- 1Department of Agricultural, Food and Forest Sciences, University of Palermo, Palermo, Italy
- 2Research Centre for Plant Protection and Certification, Council for Agricultural Research and Economics, University of Palermo, Palermo, Italy
- 3Department of Earth and Marine Sciences, University of Palermo, Palermo, Italy
Mandarin, a globally recognized fruit esteemed for its nutritional content and bioactive compounds, as well as aromatic qualities, faces the critical challenge of post-harvest shelf life impacting its marketability and appeal to consumers. This study aims to assess the efficacy of edible coatings in preserving “Tardivo di Ciaculli” mandarin (Citrus reticulata Blanco cv. Tardivo di Ciaculli). Two distinct edible coating formulations, denoted as EC1(comprising gellan gum, glycerol, calcium chloride, and distilled water) and EC2 (comprising gellan gum, glycerol, calcium chloride, distilled water, and 2% oregano essential oil), were subjected to comparative analysis against control samples (CTR). In the experimental trial, mandarin fruits were immersed in the respective edible coating solutions for approximately 5 min and promptly stored at 6 ± 1°C. Qualitative, nutraceutical, microbiological, and sensory analyses were conducted throughout the cold storage period (7, 14, and 24 days at 6 ± 1°C) and during three distinct shelf-life phases (I shelf-life: 7 days at 6 ± 1°C plus 7 days at 15 ± 1°C; II shelf-life: 14 days at 6 ± 1°C plus 7 days at 15 ± 1°C; III shelf-life: 24 days at 6 ± 1°C plus 7 days at 15 ± 1°C). Chemical analysis of the oregano essential oil in EC2 revealed the presence of 27 compounds, with carvacrol being the predominant chemical, constituting 83.42% of the total oil. The findings indicate that the application of edible coatings effectively preserved the quality parameters of mandarin fruits, minimizing weight loss and water loss. Notably, the microbiological analysis, using a culture-dependent approach, demonstrated that EC2, incorporating oregano essential oil, exhibited the capability to diminish the viability of molds throughout the entire study duration, thereby extending the shelf life of mandarin fruits.
1 Introduction
Citrus fruits, globally renowned for their diverse applications, harbor numerous phytochemicals and bioactive components with manifold health-promoting properties, serving as rich reservoirs of vital nutrients such as vitamin C, folic acid, potassium, and pectin (Rafiq et al., 2018). Among these citrus varieties, the mandarin (Citrus reticulata Blanco) stands out as one of the earliest and most distinguished, acknowledged for its nutrient richness and potential medicinal value (Wang et al., 2019).
In recent decades, there has been a remarkable surge in both global production and consumption of mandarins. From 2010 to 2020, world production escalated from 22 million to 31.6 million tons, with parallel growth in consumption from 20 to 30 million tons (USDAF, 2023). Mandarins find prominence in regions characterized by temperate summers and mild winters, notably in Mediterranean countries such as Japan, Brazil, Argentina, the United States (referred to as tangerines), and Australia (Lota et al., 2000).
However, preserving the post-harvest quality of mandarins is imperative. Necessitating adherence to good practices, such as careful handling during harvest and transportation, as well as sanitation of packing and storage facilities, is crucial to mitigate postharvest decays (Zhang et al., 2009). Temperature management emerges as a pivotal factor in preserving the quality attributes of mandarins during storage and transportation, emphasizing the need for proper storage temperatures ranging between 5 and 10°C, with shelf temperatures reaching 20°C for marketable use (Cao et al., 2019). Consideration of the storage period is equally crucial in temperature selection to ensure enhanced fruit quality during the shelf life (Van Wyk et al., 2009).
Challenges such as chilling physiopathology, manifesting at temperatures above freezing point, and harvest pitting, characterized by browning of oil glands at temperatures near 21°C, pose threats to the citrus sector. Additionally, post-harvest water loss, although not a physiological disorder, can impact quality and consumer satisfaction through marketable weight loss, wilting, shriveling, and softening. Most physiological disorders affecting citrus fruits are intricately linked to water loss (Palou et al., 2015).
While waxing is a prevalent practice in preventing these issues, the use of permeable waxes allowing sufficient gas exchange is imperative. This prevents undesirable odors due to restricted gas exchange, preventing anaerobic respiration, ethanol production, off-flavor formation, and overall quality loss (Olivas and Barbosa-Cánovas, 2009). Traditional postharvest treatments involving synthetic waxes and chemical fungicides, such as imazalil (IMZ), thiabendazole (TBZ), and sodium ortho-phenyl phenate (SOPP), have been employed to preserve fruit freshness, control decay, and extend shelf life (Palou et al., 2015).
Postharvest treatments with synthetic fungicides have been widely used for many years and are still used in citrus packing houses for preserving and maintaining the freshness of fruits (Berk, 2016). The application of chemicals fungicides is very expensive and time consuming, therefore the prolonged use of such fungicides has caused serious issues in the citrus industry: such as health and environmental troubles related to high and acute residual toxicity (Nicolopoulou-Stamati et al., 2016) and the increased resistance of molds to continuously used synthetic products. Fungal pathogens have also developed resistance strains due to extensive use of pre-and post-harvest chemical fungicides (Sánchez-Torres and Tuset, 2011). Fungal pathogens, particularly Penicillium digitatum and Penicillium italicum causing green and blue mold, respectively, contribute significantly to postharvest citrus decay (Zhang et al., 2009). The most crucial factor leading to citrus fruit not-marketability is post-harvest decay.
In response to these challenges, the food industry has explored alternatives to synthetic products for inhibiting mold growth and preserving water content in citrus fruits. Edible coatings have emerged as a promising strategy, serving as semipermeable barriers against water vapor and gases. These coatings positively influence fruit tissue metabolism by reducing respiration rates, preserving color, decreasing firmness and moisture loss, controlling microbial growth, and extending fruit quality for prolonged periods (Liguori et al., 2021).
Edible coatings are typically made from biodegradable and non-toxic materials, utilizing various biopolymer matrices such as polysaccharides, proteins, lipids, and composite materials. Polysaccharide-based materials, including pullulan, chitosan, carrageenan, starch, alginate, cellulose, pectin, gellan gum, and xanthan gum, have been employed for this purpose (Dea et al., 2011; Han, 2014; Homayouni et al., 2017; Sondari, 2019; Zubair and Ullah, 2020).
Fruit coating is a well-known practice in citrus packing houses for replacing the natural waxes that may be removed during fruit washing and handling (Palou et al., 2015). Recent efforts have focused on using natural extracts, particularly essential oils, in food preservation instead of chemicals. Carvacrol, a phytochemical compound found in Origanum vulgare L., is a major constituent of oregano essential oil (EO). Laboratory tests have demonstrated the efficacy of wax coatings enriched with carvacrol or thymol EOs in reducing green mold incidence on lemons and decreasing fruit respiration rates (Pérez-Alfonso et al., 2012). Thymol, which belongs to the bioactive compounds of oregano EO, evidenced increasing inhibitory effect against some fungi, including Aspergillus flavus (Culmone et al., 2023). As the industry moves toward sustainable practices and environmentally friendly solutions with minimal impact, this study aims to evaluate the application of natural gellan-gum products enriched with oregano EO on the pomological, physio-chemical, nutraceutical, sensory, and microbial aspects of “Tardivo di Ciaculli” mandarins.
2 Materials and methods
2.1 Oregano crop farming
The hybrid plants between “Origanum vulgare ssp. viridulum × Origanum vulgare ssp. hirtum” were collected grown in a farm located in Grotte (Agrigento, Italy; Figure 1). This area represents a model of Sicilian environmentally friendly multifunctionality for soil protection (Mammano et al., 2023). The cultivation of oregano plants was carried out according to traditionally used management methods. Propagating materials were obtained from a local wild genotype previously classified as Origanum vulgare L., and transplanted in the field in winter 2020, with plant distances of 2.0 × 0.3 m, thereby achieving a plant density of 16,660 plants ha−1.
2.2 Oregano balsamic time individuation, collecting and drying
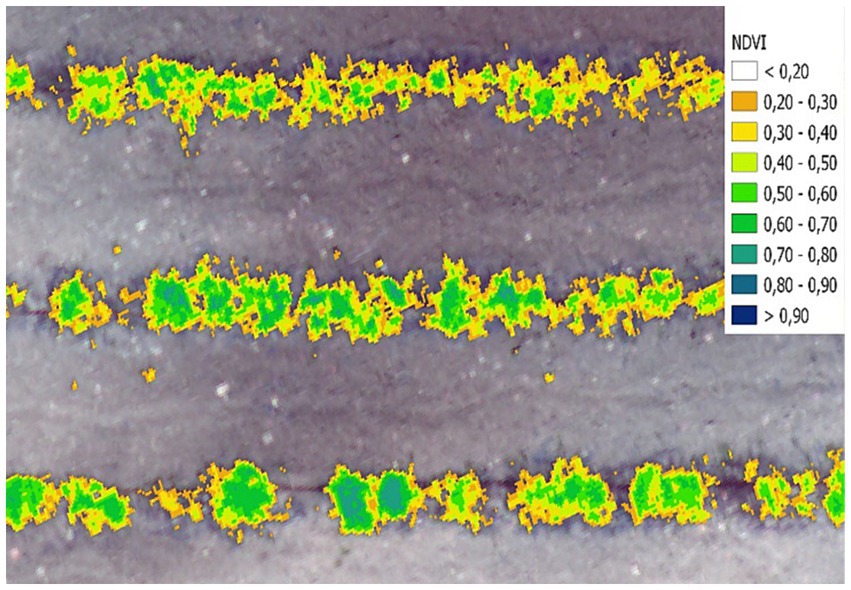
At the beginning of the flowering (25th May 2022), measurements were carried out every 10 days using a UAV DJI Phantom 4, equipped with a multispectral camera. The high-resolution multispectral images were ortho-mosaicked and thematic maps of NDVI (Normalized Difference Vegetation Index) were generated to assess the right balsamic time for oregano harvesting. Mechanical harvesting was carried out according to the technique described by Comparetti et al. (2022). The harvested oregano was then dried following the technique described by Mammano et al. (2023) and Catania et al. (2020). The employed drying technique utilizes forced convective hot air for moisture removal. It consists of passing a forced flow of dry air over the herb biomass, which is distributed over large areas in one or more layers. The drying temperature is maintained at 40°C, with a relative humidity of 25% throughout the process. The aim of this drying method is to reduce the water content of the herbs and preserve their quality by limiting microbial and enzymatic reactions (Figure 2).
2.3 Oregano essential oil extraction and chemical composition
The essential oil was extracted from dried oregano leaves and flowers as described by Garofalo et al. (2023). EOs were extracted from flowers and leaves using a 12 L volume steam distiller (Spring Extractor, Albrigi Luigi, Verona, Italy). At the end of the process, the EO were collected in 25 mL amber glass bottles with screw caps (Laboindustria, Arzergrande, Italy) and stored at 4°C.
The analysis of volatile organic components (VOC) of OEO was conducted using the solid-phase microextraction (SPME) GC–MS technique, following a dilution with hexane at a ratio of 1:100. A SPME fiber (DVB/CAR/PDMS, 50 mm, Supelco) was exposed to the diluted oils while stirring at a temperature of 60°C. After an extraction time of 5 min, the fiber was inserted into a GC spitless injector, and the volatile organic components were desorbed for 1 min at 250°C. A DB-624 capillary column (Agilent Technologies, 60 m in length, 0.25 mm in diameter, and coated with a 1.40 μm film) was utilized for chromatographic separation. The oven temperature program was set with a 5-min isotherm at 40°C, followed by a linear temperature increase of 5°C per minute up to 200°C. The temperature was held at 200°C for 2 min. The carrier gas, helium, was maintained at a flow rate of 1 mL/min. The interface temperature was set at 230°C, and mass spectra were recorded in the range of m/z 40–400 amu using the full-scan acquisition mode. Identification of individual volatile organic compounds was performed by comparing each mass spectrum with the NIST05 commercial library. The analysis was performed in triplicate, and the reported values are expressed as percentages relative to the significant peak.
2.4 Mandarins fruit samples
Mandarins, Citrus reticulata Blanco “Tardivo di Ciaculli,” collected from a commercial orchard in Palermo (38° 40‘N, 13° 23’ E, 99–104 m a.s.l.), were moved to the laboratory immediately after picking. Mandarins were hand-picked at ripe fruit stage (RF) and placed in boxes before being taken to the laboratory. Mandarins, were then washed under tap water, sanitized by immersion in 200 mg kg−1 of sodium hypochlorite for 5 min, and left to dry at room temperature (~15°C). Immediately after washing, fruit quality parameters were analyzed on 30 fruits. Only mandarin fruits with no external injuries were used for the following research and fruit processing operations were performed in sanitary conditions at 15 ± 1°C.
2.5 Coating preparation and application
The edible coating was prepared in the laboratory of the Department of Agricultural, Food and Forestry Sciences, University of Palermo. Two different edible coatings were tested and compared with the untreated sample, used as control:
• CTR: untreated fruits.
• EC1: fruits treated with edible coating based on gellan gum (special ingredients, Savona,Italy) glycerol (Lubrisolve, Long Sutton United Kingdom), calcium chloride (CaCl2), and distilled water. The edible coating solution was placed in a 1 L beaker: 935 mL distilled water; 5 g gellan gum; 30 g CaCl2; 30 mL glycerol. The solution was subjected to magnetic stirring (30°C) for 2 h and immediately afterwards was applied to the fruits.
• EC2: fruits treated with edible coating based on gellan gum specialin gredients, Savona, Italy glycerol (Lubrisolve, Long Sutton, United Kingdom), calcium chloride (CaCl2), distilled water, and high-carvacrol oregano essential oil (EC2). The edible coating solution was placed in a 1 L beaker: 933 mL distilled water; 5 g gellan gum; 30 g CaCl2; 30 mL glycerol; and 2 mL of OEO. The solution was subjected to magnetic stirring (30°C) for 2 h and immediately afterwards was applied to the fruits. This composition imparts specific characteristics to EC2, including potential antimicrobial and antioxidant properties attributed to the oregano essential oil. The thorough mixing and application of EC2 contribute to its potential in safeguarding the fruits and maintaining their freshness during storage.
The mandarin fruits were immersed in the two different edible coating solutions (EC1 and EC2) for approximately 5 min to ensure uniform coverage of the entire fruit surface, creating a consistent coating. Subsequently, both the samples treated with edible coatings and the control samples were placed in plastic commercial boxes and stored. At each sampling date, 5 samples for treatment were analyzed, with each sample comprising 3 replicates.
2.6 Quality parameters: firmness, soluble solid content, titratable acidity, pH, weight loss, and color
Quality evaluations were conducted at various stages, encompassing the harvest, cold storage periods (7, 14, and 24 days at 6 ± 1°C), and distinct shelf-life phases (I: 7 days at 6 ± 1°C plus 7 days at 15 ± 1°C; II: 14 days at 6 ± 1°C plus 7 days at 15 ± 1°C; III: 24 days at 6 ± 1°C plus 7 days at 15 ± 1°C).
During each assessment, a comprehensive analysis was performed on 15 mandarin fruits for each treatment. The examinations included both destructive and non-destructive analyses:
• Texture: Evaluated using a “Fruit Texture Analyzer” (GS-15 Qa Supplies United States) equipped with a pipette tip.
• Total Soluble Solids (TSS) Content: Determined using a digital refractometer (Atago, Tokyo).
• Titratable Acidity (TA): Measured by titrating 10 mL of mandarin juice with a 0.01 M NaOH solution and expressed as a percentage of citric acid.
• pH: Assessed with a digital pH meter (model HI8453, HANNA INSTRUMENTS, Ronchi di Villafranca Padovana, Italy).
• Weight Loss: Verified by weighing the samples during storage with a digital scale (Beurer Italia Srl, Milan, Italy). The percentage reduction in weight for each treatment (CTR, EC1, EC2) was determined based on measurements taken during cold storage on days 0, 7, 10, 14, 17, 21, 24, 27, and 30 at 6 ± 1°C. The formula used for % Weight Loss calculation was:
• Color: Measured by assessing the parameters L*, a*, and b* with a colorimeter (Minolta chroma-meter CR-400) at two opposite points of the equatorial zone of the fruits. CIE Lab* coordinates, representing lightness (L*), reddish or greenish color (a*), and yellowish or bluish color (b*), were recorded. The instrument was calibrated using a manufacturer’s plate.
2.7 Nutraceutical attributes
Total phenolic content, ascorbic acid content, and antioxidant activity of treated (EC1 and EC2) and untreated were assessed soon after coating (0 d) and during the shelf-life (I shelf-life: 7 days at 6 ± 1°C plus 7 days at 15 ± 1°C; II shelf-life: 14 days at 6 ± 1°C plus 7 days at 15 ± 1°C and III shelf-life: 24 at 6 ± 1°C plus 7 days at 15 ± 1°C). For each sampling date and experimental treatment (CTR, EC1 and EC2), three samples were randomly chosen and analyzed.
2.7.1 Fruit extract preparation
Mandarin fruit samples were frozen at −80°C pending extract preparation. Upon thawing, the samples underwent chopping, and the seeds were separated from the pulp. The pulp was homogenized, and fruit extracts were prepared following a previously described procedure with minor modifications (Kanner et al., 2001). In brief, 10 g of the whole homogenate were weighed and then extracted using 80% (v/v) methanol containing 2% formic acid. The samples were vortex-mixed for 5 min and sonicated at room temperature for 15 min (~25°C). The mixtures were allowed to stand for 2 h at room temperature. Following centrifugation (10 min at 8000× g, 4°C), the supernatants were filtered, aliquoted, and stored at −20°C. The extraction procedure was iterated to obtain three distinct technical replicates.
2.7.2 Total phenolic and content
Total phenolic content (TPC) of extracts from treated and untreated fruits was determined by the reduction of phosphotungstic–phosphomolybdic acid (Folin–Ciocalteu’s reagent) to blue pigments, in alkaline solution according to Folin–Ciocalteu’s method (Singleton and Rossi, 1965). Total phenolic content was expressed as mg gallic acid (GA) equivalent (GAE).
2.7.3 DPPH radical scavenging analysis
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging analysis was performed on 2 g of frozen tissue with 5 mL 50% (v/v) ethanol according to Wang et al. (2020). The homogenate was centrifuged at 12,000× g for 20 min at 4°C, then the supernatant was collected. The reaction mixture consisted of 0.1 mL supernatant and 1.9 mL 120 μmol L − 1 DPPH. Results were calculated using the following formula: DPPH radical scavenging activity (%) = [(A0 − A1)/A0] × 100, with A0 referring to absorbance of the control, A1 referring to absorbance of the samples. DPPH radical scavenging was expressed as a percentage.
2.7.4 Ascorbic acid content
Ascorbic acid in CTR, EC1 and EC2 samples was determined by extracting 10 g of blended fruit sample in 100 mL metaphosphoric acid (HPO3), then filtered through Whatman no 1 filter paper. A volume of 10 mL from filtered solution was determined volumetrically with the 2–6 dichlorophenol-indophenol reagent until a slightly pink coloration was observed and persisted for 15 s (Allegra et al., 2017). The reading of ascorbic acid content was expressed in mg/100 g FW.
2.8 Microbiological analyses
Edible coating samples (1 mL) were directly subjected to the serial decimal dilution into 15 mL-volume conical sterile tubes (Biosigma S.p.A., Cona, Italy) containing 9 mL of Ringer’s solution (Sigma–Aldrich, Milan, Italy), while of mandarin fruit samples (25 g) were first suspended in Ringer’s solution (225 mL) (Sigma–Aldrich) and then homogenized and diluted as reported by Liguori et al. (2021). Microbial cell suspensions were analyzed for the main microbial groups associated with the horticultural products following the approach reported by Miceli et al. (2019). Briefly, serial dilutions were plated on agar media to allow the development of: Total Mesophilic Microorganisms (TMM), pseudomonads, Listeria monocytogenes, Coagulase-Positive Staphylococci (CPS), Salmonella spp., Escherichia coli, yeasts, and molds. All agar plates were inoculated by spread plating and incubated in aerobiosis (Passafiume et al., 2022), except those for members of Enterobacteriaceae family, which were counted by pour plating on double-layered agar media to determine the microanaerobiosis conditions. Microbiological analyses of mandarin fruit samples were performed in triplicate.
2.9 Sensory analysis and visual score
Sensorial analysis and visual score were evaluated at each sampling date. Sensory analysis was carried out at the Department (SAAF) and three sections per fruit were used for each treatment.
The panel consisted of 10 people who habitually consumed mandarins and who were trained for a period of 2 weeks on the evaluation method and on the identification of the main sensory descriptors of mandarins (sweetness, juiciness, chewiness, typical tangerine flavor, off-flavor, bitterness, acidity). Because of the addition of Oregano essential oil in one of the three treatment Oregano odor, Oregano flavor and Herbaceous smell were added as descriptors. The fruits were evaluated according to the 1:10 scale of values, where the value 10 indicated maximum intensity and the value 1 indicated the absence of the descriptor. The samples were presented to the judges in randomized order and water was provided between tastings. On each sampling date, 5 boxes (3 fruits on each) CTR, EC1, EC2 fruit samples were also evaluated by each judge for the visual score. Visual appearance score resulted from the medium value of color, visible structural integrity, and visual appearance the different descriptors were quantified using a subjective 5–1 rating scale with 5 = very good, 4 = good, 3 = sufficient, 2 = poor (limit of edibility), and 1 = very poor (inedible) (Liguori et al., 2021).
2.10 Statistical analysis
An experimental randomization scheme was adopted for statistical analysis complete with 3 replications per treatment and for sampling date. The data were analyzed using one-way analysis of variance (ANOVA), and pairwise comparisons between the samples of control and treated fruits were evaluated using Tukey’s test. The differences were considered significant for p < 0.05. The statistical analysis was carried out using Systat (Systat, Chicago, IL, United States).
3 Results and discussion
3.1 Balsamic time for oregano harvesting
The collected biomass was intended for the distillation of essential oils, so it was necessary to identify the time of complete flowering. The harvested plants belonged to local ecotypes characterized by considerable genotypic variability and non-uniform flowering. The harvest time of oregano depends on the commercial use of the product:
• beginning of flowering, for herb production.
• full blooming, when the oil content is at its maximum, for essential oil production.
• at maximum blooming (higher than 50%) and plant coverage (leaves and flowers), for food use (Comparetti et al., 2022).
The NDVI index is sensitive toward crop biophysical properties like nitrogen, chlorophyll, vigor, and biomass etc. The oregano was harvested when the NDVI values of most of the plants were equal to 0.58 and high flowering was evident from visual checks (Figure 3).
3.2 Chemical analysis of oregano essential oil
The volatile profile of OEO is graphically represented in Figure 4. A comprehensive analysis identified 27 volatile compounds belonging to six phytochemical groups: monoterpenes, monoterpenoids, sesquiterpenes, ethers, ketones, and alcohols.
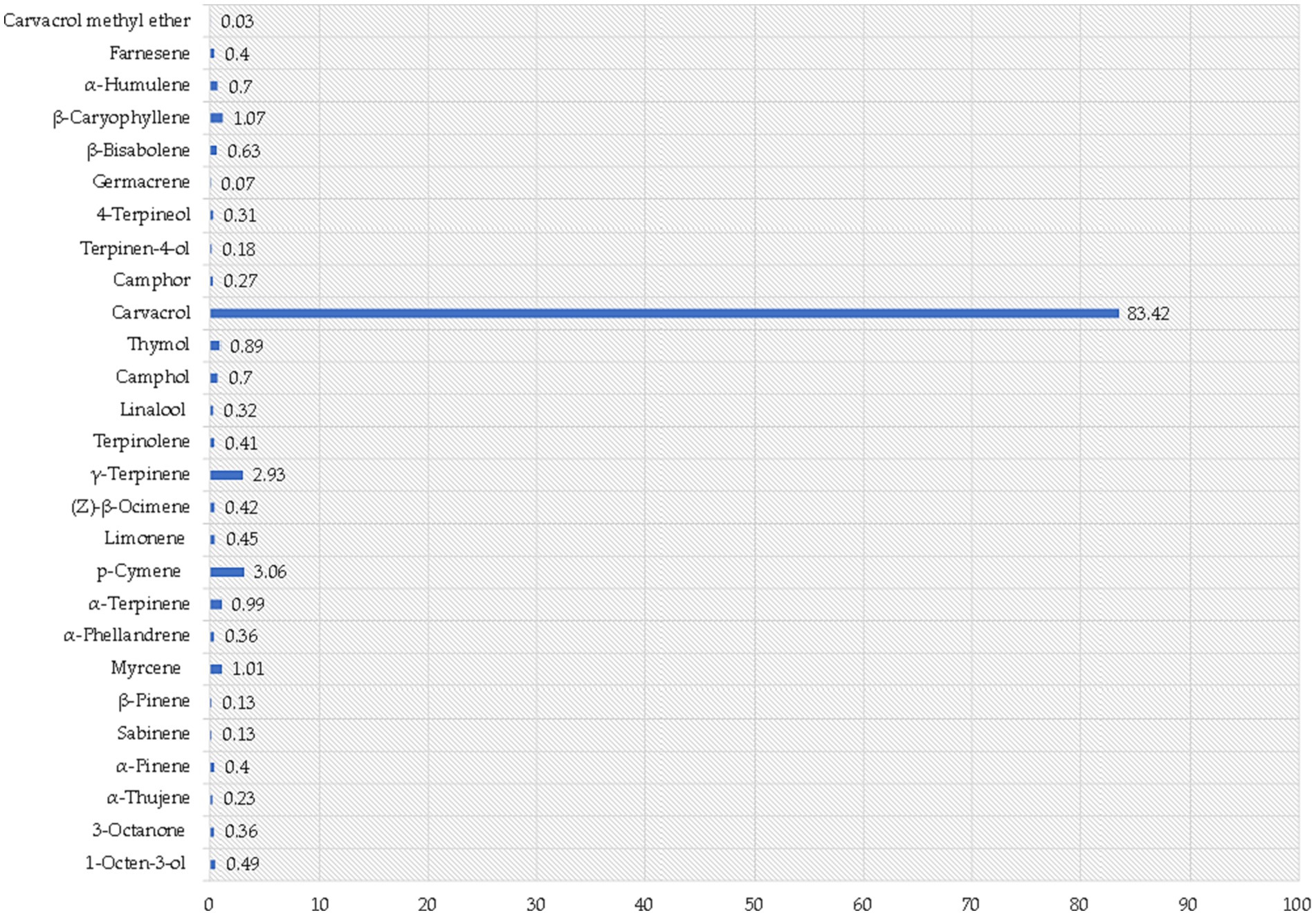
Figure 4. Volatile composition of oregano essential oil. Results indicate the mean percentage values of three measurements and are expressed as relative peak areas (peak area of each compound/total area of the significant peaks in all samples) x l00.
The most abundant group are monoterpenoids representing 86% of the total composition of the essential oil, followed by monoterpenes (10%) and sesquiterpenes (3%). The least represented groups are ketones, alcohols, and sesquiterpenols, with total abundance values below 1%. However, the most representative compounds detected was carvacrol, constituting an 83.42 ± 3.5% of the total composition, establishing monoterpenoids as the prevailing class of VOCs in OEO. Based on the classification proposed by Fleisher and Sneer (1982), our analysis indicates that the OEO under investigation corresponds to the carvacrol oregano chemotype, primarily due to its substantial carvacrol content (~80%) and similar volatile composition was observed in oregano cultivated within the Mediterranean region (Morshedloo et al., 2018; Tsitlakidou et al., 2022). This suggests that the composition of oregano essential oil is primarily determined by the oregano variety rather than the specific cultivation region. In addition to contributing to the distinctive aroma of OEO, carvacrol exhibits a wide range of bioactive properties, including antioxidative effects, inhibition of antibiotic-resistant bacteria, suppression of microbial and fungal toxins, and potential anticarcinogenic activity (Karkabounas et al., 2006; Jayakumar et al., 2012). The inhibitory effects of carvacrol on fungal growth have been attributed to its ability to disrupt cell membranes, inhibit cellular respiration, and interfere with essential enzymatic processes (Hyldgaard et al., 2012; Nostro and Papalia, 2012; Gilling et al., 2014). Therefore, given the challenges associated with food preservation and the demand for natural alternatives to synthetic chemicals, carvacrol, a major component of OEO, has emerged as a promising candidate due to its reported antimicrobial properties (Hyldgaard et al., 2012).
3.3 Quality parameters: firmness, soluble solid content, titratable acidity, pH, color, and weight loss
The effect of edible coatings implemented with oregano essential oil should be positive, particularly regarding microbial growth, as demonstrated by Barreto et al. (2016).
The firmness of mandarin fruits (CTR; EC1; EC2) exhibited no significant changes throughout both the cold storage and shelf-life periods. Notably, during the third storage period (III shelf-life), treated fruits (EC1; EC2) maintained a slightly higher firmness compared to the control sample (CTR) (Table 1). Fruit texture, a crucial quality attribute in citrus fruits, is often affected by enzymatic reactions post-harvest, leading to rapid firmness losses. The incorporation of calcium chloride in coatings, as reported in other studies (Olivas and Barbosa-Cánovas, 2005; Brasil et al., 2012; Nguyen et al., 2020), has shown positive effects on preserving firmness. Similar findings were observed in gellan-based coated fresh-cut melon by Oms-Oliu et al. (2008).
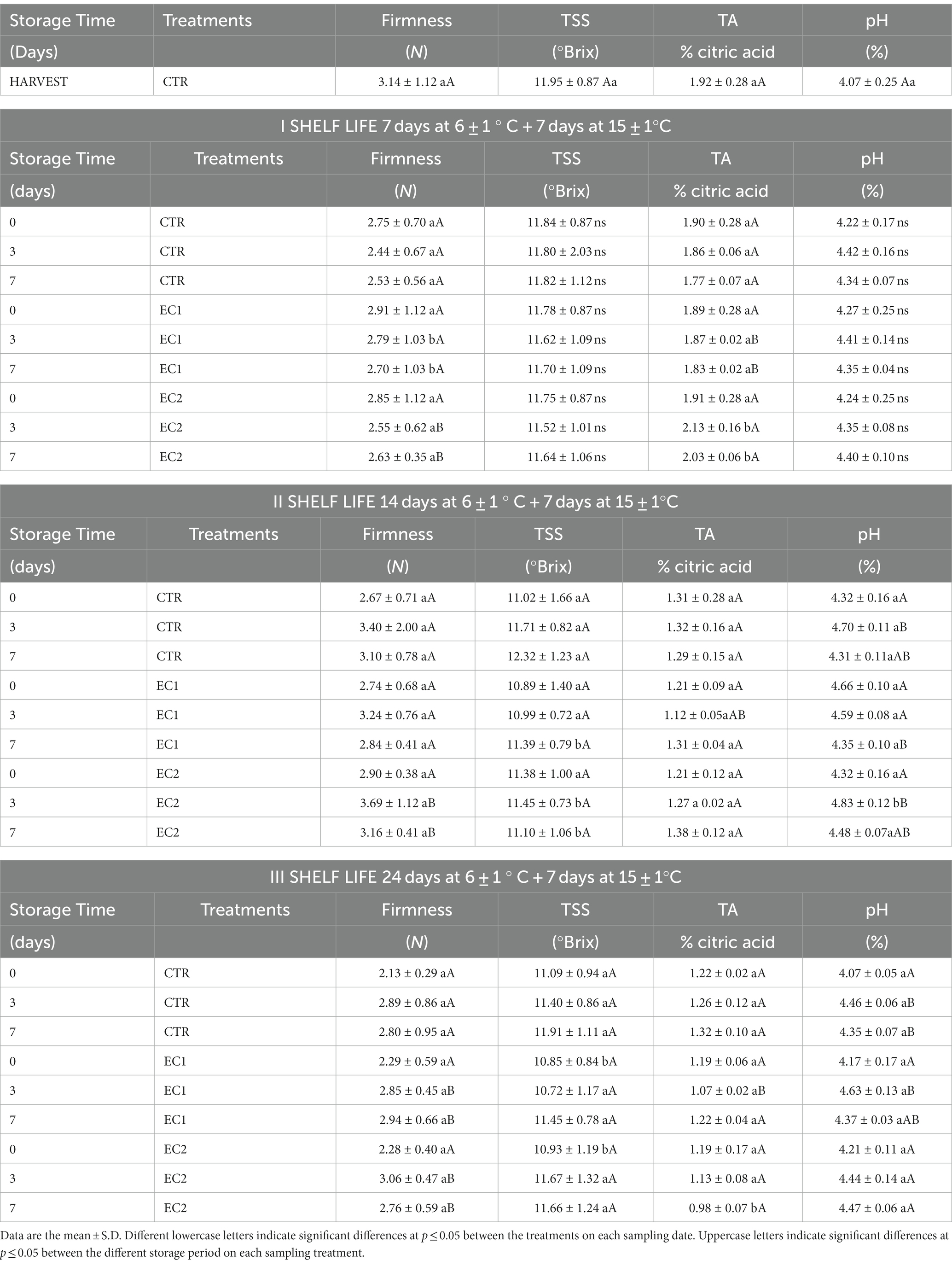
Table 1. Changes in firmness, total soluble solids (TSS) titratable acidity (TA), pH content in mandarin fruits control samples (CTR), treated mandarin fruit samples (EC1; EC2) at the harvest, during the cold storage (7, 14 and 24 days at 6 ± 1°C) and during the shelf-life (I shelf-life: 7 days at 6 ± 1°C plus 7 days at 15 ± 1°C; II shelf-life: 14 days at 6 ± 1°C plus 7 days at 15 ± 1°C and III shelf-life: 24 at 6 ± 1°C plus 7 days at 15 ± 1°C).
Total Soluble Solids (TSS) remained unchanged during the initial storage period (I shelf-life). In the second storage period (II shelf-life), an increase in TSS was noted in EC1 and the control sample (CTR), whereas EC2 exhibited constant values. Additionally, on day 7 of the second storage period, TSS values were slightly lower in treated samples (EC1; EC2) compared to the control samples. No significant differences were observed during the third storage period (III shelf-life). The rise in water loss (fresh weight loss) tends to elevate TSS concentration in fresh fruits (Ali et al., 2020). Although not statistically significant, the control samples showed a greater increase in TSS compared to the treated samples, aligning with findings in other studies (Ali et al., 2021; Bhan et al., 2022).
Both treatments saw a slight increase in titratable acidity (TA). TSS and TA are pivotal indicators influencing the taste and acceptance of citrus fruits. In the first storage period (I shelf-life), EC2 experienced a slight increase in TA, while values remained constant in EC1 and control samples. No significant changes were recorded in the second and third storage periods (II shelf-life and III shelf-life). The results suggest that the application of edible coatings did not adversely impact the TSS content and TA of the fruits, in agreement with Arnon et al., (2014) findings.
Despite some significant differences in TSS, TA, and pH values, these variations may be attributed to several factors, as natural variability, growing conditions, and complex interactions among different fruit components. The pH, a crucial quality parameter reflecting potential spoilage, did not exhibit significant differences during the three storage periods (Table 1). Monitoring pH values during handling or processing is essential for predicting the microbial composition and maintaining fruit quality (Brackett, 1987).
One of the most important mandarin fruits quality parameters is peel color, because it determines consumers’ visual perception, and influences their decision in purchases. The color of mandarin peel and pulp is principally governed by the content and composition of its carotenoid and apocarotenoid pigments (Rodrigo et al., 2013).
The color parameters L*, a*, and b* exhibited either minor or non-significant differences (Table 2). Despite a decrease in brightness over the storage period, small but significant variations were observed among the different treatments. However, these variations in color parameters could be linked to the ripening process of the fruits and field conditions, such as varying altitudes within the same orchard from which the fruits were harvested. Other research also reports that edible coatings did not affect tangerine fruit color (Gao et al., 2018).
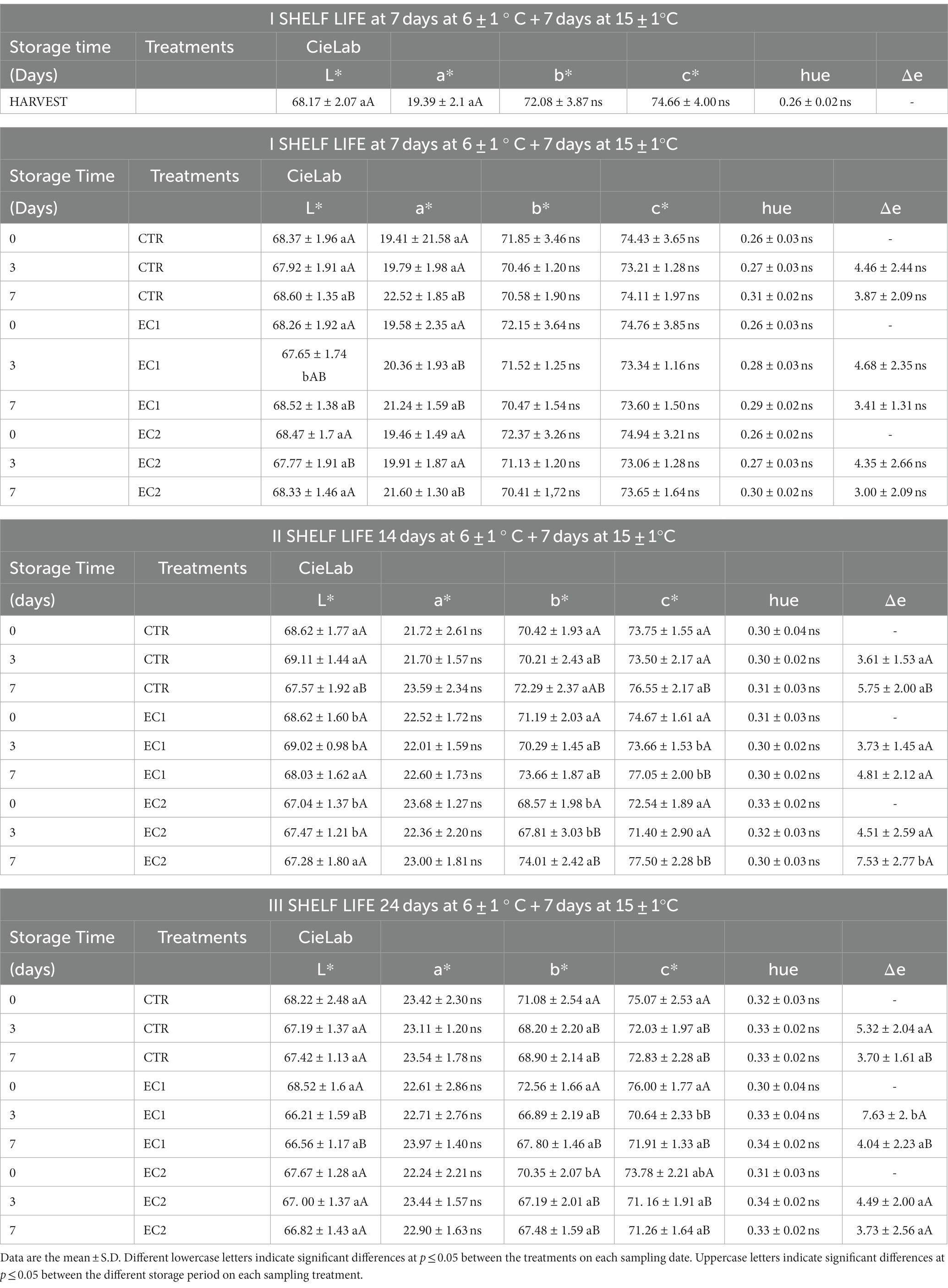
Table 2. Changes in Brightness (L*), Red/Green value (a*), Blue/Yellow value (b*) Chroma (c*), Hue (H) and Δe in mandarin fruits control samples (CTR), treated mandarin fruit samples (EC1; EC2) at the harvest, during the cold storage (7, 14 and 24 days at 6 ± 1°C) and during the shelf-life (I shelf-life: 7 days at 6 ± 1°C plus 7 days at 15 ± 1°C; II shelf-life: 14 days at 6 ± 1°C plus 7 days at 15 ± 1°C and III shelf-life: 24 at 6 ± 1°C plus 7 days at 15 ± 1°C).
Weight loss in the treated samples was lower during the cold storage period reaching a weight loss rate of 0.46 and 0.49% for EC1 and EC2, respectively (Figure 5). The lower weight loss of treated samples can be attributed to the barrier effect of edible coating, which limits gas exchange. In agreement with our results, several researchers showed that gellan-based coating formulation increased the water barrier properties of coated fresh-cut papaya (Sapper et al., 2019), melon (Olivas and Barbosa-Cánovas, 2005), pear (Oms-Oliu et al., 2008), and pineapple (Azarakhsh et al., 2014).
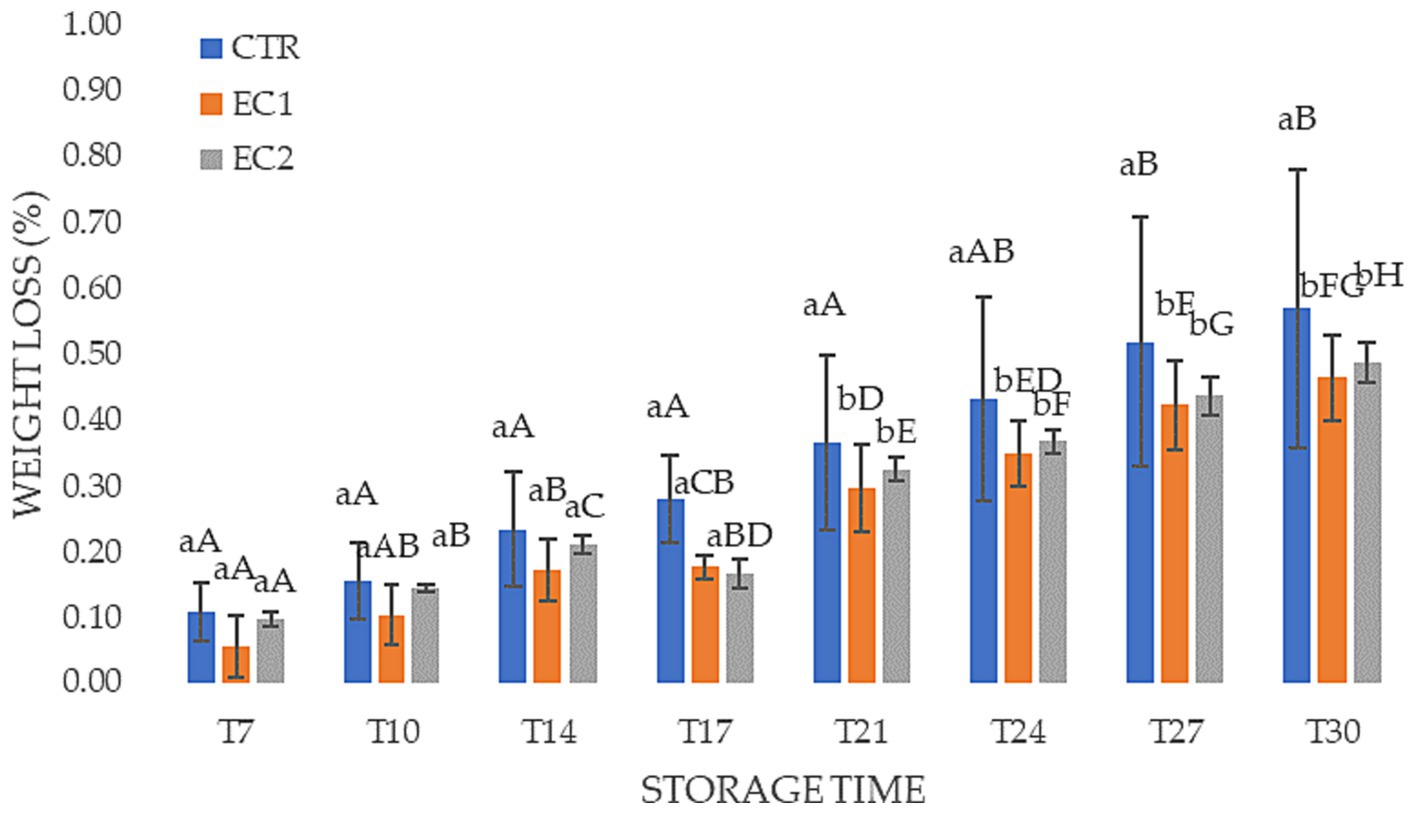
Figure 5. Changes in weight loss (%) in mandarin fruits control samples (CTR), treated mandarin fruit samples (EC1; EC2) during the cold storage at 6 ± 1°C Different lowercase letters indicate significant differences at p ≤ 0.05 between the treatments on each sampling date. Uppercase letters indicate significant differences at p ≤ 0.05 between the different survey on the same treatment on each sampling treatment. Data are the mean ± S.D.
3.4 Bioactive compounds (total phenolic content) and radical scavenging activity
The total phenolic content showed significant variations influenced by both storage time and treatment (Figure 6). A gradual decrease in total phenolic content was observed from the time of harvest to the last day of storage, regardless of treatment. The control sample exhibited the most notable decline, with a reduction of 55.68% from harvest to the last storage day (III SHELF LIFE). In contrast, the treated samples showed lower rates of decrease, with reductions of 24.52 and 20.51%, respectively, (Figure 6). Notably, during the period from day 0 to day 14 at 6 ± 1°C + 7 days at 15 ± 1°C, the total phenolic content decreased by 37.8% in the control sample, while in the treated samples, it decreased by 15.2% (EC1) and 8.79% (EC2).

Figure 6. Total phenolic content during harvest (day 0) and during the shelf-life I shelf-life: harvest plus 7 days at 15 ± 1°C; II shelf-life: 14 days at 6°C plus 7 days at 15 ± 1°C and III shelf-life: 24 days at 6°C plus 7 days at 15 ± 1°C. Data are the mean ± S.D. Different lowercase letters indicate significant differences at p ≤ 0.05 between the treatments on each sampling date. Uppercase letters indicate significant differences at p ≤ 0.05 between the different storage period on each sampling treatment.
Essential oils inherently contain natural antioxidant compounds, and their incorporation into coatings has the potential to enhance their functional properties, making them more effective in protecting fruits and vegetables. Numerous studies have explored the impact of edible coatings enriched with antioxidants, including essential oils, on the quality and preservation of both fresh and fresh-cut fruits and vegetables (Yousuf et al., 2021).
The radical scavenging activity, assessed using DPPH, exhibited a decline over the storage periods for both treated (EC1 and EC2) and untreated (CTR) fruits. The control sample experienced the most significant decrease, with a rate of 47.70% during 24 days at 6°C plus 7 days at 15 ± 1°C (Figure 7). Notably, the DPPH radical scavenging rate in EC2 (39.11%) surpassed that in EC1 (37.32%) (Figure 7). Furthermore, during the period of 24 days at 6°C plus 7 days at 15 ± 1°C, the DPPH rate decreased by 18.70% in EC2 and by 22.76% in EC1 (Figure 7). Previous studies (Robles-Sánchez et al., 2013) have demonstrated that gellan gum edible coatings can enhance antioxidant capacity. Oregano essential oil, as indicated by Jouki et al. (2014), has also shown the capability to augment the antioxidant capacity of food.
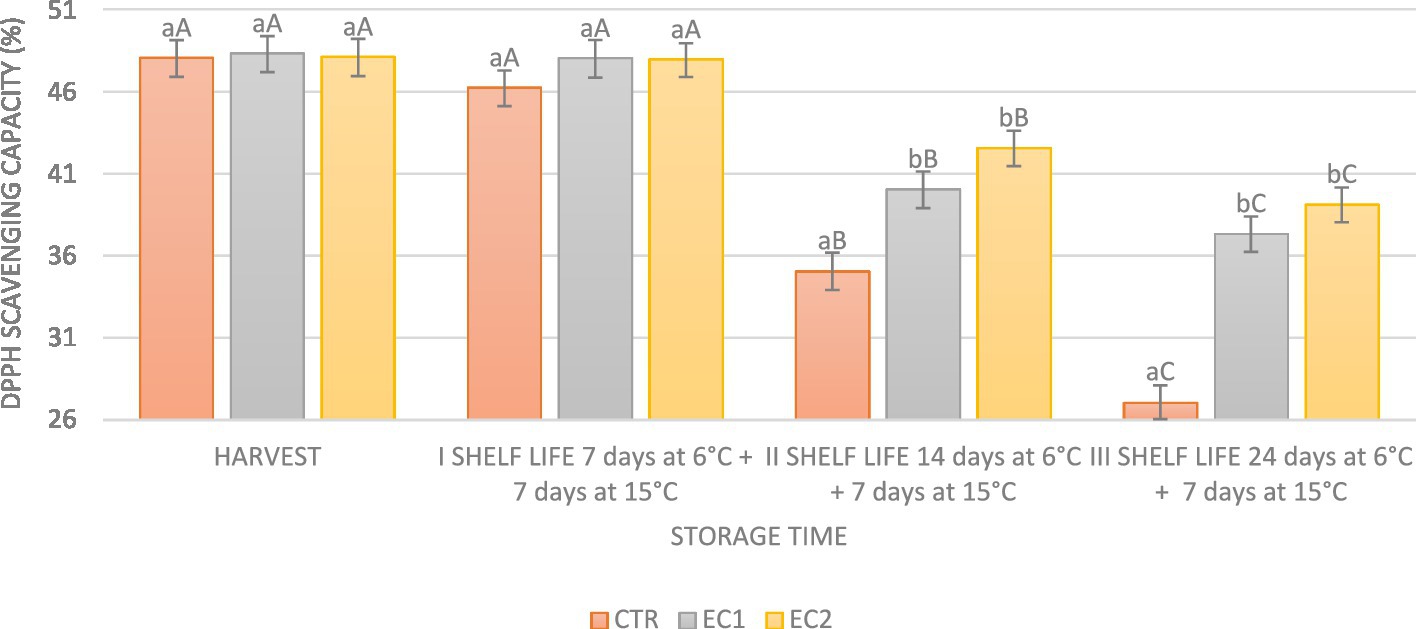
Figure 7. DPPH radical scavenging rate during harvest (day 0) and during the shelf-life I shelf-life: 7 days ±1°C; at 6 plus 7 days at 15 ± 1°C; II shelf-life: 14 days at 6°C plus 7 days at 15 ± 1°C and III shelf-life: 24 days at 6°C plus 7 days at 15 ± 1°C. Data are the mean ± S.D. Different lowercase letters indicate significant differences at p ≤ 0.05 between the treatments on each sampling date. Uppercase letters indicate significant differences at p ≤ 0.05 between the different storage period on each sampling treatment.
The ascorbic acid content (AAC) in mandarin fruits underwent notable variations influenced by the applied treatments. At day 7 ± 1°C plus 7 days at 15 ± 1°C, a substantial increase of approximately 15% in ascorbic acid content was observed in both treated and untreated fruits, initiating a subsequent decline from day 14 at 6°C plus 7 days at 15 ± 1°C. By day 14 at 6°C plus 7 days at 15 ± 1°C, the ascorbic acid content decreased by 2.08% in the control samples compared to day 0. In contrast, the treated samples exhibited an increase of 11.56% in EC1 and 9.12% in EC2 compared to day 0. On day 24 at 6°C plus 7 days at 15 ± 1°C, the increase in ascorbic acid in EC2 was 5.77%, in EC1 it was 0.93% compared to day 0, whereas a decrease of 10.4% was noted in CTR.
The initial surge in ascorbic acid levels may be associated with biosynthesis processes, while subsequent declines could result from degradation or conversion. If biosynthesis processes predominate over degradation during cold storage, an increase in ascorbic acid levels is plausible (Dzah et al., 2023). The observed tendency for the ascorbic acid content in mandarin fruits to decrease with increasing storage days aligns with findings in other studies, such as Ladaniya (2011). The primary cause of ascorbic acid reduction during storage, often attributed to oxidation, has been documented in various fruits and vegetables (Lee and Kader, 2000). Additionally, the protective role of edible coatings in mitigating the loss of ascorbic acid by limiting exposure to oxygen has been highlighted in studies such as Ali et al. (2021) (Figure 8).
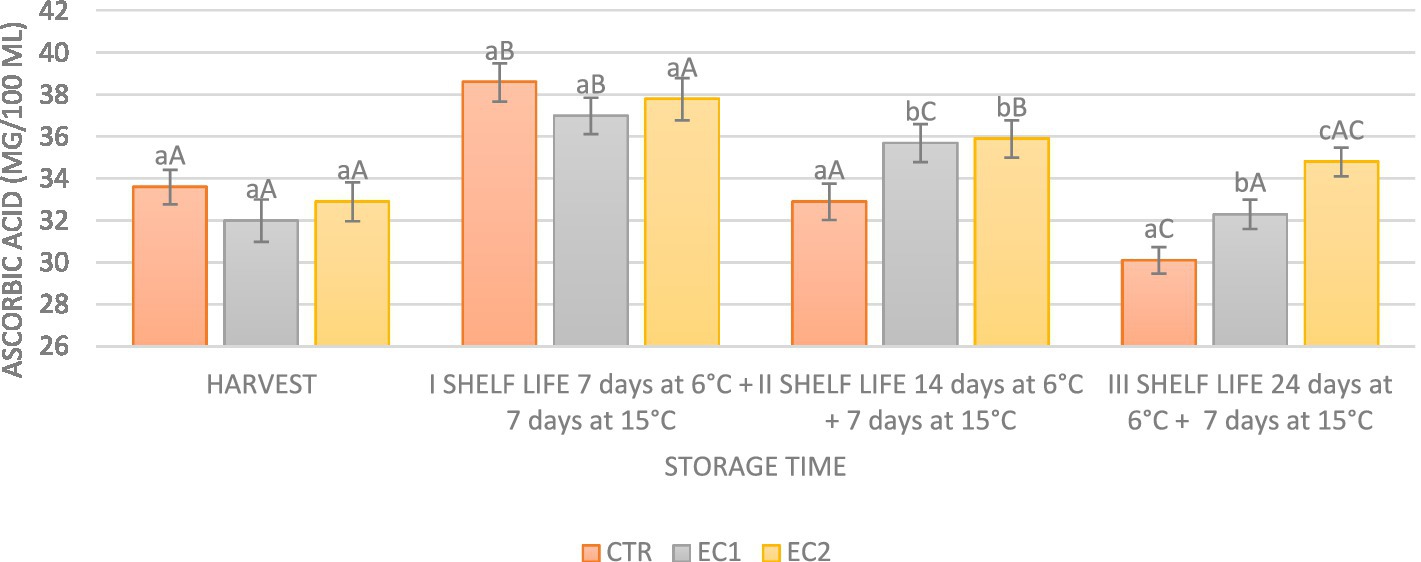
Figure 8. Ascorbic acid rate during harvest (day 0) and during the shelf-life I shelf-life: harvest plus 7 days at 15 ± 1°C; II shelf-life: 14 days at 6°C plus 7 days at 15 ± 1°C and III shelf-life: 24 days at 6°C plus 7 days at 15 ± 1°C. Data are the mean ± S.D. Different lowercase letters indicate significant differences at p ≤ 0.05 between the treatments on each sampling date. Uppercase letters indicate significant differences at p ≤ 0.05 between the different storage period on each sampling treatment.
3.5 Evolution of microbiological parameters on mandarin fruits
Mandarin fruits are susceptible to many postharvest diseases mainly determined by spoilage filamentous fungi (molds) (Zhu et al., 2019). Therefore, one effective method to increase their shelf-life is based to the application of antibacterial edible coatings. In this study, edible coating samples, control (CTR), and treated (EC1 and EC2) mandarin fruits were analyzed by culture-dependent approach for the presence of the main undesired microorganisms commonly associated with foods (Jay et al., 2009). The microbiological analysis performed on the two edible coatings did not reveal the presence of any of the microorganisms object of investigation, confirming their suitability for food applications. The presence of spoilage microorganisms (pseudomonads and yeasts), responsible to the microbial decay of horticultural products (Passafiume et al., 2023) was never found in CTR, EC1 and EC2 mandarin fruit samples. These results confirmed what previously reported by Schena et al. (2011) that bacteria and yeasts are not able to determine postharvest diseases in mandarin fruits. No foodborne pathogens such as E. coli, CPS, L. monocytogenes and Salmonella spp., associated with the consumption of fruit commodities (Leff and Fierer, 2013) developed from the cell suspensions of all mandarin fruit samples analyzed. The absence of these microorganisms is undoubtedly due to the respect of high-quality standards during the pre-and postharvest operations (Liguori et al., 2023). Significant differences (p < 0.05) were found, after 7 d at 6°C plus 7 d at 15°C ± 1°C, only for the levels of molds among trials (Figure 9).
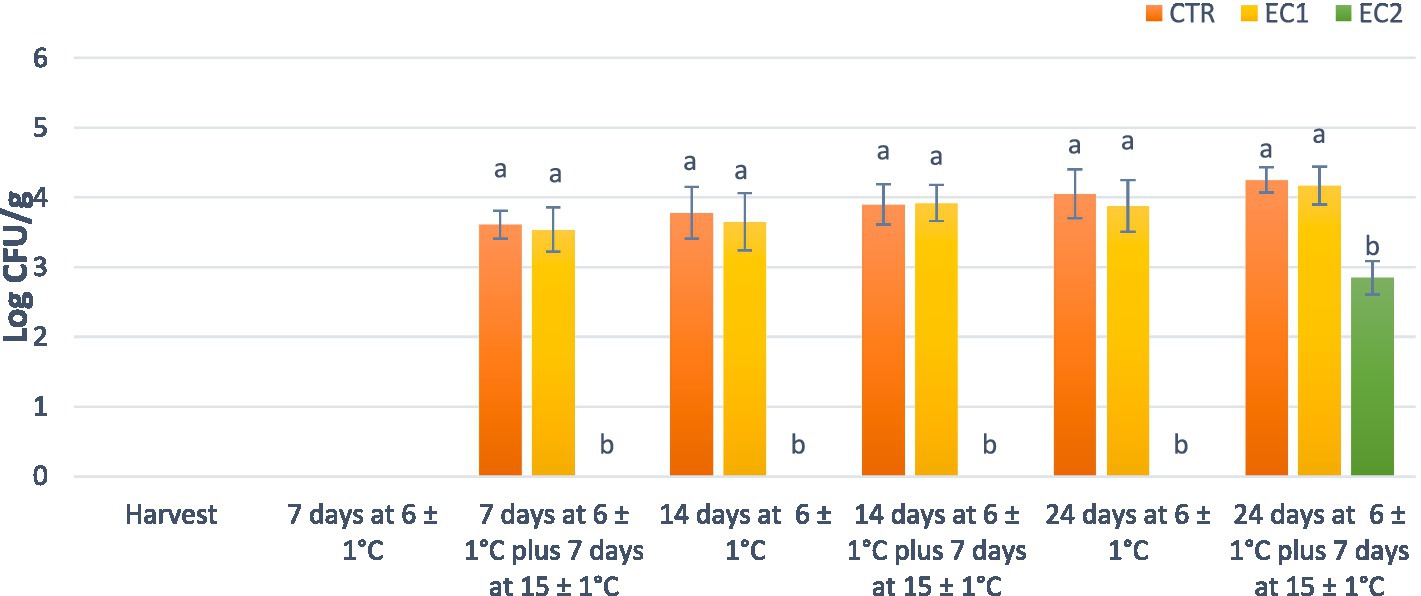
Figure 9. Cell densities of molds on control and treated mandarins fruit samples. Histograms followed by different lowercase letters are significantly different according to Tukey’s test (p < 0.05).
These microorganisms were found at about 104 CFU/g in the CTR and EC1 productions and remained constant for all shelf-life trials. Interestingly, molds were detected only after 24 d at 6°C plus 7 d at 15°C ± 1°C in EC2 treated mandarin fruits and their final levels were 2 Log cycles lower than those estimated in CTR and EC1 fruits. These differences are mainly imputable to the high concentration of carvacrol contained in the OEO used in the formulation of edible coating of the EC2 trial. Therefore, our data confirmed the antifungal properties of OEO (Leyva-López et al., 2017) even after their inclusion in edible coating formulations.
3.6 Sensory analysis and visual score
Data from sensory evaluation and visual scoring are crucial because they provide us about potential customers probable preferences for a product once they have purchased it. Mandarin fruits of all treatments (CTR; EC1; EC2) were subjected to sensory evaluation. No significant differences were found in the three treatments at the harvest, during the cold storage and during the shelf-life. The panelists rated all samples from the three case studies positively, giving a slightly higher liking rating in the treated fruits (Figures 10A–C). The following descriptors oregano odor, oregano flavor, and herbaceous hint were included in EC2 to allow the incidence of oregano essential oil to be evaluated. (Figure 10C). The “sweet” parameter was evaluated positively by the panelists; in fact, the sweetness of the fruits remained constant in all samples, and at the last shelf-life EC1 samples had one point more than the CTR and EC2 ones. The juiciness parameter remained similar in all three theses during the storage periods; as well as for chewiness. The parameter “typical tangerine flavor “had the higher score in EC2 at 24 days at 6 ± 1°C plus 7 days at 15 ± 1°C. Starting from the third storage period, the treated samples were preferred by the panelists showing 2 points more in EC2 on 24 days at 6 ± 1°C plus 7 days at 15 ± 1°C than the CTR samples. Bitter and sour parameters were similarly evaluated during the storage periods by the panelists.
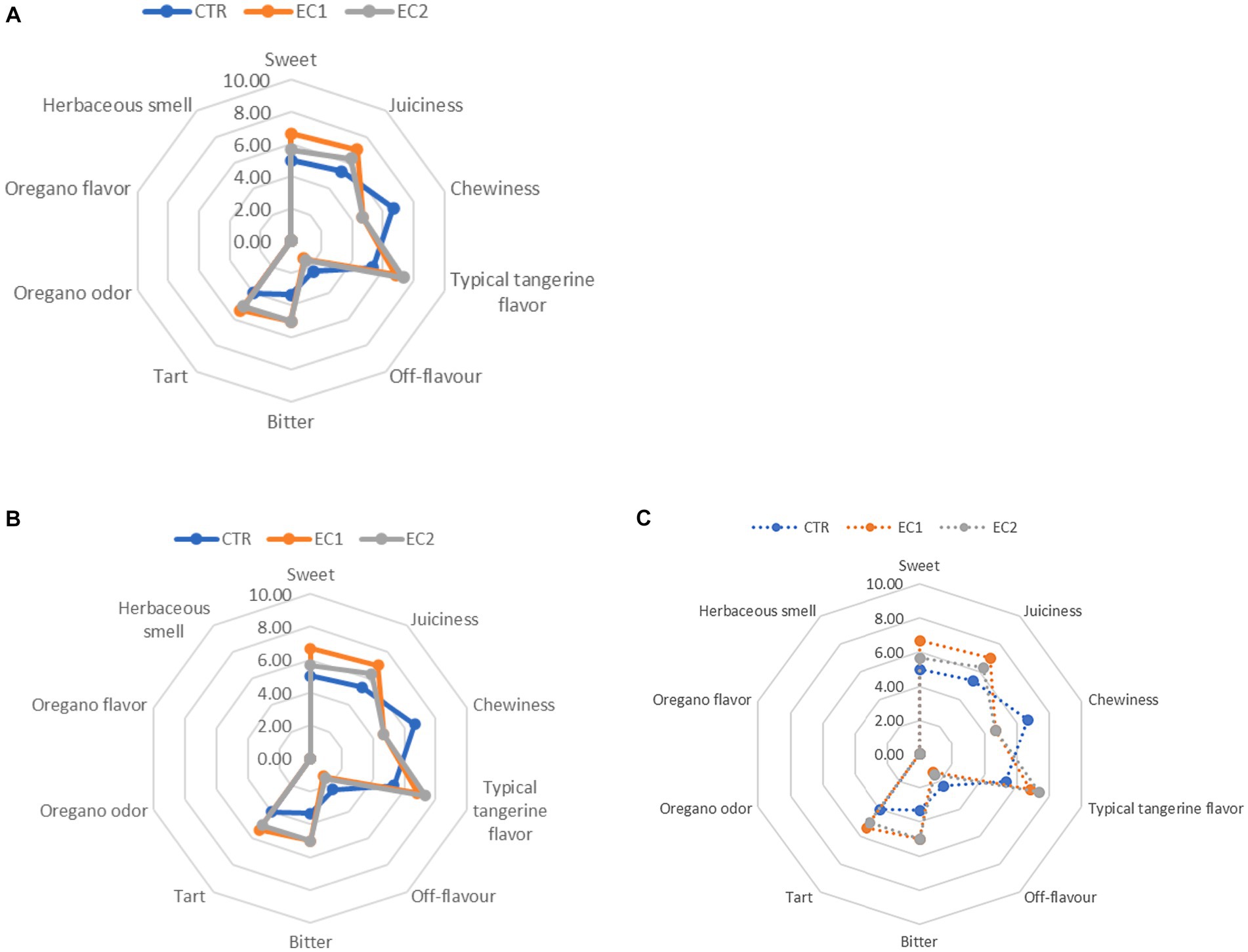
Figure 10. (A) Sensorial analysis of mandarin fruits control samples (CTR-A), treated mandarin fruit samples (EC1-B; EC2-C) at I shelf-life: 7 days at 6°C plus 7 days at 15°C; (B) Sensorial analysis of mandarin fruits control samples (CTR-A), treated mandarin fruit samples (EC1-B; EC2-C) at II shelf-life: 14 days at 6°C plus 7 days at 15°C; (C) Sensorial analysis of mandarin fruits control samples (CTR-A), treated mandarin fruit samples (EC1-B; EC2-C) at III shelf-life: 24 days at 6°C plus 7 days at 15 ± 1°C.
“Oregano odor,” “Oregano flavor” and “herbaceous smell” remained persistent until 3rd day of the second storage period (II shelf-life), following a slight increase in these descriptors between 3rd day and 7th day of the first storage period (Figure 10C). There was a similar evaluation at harvest (0 days) and 3rd day of the second storage period (II shelf-life) until these descriptors were not detected from 7th day of the second storage period (II shelf-life).
These descriptors were completely absent during the third storage period (III shelf-life 24 days at 6 ± 1°C + 7 days at 15 ± 1°C) (Figure 10C).
During the three storage periods, all treatments samples showed scores above the marketability threshold (Figure 11). Only treated samples maintained a score above 4 during the three storage periods.

Figure 11. Visual score of mandarin fruits control samples (CTR), treated mandarin fruit samples (EC1; EC2) at the harvest, during the shelf-life (I shelf-life: harvest plus 7 days at 15 ± 1°C; II shelf-life: 14 days at 6°C plus 7 days at 15 ± 1°C and III shelf-life: 24 days at 6°C plus 7 days at 15 ± 1°C).
4 Conclusion
The analysis of multispectral images acquired through UAVs offers the potential to identify the optimal collection time. Specifically, for harvested oregano intended for essential oil extraction, the balsamic time was determined when the NDVI values of most plants reached 0.58.
This study marks the first evaluation of the impact of a coating composed of gellan, glycerol, and calcium chloride enriched with essential oils of oregano featuring high carvacrol content on the shelf life of mandarin fruits. Our findings suggest that the application of edible coatings maintained the quality parameters of the fruits without inducing quality decay. Leveraging its barrier effect, the edible coating resulted in reduced weight loss of the treated fruits, a crucial aspect for preserving product quality. Additionally, the edible coatings demonstrated the ability to better preserve nutritional and nutraceutical attributes throughout the storage periods.
Notably, carvacrol exhibited inhibitory effects for 24 days at 6 ± 1°C plus 7 days at 15 ± 1°C, preventing the occurrence of green and blue mold, which are responsible for rendering the product unsuitable for commercialization. This underscores the significant value of the treatments in safeguarding the quality parameters of mandarin fruits and mitigating food waste arising from non-marketability.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
GL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing. GGr: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. GS: Data curation, Formal analysis, Investigation, Writing – original draft. GGa: Formal analysis, Investigation, Writing – original draft. RG: Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. MB: Formal analysis, Investigation, Writing – original draft. CG: Data curation, Investigation, Project administration, Resources, Writing – original draft. SO: Data curation, Formal analysis, Investigation, Resources, Writing – original draft. GF: Funding acquisition, Project administration, Resources, Writing – review & editing. MM: Funding acquisition, Project administration, Resources, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Project “VAL.INN.P.O. - Validazione di protocolli innovativi per la produzione di piante officinali di interesse nutraceutico coltivate in Sicilia.” Bando PSR Sicilia 2014/2022 – Sottomisura 16.1.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, S., Anjum, M. A., Ejaz, S., Hussain, S., Ercisli, S., Saleem, M. S., et al. (2021). Carboxymethyl cellulose coating delays chilling injury development and maintains eating quality of ‘Kinnow’mandarin fruits during low temperature storage. Int. J. Biol. Macromol. 168, 77–85. doi: 10.1016/j.ijbiomac.2020.12.028
Ali, S., Anjum, M. A., Nawaz, A., Naz, S., Ejaz, S., Sardar, H., et al. (2020). Tragacanth gum coating modulates oxidative stress and maintains quality of harvested apricot fruits. Int. J. Biol. Macromol. 163, 2439–2447. doi: 10.1016/j.ijbiomac.2020.09.179
Allegra, A., Sortino, G., Inglese, P., Settanni, L., Todaro, A., and Gallotta, A. (2017). The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’ fig (Ficus carica L.) fruit. Food Packag. Shelf. Life 12, 135–141. doi: 10.1016/j.fpsl.2017.04.010
Arnon, H., Zaitsev, Y., Porat, R., and Poverenov, E. (2014). Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol. Technol. 87, 21–26. doi: 10.1016/j.postharvbio.2013.08.007
Azarakhsh, N., Osman, A., Ghazali, H. M., Tan, C. P., and Mohd Adzahan, N. (2014). Effects of gellan-based edible coating on the quality of fresh-cut pineapple during cold storage. Food Bioprocess Technol. 7, 2144–2151. doi: 10.1007/s11947-014-1261-6
Barreto, T. A., Andrade, S. C., Maciel, J. F., Arcanjo, N. M., Madruga, M. S., Meireles, B., et al. (2016). A chitosan coating containing essential oil from Origanum vulgare L. to control postharvest mold infections and keep the quality of cherry tomato fruit. Front. Microbiol. 7:1724. doi: https://doi.org/10.3389/fmicb.2016.01724
Berk, Z. (2016). Postharvest changes in Citrus fruit processing Cambridge, MA: Academic Press, pp. 95–105.
Bhan, C., Asrey, R., Meena, N. K., Rudra, S. G., Chawla, G., Kumar, R., et al. (2022). Guar gum and chitosan-based composite edible coating extends the shelf life and preserves the bioactive compounds in stored Kinnow fruits. Int. J. Biol. Macromol. 222, 2922–2935. doi: 10.1016/j.ijbiomac.2022.10.068
Brackett, R. (1987). Microbiological consequences of minimally processed fruits and vegetables. J. Food Qual. 10, 195–206. doi: 10.1111/j.1745-4557.1987.tb00858.x
Brasil, I., Gomes, C., Puerta-Gomez, A., Castell-Perez, M., and Moreira, R. (2012). Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT Food Sci. Technol. 47, 39–45. doi: 10.1016/j.lwt.2012.01.005
Cao, J., Wang, C., Xu, S., Chen, Y., Wang, Y., Li, X., et al. (2019). The effects of transportation temperature on the decay rate and quality of postharvest Ponkan (Citrus reticulata Blanco) fruit in different storage periods. Sci. Hortic. 247, 42–48. doi: 10.1016/j.scienta.2018.12.009
Catania, P., Gaglio, R., Orlando, S., Settanni, L., and Vallone, M. (2020). Design and implementation of a smart system to control aromatic herb dehydration process. Agriculture 10:332. doi: 10.3390/agriculture10080332
Comparetti, A., Greco, C., Orlando, S., Ciulla, S., and Mammano, M. M. (2022). Comparison of mechanical, assisted and manual harvest of Origanum vulgare L. Sustain. For. 14:2562. doi: 10.3390/su14052562
Culmone, A., Mirabile, G., Tinebra, I., Michelozzi, M., Carrubba, A., Bellardi, M. G., et al. (2023). Hydrolate and EO application to reduce decay of Carica papaya during storage. Horticulturae 9:204. doi: 10.3390/horticulturae9020204
Dea, S., Ghidelli, C., Pérez-Gago, M. B., and Plotto, A. (2011). “Coatings for minimally processed fruits and vegetables” in Edible coatings and films to improve food quality. ed. J. Bai (Boca Raton, FL: CRC Press), 243–289.
Dzah, C. S., Kpodo, F. M. K., Asante-Donyinah, D., and Boateng, N. A. S. (2023). The influence of Morinda citrifolia fruit maturity level, parts and storage length on total phenols, ascorbic acid, antioxidant activity and ethylene gas emission. Food Chem. Adv. 2023:100599. doi: 10.1016/j.focha.2023.100599
Fleisher, A., and Sneer, N. (1982). Oregano spices and Origanum chemotypes. J. Sci. Food Agric. 33, 441–446. doi: 10.1002/jsfa.2740330508
Gao, Y., Kan, C., Chen, M., Chen, C., Chen, Y., Fu, Y., et al. (2018). Effects of chitosan-based coatings enriched with cinnamaldehyde on mandarin fruit cv. Ponkan during room-temperature storage. Coatings 8:372. doi: 10.3390/coatings8100372
Garofalo, G., Ponte, M., Greco, C., Barbera, M., Mammano, M. M., Fascella, G., et al. (2023). Improvement of fresh ovine “Tuma” cheese quality characteristics by application of oregano essential oils. Antioxidants, 2023 12:1293. doi: 10.3390/antiox12061293
Gilling, D. H., Kitajima, M., Torrey, J., and Bright, K. (2014). Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J. Appl. Microbiol. 116, 1149–1163. doi: 10.1111/jam.12453
Han, J. H. (2014). Edible films and coatings: a review. Innov. Food Sci. Emerg. Technol. 2014, 213–255. doi: 10.1016/B978-0-12-394601-0.00009-6
Homayouni, H., Kavoosi, G., and Nassiri, S. M. (2017). Physicochemical, antioxidant and antibacterial properties of dispersion made from tapioca and gelatinized tapioca starch incorporated with carvacrol. LWT 77, 503–509. doi: 10.1016/j.lwt.2016.12.007
Hyldgaard, M., Mygind, T., and Meyer, R. L. (2012). Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 3:12. doi: 10.3389/fmicb.2012.00012
Jay, J. M., Loessner, M. J., and Golden, D. A. (2009). Microbiologia degli alimenti. Berlin: Springer Science and Business Media.
Jayakumar, S., Madankumar, A., Asokkumar, S., Raghunandhakumar, S., Gokula Dhas, K., Kamaraj, S., et al. (2012). Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol. Cell. Biochem. 360, 51–60. doi: 10.1007/s11010-011-1043-7
Jouki, M., Yazdi, F. T., Mortazavi, S. A., and Koocheki, A. (2014). Quince seed mucilage films incorporated with oregano essential oil: physical, thermal, barrier, antioxidant, and antibacterial properties. Food Hydrocoll. 36, 9–19. doi: 10.1016/j.foodhyd.2013.08.030
Kanner, J., Harel, S., and Granit, R. (2001). Betalains-A new class of dietary cationized antioxidants. J. Agric. Food Chem. 49, 5178–5185. doi: 10.1021/jf010456f
Karkabounas, S., Kostoula, O. K., Daskalou, T., Veltsistas, P., Karamouzis, M., Zelovitis, I., et al. (2006). Anticarcinogenic and antiplatelet effects of carvacrol. Exp. Oncol. 28, 121–125.
Ladaniya, M. S. (2011). Physico-chemical, respiratory and fungicide residue changes in wax coated mandarin fruit stored at chilling temperature with intermittent warming. J. Food Sci. Technol. 48, 150–158. doi: 10.1007/s13197-010-0160-8
Lee, S. K., and Kader, A. A. (2000). Kader Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 20, 207–220. doi: 10.1016/S0925-5214(00)00133-2
Leff, J. W., and Fierer, N. (2013). Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS One 8:e59310. doi: 10.1371/journal.pone.0059310
Leyva-López, N., Gutiérrez-Grijalva, E. P., Vazquez-Olivo, G., and Heredia, J. B. (2017). Essential oils of oregano: biological activity beyond their antimicrobial properties. Molecules 22:989. doi: 10.3390/molecules22060989
Liguori, G., Gaglio, R., Greco, G., Gentile, C., Settanni, L., and Inglese, P. (2021). Effect of Opuntia ficus-indica mucilage edible coating on quality, nutraceutical, and sensorial parameters of minimally processed cactus pear fruits. Agronomy 11:1963.15. doi: 10.3390/agronomy11101963
Liguori, G., Greco, G., Gargano, F., Gaglio, R., Settanni, L., and Inglese, P. (2023). Effect of mucilage-based edible coating enriched with oregano essential oil on postharvest quality and sensorial attributes of fresh-cut loquat. Coatings 13:1387. doi: 10.3390/coatings13081387
Lota, M. L., De Rocca Serra, D., Tomi, F., and Casanova, J. (2000). Chemical variability of peel and leaf essential oils of mandarins from Citrus reticulata Blanco. Biochem. Syst. Ecol. 28, 61–78. doi: 10.1016/S0305-1978(99)00036-8
Mammano, M. M., Comparetti, A., Ciulla, S., Greco, C., and Orlando, S. (2023). A prototype of photovoltaic dryer for nutraceutical and aromatic plants. Berlin: Springer, p. 337.
Mammano, M. M., Comparetti, A., Greco, C., and Orlando, S. (2023). A model of Sicilian environmentally friendly multifunctional farm for soil protection. Berlin: Springer, p. 337.
Miceli, A., Gaglio, R., Francesca, N., Ciminata, A., Moschetti, G., and Settanni, L. (2019). Evolution of shelf-life parameters of ready-to-eat escarole (Cichorium endivia var. latifolium) subjected to different cutting operations. Sci. Hortic. 247, 175–183. doi: 10.1016/j.scienta.2018.12.023
Morshedloo, M. R., Mumivand, H., Craker, L. E., and Maggi, F. (2018). Chemical composition and antioxidant activity of essential oils in Origanum vulgare subsp. gracile at different phenological stages and plant parts. J. Food Process. Preserv. 42:e13516. doi: 10.1111/jfpp.13516
Nguyen, V. T., Nguyen, D. H., and Nguyen, H. V. (2020). Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biol. Technol. 162:111103. doi: 10.1016/j.postharvbio.2019.111103
Nicolopoulou-Stamati, P., Maipas, S., Kotampasi, C., Stamatis, P., and Hens, L. (2016). Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front. Public Health 4:148. doi: 10.3389/fpubh.2016.00148
Nostro, A., and Papalia, T. (2012). Antimicrobial activity of carvacrol: current progress and future prospectives. Recent Pat. Antiinfect. Drug Discov. 7, 28–35 (2012. doi: 10.2174/157489112799829684
Olivas, G., and Barbosa-Cánovas, G. (2005). Edible coatings for fresh-cut fruits. Crit. Rev. Food Sci. Nutr. 45, 657–670. doi: 10.1080/10408690490911837
Olivas, G. I. I., and Barbosa-Cánovas, G. (2009)Edible films and coatings for fruits and vegetables. Berlin: Springer, pp. 211–244.
Oms-Oliu, G., Soliva-Fortuny, R., and Martín-Belloso, O. (2008). Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 50, 87–94. doi: 10.1016/j.postharvbio.2008.03.005
Palou, L., Valencia-Chamorro, S., and Pérez-Gago, M. (2015). Antifungal edible coatings for fresh citrus fruit: a review. Coatings 5, 962–986. doi: 10.3390/coatings5040962
Passafiume, R., Roppolo, P., Tinebra, I., Pirrone, A., Gaglio, R., Palazzolo, E., et al. (2023). Reduction of pericarp Browning, and microbial spoilage on Litchi fruits in modified atmosphere packaging. Horticulturae 9:651. doi: 10.3390/horticulturae9060651
Passafiume, R., Tinebra, I., Gaglio, R., Settanni, L., Sortino, G., Allegra, A., et al. (2022). Fresh-cut mangoes: how to increase shelf life by using neem oil edible coating. Coatings 12:664. doi: 10.3390/coatings12050664
Pérez-Alfonso, C. O., Martínez-Romero, D., Zapata, P. J., Serrano, M., Valero, D., and Castillo, S. (2012). The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. italicum involved in lemon decay. Int. J. Food Microbiol. 158, 101–106. doi: 10.1016/j.ijfoodmicro.2012.07.002
Rafiq, S., Kaul, R., Sofi, S. A., Bashir, N., Nazir, F., and Nayik, G. A. (2018). Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 17, 351–358. doi: 10.1016/j.jssas.2016.07.006
Robles-Sánchez, R. M., Rojas-Graü, M. A., Odriozola-Serrano, I., González-Aguilar, G., and Martin-Belloso, O. (2013). Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT Food Sci. Technol. 50, 240–246. doi: 10.1016/j.lwt.2012.05.021
Rodrigo, M. J., Alquézar, B., Alós, E., Lado, J., and Zacarías, L. (2013). Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 163, 46–62. doi: 10.1016/j.scienta.2013.08.014
Sánchez-Torres, P., and Tuset, J. J. (2011). Molecular insights into fungicide resistance in sensitive and resistant Penicillium digitatum strains infecting citrus. Postharvest Biol. Technol. 59, 159–165. doi: 10.1016/j.postharvbio.2010.08.017
Sapper, M., Palou, L., Pérez-Gago, M. B., and Chiralt, A. (2019). Antifungal starch–gellan edible coatings with thyme essential oil for the postharvest preservation of apple and persimmon. Coatings 9:333. doi: 10.3390/coatings9050333
Schena, L., Strano, M. C., Sanzani, S. M., and Le Ippolito, A. (2011). Malattie degli agrumi in postraccolta. Protezione Colture 4, 30–41.
Singleton, V., and Rossi, J. (1965). Colorimetry of total polyphenols with phospho-molybdic phosphor tungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158. doi: 10.5344/ajev.1965.16.3.144
Sondari, D. (2019). Modification of sago starch for edible coating. 543. Bristol, UK: IOP Publishing, p. 012013.
Tsitlakidou, P., Papachristoforou, A., Tasopoulos, N., Matzara, A., Hatzikamari, M., Karamanoli, K., et al. (2022). Sensory analysis, volatile profiles, and antimicrobial properties of Origanum vulgare L. essential oils. Flavour Fragr. J. 37, 43–51. doi: 10.1002/ffj.3680
USDAF . (2023). Citrus: World markets and trade. Available at: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (Accessed August 28, 2023).
Van Wyk, A. A., Huysamer, M., and Barry, G. H. (2009). Extended low-temperature shipping adversely affects rind colour of ‘palmer navel’ sweet orange [Citrus sinensis (L.) Osb.] due to carotenoid degradation but can partially be mitigated by optimising post-shipping holding temperature. Postharvest Biol. Technol. 53, 109–116. doi: 10.1016/j.postharvbio.2009.04.004
Wang, F., Chen, L., Chen, H., Chen, S., and Liu, Y. (2019). Analysis of flavonoid metabolites in Citrus peels (Citrus reticulata “Dahongpao”) using UPLC-ESI-MS/MS. Molecules 24:2680. doi: 10.3390/molecules24152680
Wang, L., Shao, S., Madebo, M. P., Hou, Y., Zheng, Y., and Jin, P. (2020). Effect of nano-SiO2 packing on postharvest quality and antioxidant capacity of loquat fruit under ambient temperature storage. Food Chem. 315:126295. doi: 10.1016/j.foodchem.2020.126295
Yousuf, B., Wu, S., and Siddiqui, M. W. (2021). Incorporating essential oils or compounds derived thereof into edible coatings: effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Technol. 108, 245–257. doi: 10.1016/j.tifs.2021.01.016
Zhang, Z., Zhu, Z., Ma, Z., and Li, H. A. (2009). Molecular mechanism of azoxystrobin resistance in Penicillium digitatum UV mutants and a PCR-based assay for detection of azoxystrobin-resistant strains in packing- or store-house isolates. Int. J. Food Microbiol. 131, 157–161. doi: 10.1016/j.ijfoodmicro.2009.02.015
Zhu, H., Zhao, L., Zhang, X., Foku, J. M., Li, J., Hu, W., et al. (2019). Efficacy of Yarrowia lipolytica in the biocontrol of green mold and blue mold in Citrus reticulata and the mechanisms involved. Biol. Control 139:104096. doi: 10.1016/j.biocontrol.2019.104096
Keywords: mandarin, Tardivo di Ciaculli, edible coating, oregano essential oil, carvacrol, shelf life, physicochemical analysis, microbial decay
Citation: Liguori G, Greco G, Salsi G, Garofalo G, Gaglio R, Barbera M, Greco C, Orlando S, Fascella G and Mammano MM (2024) Effect of the gellan-based edible coating enriched with oregano essential oil on the preservation of the ‘Tardivo di Ciaculli’ mandarin (Citrus reticulata Blanco cv. Tardivo di Ciaculli). Front. Sustain. Food Syst. 8:1334030. doi: 10.3389/fsufs.2024.1334030
Edited by:
Jinyan Gong, Zhejiang University of Science and Technology, ChinaReviewed by:
Xiaoyu Jia, Tianjin Academy of Agricultural Sciences, ChinaRajat Suhag, Free University of Bozen-Bolzano, Italy
Copyright © 2024 Liguori, Greco, Salsi, Garofalo, Gaglio, Barbera, Greco, Orlando, Fascella and Mammano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Greco, Z2l1c2VwcGUuZ3JlY28wM0B1bmlwYS5pdA==; Giancarlo Fascella, Z2lhbmNhcmxvLmZhc2NlbGxhQGNyZWEuZ292Lml0
 Giorgia Liguori
Giorgia Liguori Giuseppe Greco
Giuseppe Greco Giulia Salsi1,2
Giulia Salsi1,2 Raimondo Gaglio
Raimondo Gaglio Marcella Barbera
Marcella Barbera Carlo Greco
Carlo Greco Giancarlo Fascella
Giancarlo Fascella