
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 19 July 2024
Sec. Crop Biology and Sustainability
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1428466
 Muhammad Rabiu Kabiru1,2
Muhammad Rabiu Kabiru1,2 Alfred Balenor Buernor1
Alfred Balenor Buernor1 Sara Dahhani3
Sara Dahhani3 Mohamed Hafidi4,5
Mohamed Hafidi4,5 Jibrin Mohammed Jibrin2
Jibrin Mohammed Jibrin2 Martin Jemo1*
Martin Jemo1*Supplementing soybean plants with phosphorus (P) and inoculation with effective rhizobia (Rh) strains enhance grain yield and profits and promotes sustainable agricultural practices in Nigeria. Limited field data exists on the effects of P forms (e.g., less soluble rock-P) on yield improvement with Rh or alone. We conducted a study where we grew soybeans in two agroecological zones (AEZs), i.e., Sudan (SS) and the Northern Guinea Savanna (NGS) of Nigeria. The P-treatments included no phosphorus (no-P), half the recommended amount of less soluble rock phosphate (RP), plus half the amount of water-soluble triple super phosphate (TSP). Soybean plants were subjected to one of the three different strains of Bradyrhizobia: Bradyrhizobium elkanii (Be), B. japonicum (Bj), or B. diazoefficiens strain (Bd). Control and nitrogen (40 kg N ha−1) treatments were included. The number of nodules, dry weights (DW), and shoot biomass weight were measured at flowering. A linear mixed model predicted grain yield and nodules DW variables from the managed and environmental factors, including manganese (Mn), magnesium (Mg), and the two AEZs. Soybean yield for ½RP + ½TSP gave a 27.4% relative increase to the control. Plants inoculated with the Be strain displayed the highest grain yield under the NGS soils. The linear mixed regression explained the yield and nodule variation with a trained root means square value of 0.87 and 0.82, respectively. The P sources, Rh inoculation, and the inoculated strains explained the yield variation well. Additionally, the soil-Mn content negatively impacted the yield, while the increasing soil-Mg enhanced nodule dry weight. Studies on the required Mn availability and forms in soil and the threshold concentrations of Mg for optimal N2 fixation and yield of soybeans are discussed.
After nitrogen (N), phosphorus (P) is the second most essential macronutrient for plant growth and development (Bechtaoui et al., 2021; Chongo et al., 2023). Plants rely on the P molecules to regulate their cellular metabolic functions such as photosynthesis, respiration, synthesis of nucleic acids, and energy generation (Malhotra et al., 2018; Victor Roch et al., 2019; Bhinderwala et al., 2020; Powers et al., 2020). The lack of available P alters various plant functions and impairs vigorous root system formation and development (Abdelrahman et al., 2018). To supply the plants with sufficient P and build the soil P legacy, inorganic fertilizers in water-soluble phosphate (WSP) form is a quick way to address soil P deficiency and improve crop yield. Despite their high solubility regarding the P concentration needed for crop demand, the accessibility of WSP fertilizers limits their high use by smallholder farmers with low-input production systems, such as in Northern Nigeria (Dimkpa et al., 2023; Bebeley et al., 2024). Therefore, alternative sources or P forms, such as rock P (RP), partially less soluble with WSP (soluble), may offer a promising alternative to direct RP application by improving its solubility through a slow-release process, minimizing P fixation and being affordable to soybean growers.
Several studies have investigated the additive effects of applying WSP fertilizers to enhance soybean yield and biological nitrogen fixation (BNF) in West Africa (Ronner et al., 2016; Ulzen et al., 2018; Jemo et al., 2023; Van Heerwaarden et al., 2023). The response to WSP fertilizer application was consistently positive in the context of soybean cultivation in Northern Nigeria. It could be predicted to be profitable for 67% of farmers, although variable responses were observed from farmer fields across West Africa (Van Heerwaarden et al., 2023). Fertilizing legume plants with moderate P increases the BNF process. It enhances grain yield, especially in soils with low available P, such as highly weatherable acid soil conditions (Jemo et al., 2006, 2017; Buernor et al., 2022). For the BNF process, the lack of available P in root nodules alters P-homeostasis impairs nitrogenase activity and reduces the nodule’s formation (Sulieman and Tran, 2015). In soil with ineffective soybean nodulating rhizobia (Rh), soybeans can highly benefit from inoculation with elite strains of Bradyrhizobia, selected for their high capacity N2 fixation to achieve a higher yield (Hungria et al., 2017). The inoculation process is highly profitable, especially when the legume plants are fertilized with a moderate P application rate (at 30 kg P ha−1), yield increases ranging from 452 to 815 kg ha-1, and a net economic benefit of about 400 USD ha−1 have been reported (Ronner et al., 2016; Jemo et al., 2023). However, in many African soils, the native bacterial populations are often in low numbers and ineffective in developing symbiosis, resulting in reduced BNF performance (Adjei et al., 2022).
Under natural growing conditions, crop yield prediction is challenging due to its dependency on multiple factors, such as crop genotypes, environmental factors, management practices, and their interactions (Khaki and Wang, 2019; Khaki et al., 2020). For soybean (Glycine max, L), an important cultivated cash crop in Northern Nigeria and of Africa, several impediments to higher crop yield include the low Pi status of soils (Chongo et al., 2023), poor soil quality (Serafim et al., 2019), drought stress (Jemo et al., 2017), and lack of use of improved quality seeds (Khojely et al., 2018). A further challenge to the high grain yields from soybeans is the ineffectiveness of the BNF process that allows bacteria to convert the abundant atmospheric N2 into NH3 usable by the plants (De Bruijn and Hungria, 2022). The process requires a compatible rhizobium strain and a host legume plant to be effective. However, the scarcity of commercially available formulated quality of Rh inoculants in the African market curtails implementing these best agricultural management practices and limits the adoption potential (Ronner et al., 2016; van Heerwaarden et al., 2018).
Magnesium (Mg) is an important macronutrient for plant reproductive development, affecting yield and quality. It is involved in various metabolic pathways, constitutes protein complexes, and plays a key role in activating various enzymes and genes involved in different physiological processes, such as redox reactions, photosynthesis, nutrient metabolism, and cell membrane stability to combat various environmental stresses (Ahmed et al., 2023). In legume symbiosis, low Mg availability reduces bacterial cell growth and impacts the BNF in nodules formed from symbiotic interactions between legumes and rhizobial bacteria (Peng et al., 2018). In addition to Mg, manganese (Mn) is an essential micronutrient for plant development. It involves various metabolic processes and protein complexes (Jiang et al., 2022). Mn deficiency causes instability and loss of plant vigor, resulting in reduced growth and development and, ultimately, grain yield and quality. Studies are needed to comprehensively assess the roles of Mn or Mg in sustainable soybean production across West Africa (Buernor et al., 2024).
Sustainable agricultural production requires that the current food production increase by 70%, with minimal environmental damage, to feed the ever-increasing population of 9.1 billion by the end of 2050 (Helfenstein et al., 2020; Sharma et al., 2021). In Nigeria, soybeans are an important economic crop. It accounts for about 70% of total grain legumes produced annually, and the crop is cultivated on half (50%) of the land area under the legume production (FAOSTAT, 2023). Our study has three main objectives. Firstly, we wanted to evaluate the impact of different P fertilizer forms on soybean yield variation in two agroecological zones of Northern Nigeria. Secondly, we aimed to compare the effectiveness of three formulated Rh strains in enhancing soybean nodulation and increasing yield. Finally, we explored the relationships between the measured soil factors and soybean yield variation in Northern Nigeria.
Improved soybean seeds of the Tropical Glycine max (TGx) 1835-10E developed by the International Institute of Tropical Agriculture (IITA) were used for the research (Nigerian Seed Portal Initiative, 2023). TGx 1835-10E is an early maturing, highly promiscuous variety developed from crossing TGx-1213-1D and TGx-1446-1E varieties (van Heerwaarden et al., 2018). The TGx 1835-10E variety was released in 2008 and has a potential yield of 2 t ha−1 without inoculation by compatible Rh. It is also resistant to bacterial pustules, fungal rust, and leaf spot.1
Three Rh strains, Bradyrhozobium elkanii DSM 11554 (Be), B. japonicum DSM 30140 (Bj), and B. diazoefficiens strain USDA 110 (Bd), were formulated using peat. They were used to inoculate the soybean seeds at planting. The inoculum concentration was 109 CFU g−1. B. japonicum strain is a slow-growing Rh species, highly competitive to nodulate soybean, shows high resistance to some antibiotics, and is more efficient and fixes more N2. Still, it is an exotic Rh strain in many African soils (Grönemeyer and Reinhold-Hurek, 2018). B. diazoefficiens is an established Rh strain from the United States and has been disseminated mainly in West African soil over the last three decades. B. elkanii is a widespread, geographically distributed, and highly adapted Bradyrhizobium species capable of nodulating diverse legumes in Africa (Jaiswal and Dakora, 2019).
Two P fertilizer forms, a water-soluble P as Triple Superphosphate (TSP – 46.83% P2O5) and less soluble finely ground RP (RP-18.41% P2O5) powder, were applied 14 days after sowing (DAS). The TSP was applied at 30 kg P ha−1. The 1/2 (RP + TSP) treatment was supplied at 15 kg P ha−1 each. The N treatment was applied as Urea (46% N) at the rate of 40 kg N ha−1 as a positive control. A blanket application of muriate of potash (60% KCl) at 30 kg KCl ha−1 was applied to all the treatments.
Field trials were conducted in four farmers’ sites, two in the Sudan Savanna (SS) and two in the Northern Guinea Savanna (NGS) agroecological zones of Northern Nigeria (Figure 1). The sites in the SS agroecology covered two local government areas, Tofa (110 98′N and 80 41′E), and Bunkure (110 40 N and 80 31′E). The sites in the NGS were also conducted in two local government areas, Lere (100 26′N and 80 38′46′E) and Soba (100 99′N and 80 06′E). The rainfall distribution is unimodal for both agroecological zones, raining from June to September in the SS and May to September in the NGS.
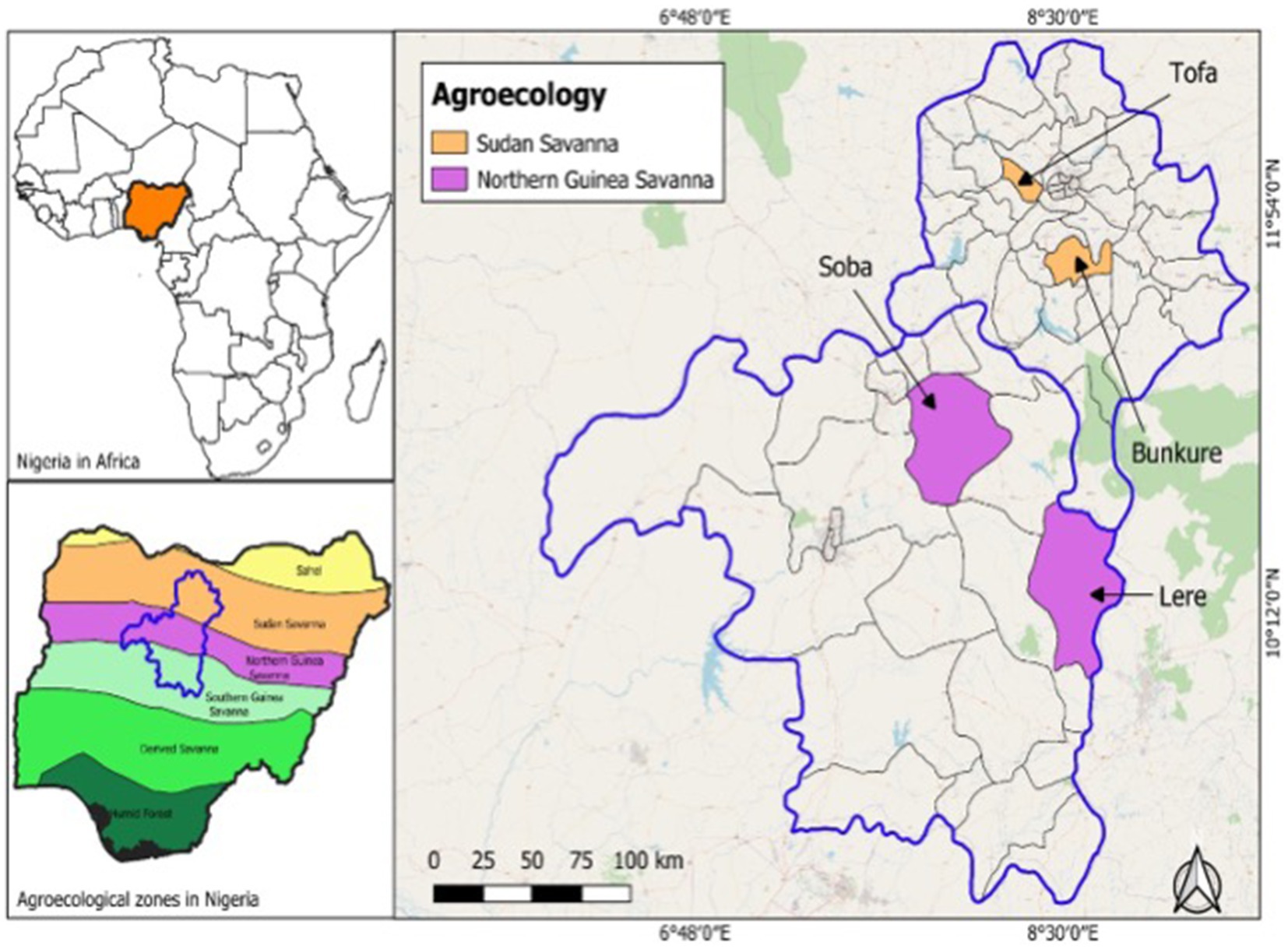
Figure 1. Map of Nigeria showing the study areas of the trials in Nigeria’s Sudan and Northern Guinea savannas agroecological zones.
A composite surface topsoil layer (0–20 cm depth) was collected in 4 replicates from each on-farm plot and transferred to the laboratory for physicochemical analyses. The soil was air-dried and sieved through a 1-mm mesh, and a fine soil fraction was analyzed for pH, P, total N, organic carbon, calcium (Ca), potassium (K), and magnesium (Mg), sand, silt, and clay contents. The pH was measured in aqueous soil suspension (1:2.5, v: v) with the Corning 125 pH meter (Corning Life Sciences, Amsterdam, Netherlands) after continuous shaking for 16 h. The Bray-I chemical extraction method was used to determine the available P content of the soil by adding 30 mL of Bray-I extractant to 3 g of the air-dried soil sample. This was followed by shaking the mixture (1:10, soil: solution) for 5 min, filtering, and calorimetrically measuring the P concentration of the filtered extract as described (Murphy and Riley, 1962). Total N content was measured as described by Novozamsky et al. (1983). The Ca, K, and Mg concentrations were measured using the Mehlich 3 method by adding 30 mL of Mehlich extractant to 3 g of the soil sample, resulting in a mixture of 1:10 (soil: solution). The mixture was then shaken for 5 min, filtered, and Ca, K, and Mg concentrations were analyzed using an atomic absorption spectrophotometer (Thermo Scientific iCE 3,300 AA Spectrometer, Thermo Scientific, United States). Lindsay and Norvell (1978) method was used for the micronutrient measurement. The zinc (Zn), iron (Fe), manganese (Mn), and copper (Cu) were extracted using 0.005MDTPA, 0.01 M CaCl2, and 0.10 M triethanolamine solution and analyzed using ICP-AES. The soil EC was measured using a suspended slurry in the ratio of 2:1 (water: soil, v/v) using standard benchtop meters as described by Rhoades (1982). The Bouyoucos-hydrometer method was used to analyze particle size fractions (Day, 1965).
The layout of the experimental design is shown in Figure 2. The experiment layout was a split-plot design with random assignments of nitrogen sources (rhizobia inoculation) at five levels of treatments: [control without inoculation, Be, Bj, and Bd strains, and positive control with the nitrogen (N) fertilizer application at 40 kg N ha−1] within the three main plots. These main plots were assigned to the P fertilizer sources at three levels [no-P, TSP, and a mixture of rock-P (low solubility) and TSP (water-soluble P)]. The P rates were at two levels (no-P and 30 kg P ha−1). The plot size measured 4 × 3 m2, with an interplot distance of 1 m. Soybean seeds were hand drilled at 2 cm depth, with an inter-row distance of 0.75 m and 0.05 m within a row. Seven days after germination, seedlings were thinned to a plant per hill to maintain a uniform population density of about 266,666 plants per hectare (27 plants per m2). The Rh inoculation was done at 10 g of peat inoculants per kg seed−1 to give a concentration of 109 CFU g−1 of rhizobia, and seeds were coated using sucrose solution as the adhesive. Fifteen (15) mL of 5% sucrose solution was added to 1 kg of the seed and stirred uniformly with a wooden spatula, followed by 10 g of the peat inoculant; the mixture was stirred again and air-dried for 15–20 min before planting. The fertilizers [TSP, ½(TSP + RP), and Urea] were applied 14 days after sowing (DAS) using a subsurface band along the plant rows.
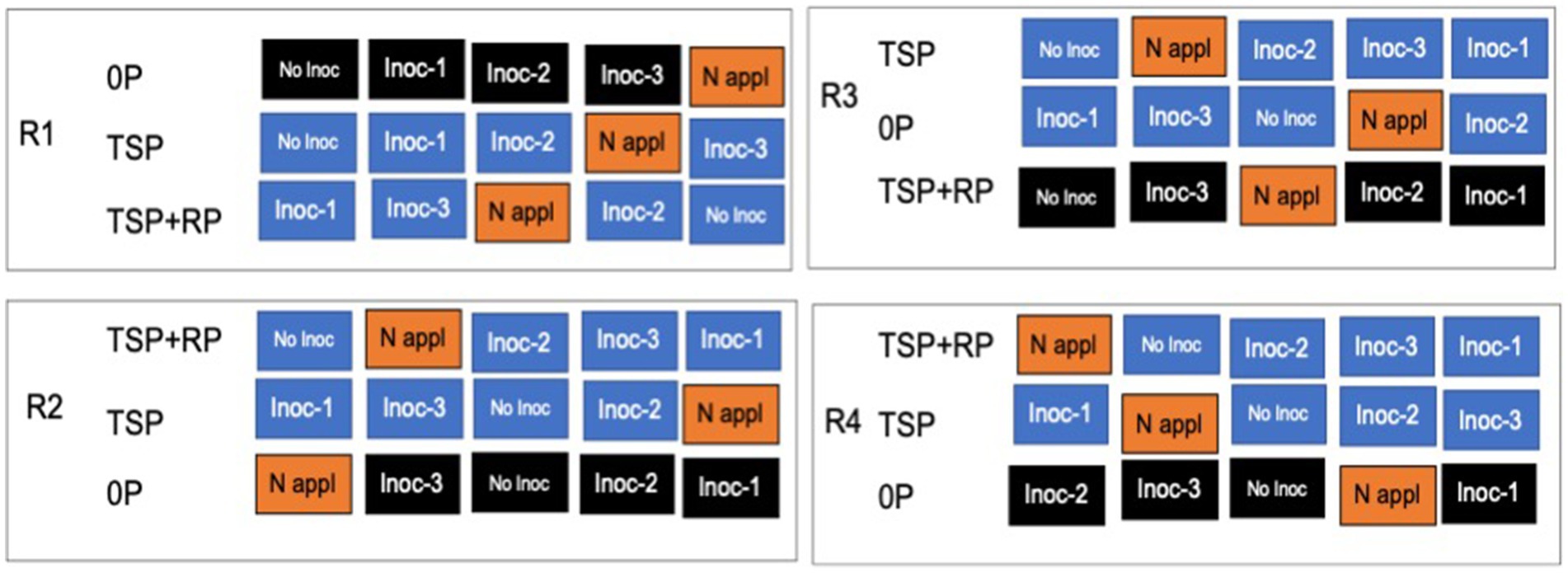
Figure 2. Layout of the experimental design implemented in farmer fields in the Sudan and Northern Guinea savannas agroecological zones of Nigeria.
Measurements were conducted following agreed protocols at flowering and grain maturity in compliance with national and international guidelines and legislation for plant material sampling. The number of nodules and dry weight (DW) were assessed at the 50% flowering stage (approximately 45–48 DAS) and the yield at grain maturity (100 DAS). Ten plants were selected from the interior borders, and the shoots were cut 5 cm above ground, soils gently removed, and washed off to remove excess soil. Fresh nodules were carefully detached from the roots and counted, and the fresh weights of shoots, roots, and nodules were determined. The nodule samples were oven-dried at 70°C for 48 h, and the DW was measured. At full grain maturity, a subplot of 3 × 2 m2 was delimited at the center of each treatment plot, the total number of plants was counted, and soybean plants with pods were harvested and threshed manually to separate the grains. The grains were further sun-dried to achieve 12% moisture of grain content, and the DW of the seeds was measured. The yield in kg ha−1 was calculated using Eq. 1:
where MC is moisture content of the grain (%) (Jemo et al., 2023).
A linear mixed model accounts for sample size imbalances and the confounding effects of uncontrolled studies. To assess the capacity of the total set of variables to explain and predict patterns of variation, we constructed these variables as factorial or categorical variables (P application, Rh inoculation treatment, Rh strains, the combined application of Rh × P), and as environmental variables (soil properties and climatic variables). Further, the R leaps package in R Studio software (RStudio Team, 2024) was implemented to select the best predictors for the linear regression model, significantly influencing grain yield and nodule dry weight. Leaps is a regression subset selection tool that performs an exhaustive search to determine the most influential predictors for our model (Wu et al., 2023). The linear function with a fixed effect trend “f (i)” with the ith level of the explanatory variables was employed. A train control was used to fit the “lm” to the trained datasets to prevent overfitting while predicting the datasets using RStudio Team (2024). The significance of each predictor to the response variable was quantified. The model prediction accuracy was evaluated in terms of root mean square error (RMSE), mean absolute error (MAE), the trained and test data coefficient of determination (R2). A Scatterplot graph was used to visualize the predicted values against the observed values in Rstudio software (RStudio Team, 2024).
The data were analyzed using descriptive and general statistical analyses using the JMP software (JMP, 2023). The effect sizes were examined for suitability for analysis using Shapiro–Wilk and Levene’s tests. A multi-way analysis of variance (MANOVA) was conducted to assess the effect of the imposed treatments on grain yield and nodule weight, nodule number, and shoot dry weight using the JMP software (JMP, 2023). The Fisher’s test was used to separate means that were different at p < 0.05 when the Fischer (F) value was significant from the ANOVA (p < 0.05). Significance levels are given by >0.05 (ns), * at p < 0.05, ** at p < 0.01, and *** at p < 0.001.
Figure 3 presents the monthly precipitation, maximum (max), and minimum (min) temperature of the planting sites in Nigeria for all sites, the SS, and NGS agroecological zones of study in the 2021 growing season. Across all the AEZs, the rainfall begins in May and ends in October, with a higher record of precipitation in July reaching over 1,000 mm (Figure 3A). The rainfall period corresponds to the planting season of the two AEZs. The SS agroecology had a shorter precipitation, while it was longer in the NGS agroecology (Figures 3B,C). The optimum temperatures required for soybean growth ranged from 20 to 30°C between July and September in the SS and June to September in the NGS agroecology (Figures 3B,C).
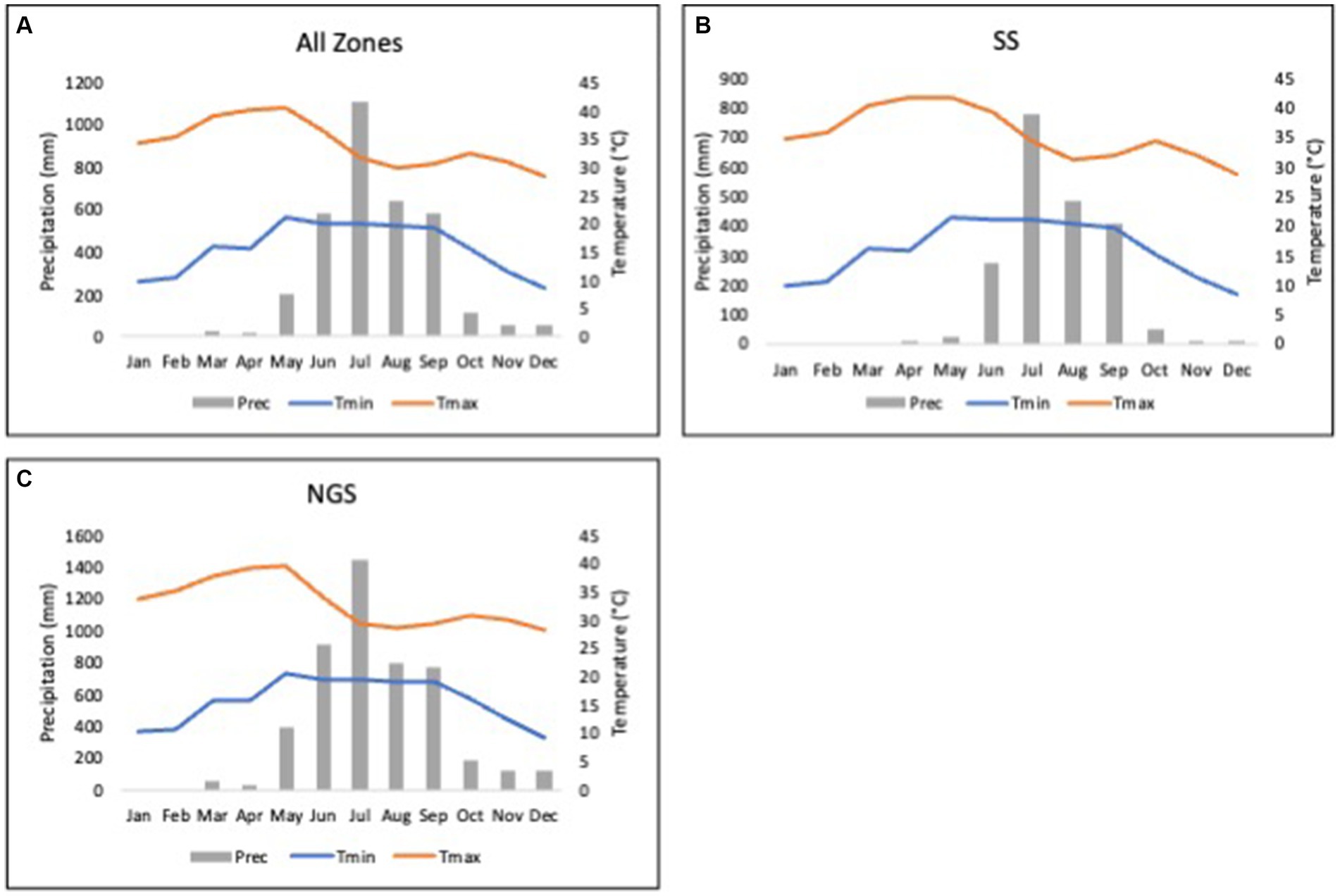
Figure 3. Climatic conditions of the study area (A) all agroecological zones, (B) Sudan savanna (SS) agroecology, and (C) northern guinea savanna (NGS) agroecology of northern Nigeria. The histogram represents the average monthly precipitation/rainfall (Prec). The curves represent the average monthly minimum (Tmin) and maximum (Tmax) temperature.
The physicochemical properties of soil in the field sites are displayed in Table 1. The average sand content of the soil was higher in the SS than in the NGS agroecology (Table 1). Soils in the NGS contained higher clay than the SS agroecology (Table 1). The average pH of soils was similar in agroecologies and slightly acidic for all soils (Table 1). In general, the average available -P in soils was low, and the Lere soils exhibited the lowest value (5.2 ± 2.6), while the Tofa site showed the highest (9.6 ± 3.2). The Tofa site had the lowest average organic carbon content than other sites, and the Lere site exhibited the lowest average N contents (Table 1). Other measured soil chemical properties are reported in Table 1.
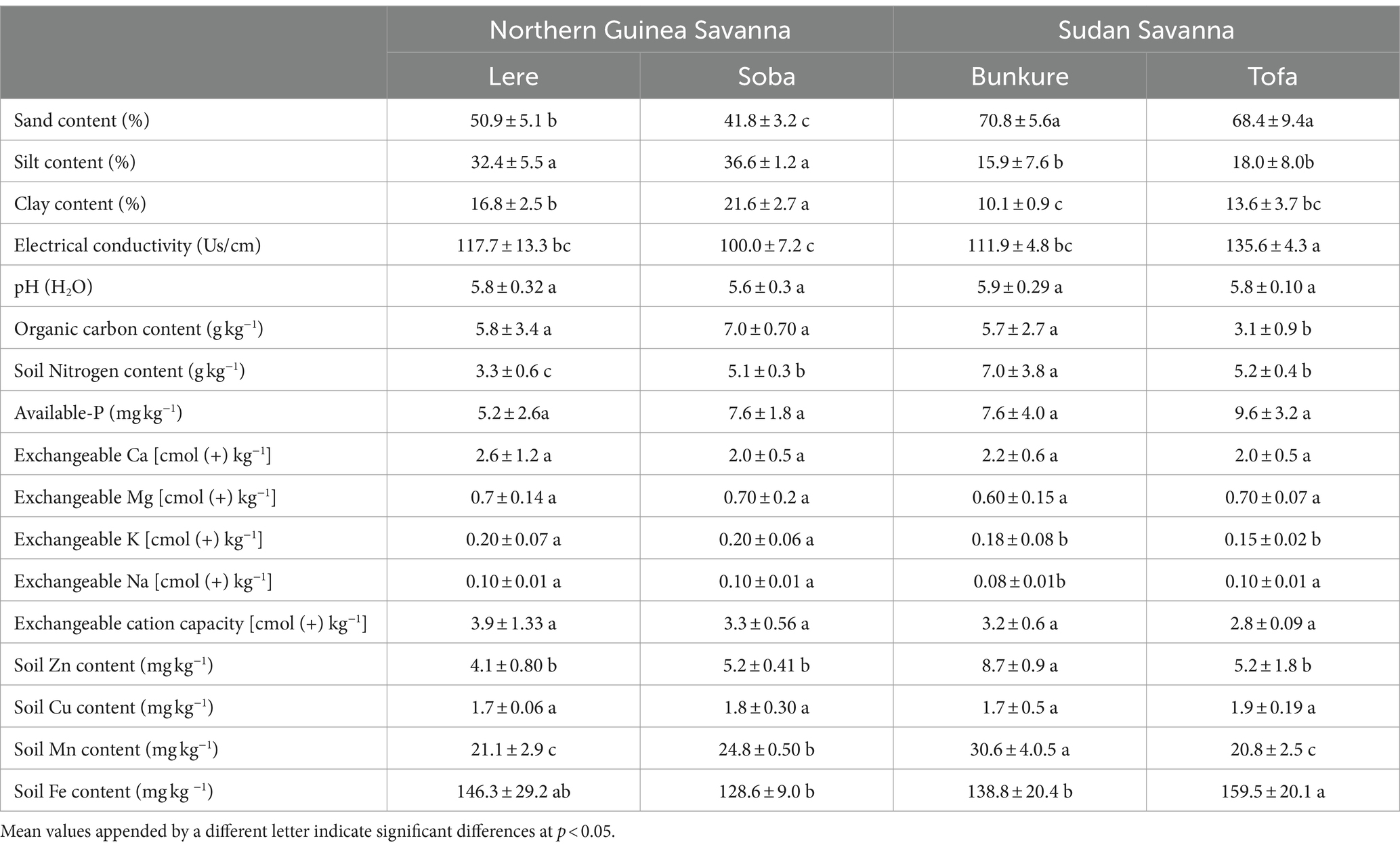
Table 1. Chemical properties of soils analyzed from the field sites in Northern Guinea (Lere and Soban) and Sudan (Bunkure and Tofa) savanna of Nigeria before field trials establishment.
The results in Table 2 show the impacts of various P fertilizer sources and Rh-inoculated strains on soybean grain yield under the two AEZs of Northern Nigeria (p > 0.001). Figures 4A–C display the growth development of soybeans under the no-P, ½RP + ½TSP, and TSP application treatments. Soybean yield was greater for the RP + TSP and TSP fertilized soybean plants than for the No-P treatment (Table 3). The P fertilizer application rate also significantly affected soybean yield, irrespective of the agroecology of the study (Table 3). The inoculated soybean treatments had higher grain yield than the uninoculated plants for the SS and NGS agroecology (Table 4). Among the Rh strains inoculated, soybean plants inoculated with the Be strain under the NGS soils displayed the highest grain yield (Table 4). triple interaction between the AEZ, P source, and inoculation treatment was significant at p < 0.05 for grain yield (Table 2). We found significant effects of the AEZ on the nodule numbers and shoot biomass DW of soybeans (Table 2). Table 2 shows that the interaction between P source and Inoculation treatment was significant for nodule DW at p < 0.05.
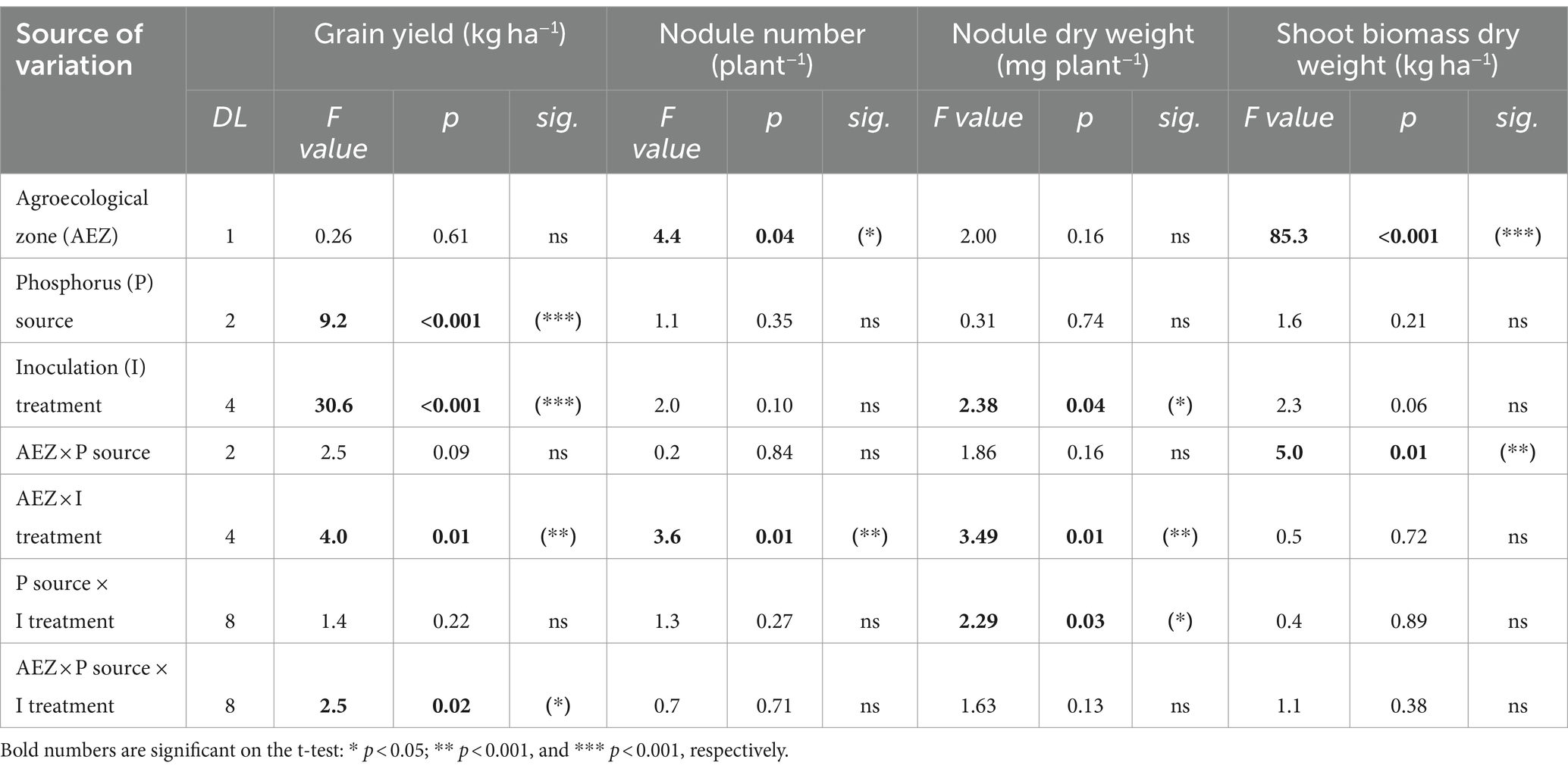
Table 2. Multi-analysis of variance (MANOVA) probabilities for the effects of agroecological zones, phosphorus (P) sources, rhizobia inoculation (I) treatments, and their interaction on the grain yield, nodule number, nodules dry weight, and shoot biomass dry weight of soybean in Northern Nigeria.

Figure 4. Growth development of soybean plants under the (A) control (no-P), (B) half rate of rock phosphate (RP) plus half rate of Triple superphosphate (TSP) [½RP + ½TSP], and (C) TSP application at 30 kg P ha−1 in the field conditions of Northern Nigeria.
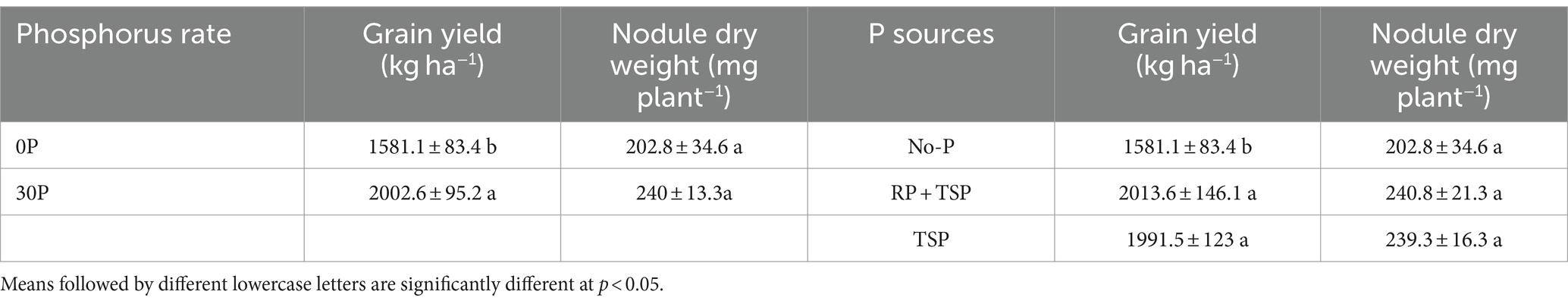
Table 3. Grain yield and nodule dry weight of soybean under phosphorus rates and sources grown in Nigeria’s Northern savanna soil conditions.
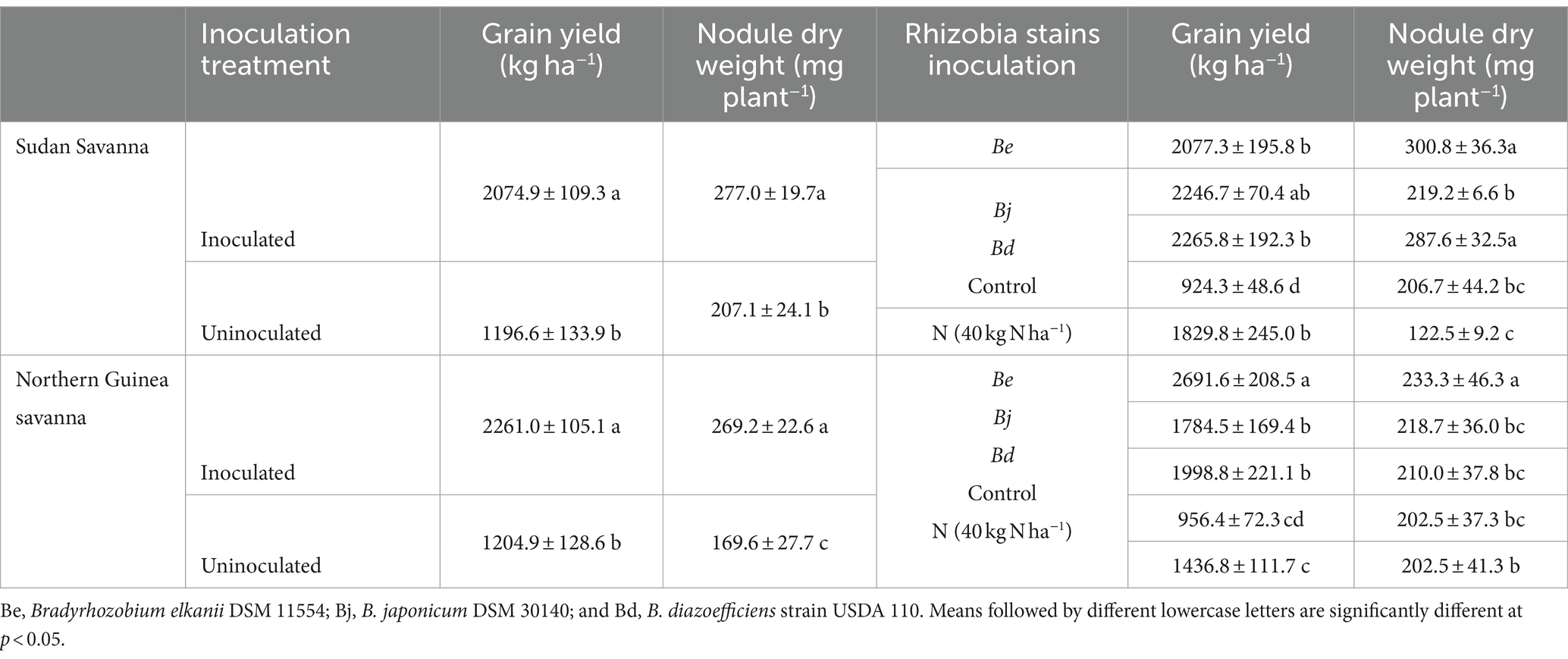
Table 4. Grain yield and nodule dry weight of soybean of the uninoculated and inoculated treatments grown in two agroecological zones of Northern savanna soil conditions of Nigeria.
We used a mixed linear regression model to analyze how moderator variables affected the yield and nodule dry weight. The analysis results, including the RMSE, MAE, trained R2, and test data R2, are presented in Figure 5. The model’s prediction accuracies were good for the grain yield and nodules’ DW with trained R2 of 0.87 and 0.82, respectively (Figures 5A,B). The RMSE of 455.1 kg ha−1 and 93.3 mg plant−1 for the grain yield and nodules DW (Figures 5A,B). The MAE values were 392.0 kg ha−1 and 81.17 mg plant−1 for the grain yield and nodule DW. The test data R2 was higher (0.56) for the grain yield than the nodule DW (Figures 5A,B).
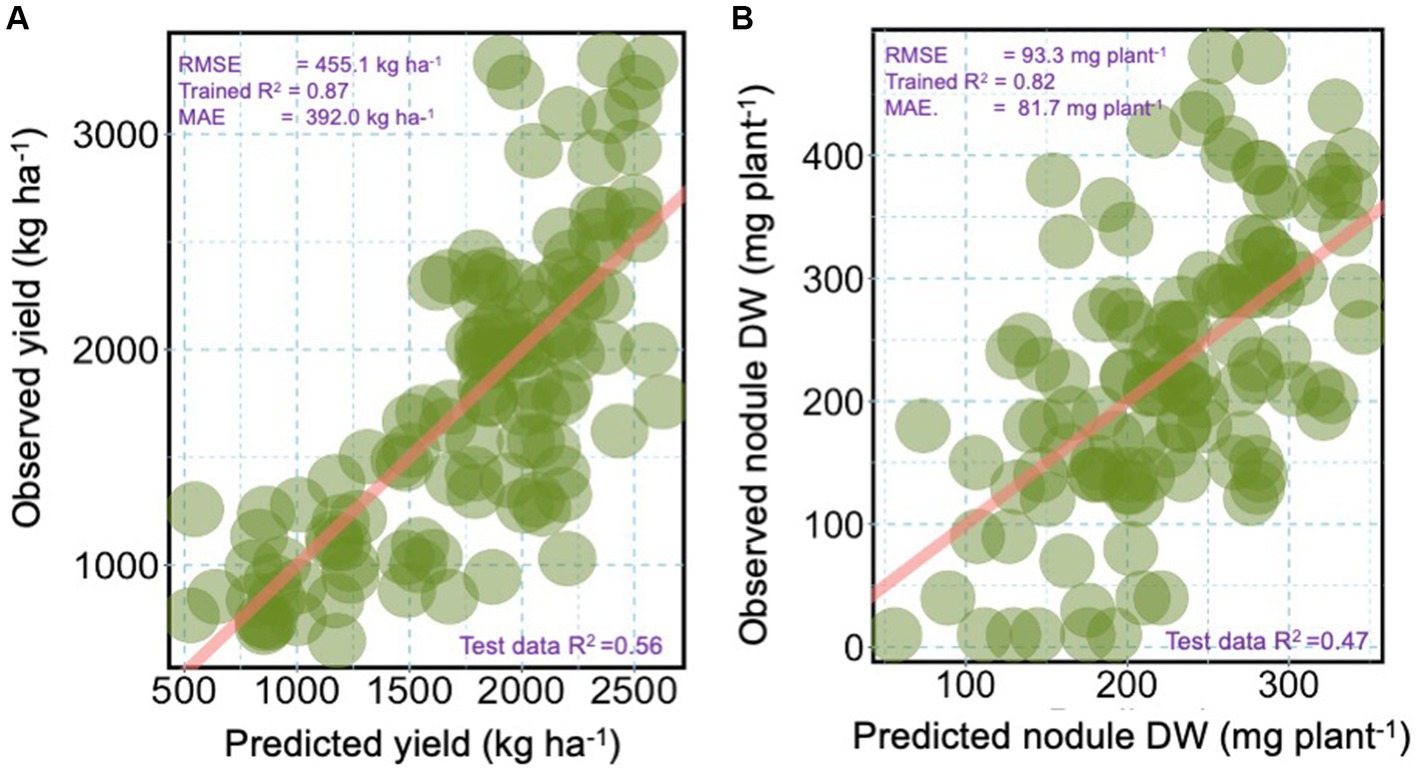
Figure 5. Scatterplots showing the relationship between observed and predicted (A) grain yield and (B) nodule dry weight (DW) from the multiple moderator variables fitted by the linear mixed model. The root square mean error (RMSE) and mean absolute error (MAE) are shown in the graphs. The trained coefficient of determination (R2) and tested R2 are also indicated. The moderator variables were log scale or arcsine-squared root-transformed and back-transformed on the x-axis and y-axis in kg ha−1 for grain and mg plant−1 for nodule DW.
The estimates, t- and p-values of the important features of the soybean yield and nodules DW variation pattern are shown in Table 5. We found that Rh inoculation (t = 4.74; p < 0.001), inoculated Rh strains, B. elkanii (t = 5.99; p < 0.001), B. japonicum (t = 3.43; p < 0.001), B. diazoefficiens (t = 3.49; p < 0.001), and the P sources positively contributed to soybean yield variation (Table 5). In contrast, the elevated July temperature (t = −1.75; p = 0.08) and soil Mn content (t = −1.76; p = 0.08) had a negative impact on the grain yield (Table 5).
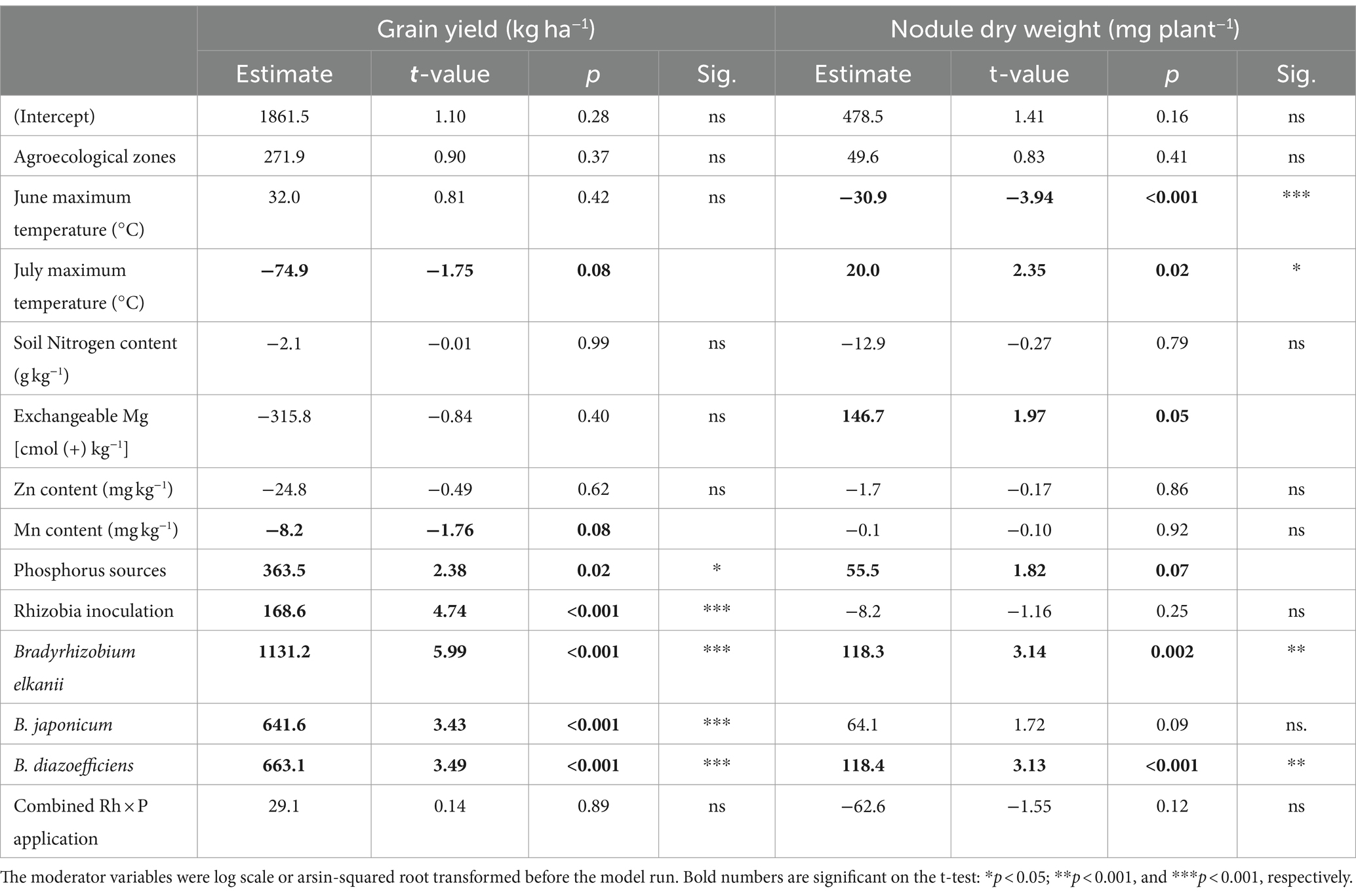
Table 5. Estimate, t-value, probability (p) values, and significance (Sig.) of the effects of multiple moderator variables on the grain yield and nodules dry weight responses of soybean from two agroecological zones of Northern Nigeria.
Among the top five variables contributing to the variation in nodule DW were the following: the elevated July temperature (t = 2.35; p = 0.02) and soil excretable Mg content (t = 1.97; p = 0.05), B. elkanii (t = 3.14; p = 0.002) and B. diazoefficiens (t = 3.13; p < 0.001). We also found that elevated temperature in June negatively impacted the nodules’ DW of soybean plants (Table 5).
There are well-documented studies of soybean responses to P fertilizers application and economic benefits across West Africa (Ronner et al., 2016; Jemo et al., 2023; Van Heerwaarden et al., 2023; Buernor et al., 2024). The inherent low available P highly justifies these positive responses across the African soil, which points out the need to supplement crops with mineral fertilizers for enhanced crop yield (Dimkpa et al., 2023). The positive effect of the WSP fetilizers are highly justifiable due to their high solubility and P concentration; their accessibility and associated costs to many farmers limit their use in low-input production systems. Unfortunately, the wide use of P fertilizer to improve legume production remains elusive, given the possible low return on P investment risks, farmer-limited resources, and yield variability across farmer field contexts of Africa. Adding half the WSP and RP rate increased soybean grain yield more than the control (Table 3). Fewer studies investigate the effects of direct RP application on soybeans to increase yield (Chongo et al., 2023; Buernor et al., 2024). The approach implemented allows legumes to use the WSP directly. At the same time, gradual acidification into the rhizosphere by WSP and rhizosphere processes increases the solubility of RP forms, aiding in breaking the P costs. The approach enhances P soil bioavailability and uptake by plants (Wang et al., 2020). Other benefits from RP direct application reside in the gradual build-up of the soil P legacy, which is critical for increased microbial activity and improved soil health (Silva et al., 2017). It is planned to investigate additional ways to reduce the cost of P fertilization practice while increasing its benefits by partially activating RP with WSP. The mechanism underpinning P regulation in legume plants has been extensively reviewed. It engages a complex set of coordinated reactions from its absorption from the soil phase to plant utilization (Bechtaoui et al., 2021). Due to P involvement in many plant metabolic functions and regulation, a decrease in its concentration triggers alternative pathways, particularly in P-dependent glycolysis and mitochondrial respiration regulation at the cytoplasmic level. P deficiency affects cell division by altering meristem division and reducing mitosis rates (Han et al., 2022). For the BNF process, the lack of available P in root nodules alters P-homeostasis, impairs nitrogenase activity and reduces the nodule’s formation (Sulieman and Tran, 2015).
Inoculation with Rh treatment and the used Rh strains increased soybean yield and favored greater nodulation (Tables 2, 4). The results are consistent with previous studies conducted on Nigerian soil conditions (Ronner et al., 2016; Jemo et al., 2023). Compared to applying N fertilizer, Rh inoculation is a cost-effective technology since it is cheap for farmers. Over 90% of farmers in Northern Nigeria have reported benefits from inoculation of about 100 kg ha−1 of soybean yield increase over the control treatments (Van Heerwaarden et al., 2023). Among the three Rh strains, inoculation with Be and Bd resulted in higher grain yield than Bj and uninoculated control under NGS agroecology. The observed differences in Rh strain performances (Be and Bd) across the two AEZs suggest the ecological adaption and competitiveness of the introduced Be and Bd strains. Nodule DW is an essential indicator for assessing the symbiotic effectiveness of an introduced Rh strain (Graham and Vance, 2003). In the NGS agroecology, the Be strains may be suited for enhancing nodulation in soybeans, while in the SS AEZ, Be and Bd resulted in more improved nodule DW, all better than their corresponding uninoculated treatments (controls). The improved nodule DW achieved with Rh inoculation corroborates the findings by Okereke et al. (2000), who reported that inoculating soybean with Bradyrhizobia spp. increased the nodule DW. In the Guinea savanna agroecology of Ghana, Buernor et al. (2024) found that soybean plants inoculated with the Bd strain had a higher number and DW of nodules per plant than other assessed Bj and Be strains, confirming the ability of soybean to select preferable Rh for nodulation. The observed effects could be attributed to differential physiological properties among the tested Rh strain (Medici et al., 2024). The authors compared four different isolates: B. diazoefficens USDA110, B. diazoefficiens USDA122, B. japonicum E109, and B. japonicum USDA6 for their physiological differences, specific genomic profiles, and found conserved physiological traits in all the studied isolates. However, the fitness, cell size, and in vitro growth widely varied across isolates and correlated with ribosomal RNA operon number, implying different responses for nodulation.
The linear mixed models were used to predict the grain yield and nodule DW based on a set of moderator variables. The main sources of variation in grain yield were the imposed moderators, and two environmental factors (maximum temperatures in July and Mn) hurt the yield (Table 5). The effects of increased temperature on soybean yield have been investigated (Tacarindua et al., 2013). According to the authors, an ambient temperature of as small as 3°C can reduce soybean dry matter from flowering to early seed filling. This happens because of a decline in leaf photosynthesis, stomatal conductance, and carbon discrimination. The present study found that soybean seed yield is the most affected parameter due to these factors. For soybean production in the North of Nigeria, this could mean a decrease in soybean yield, particularly in areas prone to droughts due to the effects of climate change. Therefore, further research is needed to study the impact of increased temperatures on soybean yield to minimize the possible negative impact of climate change and close the yield gap. We also observed that soil Mn content was among the ten top features important for predicting soybean yield, negatively impacting the grain yield (Table 5). Across field sites, the average soil Mn concentrations ranged from 20.8 to 30.6 mg kg−1, below the minimum soil concentration required to promote plant growth. Jiang et al., 2022 showed that 100 mg kg−1 of manganese oxide (MnO2) increased catalase activity in roots by 62.2% in soybean plants. The present results imply that efforts to characterize Mn availability and forms in detail are needed to close soybean yield gaps.
In addition to the managed variables, such as P sources and Rh strains inoculation, we found a positive soil Mg concentration prediction pattern on the nodule DW variation (Table 5). Mg is important in the transport of essential nutrients in plants. Mg also modulates the plant-microbe interactions by promoting bacteria growth in the rhizosphere (Nikolaou et al., 2023). Increasing evidence shows that Mg plays a crucial role in inducing and synthesizing low molecular weight substances in plants, which promote bacterial growth (Yang et al., 2023). Understanding the interactions between Mg and symbiotic bacteria is important to guide effective Mg management and promote Mg fertilization practices for sustainable soybean production in Northern Nigeria.
The relationship between the observed and predicted data for yield and nodules DW was 0.56 and 0.47, respectively (Figures 5A,B). The study results suggest that factors other than those determined may be responsible for variations in yield and nodules’ DW. Further investigations need to include the contribution of native rhizobia and its impact on the model result. One potential limitation to consider when interpreting the results of this study is that our field studies were conducted during a single-year season. This limitation may obscure any impact from off-season dynamics in interpreting our findings. However, using a multilocational approach helped to control the spatially related environmental impacts, which is more critical than seasonality. Also, due to its unimodal rainfall pattern, there is only one farming season per year in the study area.
We conducted a study to assess the effects of various phosphorus sources on soybean plants grown in Northern Nigeria. Our first focus was on water-soluble forms that are highly soluble and plant-available, as well as low-soluble forms like rock phosphate. We gave the plants different phosphorus sources at half the recommended rate at 15 kg ha−1 each and measured their impact on grain yield, nodulation, and growth parameters. We also compared the effects of rhizobia strains, particularly Bradyrhizobium elkanii DSM 11554, B. japonicum DSM 30140, and B. diazoefficiens strain USDA 110, on yield and nodulation. We implement a linear mixed model to predict the yield response and nodule variation. We found that the treatment with half rock phosphate and triple super phosphate induced higher yields than the control without any phosphorus. Among the rhizobia strains tested, soybean plants inoculated with B. elkanii DSM 11554 showed the highest grain yield under the agroecology of the northern Guinea savanna compared to other treatments. The highest nodule of dry weights were also reported on soybean plants inoculated with B. elkanii DSM 11554, irrespective of the tested agroecology. The managed variables, such as phosphorus sources, rhizobia inoculation, and inoculated strains, significantly impacted the grain yield and nodule dry weight responses. While soil Mn hurt soybean yield, the increased Magensium contributed to nodules’ dry weight variation. Therefore, additional research is needed to characterize Mg availability and forms and its effect on soybeans, improve efficiency, and close yield gaps. By delving deeper into the interactions between Magnesium and symbiotic bacteria, we can promote sustainable soybean production in Northern Nigeria. Further insights into these interactions could be the key to unlocking the full potential of soybean production in the North of Nigeria.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
MRK: Writing – review & editing, Writing – original draft, Data curation. ABB: Writing – review & editing. SD: Writing – review & editing. MH: Writing – review & editing. JMJ: Writing – review & editing. MJ: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was financially supported by a grant from OCP Africa to Mohammed VI Polytechnic (UM6P).
SD was employed by OCP Africa.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdelrahman, M., el-Sayed, M. A., Hashem, A., Abd_Allah, E. F., Alqarawi, A. A., Burritt, D. J., et al. (2018). Metabolomics and transcriptomics in legumes under phosphate deficiency in relation to nitrogen fixation by root nodules. Front. Plant Sci. 9:922. doi: 10.3389/fpls.2018.00922
Adjei, J. A., Aserse, A. A., Yli-Halla, M., Ahiabor, B. D. K., Abaidoo, R. C., and Lindstrom, K. (2022). Phylogenetically diverse Bradyrhizobium genospecies nodulate Bambara groundnut (Vigna subterranea L. Verdc) and soybean (Glycine max L. Merril) in the northern savanna zones of Ghana. FEMS Microbiol. Ecol. 98:fiac043. doi: 10.1093/femsec/fiac043
Ahmed, N., Zhang, B., Bozdar, B., Chachar, S., Rai, M., Li, J., et al. (2023). The power of magnesium: unlocking the potential for increased yield, quality, and stress tolerance of horticultural crops. Front. Plant Sci. 14:1285512. doi: 10.3389/fpls.2023.1285512
Bebeley, J. F., Kamara, A. Y., Jibrin, J. M., Tofa, A. I., Solomon, R., and Kamai, N. (2024). Effect of combined use of supplementary irrigation, manure and P fertilization on grain yield and profitability of soybean in northern Nigeria. Heliyon 10:e28749. doi: 10.1016/j.heliyon.2024.e28749
Bechtaoui, N., Rabiu, M. K., Raklami, A., Oufdou, K., Hafidi, M., and Jemo, M. (2021). Phosphate-dependent regulation of growth and stresses management in plants. Front. Plant Sci. 12:679916. doi: 10.3389/fpls.2021.679916
Bhinderwala, F., Evans, P., Jones, K., Laws, B. R., Smith, T., Morton, M., et al. (2020). Phosphorus NMR and its application to metabolomics. Anal. Chem. 92, 9536–9545. doi: 10.1021/acs.analchem.0c00591
Buernor, A. B., Kabiru, M. R., Bechtaoui, N., Jibrin, J. M., Asante, M., Bouraqqadi, A., et al. (2022). Grain legume yield responses to rhizobia inoculants and phosphorus supplementation under Ghana soils: a meta-synthesis. Front. Plant Sci. 13:877433. doi: 10.3389/fpls.2022.877433
Buernor, A. B., Kabiru, M. R., Chaouni, B., Akley, E. K., Raklami, A., Silatsa, F. B. T., et al. (2024). Soybean yield variability in northern Ghana: effects of rhizobia inoculation, P application, and soil exchangeable mg content. Plant Soil. doi: 10.1007/s11104-024-06503-2
Chongo, M., Wendt, J., Ngunjiri, M., Hafidi, M., and Jemo, M. (2023). Agronomic efficiency of activated rock phosphate granules on maize plants treated with mycorrhiza in a calcareous vertisol of Kenya. J. Soil Sci. Plant Nutr. 23, 2687–2693. doi: 10.1007/s42729-023-01225-3
Day, P. R. (1965). “Particle fractionation and particle-size analysis” in Methods of soil analysis: part 1 physical and mineralogical properties, including statistics of measurement and sampling. ed. C. A. Black (Madison, WI: American Society of Agronomy), 545–567.
De Bruijn, F. J., and Hungria, M. (2022). “Biological nitrogen fixation” in Good microbes in medicine, food production, biotechnology, bioremediation, and agriculture. eds. F. J. De Bruijn, H. Smidt, L. S. Cocolin, M. Sauer, D. Dowling, and L. Thomashow (Wiley), 466–475.
Dimkpa, C., Adzawla, W., Pandey, R., Atakora, W. K., Kouame, A. K., Jemo, M., et al. (2023). Fertilizers for food and nutrition security in sub-Saharan Africa: an overview of soil health implications. Front. Soil Sci. 3:1123931. doi: 10.3389/fsoil.2023.1123931
FAOSTAT. (2023). Food and agriculture Organization of the United Nations. Statistical database. Rome. Available at: https://openknowledge.fao.org/server/api/core/bitstreams/6e04f2b4-82fc-4740-8cd5-9b66f5335239/content.
Graham, P. H., and Vance, C. P. (2003). Legumes: importance and constraints to greater use. Plant Physiol. 131, 872–877. doi: 10.1104/pp.017004
Grönemeyer, J. L., and Reinhold-Hurek, B. (2018). Diversity of Bradyrhizobia in Subsahara Africa: a rich resource. Front. Microbiol. 9:2194. doi: 10.3389/fmicb.2018.02194
Han, Y., White, P. J., and Cheng, L. (2022). Mechanisms for improving phosphorus utilization efficiency in plants. Ann. Bot. 129, 247–258. doi: 10.1093/aob/mcab145
Helfenstein, J., Diogo, V., Bürgi, M., Verburg, P. H., Swart, R., Mohr, F., et al. (2020). “Conceptualizing pathways to sustainable agricultural intensification,” in The future of agricultural landscapes: Part I (Advances in Ecological Research; Vol. 63). eds. D. A. Bohan and A. J. Vanbergen (Academic Press Inc.), 1, 161–192.
Hungria, M., Araujo, R. S., Silva Júnior, E. B., and Zilli, J. É. (2017). Inoculum rate effects on the soybean Symbiosis in new or old fields under tropical conditions. Agron. J. 109, 1106–1112. doi: 10.2134/agronj2016.11.0641
Jaiswal, S. K., and Dakora, F. D. (2019). Widespread distribution of highly adapted Bradyrhizobium species nodulating diverse legumes in Africa. Front. Microbiol. 10:310. doi: 10.3389/fmicb.2019.00310
Jemo, M., Abaidoo, R. C., Nolte, C., Tchienkoua, M., Sanginga, N., and Horst, W. J. (2006). Phosphorus benefits from grain-legume crops to subsequent maize grown on acid soils of southern Cameroon. Plant Soil 284, 385–397. doi: 10.1007/s11104-006-0052-x
Jemo, M., Devkota, K. P., Epule, T. E., Chfadi, T., Moutiq, R., Hafidi, M., et al. (2023). Exploring the potential of mapped soil properties, rhizobium inoculation, and phosphorus supplementation for predicting soybean yield in the savanna areas of Nigeria. Front. Plant Sci. 14:1120826. doi: 10.3389/fpls.2023.1120826
Jemo, M., Sulieman, S., Bekkaoui, F., Olomide, O. A. K., Hashem, A., Abd Allah, E. F., et al. (2017). Comparative analysis of the combined effects of different water and phosphate levels on growth and biological nitrogen fixation of nine cowpea varieties. Front. Plant Sci. 8, 1–16. doi: 10.3389/fpls.2017.02111
Jiang, Y., Zhou, P., Ma, T., Adeel, M., Shakoor, N., Li, Y., et al. (2022). Effects of two Mn-based nanomaterials on soybean antioxidant system and mineral element homeostasis. Environ. Sci. Pollut. Res. 30, 18880–18889. doi: 10.1007/s11356-022-23559-8
Khaki, S., and Wang, L. (2019). Crop yield prediction using deep neural networks. Front. Plant Sci. 10:621. doi: 10.3389/fpls.2019.00621
Khaki, S., Wang, L., and Archontoulis, S. V. (2020). A CNN-RNN framework for crop yield prediction. Front. Plant Sci. 10:1750. doi: 10.3389/fpls.2019.01750
Khojely, D. M., Ibrahim, S. E., Sapey, E., and Han, T. (2018). History, current status, and prospects of soybean production and research in sub-Saharan Africa. Crop J. 6, 226–235. doi: 10.1016/j.cj.2018.03.006
Lindsay, W. L., and Norvell, W. A. (1978). Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 42, 421–428. doi: 10.2136/sssaj1978.03615995004200030009x
Malhotra, H., Vandana, S. S., and Pandey, R. (2018). “Phosphorus nutrition: plant growth in response to deficiency and excess” in Plant nutrients and abiotic stress tolerance. eds. M. Hasanuzzaman, M. Fujita, and H. Oku, et al. (Singapore: Springer), 171–190.
Medici, I. F., Bartrolí, L., Guaimas, F. F., Fulgenzi, F. R., Molina, C. L., Sánchez, I. E., et al. (2024). The distinct cell physiology of Bradyrhizobium at the population and cellular level. BMC Microbiol. 24:129.
Murphy, J., and Riley, J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 27, 31–36. doi: 10.1016/S0003-2670(00)88444-5
Nigerian Seed Portal Initiative. (2023). Available at: https://www.seedportal.org.ng
Nikolaou, C. N., Chatziartemiou, A., Tsiknia, M., Karyda, A. G., Ehaliotis, C., and Gasparatos, D. (2023). Calcium- and magnesium-enriched organic fertilizer and plant growth-promoting Rhizobacteria affect soil nutrient availability, plant nutrient uptake, and secondary metabolite production in Aloe vera (Aloe barbadensis miller) grown under field conditions. Agronomy 13:482. doi: 10.3390/agronomy13020482
Novozamsky, I., Houba, V. J. G., Van Eck, R., and Van Vark, W. (1983). A novel digestion technique for multi-element plant analysis. Commun. Soil Sci. Plant Anal. 14, 239–248. doi: 10.1080/00103628309367359
Okereke, G. U., Onochie, C. C., Onukwo, A. U., Onyeagba, E., and Ekejindu, G. O. (2000). Response of introduced Bradyrhizobium strains infecting a promiscuous soybean cultivar. World J. Microbiol. Biotechnol. 16, 43–48. doi: 10.1023/A:1008927327678
Peng, W. T., Zhang, L. D., Zhou, Z., Fu, C., Chen, Z. C., and Liao, H. (2018). Magnesium promotes root nodulation through facilitation of carbohydrate allocation in soybean. Physiol. Plant. 163, 372–385. doi: 10.1111/ppl.12730
Powers, S., Mirsky, E., Bandaranayake, A., Thavarajah, P., Shipe, E., Bridges, W., et al. (2020). Field pea (Pisum sativum L.) shows genetic variation in phosphorus use efficiency in different P environments. Sci. Rep. 10:18940. doi: 10.1038/s41598-020-75804-0
Rhoades, J. D. (1982). “Soluble salts,” in Methods of soil analysis: Part 2. agronomy monogr. 9, second ed. ed. A. L. Page (Madison, WI: ASA and SSSA), 167–178.
Ronner, E., Franke, A. C., Vanlauwe, B., Dianda, M., Edeh, E., Ukem, B., et al. (2016). Understanding variability in soybean yield and response to P-fertilizer and rhizobium inoculants on farmers’ fields in northern Nigeria. Field Crop Res. 186, 133–145. doi: 10.1016/j.fcr.2015.10.023
Serafim, M. E., Zeviani, W. M., Ono, F. B., Neves, L. G., Silva, B. M., and Lal, R. (2019). Reference values and soil quality in areas of high soybean yield in the Cerrado region, Brazil. Soil Tillage Res. 195:104362. doi: 10.1016/j.still.2019.104362
Sharma, A., Jain, A., Gupta, P., and Chowdary, V. (2021). Machine learning applications for precision agriculture: a comprehensive review. IEEE Access 9, 4843–4873. doi: 10.1109/ACCESS.2020.3048415
Silva, U. C., Medeiros, J. D., Leite, L. R., Morais, D. K., Cuadros-Orellana, S., Oliveira, C. A., et al. (2017). Long-term rock phosphate fertilization impacts the microbial communities of maize rhizosphere. Front. Microbiol. 8:1266. doi: 10.3389/fmicb.2017.01266
Sulieman, S., and Tran, L.-S. P. (2015). Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 239, 36–43. doi: 10.1016/j.plantsci.2015.06.018
Tacarindua, C. R. P., Shiraiwa, T., Homma, K., Kumagai, E., and Sameshima, R. (2013). The effects of increased temperature on crop growth and yield of soybean grown in a temperature gradient chamber. Field Crop Res. 154, 74–81. doi: 10.1016/j.fcr.2013.07.021
Ulzen, J., Abaidoo, R. C., Ewusi-Mensah, N., and Masso, C. (2018). On-farm evaluation and determination of sources of variability of soybean response to Bradyrhizobium inoculation and phosphorus fertilizer in northern Ghana. Agric. Ecosyst. Environ. 267, 23–32. doi: 10.1016/j.agee.2018.08.007
van Heerwaarden, J., Baijukya, F., Kyei-Boahen, S., Adjei-Nsiah, S., Ebanyat, P., Kamai, N., et al. (2018). Soyabean response to rhizobium inoculation across sub-Saharan Africa: patterns of variation and the role of promiscuity. Agric. Ecosyst. Environ. 261, 211–218. doi: 10.1016/j.agee.2017.08.016
Van Heerwaarden, J., Ronner, E., Baijukya, F., Adjei-Nsiah, S., Ebanyat, P., Kamai, N., et al. (2023). Consistency, variability, and predictability of on-farm nutrient responses in four-grain legumes across east and West Africa. Field Crop Res. 299:108975. doi: 10.1016/j.fcr.2023.108975
Victor Roch, G., Maharajan, T., Ceasar, S. A., and Ignacimuthu, S. (2019). The role of PHT1 family transporters in acquiring and redistributing phosphorus in plants. Crit. Rev. Plant Sci. 38, 171–198. doi: 10.1080/07352689.2019.1645402
Wang, X., Xiong, J., and He, Z. (2020). Activated dolomite phosphate rock fertilizers to reduce leaching of phosphorus and trace metals as compared to superphosphate. J. Environ. Manag. 255:109872. doi: 10.1016/j.jenvman.2019.109872
Wu, J., Jenkins, J. N., and McCarty, J. C. (2023). An adaptive sequential replacement method for variable selection in linear regression analysis. OJS 13, 746–760. doi: 10.4236/ojs.2023.135036
Keywords: features of importance, grain yield, Northern Nigeria, phosphorus sources, rhizobium
Citation: Kabiru MR, Buernor AB, Dahhani S, Hafidi M, Jibrin JM and Jemo M (2024) Soybean yield variability and predictability from applied phosphorus sources and rhizobia inoculation in Northern Nigeria. Front. Sustain. Food Syst. 8:1428466. doi: 10.3389/fsufs.2024.1428466
Received: 18 May 2024; Accepted: 03 July 2024;
Published: 19 July 2024.
Edited by:
Mohamed Ait-El-Mokhtar, University of Hassan II Casablanca, MoroccoReviewed by:
Mohamed Anli, Université des Comores, ComorosCopyright © 2024 Kabiru, Buernor, Dahhani, Hafidi, Jibrin and Jemo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Jemo, bWFydGluLmplbW9AdW02cC5tYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.