
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 10 February 2025
Sec. Agroecology and Ecosystem Services
Volume 9 - 2025 | https://doi.org/10.3389/fsufs.2025.1549061
This article is part of the Research TopicEnhancing Soil Health and Climate Resilience through Sustainable Agricultural PracticesView all articles
 Meraj Alam Ansari1,2*
Meraj Alam Ansari1,2* Amit Kumar3*
Amit Kumar3* T. P. Ahammed Shabeer4
T. P. Ahammed Shabeer4 Alok Kumar5
Alok Kumar5 Subhra Saikat Roy2,6
Subhra Saikat Roy2,6 Majhrool Hak Ansari7
Majhrool Hak Ansari7 Raghavendra Singh1,3
Raghavendra Singh1,3 Moirangthem Sangeeta2
Moirangthem Sangeeta2 Jayanta Layek3
Jayanta Layek3 Narendra Prakash1
Narendra Prakash1 Vinay K. Mishra3
Vinay K. Mishra3Perilla (Perilla frutescens (L.) Britton) is a potential but often neglected oilseed crop. It has a long cultivation history among tribal farmers in the hilly regions of North Eastern India and China. Perilla, with its rich fatty acids, essential nutrients, antioxidants, and phenolic acids, holds immense potential for climate-resilient agriculture in rainfed areas. Large-scale cultivation of perilla improves food security and offers numerous health benefits, including cardiovascular support and disease prevention, thereby enhancing human wellbeing. However, the genetic complexity of its fatty acid composition, nutrient content, and yield-associated agronomic traits remains poorly understood. To dissect the genetic basis, we evaluated 28 diverse genotypes of perilla across three growing seasons for eight agronomic traits and 16 biochemical traits, which revealed substantial phenotypic and biochemical variation. Agronomic traits displayed significant variability, with seed yield showing the highest coefficient of variation (CV), while leaf length showed the lowest CV. For oil quality traits, the maximum CV was observed for the omega-6:omega-3 ratio (12.08%) and the minimum for polyunsaturated fatty acids (PUFAs) (4.12%), with an average variation of 7.16%. Seed yield exhibited a positive correlation with copper (Cu), potassium (K), and iron (Fe), while PUFAs showed a positive correlation with omega-6 and a negative correlation with saturated fatty acids (SFAs). Principal component analysis indicated that the first two components explained 71.28% of the variance, with omega-6 and PUFAs: SFA contributing most to principal component 1 (PC1) and monounsaturated fatty acids (MUFAs) and omega-9 contributing most to principal component 2 (PC2). The genotypes RCT 30 and RCT 3 showed the highest contributions to both components (PC1 and PC2). This study provides valuable insights into the genotypic potential of perilla for enhancing its yield, oil quality, and nutrient composition, with significant implications for breeding programs aimed at developing climate-resilient varieties for the North Eastern Himalayas.
Perilla (Perilla frutescens (L.) Britton), a member of the Lamiaceae family, is an annual, aromatic, herbaceous oilseed crop native to India and China. Widely recognized for its dual applications—culinary and industrial—this crop contributes to the agricultural diversity of countries such as Japan, Korea, and India (Pandey and Bhat, 2008). In the North Eastern Himalayan (NEH) region of India, perilla is cultivated under a variety of indigenous names: ‘Bhanjira’ (Hindi), ‘Unei’ (Meghalaya), ‘Kenie’ (Nagaland), ‘Ngamum’ (Arunachal Pradesh), ‘Thoiding’ (Manipur), ‘Silam’ (Sikkim), and ‘Chhawhchi’ (Mizoram). It is also known as ‘Su-tzu’ in Chinese and ‘Kkaennip’ in Korean languages, reflecting its widespread presence across Asia. Perilla, which has traditionally been grown in kitchen gardens on shifting lands, limited large-scale production due to low economic incentives, developing market infrastructure, agronomic considerations, and a need for stronger policy support to encourage its commercial growth. There are two key varieties of Perilla frutescens: var. frutescens, which is primarily used for oil extraction, and var. crispa, preferred for its culinary and medicinal applications. The ability to cultivate perilla at diverse altitudes enhances ecological sustainability by promoting biodiversity, improving soil health, and providing a resilient crop option adaptable to various climatic conditions in the NEH region. Rich genetic diversity has been observed across the Himalayan regions, providing an excellent foundation for breeding programs that aim at improving crop traits such as oil content, fatty acid composition, and phytochemical properties (Nitta et al., 2003; Pandey and Bhat, 2008; Ansari et al., 2019).
In rainfed agriculture, where water scarcity and climate variability pose challenges, perilla’s adaptability and low input requirements make it a promising alternative. Integrating perilla into climate-resilient cropping systems can enhance productivity, resource use efficiency, and resilience to dynamic environmental conditions. The increasing global demand for high-quality oils, driven by population growth and consumer health awareness, provides an opportunity to elevate perilla as a valuable oilseed crop. Its oil is high in polyunsaturated fatty acids (PUFAs) and has a unique omega-6 to omega-3 ratio of 0.33:1, making it a desirable option for both culinary and industrial applications. Compared to flaxseed oil, perilla oil contains 31–51% oil by weight, with a composition that includes 6.7–7.6% saturated fatty acids, 14–23% oleic acid, 11–16% linoleic acid, and 50–70% linolenic acid (Food and Agricultural Organization, 1992). This composition makes perilla oil highly beneficial for heart health and positions it as a competitor in the health-conscious consumer market.
In addition to its nutritional benefits, perilla is esteemed for its medicinal properties (Singh et al., 2017). The essential oils derived from Perilla frutescens have shown efficacy in preventing and controlling the larvae of Aedes aegypti, a key vector for dengue (Pandey and Bhat, 2008; Tabanca et al., 2015). The high levels of rosmarinic acid present, particularly during the flowering and seeding stages, are a potent phenolic compound that adds further value to perilla (Liu et al., 2015; Zhang et al., 2009).
Despite its numerous benefits, the commercial cultivation of perilla in the NEH region is not gaining momentum. Currently, perilla is primarily grown for local consumption, where its seeds are roasted, ground into powder, and added to salads or traditional dishes such as laddu (ball-shaped sweet Indian cuisine). Its classification as a “semi-wild, semi-domesticated” crop has contributed to its neglect by scientific research and agricultural development programs. This underutilization of perilla is particularly evident in the lack of modern breeding efforts aimed at improving perilla’s agronomic traits. The limited focus on phytochemical variation and nutritional characterization further restricts its potential use in large-scale farming and breeding programs.
Addressing this gap in research and development, the present study seeks to explore the genotypic potential of perilla to enhance oilseed production under climate-resilient agriculture in the NEH region. Germplasm collection, characterization, and conservation efforts are critical for identifying superior genotypes with desirable traits such as yield, higher oil content, better fatty acid composition, and enhanced phytochemical properties. Previous research has revealed significant intra- and inter-population genetic variations in perilla, thereby highlighting the rich diversity available for crop improvement efforts (Singh et al., 2017). However, most studies have focused on a limited number of quality traits, often relying on market samples rather than comprehensive, field-based assessments of genetic diversity.
To harness the full potential of perilla, particularly in the face of climate change, it is essential to prioritize the crop in breeding programs aimed at enhancing both its agronomic and nutritional traits. A focus on the oil content, fatty acid composition, phenolic compounds, and antioxidant properties of improved perilla populations could lead to the development of high-yielding and nutritionally superior climate-resilient varieties suited to the NEH region. Such varieties would not only support local food security but also contribute to global agricultural diversity in the oilseed market.
The experimental material had a total of 28 genotypes, which were evaluated during the rainy season (May to October) in three consecutive years from 2015 to 2017 at the experimental farm of the ICAR Research Complex for the NEH Region, Manipur Centre. Morphological characterizations of the collected accessions were carried out at the same center. The recommended agronomic practices and plant protection measures were followed to ensure normal crop growth. Weather data are presented in Supplementary Figure 1.
The grains of all genotypes were stored in the same place and temperature to ensure equal moisture content. For recording observations on oil content and other biochemical parameters, seed grains of each accession from all three replications of the third-year (2017) crop were mixed together to prepare a composite sample. Subsequent samples were drawn randomly from each composite sample of each corresponding genotype for the estimation of oil content, fatty acids, phenolics, antioxidants, and nutritional properties.
All the extraction beakers used in oil extraction were rinsed and dried in an oven at approximately 100°C for 1 h. The extraction beakers were then allowed to cool to room temperature, and the initial weight was recorded. The perilla seeds were ground with a mortar and pestle (homogenization) for better oil extraction, which breaks fat–water emulsions and allows fat to dissolve easily in organic solvents, resulting in an increase in free fat from seed tissues. Two grams of ground sample was taken and transferred into an extraction thimble. All the samples were extracted serially in an automatic oil-extracting machine SOCS PLUS, Model SCS-6 (Pelican Equipment, Chennai, India), using petroleum ether as a solvent. Oil was extracted using 80 mL of petroleum ether (boiling point 60–80°C) at 80°C for 4 h. After 4 h, all the petroleum ether was evaporated at 120°C and then cooled in desiccators. The final constant weight of the beaker containing oil was checked after overnight drying in an oven at 60°C and was recorded. The difference between the initial and final weight is the amount of oil content (Equation 1).
The oil content in percentage was determined using the following formula:
For fatty acid analysis by gas–liquid chromatography (GLC), a Hewlett-Packard gas chromatograph, (Model 6890), with a flame ionization detector (FID), was used. The temperature levels of the injector and detector used were 260°C and 275°C, respectively. The oven temperature was programmed from 150°C holding at 1 min to 210°C at a rate of 15°C/min, followed by 210–250°C at a rate of 5°C/min for 12 min. Peaks of fatty acid methyl esters were identified by comparing their retention duration with that of known standards run under similar separation conditions. Peak integration was performed by applying HP3398A software.
For the determination of Fe, Mn, Cu, and Zn, 1.0 g plant samples were ashed in a muffle furnace at 550°C for 3 h and subsequently extracted with 2 normal (N) HCl. The extract was analyzed for Fe, Mn, Cu, and Zn (Atomic Absorption Spectrometry, AAS-200 series, PerkinElmer). We used Perkin Elmer Lumina TM Lamp AAS 200 series with part numbers N305-0121 for Cu, N305-0126 for Fe, N305-0145 for Mn, and N305-0191 for Zn. Nitrogen (N) concentration in grain was determined by micro-Kjeldahl digestion and distillation (Nelson and Sommers, 1973). To determine phosphorus (P) and potassium (K), the plant samples were ashed in a muffle furnace at 550°C for 3 h and subsequently extracted with 2 N HCl. The extract was analyzed for P (Vanadomolybdate yellow color method; Jackson, 1973) and K (Flame photometer).
Total phenolic content in ethanolic extract was assayed following the Folin–Ciocalteu method (Singleton and Rossi, 1965) using gallic acid (0–100 μg/mL) as standard. The value was expressed as mg GAE/100 g (milligrams of gallic acid equivalent per 100 g). To ascertain recovery, samples were spiked with known amounts of standard before extraction. A recovery of 98.7 ± 1.2 was obtained.
Nitric oxide was produced from sodium nitroprusside and was measured using the Greiss reaction at 540 nm, according to Marcocci et al. (1994). Subsequently, the percentage of nitric oxide radical scavenging activity (NORSA) was determined. Different concentrations of L-ascorbic acid were used as a standard. The results are expressed in milligrams of L-ascorbic acid equivalent per 100 g of the sample (mg AAE/100 g).
The descriptive statistics of morphological traits of the 28 genotypes of Perilla sp. are mentioned in Table 1. Statistically significant (p < 0.05) variations were found for all the morphological traits except leaf length (8.96–12.77 cm), leaf width (8.60–12.51 cm), and inflorescence length (7.14–10.53 cm). The maximum variation was observed for the seed yield (5.5–34.80 q/ha) with an average of 16.73 q/ha and the least variation for inflorescence length with an average of 8.93 cm. The mean and range for the hundred-seed weight (HSW) were 0.17 g and 0.13–0.25 g, respectively. The maximum and minimum coefficient of variation (CV) were found for seed yield (47.38%) and leaf length (7.87%), respectively, with an average CV of 17.98%.
The oil content and proportion of the four major fatty acids in the seed oil of the 28 genotypes of Perilla sp. are given in Table 2. There was a variation noticed in oil content (35.6–48.2% of dry seed weight) between populations with an average of 42.08%. The percentage of total saturated fatty acids (SFAs), total monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) in the seed oil ranged from 32.83 to 39.29%, 13.39–17.41%, and 44.53–53.53% of the total fatty acids, with an average of 36.64, 15.09, and 48.27%, respectively. Among the MUFAs, omega-9 (oleic acid) has a mean value of 14.83% with a range of 3.97%, while in the PUFA categories, the mean of omega-6 and omega-3 was found to be 33.31 and 14.96% with a range of 9.5 and 4.7%, respectively. The ratio of omega-6 and omega-3 and the ratio of PUFAs and SFAs in seed oil ranged from 1.87 to 2.93 and 1.17–1.63 with an average of 2.24 and 1.32, respectively. The CV was found maximum for omega-6:omega-3 (12.08%), while the minimum for PUFAs (4.12%) with an average of 7.16% for all oil quality parameters. Figure 1 represents the boxplot of oil content and fatty acid constituents. Except for omega-3 fatty acids, all traits (oil content, SFAs, omega-6, PUFAs, omega-9, and MUFAs) were positively skewed, with a greater number of genotypes showing lower values for traits clustered in the hill and the majority of extreme values being higher than the mean. For omega-3 fatty acids, genotypes having the highest values were in the highest numbers and the majority of the extreme values were lower than the mean. RCT-13 (11.85%), RCT-30 (39.88%), and RCT-30 (53.53%) were identified as outliers for omega-3, omega-6, and PUFAs, respectively. All traits, except omega-3 fatty acids, are platykurtic, with fewer values concentrated around the mean and having a lesser chance of extreme events. Omega-3 fatty acid was leptokurtic, having more values concentrated around the mean and a higher probability of extreme events.
The proportion of four major micronutrients (Fe, Mn, Zn, and Cu) and two major macronutrients (P and K) in the seeds of the 28 genotypes of Perilla sp. is given in Table 3. The mean and range of Fe, Mn, Zn, Cu, P, and K were 12.39 ppm and 6.4 ppm, 25.39 ppm and 51.28 ppm, 32.01 ppm and 13.53 ppm, 33.82 ppm and 29.45 ppm, 0.22 and 0.24%, and 0.48 and 0.41%, respectively. The CV was highest for Mn (41.34%) and lowest for Zn (8.99%), with an average of 22.5% for all nutritional parameters. The mean and range of TPC and TAC were 367.46 and 303.51 mg GAE/100 g, and 42.08 and 12.60 mg AAE/g. TPC (22.09%) exceeds the CV, which has an average of 14.72% for the two parameters.
Figure 2 depicts the correlation among the traits, with an “X” showing a non-significant correlation. Seed yield has a positive correlation with Cu, K, and Fe. Oil content shows a high and low positive correlation with TAC and TPC, respectively. Omega-3 (n-3) has a low positive and negative correlation with SFAs, omega-6 (n-6), omega-9 (n-9), MUFA, and n-6:n-3, respectively. PUFA: SFA shows a negative and positive correlation with SFAs, Mn, Fe, omega-6, PUFAs, and n-6:n-3, respectively. PUFAs have a positive and negative correlation with omega-6 and omega-3, SFAs, and Mn, respectively.
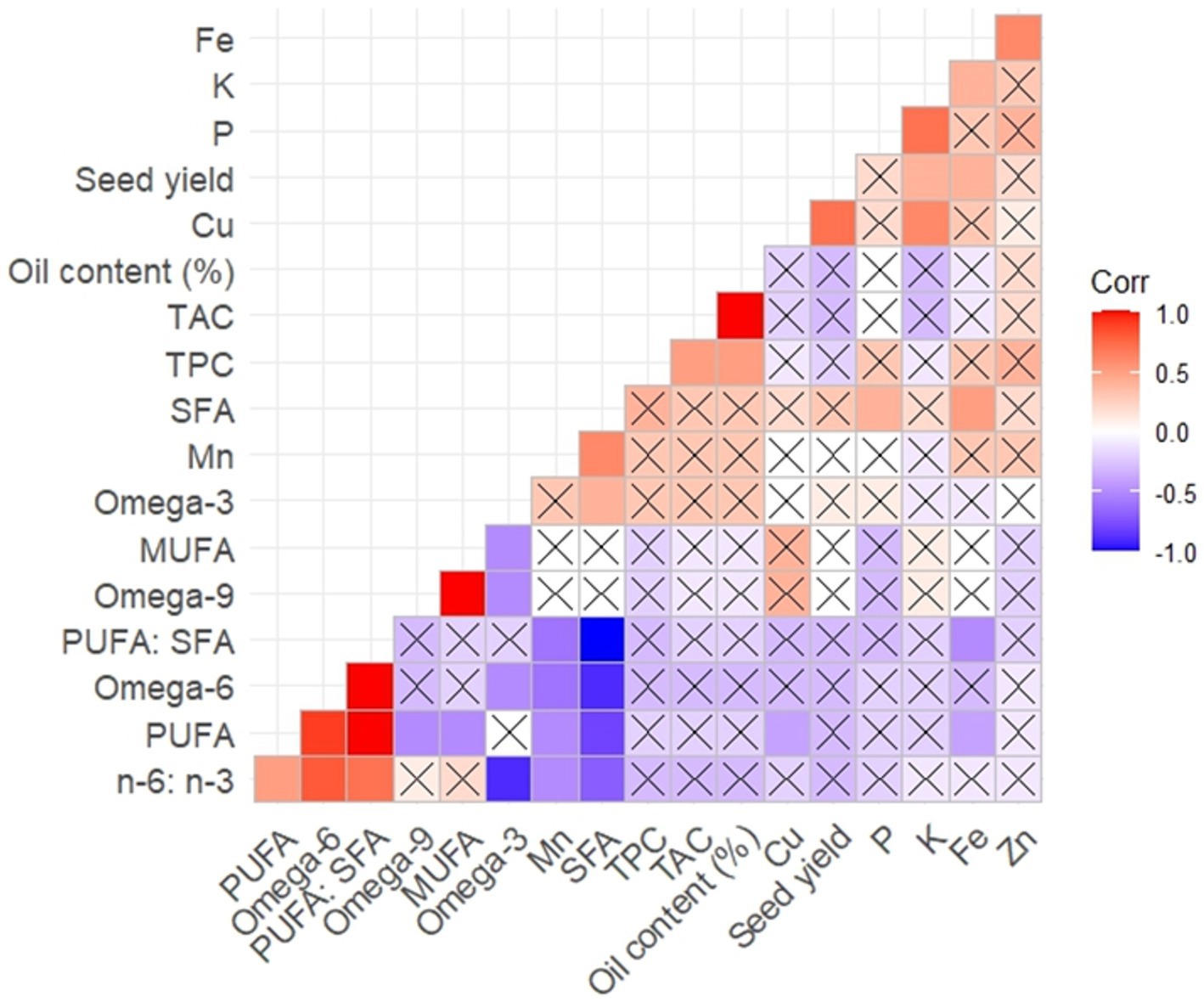
Figure 2. Correlation between seed yield and fatty acid profiles, nutrients, antioxidants and phenolics.
Figure 3 depicts the correlation among the morphological traits, with the box marked “X” showing a non-significant correlation. Seed yield is positively correlated with the number of primary branches. Hundred seed weight (100SW/HSW) is positively correlated with leaf length, leaf width, and petiole length. Inflorescence length is not correlated with any of the traits. The number of primary branches and the number of inflorescences per plant are positively correlated with plant height.
Principal component analysis (PCA) is a very useful technique for data reduction to remove interrelationships among components. Various researchers showed multivariate analysis as a valid system to deal with germplasm collection. Smith et al. (1995) analyzed germplasm preservation and utilization. They used average linkage cluster and principal component analyses and reported that the results were useful. In the present study, PCA was performed on 28 genotypes of perilla using quantitative traits. Scree plot analysis revealed that the first two most informative components accounted for 71.28% of the variance (Figure 4). Representation of different variables in different dimensions, overall representation in two dimensions, and biplot analysis are shown in Figures 5–7, respectively. Omega-6 followed by PUFA: SFA (PUFA_SFA) and SFAs have the maximum representation in PC1, while MUFAs followed by omega-9 and omega-3 have maximum representation in PC2. If we look at the overall representation of variables in PC1 and PC2, omega-6, followed by PUFAs and MUFAs, has the maximum representation. The contribution (%) of different variables in different dimensions and the overall contribution in two dimensions are depicted in Figures 8, 9, respectively. The red dashed line on the graph indicates the expected average contribution. The variables omega-6, PUFAs, MUFAs, omega-9, PUFA: SFA, SFAs, the ratio of omega-6 and omega-3 (X6_X3), and omega-3 contribute the most to dimensions 1 and 2, in descending order. The contribution (%) of different genotypes in PC1 and PC2 is depicted in Figure 10. No statistically significant contribution is observed below the red dashed line in both dimensions. RCT30 has the maximum contribution, followed by RCT-3, RCT-13, RCT-3, RCT-22, RCT-7, RCT-3, RCT-13, RCT-31, RCT-22, RCT-7, RCT-4, RCT-29, RCT-26, RCT-9, and RCT-6. The k-means cluster analysis can be used to identify the best parental combination for generating variability in various traits. A 2-D representation of a cluster in which two components explain 71.28% of the point variability is explained in Figure 11. Based on seed yield oil content, fatty acids, and micronutrients and macronutrients, 28 genotypes of perilla were grouped into four major clusters (Figure 11). The maximum number of genotypes were retained in cluster III (10) followed by clusters I (9), II (6), and IV (3). The mean performance of different characters for yield, oil content, and fatty acid traits in perilla in different clusters is shown in Figure 11. Maximum and minimum mean performance in the different clusters (in parenthesis) for oil content, SFAs, omega-9, MUFAs, omega-3, omega-6, PUFAs, ratio of omega-6 and omega-3 (omega-6:omega-3), ratio of PUFAs and SFAs (PUFA:SFA), and seed yield were found 43.72 (III) and 39.47% (IV), 38.08 (III) and 35.14% (I), 16.48 (IV) and 14.16% (I), 16.73 (IV) and 14.43% (I), 15.64 (II) and 14.14% (III), 35.53 (I) and 30.81% (IV), 50.43 (I) and 45.32% (IV), 2.41 (II) and 2.04 (III), 1.44 (I) and 1.20 (IV), and 19.78 (III) and 12.23 (II) q/ha.
Perilla, an underutilized oilseed crop, has immense potential for addressing nutritional and agricultural challenges, particularly in the context of climatic vagaries. The significant genotypic variability observed in this study, coupled with the high nutritional and oil content of specific genotypes, underscores perilla’s value as a climate-resilient crop for rainfed regions, particularly in the North Eastern Himalayas (NEH). The variability in yield-related traits, biochemical properties, and nutrient content highlights the possibility of selecting superior genotypes for future breeding programs, which are aimed at enhancing both yield and nutritional quality.
The current study revealed substantial phenotypic and biochemical variations among the 28 genotypes evaluated over three growing seasons. Significant differences were observed for all traits, indicating that there is considerable scope for improvement in perilla. The genotypes RCT-28, with the highest oil content (48.2%), and RCT-35, with the lowest (35.6%), displayed a wide range of oil yields. Previous studies have estimated that the oil content of perilla seeds falls between 37.3 and 47.8% across different accessions collected from Japan and Korea (Shin and Kim, 1994; Song et al., 2012). Similar findings were reported for Perilla frutescens by Verma et al. (2012) and Mandal et al. (2009). The seed oil content in the genotypes of perilla studied ranged from 35.6 to 48.2%, confirming the findings of Singh et al. (2017). The accessions RCT 28 (48.2%), RCT 9 (47.6%), RCT 1 (46.7%), RCT 7 (45.1%), and RCT 20 (45%) recorded more than 45% oil, making them high oil-yielding genotypes. Nine morphological parameters (Table 1), nine oil parameters (Table 2), six nutritional parameters, TPC, and TAC (Table 3) were recorded, and the mean values, range, and CV were calculated based on randomly selected 10 plants. Previous studies conducted by Bahuguna and Prasad (2014), Hussain et al. (2013), Pandey and Bhat (2008), Sharma and Hore (1994), Verma et al. (2008), and Singh et al. (2017) have reported a similar range of variation in both qualitative and quantitative traits of perilla accessions. This variability provides opportunities for selecting high oil yielding genotypes for commercial cultivation, especially in regions where oilseed crops are vital for both dietary and industrial applications. Moreover, the significant variation in micronutrient content, particularly zinc, iron, and manganese, further establishes perilla as a nutrient-rich crop. The higher manganese levels, essential for protein and carbohydrate metabolism, are a distinct advantage over other oilseed crops. The significant variation in iron and manganese content among genotypes also highlights the crop’s potential for addressing micronutrient deficiencies in human diets, particularly in rural and tribal communities where malnutrition is prevalent. The observed genetic variability in perilla enables targeted breeding for achieving enhanced climate resilience, oil quality, and disease resistance.
In this study, we identified some superior accessions that can be used in future breeding programs. K-means clustering revealed that a total of four clusters were formed (Figure 9) in which cluster 1 has nine genotypes in which RCT 30 was recorded maximum for omega-6 (39.88%), PUFAs (53.53%), omega-6: omega-3 (2.93), and PUFA: SFA (1.63). In cluster 2, a total of six genotypes were grouped out of which RCT-13 had the highest omega-6: omega-3 (2.93). Ten genotypes were grouped in cluster 3 having maximum mean values for oil content (43.72%), SFAs (38.08%), omega-3 (15.64%), and seed yield (19.78%). It has the lowest mean value for omega-6: omega-3 (RCT 4 has the least value, i.e., 1.87). In cluster 4, a total of three genotypes were grouped, in which RCT 22 displayed a maximum value of omega-9 (17.13%) and MUFAs (17.43%). This finding is consistent with previous research suggesting a lack of correlation between genetic and geographical diversity in perilla (Kang, 2004; Hussain et al., 2014; Nitta et al., 2003; Pandey and Bhat, 2008; Sa et al., 2012; Verma et al., 2008; Singh et al., 2017). It is well established that there is a close link between diet and health, with numerous studies reporting a correlation between fat intake and cardiovascular disease. Consumption of high amounts of total and saturated fats (SFAs) is associated with a greater risk of cardiovascular disease (CVD). In contrast, PUFAs may reduce the risk of CVD (Capita R and Alonso-Calleja, 2003). Powles et al. (1994) found that as the ratio of unsaturated fatty acids to saturated fatty acids (PUFA: SFA ratio) increases, so does the digestible energy (DE) value in a curvilinear manner. The seed oil of perilla is a good source of PUFAs, including a-linolenic acid (ALA), which is a significant proportion of omega-3 fatty acids when compared to other plant oils. The oil usually contains approximately 14% of the omega-6 compound (linoleic acid) and also has omega-9 (oleic acid) (Asif, 2011).
Perilla’s adaptability to the diverse climatic conditions in the NEH region, ranging from sub-tropical to temperate climates, makes it a suitable crop for rainfed agriculture in the region. The crop’s ability to grow at altitudes between 300 m and 3,500 m, coupled with its low input requirements, positions it as a promising candidate for integration into climate-resilient cropping systems. Rainfed agriculture, which dominates in the NEH, is highly vulnerable to climate variability, particularly to erratic rainfall patterns, prolonged dry spells, and soil degradation. In this context, perilla provides a sustainable alternative that can thrive under suboptimal conditions, ensuring food/oilseed and nutritional security in the face of changing climatic conditions. Climate-resilient cropping systems aim to enhance productivity while maintaining the health of ecosystems. Crops such as perilla, which can tolerate water scarcity and grow well in nutrient-deficient soils, are essential for such systems. The inclusion of perilla in intercropping or in cropping sequence with other drought-tolerant major crops can improve soil fertility, reduce pest pressure, and increase biodiversity. Additionally, perilla’s relatively short growth cycle and its ability to be cultivated on marginal and shifting lands make it ideal for farmers seeking to optimize land use sustainably. Perilla’s adaptability to suboptimal conditions, including marginal lands and erratic rainfall, makes it a valuable crop for the NEH region’s diverse agro-ecological challenges.
The high-quality oil of perilla, rich in PUFAs, particularly omega-3 and omega-6, is crucial for health. The oil has been shown to lower cholesterol levels, reduce inflammation, support brain function, and improve heart health. In regions where cardiovascular diseases and inflammatory conditions are rising due to poor dietary habits, Perilla oil offers a healthier alternative to conventional oils. The promising levels of TAC and TPC in perilla also reflect its potential health benefits, including its role in reducing oxidative stress and preventing chronic diseases. The significant variation in oil content, ranging from 35.6 to 48.2%, aligns with previous studies on perilla accessions collected from Japan and Korea. This variation provides a strong basis for selecting genotypes that can be cultivated for both nutritional and oil security in the NEH region. The genotypes identified in this study with oil content above 45%, such as RCT-28, RCT-9, RCT-1, RCT-7, and RCT-20, can be considered high oil-yielding genotypes and prioritized for future breeding programs. These genotypes not only offer high yields but also contribute to the production of high-quality oils rich in PUFAs, thereby ensuring the availability of essential nutrients for local communities.
The findings emphasize the significance of growing perilla as a climate-resilient crop in the NEH region, which is increasingly affected by climate change. With its ability to grow on marginal lands and tolerate drought, perilla is well-suited to the region’s unique agro-ecological conditions. Its cultivation provides not only a sustainable source of high-quality oils and essential nutrients but also enhances food and oil security in the face of climate variability. Integrating perilla into rainfed cropping systems can relieve the pressure on traditional crops that are more vulnerable to erratic weather patterns. By diversifying agriculture with perilla, farmers can reduce the risk of crop failure due to droughts and fluctuating rainfall, ensuring a more stable income and nutritional source. Furthermore, perilla’s adaptability to intercropping and its role in improving soil health through root systems and nutrient cycling make it a valuable component of sustainable farming systems amidst climate change.
The insights gained from this study are crucial for developing breeding programs aimed at selecting perilla genotypes with desirable traits for climate-resilient agriculture. The positive correlations observed between seed yield and nutrient content (Cu, K, and Fe) suggest that these traits can be co-selected to improve both yield and nutritional quality of perilla. Similarly, the strong representation of omega-6 and PUFAs in the PCA underscores the importance of these traits in future breeding efforts. To fully harness perilla’s potential, further research is needed to explore the genetic basis of its resilience to abiotic stresses, such as drought and nutrient-deficient soils. Molecular studies, including marker-assisted selection and genomic selection, can accelerate the development of perilla varieties that are not only high-yielding but also climate-resilient.
Perilla demonstrates significant potential as a climate-resilient oilseed crop for the rainfed regions of the North Eastern Himalayas. This study highlights substantial genotypic variability in traits such as oil content, nutrient composition, and biochemical properties, and provides a foundation for breeding programs that target both higher yields and improved nutritional quality. Perilla’s adaptability to diverse climatic conditions and its low input requirements make it an ideal candidate for sustainable agriculture in the NEH, particularly in marginal and drought-prone lands.
The high-quality oil from perilla seeds, rich in polyunsaturated fatty acids (PUFAs), particularly omega-3 and omega-6, plays a crucial role in human health. The ideal omega-6: omega-3 ratio of 1:1 to 2:1, observed in perilla oil, is associated with reduced the incidence of many chronic diseases. Additionally, the positive impact of perilla on soil health due to less nutrient-required crops, biodiversity, and its potential in intercropping systems strengthens its role in climate-resilient agriculture systems. Future breeding efforts should improve perilla climate resilience by focusing on sustainable agriculture and human health, as well as changing these findings into practical breeding and cultivation strategies for widespread adoption.
The original contributions presented in the study are included in the article/Supplementary material. For any further clarifications, readers are encouraged to contact the corresponding authors.
MAA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. AmK: Data curation, Investigation, Supervision, Writing – review & editing. TPAS: Data curation, Formal analysis, Methodology, Writing – review & editing. AlK: Data curation, Formal analysis, Writing – review & editing. SSR: Data curation, Formal analysis, Investigation, Writing – review & editing. MHA: Formal analysis, Writing – review & editing, Data curation, Software, Writing – original draft. RS: Formal analysis, Writing – review & editing. MS: Formal analysis, Writing – review & editing, Data curation. JL: Writing – review & editing, Formal analysis. NP: Investigation, Project administration, Supervision, Visualization, Writing – review & editing. VKM: Investigation, Project administration, Supervision, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the AICRP on Potential Crops.
The authors acknowledge the facilities and support received from the Joint Director ICAR RC for NEH Region, Manipur Centre, and the Director, ICAR Research Complex for NEH Region, Umiam. We also express thanks to the Director, ICAR-National Research Centre for Grapes, Pune, India for providing the facility for the fatty acids profiling analysis in the National Referral Laboratory. The authors express their gratitude to the project staff and technical officer for their assistance during the project. The authors are gratefully acknowledged to AICRP on Potential crops, ICAR-NBPGR, New Delhi for financial support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2025.1549061/full#supplementary-material
Ansari, M. A., Pandey, A., Kumar, S., Kumar, A., Sangeeta, M., Meitei, C. B., et al. (2019). Evaluation of genetic variation in Perilla for agro-morphological and quality traits. Indian J. Agric. Sci. 89, 940–945.
Asif, M. (2011). Health effects of omega-3, 6, 9 fatty acids: Perilla frutescens is a good example of plant oils. Oriental Pharm. Exp. Med. 11, 51–59. doi: 10.1007/s13596-011-0002-x
Bahuguna, A., and Prasad, B. (2014). Plant development and yield as prejudiced by Perilla (Perilla frutescens) germplasm lines in India hill condition. Res. J. Med. Plant. 8, 121–125. doi: 10.3923/rjmp.2014.121.125
Capita RAlonso-Calleja, C. (2003). Intake of nutrients associated with an increased risk of cardiovascular disease in a Spanish population. International journal of food sciences and nutrition, 54, 57–75.
Food and Agricultural Organization (1992). Minor oil crops 94 : Agricultural Services Bulletin, 107. Rome, Italy.
Hussain, S., Changkija, S., and Hore, D. K. (2013). Variability studies in some Perilla (P. frutescens L. Britton) accessions of north-east India. Int. J. Bio-Resour. Stress Manag. 4, 230–236.
Hussain, S., Changkija, S., and Hore, D. K. (2014). Genetic divergence analysis in Perilla [Perilla frutescens (L.) Britton]. Indian J. Hill Farm. 27, 10–15.
Kang, J. X. (2004). Balance of omega-6/omega-3 fatty acids is important for health: the evidence from gene transfer studies. World Rev. Nutr. Diet. 95:93.
Liu, W., Shahid, M. Q., Bai, L., Lu, Z., Chen, Y., Jiang, L., et al. (2015). Evaluation of genetic diversity and development of a core collection of wild rice (Oryza rufipogon Griff.) populations in China. PLoS One 10:e0145990. doi: 10.1371/journal.pone.0145990
Mandal, S., Suneja, P., Hussain, S., Hore, D. K., and Verma, N. (2009). Seed oil quality of Perilla frutescens (L.) Britt. genotypes. Ind. J. Plant Genet. Reso. 22, 123–128.
Marcocci, L., Maguire, J. J., Droylefaix, M. T., and Packer, L. (1994). The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Comm. 201, 748–755. doi: 10.1006/bbrc.1994.1764
Nelson, D. W., and Sommers, L. E. (1973). Determination of total nitrogen in plant material. Agron. J. 65, 109–112. doi: 10.2134/agronj1973.00021962006500010033x
Nitta, M., Lee, J. K., and Ohnishi, O. (2003). Asia Perilla crops and their weedy forms: their cultivation, utilization and genetic relationships. Econ. Bot. 57, 245–253. doi: 10.1663/0013-0001(2003)057[0245:APCATW]2.0.CO;2
Pandey, A., and Bhat, K. C. (2008). Diversity distribution and collection of genetic resources of cultivated and weedy type in Perilla frutescens (L.) Britton var frutescens and their uses in Indian Himalaya. Gene. Resour. Crop Eval. 55, 883–892. doi: 10.1007/s10722-007-9293-7
Powles, J., Wiseman, J., Cole, D. J. A., and Hardy, B. (1994). Effect of chemical structure of fats upon their apparent digestible energy value when given to young pigs. Anim. Prod. 58, 411–417.
Sa, K. J., Kim, J. A., and Lee, J. K. (2012). Comparison of seed characteristics between the cultivated and the weedy types of Perilla species. Hort. Env. Biot. 53, 310–315. doi: 10.1007/s13580-012-0031-5
Sharma, B. D., and Hore, D. K. (1994). Perilla: untapped oilseed crop of hills. J. North Eastern Counc. 14, 14–15.
Shin, H., and Kim, S. (1994). Lipid composition of Perilla seed. J. Am. Oil Chem. Soc. 71, 619–622. doi: 10.1007/BF02540589
Singh, S. K., Kole, P. C., Misra, A. K., Roy, S., Arya, L., Verma, M., et al. (2017). Characterization of Perilla frutescens (Linn.) Britt based on morphological, biochemical and STMS marker. Ind. Crop. Prod. 109, 773–785. doi: 10.1016/j.indcrop.2017.09.045
Singleton, V. L., and Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158. doi: 10.5344/ajev.1965.16.3.144
Smith, S. E., Guarino, L., Doss, A. A., and Conta, D. M. (1995). Morphological and agronomic affinities among Middle Eastern alfalfas accessions from Oman and Yemen. Crop Science. 35, 1188–94.
Song, J. Y., Lee, J. R., Oh, S., Kim, C. Y., Bae, C. H., Lee, G. A., et al. (2012). Assessment of genetic diversity and fatty acid composition of Perilla (Perilla frutescens var frutescens) germplasm. Korean J. Plant Res. 25, 762–772.
Tabanca, N., Demirci, B., Ali, A., Ali, Z., Blythe, E. K., and Khan, I. A. (2015). Essential oils of green and red Perilla frutescens as potential sources of compounds for mosquito management. Ind. Crop. Prod. 65, 36–44. doi: 10.1016/j.indcrop.2014.11.043
Verma, N., Bist, I. S., Negi, K. S., and Hore, D. K. (2008). Morphological diversity in Perilla frutescens (L.) Britton accessions from the Indian Himalayas. Pusa Agric. Sci. 31, 15–12.
Verma, N., Suneja, P., Saxena, S., and Bist, I. S. (2012). Perilla frutescens: a potential oilseed crop for the Indian Himalayas. Prog. Agric. 12, 164–168.
Keywords: antioxidants, climate resilient genotypes, fatty acids, nutritive composition, perilla, phenolics, North Eastern Himalayan region
Citation: Ansari MA, Kumar A, Ahammed Shabeer TP, Kumar A, Roy SS, Ansari MH, Singh R, Sangeeta M, Layek J, Prakash N and Mishra VK (2025) Exploring the genotypic potential of perilla (Perilla frutescens L.) for climate-resilient agriculture in the North Eastern Himalayas. Front. Sustain. Food Syst. 9:1549061. doi: 10.3389/fsufs.2025.1549061
Received: 20 December 2024; Accepted: 15 January 2025;
Published: 10 February 2025.
Edited by:
Raul Avila-Sosa, Benemérita Universidad Autónoma de Puebla, MexicoReviewed by:
Venugopalan Visha Kumari, ICAR-Central Research Institute for Dryland Agriculture, IndiaCopyright © 2025 Ansari, Kumar, Ahammed Shabeer, Kumar, Roy, Ansari, Singh, Sangeeta, Layek, Prakash and Mishra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amit Kumar, YW1pdDQxMThAZ21haWwuY29t; Meraj Alam Ansari, bWVyYWppYXJpQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.