- 1International Livestock Research Institute (ILRI), Addis Ababa, Ethiopia
- 2International Livestock Research Institute (ILRI), Bamako, Mali
- 3International Livestock Research Institute (ILRI), Ouagadougou, Burkina Faso
- 4Department of Veterinary Medicine, Woldia University, Woldia, Ethiopia
- 5International Livestock Research Institute (ILRI), Nairobi, Kenya
- 6Natural Resources Institute, University of Greenwich, London, United Kingdom
Introduction: Chicken is the most commonly consumed animal source food in street restaurants in Burkina Faso. In most of these restaurants, slaughtering, processing, and cooking practices are carried out under poor hygienic conditions.
Methods: A cross-sectional survey using a semi-structured interview was carried out to assess food safety knowledge, attitude, and hygienic practices of food handlers in street restaurants selling chicken in Burkina Faso’s capital, Ouagadougou. One hundred chicken restaurants were randomly selected, and food handlers were interviewed.
Results and discussion: Most restaurants served eat-in and takeaway chicken (66%); the remaining 34% were takeaway only; restaurants served grilled, flamed or roasted chicken. Only 11% of the food handlers had training on food hygiene and safety. Half the outlets were not regularly inspected by the authorities. Less than half (40%) slaughtered their own chickens at the restaurant: of these 85% bled chickens on bare earth. About 80% cleaned the bleeding surface immediately after slaughter with water but only 20% used water with either soap or disinfectant detergent. Eighty-two percent of them used the same cloth during slaughtering and food preparation stages. Many used the same knife in all stages of the slaughtering process. Two-thirds kept carcasses unrefrigerated at ambient temperature until cooking started. Around a quarter buried slaughter waste on-site whereas 20% disposed of it on the street near the restaurant. Only 20% had taken steps to improve food safety, and about 80% of food handlers stated that cleanliness and hygiene were not important to their customers when choosing where to eat. Almost half (42%) the food handlers continued to work when they were ill. The poor standards of hygiene observed are typical for street food and small-scale eateries in LMICs in Sub Saharan Africa. An integrated approach is required to improve the situation, including staff training, introduction of food-grade equipment and appropriate technology, behavior-change approaches, as well as worker and consumer awareness campaigns on good food safety practices. However, significant, sustained improvement in food safety will also require major upgrading of infrastructure and facilities including power and water supply, and cold chain.
1 Introduction
Across the world chicken is a widely consumed animal source food and poultry value chains are an important source of income and nutrition, particularly in low- and middle-income countries (LMICs) where production and consumption are increasing rapidly (Wong et al., 2017). However, poultry products are among those most often implicated in foodborne disease (FBD) outbreaks resulting in severe disease burden and economic losses (Thung et al., 2016; Hoffmann et al., 2017; Li et al., 2019; Mezali et al., 2019; Mpundu et al., 2019; Sapp et al., 2022; van Wagenberg and Havelaar, 2023).
The World Bank has estimated that the economic cost of FBD in LMICs at $95.2 billion per year from lost human capital (productivity), with additional costs of treating FBD at least $15 billion (Jaffee et al., 2019; Grace, 2023). These financial burdens are highest in larger, middle-income nations such as South Africa, Nigeria, and Egypt, but are also significant in other countries. African countries bear a greater per capita burden of foodborne diseases than other LMICs (Havelaar et al., 2015; Grace, 2023). This high burden has been linked to poor food handling and sanitation practices, inadequate food safety regulations, lack of financial resources to invest in safer equipment, and inadequate food-handler education; factors that jeopardize the health and nutritional status of the most vulnerable groups in those countries (Grace, 2015; Hoffmann et al., 2019). Given that most foods in LMICs are purchased in informal, traditional food systems, much the burden of FBD in LMICs is also linked to these markets (Havelaar et al., 2022).
In Burkina Faso, the poultry sector is important and growing. More than 98% of the poultry produced and consumed in the country comes from traditional systems (Schneider et al., 2010; Dione et al., 2021). The demand for poultry products in the country outstrips supply and is likely to expand with growth in population, income, and urbanization (Hoffmann et al., 2019). With this increase, the FBD burden will likely increase, unless measures are taken.
In 2017 it is estimated that at least 400,000 people, or 1 out of 50 in the total population of Burkina Faso fell ill from consuming poultry meat contaminated with Campylobacter spp. and non-typhoidal Salmonella spp., resulting in a loss of 42,600 Disability Adjusted Life Years (DALYs: one lost DALY is the equivalent of one lost year of healthy life) (Havelaar et al., 2022). The economic costs associated with just three foodborne diseases in Burkina Faso were substantial (3.0% of Gross National Income (GNI)) (van Wagenberg and Havelaar, 2023).
Chicken is the primary animal source food consumed in Ouagadougou street restaurants, with consumption in this setting being part of day-to-day life, and greater than home consumption. More than three quarters of poultry is consumed at street restaurants (called ‘maquis’) (Somda et al., 2018). In most of these restaurants, slaughtering, processing and cooking practices are carried out with poor hygienic conditions, leading to concern among market actors and consumers (Dione et al., 2021). Most customers are men or young women, with ready-to-eat chicken taken back to the home for the family. In Ouagadougou chicken was consumed on most days by middle- and high-income men, with low-income households only consuming chicken at special celebrations every few months.
However, major hygiene issues have been identified in these outlets. Poultry products on sale in Ouagadougou are heavily contaminated with diarrheagenic Escherichia coli, Salmonella spp. and Campylobacter spp. Cross-contamination is common during food processing and preparation, increasing the risk of consumer exposure to agents of FBD (Kagambèga et al., 2018; Somda et al., 2018).
Food handlers can be a major source of food contamination, particularly for ready-to-eat food, such as that served in restaurants or sold for takeaway (Ncube et al., 2020). To develop interventions to improve chicken restaurant food safety we must better understand the hygienic status, worker knowledge and perceptions towards food safety. This study was performed as part of the Pull-Push project1 to identify options for interventions to improve food safety, that could then be further investigated.
2 Materials and methods
2.1 Survey instrument and questionnaire administration
A questionnaire was developed and uploaded to note-pad mobile devices installed with open data kit (ODK) software. Trained enumerators collected data from 100 randomly selected restaurants, interviewing owners or employees. The questionnaire consisted of several questions grouped into three major sections. The first part covered background information of the participant and their working environment (demographic, regulatory, sources of live chickens, and other relevant information). The next section covered process hygiene, focusing on the participants’ slaughtering, personal hygiene, and cooking practices. The last section of the questionnaire was on personal knowledge and perception of participants towards food safety in general, and chicken handling in particular. Prior to the study, the questionnaire was piloted with 8 food handlers.
Each enumerator had a list of chicken vendors to visit with reserve outlets to replace those who did not consent to the survey or were unreachable. Enumerators clarified the objective of the work and consent was obtained from each participant.
2.2 Study area, sampling and sample size
The study was carried out in Ouagadougou, the capital of Burkina Faso. The sample size calculation was conservatively based on the outcome parameters which required the largest sample size, that is binary outcomes with a proportion of 50%, requiring 95% confidence intervals (CI) with 10% absolute precision either side of the estimate (Lemeshow et al., 1990). The required sample size was 97 outlets, which was rounded up to 100 to account for failings in data collection.
First, the city’s main roads were georeferenced, and a route was established for trained enumerators. The enumerators then surveyed this route, recording the location of each eligible chicken restaurant. Outlets located along medium and main roads throughout the city were mapped to create a sampling frame (n = 622). From this sampling frame, outlets were randomly selected (Figure 1).
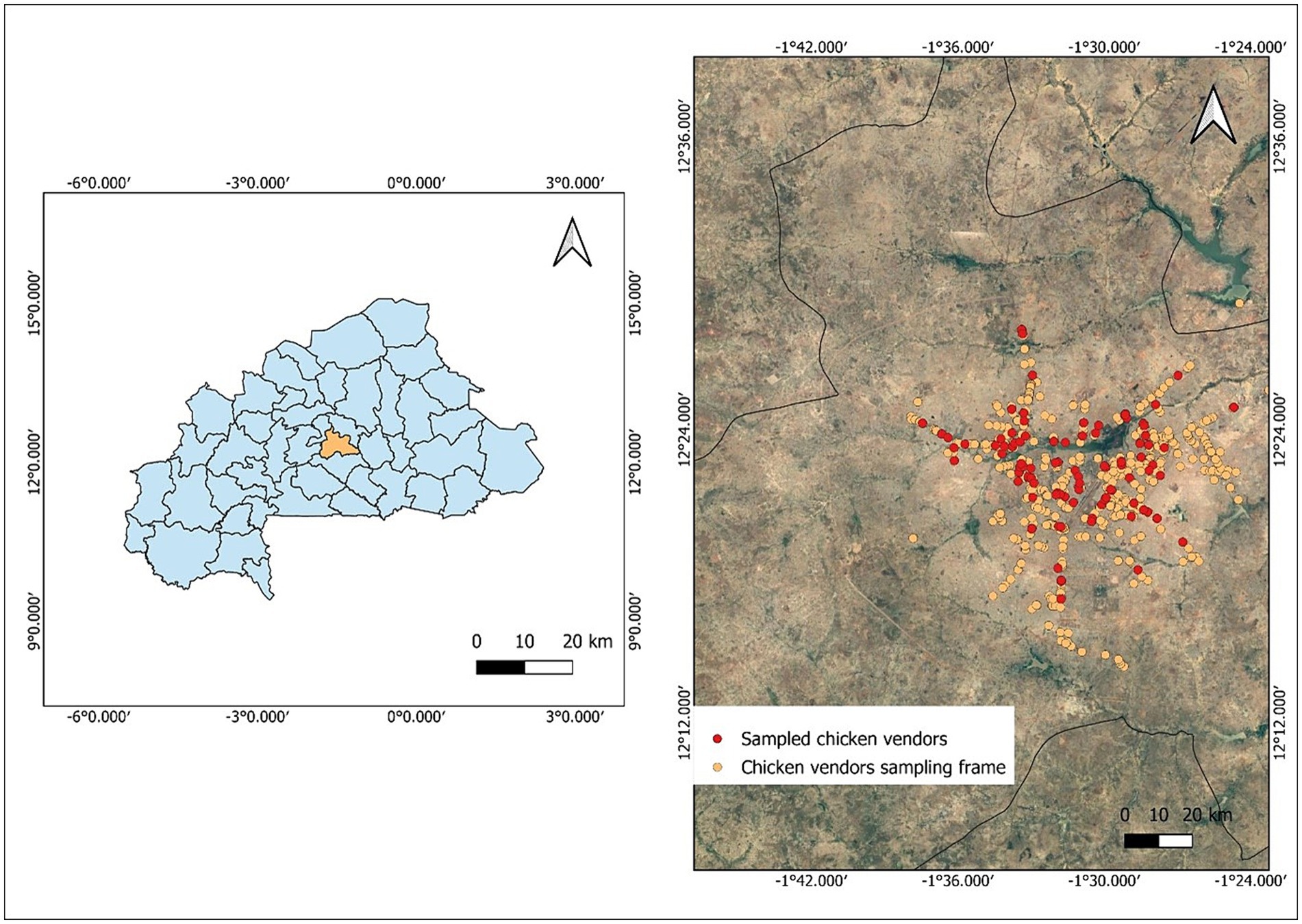
Figure 1. Map of Burkina Faso, center region and Ouagadougou city showing sampled chicken restaurants.
2.3 Data management and analysis
Once collected, data was exported to MS excel where it was cleaned, checked, and prepared for analysis. The findings were summarized with descriptive statistics using STATA version 17. Data were presented in tables (frequencies and proportions) and graphs.
3 Results
3.1 Background information of participants and the restaurants
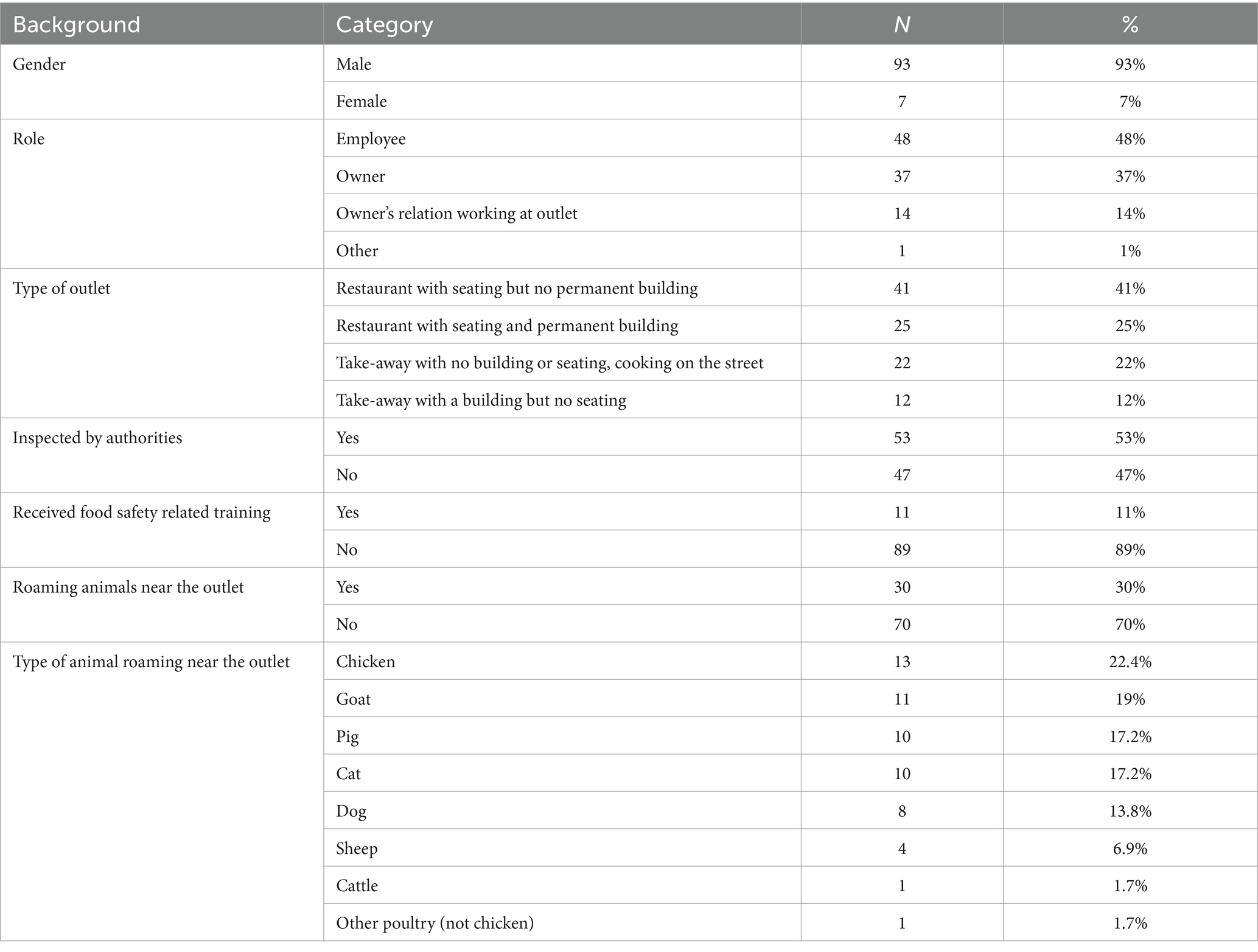
Of 100 participants, 93% were men, while 7% were women. Forty-eight percent were restaurant employees, the rest being owners or their relatives working at the outlet. Among the outlets visited, 41% were restaurants with seating but no permanent structure, 25% were restaurants with seating and a permanent structure, 22% were takeaways with no building or structure, and 11% were takeaways with a structure but no seating.
Only 11% of the respondents had previous training on food safety and related topics; 47% were not regularly inspected by the authorities. Just under a third of participants reported the presence of animals around the outlets, mostly chickens, goats, pigs, and cats (Table 1).
3.2 Price, source of carcass and live chicken markets
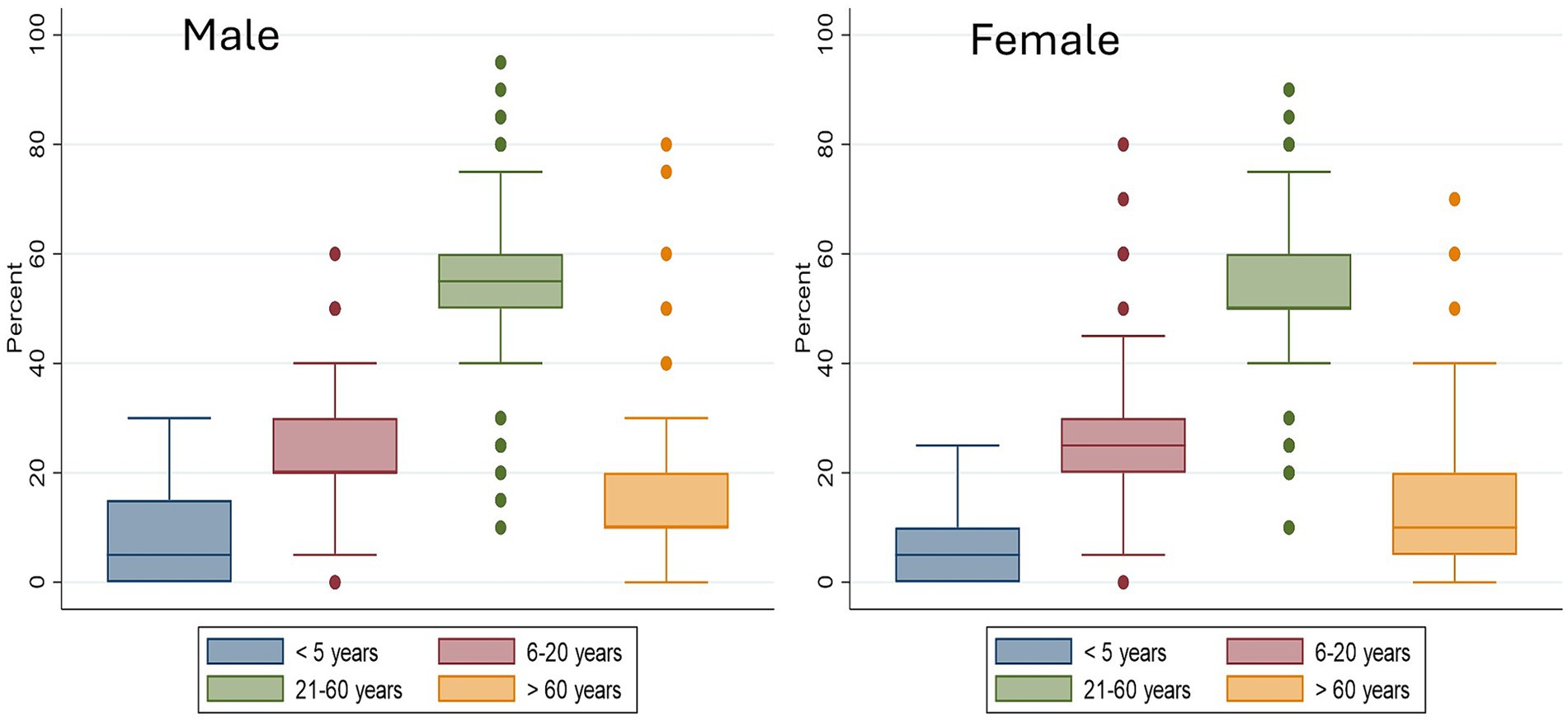
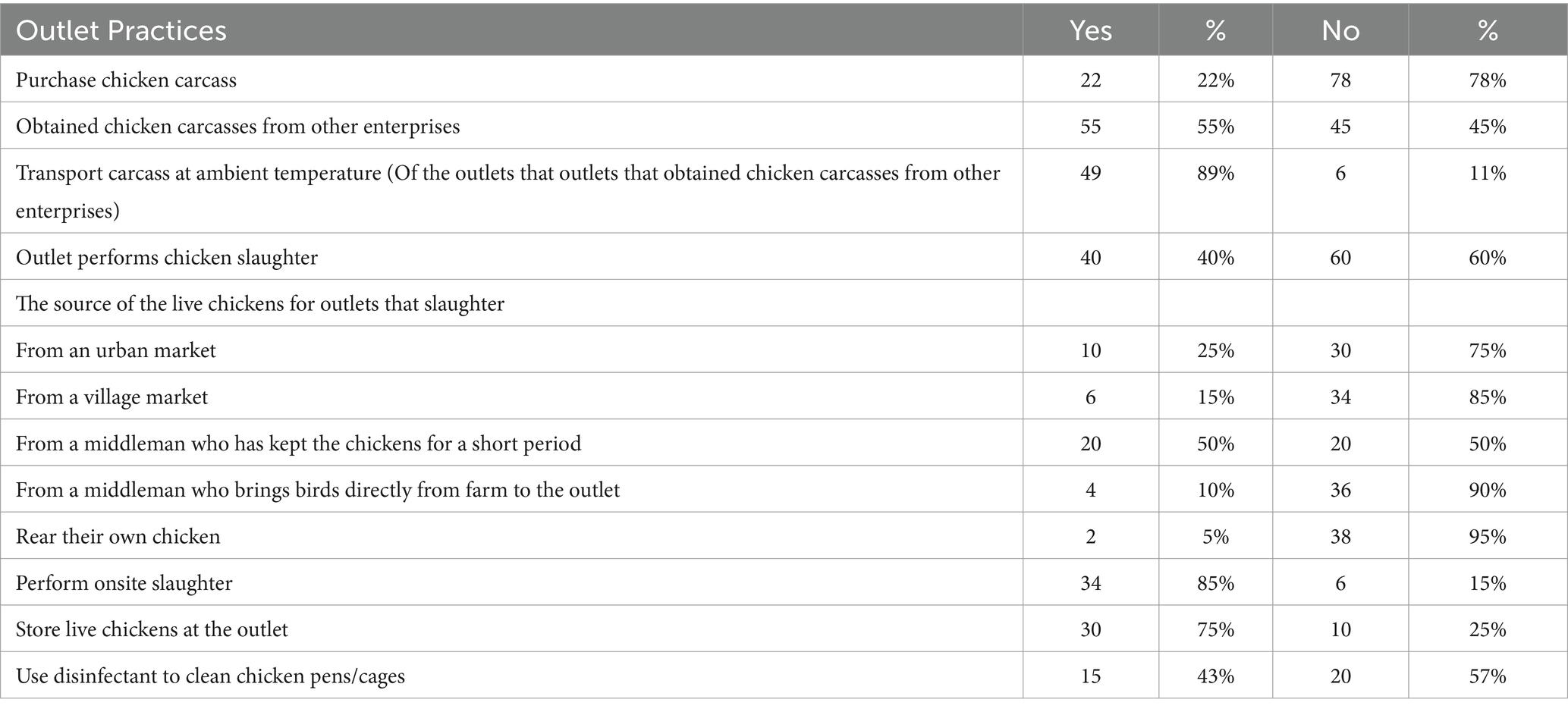
Restaurants slaughtered chickens themselves, and/or others bought carcasses from other slaughterers. Twenty-two percent of the restaurants purchased chicken carcasses, while 55% relied on other enterprises for slaughtering chickens. Of those who got chicken carcasses from other suppliers (purchased or slaughtered by another enterprise), 36 (62%) had only one supplier, while 18 (31%) had two suppliers and four (7%) had 3–4 suppliers. About 40 (40%) of the restaurants slaughtered chicken themselves, and most of these perform on-site slaughter (85%). Of those who slaughtered chicken themselves, about half of the restaurants got live chickens from a middleman who kept the chickens for a short period, the next most common source was urban markets and after that village markets. The average price of a chicken is 3,479 XOF, equivalent to 6 USD as of March 2021 exchange rate. On average establishments sell 83 servings per day and more than 300 raw chickens per week. On average participants reported that 67% of customers who visited the outlets were male. Age-wise, more than 50% of customers were between the ages of 21–60 (Figure 2).
3.3 Operational hygiene and food safety practices from collection to consumption of chicken carcass at the restaurants
3.3.1 Collection of chicken and carcass
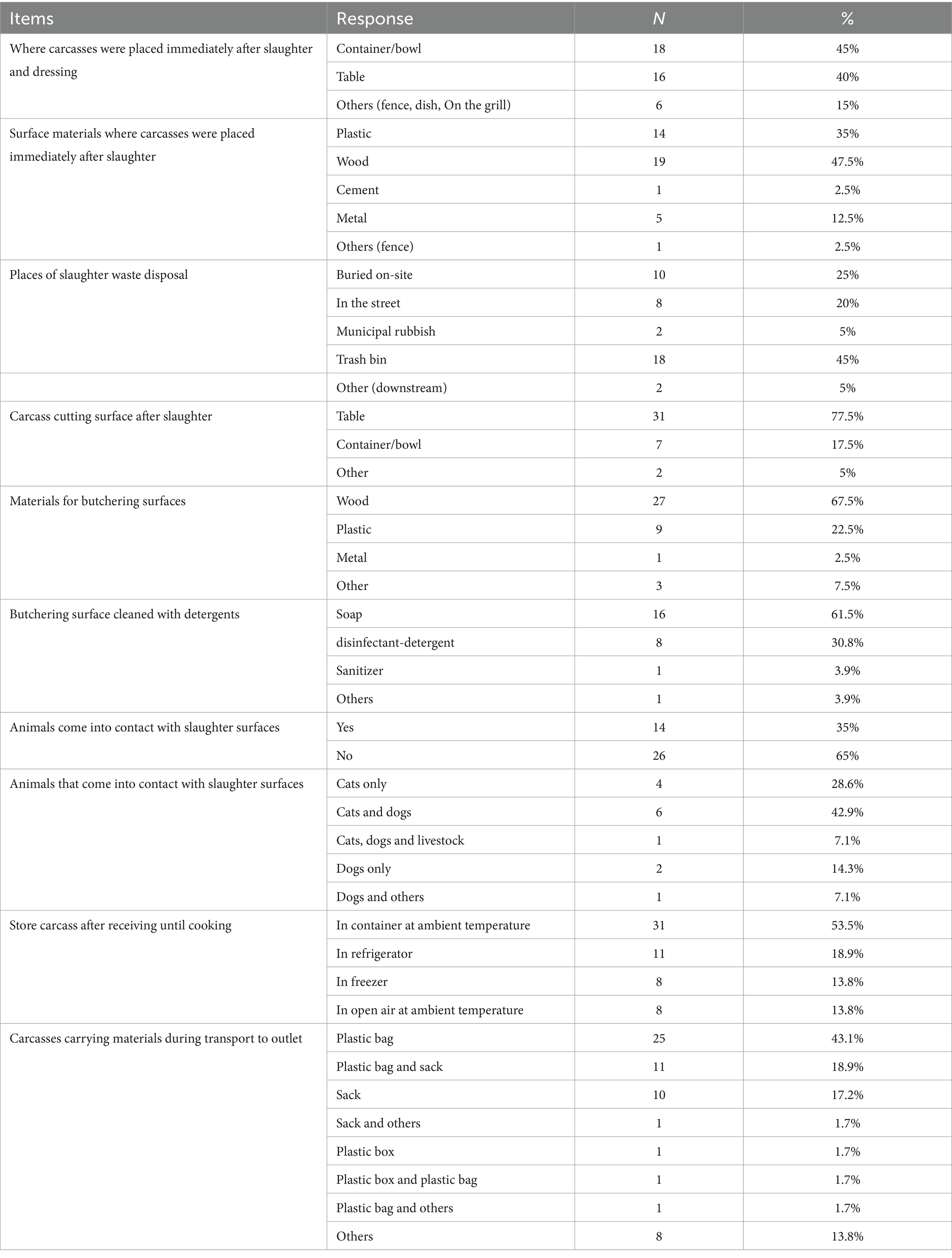
About 30 (75%) restaurants which slaughtered chickens kept live chickens at the outlet, but they did not keep chickens for many days before slaughter, on average 1.4 days, with 2.5 days a typical maximum. Around 92% of outlets claimed not to slaughter sick birds. In 97.5% of restaurants, chickens had access to feed immediately before slaughter. Restaurants, which slaughtered their birds in another establishment, commonly used plastic bags (43%) and sacks (17%) to carry carcasses to the restaurant. It took on average 30 min and a maximum of 2 h from when a chicken was slaughtered until they received the carcass. During this time, most of the restaurants (89%) transported carcasses at ambient temperature. After receiving chicken carcasses, they kept them on average for a minimum of 2.5 days, most likely for 3.5 days and maximum of 5.5 days until cooking. Only about 19 and 14% of them kept chicken carcasses in a refrigerator or freezer, respectively. The rest kept carcasses in a container or open air at ambient temperature.
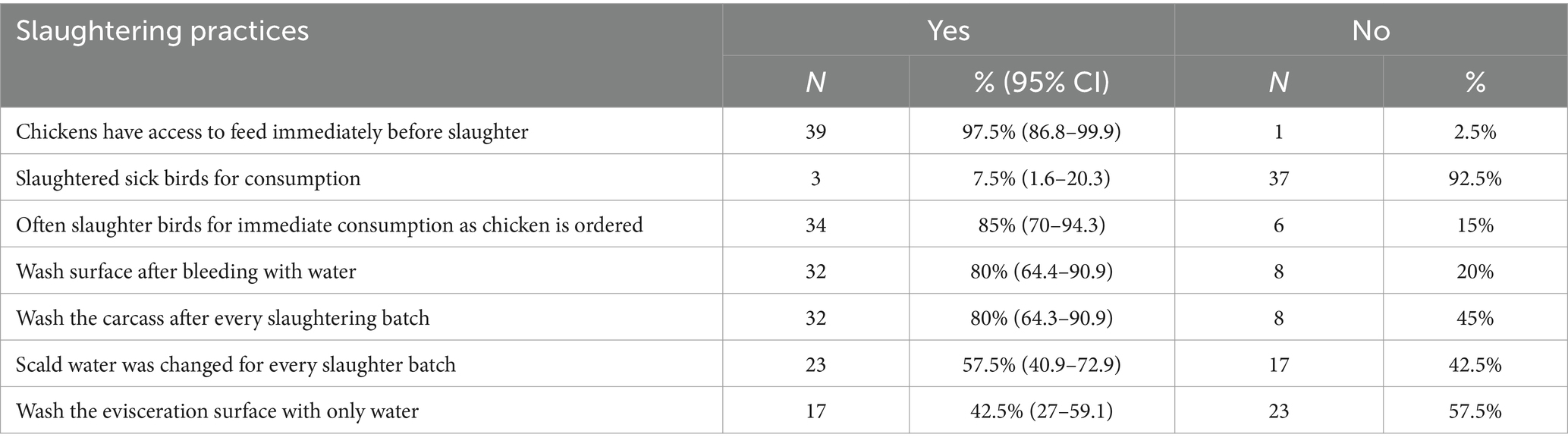
3.3.2 Slaughtering/bleeding process
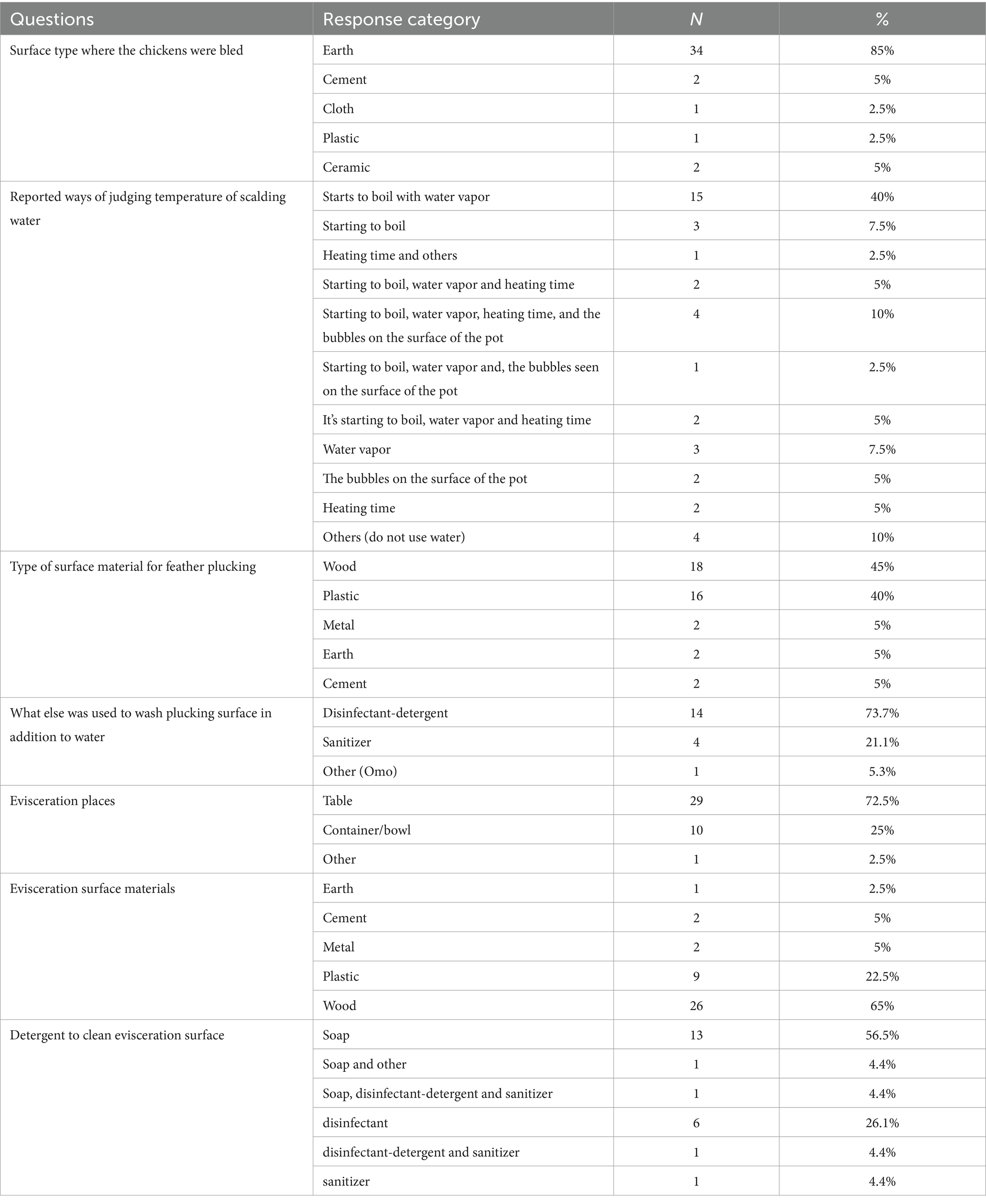
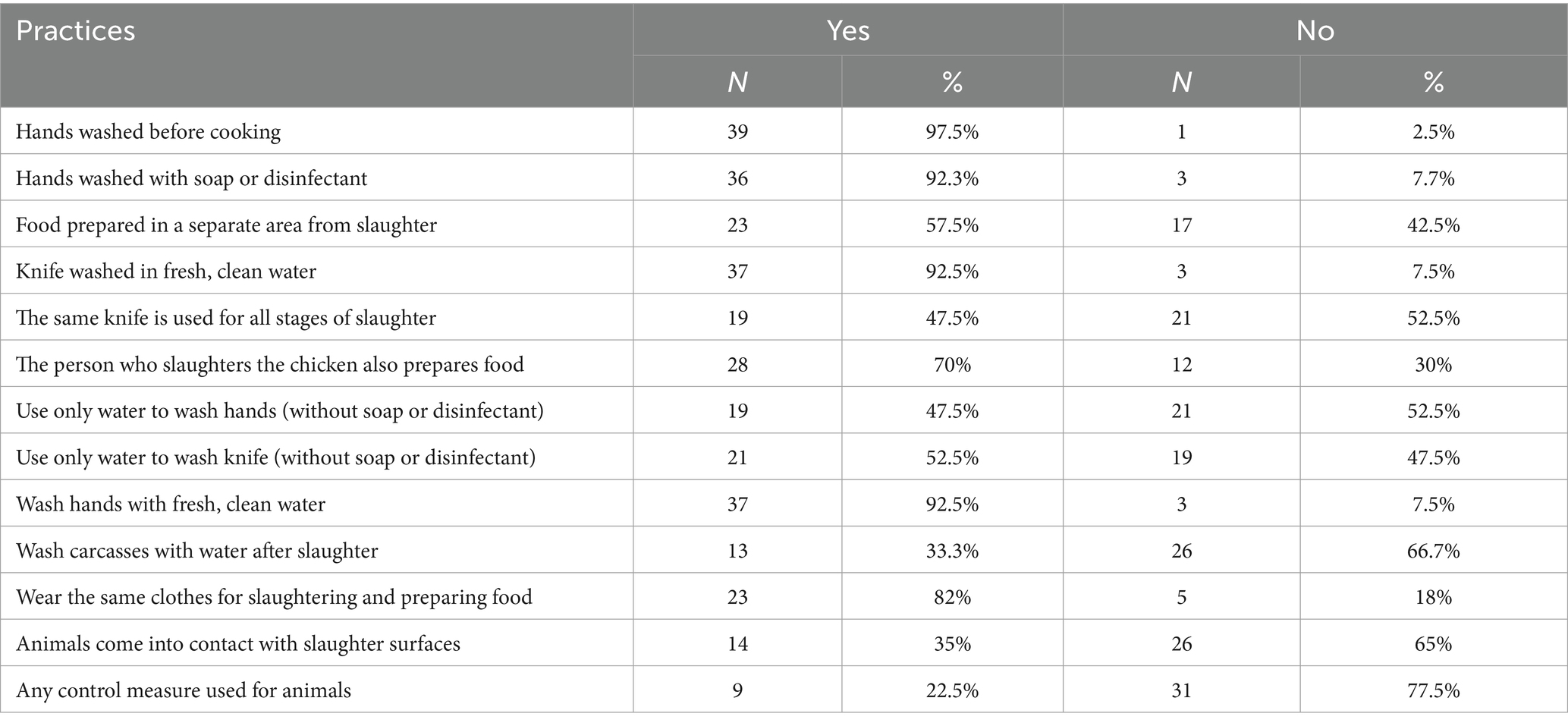
Most restaurants, 34 (85%) which slaughtered chicken performed slaughtering at the site where they prepared and sold food (Table 2). As a bleeding surface, most used bare earth, followed by cement and ceramic (Table 3). About 80% of outlets cleaned the bleeding surface immediately with water but only 8 (20%) used either soap or disinfectant-detergent (Table 4).
3.3.3 Scalding and plucking process
Following bleeding, chickens were scalded. An average of 24 chickens were slaughtered and scalded in one batch. An average of 16.5 liters of water was used in the scald tank and about 10 outlets shared the same scald water. Typically, around 17 chickens were scalded in the scald water before the water was changed. About 58% of the outlets which slaughtered chickens themselves changed scalding water for every batch of birds (Table 4). Respondents said they hot water to defeather the chickens and knew the proper temperature mainly from when the water starts to boil, and vapor appears. Most of them plucked chickens on wood and plastic surfaces. Most used disinfectant-detergent and sanitizer to clean the plucking surface (Table 3).
3.3.4 Evisceration
The majority of outlets performed evisceration on a table and in a container/bowl. The surface of table/bowl was most commonly made of wood followed by plastic. Around 43% of the outlets washed the evisceration surface with only water (Table 4), the rest using soap and disinfectants to clean the evisceration surface (Table 3). Half of the outlets washed the area of evisceration every day, but the mean was every two and half days.
3.3.5 Carcass processing
After slaughter, a carcass was placed in a bowl or on a table made of plastic or wood for further processing and kept for an average of 15 min. Respondents most commonly cut carcasses on the table, followed by in a bowl made of wood and plastic. These cutting surfaces were cleaned with soap and disinfectant-detergent. Other animals often came in contact with carcass processing surfaces, especially cats, dogs and livestock (Table 5). All respondents said they washed carcasses with water after slaughter. But 20% of them do not change the wash water for every slaughter batch and changed the wash water a minimum of once a day and a maximum of 3 times a day. When washing chicken carcasses, an average of 15 liters of water are used with on average 12 chicken carcasses washed before the water is changed.
The majority (53%) of the outlets stored carcasses at ambient temperature. Plastic bags and sacks were commonly used as carcass carrying material.
3.3.6 Frozen chicken
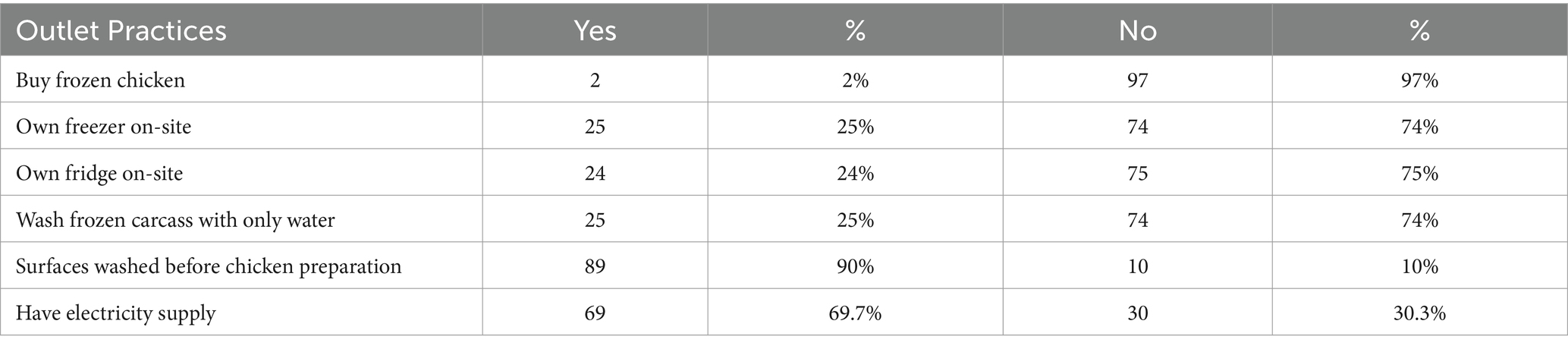
Only 2% of outlets bought frozen chicken, and 25 and 24% owned a freezer or fridge on their site, respectively.
Around 25% washed the frozen carcasses with water only before cooking. In addition, 90% washed the working area before cooking the carcass. Furthermore, 69% had access to electricity but reported power cuts every 3 days on average with power cuts lasting 6 h on average (Table 6).
3.3.7 Chicken preparation and cooking
Before cooking, respondents used on average 15 liters of water to wash the carcasses; there was a gap of 20 min between slaughter time and cooking time on average. After chicken was prepared, it took typically 15 min until it is served.
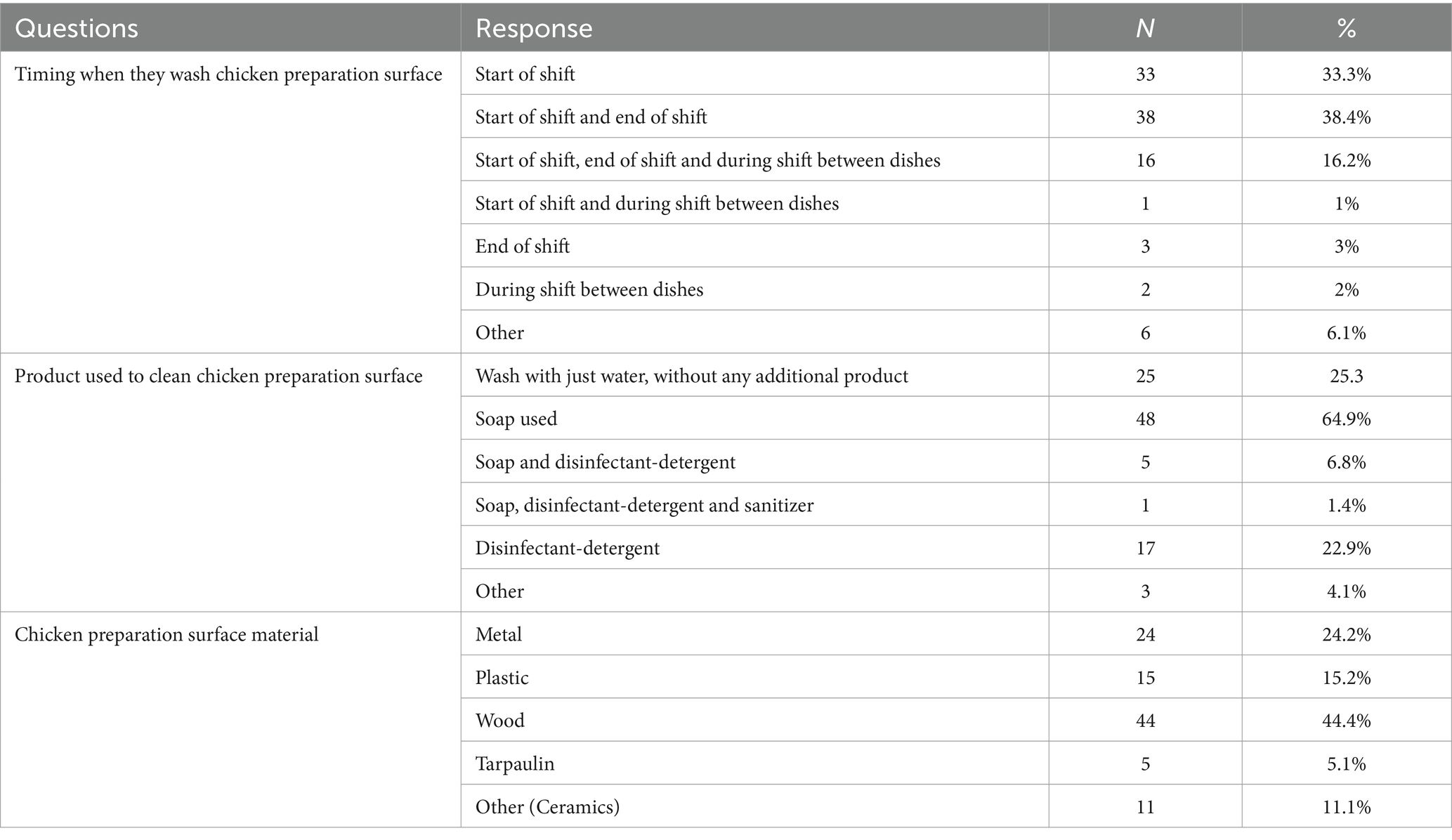
Workers typically washed chicken preparation surfaces at the start and end of the shift. Only a few of them washed in between meals. They used soap and disinfectant detergents to clean the chicken preparation surface (Table 7). Cooks knew when chicken was well cooked primarily by the appearance of the meat’s surface and by stabbing the meat (Supplementary Table S1).
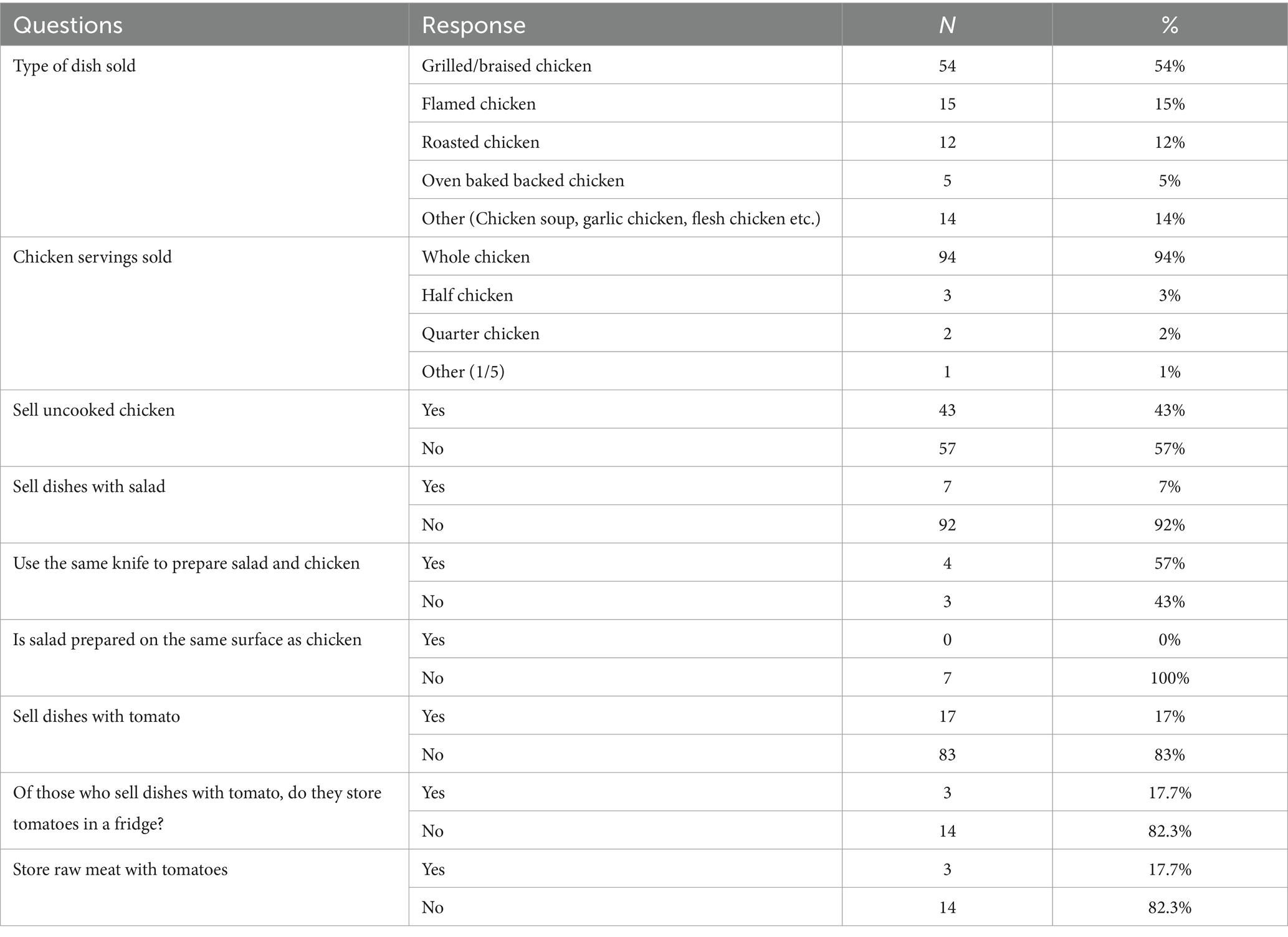
Grilled, flamed and roasted chicken were the most common dishes sold. But 43% of outlets sold raw/uncooked chicken for customers to cook at home as well. Grilled chicken was cooked over direct heat, either on a grill or a grill pan and used no other cooking medium. It was typically seasoned with herbs, spices, or marinades and cooked until the exterior was charred or marked with grill lines. Flamed chickens are processed and sold as street foods. Locally bred chickens, known as “poulet bicyclette,” are traditionally processed into flamed chickens by direct exposure to burned charcoal and wood flame. Roasted chicken are cooked in front of or over a fire or bed of coals or cooked in the oven as well. About 17% of the outlets sold dishes with raw tomatoes and 7% sold with salads (Table 8).
3.3.8 Personal hygiene and workplace
Most of the participants cleaned knives and hands during slaughter and cooking with clean water, and nearly 93% of them used cleaning products, like soap, as well. Just under half of them used the same knife in all stages of the slaughtering process. Similarly, more than 80% used the same cloth for slaughtering and food preparation (Table 9). About 95% of them claimed they have access to the required amount of water, and 100% reported access to clean water. The primary water source of the restaurants was the main public water supply, and a few of them used water from boreholes.
Most cleaned knives at the beginning and the end of the day as well as before slaughtering a batch of chickens and after slaughtering a batch. Outlets used mostly soap and disinfectant-detergents to clean knives. The majority of slaughterers claimed to wear clean clothes and aprons during slaughtering of chicken. About 28% of them wore both a clean apron and a fabric resembling an apron while working, depending on their availability (Supplementary Table S2). Most respondents washed their hands at the beginning and the end of the day, and before slaughtering a batch and after slaughtering a batch (Supplementary Table S3). Slaughter waste was mostly buried on the site, thrown away in the street or collected in trash cans (Table 5).
3.3.9 Facility hygiene
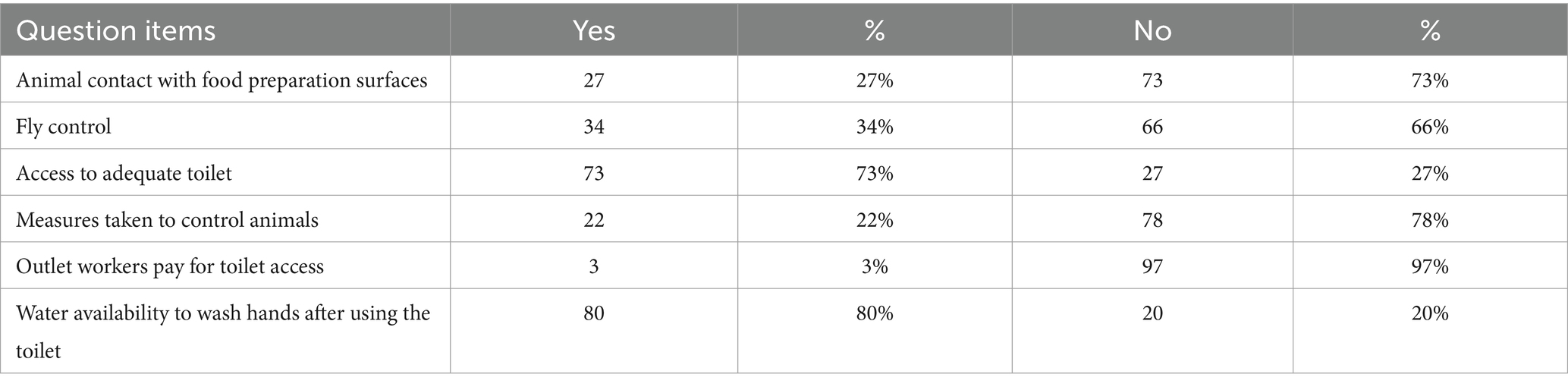
Animals did come in contact with food preparation surfaces in about quarter of the restaurants but the majority (78%) of them have no animal control plan. Moreover, 66% of them also did not control flies. About 27% of respondents reported they did not have adequate toilet facilities (Table 10).
Most restaurants used private toilets near their site or private toilets far from their restaurants. Among the animals that come and contact the chicken cooking surface, cats in 22 premises (81.5%) and dogs in 19 premises (70.4%) accounted for the highest number. Among the control measures used for these animals, fencing was most common. From those who employ fly control measures, fly spray and glass windows were used (Table 11).
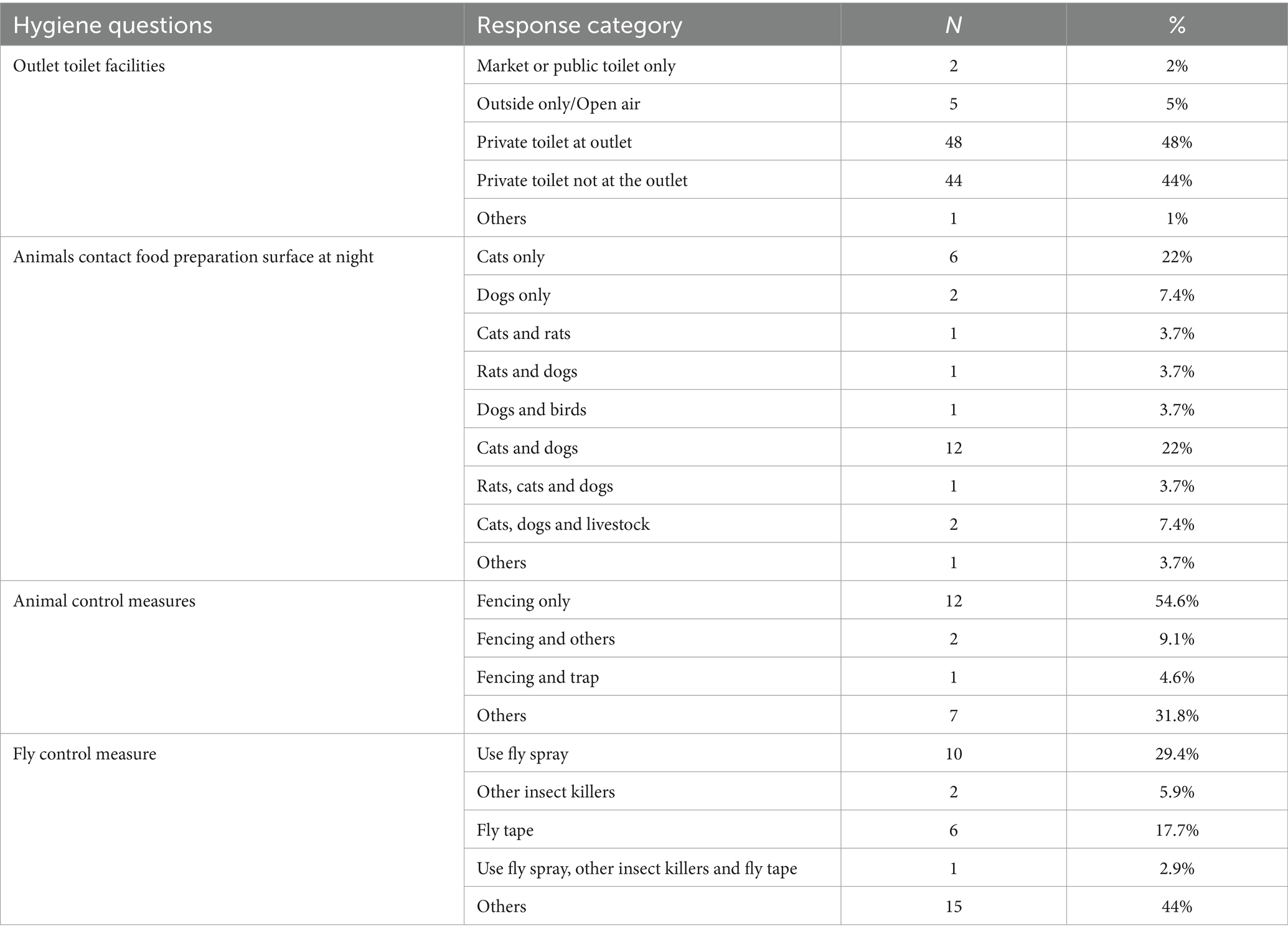
Table 11. Types of toilets accessible by the outlet and animal proximity and their control (N = 100).
3.4 Food safety perception and knowledge
About 80% of respondents thought that cleanliness and hygiene were not important to their customers when they choose where to eat chicken. Only about 20% of them had adopted an activity to improve the hygiene and safety of food in their restaurant recently. More than 80% of them were not aware of customers becoming sick from eating tomatoes and chicken. About 90% of them understood the importance of temperature in storing food (Table 12).
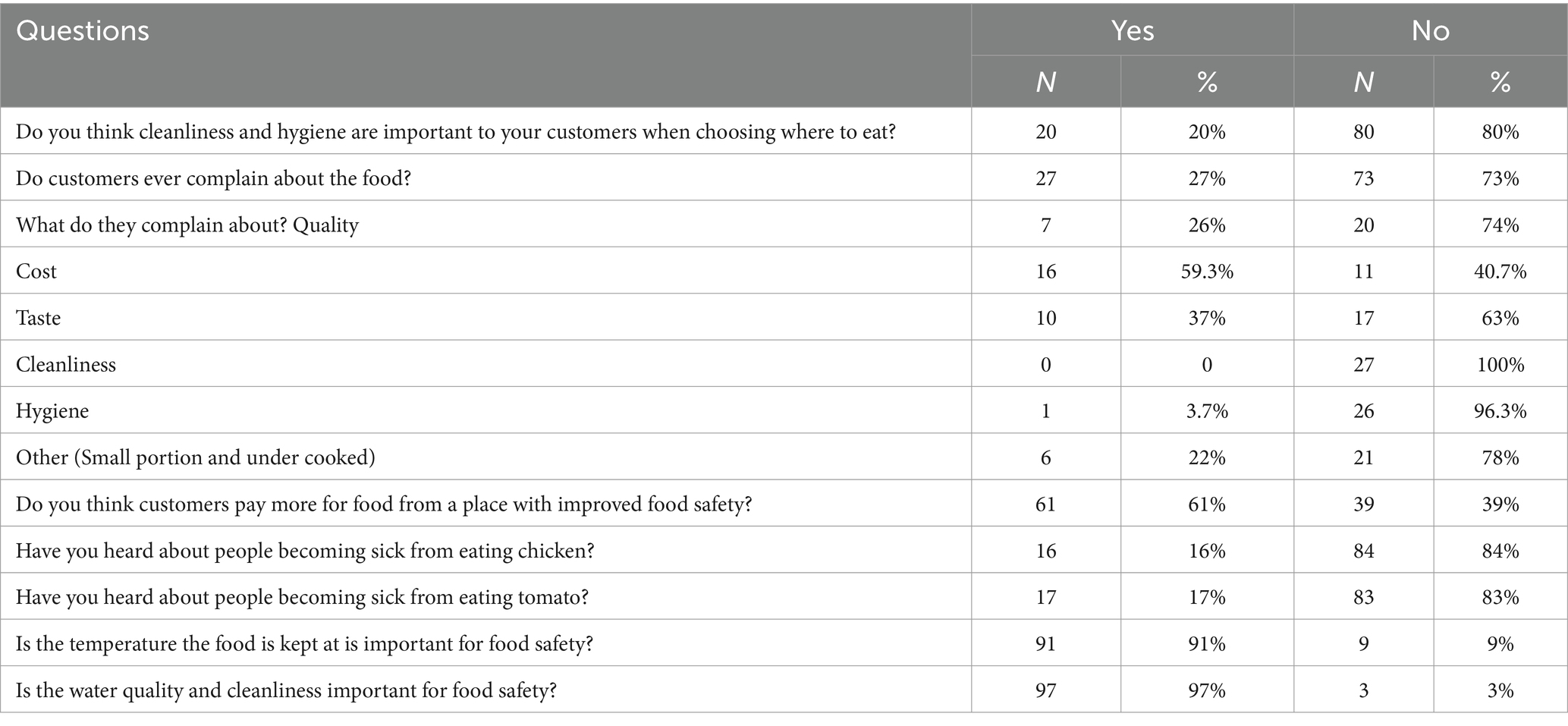
Table 12. Knowledge and perception of chicken outlet staff regarding chicken safety and hygiene in Ouagadougou (N = 100).
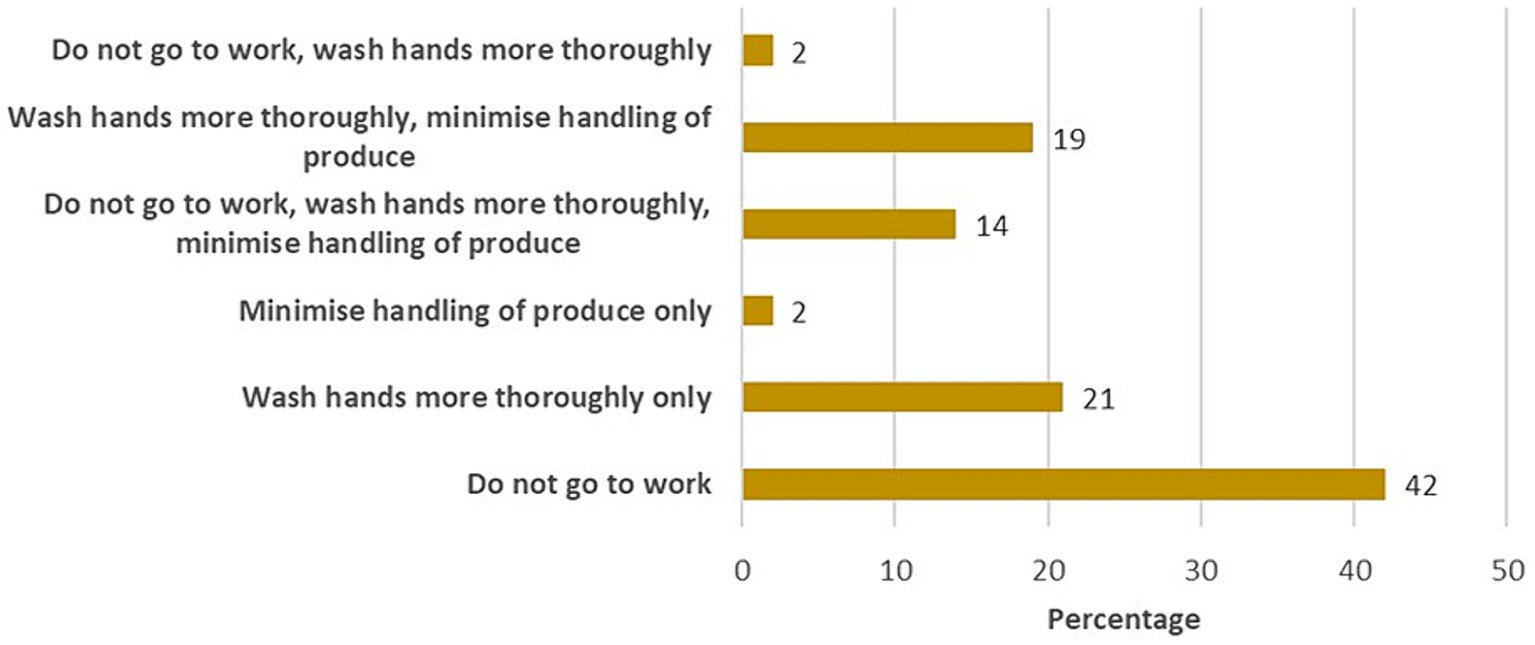
When they felt sick, 42% of outlet respondents reported they did not go to work, 21% worked but washed their hands thoroughly, and 19% washed their hands more thoroughly and minimized food handling (Figure 3). Only 27% of the restaurants received a complaint about their food. Their most frequently encountered customer complaints were the cost (37%) and taste (25.7) of a chicken.
During slaughter and food preparation stages, about quarter to a half of outlet staff think clean hands (45%), clean surfaces (45%), clean water for washing (40%), avoiding contaminating carcasses with gut contents (40%), and clean equipment (23%) were important practices to ensure chicken safety (Supplementary Tables S4, S5). Furthermore, they think that clean surfaces (77%), clean hands (44%) and clean knife/equipment (37%) were important to ensure chicken is safe during cooking (Supplementary Table S6). Respondents also reported food appearing clean (43%), food appearing fresh (33%), the origin of the food (from a producer they trust) (17%), and the stall’s appearance (7%) were the top hygiene concerns of their customers.
4 Discussion
Chicken meat can be contaminated with foodborne pathogens at various points along the supply chain including production, transport, slaughter, and meat processing and handling, posing significant health risks to consumers (Prachantasena et al., 2016; Thung et al., 2016; Kagambèga et al., 2018; Perez-Arnedo and Gonzalez-Fandos, 2019; Sun et al., 2021). In this study, we found that hygienic practices, perceptions and knowledge of workers in restaurants and takeaways selling chicken in Ouagadougou were poor, sometimes strikingly poor, with food routinely prepared in unhygienic environments with inadequate facilities and practices.
Most restaurants sold food in the street without permanent buildings, while only a quarter had a permanent site and building, mainly serving grilled, flamed and roasted chicken. Outdoor food markets are exposed to environmental contaminants, and are often dusty and windy making good hygiene a challenge (Oguttu et al., 2014; Campos et al., 2015). Permanent buildings are easier to clean and protect food from animals and dust.
The majority of restaurants lacked adequate toilet facilities with inadequate handwashing, critical for hygiene. Human faecal-oral transmission is a key pathway for foodborne pathogen spread, including via contaminated food after unhygienic handling. Half of the outlets visited had not been regularly inspected by authorities to enforce and promote good food safety practices. Furthermore, most had not received any training on food safety or good hygiene. Studies indicate that educational campaigns on food safety and hygienic practices influence people’s perception and knowledge of the subject (Adesokan et al., 2015; McFarland et al., 2019). Moreover, the presence of roaming live animals like chickens, goats, pigs and cats near the outlets, contributes to pathogen contamination (Pouokam et al., 2017; Heredia and García, 2018).
In most restaurants which do their own slaughtering, chickens had access to feed immediately before slaughter. Feed withdrawal 6 to 10 h before slaughter reduces the feed content in the digestive tract. This helps prevent gut contents spillage during carcass evisceration and contamination during processing (Xue et al., 2021).
4.1 Process hygiene (hygienic practices)
Some restaurants regularly slaughtered sick chickens for human consumption, which should not be done because sick birds are sources of foodborne zoonoses, this also reflects the poor health and welfare that birds are subjected to (Khamesipour et al., 2018). The surface where birds are slaughtered was often bare earth, making adequate cleansing a challenge or impossible, and the majority of outlets used only water to wash blood contaminated environments, when detergent or sanitizer/disinfectant are required to remove pathogen contamination (Singh et al., 2020). Furthermore, almost half of the outlets did not clean the surfaces used for bleeding after each batch of birds, cleaning only at the end of the day. This greatly increases the risk of contamination.
After bleeding, plucking was performed using hot water for scalding in equipment made of plastic and metal. Although some did not change the scalding water after every batch of birds and cleaned the tank with disinfectant-detergent, the majority did, which is a good practice because the tank is among the initial points where carcasses can be contaminated. Environmental contaminants like E. coli, Staphylococcus aureus, Pseudomonas spp., Acinetobacter spp. and many other organisms have been implicated in contaminating the carcass at plucking due to favourable temperatures (Zweifel and Stephan, 2012; Barbut, 2016; Song et al., 2021). Evisceration was performed on a table, or a bowl made of plastic or metal, and more than half of the restaurants used tap water and soap to clean the evisceration surface. Among the stages of chicken processing, evisceration is a key stage where carcasses can be contaminated with gut microbiota and environmental contaminants from the working environment unless proper hygienic measures are implemented (Schets et al., 2017; Perez-Arnedo and Gonzalez-Fandos, 2019).
Respondents indicated that they used the same knife in all slaughtering stages and the same person slaughtered, prepared and cooked the meat wearing the same clothes or apron. There should be clear separation between “dirty” stages of this process, where handler, environment and equipment contamination are likely, and stages involving handling of carcasses and meat. Handling of live birds and slaughter waste in particular should be separated, but there is also a need to reduce contamination between other steps, e.g., slaughter, defeathering, evisceration and carcass preparation.
Wearing the same clothes, and using the same uncleaned knives, with limited hand washing will transfer pathogens along the slaughter line and food preparation areas. Many only washed knives and hands at the start and end of the day, creating massive cross contamination of carcasses. Previous studies have demonstrated that the risk of contamination increases when restaurant staff wear dirty clothing while working. Poor personal hygiene can also facilitate the transmission of pathogens through food to humans. Restaurant personnel can act as mechanical vectors for Salmonella cross-contamination from raw chicken, vegetables, or the environment through utensils or other tools (Mensah et al., 2002; Cardinale et al., 2005).
Slaughter waste disposal facilities were lacking in the restaurants, and a significant number of vendors disposed slaughter waste in the streets. Slaughter waste is highly contaminated and must be separated from the slaughter environment and disposed of safely in a manner which is financially sustainable, feasible, socially and legally acceptable, and environmentally friendly (Abdel-Shafy and Mansour, 2018).
4.2 Chicken preparation and cooking
Various unhygienic practices were reported after slaughtering and during cooking and serving. Again, some restaurant workers cleaned kitchen utensils and their hands with only water, without any detergents. Even cleaning with detergent and hot water results in low reduction in microbial contamination, and disinfectants or sanitizers should be used (Scott et al., 1984). A study showed, thorough disinfection of the kitchen and utensils was linked to a reduced risk of Salmonella contamination (Cardinale et al., 2005). Washing hands with water and soap is an effective way to eliminate pathogens that may be present on hands (Centers for Disease Control and Prevention (CDC), 2021). Cross-contamination can occur when food workers touch other utensils, kitchen surfaces, or prepare other foods after handling raw chicken without properly washing their hands with soap and water (Bruhn, 2014). A recent quantitative microbial risk assessment study revealed that combining interventions at restaurants (improved hand washing plus designated kitchen utensils with improved cooking) resulted in a 75% risk reduction in pathogen contamination in Burkina Faso (Ssemanda et al., 2024).
Immediately after slaughter, respondents commonly put chicken carcasses in bowls made of plastic and on wooden tables at ambient temperature. The average time between when a chicken was slaughtered and when respondents received the carcass was 30 min, similar to another Sub-Saharan Africa study (Rwanda) (Niyonzima et al., 2018). The duration of carcass transportation under ambient temperature correlates with proliferation of Salmonella initially present on the carcasses, since the generation/doubling time for Salmonella at optimal temperature conditions (35–37°C) is known to be 25 min (Delhalle et al., 2009). Previous studies have reported bacterial growth during transportation of carcasses when temperature control was inadequate (Lo Fo Wong et al., 2002; Niyonzima et al., 2015). Keeping chicken carcasses at the proper temperature of 4°C immediately after slaughter is one of the most important steps a food handler can take to prevent growth of microorganisms that cause foodborne illness (WHO, 2006). However, only 50% of outlets had a freezer or fridge on-site, while the rest kept carcasses at room temperature; only 70% had access to electricity. Keeping chicken carcasses without a cold chain for extended periods leads to pathogenic microorganisms proliferation to unsafe levels, thereby posing a health risk to consumers (Alonso-Hernando et al., 2013).
Some of the restaurants sold chicken dishes with salad or tomatoes. As salads are not heated before consumption they are a high-risk food, vulnerable to pathogen cross-contamination. Studies have shown that chicken cross-contamination with Salmonella occurs frequently with vegetables that are not properly cleaned and disinfected (Mosupye and von Holy, 2000; Cardinale et al., 2005).
Animals such as cats and dogs were in contact with cooking and food preparation areas, with flies a regularly occurring issue with no measures to control this. This is a major concern (Nazari, 2017; Khamesipour et al., 2018).
4.3 Knowledge and perception towards chicken safety
Similar to their hygienic practices, knowledge and perception of food safety amongst outlet staff was limited. Surprisingly the majority believed cleanliness and hygiene were not important to their customers when choosing where to eat. Also, more than 80% of staff had not heard of foodborne diseases associated with eating chicken or tomato. This underscores the need to educate vendors. If their reports of consumer lack of concern are accurate, this should also be addressed. Consumer awareness campaigns, using mass-media and the involvement of key influencers, have potential to empower consumers to consistently pay attention to their own food safety behaviours while purchasing foods, and those of the retailer, leading to enhancing food safety at the informal markets (Madjdian et al., 2024).
The study also found that 42% of workers still go to work when they are ill with a stomach upset and diarrhoea, a major risk for infecting the customers. Many disease outbreaks could be prevented by ensuring that food workers do not work while they are sick. But obviously there are financial pressures working against this, for the worker and outlet.
Clean water and detergents, standardized working tables, clean protective clothes, clean cooking areas and dining areas are among the minimum requirements to ensure the safety of customers from foodborne diseases (Osbjer et al., 2015; Bintsis, 2017). However, most of these minimum requirements were not met in the chicken outlets of Ouagadougou. Effective food safety and hygienic training should focus on key behaviors that are directly linked to contamination and disease outcomes. Training combined with distribution of a food safety equipment to enable improved practices can be effective in delivering significant food safety behavior changes, and going beyond knowledge enhancement alone. For example, a chicken vendor training program in urban informal markets of Ouagadougou, Burkina Faso resulted in important behaviors to improve personal hygiene and several slaughtering and chicken preparation practices (Madjdian et al., 2024).
A limitation of this study was that the practices assessed were self-reported and social desirability bias may have led to over reporting good practices. If so, actual practices could be even worse. Despite these limitations, the results of this study remain valuable and representative, as the outlets were randomly selected, and the findings are consistent with other studies.
The results highlight the need to educate food vendors to improve food safety and hygiene knowledge and the need for equipment and infrastructure in order to allow safe food to be implemented. This is an extensive study to understand hygiene practices in one of the most important food chains of Ouagadougou and reflects handling practices of chicken and other foodstuffs in LMICs.
5 Conclusion
Like most street food outlets in Sub-Saharan Africa, chicken restaurants in Ouagadougou have poor levels of hygiene at all stages of food preparation. Restaurant workers lacked training and inspections by the authorities were limited. The working environment was contaminated, and seldom adequately cleaned, and stray animals wandered around the slaughtering and cooking areas. Furthermore, cleanable surfaces and equipment, toilets, washing, power and clean water supply were lacking. Worker perceptions and knowledge of food safety and how to ensure food is safe were poor, and they did not consider food safety important to their customers. To improve this situation an integrated approach is needed, including worker and consumer educational campaigns on good food safety practices, introduction of easy to clean food-grade surfaces, and better equipment with cleaning and disinfection materials. Upgrading of infrastructure is required including buildings along with provision of clean water and establishment of a cold chain with freezers and power supply. Assuming much needed major investment in food outlet infrastructure in unlikely in the short to medium term in most LMICs, there is a need to identify easy to implement, but effective and sustainable food safety mitigation measures, to make the best of a bad situation, and minimize the ongoing deaths and disease burden resulting from the current food safety situation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethics approval was obtained from the Institutional Research Ethics Committee of the International Livestock Research Institute (ILRI-IREC2019-36/1) and the national Ethical Committee for Health Research under reference 2020-10-220. Written informed consent from the participants was sought from the participants in this study in accordance with the national legislation and the institutional requirements.
Author contributions
BG: Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Methodology. MD: Conceptualization, Methodology, Supervision, Writing – review & editing. GI: Data curation, Investigation, Writing – review & editing, Conceptualization, Methodology. AA: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. VL: Data curation, Investigation, Writing – review & editing. DG: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. TK-J: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Bill and Melinda Gates Foundation, UK Government Foreign, Commonwealth and Development Office (FCDO)-UK Aid from the United Kingdom government (INV-008430-OPP1195588), One Health Centre in Africa (OHRECA) and the CGIAR Research Program on Agriculture for Nutrition and Health. The funder played no role in the design or conclusion of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2024.1448127/full#supplementary-material
Footnotes
1. ^“Pull-Push” Urban food markets in Africa: Incentivizing food safety using a pull-push approach.
References
Abdel-Shafy, H. I., and Mansour, M. S. M. (2018). Solid waste issue: sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 27, 1275–1290. doi: 10.1016/j.ejpe.2018.07.003
Adesokan, H. K., Akinseye, V. O., and Adesokan, G. A. (2015). Food safety training is associated with improved knowledge and Behaviours among foodservice establishments’ workers. Int. J. Food Sci. 2015, 1–8. doi: 10.1155/2015/328761
Alonso-Hernando, A., Alonso-Calleja, C., and Capita, R. (2013). Effectiveness of several chemical decontamination treatments against gram-negative bacteria on poultry during storage under different simulated cold chain disruptions. Food Control 34, 574–580. doi: 10.1016/j.foodcont.2013.05.020
Barbut, S. (2016). Poultry: Processing. Encyclopedia of Food and Health. 458–463. doi: 10.1016/b978-0-12-384947-2.00557-2
Bintsis, T. (2017). Foodborne pathogens. AIMS Microbiol. 3, 529–563. doi: 10.3934/microbiol.2017.3.529
Bruhn, C. (2014). Chicken preparation in the home: an observational study. Food Prot. Trends 34, 318–330.
Campos, J., Gil, J., Mourão, J., Peixe, L., and Antunes, P. (2015). Ready-to-eat street-vended food as a potential vehicle of bacterial pathogens and antimicrobial resistance: an exploratory study in Porto region Portugal. Int. J. Food Microbiol. 206, 1–6. doi: 10.1016/j.ijfoodmicro.2015.04.016
Cardinale, E., Perrier Gros-Claude, J. D., Tall, F., Guèye, E. F., and Salvat, G. (2005). Risk factors for contamination of ready-to-eat street-vended poultry dishes in Dakar, Senegal. Int. J. Food Microbiol. 103, 157–165. doi: 10.1016/j.ijfoodmicro.2004.12.023
Centers for Disease Control and Prevention (CDC) (2021) Handwashing: A healthy habit in the kitchen. Available at: https://www.cdc.gov/clean-hands/prevention/about-handwashing-a-healthy-habit-in-the-kitchen.html (Accessed: 22 May, 2024).
Delhalle, L., Saegerman, C., Messens, W., Farnir, F., Korsak, N., van der Stede, Y., et al. (2009). Assessing interventions by quantitative risk assessment tools to reduce the risk of human salmonellosis from fresh minced pork meat in Belgium. J. Food Prot. 72, 2252–2263. doi: 10.4315/0362-028X-72.11.2252
Dione, M. M., Diarra, S., Ilboudo, G., Konkobo-Yameogo, C., Lallogo, V. R., Roesel, K., et al. (2021) Value chain assessment of animal-source foods and vegetables in Ouagadougou, Burkina Faso–considering food safety, quality and hygiene perceptions and practices. Available at: https://hdl.handle.net/10568/117352 (Accessed December 20, 2023).
Grace, D. (2015). Food safety in low and middle income countries. Int. J. Environ. Res. Public Health 12, 10490–10507. doi: 10.3390/ijerph120910490
Grace, D. (2023). Burden of foodborne disease in low-income and middle-income countries and opportunities for scaling food safety interventions. Food Secur. 15, 1475–1488. doi: 10.1007/s12571-023-01391-3
Havelaar, A. H., Kirk, M. D., Torgerson, P. R., Gibb, H. J., Hald, T., Lake, R. J., et al. (2015). World health organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 12:e1001923. doi: 10.1371/journal.pmed.1001923
Havelaar, A. H., Sapp, A. C., Amaya, M. P., Nane, G. F., Morgan, K. M., Devleesschauwer, B., et al. (2022). Burden of foodborne disease due to bacterial hazards associated with beef, dairy, poultry meat, and vegetables in Ethiopia and Burkina Faso, 2017. Front. Sustain. Food Syst. 6:1024560. doi: 10.3389/fsufs.2022.1024560
Heredia, N., and García, S. (2018). Animals as sources of food-borne pathogens: a review. Anim. Nutr. 4, 250–255. doi: 10.1016/j.aninu.2018.04.006
Hoffmann, V., Awonon, J., and Gelli, A. (2019) Poultry production in Burkina Faso: Potential for poverty reduction and Women’s empowerment. IFPRI discussion paper 1908.
Hoffmann, S., Devleesschauwer, B., Aspinall, W., Cooke, R., Corrigan, T., Havelaar, A., et al. (2017). Attribution of global foodborne disease to specific foods: findings from a World Health Organization structured expert elicitation. PLoS One 12:e0183641. doi: 10.1371/journal.pone.0183641
Hoffmann, V., Moser, C., and Saak, A. (2019). Food safety in low and middle-income countries: the evidence through an economic lens. World Dev. 123:104611. doi: 10.1016/j.worlddev.2019.104611
Jaffee, S., Spencer, H., Laurian, U., Delia, G., and Emilie, C. (2019). The safe food imperative: Accelerating Progress in low- and middle-income countries. Washington, DC: Agriculture and Food Series; World Bank.
Kagambèga, A., Thibodeau, A., Trinetta, V., Soro, D. K., Sama, F. N., Bako, É., et al. (2018). Salmonella spp. and Campylobacter spp. in poultry feces and carcasses in Ouagadougou, Burkina Faso. Food Sci. Nutr. 6, 1601–1606. doi: 10.1002/fsn3.725
Khamesipour, F., Lankarani, K. B., Honarvar, B., and Kwenti, T. E. (2018). A systematic review of human pathogens carried by the housefly (Musca domestica L.). BMC Public Health 18:1049. doi: 10.1186/s12889-018-5934-3
Lemeshow, S., Hosmer, D. W., Klar, J., and Lwanga, S. K. (1990). Adequacy of sample size in health studies. Chichester, UK: John Wiley & Sons.
Li, M., Havelaar, A. H., Hoffmann, S., Hald, T., Kirk, M. D., Torgerson, P. R., et al. (2019). Global disease burden of pathogens in animal source foods, 2010. PLoS One 14:e0216545. doi: 10.1371/journal.pone.0216545
Lo Fo Wong, D. M. A., Hald, T., van der Wolf, J., and Swanenburg, M. (2002). Epidemiology and control measures for Salmonella in pigs and pork. Livest. Prod. Sci. 76, 215–222. doi: 10.1016/S0301-6226(02)00121-5
Madjdian, D. S., van Asseldonk, M., Ilboudo, G., Dione, M., Ouedraogo, A. A., Roesel, K., et al. (2024). Training and tool supply to enhance food safety behaviors among ready-to-eat chicken vendors in informal markets in Ouagadougou, Burkina Faso: a randomized-controlled trial. Food Control 163:110510. doi: 10.1016/j.foodcont.2024.110510
Madjdian, D. S., van Asseldonk, M., Talsma, E. F., Amenu, K., Gemeda, B. A., Girma, S., et al. (2024). Impact of a mass-media consumer awareness campaign on food safety behavior and behavioral determinants among women in Dire Dawa and Harar Ethiopia. Food Control 163:110509. doi: 10.1016/j.foodcont.2024.110509
McFarland, P., Checinska Sielaff, A., Rasco, B., and Smith, S. (2019). Efficacy of food safety training in commercial food service. J. Food Sci. 84, 1239–1246. doi: 10.1111/1750-3841.14628
Mensah, P., Yeboah-Manu, D., Owusu-Darko, K., and Ablordey, A. (2002). Street foods in Accra, Ghana: how safe are they? Bull. World Health Organ. 80, 546–554
Mezali, L., Mebkhout, F., Nouichi, S., Boudjellaba, S., and Hamdi, T. M. (2019). Serotype diversity and slaughterhouse-level risk factors related to Salmonella contamination on poultry carcasses in Algiers. J. Infect. Dev. Countr. 13, 384–393. doi: 10.3855/jidc.10450
Mosupye, F. M., and von Holy, A. (2000). Microbiological hazard identification and exposure assessment of street food vending in Johannesburg, South Africa. Int. J. Food Microbiol. 61, 137–145. doi: 10.1016/S0168-1605(00)00264-6
Mpundu, P., Mbewe, A. R., Muma, J. B., Zgambo, J., and Munyeme, M. (2019). Evaluation of bacterial contamination in dressed chickens in Lusaka abattoirs. Front. Public Health 7:19. doi: 10.3389/fpubh.2019.00019
Nazari, M. (2017). Bacterial contamination of adult house flies (Musca domestica) and sensitivity of these Bacteria to various antibiotics, Captured from Hamadan City, Iran. J. Clin. Diagnost. Res. 11, DC04–DC07. doi: 10.7860/JCDR/2017/23939.9720
Ncube, F., Kanda, A., Chijokwe, M., Mabaya, G., and Nyamugure, T. (2020). Food safety knowledge, attitudes and practices of restaurant food handlers in a lower-middle-income country. Food Sci. Nutr. 8, 1677–1687. doi: 10.1002/fsn3.1454
Niyonzima, E., Ongol, M. P., Brostaux, Y., Korsak, N., Daube, G., Kimonyo, A., et al. (2018). Meat retail conditions within the establishments of Kigali city (Rwanda): bacteriological quality and risk factors for Salmonella occurrence. Trop. Anim. Health Prod. 50, 537–546. doi: 10.1007/s11250-017-1466-6
Niyonzima, E., Ongol, M. P., Kimonyo, A., and Sindic, M. (2015). Risk factors and control measures for bacterial contamination in the bovine meat chain: a review on Salmonella and Pathogenic E.Coli. J. Food Res. 4:98. doi: 10.5539/jfr.v4n5p98
Oguttu, J. W., McCrindle, C. M. E., Makita, K., and Grace, D. (2014). Investigation of the food value chain of ready-to-eat chicken and the associated risk for staphylococcal food poisoning in Tshwane metropole South Africa. Food Control 45, 87–94. doi: 10.1016/j.foodcont.2014.04.026
Osbjer, K., Boqvist, S., Sokerya, S., Kannarath, C., San, S., Davun, H., et al. (2015). Household practices related to disease transmission between animals and humans in rural Cambodia. BMC Public Health 15:476. doi: 10.1186/s12889-015-1811-5
Perez-Arnedo, I., and Gonzalez-Fandos, E. (2019). Prevalence of Campylobacter spp. in poultry in three Spanish farms, a slaughterhouse and a further processing plant. Food Secur. 8:111. doi: 10.3390/foods8030111
Pouokam, G. B., Foudjo, B. U. S., Samuel, C., Yamgai, P. F., Silapeux, A. K., Sando, J. T., et al. (2017). Contaminants in foods of animal origin in Cameroon: a one health vision for risk management “from farm to fork”. Front. Public Health 5:197. doi: 10.3389/fpubh.2017.00197
Prachantasena, S., Charununtakorn, P., Muangnoicharoen, S., Hankla, L., Techawal, N., Chaveerach, P., et al. (2016). Distribution and genetic profiles of Campylobacter in commercial broiler production from breeder to slaughter in Thailand. PLoS One 11:e0149585. doi: 10.1371/journal.pone.0149585
Sapp, A. C., Amaya, M. P., Havelaar, A. H., and Nane, G. F. (2022). Attribution of country level foodborne disease to food group and food types in three African countries: conclusions from a structured expert judgment study. PLoS Negl. Trop. Dis. 16:e0010663. doi: 10.1371/journal.pntd.0010663
Schets, F. M., Jacobs-Reitsma, W. F., van der Plaats, R. Q. J., Heer, L. K. D., van Hoek, A. H. A. M., Hamidjaja, R. A., et al. (2017). Prevalence and types of Campylobacter on poultry farms and in their direct environment. J. Water Health 15, 849–862. doi: 10.2166/wh.2017.119
Schneider, K., Gugerty, M. K., and Plotnick, R. (2010). Poultry market in West Africa. Burkina Faso: EPAR.
Scott, E., Bloomfield, S. F., and Barlow, C. G. (1984). Evaluation of disinfectants in the domestic environment under “in use” conditions. Epidemiol. Infect. 92, 193–203.
Singh, P., Potlia, I., Malhotra, S., Dubey, H., and Chauhan, H. (2020). Hand sanitizer an alternative to hand washing—a review of literature. J. Adv. Oral Res. 11, 137–142. doi: 10.1177/2320206820939403
Somda, N. S., Bonkoungou, O. J. I., Zongo, C., Kagambèga, A., Bassolé, I. H. N., Traoré, Y., et al. (2018). Safety of ready-to-eat chicken in Burkina Faso: microbiological quality, antibiotic resistance, and virulence genes in Escherichia coli isolated from chicken samples of Ouagadougou. Food Sci. Nutr. 6, 1077–1084. doi: 10.1002/fsn3.650
Song, X., Wang, H., and Xu, X. (2021). Investigation of microbial contamination in a chicken slaughterhouse environment. J. Food Sci. 86, 3598–3610. doi: 10.1111/1750-3841.15842
Ssemanda, J. N., den Besten, H. M. W., van Wagenberg, C. P. A., and Zwietering, M. H. (2024). Quantitative assessment of food safety interventions for Campylobacter spp. and Salmonella spp. along the chicken meat supply chain in Burkina Faso and Ethiopia. Int. J. Food Microbiol. 415:110637. doi: 10.1016/j.ijfoodmicro.2024.110637
Sun, T., Liu, Y., Qin, X., Aspridou, Z., Zheng, J., Wang, X., et al. (2021). The prevalence and epidemiology of Salmonella in retail raw poultry meat in China: a systematic review and Meta-analysis. Food Secur. 10:2757. doi: 10.3390/foods10112757
Thung, T. Y., Mahyudin, N. A., Basri, D. F., Wan Mohamed Radzi, C. W. J., Nakaguchi, Y., Nishibuchi, M., et al. (2016). Prevalence and antibiotic resistance of Salmonella Enteritidis and Salmonella Typhimurium in raw chicken meat at retail markets in Malaysia. Poult. Sci. 95, 1888–1893. doi: 10.3382/ps/pew144
van Wagenberg, C. P. A., and Havelaar, A. H. (2023). Economic costs related to foodborne disease in Burkina Faso and Ethiopia in 2017. Front. Sustain. Food Syst. 7:1227430. doi: 10.3389/fsufs.2023.1227430
WHO (2006). Five keys to safer food manual. World Health Organization. Geneva, Switzerland: Department Of Food Safety, Zoonoses And Foodborne Diseases.
Wong, J. T., de Bruyn, J., Bagnol, B., Grieve, H., Li, M., Pym, R., et al. (2017). Small-scale poultry and food security in resource-poor settings: a review. Glob. Food Sec. 15, 43–52. doi: 10.1016/j.gfs.2017.04.003
Xue, G., Cheng, S., Yin, J., Zhang, R., Su, Y., Li, X., et al. (2021). Influence of pre-slaughter fasting time on weight loss, meat quality and carcass contamination in broilers. Anim. Biosci. 34, 1070–1077. doi: 10.5713/ajas.20.0560
Keywords: poultry, restaurants, food handlers, food safety, Burkina Faso
Citation: Gemeda BA, Dione M, Ilboudo G, Assefa A, Lallogo V, Grace D and Knight-Jones TJD (2024) Food safety and hygiene knowledge, attitudes and practices in street restaurants selling chicken in Ouagadougou, Burkina Faso. Front. Sustain. Food Syst. 8:1448127. doi: 10.3389/fsufs.2024.1448127
Edited by:
Nitin Dhowlaghar, The University of Tennessee, Knoxville, United StatesReviewed by:
Sandra Hoffmann, United States Department of Agriculture (USDA), United StatesAcim Heri Iswanto, Jakarta Veterans National Development University, Indonesia
Copyright © 2024 Gemeda, Dione, Ilboudo, Assefa, Lallogo, Grace and Knight-Jones. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biruk Alemu Gemeda, Yi5hLmdlbWVkYUBjZ2lhci5vcmc=
 Biruk Alemu Gemeda
Biruk Alemu Gemeda Michel Dione
Michel Dione Guy Ilboudo
Guy Ilboudo Ayalew Assefa1,4
Ayalew Assefa1,4 Delia Grace
Delia Grace Theodore J. D. Knight-Jones
Theodore J. D. Knight-Jones