- 1Fish Nutrition Lab, Department of Zoology, Government College University Faisalabad, Faisalabad, Punjab, Pakistan
- 2Department of Environmental Studies, University of California Santa Cruz, Santa Cruz, CA, United States
- 3Department of Environmental Science, College of Agriculture and Environmental Sciences, Government College University Faisalabad, Faisalabad, Punjab, Pakistan
- 4Department of Biological Sciences and Technology, China Medical University, Taichung, Taiwan
- 5Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia
Introduction: The application of therapeutic plants in aquaculture has gained considerable worldwide attention and is currently the focus of ongoing scientific research. These plants possess diverse bioactive compounds that offer safer, non-toxic, biodegradable, and biocompatible alternatives for consumers. This research assessed the efficacy of purslane extract supplementation on sunflower meal (SFM)-based diets (iso-nitrogenous, iso-lipidic, and iso-caloric) in terms of growth, improving carcass, mineral content, hematology, immune responses, and antioxidant status in Labeo catla fingerlings.
Methods: Each of the seven test diets—T0 (no extract supplementation), T1 (0.5% supplementation), T2 (1% supplementation), T3 (1.5% supplementation), T4 (2% supplementation), T5 (2.5% supplementation), and T6 (3% supplementation)—were administered two times per day at a rate equivalent to 5% of their body mass. The experiment was maintained in V-shaped steel tanks for a duration of 90 days. Each group included 15 fingerlings (N = 315; average weight: 7.36 ± 0.03 g), and each diet was administered in triplicates.
Results: Based on the results of the current study, it is clear that the T3 (1.5% supplementation) diet showed significantly improved growth (in terms of percentage weight gain), feed utilization, and whole-body protein content. In addition, the incorporation of 1.5% purslane extract in SFM-based diets significantly enhanced hematological indices, including platelets, red blood cells, hemoglobin, and white blood cells. Furthermore, all the examined diets exhibited a significant (p < 0.05) increase in mineral content. Dietary supplementation with 1.5% purslane extract exhibited significant enhancements in antioxidant defenses, including increased superoxide dismutase, catalase, and glutathione peroxide activities, alongside a marked decrease in malondialdehyde levels. Furthermore, immune parameters, such as lysozyme activity and total globulins levels, showed considerable improvements in response to the 1.5% purslane supplementation.
Discussion: This study suggested that 1.5% purslane extract supplementation is the optimum level for improving the health and physiology of L. catla fingerlings. The therapeutic benefits of purslane may be attributed to its bioactive compounds, which contribute significantly to enhancing fish performance.
1 Introduction
The goal of aquaculture is to satisfy the nutritional requirements of consumers worldwide by producing the greatest substantial amount of protein-rich food (Dawood, 2021). Furthermore, global aquaculture production is expected to reach 58.9 million tons by 2025, leading to a substantial rise in aqua feed demand to 69.5 million tons (Tacon et al., 2022). Aside from being a great protein source, fish is also an outstanding source of minerals and vitamins (Gore et al., 2021). This industry is growing at the fastest rate of any food-processing sector. Its success and participation offer to eradicate poverty and starvation while also fulfilling most of the global food demands (El-Saadony et al., 2021). Labeo catla, a surface-feeding fish, is widely used in polyculture systems, where it is cultivated alongside different species of the aquatic environment (Aslam et al., 2016). It is a kind of zooplanktivore that feeds on the surface (Saleem et al., 2022). According to 2018 statistics, C. catla production worldwide reached 3,041.3 thousand tonnes, representing 5.6% of total global fish production and securing 6th place among prominent aquaculture species (Perera et al., 2024).
The aquaculture industry requires cost-effective fish feed that meets nutritional standards, but traditional ingredients such as fish oil (FO) and fishmeal (FM) face significant constraints due to limited availability, unsustainable supply chains, high demand, and prohibitive costs (Jannathulla et al., 2019). Developing FM-free aquafeeds is crucial for sustainable aquaculture expansion. However, the prospect of rising prices and limited FM supply encouraged researchers to look for alternate protein sources. Fish feed made with plant-based protein sources has mostly substituted FM due to its accessibility, sustainability, and financial viability (Hardy, 2010; Elumalai et al., 2021). Sunflower meal (SFM) is regarded as a promising substitute for FM and a cost-effective supply of essential nutrients. Globally, it ranks as the fourth most significant plant-based protein source, after soybean, canola and cottonseed meal (Anjum et al., 2014). Its protein content is approximately 40%, which is primarily determined by the oil extraction and de-hulling processes (Mushtaq et al., 2006). Moreover, it is composed of essential amino acids, vitamin B, and minerals, making it an excellent ingredient for human nutrition and livestock feed (Wanjari and Waghmare, 2015).
Integrating functional feed supplements into aquaculture feeds offers numerous benefits (Dawood et al., 2018). These supplements, including immunostimulants, prebiotics, herbs, microelements, probiotics, and vitamins, have the potential to enhance nutritional quality, promote optimal fish health, and maximize production rate (Syanya et al., 2023). In the fisheries industry, utilizing plant supplements and their bioactive components in aqua feed is a common practice (Hoseinifar et al., 2020; Magouz et al., 2022). Specifically, incorporating medicinal herbs and their extracts into aqua feed can have a positive impact on the health and well-being of aquatic animals, enabling them to thrive and perform optimally (Awad and Awaad, 2017; Dawood, 2021). Using Portulaca oleracea, a therapeutic herb, as fish feed is a sustainable and potentially economical idea (Sourani et al., 2023). This innovation supports the principles of the circular economy by recycling waste into a valuable feedstock (Amalia et al., 2024).
Purslane, scientifically known as P. oleracea, belongs to the Portulacaceae family and has several nutritional, phytoremedial, and pharmacological characteristics (Ocampo and Columbus, 2012; Kumar et al., 2022). It has been shown that this particular plant exhibits more than 30 distinct biological activities and more than 60 therapeutic applications (Sangeetha et al., 2020). The World Health Organization has classified it as a “Global Panacea” due to its widespread utilization, making it one of the most frequently used plant species (Miao et al., 2019). An examination of phytochemicals shows that this plant has several bioactive substances, including organic acids, fatty acids, flavonoids, alkaloids, vitamins, terpenoids, and minerals (Zhou et al., 2015). Moreover, purslane extract contains ash (3.8%), fiber (0.82%), protein (4.9%), and carotenoids (40.40 mg per 100 g) (Hassan, 2014). Furthermore, dried purslane powder exhibits a notable nutritional profile, comprising 18.58% protein, 16.5% ash, 17.9% fiber, and a total carotenoid content of 110.97 mg per 100 g. The use of purslane, as an aqua feed supplement, is a promising approach to enhance the health and immunity of fish (Abdel-Razek et al., 2019; Sourani et al., 2023). Research studies have shown that several components of purslane (roots to the stem), can be used for medicinal purposes (Besong et al., 2011; Naeem and Khan, 2013). Research has explored the effects of purslane on antioxidant enzyme activity, immune responses, and growth in various fish species, including gilthead seabream, grass carp, and Nile tilapia (Abdel-Razek et al., 2019; Ahmadifar et al., 2020; Cámara-Ruiz et al., 2020).
A literature review revealed no existing studies on the use of dietary purslane extract in L. catla. However, this study aimed to investigate the potential effects of purslane extract supplemented with an SFM-based diet on the growth, body composition, hematology, whole-body mineral profile, antioxidant, and immune responses of fish.
2 Materials and methods
2.1 Experimental site
Current experiment and all laboratory analyses were carried out at the Zoology Department, GC University Faisalabad, Pakistan.
2.2 Ethical statement
The study protocols adhered to the ethical guidelines approved by the Ethics Review Committee of GC University Faisalabad, by approval Ref. No. GCUF/ERC/436. All research protocols were performed in accordance with the ARRIVE guidelines. The authors endorse that the research was conducted in an ethical and accountable manner.
2.3 Fish rearing
A total of 315 fish, with an average weight of 7.36 ± 0.03 g, were acquired from the local market and then transported to the designated experimental site. Fingerlings were transferred to an experimental tank and acclimatized to laboratory conditions for 15 days. Fingerlings were treated with 5 g/L NaCl to eliminate parasites and prevent microbial infections (Rowland and Ingram, 1991), then fed a 40% protein basal diet until satiated during the acclimatization phase (Allen and Rowland, 1992). The experimental tanks received aeration via a capillary system for 24 h every day, and water quality parameters, such as dissolved oxygen (DO) (7.5 mg/L), temperature (26–29°C), and pH (7.7), were maintained daily.
2.4 Experimental setup
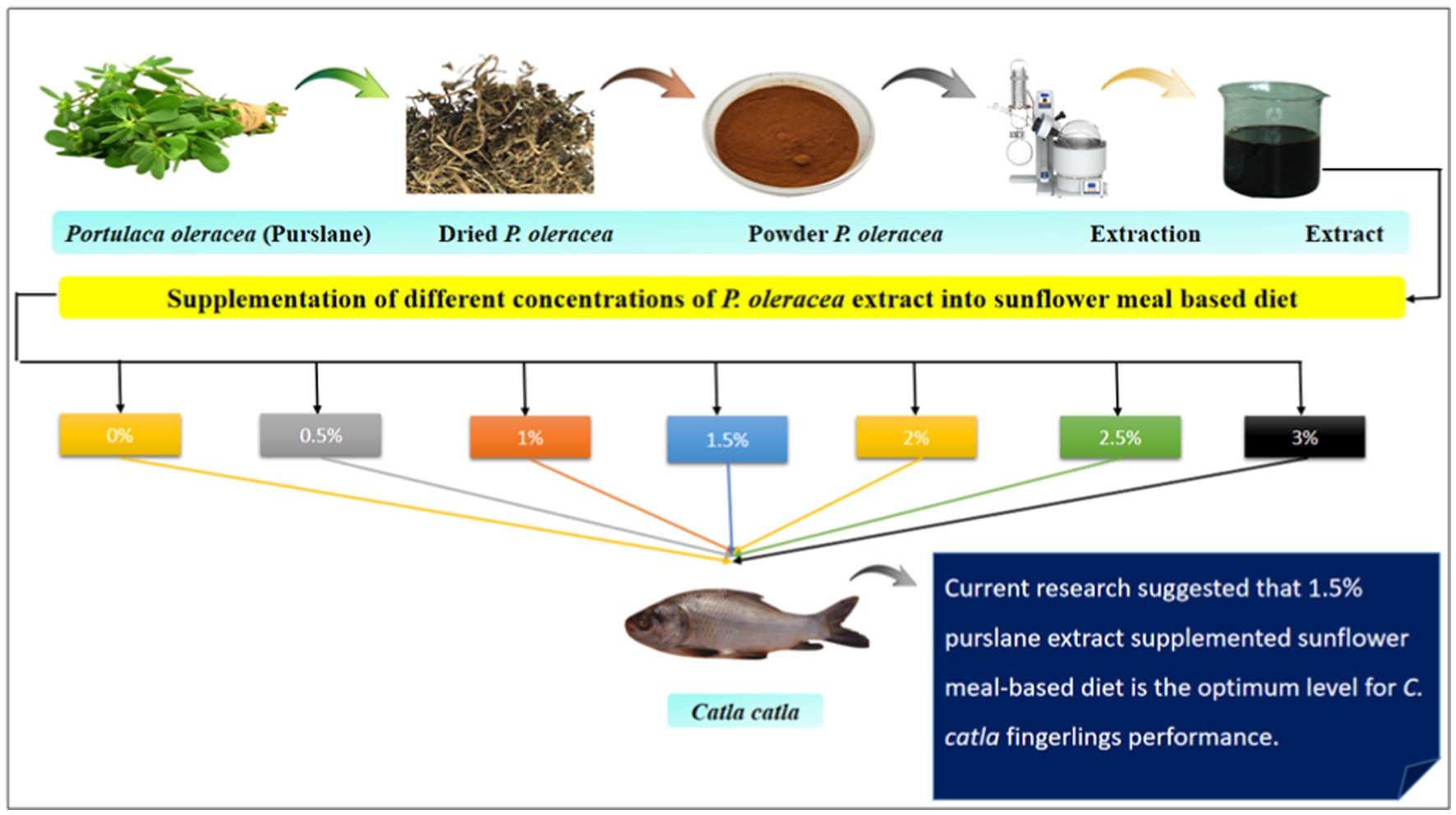
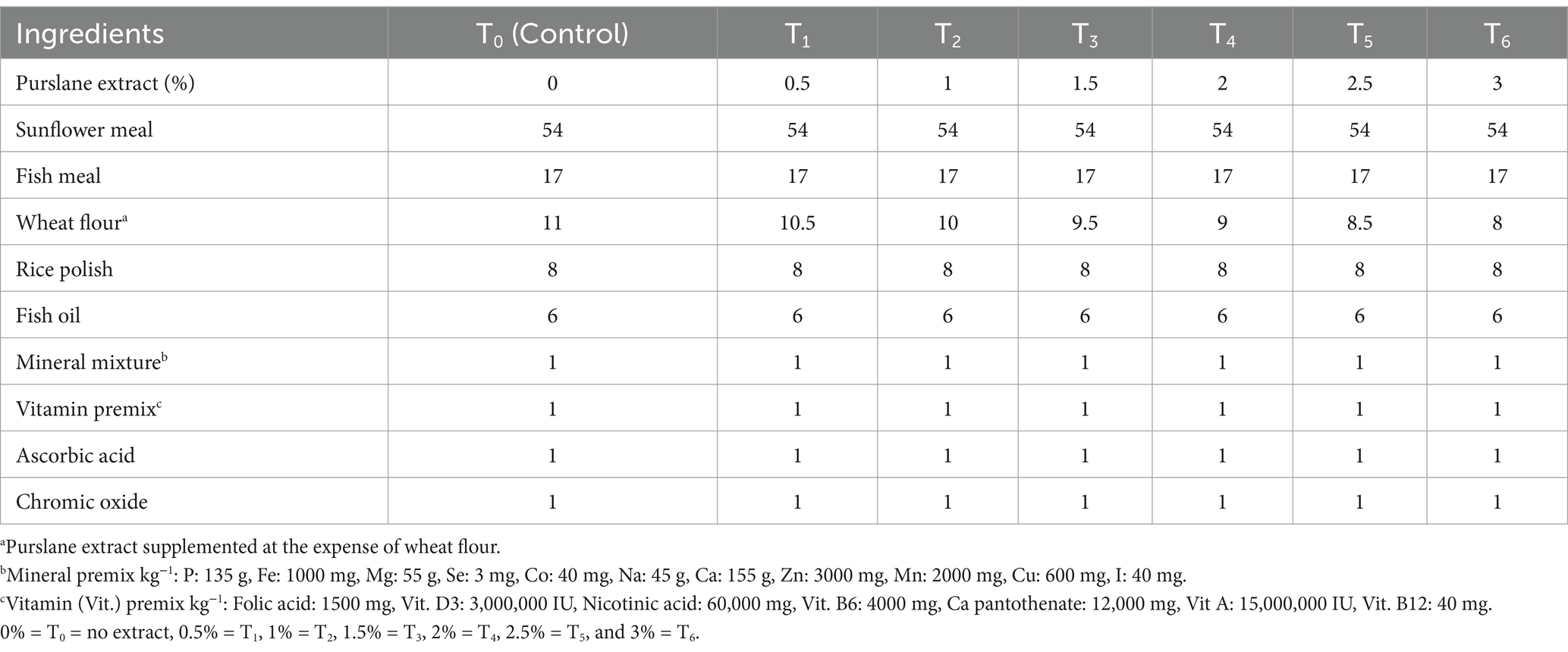
Purslane was used as a test component to prepare the test diet, and a total of seven iso-nitrogenous and iso-caloric diets were prepared for this experiment. One group served as a control T0 (no extract supplementation; only basal diet), and the others received purslane extract at different concentrations viz., T1 (0.5% supplementation), T2 (1% supplementation), T3 (1.5% supplementation), T4 (2% supplementation), T5 (2.5% supplementation), and T6 (3% supplementation), respectively. Each treatment group consisted of triplicate tanks, with 15 fish per tank. SFM-based diets supplemented with purslane extract were administered to the fingerlings two times per day at a level of 5% of the body weight of fish. Following the completion of a 2 h feeding session, any residual feed in each tank was removed using the tank valves. Each tank was thoroughly cleaned to remove residual feed particulates, then refilled with fresh water. A 90-day feeding experiment was subsequently conducted.
2.5 Purslane extract preparation
Fresh plants were collected from a farm in the district of Layyah, Kot Sultan, Pakistan. The sample was identified at the Botany Department, GCUF. Afterward, the sample was cleaned to eliminate debris, dust, and any rotting or broken components. After several days of sun-drying, the sample was crushed into a powder by using an electric grinder and stored in a cool and dry place in polythene bags (Shanker et al., 2019).
Purslane extract was obtained using the Soxhlet extraction (J.P. Selecta, s.a.; Serial #0481090) method. For this process, 2000 g of powdered purslane was loaded into the apparatus, and an ethanol solution (60% v/v) served as the extraction solvent. After extraction, the product was filtered and then concentrated to dryness using a rotary evaporator (Scilogex RE 100-S). Subsequently, the product was kept at −20°C for future use (Taghizadeh et al., 2018).
2.6 Feed processing
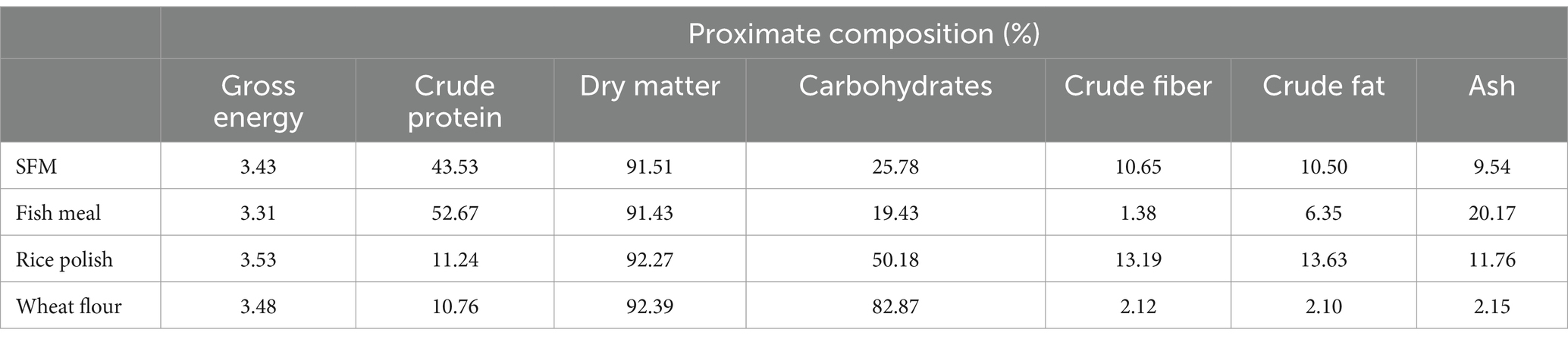
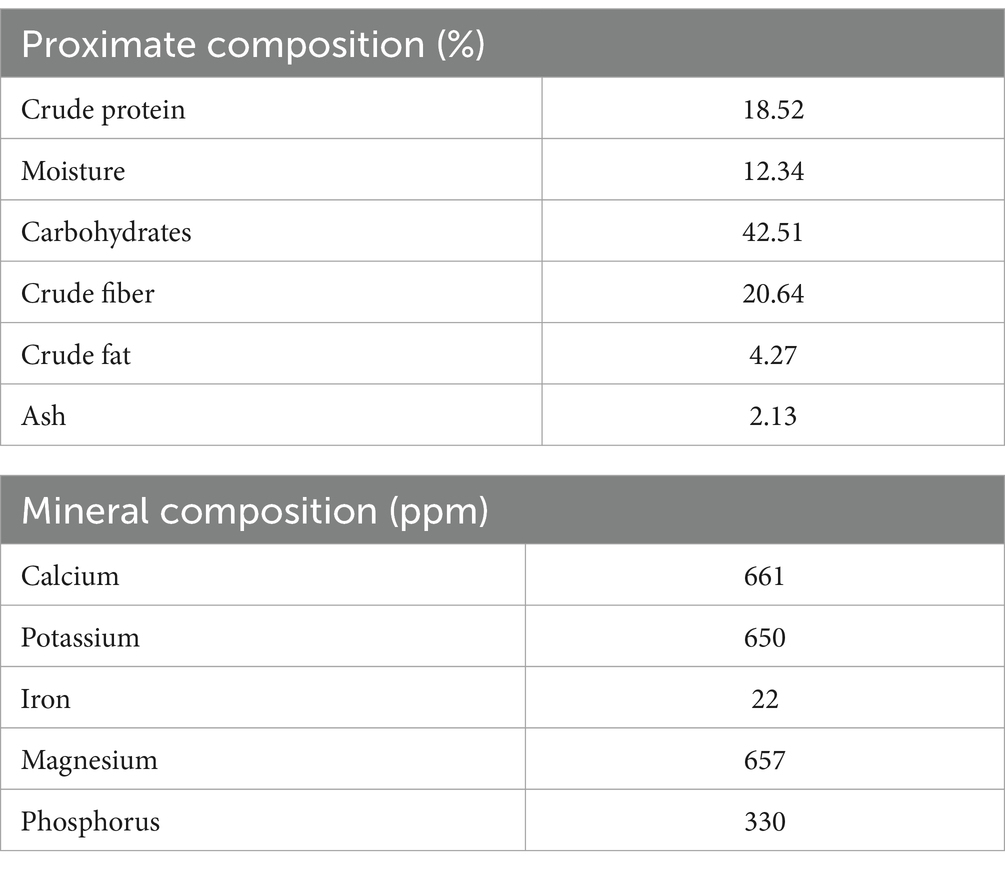
The dietary ingredients were ground and then blended with the extract for 5 minutes. Subsequently, fish oil was slowly added. The proper dough was obtained by adding 10–15% water, and diet pellets were formulated by running the dough through a pelleting machine (Lovell, 1989). The ingredient composition of test diets is presented in Table 1, and the proximate composition of different ingredients is depicted in Table 2. Table 3 shows the proximate and mineral composition of purslane extract.
2.7 Proximate analysis
Five fish were anesthetized using an overdose of anesthesia and thereafter preserved at −20°C until the study of whole-body composition. Fish carcass composition was determined following the standardized procedures outlined by AOAC (2016). To assess moisture content, the samples were subjected to oven drying at 105°C for 12 hours. The evaluation of crude protein (CP) content (N × 6.25) was conducted using a micro Kjeldahl apparatus (SCITEK, KA-DNII). The crude fiber content was analyzed by incinerating the lipid-free residues at 600°C for 2 hours, after digestion with 1.25% NaOH and 1.25% H2SO4. The ether extract (EE) was extracted using petroleum ether through the Soxlet apparatus. The overall energy content of diet samples was assessed using an adiabatic oxygen bomb calorimeter (Parr Instrument Company, 6400). Ash content was identified through a 12 h combustion in an electric furnace at 650°C (Eyela-TMF 3100).
2.8 Growth and feed utilization analysis
At the start and end of the trial, fish from each tank were bulk-weighed to evaluate growth parameters. These included weight gain (WG), specific growth rate (SGR), and percentage weight gain (WG%), and feed conversion ratio (FCR), which were calculated using standard equations as described by Faisal et al. (2024). Additionally, the following formulae were applied to determine the protein efficiency ratio (PER) and survival rate:
2.9 Hematological studies
Five fish were randomly selected from each of the experimental groups to undergo hematological analysis. Fish were immobilized using clove oil (60 mg/L) for a minimum of 5 min (Javahery et al., 2012). Using an anticoagulated syringe, the fingerlings’ caudal vein was punctured to collect blood samples for further examination. The blood was then stored in EDTA anticoagulant tubes. Hematological parameters were assessed using standard techniques. Hematocrit (Ht) levels were determined by micro-hematocrit method (Brown, 1988). While, white blood cell (WBC) and red blood cell (RBC) counts were performed using a hemocytometer(Millipore, MDH-2N1) (Blaxhall and Daisley, 1973). Derived parameters, including mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC), were calculated using established formulae by Faisal et al. (2024). In addition, the techniques developed by Wedemeyer and Yasutake (1977) were followed to assess the hemoglobin (Hb) concentration. The packed cell volume (PCV) was measured as described by Kefas et al. (2015).
2.10 Enzyme activity and immune parameters
For antioxidant analysis, 5 fish from each treatment group were dissected, and their livers were harvested for the assessment of enzymatic antioxidants, including superoxide dismutase, glutathione peroxidase and catalase, as well as malondialdehyde levels. After homogenizing the liver samples in tris buffer having 0.4 M and 7.0 pH, the samples were centrifuged for 10 min at 9400 g. The supernatant was maintained at a temperature of −20°C until further analysis. In accordance with Winterbourn et al. (1993), superoxide dismutase enzyme activity was obtained by inhibiting nitroblue-tetrazolium reduction. Catalase activity was assessed using the method described by Claiborne (1985), which measures the enzyme’s ability to catalyze the decomposition of hydrogen peroxide (H2O2) into water. Glutathione peroxidase activity was evaluated by monitoring the oxidation of glutathione by H2O2 and the resulting decrease in absorbance at 340 nm, based on the protocol of Rotruck et al. (1973). Malondialdehyde content was estimated using the chromophore detection method at 532 nm, as described by Buege and Aust (1978).
Three fish were randomly dissected from each treatment group for serum separation. To assess the lysozyme (LYZ) activity in serum, the Ellis (1999) methodology was used. Serum globulin levels (GLO) were quantified using the biochemistry analyzer (Hitachi 7600-110 Ltd).
2.11 Statistical analysis
Data analysis was conducted using one-way ANOVA (Steel et al., 1996). Co-Stat software (Version 6.303) was employed for statistical computations. Tukey’s HSD test was used to compare means, with a significance level of p < 0.05 (Snedecor and Cochran, 1991).
3 Results
3.1 Growth evaluation and survival rate
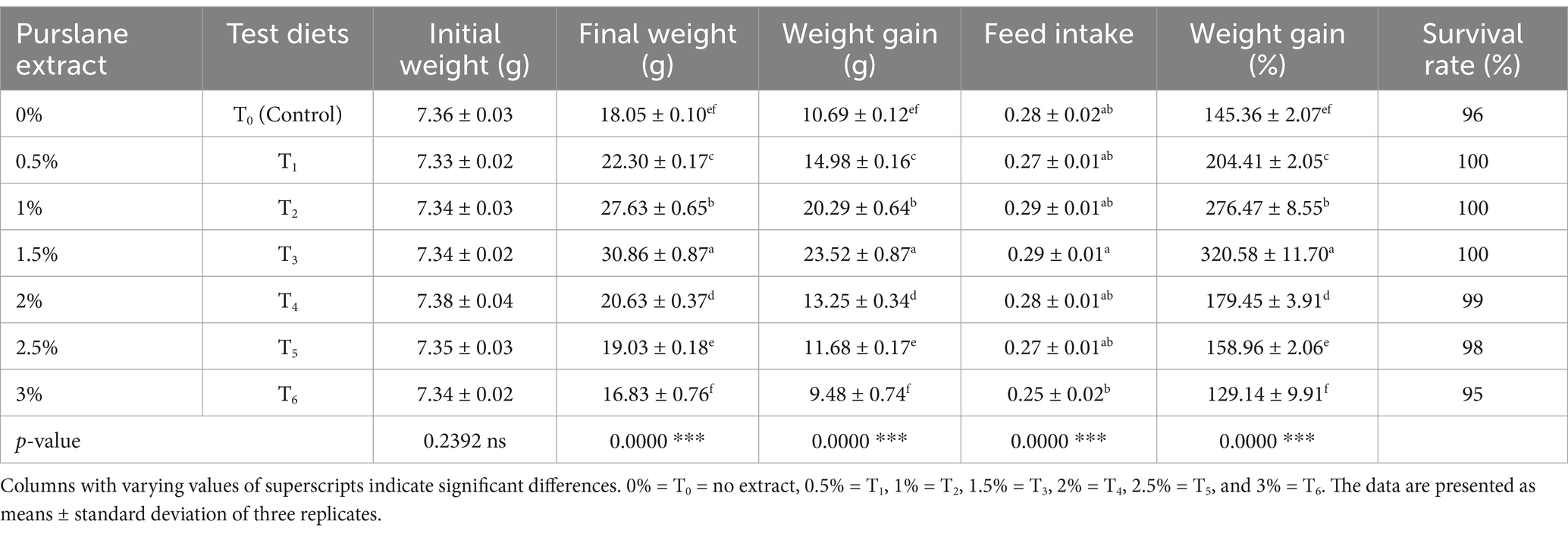
Growth indices and survival rates were measured after the experiment. The growth in terms of WG, WG%, feed intake, FCR, SGR, and PER was determined in fish fed with a purslane extract-supplemented SFM-based diet (Table 4; Figure 1). The results showed that growth indices of fish was improved significantly (p < 0.05) in all treatment groups compared to T0 and T6. Additionally, a significantly (p < 0.05) lower FCR was obtained in the T3 group throughout the treatments. Overall, the growth and feed utilization parameters were significantly enhanced up to 1.5% extract supplementation in the basal diet and decreased significantly on higher supplementation. The T1–T3 groups, fed with 0.5–1.5% purslane extract-supplemented SFM diets, demonstrated 100% fish survival.

Figure 1. Feed conversion ratio (FCR; %), protein efficiency ratio (PER; %) and specific growth rate (SGR; %) of L. catla fed varying concentrations of purslane extract. The data are presented as means ± standard deviation of three replicates.
3.2 Whole-body proximate composition
Carcass analysis of L. catla (Table 5) revealed significant variations in body composition among treatments receiving purslane extract-supplemented diets. The fat contents significantly decreased, and protein contents notably (p < 0.05) increased in the T3 group throughout the treatments. Overall, the group fed a 1.5% purslane extract-supplemented SFM-based diet showed significant enhancement compared to higher supplementation levels. There were no noticeable variations in moisture and ash contents for L. catla-fed diets based on purslane extract.
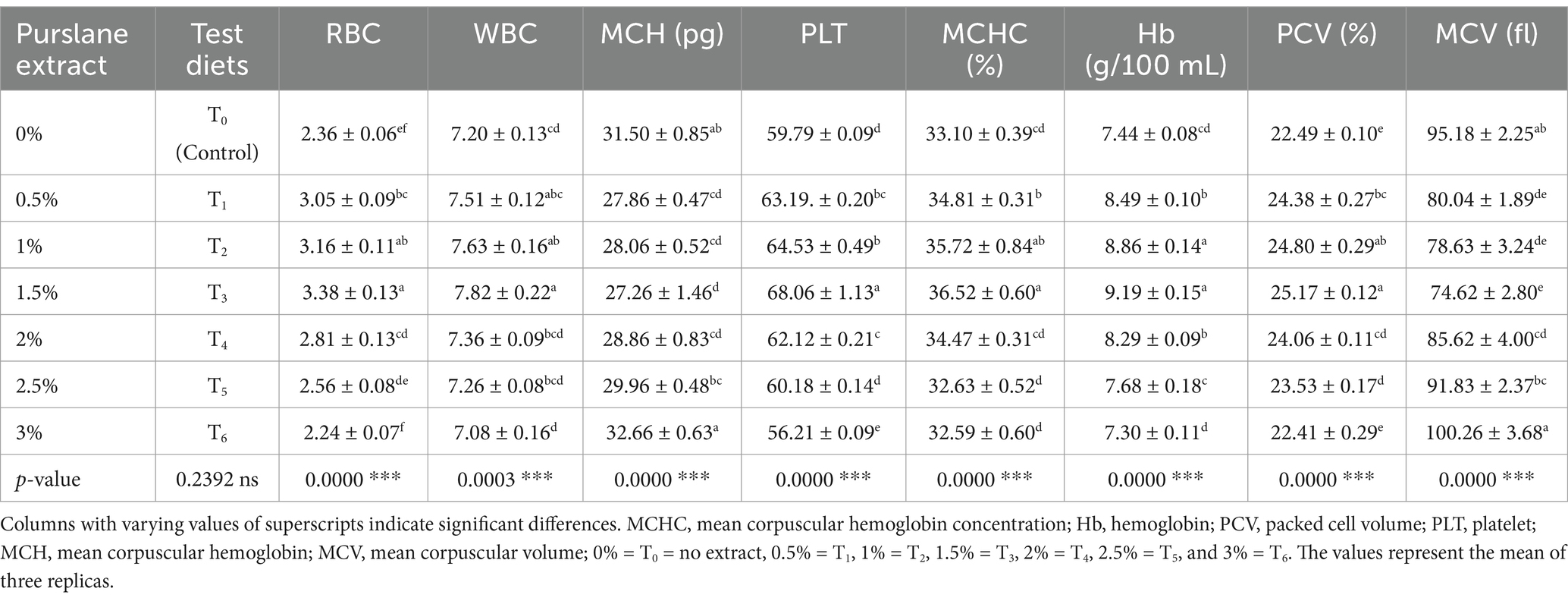
3.3 Hematology
Hematological indices were substantially increased (p < 0.05) in fish having purslane extract (Table 6). Purslane-enriched diets significantly improved the RBCs, WBCs, platelets (PLTs) count, Hb values, PCV, and MCHC in T3 (1.5% extract) relative to T0 and T6 treatments (purslane-enriched diets at 0 and 3%, respectively). However, MCH and MCV significantly (p < 0.05) decreased in fish fed with the diet having 1.5% purslane extract compared to higher treatments.
3.4 Whole-body mineralization
Table 7 shows the whole-body mineral content of the experimental groups. Purslane extract-supplemented diet significantly (p < 0.05) improved the whole-body mineral content in fish. The current results suggest that the highest concentrations of minerals—manganese (Mn), phosphorous (P), zinc (Zn), copper (Cu), sodium (Na), calcium (Ca), iron (Fe), potassium (K), and magnesium (Mg)—were found in the T3 group compared to the control and T6 treatments.

Table 7. Overall body mineralization of L. catla fed with different concentrations of purslane extract.
3.5 Antioxidant status
Figures 2A–D shows the liver antioxidant properties of L. catla-fed diets with different purslane extract concentrations for 90 days. The control group (T0), which received no purslane extract, had 85.40 U/mg catalase, 7.74 U/mg superoxide dismutase, 90.47 mU/mg glutathione peroxide, and 3.61 mg/g malondialdehyde. Purslane extract at 1.5–0.5% (T3–T1) considerably increased antioxidant enzyme activity (catalase, superoxide dismutase, and glutathione peroxide) and decreased lipid peroxidation marker (malondialdehyde). Overall, T3 (1.5% purslane extract) exhibited the highest levels of catalase (95.41 U/mg), superoxide dismutase (8.72 U/mg), and glutathione peroxide (103.52 mU/mg), along with the lowest malondialdehyde (2.88 mg/g), indicating increased oxidative stress protection. Higher dosages (T4, T5, and T6) did not sustain not maintain this increasing trend, demonstrating a reduction in antioxidant enzyme activity and an increase in malondialdehyde levels.

Figure 2. Liver antioxidant parameters of L. catla having various concentrations of purslane extract supplementation (a) Catalase (b) Superoxide dismutase (c) Glutathione peroxidase (d) Malondialdehyde. The data are presented as means ± standard deviation of three replicates.
3.6 Immune response
Figures 3A, B indicates the immunological response of L. catla when fed with graded levels of purslane in diets based on SFM for a duration of 90 days. The control group (T0) that did not receive purslane extract had the lowest LYZ activity and GLO level followed by the T6 group (3% inclusion). When purslane extract was added at concentrations of 0.5, 1, and 1.5% (T1, T2, and T3), it led to higher levels of LYZ and GLO in the serum. The highest levels were seen at 1.5% extract, with LYZ activity reaching 70.49 ± 0.18 U/mL and GLO levels at 2.00 ± 0.03 g/dL. Nevertheless, the concentrations of 2, 2.5, and 3% (T4, T5, and T6) resulted in reduction in serum LYZ and GLO levels. The group treated with the 3% extract had the lowest levels of LYZ (53.20 ± 0.10 U/mL) and GLO (1.73 ± 0.04 g/dL) compared to all other groups.

Figure 3. Immune response of L. catla having graded levels of purslane extract (a) Lysozyme (b) Globulin. The data are presented as means ± standard deviation of three replicates.
4 Discussion
The most significant variables that may affect output in the intensive aquaculture system are feeding approaches (Zaki et al., 2020). Thus, a practical approach that may enhance the overall wellbeing and growth rate of farmed fish is the utilization of functional feed additives in aqua feeds (Hoseinifar et al., 2019). Several benefits for aquatic animals have been demonstrated by using herbal plants as feed additives, including increased antioxidant activity, growth rate, feed efficiency, and immunological response (Güllü et al., 2016; Adel et al., 2020). Due to the higher concentration of bioactive chemicals in purslane, it has been utilized extensively in animal diets in recent years to enhance growth, metabolism, and disease resistance (Wang et al., 2021).
In the present study, the growth and feed utilization of L. catla fed with purslane-supplemented test diets was significantly improved, as indicated by WG, WG%, SGR, FCR, and PER values. This study aligned with Ahmadifar et al. (2020) who suggested that purslane improved the growth of grass carp when supplemented at 0.5% in the fish diet. Zenhom and Khames (2014) used 2–3% purslane seed powder in the diet of O. niloticus and concluded that growth performance was significantly enhanced when compared to the control group. The nutritional composition of purslane, particularly its omega-3 fatty acid content, plays a significant role in enhancing growth parameters (Srivastava et al., 2023). A significant positive correlation was observed between feed utilization efficiency and growth performance, with both parameters increasing with the purslane extract concentrations up to 1.5%. Moreover, the survival rate significantly improved with increasing purslane seed percentage in the diets of fish. Another research found that purslane inclusion in the diets of broilers significantly promoted the growth rate of broilers (Wang et al., 2021). Okafor et al. (2014) stated that using purslane in broiler chicken diets increased growth performance owing to purslane’s high antioxidant content. A similar study conducted on goldfish administered with purslane extract noted improved growth and survival of the fish (Şahin et al., 2021). Another research found that dietary purslane extract levels improved the growth performance of rainbow trout (Mohammadalikahni et al., 2020). Moreover, purslane plays a significant role in metabolic activities due to its rich content of vitamins E, C, and B (Srivastava et al., 2023). Furthermore, bioactive compounds in weeds have been shown to be a good appetizer for aquaculture animals (Ahmadifar et al., 2020). Conflicting results showed that growth was substantially decreased while increasing purslane levels in the diet of Nile tilapia, relative to the control (Abdel-Razek et al., 2019). The reason for conflicting results could also be the difference in culture conditions, water quality parameters, experimental periods, and fish species (Zemheri-Navruz et al., 2019).
According to the findings of this investigation, dietary purslane extract levels notably increased the carcass quality of L. catla relative to the control group. The outcomes of Zenhom and Khames (2014) also revealed that purslane seed supplementation significantly improved the carcass composition of tilapia. They demonstrated that protein content in the body was higher, while lipids were lower, as the percentage of purslane in the fish feed increased, compared to the control group. The nutritional composition of purslane, particularly its omega-3 fatty acid content, plays a significant role in enhancing growth parameters (Srivastava et al., 2023). Furthermore, purslane has the potential to improve body composition in aquaculture due to its high nutritional value, which includes proteins, minerals, omega-3 fatty acids, vitamins, and antioxidants (Ahmadifar et al., 2020).
The findings of this study showed that dietary purslane extract concentrations substantially increased the hematological parameters of L. catla relative to the control group. Mohammadalikahni et al. (2020) used purslane in the diet of rainbow trout and noted that hematological indices including RBC, WBC, and Hb were significantly improved. Moreover, Habibian et al. (2017) also suggested that supplementing dietary purslane in the diet of broiler chickens improved the Hb concentration and RBC counts. These findings were linked with flavonoids, vitamin E, phenolic acids, vitamin C, and high contents of n-3 fatty acids in purslane (Okafor et al., 2014; Varmaghany et al., 2015).
Supplementation with purslane extract at varying percentages improved the mineral content of L. catla. It may be associated with the fact that leaves of purslane contain minerals such as Mg, P, Ca, K, and Na (Srivastava et al., 2023).
Our research demonstrated that purslane improves antioxidant enzyme activity (superoxide dismutase, glutathione peroxide, and catalase) and enhances serum immunological markers (lysozyme activity and total immunoglobulin) in L. catla. The primary enzymes responsible for counteracting the generation of free oxygen radicals in cells are antioxidant enzymes, including catalase, glutathione peroxide, and superoxide dismutase (Dawood et al., 2020). Increased antioxidant enzyme activity may balance the pro-oxidants and antioxidants, leading to improved animal health (Abdel-Daim et al., 2020). Studies have shown that higher levels of GLO in blood serum are linked to enhanced health and immune function (Aly et al., 2022). In addition, LYZ is regarded as a highly responsive immunological marker in fish defense mechanisms (Burgos-Aceves et al., 2021; Rashidian et al., 2021). Our findings align with Ahmadifar et al. (2020), who reported enhanced antioxidant activity and immune response in Ctenopharyngodon idella fingerlings fed diets supplemented with 0.5% purslane extract. The outcomes of our study are consistent with the findings of Abdel-Razek et al. (2019), which showed that dietary purslane leaf powder positively affects LYZ function in Nile tilapia. According to Zhang et al. (2020), herbal extracts can enhance appetite, growth, and immune function, likely attributed to their nutrient-dense profile, which includes β-carotene, vitamins A and C, glutathione, α-tocopherol, and n-3 fatty acids that promote immunity and antioxidant activity. Purslane, in particular, contributes significantly to metabolic processes, due to the presence of its high content of vitamins E, C, and B (Srivastava et al., 2023).
5 Conclusion
Conclusively, incorporating purslane extract into L. catla feed at concentrations ranging from 0.5 to 1.5% significantly enhanced the parameters including body composition, growth performance, whole-body mineral content, hematology, antioxidant activity, and immune response. The optimal effects were observed at 1.5% purslane extract supplementation. These findings suggest that purslane extract can be an effective feed supplement for improving overall fish performance. However, future research is necessary to fully elucidate the underlying mechanisms driving these beneficial effects.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study protocols adhered to the ethical guidelines approved by the Ethics Review Committee of Government College University Faisalabad, by approval Ref. No. GCUF/ERC/436.
Author contributions
MF: Methodology, Writing – original draft. SH: Conceptualization, Formal analysis, Investigation, Supervision, Writing – original draft. PKS: Data curation, Software, Writing – review & editing. SA: Data curation, Software, Writing – review & editing. KAAG: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the University of California Santa Cruz.
Acknowledgments
The authors are grateful to HEC Pakistan (Projects No. 20-4892/NRPU/R&D/HEC/14/1145) for assisting with projects at the Zoology Department, GC University Faisalabad. The authors are also grateful to the Researchers Supporting Project Number (RSP2025R48), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Daim, M. M., Dawood, M. A., Elbadawy, M., Aleya, L., and Alkahtani, S. (2020). Spirulina platensis reduced oxidative damage induced by chlorpyrifos toxicity in Nile tilapia (Oreochromis niloticus). Animals 10:473. doi: 10.3390/ani10030473
Abdel-Razek, N., Awad, S. M., and Abdel-Tawwab, M. (2019). Effect of dietary purslane (Portulaca oleracea L.) leaves powder on growth, immunostimulation, and protection of Nile tilapia, Oreochromis niloticus against Aeromonas hydrophila infection. Fish Physiol. Biochem. 45, 1907–1917. doi: 10.1007/s10695-019-00685-8
Adel, M., Dawood, M. A., Shafiei, S., Sakhaie, F., and Shekarabi, S. P. H. (2020). Dietary Polygonum minus extract ameliorated the growth performance, humoral immune parameters, immune-related gene expression and resistance against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss). Aquaculture 519:734738. doi: 10.1016/j.aquaculture.2019.734738
Ahmadifar, E., Hoseinifar, S. H., Adineh, H., Moghadam, M. S., and Dawood, M. A. (2020). Assessing the impact of purslane (L.) on growth performance, anti-oxidative, and immune activities in grass carp. Ann. Anim. Sci. 20, 1427–1440. doi: 10.2478/aoas-2020-0042
Allen, G., and Rowland, S. (1992). Development of an experimental diet for silver perch (Bidanus bidanus). Austasia Aquac. 6, 39–40.
Aly, S. M., Abdelrazek, H., Eidaroos, N. H., Mostafa, S. I., Marzouk, S. S., Nashaat, M., et al. (2022). Effect of Oreganum (Origanum vulgare L.) essential oil on some immune parameters of the Nile tilapia (Oreochromis niloticus). Egyptian Journal of Aquatic Biol. Fish. 26. doi: 10.21608/ejabf.2022.270407
Amalia, R. L. R., Suryaningrum, L. H., Sumitro, S., Budiyanti, B., Rohmy, S., Nur, B., et al. (2024). Valorization of weed Portulaca oleracea L. as an alternative to fish feed ingredient. In BIO Web of Conferences (Vol. 87, p. 03029). EDP Sciences.
Anjum, M. A., Hussain, Z., Khan, S. H., Ahmad, N., Amer, M. Y., and Iftikhar, N. (2014). Assessment of poultry feed ingredients used in commercial compound feed. Pak. J. Life Soc. Sci. 12, 69–73.
Aslam, S., Abbas, S., Kalhoro, M. A., and Shoaib, A. (2016). Anchor worms (lernaeid parasites), Lernaea polymorpha yü and Lernaea cyprinacea (copépode: lernaeidae) on major carps at different fish farms in Punjab, Pakistan. Sci. Int. 28, 295–298.
Awad, E., and Awaad, A. (2017). Role of medicinal plants on growth performance and immune status in fish. Fish Shellfish Immunol. 67, 40–54. doi: 10.1016/j.fsi.2017.05.034
Besong, S. A., Ezekwe, M. O., and Ezekwe, E. I. (2011). Evaluating the effects of freeze-dried supplements of purslane (Portulaca oleracea) on blood lipids in hypercholesterolemic adults. Int. J. Nutr. Metab. 3, 43–49.
Blaxhall, P. C., and Daisley, K. W. (1973). Routine haematological methods for use with fish blood. J. Fish Biol. 5, 771–781. doi: 10.1111/j.1095-8649.1973.tb04510.x
Buege, J. A., and Aust, S. D. (1978). “Microsomal lipid peroxidation” in Methods in enzymology, vol. 52 (Academic press), 302–310.
Burgos-Aceves, M. A., Abo-Al-Ela, H. G., and Faggio, C. (2021). Physiological and metabolic approach of plastic additive effects: immune cells responses. J. Hazard. Mater. 404:124114. doi: 10.1016/j.jhazmat.2020.124114
Cámara-Ruiz, M., García Beltrán, J. M., Guardiola Abellán, F. A., and Esteban Abad, M. D. L. Á. (2020). In vitro and in vivo effects of purslane (Portulaca oleracea L.) on gilthead seabream (Sparus aurata L.). Agric. Food 5, 799–824.
Claiborne, A. (1985). Catalase activity. in CRC handbook of methods in oxygen radical research. ed. R. A. Greenwald (BocaRaton: CRC Press) 283–284.
Dawood, M. A. (2021). Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev. Aquac. 13, 642–663. doi: 10.1111/raq.12492
Dawood, M. A., Koshio, S., and Esteban, M. Á. (2018). Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev. Aquac. 10, 950–974. doi: 10.1111/raq.12209
Dawood, M. A., Magouz, F. I., Mansour, M., Saleh, A. A., Asely, A. M. E., Fadl, S. E., et al. (2020). Evaluation of yeast fermented poultry by-product meal in Nile tilapia (Oreochromis niloticus) feed: effects on growth performance, digestive enzymes activity, innate immunity, and antioxidant capacity. Front. Vet. Sci. 6:516. doi: 10.3389/fvets.2019.00516
Ellis, A. E. (1999). Immunity to bacteria in fish. Fish Shellfish Immunol. 9, 291–308. doi: 10.1006/fsim.1998.0192
El-Saadony, M. T., Alagawany, M., Patra, A. K., Kar, I., Tiwari, R., Dawood, M. A., et al. (2021). The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol. 117, 36–52. doi: 10.1016/j.fsi.2021.07.007
Elumalai, P., Kurian, A., Lakshmi, S., Musthafa, M. S., Ringo, E., and Faggio, C. (2021). Effect of Leucas Aspera against Aeromonas Hydrophila in Nile Tilapia (Oreochromis niloticus): immunity and gene expression evaluation. Turk. J. Fish. Aquat. Sci. 22. doi: 10.4194/TRJFAS19802
Faisal, M., Hussain, S. M., Sarker, P. K., Ali, S., Al-Ghanim, K. A., and Yousaf, Z. (2024). Utilization of Moringa oleifera leaf meal as a protein source in diets for Cirrhinus mrigala: effects on growth, body composition, and hematology. Front. Sustain. Food Syst. 8:1405614. doi: 10.3389/fsufs.2024.1405614
Gore, S. B., Balange, A. K., Nayak, B. B., Kumar, H. S., Tandale, A. T., and Xavier, K. M. (2021). Comparative analysis of unwashed and single washed mince gel from Indian major carps. J. Food Sci. Technol. 59, 377–387. doi: 10.1007/s13197-021-05024-5
Güllü, K., Acar, Ü., Kesbiç, O. S., Yılmaz, S., Ağdamar, S., Ergün, S., et al. (2016). Beneficial effects of Oral allspice, Pimenta dioica powder supplementation on the hemato-immunological and serum biochemical responses of Oreochromis mossambicus. Aquac. Res. 47, 2697–2704. doi: 10.1111/are.12717
Habibian, M., Sadeghi, G., and Karimi, A. (2017). Effects of purslane (Portulaca oleracea L.) powder on growth performance, blood indices, and antioxidant status in broiler chickens with triiodothyronine-induced ascites. Arch. Anim. Breed. 60, 315–325. doi: 10.5194/aab-60-315-2017
Hardy, R. W. (2010). Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquac. Res. 41, 770–776. doi: 10.1111/j.1365-2109.2009.02349.x
Hassan, A. (2014). Chemical and remedial effects of purslane (Portulaca oleracea) plant. Life Sci. J. 11.
Hoseinifar, S. H., Dadar, M., Van Doan, H., and Harikrishnan, R. (2019). Feed additives impacts on shellfish microbiota, health, and development. Microb. Communities Aquac. Ecosyst., 143–163. doi: 10.1007/978-3-030-16190-3_7
Hoseinifar, S. H., Sun, Y. Z., Zhou, Z., Van Doan, H., Davies, S. J., and Harikrishnan, R. (2020). Boosting immune function and disease bio-control through environment-friendly and sustainable approaches in finfish aquaculture: herbal therapy scenarios. Rev. Fish. Sci. Aquac. 28, 303–321. doi: 10.1080/23308249.2020.1731420
Jannathulla, R., Rajaram, V., Kalanjiam, R., Ambasankar, K., Muralidhar, M., and Dayal, J. S. (2019). Fishmeal availability in the scenarios of climate change: inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 50, 3493–3506. doi: 10.1111/are.14324
Javahery, S., Nekoubin, H., and Moradlu, A. H. (2012). Effect of anesthesia with clove oil in fish. Fish Physiol. Biochem. 38, 1545–1552. doi: 10.1007/s10695-012-9682-5
Kefas, M., Abubakar, K. A., and Ja'afaru, A. (2015). Haematological indices of tilapia (Oreochromis niloticus) from Lake Geriyo, Yola, Adamawa state, Nigeria. Int. J. Fish. Aquat. Stud. 3, 9–14.
Kumar, A., Sreedharan, S., Kashyap, A. K., Singh, P., and Ramchiary, N. (2022). A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon 8:e08669. doi: 10.1016/j.heliyon.2021.e08669
Magouz, F. I., El-Din, M. T. S., Amer, A. A., Gewaily, M. S., El-Dahdoh, W. A., and Dawood, M. A. (2022). A blend of herbal essential oils enhanced the growth performance, blood bio-immunology traits, and intestinal health of Nile tilapia (). Ann. Anim. Sci. 22, 751–761. doi: 10.2478/aoas-2021-0066
Miao, L., Tao, H., Peng, Y., Wang, S., Zhong, Z., El-Seedi, H., et al. (2019). The anti-inflammatory potential of Portulaca oleracea L.(purslane) extract by partial suppression on NF-κB and MAPK activation. Food Chem. 290, 239–245. doi: 10.1016/j.foodchem.2019.04.005
Mohammadalikahni, M., Shamsaie Mehrjan, M., Haghighi, M., Soltani, M., and Kamali, A. (2020). Effects of oral administration of dried purslane (Portulaca oleracea) extract on some growth indices, carcass quality and intestinal microbial flora of rainbow trout (Oncorhynchus mykiss) fry. J. Anim. Environ. 12, 229–236.
Mushtaq, T., Sarwar, M., Ahmad, G., Nisa, M. U., and Jamil, A. (2006). The influence of exogenous multienzyme preparation and graded levels of digestible lysine in sunflower meal-based diets on the performance of young broiler chicks two weeks post hatching. Poult. Sci. 85, 2180–2185. doi: 10.1093/ps/85.12.2180
Naeem, F., and Khan, S. H. (2013). Purslane (Portulaca oleracea L.) as phytogenic substance—a review. J. Herbs Spices Med. Plants 19, 216–232.
Ocampo, G., and Columbus, J. T. (2012). Molecular phylogenetics, historical biogeography, and chromosome number evolution of Portulaca (Portulacaceae). Mol. Phylogenet. Evol. 63, 97–112. doi: 10.1016/j.ympev.2011.12.017
Okafor, I. A., Ayalokunrin, M. B., and Orachu, L. A. (2014). A review on Portulaca oleracea (purslane) plant-its nature and biomedical benefits, vol. 5.
Perera, G. C., Afridin, M. R., Adikari, A. M. A. N., Heenatigala, P. P. M., Maduka, K. L. W. T., and Dunusinghe, S. B. K. (2024). Replacing the unsustainable and wild-caught fishmeal with field cricket (Gryllus bimaculatus) meal in Catla (Catla catla) fry diet: effect for growth, in vivo digestibility, carcass composition, histopathological alterations, and disease tolerance. Aquac. Int. 32, 2609–2626. doi: 10.1007/s10499-023-01288-0
Rashidian, G., Boldaji, J. T., Rainis, S., Prokić, M. D., and Faggio, C. (2021). Oregano (Origanumvulgare) extract enhances zebrafish (Danio rerio) growth performance, serum and mucus innate immune responses and resistance against Aeromonas hydrophila challenge. Animals. 11:299. doi: 10.3390/ani11020299
Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G., and Hoekstra, W. (1973). Selenium: biochemical role as a component of glutathione peroxidase. Science. 179, 588–590. doi: 10.1126/science.179.4073.588
Rowland, S. J., and Ingram, B. A. (1991). Diseases of Australian native fishes. Fish. Bull. 4, 21–23.
Şahin, D., Meryem, Ö. Z., Aral, O., Bahtiyar, M., and Taşçi, S. (2021). Growth and pigmentation of goldfish (Carassius auratus L, 1758) fed on a diet supplemented with purslane (Portulaca sp.) extract. Magnesium (Mg, ppm) 54, 1727–1725. doi: 10.17582/journal.pjz/20210318090307
Saleem, M., Iqbal, J., Shi, Z., Garrett, S. H., and Shah, M. H. (2022). Distribution and bioaccumulation of essential and toxic metals in tissues of Thaila (Catla catla) from a natural Lake, Pakistan and its possible health impact on consumers. J. Mar. Sci. Eng. 10:933. doi: 10.3390/jmse10070933
Sangeetha, S., Kiran, R. S., Abbulu, K., and Battu, S. (2020). A review on traditional herb Portulaca oleracea. World J. Pharmacol. Res. 9, 578–601. doi: 10.20959/wjpr20203-16924
Shanker, N., Maneesh Kumar, M., Juvvi, P., and Debnath, S. (2019). Moisture sorption characteristics of ready-to-eat snack food enriched with purslane leaves. J. Food Sci. Technol. 56, 1918–1926. doi: 10.1007/s13197-019-03657-1
Snedecor, G. W., and Cochran, W. G. (1991). Statistical methods. 8th Edn. Americans USA: Iowa State University Press, 503.
Sourani, Z., Shirian, S., Shafiei, S., Mosayebi, N., and Nematollahi, A. (2023). Modulation of immune-related gene expressions in zebrafish (Danio rerio) by dietary purslane (Portulaca oleracea) extract. Mar. Biotechnol. 25, 214–221. doi: 10.1007/s10126-022-10195-z
Srivastava, R., Srivastava, V., and Singh, A. (2023). Multipurpose benefits of an underexplored species purslane (Portulaca oleracea L.): a critical review. Environ. Manag. 72, 309–320. doi: 10.1007/s00267-021-01456-z
Steel, R. G. D., Torrie, J. H., and Dickey, D. A. (1996). Principles and procedures of statistics. 3rd Edn. New York. USA: McGraw Hill international Book Co. Inc., 336–352.
Syanya, F. J., Mathia, W. M., and Harikrishnan, M. (2023). Current status and trend on the adoption of fish feed additives for sustainable tilapia aquaculture production: a review. Asian J. Fish. Aquat. Res. 22, 10–25. doi: 10.9734/ajfar/2023/v22i3571
Tacon, A. G., Metian, M., and McNevin, A. A. (2022). Future feeds: suggested guidelines for sustainable development. Rev. Fish. Sci. Aquac. 30, 135–142. doi: 10.1080/23308249.2020.1860474
Taghizadeh, M., Rashidi, A. A., Taherian, A. A., Vakili, Z., and Mehran, M. (2018). The protective effect of hydroalcoholic extract of rosa canina (dog rose) fruit on liver function and structure in streptozotocin-induced diabetes in rats. J. Diet. Suppl. 15, 624–635. doi: 10.1080/19390211.2017.1369205
Varmaghany, S., Torshizi, M. A. K., Rahimi, S., Lotfollahian, H., and Hassanzadeh, M. (2015). The effects of increasing levels of dietary garlic bulb on growth performance, systolic blood pressure, hematology, and ascites syndrome in broiler chickens. Poult. Sci. 94, 1812–1820. doi: 10.3382/ps/pev148
Wang, C., Liu, Q., Ye, F., Tang, H., Xiong, Y., Wu, Y., et al. (2021). Dietary purslane (Portulaca oleracea L.) promotes the growth performance of broilers by modulation of gut microbiota. AMB Express 11, 31–11. doi: 10.1186/s13568-021-01190-z
Wanjari, N., and Waghmare, J. (2015). Phenolic and antioxidant potential of sunflower meal. Adv. Appl. Sci. Res. 6, 221–229.
Wedemeyer, G. A., and Yasutake, W. T. (1977). Clinical methods for the assessment of the effects of environmental stress on fish health, vol. 89: Department of the Interior, Fish and Wildlife Service.
Winterbourn, C. C. (1993). Superoxide as an intracellular radical sink. Free Radical Biol. Med., 14, 85–90. doi: 10.1016/0891-5849(93)90512-S
Zaki, M. A., Alabssawy, A. N., Nour, A. E. A. M., El Basuini, M. F., Dawood, M. A., Alkahtani, S., et al. (2020). The impact of stocking density and dietary carbon sources on the growth, oxidative status and stress markers of Nile tilapia (Oreochromis niloticus) reared under biofloc conditions. Aquac. Rep. 16:100282. doi: 10.1016/j.aqrep.2020.100282
Zemheri-Navruz, F., Acar, Ü., and Yılmaz, S. (2019). Dietary supplementation of olive leaf extract increases haematological, serum biochemical parameters and immune related genes expression level in common carp (Cyprinus carpio) juveniles. Fish Shellfish Immunol. 89, 672–676. doi: 10.1016/j.fsi.2019.04.037
Zenhom, M. M., and Khames, M. K. (2014). Effect of purslane seeds (Portulaca oleraceae) on growth performance, feed utilization and body composition of Nile-tilapia (Oreochromis niloticus).
Zhang, R., Wang, X. W., Liu, L. L., Cao, Y. C., and Zhu, H. (2020). Dietary oregano essential oil improved the immune response, activity of digestive enzymes, and intestinal microbiota of the koi carp, Cyprinus carpio. Aquaculture 518:734781. doi: 10.1016/j.aquaculture.2019.734781
Keywords: purslane, Labeo catla, growth, hematology, carcass analysis, immune responses
Citation: Faisal M, Hussain SM, Sarker PK, Ali S and Al-Ghanim KA (2025) Exploring the impacts of purslane extract: a novel supplement for improving physiological and immunological responses in Labeo catla fingerlings. Front. Sustain. Food Syst. 9:1462274. doi: 10.3389/fsufs.2025.1462274
Edited by:
Edward Hugh Allison, WorldFish, MalaysiaReviewed by:
Tabussam Tufail, Jiangsu University, ChinaSukham Munilkumar, Central Institute of Fisheries Education (ICAR), India
Copyright © 2025 Faisal, Hussain, Sarker, Ali and Al-Ghanim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syed Makhdoom Hussain, ZHJtYWtoZG9vbWh1c3NhaW5AZ2N1Zi5lZHUucGs=; Pallab K. Sarker, cHNhcmtlckB1Y3NjLmVkdQ==; Shafaqat Ali, c2hhZmFxYXRhbGlnaWxsQGdjdWYuZWR1LnBr
 Muhammad Faisal1
Muhammad Faisal1 Syed Makhdoom Hussain
Syed Makhdoom Hussain Pallab K. Sarker
Pallab K. Sarker Shafaqat Ali
Shafaqat Ali Khalid A. Al-Ghanim
Khalid A. Al-Ghanim