- 1ICAR Research Complex for North Eastern Hill Region, Umiam, India
- 2ICAR-Central Tobacco Research Institute, Hunsur, India
- 3ICAR-National Bureau of Plant Genetic Resources, New Delhi, India
- 4ICAR Research Complex for North Eastern Hill Region, Manipur Centre, Imphal, India
Climate change, land degradation, and shrinking land resources are major limitations for increasing crop productivity in the East Himalayan Region (EHR). Agroforestry having a plethora of complementarities is a preferable land-use option for improving agricultural productivity while conserving the natural resources. The effects of agroforestry systems with Gamhari (Gmelina arborea) (GAFS) and Alder (Alnus nepalensis) (AAFS) as tree components, on the soil nutrients, physiological characteristics, and productivity of turmeric (Curcuma longa L.), elephant foot yam (Amorphophallus paeoniifolius), and colocasia (Colocasia esculenta), were assessed in a split plot design with trees in the main plots and understorey crops in sub-plots. The hypothesis of the study was the tree components had enriched the soils and favorably influenced physiological attributes of the understorey crops, enhancing the yields and maximising systems productivities. AAFS canopy had a higher (p < 0.05) leaf area index (LAI = 2.19) than the GAFS (LAI = 1.01). AAFS recorded 32% lower (p < 0.05) photosynthetically active radiation (PAR) than sole crops under treeless conditions (TLS). ANOVA revealed significant interactions (p < 0.01) between tree systems and the understorey crops with their influence on chlorophyll content (SCMR), leaf thickness (LT), stomatal size (SS), stomatal frequency (SF), stomatal conductance (gs), photosynthetic rates (A), transpiration rates (E), intercellular CO2 concentration (Ci), instantaneous water use efficiency (iWUE) and crop yields (YLD). SCMR, SS, SF, gs, iWUE, and YLD in GAFS and AAFS increased significantly (p < 0.05) over TLS, whereas, SF and E significantly decreased (p < 0.05). Regression of physiological traits on yields showed SS (b = 0.0884, p = 0.002), gs (b = 0.00934, p = 0.018), and iWUE (b = 0.2981, p = 0.008) influenced positively, whereas SF (b = −0.0381, p = 0.019) and E (b = −2.304, p = 0.02) negatively impacted the YLD of understorey crops. Alder-turmeric system harnessed the attenuated light with better soil fertility most favorably, supporting high SCMR, low E, high A, high gs to produce higher YLD. Turmeric achieved the highest system productivity (USD 4,281 ha−1 year−1) under the AAFS. Soil pH, organic carbon and nitrogen were significantly enriched (p < 0.05) after 14 years of converting the lands to agroforestry systems. Alder-turmeric was the most effective tree-crop pairing delivering enhanced productivity, soil health and economic returns for sustainable agriculture in the EHR.
Introduction
Agricultural production is considerably affected by rapid global climate change. Frequent extreme weather events like uneven precipitation, droughts, floods, temperature fluctuations, affect crop physiology and thereby reduce the crop yields (Annie et al., 2023; Layek et al., 2023; Rangappa et al., 2024). Climate change has accelerated land degradation, impacting the sustainable growth in agricultural production to support the burgeoning population (Eekhout and de Vente, 2022; Bibi and Rahman, 2023). The climate models for Eastern Himalayan Region (EHR) indicate significant changes in temperature and precipitation (Rajbhandari et al., 2015; Choudhury et al., 2019). EHR faces severe land degradation due to unprecedented soil erosion, extensive practice of Jhum farming and wide spread low pH soils across hill terrains that pose significant challenge for achieving sustainable agriculture (Das et al., 2017; Hazarika et al., 2021). Jhum farming, a form of shifting cultivation or Swidden Agriculture is an age-old crop cultivation system practiced by the tribal farmers where the forest vegetation/biomass is slashed, dried in situ and burnt on the field. The fields are cultivated for 2 to 3 years and thereafter abandoned to regenerate before a new area is searched to repeat the process. In earlier times, the fallow period was 20 to 25 years before the lands were revisited for cultivation. Because of the expanding population in the hills and the decrease in per capita land availability, the fallow period has shortened to less than 5 years, the period too short for the lands to rejuvenate. Moreover, high-intensity rainfall and slopping land configuration lead to high soil acidity, nutrient loss, soil erosion, and associated land degradation processes. The average annual soil organic carbon loss due to soil erosion is 703 kg ha−1 year−1 in the region (Ramesh et al., 2015). About 36.6% of the total geographical area of the region is degraded; nearly double the national average of 20.17%.1 Soil loss from the hill slopes in the shifting cultivation land (Jhum farming) was reported at 147, 170, and 30 t ha−1 year−1 in the first year, second year, and abandoned Jhum, respectively (Singh and Singh, 1981). The productivity of such lands is very low. Small landholding, low productivity, and shrinking land resources due to the burgeoning population make agriculture on such lands unsustainable.
Agroforestry systems (AFS) can play an important role in adapting to degraded ecosystems in aberrant climatic conditions (de Stefano and Jacobson, 2017; Dagar and Gupta, 2020) by amending microclimates (Gomes et al., 2020). Agroforestry, which deliberately includes trees in the agricultural production system, is often advocated as a climate-smart solution. AFS provide multiple benefits in terms of improving the soil fertility and hydrological properties, enhancing land use efficiency by vertical expansion, providing insurance against sudden crop failures due to extreme climate events, and providing multifarious livelihood needs such as fuel, fodder, fiber, food, etc. (Waldron et al., 2017; Mbow et al., 2014; Masoodi et al., 2013). Many traditional AFS viz., Alder-large cardamom (Alnus nepalensis-Amomum subulatum), arecanut (Areca catechu) with pineapple (Ananas comosus) and black pepper (Piper nigrum), Khasi mandarin (Citrus reticulata)-based AFS, Parkia roxburghii based AFS, home gardens are practiced in the northeast hill region of India (Bhatt et al., 2001). However, the effectiveness of AFS depends on how the complementarities and synergies among different components are harnessed and converted into economic gain while continuing to provide ecosystem services. Complementary integration of trees and crops not only provide ecosystem services, arrest land degradation, soil fertility enhancement, prevent soil erosion, and better adapt to future climate change situations but also increase crop yield and yield stability, especially within adverse climatic conditions to enhance the livelihood of the rural/tribal population. Higher system productivity and diversified products coupled with high economic returns are essential for the acceptance and spread of the system among the resource-poor tribal farmers of the region.
Agroforestry designs (spatial and temporal) consisting of various tree species can significantly improve soil fertility, making available specific nutrients and moisture favorable for the associated understorey crops (Sinacore et al., 2017). Modification in soil and micro-climate often causes a variety of changes in the phenology (Broadhead, 2015), morphology (Buchanan et al., 2019), and physiology (Lin, 2007) of understorey crops. Interactions between trees and understorey crops are significant, making it crucial to choose the right understorey crops to pair with tree species based on a range of agronomic and physiological characteristics. The structure and density of the tree canopy significantly affect the amount and quality of light reaching the crops growing under their canopy. Agroforestry moderates the microclimates by decreasing evaporation, improving water and nutrient recycling, and enhancing soil carbon and microbial activity (Barrios et al., 2012; Kuyah et al., 2017). Investigation of the photosynthetic characteristics would help to understand the fitness of the understorey crops to the microenvironment below the tree canopy.
Moderation of micro-climate in the AFS can improve gas exchange, stomatal plasticity, moisture status, and water use efficiency by the system, depending on the tree and companion understorey crop species (Lasco et al., 2014; Arenas-Corraliza et al., 2019). Increased temperatures often cause high photorespiration in most C3 plants. Therefore, any reduction strategy by restricting high solar radiation and high temperature through shading would alleviate damage from photorespiration which could be one of the potential and key strategies for climate change adaptation. Drought and low moisture driven leaf temperature and plant water loss changes is another issue affecting crop productivity. Alternatively, adapting to shade conditions, such as AFS, without affecting the photosynthetic efficiency can lower transpiration loss and improve water use efficiency and nutrient availability for crop uptake, contributing to higher yield. Therefore, limiting transpiration while maintaining or enhancing photosynthetic rate should be the major criterion for selecting annual crops to adapt to modified micro-climate in the AFS.
Shade-tolerant species have higher plasticity in the physiological characteristics than the sun species (e.g., most C4 plants), which helps them to exploit the favorable micro-environment provided by AFS. For example, cocoa, a shade loving understorey crop, exhibited saturation between 200 and 750 μmol m−2 s−1 photosynthetically active radiation (PAR) levels with low light compensation point (LCP) in the range of 5–57 μmol m−2 s−1 (Daymond et al., 2011; Almeida et al., 2014; Ávila-Lovera et al., 2016; Tezara et al., 2016), maximum net carbon assimilation (A) rates between 1 to 8 μmol m−2 s−1 (Suárez Salazar et al., 2018). Physiological characteristics viz., growth, stem photosynthesis, and leaf-level water use efficiencies of cocoa were affected by different overstorey shade tree species (Carvalho et al., 2023). In association with Cariniana pyriformis in multi-storey cropping, cocoa exhibited a better photosynthetic ratio in all seasons. Cultivation of taro (Colocasia esculenta) and millet (Pennisetum glaucum) under Adansonia digitata and Parkia biglobosa in Burkina Faso revealed that taro produced higher biomass production under trees compared to sole cultivation by increasing its leaf area index (LAI), and thus avoiding reduction in net photosynthesis (PN), while millet showed an opposite trend, signifying taro was more efficient in effectively exploiting the tree-crop interaction in its favour to enhance productivity (Sanou et al., 2012).
Shading is important for the growth and development of intercrops like turmeric. Under bamboo canopy, turmeric performed better with light transmittance of 66 and 86% and hence required a minimum bamboo spacing of 8 × 8 m for higher production of turmeric in Kerala, India (Kittur et al., 2016). Further, they also inferred that higher LAI, lower photosynthetic active radiation (PAR), and increased root competition for nutrient uptake of the intercrop in denser bamboo spacing (4 × 4 m) resulted in decreased turmeric rhizome yield. Similarly, Dhillon et al. (2009) found that turmeric preferred partial shade for higher production without significantly reducing net photosynthesis, stomatal conductance, and transpiration. Alam et al. (2020) witnessed higher net photosynthesis in turmeric (17.40 mmol m−2 s−1) under 50% shade followed by 33% shade (15.30 mmol m−2 s−1) in the Bundelkand region of India, thus indicating 50% shade helps in increasing the above and below ground biomass production of turmeric. In 80% shade, leaf area remained significantly lower than 70% shade levels as the development and expansion of leaves reduced under sub-optimal solar radiation, adversely affecting photosynthesis, growth, and leaf formation (Sharangi et al., 2022). Full light substantially decreased its photosynthetic rate due to the high photorespiration process. The yields of rhizomes under silk tree (Albizia chinensis) stands were higher than full light, due to reduced photorespiration by Curcuma xanthorrhiza (Indonesian turmeric or temulawak) plants under low light conditions (Purnomo et al., 2018). In species with low light requirements, high irradiance may significantly increase photorespiration generating photo-inhibitory effects, decreasing electron transport, photosynthetic rates and carbon acquisition as reported in Cabralea canjerana, Cariniana estrellensis in Brazil (Calzavara et al., 2019). The transpiration (E) rates of crops were the lowest under shade than that under the open conditions. Most water use efficiency (WUE) studies use instantaneous measurements of leaf photosynthesis and transpiration, assuming they are representative of whole-plant WUE (Martorell et al., 2015). Therefore, the key to any successful agroforestry model would be the appropriate selection of components to optimize the overall system productivity (Bijakal et al., 2019).
Colocasia (Taro) and Elephant Foot Yam (EFY) were traditionally the principal food crops of most indigenous people across the major continents. They are primarily grown within subsistence farming systems in the EHR for their edible tubers and nutrient-rich leaves. They are contingency crops, providing a reliable food source in situations when the availability of other food crops is scarce. Colocasia was the original staple crop of the Nagas, the tribals of one of the EHR states called Nagaland. They are presumed responsible for the domestication and diffusion of Colocasia into other parts of the world (Mills, 1937). Elephant Foot Yam (Amorphophallus paeoniifolius) is also a nutrient-dense root crop in the diet of the rural population in many developing countries. Turmeric (Curcuma longa L.) is a perennial rhizomatous cash crop native to the Indian subcontinent and Southeast Asia. It is a valuable crop with very high culinary importance, and a constituent of many traditional Indian medicines and Ayurvedic formulations (Sasikumar, 2007; Dash et al., 2024). India is the world’s largest producer, consumer, and exporter of turmeric. India holds a 62% share of the world turmeric trade (Government of India, 2024). Turmeric is also the primary cash crop of the tribal farmers of the EHR in which they cultivate turmeric on sloping cultivable land by the Bun system, a variant of the Shifting cultivation practice. The region produces the world’s finest quality turmeric, locally known as Lakadong. This variety is distinguished by its exceptionally high curcumin content (8.5–9.0%) in the rhizome.
The yield and shade tolerance of understorey crops is regulated by specific plant traits and, the plasticity of the morpho-physiological characters to adapt to climate change. Yet data on their physiological efficiency in different tree-crop combinations within the agroforestry systems, particularly in the EHR where the practice is extensive, are insufficient. Therefore, an experiment was conducted in the mid-altitude uplands of Meghalaya, using 14 years old AFS having the Alder (Alnus nepalensis) and Gamhari (Gmelina arborea) as tree components and three shade-tolerant crops Colocasia (tuber crop), Elephant Foot Yam (tuber crop) and Turmeric (rhizomatous spice crop) as understorey crops in the tree inter-row spaces and sole crop in open conditions without any tree components were cultivated with the aim to test the hypothesis that the long-term effects of the tree components had enriched the soils of the AFS. The leaf and physiological plasticity of the understorey crops in response to the modified environment of attenuated light and better soil fertility in the AFS effectively harnessed the tree-crop synergies, enhancing total system productivity and economic returns compared to the sole cropping.
Materials and methods
Location, soil and climate of the study area
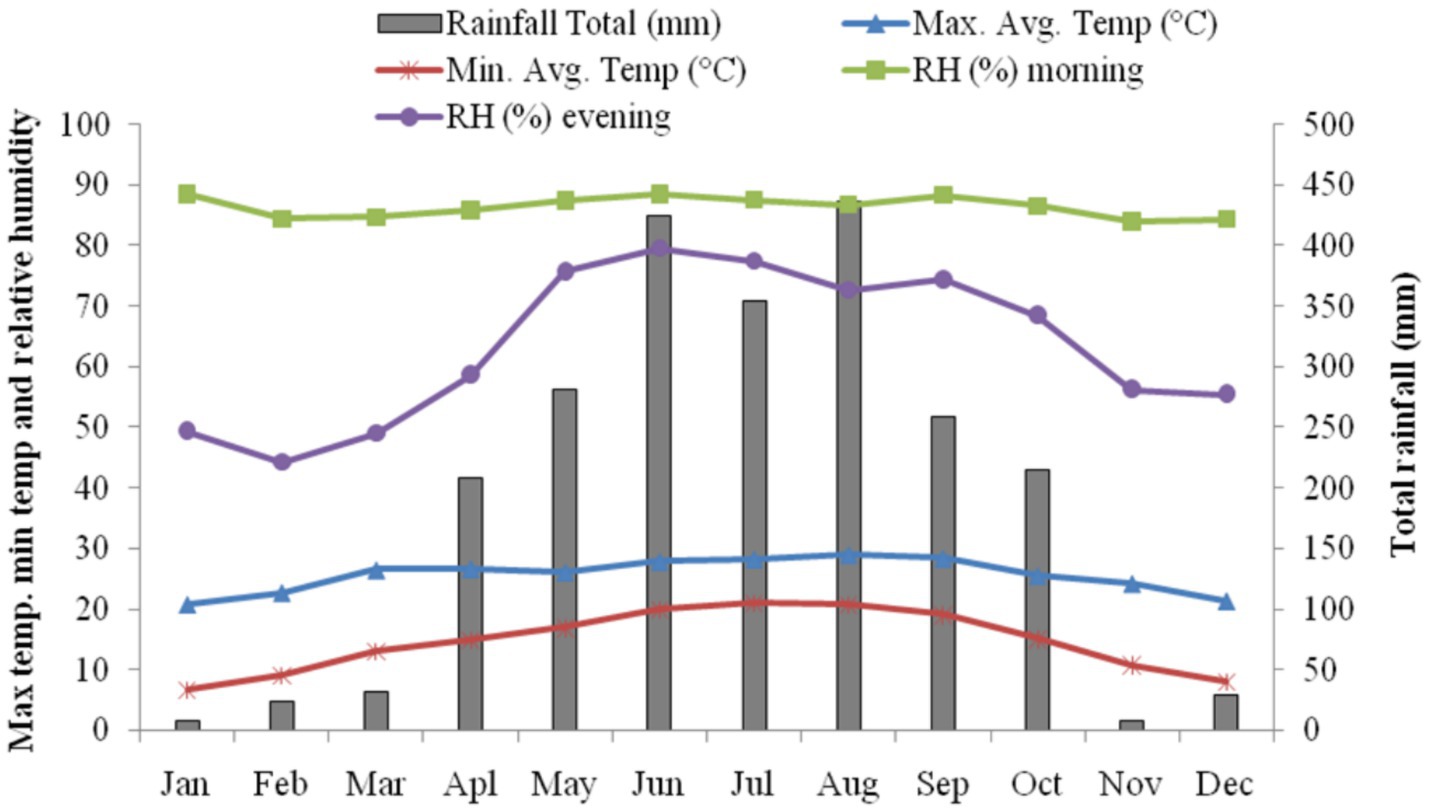
The experimental site is at the East Khasi Hills of the Meghalaya state in the North-eastern part of India (25° 40′ 45″ N latitude, 91°54′ 46″ E longitude, 993 m altitude). The soil is clay loam in texture, acidic in reaction (pH 4.36–4.76), and rich in organic matter. Long term (27 years) average annual rainfall of the locality was 2410.4 mm (±373.4); mean maximum temperature, minimum temperature, sunshine hours, relative humidity and pan evaporation were 24.81°C, 15.75°C, 5.42 h day−1, 74.40%, 1057.6 mm year−1, respectively (Choudhury et al., 2012). The mean maximum temperature during the experimental period ranged from 21.16°C to 25.94°C, whereas the mean minimum temperature ranged from 4.97°C to 12.59°C. The maximum relative humidity in morning hours was between 74.29 to 88.00%, and the minimum was 33.86 to 62.86%. The station received an annual rainfall of 2,200 mm. The pan evaporation varied from 1.93 to 3.61 mm per day. The experimental site falls under a per-humid subtropical climate. Meteorological data during the period of experimentation is depicted in Figure 1.
Layout of experiment
A field study was conducted in 14-year-old Alder and Gamhari based AFS models in the experimental farm of the Indian Council of Agricultural Research Complex for North Eastern Hill Region (ICAR RC NEH), Umiam, Meghalaya, India (Figure 2). The land with an average slope of 7.5% was converted into terraces of approximately 5 m in width. The Alder and Gamhari trees were planted on the contours along the terrace risers at a spacing of 5 m with a tree density of approximately 400 trees per ha. The Alder, and Gamhari agroforestry systems and a control without trees were considered as three treatments in the main plot. Three understorey crops: turmeric (Curcuma longa L.), elephant foot yam (Amorphophallus paeoniifolius), and Colocasia (Colocasia esculenta L.) were planted as subplot treatments during the first week of April on the terraces in alignment with the contours (Figure 3). The row-to-row and within-row spacing for turmeric, colocasia, and EFY were 40 cm × 30 cm, 50 cm × 50 cm, and 80 cm × 80 cm, respectively. About 50 cm of space was left blank on all sides of the tree trunk. A 50 cm width empty space was also left from the ridge and toe of the terrace risers. The average vertical interval between the terraces was 35 cm for manually prepared terrace risers. All three understorey crops were also grown in open fields without trees as a control treatment for comparison. Data on yield attributes were recorded on 10 randomly selected plants from the central rows, leaving a border row around each plot to nullify border effects. The average data of the plants were scaled to estimate a yield per hectare. For ease of expression, we will use the abbreviations for the Alder-based system, Gamhari-based system, and open/treeless plots as AAFS, GAFS, and TLS in the subsequent sections.
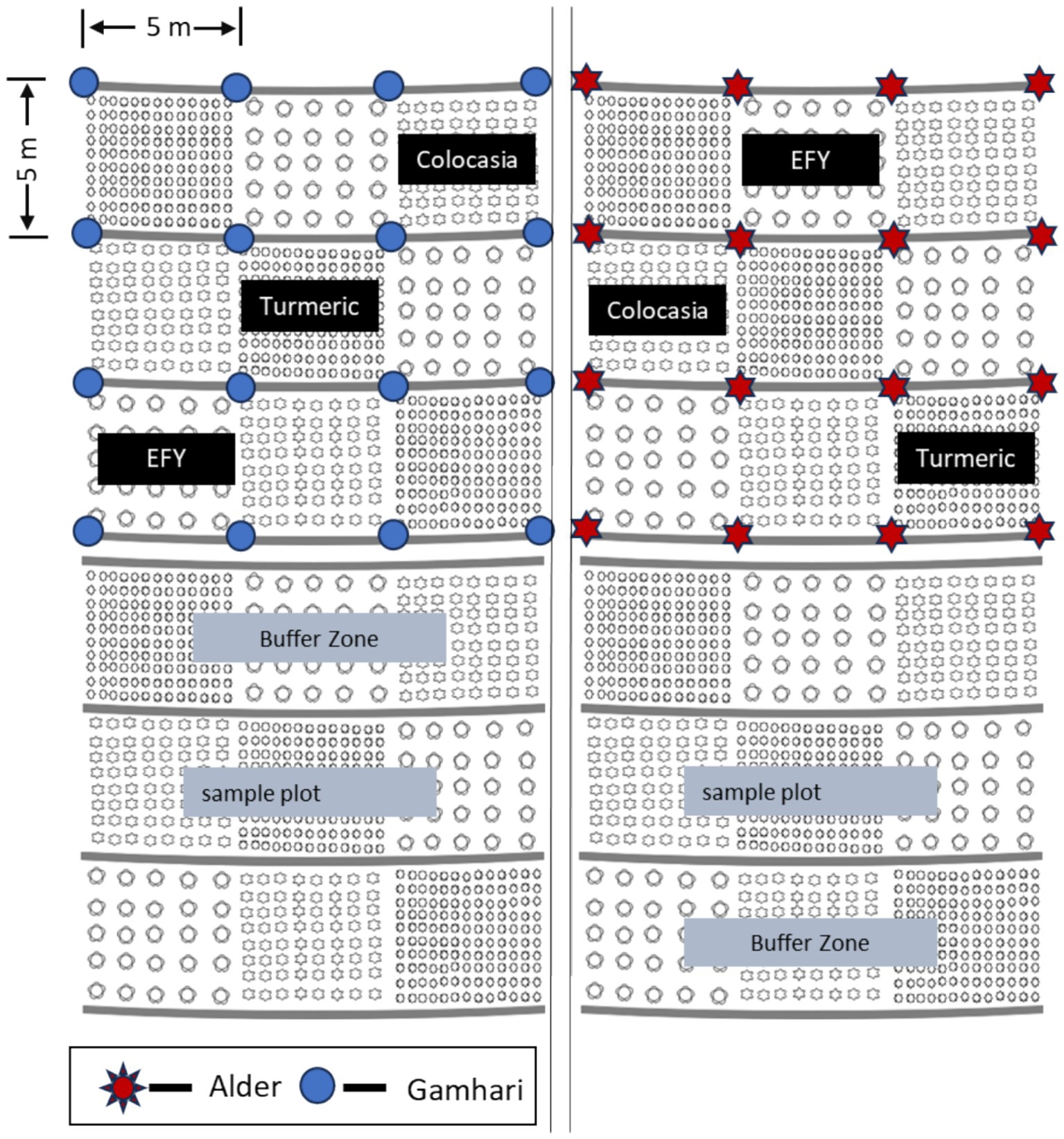
Figure 3. Layout of the experiment with trees and sole cropping as main plot treatments (n = 3) and understorey crops as sub-plots treatments (n = 3) in a split plot design.
Soil sampling and analysis
Composite soil samples in triplicate were collected from the AAFS, GAFS, and TLS at two depths (0–15 cm) and (15–30 cm) before setting up the experiment to assess the long-term effect of the tree species on soil properties. The background management in agroforestry and plots without trees were similar. Therefore, variations in the soil properties were considered to be due to the long-term effect of trees. The growth of the under-storey crops was influenced by the above ground environment created by the tree canopy and the below ground soil environment modified by the trees. The below ground effect is the accrued effect over 14 years. Therefore, we consider the effects of trees are the inclusive effect of both above and below ground environment created by the trees. The soil samples in each system- Alder, Gamhari, and TLS, were bulked separately, and composite samples for each land use were constituted. All the soil samples were air dried under shade and sieved through a 2.0 mm sieve. Soil bulk density was measured at two depths using the core sampling method with the help of a core augur (Allen et al., 1974). Soil pH was determined by a digital pH meter in soil water suspensions made of soil and distilled water in 1:2 ratio (Jackson, 1973); soil organic carbon (SOC) was estimated by Walkley and Black (1934). The soil available nitrogen content was determined by following Subbiah and Asija (1956). Initially, 20 g soil sample was added to 100 mL 0.32% potassium permanganate to which 100 mL 2.5% sodium hydroxide (NaOH) was added. The mixture was heated in a distillation unit, and the liberated ammonia (NH₃) was collected in a conical flask containing 20 mL of 4% boric acid (H3BO3) solution. The excess boric acid was titrated with standard (0.01 N NaOH) using a methyl red indicator. For determination of soil available potassium (exchangeable K), 5 g soil was diluted to 25 mL of 1 N ammonium acetate solution (1:5 ratio of soil: extractant). After 30 min of shaking, the solution was filtered. The final volume was adjusted to 25 mL with distilled water. The solution was used to determine the available potassium on a flame photometer (Jackson, 1973). Soil available phosphorus was estimated by following Bray and Kurtz (1945), where soil was extracted with the help of 0.5 M sodium bicarbonate (NaHCO3). The use of NaHCO3 helps in releasing the available soil phosphorus in the solution. This extracted solution was reacted with ammonium molybdate ((NH4)2MoO4). The phosphomolybdate complex was reduced to form “molybdenum blue” by ascorbic acid (C6H8O6). The intensity of the blue color was measured using a spectrophotometer at 600 nm wavelength.
Measurement of tree canopy parameters
LAI and mean foliage inclination of the tree canopy were recorded with the help of a Plant Canopy Analyzer (CI-100, CID, Inc., Vancouver, Washington), having a digital camera attachment and fish eye (150°) lens positioned at the end of a 0.8 m (2.62 ft.) probe. The measurements were recorded during the forenoon with the least light inclination. The software provided with the canopy analyzer divided the images into user-defined numbers of zenith and azimuthal divisions (Chen et al., 2005). Then, it analysed the fraction of sky (solar beam transmission coefficient) visible in each sector by tallying with light (monochrome). Images for each canopy were captured at five sampling points for each agroforestry system by placing the fisheye lens at the end of the probe at the base of the tree bole, and the average values were calculated.
Physiological attributes of understorey crops
The physiological parameters were recorded on three fully developed leaves per plant at the active growth stage of the understorey crops (Turmeric, Colocasia, and Elephant Foot Yam) away from the border rows. The total chlorophyll content [SPAD Chlorophyll Meter Reading (SCMR)] was measured by a SPAD meter (Konica Minolta, Japan). The photosynthetic parameters such as stomatal conductance, leaf temperature, intercellular CO2, transpiration, and photosynthetic rates were measured by a Portable Photosynthetic System (PP System), model Walz GF 3000, during the forenoon of a sunny day. The instantaneous water use efficiency at leaf level (iWUE) was calculated from the gas exchange data using the following formula (Medrano et al., 2015) (Equation 1):
The stomatal characteristics of the abaxial surface of mature leaves were analysed using thermocole-xylene, a paste prepared by dissolving thermocole (a styrene polymer) in xylene (Layek et al., 2023; Rangappa et al., 2024). A thin layer of the thermocole paste was applied to the leaf surface, allowed to dry, and then carefully peeled off using forceps to avoid damage. The peels were placed on glass coverslips with a few drops of water and mounted on a compound microscope (BX-50F, Olympus, Japan). Stomatal parameters, including stomatal density, size, and the number of guard cells, were measured in three distinct microscopic fields at 40× magnification. Stomatal frequency and stomatal index were calculated using the following formulas (Equations 2, 3):
The leaf thickness, expressed in μm, was measured by a digital vernier calliper (Mitutoyo Corporation, Japan) by clamping fresh leaves in non-destructive way (Hazarika et al., 2021).
Mean annual increment of tree volume and economics of system productivity
For estimating the volume of wood in the standing trees in the AFS, five trees per replication were randomly selected, and their diameter at breast height (diameter at 1.37 m from the ground) was recorded with the help of a calliper. The height of the trees was measured by an Abney’s level. Published allometric equations for the two species were used to estimate the volume of Individual trees.
For Alnus nepalensis, the following allometric equation was used to estimate the volume of wood per tree (Sharma and Pukkala, 1990) (Equation 4).
The equation for calculation of tree volume of Alder was originally computed from allometric equation which is commonly utilized for describing relations between tree dimensions.
For Gmelina arborea, the allometric equation used for volume estimation (Equation 6) (Carbon Stocks Report, Forest Survey of India, https://fsi.nic.in/carbon-reports) was:
The mean annual volume increment per ha was estimated by dividing the total volume per ha by the total growth period of the trees in years. The annual productivity of wood and the understorey crops was converted to monetary value based on the current market prices of the locality and summed to get the gross system productivity in US dollars (USD).
Statistical analysis
A split plot experimental design with 5 replications was performed where Alder AAFS, GAFS and TLS were treated as main plot treatments and the three understorey crops colocasia, elephant foot yam and turmeric were treated as subplots randomized within the main plots. Parameters for which the F-test was significant, LSD as a post hoc test was conducted for comparison of means at 5 percent level of significance (p = 0.05) (Gomez and Gomez, 1984). When the interactions between tree system and understorey crop species were significant, the means of the leaf, physiological and yield parameters were compared at the same level of sub-plot treatment. Statistical analysis was performed by using “agricole” package version 1.3-7 in R. Linear regressions and curve fitting were done to assess the effect of different physiological characters on yield of each crop.
Results
Physico-chemical properties of soil
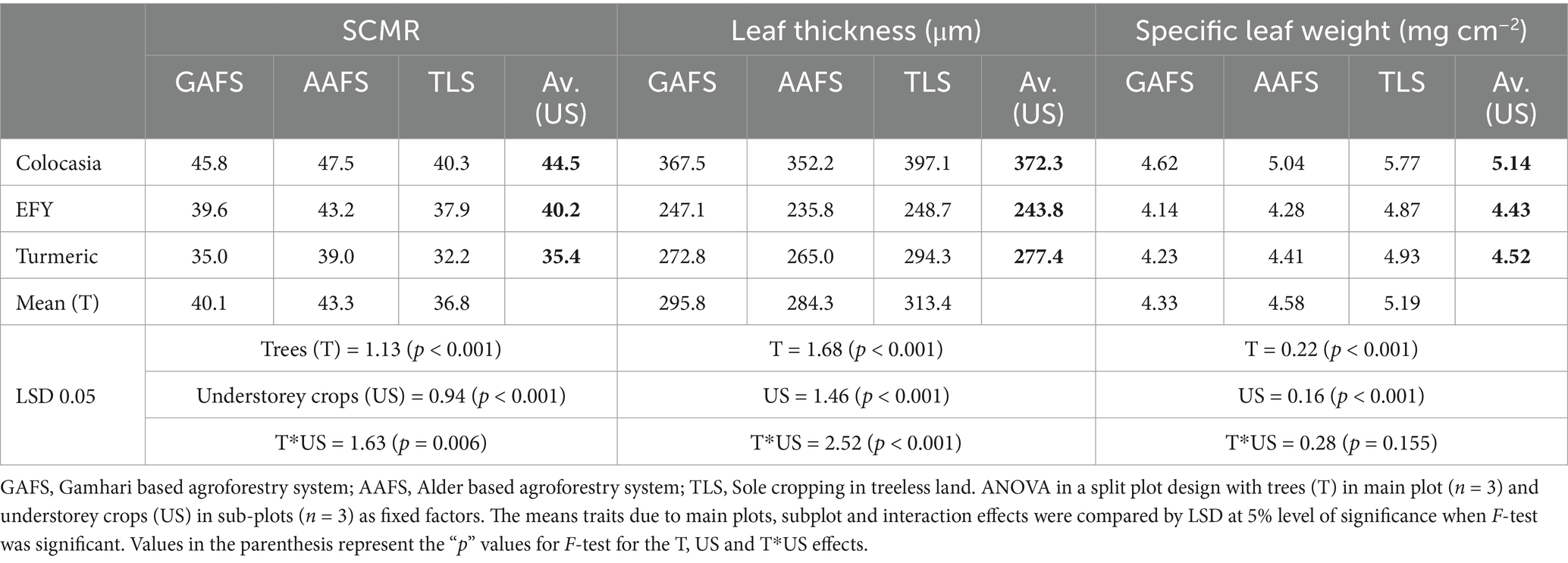
Analysis of variance of the initial soil properties revealed statistically significant variation among the land-uses for bulk density (p = 0.02), soil pH (p < 0.001), soil organic carbon (p < 0.001), available nitrogen (N) (p < 0.001), available phosphorus (P) (p < 0.001) and available potassium (K) (p = 0.004) at 0–15 cm depth (Table 1). At 0–15 cm depth, the soil bulk density in AAFS (1.22 Mg m−3) was statistically at par (p > 0.0.05) with GAFS (1.22 Mg m−3) but significantly lower (p < 0.05) than the TLS (1.27 Mg m−3). Soil pH under all the land uses were significantly different (p < 0.001) from each other, AAFS being the highest (5.21) followed by GAFS (4.88) and TLS (4.46). AAFS contained 3.64% (p > 0.05) and 17.97% (p < 0.01) higher soil organic carbon than GAFS and TLS, respectively. Similarly, soil available nitrogen in AAFS was 4.7% (p < 0.05) and 14% (p < 0.05) higher than GAFS and TLS, respectively. Available P under AAFS was significantly higher (p < 0.05) than TLS but statistically at par (p > 0.05) with GAFS. There was also significant variation (p = 0.004) in available K among the three systems. Available K between AAFS and GAFS were not significantly different, but both the AFS has significantly higher (p < 0.05) available K than that of TLS. All these soil parameters in the sub-surface layer (15–30 cm) had less numerical value than the corresponding parameters in the surface soil (0–15 cm) except for soil pH and bulk density. At 15–30 cm soil depth, ANOVA revealed significant variation among all the soil parameters except BD (p > 0.05). However, the trend of variation was similar in both depths.
Tree canopy spread and light availability
The canopy density recorded by the canopy analyzer revealed more canopy coverage for Alder (Alnus nepalensis) than for Gamhari (Gmelina arborea). Crowns of Alder and Gamhari developed uniformly in all directions (Figure 4). The LAI of Alder (LAI = 2.19) was significantly higher (p < 0.05), nearly two times, than that of Gamhari (LAI = 1.01). The mean leaf angle of the tree species that regulated the light penetration was significantly lower (p < 0.05) under the alder canopy (26.42°) than that of Gamhari (56.58°), signifying Gamhari had more horizontal orientation of leaves with the ground surface (Figure 5). ANOVA revealed significant differences (p < 0.001) in PAR among all the systems. PAR was the highest under TLS (1,258 μEi m−2 s−1) followed by Gamhari (993 μEi m−2 s−1) and Alder (858 μEi m−2 s−1). The crops under Alder received 14 and 32% less PAR (p < 0.05) than those cultivated below the canopy of Gamhari and TLS, respectively (LSD0.05 = 42.9) (Figure 5).

Figure 4. Canopy image of Alder (Alnus nepalensis) and Gamhari (Gmelina arborea) in the agroforestry systems.

Figure 5. Canopy attributes and PAR with Alder and Gamhari tree canopies. Means of MLA and LAT of the two tree species compared with Student’s t-test at 5% level of significance. ANOVA for PAR revealed significant variation (p < 0.001) and means were compared by LSD (5%). Error bars in MLA and LAI represent standard error; error bars in PAR indicate LSD (0.05).
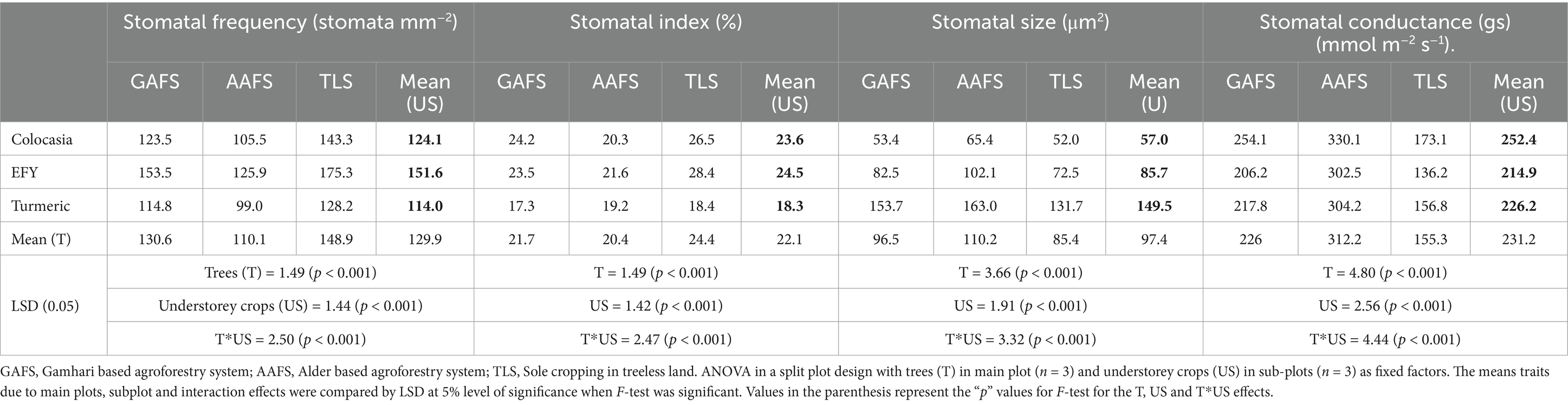
Effect of AAFS and GAFS on leaf morphological and stomatal attributes of the understorey crops
The ANOVA revealed significant differences in the leaf morphological and stomatal traits of the understorey crops (Turmeric, Colocasia and EFY) (Table 2). The AFS increased the SPAD chlorophyll meter reading (SCMR) of understorey crops in comparison to TLS. The SCMR averaged over all the understorey crops was highest in AAFS followed by GAFS and TLS (p < 0.05). Variations in the response variables of the individual understorey crops in GAFS, AAFS, and TLS were compared by comparing the subplot mean of understorey crops in the different systems. There was a statistically significant (p < 0.001) interaction between trees and the understorey crops in their influence on the leaf chlorophyll content. The highest SCMR was recorded in colocasia under AAFS (47.5), which was significantly higher (p < 0.05) than the SCMR of colocasia under GAFS (45.8) and TLS (40.3). A similar trend for SCMR (at p < 0.05) was observed for EFY and turmeric grown under AAFS and GAFS, respectively (Table 2). The AAFS and GAFS (main plot treatment effects) significantly reduced (p < 0.001) the leaf thickness of the understorey crops. But there was also a significant interaction (p < 0.001) effect between tree systems and understorey crops on the leaf thickness. The reduction of leaf thickness (p < 0.05) in the AAFS compared to TLS in colocasia, turmeric and EFY were 11.3, 10 and 5.2%, respectively.
A wide variation was observed in stomatal features among the understorey crops (Figure 6). In all crops the abaxial surface had much more stomata than the adaxial leaf surface. The stomatal openings of EFY were narrower than turmeric and colocasia. The overall effects of trees (main plot effects) and tree-crop interaction, as revealed by ANOVA, was highly significant (p < 0.001) on the stomatal frequency (SF) and stomatal size (SS), stomatal index (SI) and stomatal conductance (gs) of colocasia, EFY and turmeric. The mean SF of the understorey crops in AAFS and GAFS were 26.0 and 12.3% lower (p < 0.05) than TLS (Table 3). For the individual crops under different systems, the reduction was more pronounced in EFY where the reduction under AAFS in comparison to TLS (p < 0.05) was 28%. The SS followed an opposite trend, the highest under AAFS (110.2 μm2) and the lowest under TLS (85.4 μm2). Among the land-use systems for an understorey crop, turmeric under Alder (AAFS) had the largest stomata (163 μm2), significantly higher (p < 0.05) than the Gamhari (GAFS) and TLS. The lowest SS (52.0 μM2) was recorded in colocasia in TLS. The SI of all the understorey crops decreased significantly (p < 0.05) in tree-based systems with reference to sole crops (TLS) except in turmeric where the decrease was statistically non-significant (p > 0.05). The value of stomatal conductance of all the three understorey crops were higher to a tune of 101.0 and 38.9% (p < 0.05) under AAFS (Alder tree) than GAFS and TLS (pure crop). The stomatal conductance in colocasia under different systems were statistically different (p < 0.001) and were in the order of AAFS (330.1 mmol m−2 s−1) > GAFS (254.1 mmol m−2 s−1) > TLS (173.1 mmol m−2 s−1) (p < 0.05). EFY and turmeric also followed similar trends (p < 0.05). It was in the order of 302.5 mmol m−2 s−1, 206.2 mmol m−2 s−1 and 136.2 mmol m−2 s−1 under AAFS, GAFS and TLS, respectively. For turmeric, stomatal conductance was highest in AAFS (304.2 mmol m−2 s−1) followed by (p < 0.05) GAFS (217.8 mmol m−2 s−1) and TLS (156.8 mmol m−2 s−1) (Table 3).
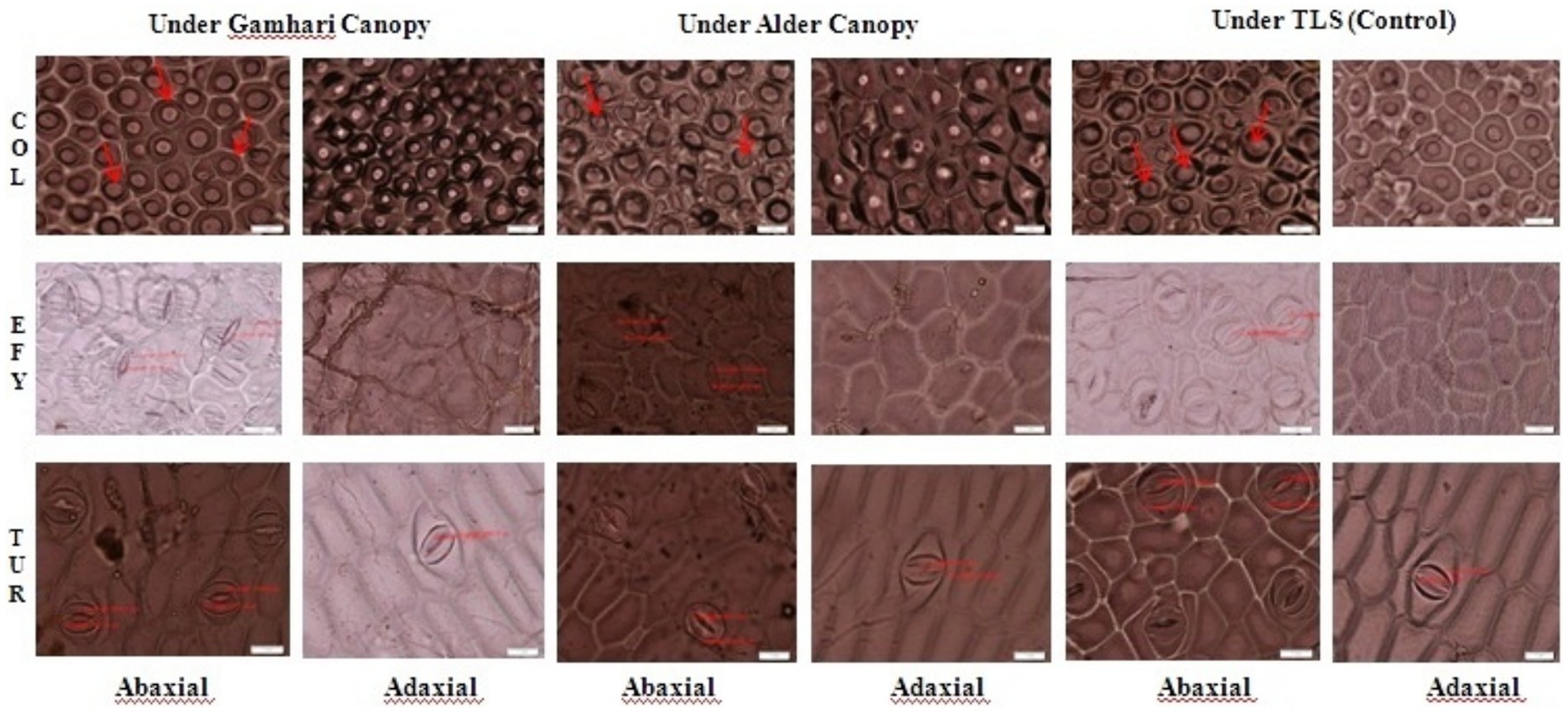
Figure 6. Stomatal attributes of different ground crops grown under the tree canopy of Alder, Gamhari and TLS (control) conditions. COL, Colocasia; EFY, Elephant Foot Yam; TUR, Turmeric.
Photosynthetic traits of understorey crops as influenced by tree species
ANOVA revealed all three effects, i.e., main plot, subplot and interaction effects were significant (p < 0.01) for transpiration (E) and photosynthetic rates (A) and intercellular CO2 concentration (Ci) (Table 4 and Figure 7). The transpiration rates (E) of all the understorey crops in TLS significantly decreased (p < 0.001 for main plots) when they were cultivated under the tree-based land use systems. Tree-crop interactions estimated in the ANOVA was highly significant (p < 0.001) for E of the understorey crops. The highest transpiration rate (2.66 mmol m−2 s−1) was in colocasia grown in TLS which decreased under the systems integrated with Gamhari (2.44 mmol m−2 s−1) and Alder (2.05 mmol m−2 s−1) trees (p < 0.05). EFY and turmeric followed a similar trend. The interaction between the land-uses and understorey crops was significant (p < 0.001) with a moderating influence on the leaf temperature of colocasia, EFY and turmeric. Leaf temperature was reduced (p < 0.05) by approximately 4°C in Colocasia and turmeric and 2°C in EFY under AAFS compared to TLS. The effect of the agroforestry systems on the intercellular CO2 was also similar which decreased in the order of TLS > GAFS > AAFS (p < 0.05). However, the difference between GAFS and AAFS was not statistically significant (p > 0.05). When we compared the intercellular CO2 concentration values of the individual crops under these three land use systems, only the Ci of turmeric in AAFS was significantly higher (p < 0.05) than the TLS. The photosynthetic rate (A) followed an opposite trend. On average, the photosynthetic rate of crops grown under Alder trees was 27.5 and 19.3% higher (p < 0.05) than those in TLS and GAFS (Table 4). A similar trend with statistically significant differences (p < 0.05) was maintained in the photosynthetic rate of Colocasia, EFY, and Turmeric across the land use systems. There was a significant difference (p < 0.001) in instantaneous water use efficiency (iWUE) among all three understorey crops cultivated with and without trees, i.e., pure crops in open conditions (Figure 7). ANOVA revealed significant interaction (p < 0.001) between the land-uses (Alder, Gamhari and sole cropping) and the understorey crops for water use efficiency. The highest iWUE was recorded in all the three understorey crops colocasia (12.82 mol of CO2 per mmol of H2O), EFY (12.01 mol of CO2 per mmol of H2O), and turmeric (8.0 mol of CO2 per mmol of H2O) under AAFS. The corresponding values in the open cultivation as pure crops (7.78, 8.5, and 6.2 mol of CO2 per mmol of H2O, respectively) were significantly (p < 0.05) less. Both AAFS and Gamhari GAFS positively impacted the tuber yield of colocasia, EFY, and rhizome yield of turmeric. Between the AAFS and GAFS, AAFS had a more pronounced effect in increasing the yield of the understorey crops. The overall yield improvement of the understorey crops under AAFS and GAFS was 24.31 and 11.84%, respectively over the pure crop (TLS) (p < 0.05). There was a significant interaction (p < 0.001) between the land-uses and understorey crops, in their influence on the corm, tuber, and rhizome yield of colocasia, EFY and turmeric, respectively. Among the three crops, turmeric recorded the highest yield under the alder tree canopy (Figure 7).
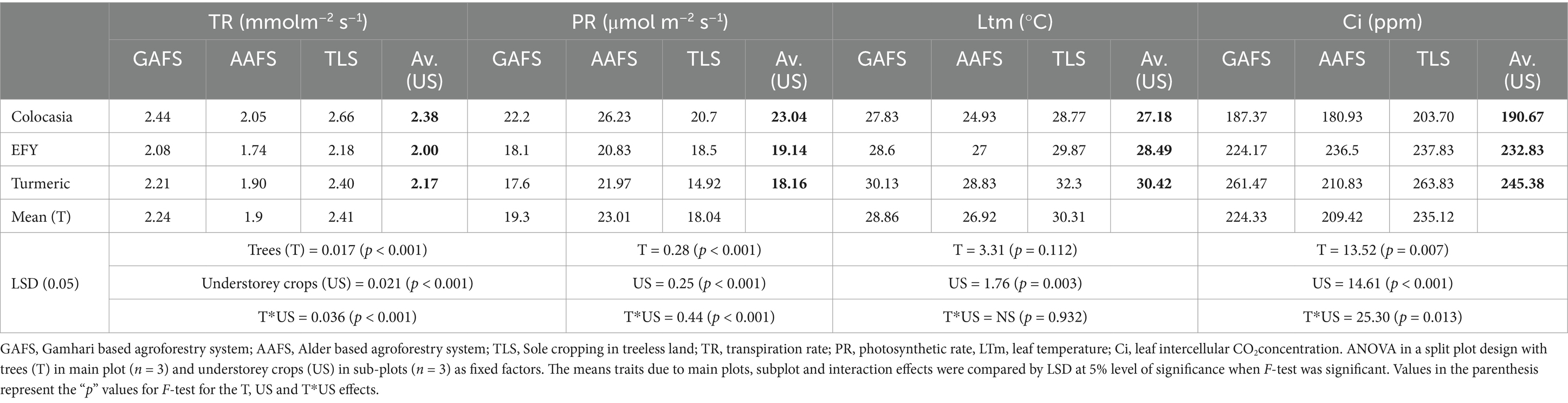
Table 4. Photosynthetic traits of understorey crops grown under tree canopies of Gamhari and Alder trees.
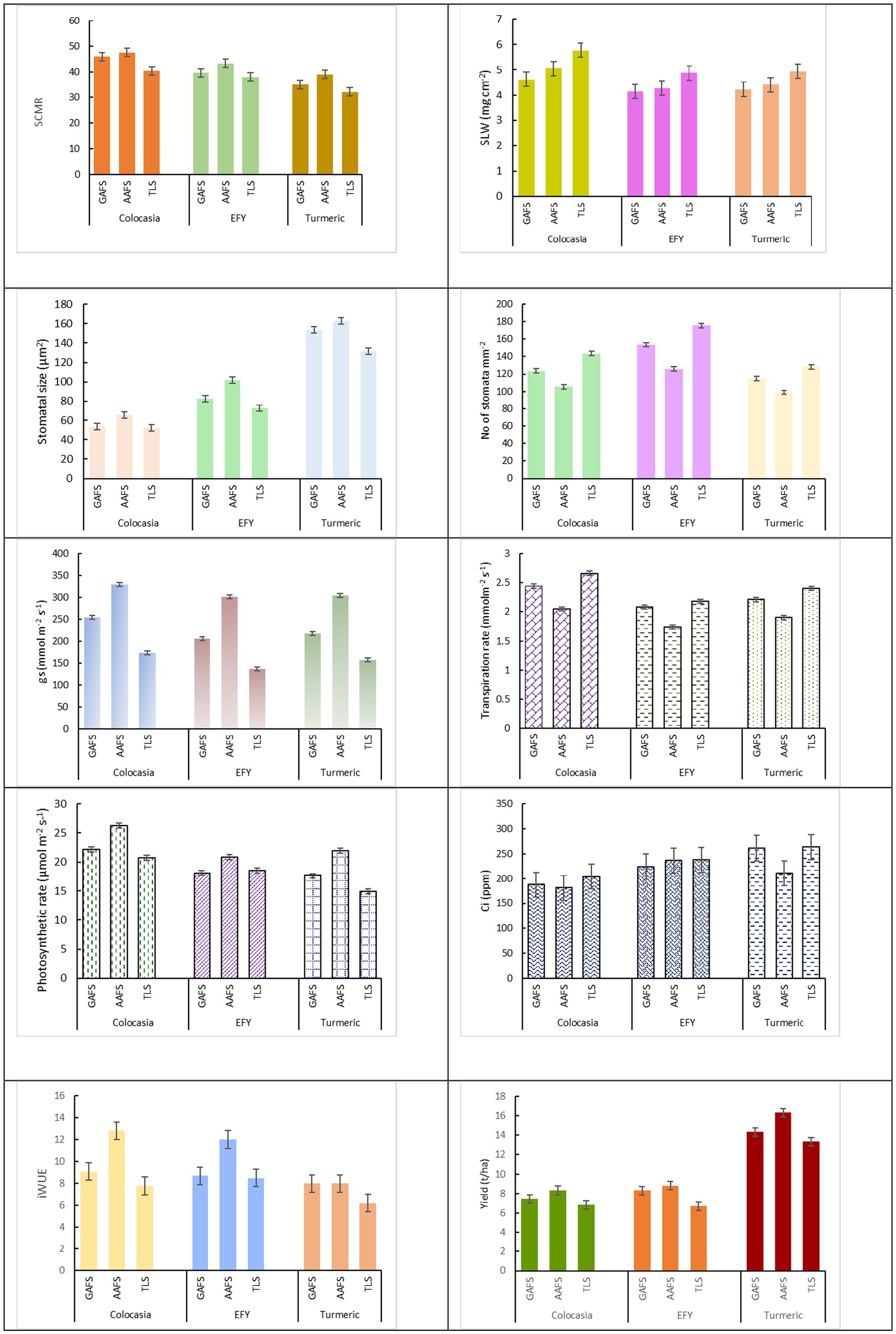
Figure 7. Graphical comparison of physiological characteristics of understorey crops under different agroforestry systems. Results of an ANOVA with tree (main plot-T) (n = 3) and understorey crops (sub-plot-US) (n = 3). The error bars represent LSD (0.05) of T*S. GAFS, Gamhari based agroforestry system; AAFS, Alder based agroforestry system; TLS, Sole crops in treeless land.
Effect of physiological traits on tuber/rhizome yield of the understorey crops
To understand the relationships of the physiological parameters with rhizome/tuber yields of the understorey crops, the yield of each crop was regressed over the physiological parameters separately. Regression coefficients (byx) of physiological parameters (x) on yield (y) of individual understorey crops were positive and significant for Stomatal size (SS), Stomatal conductance (gs), Photosynthetic rate (PR) and instantaneous water use efficiency (iWUE) (Table 5 and Figure 8). For SCMR, the regression coefficients were significant for colocasia (b = 0.1514, p = 0.032) and turmeric (b = 0.41, p = 0.002) but not for EFY (b = 0.1710, p = 0.217). For all these parameters the coefficients for turmeric were higher than that of colocasia and EFY. Regression coefficients were negative and statistically significant for stomatal frequency (SF) of colocasia (b = −0.0381, p = 0.019), EFY (b = −0.0419, p = 0.003), and turmeric (b = −0.1026, p = 0.001); transpiration rate (TR) in colocasia (b = −2.304, p = 0.02), EFY (b = −3.79, p = 0.02), and turmeric (b = −6.13, p = 0.001); intercellular CO2 concentration (Ci) for colocasia (b = −0.0374, p = 0.006), and turmeric (b = −0.0285, p = 0.043) except Ci for EFY (b = −0.0053, p = 0.799), and SI for colocasia (b = −0.1418, p = 0.071), and turmeric (b = 0.079, p = 0.793).
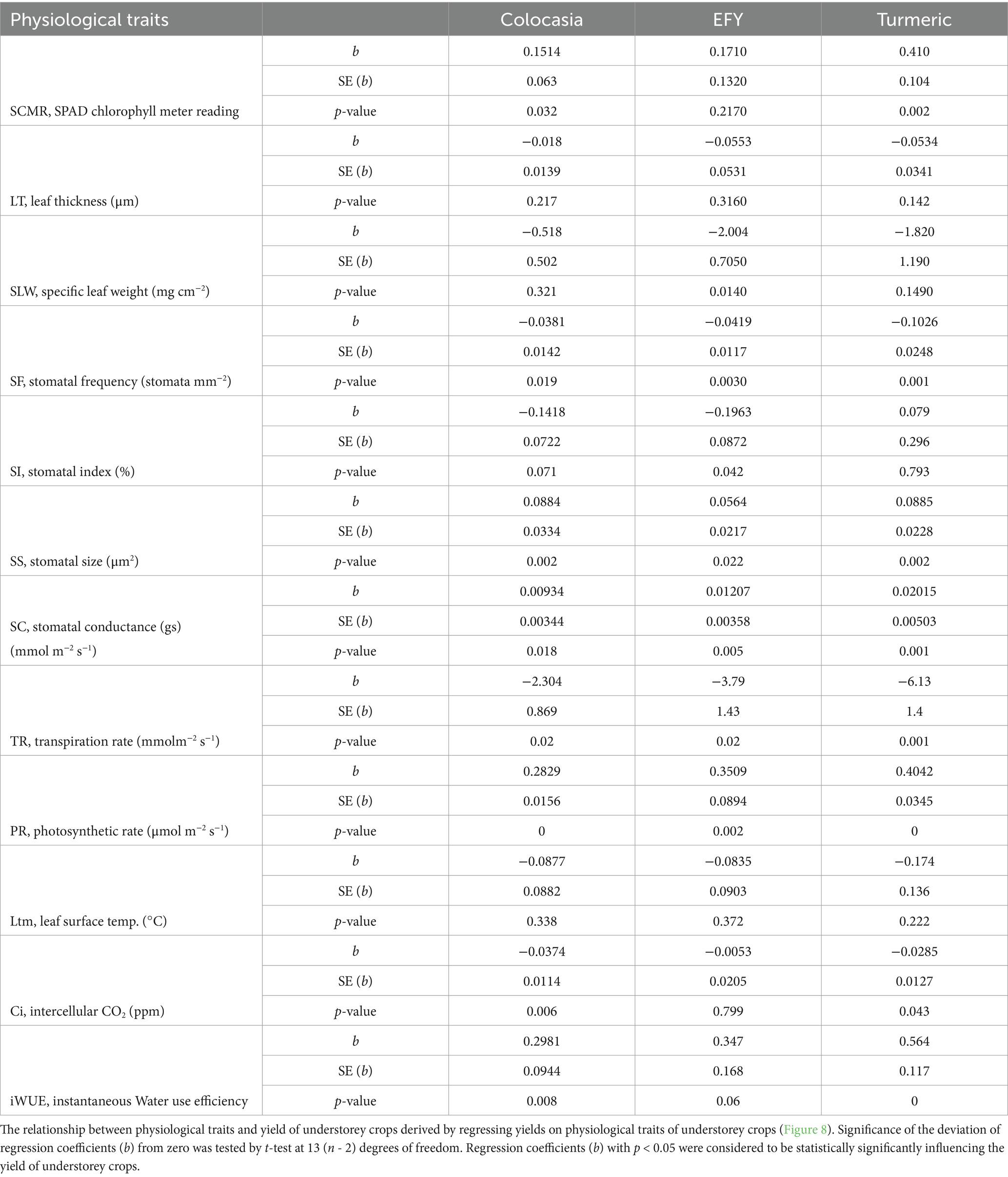
Table 5. Regression coefficients (n = 15) of crop yields over physiological traits of understorey crops.
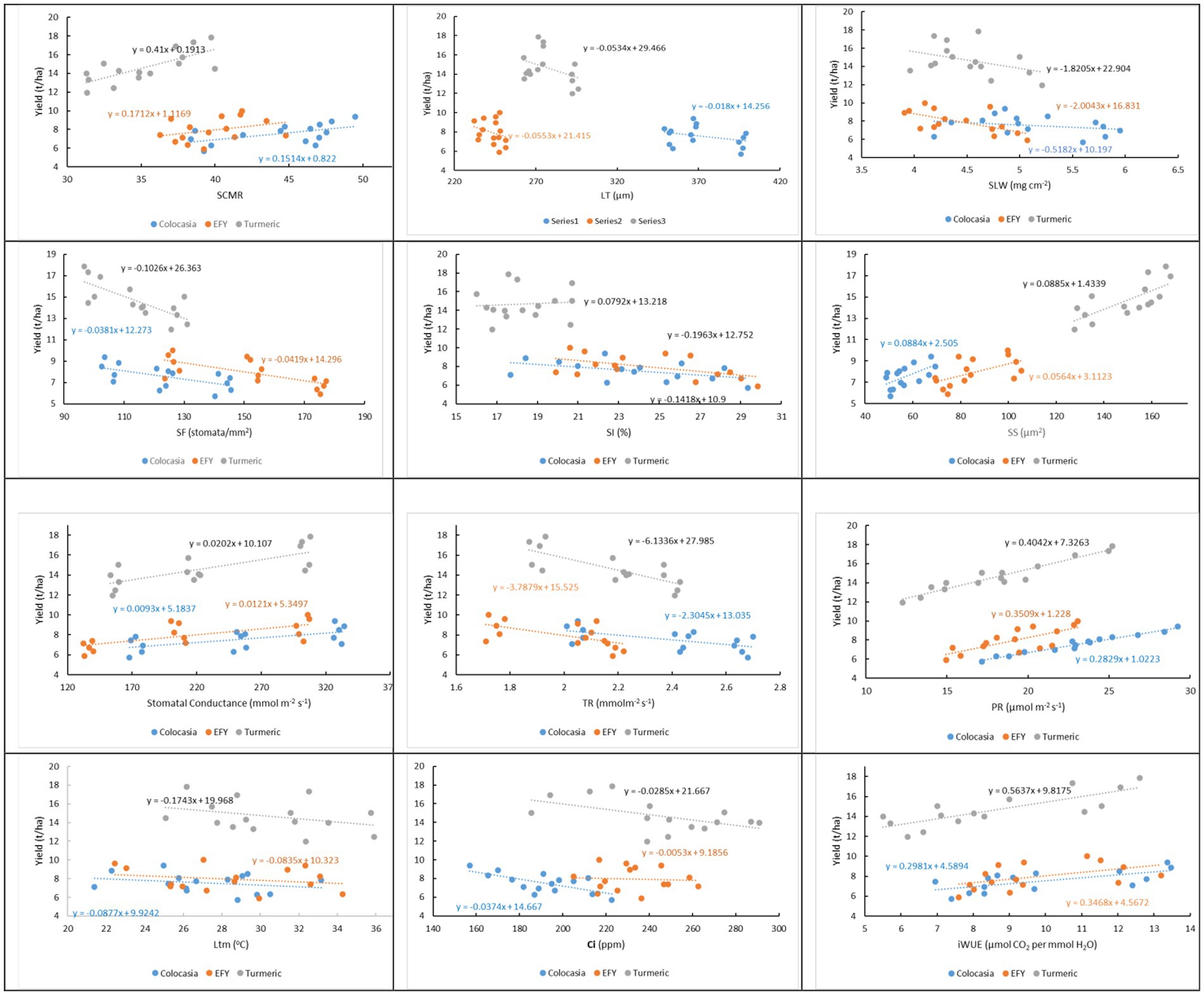
Figure 8. The relationship between physiological traits and yield of understorey crops depicted by regression equations for each understorey crop (n = 15). The slope and intercept indicate the nature and extent of the effect of the physiological traits on the yields of understorey crops. Significance of regression coefficients are detailed in Table 5. SCMR, SPAD chlorophyll meter reading (SCMR); LT, leaf thickness (μm); SLW, specific leaf weight (mg cm−2); SF, stomatal frequency (stomata mm−2); SI, stomatal index (%); SS, stomatal size (μm2); SC, stomatal conductance (gs) (mmol m−2 s−1); TR, transpiration rate (mmolm−2 s−1); PR, photosynthetic rate (μmol m−2 s−1), Ltm, leaf surface temp. (°C); Ci, intercellular CO2 (ppm); iWUE, instantaneous water use efficiency.
Comparison of total system productivities: agroforestry vs. sole crops
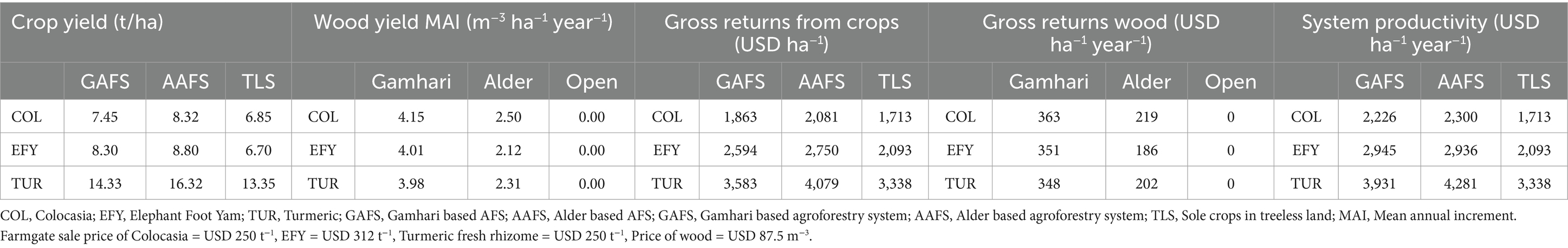
In AFS, the total productivity and economic returns from the complete system are more relevant to the farmers than the productivity of the individual component crops. From the tree component, the mean annual productivity/mean annual increment of wood from Gamhari was nearly doubles that of the Alder. However, the total system productivity in terms of economic returns (USD) was maximum from the AAFS having turmeric as an understorey crop (USD 4,281 per ha); the least income was derived from the sole crops (TLS) (Table 6). Total gross income per ha from sole crops was USD 1,713, 2,093, and 3,338 for colocasia, EFY, and turmeric, respectively. The gross income from the economically best productive system [AAFS (Alder + Turmeric)], was 28, 104, and 150% more than the sole turmeric, EFY, and colocasia, respectively.
Discussion
Alder and Gamhari improved soil fertility of agroforestry systems
Soil nitrogen increased significantly in the AAFS compared to the GAFS and TLS. Similar trends were observed for available phosphorous, potassium, and soil organic carbon. Alder trees fix atmospheric nitrogen through Frankia, gram-positive nitrogen-fixing bacteria that form actinorhizal symbiosis (Naik et al., 2023; Yuan et al., 2023). Alder trees were reported to have fixed 155 kg of nitrogen per hectare per annum in an alder (Alnus nepalensis)-large cardamom (Ammomum subulatum) AFS in the Eastern Himalayas (Sharma et al., 2002). In the acid soils, an increase in soil pH under Alder by 0.75 above TLS and 0.33 above GAFS would favor the availability of soil nutrients like N, P, and K. Because of higher LAI, the contribution of leaf litter by Alder to the soil would be higher than that of Gamhari. Saha et al. (2012) had conducted the leaf litter fall and decomposition studies of A. nepalensis and G. arborea in Eastern Himalayas (Meghalaya) and recorded that A. nepalensis produced more the leaf litter (≈9%) and fine root biomass (≈3%) than G. arborea. Apart from these, leaf litter decomposition was more faster A. nepalensis than G. arborea. Subsurface soil had a higher bulk density and lower soil nutrient levels (OC, N, P, K) than the surface layer. Dhyani and Tripathi (2000) and Lenka et al. (2012) reported lower soil nutrient values in the sub-surface soil in the EHR, and they suggested the reduced fine root turnover, aeration, and microbial activity in the sub-surface layers were the principal causes of such differences. Chen et al. (2023) demonstrated soil temperature, litter depth, and the availability of nitrogen and calcium in the soil were strongly influenced by the composition of the canopy.
Tree canopy architecture influenced light availability to understorey crop
The LAI of Alder was twice that of Gamhari, but its mean leaf angle was lower than Gamhari. Lesser leaf angles allow more solar radiations to pass through the canopy as the leaves are oriented more towards vertical plane. Despite having less mean leaf angle than Gamhari, Alder canopy allowed 14% less PAR to reach the understorey crops than the canopy of Gamhari. Higher LAI of Alder might have compensated for the effect of low mean leaf angle and created comparatively more shade effect. Plant canopies intercept and reduce the irradiance exponentially below their canopy. The extent of attenuation depends on the orientation, arrangement, and the total amount of leaves, canopy stratification, and the structural complexities in the canopy (Lambers et al., 2008; Jucker et al., 2014; Zhang and Chen, 2015).
Effect of trees on leaf stomatal and photosynthetic traits of the understorey crops
Plants tend to acclimate to the low irradiance at several leaf plasticity features. Leaf plasticity of a single trait may not fulfil the acclimation requirements to maintain the normal growth and development of a plant. They may alter the leaf anatomy to adapt to change, partition the biomass invested in different plant parts, or change the relative nitrogen investment among photosynthetic components. To increase their light interception, plants generally try to acclimate to low-light conditions by increasing the SLA and Chlorophyll content (Evans and Poorter, 2001). In our experiment the SCMR of the understorey crops increased significantly under the Alder canopy which allowed less PAR through its canopy compared to Gamhari. Elango et al. (2023) and Chen et al. (2021) have indicated that leaves in shaded environments often optimize light absorption by increasing the chlorophyll content per leaf area per unit mass. Sawitri et al. (2020) reported higher chlorophyll concentration in Yam leaves under low irradiation of Tectona grandis based agroforestry system compared to pure yam cultivated under full sun light. Leaf thickness and SLW were reduced in all the understorey crops under AAFS and GAFS, implying that the leaves became thinner and that the specific leaf area (area per unit mass of leaf), which is the reciprocal of specific leaf weight, increased. Many studies reported significant correlations between SLA and relative growth rate (RGR) in plants. However, SLA by itself may not enhance photosynthesis in leaf nitrogen and chlorophyll limiting situations (Osone et al., 2008). Soil nitrogen and phosphorous in the tree-based systems were higher than the sole cropping. The pH moderation in acidic soil also increased the availability of soil nutrients to the understorey crops. Higher availability of N helps to divert N towards chlorophyll synthesis, enhances light capture, and compensates for photosynthetic capacity (Poorter et al., 2019). Therefore, the cumulative effect of higher SLA, soil nitrogen, and chlorophyll might have a direct positive impact on leaf photosynthesis. The effect of N in increasing leaf chlorophyll was more pronounced in colocasia and EFY than in turmeric in shaded environment. In the AFS, the availability of P also increased. Adequate P supports enhancing photosynthesis, strongly influencing stomatal conductance and respiration (Li et al., 2016). Plants that tolerate shade often expand their specific leaf area, resulting in better light absorption (Charlotte et al., 2013). Certain plants adapt their epidermal cells into lens-shaped formations to boost light absorption and distribution, thereby reducing the transmission and reflection of excessive light. Plants may arrange their mesophyll cells in an isobilateral fashion to expand the area available for photosynthesis (Mathur et al., 2017). An increase in the SLA significantly boosts carbon acquisition, potentially leading to increased biomass production and higher yields (Melis and Harvey, 1981). Lower nitrogen and carbon investment per unit leaf surface of shade leaves may boost the carbon gain of plants in low-light environments, increasing net leaf area and light capture (Sims and Pearcy, 1989). The understorey crops evaluated in the present study might have the capability to achieve higher carbon gain in a low-light environment. We observed less leaf thickness in Colocasia (352.0 μm), EFY (235.8 μm), and Turmeric (265.0 μm) under the Alder tree canopy than in the TLS. Leaves grown in high light intensities (sun leaves) are thick and dense, having a larger mesophyll volume fraction and cell surface area than leaves grown at lower light intensities (Poorter et al., 2019). Yang et al. (2019) reported shading by trees decreased leaf thickness, leaf dry matter content (LDMC), leaf mass per unit area (LMA), and enhanced chlorophyll content in Dactylis glomerata. Faridah et al. (2017) reported thinner leaves in EFY under forest tree species with higher shading effects. About 47% higher average leaf area under a yellow shade net than the open field was reported by Harish et al. (2022). Therefore, the leaves of the understorey crops in the AFS were not thin enough to reduce the photosynthetic efficiency significantly as it was adequately compensated by higher chlorophyll content. Chlorophyll in Colocasia and Turmeric leaves revealed greater leaf plasticity than elephant foot yam in the agroforestry systems.
In the AFS, the SF of all the understorey crops decreased, whereas the size of the SS and their stomatal conductance increased. The stomatal conductance is a major determinant of crop yield by impacting photosynthesis and water use. The stomatal morphology, including stomatal size, density, and spacing, has been widely accepted as the determinants of stomatal conductance (Fanourakis et al., 2015). In our study, colocasia showed the highest stomatal conductance under AAFS compared to other understorey crops. The SS and stomatal conductance in turmeric were higher in agroforestry than in the TLS. Stomatal density in leaves of Yam cultivated in Tectona grandis-based AFS receiving 50% of the PAR under full sunlight, was reduced by 45% without any trade-off with tuber yield (Sawitri et al., 2020). Zhang et al. (2019) suggested that larger size and lower density of stomata may promote the initial stomatal conductance at low light. The enhanced stomatal conductance and photosynthesis may have facilitated greater CO2 absorption, leading to an increase in yields (Yan et al., 2012; Hajong et al., 2022). Derebe et al. (2019) reported very high stomatal conductance (0.86 mol m−2 s−1) for Colocasia esculenta in a shaded environment in Ethiopia. Under a low-light environment, high soil nutrient availability, and stomatal conductance are strongly correlated with the photosynthetic rate as they influence the rate of CO2 fixation and assimilation by leaf mesophyll tissue (Li et al., 2022). Under AFS, the supply of all major nutrients like N and P was higher than the sole crops of TLS. The leaf temperature and the transpiration rates of the understorey crops in the agroforestry systems were lower than in the open conditions. The transpiration rate and stomatal conductance of EFY decreased more compared to colocasia and turmeric. Altered SLA and chlorophyll pigments allowed the leaves to absorb enough irradiance/PAR to maintain a high photosynthetic rate. In all the understorey crops in agroforestry systems, transpiration rate were lesser than the sole crops in TLS which might be due to the cooling effects of the tree canopies. The reduced transpiration rate was reported to have highest positive effects on a few crops, increasing the yield up to 0.2 t ha−1 (Sinclair, 2018).
The present study observed an increased photosynthetic rate in the understorey crops beneath the alder canopy, which may be linked to leaf metabolic activity that occurred alongside leaf plasticity in various structures and improved efficiency in utilizing low light conditions (Chen et al., 2007; Vile et al., 2005). The iWUE was better under the alder canopy than the Gamhari and sole crops which may be attributed to lower transpiration rate and higher levels of leaf chlorophyll and photosynthesis. Turmeric could be grown as a shade-tolerant crop (partial shade regimes from 33 to 50%) under light-limiting environments like agroforestry systems. The Photosynthesis rate of the all the understorey crops which are C3 plants, significantly declined under full sunlight. When grown in the agroforestry systems under low light conditions, CO2 uptake and conductance increased. Despite 32% reduction in PAR under the Alder canopy, the PR in colocasia, EFY, and turmeric increased compared to the sole crops in TLS. As light intensity increased to 100% under open conditions (~1,300 μmol m−2 s−1), the photosynthesis decreased, possibly due to higher photorespiration, higher leaf temperature, lower chlorophyll, and lower iWUE. In most shade-tolerant plants, the leaves are at risk of photo-inhibition damage from the high irradiance (Purnomo et al., 2018; Lambers et al., 2008). The threshold PPFD for a species depends on many stress factors like temperature, leaf water potential, leaf nitrogen, etc., (Peltoniemi et al., 2012). Metabolic reprogramming and adjustment in mitochondria and chloroplast were demonstrated in coffee under high light (up to 1,000 μmol photons m−2 s−1) with considerable rates of energy dissipation through photorespiration and efficient photosynthesis (Martins et al., 2014). Chowdhury et al. (2009) reported in PPFD of 800 to 900 μmol m−2 s−1for higher photosynthetic efficiency in Colocasia esculenta. After 900 μmol m−2 s−1, the Fv/Fm ratio started decreasing along with ΦPSII and reached Fv/Fm = 0.68 and ΦPSII = 0.39 at PPDF 1,310 μmol m−2 s−1.
Alam et al. (2020) investigated the physiological attributes that endow turmeric (Curcuma longa) the ability to adapt to shaded environments. They noted that turmeric plants reached a higher CO2 assimilation rate at moderate Photosynthetic Photon Flux Density (PPFD of 704.0 μmol m−2 s−1under 50% shade), exhibited a high photosynthesis rate and PSII quantum yield. Plants display a low coefficient of non-photochemical quenching, maintaining steady photochemical quenching, and an ideal thylakoid electron transport rate in low-light scenarios. Saturation of photosynthetic capacity at low light levels (~ 400 μmol m−2 s−1) was suggested to be responsible for the better performance of cocoa (Theobroma cacao), a naturally shade-loving tree in the tropics (Blaser-Hart et al., 2021). In colocasia, EFY, and turmeric, similar physiological mechanisms could be responsible for adaptation to low light conditions.
Trees improved the yields of understorey crops and system productivity
In general, crop yields were higher beneath the canopy of Alder trees than in the TLS. Accumulation of SOC, N, and P was higher in systems based around Alder trees than in those with Gamhari or TLS. Positive modifications of micro-climate created congenial environments for the understorey crops to enhance their productivity. A meta-analysis by Laub et al. (2022) inferred that beneficial effects could accrue up to a 40% reduction in solar radiation in berries, fruits, and vegetables. Slattery et al. (2013) reported shading increased the plant energy conversion efficiency, offsetting yield depressions in a wide range of species. Indirect benefits of shading are lower transpiration, low surface temperature and evaporation, leaf temperature, and higher water use efficiency, leading to higher yield. The enhanced yield of the agronomic crops could be due to the improved availability of soil carbon and macronutrients, particularly nitrogen and phosphorus, as well as the optimum transmission of PAR to the vegetation beneath the alder canopy compared to areas without canopy cover (Chauhan et al., 2013; Beatty, 1984). Shade-tolerant plants are adapted to use soil nutrients and moisture more efficiently than shade-intolerant crops in low light environments. Photosynthesis serves as the fundamental process underpinning the biomass and yield of crop plants, with any subtle shifts in the photosynthetic rate of a plant mirroring the impact of environmental stressors on its photosynthetic function (Sun et al., 2013).
Demand and supply of photosynthates strongly influence carbon partitioning in a plant. Carbon export to the different sinks including the belowground storage organs is strongly correlated with the rate of photosynthesis in resources non-limiting situations. A higher yield implies that a large portion of the photosynthates has been diverted to the sink (Lemoine et al., 2013). In the case of colocasia, during the corm expansion stage, the sink demand increases and photosynthates are preferentially transferred to the corm instead of above-ground parts. The partition coefficient (ratio of root to crop growth) increases from 0.40 after 90 days of planting (DAP) to 0.77 at 120 DAP during the rapid corm expansion phase (Manrique, 1994). Such strong sink demand for photosynthates in colocasia was supported by the high rate of photosynthesis, higher stomatal conductance, and lower transpiration under a higher supply of soil nitrogen. In yams, the tuber weight began to increase 90 DAP. Partitioning of dry matter to leaves and vines declined by 120 DAP, thereafter, it increased to tubers from 130 days to 210 DAPs (Ravi et al., 2022). Therefore, supply from the source supported by higher rates of photosynthesis, stomatal conductance and high sink demand in the belowground corms and tuber are the major drivers of carbon partitioning.
Crop growth and yield are closely linked with soil nutrient availability especially major nutrients like N, P and K thus influencing the crop growth performance and productivity (DeMalach et al., 2017). Sharangi et al. (2022) and Kittur et al. (2016) reported the increase yield of turmeric is expected with increasing abundance of mineral elements and quality light available under tree canopies. Turmeric exhibited the highest rhizome yield under the tree canopy relative to other crops, owing to its inherent shade tolerance capacity (Chauhan et al., 2013). A combined application of shade and nutrition increased the yield of turmeric to one and half times of open field full sunlight conditions (Padmapriya et al., 2007). Turmeric preferred partial shade for higher production without significant reduction in net photosynthesis, stomatal conductance, and transpiration rate (Dhillon et al., 2009). Alam et al. (2020) witnessed higher net photosynthesis of turmeric (17.40 mmol m−2 s−1) under 50% shade followed by 33% shade (15.30 mmol m−2 s−1) with increased above and below ground biomass production. But too dense canopies might hamper the growth and development of intercrop like turmeric. Sharangi et al. (2022) showed under 80% shade level, leaf area remained significantly lower than 50 and 70% shade levels as development of leaf area was significantly reduced with receipt of sub-optimal solar radiation which ultimately suppresses photosynthesis, growth and leaf formation.
The response of the understorey crops (Turmeric, Colocasia, and EFY) to soil fertility and shading effects was quite variable. Studies indicate that variations in shade tolerance among plant species may influence adaptation to soil nitrogen and its utilization efficiency (Huang et al., 2015; Jin et al., 2023), which may facilitate the coexistence of companion species. Faridah et al. (2017) reported higher tuber formation efficiency in elephant foot yam (Amorphophallus oncophyllus) under the Acacia mangium stand, which had less light penetration than other stands. The productivity of Alocasia macrorrhiza and Colocasia esculenta decreased with high photon flux density in open fields (Sims and Pearcy, 1989). The mean annual productivity of Gamhari was nearly double that of the Alder. However, the total system productivity of the AAFS was more than the GAFS. It may be due to the higher positive influence of Alder on the understorey crops than Gamhari. The system productivity was highest for the Alder + Turmeric system with an annual gross return of USD 4281 per ha.
Conclusion
The long-term effects of the Alder and Gamhari trees on the land use improved the soil’s organic carbon, nitrogen, and phosphorous. The Alder-based systems could increase soil pH up to 5.21, which would play a crucial role in addressing the soil acidity problem as most of the soil in the region has 4.5 soil pH. Attenuated light intensity coupled with better soil fertility in the agroforestry systems induced a strong photo-acclimation response, favourably adjusting leaf and physiological traits in colocasia, elephant foot yam, and turmeric to enhance the rhizome/tuber productivity. The leaf chlorophyll content (SCMR), stomatal size, stomatal frequency, stomatal conductance, and instantaneous water use efficiency of the understorey crops increased while the transpiration rate and stomatal frequency decreased in the agroforestry systems. The findings indicated that the Alder (Alnus nepalensis) trees were more beneficial than Gamhari (Gmelina arborea) in improving the physiological efficiency and productivity of all the understorey crops. The co-cultivation of turmeric with Alder produced maximum system productivity in terms of monetary value (USD 4,281 ha−1 year−1) because of higher rhizome yield and better market price of turmeric. Therefore, the Alder-Turmeric agroforestry system can be leveraged for better soil health management, reducing land degradation, and achieving higher system productivity on the hilly and sloping terrain of the Eastern Himalayas.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
KR: Conceptualization, Investigation, Methodology, Resources, Writing – original draft. NRS: Data curation, Formal analysis, Methodology, Resources, Writing – original draft. RJJ: Conceptualization, Investigation, Methodology, Writing – original draft. KPM: Conceptualization, Investigation, Methodology, Writing – review & editing. PC: Formal analysis, Writing – review & editing. BUC: Data curation, Formal analysis, Resources, Writing – review & editing. PM: Data curation, Formal analysis, Resources, Writing – review & editing. SD: Data curation, Formal analysis, Resources, Writing – review & editing. LJC: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. ND: Data curation, Formal analysis, Writing – review & editing. NUS: Data analysis, Writing – review & editing. SH: Data curation, Formal analysis, Supervision, Writing – review & editing. YBK: Formal analysis, Software, Writing – review & editing. VKM: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors pay tribute to the late Dr. A. Venkatesh, PS and former in-charge of the agroforestry section, who initiated the experiment under the institute project Development of self-sustainable integrated farming system through crop, livestock, and forestry interventions under rainfed conditions for productivity enhancement (IXX08985). The authors are grateful to Dr. S. V. Ngachan, the former Director of the Indian Council of Agricultural Research (ICAR)-Research Complex for North Eastern Hill Region, Umiam, Meghalaya, India, for his guidance and support in conducting this study. The research facilities used under the NICRA (National Innovations in Climate Resilient Agriculture) project are sincerely acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Alam, B., Chaturvedi, M., Singh, R., Newaj, R., and Dhyani, S. (2020). Physiological determinants for adaptive potential of turmeric (Curcuma longa) for its growth and yield under different regimes of shade in semi-arid region of Central India. Indian J. Agrofor. 16, 25–29. Available at: https://epubs.icar.org.in/index.php/IJA/article/view/103411
Allen, S. E., Grimshaw, H. M., Parkinson, J. A., and Quarnby, C. (1974). Chemical analysis of ecological materials. Oxford: Blackwell Scientific, 565.
Almeida, A. A. F., Gomes, F. P., Araujo, R. P., Santos, R. C., and Valle, R. R. (2014). Leaf gasexchange in species of the Theobroma genus. Photosynthetica 52, 16–21. doi: 10.1007/s11099-013-0048-8
Annie, M., Pal, R., Kumar, G., Gawai, A. S., and Sharma, A. (2023). Assessing the impact of climate change on agricultural production using crop simulation model. Int. J. Environ. Clim. Change 13, 538–550. doi: 10.9734/ijecc/2023/v13i71906
Arenas-Corraliza, M. G., Rolo, V., López-Díaz, M. L., and Moreno, G. (2019). Wheat and barley can increase grain yield in shade through acclimation of physiological and morphological traits in Mediterranean conditions. Sci. Rep. 9:9547. doi: 10.1038/s41598-019-46027-9
Ávila-Lovera, E., Coronel, I., Jaimez, R., Urich, R., Pereyra, G., Araque, O., et al. (2016). Ecophysiological traits of adult trees of Criollo cocoa cultivars (Theobroma cacao L.) from a germplasm bank in Venezuela. Exp. Agric. 52, 137–153. doi: 10.1017/S0014479714000593
Barrios, E., Sileshi, G. W., Shepherd, K., and Sinclair, F. (2012). “Agroforestry and soil health: linking trees, soil biota, and ecosystem services” in Soil ecology and ecosystem services. ed. D. H. Wall (Oxford: Oxford University Press), 315–330.
Beatty, S. W. (1984). Influence of micro-topography and canopy species on spatial patterns of forest understorey plants. Ecology 65, 1406–1419. doi: 10.2307/1939121
Bhatt, B. P., Singh, R., Misra, L. K., Tomar, J. M. S., Singh, M., Chauhan, D. S., et al. (2001). “Agroforestry research and practices: an overview” in Steps towards modernization of agriculture in NEH region. eds. N. D. Verma and B. P. Bhatt (Meghalaya: ICAR Publication), 365–392.
Bibi, F., and Rahman, A. (2023). An overview of climate change impacts on agriculture and their mitigation strategies. Agriculture 13:1508. doi: 10.3390/agriculture13081508
Bijakal, B. S., Kaur, N., and Gill, R. I. S. (2019). Evaluation of turmeric (Curcuma longa L.) varieties for yield and quality parameters under poplar-based agroforestry system. Indian J. Agrofor. 21, 29–34. Available at: https://epubs.icar.org.in/index.php/IJA/article/view/96520
Blaser-Hart, W. J., Hart, S. P., Oppong, J., Kyereh, D., Yeboah, E., and Six, J. (2021). The effectiveness of cocoa agroforests depends on shade-tree canopy height. Agric. Ecosyst. Environ. 322:107676. doi: 10.1016/j.agee.2021.107676
Bray, R. H., and Kurtz, L. T. (1945). Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 59, 39–46. doi: 10.1097/00010694-194501000-00006
Broadhead, J. (2015). “Competition and phenology in agroforestry” in Tree-crop interactions: agroforestry in a changing climate. eds. C. K. Ong, C. Black, and J. Wilson (Wallingford: CABI).
Buchanan, S., Isaac, M. E., Van den Meersche, K., and Martin, A. R. (2019). Functional traits of coffee along a shade and fertility gradient in coffee agroforestry systems. Agrofor. Syst. 93, 1261–1273. doi: 10.1007/s10457-018-0239-1
Calzavara, A. K., Bianchini, E., Pimenta, J. A., Oliveira, H. C., and Stolf-Moreira, R. (2019). Photosynthetic light-response curves of light-demanding and shade-tolerant seedlings of neotropical tree species. Photosynthetica 57, 470–474. doi: 10.32615/ps.2019.061
Carvalho, F. E. L., Escobar-Pachajoa, L. D., Camargo, I. D., Rojas-Molina, J., Jaimes-Suárez, Y. Y., and Rivera-Meneses, J. J. (2023). The interspecific interactions in agroforestry systems enhance leaf water use efficiency and carbon storage in cocoa. Environ. Exp. Bot. 205:105119. doi: 10.1016/j.envexpbot.2022.105119
Charlotte, M. M., Gommers, E. J. W., Visser, K. R., St Onge, L. A. C. J., Voesenek, P., and Pierik, R. (2013). Shade tolerance: when growing tall is not an option. Trends Plant Sci. 18, 65–71. doi: 10.1016/j.tplants.2012.09.008
Chauhan, S. K., Dhillon, W. S., Singh, N., and Sharma, R. (2013). Physiological behaviour and yield evaluation of agronomic crops under agri-horti-silviculture system. Int. J. Plant Res. 3, 1–8. doi: 10.5923/j.plant.20130301.01
Chen, J. M., Leblanc, S. G., Miller, J. B., and Deering, D. W. (2005). Methodology comparison for canopy structure parameters extraction from digital hemispherical photography in boreal forests. Agric. For. Meteorol. 129, 187–207. doi: 10.1016/j.agrformet.2004.09.006
Chen, J., Wu, S., Dong, F., Li, J., Zeng, L., Tang, J., et al. (2021). Mechanisms underlying the shading-induced chlorophyll accumulation in tea leaves. Front. Plant Sci. 12:779819. doi: 10.3389/fpls.2021.779819
Chen, Y., Yuan, L. P., Wang, X. H., Zhang, D. Y., Deng, Q. Y., Zhao, B. R., et al. (2007). Relationship between grain yield and leaf photosynthetic rate in super hybrid rice. J. Physiol. Mol. Biol. 33, 235–243.
Chen, J., Zhu, J., Wang, Z., Xing, C., Chen, B., Wang, X., et al. (2023). Canopy gaps control litter decomposition and nutrient release in subtropical forests. Forests 14:673. doi: 10.3390/f14040673
Choudhury, B. U., Das, A., Ngachan, S. V., Slong, A., Bordoloi, L. J., and Choudhury, P. (2012). Trend analysis of long-term weather variables in mid-altitude of Meghalaya, North-East India. J. Agric. Phys. 12, 12–22.
Choudhury, B. A., Saha, S. K., Konwar, M., Sujith, K., and Deshamukhya, A. (2019). Rapid drying of Northeast India in the last three decades: climate change or natural variability? J. Geophys. Res. Atmos. 124, 227–237. doi: 10.1029/2018JD029625
Chowdhury, S. R., Kumar, A., and Sahoo, N. (2009). Diurnal changes in chlorophyll fluorescence and light utilization in Colocasia esculenta leaves grown in marshy waterlogged area. Biol. Plant. 53, 167–170. doi: 10.1007/s10535-009-0027-x
Dagar, J. C., and Gupta, S. R. (2020). “Agroforestry developments for degraded landscapes: a synthesis” in Agroforestry for degraded landscapes. eds. J. C. Dagar, S. R. Gupta, and D. Teketay (Singapore: Springer), 447–458.
Das, A., Ghosh, P. K., Lal, R., Saha, R., and Ngachan, S. V. (2017). Soil quality effect of conservation practices in maize–rapeseed cropping system in Eastern Himalaya. Land Degrad. Dev. 28, 1862–1874. doi: 10.1002/ldr.2325
Dash, U., Gupta, B., Bhardwaj, D. R., Sharma, P., Kumar, D., Chauhan, A., et al. (2024). Tree spacings and nutrient sources effect on turmeric yield, quality, bio-economics and soil fertility in a poplar-based agroforestry system in Indian Himalayas. Agrofor. Syst. 98, 911–931. doi: 10.1007/s10457-024-00962-3
Daymond, A. J., Tricker, P. J., and Hadley, P. (2011). Genotypic variation in photosynthesis in cacao is correlated with stomatal conductance and leaf nitrogen. Biol. Plant. 55, 99–104. doi: 10.1007/s10535-011-0013-y
de Stefano, A., and Jacobson, M. G. (2017). Soil carbon sequestration in agroforestry systems: a meta-analysis. Agrofor. Syst. 92, 285–299. doi: 10.1007/s10457-017-0147-9
DeMalach, N., Zaady, E., and Kadmon, R. (2017). Light asymmetry explains the effect of nutrient enrichment on grassland diversity. Ecol. Lett. 20, 60–69. doi: 10.1111/ele.12706
Derebe, A. D., Gobena Roro, A., Tessfaye Asfaw, B., Worku Ayele, W., and Hvoslef-Eide, A. K. (2019). Effects of solar UV-B radiation exclusion on physiology, growth and yields of taro (Colocasia esculenta (L.)) at different altitudes in tropical environments of Southern Ethiopia. Sci. Hortic. 256:108563. doi: 10.1016/j.scienta.2019.108563
Dhillon, W. S., Chauhan, S. K., and Singh, N. S. (2009). Physiology and yield of turmeric under poplar canopy. Asia-Pac. Agrofores. Newslett. 35, 5–6.
Dhyani, S. K., and Tripathi, R. S. (2000). Biomass and production of fine and coarse roots of trees under agrisilvicultural practices in north-east India. Agrofor. Syst. 50, 107–121. doi: 10.1023/A:1006439018621
Eekhout, J. P. C., and de Vente, J. (2022). Global impact of climate change on soil erosion and potential for adaptation through soil conservation. Earth Sci. Rev. 226:103921. doi: 10.1016/j.earscirev.2022.103921
Elango, T., Anburaj, J., Haripriya, D., Santhosh, A., Rajakumar, G., Kavya, P., et al. (2023). Influence of shading intensity on chlorophyll, carotenoid and metabolites biosynthesis to improve the quality of green tea: a review. Energy Nexus 12:100241. doi: 10.1016/j.nexus.2023.100241
Evans, J. R., and Poorter, H. (2001). Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 24, 755–767. doi: 10.1046/j.1365-3040.2001.00724.x
Fanourakis, D., Giday, H., Milla, R., Pieruschka, R., Kjaer, K. H., Bolger, M., et al. (2015). Pore size regulates operating stomatal conductance, while stomatal densities drive the partitioning of conductance between leaf sides. Ann. Bot. 115, 555–565. doi: 10.1093/aob/mcu247
Faridah, E., Budiadi, R., and Rahmadaniarti, A. (2017). Growth adaptation of elephant foot yam (Amorphophallus oncophyllus Prain) under different forest stand species. A. Isnansetyo and T. Nuringtyas. Proceeding of the 1st International Conference on Tropical Agriculture. Cham: Springer.
Gomes, L. C., Bianchi, F. J. J. A., Cardoso, I. M., Fernandes, R. B. A., Filho, E. I. F., and Schulte, R. P. O. (2020). Agroforestry systems can mitigate the impacts of climate change on coffee production: a spatially explicit assessment in Brazil. Agric. Ecosyst. Environ. 294:106858. doi: 10.1016/j.agee.2020.106858
Gomez, K. A., and Gomez, A. A. (1984). Statistical procedures for agricultural research. New York: John Wiley.
Government of India. (2024). Press release. Available at: https://pib.gov.in/PressReleaseIframePage.aspx?PRID=1964083 (Accessed August 22, 2024).
Hajong, S., Rangappa, K., Dasaiah, H. G., Moirangthem, P., Saikia, U. S., Bhattacharjee, B., et al. (2022). Genotypic variability and physio-morphological efficiency of buckwheat (Fagopyrum spp.) under moisture stress at mid-altitudes of Meghalaya (India). Crop Pasture Sci. 74, 204–218. doi: 10.1071/CP22062
Harish, B. S., Umesha, K., Venugopalan, R., and Maruthi Prasad, B. N. (2022). Photo-selective nets influence physiology, growth, yield and quality of turmeric (Curcuma longa L.). Ind. Crop. Prod. 186:115202. doi: 10.1016/j.indcrop.2022.115202
Hazarika, S., Nabam, A., Thakuria, D., Kataki, S., and Krishnappa, R. (2021). Lime equivalence of organic manures and scope of their utilization as acid soil amendments. Arch. Agron. Soil Sci. 67, 660–674. doi: 10.1080/03650340.2020.1749266
Huang, W., Zhang, S. B., Zhang, J. L., and Hu, H. (2015). Photoinhibition of photosystem I under high light in the shade-established tropical tree species Psychotriarubra. Front. Plant Sci. 6:801. doi: 10.3389/fpls.2015.00801
Jin, M.-Y., Johnson, D. J., Jin, G.-Z., Jin, G.-Z., Guo, Q.-X., and Liu, Z.-L. (2023). Soil water content and nitrogen differentially correlate with multidimensional leaf traits of two temperate broadleaf species. Plant Divers. 45, 694–701. doi: 10.1016/j.pld.2023.03.001
Jucker, T., Bouriaud, O., Avacaritei, D., Dănilă, I., Duduman, G., Valladares, F., et al. (2014). Competition for light and water play contrasting roles in driving diversity-productivity relationships in Iberian forests. J. Ecol. 102, 1202–1213. doi: 10.1111/1365-2745.12276
Kittur, B. H., Sudhakara, K., Mohan Kumar, B., Kunhamu, T. K., and Sureshkumar, P. (2016). Bamboo-based agroforestry systems in Kerala, India: performance of turmeric (Curcuma longa L.) in the subcanopy of differentially spaced seven-year-old bamboo stand. Agrofor. Syst. 90, 237–250. doi: 10.1007/s10457-015-9849-z
Kuyah, S., Oborn, I., and Jonsson, M. (2017). “Regulating ecosystem services delivered in agroforestry systems” in Agroforestry. eds. J. C. Dagar and V. P. Tewari (Singapore: Springer), 797–815.
Lambers, H., Chapin, F. S., and Pons, T. L. (2008). Plant physiological ecology. New York: Springer.
Lasco, R. D., Delfino, R. J. P., and Espaldon, M. L. O. (2014). Agroforestry systems: helping smallholders adapt to climate risks while mitigating climate change. WIREs Clim. Change 5, 825–833. doi: 10.1002/wcc.301
Laub, M., Pataczek, L., Feuerbacher, A., Zikeli, S., and Högy, P. (2022). Contrasting yield responses at varying levels of shade suggest different suitability of crops for dual land-use systems: a meta-analysis. Agron. Sustain. Dev. 42:51. doi: 10.1007/s13593-022-00783-7
Layek, J., Rangappa, K., Das, A., Ansari, M. A., Choudhary, S., Rajbonshi, N., et al. (2023). Evaluation of millets for physio-chemical and root morphological traits suitable for resilient farming and nutritional security in Eastern Himalayas. Front. Nutr. 10:1198023. doi: 10.3389/fnut.2023.1198023
Lemoine, R., La Camera, S., Atanassova, R., Dédaldéchamp, F., Allario, T., Pourtau, N., et al. (2013). Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 4:272. doi: 10.3389/fpls.2013.00272
Lenka, N. K., Dass, A., Sudhishri, S., and Patnaik, U. S. (2012). Soil carbon sequestration and erosion control potential of hedgerows and grass filter strips in sloping agricultural lands of eastern India. Agric. Ecosyst. Environ. 158, 31–40. doi: 10.1016/j.agee.2012.05.017
Li, S., Tan, T., Fan, Y., Raza, M. A., Wang, Z., Wang, B., et al. (2022). Responses of leaf stomatal and mesophyll conductance to abiotic stress factors. J. Integr. Agric. 21, 2787–2804. doi: 10.1016/j.jia.2022.07.036
Li, H., Yang, Y., Zhang, H., Chu, S., Zhang, X., Yin, D., et al. (2016). A genetic relationship between phosphorus efficiency and photosynthetic traits in soybean as revealed by QTL analysis using a high-density genetic map. Front. Plant Sci. 7:924. doi: 10.3389/fpls.2016.00924
Lin, B. B. (2007). Agroforestry management as an adaptive strategy against potential microclimate extremes in coffee agriculture. Agric. Meteorol. 144, 85–94. doi: 10.1016/j.agrformet.2006.12.009
Manrique, L. A. (1994). Nitrogen requirements of taro. J. Plant Nutr. 17, 1429–1441. doi: 10.1080/01904169409364817
Martins, S. C., Araújo, W. L., Tohge, T., Fernie, A. R., and Da Matta, F. M. (2014). In high-light-acclimated coffee plants, the metabolic machinery is adjusted to avoid oxidative stress rather than to benefit from extra light enhancement in photosynthetic yield. PLoS One 9:e94862. doi: 10.1371/journal.pone.0094862
Martorell, S., Medrano, H., Tomás, M., Escalona, J. M., Flexas, J., and Díaz-Espejo, A. (2015). Plasticity of vulnerability to leaf hydraulic dysfunction during acclimation to drought in grapevines: an osmotic-mediated process. Physiol. Plant. 153, 381–391. doi: 10.1111/ppl.12253
Masoodi, T. H., Masoodi, N. A., Gangoo, S. A., Mushtaq, S. M., and Ahmad, H. (2013). Comparative field performance of some agricultural crops under a canopy of Populusdeltoides and Ulmus wallichiana. J. For. Res. 24, 783–790. doi: 10.1007/s11676-013-0417-y
Mathur, S., Jain, L., and Jajoo, A. (2017). Photosynthetic efficiency in sun and shade plants. Photosynthetica 56, 354–365. doi: 10.1007/s11099-018-0767-y
Mbow, C., Van, N. M., Luedeling, E., Neufeldt, H., Minang, P. A., and Kowero, G. (2014). Agroforestry solutions to address food security and climate change challenges in Africa. Curr. Opin. Environ. Sustain. 6, 61–67. doi: 10.1016/j.cosust.2013.10.014
Medrano, H., Magdalena, T., Sebastià, M., Jaume, F., Esther, H., Joan, R., et al. (2015). From leaf to whole-plant water use efficiency (WUE) in complex canopies: limitations of leaf WUE as a selection target. Crop J. 3, 220–228. doi: 10.1016/j.cj.2015.04.002
Melis, A., and Harvey, G. W. (1981). Regulation of photosystem stoichiometry, chlorophyll a and chlorophyll b content and relation to chloroplast ultrastructure. Biochim. Biophys. Acta 637, 138–145. doi: 10.1016/0005-2728(81)90219-X
Naik, H., Chandarana, K. A., Gamit, H. A., Chandwani, S., and Amaresan, N. (2023). “Nutrition and cultivation strategies of core rhizosphere microorganisms” in Rhizobiome: ecology, management and application (Amsterdam: Elsevier), 209–231.
Osone, Y., Ishida, A., and Tateno, M. (2008). Correlation between relative growth rate and specific leaf area requires associations of specific leaf area with nitrogen absorption rate of roots. New Phytol. 179, 417–427. doi: 10.1111/j.1469-8137.2008.02476.x
Padmapriya, S., Chezhiyan, N., and Sathiyamurthy, V. A. (2007). Effect of shade and integrated nutrient management on biochemical constituents of turmeric (Curcuma longa L.). J. Hortic. Sci. 2, 123–129. doi: 10.24154/jhs.v2i2.619
Peltoniemi, M. S., Duursma, R. A., and Medlyn, B. E. (2012). Co-optimal distribution of leaf nitrogen and hydraulic conductance in plant canopies. Tree Physiol. 32, 510–519. doi: 10.1093/treephys/tps023
Poorter, H., Niinemets, Ü., Ntagkas, N., Siebenkäs, A., Mäenpää, M., Matsubara, S., et al. (2019). A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 223, 1073–1105. doi: 10.1111/nph.15754
Purnomo, D., Budiastuti, M. S., Sakya, A. T., and Cholid, M. I. (2018). The potential of turmeric (Curcuma xanthorrhiza) in agroforestry system based on silk tree (Albizia chinensis). IOP Conf. Ser.: Earth Environ. Sci. 142:012034. doi: 10.1088/1755-1315/142/1/012034
Rajbhandari, R., Shrestha, A. B., Kulkarni, A., Patwardhan, S. K., and Bajracharya, S. R. (2015). Projected changes in climate over the Indus River basin using a high-resolution regional climate model (PRECIS). Clim. Dyn. 44, 339–357. doi: 10.1007/s00382-014-2183-8
Ramesh, T., Manjaiah, K. M., Mohopatra, K. P., Rajasekar, K., and Ngachan, S. V. (2015). Assessment of soil organic carbon stocks and fractions under different agroforestry systems in subtropical hill agroecosystems of north-east India. Agrofor. Syst. 89, 677–690. doi: 10.1007/s10457-015-9804-z
Rangappa, K., Choudhury, B. U., Kumar, A., Das, S. P., Ayam, G., Hazarika, S., et al. (2024). Comparative stress physiological analysis of indigenous rice cultivars of Eastern Himalayan Region under elevated temperature of changing climate. Plant Physiol. Rep. 29, 535–551. doi: 10.1007/s40502-024-00796-2
Ravi, V., More, S. J., Raju, S., Muthuraj, R., and Suja, G. (2022). Assessment of photosynthetic efficiency of greater yam and white yam subjected to elevated carbon dioxide. S. Afr. J. Bot. 145, 397–404. doi: 10.1016/j.sajb.2021.12.041
Saha, R., Chaudhary, R. S., and Somasundaram, J. (2012). Soil health management under hill agroecosystem of north East India. Appl. Environ. Soil Sci. 2012:696174. doi: 10.1155/2012/696174
Sanou, J., Bayala, J., Bazei, P., and Teklehaimanot, Z. (2012). Photosynthesis and biomass production by millet (Pennisetum glaucum) and taro (Colocasia esculenta) grown under (Adansonia digitata) and nere (Parkia biglobosa) in an agroforestry system of Burkina Faso (West Africa). Exp. Agric. 48, 283–300. doi: 10.1017/S0014479712000014
Sasikumar, B. (2007). Genetic resources of Curcuma: diversity, characterization and utilization. Plant Genetic Resour. 3, 230–251. doi: 10.1079/PGR200574
Sawitri,, Primananda, E., and Budiadi,. (2020). Morpho-anatomical adaptation of lesser yam (Dioscoreaesculenta) on different planting patterns and relative light intensity in Java community forest. IOP Conf. Ser.: Earth Environ. Sci. 449:012009. doi: 10.1088/1755-1315/449/1/012009
Sharangi, A. B., Gowda, M. P., and Das, S. (2022). Responses of turmeric to light intensities and nutrients in a forest ecosystem: retrospective insight. Trees For. People 7:100208. doi: 10.1016/j.tfp.2022.100208
Sharma, E., and Pukkala, T. (1990). Volume equations and biomass prediction of forest trees in Nepal. Babar Mahal, Kathmandu: Ministry of Forests and Soil Conservation, 1–16.
Sharma, G., Sharma, R., Sharma, E., and Singh, K. K. (2002). Performance of an age series of Alnus cardamom plantations in the Sikkim Himalaya: nutrient dynamics. Ann. Bot. 89, 273–282. doi: 10.1093/aob/mcf036
Sims, D. A., and Pearcy, R. W. (1989). Photosynthetic characteristics of a tropical forest understorey herb, Alocasia macrorrhiza, and a related crop species, Colocasia esculenta grown in contrasting light environments. Oecologia 79, 53–59. doi: 10.1007/BF00378239
Sinacore, K., Hall, J. S., Potvin, C., Royo, A. A., Ducey, M. J., and Ashton, M. S. (2017). Unearthing the hidden world of roots: root biomass and architecture differ among species within the same guild. PLoS One 12:e0185934. doi: 10.1371/journal.pone.0185934
Sinclair, T. R. (2018). Effective water use required for improving crop growth rather than transpiration efficiency. Front. Plant Sci. 9:1442. doi: 10.3389/fpls.2018.01442
Singh, A., and Singh, M. D. (1981). Effect of various stages of shifting cultivation on soil erosion from steep hill slopes. Indian Forester. 106, 115–121.
Slattery, R. A., Ainsworth, E. A., and Ort, D. R. (2013). A meta-analysis of responses of canopy photosynthetic conversion efficiency to environmental factors reveals major causes of yield gap. J. Exp. Bot. 64, 3723–3733. doi: 10.1093/jxb/ert207
Suárez Salazar, J. C., Melgarejo, L. M., Casanoves, F., di Rienzo, J. A., Da Matta, F. M., and Armas, C. (2018). Photosynthesis limitations in cacao leaves under different agroforestry systems in the Colombian Amazon. PLoS One 13:e0206149. doi: 10.1371/journal.pone.0206149
Subbiah, B. V., and Asija, G. L. (1956). A rapid procedure for the estimation of available nitrogen in the soils. Curr. Sci. 8, 259–260.
Sun, J., Gu, J., Zeng, J., Han, S., Song, A., Chen, F., et al. (2013). Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci. Hortic. 161, 249–258. doi: 10.1016/j.scienta.2013.07.015
Tezara, W., Urich, R., Jaimez, R., Coronel, I., Araque, O., Azocar, C., et al. (2016). Does Criollo cocoa have the same ecophysiological characteristics as Forastero? Bot. Sci. 94, 563–574. doi: 10.17129/botsci.552
Vile, D., Garnier, E., Shipley, B., Laurent, G., Navas, M. L., Roumet, C., et al. (2005). Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann. Bot. 96, 1129–1136. doi: 10.1093/aob/mci264
Waldron, A., Garrity, D., Malhi, Y., Girardin, C., Miller, D. C., and Seddon, N. (2017). Agroforestry can enhance food security while meeting other sustainable development goals. Trop. Conserv. Sci. 10, 1–6. doi: 10.1177/1940082917720667
Walkley, A., and Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Yan, F., Sun, Y., Song, F., and Liu, F. (2012). Differential responses of stomatal morphology to partial root-zone drying and deficit irrigation in potato leaves under varied nitrogen rates. Sci. Hortic. 145, 76–83. doi: 10.1016/j.scienta.2012.07.026
Yang, M., Liu, M., Lu, J., and Yang, H. (2019). Effects of shading on the growth and leaf photosynthetic characteristics of three forages in an apple orchard on the Loess Plateau of eastern Gansu, China. Peer J. 7:e7594. doi: 10.7717/peerj.7594
Yuan, Y., Chen, Z., Huang, X., Wang, F., Guo, H., Huang, Z., et al. (2023). Comparative analysis of nitrogen content and its influence on actinorhizal nodule and rhizospheric microorganism diversity in three Alnus species. Front. Microbiol. 14:1230170. doi: 10.3389/fmicb.2023.1230170
Zhang, Y., and Chen, H. Y. (2015). Individual size inequality links forest diversity and above-ground biomass. J. Ecol. 103, 1245–1252. doi: 10.1111/1365-2745.12425
Keywords: PAR, physiological traits, stomatal conductance, tree canopies, understorey crops
Citation: Rangappa K, Singh NR, Janyanaik Joga R, Mohapatra KP, Chandra P, Choudhury BU, Moiranghtem P, Debnath S, Chanu LJ, Devi NP, Singh NU, Hazarika S, Kumar YB and Mishra VK (2025) Tree crop interactions, productivity and physiological efficiency of understorey crops in Alnus nepalensis and Gmelina arborea based agroforestry systems in Eastern Himalayas. Front. Sustain. Food Syst. 9:1494371. doi: 10.3389/fsufs.2025.1494371
Edited by:
Gaetano Alessandro Vivaldi, University of Bari Aldo Moro, ItalyReviewed by:
Syam Viswanath, Kerala Forest Research Institute, IndiaDennis Shibonje Ashilenje, Mohammed VI Polytechnic University, Morocco
Copyright © 2025 Rangappa, Singh, Janyanaik Joga, Mohapatra, Chandra, Choudhury, Moiranghtem, Debnath, Chanu, Devi, Singh, Hazarika, Kumar and Mishra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kamal Prasad Mohapatra, a3BtYmJzckBnbWFpbC5jb20=; Nongmaithem Raju Singh, cmFqdWZvcmVzdHJ5QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Krishnappa Rangappa
Krishnappa Rangappa Nongmaithem Raju Singh
Nongmaithem Raju Singh Rajappa Janyanaik Joga
Rajappa Janyanaik Joga Kamal Prasad Mohapatra
Kamal Prasad Mohapatra Puran Chandra1,3
Puran Chandra1,3 Burhan U. Choudhury
Burhan U. Choudhury Prabha Moiranghtem
Prabha Moiranghtem Ningthoujam Peetambari Devi
Ningthoujam Peetambari Devi Samarendra Hazarika
Samarendra Hazarika Vinay Kumar Mishra
Vinay Kumar Mishra