
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 16 November 2021
Sec. Gastroenterology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.784240
This article is part of the Research Topic Acute-On-Chronic Liver Failure: Natural History, Mechanism, And Treatment View all 20 articles
Background and Aims: Granulocyte colony-stimulating factor (G-CSF) has been proposed as a therapeutic option for patients with acute-on-chronic liver failure (ACLF). However, its clinical efficacy remains debatable. This study aimed to synthesize available evidence on the efficacy of G-CSF in ALCF.
Methods: The Cochrane Library, CNKI, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov were searched from inception until September 2021. After qualitative evaluation of the included literature, the included studies were analyzed.
Results: Seven studies were included in this meta-analysis. Overall, G-CSF therapy was not associated with a reduced risk of death (30-day survival, OR = 1.55, 95% CI: 1.00, 2.38, P = 0.05; 60-day survival, OR = 1.50, 95% CI: 0.95, 2.36, P = 0.08; 90-day survival, OR = 1.61, 95% CI: 0.99, 2.62, P = 0.05) or complication including occurrence of infections infection (OR = 0.66, 95% CI: 0.41, 1.05, P = 0.08), bleeding (OR = 1.50, 95% CI: 0.58, 3.89, P = 0.41), and hepatorenal syndrome (OR = 0.56, 95% CI: 0.25, 1.24, P = 0.15). Moreover, it had no obvious beneficial effects on the model of end-stage liver disease score (30-day SMD = −3.31, 95%CI: −7.42, 0.81, P = 0.12; 60-day SMD = −1.23, 95% CI: −5.21, 2.75, P = 0.54; 90-day SMD = −2.29, 95%CI: −4.94, 0.37, P = 0.09). Sensitivity analyses showed that patients in Asia had improved survival (30-day OR = 2.76, 95%CI: 1.43, 5.35, P = 0.003; 60-day OR = 2.83, 95% CI: 1.39, 5.73, P = 0.004; 90-day OR = 2.92, 95% CI: 1.34, 6.36, P = 0.007).
Conclusions: Our findings suggest that, currently, G-CSF cannot be recommended for the treatment of ACLF.
Acute-on-chronic liver failure (ACLF) is a kind of short-term liver damage due to acute causes of chronic liver disease; it is accompanied by the failure of one or more extrahepatic organs, and the short-term risk of death is high (1). Currently, there is no specific treatment for ACLF, and liver transplantation is the definitive treatment for ACLF. However, many patients cannot benefit from liver transplantation because of limited organ availability, high cost, transplant-related complications, and lifetime immunity-related side effects (2, 3). Therefore, alternative treatment strategies for liver transplantation are being sought.
Granulocyte colony-stimulating factor (G-CSF) is a glycoprotein that can mobilize immune cells, stimulate bone marrow and stem cell production, and it has immune regulation and regeneration capabilities. Previous studies have been reported encouraging results on the use of G-CSF in animal models. G-CSF is found to mobilize hematopoietic stem cells, induce liver regeneration, and improve survival. However, Engelmann et al. (4) found that G-CSF increased Toll-like receptor-mediated inflammation, which led to an increase in mortality. In human studies, a few small randomized clinical trials have demonstrated not only improvement in liver function with G-CSF but also significant survival benefit compared with standard medical therapy for ACLF (5–7). On the contrary, other clinical trials reported that the use of G-CSF in ACLF patients did not result in survival benefits (4, 8), which has caused widespread concern. Therefore, in this study, we conducted a meta-analysis of randomized controlled trials (RCTs) to compare the risk of death and infection between ACLF patients treated with G-CSF and ACLF patients who did not receive G-CSF.
We searched for RCTs involving ACLF patients treated with G-CSF from electronic medical databases, including the Cochrane Library, CNKI, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov, from inception to September 14, 2021. Key search terms were “granulocyte colony-stimulating factor,” “acute-on-chronic liver failure,” “hepatic insufficiency,” “end stage liver disease,” and “randomized controlled trial.” MeSH terms and free-text terms, as well as variations of root words, were combined within each database. No language restrictions were applied during the search. The reference lists of eligible articles and relevant review articles were also checked to identify additional studies. The detailed search strategies are outlined in Figure 1.
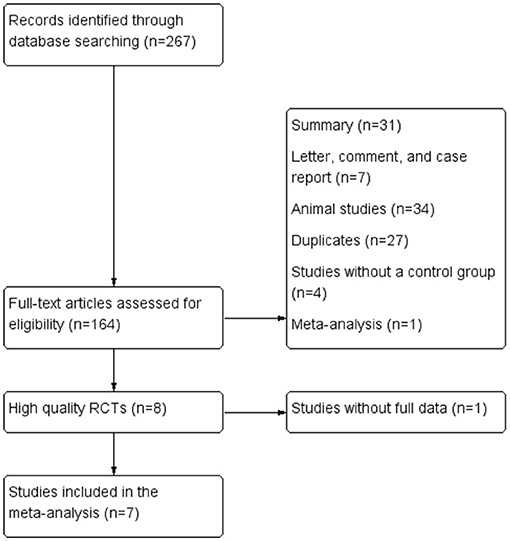
Figure 1. Flow chart of the selection of studies for inclusion in the meta-analysis. RCT, randomized controlled trial.
The inclusion criteria were as follows: (1) RCTs, (2) patients diagnosed with ACLF, (3) patients in the experimental group received G-CSF therapy, and patients in the control group received conventional treatment, and (4) availability of clinical outcomes. The primary outcomes were short-term survival rate. The secondary outcomes included the scores of Model for End-Stage Liver Disease (MELD), risk of bleeding, occurrence of infections and hepatorenal syndrome. The exclusion criteria were (1) animal-based review articles or case reports, and (2) research in which valid data could not be extracted from the full text.
Data extraction was performed independently by two authors using standardized data collection forms, and disagreements were resolved through discussion with another author. The following details were extracted from the included studies using a predefined data form: study first author, year of publication, country, and the number of participants in the experimental and control groups, the specific etiology of liver failure, the dose, administration, and duration of G-CSF treatment. Each trial was assessed using the Cochrane risk of bias tool. The standard criteria included the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias.
Review Manager (version 5.4) software was used for the data merging and processing. For categorical variables in the study, the standardized mean difference (SMD) and odds ratio (OR) was used as the effect index to calculate the combined value and 95% confidence interval (CI). The Mantel-Haenszel test was used to test the heterogeneity of the included studies. If I2 ≤ 50% (P ≥ 0.05), indicating that the differences in the studies were not statistically significant, the fixed-effects model was used for analysis. If I2 > 50% (P < 0.05), indicating that there was significant heterogeneity, the random-effects model was used. Obvious clinical heterogeneity was evaluated by removing a single study and repeating the meta-analysis. Statistical significance was set as P < 0.05. Publication bias was evaluated with a funnel plot.
Following the search strategy described in Figure 1, 267 studies were initially identified based on the assessment of the titles and abstracts. We excluded 260 studies considering the predefined criteria. Seven eligible studies were finally included in the meta-analysis (5, 6, 8–12).
Seven studies involving 479 ACLF patients included G-CSF treatment (n = 240) and control (n = 239) groups. The baseline characteristics, including the study design, treatment methods for each group, sample sizes of each group (Table 1).
The details of the risk of bias tool are shown in Figure 2.
Seven studies were included in the analysis of survival rate, compared with conventional treatment, G-CSF therapy was not associated with a reduced risk of death (30-day survival, OR = 1.55, 95% CI: 1.00, 2.38, P = 0.05; 60-day survival, OR = 1.50, 95% CI: 0.95, 2.36, P = 0.08; 90-day survival, OR = 1.61, 95% CI: 0.99, 2.62, P = 0.05) (Figure 3).
The scores of Model for End-Stage Liver Disease (MELD) in ACLF patients for meta-analysis were available from three studies (Figure 4). Before and after treatment, no significant difference was observed between the experimental and control groups (Before treatment: SMD = −1.36, 95% CI: −3.05, 0.32, P = 0.11; After treatment: 30-day SMD = −3.31, 95%CI: −7.42, 0.81, P = 0.12; 60-day SMD = −1.23, 95% CI: −5.21,2.75, P = 0.54;90-day SMD = −2.29, 95%CI: −4.94,0.37, P = 0.09).
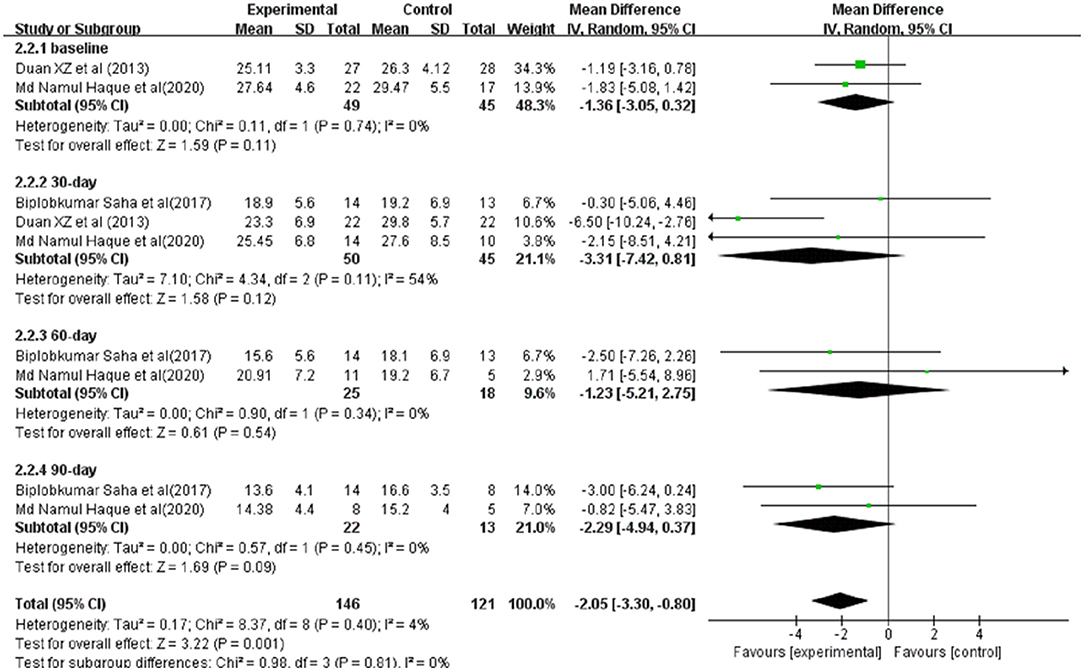
Figure 4. Improvement in clinical severity indices between patients with ACLF. Treated by G-CSF and controls.
Secondary infections were reported in 5 studies involving 416 ACLF patients, 209 were in the G-CSF treatment group and 207 were in the control group. Owing to the low heterogeneity (I2 = 33%), a fixed-effects model was adopted. The meta-analysis showed that patients receiving G-CSF did not have a significantly reduced risk of infections compared with traditional treatment (OR = 0.66, 95% CI: 0.41, 1.05, P = 0.08) (Figure 5).
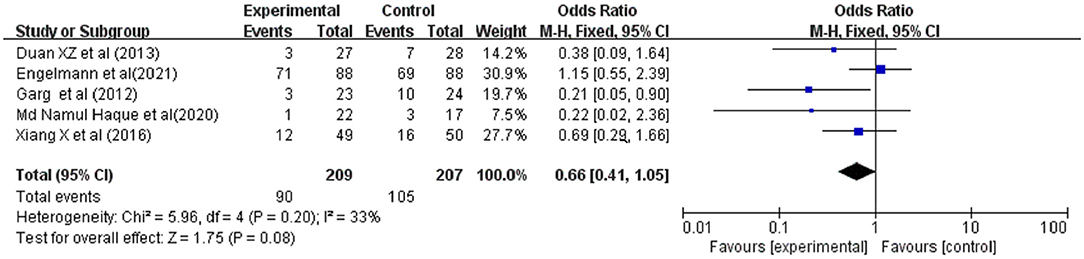
Figure 5. Pooled estimate rate for infection between patients with ACLF. Treated by G-CSF and controls.
Bleeding was reported in 4 studies involving 162 ACLF patients, 83 were in the G-CSF treatment group and 79 were in the control group. Owing to the low heterogeneity (I2 = 9%), a fixed-effects model was adopted. The meta-analysis showed that compared with traditional treatment, patients receiving G-CSF did not have a significantly reduced risk of bleeding (OR = 1.50, 95% CI: 0.58, 3.89, P = 0.41) (Figure 6).
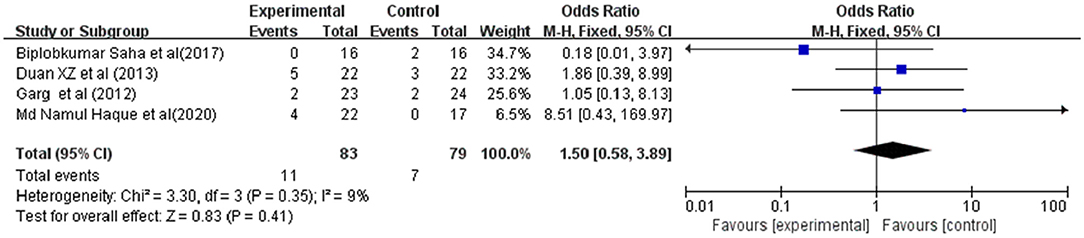
Figure 6. Pooled estimate rate for bleeding between patients with ACLF. Treated by G-CSF and controls.
Hepatorenal syndrome (HRS) were reported in 4 studies involving 214 ACLF patients, 109 were in the G-CSF treatment group and 105 were in the control group. Owing to the low heterogeneity (I2 = 0%), a fixed-effects model was adopted. The meta-analysis showed that compared with traditional treatment, patients receiving G-CSF did not have a significantly reduced risk of HRS (OR = 0.56, 95% CI: 0.25, 1.24, P = 0.15) (Figure 7).
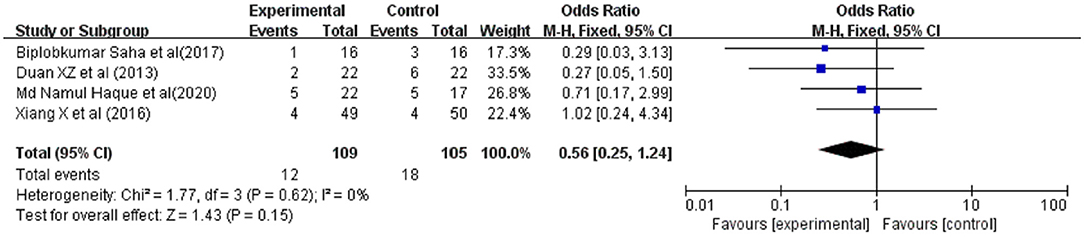
Figure 7. Pooled estimate rate for Hepatorenal syndrome between patients with ACLF. Treated by G-CSF and controls.
The sensitivity analysis showed that after excluding European studies, 4 Asian studies remained with low heterogeneity (30-day and 60-day I2 = 0%; 90-day I2 = 9%); considering these Asian studies, the patients' survival rate improved after the injection of G-CSF (30-day OR = 2.76, 95%CI: 1.43, 5.35, P = 0.003;60-day OR = 2.83, 95% CI: 1.39, 5.73, P = 0.004) (Figures 8A–C).
Funnel plots of survival rate meta-analyses demonstrated asymmetry and suggested the presence of publication bias (Figures 9A–C).
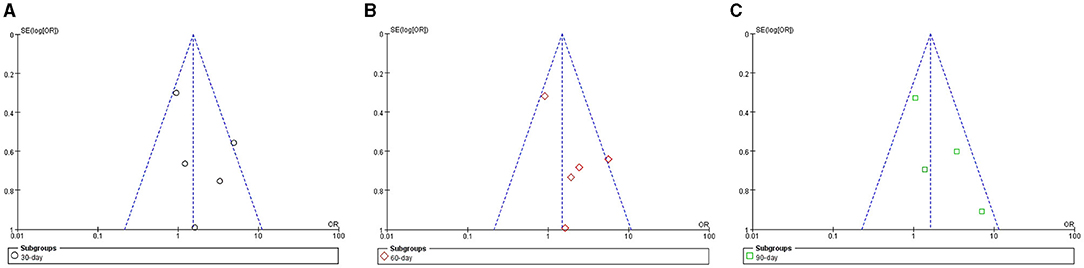
Figure 9. Funnel plot of meta-analyses for survival rate in ACLF patients. (A) 30-day; (B) 60-day; (C) 90-day.
ACLF is characterized by organ failure and high short-term mortality. Currently, there are no specific therapies for ACLF. Liver transplantation is the ultimate treatment for those who are acceptable candidates, but is limited by organ shortage and high frequency of contraindications in this group of patients (2, 3). Previous studies have shown that G-CSF can reduce the short-term mortality of ACFL patients (13). Yet the newly published clinical trial does not seem to support this conclusion (8). In this study, we conducted a comprehensive meta-analysis of 7 RCTs to evaluate the efficacy and safety of G-CSF in the treatment of ACLF patients. We failed to find a significant beneficial effect of G-CSF for patients with ACLF, unlike noted in a previous meta-analysis (14). Through sensitivity analysis of sources of heterogeneity, we found that etiologies of ACLF differ by region, with reactivation of hepatitis B more common in Asia, whereas alcoholic hepatitis are reportedly more common in European. We speculate the ultimate treatment outcome of patient may depend on the etiology of ACLF. In addition, different ACLF diagnostic criteria have led to considerable regional differences in ACLF recognition, onset and treatment timing, and final prognosis. In this meta-analysis, the admission criteria for ACLF patients in the Asian study were in accordance with APASL, and the admission criteria for patients in the European study were in accordance with the EASL-CLIF criteria. An intriguing and important finding of this study was significantly different disease progression among patients with ACLF at enrollment defined by APASL or EASL-CLIF Consortium. This may also affect the therapeutic effect of G-CSF on different ACLF patients.
Currently, it has been shown that G-CSF stimulates liver regeneration. G-CSF can stimulate bone marrow to release stem cells (CD34+), which could migrate to liver and differentiate into mature hepatocytes. It can also reduce the production of interferon-gamma, improve the local microenvironment of liver, promote liver repair, and improve liver injury. This translates into improved liver function, decreased risk of complications of liver disease, reduced risk of infections, and improved survival (15). The effect of G-CSF on liver regeneration may explain the survival benefit which was observed in Asian studies. The role of G-CSF in the latter stages of ACLF is limited due to the exhausted and destructed state of bone marrow ecology. In the Asian regional study included in this meta-analysis, G-CSF was generally used in the early stages of ACLF. In the included European RCTs trial, we found that ~70% of patients had cardiopulmonary failure and severe sepsis at the time of enrollment (8), which means they were in the end-stage of ACLF. Whether this condition affects our final results needs further exploration. In addition, studies have found that G-CSF requires a non-inflammatory environment to exert its protective effects on the liver. ACLF is characterized by increased white blood cell counts and plasma C-reactive protein levels. Patients often have a strong systemic inflammatory response (8, 16), and we speculate that G-CSF does not play a beneficial role in ACLF patients. Moreover, there were fewer studies in the European region in this study. Thus, more European clinical trials are needed to determine whether current results from the included European region trials were non-comprehensive. There were no clear conclusions concerning the usefulness of G-CSF in those with ACLF, although survival benefits were observed in Asian patients compared to European patients. The conflicting results between Asian and European studies led to a high degree of overall heterogeneity in the analysis, and it is unclear whether this difference can be explained by ethnic differences or patient selection. Based on our results, we do not recommend G-CSF as a definitive treatment for patients with ACLF.
The Model for End-Stage Liver Disease (MELD) has been established as a reliable indicator of short-term survival in patients with end-stage liver disease. In this meta-analysis, we comprehensively analyzed the clinical severity indices of ACLF. The results suggested that G-CSF therapy may not improve MELD scores, unlike what is noted in a previous meta-analysis (17). Also, patients with G-CSF therapy did not achieve significantly lower bleeding risk and the occurrence of HRS compared with standard medical treatment. ACLF has marked pathophysiological features, namely, susceptibility to infection. Moreover, bacterial infection is a major challenge for its treatment (18, 19). G-CSF is an immunomodulatory glycoprotein that exerts anti-inflammatory and immunomodulatory effects in the body, thereby reducing the occurrence of bacteremia and infection. This benefit of G-CSF may be particularly important in patients with ACLF. Nonetheless, in this meta-analysis, there was no significant difference in the risk of infection among patients receiving treatment. We found that in the study of Engelmann et al. almost 40% of patients had bacterial infection at enrollment (8). The role of G-CSF for ACLF patients with severe bacterial infection is debatable. G-CSF is helpful to prevent development of bacterial infection, but is not beneficial to treat it. It is important to clarify whether this affected the final results.
Several limitations of this meta-analysis should be mentioned. First of all, the high heterogeneity in some aggregate estimation results may have hindered the establishment of reliable conclusions and recommendations. Sensitivity analyses indicated that regions and different types of liver disease may be the main cause of heterogeneity. However, it is unclear whether other factors would lead to the results of the study, such as the degree of ACLF progress, differences between studies, and the dose and duration of G-CSF injections. Moreover, some data cannot be obtained from each study, resulting in limited strength of evidence for the results obtained. Secondly, the total sample size is small, which may affect the reliability of the analysis results to a certain extent, and has limited significance for clinical guidance. Lastly, different trials use different outcome parameters at different measurement time points to evaluate the treatment effect, making it difficult to use a limited statistical sample size at a specific time point to summarize reliable results.
No clear conclusion could be drawn regarding the usefulness of G-CSF in ACLF, although survival benefits were observed in Asian patients. The conflicting results between regions and different etiology of liver disease lead to a high degree of overall heterogeneity in the analysis. It is unclear whether these differences can be explained by ethnic differences or different liver failure causes. Moreover, different diagnostic criteria for ACLF caused different patient prognosis (20). We need more RCTs and high-quality literature are required to clarify the usefulness of G-CSF for ACLF treatment. In conclusion, based on our results, we do not recommend G-CSF as a definitive treatment for patients with ACLF.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
XH, YL, and CZ conceived and designed the study. XH, HY, and JC selected the studies, collected the data, and drafted and revised the paper. XH, RL, and JL analyzed data. All authors interpreted the results, read, and approved the final version of the manuscript.
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81770591 and 81800778), Science and Technology Plan of Hainan Province (Clinical Research Center) (LCYX202103), and Hainan Province Clinical Medical Center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACLF, acute-on-chronic liver failure; CI, confidence interval; G-CSF, granulocyte colony-stimulating factor; OR, odds ratio; RCT, randomized controlled trial.
1. Allen AM, Kim WR, Moriarty JP, Shah ND, Larson JJ, Kamath PS. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology. (2016) 64:2165–72. doi: 10.1002/hep.28812
2. Benítez C, Wolff R. Current status and future challenges of liver transplantation programs in Chile. Liver Transpl. (2018) 24:1757–61. doi: 10.1002/lt.25332
3. Belli LS, Duvoux C, Artzner T, Bernal W, Conti S, Cortesi PA, et al. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: results of the ELITA/EF-CLIF collaborative study (ECLIS). J Hepatol. (2021) 75:610–22. doi: 10.1016/j.jhep.2021.03.030
4. Engelmann C, Martino VD, Kerbert AJC, Weil-Verhoeven D, Aehling NF, Herber A, et al. The current status of granulocyte-colony stimulating factor to treat acute-on-chronic liver failure. Semin Liver Dis. (2021) 41:298–307. doi: 10.1055/s-0041-1723034
5. Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. (2012) 142:505–512.e1. doi: 10.1053/j.gastro.2011.11.027
6. Duan XZ, Liu FF, Tong JJ, Yang HZ, Chen J, Liu XY, et al. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol. (2013) 19:1104–10. doi: 10.3748/wjg.v19.i7.1104
7. Verma N, Kaur A, Sharma R, Bhalla A, Sharma N, De A, et al. Outcomes after multiple courses of granulocyte colony-stimulating factor and growth hormone in decompensated cirrhosis: a randomized trial. Hepatology. (2018) 68:1559–73. doi: 10.1002/hep.29763
8. Engelmann C, Herber A, Franke A, Bruns T, Schiefke I, Zipprich A, et al. Granulocyte-Colony Stimulating Factor (G-CSF) to treat acute-on-chronic liver failure, a multicenter randomized trial (GRAFT study). J Hepatol. (2021). doi: 10.1016/j.jhep.2021.07.033
9. Saha BK, Mahtab MA, Akbar SMF, Noor-E-Alam SM, Mamun AA, Hossain SMS, et al. Therapeutic implications of granulocyte colony stimulating factor in patients with acute-on-chronic liver failure: increased survival and containment of liver damage. Hepatol Int. (2017) 11:540–6. doi: 10.1007/s12072-017-9814-1
10. Sharma S, Lal SB, Sachdeva M, Bhatia A, Varma N. Role of granulocyte colony stimulating factor on the short-term outcome of children with acute on chronic liver failure. J Clin Exp Hepatol. (2020) 10:201–10. doi: 10.1016/j.jceh.2019.10.001
11. Xu X, Liu XY, Chen J, Xiao L, Tong JJ, Guan CD, et al. Randomized controlled clinical study of granulocyte colony stimulating factor in the treatment of chronic hepatitis B-related acute liver failure. Infect Dis Inf. (2016) 29:279–83.
12. Haque MN, Al-Mahtab M, Das DC, Mohammad NS, Mamun AA, Khan MSI, et al. Effect of granulocyte colony-stimulating factor and erythropoietin on patients with acute-on-chronic liver failure. Euroasian J Hepatogastroenterol. (2020) 10:64–7. doi: 10.5005/jp-journals-10018-1330
13. Simonetto DA, Shah VH, Kamath PS. Improving survival in ACLF: growing evidence for use of G-CSF. Hepatol Int. (2017) 11:473–5. doi: 10.1007/s12072-017-9834-x
14. Chavez-Tapia NC, Mendiola-Pastrana I, Ornelas-Arroyo VJ, Noreña-Herrera C, Vidaña-Perez D, Delgado-Sanchez G, et al. Granulocyte-colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Ann Hepatol. (2015) 14:631–41. doi: 10.1016/S1665-2681(19)30757-4
15. Rathi S, Hussaini T, Yoshida EM. Granulocyte colony stimulating factor: a potential therapeutic rescue in severe alcoholic hepatitis and decompensated cirrhosis. Ann Hepatol. (2021) 20:100211. doi: 10.1016/j.aohep.2020.04.011
16. Gustot T. Beneficial role of G-CSF in acute-on-chronic liver failure: effects on liver regeneration, inflammation/immunoparalysis or both? Liver Int. (2014) 34:484–6. doi: 10.1111/liv.12356
17. Chen P, Wang YY, Chen C, Guan J, Zhu HH, Chen Z. The immunological roles in acute-on-chronic liver failure: an update. Hepatobiliary Pancreat Dis Int. (2019)18:403–11. doi: 10.1016/j.hbpd.2019.07.003
18. Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic liver failure. Lancet. (2015) 386:1576–87. doi: 10.1016/S0140-6736(15)00309-8
19. Zhang Q, Li Y, Han T, Nie C, Cai J, Liu H, Liu Y. Comparison of current diagnostic criteria for acute-on-chronic liver failure. PLoS ONE. 10:e0122158. doi: 10.1371/journal.pone.0122158
Keywords: granulocyte colony-stimulating factor, acute-on-chronic liver failure, end stage liver disease, hepatic insufficiency, randomized controlled trial
Citation: Hou X, Li Y, Yuan H, Cai J, Liu R, Li J and Zhu C (2021) Therapeutic Effect and Safety of Granulocyte Colony-Stimulating Factor Therapy for Acute-On-Chronic Liver Failure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Med. 8:784240. doi: 10.3389/fmed.2021.784240
Received: 27 September 2021; Accepted: 26 October 2021;
Published: 16 November 2021.
Edited by:
Yu-Chen Fan, Shandong University, ChinaReviewed by:
Yu Shi, Zhejiang University, ChinaCopyright © 2021 Hou, Li, Yuan, Cai, Liu, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanlong Zhu, Y2h1YW5sb25nQHlhaG9vLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.