
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 29 October 2021
Sec. Atherosclerosis and Vascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.728724
 Ceren Eyileten1
Ceren Eyileten1 Joanna Jarosz-Popek1
Joanna Jarosz-Popek1 Daniel Jakubik1
Daniel Jakubik1 Aleksandra Gasecka2
Aleksandra Gasecka2 Marta Wolska1
Marta Wolska1 Marcin Ufnal3*
Marcin Ufnal3* Marek Postula1
Marek Postula1 Aurel Toma4
Aurel Toma4 Irene M. Lang4
Irene M. Lang4 Jolanta M. Siller-Matula1,4*
Jolanta M. Siller-Matula1,4*To investigate the association of liver metabolite trimethylamine N-oxide (TMAO) with cardiovascular disease (CV)-related and all-cause mortality in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention. Our prospective observational study enrolled 292 patients with ACS. Plasma concentrations of TMAO were measured during the hospitalization for ACS. Observation period lasted seven yr in median. Adjusted Cox-regression analysis was used for prediction of mortality. ROC curve analysis revealed that increasing concentrations of TMAO levels assessed at the time point of ACS significantly predicted the risk of CV mortality (c-index=0.78, p < 0.001). The cut-off value of >4 μmol/L, labeled as high TMAO level (23% of study population), provided the greatest sum of sensitivity (85%) and specificity (80%) for the prediction of CV mortality and was associated with a positive predictive value of 16% and a negative predictive value of 99%. A multivariate Cox regression model revealed that high TMAO level was a strong and independent predictor of CV death (HR = 11.62, 95% CI: 2.26–59.67; p = 0.003). High TMAO levels as compared with low TMAO levels were associated with the highest risk of CV death in a subpopulation of patients with diabetes mellitus (27.3 vs. 2.6%; p = 0.004). Although increasing TMAO levels were also significantly associated with all-cause mortality, their estimates for diagnostic accuracy were low. High TMAO level is a strong and independent predictor of long-term CV mortality among patients presenting with ACS.
Acute coronary syndrome (ACS) remains a leading cause of mortality worldwide (1). Despite development of pharmacological treatment and percutaneous coronary intervention (PCI) (2–5), patients who experienced ACS are at high risk of future cardiovascular events and death (6–8). Identification of reliable predictive tools could potentially improve the risk stratification (9). Numerous studies revealed that intestinal microbial organisms (microbiota) and its metabolites, as TMA (oxidize trimethylamine) may play a pathogenic role in a wide range of diseases, including the onset and progression of cardiovascular disease (CVD) and ACS (10). By oxidation of TMA in the liver originates TMAO (trimethylamine-N-oxide). An additional, direct source of TMAO in humans is TMAO-rich seafood (11). Elevated concentration of circulating TMAO has been associated with increased risk of CVD and major adverse cardiac events (MACE), including myocardial infarction (MI), stroke, major bleeding and all-cause mortality (12). Though, data on the effect of TMAO on the circulatory system are conflicting (11, 13–15).
A population of special interest in the context of bacterial metabolites are patients with diabetes mellitus (DM) (16, 17). Patients with DM are at a higher risk of developing micro- and macrovascular complications such as chronic kidney disease (CKD) or atherosclerosis than those without these risk factors (16, 17). Because the number of patients with DM continues to increase, there is a need for new CV risk prediction markers in order to reduce DM-related complications (18, 19). Elevated intake of TMAO precursors in the diet shows an association with a higher risk of developing DM (20). Moreover, recent studies revealed that DM contributes to even a 10-fold increase in TMAO levels, indicating a potential role of this metabolite as a predictor of insulin resistance and impaired glucose tolerance (21). It has been shown that the all-cause and CV-related mortality among DM individuals is up to 4 - fold higher compared to patients without DM (14).
Herein, our objective was to investigate the association of TMAO with CV-related and all-cause mortality in patients with ACS undergoing percutaneous coronary intervention (PCI) with a special focus on patients with DM.
This is a prospective observational study, which included consecutive ACS patients and blood sampling was done between July 2012 until July 2016 at the Medical University of Vienna. The Ethics Committee of the Medical University of Vienna approved the study protocol in accordance with the Declaration of Helsinki. In summary, the study aimed to investigate short-term and long-term clinical outcomes in consecutive patients after ACS and PCI who were treated with potent platelet inhibitors as ticagrelor and prasugrel. Inclusion criteria were comprised ACS at admission, provision of written informed consent before study entry, age >18 yr and planned treatment with potent platelet inhibitors. Patients were excluded when participating in interventional trials. Follow-up information was obtained by contacting patients every three months within the first year. Further, data regarding long-term mortality was acquired through queries of the Austrian death registry - most recently, in June 2020. For this analysis we only considered patients, where blood biomarkers were assessed during hospitalization for ACS (22–24).
Primary efficacy endpoint was long-term CV death. Incidence of MACE within one year after discharge and long-term all-cause mortality were regarded as our secondary endpoint. MACE was determined as non-fatal MI, non-fatal stroke and CV death. The composite endpoint was defined in accordance with the current universal criteria (25, 26). Stroke was defined as an abrupt onset of a focal neurologic deficit, generally distributed in the territory of a single brain artery (including the retinal artery), and that is not attributable to an identifiable non-vascular cause (i.e., brain tumor or trauma). Stroke definition reflects the Statement for Healthcare Professionals From the American Heart Association/American Stroke Association (27), that incorporates the World Health Organization (WHO) definition of stroke (28). Myocardial infarction (MI) was defined according to the latest version of the Universal Definition (29). CV deaths include deaths that result from an acute myocardial infarction, sudden cardiac death, death due to heart failure, death due to stroke, death due to CV procedures, death due to CV hemorrhage, and death due to other CV causes according (26).
Venous blood samples were drawn (VACUETTE® CAT Serum Sep Clot Activator; 8 mL). The evaluation of blood plasma metabolites concentration was done in December 2019 at the Medical University of Warsaw. The plasma concentration of TMAO was evaluated using a Waters Acquity Ultra Performance Liquid Chromatography coupled with a Waters TQ-S Triple-Quadrupole Mass Spectrometer as we have previously described (30, 31). In short, chromatographic separation was performed using a Waters HILIC column (1.7 μm, 2.1 mm × 50 mm) thermostatted at 70°C. Mobile phase A was Mili-Q water with an addition of 1 mL of 25% NH4OH per 1,000 mL of water, and mobile phase B was 1 mL of formic acid in 1,000 mL of acetonitrile. The flow rate of the mobile phase was set at 0.5 mL/min. The total time of separation was 1.7 min. The mass spectrometer operated in multiple-reaction monitoring (MRM)-positive electrospray ionization (ESI+) mode. The calibration curve ranges were 0.02–20 μg/mL for TMAO. Mean R2 coefficients of a calibration curves from 6 calibrators was not lower than 0.99.
Risk factors, clinical data and categorical variables are presented as percentages of patients and were compared using χ2 or Fisher's exact tests, as appropriate. Continuous data are expressed as mean ± standard deviation (SD) or median and interquartile range depending on the data distribution and compared using Student's t test, Mann-Whitney U test for two independent samples. The distribution of data was checked with the Kolmogorov-Smirnov test. The Kaplan-Meier method was utilized for construction of survival curves. The log-rank test was applied to evaluate differences between groups. Proportional Cox-regression analysis was used to adjust for confounding factors. Potential confounders (prior MI, diabetes, age, gender, diabetes, hemoglobin and creatinine levels at admission) were entered into the Cox model on the basis of known clinical relevance or significant association observed at univariate analysis. Effect estimates were presented as hazard ratios (HR) and 95% CI. All tests were two-sided, a p-value < 0.05 was considered statistically significant. Calculations were performed using SPSS version 22.0 (IBM Corporation, Chicago, USA). Based on a 16% long-term mortality in the high TMAO group as compared to 1% in the low TMAO group, we calculated that with 290 patients (3:1 sampling ratio), our analysis had 99% power with a two-sided alpha value of < 0.05.
Patient demographics, concomitant medication, laboratory results and ACS data are summarized in Table 1. Overall, the majority of the participants were male (80%). Out of 344 patients included, blood samples for analyses presented in this study were unavailable for 52 subjects. Among the 292 patients included, 13 (5%) patients died within a median observation time of 84 months (7 years). The most frequent diagnosis at admission has been ST Elevation Myocardial Infarction (STEMI) with 63 % in total. CV risk factors, such as hypertension (63%), dyslipidemia (56%) and history of smoking (74%) were common in most patients. DM has been diagnosed in 20% of the cohort. Peripheral artery disease (PAD) and cerebrovascular disease were reported in 6 and 4%, respectively. Acetylsalicylic acid (ASA) was administered to 100% of the study population. Patients who underwent repeated revascularization (non-target or target vessel) had significantly higher TMAO concentration than patients who did not need revascularization (p = 0.029) (Figure 1). Patients who died had 3- fold higher TMAO levels as compared with patients who survived (p < 0.001; Figure 2A). Also, patients with DM had 1.4- fold higher TMAO levels than patients without DM (p < 0.001; Figure 2B).
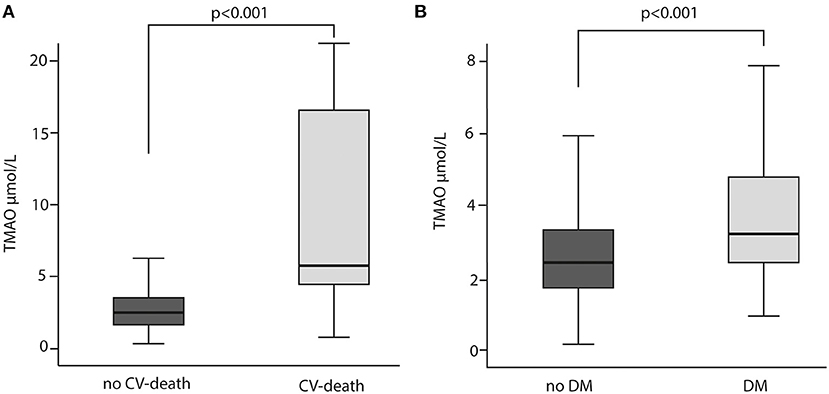
Figure 2. TMAO levels in relation to cardiovascular (CV) death (A) CV death and diabetes mellitus (DM) (B) Mann-Whitney U test was used for the comparison.
ROC curve analysis has shown that TMAO cut-off of >4 μmol/L (high TMAO group) had a c-index of 0.78 (95% (CI): 0.61-0.95; p = 0.001; Figure 2A, Table 2) for prediction of long-term CV death, which was associated with a 80% sensitivity, 85% specificity, 99% positive predictive value, 16% negative predictive value, and 4.2 positive likelihood ratio (Figure 3A, Table 2).
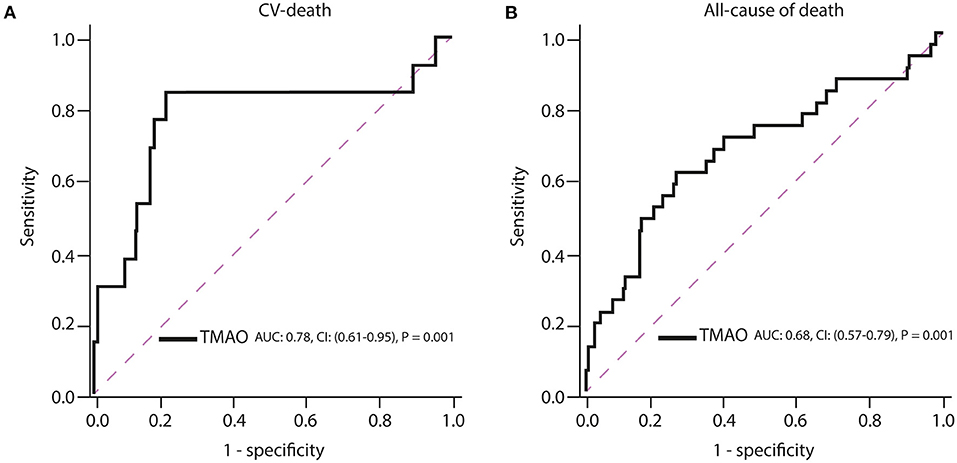
Figure 3. Receiver operating characteristic (ROC) curves of TMAO for prediction of (A) cardiovascular death and (B) all-cause of death. AUC, area under the curve; CI, confidence interval.
Increasing TMAO levels were significantly associated with all-cause mortality, but their c-index estimates were low (TMAO: c-index = 0.68; 95% (CI): 0.57–0.79; p = 0.001; Figure 3B). The measures for diagnostic accuracy of TMAO for all-cause mortality were lower than for CV mortality (Tables 2, 3).
Based on the best TMAO accuracy cut-off, the study population (292 patients) was divided into two subgroups: low TMAO (mean ± standard deviation [SD]: 2.2 ± 0.85 μmol/L), including 225 (77%) patients and high TMAO (8.98 ± 11.46 μmol/L), counting 67 (23%) patients. Mean age in patients assigned to the low TMAO subgroup was 56 ± 11 yr and 66 ± 14 years in patients of the high TMAO subgroup (p < 0.0001). Likewise, DM (17 vs. 33%; p = 0.006), PAD (3 vs. 12%; p = 0.009), prior MI (19 vs. 31%; p = 0.039), prior PCI (9 vs. 18%; p = 0.035) were more frequent in patients with high TMAO than with low TMAO. Interestingly, smokers had more often low TMAO than high TMAO levels (77 vs. 63%; p = 0.017). Moreover, beta-blockers (BB) were applied more often in the low TMAO subgroup rather than in the high TMAO subgroup (96 vs. 86%; p = 0.009). Contrarily, patients with high TMAO levels were administered more frequent calcium channel-blockers (CCB) (15 vs. 7%; p = 0.033), and antidiabetic drugs (23 vs. 12%; p = 0.018). Regarding laboratory data, platelets and hemoglobin levels were higher in patients with low TMAO than high TMAO (246.4 ± 67 × 109/L vs. 225 ± 75 × 109/L; p = 0.011 and 14.6 ± 1.6 g/dL vs. 13.9 ± 2.5 g/dL; p = 0.011, respectively) concentrations. Creatinine values were higher in patients with high TMAO levels (1.1 ± 0.6 vs. 0.9 ± 1.2; p < 0.001).
The primary endpoint of long-term CV death occurred in 13 (5%) out of 292 patients, for whom the TMAO levels were available. Eleven of these patients who died had high TMAO values and 2 patients had low TMAO values (Table 4). Higher plasma TMAO level was associated with a crude 21.3-fold increased CV-related mortality risk (HR = 21.33, 95%CI: 4.71–96.48; p < 0.001; Table 5). For following adjustments, we used a multivariate Cox regression model to identify independent variables for long-term CV death (Table 6). Patients with high TMAO levels had 11.6-fold higher risk to experience CV death within seven yr as compared to those with lower TMAO values in the multivariate Cox regression model (adjusted HR = 11.62, 95% CI: 2.26–59.67; p = 0.003; Table 6). Kaplan-Meier survival analysis showed a significant separation of curves between patients with high and low TMAO levels (p < 0.001) (Figure 4A). The highest risk to die was found in a subgroup of patients with DM and high TMAO levels (DM high vs. low TMAO: 27.3 vs. 2.6%, p = 0.004; none DM: 11.1 vs. 0.5%; p < 0.001; Figure 4B). Additionally, Kaplan-Meier survival analysis showed a significant separation of curves between patients with high and low TMAO levels for MACE (p < 0.001) (Figure 4C).
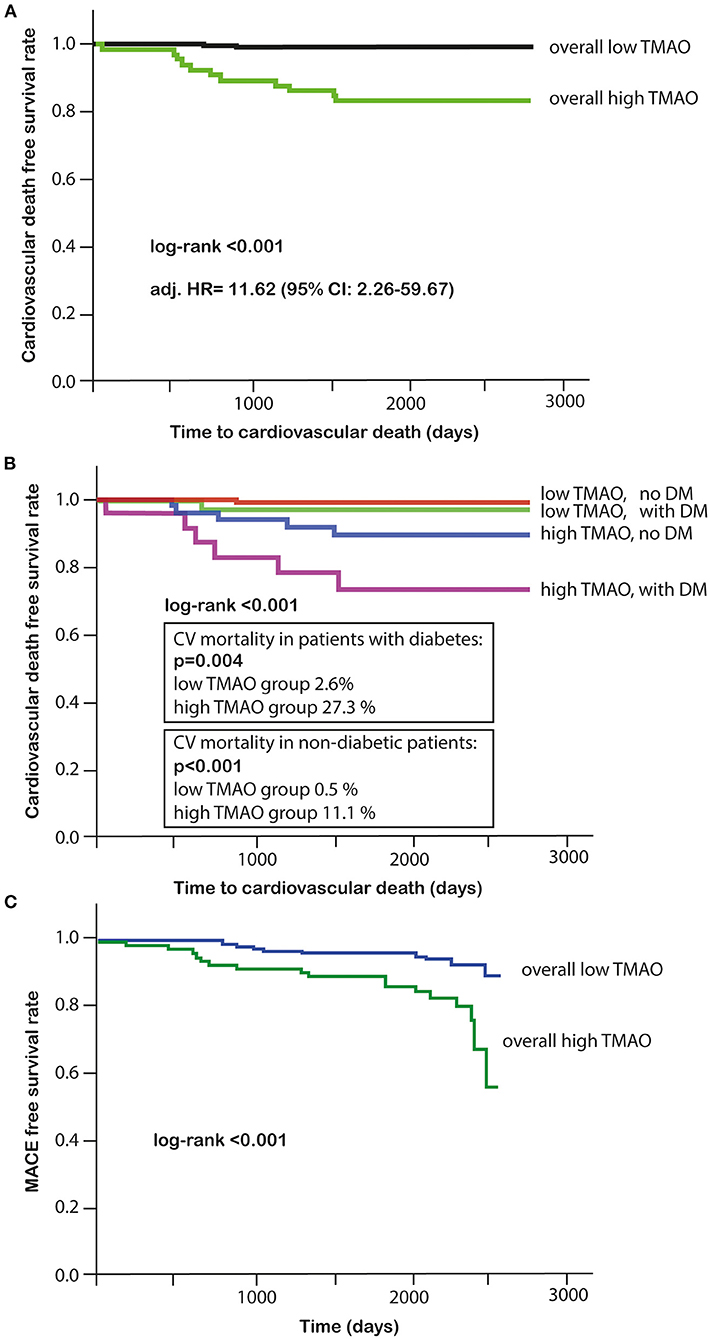
Figure 4. (A) Kaplan-Meier survival analysis for cardiovascular death in high or low TMAO subgroups; (B) Kaplan-Meier survival analysis in DM and high or low TMAO subgroups; (C) Kaplan-Meier survival analysis for MACE in high or low TMAO subgroups.
The present study was conducted to evaluate the association of and TMAO with CV-related and all-cause mortality in patients with ACS. It demonstrates that high concentration of TMAO in the plasma at the time of ACS is associated with 12-fold increased long-term CV mortality. Additionally, high concentration of TMAO in patients with DM is associated with a very high, 27% incidence of seven-yr CV mortality.
TMAO has recently been extensively investigated for its prognostic value. High levels of plasma TMAO were found to be associated with poor prognostic outcomes in several conditions, including CAD, HF, PAD, COPD, CKD and psoriasis (32–38). Moreover, some previous reports have also shown the association between TMAO and CV events in patients with ACS (39, 40). A number of studies and meta-analyses confirmed the association between higher concentration of TMAO and higher risk of MACE, including CV and all-cause death and mortality during follow-up periods of up to 4 years (14, 21, 41–46). Interestingly, higher levels of TMAO were related to increased incidence of all-cause mortality in a dose-dependent manner, namely 7.6% per each 10 μmol/L increment of TMAO in a median follow-up period of 4.3 yr (47). In our study, focusing on a very homogenous population of ACS patients, elevated levels of TMAO (≥4 μmol/L) were associated with a 12-times higher risk of CV death during the longest to our knowledge follow-up time of seven yr. Of interest, our study also provides novel evidence that high levels of TMAO were detected more frequently in patients with a history of MI or previous PCI as well as with post-PCI revascularization. Another important finding in our study is the strong association between DM and high levels of TMAO, which is in line with other studies (14, 48, 49). Recently, it has been demonstrated that circulating TMAO levels were independently associated with age, BMI, and diabetes status in animal model (50). It should be pointed out that although our patients had an optimal medical treatment at discharge (statins, BB, ACE-I, potent platelet inhibition), the residual risk with high TMAO is an independent predictor.
Our study shows that TMAO cut-off of 4 μmol/L was associated with a very high (99%) negative predictive value, 85% sensitivity, 80% specificity and an acceptable c-index of 0.78, underlying the effectiveness of TMAO as a predictive tool. Further studies are now necessary to investigate whether specific treatments or lifestyle modifications according to this cut-off would improve patient outcome.
It is interesting to speculate, which underlying mechanisms might be responsible for the association between high plasma TMAO and increased CV death. There is an ongoing debate whether TMAO is a mediator or merely a marker of cardiovascular pathology. Experimental data on cardiovascular effects of TMAO provide contradictory results (51–54). TMAO has been suggested to promote development of atherosclerotic plaque, alternate macrophage and endothelial cell phenotype and promote platelet reactivity (52, 55, 56). TMAO has also been implicated in the regulation of glucose, sterol and lipid metabolism which are associated with CAD (57–59). Notably, some studies not only show no negative effects related to TMAO, but also positive effects are observed (60, 61). Namely, in animal model high TMAO is suggested to decelerate aortic lesion formation and therefore may play a protective role in atherosclerosis progression (53). Additionally, TMAO fed rats presented not only lower systolic and diastolic blood pressure, but also downward trend of plasma NT-proBNP and raised LVEF (62, 63).
In this study, we have found that higher levels of TMAO were associated with increased all-cause mortality. However, plasma TMAO estimates for diagnostic accuracy were low. We also demonstrated that higher creatinine levels were associated with increased levels of plasma TMAO, a finding also observed by others (64). Nevertheless, in the multivariate analysis, only TMAO levels were revealed as an independent predictor of CV mortality.
Accumulating evidence suggests that concomitant medication is associated with higher plasma levels of TMAO. A significant increase in TMAO levels was observed among subjects using beta-blockers (BB), calcium channel blockers (CCB), statins and diuretics, but not metformin in one study (59). Indeed, patients with high TMAO levels in our study were also administered more frequently CCB and antidiabetic drugs, which might only specify DM disease associated treatment. In contrast to the published observations, BB use corresponded with low rather than high levels of TMAO in our study. Summing up, it is unclear whether specific or concomitant treatment can modulate TMAO levels. Therefore, further studies are needed to clarify this issue.
Building on the results of the present study we postulate that TMAO is a more accurate biomarker of CV pathology. It should be also underlined that most clinical studies investigating that issue have been carried out in patients with known CVD and in high-risk patients. Still, no standardized normal level and life-trend of TMAO has been studied in the general population and no prospective cohort study has been carried out in patients at different levels of cardiovascular risk.
The major limitation is the observational design of the study. Despite efforts to adjust for baseline differences using multivariate Cox regression analysis, a potential bias cannot be ruled out. Moreover, dietary consumption of TMAO precursors such as choline, betaine, L-carnitine may be influenced by sex, age, health condition or glycemic status. As we did not have the data of the daily diets of the patients, we also cannot exclude the potential influence of the used medications during hospitalization and diets by studied subjects. The paper does not distinguish between target and not target vessel revascularization due to the limitations of the database. Additionally, plasma sampling shortly after trauma or any other intervention may influence the concentration of TMAO (49, 59).
In the current study, we demonstrated that in patients experiencing ACS, who were referred to PCI, the concentration of TMAO is independently associated with long-term CV mortality: patients with high TMAO levels (>4 μmol/L) were at 11-times higher risk to experience CV-related death than those with low TMAO. Furthermore, TMAO with a similar accuracy predicted all-cause death. Additionally, individuals suffering from DM had a higher concentration of TMAO, which was independently associated with increased risk of CV mortality. Future studies are necessary to delineate whether TMAO represents a pure marker of disease severity or a modifiable risk factor.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Medical University of Vienna approved the study protocol in accordance with the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
MU evaluation of TMAO plasma concentrations. CE, JJ-P, DJ, AG, MW, MU, AT, IL, JS-M, and MP writing—original draft preparation. CE, DJ, MU, JS-M, and MP writing—review and editing. CE visualization. MU, JS-M, and MP supervision. All authors contributed to the article and approved the submitted version.
This work was implemented with CEPT infrastructure financed by the European Union-the European Regional Development Fund within the Operational Program Innovative economy for 2007–2013.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This paper was written as a part of cooperation of the international scientific group I-COMET (International Cardiovascular and Cardiometabolic Research Team, www.icomet.science).
1. Haider A, Bengs S, Luu J, Osto E, Siller-Matula JM, Muka T, et al. Sex and gender in cardiovascular medicine: presentation and outcomes of acute coronary syndrome. Eur Heart J. (2020) 41:1328–36. doi: 10.1093/eurheartj/ehz898
2. Jezewski MP, Kubisa MJ, Eyileten C, De Rosa S, Christ G, Lesiak M, et al. Bioresorbable vascular scaffolds—dead end or still a rough diamond? J Clin Med. (2019) 8:2167. doi: 10.3390/jcm8122167
3. Eyileten C, Soplinska A, Pordzik J, Siller-Matula JM, Postuła M. Effectiveness of antiplatelet drugs under therapeutic hypothermia: a comprehensive review. Clin Pharmacol Ther. (2019) 106:993–1005. doi: 10.1002/cpt.1492
4. Gasecka A, Konwerski M, Pordzik J, Soplińska A, Filipiak KJ, Siller-Matula JM, et al. Switching between P2Y antagonists - From bench to bedside. Vascul Pharmacol. (2019) 115:1–12. doi: 10.1016/j.vph.2019.01.003
5. Komosa A, Lesiak M, Krasiński Z, Grygier M, Siniawski A, Skorupski W, et al. Optimal timing of P2Y12 inhibitor loading in patients undergoing PCI: a meta-analysis. Thromb Haemost. (2019) 119:1000–20. doi: 10.1055/s-0039-1683421
6. Gue YX, Spinthakis N, Farag M, Kubica J, Siller-Matula JM, Srinivasan M, et al. Impact of preadmission morphine on reinfarction in patients with ST-elevation myocardial infarction treated with percutaneous coronary intervention: a meta-analysis. Clin Pharmacol Ther. (2020) 108:54–62. doi: 10.1002/cpt.1798
7. Kubisa M, Jezewski MP, Gasecka A, Siller-Matula J, Postuła M. Ticagrelor – toward more efficient platelet inhibition and beyond. Ther Clin Risk Manag. (2018) 14:129–40. doi: 10.2147/TCRM.S152369
8. Eyileten C, Postula M, Jakubik D, Toma A, Mirowska-Guzel D, Patti G, et al. Non-Vitamin K Oral Anticoagulants (NOAC) versus Vitamin K Antagonists (VKA) for atrial fibrillation with elective or urgent percutaneous coronary intervention: a meta-analysis with a particular focus on combination type. J Clin Med. (2020) 9:1120. doi: 10.3390/jcm9041120
9. Siller-Matula J, Lang IM, Schoergenhofer C, Roest M, Jilma B. Interdependence between osteoprotegerin and active von Willebrand factor in long-term cardiovascular mortality prediction in patients undergoing percutaneous coronary intervention. Thromb Haemost. (2017) 117:1730–8. doi: 10.1160/TH17-02-0087
10. Tang WHW, Wilson Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. (2017) 120:1183–96. doi: 10.1161/CIRCRESAHA.117.309715
11. Cheung W, Keski-Rahkonen P, Assi N, Ferrari P, Freisling H, Rinaldi S, et al. A metabolomic study of biomarkers of meat and fish intake. Am J Clin Nutr. (2017) 105:600–8. doi: 10.3945/ajcn.116.146639
12. Mittermayer F, Krzyzanowska K, Exner M, Mlekusch W, Amighi J, Sabeti S, et al. Asymmetric dimethylarginine predicts major adverse cardiovascular events in patients with advanced peripheral artery disease. Arterioscler Thromb Vasc Biol. (2006) 26:2536–40. doi: 10.1161/01.ATV.0000242801.38419.48
13. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. (2013) 368:1575–84. doi: 10.1056/NEJMoa1109400
14. Tang WW, Wang Z, Li XS, Fan Y, Li DS, Wu Y, et al. Increased Trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. (2017) 63:297–306. doi: 10.1373/clinchem.2016.263640
15. Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. (2015) 116:448–55. doi: 10.1161/CIRCRESAHA.116.305360
16. Winther SA, Øllgaard JC, Tofte N, Tarnow L, Wang Z, Ahluwalia TS, et al. Utility of plasma concentration of Trimethylamine N-oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Diabetes Care. (2019) 42:1512–20. doi: 10.2337/dc19-0048
17. Königsbrügge O, Schmaldienst S, Auinger M, Klauser-Braun R, Lorenz M, Tabernig S, et al. Antithrombotic agents for primary and secondary prevention of cardiovascular events in patients with end-stage renal disease on chronic hemodialysis. Atherosclerosis. (2020) 298:1–6. doi: 10.1016/j.atherosclerosis.2020.02.011
18. Tripolt NJ, Kolesnik E, Pferschy PN, Verheyen N, Ablasser K, Sailer S, et al. Impact of EMpagliflozin on cardiac function and biomarkers of heart failure in patients with acute MYocardial infarction—The EMMY trial. Am Heart J. (2020) 221:39–47. doi: 10.1016/j.ahj.2019.12.004
19. Gelbenegger G, Postula M, Pecen L, Halvorsen S, Lesiak M, Schoergenhofer C, et al. Aspirin for primary prevention of cardiovascular disease: a meta-analysis with a particular focus on subgroups. BMC Med. (2019) 17:1–6. doi: 10.1186/s12916-019-1428-0
20. Shan Z, Sun T, Huang H, Chen S, Chen L, Luo C, et al. Association between microbiota-dependent metabolite trimethylamine- N -oxide and type 2 diabetes. Am J Clin Nutr. (2017) 106:888–94. doi: 10.3945/ajcn.117.157107
21. Dambrova M, Latkovskis G, Kuka J, Strele I, Konrade I, Grinberga S, et al. Diabetes is associated with higher trimethylamine N-oxide plasma levels. Exp Clin Endocrinol Diabetes. (2016) 124:251–6. doi: 10.1055/s-0035-1569330
22. Winter M-P, von Lewinski D, Wallner M, Prüller F, Kolesnik E, Hengstenberg C, et al. Incidence, predictors, and prognosis of premature discontinuation or switch of prasugrel or ticagrelor: the ATLANTIS - SWITCH study. Sci Rep. (2019) 9:8194. doi: 10.1038/s41598-019-44673-7
23. Siller-Matula JM, Akca B, Neunteufl T, Maurer G, Lang IM, Kreiner G, et al. Inter-patient variability of platelet reactivity in patients treated with prasugrel and ticagrelor. Platelets. (2016) 27:373–7. doi: 10.3109/09537104.2015.1095874
24. Siller-Matula JM, Hintermeier A, Kastner J, Kreiner G, Maurer G, Kratochwil C, et al. Distribution of clinical events across platelet aggregation values in all-comers treated with prasugrel and ticagrelor. Vascul Pharmacol. (2016) 79:6–10. doi: 10.1016/j.vph.2016.01.003
25. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). Glob Heart. (2018) 72:2231–64. doi: 10.1016/j.jacc.2018.08.1038
26. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, et al. 2017 Cardiovascular and stroke endpoint definitions for clinical trials. Circulation. (2018) 137:961–72. doi: 10.1161/CIRCULATIONAHA.117.033502
27. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJB, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
28. Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. (1980) 58:113–30.
29. Alpert JS, Thygesen K, Jaffe A, White HD. The universal definition of myocardial infarction: a consensus document. Heart. (2008) 94:1335–41. doi: 10.1136/hrt.2008.151233
30. Jaworska K, Hering D, Mosieniak G, Bielak-Zmijewska A, Pilz M, Konwerski M, et al. TMA, a forgotten uremic toxin, but not TMAO, is involved in cardiovascular pathology. Toxins. (2019) 11:490. doi: 10.3390/toxins11090490
31. Jaworska K, Huc T, Samborowska E, Dobrowolski L, Bielinska K, Gawlak M, et al. Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PLoS ONE. (2017) 12:e0189310. doi: 10.1371/journal.pone.0189310
32. Missailidis C, Hällqvist J, Qureshi AR, Barany P, Heimbürger O, Lindholm B, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS ONE. (2016) 11:e0141738. doi: 10.1371/journal.pone.0141738
33. Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Wilson Tang WH, et al. Intestinal microbiota-generated metabolite trimethylamine- N- oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc. (2016) 5:e002816. doi: 10.1161/JAHA.115.002816
34. Trøseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. (2015) 277:717–26. doi: 10.1111/joim.12328
35. Roncal C, Martínez-Aguilar E, Orbe J, Ravassa S, Fernandez-Montero A, Saenz-Pipaon G, et al. Trimethylamine-N-oxide (TMAO) predicts cardiovascular mortality in peripheral artery disease. Sci Rep. (2019) 9:15580. doi: 10.1038/s41598-019-52082-z
36. Ottiger M, Nickler M, Steuer C, Bernasconi L, Huber A, Christ-Crain M, et al. Gut, microbiota-dependent trimethylamine-N-oxide is associated with long-term all-cause mortality in patients with exacerbated chronic obstructive pulmonary disease. Nutrition. (2018) 45:135–41.e1. doi: 10.1016/j.nut.2017.07.001
37. Zhou X, Jin M, Liu L, Yu Z, Lu X, Zhang H. Trimethylamine N-oxide and cardiovascular outcomes in patients with chronic heart failure after myocardial infarction. ESC Heart Failure. (2020) 7:189–94. doi: 10.1002/ehf2.12552
38. Sikora M, Kiss N, Stec A, Giebultowicz J, Samborowska E, Jazwiec R, et al. Trimethylamine N-oxide, a gut microbiota-derived metabolite, is associated with cardiovascular risk in psoriasis: a cross-sectional pilot study. Dermatol Ther. (2021) 13:1–3. doi: 10.1007/s13555-021-00547-3
39. Li XS, Obeid S, Wang Z, Hazen BJ Li L, Wu Y, et al. Trimethyllysine, a trimethylamine N-oxide precursor, provides near- and long-term prognostic value in patients presenting with acute coronary syndromes. Eur Heart J. (2019) 40:2700–9. doi: 10.1093/eurheartj/ehz259
40. Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Räber L, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. (2017) 38:814–24. doi: 10.1093/eurheartj/ehw582
41. Heianza Y, Ma W, Manson JE, Rexrode KM Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. (2017) 6:e004947. doi: 10.1161/JAHA.116.004947
42. Qi J, You T, Li J, Pan T, Xiang L, Han Y, et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. (2018) 22:185–94. doi: 10.1111/jcmm.13307
43. Yao M-E, Liao P-D, Zhao X-J, Wang L. Trimethylamine-N-oxide has prognostic value in coronary heart disease: a meta-analysis and dose-response analysis. BMC Cardiovasc Disord. (2020) 20:7. doi: 10.1186/s12872-019-01310-5
44. Croyal M, Saulnier P-J, Aguesse A, Gand E, Ragot S, Roussel R, et al. Plasma Trimethylamine N-oxide and risk of cardiovascular events in patients with type 2 diabetes. J Clin Endocrinol Metab. (2020) 105:2371–80. doi: 10.1210/clinem/dgaa188
45. Winther SA, Øllgaard JC, Hansen TW, von Scholten BJ, Reinhard H, Ahluwalia TS, et al. Plasma trimethylamine N-oxide and its metabolic precursors and risk of mortality, cardiovascular and renal disease in individuals with type 2-diabetes and albuminuria. PLoS ONE. (2021) 16:e0244402. doi: 10.1371/journal.pone.0244402
46. Gencer B, Li XS, Gurmu Y, Bonaca MP, Morrow DA, Cohen M, et al. Gut microbiota-dependent trimethylamine N-oxide and cardiovascular outcomes in patients with prior myocardial infarction: a nested case control study from the PEGASUS-TIMI 54 trial. J Am Heart Assoc. (2020) 9:e015331. doi: 10.1161/JAHA.119.015331
47. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. (2017) 38:2948–56. doi: 10.1093/eurheartj/ehx342
48. Schugar RC, Shih DM, Warrier M, Helsley RN, Burrows A, Ferguson D, et al. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the Beiging of white adipose tissue. Cell Rep. (2017) 20:279. doi: 10.1016/j.celrep.2017.06.053
49. Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, et al. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS ONE. (2014) 9:e114969. doi: 10.1371/journal.pone.0114969
50. Cardona A, O'Brien A, Bernier MC, Somogyi A, Wysocki VH, Smart S, et al. Trimethylamine N-oxide and incident atherosclerotic events in high-risk individuals with diabetes: an ACCORD trial post hoc analysis. BMJ Open Diab Res Care. (2019) 7:e000718. doi: 10.1136/bmjdrc-2019-000718
51. Geng J, Yang C, Wang B, Zhang X, Hu T, Gu Y, et al. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed Pharmacother. (2018) 97:941–7. doi: 10.1016/j.biopha.2017.11.016
52. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. (2011) 472:57–63. doi: 10.1038/nature09922
53. Collins HL, Drazul-Schrader D, Sulpizio AC, Koster PD, Williamson Y, Adelman SJ, et al. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE–/– transgenic mice expressing CETP. Atherosclerosis. (2016) 244:29–37. doi: 10.1016/j.atherosclerosis.2015.10.108
54. Aldana-Hernández P, Leonard K-A, Zhao Y-Y, Curtis JM, Field CJ, Jacobs RL. Dietary choline or trimethylamine N-oxide supplementation does not influence atherosclerosis development in Ldlr-/- and Apoe-/- male mice. J Nutr. (2020) 150:249–55. doi: 10.1093/jn/nxz214
55. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. (2016) 165:111–24. doi: 10.1016/j.cell.2016.02.011
56. Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc. (2016) 5:e002767. doi: 10.1161/JAHA.115.002767
57. Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. (2015) 56:22–37. doi: 10.1194/jlr.M051680
58. Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. (2014) 118:476–81. doi: 10.1016/j.jbiosc.2014.03.001
59. Obeid R, Awwad HM, Rabagny Y, Graeber S, Herrmann W, Geisel J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. (2016) 103:703–11. doi: 10.3945/ajcn.115.121269
60. Querio G, Antoniotti S, Levi R, Gallo MP. Trimethylamine N-oxide does not impact viability, ROS production, and mitochondrial membrane potential of adult rat cardiomyocytes. Int J Mol Sci. (2019) 20:3045. doi: 10.3390/ijms20123045
61. Oakley CI, Vallejo JA, Wang D, Gray MA, Tiede-Lewis LM, Shawgo T, et al. Trimethylamine–oxide acutely increases cardiac muscle contractility. Am J Physiol Heart Circ Physiol. (2020) 318:H1272–82. doi: 10.1152/ajpheart.00507.2019
62. Huc T, Drapala A, Gawrys M, Konop M, Bielinska K, Zaorska E, et al. Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am J Physiol Heart Circ Physiol. (2018) 315:H1805–20. doi: 10.1152/ajpheart.00536.2018
63. Gawrys-Kopczynska M, Konop M, Maksymiuk K, Kraszewska K, Derzsi L, Sozanski K, et al. TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. Elife. (2020) 9:e57028. doi: 10.7554/eLife.57028
Keywords: trimethylamine N-oxide, acute coronary syndrome, type 2 diabetes mellitus, cardiovascular mortality, cardiovascular disease
Citation: Eyileten C, Jarosz-Popek J, Jakubik D, Gasecka A, Wolska M, Ufnal M, Postula M, Toma A, Lang IM and Siller-Matula JM (2021) Plasma Trimethylamine-N-Oxide Is an Independent Predictor of Long-Term Cardiovascular Mortality in Patients Undergoing Percutaneous Coronary Intervention for Acute Coronary Syndrome. Front. Cardiovasc. Med. 8:728724. doi: 10.3389/fcvm.2021.728724
Received: 21 June 2021; Accepted: 04 October 2021;
Published: 29 October 2021.
Edited by:
Simon W. Rabkin, University of British Columbia, CanadaReviewed by:
Jes-niels Boeckel, University Hospital Leipzig, GermanyCopyright © 2021 Eyileten, Jarosz-Popek, Jakubik, Gasecka, Wolska, Ufnal, Postula, Toma, Lang and Siller-Matula. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jolanta M. Siller-Matula, Jolanta.siller-matula@meduniwien.ac.at; Marcin Ufnal, mufnal@wum.edu.pl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.