- 1National Institute of Food Science and Technology, University of Agriculture, Faisalabad, Pakistan
- 2Department of Genetics, Department of Animal Science and Technology, University of Oradea, Oradea, Romania
- 3Department of Mathematics and Statistics, University of Agriculture, Faisalabad, Pakistan
- 4Division of Livestock Products Technology, SKUAST-J, Jammu, India
- 5Department of Food Science and Nutrition, College of Food and Agriculture, King Saud University, Riyadh, Saudi Arabia
Introduction: This present research was designed to investigate the anti-inflammatory and immune-modulatory effects of a 50% hydroethanolic extract of “Gola” guava fruit (GF50%) and guava leaf (GL50%) against papain-induced knee osteoarthritis (KOA).
Methods: Sixty Sprague–Dawley rats were divided into five groups (10 rats/ group): T0 (negative control), T1 (positive control), T2 (200 mg/kg GF50%), T3 (400 mg/kg GF50%), T4 (200 mg/kg GL50%), and T5 (400 mg/kg GL50%). Physical parameters were evaluated throughout the trial, while biochemical, histopathological, and radiographic analyses were performed at 0, 15, and 30 days. The histopathological and radiographic analyses were evaluated using the Osteoarthritis Research Society International (OARSI) score and Kellgren–Lawrence (KL) classification systems, respectively.
Results and discussion: The T1 group demonstrated a significant increase in knee diameter, confirming successful OA induction. The T5 group maintained a significantly lower body weight at day 30, and the T3 group exhibited the highest weight gain. The high dose of GL50% (400 mg/ kg) effectively reduced knee inflammation and significantly downregulated myeloperoxidase (MPO), interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). In contrast, it significantly (p < 0.001) upregulated the serum and knee capsule tissue superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). In addition, histopathological and X-ray examinations also confirmed the chondroprotective potential of GL50% extract against OA. Consequently, 400 mg/kg GL50% exhibited anti-inflammatory and chondroprotective potential by lowering oxidative stress and pro-inflammatory cytokines and elevating antioxidant status. These findings could provide a theoretical basis for understanding the mechanism and potential medicinal value of guava fruit and leaf in treating KOA.
1 Introduction
Osteoarthritis (OA) is a prevalent joint disorder characterized by structural and functional changes in joint tissues, including the formation of osteophytes, degradation of cartilage, and remodeling of bone. These alterations contribute to symptoms, such as pain, stiffness, swelling, and restricted joint mobility, impacting overall joint function (Allen et al., 2022; Chen, 2022). OA is the most common disability-causing condition, and its prevalence is increasing steadily, which indicates a high social burden and increases the challenges to public health. OA affected more individuals globally than any other condition between 1990 and 2019. According to Global Burden of Disease research, the crude incidence rate of OA increased by 102% between 1990 and 2017 (Quicke et al., 2022). Estimates indicate that 303 million individuals globally suffer from OA, with 61.2 million cases reported in China in 2017 (Collaborators, 2018), and in 2019, it was the 15th leading cause of years lived with disability (YLD) globally, accounting for 2% of the total global YLDs (Hunter et al., 2020).
Although OA is one of the most common and widespread joint diseases, no promising therapy is still available (Tong et al., 2022). The primary pharmacological intervention for OA mainly revolves around acetaminophen/paracetamol, oral non-steroidal anti-inflammatory medicines (NSAIDs), and intra-articular corticosteroids (Nelson et al., 2014). The long-term use of these drugs is often associated with significant side effects and toxicities (Tong et al., 2022), and it remains a significant challenge for healthcare professionals. However, the perspicacious key underlying pathways that aggravate this disease propose a plethora of bioactive agents for tackling the OA-associated complications. Nutritional approaches are gaining attention in OA prevention. Over the past 20 years, several investigations have reported that polyphenols have an advantageous function in the management of different diseases, e.g., cancer, ulcer, OA hypercholesterolemia, and toxicity, alleviating oxidative stress (Ansari et al., 2020; Ara et al., 2022; Degla et al., 2022; Mubashir et al., 2022; Nworah et al., 2022; Salsabil et al., 2022; Sirše, 2022; Swantara et al., 2022; Widowati et al., 2022; Choudhary and Tahir, 2023). Gola guava fruit and leaf (Psidium guajava L.), a member of the family Myrtaceae, possess untapped phytochemicals, e.g., gallic acid, kaempferol, ellagic acid, quercetin, chlorogenic acid, ferulic acid, ascorbic acids, m-coumaric acid, p-coumaric acid, syringic acid, and vitamin C (Nuerjiang et al., 2023). These compounds hold anti-inflammatory and antioxidant potential to repress the reactive oxygen species (ROS) and pro-inflammatory cytokine production in chondrocytes, cartilage explants, and animal OA models (Ansari et al., 2020; Sirše, 2022). Due to their potential to mitigate the OA, guava fruit and leaf antioxidant compounds are under consideration (Shahid et al., 2022a). Several studies have shown that a 50% hydro-ethanolic solvent is more appropriate to extract the bioactive compounds (ellagic acid, gallic acid, quercetin, kaempferol, chlorogenic acid, ferulic acid, ascorbic acids, m-coumaric acid, p-coumaric acid, syringic acid, and vitamin C) because of its higher capacity for extracting guava bioactive components than any other solvents (Seo et al., 2014; Tanideh et al., 2017). Another current study suggested that a 50% hydroethanolic solvent is more effective in extracting the bioactive compounds from guava fruit and leaf compared with a 70% hydroethanolic extract (Shahid et al., 2022b). Thus, this study was designed to evaluate the anti-inflammatory and immune-modulatory potential of a 50% hydroethanolic extract of white “Gola” guava fruit (GF50%) and guava leaf (GL50%) at various concentrations using a papain-induced osteoarthritis Sprague–Dawley rat model after considering this evidence. Several analyses were performed in this study, including serum and knee tissue superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and myeloperoxidase (MPO), to assess the oxidant and antioxidant levels. The immunomodulatory effects of GL50% and GF50% were assessed by evaluating interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) levels. Radiography and histopathological examinations were also performed at 0, 15, and 30 days of experiment, to examine the damage and recovery in the knee joint.
2 Materials and methods
2.1 Extract preparation
The hydroethanolic extracts of white “Gola” guava fruit and leaf of were prepared with distilled water and ethanol, with a concentration of 50%. The white “Gola” fruit and leaf were washed, diced, or sliced, and then dried in a dehydrator (Model R-5A, Commercial Dehydrator Systems, INC., United States) at 54°C for 48 h. The dried “Gola” guava fruit and leaf were finely ground using a blender (Model 31BL92, New Hartford, CT) (Patel et al., 2016). Then, 100 g of dried powder samples of the plant material was soaked in a mixture of ethanol and water with a volume ratio of 50:50. After 4 days, the samples were filtered using Whatman filter paper No. 4, and the resulting filtrates were concentrated at a temperature of 50°C using a rotary evaporator (VP-30, LabTech, Inc., Sorisole, Northern Italy). The resulting filtrates were freeze dried and stored at a − 18°C until further examination (Seo et al., 2014). Using this approach, the extracts were prepared and labeled as GF50% (4.76% yield) and GL50% (4.13% yield).
2.2 In vivo study
The Sprague–Dawley rats, weighing between 150 and 160 grams, were obtained and accommodated within the animal facility at the National Institute of Food Science and Technology, University of Agriculture Faisalabad, Pakistan. These rats were maintained at a consistent room temperature of 27 ± 2°C, following a 12-h light and 12-h dark cycle. Rats were acclimatized for the first week before being given intervention the following week. The biochemical parameters were tested on the 0th, 15th, and 30th days of experiment, while the physical parameters were evaluated throughout the trial. Rats were handled following the National Biosafety Regulations of 2005, Punjab Biosafety of 2014, Punjab Animal Health Act of 2019, and Bioethics Protocol guidelines (Tauser, 2003; Gupta et al., 2008). The study was approved by the Institutional Biosafety and Bioethical Committee (IBC, Ethical Issue No. 7990), UAF, Pakistan.
2.2.1 Induction of osteoarthritis
A 4% papain solution (Sigma, United States) with a 0.03 M cysteine solution (Sigma, USA) was prepared in distilled water for the OA model. In total, 0.2 mL of 4% papain was mixed with 0.1 mL of 0.03 mol L-1 L-cysteine and allowed to stand for half an hour. Then, 20 mL of papain-mixed solution was injected into the articular cavity of both knees on the 1st, 4th, and 7th days to induce OA (Murat et al., 2007).
2.2.2 Treatment plan
Sixty subjects were collected randomly and separated into groups (10 rats/group). Group T0 served as a control group. The experimental group was further divided into five groups: T1, T2, T3, T4, and T5, and all groups had osteoarthritic subjects. The T1 group contained osteoarthritic rats who were fed on a regular diet, while T2 subjects were given 200 mg/kg GF50% orally. The T3 group subjects were fed 400 mg/kg GF50%; the T4 group was treated with 200 mg/kg GL50% orally. T5 group subjects were given orally 400 mg/kg GL50%. All these groups were treated for 30 days and fed the standard diet with GF and GL extracts, except the control groups who were fed only the regular diet. Baseline values of the rats’ biochemical parameters after acclimatization were determined by decapitating the three animals. Three rats from each group were sacrificed to get the midline values for the biochemical parameters.
2.2.3 Body weight
The rats were weighed at the beginning of the trial and every third day using an electronic balance (AW320, Shimadzu, Tokyo, Japan) (Albus, 2012).
2.2.4 Knee diameter
The knee inflammation and anti-inflammatory effects of the extract were determined by measuring the knee diameter of every rat after 3 days using a digital caliper (Mitutoyo Absolute Digimatic 150 mm, Japan). The hair around the knee joints was trimmed to ensure accurate palpation of the knee. Then, the joints were disinfected before measuring the diameter, and the diameter of the osteoarthritic knee joint was measured and compared with the healthy rat’s knee joint diameter (Bar-Yehuda et al., 2009).
2.2.5 Serum and tissue oxidant/antioxidant assessment
Serum and knee tissue samples from the rats were obtained in order to assess the levels of antioxidant enzymes, specifically SOD, CAT, and GPx. The experimental rat samples were carefully excised and homogenized in a 50 mM phosphate buffer with a pH of 7.4. The homogenates were then subjected to centrifugation (Eppendorf® Centrifuge 5,804, Hamburg, Germany) at 5,000 rpm for 20 min, followed by cooling at a temperature of 4°C. The resulting supernatants were transferred to Eppendorf tubes and stored at −40°C for subsequent analysis (Wu et al., 2022). The SOD activity of serum (U/ml) and knee tissues (U/mg) was estimated by following the method described by Dawood et al. (2020). The catalase activity of the rat’s serum (U/ml) and knee tissues (U/mg) was assessed based on hydrogen peroxide (H2O2) decomposition (Sinha, 1972). The serum GPx (U/ml) activity was assessed using the method described by Pilarczyk et al. (2012). According to the protocol, MPO (mU/mg) activity in knee was assessed using H2O2 solution (De Young et al., 1989). The absorbance of the samples was measured via UV–VIS spectrophotometer (PG Instruments, T80, United Kingdom).
2.2.6 Immunochemical analysis
Several blood biomarkers, such as TNF-α, IL-1β, and IL-6, were assessed using the sandwich enzyme-linked immunosorbent assay (ELISA) method (Zhao et al., 2015). For that analysis, blood samples obtained from the secondary aorta subjected to centrifugation at 100 × g for 15 min, and the resulting supernatants were used immediately to quantify TNF-α (pg/ml), IL-1β (pg/ml), and IL-6 (pg/ml) using ELOSA DSX best 2000® microtiter plate and the ELISA kits (MyBioSource, Inc.).
2.2.7 Radiography analysis
An X-ray examination was performed on osteoarthritic and control groups to examine changes in the knee joint on 0th, 15th, and 30th days of the experiment. The anesthetized rats were moved onto the animal bed and performed X-ray imaging in their dorsal recumbent position, and the results were evaluated according to the Kellgren–Lawrence (KL) classification system, which consisted of four grades (0–4): grade 0 (normal joint), grade 1 (doubtful), grade 2 (minimal), grade 3 (moderate), and grade 4 (severe) (Luijkx and Pai, 2016).
2.2.8 Histopathological examination
The joints were removed for histopathological examination and fixed in 4% paraformaldehyde at room temperature for 2 h. Then, decalcification was performed using a 10% ethylenediaminetetraacetic acid (EDTA) solution. Decalcification took 4 weeks and was performed at room temperature. From knee joints, 5 μm thick serial sections were cut after dehydrating and embedding these sections in paraffin. Before staining, paraffin wax was removed by xylene and xylene substitutes which enable Hematoxylin–Eosin (H&E) (Muto, Japan) staining of tissues in the aqueous hematoxylin solution (Feldman and Wolfe, 2014). By fluorescent microscopy (BX51, Olympus, Japan), images were captured at 40X, and the damage to the knees due to OA was assessed by the Osteoarthritis Research Society International (OARSI) scoring system (Gerwin et al., 2010) (Supplementary Data 1).
2.3 Statistical analysis
The experimental data in this study were the average of triplicate replications and were analyzed by applying two-way analysis of variance (ANOVA), and the results were reported as mean ± standard deviation. Tukey’s HSD test was employed to determine the statistical difference among groups. SPSS 20.0 software (IBM Corp., Armonk, NY, United States) and GraphPad Prism 5.04 (GraphPad Software Inc., USA) were used to evaluate the data at a p-value of <0.05. Additionally, the effect size was expressed as standardized mean differences (Cohen’s d) (Montgomery, 2019).
3 Results
3.1 Body weight
The effect of treatments on the body weight of papain-induced osteoarthritis rats was observed. As shown in Figures 1A,B, statistically highly significant differences (p < 0.01) in body weight were found across all groups. The body weight was markedly increased in the T3 group followed by the T2 group. A significant decrease in weight was observed in the T5 group, followed by the T4 group. However, no significant difference in body weight was observed between the negative and positive control groups.
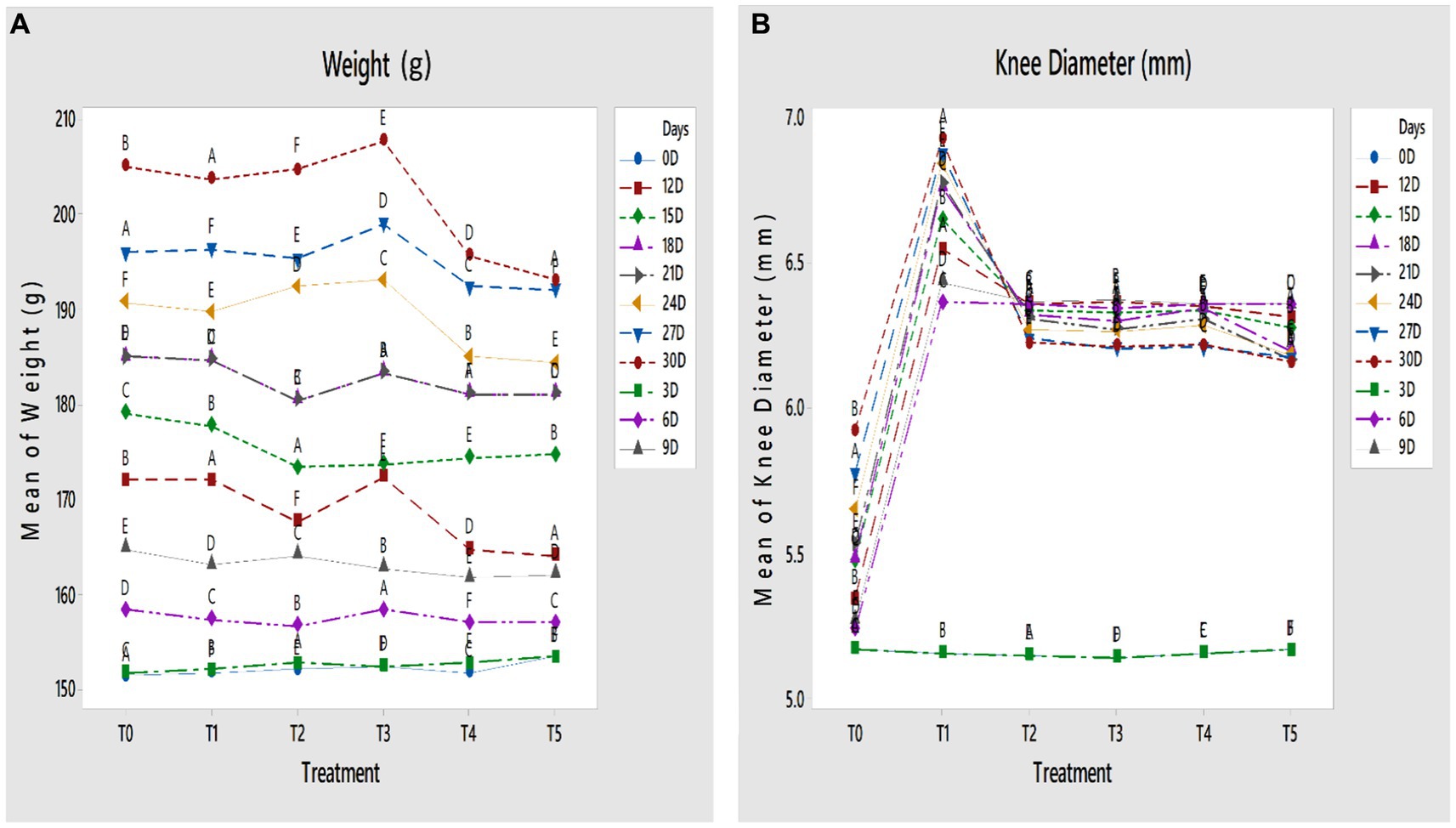
Figure 1. (A,B) Effect of GL50% and GF50% extracts on (A) weight (g) and (B) knee diameter (mm) of papain-induced osteoarthritis rats. T0: control group, T1: positive group, T2: 200 mg/kg GF50%, T3: 400 mg/kg GF50%, T4: 200 mg/kg GL50%, T5: 400 mg/kg GL50%. All values are mean ± SD of 10 rats in each group. Dissimilar letters indicate a significant difference (p < 0.05).
3.2 Knee diameter
The knee diameter of papain-induced osteoarthritis rats was measured throughout the study, to evaluate the anti-osteoarthritic potentiality of GF50% and GL50%. Highly significant differences were found among all treatment groups (p < 0.01). The knee joint diameter in the T5 group significantly decreased compared with other treatment groups at the end of the study. The knee joint diameter of the T4 group was significantly smaller than the T2 and T3 groups at the end of the study, as shown in Figures 1A,B.
3.3 Serum and tissue oxidant/antioxidant assessment
As presented in Table 1, the results show that papain significantly plummeted the serum antioxidants level (sSOD, sCAT, and sGPX). Non-significant differences were found in the negative control group, whereas significant differences were found among all treatment groups (p < 0.01). A significantly higher (p < 0.001) level of serum SOD, CAT, and GPX was found in the T5 group than other treatment groups. The T4 group has lower serum antioxidant level than the T5 group. In contrast, the T2 group exhibited a plummet level of serum antioxidants compared with the T3 group. A similar trend of the antioxidant and oxidant contents for the knee capsule tissue has been found. Figures 2A,B presented a significantly high (p < 0.001) level of knee MPO and significantly low level of knee SOD in the T1 group. The knee capsule tissue SOD significantly (p < 0.001) leaped while knee capsule MPO significantly decreased than other treatments.
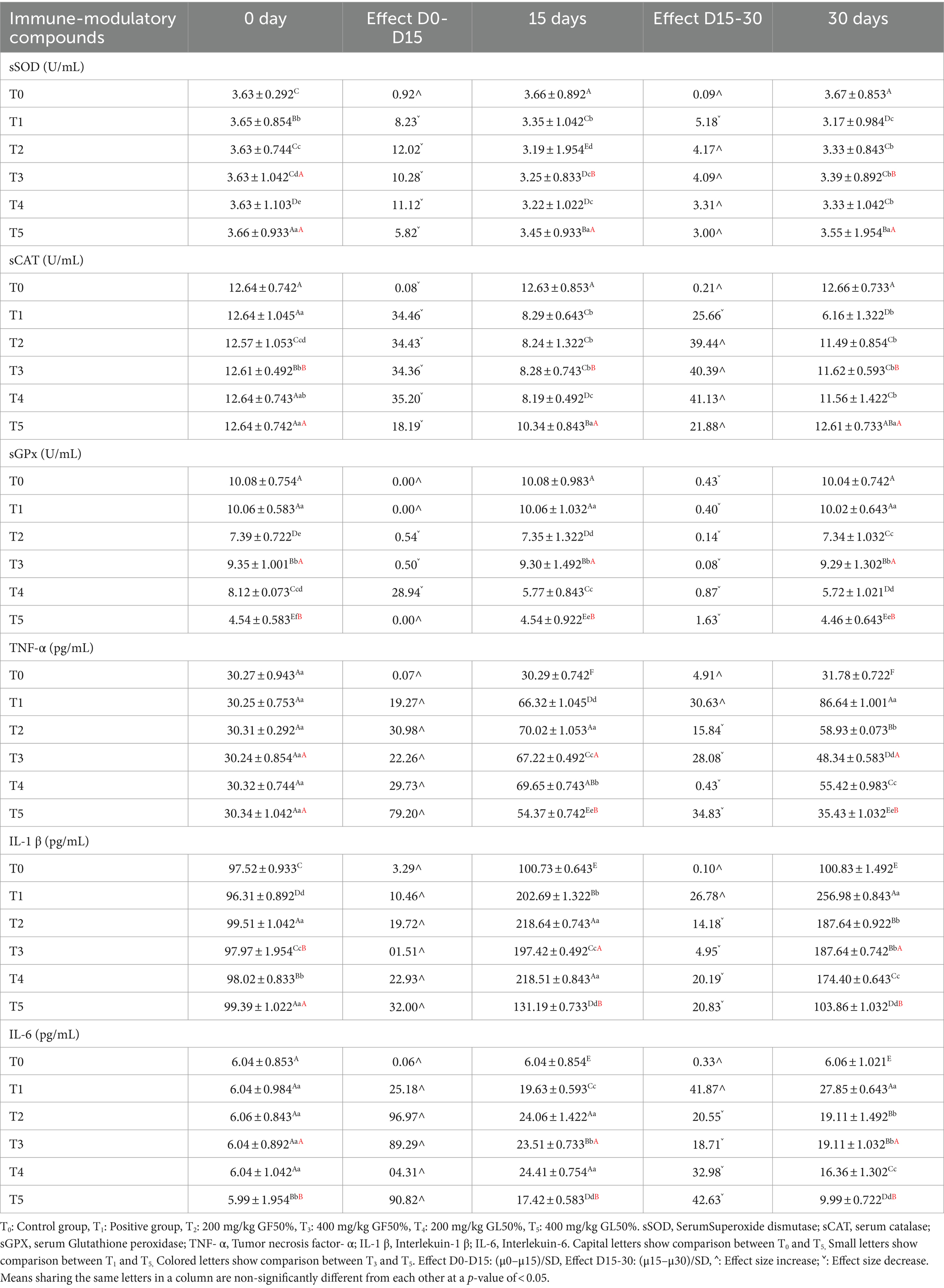
Table 1. Serum values (mean ± SD) of SOD (U/ml), CAT (U/ml), GPX (U/ml), TNF- α (pg/ml), IL-1 β (pg/ml), and IL-6 (pg/mL) of papain-induced osteoarthritis rats treated with GL50% and GF50% at different doses.
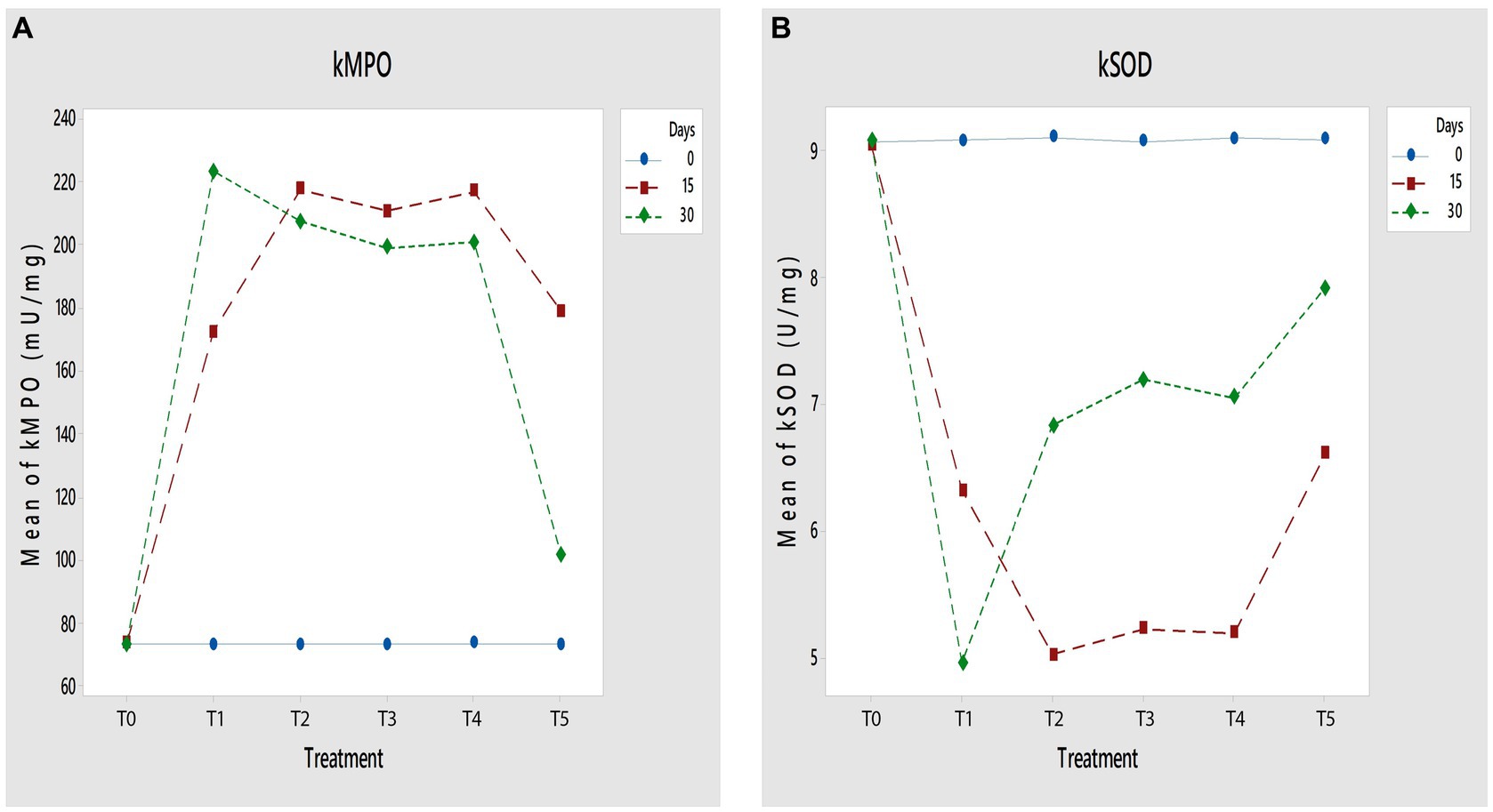
Figure 2. (A,B) Effect of GL50% and GF50% extracts on (A) kMPO (mU/mg) and (B) kSOD (U/mg) of papain-induced osteoarthritis rats. T0: control group, T1: positive group, T2: 200 mg/kg GF50%, T3: 400 mg/kg GF50%, T4: 200 mg/kg GL50%, T5: 400 mg/kg GL50%. All values are mean ± SD of 10 rats in each group.
3.4 Immunochemical analysis
As presented in Table 1, the results show a significantly high (p < 0.001) activity of TNF-α, IL1-β, and IL-6 in the T1 group compared with the positive control group. TNF-α, IL1-β, and IL-6 concentrations in the T5 group were significantly lower compared with other treatment groups. The intra-comparison of the T2 and T3 groups manifested that the T3 group exhibited a plummet level of TNF-α, IL1-β, and IL-6 compared with the T2 group, and the T5 group has significantly lower concentrations of TNF-α, IL1-β, and IL-6 than the T4 group.
3.5 Radiography analysis
The radiographic analysis (Figures 3, 4) conducted on the 15th and 30th days of the experiment revealed that the knee joints of the T0 group were in a healthy condition with normal articular surfaces, graded as “0” according to the KL classification system. Groups T1, T2, and T3 exhibited the development of osteophytes and marked joint space narrowing (JSN), and these radiographs were graded as “4.” Group T4 has multiple osteophytes and was graded as “3.” Group T5 has mild damage with possible JSN, and this radiograph is graded as “2.” The radiographs of groups T2 and T3 on day 30 showed a minor improvement and were graded as “4.” The overall joint health and medical conditions in groups T4 and T5 showed substantial improvement at the end of the study. The group T4 was graded as “3.” In group T5, the bone surface returned to a nearly normal state, yet it exhibited possible JSN and minor reactive alterations and was graded less than “1” but not “0,” according to the KL classification.
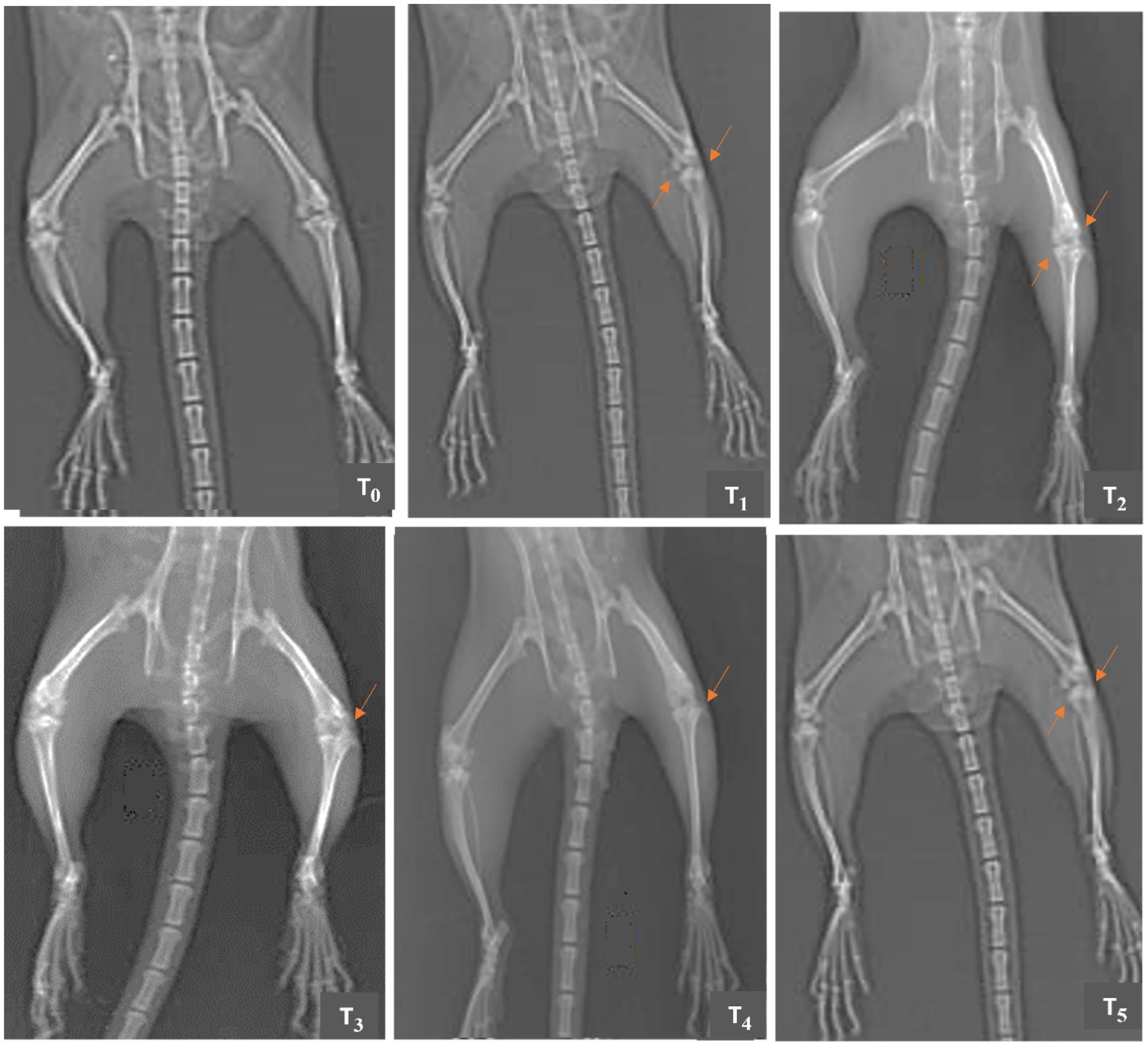
Figure 3. Comparative X-rays of papain-induced osteoarthritis rats treated with GL50% and GF50% on day 15 of the experimental study. T0 (control group) has no structural changes or joint space narrowing (JSN); T1 (positive group) has marked JSN, severe sclerosis, and osteophytes; T2 (200 mg/kg GF50%) has structural deformities, severe sclerosis, and JSN; T3 (400 mg/kg GF50%) has definite JSN, moderate osteophytes, and sclerosis; T4 (200 mg/kg GL50%) has osteophytes, sclerosis, and definite JSN; T5 (400 mg/kg GL50%) has JSN and osteophyte.

Figure 4. Comparative X-rays of papain-induced osteoarthritis rats treated with GL50% and GF50% on day 30 of the experimental study. T0 (control group) has no radiographic features of OA; T1 (positive group) has large osteophytes, marked JSN, and severe sclerosis; T2 (200 mg/kg GF50%) has osteophytes, structural deformities, definite JSN, and severe sclerosis; T3 (400 mg/kg GF50%) also has definite JSN, osteophytes, and sclerosis; T4 (200 mg/kg GL50%) has multiple osteophytes, possible JSN, and sclerosis; T5 (400 mg/kg GL50%) has doubtful JSN.
3.6 Histopathology
Histopathologic samples collected on the 15th and 30th days of the study were observed to evaluate the osteoarthritis-related lesions (Figures 5, 6), respectively. On the 15th day of the experiment, the T0 slide indicated the knee joint as entirely normal with smooth articular surfaces, and this slide scored “0” according to the OARSI score system. In the T1 group, evident basophilic infiltration, chondrocyte proliferation, and irregular areas of fibro hyaline cartilage, marked by the black arrowhead, were observed. According to the OARSI score system, the damage is almost 75%; thus, the T1 slide can be scored as “5”. Group T2 slide showed basophilia and fissures in the cartilaginous matrix. Osteophyte formation is also evident in this group and is scored as “5”. The irregularly organized cluster of some newly deposited chondrocytes is conspicuous in the T3 group, with vertical clefts in the calcified cartilage extending to 50% of the articular cartilage. This slide is scored as “4”. The newly deposited chondrocytes in an irregular pattern corroborate the regeneration of cells in the T4 group with fissures, and this slide is scored as “4”. The slide of the T5 group is scored as “2” as minimal degradation is evident with newly formed chondrocyte depositions and arrangements. The cartilage cell development over the calcified region is better in this slide than in all previous slides.
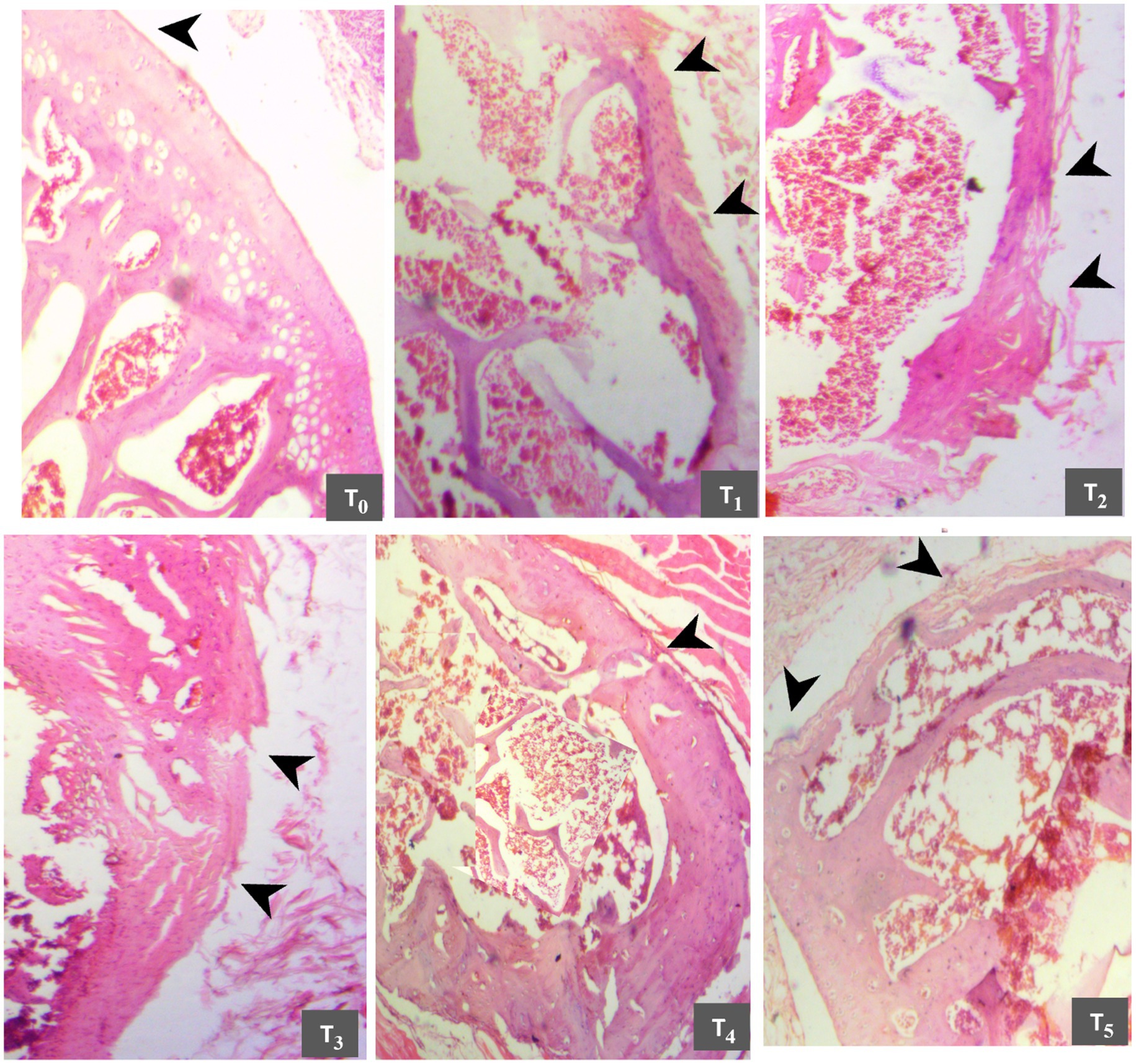
Figure 5. Comparative histomicrographs of papain-induced osteoarthritis rats treated with GL50% and GF50% on day 15 of the experimental study. T0 (control group): Normal articular structure with intact surface and chondrocytes; T1 (positive group): Complete cartilage erosion, severe proteoglycan loss, and matrix discontinuity; T2 (200 mg/kg GF50%): hypocellularity in transitional and radial zones, and clefts until tide mark; T3 (400 mg/kg GF50%,): Cartilage erosion, fissure, and proteoglycan loss; T4 (200 mg/kg GL50%,): proteoglycan loss and cartilage loss; T5 (400 mg/kg GL50%): Small fibrillations without loss of cartilage.
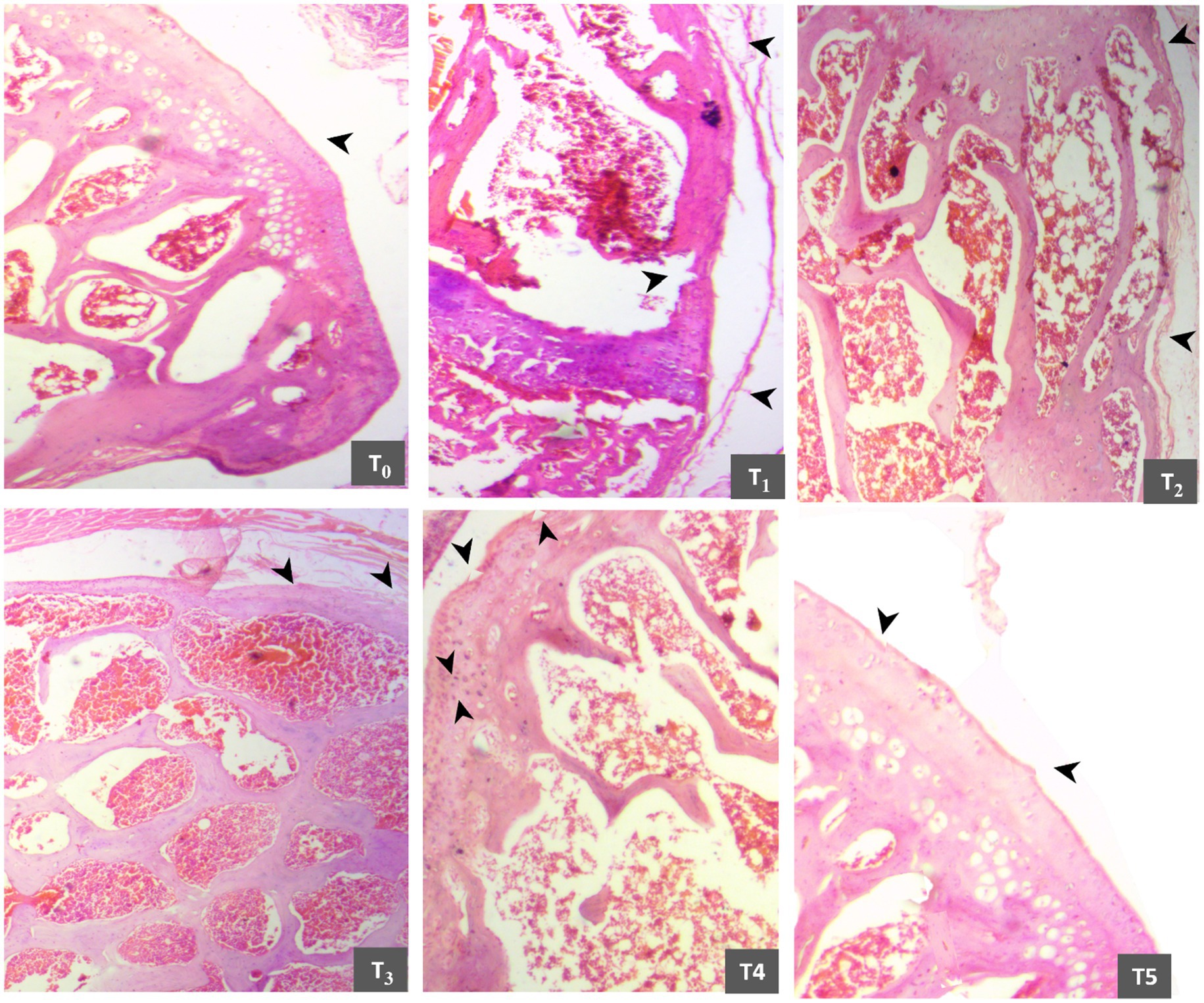
Figure 6. Comparative histomicrographs of papain-induced osteoarthritis rats treated with GL50% and GF50% on day 30 of the experimental study. T0 (control group): smooth surface and regular chondrocytes; T1 (positive group): matrix discontinuity, disorganized chondrocytes, cartilage, and proteoglycan loss; T2 (200 mg/kg GF50%): matrix discontinuity and cartilage loss; T3 (400 mg/kg GF50%,): fibrillation, fissures, and inflammatory infiltration; T4 (200 mg/kg GL50%): Fibrillation and fissures; T5 (400 mg/kg GL50%): intact surface with minor superficial fibrillation.
On the 30th day of research, the score remained the same for the T0 group. However, the score for the T1 group increased from “5” to “6”, and the score for the T2 group did not change. The T3 group showed healing, but its score did not decline from “4”. The T4 group slide received a score of “3”. While tremendous histopathological improvement is prominent in the T5 group, it secured a score close to “0” (Supplementary Data 2).
4 Discussion
In the current study, 50% hydroethanolic “Gola” guava fruit and leaf extracts were prepared and their anti-inflammatory and immunomodulatory activities were evaluated against the papain-induced knee OA (KOA) rat model. Papain injection is the most common and popular drug used for establishing the OA animal model analogous to OA in humans. The advantages of this model are high success rate, quick modeling time, and strong repeatability (Cheng et al., 2019). Excessive weight gain, or obesity, is a decisive risk factor for OA. The OARSI recommendations for KOA observed that weight loss was suggested as a primary treatment in 13 worldwide recommendations (Wu et al., 2022). A high body mass index is a risk factor for KOA and OA (King and March, 2013). Because of this close relationship between weight and KOA, weight gain and loss patterns were observed in this study. A significant difference in the body weight of osteoarthritic rats fed on GL and GF extracts was observed, as agreed with the previous study, which reported that low weight was observed in the group fed on guava leaf extract (Kawasaki et al., 2018). These findings concord with the result of our study, as high weight gain was not observed in rats fed on GL extract compared with groups fed on GF extract (T3 and T2), which showed the weight gain trend. The findings of another study justified the low weight gain trend in rats (T4 and T5) fed on GL extract (Rahman et al., 2013). This previous study incorporated the GL meal into the broiler’s diet to assess its effect on broiler growth and found that 2.5 to 4.5% GL meal significantly decreased broiler fat. Oliveira et al. (2018) investigated the effect of guava fruit byproducts on broiler weight. After 7 days, the trial showed a significant weight gain. Adding guava fruit waste to a broiler diet linearly boosted weight gain. As guava fruit byproduct integration increased, the thiobarbituric acid reactive substances (TBARSs) declined to 0.72%, showing enhanced lipid stability in thigh meat (Oliveira et al., 2018). These findings concord with the current study results, as the rats of T3 and T2 groups fed on “Gola” guava fruit extracts demonstrated the highest weight gain at the end of the study.
Inflammation of joints is a clinical feature of OA, and now more than ever, the role of inflammation in the development and progression of OA is widely accepted. In many studies on OA, joint diameter is used as an evaluator parameter to assess the anti-inflammatory effect of the active ingredient (Möller et al., 2019). The current study used papain injections in the articular cavity to induce OA. The knee diameter of the T0 group was significantly smaller compared with the T1 group, which showed that T1 recapitulated the OA features, manifesting the successful induction of OA through papain injections (Murat et al., 2007). After 30 days of extract administration, rats fed on GL (T5 and T4) had a significantly smaller knee diameter than rats fed on GF (T2 and T3). The intra-comparison of the groups T4 (200 mg/kg GF50%) and T5 (400 mg/kg GL50%) manifested that a high dose of GL50% 400 mg/kg was more effective in reducing the inflammation than 200 mg/kg GL50%. The results of this study agree with the previous study, which manifested the anti-inflammatory properties of guava leaf extract (Weni et al., 2011). In vitro investigations have demonstrated the capacity of guava leaf extract to diminish the expression of pro-inflammatory mediators induced by lipopolysaccharide, suggesting its potential benefits for enhancing biological cell activity (Choi et al., 2008). The result of the current study corroborated that GL50% at a high dose (400 mg/kg) has significantly higher anti-inflammatory potential than GF50%, and the justification for this result is that guava leaves have a higher polyphenol content (gallic acid, ellagic acid, etc.) than guava fruit (Zhao et al., 2022), which signifies the anti-inflammatory properties by suppressing the expression of the cyclooxygenase-2 (COX-2) enzyme (Umesalma and Sudhandiran, 2010).
Due to the eccentric nature of OA, the pathophysiological basis of OA is not yet fully understood, although the incrimination of reactive oxygen species (ROS) in its pathogenesis is evident. The adverse effects of ROS are usually blocked by the natural antioxidant defense system of the body, including SOD, CAT, and GPx. However, in the pathological state of OA, either the antioxidant levels deplete or/and excess ROS accumulates in cartilage and synovium, disrupting the equilibrium between antioxidants and oxidative stress level (Gui et al., 2022). The result of the study showed that rats in the group T5 that were fed a high dose of GL extract (400 mg/kg) have elevated serum and joint capsule SOD, CAT, and GPx than the rats fed on a lower dose of GL extract (T4) and GF extract (T2, and T3). The restoration of antioxidant level to the standard levels in the T5 group indicates that a 400 mg/kg GL50% extract has a high antioxidant activity. The findings of this study similar to numerous other studies signify that ellagic acid, quercetin, and gallic acid are the principal antioxidants in guava leaves that repress oxidative stress and inflammation (Vijayababu et al., 2006; Naseer et al., 2018; Sampath Kumar et al., 2021). Wen et al. (2015) concluded this study that gallic acid ascends the serum SOD level and has a chondroprotective role that can be used as a potential drug for OA. A review conducted in 2019 elucidated the role of gallic acid in oxidative stress reported that gallic acid significantly alleviates the oxidative stress and elevates the GPx level (Gao et al., 2019). Oral administration of quercetin repressed the bone loss of diabetic osteopenia by significantly increasing urinary deoxypyridinoline, serum GPx, SOD, CAT, alkaline phosphatase activity, and osteocalcin in diabetic rats (Liang et al., 2011).
One of the decisive pro-inflammatory cytokines involved in the pathogenesis of OA is IL-1β, which activates the major signaling pathway NFκB, and then, NFκB activates the synthesis of various pro-inflammatory cytokines, such as TNF-α and IL-6 (Grässel and Aszodi, 2019; Sirše, 2022; Socol et al., 2022) According to Vincent (2020), a number of therapeutic research studies have shown that blocking the IL-6 declined the OA progression. The current research outcomes ascertained the potential of “Gola” guava leaf extract to repress the IL-1β, IL-6, and TNF-α that are crucial in OA pathogenesis. A significant increase in inflammatory cytokines was discernible in the group T1 on the 15th and 30th day of the study. The results of this study are similar to previous studies. El-Seweidy et al. demonstrated that TNF- α expression is high in osteoarthritic rats compared with the negative control groups (El-Seweidy et al., 2016). These findings supported the current study’s TNF- α expression result, which was higher in osteoarthritic rats than in the negative control group. These findings supported the current study’s TNF- α expression result, which was higher in osteoarthritic rats than in the negative control group. While the rats fed a high dose of GL50% (400 mg/kg) had significantly lower expressions of TNF-α, IL1-β, and IL-6 than the rats fed a lower dose of GL50% (T4) and GF50% (T2, and T3), indicating the potential of “Gola” guava leaf extract (400 mg/kg) in attenuating the inflammatory cytokines. The result of the study similar to a previous study manifested that guava leaf extract significantly suppressed the lipopolysaccharide (LPS)-induced pro-inflammatory cytokine expression in RAW 264.7 macrophages. Furthermore, guava leaf extract also suppressed TNF-α, IL1-β, and IL-6 significantly in different animal models of inflammation (Jang et al., 2014). Another author stated that gallic acid can significantly alleviate TNF- α and IL-6 expression and protect against inflammation (Wu et al., 2022). Quercetin, gallic acid, and ellagic acid repress the inflammatory cytokines, but particularly ellagic acid suppresses IL-6 and TNF- α expression in LPS-treated RAW 264.7 cells (Seo et al., 2016).
The radiographs of rats at 0th, 15th, and 30th days of the experiment manifested the healing potential of “Gola” guava leaf extract for OA-affected joints. A greater degree of healing was observed for T5 than T4, T3, and T2 groups. The result of the current study is in accordance with previous studies that “Gola” guava leaf extract has ameliorative potential to alleviate OA progression (Kakuo et al., 2018; Kawasaki et al., 2018).
A histological assay is crucial for evaluating the changes and degradation of the joint tissue caused by degenerative joint diseases in animal and human models. The histological characteristics of diseases are critical and well suited for demonstrating the alteration in the early stage of OA (Grässel and Aszodi, 2019). For accurate histological assessment, the staining method is crucial. Various staining methods are recommended, but we used Hematoxylin and Eosin (H&E) because it is sensitive enough to demarcate the chondrocytes, tidemark, and other important features critical to accurately assess the articular cartilage damage (McNulty et al., 2011). In this current study, on the 15th and 30th days of the research, the cartilage matrix can be observed as normal across the slide, which is also marked with an arrowhead in T0 slide. In the T1 group, the severe basophilia and chondrocyte proliferation were profound in this slide; the black arrowhead marked these small, packed cyst-like enclosures. The calcified cartilaginous tissues are prominent in this slide. These findings supported the previous study that articular cartilage destruction aggravates in papain-induced OA rats over time (Cheng et al., 2019). Another study also aligned with the current findings and reported that papain caused condyle deformation, middle-layer chondrocyte disorientation, deep zone proliferation, and increased extracellular matrix which worsen with time (Molinet et al., 2020). The T3 and T4 slides showed irregularly arranged clusters of some locally deposited chondrocytes, upregulated cellular growth, and ossification, whereas the T4 slide showed greater healing than the T3 slide. However, the chronicity of this disease was not fully cured with these extracts. The T5 slide has highly irregularly distributed subchondral bony tissue development, which indicated significant healing and recovery in that slide on the 15th day. On the 30th day, this slide depicted the preeminent improvement compared with all other slides. The papain-induced KOA substantially improved with a higher dose of “Gola” guava leaf extract, and on the 30th day of the experiment, the structure of that slide is analogous to the negative control slide. Damage and regeneration for that slide can be rated slightly lower than “1” but not “0” on the OARSI scale (Supplementary Data 3).
This improvement corroborates that “Gola” guava leaf extract exhibited a dose-related effect for healing of OA-affected structures based on both histopathological and radiological features; a greater degree of healing was observed after 400 mg/kg treatment than that after 200 mg/kg treatment. In another study, the same trend has been observed in KOA histograms that guava leaf extract enhances the regeneration of chondrocytes and cartilage tissues (Kawasaki et al., 2018). Based on the findings of that study, it can be deduced that GL50% at a high dose can suppress inflammation by repressing the inflammatory cytokines, reducing the activation of the immune response, and elevating the natural antioxidants more than GF50%. The explanation for the result is that guava leaves have a higher polyphenol content than guava fruits (Gerwin et al., 2010). Shahid et al. (2022b) also found that 50% “Gola” guava leaf extract has higher antioxidant and polyphenol contents than 50% “Gola” guava fruit extract.
5 Conclusion and future perspective
The findings of this study suggest that GL50% has higher therapeutic and restorative effects than GF50% in treating papain-induced OA in rats because of its higher antioxidant and polyphenol contents than GF. GL50% at a high dose (400 mg/kg) showed that chondroprotective effects were mediated by the modulation of pro-inflammatory mediators (TNF-α, IL-1β, and IL-6) and antioxidant/oxidative stress markers (SOD, CAT, GPx, and MPO) without endangering hepatic or renal health (Supplementary Table S1). However, it should be noted that this study has examined only Sprague–Dawley rat models; therefore, at present, it is not clear if there are unwanted side effects in practical clinic applications. Due to time and funding constraints, we did not study the potential of “Gola” guava fruit and leaf extract to mitigate the inflammatory cytokines in synovial fluid and its effect on gene expression in the OA models. Therefore, further studies are required to investigate the effects of guava fruit and leaf extract in modulating gene expression pathways involved in OA and evaluate bioavailability, pharmacokinetic activities, and interaction of active compounds with other compounds. Future studies of guava fruit and leaf extract should focus on their synergistic effects in combination with other conventional therapies. These investigations are crucial to understand the potential benefits and limitations of these extracts for OA treatment in humans, which can pave the way for the formulation of functional foods and drugs from “Gola” guava leaf extract.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Institutional Biosafety and Bioethics Committee, the animal efficacy trial was carried out in the NIFSAT, UAF and local community of Faisalabad with the approval of the Institutional Biosafety and Bioethics Committee (D#7990/ORIC) UAF, Pakistan. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AS: Formal analysis, Investigation, Writing – original draft. MI-U-R: Conceptualization, Supervision, Writing – review & editing. CS: Conceptualization, Data curation, Writing – review & editing. CM: Writing – review & editing. FC: Writing – review & editing. HM: Formal analysis, Software, Writing – original draft. ZB: Writing – review & editing. SH: Validation, Writing – review & editing. RA: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. University of Oradea provided funding for publication of this research. This research was supported by the National Institute of Food Science and Technology of University of Agriculture Faisalabad, Pakistan.
Acknowledgments
The authors also appreciate the support from the Researchers Supporting Project number (RSPD2024R1073), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2024.1442219/full#supplementary-material
References
Albus, U. (2012). Guide for the care and use of laboratory animals (8th edn). 46: 267–268. Available at: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf
Allen, K. D., Thoma, L. M., and Golightly, Y. M. (2022). Epidemiology of osteoarthritis. Osteoarthr. Cartil. 30, 184–195. doi: 10.1016/j.joca.2021.04.020
Ansari, M. Y., Ahmad, N., and Haqqi, T. M. (2020). Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed. Pharmacother. 129:110452. doi: 10.1016/j.biopha.2020.110452
Ara, C., Arshad, A., Faheem, M., Khan, M., and Shakir, H. A. (2022). Protective potential of aqueous extract of Allium cepa against tartrazine induced reproductive toxicity. Pak. Vet. J. 42, 358–363. doi: 10.29261/pakvetj/2022.029
Bar-Yehuda, S., Rath-Wolfson, L., Del, V. L., Ochaion, A., Cohen, S., Patoka, R., et al. (2009). Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis Rheum. 60, 3061–3071. doi: 10.1002/art.24817
Chen, D. (2022). Osteoarthritis: a complicated joint disease requiring extensive studies with multiple approaches. J. Ortho. Transl. 32:130. doi: 10.1016/j.jot.2022.02.009
Cheng, F., Yan, F. F., Liu, Y. P., Cong, Y., Sun, K. F., and He, X. M. (2019). Dexmedetomidine inhibits the NF-κB pathway and NLRP3 inflammasome to attenuate papain-induced osteoarthritis in rats. Pharm. Biol. 57, 649–659. doi: 10.1080/13880209.2019.1651874
Choi, S., Hwang, J., Park, S., Jin, Y., Ko, H., Moon, S., et al. (2008). Fermented guava leaf extract inhibits LPS-induced COX-2 and iNOS expression in mouse macrophage cells by inhibition of transcription factor NF-κB. Phytother. Res. 22, 1030–1034. doi: 10.1002/ptr.2419
Choudhary, A. N., and Tahir, F. (2023). The therapeutic effect of Gymnema sylvestre extract against hyperglycemia: in vivo study. Agrobiol. Rec. 14, 50–58. doi: 10.47278/journal.abr/2023.038
Collaborators, G. B. D. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 392, 1789–1858. doi: 10.1016/S0140-6736(18)32279-7
Dawood, M. A. O., Eweedah, N. M., El-Sharawy, M. E., Awad, S. S., Van Doan, H., and Paray, B. A. (2020). Dietary white button mushroom improved the growth, immunity, antioxidative status and resistance against heat stress in Nile tilapia (Oreochromis niloticus). Aquaculture 523, 735229–735238. doi: 10.1016/j.aquaculture.2020.735229
De Young, L. M., Kheifets, J. B., Ballaron, S. J., and Young, J. M. (1989). Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 26, 335–341. doi: 10.1007/BF01967298
Degla, L. H., Kuiseu, J., Olounlade, P. A., Attindehou, S., Hounzangbe-Adote, M. S., Edorh, P. A., et al. (2022). Use of medicinal plants as alternative for the control of intestinal parasitosis: assessment and perspectives. Agrobiol Rec. 7, 1–9. doi: 10.47278/journal.abr/2021.011
El-Seweidy, M. M., Sousou, I. A., Elswefy, S. E., and Mashhour, M. M. (2016). Omega 3-fatty acids, atorvastatin as modulators for inflammatory pattern versus diclofenac in osteoarthritis induced in experimental rats. Afr. J. Pharm. Pharmacol 10, 472–479. doi: 10.5897/AJPP2016.4558
Feldman, A. T., and Wolfe, D. (2014). Tissue processing and hematoxylin and eosin staining. Histopathology 1180, 31–43. doi: 10.1007/978-1-4939-1050-2_3
Gao, J., Hu, J., Hu, D., and Yang, X. (2019). A role of gallic acid in oxidative damage diseases: a comprehensive review. Nat. Prod. Commun. 14:1934578X19874174. doi: 10.1177/1934578X1987417
Gerwin, N., Bendele, A. M., Glasson, S., and Carlson, C. S. (2010). The OARSI histopathology initiative–recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 18, S24–S34. doi: 10.1016/j.joca.2010.05.030
Grässel, S., and Aszodi, A. (2019). Osteoarthritis and cartilage regeneration: focus on pathophysiology and molecular mechanisms. Int. J. Mol. Sci. 20, 6156–6167. doi: 10.3390/ijms20246156
Gui, T., Luo, L., Chhay, B., Zhong, L., Wei, Y., Yao, L., et al. (2022). Superoxide dismutase-loaded porous polymersomes as highly efficient antioxidant nanoparticles targeting synovium for osteoarthritis therapy. Biomaterials 283, 121437–121462. doi: 10.1016/j.biomaterials.2022.121437
Gupta, K., Karihaloo, J.L., and Khetarpal, R.K. (2008). Biosafety regulations of Asia-Pacific countries. Pp. 108. Bangkok: Asia-Pacific Association of Agricultural Research Institutions; Asia-Pacific consortium on agricultural biotechnology, New Delhi and food and agricultural Organization of the United Nations, Rome.
Hunter, D. J., March, L., and Chew, M. (2020). Osteoarthritis in 2020 and beyond: a lancet commission. Lancet 396, 1711–1712. doi: 10.1016/S0140-6736(20)32230-3
Jang, M., Jeong, S. W., Cho, S. K., Ahn, K. S., Lee, J. H., Yang, D. C., et al. (2014). Anti-inflammatory effects of an ethanolic extract of guava (Psidium guajava L.) leaves in vitro and in vivo. J. Med. Food 17, 678–685. doi: 10.1089/jmf.2013.2936
Kakuo, S., Fushimi, T., Kawasaki, K., Nakamura, J., and Ota, N. (2018). Effects of Psidium guajava Linn. Leaf extract in Japanese subjects with knee pain: a randomized, double-blind, placebo-controlled, parallel pilot study. Aging Clin. Exp. Res. 30, 1391–1398. doi: 10.1007/s40520-018-0953-6
Kawasaki, K., Fushimi, T., Nakamura, J., and Ota, N. (2018). Guava leaf extract suppresses osteoarthritis progression in a rat anterior cruciate ligament transection model. Food Sci. Nutr. 6, 800–805. doi: 10.1002/fsn3.601
King, L. K., and March, L. (2013). Obesity & osteoarthritis. Indian J. Med. Res. 138, 185–193. Available at: https://pubmed.ncbi.nlm.nih.gov/24056594/
Liang, W., Luo, Z., Ge, S., Li, M., Du, J., Yang, M., et al. (2011). Oral administration of quercetin inhibits bone loss in rat model of diabetic osteopenia. Eur. J. Pharmacol. 670, 317–324. doi: 10.1016/j.ejphar.2011.08.014
Luijkx, T., and Pai, V. (2016). Kellgren and Lawrence system for classification of osteoarthritis of knee. Datum pristupa. 7. Dostupno na adresi: http://radiopaedia.org/articles/kellgren-and-lawrencesystem-for-classification-of-osteoarthritis-of-knee
McNulty, M. A., Loeser, R. F., Davey, C., Callahan, M. F., Ferguson, C. M., and Carlson, C. S. (2011). A comprehensive histological assessment of osteoarthritis lesions in mice. Cartilage 2, 354–363. doi: 10.1177/1947603511402665
Molinet, M., Alves, N., Vasconcelos, A., and Deana, N. F. (2020). Comparative study of osteoarthritis induced by monoiodoacetate and papain in rabbit temporomandibular joints: macroscopic and microscopic analysis. Folia Morphol. (Warsz) 79, 516–527. doi: 10.5603/FM.a2019.0104
Möller, K. Ä., Klein, S., Seeliger, F., Finn, A., Stenfors, C., and Svensson, C. I. (2019). Monosodium iodoacetate-induced monoarthritis develops differently in knee versus ankle joint in rats. Neurobiol. Pain 6, 100036–100044. doi: 10.1016/j.ynpai.2019.100036
Montgomery, D. C. (2019). Design and analysis of experiments. 10th Edn: John wiley & sons. Available at: https://www.researchgate.net/profile/Farshad-Fattahi/post/Need-the-procedure-for-critical-limit-fixation/attachment/59d6459179197b80779a0aa7/AS%3A453901993418752%401485230076186/download/Douglas-C.-Montgomery-Design-and-Analysis-of-Experiments-Wiley-2012.pdf
Mubashir, A., Ghani, A., and Mubashar, A. (2022). Common medicinal plants effective in peptic ulcer treatment: a nutritional review. Int. J. Agric. Biosci. 11, 70–74. doi: 10.47278/journal.ijab/2022.010
Murat, N., Karadam, B., Ozkal, S., Karatosun, V., and Gidener, S. (2007). Quantification of papain-induced rat osteoarthritis in relation to time with the Mankin score. Acta Orthop. Traumatol. Turc. 41, 233–237. Available at: https://scholar.google.com/scholar_lookup?hl=en&volume=41&publication_year=2007&pages=233-237&journal=Acta+Orthopaedica+et+Traumatologica+Turcica&issue=3&author=N.+Murat&author=B.+Karadam&author=S.+Ozkal&author=V.+Karatosun&author=S.+Gidener&title=Quantification+of+papain%E2%80%90induced+rat+osteoarthritis+in+relation+to+time+with+the+Mankin+score
Naseer, S., Hussain, S., Naeem, N., Pervaiz, M., and Rahman, M. (2018). The phytochemistry and medicinal value of Psidium guajava (guava). Clin. Phytoscience 4, 1–8. doi: 10.1186/s40816-018-0093-8
Nelson, A. E., Allen, K. D., Golightly, Y. M., Goode, A. P., and Jordan, J. M. (2014). A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the US bone and joint initiative. Semin. Arthritis Rheum. 43, 701–712. doi: 10.1016/j.semarthrit.2013.11.012
Nuerjiang, M., Li, Y., Yue, X., Kong, B., Liu, H., Wu, K., et al. (2023). Analysis of inhibition of guava (Psidium guajava L.) leaf polyphenol on the protein oxidative aggregation of frozen chicken meatballs based on structural changes. Food Res. Int. 164, 112433–112447. doi: 10.1016/j.foodres.2022.112433
Nworah, F. N., Chukwuma, I. F., Nwanelo, V. O., Osuji, D. O., Onyeso, S. S., Iwuji, G. O., et al. (2022). Gastroprotective effect of aqueous Achatina achatina L. (snail) slime extract on indomethacin-and acidified ethanol-induced ulceration in wistar albino rats. Pak. Vet. J. 42, 571–575. doi: 10.29261/pakvetj/2022.071
Oliveira, M. D. D., Mello, H. H. D. C., Stringhini, J. H., Mascarenhas, A. G., Arnhold, E., Conceição, E. C. D., et al. (2018). Antioxidant effect of the guava byproduct in the diet of broilers in the starter phase. Rev. Bras. Zootec. 47, 1–8. doi: 10.1590/rbz4720160290
Patel, P., Sunkara, R., Walker, L. T., and Verghese, M. (2016). Effect of drying techniques on antioxidant capacity of guava fruit. Food Nutr. Sci. 7, 544–554. doi: 10.4236/fns.2016.77056
Pilarczyk, B., Jankowiak, D., Tomza-Marciniak, A., Pilarczyk, R., Sablik, P., Drozd, R., et al. (2012). Selenium concentration and glutathione peroxidase (GSH-Px) activity in serum of cows at different stages of lactation. Biol. Trace Elem. Res. 147, 91–96. doi: 10.1007/s12011-011-9271-y
Quicke, J. G., Conaghan, P. G., Corp, N., and Peat, G. (2022). Osteoarthritis year in review 2021: epidemiology & therapy. Osteoarthr. Cartil. 30, 196–206. doi: 10.1016/j.joca.2021.10.003
Rahman, Z., Siddiqui, M. N., Khatun, M. A., and Kamruzzaman, M. (2013). Effect of guava (Psidium guajava) leaf meal on production performances and antimicrobial sensitivity in commercial broiler. J. Nat. Prod. 6, 177–187. Available at: https://www.researchgate.net/publication/330556317_Effect_of_Guava_Psidium_guajava_leaf_meal_on_production_performances_and_antimicrobial_sensitivity_in_commercial_broiler
Salsabil, S. S., Ardana, V. P., Larastiyasa, R. R. P. B., Pratiwi, I. W., Widianti, R. A., and Pratama, A. M. (2022). Nanoparticles of Kirinyuh (Chromolaena odorata (L.) RM King & H. Rob.) leaves extract as a candidate for natural remedies lowering hypercholesterol: In Silico and in vivo study. Pakistan Vet. J. 42, 397–403. doi: 10.29261/pakvetj/2022.023
Sampath Kumar, N. S., Sarbon, N. M., Rana, S. S., Chintagunta, A. D., Prathibha, S., Ingilala, S. K., et al. (2021). Extraction of bioactive compounds from Psidium guajava leaves and its utilization in preparation of jellies. AMB Express 11, 36–39. doi: 10.1186/s13568-021-01194-9
Seo, C. S., Jeong, S. J., Yoo, S. R., Lee, N. R., and Shin, H. K. (2016). Quantitative analysis and in vitro anti-inflammatory effects of gallic acid, ellagic acid, and quercetin from radix sanguisorbae. Pharmacogn. Mag. 12, 104–108. doi: 10.4103/0973-1296.177908
Seo, J., Lee, S., Elam, M. L., Johnson, S. A., Kang, J., and Arjmandi, B. H. (2014). Study to find the best extraction solvent for use with guava leaves (Psidium guajava L.) for high antioxidant efficacy. Food Sci. Nutr. 2, 174–180. doi: 10.1002/fsn3.91
Shahid, A., Inam-ur-Raheem, M., Aadil, R. M., and Israr, B. (2022b). Phytochemical screening and in vitro radical scavenging activities of “gola” guava fruit and leaf extracts. J. Food Proc. Preserv. 46, 16989–17000. doi: 10.1111/jfpp.16989
Shahid, A., Inam-ur-Raheem, M., Nawaz, M. Y., Rashid, M. H., Oz, F., Proestos, C., et al. (2022a). Diet and lifestyle modifications: an update on non-pharmacological approach in the management of osteoarthritis. J. Food Proc. Preserv. 46, 16786–16813. doi: 10.1111/jfpp.16786
Sinha, A. K. (1972). Colorimetric assay of catalase. Anal. Biochem. 47, 389–394. doi: 10.1016/0003-2697(72)90132-7
Sirše, M. (2022). Effect of dietary polyphenols on osteoarthritis—molecular mechanisms. Life 12, 436–454. doi: 10.3390/life12030436
Socol, C. T., Chira, A., Martinez-Sanchez, M. A., Nuñez-Sanchez, M. A., Maerescu, C. M., Mierlita, D., et al. (2022). Leptin signaling in obesity and colorectal cancer. Int. J. Mol. Sci. 23, 4713–4734. doi: 10.3390/ijms23094713
Swantara, M. D., Rita, W. S., Dira, M. A., and Agustina, K. K. (2022). Effect of the methanol extract of annona squamosa Linn leaf on cervical cancer. Int. J. Vet. Sci. 12, 295–301. doi: 10.47278/journal.ijvs/2022.187
Tanideh, N., Zare, Z., Jamshidzadeh, A., Lotfi, M., Azarpira, N., Sepehrimanesh, M., et al. (2017). Hydroethanolic extract of Psidium guajava leaf for induced osteoarthritis using a guinea pig model. Biotech. Histochem. 92, 417–424. doi: 10.1080/10520295.2017.1308013
Tauser, G. (2003). Bioethical principles concerning laboratory animals’ use for biomedical research. Revista Romana de Bioetica. 1, 1–8. Available at: https://www.proquest.com/scholarly-journals/bioethical-principles-concerning-laboratory/docview/1416787028/se-2
Tong, L., Yu, H., Huang, X., Shen, J., Xiao, G., Chen, L., et al. (2022). Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 10, 60–77. doi: 10.1038/s41413-022-00226-9
Umesalma, S., and Sudhandiran, G. (2010). Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-κB, iNOS, COX-2, TNF-α, and IL-6 in 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin. Pharmacol. Toxicol. 107, 650–655. doi: 10.1111/j.1742-7843.2010.00565.x
Vijayababu, M. R., Kanagaraj, P., Arunkumar, A., Ilangovan, R., Dharmarajan, A., and Arunakaran, J. (2006). Quercetin induces p53-independent apoptosis in human prostate cancer cells by modulating Bcl-2-related proteins: a possible mediation by IGFBP-3. Oncol. Res. 16, 67–74. doi: 10.3727/000000006783981224
Vincent, T. L. (2020). Of mice and men: converging on a common molecular understanding of osteoarthritis. Lancet Rheumatol. 2, e633–e645. doi: 10.1016/S2665-9913(20)30279-4
Wen, L., Qu, T. B., Zhai, K., Ding, J., Hai, Y., and Zhou, J. L. (2015). Gallic acid can play a chondroprotective role against AGE-induced osteoarthritis progression. J. Orthop. Sci. 20, 734–741. doi: 10.1007/s00776-015-0718-4
Weni, L., Harliansyah, H., and Widayanti, W. (2011). Anti-inflammatory activity of the extract of guava leaves (Psidium guajava L.) in the rat (Rattus norvegicus L). Indonesian J. Cancer Chemoprev. 2, 169–172. doi: 10.14499/indonesianjcanchemoprev2iss1pp169-172
Widowati, W., Prahastuti, S., Hidayat, M., Hasiana, S. T., Wahyudianingsih, R., Afifah, E., et al. (2022). Protective effect of ethanolic extract of Jati Belanda (Guazuma ulmifolia L.) by inhibiting oxidative stress and inflammatory processes in cisplatin-induced nephrotoxicity in rats. Pak. Vet. J. 42, 376–382. doi: 10.29261/pakvetj/2022.050
Wu, Y., Li, K., Zeng, M., Qiao, B., and Zhou, B. (2022). Serum metabolomics analysis of the anti-inflammatory effects of gallic acid on rats with acute inflammation. Front. Pharmacol. 13, 1–11. doi: 10.3389/fphar.2022.830439
Zhao, M., Li, Y., Bai, X., Feng, J., Xia, X., and Li, F. (2022). Inhibitory effect of guava leaf polyphenols on advanced glycation end products of frozen chicken meatballs (− 18° C) and its mechanism analysis. Food Secur. 11, 2509–2524. doi: 10.3390/foods11162509
Keywords: osteoarthritis, guava leaf, hydroethanolic extract, inflammatory biomarkers, cartilage osteoarthritis, cartilage
Citation: Shahid A, Inam-Ur-Raheem M, Socol CT, Maerescu CM, Criste FL, Murtaza HB, Bhat ZF, Hussain S and Aadil RM (2024) Investigating the anti-inflammatory and immune-modulatory effects of “Gola” guava fruit and leaf extract in alleviating papain-induced knee osteoarthritis. Front. Sustain. Food Syst. 8:1442219. doi: 10.3389/fsufs.2024.1442219
Edited by:
Anwar Ali, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Mohammad Shabaz, Model Institute of Engineering and Technology, IndiaSanju Bala Dhull, Chaudhary Devi Lal University, India
Muhammad Waseem, Islamia University of Bahawalpur, Pakistan
Copyright © 2024 Shahid, Inam-Ur-Raheem, Socol, Maerescu, Criste, Murtaza, Bhat, Hussain and Aadil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rana Muhammad Aadil, bXVoYW1tYWQuYWFkaWxAdWFmLmVkdS5waw==; Claudia Terezia Socol, Y2xhdXNvY29sQHlhaG9vLmNvbQ==; Muhammad Inam-Ur-Raheem, cmFoZWVtdWFmQHVhZi5lZHUucGs=
 Arashi Shahid1
Arashi Shahid1 Claudia Terezia Socol
Claudia Terezia Socol Cristina Maria Maerescu
Cristina Maria Maerescu Florin Leontin Criste
Florin Leontin Criste Zuhaib F. Bhat
Zuhaib F. Bhat Shahzad Hussain
Shahzad Hussain Rana Muhammad Aadil
Rana Muhammad Aadil