
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 29 October 2021
Sec. Heart Failure and Transplantation
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.656536
 Yuxi Sun1†
Yuxi Sun1† Jinping Si1
Jinping Si1 Jiaxin Li1
Jiaxin Li1 Mengyuan Dai1
Mengyuan Dai1 Emma King2
Emma King2 Xinxin Zhang1
Xinxin Zhang1 Yanli Zhang1
Yanli Zhang1 Yunlong Xia1
Yunlong Xia1 Gary Tse1,2,3*
Gary Tse1,2,3* Ying Liu1*
Ying Liu1*Aims: HFA-PEFF score has been proposed for diagnosing heart failure with preserved ejection fraction (HFpEF). Currently, there are only a limited number of tools for predicting the prognosis. In this study, we evaluated whether the HFA-PEFF score can predict mortality in patients with HFpEF.
Methods: This single-center, retrospective observational study enrolled patients diagnosed with HFpEF at the First Affiliated Hospital of Dalian Medical University between January 1, 2015, and April 30, 2018. The subjects were divided according to their HFA-PEFF score into low (0–2 points), intermediate (3–4 points), and high (5–6 points) score groups. The primary outcome was all-cause mortality.
Results: A total of 358 patients (mean age: 70.21 ± 8.64 years, 58.1% female) were included. Of these, 63 (17.6%), 156 (43.6%), and 139 (38.8%) were classified into the low, intermediate, and high score groups, respectively. Over a mean follow-up of 26.9 months, 46 patients (12.8%) died. The percentage of patients who died in the low, intermediate, and high score groups were 1 (1.6%), 18 (11.5%), and 27 (19.4%), respectively. A multivariate Cox regression identified HFA-PEFF score as an independent predictor of all-cause mortality [hazard ratio (HR):1.314, 95% CI: 1.013–1.705, P = 0.039]. A Cox analysis demonstrated a significantly higher rate of mortality in the intermediate (HR: 4.912, 95% CI 1.154–20.907, P = 0.031) and high score groups (HR: 5.291, 95% CI: 1.239–22.593, P = 0.024) than the low score group. A receiver operating characteristic (ROC) analysis indicated that the HFA-PEFF score can effectively predict all-cause mortality after adjusting for age and New York Heart Association (NYHA) class [area under the curve (AUC) 0.726, 95% CI 0.651–0.800, P = 0.000]. With an HFA-PEFF score cut-off value of 3.5, the sensitivity and specificity were 78.3 and 54.8%, respectively. The AUC on ROC analysis for the biomarker component of the score was similar to that of the total score.
Conclusions: The HFA-PEFF score can be used both to diagnose HFpEF and predict the prognosis. The higher scores are associated with higher all-cause mortality.
Heart failure (HF) represents the final common pathway of different cardiac diseases. It is a rising global epidemic with an estimated prevalence of >37.7 million individuals globally (1). The patients with HF experience various symptoms, including dyspnea, poor exercise tolerance, and fluid retention, and have a poor long-term prognosis. The latest European Society of Cardiology (ESC) guidelines divided HF involving left ventricular ejection fraction (LVEF) into HF with reduced ejection fraction (HFrEF), HF with mid-range ejection fraction (HFmrEF), and HF with preserved ejection fraction (HFpEF) (2). Previous work reported that in the general population aged ≥ 60 years, 4.9% were identified as having HFpEF in Europe (3). This number is expected to increase with an aging population (4). Indeed, the HFpEF is usually considered to evolve from a combination of risk factors and comorbidities, including advanced age, female gender, obesity, hypertension, diabetes mellitus, renal dysfunction, anemia, iron deficiency, sleep disorders, and chronic obstructive pulmonary disease (5–8). Major mechanisms affecting the myocardium in HFpEF include left atrial hypertension, pulmonary hypertension, plasma volume expansion, systemic microvascular inflammation, cardiometabolic functional abnormalities, and cellular/extracellular structural abnormalities (9). It must be pointed out that before the publication of the PARALLAX trial, there was no convincing evidence-based strategy that has been shown to improve prognosis in patients with HFpEF.
Pieske et al. proposed the HFA-PEFF diagnostic algorithm for HFpEF in 2019 (10). Two points were allocated for major criteria and one point was allocated for minor criteria in the functional, morphological, and biomarker domains. The calculations and interpretations are as follows: ≤ 1 point (HFpEF unlikely); 2–4 points (diagnostic uncertainty and need further evaluation) and ≥5 points (definite HFpEF). While this score has been well-validated for the diagnosis of HFpEF, its relevance to the prognosis remains unclear. Therefore, we further investigated the predictive value of the score in this cohort study of the patients with HFpEF.
The HFA-PEFF diagnostic algorithm contains four consecutive steps. Step 1: initial workup, including assessment of the symptoms and signs of HF, the comorbidities or risk factors, ECG, echocardiography, natriuretic peptides, 6 min walking test, or cardiopulmonary exercise testing. Step 2: diagnostic workup, including echocardiography, and natriuretic peptide score. Step 3: advanced workups (functional testing in the case of uncertainty), such as exercise stress echocardiography and invasive hemodynamic measurements. Step 4: etiological workup, comprising cardiovascular magnetic resonance, cardiac or non-cardiac biopsies, scintigraphy or CT or PET, genetic testing, and specific laboratory tests. The specific echocardiographic variables include mitral annular early diastolic velocity (e'), left ventricular (LV) filling pressure (E/e'), left atrial volume index, LV mass index, LV relative wall thickness, tricuspid regurgitation velocity, LV global longitudinal systolic strain, and natriuretic peptide levels. Additionally, they recommended a scoring system based on the functional, morphological and biomarker domains (Table 1). If any major criterion from this domain is positive, this domain can contribute 2 points; if no major but any minor criterion is positive, this domain contributes 1 point. If several major criteria within a domain are positive, this domain still contributes 2 points; and if no major, but several minor criteria are positive the contribution is still 1 point. Notably, the HFA-PEFF score can be calculated even if all the parameters are not obtained, which can add to the utility of the score in clinical practice.
This study was a retrospective, single-center, observational study, and was approved by the institutional review board of Dalian Medical University, Liaoning, China. All the procedures were conducted in accordance with the Declaration of Helsinki and its amendments. In this study, 856 consecutive patients with HFpEF were hospitalized at the First Affiliated Hospital of Dalian Medical University between January 1, 2015, and April 30, 2018. The exclusion criteria were missing echocardiographic results, loss to follow-up, severe valvular heart disease, or end-stage renal failure. The cohort was divided into three groups based on the score: low (0–2 points), intermediate (3–4 points), and high (5–6 points) score groups.
Heart failure with preserved ejection fraction was defined according to the ESC guidelines: (i) symptoms or signs; (ii) LVEF ≥ 50%; (iii) elevated levels of natriuretic peptides, and at least one of the additional criteria: (1) relevant structural heart disease (LV or left atrial enlargement); (2) diastolic dysfunction (2). Hypertension was defined as a recorded blood pressure ≥ 140/90 mmHg or any prescription of antihypertensive medications (11). Diabetes mellitus was defined as treatment with any antidiabetic medication, fasting plasma glucose ≥ 7 mmol/L, or HbA1C ≥ 6.5%, or the presence of symptoms of diabetes and a random plasma glucose concentration ≥ 11.1 mmol/L (12).
Details of the clinical characteristics, drug therapy, comorbidities, biomarker assessment, arrhythmias, and echocardiography findings were collected and recorded from Yidu Cloud. The laboratory indicators were measured on admission, with a requirement to fast for more than 8 h before venous blood collection. All the subjects underwent dynamic electrocardiography to record various arrhythmias. Echocardiography was performed with the patients at rest, before discharge by the experienced cardiologists.
The primary outcome was all-cause mortality. After discharge, all the patients were required to return to the outpatient department regularly. If the patients did not attend their scheduled clinic appointments, they were interviewed by telephone annually. The cutoff was November 30, 2018, or the occurrence of death.
Statistical analysis was performed with Statistical Package for Social Sciences, version 24.0 (SPSS Inc., Chicago, IL, USA). Counting data were expressed as percentages (%), and the chi-squared test was used for comparison among the three groups. Measurement data with non-normal distribution were expressed as the median (interquartile range), and the Kruskal–Wallis test was used to assess the differences among the groups. The quantitative variables of normal distribution were expressed as arithmetic means ± SDs (x ± s), and ANOVA was used for between-group comparisons. A Kaplan–Meier analysis was performed to calculate the cumulative incidence of all-cause mortality, and the log-rank test was used to compare the differences. A Cox regression analysis was used to investigate the risk factors of adverse events in the patients with HFpEF. The HRs with 95% CIs were presented. A ROC curve was constructed from logistic regression to assess the availability of the HFA-PEFF score to predict the all-cause mortality in the patients with HFpEF. All the values were two-tailed, and a p-value <0.05 was considered statistically significant.
A total of 856 consecutive patients with HFpEF who were hospitalized at the First Affiliated Hospital of Dalian Medical University between January 1, 2015, and April 30, 2018, were initially identified. Of these, 498 patients were excluded due to missing echocardiographic results (n = 248), loss to follow-up (n = 110), severe valvular heart disease (n = 75), or end-stage renal failure (n = 65). In the remaining cohort, the percentages of the low, intermediate, and high score groups were 63 (17.6%), 156 (43.6%), and 139 (38.8%), respectively. The study flowchart is shown in Figure 1.
The mean age of the subjects was 70.21 ± 8.64 years, with 58.1% female. The baseline characteristics are shown in Table 2. Overall, the patients in the high score group had a faster heart rate (P < 0.001), were more likely to have a history of atrial fibrillation (AF)/flutter (P < 0.001), more often took medications, such as loop diuretics (P = 0.039) and digoxin (P = 0.025). In contrast, the patients in the low score group had a heavier burden of myocardial infarction (MI) (P = 0.020), higher surgical rates of percutaneous coronary intervention (PCI) (P = 0.043), and more often took medicines, such as statin (P = 0.011) and antiplatelet drugs (P < 0.001). In terms of laboratory data, the patients in the high score group had a higher level of B-type natriuretic peptide (BNP) (P < 0.001), high sensitivity troponin (hs-TNI) (P < 0.001), and serum potassium (P = 0.025) but lower level of hemoglobin (P = 0.010), triglyceride (P = 0.013), cholesterol (P = 0.002), high density lipoprotein cholesterol (HDL-C) (P < 0.001), and low density lipoprotein cholesterol (LDL-C) (P = 0.023) compared with those in the low and intermediate score groups. Interestingly, the discrepancy in the natriuretic peptides was mainly observed in the patients with sinus rhythm (SR) rather than those with AF. Regarding echocardiographic findings, the high score group had higher values of left atrial volume index (LAVI) (P < 0.001), LV wall thickness (P = 0.01), LV end diastolic diameter (P < 0.001), mitral doppler early velocity/mitral annular early velocity (E/e') (P < 0.001), and lower early to late diastolic transmitral flow velocity (E/A) (P < 0.001) than the low and intermediate groups. Furthermore, the patients with AF had higher LAVI compared with those with SR. By contrast, no statistical differences among the three groups were observed for left ventricular mass index (LVMI) and relative wall thickness (RWT). In the subgroup analysis of LVMI, the intermediate group had the highest value than the low and high score groups in the male (P = 0.005), mainly due to the higher frequency of hypertrophic cardiomyopathy.
In the functional domain which included echocardiographic findings, nearly 50% of the patients received 1 point, whereas the majority of patients in the cohort met the major criterion in both the morphological and biomarker domains (Figure 2).
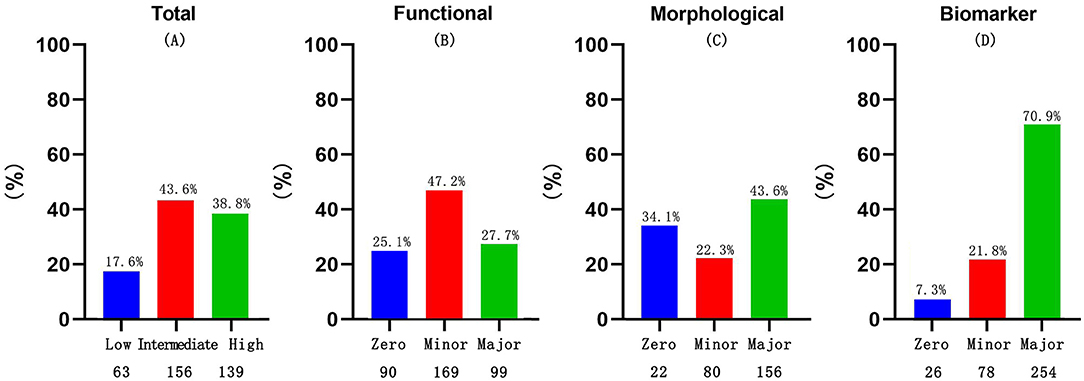
Figure 2. Discriminates between the major and minor criteria for total HFA-PEFF score categories (A), functional (B), morphological (C) and biomarker sub-scores (D), among the cohort.
The patients were followed up for an average of 26.9 ± 11.1 months. Of the cohort, 46 patients died (12.8%), with rates for the low, intermediate, and high score groups of 1 (1.6%), 18 (11.5%), and 27 (19.4%), respectively. A Kaplan–Meier analysis was performed and the log-rank test showed the mortality rate of the high score group was significantly higher than the low (1.6%) and intermediate score groups (11.5%) (P < 0.001) (Figure 3). During the follow-up period, the mortality rate of the three groups significantly differed at 12 and 24 months following the discharge (P = 0.025) (Supplementary Table 1).
The multivariate Cox regression demonstrated that age (HR: 1.086, 95% CI: 1.029–1.146, P = 0.003), diabetes (HR: 2.915, 95% CI: 1.428–5.948, P = 0.003), UA (HR: 1.004, 95% CI: 1.002–1.006, P = 0.000), d-dimer (HR: 1.000, 95% CI: 1.000–1.000, P = 0.003), and HFA-PEFF score (HR: 1.314, 95% CI: 1.013–1.705, P = 0.039) were significant predictors of the all-cause mortality after being fully adjusted (Table 3). The intermediate score group (HR: 4.912, 95% CI 1.154–20.907, P = 0.031) and high score group (HR: 5.291, 95% CI: 1.239–22.593, P = 0.024) showed a higher risk of all-cause death compared with the low score group after adjustment (Table 4).
Receiver operating characteristics analysis was constructed to evaluate the availability of HFA-PEFF score to predict the all-cause mortality in the patients with HFpEF, with an area under the curve (AUC) of 0.726 (95% CI: 0.651–0.080, P = 0.000) after adjusting for age and New York Heart Association (NYHA) class. The sensitivity and specificity of the HFA-PEFF score at the cut-off of 3.5 were 78.3 and 54.8%, respectively. Additionally, the functional sub-score showed relatively little prognostic value, whereas the biomarker sub-score reached an area under the curve similar to that of the total score, which was considered as the most significant parameter among the three sub-scores (Figure 4).
The main findings of this study are that: (1) HFA-PEFF score is not only a valuable diagnostic tool for HFpEF but can also effectively predict the prognosis. (2) A Cox regression analysis identified the HFA-PEFF score as an independent predictor of the all-cause mortality in the patients with HFpEF, and higher scores are associated with a higher incidence of adverse events. (3) The optimum cut-off value of the HFA-PEFF score is 3.5 for all-cause mortality.
To date, the mortality of patients with HFpEF has been investigated in the clinical trials (13–16), observational studies (17, 18), and meta-analyses (19). However, the estimates of mortality varied considerably mainly due to the difference in the study design, baseline risk of the study population, and EF cut-off value used to define HFpEF (20). In-hospital, the mortality ranges from 2.4 (21) to 4.9% (22), with slightly higher 30-day (5%) (17) and 60–90 day (9.5%) (23) mortality. By 1-year post-diagnosis, the mortality varied from 20 to 29% (17, 19, 22, 24). By 5 years, approximately half of all the patients with HFpEF have died, with an estimated mortality ranging from 53 to 74% (19, 24–26). Currently, the cardiovascular causes remain a significant contributor to the mortality in patients with HFpEF (27). In this research, only 12.8% of patients with HFpEF died over ~2 years of follow-up, which is lower than the percentage reported elsewhere. This discrepancy may be attributed to the different baseline characteristics of prior cohorts, namely, older age, higher comorbidity burden, and worse cardiac function with frailer patients. These characteristics would predispose to the patients to higher rates of death (28–33).
Heart failure with preserved ejection fraction is often accompanied by multiple cardiac and non-cardiac comorbidities, making the diagnosis and treatment more difficult. These comorbidities are often associated with a higher incidence of various adverse outcomes in HFpEF. In this cohort, the enrolled subjects also suffered a high burden of various comorbidities: hypertension (80.7%), diabetes (48.9%), AF (53.7%), and MI (23.5%). Surprisingly, these common comorbidities showed no positive correlation with all-cause death after being fully adjusted, except for diabetes. AF and HFpEF commonly coexist and share common epidemiology, pathophysiology, pathogenesis, and risk factors (34). Both the prevalence and incidence of AF are associated with the increased mortality in HFpEF (35). In this research, AF was not positively correlated with the all-cause mortality after adjustment, which is probably due to the relatively small sample and short follow-up period. Diabetes mellitus can increase the risk of morbidity and mortality in patients with chronic HF, and the negative prognostic association may be greater in HFpEF than in HFrEF. Diabetes mellitus in HFpEF may be accompanied by poorer functional status and lower exercise capacity, increased markers of inflammation/fibrosis/endothelial dysfunction, worse congestion, higher LV filling pressures, and increased mortality and HF admissions. The potential pathophysiological mechanisms include sodium retention, volume overload, metabolic disorders, systemic inflammation, poor skeletal muscle function, impaired peripheral oxygen delivery, and chronotropic incompetence (36).
A number of factors could affect the prognosis of HFpEF (37–41). LV diastolic dysfunction plays a central role in the pathophysiology of HFpEF, defined as an impairment in relaxation or an increase in stiffness. LV diastolic dysfunction can cause an elevation in LV filling pressure and promote the symptoms of dyspnea, can impair exercise capacity, and increase the risk of hospitalization and death (42, 43). In addition to LV diastolic dysfunction, multiple non-diastolic abnormalities may influence the prognosis of HFpEF, such as pulmonary hypertension (PH), right ventricular (RV) dysfunction, elevated plasma natriuretic peptide levels, and increased red cell distribution width (RDW). PH is common in HFpEF, seen in nearly 80% of the patients, and mortality is increased in this cohort (44). Pulmonary congestion is an important factor causing PH. Coiro et al. reported that the development of pulmonary congestion during exercise, as easily assessed by lung ultrasonography, is an independent predictor of cardiovascular death and heart failure rehospitalization in patients with HFpEF. In their research, the patients with B-line change > 10 or peak B-lines > 10, a semiquantitative indicator evaluating increased extravascular lung water, experienced a higher risk of adverse outcomes during the follow-up (45). Continued stimulation of PH can inescapably lead to RV systolic dysfunction, which is common and associated with adverse outcomes in patients with HFpEF (44). Recent research demonstrated the response of the coupling of RV to PH is significantly important and can be assessed by the ratio of tricuspid annular plane systolic excursion (TAPSE) and right ventricular systolic pressure (RVSP), a lower ratio always implies adverse events in HFpEF (46). The natriuretic peptides biomarkers (BNP and NT-proBNP) are hormones released in response to the increased cardiomyocyte stretch. The normal levels have been traditionally used to exclude the diagnosis of HF for patients presenting with dyspnea. Increased levels, by contrast, have been associated with a worse prognosis in HF. For example, elevated levels of BNP are associated with higher risks of mortality and HF rehospitalization both in the inpatients and outpatients over a follow-up of 12 months (47). RDW represents the variability of sizes of circulating erythrocytes and can be measured in a complete blood count. Recently, RDW has received more attention and has been known as an available biomarker to evaluate various cardiovascular disorders (48–51). A higher level of RDW has been associated with increased all-cause mortality in patients with acute HF with preserved but not with reduced LVEF (52). In addition, Imai et al. reported RDW levels at admission can independently predict the poor outcomes caused by non-cardiac events in the patients with acute decompensated HFpEF (53).
Before the publication of the PARALLAX trial, there were no definitive treatments proven to improve the prognosis of patients with HFpEF (54, 55). In 2020, ESC recommended the use of sacubitril/valsartan for HF across the full ejection fraction spectrum. The investigators found that sacubitril/valsartan at the target dose reduced the incidence of the first hospitalization due to HF and composite of time to death due to cardiac failure or HF hospitalization, as well as delaying estimated glomerular filtration rate (eGFR) decline. Additionally, regular sacubitril/valsartan use significantly reduced the level of NT-proBNP from the fourth week after taking the drug, indicating improvement of cardiac function. While these findings are encouraging, the subjects involved in the study were those with ejection fraction > 40% rather than ≥50%. Aside from the pharmacological treatment, there is evidence that aerobic exercise can improve the peak oxygen consumption and quality of life in patients with HFpEF (56). Recently, Ge et al. introduced a clinical phenotypic classification of HFpEF (57), which provides a better understanding of the risk factors, etiology, pathophysiology, and clinical course of HFpEF and contributes to guiding the targeted treatment. In the near future, targeting treatment to etiology and comorbidities may be another good choice in the treatment of patients with HFpEF (58). Machine learning (ML) algorithms have been used to identify the latent features otherwise not amenable to detection by the conventional techniques, and they have demonstrated promising utility for the diagnosis, management, and prediction of endpoints in HF. It is anticipated that they are more easily translated for clinical use in the near future (59).
Nevertheless, this study has several limitations. First, this was a retrospective, single-center study with a small number of subjects. Second, some functional parameters, such as mitral annular early diastolic velocity (e'), tricuspid regurgitation velocity, and LV global longitudinal systolic strain, were not routinely performed in our center and were only available in a small number of patients. Thus, these functional variables could not be explored as the potential prognostic factors, which may influence the predictive accuracy of functional sub-score.
The HFA-PEFF score can be used to assess prognosis in the patients with HFpEF, and higher scores are associated with higher all-cause mortality.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Dalian Medical University. The Ethics Committee waived the requirement of written informed consent for participation.
YS was responsible for collecting clinical data and writing the paper. JS and JL assisted YS in collecting data and conducting telephone follow-up. MD, YZ, and XZ were responsible for the statistical analysis. EK helped address reviewers' comments. YX, YL, and GT were responsible for revising the paper and determining the research direction. All authors were involved in the drafting or revision of the manuscript.
This work was supported in part by the National Science Foundation of China (No. 82170385, Recipient: YL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.656536/full#supplementary-material
1. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. (2011) 8:30–41. doi: 10.1038/nrcardio.2010.165
2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
3. Van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. (2016) 18:242–52. doi: 10.1002/ejhf.483
4. Seferovic PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2018) 20:853–72. doi: 10.1002/ejhf.1170
5. Little WC, Zile MR. HFpEF: cardiovascular abnormalities not just comorbidities. Circ Heart Fail. (2012) 5:669–71. doi: 10.1161/CIRCHEARTFAILURE.112.972265
6. Triposkiadis F, Giamouzis G, Parissis J, Starling RC, Boudoulas H, Skoularigis J, et al. Reframing the association and significance of co-morbidities in heart failure. Eur J Heart Fail. (2016) 18:744–58. doi: 10.1002/ejhf.600
7. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC heart failure long-term registry. Eur J Heart Fail. (2017) 19:1574–85. doi: 10.1002/ejhf.813
8. Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. (2018) 138:198–205. doi: 10.1161/CIRCULATIONAHA.118.034271
9. Lam CSP, Voors AA, De Boer RA, Solomon SD, Van Veldhuisen DJ. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J. (2018) 39:2780–92. doi: 10.1093/eurheartj/ehy301
10. Pieske B, Tschope C, De Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. (2019) 40:3297–317. doi: 10.1093/eurheartj/ehz641
11. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. (2016) 134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912
12. Petersmann A, Muller-Wieland D, Muller UA, Landgraf R, Nauck M, Freckmann G, et al. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. (2019) 127:S1–7. doi: 10.1055/a-1018-9078
13. Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. (2010) 121:1393–405. doi: 10.1161/CIRCULATIONAHA.109.909614
14. Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. (2013) 309:781–91. doi: 10.1001/jama.2013.905
15. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. (2013) 309:1268–77. doi: 10.1001/jama.2013.2024
16. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. (2014) 370:1383–92. doi: 10.1056/NEJMoa1313731
17. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. (2006) 355:260–9. doi: 10.1056/NEJMoa051530
18. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. (2015) 175:996–1004. doi: 10.1001/jamainternmed.2015.0924
19. Meta-Analysis Global Group in Chronic Heart Failure. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. (2012) 33:1750–7. doi: 10.1093/eurheartj/ehr254
20. Somaratne JB, Berry C, Mcmurray JJ, Poppe KK, Doughty RN, Whalley GA. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail. (2009) 11:855–62. doi: 10.1093/eurjhf/hfp103
21. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. (2012) 126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770
22. Perez De Isla L, Canadas V, Contreras L, Almeria C, Rodrigo JL, Aubele AL, et al. Diastolic heart failure in the elderly: in-hospital and long-term outcome after the first episode. Int J Cardiol. (2009) 134:265–70. doi: 10.1016/j.ijcard.2007.12.059
23. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J Am Coll Cardiol. (2007) 50:768–77. doi: 10.1016/j.jacc.2007.04.064
24. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. (2006) 355:251–9. doi: 10.1056/NEJMoa052256
25. Tribouilloy C, Rusinaru D, Mahjoub H, Souliere V, Levy F, Peltier M, et al. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. (2008) 29:339–47. doi: 10.1093/eurheartj/ehm554
26. Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. (2009) 119:3070–7. doi: 10.1161/CIRCULATIONAHA.108.815944
27. Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, et al. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol. (2017) 69:556–69. doi: 10.1016/j.jacc.2016.10.078
28. Tse G, Gong M, Wong SH, Wu WKK, Bazoukis G, Lampropoulos K, et al. Frailty and clinical outcomes in advanced heart failure patients undergoing left ventricular assist device implantation: a systematic review and meta-analysis. J Am Med Dir Assoc. (2018) 19:255–61.e251. doi: 10.1016/j.jamda.2017.09.022
29. Zhang Y, Yuan M, Gong M, Tse G, Li G, Liu T. Frailty and clinical outcomes in heart failure: a systematic review and meta-analysis. J Am Med Dir Assoc. (2018) 19:1003–8.e1001. doi: 10.1016/j.jamda.2018.06.009
30. Zhang Y, Yuan M, Gong M, Li G, Liu T, Tse G. Associations between prefrailty or frailty components and clinical outcomes in heart failure: a follow-up meta-analysis. J Am Med Dir Assoc. (2019) 20:509–10. doi: 10.1016/j.jamda.2018.10.029
31. Carnicelli AP, Clare R, Chiswell K, Lytle B, Bjursell M, Perl S, et al. Comparison of characteristics and outcomes of patients with heart failure with preserved ejection fraction with versus without hyperuricemia or gout. Am J Cardiol. (2020) 127:64–72. doi: 10.1016/j.amjcard.2020.04.026
32. Macdonald MR, Tay WT, Teng TK, Anand I, Ling LH, Yap J, et al. Regional variation of mortality in heart failure with reduced and preserved ejection fraction across asia: outcomes in the ASIAN-HF registry. J Am Heart Assoc. (2020) 9:e012199. doi: 10.1161/JAHA.119.012199
33. Temma T, Nagai T, Watanabe M, Kamada R, Takahashi Y, Hagiwara H, et al. Differential Prognostic impact of atrial fibrillation in hospitalized heart failure patients with preserved ejection fraction according to coronary artery disease status- report from the Japanese Nationwide Multicenter Registry. Circ J. (2020) 84:397–403. doi: 10.1253/circj.CJ-19-0963
34. Sun Y, Wang N, Li X, Zhang Y, Yang J, Tse G, et al. Predictive value of H2 FPEF score in patients with heart failure with preserved ejection fraction. ESC Heart Fail. (2021) 8:1244–52. doi: 10.1002/ehf2.13187
35. Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. (2013) 128:1085–93. doi: 10.1161/CIRCULATIONAHA.113.001475
36. Mchugh K, Devore AD, Wu J, Matsouaka RA, Fonarow GC, Heidenreich PA, et al. Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:602–11. doi: 10.1016/j.jacc.2018.11.033
37. Meng Y, Zhu S, Xie Y, Zhang Y, Qian M, Gao L, et al. Prognostic value of right ventricular 3D speckle-tracking strain and ejection fraction in patients with HFpEF. Front Cardiovasc Med. (2021) 8:694365. doi: 10.3389/fcvm.2021.694365
38. Okuno K, Naito Y, Asakura M, Sugahara M, Horimatsu T, Yasumura S, et al. Anemia has an impact on prognosis in heart failure with preserved ejection fraction with mild chronic kidney disease. Int J Cardiol Heart Vasc. (2021) 34:100796. doi: 10.1016/j.ijcha.2021.100796
39. Pagel PS, Tawil JN, Boettcher BT, Izquierdo DA, Lazicki TJ, Crystal GJ, et al. Heart failure with preserved ejection fraction: a comprehensive review and update of diagnosis, pathophysiology, treatment, and perioperative implications. J Cardiothorac Vasc Anesth. (2021) 35:1839–59. doi: 10.1053/j.jvca.2020.07.016
40. Zhang X, Sun Y, Zhang Y, Chen F, Zhang S, He H, et al. Heart failure with midrange ejection fraction: prior left ventricular ejection fraction and prognosis. Front Cardiovasc Med. (2021) 8:697221. doi: 10.3389/fcvm.2021.697221
41. Zhou Q, Li P, Zhao H, Xu X, Li S, Zhao J, et al. Heart failure with mid-range ejection fraction: a distinctive subtype or a transitional stage? Front Cardiovasc Med. (2021) 8:678121. doi: 10.3389/fcvm.2021.678121
42. Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imaging. (2020) 13:245–57. doi: 10.1016/j.jcmg.2018.12.034
43. Palazzuoli A, Caravita S, Paolillo S, Ghio S, Tocchetti CG, Ruocco G, et al. Current gaps in HFpEF trials: time to reconsider patients' selection and to target phenotypes. Prog Cardiovasc Dis. (2021) 67:89–97. doi: 10.1016/j.pcad.2021.03.007
44. Mohammed SF, Hussain I, Abouezzeddine OF, Takahama H, Kwon SH, Forfia P, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. (2014) 130:2310–20. doi: 10.1161/CIRCULATIONAHA.113.008461
45. Coiro S, Simonovic D, Deljanin-Ilic M, Duarte K, Carluccio E, Cattadori G, et al. Prognostic value of dynamic changes in pulmonary congestion during exercise stress echocardiography in heart failure with preserved ejection fraction. Circ Heart Fail. (2020) 13:e006769. doi: 10.1161/CIRCHEARTFAILURE.119.006769
46. Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. (2013) 305:H1373–81. doi: 10.1152/ajpheart.00157.2013
47. Carnes J, Gordon G. Biomarkers in heart failure with preserved ejection fraction: an update on progress and future challenges. Heart Lung Circ. (2020) 29:62–8. doi: 10.1016/j.hlc.2019.05.180
48. Weymann A, Ali-Hasan-Al-Saegh S, Sabashnikov A, Popov AF, Mirhosseini SJ, Liu T, et al. Prediction of new-onset and recurrent atrial fibrillation by complete blood count tests: a comprehensive systematic review with meta-analysis. Med Sci Monit Basic Res. (2017) 23:179–222. doi: 10.12659/MSMBR.903320
49. Shao Q, Korantzopoulos P, Letsas KP, Tse G, Hong J, Li G, et al. Red blood cell distribution width as a predictor of atrial fibrillation. J Clin Lab Anal. (2018) 32:e22378. doi: 10.1002/jcla.22378
50. Weymann A, Ali-Hasan-Al-Saegh S, Popov AF, Sabashnikov A, Mirhosseini SJ, Liu T, et al. Haematological indices as predictors of atrial fibrillation following isolated coronary artery bypass grafting, valvular surgery, or combined procedures: a systematic review with meta-analysis. Kardiol Pol. (2018) 76:107–18. doi: 10.5603/KP.a2017.0179
51. Bazoukis G, Letsas KP, Vlachos K, Saplaouras A, Asvestas D, Tyrovolas K, et al. Simple hematological predictors of AF recurrence in patients undergoing atrial fibrillation ablation. J Geriatr Cardiol. (2019) 16:671–5. doi: 10.11909/j.issn.1671-5411.2019.09.008
52. Sotiropoulos K, Yerly P, Monney P, Garnier A, Regamey J, Hugli O, et al. Red cell distribution width and mortality in acute heart failure patients with preserved and reduced ejection fraction. ESC Heart Fail. (2016) 3:198–204. doi: 10.1002/ehf2.12091
53. Imai R, Uemura Y, Okumura T, Takemoto K, Uchikawa T, Koyasu M, et al. Impact of red blood cell distribution width on non-cardiac mortality in patients with acute decompensated heart failure with preserved ejection fraction. J Cardiol. (2017) 70:591–7. doi: 10.1016/j.jjcc.2017.03.010
54. Gallego-Colon E, Bonaventura A, Vecchié A, Cannatà A, Fitzpatrick CM. Cardiology on the cutting edge: updates from the European Society of Cardiology (ESC) Congress 2020. BMC Cardiovasc Dis. (2020) 20:448. doi: 10.1186/s12872-020-01734-4
55. Wachter R, Shah SJ, Cowie MR, Szecsody P, Shi V, Ibram G, et al. Angiotensin receptor neprilysin inhibition versus individualized RAAS blockade: design and rationale of the PARALLAX trial. ESC Heart Fail. (2020) 7:856–64. doi: 10.1002/ehf2.12694
56. Gomes-Neto M, Duraes AR, Conceicao LSR, Roever L, Liu T, Tse G, et al. Effect of aerobic exercise on peak oxygen consumption, VE/VCO2 slope, and health-related quality of life in patients with heart failure with preserved left ventricular ejection fraction: a systematic review and meta-analysis. Curr Atheroscler Rep. (2019) 21:45. doi: 10.1007/s11883-019-0806-6
57. Ge J. Coding proposal on phenotyping heart failure with preserved ejection fraction: a practical tool for facilitating etiology-oriented therapy. Cardiol J. (2020) 27:97–8. doi: 10.5603/CJ.2020.0023
58. Lakhani I, Leung KSK, Tse G, Lee APW. Novel mechanisms in heart failure with preserved, midrange, and reduced ejection fraction. Front Physiol. (2019) 10:874. doi: 10.3389/fphys.2019.00874
Keywords: predictive value, all-cause mortality, HFpEF, HFA-PEFF score, prognosis
Citation: Sun Y, Si J, Li J, Dai M, King E, Zhang X, Zhang Y, Xia Y, Tse G and Liu Y (2021) Predictive Value of HFA-PEFF Score in Patients With Heart Failure With Preserved Ejection Fraction. Front. Cardiovasc. Med. 8:656536. doi: 10.3389/fcvm.2021.656536
Received: 21 January 2021; Accepted: 04 October 2021;
Published: 29 October 2021.
Edited by:
Gianluigi Savarese, Karolinska Institutet (KI), SwedenReviewed by:
Stefano Coiro, Hospital of Santa Maria Della Misericordia in Perugia, ItalyCopyright © 2021 Sun, Si, Li, Dai, King, Zhang, Zhang, Xia, Tse and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Liu, eWluZ2xpdS5tZWRAZ21haWwuY29t; Gary Tse, Z2FyeXRzZTg2QGdtYWlsLmNvbQ==
†First author
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.