
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 09 February 2024
Sec. Crop Biology and Sustainability
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1345259
 Qurat-Ul-Ain Raza1
Qurat-Ul-Ain Raza1 Abdur Rehim1*
Abdur Rehim1* Muhammad Amjad Bashir1,2,3
Muhammad Amjad Bashir1,2,3 Hafiz Muhammad Ali Raza1,2
Hafiz Muhammad Ali Raza1,2 Muhammad Aon1
Muhammad Aon1 Yucong Geng4
Yucong Geng4 Mahmoud Moustafa5
Mahmoud Moustafa5 Mohammed O. Alshaharni5
Mohammed O. Alshaharni5 Haider Ali6
Haider Ali6 Rosa Sanchez Lucas6
Rosa Sanchez Lucas6Vegetables are important agricultural products with numerous health benefits. Excessive chemical fertilization to meet the food challenge has resulted in environmental and soil health hazards. Considering this aspect, the current study was conducted with the aim to introduce biostimulants as an alternative to chemical fertilizers to improve soil quality, crop quality, and yield. In the first experiment, the response of radish was noted against the application of glycine (GLY), aspartic acid (ASP), lysine (LYS), vitamin B complex (VBC), and chemical fertilizers (CF). The biostimulants were sourced from Sigma Aldrich and Martin Dow Market Ltd. The results indicated that ASP has significantly improved the phenolic contents in shoot (1.01%) and root (12.23%) compared with CF. Additionally, total protein was significantly increased in shoot with GLY (251.81%) and in root with ASP (57.06%). Shoot ascorbic acid contents were significantly improved with ASP (179.90%), VBC (159.91%), and LYS (139.92%). Plant fresh and dry weight was improved with VBC (478.31%) and ASP (364.73%). The N and P concentrations in radish root were higher in VBC (25.93%) and LYS (100%). Soil organic matter was improved ASP (61.51%), followed by VBC (60.13%). Soil available P concentration was also enhanced with LYS (40.43%), ASP (31.20%), and VBC (23.19%). The second experiment was focused on identifying the response of turnip crop against the following treatments: chemical fertilizers (CF), Isabion® (ISA), 25% CF + LYS + GLY (CLG), 25% CF + ASP + GLY (CAG), and 25% CF + ASP + LYS (CAL). The biostimulants were sourced from Sigma Aldrich Syngenta, Pakistan. The results denoted that CAL and ISA significantly improved the phenolic contents in turnip shoot and root. The ascorbic acid in turnip shoot was improved with CAL (19.27%), CAG (18.13%), ISA (17.68%), and in root with CLG (26.96%). The P concentration in turnip shoot was significantly higher in ISA (19.25%), CLG (16.85%), and CAG (12.26%). Soil total N was improved in all treatments. ISA improved the available P concentration, whereas CF (67.87 mg kg−1) followed by ISA (65.93 mg kg−1) improved the soil available K. Both studies conclude that biostimulants capable of improving vegetable quality.
Vegetables are an essential agricultural product for the vegetable industry and people's livelihood. They are a rich source of nutrient and has numerous health benefits, such as blood pressure and cardio-activity, mental health, hormonal production, anti-inflammation, and anti-cancer benefits (Buturi et al., 2021). Radish (Raphanus sativus L.) is a tap root vegetable with a white fleshy edible root, rich in carbohydrates, proteins, and minerals cultivated globally. It is the second-largest vegetable crop in China and is cultivated mainly in Indo-Pak (Yousaf et al., 2021). Turnip (Brassica rapa L.) is also a root vegetable and forage crop, indigenous to Asia, Europe, the Near East, and Russia (Sun, 2015). In 2020–21, radish and turnip were cultivated on 7,231 and 9,609 hectares of land, with an annual production of 113,074 and 167,065 tons in Pakistan, respectively (Government of Pakistan, 2021).
The world population is growing, estimated to reach 9.7 billion by 2050, posing a critical challenge to meet food demand. This demand could be fulfilled with a 70% increase in food production (Del Buono, 2021). Commonly, chemical fertilizers are adopted to achieve high crop yield and agricultural production. However, it has also been reported that only 30–40% of the fertilizers are utilized by the plants, whereas the remaining is left behind, causing environmental pollution and associated hazards (Liu et al., 2023). Considering all these factors, the scientific community has focused on developing sustainable production technology that can enhance the yield and quality of agricultural products. In this aspect, biostimulants are a modern and eco-friendly approach toward sustainable agriculture (Radkowski et al., 2021). The status of biostimulants can determine its significance as it is one of the largest growing industries that has reached a market of USD 2.5 billion in 2019 (Raza et al., 2023) and is expected to reach 4.9 billion by 2025 (Shahrajabian et al., 2021). This trend offers a valuable approach to minimizing the application of chemical fertilizers.
Biostimulants are organic or inorganic substances that can improve crop performance and productivity by influencing plant nutrient use efficiency, developing tolerance against biotic and abiotic stresses, improving quality, and enhancing nutrient availability in the rhizosphere (Bashir et al., 2021). It contains biologically active substances, including proteins, amino acids, vitamins, nutritional elements, hormones, and other compounds. Biostimulants have also emerged as enzyme activators, causing changes to physiological and biochemical processes, influencing hormonal activity, and acting as a chelating agent of other minerals (Al-Karaki and Othman, 2023). Moreover, biostimulants can affect the primary and secondary metabolism in plants by improving photosynthetic activities and activating biosynthetic pathways (Franzoni et al., 2022).
The positive effects of biostimulants on various horticultural crops have been reported. A study demonstrated that biostimulants improved photosynthetic activity and inhibited chlorophyll degradation, enhancing radish crop production (Raza et al., 2022). Biostimulants, in addition to vitamin B12 and coenzyme Q10, enhanced the root and shoot biomass of red radish (Rehim et al., 2021). Chitosan has also been reported to influence hypocotyl and root elongation and improve organ development in radish (Sarhang et al., 2023). Moreover, biostimulants positively influenced the various nutraceutical parameters and reduced the nitrate content in radish and turnip microgreens (Toscano et al., 2023). The application of the biostimulant has also been reported to increase the phenolic compounds concentrations in barley grains (Nowak et al., 2023).
To explore the efficacy of biostimulants for radish and turnip crop performance, quality, and nutritional value, and its impact on soil properties the current study was planned. Two pot experiments were performed, and sole and mixed biostimulants were applied with reduced or null fertilization. We hypothesized that using biostimulants would reduce the application of chemical fertilizers and become an alternative and eco-friendly approach to sustainable agriculture. The objectives of our study were to (i) assess the influence of biostimulants on radish and turnip performance, quality, and nutritional value, (ii) identify its effects on soil properties, and (iii) reduce or eliminate the dependency on chemical fertilizers.
Two pot trials were conducted to explore the role of biostimulants in vegetable performance and production. The first study was conducted in the research area of the Department of Soil Science, Faculty of Agricultural Sciences and Technology, BZU, Multan (30.258°E, 71.515°N), Pakistan, from September to December 2021. The second experiment was conducted at the experimental area of the College of Agriculture, BZU, Bahadur Sub-campus (now University of Layyah) Layyah (30.97°E, 70.96°N) Pakistan, from October to January 2021. Details of both trials are given in Table 1. This study was supported by Higher Education Commission (HEC) Pakistan under the indigenous Ph.D. 5000 fellowship program. Qurat-Ul-Ain Raza [520 (PH-II) 2AV6-075/HEC/IS/2020] acknowledges HEC for providing financial support to fulfill her degree requirements at BZU.
This experiment aims to identify the potential of biostimulants in the absence of chemical fertilization. Pots were filled with 15 kg soil, and six radish seeds were sown on September 24, 2021. Later, thinning was done, and two healthy plants were kept in each pot. Following a completely randomized design (CRD), five treatments with three replications were maintained. Tested treatments are given in Table 2. The foliar application of treatments was done using a hand sprayer four times during the crop cycle at 10-day intervals. Plants were irrigated, and manual weed eradication was performed throughout the experiment. The crop was harvested on December 9, 2021.
This experiment aims to identify the role of biostimulants with reduced chemical fertilization. Pots were filled with 8 kg soil, and turnip seeds were sown on October 21, 2021. Agricultural practices and experimental design were similar to the first experiment (Table 2). The crop was harvested on January 13, 2022.
The fresh plant sample (shoot and root) was homogenized using ethanol (10 ml, 80%) and then centrifuged (10 min, 4,500 rpm). The supernatant was diluted with deionized water, and Folin-Ciocalteu (FC) reagent was added. After 20 min, 20% sodium carbonate was added, and the sample was mixed using a vortex mixer; then, the samples were stored in the dark for 60 min. The absorbance was measured at 765 nm using a spectrophotometer, and a calibration curve was prepared (Brighente et al., 2007).
The fresh plant sample of root and shoot was homogenized with deionized water (25 ml) using a motor and pestle and then centrifuged (10 min, 4,500 rpm) (Lu et al., 2008). The supernatant was added to an alkaline copper solution, shaken, and left for 10 min. Later, the FC reagent was added, and samples were kept in the dark for 30 min at normal temperature. A spectrophotometer was used to measure the absorbance at 660 nm wavelength. The standard solutions were prepared using bovine serum albumin (Waterborg, 2009).
The fresh plant root and shoot samples were homogenized using oxalic acid (4%, 25 ml) using a motor and pestle and centrifuged (10 min, 4,500 rpm). The supernatant was titrated against Tillmans' reagent until the pink color was developed and sustained for 30 s (Godlewska et al., 2021).
The plant root and shoot samples were oven-dried at 65°C until constant weight was achieved. The samples were ground using an electrical grinder and then digested to determine total N, P, and K concentrations. N concentration in plant samples was estimated by the Kjeldahl N determination method (Kjeldahl, 1883). While P was measured using the colorimetric method using a spectrophotometer (McGeorge, 1954), and K concentration was estimated by a photometric method using a flame photometer (Reitemeier, 1963), respectively.
Harvested plants were washed and cleaned using distilled water to remove soil and other dust particles. Plant fresh weight (shoot + root) was measured using a digital weighing balance. Afterwards, plant samples were oven-dried at 65°C until constant temperature was achieved to determine the dry weight of the samples.
Soil sample from each pot was collected, ground, sieved, and stored for analysis. Soil pH, electrical conductivity, soil organic matter (Walkley, 1947), total N (Jackson, 1960), extractable P (Olsen and Sommers, 1982), and K (Richards, 1954) were estimated.
All the data sets were reported as average ± standard deviation (n = 3), and a complete randomized design was used to analyze variance (ANOVA). To compare the results, the Fisher's Least Significant Difference test (p-value = 0.05) was used (Calinski et al., 1981). Statistix 9® was used for statistical analysis. For graphical representation and data processing, Microsoft Excel 2016 and R Studio (2022.12.0) were used.
Comparative to CF, the application of ASP significantly enhanced the total phenolic compounds in radish shoot (1.01%) and root (12.23%). Other treatments significantly reduced the total phenolic compounds compared to chemical fertilizers (Figure 1A). Moreover, biostimulants enhanced the total protein contents in radish. The results revealed the total protein contents in radish shoots were significantly increased with the application of GLY (251.81%). Statistically similar results were found with other treatments, but all improved the protein content compared to CF. On the contrary, ASP improved the protein content in the root (57.06%; Figure 1B). In addition, biostimulants also influenced the ascorbic acid content in plants. In radish shoots, ascorbic acid was improved with the use of ASP (179.90%), VBC (159.91%), and LYS (139.92%). All the treatments were alike except LYS, which significantly reduced the ascorbic acid contents in radish root (28.99%) compared to CF (Figure 1C).
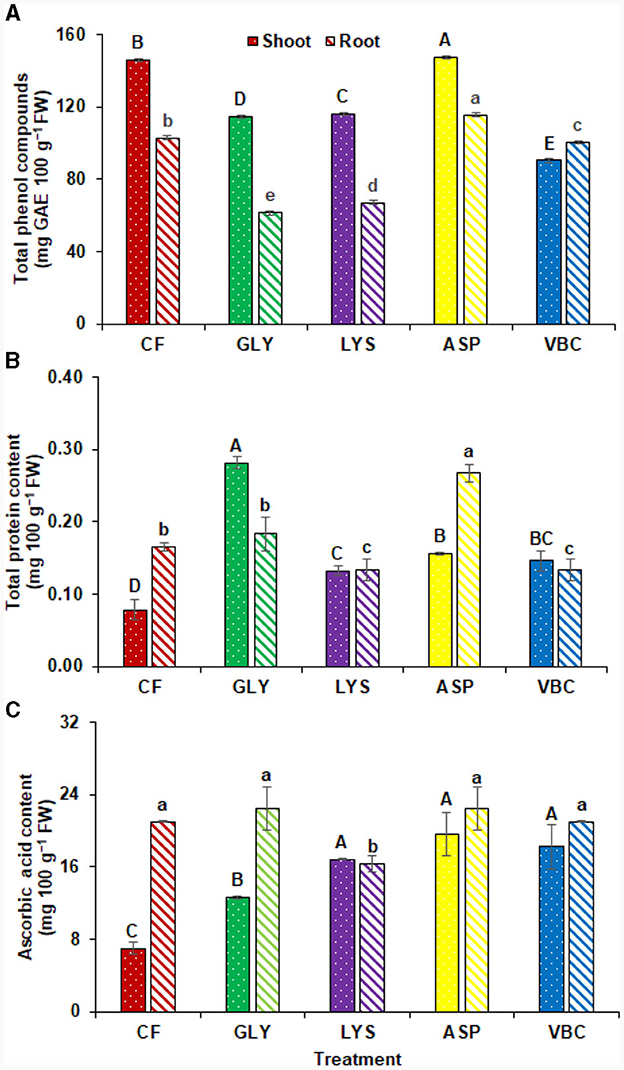
Figure 1. (A) Total phenolic compounds, (B) total protein content, (C) ascorbic acid content with the application of chemical fertilizers (CF), glycine (GLY), lysine (LYS), aspartic acid (ASP), and vitamin B complex (VBC). Lettering indicates a significant difference among means.
The nutritional value of the vegetables was also improved with the application of biostimulants. The results elaborated that all biostimulants increased the N concentration in the radish shoot except LYS. In addition, VBC (25.93%) and GLY (18.51%) significantly enhanced, while LYS significantly reduced the N concentration in the radish root (Figure 2A). Moreover, P concentration was significantly improved in radish shoot with CF (275.71 mg kg−1) followed by LYS (201.88 mg kg−1), VBC (181.58 mg kg−1), GLY (156.38 mg kg−1), and ASP (148.46 mg kg−1). In addition, radish root, LYS showed the highest P concentration (301.92 mg kg−1) followed by ASP (234.00 mg kg−1), GLY (185.92 mg kg−1), VBC (170.58 mg kg−1), and CF (150.96 mg kg−1). This indicates that LYS significantly improved P concentration (100%) in radish compared to CF, followed by ASP, GLY, and VBC with 55.01, 23.16, and 13.00%, respectively (Figure 2B). For radish shoot, K concentration was highest with VBC (270.64%) and LYS (260.70%), followed by ASP (237.38%) and GLY (219.04%) as compared to CF. Whereas LYS significantly reduced the K concentration in ASP (17.83%) compared to CF, all other treatments were similar in this aspect (Figure 2C).
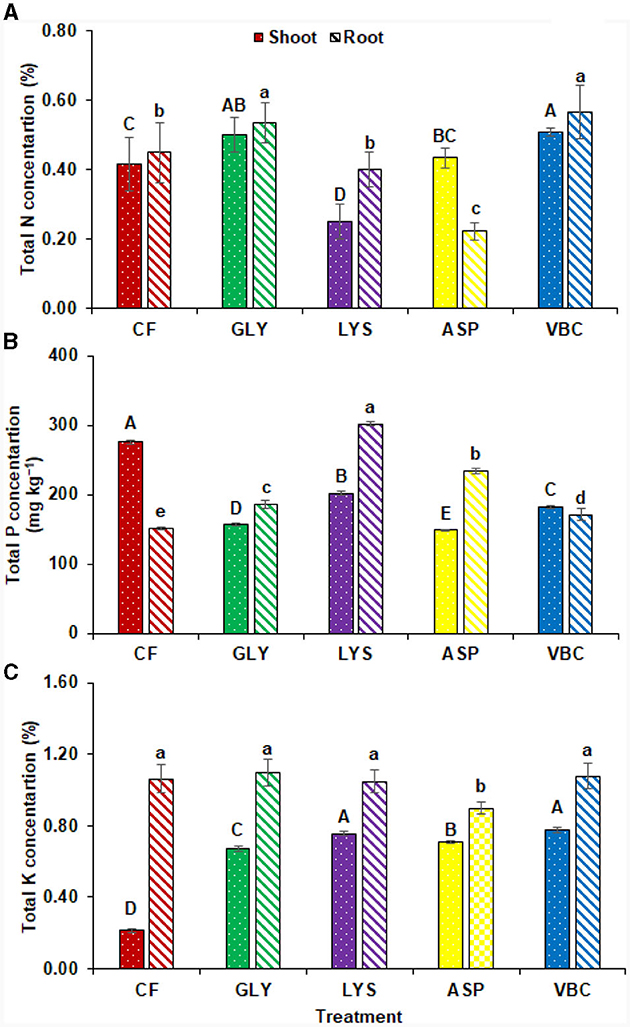
Figure 2. (A) Total nitrogen concentration, (B) total phosphorus concentration, (C) total potassium concentration with the application of chemical fertilizers (CF), glycine (GLY), lysine (LYS), aspartic acid (ASP), and vitamin B complex (VBC). Lettering indicates a significant difference among means.
The application of biostimulants significantly improved the plant's fresh and dry weights (Table 3). Plant fresh weight was improved in radish with VBC (478.31%), followed by ASP (472.82%), GLY (284.63%), and LYS (264.02%). Moreover, plant dry weight was also significantly enhanced with the foliar application of ASP (364.73), followed by LYS (337.90%), VBC (364.73%), and GLY (212.50%) compared to the CF.
Comparing the treatment effects on soil properties revealed that the foliar application of biostimulants influenced the soil pH and EC, but the results were non-significant. Moreover, soil organic matter was significantly improved in all treatments except CF. Soil organic matter was highest with the application of ASP (61.51%), followed by VBC (60.13%), LYS (59.57%), and GLY (42.35%) as compared to CF. Likewise, the total N concentration was also positively enhanced in the soil. ASP (61.51%), VBC (60.13%), and LYS (59.57%) were significantly higher, followed by GLY (42.35) as compared to CF. Furthermore, soil available P concentration was significantly improved with the application of LYS (40.43%), followed by ASP (31.20%) and VBC (23.19%). Soil K concentration was significantly highest in CF (270.09 mg kg−1), and all other treatments significantly reduced the soil available K concentration (Table 3).
The application of biostimulants significantly improved the turnip quality. Our study revealed that CAL (22.09%) significantly enhanced the phenolic compounds in turnip shoot, followed by CAG (15.40%) compared to CF. Whereas ISA (31.78%) showed the highest phenolic compounds in turnip root, followed by CAG (22.46%) compared to CF (Figure 3A). Total protein in turnip shoot was improved with CLG (246.97%), followed by CAG (223.48%), ISA (204.55%), and CAL (130.30%) compared to CF. All biostimulants contributed positively and improved protein content in turnip root. CAG and CAL showed similar results (Figure 3B). Compared to CF, ascorbic acid was improved with CAL (19.27%), CAG (18.13%), and ISA (17.68%) in turnip shoot. Moreover, CLG (26.96%) improved the ascorbic acid in turnip root, followed by CAG (10.87%) and CAL (0.97%) as compared to CF (Figure 3C).

Figure 3. (A) Total phenolic compounds, (B) total protein content, (C) ascorbic acid content with the application of chemical fertilizers (CF), isabion (ISA), 25% CF + lysine + glycine (CLG), 25% CF + aspartic acid + glycine (CAG), and 25%CF + aspartic acid + lysine (CAL). Lettering indicates a significant difference among means.
Biostimulants significantly improved the nutritional attributes of turnip crops. ISA (19.25%), CLG (16.85%), and CAG (12.26%) significantly improved P concentration in turnip shoot. Whereas CF showed the highest P concentration in turnip root (Figure 4A). K concentration in turnip shoot was significantly improved with CAG (104.27%), CAL (92.55%), CLG (85.24%), and ISA (42.56%) as compared to CF. In turnip root, CLG (162.13%) and ISA (139.92%) improved K concentration, followed by CAL (71.43%) and CAG (66.32%) compared to CF (Figure 4B).
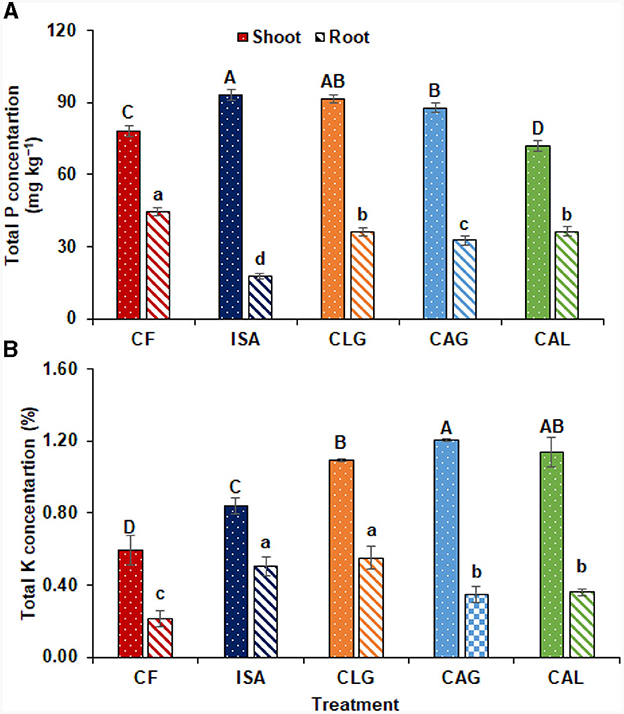
Figure 4. (A) Total P concentration, (B) total K concentration with the application of chemical fertilizers (CF), isabion (ISA), 25% CF + lysine + glycine (CLG), 25% CF + aspartic acid + glycine (CAG), and 25%CF + aspartic acid + lysine (CAL). Lettering indicates a significant difference among means.
Turnip plant fresh weight was enhanced in CLG (5.84%), while the other treatments, ISA (67.00 g), CAG (50.00 g), and CAL (47.67 g), showed negative results as compared to CF (102.67 g), respectively. Moreover, turnip plant dry weight was highest in CF (14.72 g), followed by BLG (11.48 g), CAG (8.51 g), CAL (6.16 g), and ISA (7.83 g; Table 3).
The application of biostimulants showed non-significant changes in soil pH and EC. Soil organic matter also exhibited minor changes, but the results were non-significant. Soil total N was improved in all treatments significantly except CF. ISA improved the soil P concentration, while other treatments were non-significant with respect to CF. Soil K was improved with CF (67.87 mg kg−1) followed by ISA (65.93 mg kg−1), and all other treatments reduced the available K concentration in soil compared to CF.
Heat map indicated the influence of biostimulants on various parameters. Blue color specifies a negative correlation, yellow color indicates a positive correlation, and purple color indicates a neutral association with the parameters. The higher the color intensity, the stronger will be the correlation. In experiment 1, various parameters show a negative influence of the use of CF on radish crops. Whereas the second experiment showed a neutral influence of biostimulants on turnip crop (Figure 5). It justifies that biostimulant effects vary with specie, crop, climate, and dose.
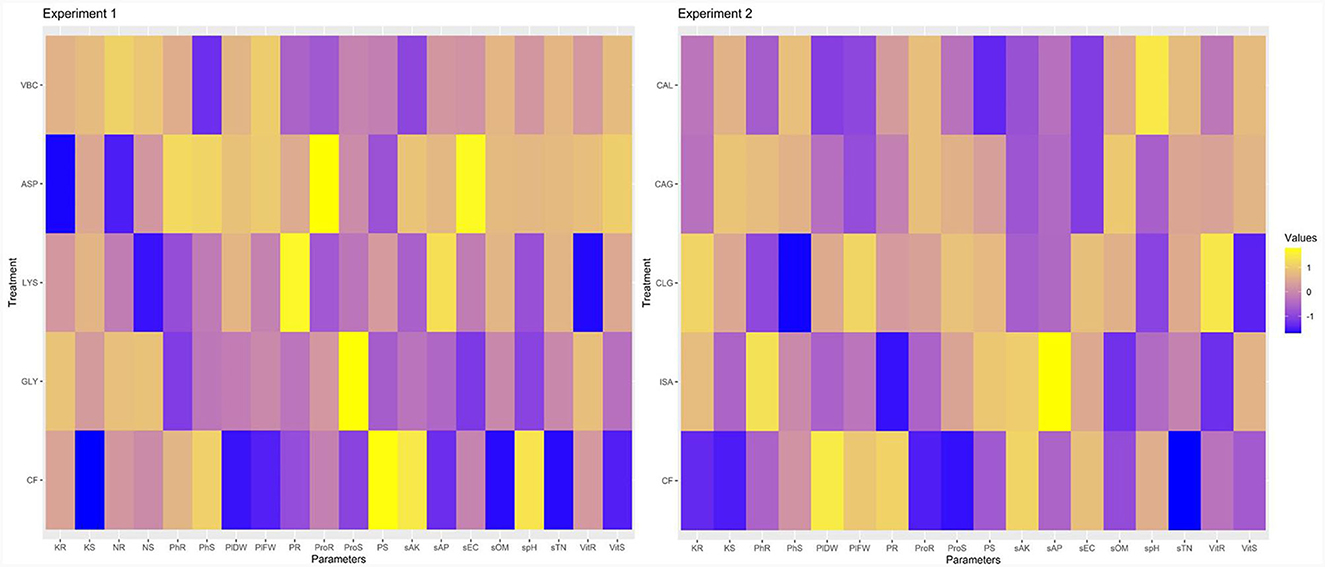
Figure 5. Heatmap indicated the influence of biostimulant on radish and turnip. Herein PhS is phenolic compounds in shoot, PhR is phenolic compounds in roots, ProS is protein in shoot, ProR is protein in shoot, VitS is ascorbic acid in shoot, VitR is ascorbic acid in root, NS and NR is nitrogen in shoot and root, PS and PR is phosphorus in shoot and root, KS and KR is potassium in root and shoot, spH is soil pH, sEC is soil, sOM is soil organic matter, sTN is soil total N, sAP is soil available phosphorus, and sAK is soil available K.
Biostimulants have played a significant role in improving radish and turnip performance. Literature reported that the plants subjected to amino acids respond positively and enhance plant development; therefore, amino acid application has widely been adopted to improve horticultural crop production (Khan et al., 2019). Moreover, exogenously applied glycine also improves yield and boosts the growth and physiological traits of plants (Ali et al., 2020). Previous studies also reported that biostimulants combined with coenzyme Q10 and vitamin B12 have the potential to improve radish biomass (Rehim et al., 2021), and commercial biostimulants improved the protein content in bean (Phaseolus vulgaris L.) (Kocira et al., 2020). Considering the above, our study focused on the sole application of biostimulants in the absence of chemical fertilizers, as well as combining biostimulatory agents with chemical fertilizers to find their antagonistic or synergistic effects.
The findings of our studies elaborated that foliar application of amino acids improved the phenolic compounds, proteins, and vitamin contents in radish and turnip. It could be associated with the amino acids-based treatments that are building blocks of protein and a precursor for phyto-hormones (Kocira et al., 2020). The ascorbic acid content is reduced in the presence of high N application (Paradiković et al., 2011), so it may be hypothesized that amino acids are a reduced form of N and might be a reason for higher ascorbic acid content in plants. Another study also presumes that the building of ascorbic acid might be linked with the process of sugar or glucose synthesis in plants (Khan et al., 2019). Another study (Paradiković et al., 2011), also reported that biostimulants are involved in synthesizing proteins and phenolic compounds.
In addition, amino acids have carboxylic and amine groups that act as buffering compounds, maintain pH, and ensure the proper functioning of plant cell (Souri, 2016). Amino acid also plays numerous roles in plant metabolism; therefore, their exogenous application aids plant growth and enhance quality products (Teixeira et al., 2017; Khan et al., 2019; Alfosea-Simón et al., 2021; Rehim et al., 2021). Glycine is the most commonly used biostimulant because the plant's leaves easily absorb it. It is also considered the most suitable and safe approach to sustainable agriculture and alternative to chemical fertilizers (Zargar Shooshtari et al., 2020). Aspartic acid is metabolized to produce lysine and other amino acids (Alfosea-Simón et al., 2021) and has been reported to improve plant growth under stress conditions with unknown mechanisms (Rizwan et al., 2017; Alfosea-Simón et al., 2020). However, it has been observed that amino acids have a role in influencing plant growth and antioxidant activities depending upon the formulation and the surrounding growth conditions (Rizwan et al., 2017). Moreover, the application of N has also been reported to improve protein content in wheat primarily due to the accumulation of gliadins and glutenins (Zhang et al., 2017).
Nutrient concentrations in plant root and shoot were also enhanced by applying biostimulants. It might be associated with improved nutrient uptake, a characteristic of biostimulants (Bashir et al., 2021). Furthermore, glycine has also been reported to mitigate the uptake of sodium (Na), enhancing the uptake of other nutrients and facilitating improved plant growth. Additionally, the application of reduced forms of nitrogen, such as amino acids, may serve as an excellent energy source for soil microorganisms and could play a role in improving soil life and microorganisms' activity (Zargar Shooshtari et al., 2020). Amino acids also play an essential role in N assimilation, translocation, and accumulation in all parts of the plants (Noroozlo et al., 2019). Glycine has better potential to release N than chemical fertilizer, i.e., urea (McCoy et al., 2020). Plants can biosynthesis their vitamins except vitamin B12, whereas vitamin B6 acts as a cofactor for the synthesis of specific enzymes that are involved in the biosynthesis and catabolism of amino acids and various other plant-specific pathways (Vanderschuren et al., 2013). The vitamin B complex used in our study includes B1, 6, and 12, which fulfills the plant's requirement and enhances its performance.
Our studies reported that the plant's fresh and dry weight was significantly improved with the application of biostimulants. Various scientific studies also reported the positive influence of biostimulants on yielding plants. It might be associated with the mixture of amino acids, vitamins, humic acids, and other substances present in biostimulants. In addition, the higher plant fresh weight might be linked with better leaf area, higher photosynthetic activity, and improved chlorophyll content. All these conditions enable plants to improve their nutritional value (Drobek et al., 2019). Our previous studies also demonstrated that amino acids and vitamins based biostimulants improve the gaseous exchange attributes in plants (Raza et al., 2022). Also, glycine is an important component for normal cell growth and elongation (Noroozlo et al., 2019).
Our studies revealed that biostimulants has a potential to improved production, and biochemical and nutritional quality of radish and turnip crop. It also has a promising role in mitigating the dependency on chemical fertilizers. Biostimulants are capable of providing good quality agricultural products to meet the increasing food demand. However, the molecular mechanism behind the activity of plants under the influence of biostimulants needs to studied to find the possible mechanism. Plants utilize amino acids and other biostimulants according to their nutritional requirements, environmental condition, developmental cues, and genetic makeup. The recommendation of biostimulants can been made after multiple trials because plant shows a specie and environment specific responses toward them. Plant and environmental scientist has developed an interest in exploring the efficacy of biostimulants, their potential and their suitability for plant growth (Khan et al., 2019). However, field trials and long-term experiments need to be conducted to identify optimal doses, application time, and recommendations to the farming community for other agronomic and horticultural crops.
Our studies elaborated that the foliar application of biostimulants with reduced or complete absence of chemical fertilizers can achieve quality products, improve crop yield as well as soil quality. This identifies the potential of biostimulants to replace chemical fertilizers. However, the long-term studies, field experiments and advance molecular researches are needed before any general recommendations. In addition, the biostimulants are specific for crops, dose, application time, and method, and climatic conditions therefore our results might differ from other reported results. In a nutshell, our research state that small-scale farmers and organic vegetable gardeners can implement biostimulants and grow radish and turnip crops with less use of fertilization. Further research to find its mechanism of action and its economic analysis is an open question.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Q-U-AR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Visualization, Writing—original draft. AR: Conceptualization, Data curation, Investigation, Resources, Supervision, Validation, Writing—review & editing. MB: Conceptualization, Data curation, Investigation, Software, Writing—review & editing. HR: Data curation, Methodology, Writing—review & editing. MA: Software, Visualization, Writing—review & editing. YG: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing—review & editing. MM: Writing—review & editing, Methodology, Funding acquisition. MOA: Writing—review & editing, Methodology, Conceptualization. HA: Writing—review & editing, Conceptualization, Funding acquisition. RL: Writing—review & editing, Conceptualization, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through large group Research Project under grant number RGP2/304/44. Authors are thankful to University of Birmingham for supporting with APC through their central open access budget.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alfosea-Simón, M., Simón-Grao, S., Zavala-Gonzalez, E. A., Cámara-Zapata, J. M., Simón, I., Martínez-Nicolás, J. J., et al. (2020). Application of biostimulants containing amino acids to tomatoes could favor sustainable cultivation: implications for tyrosine, lysine, and methionine. Sustainability 12, 1–19. doi: 10.3390/su12229729
Alfosea-Simón, M., Simón-Grao, S., Zavala-Gonzalez, E. A., Cámara-Zapata, J. M., Simón, I., Martínez-Nicolás, J. J., et al. (2021). Physiological, nutritional and metabolomic responses of tomato plants after the foliar application of amino acids aspartic acid, glutamic acid and alanine. Front. Plant Sci. 11:581234. doi: 10.3389/fpls.2020.581234
Ali, S., Abbas, Z., Seleiman, M. F., Rizwan, M., Yavaş, I., Alhammad, B. A., et al. (2020). Glycine betaine accumulation, significance and interests for heavy metal tolerance in plants. Plants 9, 1–23. doi: 10.3390/plants9070896
Al-Karaki, G. N., and Othman, Y. (2023). Effect of foliar application of amino acids biostimulants on growth, macronutrient, total phenols contents and antioxidant activity of soilless grown lettuce cultivars. South Afr. J. Bot. 154, 225–231. doi: 10.1016/j.sajb.2023.01.034
Bashir, M. A., Rehim, A., Raza, Q.-U.-A., Muhammad Ali Raza, H., Zhai, L., Liu, H., et al. (2021). “Biostimulants as plant growth stimulators in modernized agriculture and environmental sustainability,” in Technology in Agriculture, eds. F. Ahmad and M. Sultan (London: IntechOpen).
Brighente, I. M. C., Dias, M., Verdi, L. G., and Pizzolatti, M. G. (2007). Antioxidant activity and total phenolic content of some Brazilian species. Pharm. Biol. 45, 156–161. doi: 10.1080/13880200601113131
Buturi, C. V., Mauro, R. P., Fogliano, V., Leonardi, C., and Giuffrida, F. (2021). Mineral biofortification of vegetables as a tool to improve human diet. Foods 10:223. doi: 10.3390/foods10020223
Calinski, T., Steel, R. G. D., and Torrie, J. H. (1981). Principles and procedures of statistics: a biometrical approach. Biometrics 37:859. doi: 10.2307/2530180
Del Buono, D. (2021). Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 751:141763. doi: 10.1016/j.scitotenv.2020.141763
Drobek, M., Frac, M., and Cybulska, J. (2019). Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress-a review. Agronomy 9:335. doi: 10.3390/agronomy9060335
Franzoni, G., Cocetta, G., Prinsi, B., Ferrante, A., and Espen, L. (2022). Biostimulants on crops: their impact under abiotic stress conditions. Horticulturae 8:189. doi: 10.3390/horticulturae8030189
Godlewska, K., Pacyga, P., Michalak, I., Biesiada, A., Szumny, A., Pachura, N., et al. (2021). Systematic investigation of the effects of seven plant extracts on the physiological parameters, yield, and nutritional quality of radish (Raphanus sativus var. sativus). Front. Plant Sci. 12:651152. doi: 10.3389/fpls.2021.651152
Government of Pakistan (2021). Ministry of National Food Security and Research Economic Wing Islamabad. Available online at: http://www.mnfsr.gov.pk/Detail/NzJlNWE2N2MtNzY5Ni00ZWUwLWFkNTItZjk4MmM4MWY1MWE1 (accessed May 12, 2023).
Khan, S., Yu, H., Li, Q., Gao, Y., Sallam, B. N., Wang, H., et al. (2019). Exogenous application of amino acids improves the growth and yield of lettuce by enhancing photosynthetic assimilation and nutrient availability. Agronomy 9:266. doi: 10.3390/agronomy9050266
Kjeldahl, J. (1883). New method for the determination of nitrogen in organic matter. Zeitschrift Anal. Chem. 22, 366–382. doi: 10.1007/BF01338151
Kocira, A., Lamorska, J., Kornas, R., Nowosad, N., Tomaszewska, M., Leszczyńska, D., et al. (2020). Changes in biochemistry and yield in response to biostimulants applied in bean (Phaseolus vulgaris L.). Agronomy 10:189. doi: 10.3390/agronomy10020189
Liu, X., Amjad Bashir, M., Geng, Y., Raza, Q.-U.-A., Rehim, A., Aon, M., et al. (2023). Assessment of nutrient leaching losses and crop uptake with organic fertilization, water saving practices and reduced inorganic fertilizer. Phyton (B. aires) 92, 1555–1570. doi: 10.32604/phyton.2023.026735
Lu, Z.-L., Liu, L. W., Li, X. Y., Gong, Y. Q., Hou, X. L., Zhu, X. W., et al. (2008). Analysis and evaluation of nutritional quality in Chinese radish (Raphanus sativus L.). Agric. Sci. China 7, 823–830. doi: 10.1016/S1671-2927(08)60119-4
McCoy, R. M., Meyer, G. W., Rhodes, D., Murray, G. C., Sors, T. G., and Widhalm, J. R. (2020). Exploratory study on the foliar incorporation and stability of isotopically labeled amino acids applied to turfgrass. Agronomy 10:358. doi: 10.3390/agronomy10030358
McGeorge, W. T. (1954). Diagnosis and improvement of saline and alkaline soils. Soil Sci. Soc. Am. J. 18:348. doi: 10.2136/sssaj1954.03615995001800030032x
Noroozlo, Y. A., Souri, M. K., and Delshad, M. (2019). Effects of foliar application of glycine and glutamine amino acids on growth and quality of sweet basil. Adv. Hort. Sci. 33, 495–501.
Nowak, R., Szczepanek, M., Błaszczyk, K., Kobus-Cisowska, J., Przybylska-Balcerek, A., Stuper-Szablewska, K., et al. (2023). Impact of the farming system and amino-acid biostimulants on the content of carotenoids, fatty acids, and polyphenols in alternative and common barley genotypes. Agronomy 13:1852. doi: 10.3390/agronomy13071852
Olsen, S. R., and Sommers, L. E. (1982). “Phosphorus,” in Methods of soil analysis, Argon No 9, Part 2: Chemical and Microbiological Properties, 2nd Edn (Madison, WI: Am Soc Agron), 403–430.
Paradiković, N., Vinković, T., Vinković Vrček, I., Žuntar, I., Bojić, M., and Medić-Šarić, M. (2011). Effect of natural biostimulants on yield and nutritional quality: an example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 91, 2146–2152. doi: 10.1002/jsfa.4431
Radkowski, A., Radkowska, I., Bocianowski, J., Cyplik, A., Wolski, K., and Bujak, H. (2021). Effect of amino acids and effective microorganisms on meadow silage chemical composition. Agronomy 11:1198. doi: 10.3390/agronomy11061198
Raza, Q., Bashir, M. A., Rehim, A., Geng, Y., Raza, H. M. A., Hussain, S., et al. (2023). Identifying the role of biostimulants in turnip (Brassica rapa L.) production compared with chemical fertilization. Sustainability 15:11851. doi: 10.3390/su151511851
Raza, Q.-U.-A., Bashir, M. A., Rehim, A., Ejaz, R., Raza, H. M. A., Shahzad, U., et al. (2022). Biostimulants induce positive changes in the radish morpho-physiology and yield. Front. Plant Sci. 13:2475. doi: 10.3389/fpls.2022.950393
Rehim, A., Amjad Bashir, M., Raza, Q.-U.-A., Gallagher, K., and Berlyn, G. P. (2021). Yield enhancement of biostimulants, vitamin B12, and CoQ10 compared to inorganic fertilizer in radish. Agronomy 11:697. doi: 10.3390/agronomy11040697
Reitemeier, R. F. (1963). Methods of analysis for soils, plants, and waters. Soil Sci. Soc. Am. J. 27, iv–iv. doi: 10.2136/sssaj1963.03615995002700010004x
Richards, L. A. (Ed.). (1954). Diagnosis and Improvement of Saline and Alkali Soils (No. 60). US Government Printing Office.
Rizwan, M., Ali, S., Zaheer Akbar, M., Shakoor, M. B., Mahmood, A., Ishaque, W., et al. (2017). Foliar application of aspartic acid lowers cadmium uptake and Cd-induced oxidative stress in rice under Cd stress. Environ. Sci. Pollut. Res. 24, 21938–21947. doi: 10.1007/s11356-017-9860-1
Sarhang, M., Acemi, A., and Türker-Kaya, S. (2023). Comparison of the effects of cytokinins and chitosan on in vitro seed germination and organ development in radish. Plant Cell Tissue Organ Cult. 154, 29–41. doi: 10.1007/s11240-023-02507-5
Shahrajabian, M. H., Chaski, C., Polyzos, N., and Petropoulos, S. A. (2021). Biostimulants application: a low input cropping management tool for sustainable farming of vegetables. Biomolecules 11:698. doi: 10.3390/biom11050698
Souri, M. K. (2016). Aminochelate fertilizers: the new approach to the old problem; a review. Open Agric. 1, 118–123. doi: 10.1515/opag-2016-0016
Sun, R. (2015). “Economic/academic importance of Brassica rapa,” in The Brassica rapa Genome, ed. Z. de Ruiter (Berlin: Springer), 1–15.
Teixeira, W. F., Fagan, E. B., Soares, L. H., Umburanas, R. C., Reichardt, K., and Neto, D. D. (2017). Foliar and seed application of amino acids affects the antioxidant metabolism of the soybean crop. Front. Plant Sci. 8:245273. doi: 10.3389/fpls.2017.00327
Toscano, S., Romano, D., and Patanè, C. (2023). Effect of application of biostimulants on the biomass, nitrate, pigments, and antioxidants content in radish and turnip microgreens. Agronomy 13:145. doi: 10.3390/agronomy13010145
Vanderschuren, H., Boycheva, S., Li, K, Te Szydlowski, N., Gruissem, W., and Fitzpatrick, T. B. (2013). Strategies for vitamin B6 biofortification of plants: A dual role as a micronutrient and a stress protectant. Front. Plant Sci. 4:48827. doi: 10.3389/fpls.2013.00143
Walkley, A. (1947). A critical examination of a rapid method for determining organic carbon in soils—effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 63, 251–264. doi: 10.1097/00010694-194704000-00001
Waterborg, J. H. (2009). “The lowry method for protein quantitation,” in The Protein Protocols Handbook, ed. J. Walker (Totowa, NJ: Humana Press), 7–10.
Yousaf, M., Bashir, S., Raza, H., Shah, A. N., Iqbal, J., Arif, M., et al. (2021). Role of nitrogen and magnesium for growth, yield and nutritional quality of radish. Saudi J. Biol. Sci. 28, 3021–3030. doi: 10.1016/j.sjbs.2021.02.043
Zargar Shooshtari, F., Souri, M. K., Hasandokht, M. R., and Jari, S. K. (2020). Glycine mitigates fertilizer requirements of agricultural crops: case study with cucumber as a high fertilizer demanding crop. Chem. Biol. Technol. Agric. 7, 1–10. doi: 10.1186/s40538-020-00185-5
Keywords: amino-acids, quality, nutrition, radish, turnip, vitamins
Citation: Raza Q-U-A, Rehim A, Bashir MA, Raza HMA, Aon M, Geng Y, Moustafa M, Alshaharni MO, Ali H and Lucas RS (2024) Identifying the abilities of biostimulants to improve vegetable production compared with conventional fertilizer. Front. Sustain. Food Syst. 8:1345259. doi: 10.3389/fsufs.2024.1345259
Received: 27 November 2023; Accepted: 29 January 2024;
Published: 09 February 2024.
Edited by:
Matteo Balderacchi, Independent Researcher, Piacenza, ItalyReviewed by:
Lamine Baba-Moussa, University of Abomey-Calavi, BeninCopyright © 2024 Raza, Rehim, Bashir, Raza, Aon, Geng, Moustafa, Alshaharni, Ali and Lucas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdur Rehim, YWJkdXIucmVoaW1AYnp1LmVkdS5waw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.