
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 02 October 2024
Sec. Nutrition and Sustainable Diets
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1440053
 Khoula Begum1
Khoula Begum1 Imran Khan2*
Imran Khan2* Asif Wali3
Asif Wali3 Rokayya Sami4*
Rokayya Sami4* Huda Aljumayi4
Huda Aljumayi4 Suzan A. Abushal5
Suzan A. Abushal5 Reham M. Algheshairy6
Reham M. Algheshairy6 Hend F. Alharbi6
Hend F. Alharbi6 Faris J. Tayeb7
Faris J. Tayeb7 Zeyad M. Alharbi7
Zeyad M. Alharbi7 Rasha A. Al-Eisa8
Rasha A. Al-Eisa8 Awatif M. Almehmadi9
Awatif M. Almehmadi9 Mahmoud Helal10
Mahmoud Helal10 Faez F. Alshehri11
Faez F. Alshehri11Buckwheat grains contain bioactive components with known effects on cardio-metabolic biomarkers. Previous research on oxidative stress markers made exclusively from wheat flour or two-hour postprandial levels of antioxidant status, plasma total polyphenols, and advanced glycation end products (AGEs) were considerably higher than fasting values after consuming buckwheat-incorporated bread (BWB), including clinical, animal, and epidemiological studies, suggesting the potential of buckwheat intake to influence parameters such as polyphenol intake, antioxidant status, insulin levels, and oxidative stress markers. However, the specific impact of buckwheat-containing bread on these biomarkers in individuals with type-II diabetes remains a significant gap in our understanding. Our current work aims to address this gap by exploring the effects of buckwheat-containing bread on insulin levels, polyphenol intake, antioxidant status, and markers of oxidative stress. In a randomized crossover study, 16 individuals with type-II diabetes were assigned to consume control bread made exclusively from wheat flour or bread incorporating 50% buckwheat flour. The research followed a crossover design, with a period of one to two washout interventions. Test breads were given at breakfast following a 12 h fast the previous night. After 2 h of bread intake, blood samples were collected at baseline (fasting). The two-hour postprandial levels of antioxidant status, plasma total polyphenols, and advanced glycation end products (AGEs) were significantly higher than fasting values after consuming buckwheat-incorporated bread (BWB). This suggests that the buckwheat-containing bread improved plasma total polyphenol levels and total antioxidant status. Our research concludes that the intake of bread containing buckwheat positively influences polyphenols in plasma and the status of antioxidants. This suggests that incorporating buckwheat into bread may have favorable effects on biomarkers associated with cardio-metabolic health in individuals with type-II diabetes. By addressing these knowledge gaps, our research aims to inform and engage the scientific community in further exploration of the potential health benefits of buckwheat-containing bread.
Worldwide, cardiovascular disease (CVD), which account for the majority of current deaths, are becoming infectious diseases and a primary cause of morbidity and mortality (WHO, 2013; Tsao et al., 2022). Oxidative stress plays a significant part in the etiology of several illnesses, such as heart disease and insulin resistance (Sarafidis and Grekas, 2007). Oxidative stresses increase due to reactive oxygen and nitrogen species overpowering the antioxidant defense mechanism. These reactive species oxidize biochemically significant macromolecules, leading to tissue damage, injury, cell death, and chronic illness (Trevisan et al., 2001). Enzymatic and non-enzymatic antioxidants comprise the human antioxidant system, protecting the body from harm caused by reactive oxygen species (ROS). Antioxidants can be produced internally or externally through food or other means. Dietary antioxidant ingestion may enhance endogenous antioxidant synthesis (Liu et al., 2018). Finding foods high in antioxidants may be essential to helping prevent illnesses linked to oxidative stress. There is an increased interest in functional food consumption, which offers health importance beyond primary nutrition. Foods rich in antioxidants can reduce the chances of postprandial oxidative stress, which can slow the onset of many chronic illnesses (Haddad et al., 2014). The promotion and maintenance of good health are influenced by nutrition, diet, and social factors that affect the global risk profile and can contribute to the development of chronic illnesses (Kennedy, 2006). Hence, the main objective of nutritional research is to prevent chronic diseases rather than address dietary shortages (WHO, 2013). In most countries and regions, cereals and pseudo-cereals constitute the primary dietary category and a significant energy source for people. Fagopyrum esculentum, often known as buckwheat, is a gluten-free pseudo cereal that is becoming more and more recognized as a potentially beneficial food due to its high nutrients as minerals, dietary fiber, lipids, and protein (Zhang et al., 2012; Gimenez-Bastida and Zielinski, 2015). Buckwheat has been associated with increased resistance to oxidation and decreased glucose levels, which are associated with cardiovascular, cancer-preventing, and controlling diabetes. The research has predominantly concentrated on animal studies, with limited investigations conducted on humans thus far (Zhang et al., 2012; Gimenez-Bastida and Zielinski, 2015). Notably, significant research has linked regular consumption of buckwheat as a staple food to reducing the incidence of dyslipidemia, hypertension, and hyperglycemia (Zhang et al., 2007). Our hypothesis posited that consuming wheat bread enriched with buckwheat flour would enhance antioxidant levels and impact oxidative stress markers with type-II diabetes. Consequently, the current study aims to ascertain the immediate effects of white bread enriched with buckwheat flour on polyphenols in plasma and oxidative stress biomarkers.
Advertisements in the current investigation were used to recruit 16 human individuals with diabetes. Each participant gave written permission to participate in the study. The study included diabetic participants with fasting blood glucose levels >110 mg/dL and an age >30 years, as per the inclusion criteria. On the other hand, the trial included individuals with hyperlipidemia, a history of gastrointestinal disorders, eating disorders, endocrine disorders, smokers, pregnant or lactating individuals, and those who skipped breakfast. These individuals needed to meet certain criteria: they had to be diabetic, over 30 years old, and have a fasting glucose level of over 110 mg/dL. Participants taking any medicine that would affect their appetite, weight, or dietary sensitivities were not allowed to participate. Furthermore, they are free to exit the research at any moment (Hajira and Khan, 2022; Khan et al., 2015). The study followed the principles outlined under the Ethics (HN-HREC/2018–0021) from Agriculture University.
A cross-over, randomized controlled trial was carried out. A washout period of 1–2 weeks was permitted between testing sessions to lessen the carryover impact. The two test breads (control or treatment) were given to subjects at breakfast during each testing session, which were almost 108 g of BWB and 103 g of white bread. The test bread was distributed in a random number using computer-generated envelopes that the principal investigator examined one at a time. The participants were allowed regular meals and prohibited from heavy physical activity during this research period. Moreover, the individuals were asked to eat comparable items the day before each visit to reduce variance further. The patients continued their fast after eating their meal before 10:00 p.m. They might, however, continue to consume water until the next morning. As soon as the subjects entered the laboratory, baseline data such as height, weight, and waist circumference were taken. The participants were told to remain alert while at rest for 5 min. The test bread was then served to the individuals along with water. The individuals were allowed 10 min to end their meals. Venous blood samples were taken from the participants during the baseline fasting and 2 h after consuming bread, and 2 h after the test. Considering that polyphenols have a peak absorption period of 90–150 min, a single two-hour postprandial time point was obtained, according to the Torabian et al. (2009).
Wheat flour was brought from a nearby local store. The seeds were washed to remove any undesirable objects, such as rocks, stones, and other materials, and buckwheat seeds were obtained from the Pakistan Council of Scientific and Industrial Research, Skardu, Pakistan. The seeds were washed to remove undesirable objects, such as rocks, stones, and other materials. Then, they were ground in a professional grinder and kept in plastic bags until needed.
In the current research, two different kinds of test breads were employed. The CB (control bread) is made from 100% wheat flour. Fifty percent of buckwheat flour was used instead of wheat flour to make buckwheat-containing bread (BWB). This degree of replacement was chosen based on the results of prior consumer acceptance research carried out in the laboratory. Additional components for bread making were 5 g (oil), 1 g (salt), 3 g (yeast), 6 g (sugar), and 70 mL (water). The straight dough method was used to make the bread. In addition to the 100 g of composite flour, other baking ingredients were used. To make a soft and uniform dough, the ingredients were hand-kneaded for 10 min. The dough was then fermented for 30 min while remaining in baking pans. Using an electric oven at a temperature of 220°C for 30 min, the bread was baked. A similar process was also used to make the CB. The nutritional composition of buckwheat incorporated bread was (105 g), water (245 g), total weight (350 g), energy value (304 kcal), carbohydrates (50 g), protein (9.16 g), fats (6.05 g), dietary fiber (3.47 g), and total phenolics (135.4 mgGAE/100 g), respectively. Similarly, control bread was (103 g), water content (247 g), total weight (350 g), energy value (288 kcal), carbohydrates (50 g), protein value (10.6 g), fat content (5.14 g), dietary fiber (1.52 g), and total phenolic (36.08 mgGAE/100 g). Both test breads were almost similar nutritional values except for dietary fiber and phenolics, which increased in BWB compared to control bread.
A digital scale was used to record the subject’s weight while they were in light clothes and no shoes. The height measurement was detected by using a stadiometer. The body mass index (BMI) was computed by measurements of body weight and height. The waist circumference was taken by using a measuring tape (non-stretchable) halfway between the lower rib borders and iliac crests. Blood pressure was measured in a horizontal posture using a manual mercurial sphygmomanometer (600 Yamasu, Tokyo, Japan).
A certified phlebotomist obtained aseptic blood samples (5 mL) from each participant by inserting a needle into ETDA tubes that had already been labeled. Plasma was extracted from blood samples by centrifuging for 15 min at 3,000 g. Following individual plasma separation, it was stored at −80°C in cryo-vials with the proper labels until analysis.
Blood was taken from a warmed fingertip through a lancet device following testing methods and glycemic recommendations (Brouns et al., 2005). Before the prick, the individual’s fingers are heated to facilitate blood flow; capillary blood samples are taken without pinching the finger to prevent plasma dilution. Using a glucometer blood glucose analyzer (Accu-check), blood was taken in a series starting at baseline before eating bread and therefore at 30,45,60,90, and 120 min. To reduce “intra-subject-variation,” the same glucometer was used throughout the study. A recording sheet was used to record the blood values.
The laboratory measured the plasma insulin level in diabetic patients using an electrochemi-luminescence immunoassays (ELISA) analyzer (Cobas e411, Mannheim, Germany). In the current method, the sample analyte competed with the ruthenium-labeled analog. An electrochemical luminescence signal was observed after adding voltage. During the first stage, ruthenium generated from sandwich complex and monoclonal insulin-specific antibodies labeled with biotinylated monoclonal were incubated for 9 min using a 20 μL plasma sample. Subsequently, streptavid in-coated microparticles were magnetically attached to the electrode surface by an applied voltage, and the chemiluminescent emission was observed. Following that, unbound reagents were removed before the voltage was applied. In the electrochemical luminescence immunoassay (ECLIA), the manufacturer uses a master standard curve and 2-point calibration via the reagent barcode for the quantitative measurement of human insulin. The 0.20-1000 IU/mL measurement range was used. The findings were expressed in μU/ml.
We assessed the amount of polyphenols using the Folin–Ciocalteu technique (Khan et al., 2015). The polyphenols were extracted using 200 μL of plasma and 400 μL HCl (1 M). The resultant liquid was vortexed for 1 min and incubated for 30 min at 37°C followed by adding 400 μL2 Msodium hydroxide in 75%methanol and then incubated for 30 min at 37°C. Subsequently, 400 μL of 0.75 M HPO3was added and centrifuged at (1,700 g × 10 min, 4°C) for 2 min. The supernatants were collected in Eppendrop tubes and stored in the refrigerator in the dark.400 μL of acetone water (1:1 v/v) was added to the pellets to remove any remaining polyphenols. Then the mixture was vortexed for 1 min, and centrifuged at (1700 g × 10 min, 4°C). Finally, the two supernatants were combined and centrifuged one more at 4°C for 1700 g × 5 min. Before analyzing the total polyphenols in the plasma, the final supernatant was gathered and kept cold. One hundred μl of the final supernatant was combined with 500 μL of the Folin–Ciocalteu reagent (0.2 N) and 400 μl of Na2CO3 solution for the test. The resultant combination was left to stand at normal temperature in the dark for 90 min. The microplate reader (Mutiskan, Scientific, USA) measured the absorbance at 750 nm. A total 100 μL of milli-Q water was present in the blank solution. Gallic acid equivalents (GAE) per liter were measured using a calibration curve with an acid concentration ranging from 0 to 500 mg/L.
Plasma antioxidants were measured by using an ELISA kit (E2199Hu, T-AOC kit, China). Approximately 120 μL of standard (96 U/mL) was combined with 120 μL to generate a 48 U/mL stock solution. The mixture was then gently stirred for 15 min, while two serial dilutions of the standard stock solution (48 U/mL) produced the following results: 24 U/mL, 12 U/mL, 6 U/mL, and 3 U/mL, respectively. Diluent (0 U/mL) was used as the reference point for zero. In the 96-well microplate, 40 μL of sample and 50 μL ofthe standard were put into put into the corresponding wells for the experiment. The sample wells received 10 microliters of anti-TOC antibody, which was added to them. Subsequently, 50 μL streptavidin-horseradish was added to both the standard and sample wells. All that’s in the blank well is regular diluent. The plate was sealed, let to sit at 37°C for an hour, and then sealed again. The plate was cleaned five times using a close-sealing wash buffer, soaking for 30 to 60 s each time. Following washing, 50 μL of solution A and 50 μL of solution B were added to the wells, causing the color to change in proportion to the level of antioxidant status in human plasma. After sealing the plate with a fresh sealer, it was incubated at 37°C for 10 min. Ultimately, each well received 50 μL of an acidic solution. Thermo Fisher Scientific, USA’s Mutiskan GO microplate reader was utilized to measure absorbance at 450 nm.
The plasma TBARS ratio was evaluated by using a commercially available ELISA kit (E3642Hu, TBARS Kit, China), per the manufacturer’s instructions. Standards wells were then filled with the standards after all chemicals and solutions were prepared following the instructions. The sample wells were then filled with 40 μL of sample and the anti-TBARS antibody. The sample and standard wells were the only ones to receive the addition of streptavidin - HRP 50 μL. The plate was covered and incubated at 37°C for 60 min. Following the incubation period, a multichannel pipette was used to remove the plate sealer and clean it using a buffer. After adding 50 μL of each solution, the solutions were kept at 37°C in the dark for 10 min. To end the reaction and show that the color has changed from blue to yellow, 50 μL of an acidic stop solution was utilized. The absorbance was measured at 450 nm.
An ELISA kit measured advanced glycation end products (AGEs) in humans. Supplied and manufactured in Birmingham, England, E 0003 Hu, at 419 Harborne Road, Edgbaston, by a bioassay technology laboratory. When lipids or proteins are exposed to sugar, they are glycated, resulting in AGEs. All reagents and the standard were prepared at room temperature by the instructions. Then 50 μL standard solution to the standard well, no antibody was needed; the standard solution already included biotinaylated antibodies. After adding 40 μL samples to each well, 10 μL anti AGEs were added, and 50 μL streptavidin-HRP was applied to both the standard and sample wells. After that, cover it with sealer and let it sit at 37 degrees for 60 min. After 60 min, remove all sealers and use a wash buffer to wash the plate. Then, each well was filled with 50 μL of substrate solution A and 50 μL of substrate solution B. After that, incubate the plate in the dark for 10 min at 37°C with a new sealer. The blue color turned yellow as soon as the stop solution was applied to each well. In 10 min following the addition of the stop solution, the optical density (OD value) was determined at 450 nm.
The statistical analysis was carried out using SPSS (Version 21, SPSS In, Chicago, IL, USA) as a statistical analysis tool. The Kolmogorov–Smirnov and Levenes tests were applied to measure data normality and homogeneity. A paired t-test and independent sample t-test were used to evaluate (baseline vs. 2 h values for each test bread) and net change (post-meal–pre-meal) for both group comparisons.
A total of 16 individuals were chosen for the present study. There were no unintended consequences noted. The mean age (years) of diabetic subjects were 46.60 ± 8.63, the average weight (kg) of subjects were 90.39 ± 12.74, height (cm) were 172.50 ± 3.89, body mass index (kg/m2) were 30.47 ± 4.88, waist circumference (cm) were 105.60 ± 11.26, fasting blood glucose (mg/dl) were 155.75 ± 35.21, systolic blood pressure (mm Hg) were 133.00 ± 12.08, while diastolic blood pressure (mm Hg) were 94.90 ± 10.81, respectively.
Table 1 show both the bread value from baseline and after 120 min of consumption. Plasma polyphenol and total antioxidant levels were significantly higher after 2 h of consumption of 50% BWB compared to the baseline level (0 min). TBARS and AGE levels were reduced after 120 min of consumption of 50% BWB compared to the baseline level (0 min).
Figures 1A,B displays the postprandial glucose responses together with the corresponding incremental area of the curve. In diabetic patients, there was an overall impact of time (p < 0.001), although no treatment (p = 0.320) and time × treatment (p < 0.982) were seen on postprandial blood glucose levels. Additionally, there was no discernible change in iAUC (p = 0.267) between bread containing buckwheat and the control group.
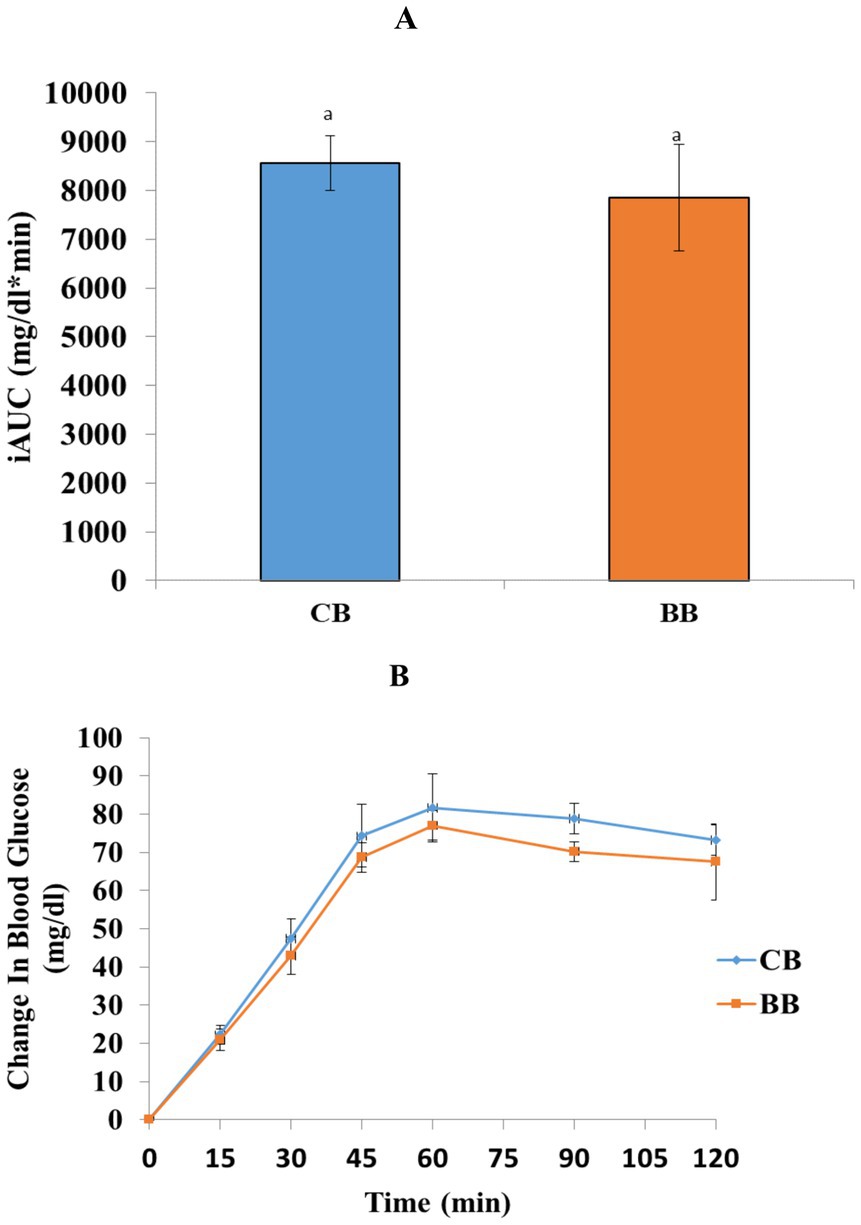
Figure 1. (A,B) Changes in blood glucose mean (±standard error of the mean), incremental areas under the curves (iAUCs), and Two-way repeated measure ANOVA with Bonferroni adjustment values are not substantially different at any time point (p < 0.05). Per paired t-test (p < 0.05), there is no significant difference seen in the vertical bars.
Figure 2 shows the levels of plasma insulin. At baseline, CB and BWB had plasma insulin levels of 17.18 and 18.95 pmoL/L, respectively. In CB, this level rose to 26.8 at 1 h and 27.76 at 2 h postprandial (PP). The BWB insulin IAUC (Figure 2) value was higher than the insulin iAUC (pmol/min) response of the control bread. Nevertheless, with p > 0.05, the treatment and treatment × time interaction effects were not significant.
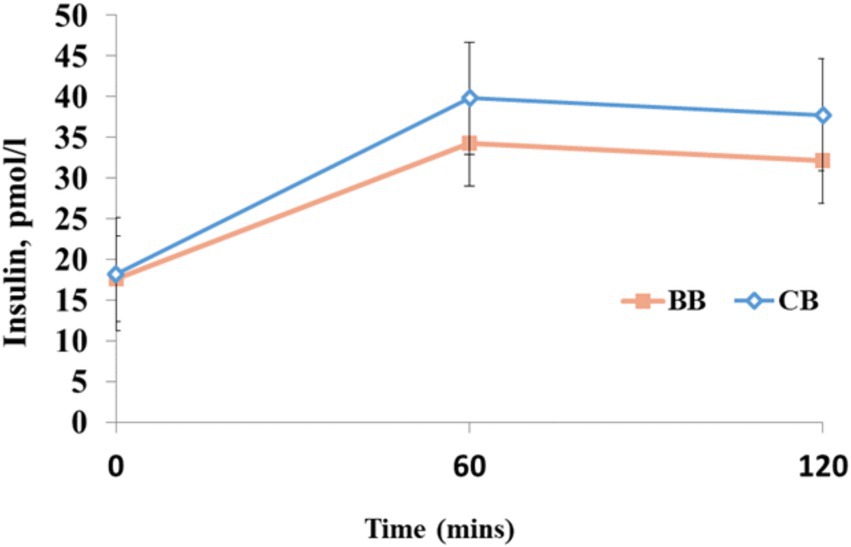
Figure 2. Values are not significantly different at each time point. Post-hoc pairwise comparison using, Two-way ANOVA with Bonferroni adjustment p < 0.05.
Figure 3 displays the individual’s levels of polyphenols at baseline and 2 h after consuming bread (control and test bread). At 120 min in CB, the total polyphenolic concentration in blood plasma gradually dropped. For test bread (BWB), the plasma polyphenolic level rose throughout this period. However, the net change was also significantly increased for 50% BWB compared to CB.
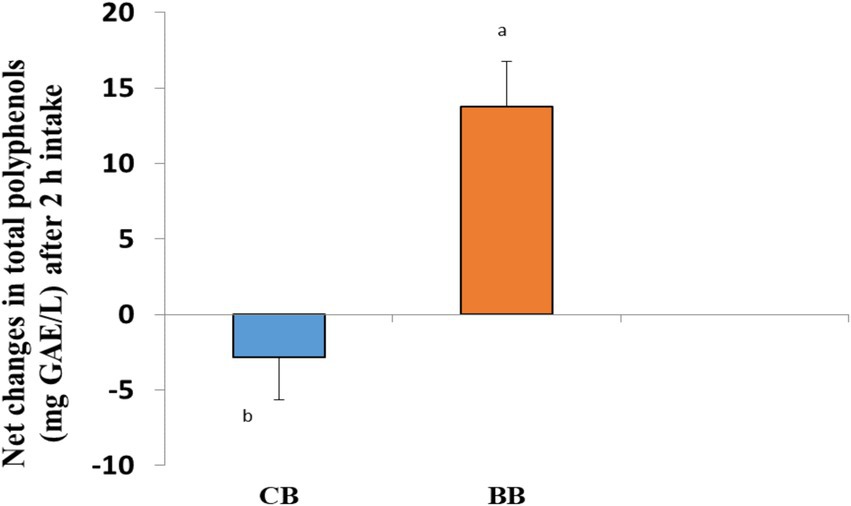
Figure 3. Plasma total polyphenols consumption of test breads shows a noticeable difference between bars with different letters. p < 0.05 for the independent sample t-test.
Two hours after consuming 50% BWB, The plasma antioxidant status increased dramatically (p = 0.031) from the baseline (0 min) levels. The Net changes for 50% BWB were substantially greater (p = 0.047) compared to CB (Figure 4).
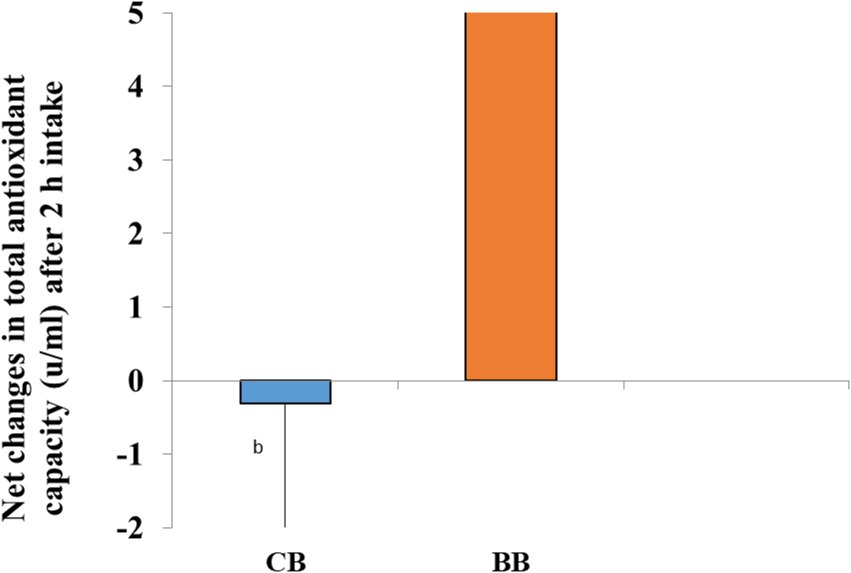
Figure 4. After consuming test breads bars with different letters are statistically significant. Independent sample t-test, p < 0.05.
After consuming test bread for 2 h, the plasma TBARS level did not change significantly (p > 0.05) in comparison to the matching baseline level (0 min). Additionally, after consuming 50% BWB, the net postprandial change in TBARS levels decreased, but it was not statistically different from CB (Figure 5).
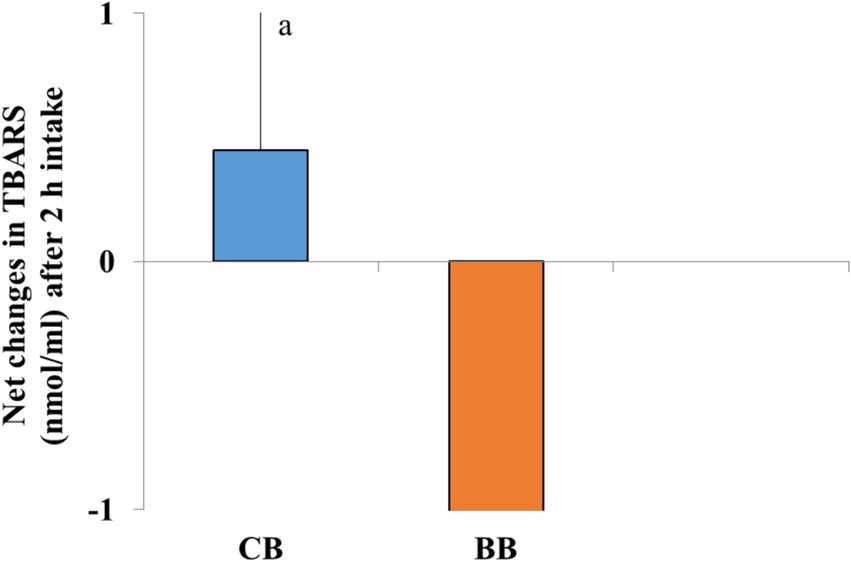
Figure 5. After consuming bread, Bars are not significantly different. Independent sample t-test, p < 0.05.
Advance glycation in plasma was increased significantly (p = 0.026) after 2 h of consumption of 50% BWB compared with the baseline values (0 min). Statistically, net changes were statistically different for 50% BWB compared with CB (Figure 6).
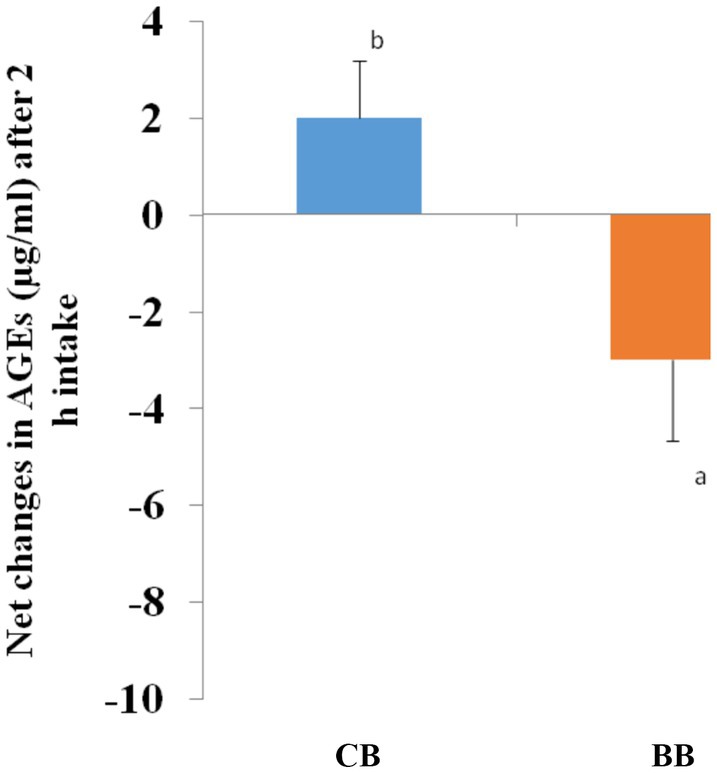
Figure 6. Following the consumption of bread, Bars with distinct letter representations are statistically noteworthy. p < 0.05 for an independent sample (t-test).
The accumulating scientific evidence indicates a growing likelihood that a diet rich in buckwheat may confer protective effects against oxidative stress. Therefore, the objective of the current research work was to find out how BWB affected a cohort of diabetes participant’s glycemic profile, insulin, and specific biochemical markers of oxidative stress. Improvements in plasma insulin levels and glycemic responses were noted in the cross-over randomized research design. Furthermore, the addition of buckwheat augmented indicators of oxidative stress, such as plasma polyphenols, antioxidant capacity, and biomarkers of lipid peroxidation (TBARS), advanced glycation products (AGEs). For the first time, the impact of buckwheat-infused bread on these biomarkers was examined in diabetes patients. But in the current investigation, the notion was only validated in terms of plasma antioxidant status. The results were linked to the increased plasma total polyphenols, T-AOC, and TBARS levels when compared with CB. After consuming BWB, there may be a rise in plasma total polyphenols because of its higher phenolic content than CB. The majority of studies done so far have focused on cell lines and animal models to determine the effects of different buckwheat extracts or particular molecular components; nevertheless, human studies have also been undertaken. The current research has made a significant contribution, especially in terms of examining any potential anti-inflammatory effects. It was relevant to note that after consuming buckwheat-enriched wheat bread as the primary source of carbohydrates in the diet, the aforementioned parameters could be examined.
Several studies have investigated polyphenol levels in food and blood plasma by analyzing individual foods or combinations thereof. Several studies observed that a diet high in polyphenols (one/two servings/day) raised its plasma level in 30–60 min (Fuhrman et al., 2005; Henning et al., 2004; Covas et al., 2003). In a similar vein, a diet high in polyphenols not only increased plasma polyphenol levels but also increased antioxidant capability (Langley-Evans, 2000). There is a clear correlation between polyphenols and antioxidants ability to modify blood lipid levels in a way that lowers cardiovascular risk by providing antioxidant protection (Maguire et al., 2004).
Eating whole grains is linked to lower glucose concentrations and is indirectly correlated with resistance to insulin, suggesting that cereal foods and their constituents may be able to regulate glucose and insulin homeostasis. There is a risk of developing CVD with both insulin resistance and hyperglycemia (Uwaifo and Ratner, 2003), and it has been demonstrated that dietary polyphenols have strong antioxidant potentials to break down ROS, such as SOD, CAT, GPx, and GRd (Han et al., 2007; Hallfrisch and Behall, 2000). Since the benefits of buckwheat have not been well-supported by research on animals and may not apply to humans, it is shown as a low glycemic index food; In contrast, buckwheat-supplemented meals were linked to a substantial 0.85 mmol/L drop in blood glucose concentration (p < 0.001), according to an analysis of nine clinical studies. Numerous possible approaches can be used to modify blood glucose concentrations (Steffen et al., 2003). Buckwheat has a variety of bioactive phytochemicals that are well recognized for their capacity to improve an animal’s ability to metabolize glucose or insulin, such as D-Chiro-Inositol and other polyphenols (Fonteles et al., 2000). Furthermore, buckwheat’s low glycemic index was found to be influenced by resistance starch, according to research (Skrabanja et al., 2001).
These findings, regarding the anti-hyperglycemia potential of buckwheat, were consistent with previous studies. The fact that antioxidants may effectively lower oxidative stress suggests that taking dietary supplements or ingesting natural antioxidants may be advantageous (Urquiaga and Leighton, 2000). Antioxidant supplementation may thereby lower oxidative stress in diabetic animals. Additionally dietary polyphenol supplements may have a role in the management of diabetes. By chelating metal ions, scavenging free radicals, and blocking Xanthine Oxidase and lipid peroxidation (Larocca et al., 1995). Numerous tissues can be affected by oxidative stress, which is associated with underlying variables in the etiology of diabetes (Kakkar et al., 1998). High blood sugar is a hallmark of diabetes for an extended period of time. One important element linked to a rise in glucose levels is the development of non-enzymatic protein glycation, which promotes the formation of AGEs (Negre-Salvayre et al., 2009). The key factor contributing to early death in diabetics is increased arterial atherosclerosis, which is primarily identifiable by elevated arterial atherosclerosis. Strategies to lessen AGE-induced damage to heart tissue have been thought of (Susic et al., 2004).
Contrary to CB, consumption of BWB bread reduces AGE activity in the current investigation. Cereal polyphenols have the potential to both prevent and treat type II diabetes by preventing the production of glycation. The presence of AGEs can lead to various negative effects such as dysfunction of autophagy, stress of oxidation, carbonyl stress, inflammation, and gut microbiota. Cereal polyphenols have been shown to be effective as a non-pharmacologic intervention in reducing T2D and anti-AGEs. Moreover, unchecked and continuous exposure to hyperglycemia was an alarming factor in the development and buildup of AGEs in diabetes. Cereal polyphenols may enhance the way that type 2 diabetes and AGEs interact (Dong et al., 2023).
In the present research, a considerable increase in blood total antioxidants is correlated with greater plasma polyphenol levels. Oral intake of foods high in phenol has been shown to enhance the antioxidant capacity of humans (Torabian et al., 2009). The current study’s findings indicate a favorable association between plasma polyphenols and T-AOC. Therefore, the high phenolic content of 50% BWB may be the cause of the rise in plasma T-AOC following ingestion.
In the current study, a rise in antioxidants was associated with a lower quantity of oxidized lipids measured by TBARS levels decreased following consumption of 50% BWB. This contradicts the findings of (Evans et al., 2000), who reported that buckwheat-enriched food consumption can increase circulatory antioxidants. This is in line with earlier research that showed the effectiveness of short-term treatments on these parameters using 30% buckwheat-enriched bread (Bojňanská et al., 2009). Conversely, in animal studies, a buckwheat diet showed lower TBARS levels and increased GPx activity with no effect on plasma total antioxidant levels (Bojňanská et al., 2009). The differential in polyphenol content between whole grain flour and different fractions utilized to combat oxidative stress may be the cause of this non-significant impact. Furthermore, differences in the buckwheat varieties and cultivation techniques may result in variances in the nutritional values and health advantages (Gianotti et al., 2011).
The current research suggests that bread containing up to 50% buckwheat flour, as opposed to wheat flour, exhibited elevated polyphenol levels, thereby enhancing bread’s antioxidant capacity and endogenous antioxidant defense enzymes. Consequently, the consumption of buckwheat-containing bread is recommended as a dietary source of antioxidants to promote improved overall health and cardio-metabolic conditions. The current research is limited by its short intervention duration and the collection of blood samples post-intervention. Therefore, a longer duration of research with more post-intervention blood samples is suggested for future studies. Furthermore, other related markers of heart health should be investigated in future research. Another restriction of the current investigation is that it requires registration in a clinical trial registry.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Agriculture University (HN-HREC/2018-0021). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
KB: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. IK: Data curation, Formal analysis, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. AW: Data curation, Formal analysis, Methodology, Resources, Writing – original draft, Writing – review & editing. RS: Conceptualization, Formal analysis, Investigation, Project administration, Validation, Writing – original draft, Writing – review & editing. HA: Funding acquisition, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SA: Conceptualization, Data curation, Investigation, Software, Supervision, Writing – original draft, Writing – review & editing. RA: Formal analysis, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. HFA: Data curation, Formal analysis, Funding acquisition, Resources, Writing – original draft, Writing – review & editing. FT: Data curation, Formal analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. ZA: Conceptualization, Investigation, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. RA-E: Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. AA: Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. MH: Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. FA: Conceptualization, Data curation, Software, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Taif University, Saudi Arabia (Project no. TU-DSPP-2024-79).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bojňanská, T., Frančáková, H., Chlebo, P., and Vollmannová, A. (2009). Rutin content in buckwheat enriched bread and influence of its consumption on plasma Total antioxidant status. Potravinářské vědy 27, S236–S240. doi: 10.17221/967-CJFS
Brouns, F., Bjorck, I., Frayn, K. N., Gibbs, A. L., Lang, V., Slama, G., et al. (2005). Glycaemic index methodology. Nutr. Res. Rev. 18, 145–171. doi: 10.1079/NRR2005100
Covas, M., Miró-Casas, E., Fitó, M., Farré-Albadalejo, M., Gimeno, E., Marrugat, J., et al. (2003). Bioavailability of tyrosol, an antioxidant phenolic compound present in wine and olive oil, in humans. Drugs Exp. Clin. Res. 29, 203–206
Dong, L., Li, Y., Chen, Q., Liu, Y., Wu, Z., Pan, D., et al. (2023). Cereal polyphenols inhibition mechanisms on advanced glycation end products and regulation on type 2 diabetes. Crit. Rev. Food Sci. Nutr. 12, 1–19. doi: 10.1080/10408398.2023.2213768
Evans, J. L., Balkan, B., Chuang, E., and Rushakoff, R. J. (2000). Oral and injectable (non-insulin) pharmacological agents for type 2 diabetes. South Dartmouth, MA: Endotext.
Fonteles, M., Almeida, M., and Larner, J. (2000). Antihyperglycemic effects of 3-O-methyl-D-chiro-inositol and D-chiro-inositol associated with manganese in streptozotocin diabetic rats. Horm. Metab. Res. 32, 129–132. doi: 10.1055/s-2007-978606
Fuhrman, B., Volkova, N., Coleman, R., and Aviram, M. (2005). Grape powder polyphenols attenuate atherosclerosis development in apolipoprotein E deficient (E0) mice and reduce macrophage atherogenicity. J. Nutr. 135, 722–728. doi: 10.1093/jn/135.4.722
Gianotti, A., Danesi, F., Verardo, V., Serrazanetti, D. I., Valli, V., Russo, A., et al. (2011). Role of cereal type and processing in whole grain in vivo protection from oxidative stress. Front. Biosci. 16, 1609–1618. doi: 10.2741/3808
Gimenez-Bastida, J. A., and Zielinski, H. (2015). Buckwheat as a functional food and its effects on health. J. Agric. Food Chem. 63, 7896–7913. doi: 10.1021/acs.jafc.5b02498
Haddad, E. H., Gaban-Chong, N., Oda, K., and Sabaté, J. (2014). Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr. J. 13, 1–9. doi: 10.1186/1475-2891-13-4
Hajira, B., and Khan, I. (2022). Effect of sorghum and barley-containing bread on plasma total polyphenols, antioxidant status and inflammation in healthy subjects. J. Food Sci. Technol. 59, 4935–4944. doi: 10.1007/s13197-022-05582-2
Hallfrisch, J., and Behall, K. M. (2000). Mechanisms of the effects of grains on insulin and glucose responses. J. Am. Coll. Nutr. 19, 320S–325S. doi: 10.1080/07315724.2000.10718967
Han, X., Shen, T., and Lou, H. (2007). Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 8, 950–988. doi: 10.3390/i8090950
Henning, S. M., Niu, Y., Lee, N. H., Thames, G. D., Minutti, R. R., Wang, H., et al. (2004). Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am. J. Clin. Nutr. 80, 1558–1564. doi: 10.1093/ajcn/80.6.1558
Kakkar, R., Mantha, S. V., Radhi, J., Prasad, K., and Kalra, J. (1998). Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clin. Sci. 94, 623–632. doi: 10.1042/cs0940623
Kennedy, E. T. (2006). Evidence for nutritional benefits in prolonging wellness. Am. J. Clin. Nutr. 83, 410S–414S. doi: 10.1093/ajcn/83.2.410S
Khan, I., Yousif, A. M., Johnson, S. K., and Gamlath, S. (2015). Acute effect of sorghum flour-containing pasta on plasma total polyphenols, antioxidant capacity and oxidative stress markers in healthy subjects: a randomised controlled trial. Clin. Nutr. 34, 415–421. doi: 10.1016/j.clnu.2014.08.005
Langley-Evans, S. C. (2000). Consumption of black tea elicits an increase in plasma antioxidant potential in humans. Int. J. Food Sci. Nutr. 51, 309–315. doi: 10.1080/096374800426902
Larocca, L. M., Teofili, L., Sica, S., Piantelli, M., Maggiano, N., Leone, G., et al. (1995). Quercetin inhibits the growth of leukemic progenitors and induces the expression of transforming growth factor-beta 1 in these cells. Blood 85, 3654–3661. doi: 10.1182/blood.V85.12.3654.bloodjournal85123654
Liu, Z., Ren, Z., Zhang, J., Chuang, C. C., Kandaswamy, E., Zhou, T., et al. (2018). Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 9:477. doi: 10.3389/fphys.2018.00477
Maguire, L., O’Sullivan, S. M., Galvin, K., O’Connor, T. P., and O’Brien, N. M. (2004). Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int. J. Food Sci. Nutr. 55, 171–178. doi: 10.1080/09637480410001725175
Negre-Salvayre, A., Salvayre, R., Augé, N., Pamplona, R., and Portero-Otín, M. (2009). Hyperglycemia and glycation in diabetic complications. Antioxid. Redox Signal. 11, 3071–3109. doi: 10.1089/ars.2009.2484
Sarafidis, P. A., and Grekas, D. M. (2007). Insulin resistance and oxidant stress: an interrelation with deleterious renal consequences? J. Cardiometab. Syndr. 2, 139–142. doi: 10.1111/j.1559-4564.2007.06666.x
Skrabanja, V., Liljeberg Elmståhl, H. G. M., Kreft, I., and Björck, I. M. E. (2001). Nutritional properties of starch in buckwheat products: studies in vitro and in vivo. J. Agric. Food Chem. 49, 490–496. doi: 10.1021/jf000779w
Steffen, L. M., Jacobs, D. R., Murtaugh, M. A., Moran, A., Steinberger, J., Hong, C. P., et al. (2003). Whole grain intake is associated with lower body mass and greater insulin sensitivity among adolescents. Am. J. Epidemiol. 158, 243–250. doi: 10.1093/aje/kwg146
Susic, D., Varagic, J., Ahn, J., and Frohlich, E. (2004). Collagen cross-link breakers: a beginning of a new era in the treatment of cardiovascular changes associated with aging, diabetes, and hypertension. Cardiovasc. Hematol. Disord. Drug Targets 4, 97–101. doi: 10.2174/1568006043481347
Torabian, S., Haddad, E., Rajaram, S., Banta, J., and Sabaté, J. (2009). Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J. Hum. Nutr. Diet. 22, 64–71. doi: 10.1111/j.1365-277X.2008.00923.x
Trevisan, M., Browne, R., Ram, M., Muti, P., Freudenheim, J., Carosella, A. M., et al. (2001). Correlates of markers of oxidative status in the general population. Am. J. Epidemiol. 154, 348–356. doi: 10.1093/aje/154.4.348
Tsao, C. W., Aday, A. W., Almarzooq, Z. I., Alonso, A., Beaton, A. Z., Bittencourt, M. S., et al. (2022). Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 145, e153–e639. doi: 10.1161/CIR.0000000000001052
Urquiaga, I., and Leighton, F. (2000). Plant polyphenol antioxidants and oxidative stress. Biol. Res. 33, 55–64. doi: 10.4067/S0716-97602000000200004
Uwaifo, G. I., and Ratner, R. E. (2003). The roles of insulin resistance, hyperinsulinemia, and thiazolidinediones in cardiovascular disease. Am. J. Med. 115, 12–19. doi: 10.1016/j.amjmed.2003.08.009
WHO (2013). Cardiovascular diseases (CVDs): fact sheet no. 317, Vol. 20. Geneva: Media Centre. World Health Organization, 3.
Zhang, H.-W., Zhang, Y. H., Lu, M. J., Tong, W. J., and Cao, G. W. (2007). Comparison of hypertension, dyslipidaemia and hyperglycaemia between buckwheat seed-consuming and non-consuming Mongolian-Chinese populations in Inner Mongolia, China. Austr. Soc. Med. Res. Proc. Austr. Soc. Med. Res. 34, 838–844. doi: 10.1111/j.1440-1681.2007.04614.x
Keywords: buckwheat bread, polyphenols, oxidative stress, total antioxidant capacity, type-II diabetic individuals
Citation: Begum K, Khan I, Wali A, Sami R, Aljumayi H, Abushal SA, Algheshairy RM, Alharbi HF, Tayeb FJ, Alharbi ZM, Al-Eisa RA, Almehmadi AM, Helal M and Alshehri FF (2024) Buckwheat containing-bread: a scientific inquiry into insulin, polyphenols, antioxidants status, and oxidative stress markers in type-II diabetic individuals. Front. Sustain. Food Syst. 8:1440053. doi: 10.3389/fsufs.2024.1440053
Received: 28 May 2024; Accepted: 23 September 2024;
Published: 02 October 2024.
Edited by:
Reza Rastmanesh, American Physical Society, United StatesReviewed by:
Seydi Yıkmış, Namik Kemal University, TürkiyeCopyright © 2024 Begum, Khan, Wali, Sami, Aljumayi, Abushal, Algheshairy, Alharbi, Tayeb, Alharbi, Al-Eisa, Almehmadi, Helal and Alshehri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Imran Khan, aS5raGFuMUBzcXUuZWR1Lm9t; Rokayya Sami, cm9rYXl5YUB5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.