
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 12 September 2024
Sec. Crop Biology and Sustainability
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1388916
This article is part of the Research Topic Innovative Solutions For Next-Generation Fertilizers View all 13 articles
 Rajan Bhatt1*†
Rajan Bhatt1*† Patricia Imas2
Patricia Imas2 Adi Perelman2
Adi Perelman2 Krishan K. Verma3
Krishan K. Verma3 Laila A. Al-Shuraym4
Laila A. Al-Shuraym4 Samy Sayed5
Samy Sayed5 Ahmed Gaber6
Ahmed Gaber6 Akbar Hossain7*
Akbar Hossain7*Introduction: In semiarid tropical locations, polyhalite (K2Ca2Mg(SO4)4H2O) and muriate potash (KCl) were tested for their ability to increase cane growth, yield, and recovery at potash (K)- and calcium (Ca)-deficient sites.
Methods: The treatments involved control plots with no potash fertilizer (T1); T2 and T3 applied potassium through (muriate potash) MOP only at 80 and 120 kg K2O ha−1, whereas T4 and T5 applied potassium with half of MOP and polyhalite at 80 and 120 kg K2O ha−1, respectively.
Results and discussion: At 35 days after harvest (DAH), T2 (10.82%), T3 (24.1%), T4 (34.9%), and T5 (34.9%) had a greater ratoon resprouting rate than did the control treatment, where it was just 37.0 out of 100 harvested canes. At 308 DAH, T2 (−5.9%), T3 (−5.7%), and T5 (−6.6%) presented greater leaf chlorophyll contents than did T1. The K-fertilized plots yielded 64.31 t ha−1 in T2 and 65.97 t ha−1 in T5, whereas the control plot yielded 61.5 t ha−1. Compared with the control plots, the T5 plots experienced fewer stalk borer (−28.6%), top borer (−23.3%), and early shoot borer (−23.3%) attacks. T2, T4, and T5 presented higher percentages of commercial cane sugar (CCS) (6.82, 8.83, and 8.74%, respectively) than did the control plots. T1 and T3 had similar CCSs (10.99 and 11.33%, respectively). The CCS weight per area ranged from 7.98 to 8.47 t ha−1 near maturity. T4 (8.59 t ha−1) and T5 (8.60 t ha−1) had significantly greater values than did T1–T3. Compared with the control, the applied potassium fertilizer increased the economic output by 8,711, 11,687, 13,485, and 13,857 INR ha−1 in the T2, T3, T4, and T5 plots, respectively. The higher cost of polyhalite than MOP has reduced its economic advantages. Thus, the T4 plots outperformed the other treatments in terms of growth, yield, and quality indices, but their higher values (120 kg K2O ha−1) were statistically equivalent.
Conclusion: Finally, the study concluded that MOP and polyhalite at a 50% ratio of 80 kg K2O ha−1 may help improve sugarcane growth, yield, and quality in semiarid tropical locations.
As a major industrial crop, sugarcane (Saccharum spp.) is cultivated in semiarid regions ranging from 36.7°N to 31.0°S in tropical to subtropical regions of the equator (Bhatt et al., 2021c; Choudhary and Singh, 2016; Bhatt, 2020). Generally, sugarcane is planted for two purposes: to extract sugar, which accounts for 75% of global sugar consumption, and to produce ethanol, which is blended into gasoline (O’Hara et al., 2009; Singh et al., 2011; Bhatt and Singh, 2021). In Punjab, India, the cane area is 91,000 ha, with a land productivity of 800 qt ha−1 and a sugar recovery of <10% (PAU, 2022). Among the different claimed reasons for the lower sugar recovery in the region, the major ones are unbalanced fertilizer use with little attention given to potash, upcoming water-stressed conditions, higher insect pest and disease incidence, and poor quality of cane seeds offered to cane farmers, which restricts sugarcane productivity and quality in the region (Bhatt et al., 2021a; Bhatt et al, 2021c).
However, an important barrier to reaching the potential yield of high-quality crops is the uneven use of fertilizers, especially potash (Bhatt et al., 2021b). According to one previous study, 2.08 qt ha−1 nitrogen, 0.53 qt ha−1 phosphorus, 2.80 qt ha−1 potassium, and 0.30 qt ha−1 S, as well as relatively low levels of other elements, are required for every 1,000 qt of sugarcane produced (Shukla et al., 2017).
Sugarcane is grown in Indian Punjab on soils that are already low in sulfur (S), magnesium (Mg), calcium (Ca), and potassium (K). Very little soil fertilizer containing potassium is used. Therefore, both sugarcane productivity and recovery are lower than those in neighboring states with solid potash recommendations. To reduce the risk of lodging, insect pests, and disease susceptibility in the canes, potash is needed. The various pools of K present in the soils are as follows: 90–98% of the total is mineral K, 1–2% is exchangeable K, 1–10% is non-exchangeable K, and 0.1–0.2% is soil-soluble K. Potassium ions move across pools in the soil system when the pool equilibrium is changed (Barber, 1995; Wakeel and Ishfaq, 2022). The soluble and exchangeable potassium pools in the soil re-equilibrate quickly. After being transferred from clay exchange sites during flooding, some soils can lose a significant quantity of potassium. In soils with limited cation exchange capacity, potash (K) leakage is a serious problem (Singh et al., 2004; Fageria et al., 1990).
In agricultural soils for enhancing both water and land productivity, muriate potash (KCl) has long been used. to meet crop potassium demands (Bhatt and Singh, 2021). The sustainable dose of MOP in sugarcane agriculture has not been standardized or recommended in the region (Bhatt and Singh, 2021; Bhatt et al., 2021b). However, Bhatt et al. (2021b) attempted to treat plant canes in the region but, to date, have not been tested for ratoon canes. Sugarcane lands where canes have been preferred for decades have been reported to be deficient in nutrients, particularly potash, owing to their high uptake and almost no supply from the outside environment. “Polyhalite” (K2Ca2Mg(SO4)4H2O) is a multinutrient fertilizer (AngloAmerican, 2016) that provides us with many nutrients, viz. 14% K, 17% Ca, 6% MgMg, and 48% SS (AngloAmerican, 2016). This nutrient organic fertilizer is being removed 1.2 km deep from the sea of the northeastern coast of England (Garnett, 2021), which has few ecological implications (Pavinato et al., 2020). Compared with typical fertilizer, polyhalite acts as a slow-release nutrient (Vale, 2016), which further leads to less leaching losses and reduces its footprint (Bhatt and Singh, 2021). Previously, many workers evaluated its efficiency in many crops, viz. Zea mays, L. (maize) (Fraps, 1932; Tien et al., 2020); Sorghum bicolor, L. (sorghum) (Barbarick, 1991); Actinidia deliciosa (kiwifruit) (Zhao et al., 2020); Solanum tuberosum (potato) (Garnett, 2021); Solanum Lycopersicum (tomato) (Sacks et al., 2017); Brassica oleracea var. capitata (cabbage) (Tien et al., 2021). In comparison to other forms of K fertilizers, including KCl, polyhalite has been shown to produce soil K that is retained by plants for an extended period of time (Lewis et al., 2020). To date, the efficiency of polyhalite in the ratoon crop in semiarid regions of sugarcane (Saccharum officinarum) has not been examined (Bhatt et al., 2020), although Bhatt et al. (2021a) evaluated the performance of this plant crop. In the present study, halite, which is composed of hydrated K, Ca, Mg, and S, was studied as a partial substitute for MOP fertilizer.
Polyhalite supplies Ca for membrane stability (Steward, 1974; Kirkby and Pilbeam, 1984; White and Broadley, 2003), as do numerous signal transduction pathways and initiation (Steward, 1974; Kirkby and Pilbeam, 1984; White and Broadley, 2003; Monshausen, 2012). Furthermore, because Ca is transferred in vegetation via xylem sap, canes are unable to remobilize Ca from older tissues, increasing the importance of polyhalite in our Ca-deficient plots, which resulted in greater growth and yield parameters. The Mg provided by polyhalite is necessary for photosynthesis and glucose partitioning (Cakmak and Yazici, 2010; Farhat et al., 2016; Gransee and Führs, 2013). However, S also enhances cane land productivity (Khan and Mobin, 2005; Kovar and Grant, 2011) by commonly interacting with nitrogen (Jamal et al., 2010). Balanced nutrient utilization is required to sustainably increase sugarcane production and quality; ignorance of the optimum nutrient balance could be detrimental (Bhatt, 2020; Bhatt and Singh, 2021). K is important for the morphological and biochemical activity of sugarcane plants: it controls stomatal opening, translocates plant assets from all around the plant, diminishes the prevalence and mortality of insect pest attacks, encourages root development, and enhances nutrient, pesticide, and moisture efficiency improvements while also decreasing crop inputs in agriculture to reduce their respective footprints (Bhatt et al., 2021a; Bhatt et al., 2021b). When K levels are low, photosynthesis products (Hartt, 1969) and their transportation in cane plants are significantly hampered (Quampah et al., 2011). While Bhatt et al. (2021b) attempted to corroborate these findings in the plant crop, the present study also conducted experiments in the ratoon crop using different combinations of MOP and multinutritional fertilizer polyhalite during the 2021–2022 ratoon season. The objectives of this study were to determine (1) a sustainable potash dose for increasing ratoon cane development and land productivity, (2) which was associated with the lowest incidence of insect pests, and (3) which was associated with the greatest cost benefits.
Investigations were approved from March 2021 to March 2022 during the sugarcane ratoon season at the experimental farm of the PAU–Regional Research Station, Kapurthala, Punjab, India, which is situated at 31° 23.032′ N and 75° 21.647′ E, with an elevation of 0.225 km above sea level. The ratoon cane crop regenerated from spring 2021 to 2022 after the plant cane crop was harvested on 22 March 2021. The meteorological data during the experiment period from April 2021 to March 2022 are provided in Supplementary Figure S1.
Standard procedures were used to gather representative, repeated soil samples from the site (Bhatt and Sharma, 2014). In March 2021, following the harvesting of sugarcane seed crops via a posthole auger (with an inner diameter of 7.2 cm), 10 surface (0–15 cm) soil samples were collected. The samples of soil were left in the shade for 48 h to dry. Throughout the sampling depths, large roots, trash, and stones were carefully removed from the samples that were obtained. The samples of soil were laid out on an uncontaminated piece of cloth and allowed to dry in the shade for 48 h. After the drying process was finished, the soil clods were broken up with a wooden hammer and passed through a 2 mm sieve. The materials were subsequently placed in sterile polythene bags and appropriately labeled for evaluation of their chemical and physical properties. The texture of the soil was estimated via the feel method. Standard procedures were followed to estimate the pH and EC of the soil in a 1:2 soil:water mixture (Jackson, 1967). The soil organic carbon content was measured via Walkley and Black wet digestion and the fast titration method (Walkley and Black, 1934). The available K and phosphorus (P) contents were measured via 1 N ammonium acetate (pH 7) extract and 0.5 M NaHCO3 extract (Olsen, 1954), respectively (Jackson, 1967). To determine the Ca and Mg contents in the soils, the EDTA method was used (Barrows and Simpson, 1962). The results of the analysis revealed that 65–68% of the samples from the investigated location were loaded with coarse sand and 11–33% with clay, and the topsoil was low in K, Ca, and SOC (%) but high in P and Mg (Table 1).
The groundwater level at the testing site was 26 m. The quality of the irrigation water used on the crop was determined in triplicate, and the findings are displayed in Table 2.
Nitrogen fertilizers were applied to all the plots at the regionally recommended dose (RRD) (PAU, 2022). Potash fertilizers (K2O ha−1) were broadcast under different treatments: T2: MOP alone or in combination with polyhalite (K2Ca2Mg(SO4)4H2O) under different doses; T1: 0 kg of K2O ha−1; T2: 80 kg of K2O ha−1 as muriate potash; T3: 120 kg of K2O ha−1 as muriate potash; T4: 80 kg of K2O ha−1 as muriate potash + polyhalite (50% each); T5: 120 kg of K2O ha−1 as muriate potash + polyhalite (50% each). The detailed treatments of the present ratoon sugarcane experiments are summarized in Table 3.
The above combinations were distributed in randomized block designs in 15 plots measuring 6 m × 4.5 m in length, with three replicates, as was done earlier for plant crops, as shown in Figure 1.
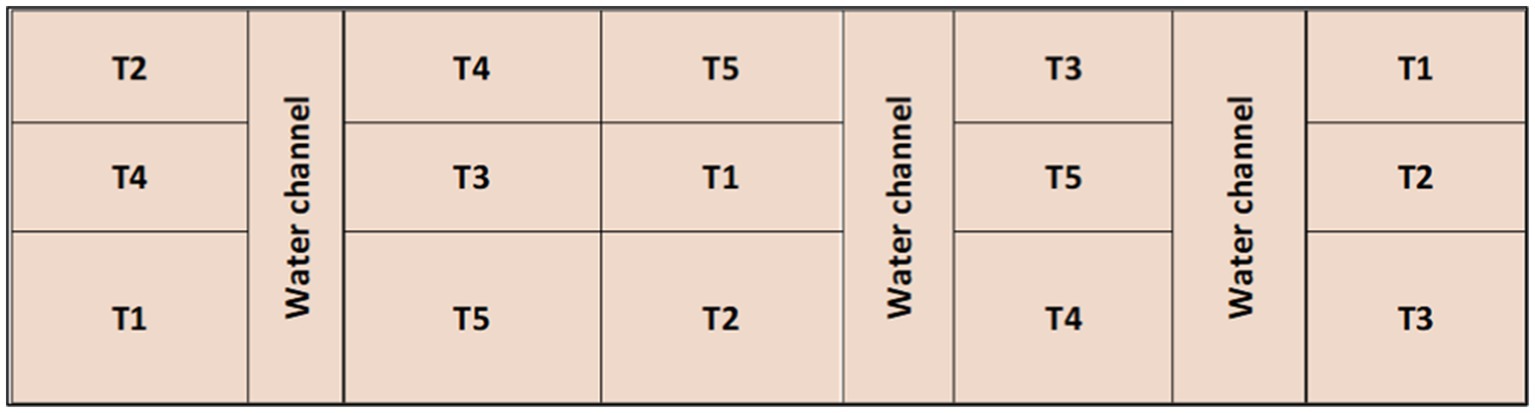
Figure 1. Layout of the experiment carried out at RRS, Kapurthala [T1: 0 kg of K2O ha−1; T2: 80 kg of K2O ha−1 as a muriate potash (KCl); T3: 120 kg of K2O ha−1 as a muriate potash; T4: 80 kg of K2O ha−1 as a muriate potash + polyhalite (50% each); T5: 120 kg of K2O ha−1 as a muriate potash + polyhalite (50% each)].
On 26 March 2021, the earlier sugarcane crop of CoPb 93 was harvested for the regrowth of the next ratoon crop in the present study. The best-practice agronomic approaches for ratoon cane production were adopted on the basis of the recommendation of Punjab Agricultural University, Ludhiana (PAU, 2022), and the crop stand is shown in Figure 2.
The proportion of resprouted setts that germinated in each plot 35 days after the plant crop was harvested in each treatment was calculated (Bhatt and Singh, 2021; Bhatt et al., 2021b). To determine the number of tillers in each treatment plot, the total number of tillers from a 5 m2 area was physically counted at 210 and 310 days after harvesting (DAH) of seed crops (Bhatt and Singh, 2021). A total of 347 DAHs for the milling of sugarcane stalks were reported. From the entire plot area, canes that were suitable for milling were visually examined and numbered. 1,000 ha−1 was the unit of expression used to describe the ends (Bhatt and Singh, 2021; Bhatt et al., 2021b). For each plot, five sugarcane stalks were randomly selected and marked. At 128, 144, 172, and 217 days after harvest (DAH), a ruler was used to measure the distance between the highest growth point of the stalks and the soil surface.
Using Vernier calipers, the cane girths of five randomly selected and tagged sugarcane stalks were measured at 116, 171, 198, and 280 DAH. The stalk diameter was calculated by averaging the stalk diameter measurements at the cane’s head, center, and lower ends (Bhatt and Singh, 2021; Bhatt et al., 2021b). At 170, 218, 280, and 315, five randomly selected disease-free tagged sugarcane stalks were used for recording the total number of nodes and the average of the five nodes considered. At 238, 277, and 308 DAH, a SPAD-502+ chlorophyll meter was used to measure the leaf chlorophyll content under the different treatments. Additionally, when the sugarcane stalks from each experimental plot were harvested by hand and weighed via a field scale, the weight of all the stalks was recorded as t ha−1, representing the cane yield.
At the 10th and 12th months, five disease-free ratoon stalks were randomly selected and removed from each plot for analysis of their juice quality parameters at the Biochemistry Laboratory of the PAU–Regional Research Station, Kapurthala, Punjab, India. Using a cane crusher, the juice was extracted and tested for quality through established procedures (Meade and Chen, 1977). Using a digital refractometer (Optics Technology Delhi 34), the Brix and sugar percentage in the juice were determined as described previously (Meade and Chen, 1977). The following formula (Equation i) was used to estimate the percentage of commercial cane sugar (CCS) consumed:
(Equation i) has crushing and multiplication factors of 0.74 and 0.4, respectively.
The following formula (Equation ii) was used to determine the CCS content in t ha−1 via the cane yield and the percentage of total CCS.
During the 2021–2022 ratoon season, several insect pests of sugarcane were thoroughly documented. These pests, which included the early shoot borer (Chilo infuscatellus), top borer (Scirpophaga excerptalis), and stalk borer (Chilo auricilius), had a detrimental effect on sugarcane yield. In June, the top borer population was counted, the early shoot borer population was counted after 60 DAH in May, and the stalk borer population was counted from 100 plants at harvest to evaluate the impact of irrigation and potash doses on the incidence of insect pests on sugarcane. The % incidence of early shoot borer has been estimated by using the following formula (Equation iii):
In June, July, and August, the top borer percentage incidence (Equation iv) was recorded, and the cumulative incidence was computed.
The percentage of aged stalks at the time of harvest was recorded.
The benefits of the different treatments were calculated via the costs of MOP and polyhalite (as applied) as well as the MSP (Bhatt and Singh, 2021; Bhatt et al., 2021b; Kumar et al., 2019) via the following (Equation vi):
The online OPSTAT tool was used to assess cane growth, quality, and insect pest data. p < 0.05 indicated statistical significance. The correlations between experimental treatment quality measures were also examined via R (Olivoto and Dal’Col Lúcio, 2020).
During 2021–2022, the resprouting time, height, girth, number of nodes per plant, number of millable canes, number of tillers per plant, chlorophyll content, and cane weight were greater on the advanced side of the K-fertilized plots than in the control plots (Figure 3; Table 4).
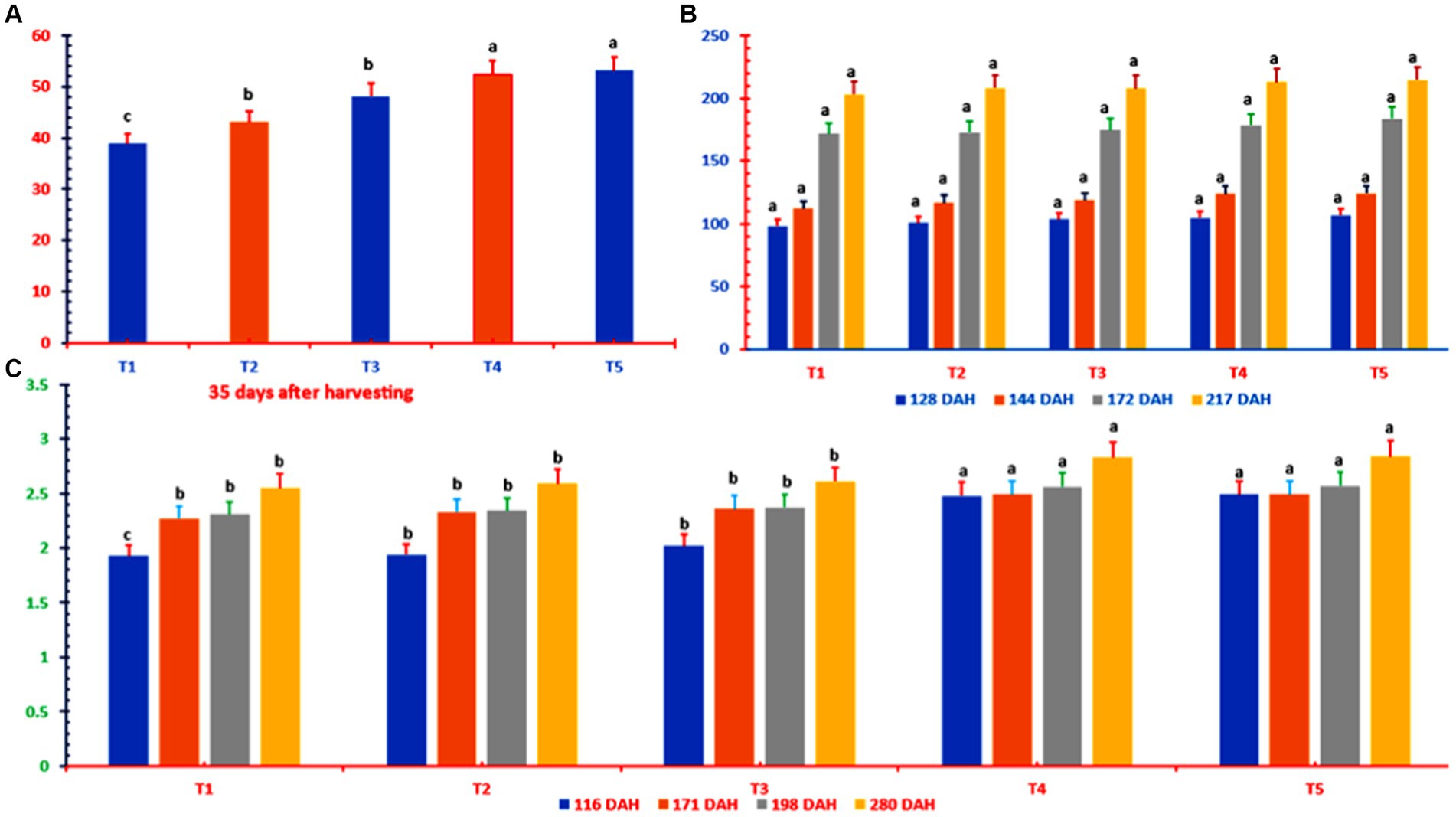
Figure 3. Ratoon sugarcane resprouting (A), height (B), and girth (C) at different K doses and sources. DAH, days after harvesting; T1 = 0 kg of K2O ha−1; T2 = 80 kg of K2O ha−1 as muriate of potash; T3 = 120 kg of K2O ha−1 as muriate of potash; T4 = 80 kg of K2O ha−1 as muriate potash + polyhalite (50% each); T5 = 120 kg of K2O ha−1 as muriate potash + polyhalite (50% each). In column bars, means with similar letter(s) are identical as per LSD0.05.
The irrigation water used to irrigate the canes is a good standard (Table 2). At 35 DAH, the resprouted ratoon buds were greater in the T2 (through 10.82%), T3 (through 24.1%), T4 (through 34.9%), and T5 (through 34.9%) treatments than in the control treatment (Figure 3). At 128 DAH, there was no discernible difference in ratoon cane length between the K treatment and the control treatment. There were no changes in stalk height between any of the treatments at 144 DAH (T3, T4, and T5 had stalks greater than those in the control plots) or 172 DAH (the stalks in the T5 canes were taller than those in the control plots). By 217 DAH, however, there were no appreciable differences in stalk height across any of the treatments.
The sugarcane stalk widths for T4 and T5 were greater than those for T1, T2, or T3 (Figure 3). At 116 DAH, the difference in stalk diameter was greatest, with an increase over that in T1 of up to 29% in T5. At 280 DAH, compared with the control treatment, T5 resulted in the greatest increase in stalk diameter at 11%. Similarly, the T4 and T5 treatments resulted in significantly greater changes from the baseline treatment across all three growth and yield measures and greater changes in stalk diameter than did the T1, T2, and T3 treatments. However, the T4 and T5 treatment plots were significantly comparable (Figure 3).
Table 4 clearly shows that the number of nodes or tillers per ratoon cane did not change over time in any way that could be distinguished between the treatments. The K-treated plots had more millable cane per treatment at 347 DAH than did the control plots, where no potash was applied (Table 4). T4 and T5, which were MOP plus polyhalite treatments, had 36–39% more NMC than did T1, whereas T2 and T3, which were MOP-only treatments, had 12–18% more NMC than did T1.
The SPAD meter results indicated that at 238, 277, and 308 DAH, the chlorophyll content of the leaves was lower in all the K treatments than in the reference point control plots (Table 4). The leaf chlorophyll concentrations at 308 DAH were lower in T2 (−5.9%), T3 (−5.7%), and T5 (−6.6%) than in T1, with T4 (−14.2%) having the lowest concentration.
The baseline treatment yielded an average of 61.5 t ha−1. All the potash-fertilized plots yielded yields greater than the baseline yield, ranging from 64.31 t ha−1 in T2 to 65.97 t ha−1 in T5, with the T4 and T5 plots reporting the highest yields, followed by the T3 and T2 plots. This was true regardless of the type of K fertilizer used. The T2, T3, T4, and T5 plots yielded 4.6, 6.1, 7.1, and 7.3% more pollen, respectively, than did the control T1 plot (Table 4).
The T4 and T5 treatment plots presented greater purities (3.2 and 4.3%, respectively) than did the T1 control treatment after 10 months of cane crop growth (Table 5). In terms of purity, however, T3 did not differ considerably from T1. The T1 and T3 treatments had statistically similar Pols, whereas the T2 (5.6%), T4 (6.8%), and T5 (7.5%) treatments had greater Pols than did the T1 control. The percentages of commercial cane sugar (CCS) in T1 and T3 were similar (10.987 and 11.332%, respectively), but the percentage of CCS was greater in T2 (6.82%), T4 (8.83%), and T5 (8.74%) than in the control plots.
For all the quality parameters at the 10th month, the T4 and T5 plots presented similar values, which were greater than those of the control T1 plot; however, at a higher dose in the T5 plot, there was a 3% decrease in the amount of extracted sugar. However, the T2 and T4 plots received 80 kg K2O ha−1 potash alone or in combination with 40 kg K2O ha−1 and 40 kg polyhalite ha−1, respectively, while the juice quality parameters were similar. However, on the higher side, viz. like in T3 and T5, 120 kg K2O ha−1 had some benefit from the T2 and T4 plots in terms of quality, but all five quality metrics were similar (Table 5).
Table 6 shows that T2, T4, and T5, which were significantly equivalent in purity (by 2.81, 2.87, and 0.81%, respectively), had germinated from ratoon sugarcane after 12 months compared with T1 and T3. On the other hand, the Brix values of the T2, T3, T4, and T5 plots were 4.58, 0.14, 3.66, and 0.73% lower than those of the T1 control plots, respectively (Table 6). In treatments T1 through T5, the CCS percentage (ranging from 12.99 to 13.05%) was comparable across all the treatment plots. A comparison of the T2 and T3 plots to the T1 plot revealed that the average values were − 0.62 and − 0.10%, respectively, but the increases in the T4 and T5 plots were + 0.46 and + 0.38%, respectively. In terms of weight per area, the CCS ranged from 7.98 to 8.47 t ha−1, with T4 (8.59 t ha−1) and T5 (8.60 t ha−1) having significantly greater CCSs than did any of the T1–T3 samples.
Furthermore, Table 6 shows that T2 (20.83O) and T4 (21.03O) had lower Brix values than did the T1 control (21.83°). The percentage of sugar extracted did not differ significantly between treatments, ranging from 48.82% in T1 to 50.66% in T4, as previously reported (Filho, 1985; Wood, 1990; Chapman, 1980). In terms of the quality measures investigated, the T2 and T4 plots with 80 kg ha−1 K exhibited a substantial difference from the T1 control compared with the treatments with 120 kg ha−1 K fertilizer applied, as previously reported (Sudama et al., 1998; Singh et al., 1999).
Compared with the control treatment, all K treatments reduced early shoot borer (Chilo infuscatellus) attack, as T5 had the greatest reduction (−25.8%), whereas T2 and T3 had the least reduction (−16.1%) (Figure 4).
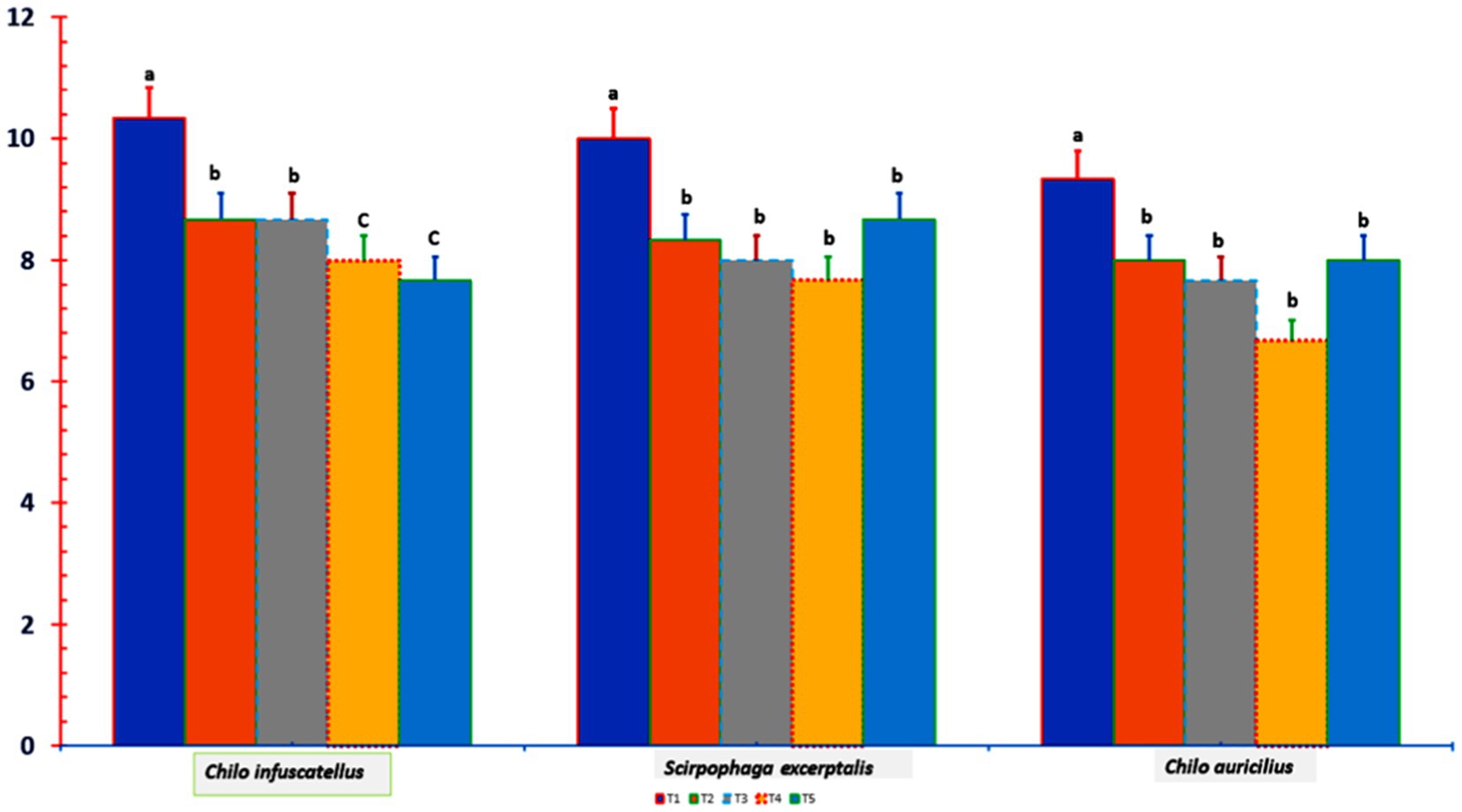
Figure 4. Insect pest incidence in ratoon canes at different K doses and sources. In all the treatments, the recommended dose of non-K fertilizers was applied. DAH, days after harvesting; T1 = 0 kg of K2O ha−1; T2 = 80 kg of K2O ha−1 as muriate potash; T3 = 120 kg of K2O ha−1 as muriate; T4 = 80 kg of K2O ha−1 as muriate potash + polyhalite (50% each); T5 = 120 kg of K2O ha−1 as muriate potash + polyhalite (50% each; three replicates). In column bars, means with similar letter(s) are identical as per LSD0.05.
While the stalk borer (Chilo auricilius) and top borer (Scirpophaga excerptalis) were less common in T4 than in the control, there was no discernible difference in their occurrence between any of the K2O treatments and the control (Figure 4). Compared with those in the control plots, T5 presented the fewest early shoot-borer attacks (−23.3%), whereas T4 presented the lowest incidence of stalk borer attacks (−28.6%) and top borer assaults (−23.3%). Among the K2O treatments, T3 had the highest frequency of stem borer infestation and the greatest occurrence of attack from all three insect pests. The early shoot borer (Chilo infuscatellus) and top borer (Scirpophaga excerptalis), at 0.0, −8.0, and 13.4%, and the stalk borer (Chilo auricilius), at −4.2, −13.0, and 20.0%, respectively, were significantly lower in the T2, T3, T4, and T5 plots (Figure 4). The T4 treatment (80 kg K2O ha−1) had the lowest incidence of insect pest infestations, despite being significantly comparable to the other potash treatments.
Correlation analysis between quality variables revealed that Brix was positively correlated with all the other quality parameters, with much stronger correlations with CCS (%) and Pol. Furthermore, CCS (%) had a stronger and more positive relationship with Brix, Pol, purity, and extractable %age. The extractable %age and purity were reported to have strong positive relationships with CCS (%) and Pol, respectively. Finally, Pol was reported to have a strong positive relationship with all the other quality parameters by December (Table 7).
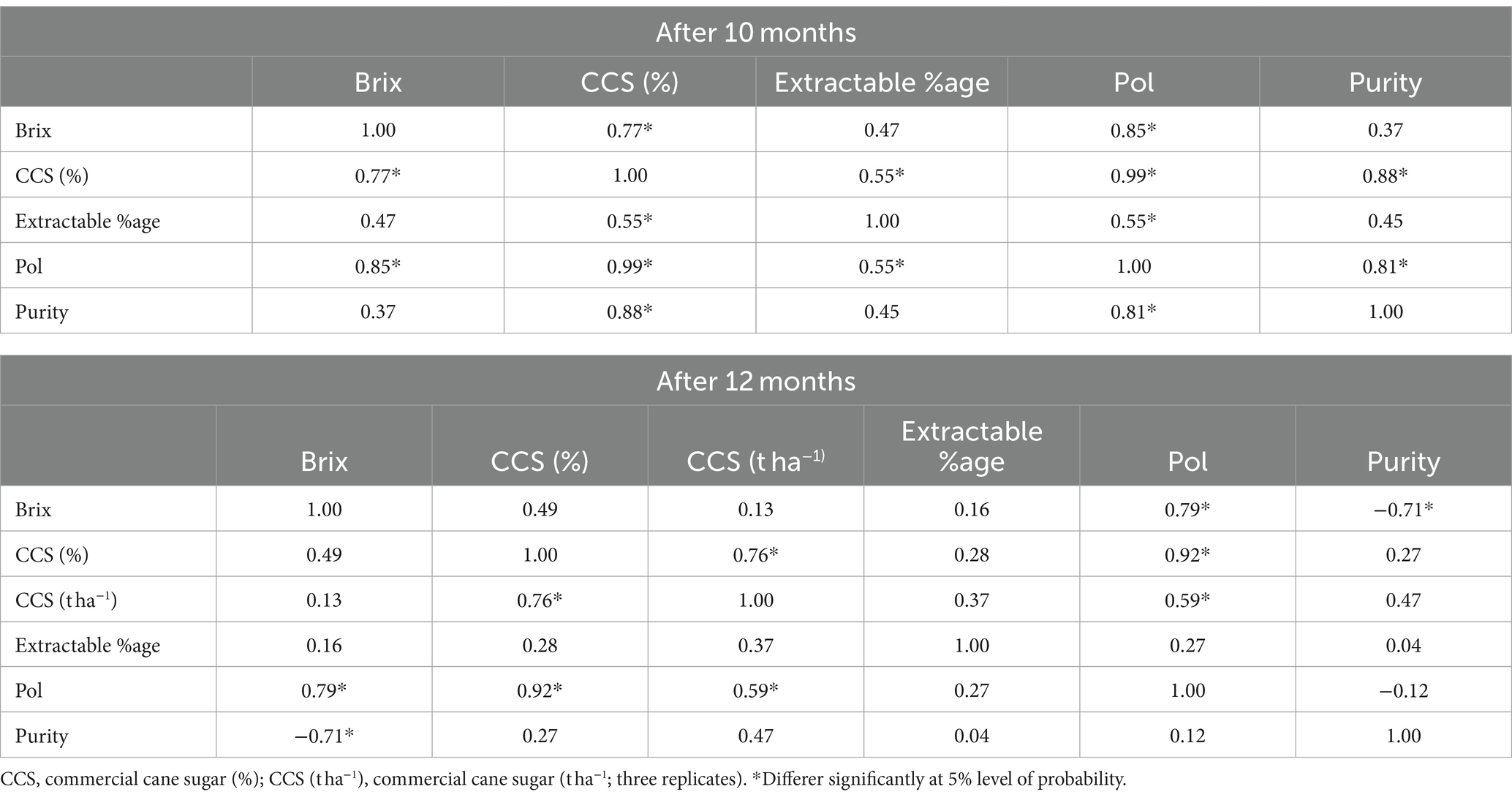
Table 7. Investigation of correlations between various quality indices of ratoon sugarcane plants at 10 and 12 months of age with various K sources and dosages.
For the ratoon crop at 12 months, Brix was positively but weakly related to CCS (%), CCS (t ha−1), and extractable %age but was strongly related to pol (0.79); however, it was strongly but negatively related to purity (−0.71), which was not observed after the 10th month. CCS (%) had both positive and strong relationships with CCS (t ha−1) (0.76) and pol (0.92) but had weak relationships with purity (0.27) and extractable %age (028). However, Pol had positive relationships with Brix (0.79), CCS (%) (0.92), and CCS (t ha−1) (0.59) (Table 7). However, after the 12th month, the extractable %age also had a weaker relationship with the other quality parameters.
The most costly K fertilizers were found in T5 (120 kg K2O ha−1 applied as KCl and K2Ca2Mg(SO4)4H2O combined; Table 8), whereas the least expensive fertilizers were found in T2 (80 kg K2O ha−1 applied as KCl alone). T5 yielded 65.97 t ha−1, the highest output, whereas T1 yielded 61.5 t ha−1. For T2, T3, T4, and T5, the applied K fertilizer produced economic gains of 117.8, 158.1, 182.4, and 187.4 US $ ha−1, respectively (Table 8).
The benefit-to-cost ratios of T2 (3.44) and T3 (3.08) were the highest, whereas those of T4 (2.43) and T5 (2.43) were the lowest (1.66). Although polyhalite, which is imported from England, is more expensive than other nutrients, it is still a great multinutrient fertilizer for soils that are low in both Ca and K. Benefit reductions of 10.5 and 31.6% were observed after switching from T2 to T3 and from T4 to T5, respectively. Higher K fertilizer broadcasting did not enhance farmers’ economic benefits (Table 8), because greater production costs are associated with greater K fertilizer application. These costs were lower for the combined KCL and K2Ca2Mg(SO4)4H2O treatments than for the KCl treatment alone. The advantages were not increased but rather diminished by switching from T2 to T3 and then from T4 to T5 (Table 8). This could have been due to increased insect pest infestations, yield responses, and higher fertilizer prices.
In the deficient K soils, broadcasted potash resulted in enhanced development and land productivity of the ratoon sugarcane plants (Figure 3; Table 4) compared with those in the unfertilized control plots because of the role of the potash in controlling stomatal openings under stressed conditions and sugar translocation (Wood and Schroeder, 2004). Potash plays a role in increasing the rhizosphere zone of cane plants, which helps reduce the negative effects of water stress (Bhatt and Singh, 2021; Bhatt et al., 2020;Bhatt et al.,2021b). Furthermore, the effective H+/K+ symport—a protein that simultaneously transports H+ and K+ ions across root cell membranes—conveys that K absorption is more efficient than Ca or Mg (Bhatt et al., 2020; Wood and Schroeder, 2004). Potash also catalyzes enzymes, enhances the efficiency of applied inputs such as water and fertilizers such as N fertilizers through interactive effects (Korndörfer and Oliveira, 2005), and improves the extraction of moisture and minerals from soils (Singh et al., 1999; Wood and Schroeder, 2004; Korndörfer and Oliveira, 2005; Schultz et al., 2010; Kwong, 2002; Ashraf et al., 2008; Bhatt et al, 2021c).
Both 80 kg K2O ha−1 pure muriate potash and 120 kg K2O ha−1 pure muriate potash improved the sugarcane yield when mixed with polyhalite at a 50% ratio. Polyhalite augmented the production of dry matter. In combination with polyhalite and muriate potash treatments, there is less competition between Cl− and SO42− for absorption by plant roots. In sole muriate potash treatments, on the other hand, there can be more severe competition due to the presence of Cl− and the lack of SO42− in the soil (Huber et al., 2012; Dordas, 2008). With respect to isolated potash deposits, this competition could be even worse. Ca2+, K+, and SO42− accumulate in the soil in treatments where polyhalite + MOP are both accessible, leading to enhanced cane development and growth. Furthermore, K in KCl binds to clay granules in soils more firmly than does K from polyhalite because of competition between monovalent (K+) and divalent (Ca2+ and Mg2+) cations; as a result, potash from later sources is more easily accessible. Crop performance is impacted by synchronizing the availability of nutrients with periods of crop nutrient need, as well as by inconsistency in the accessibility of Ca, Mg, and S, particularly in treatments where only MOP is present (Pavuluri et al., 2017). At our Ca deficiency site, polyhalite supplied a regular flow of Ca, Mg, and S, which improved sugarcane development and land productivity in the region (Figure 4; Table 4) (Smith et al., 1987; Clark and Smith, 1988).
Reportedly, higher numbers of millable canes at 315 and 347 DAH, chlorophyll concentrations at 238, 277, and 308 DAH, and land productivity (Table 4), followed by quality parameters at the 10th and 12th months (Tables 5, 6), have been regularly reported in control plots than K-fertilized plots. The enhancements reached a maximum when both polyhalite and muriate potash (50% each) were applied at 80 K2O ha−1, which was also found to be statistically equivalent to 120 K2O ha−1 plots. Furthermore, lower stomatal conductance and higher mesophyll resistance led to a decrease in starch synthase, nitrate reductase, invertase, phosphofructokinase, sucrose phosphate synthase, β-amylase, and pyruvate kinase. However, deficient K supply resulted in wilting and inadequate photosynthetic translocation from leaves to cane stems under stressful conditions, which also reported a higher prevalence of insect pests (Bhatt and Singh, 2021; Bhatt et al., 2021a; Bhatt et al., 2021b).
Under T4 and T5 treatments loaded with polyhalite fertilizer, cane plants receive relatively high levels of K, Ca, and Mg, which increases the storage lifetime of reaped ratton plants and decreases losses that occur after harvesting (Yermiyahu et al., 2019). When loaded with a low chloride concentration, polyhalite behaves as a slow-release fertilizer and is further reported to have a relatively high use efficiency (Yermiyahu et al., 2019; Herrera et al., 2022), lowering the danger of saline conditions and indigenous soil K loss. Ca effects of polyhalite are similar to those of gypsum, which are recognized to be significant for sugarcane output (Bhatt et al., 2020; Bhatt et al., 2021b). Because polyhalite is >10 times more soluble than gypsum, the calcium would migrate to the subsurface more quickly than it does for gypsum. Ca would be applied yearly with polyhalite in place of applying gypsum every 5–7 years to reduce subsoil acidity.
Additionally, the benefit-to-cost ratio decreased when the potash dose increased from 40 kg K2O ha−1. This occurred because of the greater fertilizer costs (Table 8), lower yields (Table 4), and greater incidence of insect pests (Figure 4). Cane growth and land productivity were both increased by the addition of Ca, Mg, and S, which are low in these nutrients, to the experimental soil.
For combinations of muriate potash and polyhalite, even at 80 kg K2O ha−1, ratoon cane performance standards were superior to those of muriate potash applied alone, even if the dose was the same (Tables 5, 6). This is because polyhalite provides a more steady supply of essential nutrients, including K+ and Ca2+, which further enhances various juice quality metrics. Compared with Ca2+ or Mg2+, K+ adsorbs less strongly to mineral soil surfaces, and the overall adsorption capacity of soil increases with increasing clay mineral concentration (Mengel and Haeder, 1977; Rabindra and Kumaraswamy, 1978).
In control plots, insufficient potassium upsets the water balance, resulting in poor growth and reduced sucrose accumulation. Potassium aids in maintaining cell turgor pressure, which is necessary for optimum growth. Potassium stimulates several enzymes essential for starch synthesis, protein synthesis, and photosynthesis. The plant’s capacity to create and store carbohydrates, which are subsequently transformed into sucrose, is improved by this activation (Elwan et al., 2008). These metabolic processes are less effective in the absence of sufficient potassium, which lowers the sugar concentration and degrades the quality of the harvested cane in control plots. The movement of nutrients and photosynthates, or the byproducts of photosynthesis, from the leaves to the stalks, where they are stored as sucrose, is another process in which potassium is essential (Bhatt and Singh, 2021; Bhatt et al., 2021b; Wood and Schroeder, 2004).
The plant nutrient balance increases crop resistance to most pests and diseases simply because a healthy ratoon cane is less vulnerable to assault (Huber et al., 2012; Dordas, 2008). A single treatment of MOP at 80 kg K2O ha−1 reduced the number of early shoot borers (Chilo infuscatellus), top borers (Scirpophaga excerptalis), and stalk borers (Chilo auricilius), albeit not significantly. These reductions were further achieved by pairing MOP and polyhalite at the same dose (Figure 4). Potash (potassium) strengthens plant cell walls, increasing their resistance to pest penetration and reducing insect–pest attacks in sugarcane (Hartt, 1969). Additionally, potassium improves the general health of the plant by strengthening its defense systems, such as the synthesis of chemicals that ward off pests. Furthermore, plants that receive enough potassium from their diet experience less stress, which makes them less susceptible to pest infestations. Potassium increases the plant’s capacity to heal from wounds, which lessens the possibility that pests may spread and do serious damage. Reduced pest attacks are the result of stronger physical barriers and improved biochemical defenses in sugarcane that has received potassium fertilization (Bhatt and Singh, 2021; Bhatt et al., 2021b; Elwan et al., 2008; Shukla et al., 2009; Bhatt et al, 2021c).
From the results and discussion of the study, it can be concluded that in semiarid tropical soils, among the different nutrients involved in sugarcane cultivation, K+, Ca2+, Mg2+, and SO42− are among those in short supply, owing in part to agricultural intensification. Sugarcane output and juice quality are hampered by a shortage of these critical nutrients. The traditionally used muriate potash is inadequate for addressing this issue and meeting plant needs. Polyhalite has potential for use in the sugarcane production region of North India. We discovered that applying muriate potash alone at 80 kg K2O ha−1 improved ratoon cane performance and enhanced benefits, and these enhancements were further significantly amplified when muriate potash was mixed with polyhalite in deficient soils. However, interestingly, these enhancements in ratoon cane plants were reduced when a higher dose of 120 kg K2O ha−1 was tested at the site, which also reduced the benefits due to the higher costs of additional fertilizers than the reported benefits. The addition of Ca to soils that are low in Ca is one of the anticipated benefits of blending polyhalite with muriate potash at 50%. Further attempts are needed to determine the optimal amounts of important nutrients, such as K, Ca, Mg, and S, for developing balanced fertilization schedules with special emphasis on different sources of potash for improving sugarcane performance and the livelihoods of cane farmers in the region.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any identifiable images or data included in this article.
RB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft. PI: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft. AP: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. KV: Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – original draft. LA-S: Data curation, Formal analysis, Funding acquisition, Project administration, Software, Writing – review & editing. SS: Data curation, Formal analysis, Funding acquisition, Software, Writing – review & editing. AG: Data curation, Formal analysis, Funding acquisition, Software, Writing – review & editing. AH: Data curation, Formal analysis, Funding acquisition, Resources, Software, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Potash Research Institute of India, Gurgaon, and the International Potash Institute, Switzerland, through Project No. Misc. 168 (PC 5034), “Assessment of POLYHALITE in improving yield and quality of sugarcane in Punjab, India,” and was also partially funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R365), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2024.1388916/full#supplementary-material
AngloAmerican. (2016) POLY4 brochure. Available at: https://uk.angloamerican.com/our-fertiliser (Accessed December 21, 2016).
Ashraf, M. Y., Hussain, F., Akhter, J., Gul, A., Ross, M., and Ebert, G. (2008). Effect of different sources and rates of nitrogen and supra optimal level of potassium fertilization on growth, yield and nutrient uptake by sugarcane. Grown under saline conditions. Pak. J. Bot. 40, 1521–1531.
Barbarick, K. A. (1991). Polyhalite application to sorghum–sudangrass and leaching in soil columns. Soil Sci. 151, 159–166. doi: 10.1097/00010694-199102000-00005
Barber, S. A. (1995). Soil nutrient bioavailability: A mechanical approach. 2nd Edn. New York: Wiley. 432.
Barrows, H. L., and Simpson, E. C. (1962). An EDTA method for the direct routine determination of calcium and magnesium in soils and plant tissue. Soil Sci. Soc. Am. J. 26, 443–445. doi: 10.2136/sssaj1962.03615995002600050012x
Bhatt, R. (2020). “Resources management for sustainable sugarcane production” in Resources use efficiency in agriculture. eds. S. Kumar, S. R. Meena, and K. M. Jhariya (Singapore: Springer), 650–685. doi: 10.1007/978-981-15-6953-1_18
Bhatt, R., Oliveira, M. W., and Silva, V. S. G. (2021a). Sugarcane nutrition for food and environmental security. Brazil J. Dev. 7, 64431–64467. doi: 10.34117/bjdv7n6-701
Bhatt, R., and Sharma, M. (2014). Importance of soil testing and techniques of soil sampling. England: Lap Lambert Academic Publishing, 1–48.
Bhatt, R., and Singh, P. (2021). Sugarcane response to irrigation and potash levels in subtropics. Agric. Res. J. 58, 709–715. doi: 10.5958/2395-146X.2021.00100.9
Bhatt, R., and Singh, M. (2021). Comparative efficiency of polymer–coated urea for lowland rice in semiarid tropics. Commun. Soil Sci. Plant Anal. 52, 2331–2341. doi: 10.1080/00103624.2021.1925689
Bhatt, R., Singh, P., Ali, O. M., Latef, A. A. H. A., Laing, A. M., and Hossain, A. (2021b). Yield and quality of ratoon sugarcane are improved by applying potassium under irrigation to potassium deficient soils. Agronomy 11:1381. doi: 10.3390/agronomy11071381
Bhatt, R., Singh, P., Hussain, A., and Tamsina, J. (2021c). Rice–wheat system in the northwest indo-Gangetic plains of South Asia: issues and technological interventions for increasing productivity and sustainability. Paddy Water Environ. 19, 345–365. doi: 10.1007/s10333-021-00846-7
Bhatt, R., Singh, P., and Kumar, R. (2020). Assessment of polyhalite in improving yield and quality of sugarcane in Punjab, India. Gurgaon: Project submitted to Indian Potash Limited (IPL).
Cakmak, I., and Yazici, A. M. (2010). Magnesium: a forgotten element in crop production. Better Crops 94, 23–25.
Chapman, L. S. (1980) Long term responses in cane yields and soil analyses from potassium fertilizer. In: Proceedings of the 1980 conference of the Australian Society of Sugar Cane Technologists, pp. 63–68.
Choudhary, H. R., and Singh, R. K. (2016). Effect of sequential application of herbicides on weeds and productivity of spring–planted sugarcane (Saccharum officinarum L.). Bioscan 11, 687–690.
Clark, C. J., and Smith, G. S. (1988). Seasonal accumulation of mineral nutrients by kiwifruit 2. Fruit. New Phytol. 108, 399–409. doi: 10.1111/j.1469-8137.1988.tb04180.x
Dordas, C. (2008). Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 28, 33–46. doi: 10.1051/agro:2007051
Elwan, E. A., Abazied, A. A., Youssef, L. A., and Sakr, H. E. A. (2008). Influence of some agricultural practices on the infestation with lesser sugarcane borer, Chilo Agamemnon Bles. in the autumn plant cane and its 1st ratoon in upper Egypt. Egypt. J. Agric. Res. 86, 1801–1826.
Fageria, N. K., Baligar, V. C., Wright, R. J., and Carvalho, J. R. P. (1990). Lowland rice response to potassium fertilization and its effect on N and P uptake. Fert. Res. 21, 157–162. doi: 10.1007/BF01087425
Farhat, N., Elkhouni, A., Zorrig, W., Smaoui, A., Abdelly, C., and Rabhi, M. (2016). Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant 38:145. doi: 10.1007/s11738-016-2165-z
Filho, J. O. (1985). “Potassium nutrition of sugarcane” in Potassium in agriculture. ed. R. D. Munson (Madison, WI, USA: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America), 1045–1062.
Fraps, G. S. (1932). Availability to plants to potash in polyhalite. College Station, Texas: Texas Agricultural Experiments Station Bulletin No. 449.
Garnett, S. (2021) The potential of polyhalite fertilizers to enhance potato yield and quality in the United Kingdom. E-ifc, International Potash Institute; 63, pp. 18–27.
Gransee, A., and Führs, H. (2013). Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 368, 5–21. doi: 10.1007/s11104-012-1567-y
Hartt, C. E. (1969). Some effects of potassium upon the amounts of protein and amino forms of nitrogen, sugars and enzyme activity of sugarcane. Plant Physiol. 9, 452–490. doi: 10.1104/pp.9.3.452
Herrera, W. F. B., Arruda, B., De Carvalho, H. W. P., and Pavinato, P. S. (2022). Improving potassium use efficiency of sugarcane through the use of polyhalite. CABI Agric. Biosci. 3, 1–11. doi: 10.1186/s43170-022-00124-4
Huber, D., Römheld, V., and Weinmann, M. (2012). “Relationship between nutrition, plant diseases and pests” in Marschner’s mineral nutrition of higher plants (Academic Press), 283–298.
Jamal, A., Moon, Y. S., and Abdin, M. Z. (2010). Sulfur – a general overview and interaction with nitrogen. Australian J. Crop Sci. 4, 523–529.
Khan, N. A., and Mobin, M. (2005). Samiullah, the influence of gibberellic acid and sulfur fertilization rate on growth and S–use efficiency of mustard (Brassica juncea). Plant Soil 270, 269–274. doi: 10.1007/s11104-004-1606-4
Kirkby, E. A., and Pilbeam, D. J. (1984). Calcium as a plant nutrient. Plant Cell Environ. 7, 397–405. doi: 10.1111/j.1365-3040.1984.tb01429.x
Korndörfer, G. H., and Oliveira, L. A. (2005). “Potassium in sugarcane crops” in Potassium in Brazilian agriculture. eds. T. Yamada and T. L. Roberts (Piracicaba: Esalq/USP).
Kovar, J. L., and Grant, C. A. (2011) Nutrient cycling in soils: Sulfur. Publications from USDA–ARS/UNL Faculty; Paper 1383.
Kumar, A., Babar, L., Mohan, N., and Bansal, S. K. (2019). Effect of potassium application on yield, nutrient uptake and quality of sugarcane and soil health. Indian J. Fertil. 15, 782–786.
Kwong, K. F. (2002) The effects of potassium on growth, development, yield and quality of sugarcane. In Potassium for sustainable crop production and food security, proceedings of the first National Potash Symposium, Dar Es Salaam, Tanzania, 28–29 July 2015; International Potash Institute: Zug, Switzerland; pp. 430–444.
Lewis, T. D., Hallett, P. D., Paton, G. I., and Harrold, L. (2020). Retention and release of nutrients from polyhalite to soil. Soil Use Manag. 36, 117–122. doi: 10.1111/sum.12548
Meade, G. P., and Chen, J. C. P. (1977). Can Sugar Handbook. 10th Edn. New York: Wiley–Interscience, Publication, 405.
Mengel, K., and Haeder, H. E. (1977). Effect of potassium supply on the rates of phloem sap exudation and the composition of phloem sap of ricinis communis. Plant Physiol. 59, 282–284. doi: 10.1104/pp.59.2.282
Monshausen, G. B. (2012). Visualizing Ca2+ signatures in plants. Curr. Opin. Plant Biol. 15, 677–682. doi: 10.1016/j.pbi.2012.09.014
O’Hara, I. M., Edye, L. A., and Doherty, W. (2009) Towards a commercial lignocellulosic ethanol industry in Australia: the Mackay renewable bio commodities pilot plant. In Proceedings of the Australian Society of Sugarcane Technologists, Balina, Australia, 5–8, 31, pp. 11–17.
Olivoto, T., and Dal’Col Lúcio, A. (2020). Metan, an R package for multi-environment trial analysis. Methods Ecol. Evol. 11, 783–789. doi: 10.1111/2041-210X.13384
Olsen, S. R. (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate (No. 939); US Department of Agriculture.
PAU. (2022) Package of practices for crops of Punjab–Kharif; Punjab Agricultural University: Ludhiana, India; pp. 55–66.
Pavinato, P. S., Corá, J. E., Santos, C., Herrera, A., Pavuluri, W. F. B., and Pierce, F. J. (2020). Sugarcane response to polyhalite fertilizer in Brazilian Oxisols. Agron. J. 112, 5264–5278. doi: 10.1002/agj2.20452
Pavuluri, K., Malley, Z., Mzimbiri, M. K., Lewis, T. D., and Meakin, R. (2017). Evaluation of polyhalite in comparison to muriate for corn grain yield in the southern highlands of Tanzania. African J. Agron. 5, 325–332.
Quampah, A., Wang, R. M., Shamsi, I. H., Jilani, G., Zhang, Q., Hua, S., et al. (2011). Improving water productivity by potassium application in various rice genotypes. Int. J. Agric. Biol. 13, 9–17.
Rabindra, B., and Kumaraswamy, S. (1978). Potash and eye spot disease in sugarcane. Ind. Potash J. 3, 15–18.
Sacks, M., Gantz, S., Mezuman, U., Peled, L., and Imas, P. (2017) Polyhalite – a multinutrient fertilizer preventing ca. and mg deficiencies in greenhouse tomatoes under desalinized irrigation water. E-ifc, International Potash Institute; 51, pp. 24–30.
Schultz, N., Lima, E., Pereira, M. G., and Zonta, E. (2010). Residual effects of nitrogen, potassium and vinasse, fertilization on cane plant and ratoon harvested with and without straw burning. Rev. Bras. Ci Solo 34, 811–820. doi: 10.1590/S0100-06832010000300023
Shukla, S. K., Solomon, S., Sharma, L., Jaiswal, V. P., Pathak, A. D., and Singh, P. (2017). Green technologies for improving cane sugar productivity and sustaining soil fertility in the sugarcane–based cropping system. Sugar Tech. 21, 186–196. doi: 10.1007/s12355-019-00706-z
Shukla, S. K., Yadav, R. L., Singh, P. N., and Singh, I. (2009). Potassium nutrition for improving stubble bud sprouting, dry matter partitioning, nutrient uptake and winter initiated sugarcane (Saccharum spp. hybrid complex) ratoon yield. Eur. J. Agron. 30, 27–33. doi: 10.1016/j.eja.2008.06.005
Singh, K. D. N., Mishra, G. K., and Ojha, J. B. (1999). Effect of potassium on yield and quality of sugarcane in calciothents. Ind. Sugar 49, 499–507.
Singh, J., Singh, R. D., Anwar, S. I., and Solomon, S. (2011). Alternative sweeteners production from sugarcane in India: lump sugar (Jaggery). Sugar Tech. 13, 366–371. doi: 10.1007/s12355-011-0110-4
Singh, B., Singh, Y., Imas, P., and Xie, J. (2004). Potassium nutrition of the rice–wheat cropping system. Adv. Agron. 81, 203–259. doi: 10.1016/S0065-2113(03)81005-2
Smith, G. S., Clark, C. J., and Henderson, H. V. (1987). Seasonal accumulation of mineral nutrients by kiwifruit I. Leaves. New Phytol. 106, 81–100. doi: 10.1111/j.1469-8137.1987.tb04793.x
Steward, F. C. (1974). Mineral nutrition of plants: principles and perspectives, Emanuel Epstein. Q. Rev. Biol 49, 353–354. doi: 10.1086/408215
Sudama, S., Tiwari, T. N., Srivastava, R. P., Singh, G. P., and Singh, S. (1998). Effect of potassium on stomatal behaviour, yield and juice quality of sugarcane under moisture stress conditions. Ind. J. Plant Physiol. 3, 303–305.
Tien, T. M., Trang, T. T. T., Ha, P. T. N., Chien, D. T., Thai, T. T., Thang, D. T., et al. (2020) Polyhalite effects on winter maize crop performance on degraded soil in northern Vietnam. E-ifc, International Potash Institute; 62, pp. 3–12.
Tien, T. M., Trang, T. T. T., Ha, P. T. N., and Thu, T. T. M. (2021) Effects of polyhalite application on yield and quality of cabbage grown on degraded soils in northern Vietnam. E-ifc, International Potash Institute; 63, pp. 3–10.
Vale, F. (2016) Calcium and magnesium movement in soil profile with polyhalite as potassium fertilizer for soybean crop. Proceedings of FERTBIO 2016, Goiana, Brazil, October 16–20, 2016.
Wakeel, A., and Ishfaq, M. (2022). “Future research perspectives” in Potash use and dynamics in agriculture (Singapore: Springer), 109–119.
Walkley, A., and Black, I. A. (1934). An estimation of the method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
White, P. J., and Broadley, M. R. (2003). Calcium in plants. Ann. Bot. 92, 487–511. doi: 10.1093/aob/mcg164
Wood, R. A. (1990). The roles of nitrogen, phosphorus and potassium in the production of sugarcane in South Africa. Fertil. Res. 26, 89–98. doi: 10.1007/BF01048746
Wood, A. W., and Schroeder, B. L. (2004) Potassium: a critical role in sugarcane production, particularly in drought conditions. In Proceedings of the Australian Society of Sugarcane Technologists, Brisbane, Australia, 4–7 May 2004. Available at: http://www.cabdirect.org/abstracts/20043079912.html (accessed May 7, 2020).
Yermiyahu, U., Zipori, I., Omer, C., and Beer, Y. (2019) Solubility of granular polyhalite under laboratory and field conditions. E-ifc, International Potash Institute; 58, pp. 3–9.
Keywords: polyhalite, sugarcane, productivity, quality, insect pest
Citation: Bhatt R, Imas P, Perelman A, Verma KK, Al-Shuraym LA, Sayed S, Gaber A and Hossain A (2024) Polyhalite improves growth, yield, and quality and reduces insect pest incidence in sugarcane (Saccharum officinarum L.) in the semiarid tropics. Front. Sustain. Food Syst. 8:1388916. doi: 10.3389/fsufs.2024.1388916
Received: 20 February 2024; Accepted: 15 August 2024;
Published: 12 September 2024.
Edited by:
Mohamed Ait-El-Mokhtar, University of Hassan II Casablanca, MoroccoReviewed by:
Marco E. Mng’ong’o, Mbeya University of Science and Technology, TanzaniaCopyright © 2024 Bhatt, Imas, Perelman, Verma, Al-Shuraym, Sayed, Gaber and Hossain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajan Bhatt, cmFqYW5zb2lsc0BwYXUuZWR1; Akbar Hossain, YWtiYXJob3NzYWlud3JjQGdtYWlsLmNvbQ==
†ORCID: Rajan Bhatt, https://orcid.org/0000-0001-9977-9955
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.