- 1School of Life Sciences, Guizhou Normal University, Guiyang, China
- 2School of Karst Science, Guizhou Normal University, Guiyang, China
- 3Colloge of Animal Science, Guizhou University, Guiyang, China
Alfalfa (Medicago sativa L.) plays an important role in the development of animal husbandry in the karst region of southwestern China, and karst environmental stress has a significant impact on the germination of alfalfa seeds. This study subjected alfalfa seeds to calcium salt stress (0–100 mM), drought stress (0–0.53 MPa), and pH stress (pH 3–9). Germination indicators (germination rate, germination potential, germination index, and vigor index), seedling morphological indicators (shoot length and root length), and biomass indicators were measured to assess seed stress resistance. The results showed that mild drought stress (5% PEG solution) and weak alkaline stress (pH 8) promoted seed germination, and a 20 mM CaCl2 solution significantly increased the germination rate. The root system of seedlings was more sensitive to the three types of stress. Under moderate calcium stress (40 mM), only a slight decrease in tissue water content was observed. Under moderate drought stress (10% PEG), fresh weight and tissue water content decreased, but dry weight significantly increased. Under alkaline stress (pH 9), both biomass indicators and tissue water content increased. This study provides a theoretical reference for selecting plants suitable for cultivation in karst environments.
1 Introduction
Medicago sativa L., commonly known as alfalfa, is an important source of feed for animal husbandry worldwide, characterized by superior nutritional properties, including high levels of crude protein, secondary metabolites, and macro and trace minerals (Zhou et al., 2024; Suwignyo et al., 2023). Due to its ability to thrive in various ecological environments and its excellent adaptability (Bagavathiannan and Van Acker, 2009; Sim et al., 2017), it is often referred to as the “king of forage.”
Alfalfa is widely cultivated in China and serves as an important forage source in the southwestern region. The southwestern region of China (102–111° E, 23–32° N), mainly in the provinces of Yunnan, Guizhou, and Guangxi, is characterized by extensive karst landforms. This area belongs to the subtropical and tropical humid climate zones and is one of the three major concentrated karst distribution areas in the world. Karst landscapes typically consist of discontinuous thin soils overlying soluble carbonate bedrock, such as limestone or dolomite. These soils are alkaline calcareous soils rich in calcium ions (Wang et al., 1999), have extremely poor water retention capacity, frequently experience temporary droughts (Deng et al., 2018), and have pH values between 7.28 and 8.35 (Yun et al., 2016). These factors significantly affect plant growth and development. Therefore, selecting crops suitable for the local climate and soil conditions is crucial for agricultural production in karst regions.
Excessive soil calcium levels can be a significant factor affecting the growth and development of alfalfa. High concentrations of calcium can interfere with the normal physiological and biochemical functions of plant cells (Dodd et al., 2010; Ribis et al., 2023), inhibit seed germination (Zhan and Huang, 2016), and affect photosynthesis (Wang et al., 2022), thereby inhibiting plant growth and development. Although calcium is an essential nutrient for plants, playing important roles in stabilizing cell walls (Sarfraz et al., 2024), maintaining intracellular ion balance, providing osmotic protection, and ensuring the integrity of cell membrane structures (Gilliham et al., 2011; Dayod et al., 2010), excessive calcium can disrupt these processes. As a second messenger that links extracellular signals with intracellular physiological and biochemical reactions, calcium participates in processes such as plant growth and development, photosynthetic respiration, and gene regulation of related enzymes (White and Broadley, 2003; Weinl et al., 2008). However, an overload of calcium can reduce the ability of plants to withstand environmental stress (Han et al., 2020), thereby impairing plant stress resistance.
Drought stress is considered the most unfavorable factor limiting plant survival, growth, and productivity (Zhao et al., 2022; Rao et al., 2024), severely restricting the germination of alfalfa seeds and the growth of seedlings (Ross and Hegarty, 1980). When there is a water deficit, plants cope by regulating various physiological and biochemical processes (Huang et al., 2022; Wang et al., 2024). For example, they upregulate abscisic acid production to induce stomatal closure, reducing water loss and enhancing drought tolerance (Rao et al., 2024; Schroeder et al., 2001); they maintain water balance through osmotic regulation (Quan et al., 2016) to avoid low leaf turgor pressure and damage to cell membranes (Ma et al., 2012).
Maintaining a constant pH is essential for the normal growth and development of plants. Excessive acidity or alkalinity in the environment can weaken plant stress resistance, affect normal growth, and reduce production capacity (Prakash et al., 2024; Yin et al., 2022). In soils with high acidity, the ultrastructure of plant cells can be damaged, and chlorophyll content and photosynthetic efficiency significantly decrease (Yin et al., 2022). Studies have shown that he biomass of alfalfa significantly decreases with decreasing pH, indicating that soil acidity severely limits the cultivation of alfalfa (Li et al., 2010). Conversely, high pH alkaline conditions can reduce the plasticity of plant cell walls, leading to limited cell growth and expansion. Excessive pH can also affect the rate of ATP synthesis in cells, disrupt the material and energy balance of plants, and ultimately impair normal growth and development (Wu, 2020).
Currently, there is limited research on the adaptability of alfalfa seed germination and seedling growth under calcium salt stress, drought stress, and alkaline soil conditions. In this study, we comprehensively analyze the effects of high calcium stress, drought stress, and pH stress on alfalfa seed germination and seedling growth. The aim is to provide basic data and technical references for the screening and evaluation of suitable forage, green manure crops, and soil and water conservation crops in karst areas.
2 Materials and methods
2.1 Plant materials
The experiment utilized alfalfa (Medicago sativa L.) seeds of the variety ‘Bara 416WET,’ obtained from Bailu (China). This high-yield, fibrous-root type cultivar is characterized by strong disease resistance, rapid regeneration, and tolerance to moisture stress.
2.2 Experimental design
Uniform and plump alfalfa seeds were selected and disinfected with a 1% sodium hypochlorite (NaClO) solution for 10 min, then rinsed thoroughly with distilled water and air-dried. The seeds were placed in 9 cm diameter Petri dishes lined with double-layer filter paper. Thirty seeds were evenly distributed in each dish. The filter paper was moistened with approximately 5 mL of the respective treatment solutions. The dishes were covered and incubated at 25°C under a photoperiod of 16 h light and 8 h dark. Each petri dish was considered one replicate, and four replicates were prepared for each treatment. Germination was monitored daily at 6 p.m., and water loss due to evaporation was compensated using the weighing method. For drought and calcium salt stress treatments, deionized water was used as the control, while for pH stress treatments, a medium with a pH of 7 served as the control.
2.3 Preparation of treatment solutions
2.3.1 Calcium stress
To simulate calcium-rich soil conditions characteristic of karst environments, calcium chloride (CaCl2) solutions with concentrations of 0, 5 mM, 10 mM, 25 mM, 50 mM, 100 mM, and 150 mM were prepared. These solutions were designed to incrementally increase calcium ion (Ca2+) concentration, effectively representing high-calcium karst soils.
2.3.2 Drought stress
Polyethylene glycol (PEG6000) solutions with concentrations of 0, 5, 7, 10, 13, 15, and 20% were prepared, corresponding to osmotic potentials of 0, −0.06, −0.09, −0.17, −0.26, −0.32, and −0.53 MPa, respectively. PEG6000, a common osmotically active compound, was used to reduce water potential and simulate various levels of drought stress.
2.3.3 pH stress
To simulate soil pH variability in karst regions, the pH of the germination medium was adjusted to values of 3, 4, 5, 6, 7, 8, and 9 using hydrochloric acid (HCl) or sodium hydroxide (NaOH). pH levels were monitored using pH test strips, and the desired values were achieved by incrementally adding HCl or NaOH while continuously measuring.
2.4 Seed germination indicators
Seed germination potential and germination rate were determined following the Chinese National Standard GB/T 2930.4–2001, using radicle emergence as the criterion:
Germination index: calculate the germination index on the 14th day of cultivation, with germination index (GI) = ∑ (Gt/Dt), where Gt is the number of sprouts on the t-th day; Dt is the corresponding germination day;
Vitality index (VI) = germination index x shoot length.
2.5 Measurement of seedling growth
On the 14th day of cultivation, the embryo length and embryonic root length of alfalfa seedlings from each treatment were measured using a millimeter ruler with an accuracy of 0.1 cm.
For fresh and dry weight measurements, five seedlings were randomly selected from each Petri dish. Surface moisture was removed using filter paper. The fresh weight was measured using an analytical balance with a precision of 0.0001 g. The seedlings were then dried to constant weight in an oven at 80°C, and the dry weight was recorded.
2.6 Data analysis
Data were organized and plotted using Microsoft Excel 2021 and Origin 2022. Statistical analyses were performed using SPSS 27.0 software. One-way analysis of variance (ANOVA) was conducted, and Tukey’s multiple range test was used for comparisons among means at the 5% significance level (p < 0.05). Results were expressed as mean ± standard error (SE).
3 Results
3.1 Effects of different stresses on alfalfa seed germination
As shown in Table 1, compared with the control (0 mM CaCl2), a calcium salt concentration of 20 mM significantly promoted germination rate (GR%) and germination potential (GP%), increasing them by 13.33 and 11.67%, respectively, while the vigor index (VI) increased by 10%. High concentrations of calcium salt (CaCl2 concentration ≥ 80 mM) had a significant inhibitory effect on the germination index (GI). These results indicate that a calcium salt concentration of 20 mM is optimal for alfalfa seed germination.
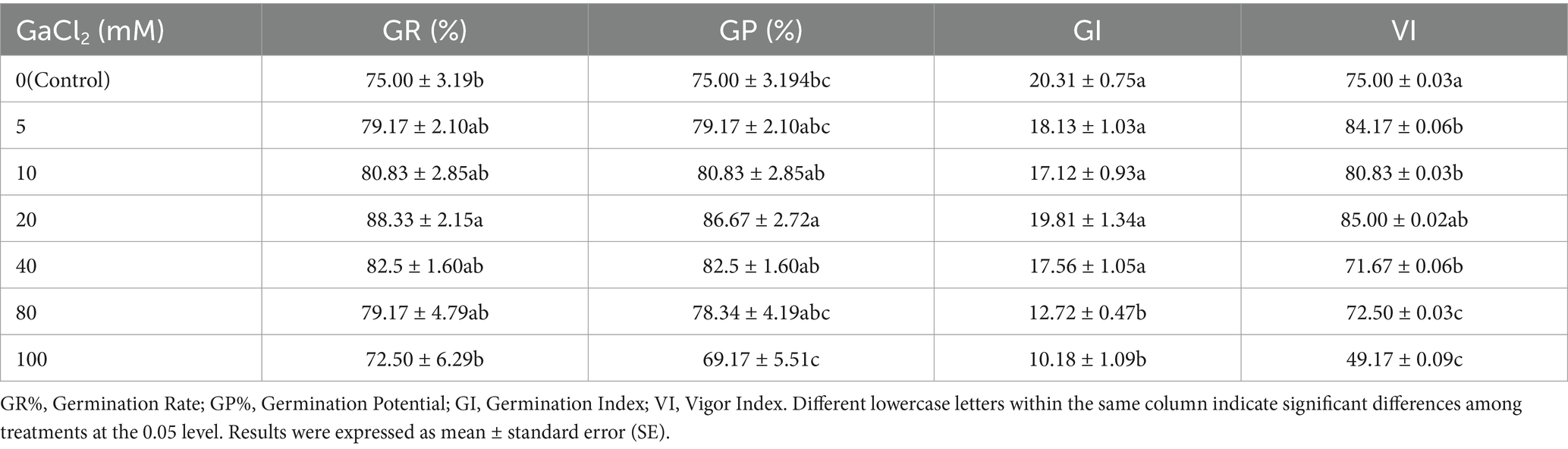
Table 1. Effects of different concentrations of calcium salt stress on alfalfa seed germination (Tukey test).
PEG6000 was used to simulate drought stress at different osmotic potentials. As shown in Table 2, compared to the control (0% PEG6000), a PEG6000 concentration of 20% significantly inhibited all germination indicators. PEG6000 concentrations ≥10% significantly inhibited GI and VI, while concentrations ≥7% significantly inhibited VI. At a PEG6000 concentration of 5%, GR%, GP%, and GI reached their maximum values, increasing by 11.67, 9.17, and 0.99%, respectively, compared to the control. This suggests that a PEG6000 concentration of 5% is most conducive to seed germination.
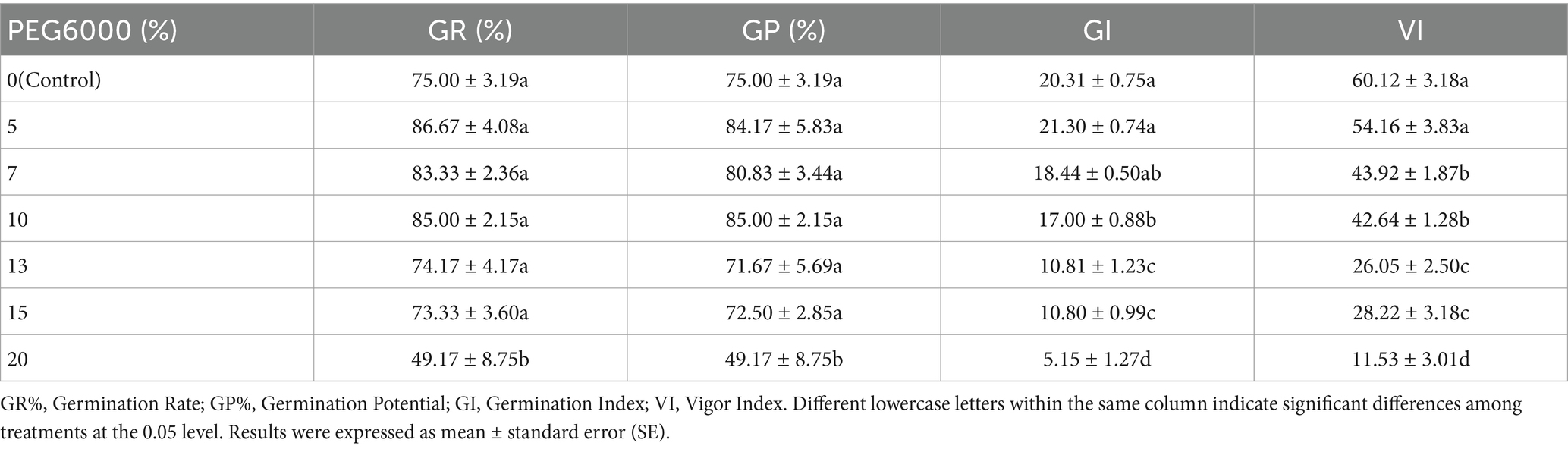
Table 2. Effects of different concentrations of PEG6000 under drought stress on alfalfa seed germination (Tukey test).
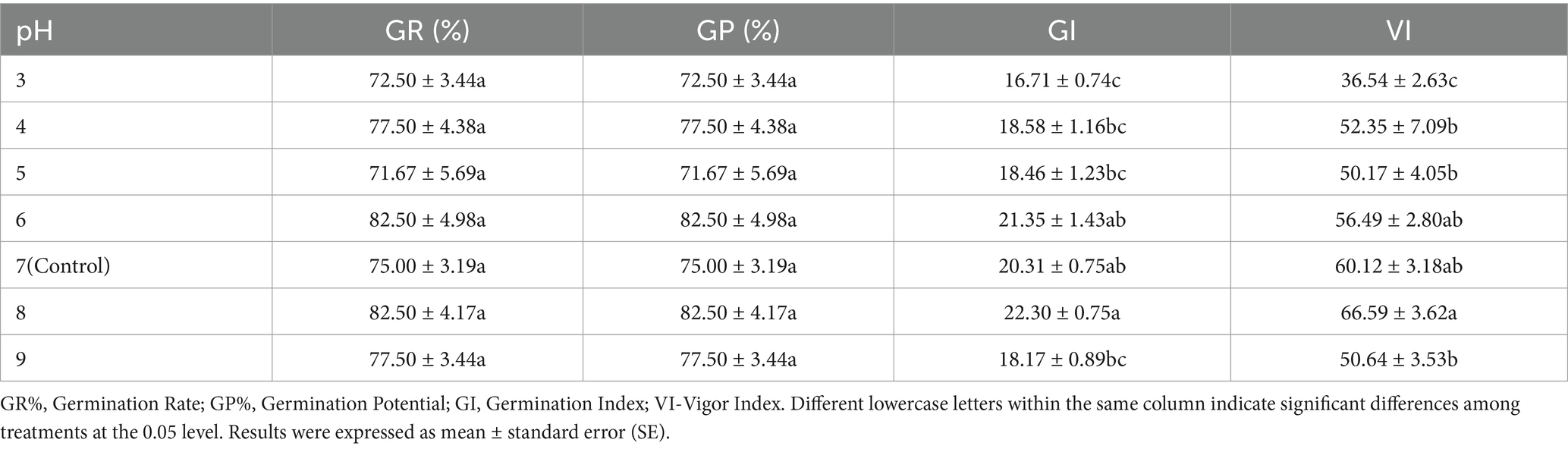
As shown in Table 3, certain pH treatments promoted alfalfa seed germination. At pH 6, GR%, GP%, and GI were enhanced compared to the control (pH 7), although VI was slightly inhibited. At pH 8, all germination index values increased; compared to the control, GR% and GP% increased by 10%, GI increased by 1.99%, and VI increased by 6.47%. Therefore, pH 8 appears to be the optimal pH for seed germination.
Based on the analyses of the effects of calcium salt, drought, and pH treatments on alfalfa seed germination, it was determined that a 20 mM CaCl2 solution is most beneficial for seed germination. Additionally, low concentrations of polyethylene glycol (5% PEG6000) and distilled water adjusted to pH 8 can effectively promote seed germination.
3.2 Effects of different stresses on alfalfa seedlings
Figure 1 and Supplementary Table S1 show that calcium salt stress inhibits the elongation of both the shoot and the root. The root is more sensitive to calcium salt stress than the shoot, with high concentrations of calcium salt (80 mM) having a significant negative impact on radicle growth.
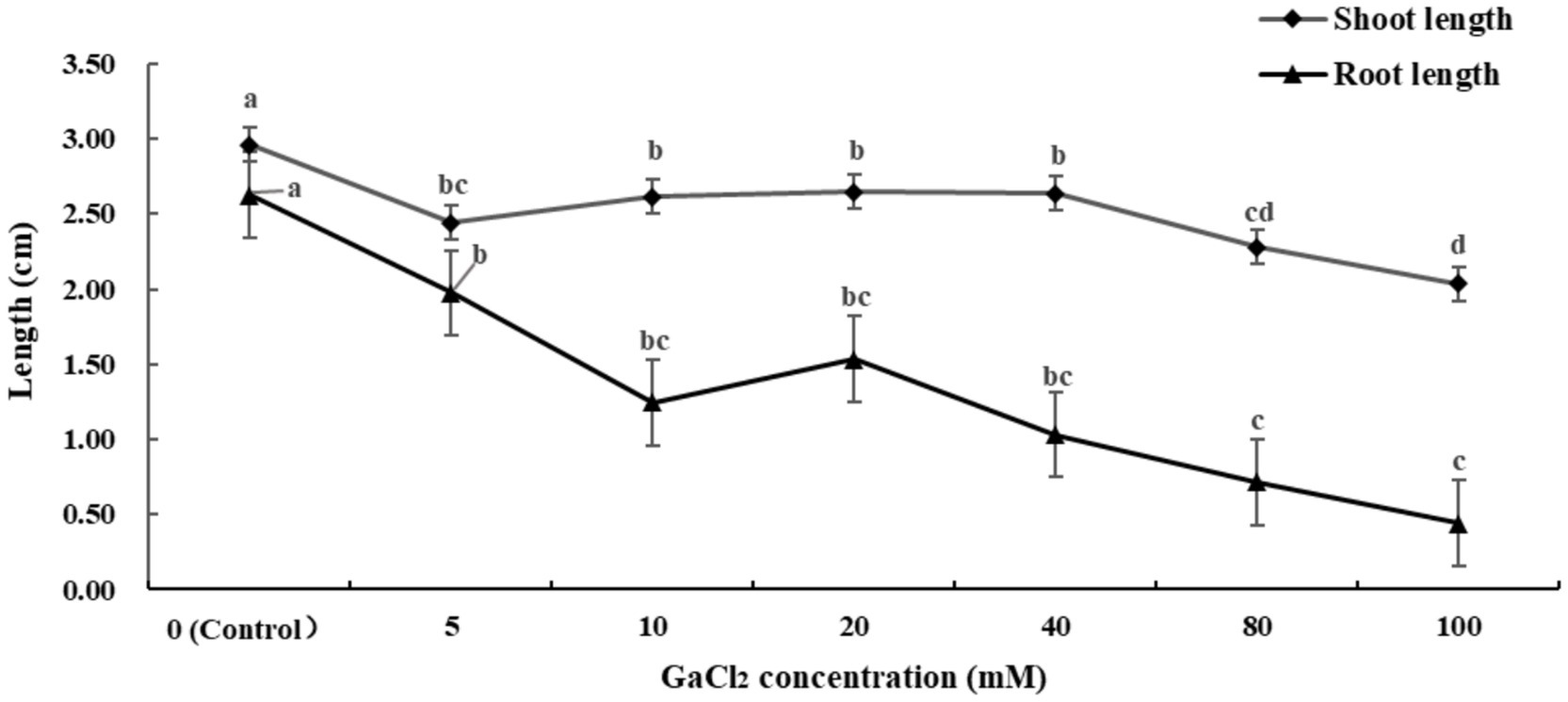
Figure 1. Effects of different concentrations of calcium salt stress on alfalfa seedlings (Tukey test).
As presented in Figure 2 and Supplementary Table S2, the growth of alfalfa shoots and roots is less affected by fluctuations in drought stress. Low concentrations of PEG6000 promote the growth of roots, while high concentrations have the opposite effect. When the PEG6000 concentration was 5 and 7%, root length increased by 17.5 and 8.75%, respectively, compared to the control. Higher concentration treatments had inhibitory effects on root growth, but the differences were not significant compared to the control.
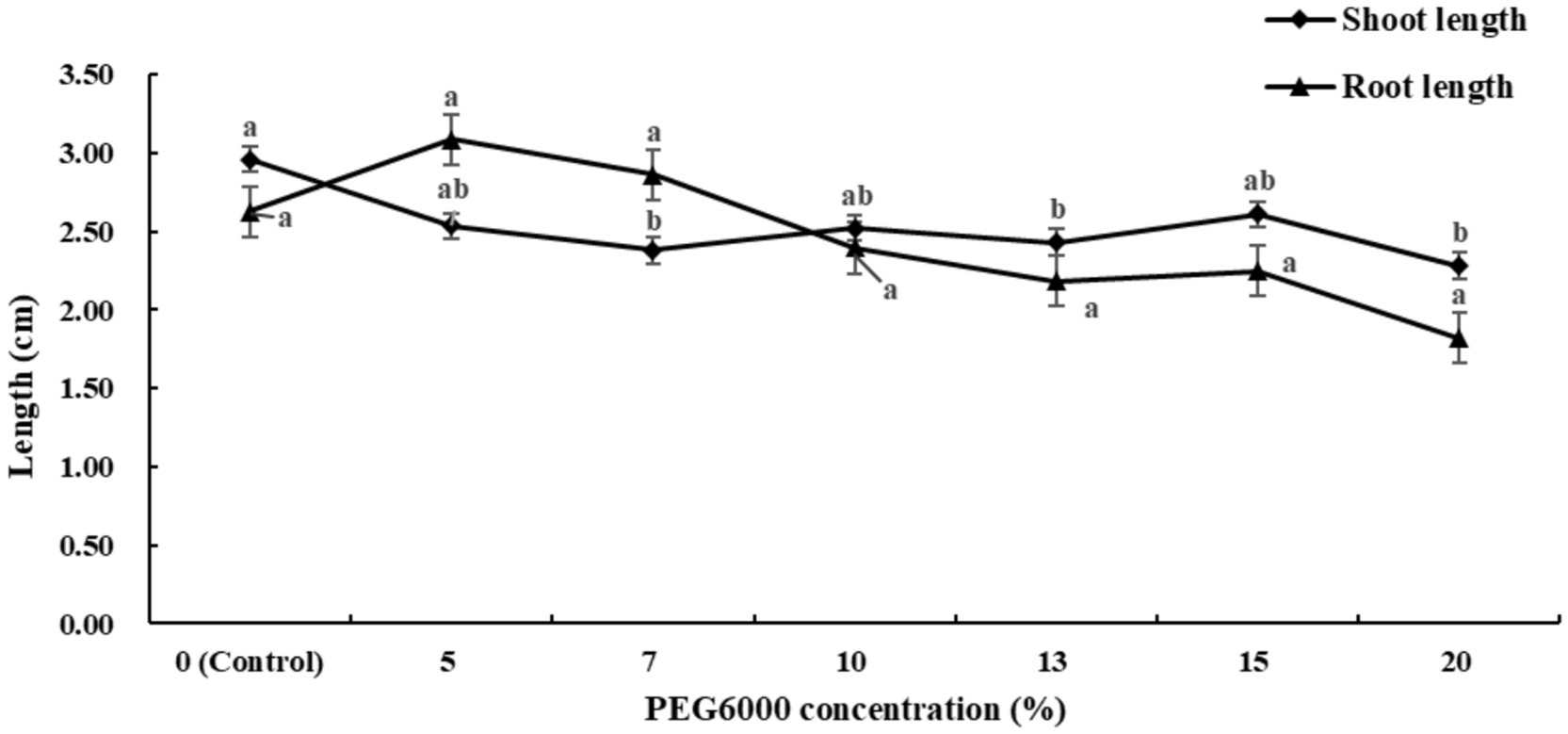
Figure 2. Effects of different concentrations of PEG6000 drought stress on alfalfa seedlings (Tukey test).
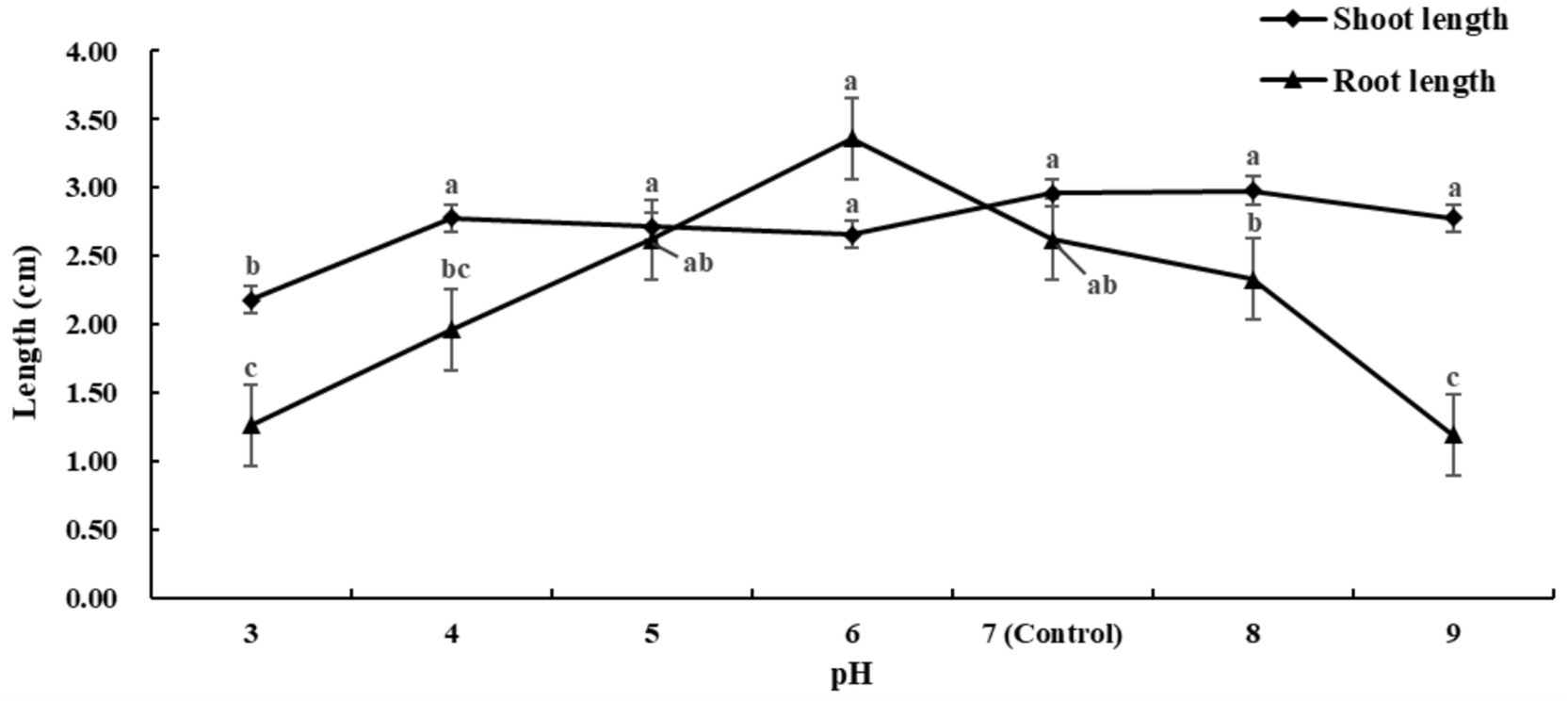
Figure 3 and Supplementary Table S3 indicate that roots are more susceptible to pH changes. At pH 8, shoot growth was promoted, with a 0.68% increase in shoot length compared to the control (pH 7). At pH 6, root growth was more significantly promoted than with 5% PEG6000, showing a 27.76% increase compared to the control. The data show no significant difference in shoot length between the pH 4–6 and pH 8–9 groups compared to the control, nor in root length between the pH 5–6 and pH 8 groups.
Based on the effects of calcium salt stress, drought stress, and pH stress on the growth of alfalfa shoots and roots, we conclude that pH 8 promotes shoot growth, while low concentrations of PEG6000 (5 and 7%) and distilled water at pH 6 promote root growth, with pH 6 having a more significant effect on roots.
3.3 Effects of different stresses on biomass and tissue moisture content of alfalfa seedlings
The changes in biomass and tissue moisture content of ‘Bara 416WET’ seedlings under different concentrations of calcium stress are shown in Figure 4. As the concentration of calcium stress increased, the fresh weight, dry weight, and tissue moisture content of alfalfa seedlings showed a trend of first decreasing, then increasing, and then decreasing again. Among the experimental groups, the fresh weight and dry weight reached their maximum values at 40 mM CaCl2, increasing by 18.79 and 18.18%, respectively, compared to the control. The tissue moisture content decreased slightly by 0.17% compared to the control.
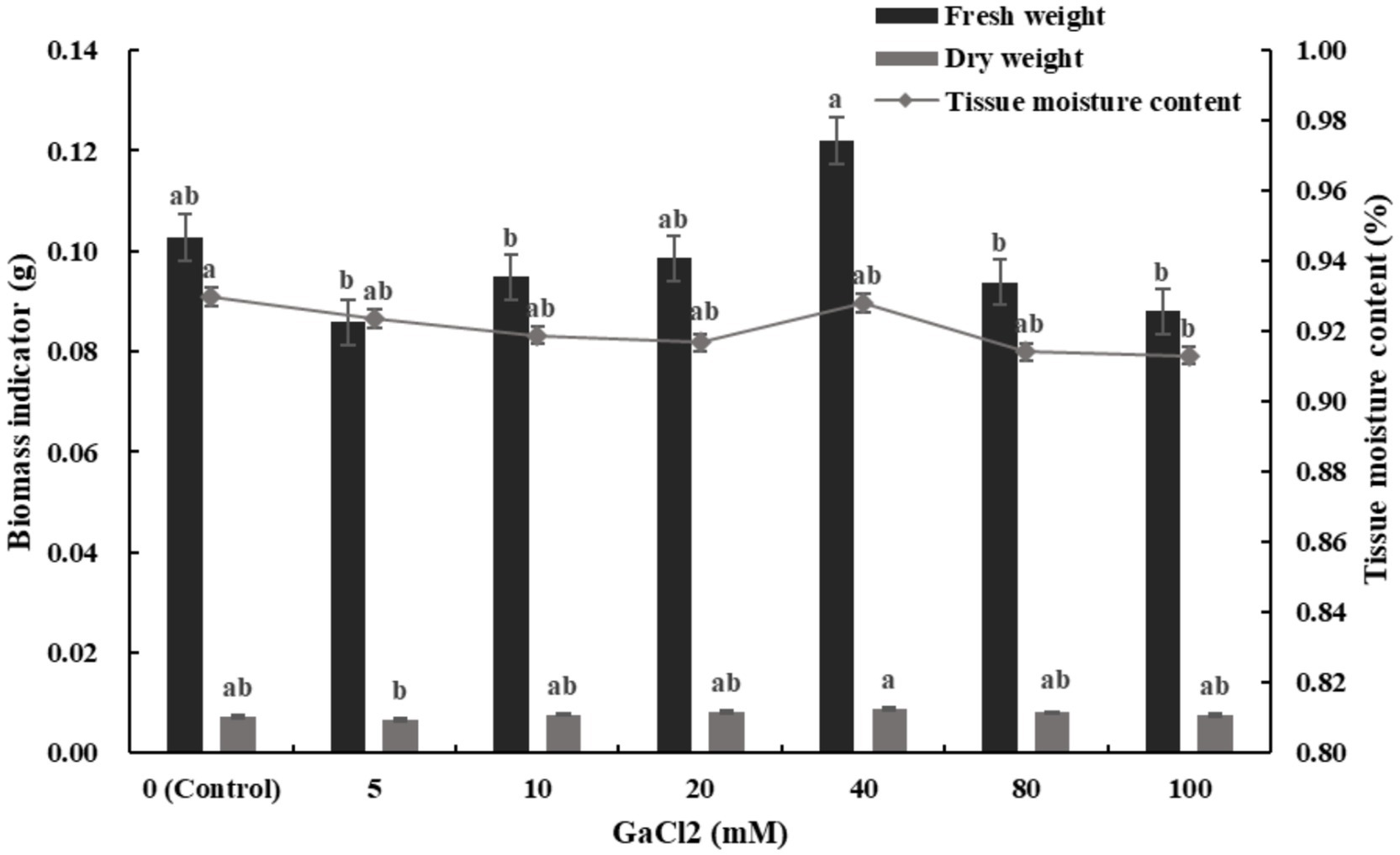
Figure 4. Effects of different concentrations of calcium salt stress on biomass and tissue moisture content of alfalfa seedlings (Tukey test).
Figure 5 shows the changes in biomass and tissue moisture content of alfalfa seedlings under different levels of drought stress. Under various concentrations of PEG6000, the fresh weight and tissue moisture content were lower than the control, but the dry weight was slightly higher. At 10% PEG6000, the dry weight reached its maximum value, increasing by 17.24% compared to the control. Correspondingly, the fresh weight and tissue moisture content decreased by 25.43 and 2.96%, respectively.
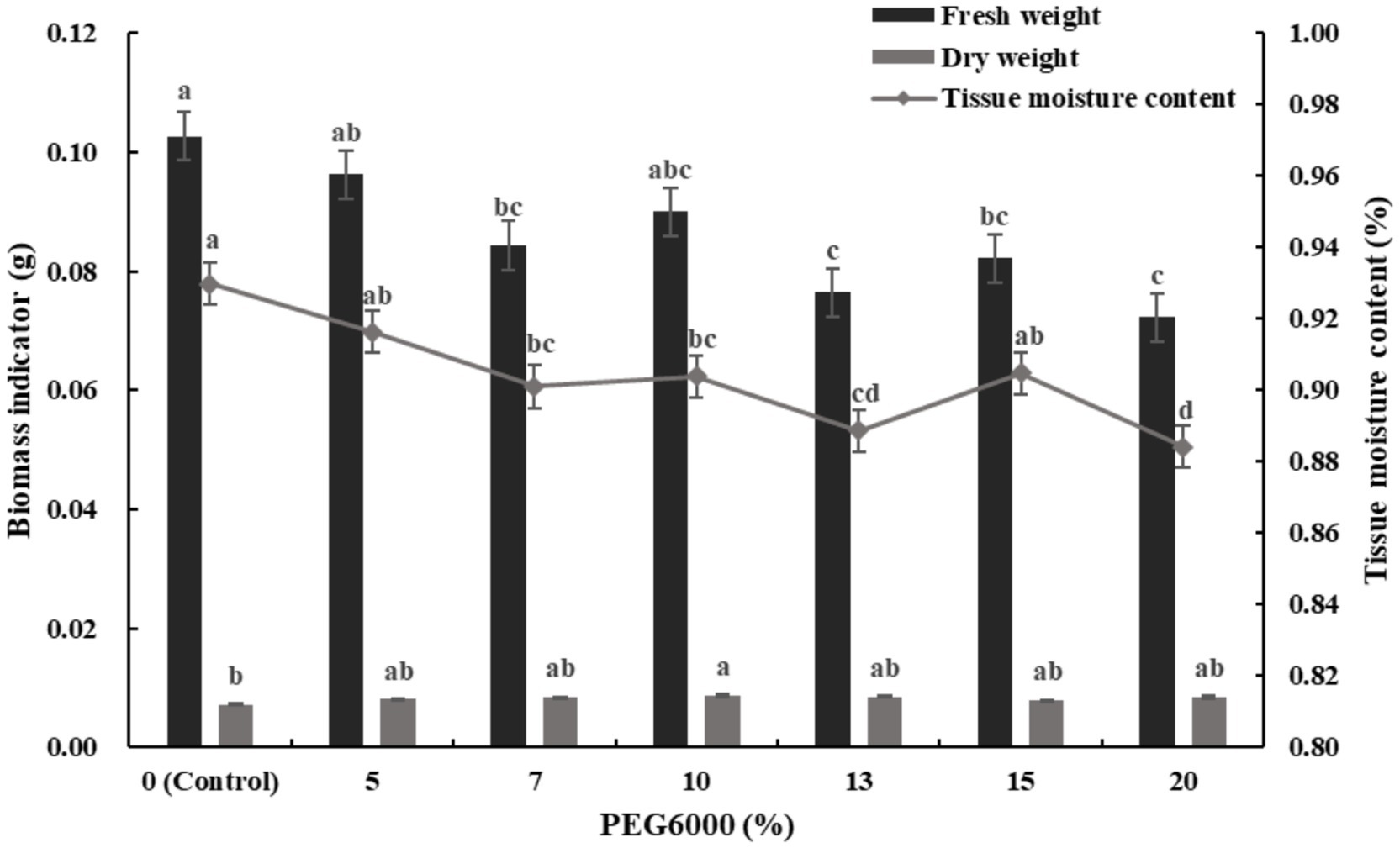
Figure 5. Effects of different concentrations of PEG6000 drought stress on biomass and tissue moisture content of alfalfa seedlings (Tukey test).
Figure 6 illustrates the changes in seedling biomass and tissue moisture content under different pH levels. At pH 9, both dry weight and fresh weight reached their maximum increases of 5.26 and 15.54%, respectively, relative to the control. The corresponding tissue moisture content was relatively high, increasing by 0.72%, only 0.05% lower than the maximum value of 93.7% observed at pH 6. The fresh weight at pH 6 increased by 7.14% compared to the control (pH 7).
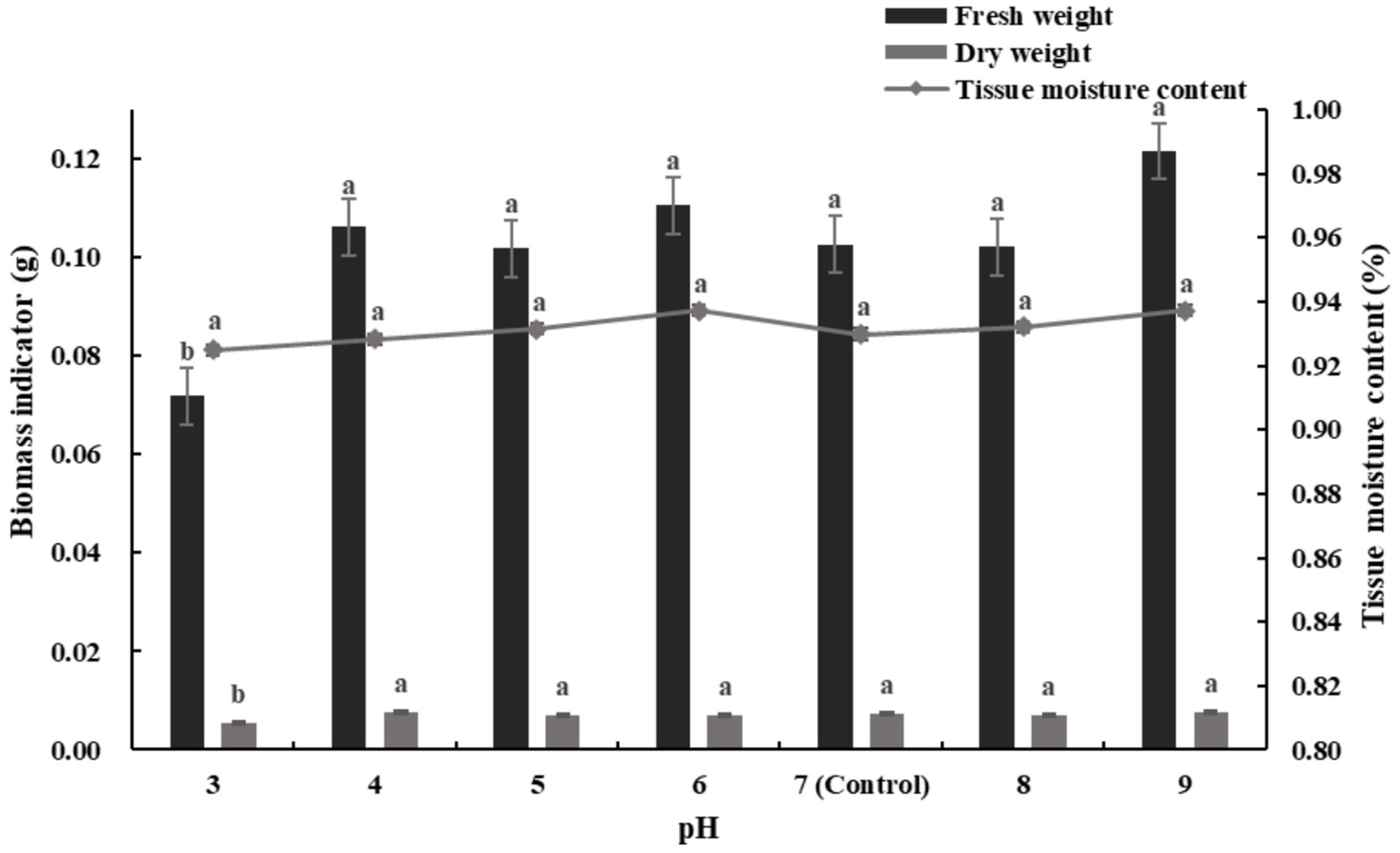
Figure 6. Effects of different pH stresses on biomass and tissue moisture content of alfalfa seedlings (Tukey test).
3.4 Correlation analysis between germination and seedling indicators under different stresses
Figure 7 shows the correlation between alfalfa seed germination and various seedling indicators under calcium salt stress. CaCl2 concentration was significantly negatively correlated with GI, VI, shoot length, and root length, which is consistent with the results shown in Table 1 and Figure 1. Additionally, there was no significant correlation between calcium concentration and GP%, GR%, tissue water content, fresh weight, and dry weight. The correlation analysis between drought stress and various indicators during the germination stage is shown in Figure 8. The PEG6000 concentration was significantly negatively correlated with GP%, GR%, GI, VI, fresh weight, tissue moisture content, and shoot length. Figure 9 presents the correlation analysis between germination and seedling growth indicators under pH stress. The pH was significantly positively correlated with GI, dry weight, and tissue moisture content; it was also significantly positively correlated with VI, fresh weight, and shoot length.
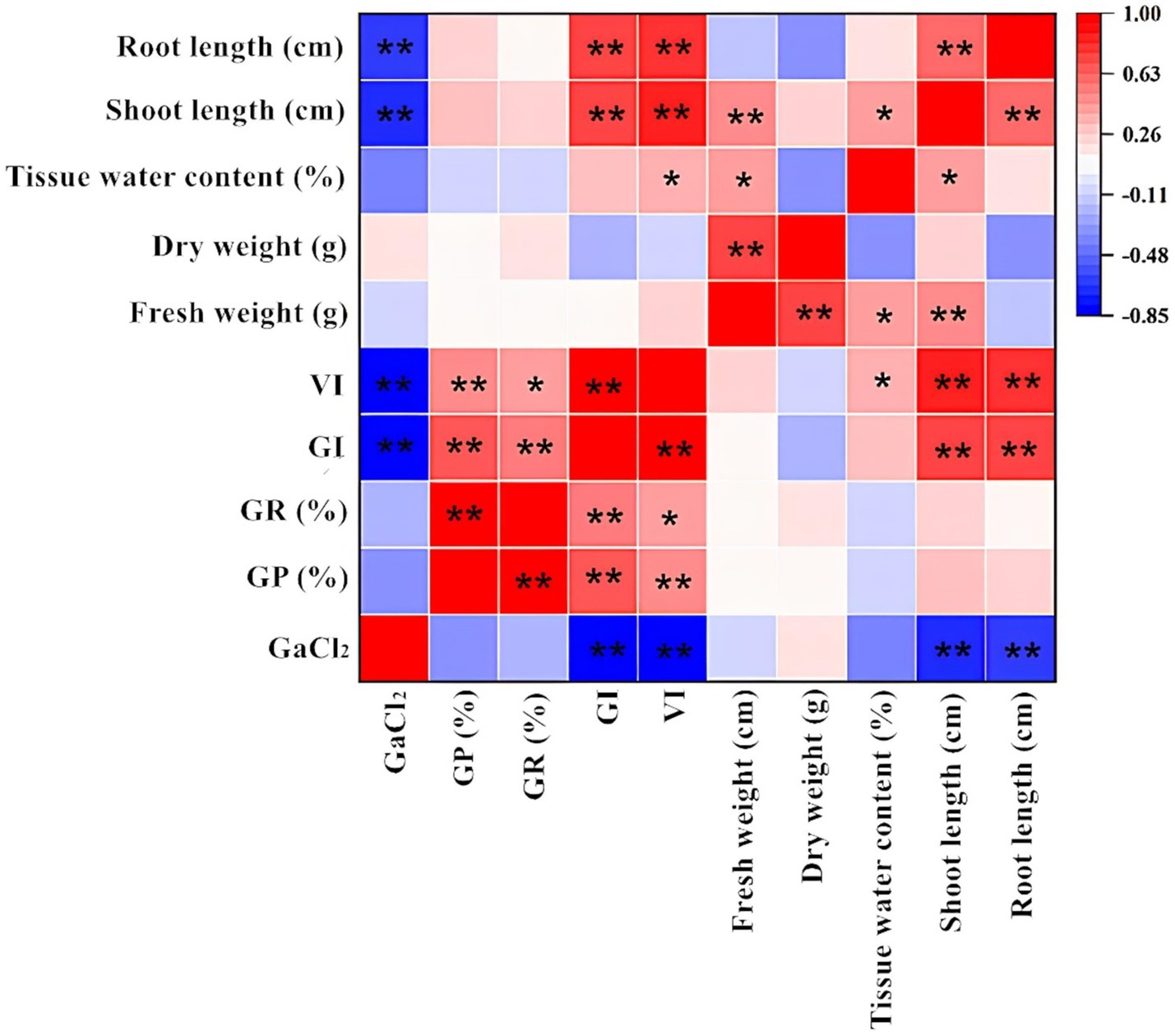
Figure 7. Correlation analysis between germination indicators, seedling morphology indicators, and seedling biomass indicators under calcium salt stress (Tukey test). GR (%), Germination Rate (%); GP (%), Germination Potential (%); GI, Germination Index; VI, Vigour Index. The “*” indicates significant differences between treatments at the 0.05 level, “**” indicates a highly significant difference between treatments at the 0.01 level in the figure.
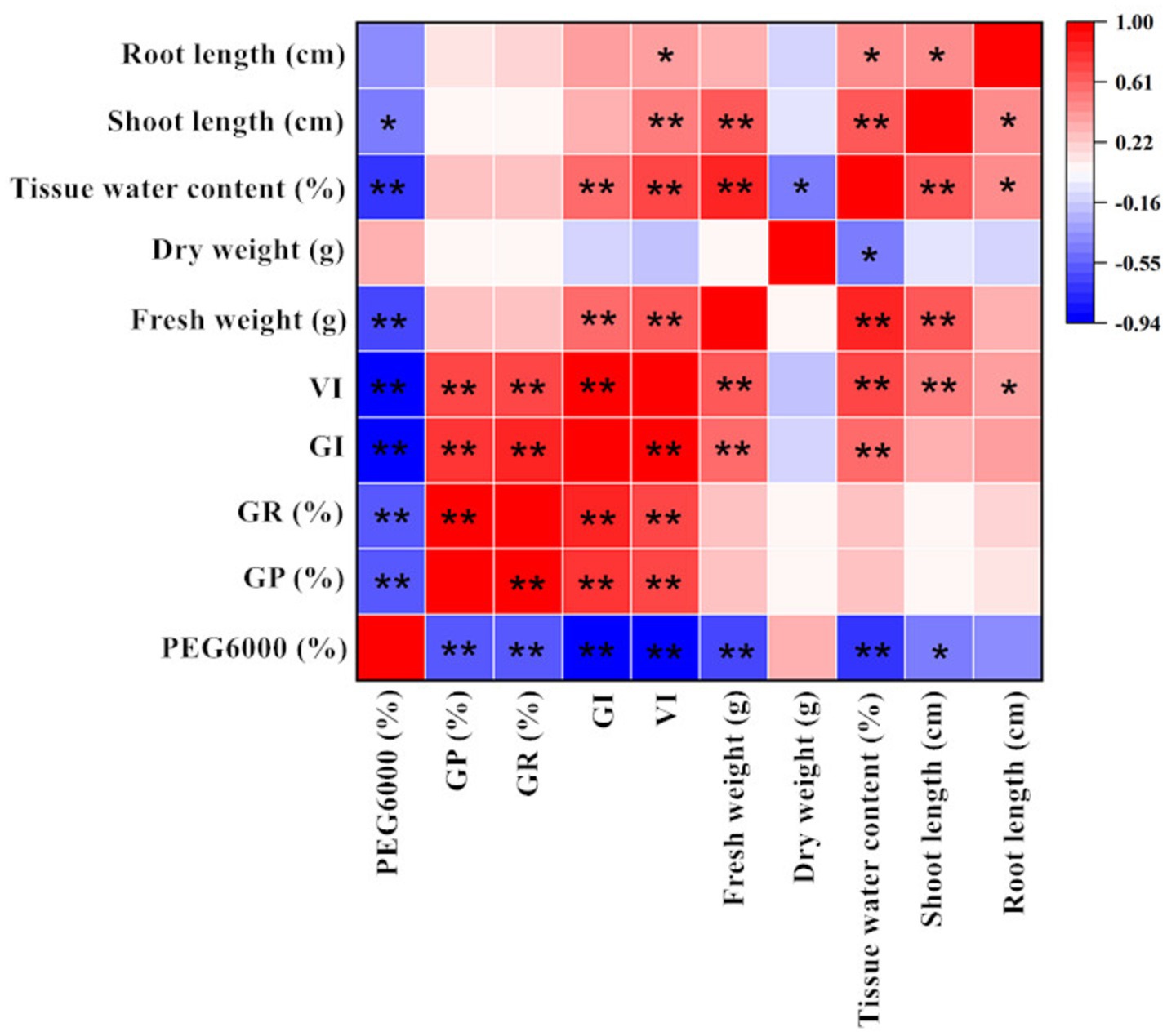
Figure 8. Correlation analysis between germination indicators, seedling morphology indicators, and seedling biomass indicators under drought stress (Tukey test). GR (%), Germination Rate (%); GP (%), Germination Potential (%); GI-Germination Index; VI-Vigour Index. The “*” indicates significant differences between treatments at the 0.05 level, “**” indicates a highly significant difference between treatments at the 0.01 level in the figure.
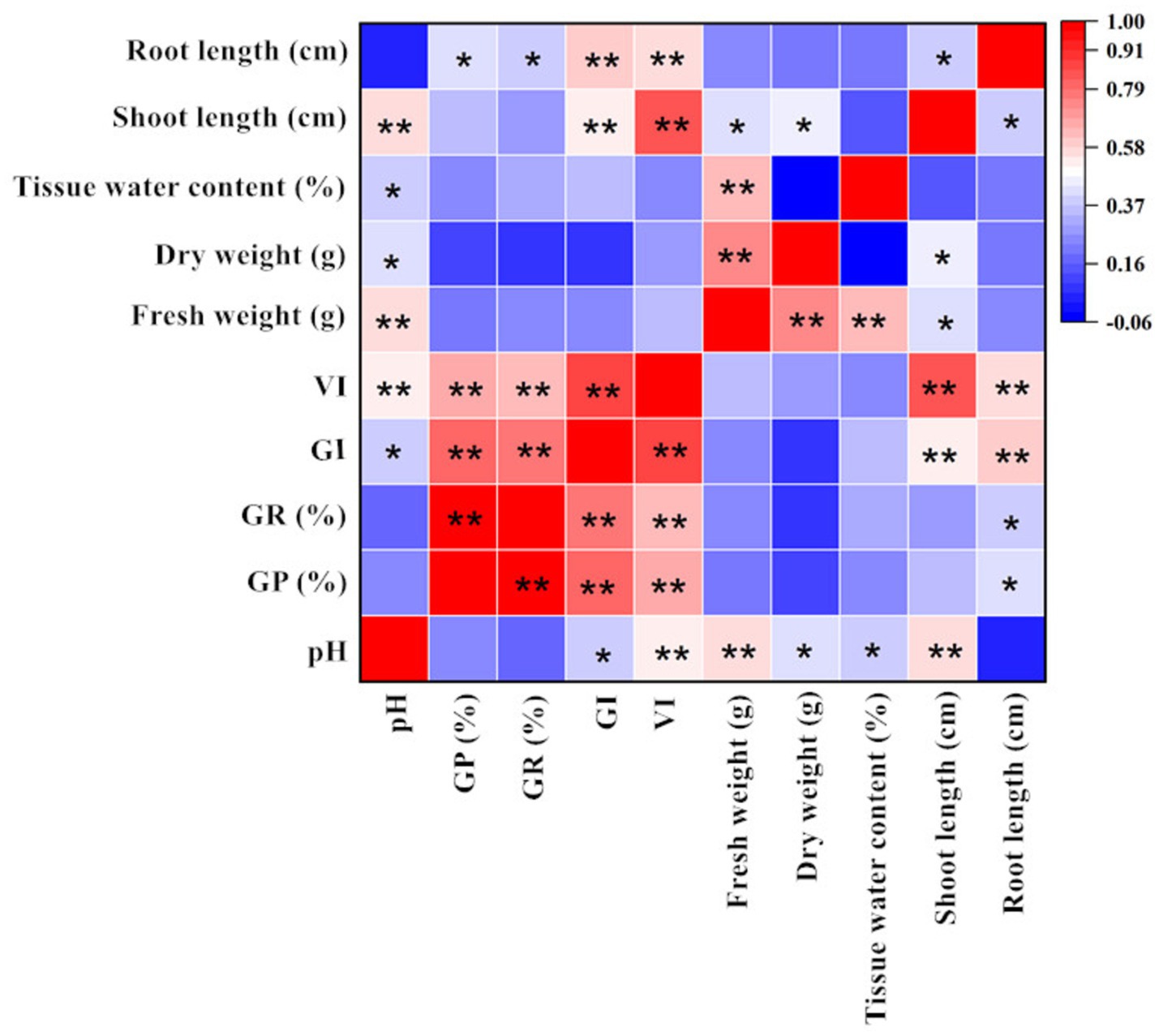
Figure 9. Correlation analysis between germination indicators, seedling morphology indicators, and seedling biomass indicators under pH stress (Tukey test). GR (%)-Germination Rate (%); GP (%)-Germination Potential (%); GI-Germination Index; VI-Vigour Index. The “*” indicates significant differences between treatments at the 0.05 level, “**” indicates a highly significant difference between treatments at the 0.01 level in the figure.
It is noteworthy that under calcium salt, drought, and pH stress, there was no significant correlation between root length and fresh weight or dry weight. In contrast, shoot length was highly or significantly correlated with fresh weight across all treatments and was significantly correlated with dry weight only under pH stress. These results confirm the consistency of the data analysis presented in Sections 3.2 and 3.3. The correlation matrix graphs indicate a certain degree of correlation between each indicator, resulting in overlapping growth effects. Therefore, evaluating the effects of different treatments on alfalfa seed germination and seedling growth should be based on multiple indicators to ensure reliability.
4 Discussion
Alfalfa, known for its high nutritional and ecological value, plays an important role in the development of animal husbandry and the improvement of ecological environments in Southwest China. The calcium-rich, alkaline, and arid karst soils characteristic of this region are significant stress factors that limit plant growth. Generally, plant seeds are more sensitive during germination and seedling stages and are highly susceptible to adverse environmental stresses. Therefore, many studies on stress and adversity have focused on seed germination and seedling stages (Gao et al., 2024; Seleiman et al., 2024; Long et al., 2024).
4.1 Germination and seedling growth of alfalfa under calcium salt stress
An appropriate concentration of CaCl2 solution can significantly improve seed germination rate, germination vigor, and vigor index, positively impacting seed germination quality. Our results showed that germination indicators of alfalfa first increased and then decreased with the rise in calcium ion concentration. This may be because low salt concentrations promote water absorption to some extent, accelerating the seed germination process (Cornacchione and Suarez, 2017). However, when the salt concentration exceeds a certain threshold, osmotic stress occurs, making it difficult for cells to absorb water. Excessive ions entering the cells can cause toxicity, leading to membrane damage, decreased seed vitality, and even cell death (Carillo et al., 2019).
The experimental results indicated that calcium salt stress inhibited the growth of both the aboveground parts and root systems of alfalfa, with a significantly greater effect on root length than on seedling height. Wang et al. (2023) also found in their studies on salt tolerance evaluation of alfalfa varieties during germination that embryonic root length was more sensitive to salt stress than shoot length. Under moderate calcium stress (40 mM), the biomass and tissue water content of alfalfa reached their peak, while other calcium stress concentrations caused a relatively small decrease in these parameters, with no significant difference compared to the control (Supplementary Table S4). This suggests that this alfalfa variety can maintain a high growth rate and biomass accumulation in calcium-rich environments, demonstrating a strong tolerance to calcium salts. Zhou et al. (2023) reported that varieties with stronger salt tolerance might possess better salt regulation mechanisms, such as more efficient salt excretion or ion balance regulation.
4.2 Germination and seedling growth under drought stress
The ability of plants to maintain good water status and effectively utilize available resources is crucial for their growth and survival in water-scarce environments. Our study revealed that alfalfa seeds showed no significant difference in germination rate and vigor under PEG6000 concentrations ranging from 5 to 15%. However, at a PEG6000 concentration of 20%, the germination rate significantly decreased, consistent with the findings of Liu et al. (2018). The germination index and vigor index under mild drought (5% PEG6000) did not differ significantly from those under adequate water conditions. In fact, the germination rate under 5 to 10% PEG6000 was higher than that under the control treatment, aligning with the research of Yu et al. on alfalfa germination under certain drought stress (Yu et al., 2012). Slight differences in drought resistance among different varieties were observed.
Our study also showed that drought stress reduced the growth of alfalfa shoots and had a “low promotion and high inhibition” effect on root growth. Low concentrations of drought stress (5 and 7% PEG6000) promoted root growth, while higher concentrations inhibited it. Although root growth was not significantly affected compared to the control, root length decreased with increasing PEG6000 concentration (Supplementary Table S2). This may be due to the enhanced absorption capacity of the root system under water deficit conditions (Huang et al., 2024), leading to an increased root-to-shoot ratio (Liu et al., 2004; Turner and Begg, 1981). Han Zhishun et al. also found in their study on the effects of drought stress on the morphological and physiological characteristics of different alfalfa varieties that root length first increased and then decreased (Han et al., 2020). Interestingly, under water-deficient conditions, the fresh weight and tissue moisture content of alfalfa seedlings were lower than those under adequate water, but the dry weight increased. This indicates that while the overall growth of alfalfa seedlings is inhibited under drought stress, they exhibit strong tolerance to water-deficient environments.
4.3 Germination and seedling growth under pH stress
Our results indicate that extreme pH values inhibit alfalfa germination, possibly due to high acidity or alkalinity damaging cell membranes, causing cellular system disorders, and inhibiting germination. Alfalfa seeds exhibited good tolerance to both acidic and alkaline conditions, with distilled water at pH 6 and pH 8 promoting germination. We found that pH 4–9 had no significant effect on the aboveground parts of seedlings, while the roots were more sensitive to pH changes. Weakly acidic conditions (pH 6) were beneficial for root elongation, whereas weakly alkaline conditions (pH 8) slightly inhibited root growth.
Alkaline stress had no significant effect on the fresh weight, dry weight, and tissue moisture content of alfalfa seedlings. The key for plants to cope with high pH lies in the regulatory role of the root system, mainly reflected in the synthesis and secretion of organic acids. Plants synthesize organic acids by absorbing large amounts of inorganic anions (Cl−, SO42−, NO3−) to balance cation accumulation, maintaining ion balance and pH stability (Liu and Shi, 2010). Organic acids serve as precursors for the synthesis of many compounds and are important intermediates in substance and energy metabolism, playing crucial roles in plant responses to environmental changes (José et al., 2000). Additionally, research has shown that the regulation of organic acid metabolism may play different roles under different types of stress (Chen et al., 2011).
5 Conclusion
This study simulated the effects of karst soil stress-including high calcium stress, drought stress, and pH stress-on the germination and seedling growth of alfalfa cultivar ‘Bara 416WET’. The results indicate that a 5% PEG6000 solution and distilled water adjusted to pH 8 can effectively promote alfalfa seed germination, but the optimal treatment is a 20 mM CaCl2 solution. Seedlings were more sensitive to the three types of stress, especially the roots. Under moderate calcium salt stress (40 mM), alfalfa showed only a slight decrease in tissue water content. Under moderate drought stress (10% PEG6000), dry weight increased significantly, but fresh weight and tissue moisture content decreased notably. In an alkaline environment (pH 9), biomass indicators and tissue moisture content increased to varying degrees. These results provide a theoretical foundation for the early identification and selection of forage varieties with tolerance to calcium, drought, and pH stresses, offering valuable insights for improving forage cultivation in karst regions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZZ: Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. JL: Methodology, Writing – review & editing, Conceptualization, Investigation. YY: Formal analysis, Supervision, Writing – review & editing. YG: Supervision, Writing – review & editing. XW: Investigation, Writing – review & editing. HH: Writing – review & editing, Supervision, Conceptualization. RW: Writing – review & editing, Supervision, Conceptualization. WP: Formal analysis, Funding acquisition, Software, Writing – review & editing. LZ: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of China (32260351, 32260351) and Guizhou provincial science and technology projects of China (Qiankehejichu-ZK[2024]yiban446, Qiankehepingtairencai-GCC[2022]022-1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2024.1510596/full#supplementary-material
References
Bagavathiannan, M. V., and Van Acker, R. C. (2009). The biology and ecology of feral alfalfa (Medicago sativa L.) and its implications for novel trait confinement in North America. Crit. Rev. Plant Sci. 28, 69–87. doi: 10.1080/07352680902753613
Carillo, P., Cirillo, C., Veronica, D. M., Arena, C., Stefania, D. P., and Rouphael, Y. (2019). Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis wild. Trained to different canopy shapes. Agr. Water Manage. 212, 12–22. doi: 10.1016/j.agwat.2018.08.037
Chen, W., Feng, C., Guo, W., Shi, D., and Yang, C. (2011). Comparative effects of osmotic-, salt-and alkali stress on growth, photosynthesis, and osmotic adjustment of cotton plants. Photosynthetica 49, 417–425. doi: 10.1007/s11099-011-0050-y
Cornacchione, M. V., and Suarez, D. L. (2017). Evaluation of alfalfa (Medicago sativa L.) populations’ response to salinity stress. Crop. Sci. 57, 137–150. doi: 10.2135/cropsci2016.05.0371
Dayod, M., Tyerman, S. D., Leigh, R. A., and Gilliham, M. (2010). Calcium storage in plants and the implications for calcium biofortification. Protoplasma 247, 215–231. doi: 10.1007/s00709-010-0182-0
Deng, Y., Wang, S., Bai, X., Tian, Y., Wu, L., Xiao, J., et al. (2018). Relationship among land surface temperature and LUCC, NDVI in typical karst area. Sci. Rep. 8:641. doi: 10.1038/s41598-017-19088-x
Dodd, A. N., Kudla, J., and Sanders, D. (2010). The language of calcium signaling. Annu. Rev. Plant Biol. 61, 593–620. doi: 10.1146/annurev-arplant-070109-104628
Gao, Y., Zhang, Y., Wang, P., and Zhao, L. (2024). Structure and diversity of endophytic bacteria in maize seeds and germinating roots. Microorganisms 12:1348. doi: 10.3390/microorganisms12071348
Gilliham, M., Dayod, M., Hocking, B., Xu, B., Conn, S. J., Kaiser, B. N., et al. (2011). Calcium delivery and storage in plant leaves: exploring the link with water flow. J. Exp. Bot. 62, 2233–2250. doi: 10.1093/jxb/err111
Han, Z., Zhen, M., Liang, X., Kang, J., and Chen, Y. (2020). Effects of drought stress on morphological and physiologicalcharacteristics of different alfalfa cultivars. Chin. J. Grassland 42, 37–43. doi: 10.16742/j.zgcdxb.20190177
Huang, H., Wang, X., Li, J., Gao, Y., Yang, Y., Wang, R., et al. (2024). Trends and directions in oat research under drought and salt stress: a bibliometric analysis (1993-2023). Plan. Theory 13:1902. doi: 10.3390/plants13141902
Huang, L., Zhao, X., Sun, X., Zhao, L., and Wang, P. (2022). Differential physiological, transcriptomic, and metabolomic responses of Paspalum wettsteinii under high-temperature stress. Front. Plant Sci. 13:865608. doi: 10.3389/fpls.2022.865608
José, L. B., Nieto-Jacobo, M. A. F., Verenice, R. R. R. G., and Lui, S.-E. (2000). Organic acid metabolism in plants: from adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 160, 1–13. doi: 10.1016/S0168-9452(00)00347-2
Li, J., Shi, S., and Zhang, S. (2010). Effects of the pH value of an acid environment on early growth and physiology of Medicago sativa. Acta Agrestia Sinica 19, 47–54. doi: 10.11686/cyxb20100207
Liu, J., Du, J., Wang, Z., Cui, L., and Zhao, Y. (2018). Responses of leaf n and p re-sorption in grasses of three functional groups to fertilization. Chin. J. Grassland 40, 27–34. doi: 10.16742/j.zgcdxb.2018-03-05
Liu, H., Li, F., and Xu, H. (2004). Deficiency of water can enhance root respiration rate of drought-sensitive but not drought-tolerant spring wheat. Agric. Water Manag. 64, 41–48. doi: 10.1016/S0378-3774(03)00143-4
Liu, J., and Shi, D. C. (2010). Photosynthesis, chlorophyll fluorescence, inorganic ion and organic acid accumulations of sunflower in responses to salt and salt-alkaline mixed stress. Photosynthetica 48, 127–134. doi: 10.1007/s11099-010-0017-4
Long, S., Xie, W., Zhao, W., Liu, D., Wang, P., and Zhao, L. (2024). Effects of acid and aluminum stress on seed germination and physiological characteristics of seedling growth in Sophora davidii. Plant Signal. Behav. 19:891. doi: 10.1080/15592324.2024.2328891
Ma, Q., Yue, L., Zhang, J., Wu, G., Bao, A., and Wang, S. (2012). Sodium chloride improves photosynthesis and water status in the succulent xerophyte zygophyllum xanthoxylum. Tree Physiol. 32, 4–13. doi: 10.1093/treephys/tpr098
Prakash, J., Agrawal, S. B., and Agrawal, M. (2024). Elucidating the ramifications of simulated acid rain on palak (Beta vulgaris l.) cultivars: insights from morphological, physiological, biochemical and quality analyses. Water Air Soil Poll. 235:596. doi: 10.1007/s11270-024-07409-6
Quan, W., Liu, X., Wang, H., and Chan, Z. (2016). Comparative physiological and transcriptional analyses of two contrasting drought tolerant alfalfa varieties. Front. Plant Sci. 6:256. doi: 10.3389/fpls.2015.01256
Rao, X., Zhang, Y., Gao, Y., Zhao, L., and Wang, P. (2024). Influence of exogenous abscisic acid on germination and physiological traits of sophora viciifolia seedlings under drought conditions. Appl. Sci. 14:4359. doi: 10.3390/app14114359
Ribis, J. W., Melo, L., Shrestha, S., Giacalone, D., Rodriguez, E. E., Shen, A., et al. (2023). Single-spore germination analyses reveal that calcium released during clostridioides difficile germination functions in a feedforward loop. Msphere 8, e00005–e00023. doi: 10.1128/msphere.00005-23
Ross, H. A., and Hegarty, T. W. (1980). Action of growth regulators on lucerne germination and growth under water stress. New Phytol. 85, 495–501. doi: 10.1111/j.1469-8137.1980.tb00764.x
Sarfraz, R., Priyadarshani, S. V. G. N., Fakhar, A., Khan, M. I., Hassan, Z. U., Pil, J. K., et al. (2024). Unlocking plant defense: exploring the nexus of biochar and Ca2+ signaling. Plant Stress. 14:100584. doi: 10.1016/j.stress.2024.100584
Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M., and Waner, D. (2001). Guard cell signal transduction. Annu. Rev. Plant Physiol. Mol. Biol. 52, 627–658. doi: 10.1146/annurev.arplant.52.1.627
Seleiman, M. F., Ali, N., Nungula, E. Z., Gitari, H. I., Alhammad, B. A., and Battaglia, M. L. (2024). Enhancing germination and seedling growth of barley using plasma-activated water (PAW) with neutralized pH. Cogent Food Agr. 10:162. doi: 10.1080/23311932.2024.2390162
Sim, R. E., Brown, H. E., Teixeira, E. I., and Moot, D. J. (2017). Soil water extraction patterns of lucerne grown on stony soils. Plant Soil 414, 95–112. doi: 10.1007/s11104-016-3112-x
Suwignyo, B., Eprilia, A. R., and Helmiyati, S. (2023). The profile of tropical alfalfa in Indonesia: a review. Saudi J. of Biol. Sci. 30:103504. doi: 10.1016/j.sjbs.2022.103504
Turner, N. C., and Begg, J. E. (1981). Plant-water relations and adaptation to stress. Plant Soil 58, 97–131. doi: 10.1007/BF02180051
Wang, J., Chai, R., Chen, L., Feng, Y., Zhu, M., Ma, H., et al. (2023). Evaluationon salt tolerance of 29 alfalfa cultivars during germination. Acta Agrestia Sinica 31, 2722–2729. doi: 10.11733/j.issn.1007-0435.2023.09.016
Wang, S., Ji, H., Ouyang, Z., Zhou, D., Zhen, L., and Li, T. (1999). Preliminary study on weathering and pedogenesis of carbonate rock. Sci. China Ser. D. 42, 572–581. doi: 10.1007/BF02877784
Wang, S., Leus, L., Lootens, P., Johan, V. H., and Van Labeke, M.-C. (2022). Germination kinetics and chlorophyll fluorescence imaging allow for early detection of alkalinity stress in rhododendron species. Horticulturae 8:823. doi: 10.3390/horticulturae8090823
Wang, X., Yang, J., Gao, Y., Li, J., Yang, Y., and Wang, P. (2024). Allocation, morphology, physiology: multiple aspects of above-and below-ground responses to water table stress, duration of drainage in alpine wetland plants (carex muliensis). Plant Soil. 2024, 1–16. doi: 10.1007/s11104-024-06701-y
Weinl, S., Held, K., Schluecking, K., Steinhorst, L., Kuhlgert, S., Hippler, M., et al. (2008). A plastid protein crucial for Ca2+ regulated stomatal responses. New Phytol. 179, 675–686. doi: 10.1111/j.1469-8137.2008.02492.x
White, P. J., and Broadley, M. R. (2003). Calcium in plants. Ann. Bot. 92, 487–511. doi: 10.1093/aob/mcg164
Wu, S. (2020). Study on the mechanism of calcium, iron and zinc nutrition formation in alfalfa. Master’s thesis. Inner Mongolia University.
Yin, L., Wei, M., Wu, G., and Ren, A. (2022). Epichloë endophytes improved Leymus chinensis tolerance to both neutral and alkali salt stresses. Front. Plant Sci. 13:968774. doi: 10.3389/fpls.2022.968774
Yu, R., Du, X., Chen, C., Zhou, X., and Shi, G. (2012). Effect of PEG stress on seed germination and seeding physiology of three legumes. Agric. Res. Arid Areas. 30, 99–103. doi: 10.19707/j.cnki.jpa.2020.02.004
Yun, Y., Wang, H., Man, B., Xiang, X., Zhou, J., Qiu, X., et al. (2016). The relationship between pH and bacterial communities in a single karst ecosystem and its implication for soil acidification. Front. Microbiol. 7:1955. doi: 10.3389/fmicb.2016.01955
Zhan, N., and Huang, L. (2016). Effects of ca on pollen germination of three species. Silvae Genet. 65, 11–16. doi: 10.1515/sg-2016-0012
Zhao, X., Huang, L., Sun, X., Zhao, L., and Wang, P. (2022). Transcriptomic and metabolomic analyses reveal key metabolites, pathways and candidate genes in Sophora davidii (franch.) skeels seedlings under drought stress. Front. Plant Sci. 13:785702. doi: 10.3389/fpls.2022.785702
Zhou, Z., Li, J., Gao, Y., Wang, X., Wang, R., Huang, H., et al. (2024). Research on drought stress in Medicago sativa L. from 1998 to 2023: a bibliometric analysis. Front. Plant Sci. 15:1406256. doi: 10.3389/fpls.2024.1406256
Keywords: karst adversity, Medicago sativa L., seed germination, environmental stress, alfalfa cultivation
Citation: Zhou Z, Li J, Yang Y, Gao Y, Wang X, Huang H, Wang R, Wang P and Zhao L (2024) Effects of karst environmental stresses on seed germination and seedling growth of alfalfa (Medicago sativa L.). Front. Sustain. Food Syst. 8:1510596. doi: 10.3389/fsufs.2024.1510596
Edited by:
Raju Datla, Global Institute for Food Security (GIFS), CanadaReviewed by:
Marta Joanna Monder, Warsaw University of Life Sciences, PolandAdriana F. Sestras, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Copyright © 2024 Zhou, Li, Yang, Gao, Wang, Huang, Wang, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Puchang Wang, d2FuZ3B1Y2hhbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Zijun Zhou
Zijun Zhou Junqin Li
Junqin Li Yuting Yang1
Yuting Yang1 Yang Gao
Yang Gao Xiangtao Wang
Xiangtao Wang Puchang Wang
Puchang Wang Lili Zhao
Lili Zhao