
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 24 January 2025
Sec. Nutrition and Sustainable Diets
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1525572
This article is part of the Research Topic Bioactive Compounds from Medicinal Mushrooms and Plants - Extraction and Potential Application in Foods View all 8 articles
 Malak Zirari1*
Malak Zirari1* Marouane Aouji2
Marouane Aouji2 Wissal Baghdad3
Wissal Baghdad3 Mohammed Er-rajy4
Mohammed Er-rajy4 Hamada Imtara5*
Hamada Imtara5* Feras Abujaber5
Feras Abujaber5 Otmane Elharrati3
Otmane Elharrati3 Omar M. Noman6
Omar M. Noman6 Mahmoud Tarayrah7
Mahmoud Tarayrah7 Driss Hmouni2
Driss Hmouni2 Nouredine El Mejdoub1
Nouredine El Mejdoub1Introduction: Morocco is renowned for its rich plant biodiversity, although many plants are underutilized. Consequently, the present study was conducted to assess the nutritional composition, bioactive constituents, antioxidant properties, and acute oral toxicity of Abies marocana's woody biomass.
Methods: The nutritional value of the twigs and cones was determined using the AOAC method, while mineral components were examined through ICP-OES. To search for phytochemicals in the methanolic extracts, a number of established techniques were applied, and evaluate their antioxidant activity, while the volatile content was determined using the GC-MS method. The acute oral toxicity test was carried out following the guidelines of OECD 423.
Results and discussion: Proximate analysis revealed a variety of components in different parts, including moisture, ash, fiber, protein, carbohydrates, and fat. Cones and twigs were found to be rich in mineral elements, as well as phenols, flavonoids, tannins, and phytosterols. Chromatographic analysis identified linoleic acid as the main component in twig extract and 2-Bornanone as the primary constituent in cone extract. The methanolic extracts of A. marocana displayed strong antioxidant properties through notable DPPH scavenging activity, with no mortality observed in rats even at doses exceeding 1,000 mg.kg−1, indicating potential for medicinal, cosmetic, or dietary uses. A molecular docking study of the five main compounds of both plants showed that they aligned and interacted with the binding sites of selected proteins, demonstrating significant antioxidant activity.
Recently, the byproducts originating from activities in the forestry sector, namely logging and the production of timber, encompass a rich repository of antioxidants found in plants, which can be obtained and employed. Several investigations have highlighted the considerable promise of these innate antioxidants across diverse domains (Bouras et al., 2016; Tálos-Nebehaj et al., 2017).
These domains involve the advancement of natural food additives (Gyawali and Ibrahim, 2014), medical and medical-related commodities (Watson et al., 2018) along with natural growth regulators (Popa et al., 2002). Remarkably, recent focus has been given to botanical extracts owing to their exceptional antioxidative, stabilizing, and enveloping impacts in the production of silver nanoparticles (Fahimirad et al., 2019). Indeed, woody biomass has garnered significant scientific interest as it is regarded as a promising reservoir of bioactive phytochemicals, termed extractives. The distribution of these natural compounds within tree tissues is not uniform. Specific tree components such as knots and bark have been identified as abundant and cost-effective sources of polyphenols according to Vek et al. (2021).
Conifers, which belong to the order Coniferales of the Gymnosperms, are an extensive assemblage of coniferous trees and shrubs that bear cones and produce resin. The order Coniferales encompasses seven distinct conifer families, which are further categorized into a total of 67 genera. This assemblage of genera encompasses over 615 extant species (Auders and Spicer, 2012). Miller (1691–1771) gave the first description of Abies in 1754. With all of its species spread over the Northern Hemisphere, this genus the second biggest in the Pinaceae family is thought to have a higher degree of complexity than other genera in the same family (Nikolić et al., 2021). The Abies genus is believed to encompass 52 acknowledged species, in addition to 58 unidentified species awaiting classification (WFO, 2023).
Various folklore accounts exist concerning the potential therapeutic effects of specific species in this genus against conditions such as stomachache, indigestion, colds, as well as vascular, venereal and pulmonary diseases (Yeşilada et al., 1995). Besides, Bowers et al. (1965) have recorded a range of activities displayed by different species within this genus, including antitumor, antibacterial, antifungal, anti-ulcerogenic and anti-inflammatory. The only place to find A. marocana, a species that is native to Morocco, is in the Rif area, between 1,400 and 2,000 meters above sea level (Zirari et al., 2024a). The various constituents of A. marocana, such as its needles, branches, and cones, have garnered considerable interest in the pharmaceutical sector due to the existence of bioactive compounds, as emphasized by Zirari et al. (2024b).
Because these foods are rich in vital nutrients such vitamins, minerals, and phytochemicals, including them in meals may provide a sustainable approach to nutrition, especially in places where traditional food sources are limited or non-existent (Zirari et al., 2024a). Recently, a novel extract obtained from the bark of Abies alba, which has previously exhibited antioxidant properties in scientific investigations (Benković et al., 2014), under the brand names AlbiPhenol® and Abigenol® has been presented to the market (Leone et al., 2022). The upper parts of A. marocana harbor specific components, such as terpenes, that may pose toxic or irritant effects when present in substantial amounts. Zirari et al. (2024b) have presented data indicating that the volatile fraction of A. marocana twigs and cones is notably rich in terpene compounds. Given the limited data available on this subject, the primary aim of our current investigation is to assess the safety of A. marocana extracts as a dietary supplement. In line with this, the present investigation endeavors to enhance the value of A. marocana woody biomass extracts by exploring their nutritional value, phytochemical composition, encompassing antioxidant activity, as well as their toxicity.
The application of molecular bonding has already facilitated a reduction in both the financial resources and temporal demands associated with research into the mechanisms of activity by expediting the alignment of experimental studies with the optimal active substance in a manner that surpasses traditional research methodologies (Stanzione et al., 2021). This approach yields insights into the interactions between pharmacological agents and their corresponding receptors, thereby enabling the anticipation of the spatial orientation of drug candidates upon their association with target proteins (Lee and Kim, 2019). Furthermore, this methodology streamlines systemic analysis by facilitating the non-covalent introduction of a molecule into the binding site of a macromolecular target, resulting in precise binding at the active sites of individual ligands (Trott and Olson, 2010). The execution of this technique allows for the prediction of binding modes pertaining to peptides, small molecules, and ligands in relation to their respective receptors, including enzymes.
Moreover, an all-encompassing scrutiny of the bioactive components of the twigs and cones was executed employing GC-MS. The aim of this particular investigation is to furnish a more comprehensive insight into the possible health benefits and medical uses that can be obtained from this particular plant. Today, researchers are increasingly relying on computational approaches, particularly molecular docking simulations. These tools are used to assess the potential efficacy of identified molecules and predict how they may exert an antioxidant effect.
All the chemicals employed in the research were obtained from Sigma Aldrich, demonstrating a superior level of purity equal to or exceeding 98%.
The twigs and cones of A. marocana were gathered from a single genotype on the Chouihate mountain (Figure 1), located in Chefchaouen (35°11′05.6” N 5°13′47.9” W), a city positioned in the northern part of Morocco, at an altitude of 1,785 m. The specimen was classified at the Scientific Research Center in Rabat. Following this, a thorough cleansing procedure was carried out on them using water, after which they were dried for a period of 3 days at a regulated temperature of (45 ± 2°C). The dried woody biomass was then ground into powder form, employing an electric grinding apparatus, and subsequently stored in a refrigerator for future examination.
The proximate composition of the samples was determined using standard analytical methods. Moisture, ash, and crude fiber content were assessed according to the protocols outlined by the Association of Analytical Chemists (AOAC, 2016). Protein levels was determined by measuring nitrogen content using the Kjeldahl method (Kjeldahl, 1983). Lipid content was measured using AOAC method (AOAC, 1995). Carbohydrate levels was measured using the differential method (Hussain et al., 2009). Crude protein, crude fat, and total carbohydrate values were multiplied by the Atwater coefficients to get the total energy content, the result was expressed in kilocalories per 100 g of sample (Merrill and Watt, 1973; Shad et al., 2013).
The resultant residue of white ash, was dissolved in a concentrated solution of nitric acid HNO3 (25%) and subsequently passed through a filtration process. The resultant solution underwent an analysis to determine the specific major mineral composition. The concentration of various ions in the woody biomass was evaluated using inductively coupled plasma optical emission spectrometry (ICP-OES) (Skujins, 1998). The experimental conditions applied for the ICP-OES analysis were as follows: the utilized equipment was the Perkin Elmer Optima 8,000 model, operating at an RF power of 1,500 watts, a plasma gas flow rate (Ar) of 8 L·min−1, and an auxiliary gas flow rate (Ar) of 0.2 L·min−1. Other parameters considered were the axial view size, the copying and playback duration of 45 min, and a copying time of 15 min.
Twenty five g of the various powder (twigs and cones) underwent delipidation using petroleum ether and were subjected to extraction in a Soxhlet apparatus employing methanol (250 mL; 40°C/8 h) (Zirari et al., 2024a,b). Subsequently, the obtained extract underwent concentration (35°C) and storage at a temperature of 4°C for subsequent analysis. Furthermore, the determination of the crude extract yield was conducted following the procedure outlined in NM ISO 734 (BNS EN ISO 734-1, 2020).
Phytochemical analysis was conducted in order to discern the primary classes of secondary metabolites present in our samples that were accountable for the potential bioactivities. The conventional identification reagents facilitated the recognition of the subsequent chemical classifications: flavonoids, alkaloids, polyphenols, tannins, proteins, anthocyanins, reducing compounds, essential oils, cardiac glycosides, terpenes, and sterols (Harborne, 1998; Sofowora, 1993; Trease and Evans, 1989).
The levels of polyphenols, flavonoids, and tannins were assessed through the utilization of distinct methodologies, namely the approach developed by Cheok et al. (2013) the method outlined by Lamaison and Carnat (1990), and the technique introduced by Hagerma (2002). These assessments were conducted using specific reagents such as Folin-Ciocalteu, AlCl3, and vanillic acid.
Phytosterol content was quantified using the methodology outlined by Shahar et al. (2023) which uses Liebermann reagent.
Samples were individually analyzed utilizing a workstation system that was equipped with a BR-5ns FS capillary column (60 m × 0.32 mm ID × 0.25 μm) in combination with mass spectrometry (BRUKER 456-GC EVOQ). A high-purity helium gas served as the carrier, flowing at a rate of 1.7 mL·min−1. The sample injection temperature was kept constant at 250°C, with the initial oven temperature starting at 40°C and gradually increasing to 260°C at a rate of 8°C·min−1. Extracts were gathered through a syringe and introduced into the injector with a split ratio of 40:1. A wide range of full-scan mass spectra covering from 40–550 AMU was generated to gather comprehensive data. The ion source temperature was set at 230°C, while the quadrupole temperature remained at a constant 150°C. Following self-tuning, the electron multiplier voltage was set to 1,100 V, with a solvent delay of 3 min (Zirari et al., 2024b). Compound identification and characterization within the various raw extracts were based on their retention times in gas chromatography. The obtained mass spectra were cross-referenced with standards in the Mass Spectral Library (NISTII), and the findings were presented as a percentage of the peak area.
The evaluation of the antioxidant capacity was carried out by examining the overall total antioxidant activity (TAC) and the DPPH radical scavenging activity, according to the methods described in a previous study conducted by Zirari et al. (2024a). Ascorbic acid served as the standard reference material.
The ethical institutional committee of the Faculty of Sciences at Ibn Tofail University in Kenitra, Morocco, granted approval for the protocol. An assessment of the LD50 of methanolic extracts from A. marocana was conducted using 25 adults female Wistar albino rats (Laboratory Biology and Health, Faculty of Sciences, Ibn Tofail University, Kenitra, Morocco), following the protocols outlined by the OECD 423 (OECD, 2001). Prior to commencing the study, a fasting period of 4 h was observed for the rats, who were then divided into five groups, with each group consisting of 5 female rats (OECD, 2001; Aouji et al., 2023). Of these groups, four were subjected to oral doses of twigs and cones methanolic extracts at levels of 500 and 1,000 mg.kg−1, while the control group was given distilled water at a dose of 10 mL.kg−1. Behavioral monitoring was performed 1-h post-administration. Throughout the study, the rats were provided with equal amounts of hydration and daily food. Following extract administration, observations on mortality rates, toxicity symptoms, changes in body weight, food consumption, respiration patterns, and convulsions were carried out within the initial hours post-gavage and subsequently on a daily basis for a duration of 14 days.
The sacrifice procedure was performed on the rats under anesthesia induced by 7% Chloral hydrate subsequent to the completion of the 14th day. Following this, the organs such as the kidneys, liver, heart, and spleen were weighed after thorough cleansing with 0.09% normal saline and drying by blotting with tissue paper. Subsequently, the relative weight of each organ was calculated using a specific equation (Tcheutchoua et al., 2022).
Where, Wr is the relative organ weight, W0 is the organ weight (g) and Wb is the rat body weight (g).
The molecular docking technique is equipped with tools that facilitate the understanding the interactions between a presumed rigid active site and a ligand molecule, making it a valuable approach in drug discovery (Akinsola et al., 2021). Molecular docking investigations were conducted to evaluate the interactions of compounds, identified through GC-MS based on major peak areas, focusing on antioxidant activity (Daouk et al., 2014).
As shown by GC-MS, the two plants have 5 major molecules. The five major ligands, 2-Bornanone, Linoelaidic acid, 13-Octadecenoic acid, methyl ester Linoleic Acid, and Stearic acid were downloaded from the PubChem database, and biomacromolecules (Code ID: 2CDU) were acquired in protein data bank (PDB) format (Trumbo et al., 2002). Ligands were prepared as PDB files to facilitate molecular research using various software tools, including Discovery Studio and ChemOffice (Pamela et al., 2005). In addition, Discovery Studio was used to prepare the proteins obtained from the PDB base.
The ligands' structures were docked into the enzyme's active site using AutoDockTools (Pearson et al., 1999). The interactions between enzyme active-site residues and ligand molecules were analyzed using Discovery Studio Visualizer, which included examining 2D interactions (Salem et al., 2020).
Using IBM Corp.'s Armonk, New York SPSS version 27, a one-way analysis of variance (ANOVA) and a Tukey post hoc analysis were performed to assess the statistical significance of the results at a significance level of α = 5%. The significance threshold was established in accordance with the results, which were presented as mean ± standard deviation.
Figure 2 shows the approximate composition of A. marocana twigs and cones. The moisture content of the twigs and cones (10.586 ± 0.175% and 12.142 ± 0.127% respectively) exhibited a notable discrepancy. This diminished moisture level offers a beneficial aspect by enhancing the longevity of the specimens (Kris-Etherton et al., 2002). The recorded moisture content closely approximated the moisture content of pine wood as documented by Jones et al. (1985). The protein content of the cones (2.584 ± 0.022%) was markedly higher in comparison to the protein content of the twigs (1.753 ± 0.027%). While the plant serves as a moderate protein source, it falls short when juxtaposed with the recommended dietary allowance for protein, set at 56 g for individuals weighing 70 kg and 46 g for adults weighing 50 kg (Karoly, 2011). Plant-derived proteins are commonly regarded as lower in quality, yet when integrated with other protein sources such as animal-derived proteins, they can culminate in a sufficient nutritional profile (Scientific Advisory Committee on Nutrition, 2008). According to Oladiji and Mih (2005), plants are considered good protein sources if their protein caloric value is more than 12%.
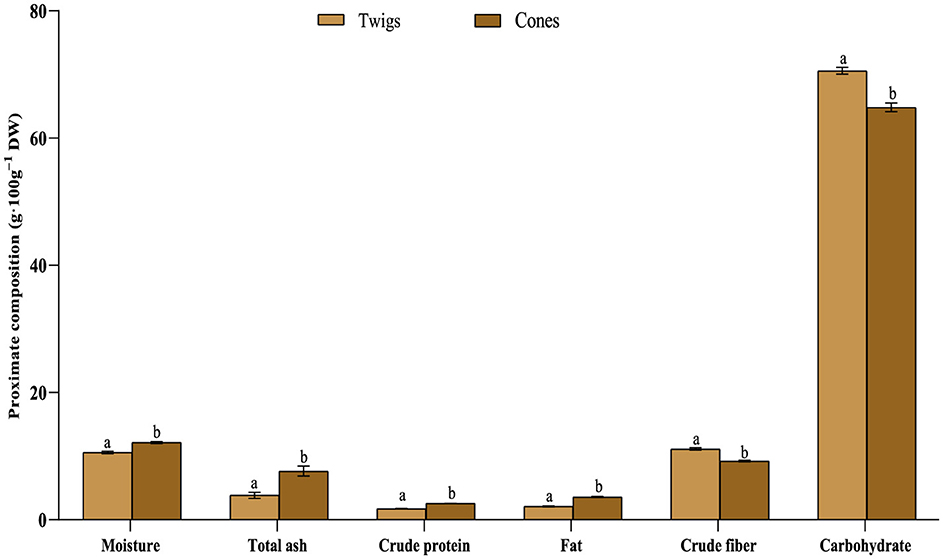
Figure 2. A. marocana woody biomass's nutritional content. The letters a and b show the significant difference (p < 0.05) in this case.
The ash content of the cones (7.640 ± 0.788%) exhibited a higher level in comparison to the twigs (3.849 ± 0.471%), indicating an elevated mineral concentration in the cones of A. marocana. Soil fertility, moisture content, and growth temperature are the main environmental factors that influence the nutritional makeup of plants grown in different regions. The fat content in the cones (4.052 ± 0.046 %) surpassed that of the twigs (2.105 ± 0.085%). Previous research has identified fat concentrations reaching 11.027 mg.g−1 DW and 12.810 mg.g−1 DW in A. alba and Picea abies heartwood (Onwuka, 2005). A dietary regime providing 1%−2% of its caloric intake as lipids is deemed adequate for human consumption, since consuming too much fat has been connected to the development of obesity and cardiovascular diseases (Emebu and Anyika, 2011). Furthermore, lipids play a crucial role in the diet by serving as a valuable energy source, facilitating the transportation of fat-soluble vitamins, contributing to essential cellular functions, and providing insulation and protection for internal tissues (Scientific Advisory Committee on Nutrition, 2008; Ejelonu et al., 2011).
A useful indicator of non-digestible carbohydrates and lignin is crude fiber, which may be found in food or plants (Ucar and Ucar, 2008). The percentage of fiber in twigs (11.140 ± 0.174%) exceeded that in cones (9.231 ± 0.120%). Various research studies have indicated that an escalation in fiber intake could potentially reduce the prevalence of gastrointestinal disorders and various ailments including diabetes, colon cancer, hypertension, obesity, and a variety of other digestive disorders (Jyske et al., 2020). This is primarily due to its role in facilitating the digestion and absorption of glucose and lipids. Despite the fact that crude fiber promotes digestibility, excessive levels of its presence can lead to gastrointestinal disturbances and reduced nutrient utilization (Lima Rojas, 2013) owing to its high cellulose content and limited digestibility of lignin in humans (Anita et al., 2006).
The carbohydrate content of the twigs (70.567 ± 0.543%) was observed to be higher compared to the cones (64.350 ± 0.710%), possibly attributed to lower levels of fiber (Figure 2). Carbohydrates play a crucial role in providing the energy necessary for bodily functions, serving as essential nutrients for a balanced diet (Achi et al., 2017). They are responsible for fueling various cells in the body including those in the brain, muscles, and blood (Thomas and Krishnakumari, 2015). These results align with previous studies on black pine cones indicating a carbohydrate content of 67.8% (Robert et al., 2003).
The energy content of the twigs (329.490 ± 2.022 Kcal.100g−1) surpasses that of the cones (324.692 ± 3.424 Kcal.100g−1), potentially attributed to the elevated carbohydrate levels in the twigs. These results are in accordance with the outcomes documented by Pathak and Kapil (2004), for Picea abies needles. To summarize, the results presented herein underscore that A. marocana twigs and cones have the potential to act as a valuable reservoir of vital nutrients and energy, rendering them suitable for enriching the dietary intake and overall wellbeing of individuals.
The importance of minerals for human nutrition is widely recognized, as they also play a major role in supporting overall physical and mental health. In fact, a varied range of vital micro and macro components, often denoted as trace elements, are essential for a multitude of physiological processes (Shahar et al., 2023). The mineral composition of the woody biomass of A. marocana was assessed and documented in Table 1. The twigs exhibit a higher concentration of Ca (4,893.377 ± 1.271 mg.Kg−1), K (3,564.556 ± 1.071 mg.Kg−1), Al (97.077 ± 1.879 mg.Kg−1), Na (248.875 ± 0.104 mg.Kg−1), and Fe (53.306 ± 1.588 mg.Kg−1) compared to the cones; 1,075.379 ± 2.852 mg.Kg−1, 3,045.399 ± 20.334 mg.Kg−1, 76.535 ± 1.682 mg.Kg−1, 189.225 ± 5.288 mg.Kg−1, and 38.659 ± 0.599 mg.Kg−1 respectively. The results align with the research conducted by Lima Rojas (Vunchi et al., 2011), which examined the mineral composition of the bark, sapwood, and heartwood of A. religiosa. Minerals play a crucial role in supporting the proper functioning of tissues and act as secondary messengers in various biochemical processes (Martínez-Gómez et al., 2022). Iron is an essential element in proteins and acts as a catalyst for specific enzymes like cytochrome oxidase (Do et al., 2014), and it is vital for the synthesis of hemoglobin (Parkash et al., 2015). It plays a role in energy transfer within the plant and also regulates obesity by promoting the oxidation of biomolecules (Do et al., 2014). Sodium is predominantly found in A. marocana twigs, functioning as a primary cation in extracellular and intracellular fluids, contributing to the maintenance of electrolyte balance in the body (Herodež et al., 2003). Nonetheless, the levels of Mg (2,943.800 ± 55.119 mg.Kg−1), Mn (25.580 ± 0.185 mg.Kg−1), Zn (13.983 ± 2.748 mg.Kg−1), and P (1,518.333 ± 32.532 mg.Kg−1) in the cones are notably higher than those in the twigs, as indicated in Table 1. Zinc plays a crucial role in protein synthesis, DNA replication, cellular differentiation, immune function, and reproductive processes (Ameen et al., 2021). Calcium is a fundamental constituent of bones and teeth, essential for blood coagulation, muscle contraction, and serves as a cofactor in enzyme reactions (Benouchenne et al., 2020). These findings are in agreement with the outcomes reported by Kumari et al. (2017) for the woods of Abies and Pinus.
In order to remove active chemicals from plant materials and reduce the interference of other substances, the extraction phase plays a critical role (Milner, 1994). By employing the Soxhlet extraction method and using methanol as the extracting agent, the successful isolation of bioactive compounds from the woody biomass of A. marocana was accomplished. The solid extract's yield percentage was determined as (g extract.100 g−1 dried plant material) and depicted in Figure 3A. Observably, the yield ranges from 15.900 ± 0.003% in cones to 21.951 ± 0.001% in twigs. This result exceeds the findings documented by Bartnik et al. (2020), in their investigation on A. webbiana. The methanol extract of A. webbiana aerial parts was produced through the Soxhlation process, resulting in a yield of 16.21%. Notably, the extraction yield is influenced by the temperature at which the extraction occurs, the ratio of solvent to sample used during the extraction process, and the size of the sample's particles (Schoss et al., 2022).
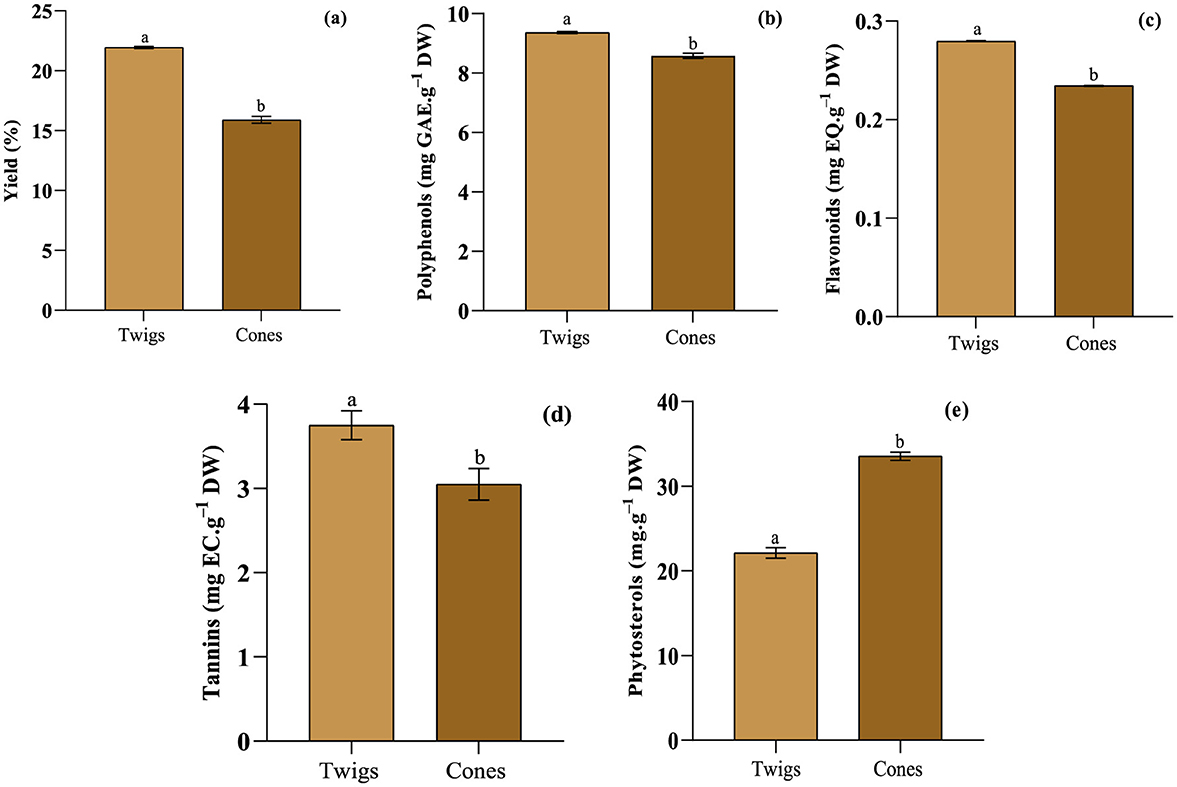
Figure 3. Summary of yield (A), total phenolic (B), flavonoid (C), tannin (D), and phytosterol (E) content of A. marocana methanolic extracts. The significant difference (p < 0.05) is illustrated by the letters a, and b.
Table 2 displays the results of the phytochemical screening. It was observed in the results that phenolic compounds, terpenes, essential oils, and sterols are present in the woody biomass of this plant. Notably, the different aerial parts are rich in polyphenols, flavonoids, and tannins. Conversely, twigs exhibit higher levels of reducing compounds and cardiac glycosides compared to cones. These bioactive constituents are renowned for their diverse modes of action and their capacity to display antioxidant and antimicrobial attributes (Semerci et al., 2020). In contrast, sterols, terpenes, proteins, and essential oils are detected in relatively low concentrations across all plant parts. However, the various sections of A. marocana lack alkaloids and anthocyanins. These outcomes align with previous phytochemical analyses, which have illustrated similar patterns. Previous investigations have indicated that the methanolic extract of A. numidica aerial parts is abundant in flavonoids and tannins, with traces of anthocyanins. Nevertheless, alkaloids, coumarins, saponins, and terpenes are not present (Shi et al., 2022). These differences may be attributed to variations in geographical and environmental conditions.
Plants are a significant source of phenolic compounds stemming from diverse sources and exhibiting various functionalities. A large proportion of these compounds are bioactive substances of plant origin, demonstrating antiviral, anti-cancer, and antibacterial properties (Vu et al., 2019). The outcomes of the analyses conducted were depicted in Figure 3. The A. Marocana twig's methanolic extract displayed the highest total phenolic compounds (TPC) content, with an average of 9.363 ± 0.031 mg GAE.g−1 DW. Polyphenols, as secondary metabolites, are synthesized by plants as a defense mechanism against external organisms. The consumption of dietary polyphenols has been proven to have significant impacts on human health. A substantial intake of polyphenols has been associated with reduced risks of various chronic ailments such as cancer, cardiovascular diseases, chronic inflammation, and degenerative conditions (Wajs-Bonikowska et al., 2013). This recorded value surpasses the one reported by Bartnik et al. (2020), for Picea Abies heartwood (3.28 mg.g−1 DW) and sapwood (2.44 mg.g−1 DW) but falls short of the level detected in Abies alba Mill Branches (28.7 mg.g−1 DW) (Schoss et al., 2022). Additionally, the methanolic extract of twigs exhibited a remarkable content of total flavonoid compounds (TFC) at 0.280 ± 0.001 mg EQ.g−1 DW and a noteworthy level of total condensed tannins (TTC) totaling 3.750 ± 0.171 mg EC.g−1 DW. Conversely, the methanolic extract of A. marocana cones revealed a lower TPC content averaging 8.583 ± 0.086 mg GAE.g−1 DW. Similarly, the TFC content in the cones was relatively lower, measuring 0.234 ± 0.001 mg EQ.g−1 DW. Moreover, the TTC content in cones was lower, at 3.048 ± 0.188 mg EC.g−1 DW. Semerci et al. (2020), determined the total phenolic constituents in the methanolic extracts of certain female gymnosperm plant cones, with TPC values ranging from 69 to 220 mg GAE.100 g−1. Several factors, such as geographical and climatic conditions, plant maturation, shelf life, and extraction techniques, can significantly impact the concentrations of phenolic compounds (Shi et al., 2022).
Phytosterols exhibit characteristics linked to the decrease in cholesterol levels, particularly triglycerides and low-density lipoprotein cholesterol. Phytosterols have been scientifically validated to possess various health-promoting properties such as anti-inflammatory, antioxidant, and anticancer effects (Vu et al., 2019). The current investigation offers proof that the overall phytosterol content in the cones is found in a relatively elevated concentration (33.547 ± 0.489 mg.g−1 DW) compared to the twigs (22.126 ± 0.640 mg.g−1 DW) (Figure 3E). Wajs-Bonikowska et al. (2013), observed that the mean quantity of phytosterols in the seed of Abies koreana is 414 ± 95.6 μg.100 g−1, with the key phytosterols identified as ergosta-8,24(28)-dien-3-ol and β-sitosterol, present at comparable levels: 153 and 151 μg.100g−1, respectively. The application of phytosterols in the formulation of functional foods is aimed at improving their cholesterol-lowering properties (Shahar et al., 2023). Dietary intake of phytosterols has a beneficial nutritional influence as they aid in lowering cholesterol levels in the bloodstream. Consequently, the inclusion of phytosterol-rich foods is essential in a balanced diet (Velasco et al., 2014). Therefore, multiple recommendations advocate the consumption of 2 g/day of plant sterols and/or stanols to reduce LDL-cholesterol levels (Cabral and Klein, 2017). The above-ground components of A. marocana can be considered as a promising asset.
The chemical constituents, along with their corresponding retention time (RT) and percentage composition of bioactive compounds (%), are delineated in Table 3, while the GC-MS chromatogram attained for the methanolic extracts is depicted in Figure 4. The visualization in Figure 4 reveals both common and distinct peaks in each extract. A total of 48 components, constituting 89.532 % of the overall chemical composition, were identified in the methanolic extract of twigs, with linoleic acid (26.270%), linoelaidic acid (11.747%), and stearic acid (11.384%) being the primary constituents. Research has indicated the antibacterial properties of stearic, oleic, linoleic, and linolenic acids (Wajs-Bonikowska et al., 2013), while linoelaidic acid demonstrates anticancer effects (Dutta et al., 2023). Predominant fractions of this extract comprise fatty acids and their derivatives (65.031%), ketones (6.574%), and alcohols (6.163%). Furthermore, significant quantities of phytosterols, specifically Ergost-5-en-3-yl acetate (1.226%), Stigmastan-3,5-diene (0.786%), and Cholesta-3,5-diene (0.537%), were also detected.
Nevertheless, the analysis via GC-MS of the cone extract components revealed a representation of 95.730% of the total chemical composition. The primary components identified in the cone extract encompassed 2-Bornanone (22.321%), 13-Octadecenoic acid, methyl ester (11.561%), Linoelaidic acid (7.084%), linoleic acid (6.643%), cis-Vaccenic acid (6.529%), and stearic acid (5.727%), with others present in lesser proportions. Notably, 2-Bornanone, characterized by aromatic properties, exhibits diverse attributes such as anti-tumor, analgesic antibacterial, anti-inflammatory sedative, fungicide, and anticancer activities. Previous studies by George and Britto (2015), have highlighted its potential in combating cancer. Additionally, cis-vaccenic acid, an omega-7 fatty acid, is recognized for its antibacterial and hypolipidemic effects in rats (Hamazaki et al., 2016). These findings align with those of Zirari et al. (2024b), who previously identified a cluster of natural terpenoids, fatty acids, and derivatives in the etheric extract of A. marocana cones.
The outcomes suggest that extracts from A. marocana twigs and cones are abundant in valuable phytochemical compounds with inherent biological activities. The significance of discerning phytobioconstituents extends beyond the pharmaceutical sector to encompass health industries engaged in the production of dietary supplements for overall wellbeing and various products, including the cosmetic industry.
The methodology's fundamental idea is based on the botanical samples' ability to reduce molybdenum (IV) to molybdenum (V), which results in the creation of a phosphate/molybdenum (V) complex in acidic settings. There is a noticeable green tint to this compound (Moonmun et al., 2017). The TAC was quantified in terms of milligrams of ascorbic acid equivalent per gram of dry weight (mg EAA.g−1 DW). According to Table 4, it is evident that the total antioxidant capability of botanical extracts varies significantly based on the specific plant component utilized. Notably, the extract derived from cones demonstrated a superior antioxidant capacity (63.007 ± 0.847 mg EAA.g−1 DW) in comparison to that obtained from twigs (56.583 ± 0.343 EAA.g−1 DW). Hence, the elevated antioxidant potential observed in A. marocana extracts indicates a rich presence of bioactive antioxidant constituents. Numerous research endeavors have proposed that flavonoids and polyphenolic compounds serve as the primary agents responsible for the phosphomolybdate scavenging efficacy of medicinal plants (Oueslati et al., 2012). These conclusions suggest that the antioxidant prowess of the above-ground portions can be linked to the existence of phenolic compounds and flavonoids within the methanolic extracts of the plant.
The DPPH free radical scavenging activity is commonly utilized due to its simplicity and rapidity, making it the preferred approach for assessing the antioxidant capacity of both natural and synthetic compounds (Angeli et al., 2021). Evaluation of antioxidant activity was conducted using IC50 values, which are detailed in Table 4. The examination of extracts indicated significant scavenging capabilities when assessed with DPPH. Notably, this scavenging potential was notably affected by the plant organ, with cones exhibiting greater efficacy compared to twigs. Specifically, the IC50 value from the cone extract was 354.642 ± 42.007 μg.mL−1, while twigs displayed an IC50 of 396.860 ± 10.618 μg.mL−1. The observed DPPH activity inhibition is likely attributed to electron transfer from the plant's phytoconstituents (Chukwuma et al., 2020). Polyphenols have been identified as potent antioxidants capable of counteracting free radicals by electron or hydrogen atom donation (Batool et al., 2019). In a recent study, Latos-Brozio et al. (2021), determined that ethanolic cone extracts from various fir species exhibited IC50 values of 11.85 ± 0.59 mg.mL−1 for Douglas fir, 13.82 ± 0.69 mg.mL−1 for Scots pine, and 15.43 ± 0.77 mg.mL−1 for Korean fir. Additionally, Zirari et al. (2024b), investigated the etheric extracts of Abies marocana twigs and cones, reporting IC50 values of 3,844 ± 55.95 μg.mL−1 and 4,496 ± 31.00 μg.mL−1, respectively.
To determine the lethal dose 50 (LD50), evaluations were carried out on methanolic extracts obtained from cones and twigs of A. marocana through acute oral toxicity assessments. The approach utilized was the adjustment technique delineated by the OECD, as specified in line 423 (OECD, 2001). When rats were orally administered with the aforementioned A. marocana extracts at 500 and 1,000 mg.kg−1 bw, there were no signs of acute toxicity observed, particularly the decrease in responsiveness to different stimuli. These stimuli encompassed pain and noise sensitivity, reduced mobility, fecal softening, changes in behavior, weight loss, and potential mortality within a 14-day period. The behavior exhibited by the rats during this timeframe mirrored that of the control group. The LD50 for both extracts were established to be above 1,000 mg.kg−1, as depicted in Table 5. Figure 5 shows how the rats' body weights increased gradually during the allotted time. There was no statistically significant difference in the body weight of the rats administered the extracts when compared to the control group. These results indicate that the oral intake of the extracts does not hinder the standard growth of rats, thus indicating the absence of behavioral toxicity. According to the guidelines of the Globally Harmonized System of Classification and Labeling of Chemicals (OECD, 2001), the two extracts are classified under the 5th category and are considered non-toxic via the oral pathway. Nayak et al. (2004), observed that the LD50 values for methanol, chloroform, and petroleum ether extracts of A. webbiana leaves were 986, 1,387, and above 3,200 mg.kg−1, respectively. Additionally, the acute toxicity assessments of the methanolic extracts of Abies webbiana Lindl. revealed no fatalities in mice after the oral administration of a 2,000 mg.kg−1 dose of the methanolic extracts obtained from the aerial parts this plant. No instances of mortality or adverse reactions were documented during the study period; hence, the dosage is categorized as “unclassified” based on the toxicity scale (Parkash et al., 2015).
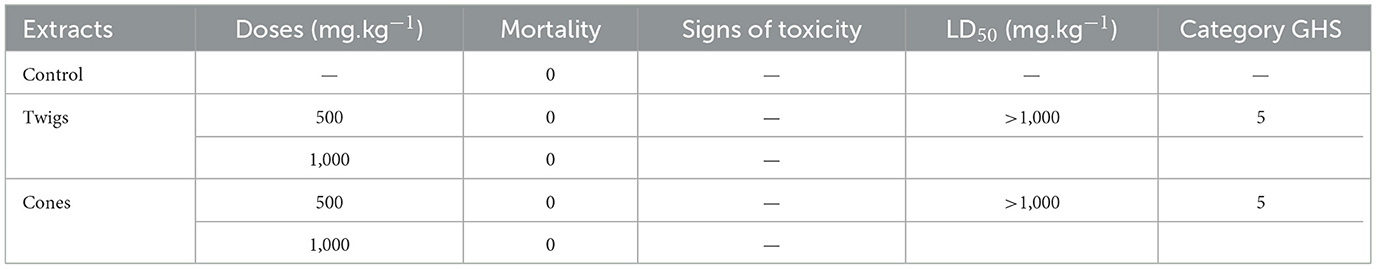
Table 5. Study of the acute toxicity of methanolic extracts of A. marocana administered by gavage to rats.

Figure 5. Influence of methanolic extracts of A. marocana twigs (A) and cones (B) on the weight change of rats during 14 days.
Relative organ weight is commonly employed to assess the level of toxic damage to a particular organ. The relative weights of the rat organs are depicted in Table 6. Notable discrepancies in weight, color, or texture compared to the control group of specific organs were not observed, suggesting that the administration of these extracts did not affect their normal growth. In a study conducted by Yadav et al. (2016), it was noted that flavonoids extracted from A. webbiana leaves did not demonstrate any adverse effects on the liver and kidneys. Conversely, they displayed a substantial protective effect on these organs.
Computer-aided drug design utilizes molecular docking as a key method to understand the interactions between protein receptors and ligands, offering essential insights into binding affinities, interaction mechanisms, and opportunities to enhance drug efficacy and selectivity.
NAD(P)H Oxidase is an enzyme whose unique function is the production of reactive oxygen species at physiological concentrations (Magnani and Mattevi, 2019). Moreover, its activity can be overregulated in certain pathological conditions such as diabetes, cancer and COVID-19 (Santos et al., 2021). Several molecules have demonstrated an ability to inhibit enzymatic hyperactivity, leading to a reduction in the excessive production of reactive oxygen species. However, their inhibitory action turns out to be nonspecific (Augsburger et al., 2019).
The oxidant activity of the various extracts from our two parts shows that the main compounds identified by GC-MS have an antioxidant effect against NAD(P)H Oxidase (PDB ID: 2CDU). We performed molecular docking of these compounds, which revealed that all of them exhibited high negative binding energy values.
The results of the molecular docking of three molecules identified in the cones plant with the target proteins are shown in Figure 6.
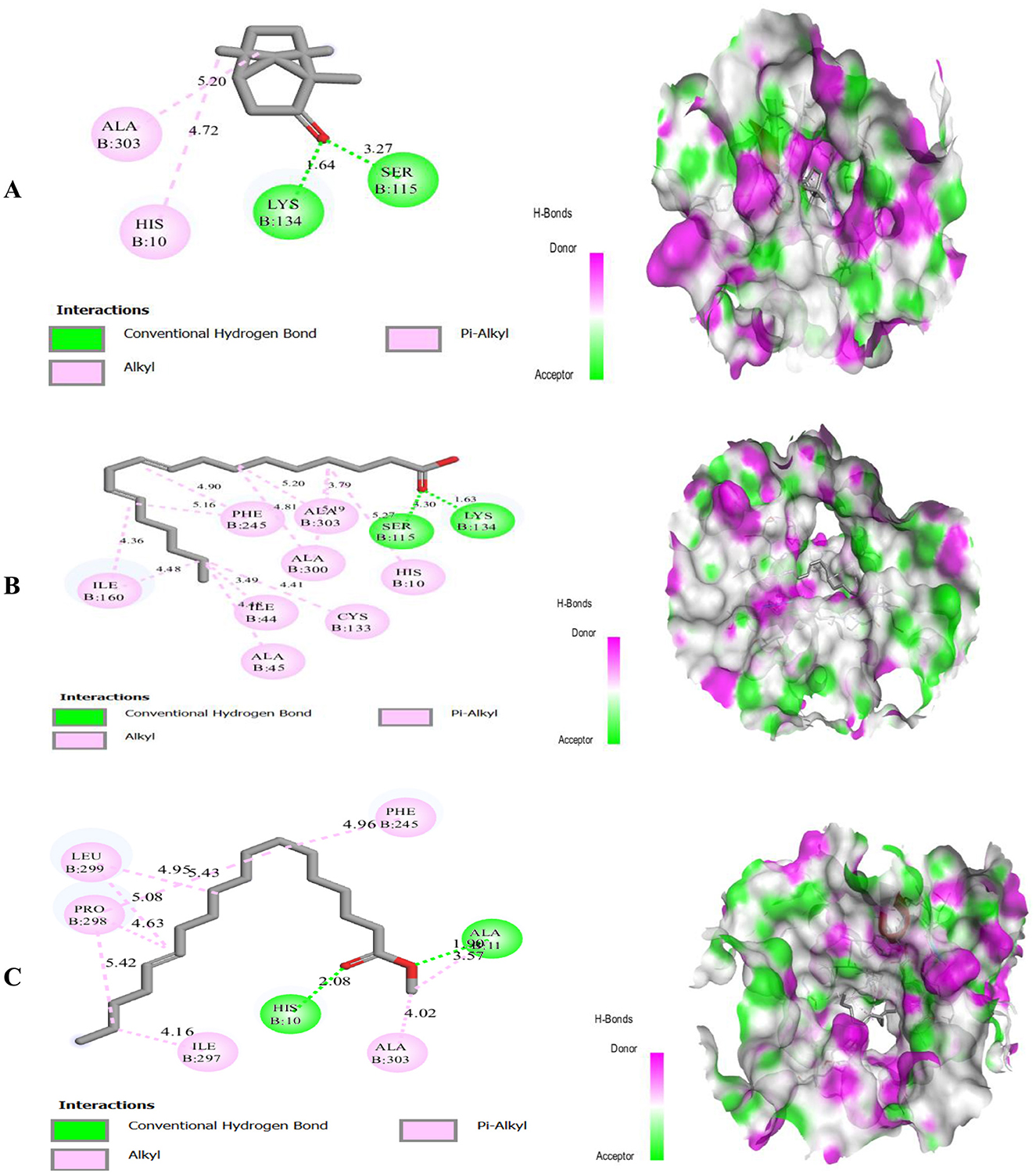
Figure 6. Molecular docking of three ligands, 2-Bornanone (A), Linoelaidic acid (B), and 13-Octadecenoic acid, methyl ester (C), with the 2CDU target protein.
Figure 6 shows the results of the molecular docking study showed the 2-Bornanone compound exhibited two hydrogen bonding interactions with the protein residues Ser-115 and Lys-134, with a distance >3.27 and 1.64 Å respectively, and also two Pi-alkyl and alkyl bonds with the protein residues Ala-303, and His-10.
Also, the results of the molecular docking study are shown in Figure 6, which demonstrated that the Linoelaidic acid compound exhibited a two-hydrogen bond interaction with protein residues Ser-115, and Lys-184 with a distance >3.30 and 1.63 Å respectively, as well as eight alkyl bonds with the protein residues Ala-303, Ala-300, His-10, Phe-245, Ile-44, Ala-45, Ile-160, and Cys-133.
Also, the results of the molecular docking study are shown in Figure 6, which demonstrated that the 13-Octadecenoic acid, methyl ester compound exhibited a two-hydrogen bond interaction with protein residues Ala-11, and His-10 with a distance >1.90 and 2.08 Å respectively, as well as five alkyl bonds with the protein residues Phe-245, Ala-303, Ile-297, Leu-299, and Pro-298.
The results suggest that 2-Bornanone, Linoelaidic acid, and 13-Octadecenoic acid, methyl ester are potential inhibitors of antioxidant receptor.
After the complete description of three molecules identified in plant cones, a complementary study was carried out on the three most identified compounds in plant twigs. The results of the molecular docking of three molecules identified in plant twigs with target proteins are shown in Figure 7.
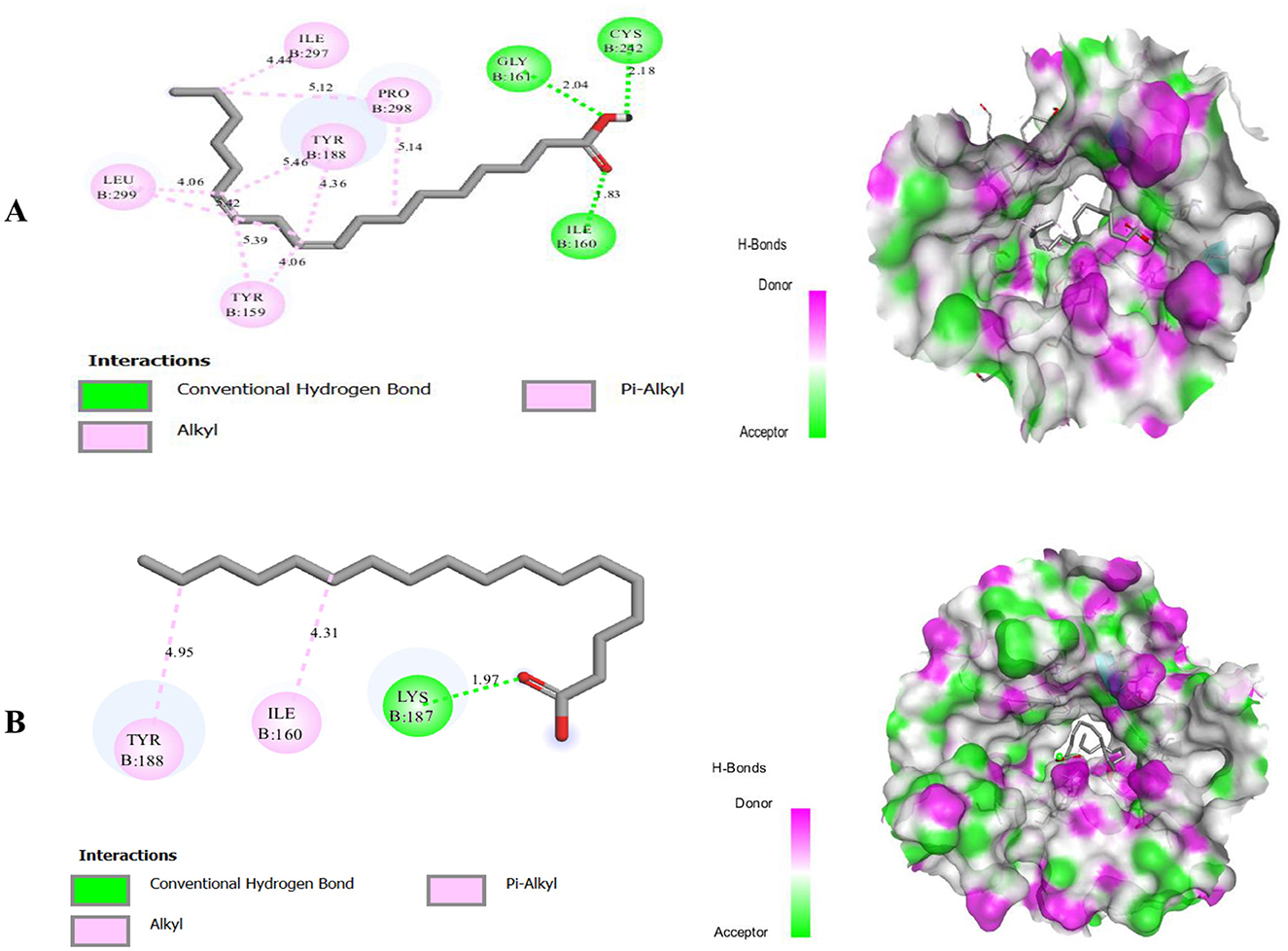
Figure 7. Molecular docking of two ligands, Linoleic Acid (A) and Stearic acid (B), with the 2CDU target protein.
Figure 7 presents the results of the molecular docking study, revealing that the Linoleic Acid compound formed two hydrogen bonds with the protein residues Gly-161, Cys-242, and Ile-160, at distances of 3.04 Å, 2.18 Å, and 1.83 Å, respectively. Additionally, it established five Pi-alkyl and alkyl interactions with the residues Ile-297, Pro-298, Tyr-188, Tyr-159, and Leu-299.
We also present the results of the molecular docking study are shown in Figure 7, which demonstrated that the Stearic acid compound exhibited a one hydrogen bond interaction with protein residues Lys-187 with a distance >1.97 Å, as well as two alkyl bonds with the protein residues Ile-160 and Tyr-188. For the compound Linoelaidic acid it's the same description of plant cones results.
After an in-depth molecular docking study of the five most identified compounds in two plants (Twigs and Cones), we found that these compounds interacted and correlated with the binding sites of selected proteins. Consequently, it can be concluded that these compounds exhibit significant antioxidant activities.
The current study delved into the nutritional and chemical constituents, antioxidative characteristics, and acute oral toxicity of twigs and cones of Abies marocana. Analysis outcomes revealed significant levels of proximate composition and minerals in the woody biomass, suggesting its potential nutritional value. Assessment of phytochemical content demonstrated the existence of diverse secondary metabolites possessing medicinal attributes. These results advocate for further inquiry into the modes of action of these bioactive compounds and their plausible physiological effects such as anti-inflammatory and anticarcinogenic properties. Moreover, the methanolic extracts of the twigs and cones exhibited antioxidative capacities linked to flavonoids, polyphenols, phytosterols, and fatty acids. Regarding acute toxicity, it was observed that the methanolic extracts of A. marocana did not result in mortality in rodents. Subsequent research endeavors could enhance these findings by scrutinizing the chronic oral toxicity and pharmacodynamic characteristics of these extracts. A molecular docking study of the five most important compounds in both plants revealed that these compounds interacted and aligned with the binding sites of various selected proteins. We can therefore conclude that these compounds have significant antioxidant activities.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The animal study was approved by the protocols outlined by the OECD 423. The study was conducted in accordance with the local legislation and institutional requirements.
MZ: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. MA: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. WB: Resources, Writing – review & editing. ME-r: Formal analysis, Resources, Writing – review & editing. HI: Conceptualization, Data curation, Formal analysis, Resources, Writing – review & editing. FA: Data curation, Writing – review & editing. OE: Resources, Writing – review & editing. ON: Resources, Writing – review & editing. MT: Data curation, Writing – review & editing. DH: Formal analysis, Writing – review & editing. NM: Formal analysis, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the King Saud University, Researchers Supporting Project Number (RSPD2025R1087), King Saud University, Riyadh, Saudi Arabia.
The authors extend their appreciation to the King Saud University, Researchers Supporting Project Number (RSPD2025R1087), King Saud University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Achi, N. K., Onyeabo, C., Ekeleme-Egedigwe, C. A., and Onyeanula, J. C. (2017). Phytochemical, proximate analysis, vitamin and mineral composition of aqueous extract of ficus capensis leaves in south eastern Nigeria. J. App. Pharm. Sci. 7, 117–122. doi: 10.7324/JAPS.2017.70319
Akinsola, A. F., Olatunde, O. C., Osasona, I., Sekayo, O. F., and Omotayo, F. O. (2021). Nutritional evaluation of brillantaisia patula leaves. Asian Plant Res. J. 8, 63–73. doi: 10.9734/aprj/2021/v8i430186
Ameen, O. A., Hamid, A. A., Yusuf, Q., Njoku, O. G., Oseni, T. O., Jamiu, W., et al. (2021). Quantitative and qualitative assessment of phytochemicals in methanolic extracts of hurricane weed (Phyllanthus amarus Schumach. and Thonn) Plant. J. Appl. Sci. Environ. Manag. 25, 159–165. doi: 10.4314/jasem.v25i2.4
Angeli, L., Imperiale, S., Ding, Y., Scampicchio, M., and Morozova, K. (2021). A novel stoichio-kinetic model for the DPPH• assay: the importance of the side reaction and application to complex mixtures. Antioxidants 10:1019. doi: 10.3390/antiox10071019
Anita, B. S., Akpan, E. J., and Okon, P. A. (2006). Nutritive and antinutritive evaluation of sweet potatoes (Ipomoea batatas) leaves. Pak J. Nutr. 5, 166–168. doi: 10.3923/pjn.2006.166.168
AOAC (1995). Association of Official Analytical Chemists International, Official Methods of Analysis, 16th edition, New York, USA: AOACInternational.
AOAC (2016). Association of Official Analytical Chemists International, Official Methods of Analysis, 20th edition, Rockville, MD, USA: AOACInternational.
Aouji, M., Imtara, H., Rkhaila, A., Bouhaddioui, B., Alahdab, A., Parvez, M. K., et al. (2023). Nutritional composition, fatty acids profile, mineral content, antioxidant activity and acute toxicity of the flesh of Helix aspersa müller. Molecules 28:6323. doi: 10.3390/molecules28176323
Auders, A. G., and Spicer, D. P. (2012). Royal Horticultural Society Encyclopedia of Conifers: A Comprehensive Guide to Cultivars and Species. Nicosia, Cyprus: Kings blue Publishing Limited.
Augsburger, F., Filippova, A., Rasti, D., Seredenina, T., Lam, M., Maghzal, G., et al. (2019). Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 26:101272. doi: 10.1016/j.redox.2019.101272
Bartnik, C., Nawrot-Chorabik, K., and Woodward, S. (2020). Phenolic compound concentrations in Picea abies wood as an indicator of susceptibility towards root pathogens. For. Pathol. 50:e12652. doi: 10.1111/efp.12652
Batool, R., Khan, M. R., Sajid, M., Ali, S., and Zahra, Z. (2019). Estimation of phytochemical constituents and in vitro antioxidant potencies of Brachychiton populneus (Schott and Endl.) R. Br. BMC Chem. 13, 1–15. doi: 10.1186/s13065-019-0549-z
Benković, E. T., Grohar, T., Žigon, D., Švajger, U., Janeš, D., Kreft, S., et al. (2014). Chemical composition of the silver fir (Abies alba) bark extract Abigenol® and its antioxidant activity. Ind. Crops Prod. 52, 23–28. doi: 10.1016/j.indcrop.2013.10.005
Benouchenne, D., Bellil, I., Akkal, S., Bensouici, C., and Khelifi, D. (2020). LC–MS/MS analysis, antioxidant and antibacterial activities of Algerian fir (Abies numidica de LANNOY ex CARRIÈRE) ethylacetate fraction extracted from needles. J. King Saud Univ. Sci. 32, 3321–3327. doi: 10.1016/j.jksus.2020.09.017
BNS EN ISO 734-1 (2020). Oilseed meals - Determination of oil content - Part 1: Extraction method with hexane (or light petroleum). Technical Report.
Bouras, M., Grimi, N., Bals, O., and Vorobiev, E. (2016). Impact of pulsed electric fields on polyphenols extraction from Norway spruce bark. Ind. Crops Prod. 80, 50–58. doi: 10.1016/j.indcrop.2015.10.051
Bowers, W. S., Thompson, M. J., and Uebel, E. C. (1965). Juvenile and gonadotropic hormone activity of 10, 11-epoxyfarnesenic acid methyl ester. Life Sci. 4, 2323–2331. doi: 10.1016/0024-3205(65)90256-0
Cabral, C. E., and Klein, M. R. S. T. (2017). Phytosterols in the treatment of hypercholesterolemia and prevention of cardiovascular diseases. Arq. Bras. Cardiol. 109, 475–482. doi: 10.5935/abc.20170158
Cheok, C. Y., Chin, N. L., Yusof, Y. A., Talib, R. A., and Law, C. L. (2013). Optimization of total monomeric anthocyanin (TMA) and total phenolic content (TPC) extractions from mangosteen (Garcinia mangostana Linn.) hull using ultrasonic treatments. Ind. Crops Prod. 50, 1–7. doi: 10.1016/j.indcrop.2013.07.024
Chukwuma, I. F., Nkwocha, C. C., Ezeanyika, L. U., and Ogugua, V. N. (2020). Phytochemical investigation and in vitro antioxidant potency of root bark of Brenania brieyi fractions. Trop. J. Natur. Prod. Res. 4, 970–975. doi: 10.26538/tjnpr/v4i11.21
Daouk, E., Van de Steene, L., Paviet, F., and Salvador, S. (2014). “Oxidative pyrolysis of a large wood particle: Effects of oxygen concentration and of particle size,” in ICONBM – International Conference on Biomass, Pts 1 and 2 (Florence, Italy), 73–78.
Do, Q. D., Angkawijaya, A. E., Tran-Nguyen, P. L., Huynh, L. H., Soetaredjo, F. E., Ismadji, S., et al. (2014). Effect of extraction solvent on total phenol content, total favonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug. Anal. 22, 296–302. doi: 10.1016/j.jfda.2013.11.001
Dutta, A., Panchali, T., Khatun, A., Jarapala, S. R., Das, K., Ghosh, K., et al. (2023). Anti-cancer potentiality of linoelaidic acid isolated from marine Tapra fish oil (Ophisthopterus tardoore) via ROS generation and caspase activation on MCF-7 cell line. Sci. Rep. 13:14125. doi: 10.1038/s41598-023-34885-3
Ejelonu, B. C., Lasisi, A. A., Olaremu, A. G., and Ejelonu, O. C. (2011). The chemical constituents of calabash (Crescentia cujete). Afr. J. Biotechnol. 10, 19631–19636. doi: 10.5897/AJB11.1518
Emebu, P. K., and Anyika, J. U. (2011). Proximate and mineral composition of kale (Brassica oleracea) Grown in Delta State, Nigeria. Pakistan J. Nutr. 10, 190–194. doi: 10.3923/pjn.2011.190.194
Fahimirad, S., Ajalloueian, F., and Ghorbanpour, M. (2019). Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicol. Environ. Saf. 168, 260–278. doi: 10.1016/j.ecoenv.2018.10.017
George, M., and Britto, S. J. (2015). Phytochemicaland antioxidant studies on the essential oil of the rhizome of Curcuma aeruginosa Roxb. Int. Res. J. Pharm 6, 573–579. doi: 10.7897/2230-8407.068113
Gyawali, R., and Ibrahim, S. A. (2014). Natural products as antimicrobial agents. Food Control 46, 412–429. doi: 10.1016/j.foodcont.2014.05.047
Hamazaki, K., Suzuki, N., Kitamura, K. I., Hattori, A., Nagasawa, T., Itomura, M., et al. (2016). Is vaccenic acid (18: 1t n-7) associated with an increased incidence of hip fracture? An explanation for the calcium paradox. Prostagl. Leukotr. Essent. Fatty Acids 109, 8–12. doi: 10.1016/j.plefa.2016.04.001
Harborne, A. J. (1998). Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. New York: Springer Science and Business Media.
Herodež, Š. S., Hadolin, M., Škerget, M., and Knez, Ž. (2003). Solvent extraction study of antioxidants from Balm (Melissa officinalis L.) leaves. Food Chem. 80, 275–282. doi: 10.1016/S0308-8146(02)00382-5
Hussain, J., Khan, A. L., Rehman, N., Zainullah, K. F., Hussain, S. T., Shinwari, Z. K., et al. (2009). Proximate and nutrient analysis of selected medicinal plantspecies of Pakistan. Pakistan J. Nut. 8, 620–624. doi: 10.3923/pjn.2009.620.624
Jones, M. M., Johnson, D. O., Netterville, J. T., Wood, J. I., and Joesten, M. (1985). Chemistry and Society 5th ed. New York, USA: Sanders College Publishers, 521–577.
Jyske, T., Järvenpää, E., Kunnas, S., Sarjala, T., Raitanen, J. E., Mäki, M., et al. (2020). Sprouts and needles of Norway spruce (Picea abies (L.) Karst.) as Nordic specialty-consumer acceptance, stability of nutrients, and bioactivities during storage. Molecules 25:4187. doi: 10.3390/molecules25184187
Kris-Etherton, P. M., Hecker, K. D., Bonanome, A., Coval, S. M., Binkoski, A. E., Hilpert, K. F., et al. (2002). Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 9, 71–88. doi: 10.1016/S0002-9343(01)00995-0
Kumari, A., Parida, A. K., Rangani, J., and Panda, A. (2017). Antioxidant activities, metabolic profiling, proximate analysis, mineral nutrient composition of Salvadora persica fruit unravel a potential functional food and a natural source of pharmaceuticals. Front. Pharmacol. 8:232579. doi: 10.3389/fphar.2017.00061
Lamaison, J. L., and Carnat, A. (1990). Teneur en principaux flavonoides des fleurs et des feuilles de Crataegus monogyna Jacq. et de Crataegus laevigata (Poiret) DC. (Rosacea). Pharmac. Acta Helv. 65, 315–320.
Latos-Brozio, M., Masek, A., Chrzescijanska, E., Podsedek, A., and Kajszczak, D. (2021). Characteristics of the polyphenolic profile and antioxidant activity of cone extracts from conifers determined using electrochemical and spectrophotometric methods. Antioxidants 10:1723. doi: 10.3390/antiox10111723
Lee, K., and Kim, D. (2019). In-silico molecular binding prediction for human drug targets using deep neural multi-task learning. Genes 10:906. doi: 10.3390/genes10110906
Leone, K., Micheletto, M., Di Maira, G., Tedesco, E., Benetti, F., Zaloker, U., et al. (2022). Role of a novel silver fir (Abies alba) extract, Abigenol®/AlbiPhenol®, in modulating cardiovascular disorders: key factors. Antioxidants 11:618. doi: 10.3390/antiox11040618
Lima Rojas, L. (2013). Evaluación de la composición química y propiedades físicas de madera y corteza de cuatro coníferas para la producción de bioenergía. Doctoral dissertation, Universidad Autónoma de Nuevo León.
Magnani, F., and Mattevi, A. (2019). Structure and mechanisms of ROS generation by NADPH oxidases. Curr. Opin. Struct. Biol. 59, 91–97. doi: 10.1016/j.sbi.2019.03.001
Martínez-Gómez, O., Pintor-Ibarra, L. F., Rutiaga-Quiñones, J. G., and Corona-Terán, J. (2022). Chemical composition and energy evaluation of abies spp. and pinus spp. sawdust collected as a byproduct of the primary wood sawing. South-east Eur. Forest. 13, 89–96. doi: 10.15177/seefor.22-08
Merrill, A. L., and Watt, B. K. (1973). Energy Value of Foods-Basis and Derivation. St. Louis: USDA Handbook.
Milner, J. A. (1994). “Reducing the risk of cancer,” in Functional Foods: Designer Foods, Pharmafoods, Nutraceuticals (Boston). doi: 10.1007/978-1-4615-2073-3_3
Moonmun, D., Majumder, R., and Lopamudra, A. (2017). Quantitative phytochemical estimation and evaluation of antioxidant and antibacterial activity of methanol and ethanol extracts of Heliconia rostrata. Indian J. Pharm. Sci. 79, 79–90. doi: 10.4172/pharmaceutical-sciences.1000204
Nayak, S. S., Ghosh, A. K., Debnath, B., Vishnoi, S. P., and Jha, T. (2004). Synergistic effect of methanol extract of Abies webbiana leaves on sleeping time induced by standard sedatives in mice and anti-inflammatory activity of extracts in rats. J. Ethnopharmacol. 93, 397–402. doi: 10.1016/j.jep.2004.04.014
Nikolić, J. S., Zlatković, B. K., Jovanović, S. C., Stojanović, G. S., Marin, P. D., and Mitić, Z. S. (2021). Needle volatiles as chemophenetic markers in differentiation of natural populations of Abies alba, A. x borisii-regis, and A. cephalonica. Phytochemistry 183:112612. doi: 10.1016/j.phytochem.2020.112612
OECD (2001). Guidance Document on Acute Oral Toxicity Testing; OECD Series on Testing and Assessment. Paris, France: OECD. ISBN 978-92-64-07841-3
Oladiji, A. T., and Mih, F. O. (2005). Proximate composition mineral and phytochemical constituents of Eleusine coracana (finger millet). Afr. J. Biotechnol. 4, 1440–1441.
Onwuka, G. I. (2005). Food Analysis and Instrumentation, Proximate Composition of Food Minerals 1st Edition. Nigeria: Naplithali print, a Division of HG support Nigerian Ltd, 114.
Oueslati, S., Ksouri, R., Falleh, H., Pichette, A., Abdelly, C., Legault, J., et al. (2012). Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 132, 943–947. doi: 10.1016/j.foodchem.2011.11.072
Pamela, C. C., Richard, A. H., and Denise, R. F. (2005). Lippincott's Illustrated Reviews Biochemistry 3rd Ed, Philadelphia: Lippincott Williams and Wilkins, 335–388.
Parkash, O., Kumar, D., and Kumar, S. (2015). Screening of methanol extract and ethyl acetate fraction of Abies webbiana Lindl. for neuropharmacological activities. Indian J. Pharm. Sci. 77:536. doi: 10.4103/0250-474X.169039
Pathak, P., and Kapil, U. (2004). Role of trace elements zinc, copper and magnesium during pregnancy and its outcome. Indian J. Paediatr. 71, 1003–1005. doi: 10.1007/BF02828116
Pearson, D., Berko, A., and Tayie, E. (1999). Proximate analysis of some underutilized Ghanaian vegetables. Ghana J. Sci. 39, 91–92.
Popa, V. I., Agache, C., Beleca, C., and Popa, M. (2002). Polyphenols from spruce bark as plant growth regulator. CropRes. 24, 398–406.
Robert, K. M., Daryl, K. G., Peter, A. M., and Victor, W. R. (2003). “Harper's illustrated biochemistry,” in Benders and Mayes Vitamins and Minerals (New York: Lange Medical Books/McGraw-Hill, Medical Publishing Division), 496.
Salem, M. Z. M., Nasser, R. A., Zeidler, A., Elansary, H. O., Aref, I. M., Böhm, M., et al. (2020). Methylated fatty acids from heartwood and bark of Pinus sylvestris, Abies alba, Picea abies, and Larix decidua: effect of strong acid treatment. BioResources 10, 7715–7724. doi: 10.15376/biores.10.4.7715-7724
Santos, A. F., Povoa, P., Paixao, P., Mendonça, A., and Taborda-Barata, L. (2021). Changes in glycolytic pathway in SARS-COV 2 infection and their importance in understanding the severity of COVID-19. Front. Chem 9:685196. doi: 10.3389/fchem.2021.685196
Schoss, K., Benedeti,č, R., and Kreft, S. (2022). The phenolic content, antioxidative properties and extractable substances in silver fir (Abies alba Mill.) branches decrease with distance from the trunk. Plants 11:333. doi: 10.3390/plants11030333
Scientific Advisory Committee on Nutrition (2008). Draft SCAN position statement on dietary fiber and health and the dietary fiber definition. SACN/08/20. Available at: https://assets.publishing.service.gov.uk/media/5a7cfaf5ed915d28e9f39533/SACN_Draft_position_statement_on_dietary_fibre_and_health_and_dietary_fibre_definition_2008.pdf
Semerci, A. B., Inceçayir, D., Konca, T., Tunca, H., and Tunç, K. (2020). Phenolic constituents, antioxidant and antimicrobial activities of methanolic extracts of some female cones of gymnosperm plant. Indian J. Biochem. Biophys. 57, 298–303.
Shad, A., Shah, H. U., and Bakht, L. (2013). Ethnobotanical assessment and nutritive potential of wild food plant. J. Animal Plant Sci. 23, 92–97.
Shahar, B., Indira, A., Santosh, O., Dolma, N., and Chongtham, N. (2023). Nutritional composition, antioxidant activity and characterization of bioactive compounds from Thymus serpyllum L. an underexploited wild aromatic plant. Measur. Food 10:100092. doi: 10.1016/j.meafoo.2023.100092
Shi, L., Zhao, W., Yang, Z., Subbiah, V., and Suleria, H. A. R. (2022). Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 29, 81112–81129. doi: 10.1007/s11356-022-23337-6
Skujins, S. (1998). Handbook for ICP-AES (Varian-vista), a short guide to vista series ICP-AES operation, Version 1.0. Varian International AG.
Sofowora, A. (1993). Medicinal Plants and Traditional Medicine in Africa. Ibadan, Nigeria: Spectrum Books Limited, 1–153.
Stanzione, F., Giangreco, I., and Cole, J. C. (2021). Use of molecular docking computational tools in drug discovery. Prog. Med. Chem. 60, 273–343. doi: 10.1016/bs.pmch.2021.01.004
Tálos-Nebehaj, E., Hofmann, T., and Albert, L. (2017). Seasonal changes of natural antioxidant content in the leaves of Hungarian forest trees. Ind. Crops Prod. 98, 53–59. doi: 10.1016/j.indcrop.2017.01.011
Tcheutchoua, Y. C., Bilanda, D. C., Mengue Ngadena, Y. S., Djomeni Dzeufiet, P. D., Owona, P. E., Fifen, R. N., et al. (2022). Acute and subchronic toxicity studies on the aqueous extract of the plant mixture (Bidens pilosa and Cymbopogon citratus aerial parts) in rat model. J. Toxicol. 2022:1998433. doi: 10.1155/2022/1998433
Thomas, R. A., and Krishnakumari, S. (2015). Proximate analysis and mineral composition of Myristica fragrans seeds. J. Pharmacogn. Phytochem. 3, 39–42.
Trease, G. E., and Evans, W. C. (1989). Textbook of Pharmacology. 13th Ed. London: Macmillan Publishers Limited, 343–383.
Trott, O., and Olson, A. J. (2010). AutoDock Vina: Améliorer la vitesse et la précision de l'amarrage avec une nouvelle fonction de notation, une optimisation efficace et le multithreading. J. Comput. Chem. 31, 455–461. doi: 10.1002/jcc.21334
Trumbo, P., Schlicker, S., Yates, A. A., and Poos, M. (2002). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. (Commentary). J. Am. Diet. Assoc. 102, 1621–1631. doi: 10.1016/S0002-8223(02)90346-9
Ucar, M. B., and Ucar, G. (2008). Lipophilic extractives and main components of black pine cones. Chem. Nat. Comp. 44, 380–383. doi: 10.1007/s10600-008-9071-6
Vek, V., KerŽič, E., Poljanšek, I., Eklund, P., Humar, M., Oven, P., et al. (2021). Wood extractives of silver fir and their antioxidant and antifungal properties. Molecules 26:6412. doi: 10.3390/molecules26216412
Velasco, L., Fernández-Cuesta, Á., and Fernández-Martínez, J. M. (2014). New sunflower seeds with high contents of phytosterols. OCL 21:D604. doi: 10.1051/ocl/2014036
Vu, D. C., Lei, Z., Sumner, L. W., Coggeshall, M. V., and Lin, C. H. (2019). Identification and quantification of phytosterols in black walnut kernels. J. Food Comp. Analy. 75, 61–69. doi: 10.1016/j.jfca.2018.09.016
Vunchi, M. A., Umar, M. A., King, A. A., Liman, G. J., and Aigbe, C. O. (2011). Proximate, vitamins and mineral composition of vitex doniana (black plum) fruit pulp. Nigerian J. Basic Appl. Sci. 19, 97–101. doi: 10.4314/njbas.v19i1.69352
Wajs-Bonikowska, A., Olejnik, K., Bonikowski, R., and Banaszczak, P. (2013). Analysis of volatile components, fatty acids, and phytosterols of Abies koreana growing in Poland. Nat. Prod. Commun. 8:1934578X1300800928. doi: 10.1177/1934578X1300800928
Watson, R. R., Preedy, V. R., and Zibadi, S. (2018). Polyphenols: Prevention and Treatment of Human Disease. London: Academic Press.
WFO (2023). World Flora online. Available at: http://www.worldfloraonline.org (accessed July 12, 2023).
Yadav, D. K., Ali, M., Ghosh, A. K., and Kumar, B. (2016). Isolation of flavonoid from Abies webbiana leaves and its activity. Pharmac. J. 8, 341–345. doi: 10.5530/pj.2016.4.6
Yeşilada, E., Honda, G., Sezik, E., Tabata, M., Fujita, T., Tanaka, T., et al. (1995). Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J. Ethnopharmacol. 46, 133–152. doi: 10.1016/0378-8741(95)01241-5
Zirari, M., Aouji, M., Imtara, H., Hmouni, D., Tarayrah, M., Noman, O. M., et al. (2024a). Nutritional composition, phytochemicals, and antioxidant activities of Abies marocana Trab. needles. Front. Sustain. Food Syst. 8:1348141. doi: 10.3389/fsufs.2024.1348141
Keywords: Abies marocana, nutritional value, woody biomass, antioxidant activity, acute oral toxicity, molecular docking
Citation: Zirari M, Aouji M, Baghdad W, Er-rajy M, Imtara H, Abujaber F, Elharrati O, Noman OM, Tarayrah M, Hmouni D and Mejdoub NE (2025) In-depth study of the nutritional composition, phytochemicals, antioxidant activity, molecular docking interactions, and toxicological evaluation of Abies marocana Trab. woody biomass. Front. Sustain. Food Syst. 8:1525572. doi: 10.3389/fsufs.2024.1525572
Received: 09 November 2024; Accepted: 26 December 2024;
Published: 24 January 2025.
Edited by:
Dunja Miletić, University of Belgrade, SerbiaReviewed by:
Inas Youssef Younis, Cairo University, EgyptCopyright © 2025 Zirari, Aouji, Baghdad, Er-rajy, Imtara, Abujaber, Elharrati, Noman, Tarayrah, Hmouni and Mejdoub. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Malak Zirari, bWFsYWsuemlyYXJpQHVpdC5hYy5tYQ==; Hamada Imtara, SGFtYWRhLmltdGFyYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.