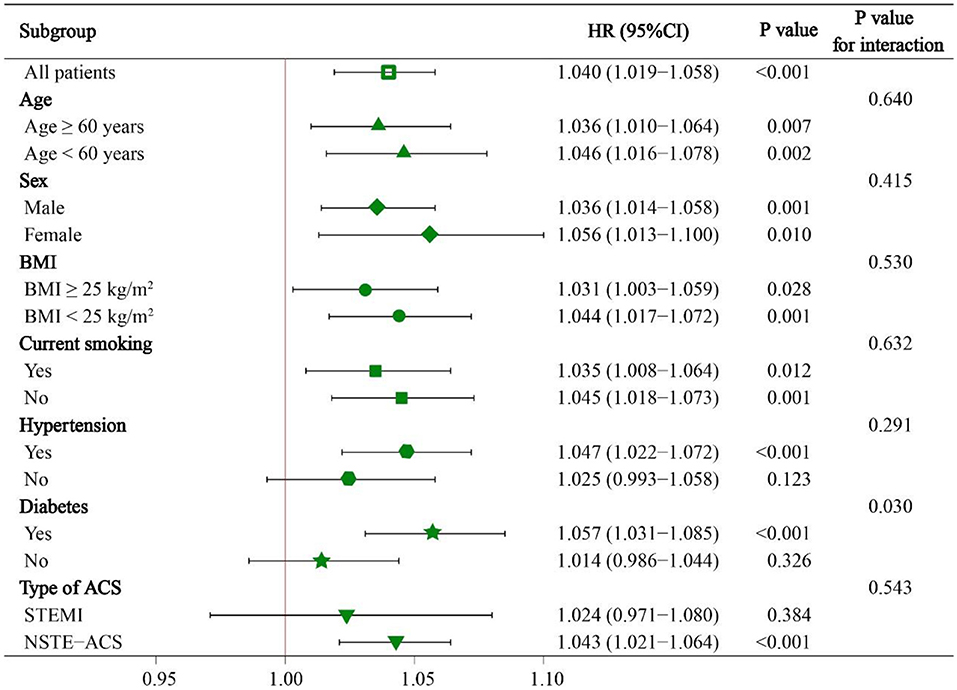
Figure 5. Subgroup analyses of continuous NRI for MACE. HR was evaluated by 1-point decrease of NRI. HR, hazard ratio; CI, confidence interval; BMI, body mass index; STEMI, ST-segment elevation myocardial infarction; NSTE-ACS, non-ST-segment elevation acute coronary syndrome.
Compared with the baseline GRACE score, the addition of NRI had a significant increase in C-statistic from 0.524 (95%CI 0.493–0.556) to 0.565 (95%CI 0.534–0.596) (P = 0.006), and significant improvement in reclassification as assessed by the cNRI (0.070, 95%CI 0.010–0.135, P = 0.010) and IDI (0.005, 95% CI 0.001–0.014, P < 0.001) (Table 4). Supplementary Table 3 shows the model performance after the addition of NRI to the baseline model in overall population.
We then conducted further analyses to investigate the predictive value of NRI among diabetes subjects. Compared with non-malnourished patients with diabetes, malnourished patients with diabetes had nearly 2-fold high adjusted risk of MACE [HR for mild and moderate-to-severe nutritional risk respectively: 1.601 (95%CI 1.030–2.489) and 1.977 (95%CI 1.283–3.046)] (Table 5). Compared with the baseline GRACE score, the addition of NRI had a more significant increase in C-statistic from 0.504 (95%CI 0.461–0.548) to 0.595 (95%CI 0.555–0.636) (P < 0.001), and more significant improvement in reclassification as assessed by the cNRI (0.176, 95%CI 0.062-0.278, P < 0.001) and IDI (0.022, 95% CI 0.003–0.052, P < 0.001) in the diabetes subgroup (Table 6). The model performance after the addition of NRI to the baseline model in diabetic population is shown in Supplementary Table 4. In addition, Supplementary Tables 5, 6 show the relationships between MACE and NRI in the overall population and diabetic population when the first event was selected for the primary endpoint analysis. NRI was an independent predictor of MACE whether the most severe event or the first event was used as the endpoint event.
In the present study, we noticed a significant association of NRI with CV outcomes. Compared with those with normal nutritional status, patients with malnutrition as defined by NRI had a higher risk of MACE. Even after adjustment for as many potential confounders as possible, NRI remained an independent predictor of MACE. The addition of NRI significantly improved the ability of the GRACE score to predict MACE. Intriguingly, in the diabetes subgroup, malnutrition was associated with relatively higher adjusted risk of MACE, and the GRACE score combined with NRI seemed to have better predictive ability than that in the overall population. Therefore, the present study supported the utility of NRI in predicting CV outcomes and improving the predictive ability of the model containing the GRACE score among patients with ACS.
Several reliable risk scoring models have been developed to assist clinicians in risk stratification, such as the GRACE (18), TIMI (Thrombolysis in Myocardial Infarction) (19), and CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) scores (20). Of them, the GRACE score is relatively easy to assess, and has been widely accepted as a powerful predictor of adverse CV outcomes after ACS at different time points up to 4 years (2, 18, 21). Malnutrition is common in patients with ACS and is associated with a poor prognosis regardless of GRACE score, BMI, LVEF, coronary revascularization, optimal medical treatment, and other risk factors (7). It is worth noting that variables required for nutritional status calculation are widely available, and malnutrition appears to be a potentially modifiable risk and therapeutic target.
NRI is a nutritional assessment score that includes ALB as a visceral protein element and actual weight relative to ideal weight as an anthropometric element, both of which are predictors of clinical outcomes in patients with CAD, HF, or diabetes (22, 23). However, albuminemia alone does not appear to be a reliable indicator of nutritional status, as it may be related to inflammation or hydration status rather than malnutrition (24). Hydration status is negatively correlated with ALB concentration, while positively correlated with body weight (8). The combination of both components (i.e., ALB and body weight) in the NRI counteracts the effect of hydration status on nutritional assessment. As to the NRI formula, if the current weight was higher than the ideal weight, we set weight ratio as one, which leads to a higher weighting for albumin than for weight. Otherwise, malnourished patients with overweight would not have been sensitively diagnosed. BMI is often used to define obesity, but it cannot fully reflect the nutritional status. In our study, we found that many obese patients had malnutrition and hypoalbuminemia. The study of Roubín et al. (7) showed that malnutrition was prevalent even in patients with overweight and obesity: a substantial proportion of patients with a BMI of ≥ 25 kg/m2 were malnourished (58% with the NRI). In the study of Sze et al. (25), one-half of heart failure patients with a BMI of ≥ 30 kg/m2 were malnourished as defined by Controlling Nutritional Status (CONUT) scores (another good indicator for nutritional status). These two studies suggest that malnutrition in obese people mainly manifested in low serum albumin levels, which has been shown to reflect active systematic inflammation (26–28). As we known, obesity is associated with active systemic inflammation (29). Of note, inflammation has been shown to reduce serum albumin through several possible mechanisms, including downregulation of synthesis, increased catabolism, and increased vascular permeability (30, 31). These may explain why many obese patients in our study have malnutrition and hypoalbuminemia.
Nutritional status is affected by many factors, and malnourished patients often have complex clinical conditions. In this study, NRI remained strongly associated with MACE after adjustment for multiple potential confounders, such as clinical variables, coronary revascularization, and optimal medical treatment. The two components of NRI are widely used and easily collected in the clinical practice. Therefore, NRI could be considered as a feasible and convenient tool to help predict CV outcomes. In fact, NRI was originally used to assess the nutritional status of elderly patients, who are more likely to experience unconscious weight loss (8). Although our study included patients of all ages, we also conducted an age subgroup analysis. We found no difference in the predictive value of NRI for MACE between the younger and older groups, which was consistent with the results of previous studies (15, 32).
Previous studies supported NRI-defined malnutrition as a reliable predictor of adverse CV events in many patient groups, such as patients with acute or chronic HF (12, 33, 34), patients undergoing aortic valve replacement (13, 35), and patients with other systemic diseases (14, 15, 36). Furthermore, several other studies showed that in patients with stable CAD or ACS, lower NRI levels (the lower the NRI levels, the greater the nutritional risk) were associated with in-hospital and long-term adverse CV events after PCI (11, 32, 37–39); however, these studies did not specifically investigate the prognostic value of malnutrition in ACS patients with diabetes. Since a high prevalence of diabetes-related complications and comorbidities may further impair nutritional status (40). Compared with those without diabetes, people with diabetes are more likely to suffer from malnutrition due to the diabetes itself, injuries, medications, and other factors affecting metabolism (41), which suggests that malnutrition may contribute to a higher risk of adverse CV events in ACS patients with diabetes vs. without diabetes.
Malnutrition is a complex pathological condition, and it is difficult to explain how malnutrition affects CV outcomes in patients with ACS from the results of this observational study. We believe that one of the potential mechanisms is malnutrition-inflammation-atherosclerosis (MIA) syndrome (42). ACS is the result of atherosclerotic plaque rupture causing by the chronic inflammatory response. Meanwhile, patients with diabetes are more likely to have higher levels of inflammatory markers such as C-reactive protein (43, 44), which may increase the burden of atherosclerosis (45). Thus, when malnutrition is present in patients with ACS and diabetes, hypoalbuminemia may be the result of the combination of malnutrition and inflammation. Malnutrition may be driven by inflammatory cytokines and is characterized by chronic inflammation with an increase in insulin resistance, reduction of appetite, production of catabolic cytokines, and muscle catabolism (46). While increased insulin resistance may in turn inhibit the entry of nutrients into cells and accelerate atherosclerosis (46). Another underlying mechanism of poor prognosis due to malnutrition is protein-energy malnutrition (PEM). PEM refers to a persistent state of inadequate food and nutrient intake, leading to changes in body weight, composition, and functioning (47). The association of PEM with poor prognosis in patients with acute MI, acute ischemic stroke, and HF has been demonstrated (48–50). One study showed that pre-existing PEM impaired the body's healing capability after injury, resulting in devastating clinical outcomes among patients with acute MI (51). We consider that for patients with ACS and diabetes, those who are undernourished may typically have lower cardiac and systemic muscle and nutrient reserves and may suffer more severe myocardial damage due to weaker baseline cardiac function and limited capacity to repair.
Previous studies have identified several potential risk factors beyond the GRACE score predicting model which enhance the predictive power for CV events after ACS, such as B-type natriuretic peptide (52), neutrophil count (53), and 2-h post-load glucose (54). The combination of NRI and the GRACE score produced a stronger predictive value, which improved the ability of model discrimination and risk reclassification. Our findings suggest that clinicians can apply nutritional status in combination with the GRACE score to identify higher-risk patients with ACS and diabetes and take effective measures to improve their clinical outcomes. In addition, our findings support the necessity and benefits for physicians to integrate the recognition of malnutrition in the clinical practice. Malnutrition screening in patients with ACS and diabetes can identify patients at high residual risk of CV events, who may benefit from optimized secondary prevention therapy and appropriate nutritional supplements. A recent randomized controlled trial which recruited 2,088 medical inpatients at nutritional risk found that the use of individualized nutritional support during the hospital stay improved clinical outcomes, compared with the use of standard hospital food (55). The strong evidence that individualized nutritional support can improve the prognosis of patients with HF is worth learning (56). The benefits of eating plans adjusted by the patient's preference, food fortifications, and oral nutritional supplements have been proved (57, 58). Dietary counseling and educational interventions after discharge should also be provided in the outpatient clinic. Hence, a rehabilitation unit needs physicians to cooperate with dietitians, nurses, care workers, and other professionals involved in the caring process.
This is a single-center observational study with the subsequent limitation to its nature. First, we only assessed the relationship between NRI at admission and CV outcomes and did not focus on changes in nutritional status during the follow-up. Second, the threshold of malnutrition defined by NRI is vague, and different studies set different grading criteria. Hence, there is no authoritative grading reference at present. Third, due to a limited sample size, the range of NRI was relatively small, which may affect the estimation of the relationship between NRI and CV outcomes. Fourth, all patients in this study were Chinese, so these results should be interpreted with caution and generalized to other ethnic groups since dissimilar metabolic levels exist among different races. Fifth, CKD and albuminuria might affect albumin in the blood, and glomerular hyperfiltration is one of important features in diabetic patients. In terms of kidney function, we could only obtain serum creatinine levels from the original cohort. In addition, markers of kidney injury such as albuminuria, abnormal urinary sediment, histological abnormalities, and imaging abnormalities were not routinely detected at our CV center. Therefore, we could not make an accurate diagnosis of CKD and further analyze the effect of CKD on NRI. Albuminuria was only detected qualitatively rather than quantitatively in most patients, so we could not accurately analyze the effect of albuminuria on NRI. Sixth, liver diseases might affect the clinical outcomes, or be associated with hypoalbuminemia; however, it was not taken into account in our study since indicators of liver function and information about liver diseases were not collected in the original cohort. To be clear, deferred PCI for patients with significant liver dysfunction is usually considered at our CV center unless the life-threatening conditions, so few patients with significant liver dysfunction were included in our study. Seventh, malnutrition may influence CV outcomes by promoting inflammation. Therefore, we include hs-CRP, lymphocytes, neutrophils, and monocytes into the analysis. Unfortunately, proinflammatory cytokines were not routinely measured at our CV center, so we could not analyze the correlation between proinflammatory cytokines and malnutrition or prognosis.
Malnutrition as defined by NRI was independently and strongly associated with a higher risk of MACE in ACS patients who underwent PCI, especially in individuals with diabetes. NRI also improved the predictive ability of the GRACE score based prognostic models. Clinical trials are needed to determine whether improving nutritional status can improve CV outcomes in these patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Committee of Beijing Anzhen Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
X-TM and Q-YS wrote the first draft of the manuscript. X-TM also made important contributions to the revision of the manuscript. X-TM, Q-YS, Q-XL, Z-QY, K-NH, JL, HS, X-LL, Y-JZ, and Z-JW were involved in the conception and design of the study and the collection, analysis, interpretation of the data, and reviewed the final manuscript. All authors read and approved the final manuscript.
Project funded by National Key Research and Development Program of China (2017YFC0908800, Y-JZ), Beijing Municipal Administration of Hospitals' Mission plan (SML20180601, Y-JZ), China Postdoctoral Science Foundation (2021M692253, X-TM), Beijing Postdoctoral Research Foundation (2021-ZZ-023, X-TM), Beijing Municipal Health Commission (Jing 19-15, HS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YC declared a shared affiliation, with the authors X-TM, Q-YS, Q-XL, Z-QY, K-NH, JL, HS, X-LL, Y-JZ, and Z-JW to the handling editor at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.773200/full#supplementary-material
ACEI, angiotensin converting enzyme inhibitor; ACS, acute coronary syndrome; ALB, serum albumin; ARB, angiotensin II receptor blocker; BMI, body mass index; CAD, coronary artery disease; CADILLAC, Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications; CI, confidence interval; CKD, chronic kidney disease; cNRI, continuous net reclassification improvement; CONUT, Controlling Nutritional Status; CV, cardiovascular; FPG, fasting plasma glucose, GRACE, Global Registry of Acute Coronary Events; HDL-C, high-density lipoprotein-cholesterol; HF, heart failure; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; IDI, integrated discrimination improvement; IQR, interquartile range; LDL-C, low-density lipoprotein-cholesterol; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; MI, myocardial infarction; MIA, malnutrition inflammation-atherosclerosis; NRI, nutritional risk index; NSTEMI, non-ST segment elevation myocardial infarction; NSTE-ACS, non-ST segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; PEM, protein-energy malnutrition; STEMI, ST segment elevation myocardial infarction; SYNTAX, SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery; TC, serum total cholesterol; TG, Triglycerides; TIMI, Thrombolysis in Myocardial Infarction.
1. Fox KAA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. (2006) 333:1091. doi: 10.1136/bmj.38985.646481.55
2. Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. (2004) 291:2727–33. doi: 10.1001/jama.291.22.2727
3. Lane JS, Magno CP, Lane KT, Chan T, Hoyt DB, Greenfield S. Nutrition impacts the prevalence of peripheral arterial disease in the United States. J Vasc Surg. (2008) 48:897–904. doi: 10.1016/j.jvs.2008.05.014
4. Lee MY, Lin KD, Chang YH, Hsiao PJ, Shin SJ. Albuminuria is the stronger risk factor for peripheral arterial disease than eGFR decline in a type 2 diabetic Taiwanese population. Kidney Blood Press Res. (2010) 33:352–9. doi: 10.1159/000317524
5. Kumakura H, Kanai H, Hojo Y, Iwasaki T, Ichikawa S. Long-term survival and fate of the leg in de novo intermittent claudication. Eur Heart J Qual Care Clin Outcomes. (2017) 13:208–15. doi: 10.1093/ehjqcco/qcw057
6. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. (1997) 349:1050–3. doi: 10.1016/S0140-6736(96)07015-8
7. Raposeiras Roubín S, Abu Assi E, Cespón Fernandez M, Barreiro Pardal C, Lizancos Castro A, Parada JA, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol. (2020) 76:828–40. doi: 10.1016/j.jacc.2020.06.058
8. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent J-P, Nicolis I, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
9. Cereda E, Zagami A, Vanotti A, Piffer S, Pedrolli C. Geriatric nutritional risk index and overall-cause mortality prediction in institutionalised elderly: a 3-year survival analysis. Clin Nutr. (2008) 27:717–23. doi: 10.1016/j.clnu.2008.07.005
10. Cereda E, Pusani C, Limonta D, Vanotti A. The ability of the geriatric nutritional risk index to assess the nutritional status and predict the outcome of home-care resident elderly: a comparison with the mini nutritional assessment. Br J Nutr. (2009) 102:563–70. doi: 10.1017/S0007114509222677
11. Yoo SH, Kook HY, Hong YJ, Kim JH, Ahn Y, Jeong MH. Influence of undernutrition at admission on clinical outcomes in patients with acute myocardial infarction. J Cardiol. (2017) 69:555–60. doi: 10.1016/j.jjcc.2016.05.009
12. Minamisawa M, Seidelmann SB, Claggett B, Hegde SM, Shah AM, Desai AS, et al. Impact of malnutrition using geriatric nutritional risk index in heart failure with preserved ejection fraction. JACC Heart Fail. (2019) 7:664–75. doi: 10.1016/j.jchf.2019.04.020
13. Shibata K, Yamamoto M, Kano S, Koyama Y, Shimura T, Kagase A, et al. Importance of geriatric nutritional risk index assessment in patients undergoing transcatheter aortic valve replacement. Am Heart J. (2018) 202:68–75. doi: 10.1016/j.ahj.2018.04.021
14. Cheng N, Dang A, Lv N, He Y, Wang X. Malnutrition status in patients of very advanced age with nonvalvular atrial fibrillation and its impact on clinical outcomes. Nutr Metab Cardiovasc Dis. (2019) 29:1101–9. doi: 10.1016/j.numecd.2019.06.021
15. Lin T-Y, Hung S-C. Geriatric nutritional risk index is associated with unique health conditions and clinical outcomes in chronic kidney disease patients. Nutrients. (2019) 11:2769. doi: 10.3390/nu11112769
16. Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. (2020) 19:31. doi: 10.1186/s12933-020-01006-7
17. Cereda E, Pedrolli C. The geriatric nutritional risk index. Curr Opin Clin Nutr Metab Care. (2009) 12:1–7. doi: 10.1097/MCO.0b013e3283186f59
18. Tang EW, Wong C-K, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J. (2007) 153:29–35. doi: 10.1016/j.ahj.2006.10.004
19. Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. (2000) 102:2031–7. doi: 10.1161/01.CIR.102.17.2031
20. Halkin A, Singh M, Nikolsky E, Grines CL, Tcheng JE, Garcia E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol. (2005) 45:1397–405. doi: 10.1016/j.jacc.2005.01.041
21. Bradshaw PJ, Ko DT, Newman AM, Donovan LR, Tu JV. Validity of the GRACE (global registry of acute coronary events). Acute coronary syndrome prediction model for six month post-discharge death in an independent data set. Heart. (2006) 92:905–9. doi: 10.1136/hrt.2005.073122
22. Liu M, Chan C-P, Yan BP, Zhang Q, Lam Y-Y, Li R-J, et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. (2012) 14:39–44. doi: 10.1093/eurjhf/hfr154
23. Tobias DK, Pan A, Jackson CL, O'Reilly EJ, Ding EL, Willett WC, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. (2014) 370:233–44. doi: 10.1056/NEJMoa1304501
24. Ballmer PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr. (2001) 20:271–3. doi: 10.1054/clnu.2001.0439
25. Sze S, Pellicori P, Kazmi S, Rigby A, Cleland JGF, Wong K, et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Heart Fail. (2018) 6:476–86. doi: 10.1016/j.jchf.2018.02.018
26. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
27. Kim S, McClave SA, Martindale RG, Miller KR, Hurt RT. Hypoalbuminemia and clinical outcomes: what is the mechanism behind the relationship? Am Surg. (2017) 83:1220–7. doi: 10.1177/000313481708301123
28. Sheinenzon A, Shehadeh M, Michelis R, Shaoul E, Ronen O. Serum albumin levels and inflammation. Int J Biol Macromol. (2021) 184:857–62. doi: 10.1016/j.ijbiomac.2021.06.140
29. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. (2010) 2010:289645. doi: 10.1155/2010/289645
30. Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest. (1987) 79:1635–41. doi: 10.1172/JCI113000
31. Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. (1985) 1:781–4. doi: 10.1016/S0140-6736(85)91447-3
32. Wada H, Dohi T, Miyauchi K, Doi S, Naito R, Konishi H, et al. Prognostic impact of the geriatric nutritional risk index on long-term outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol. (2017) 119:1740–5. doi: 10.1016/j.amjcard.2017.02.051
33. Honda Y, Nagai T, Iwakami N, Sugano Y, Honda S, Okada A, et al. Usefulness of geriatric nutritional risk index for assessing nutritional status and its prognostic impact in patients aged ≥65 years with acute heart failure. Am J Cardiol. (2016) 118:550–5. doi: 10.1016/j.amjcard.2016.05.045
34. Narumi T, Arimoto T, Funayama A, Kadowaki S, Otaki Y, Nishiyama S, et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J Cardiol. (2013) 62:307–13. doi: 10.1016/j.jjcc.2013.05.007
35. Goldfarb M, Lauck S, Webb JG, Asgar AW, Perrault LP, Piazza N, et al. Malnutrition and mortality in frail and non-frail older adults undergoing aortic valve replacement. Circulation. (2018) 138:2202–11. doi: 10.1161/CIRCULATIONAHA.118.033887
36. Matsuo Y, Kumakura H, Kanai H, Iwasaki T, Ichikawa S. The geriatric nutritional risk index predicts long-term survival and cardiovascular or limb events in peripheral arterial disease. J Atheroscler Thromb. (2020) 27:134–43. doi: 10.5551/jat.49767
37. Katayama T, Hioki H, Kyono H, Watanabe Y, Yamamoto H, Kozuma K. Predictive value of the geriatric nutritional risk index in percutaneous coronary intervention with rotational atherectomy. Heart Vessels. (2020) 35:887–93. doi: 10.1007/s00380-020-01558-4
38. Kunimura A, Ishii H, Uetani T, Aoki T, Harada K, Hirayama K, et al. Impact of geriatric nutritional risk index on cardiovascular outcomes in patients with stable coronary artery disease. J Cardiol. (2017) 69:383–8. doi: 10.1016/j.jjcc.2016.09.004
39. Cheng L, Rong J, Zhuo X, Gao K, Meng Z, Wen X, et al. Prognostic value of malnutrition using geriatric nutritional risk index in patients with coronary chronic total occlusion after percutaneous coronary intervention. Clin Nutr. (2021) 40:4171–9. doi: 10.1016/j.clnu.2021.01.042
40. Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. (2007) 50:18–25. doi: 10.1007/s00125-006-0491-1
41. Armstrong DG, Hanft JR, Driver VR, Smith APS, Lazaro-Martinez JL, Reyzelman AM, et al. Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a prospective randomized controlled trial. Diabet Med. (2014) 31:1069–77. doi: 10.1111/dme.12509
42. Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant. (2000) 15:953–60. doi: 10.1093/ndt/15.7.953
43. Effoe VS, Correa A, Chen H, Lacy ME, Bertoni AG. High-sensitivity C-reactive protein is associated with incident type 2 diabetes among African Americans: the Jackson heart study. Diabetes Care. (2015) 38:1694–700. doi: 10.2337/dc15-0221
44. Abe M, Okada K, Maruyama T, Maruyama N, Matsumoto K, Soma M. Relationship between erythropoietin responsiveness, insulin resistance, and malnutrition-inflammation-atherosclerosis (MIA) syndrome in hemodialysis patients with diabetes. Int J Artif Organs. (2011) 34:16–25. doi: 10.5301/IJAO.2011.6314
45. Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. (2004) 29:439–93. doi: 10.1016/S0146-2806(04)00074-X
46. Merker M, Felder M, Gueissaz L, Bolliger R, Tribolet P, Kägi-Braun N, et al. Association of baseline inflammation with effectiveness of nutritional support among patients with disease-related malnutrition: a secondary analysis of a randomized clinical trial. JAMA Netw Open. (2020) 3:e200663. doi: 10.1001/jamanetworkopen.2020.0663
47. Marshall S. Protein-energy malnutrition in the rehabilitation setting: evidence to improve identification. Maturitas. (2016) 86:77–85. doi: 10.1016/j.maturitas.2016.01.014
48. Adejumo OL, Koelling TM, Hummel SL. Nutritional Risk Index predicts mortality in hospitalized advanced heart failure patients. J Heart Lung Transplant. (2015) 34:1385–9. doi: 10.1016/j.healun.2015.05.027
49. Gariballa SE, Parker SG, Taub N, Castleden CM. Influence of nutritional status on clinical outcome after acute stroke. Am J Clin Nutr. (1998) 68:275–81. doi: 10.1093/ajcn/68.2.275
50. Kang MK, Kim TJ, Kim Y, Nam K-W, Jeong H-Y, Kim SK, et al. Geriatric nutritional risk index predicts poor outcomes in patients with acute ischemic stroke - Automated undernutrition screen tool. PLoS One. (2020) 15:e0228738. doi: 10.1371/journal.pone.0228738
51. Adejumo AC, Adejumo KL, Adegbala OM, Enwerem N, Ofosu A, Akanbi O, et al. Inferior outcomes of patients with acute myocardial infarction and comorbid protein-energy malnutrition. JPEN J Parenter Enteral Nutr. (2020) 44:454–62. doi: 10.1002/jpen.1680
52. Carvalho LSF, Bogniotti LAC, de Almeida OLR, E Silva JCQ, Nadruz W, Coelho OR, et al. Change of BNP between admission and discharge after ST-elevation myocardial infarction (Killip I) improves risk prediction of heart failure, death, and recurrent myocardial infarction compared to single isolated measurement in addition to the GRACE score. Eur Heart J Acute Cardiovasc Care. (2019) 8:643–51. doi: 10.1177/2048872617753049
53. Zhang S, Wan Z, Zhang Y, Fan Y, Gu W, Li F, et al. Neutrophil count improves the GRACE risk score prediction of clinical outcomes in patients with ST-elevation myocardial infarction. Atherosclerosis. (2015) 241:723–8. doi: 10.1016/j.atherosclerosis.2015.06.035
54. Chattopadhyay S, George A, John J, Sathyapalan T. Adjustment of the GRACE score by 2-hour post-load glucose improves prediction of long-term major adverse cardiac events in acute coronary syndrome in patients without known diabetes. Eur Heart J. (2018) 39:2740–5. doi: 10.1093/eurheartj/ehy233
55. Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet (London, England). (2019) 393:2312–21. doi: 10.1016/S0140-6736(18)32776-4
56. Hersberger L, Dietz A, Bürgler H, Bargetzi A, Bargetzi L, Kägi-Braun N, et al. Individualized nutritional support for hospitalized patients with chronic heart failure. J Am Coll Cardiol. (2021) 77:2307–19. doi: 10.1016/j.jacc.2021.03.232
57. Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009:CD003288. doi: 10.1002/14651858.CD003288.pub3
Keywords: nutritional risk index (NRI), GRACE score, acute coronary syndrome (ACS), percutaneous coronary intervention, diabetes
Citation: Ma X-T, Shao Q-Y, Li Q-X, Yang Z-Q, Han K-N, Liang J, Shen H, Liu X-L, Zhou Y-J and Wang Z-J (2021) Nutritional Risk Index Improves the GRACE Score Prediction of Clinical Outcomes in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 8:773200. doi: 10.3389/fcvm.2021.773200
Received: 09 September 2021; Accepted: 25 November 2021;
Published: 16 December 2021.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Yujing Cheng, Capital Medical University, ChinaCopyright © 2021 Ma, Shao, Li, Yang, Han, Liang, Shen, Liu, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Jian Wang, emp3YW5nMTk3NUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.