
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 05 February 2024
Sec. Sustainable Food Processing
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1356705
 Rongzheng Huang1†
Rongzheng Huang1† Bingxin Cai1†
Bingxin Cai1† Yongcheng Chen1
Yongcheng Chen1 Xiaokai Zheng1
Xiaokai Zheng1 Jianqi Yang2
Jianqi Yang2 Chunhui Ma1
Chunhui Ma1 Xuzhe Wang1*
Xuzhe Wang1* Fanfan Zhang1*
Fanfan Zhang1*Background: In this study, we aimed to address the low utilization of straw and poor fermentation quality of paper mulberry silage (under natural fermentation conditions). Straw was combined with paper mulberry for ensiling, and the fermentation characteristics, bacterial community, and metabolite composition of the mixed straw and paper mulberry silage were investigated. Four treatment groups were established: corn-straw treatment 2 (3:7 ratio of corn straw to paper mulberry), corn-straw treatment 3 (5:5 ratio of corn straw to paper mulberry), wheat-straw treatment 2 (3:7 ratio of wheat straw to paper mulberry), wheat-straw treatment 3 (5:5 ratio of wheat straw to paper mulberry), and a control group (ensiling of paper mulberry alone).
Results: The control group demonstrated the highest pH and ammonia (AN) and acetic acid (AA) content compared with all the treatment groups. Corn-straw treatment 2 had the highest lactic acid content (54.70 g/kg dry weight) compared with the control and other treatment groups. The relative abundance of Enterobacter (7.085%) was the lowest in the control than in the other treatment groups (p < 0.05). The relative abundance of Enterococcus was higher in both the control and wheat-straw treatment 2 (22.03% and 21.29%, respectively) than in other treatment groups. The relative abundance of Lactococcus was highest in wheat-straw treatment 3 (15.83%) compared with the control and other treatment groups. Corn-straw treatments 2 and 3 demonstrated the same metabolite composition but were clearly different from the wheat-straw treatment 2, wheat-straw treatment 3, and the control. Diacetoxyscirpenol (DAS) belongs to the Fusarium metabolite type A trichothecenes, which were not detected in corn or wheat silage. DAS was downregulated in the wheat-straw treatment 3 and both corn-straw treatments compared with the control, which indicates that the addition of straw decreased mycotoxin production. Lactococcus was significantly and positively correlated with gluconic acid content (R2 = 0.5166).
Conclusion: Our results suggest that straw treatment can improve the nutritional value of paper mulberry silage by decreasing mycotoxin production, pH value, and AN content and increasing lactic acid production.
Paper mulberry is a new source of animal feed used during feed shortages, and due to its high nutritional value, biological content, high production capacity, and low cultivation and production costs, it has rapidly developed in the livestock industry (Du et al., 2021). Ensiling is a superior method of preserving forage nutrition (McDonald et al., 1991; Kan et al., 2023). Recently, paper mulberry silage has been used to replace part of the alfalfa hay in the diet of ruminants. However, similar to alfalfa, naturally ensiled paper mulberry results in low silage fermentation because of its low dry matter (DM), high buffering capacity, low water-soluble carbohydrate (WSC) content, and relatively low number of lactic acid bacteria (LAB) (Hao et al., 2022). Straw is a residue of vegetative parts of grains such as rice and wheat, but the utilization of straw for ruminant animal feed is limited because of its low protein content, phenolic properties, and high silica and lignin levels (Oladosu et al., 2016). Approximately 90.49% of the global rice straw is produced in Asia, but only 20% of the total crop residue is utilized (Lu et al., 2022). Paper mulberry silage showed the same effects as alfalfa hay on dairy goat performance, such as reduced feed costs and increased antioxidant capacity in animals (Zhang et al., 2022). Replacing alfalfa hay with paper mulberry silage in the diet of dairy cows had no effect on their dry matter intake or milk yield but improved their antioxidative capacity (Wu et al., 2022). Thus, paper mulberry silage is important in the field of ruminant feed. In addition, ensiling paper mulberry alone typically results in extensive proteolysis, an unfavorable odor, and high butyric acid content (Kadam et al., 2000; Dong et al., 2020). Ensiled alfalfa mixed with straw has beneficial effects on fermentation quality, such as decreased pH but increased lactic acid content, number of LAB, and in vitro digestibility of dry matter (Wang et al., 2018). Ensiling straw and forage together may improve straw nutrition and the efficient utilization of crop residues.
Ensiling straw with paper mulberry could solve the problems of poor paper mulberry quality after the natural fermentation and lack of straw utilization; however, few studies have focused on ensiling straw with paper mulberry. Therefore, in this study, we investigated the fermentation characteristics, bacterial community, and associated metabolites composition of straw and paper mulberry silage. This study provides new insights into resource utilization in the livestock industry.
Paper mulberry (Broussonetia papyrifera L.), wheat, and corn straw were harvested on 30 July 2022 from a tree-planting demonstration base in Xinjiang, China (44.20 N, 83.51 E; 450 m altitude). The samples were dried to an approximate fresh weight of 300 g/kg after wilting and then chopped into 2 cm stalks using a forage cutter. Five groups were established: control (100% paper mulberry); paper mulberry mixed with wheat-straw at a 5:5 ratio (M3) and 7:3 ratio (M2); paper mulberry mixed with corn straw at a 5:5 ratio (Y3) and 7:3 ratio (Y2). Each combination was mixed manually. Subsequently, samples weighing 1 kg were packed into polyethylene plastic bags and equipped with a one-way air extraction valve (23 × 30 cm). The bags were sealed using a vacuum sealer and stored at 24°C for 60 days of fermentation. Five replicates were created for each treatment.
After 60 days of ensiling, samples (200 g) were dried at 65°C for 48 h and then ground and filtered with a 1-mm sieve to determine DM content. Total nitrogen (TN) was determined using an automatic Kjeldahl nitrogen analyzer (K9840, Hanon Co. Ltd., Shandong, China), and the cured protein was calculated according to the method of the Association of Official Agricultural Chemists (AOAC). The WSC content was determined as previously described (McDonald and Henderson, 1964).
Fresh silage samples (20 g) were used to analyze the fermentation characteristics. After using four cheesecloth layers to filter the water-silage mixture (1:9 v/v), the pH was measured using a portable pH meter (PHS-3C, Instrument and Electrical Science Instrument Co. Ltd., Shanghai, China), and the supernatant was collected for ammonium (AN) and organic acid content analysis, according to previously described methods (Weatherburn, 1967; Huang et al., 2022b). In brief, the supernatant was filtered with a 0.22-μm dialyzer and then analyzed using high-performance liquid chromatography (HPLC) (1,200 series, Agilent Technologies, Inc., Waldbronn, Germany) with a C18 column (150 × 4.6 mm, FMF-5559-EONU, FLM Scientific Instrument Co., Ltd., Guangzhou, PR China) to measure the organic acid content. Na₂HPO₄ (1 mM) was used for the analysis of the mobile phase with a flow rate of 0.6 mL·min−1, and an injection volume of 20 μL at 50°C.
The total DNA of each sample was extracted using a commercial DNA Kit (FastDNA® Spin Kit for Soil, MP Biomedicals, Irvine, CA, United States). Primers targeting the V3-V4 regions of 16S rDNA (338F: ACTCCTACGGGAGGCAGCAG; 806R: GGACTACHVGGGTWTCTAAT) were used for PCR amplification, as described in a previous study (Ni et al., 2017). Amplicons were extracted, purified, and analyzed as previously described (Su et al., 2019). Three replicates were conducted for each sample, and a mixture of the three replicates for each sample was sequenced. The PCR reaction mixture included 4 μL of 5 × Fast Pfu buffer, 2 μL 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL Fast Pfu polymerase, 10 ng of template DNA, and ddH2O until a final volume of 20 μL was reached. PCR amplification cycling conditions were as follows: initial denaturation at 95°C for 3 min, followed by 27 cycles of denaturing at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 45 s, single extension at 72°C for 10 min, and ended at 4°C.
Silage samples weighing 50 mg were collected, and the metabolites were extracted using a 400 μL methanol and water (4:1, v/v) solution. The mixture was allowed to settle at −20°C and then treated with a high-throughput tissue crusher Wonbio-96c (Shanghai Wanbo Biotechnology Co., Ltd., Shanghai, China) at 50 Hz for 6 min. Subsequently, the samples were vortexed for 30 s, ultrasonicated at 40 kHz for 30 min at 5°C, and then stored at −20°C for 30 min to allow the proteins to precipitate. The samples were then centrifugated at 13,000 × g at 4°C for 15 min, and the supernatant was collected for LC–MS/MS analysis. The specific method used for LC–MS/MS analysis has been described previously (Huang et al., 2022a).
All statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, United States). The characteristics data of paper mulberry silage were analyzed using one-way analysis of variance (ANOVA). Significant differences between treatments were determined using Tukey’s test at p < 0.05. The bacterial community and metabolites were analyzed using the free online MajorBio Cloud Platform (see Table 1).1
As shown in Table 2, the content of DM in Y3 and M3 was higher than that in the other treatment groups (25.69% and 24.63%, respectively; p < 0.05) and was the lowest in the control (16.77%; p < 0.05). CP content was the highest in the control group (p < 0.05), followed by Y2 and Y3 (p < 0.05) and M2 and M3 (p < 0.05). All treatment groups showed higher WSC content than the control (p < 0.05), but there was no significant difference among the treatment groups (p > 0.05). NDF content was the highest in M3 (p < 0.05), followed by M2, Y3 (p < 0.05), Y2, and the control (p < 0.05). All treatment groups demonstrated a higher ADF content than the control group (p < 0.05), but there was no significant difference among the treatment groups (p > 0.05).
As shown in Table 3, the control had the highest pH (p < 0.05), followed by Y2, M2, and M3; however, there was no difference among the three treatment groups (Y2, M2, and M3, p > 0.05) and Y2 (p < 0.05). The ammonia (AN) content (1.91% TN) was higher in control than the other treatment groups (p < 0.05); Y2 and M2 were higher than Y3 and M3 (p < 0.05). The LA content was higher in the Y3 group (54.70 g/kg DM) compared with the control and the other treatment groups (p < 0.05), and Y2 and M3 had higher LA content than M2 and the control (p < 0.05). The AA content was higher in the control group than in the other treatment groups (p < 0.05) and was lowest in Y3 (p < 0.05). PA was detected only in the control group.
As shown in Table 4, the control group had the highest Shannon index value (p < 0.05), followed by M3 (p < 0.05), Y2, Y3, and M2 (p < 0.05); however, there was no difference among the three treatment groups (Y2, Y3, and M2, p > 0.05). The control group had the highest Chao index value (p < 0.05), followed by Y2, Y3, and M2 (p < 0.05); however, there was no difference among the three treatment groups (Y2, Y3, and M2, p > 0.05) and M3 (p < 0.05).
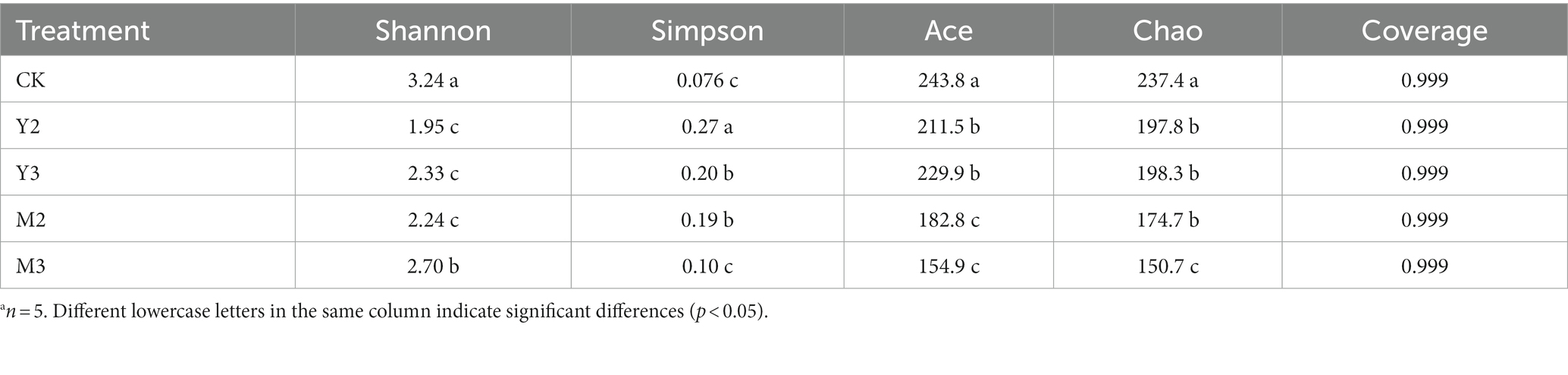
Table 4. Alpha diversity of bacterial community after 60 days of ensilationa.
As shown in Figure 1, there was no clear difference between Y2 and Y3; however, there was a clear difference between the treatment and control groups (p < 0.05).
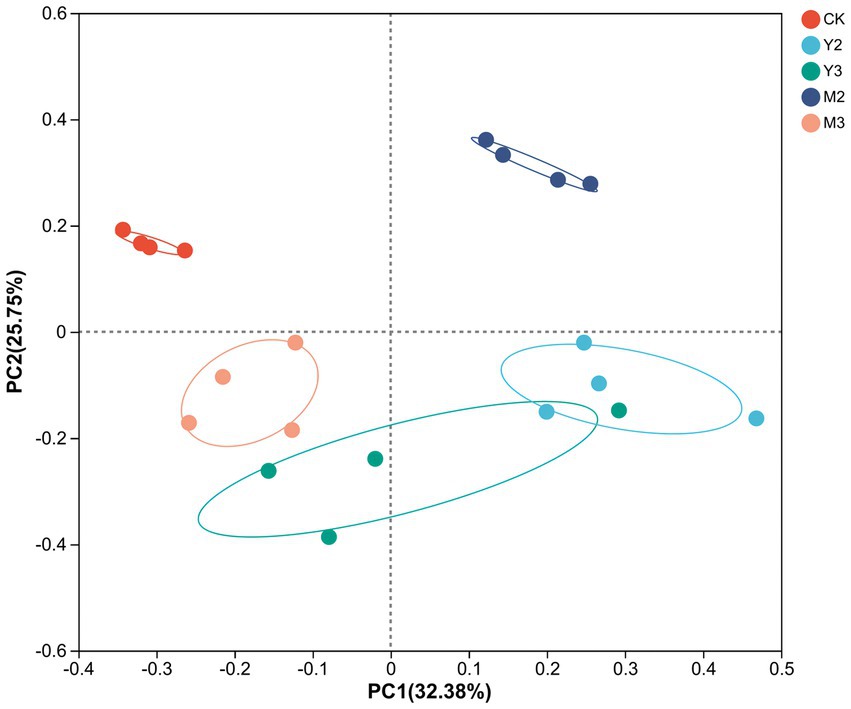
Figure 1. Principal coordinates analysis (PCoA) based on the operational taxonomic unit (OTU) level of silage. CK: control (paper mulberry); Y2 (corn straw mixed with paper mulberry at a 3:7 ratio) (based on fresh weight); Y3 (corn straw mixed with paper mulberry at a 5:5 ratio); M2 (wheat straw mixed with paper mulberry at a 3:7 ratio); M3 (wheat straw mixed with paper mulberry at a 5:5 ratio).
As shown in Figure 2A, at the phylum level, Proteobacteria were dominant in all groups, followed by Proteobacteria and Cyanobacteria. Firmicutes (55.07–78.87%) were dominant in the treatment and control groups but showed no difference between the groups (p > 0.05). Proteobacteria had the highest relative abundance in the Y2 group (39.75%, p < 0.05), followed by Y3 and M2 (29.71 and 28.97%, respectively, p < 0.05), but had the lowest relative abundance in the control group (9.26%). The relative abundance of Cyanobacteria was 4.29%–6.72% and showed no difference between each treatment group (p > 0.05).
At the genus level, Lactobacillus was the dominant genus in all groups, followed by Enterobacter, Enterococcus, and Weissella (Figure 2B). The relative abundance of Lactobacillus was 26.83–44.57% and showed no difference between the treatment groups (p > 0.05). The highest relative abundance of Enterobacter was observed in the Y2 group (35.69%), followed by M2 and Y3 (26.12% and 23.51%, respectively, p < 0.05), and the lowest was observed in the control group (7.09%). The highest relative abundance of Enterococcus was observed in the control and M2 groups (22.03% and 21.29%, respectively; p < 0.05), followed by M3 (9.08%; p < 0.05), and the lowest was observed in Y2 and Y3 (3.95% and 3.41%, respectively; p < 0.05). The highest relative abundance of Weissella was observed in the M3 group (11.92%), followed by the Y3, control (10.05% and 8.89%, respectively; p < 0.05), M2, and Y2 groups (1.17% and 0.95%, respectively; p < 0.05).
As shown in Figure 3, there was no clear distance between the Y2 and Y3 treatment groups; the treatment and control groups both exhibited a clear distance (p < 0.05).
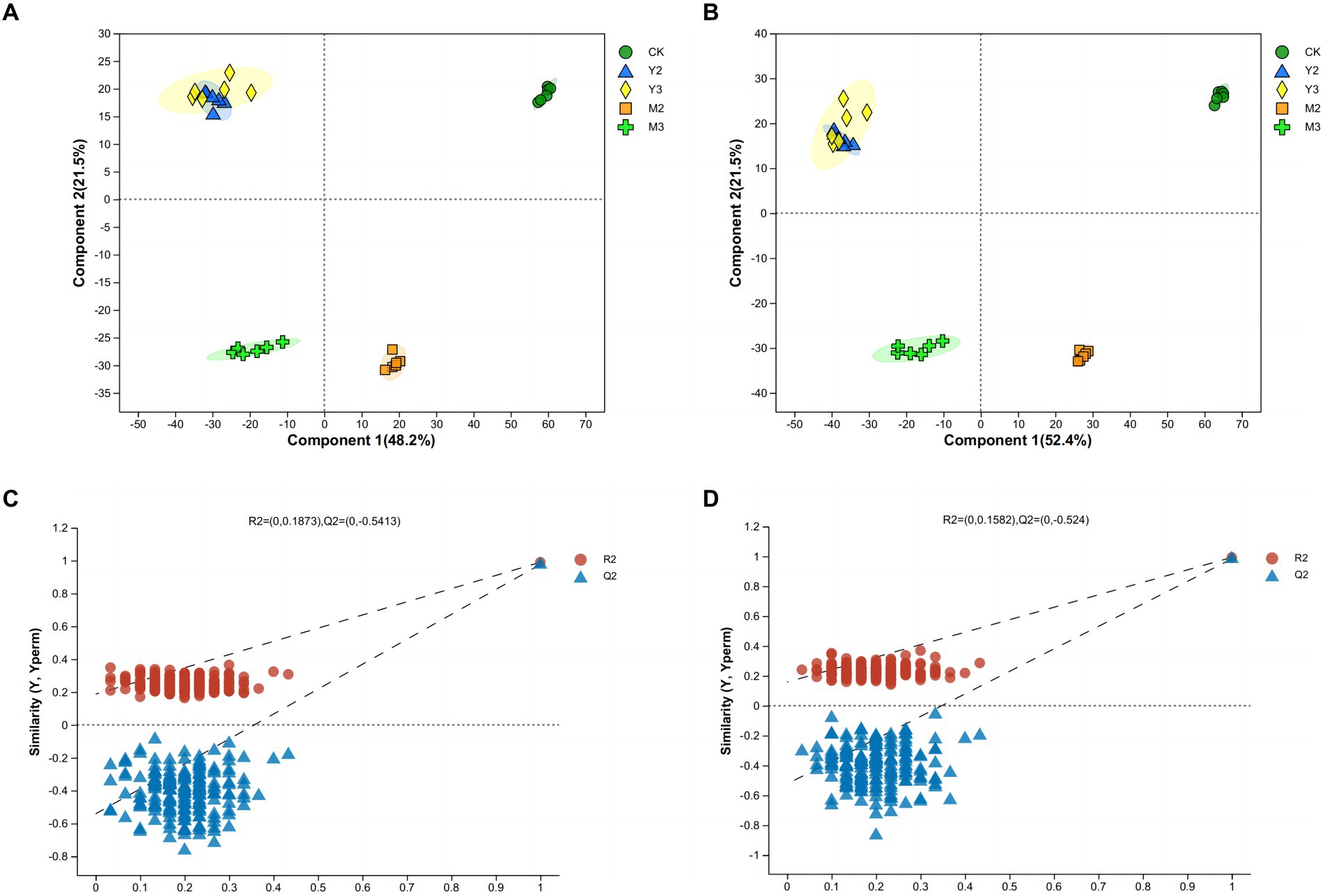
Figure 3. Principal component analysis (PCA) and orthogonal projections to latent structures-discriminant analysis (OPLS-DA) of paper mulberry silage. (A) Score scatter plot of the PCA model for the positive ion of metabolites. (B) Score scatter plot of the PCA model for the negative ion of metabolites. (C) Permutation test of the OPLS-DA model for the positive ion of metabolites. (D) Permutation test of the OPLS-DA model for the negative ion of metabolites.
As shown in Figure 4A, in the category of compounds with biological roles, amino acids (20 metabolites) were dominant, followed by monosaccharides (16 metabolites). Flavonoids (61 metabolites) were the dominant metabolites in the phytochemical category (Figure 4B). PK12 flavonoids (93 metabolites), followed by FA01 fatty acids and their conjugates (60 metabolites), were the dominant metabolites in the lipid category (Figure 4C).
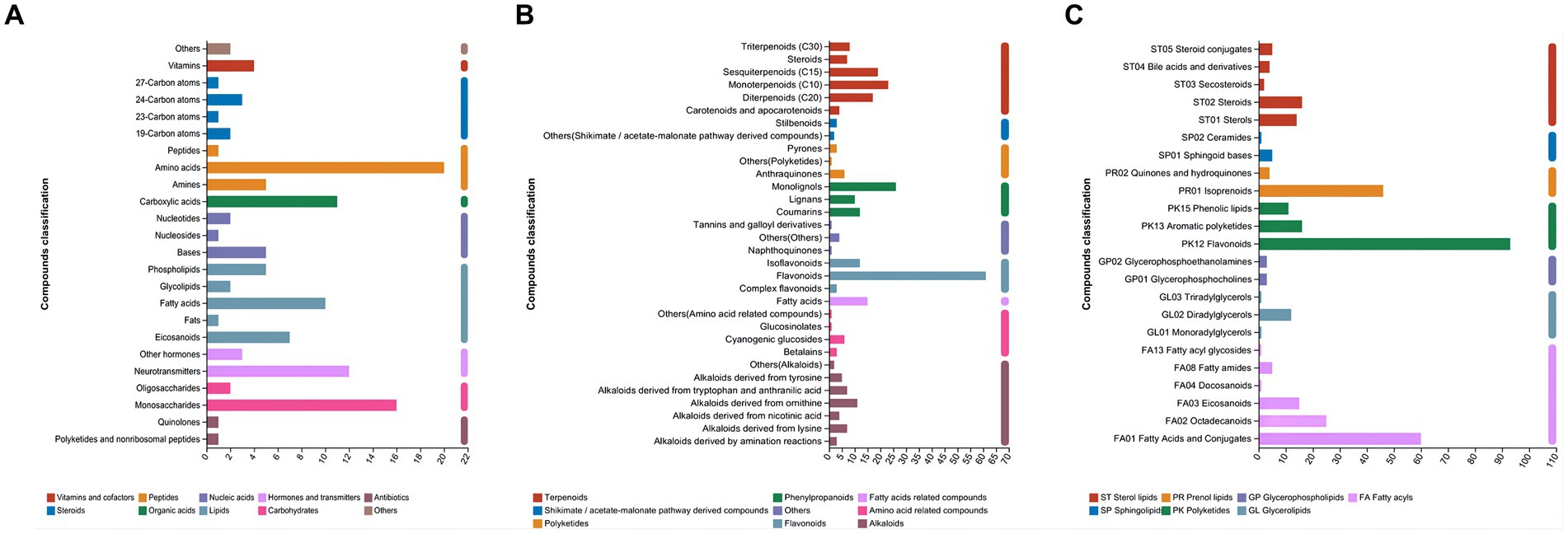
Figure 4. Different metabolites annotated by Kyoto Encyclopedia of Genes and Genomes (KEGG) in paper mulberry silage. (A) Compounds with biological roles, (B) phytochemical compounds, and (C) lipids.
A total of 274 and 740 metabolites were upregulated and downregulated in the Y treatment (combined metabolites of the Y2 and Y3 treatment groups, based on PCA analysis) compared with the control, respectively; 184 and 707 metabolites were upregulated and downregulated in the M3 treatment group, respectively, compared with the control group; 252 and 753 metabolites were upregulated and downregulated in the M2 treatment group, respectively, compared with the control; 447 and 383 metabolites were upregulated and downregulated in the M3 treatment group, respectively, compared with the Y treatment groups; 341 and 366 metabolites were upregulated and downregulated in the M2 treatment group, respectively, compared with the M3 treatment group.
As shown in Figures 5A–F, compared with control, six metabolites were downregulated including acetylvulgaroside, propanoic acid, and diacetoxyscirpenol, but 24 metabolites were upregulated including grossamide, methylarbutin, and glucaric acid, in the Y treatment groups; 7 metabolites, including enterodiol, solamargine, and malonyltryptophan, were downregulated and 23 were upregulated, including glucaric acid, apiin, and benomyl, in the M2 treatment group; 6 metabolites were downregulated, including cyclopentenyl cytosine, moracin P, and diacetoxyscirpenol and 23 metabolites were upregulated, including anserine, ftivazide, and tricin, in the M3 treatment group. Compared with the Y treatment group, 17 metabolites were downregulated, including pirfenidone, ascorbic acid, and muramic acid, and 13 metabolites were upregulated, including benomyl, alanylphenylalanine, and diacetoxyscirpenol, in the M2 treatment group; 21 metabolites were downregulated, including aniracetam, swertiamarin, and moupinamide, and 9 metabolites were upregulated, including nefiracetam, psilocybine, and benomyl, in the M3 treatment group. Compared with M2, 18 metabolites were downregulated, including combretastatin, methylphenidate, and umbelliferone, and 12 metabolites were upregulated, including flavazide, lepidimoic acid, and carmofur, in the M3 treatment group.
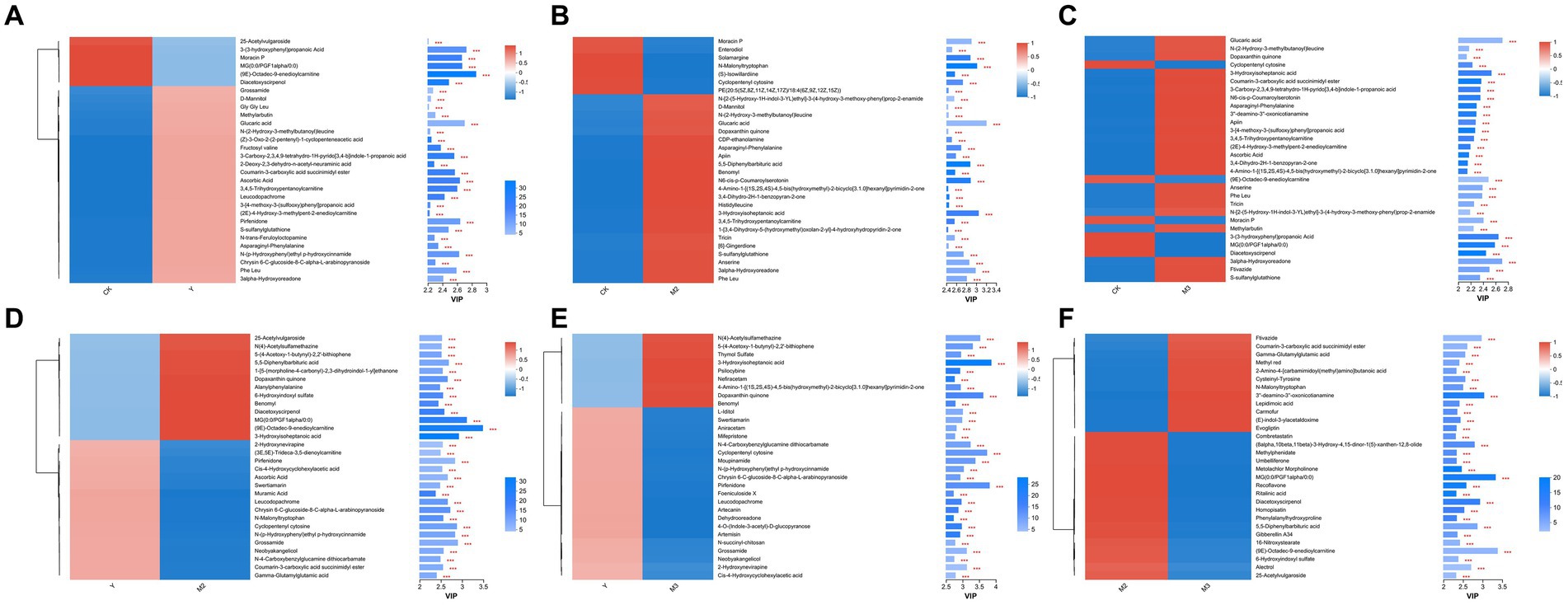
Figure 5. Heatmap of the differentially accumulated metabolites in paper mulberry silage. (A) CK vs. Y, (B) CK vs. M2, (C) CK vs. M3, (D) Y vs. M2, (E) Y vs. M3, and (F) M2 vs. M3.
As shown in Figure 6, Enterococcus and Caproiciproducens demonstrated the highest correlation with the metabolites among the observed bacteria. Enterococcus was significantly negatively correlated with 28 metabolites, including quinic acid, apigenin, and genistein (p < 0.05), and positively correlated with 15 metabolites, including floionolic acid, avocadene, and indoleacrylic acid (p < 0.05). Caproiciproducens had a significant negative correlation with 27 metabolites and a positive correlation with 11 metabolites (p < 0.05). Lactococcus was significantly positively correlated with 11 metabolites, including schaftoside, gluconic acid, and proline, and negatively correlated with ceanothine B, epsilon-caprolactam, hydroxyhexadecanedioic acid, dihydroxyphenylpropanoate, and N-acetyl-dl-leucine (p < 0.05). Enterobacter was significantly negatively correlated with 4-hydroxystyrene, styrene, methylbutanoic acid, sorbitan laurate, and avocadene and positively correlated with seven metabolites, including quinic acid, vicenin 2, and tricarballylic acid (p < 0.05). Weissella was significantly negatively correlated with epsilon-caprolactam and hydroxyhexadecanedioic acid (p < 0.05).
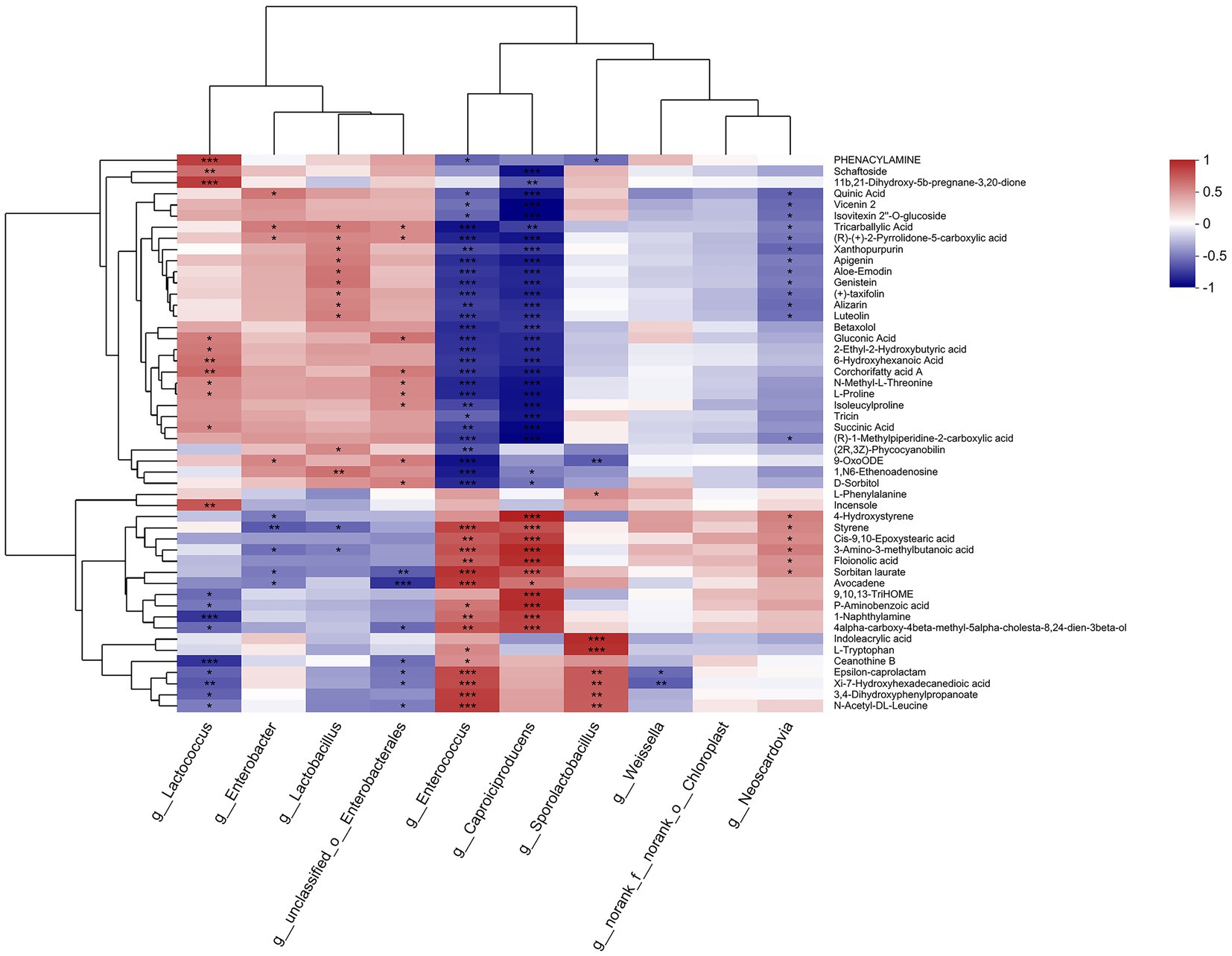
Figure 6. Heatmap of the correlation analysis between bacteria (top 10 in relative abundance) and metabolites (top 50 in relative abundance) in paper mulberry silage.
Generally, LAB converts WSC into organic acids such as LA and AA (Li et al., 2019). The results of this study showed that the addition of straw increased the LA content (except in the M2 treatment) but decreased AA and PA (undetected in any mixed silage). The results indicated that ensiled paper mulberry alone could enhance WSC conversion by LAB, but the organic acid composition could change compared with a mixture of straw and paper mulberry. Furthermore, an LA to AA ratio > 3 indicates homolactic fermentation, whereas an LA to AA ratio < 3 indicates heterolactic fermentation (Ali et al., 2020). In the present study, the LA to AA ratio was <3 when mixed at a 3:7 ratio of wheat straw and paper mulberry, but >3 at a 5:5 ratio; however, the LA to AA ratio remained >3 at any ratio of paper mulberry with corn straw. Our results suggest that the addition of corn straw mixed with paper mulberry predominantly undergoes homolactic fermentation during silage; however, this capacity is dose-dependent when wheat straw and paper are combined. The AN production is mainly attributed to bacterial (Enterobacter and Clostridia) enzyme activity, but bacterial activity is inhibited at pH < 4.5 (McDonald et al., 1991). In this study, both the control (paper mulberry alone) and treatment groups (paper mulberry mixed with straw) had a pH > 4.5, with the mixed groups showing a lower pH than the control (p < 0.05), which suggests that the addition of corn or wheat straw may inhibit protein degradation, resulting in a decrease in AN production, which is partly correlated with a decrease in pH. Usually, the AN content accounts for <5% of total N in high-quality grass silage (Huhtanen et al., 2002). In addition, during rumen protein synthesis, microbes preferentially use non-protein nitrogen (NPN) from fresh forages compared with NPN from silage (Wetherall et al., 1995). The AN content was >5% in both the control and treatment groups after ensilation, which indicates that the quality of silage was average.
Generally, bacterial diversity is negatively correlated with fermentation quality; thus, Lactobacillus can become the dominant genus during prolonged ensiling in well-fermented silage because of its tolerance to the acidic environment and production of organic acid to decrease pH, which indicates that low bacterial diversity reflects a good fermentation level (Li et al., 2019). In this study, the addition of corn or wheat straw significantly improved fermentation owing to decreased bacterial diversity in silage, and wheat straw had a greater capacity to affect the bacterial community during paper mulberry ensiling than corn straw.
Firmicutes were dominant in both the control and treatment groups. Proteobacteria were more abundant in the treatment groups than in the control group. The primary phyla shifted from Proteobacteria to Firmicutes after ensiling (Xiao et al., 2022).
Lactobacillus was dominant in both the control and treatment groups. Enterobacter was the least abundant genus in the control group. The addition of straw to paper mulberry silage increased the relative abundance of Enterobacter. However, the Enterobacter activity is inhibited when the pH is <4.5 (Muck, 2010). In the present study, the pH of both the control and treatment groups was >4.5, thus the pH did not affect the relative abundance of Enterobacter. Paper mulberry contains various flavonoids that have strong inhibitory activities against several bacteria, including Enterobacter aerogenes (Chen et al., 2022). Theoretically, the proportion of antibacterial substances should be diluted with the addition of straw, leading to an increase in the relative abundance of Enterobacter as the straw ratio increases; however, our results did not reflect this. This difference is partly related to LAB, which can release flavonoids from plants or transform them into different metabolites, inducing other biological activities, such as antibacterial activity during fermentation (Huynh et al., 2014). These results suggest that the dilution effects of straw on the antibacterial activity of paper mulberry silage are not dose-dependent.
The relative abundance of Enterococcus was higher in the control and wheat straw-treated groups but lower in the corn straw-treated groups, indicating that corn straw demonstrated a greater capacity to inhibit Enterococcus activity. The relative abundance of Lactococcus was the highest in the treatment groups (M3 and Y3; relative abundance of 15.83%). Enterococcus and Lactococcus both show low tolerance to low pH environments (< 5) (McDonald et al., 1991). In this study, the pH of all treatment groups was approximately 5.0, except for corn straw (Y2, pH = 4.6) and the control (pH = 5.89), indicating that pH cannot be the main reason for the activity of these two bacteria. Similar results were observed in a previous study on paper mulberry silage, in which the bacterial activity was attributed to a high DM content, resulting in the rapid formation of anaerobic conditions during ensiling (He et al., 2021). In this study, DM content increased with increasing straw ratio, and only Lactococcus activity increased as the straw ratio increased, especially with the addition of wheat straw. The underlying mechanism promoting the difference between Enterococcus and Lactococcus requires further investigation. The relative abundance of Weissella was lowest in both wheat straw and corn straw treatments (M3 and Y3), but no difference between the control and other treatment groups (p > 0.05) was observed, indicating that the addition of corn or wheat straw to paper mulberry may inhibit the activity of Weissella to a certain level. In the present study, the relative abundance of Caproiciproducens was higher in the control than in all the other treatment groups, indicating that the addition of straw significantly decreased the relative abundance of Caproiciproducens, with no correlation to the material (corn or wheat) or dose. Caproiciproducens degrades protein, producing ammonia and butyric acid (BA), which accumulate in the silage (Jia et al., 2021). However, the present study did not detect BA likely because Enterobacter and Clostridia are the two main bacteria that degraded proteins (McDonald et al., 1991), and Enterobacter may have been performing most of the protein degradation, resulting in ammonia accumulation. The relative abundance of Sporolactobacillus was highest in the wheat straw-treated group (M3) compared with both the control and other treatment groups, indicating that wheat straw likely promotes the growth of Sporolactobacillus during ensiling. Sporolactobacillus is highly resistant to acidic environments and typically appears during the late stage of ensiling and improves silage fermentation (Li et al., 2019). Thus, our results indicate that the addition of straw, especially wheat straw, can improve the fermentation quality of paper mulberry silage by facilitating LAB proliferation and inhibiting undesirable bacteria.
Different ratios of corn straw and paper mulberry after ensilation showed the same metabolite composition but had clear differences with the other treatments, indicating that wheat straw can significantly alter the metabolite composition in paper mulberry silage. The corn and wheat straw mixed with paper mulberry silage had a significant capacity to inhibit metabolite production. The differential metabolites (DEMs) between each group were mostly those involved in amino acid metabolism, such as tryptophan, tyrosine, and histidine, followed by the ABC transport pathways. The DEMs between the control and wheat straw-treated groups (M3) were mostly those involved in ABC transport (15 metabolites) and tyrosine and tryptophan metabolism (both had 16 metabolites) when compared with the wheat straw-treated group (M2). These results indicate that wheat straw combined with paper mulberry can alter the metabolite pathway during ensiling in a dose-dependent manner. Among the 30 most abundant metabolites, based on variable importance in projection (VIP > 2) analysis, several DEMs (including moracin P, enterodiol, and solamargine) were downregulated and 23 metabolites were upregulated in the wheat straw-treated group (M3) compared with the control; 5 metabolites including moracin P, cyclopentenyl cytosine, 3-(3-hydroxyphenyl) propanoic acid, prostaglandinf1, and diacetoxyscirpenol were downregulated and 25 metabolites were upregulated in the wheat straw-treated group (M2) compared with the control. In addition, moracin P was downregulated in the corn straw-treated groups (Y2 and Y3) compared with the control. Moracin P was the only metabolite that was downregulated in the wheat straw-treated group (in a dose-dependent manner). Moracin P was extracted from Ficus sagittifolia (Moraceae family) and demonstrated antibacterial activity against various bacteria such as E. coli and P. aeruginosa (Taiwo et al., 2023). Paper mulberry belongs to the Moraceae family, and our results showed that the addition of corn or wheat straw increased the relative abundance of Enterobacter. Thus, these results suggest that the addition of corn or wheat straw can reduce the antibacterial effects of paper mulberry by decreasing the content of antibacterial substances after ensilation. DAS was downregulated in the wheat straw-treated group (M2) and corn straw-treated groups (Y2 and Y3), but there was no difference in the wheat straw-treated group (M3) compared with the control (p > 0.05). Notably, DAS was downregulated in the wheat straw-treated group (M2) and corn straw-treated group (Y2 and Y3) compared with the wheat straw-treated group (M3). DAS belongs to the Fusarium metabolite type A trichothecenes (Ulrich et al., 2021) but is not detected in corn or wheat silage (Driehuis et al., 2008). The results of the present study suggest that the addition of corn or wheat straw decreases mycotoxin production during paper mulberry ensiling. Several peptide segments were upregulated in the straw-treated groups (such as glycine–glycine-leucine, asparaginyl-phenylalanine, and phenylalanine-leucine in corn straw-treated groups and asparaginyl-phenylalanine in wheat straw-treated groups) compared with paper mulberry ensilation alone. These results suggest that the addition of straw may decrease protein degradation to a certain extent. Glucaric acid was upregulated in both corn and wheat straw-treated groups compared with paper mulberry ensilation alone; however, no significant difference was observed between the straw-treated groups (p > 0.05). Glucaric acid, a promising high-value monomer in the manufacturing industry, is produced by microbes such as Saccharomyces cerevisiae and Pseudomonas syringae (Alonso et al., 2015). Ascorbic acid (vitamin C) was upregulated in both corn straw and wheat straw-treated groups (Y2 and M2) compared with the control; however, no difference was observed between the control and wheat straw-treated groups (Y2 and Y3, p > 0.05), indicating that corn straw enhanced the vitamin content of paper mulberry silage better than wheat straw.
Lactococcus showed a significant positive correlation with succinic acid (R2 = 0.4730, p < 0.05), whereas Enterococcus and Caproiciproducens both showed significantly negative correlations (R2 = −0.6549 and −0.8705, respectively, p < 0.05). Succinic acid is involved in the tricarboxylic acid (TCA) pathway as an intermediate product. Theoretically, it should be degraded, but Lactococcus cannot metabolize succinic acid because it possesses an incomplete TCA pathway (Cao et al., 2021). In addition, the pycA genes of Lactococcus lactis can produce succinic acid from different carbon sources (alone or combined), such as fructose, sucrose, and glucose (Wang et al., 2011). Thus, succinic acid accumulation was positively correlated with Lactococcus. However, Enterococcus flavescens is capable of producing succinic acid in the pH range of 4.0–9.0 via the phosphoenol pyruvate carboxykinase (PPCK) pathway (Agarwal et al., 2007). Therefore, the mechanism underlying the negative correlation between Enterococcus and succinic acid requires further investigation. Styrene was significantly negatively correlated with Enterobacter and Lactobacillus (R2 = −0.5808 and −0.5386, respectively; p < 0.05) but positively correlated with Enterococcus and Caproiciproducens (R2 = 0.7432 and R2 = 0.6927, respectively; p < 0.05). Styrene showed bactericidal activity that was stronger against gram-negative bacteria, such as E. coli, than gram-positive bacteria, such as Staphylococcus aureus (Tashiro, 2001), indicating that styrene can inhibit the activity of Enterobacter and Lactobacillus in silage.
Several amino acids, such as phenylalanine, tryptophan, and proline, were significantly correlated with bacteria. One peptide (isoleucylproline) had a significantly negative correlation with both Enterococcus and Caproiciproducens (R2 = −0.6433 and R2 = −0.8364, respectively, p < 0.05). Phenylalanine showed a significant positive correlation with only Sporolactobacillus (R2 = 0.4465, p < 0.05). Proline was significantly positively correlated with Lactococcus (R2 = 0.4588, p < 0.05) but negatively correlated with Enterococcus and Caproiciproducens (R2 = −0.7560 and R2 = −0.8406, respectively, p < 0.05). Tryptophan was significantly positively correlated with Enterococcus and Sporolactobacillus (R2 = 0.4740 and R2 = 0.8213, respectively; p < 0.05). Lactococcus had peptidase activities such as dipeptidase (pepV) and prolinase (pepP) (Christensen et al., 1999), which could explain its positive correlation with proline. Enterococcus also exhibits peptidase activity against glycine-proline and glutamate-4-nitroanilide (Sarantinopoulos et al., 2001). These results suggest that Lactococcus degraded peptide segments into proline, and Enterococcus degraded peptide segments into tryptophan in paper mulberry silage. Gluconic acid was significantly positively correlated with Lactococcus (R2 = 0.5166, p < 0.05) but negatively correlated with Enterococcus and Caproiciproducens (R2 = −0.7284 and R2 = −0.7484, respectively, p < 0.05). Gluconic acid, produced through the catalytic reaction of 2,5-diketo-D-gluconic acid reductase (2, 5-DKG reductase), is a direct precursor (lactone) of ascorbic acid (vitamin C), and Lactococcus can express this enzyme (Kaswurm et al., 2013). This study showed that the relative abundance of Lactococcus was higher in the wheat straw-treated groups with high levels of vitamin C and that the wheat straw treatments (M2 and M3) enhanced the silage nutrition of paper mulberry by facilitating Lactococcus activity.
Mixing straw with paper mulberry for ensilation decreases mycotoxin production, pH, and AN content but increases lactic acid production. Homolactic fermentation was dominant with the addition of corn straw to paper mulberry silage but was dose-dependent with the addition of wheat straw (the same capacity was observed at a 5:5 ratio). The relative abundance of Enterobacter increased and that of Lactobacillus was not affected by the addition of straw. The relative abundance of Enterococcus and Lactococcus increased with the addition of straw, especially at higher wheat straw treatment levels (M3). Straw treatment improved the nutritional value of paper mulberry silage.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
RH: Conceptualization, Writing – original draft, Writing – review & editing. BC: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Writing – original draft. YC: Software, Writing – original draft. XZ: Writing – original draft. JY: Writing – original draft. CM: Conceptualization, Investigation, Writing – original draft. XW: Conceptualization, Visualization, Writing – review & editing. FZ: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the South Xinjiang Key Industry Innovation and Development Support Plan Project (grant no. 2022DB017) and the China Agriculture Research System of the MOF and MARA (grant no. CARS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agarwal, L., Isar, J., Meghwanshi, G. K., and Saxena, R. K. (2007). Influence of environmental and nutritional factors on succinic acid production and enzymes of reverse tricarboxylic acid cycle from Enterococcus flavescens. Enzym. Microb. Technol. 40, 629–636. doi: 10.1016/j.enzmictec.2006.05.019
Ali, N., Wang, S. R., Zhao, J., Dong, Z. H., Li, J. F., Nazar, M., et al. (2020). Microbial diversity and fermentation profile of red clover silage inoculated with reconstituted indigenous and exogenous epiphytic microbiota. Bioresour. Technol. 314:123606. doi: 10.1016/j.biortech.2020.123606
Alonso, S., Rendueles, M., and Diaz, M. (2015). Microbial production of specialty organic acids from renewable and waste materials. Crit. Rev. Biotechnol. 35, 497–513. doi: 10.3109/07388551.2014.904269
Cao, W. F., Aubert, J., Maillard, M. B., Boissel, F., Leduc, A., Thoms, J. L., et al. (2021). Fine-tuning of process parameters modulates specific metabolic bacterial activities and aroma compound production in semi-hard cheese. J. Agric. Food Chem. 69, 8511–8529. doi: 10.1021/acs.jafc.1c01634
Chen, Y. R., Wang, L., Liu, X., Wang, F. L., An, Y., Zhao, W., et al. (2022). The genus Broussonetia: An updated review of Phytochemistry, Pharmacology and Applications. Molecules 27:5344. doi: 10.3390/molecules27165344
Christensen, J. E., Dudley, E. G., Pederson, J. A., and Steele, J. L. (1999). Peptidases and amino acid catabolism in lactic acid bacteria. Anton. Leeuw. Int. J. Gen. Mol. Microbiol. 76, 217–246. doi: 10.1023/A:1002001919720
Dong, L. F., Zhang, H. S., Gao, Y. H., and Diao, Q. Y. (2020). Dynamic profiles of fermentation characteristics and bacterial community composition of Broussonetia papyrifera ensiled with perennial ryegrass. Bioresour. Technol. 310:123396. doi: 10.1016/j.biortech.2020.123396
Driehuis, F., Spanjer, M. C., Scholten, J. M., and Giffel, M. C. T. (2008). Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Addit. Contam. Part B Surveill. 1, 41–50. doi: 10.1080/19393210802236927
Du, Z., Sun, L., Chen, C., Lin, J., Yang, F., and Cai, Y. (2021). Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed Sci. Technol. 275:114766. doi: 10.1016/j.anifeedsci.2020.114766
Hao, J., Sun, W. T., Wu, C. R., Zhang, M. Z., Xia, G. H., Zheng, Y. L., et al. (2022). Fermentation quality, bacterial community, and aerobic stability of perennial recut Broussonetia papyrifera silage with different additives and wilting time. Fermentation 8:262. doi: 10.3390/fermentation8060262
He, Q., Zhou, W., Chen, X. Y., and Zhang, Q. (2021). Chemical and bacterial composition of Broussonetia papyrifera leaves ensiled at two ensiling densities with or without Lactobacillus plantarum. J. Clean. Prod. 329:129792. doi: 10.1016/j.jclepro.2021.129792
Huang, R. Z., Wang, X. Z., Ma, C. H., and Zhang, F. F. (2022a). Effects of intrinsic tannins on proteolysis dynamics, protease activity, and metabolome during sainfoin ensiling. Front. Microbiol. 13:976118. doi: 10.3389/fmicb.2022.976118
Huang, R. Z., Zhang, F. F., Wang, T., Zhang, Y. L., Li, X., Chen, Y. C., et al. (2022b). Effect of intrinsic tannins on the fermentation quality and associated with the bacterial and fungal Community of Sainfoin Silage. Microorganisms 10:844. doi: 10.3390/microorganisms10050844
Huhtanen, P., Khalili, H., Nousianinen, J. I., Rinne, M., Jaakkola, S., Heikkila, T., et al. (2002). Prediction of relative intake potential of grass silage bydairy cows. Livest. Prod. Sci. 73, 111–130. doi: 10.1016/S0301-6226(01)00279-2
Huynh, N. T., Van Camp, J., Smagghe, G., and Raes, K. (2014). Improved release and metabolism of flavonoids by steered fermentation processes: a review. Int. J. Mol. Sci. 15, 19369–19388. doi: 10.3390/ijms151119369
Jia, T. T., Yun, Y., and Yu, Z. (2021). Propionic acid and sodium benzoate affected biogenic amine formation, microbial community, and quality of oat silage. Front. Microbiol. 12:750920. doi: 10.3389/fmicb.2021.750920
Kadam, K. L., Forrest, L. H., and Jacobson, W. A. (2000). Rice straw as a lignocellulosic resource: collection, processing, transportation, and environmental aspects. Biomass Bioenergy 18, 369–389. doi: 10.1016/S0961-9534(00)00005-2
Kan, Y. F., Li, J. Z., Zhang, S. F., and Gao, Z. H. (2023). Novel bridge assistance strategy for tailoring crosslinking networks within soybean-meal-based biocomposites to balance mechanical and biodegradation properties. Chem. Eng. J. 472:144858. doi: 10.1016/j.cej.2023.144858
Kaswurm, V., Nguyen, T. T., Maischberger, T., Kulbe, K. D., and Michlmayr, H. (2013). Evaluation of the food grade expression systems NICE and pSIP for the production of 2,5-diketo-D-gluconic acid reductase from Corynebacterium glutamicum. AMB Express 3:7. doi: 10.1186/2191-0855-3-7
Li, P., Zhang, Y., Gou, W. L., Cheng, Q. M., Bai, S. Q., and Cai, Y. M. (2019). Silage fermentation and bacterial community of bur clover, annual ryegrass and their mixtures prepared with microbial inoculant and chemical additive. Anim. Feed Sci. Technol. 247, 285–293. doi: 10.1016/j.anifeedsci.2018.11.009
Lu, L. N., Zhai, X. G., Li, X. L., Wang, S. S., Zhang, L. J., Wang, L. Y., et al. (2022). Met1-specific motifs conserved in OTUB subfamily of green plants enable rice OTUB1 to hydrolyse Met1 ubiquitin chains. Nat. Commun. 13:4672. doi: 10.1038/s41467-022-32364-3
McDonald, P., and Henderson, A. R. (1964). Determination of water-soluble carbohydrates in grass. J. Sci. Food Agric. 15, 395–398. doi: 10.1002/jsfa.2740150609
McDonald, P., Henderson, A. R., and Herson, S. (1991). The biochemistry of silage. Kingston, Kent, UK: Chalcombe Publications.
Muck, R. E. (2010). Silage microbiology and its control through additives. Rev. Bras. Zootec. 39, 183–191. doi: 10.1590/S1516-35982010001300021
Ni, K. K., Wang, F. F., Zhu, B. G., Yang, J. X., Zhou, G. A., Pan, Y., et al. (2017). Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 238, 706–715. doi: 10.1016/j.biortech.2017.04.055ali
Oladosu, Y., Rafii, M. Y., Abdullah, N., Magaji, U., Hussin, G., Ramil, A., et al. (2016). Fermentation quality and additives: a case of rice straw silage. Biomed. Res. Int. 2016:7985167. doi: 10.1155/2016/7985167
Sarantinopoulos, P., Andrighetto, C., Georgalaki, M. D., Rea, M. C., Lombardi, A., Cogan, T. M., et al. (2001). Biochemical properties of enterococci relevant to their technological performance. Int. Dairy J. 11, 621–647. doi: 10.1016/S0958-6946(01)00087-5
Su, R. N., Ni, K. K., Wang, T. W., Yang, X. P., Zhang, J., Liu, Y. Y., et al. (2019). Effects of ferulic acid esterase-producing Lactobacillus fermentum and cellulase additives on the fermentation quality and microbial community of alfalfa silage. PeerJ 7:e7712. doi: 10.7717/peerj.7712
Taiwo, O. M., Olaoluwa, O. O., Aiyelaagbe, O. O., and Schmidt, T. J. (2023). Chemical constituents from Ficus sagittifolia stem bark and their antimicrobial activities. Plan. Theory 12:2801. doi: 10.3390/plants12152801
Tashiro, T. (2001). Antibacterial and bacterium adsorbing macromolecules. Macromol. Mater. Eng. 286, 63–87. doi: 10.1002/1439-2054(20010201)286:2<63::AID-MAME63>3.0.CO;2-H
Ulrich, S., Gottschalk, C., Biermaier, B., Bahlinger, E., Twaruzek, M., Asmussen, S., et al. (2021). Occurrence of type a, B and D trichothecenes, zearalenone and stachybotrylactam in straw. Arch. Anim. Nutr. 75, 105–120. doi: 10.1080/1745039X.2021.1877075
Wang, S. R., Li, J. F., Dong, Z. H., Chen, L., and Shao, T. (2018). Inclusion of alfalfa improves nutritive value and invitro digestibility of various straw-grass mixed silages in Tibet. Grass Forage Sci. 73, 694–704. doi: 10.1111/gfs.12365
Wang, J., Zhu, J. F., Bennett, G. N., and San, K. Y. (2011). Succinate production from different carbon sources under anaerobic conditions by metabolic engineered Escherichia coli strains. Metab. Eng. 13, 328–335. doi: 10.1016/j.ymben.2011.03.004
Weatherburn, M. W. (1967). Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39, 971–974. doi: 10.1021/ac60252a045
Wetherall, J. A., Armstrong, D. G., Finlayson, H. J., and Rooke, J. A. (1995). Reduction of proteolysis during ensilage of perennial ryegrass by protease inhibitors. J. Sci. Food Agric. 68, 497–505. doi: 10.1002/jsfa.2740680414
Wu, Z. H., Liang, C. Y., Huang, R. C., Ouyang, J. L., Zhao, L. S., and Bu, D. P. (2022). Replacing alfalfa hay with paper mulberry (Broussonetia papyrifera L.) silage in diets do not affect the production performance of the low lactating dairy cows. Anim. Feed Sci. Technol. 294:115477. doi: 10.1016/j.anifeedsci.2022.115477
Xiao, Y. Z., Sun, L., Wang, Z. J., Wang, W., Xin, X. P., Xu, L. J., et al. (2022). Fermentation characteristics, microbial compositions, and predicted functional profiles of forage oat ensiled with Lactiplantibacillus plantarum or Lentilactobacillus buchneri. Fermentation Basel 8:707. doi: 10.3390/fermentation8120707
Zhang, J., Wei, Q., Li, Q., Liu, R., Tang, L., Song, Y., et al. (2022). Effects of hybrid Broussonetia papyrifera silage on growth performance, visceral organs, blood biochemical indices, antioxidant indices, and carcass traits in dairy goats. Anim. Feed Sci. Technol. 292:115435. doi: 10.1016/j.anifeedsci.2022.115435
Keywords: corn straw, wheat straw, silage, paper mulberry, bacteria, metabolites
Citation: Huang R, Cai B, Chen Y, Zheng X, Yang J, Ma C, Wang X and Zhang F (2024) Bacterial community structure and metabolites after ensiling paper mulberry mixed with corn or wheat straw. Front. Sustain. Food Syst. 8:1356705. doi: 10.3389/fsufs.2024.1356705
Received: 16 December 2023; Accepted: 10 January 2024;
Published: 05 February 2024.
Edited by:
Muhammad Saqlain Zaheer, Khwaja Fareed University of Engineering and Information Technology (KFUEIT), PakistanReviewed by:
Akhtar Hameed, Muhammad Nawaz Shareef University of Agriculture, PakistanCopyright © 2024 Huang, Cai, Chen, Zheng, Yang, Ma, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fanfan Zhang, emhhbmdmYW5mYW5Ac2h6dS5lZHUuY24=; Xuzhe Wang, d2FuZ3h1emhlMTIzQHNvaHUuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.