
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 30 March 2023
Sec. Sustainable Food Processing
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.979943
This article is part of the Research Topic Emerging Non-Thermal Technology Applications for Sustainable Food Processing View all 6 articles
Thermal processing is widely used in juice production to ensure microbial safety and to extend juice shelf life; however, it can have an impact on quality attributes such as color and nutritional content. UV-C irradiation and high-pressure processing (HPP) are non-thermal processing methods which causes little impact on the quality of fruit juice compared to conventional heat treatment (CHT). The objective of this study was to investigate the effect of combining UV-C and HPP (UV-C + HPP) treatments on microbial loads and quality of “Nanglae” pineapple juice during cold storage at 5 ± 1°C for 91 days. The treatments were as follows: (1) no treatment; (2) conventional heat treatment (80 ± 5°C, 10 min); and (3) a combination of UV-C (3 kJ/m2) and HPP (600 MPa, 5 min) treatments. The combined treatments of UV-C and HPP reduced the numbers of viable cells of aerobic microorganisms to less than the quantification limit of 1.48 log CFU/mL and yeasts and molds to <1.18 log CFU/mL throughout the 91-day storage period. Pineapple juice treated with CHT contained yeasts and molds exceeding the quantification limit of 1.18 log CFU/mL after 63 days of storage. The UV-C + HPP treatment preserved carotenoids and protein levels comparable to those found in fresh pineapple juice over 91 days of storage, whereas the CHT significantly decreased these values. Throughout the storage period, ascorbic acid levels in the CHT were slightly lower than in the combined treatments. These results clearly demonstrate that the combination of UV-C and HPP can ensure the safety of “Nanglae” pineapple juice while also retaining bioactive compounds. Combining these two technologies could be a new approach to producing healthy and safe juices.
Fresh fruits and vegetables, as well as minimally processed products, are increasingly popular for health reasons. Pineapple (Ananas comosus L. Merr) is high in calcium, potassium, carbohydrates, crude fiber, and minerals. These nutrients are beneficial to the digestive system and diet. Furthermore, pineapple contains bromelain, a group of enzymes that promote protein digestion and reduces inflammation. Pineapple also contains bioactive compounds such as vitamin C, polyphenols, and carotenoids that help prevent diseases, e.g., cancer, heart disease, diabetes, tooth decay, and obesity, as well as some pathogenic microorganisms (Ferreira et al., 2016). The pineapple cultivar “Nanglae” is a Chiang Rai Geographical Indication (GI) that is commonly consumed fresh; however, increased pineapple production for fresh consumption leads to oversupply and lower prices. The price of “Nanglae” pineapple sold as fresh fruit could be reduced by up to 50% during the peak season (Thanwa Aree, personal communication, 26 Jan 2022). Processed pineapple is an option for mitigating this problem while also increasing the value of pineapple. The distinct aroma and juiciness of “Nanglae” pineapple make it ideal for juice production and the development of a refreshing and healthy beverage. Conventional heat treatment (CHT) is commonly used during juice production to kill pathogens and extend its shelf life of the juice; pasteurization at temperatures lower than 100°C is commonly used for this purpose (Deak, 2014). However, thermal processing can cause a loss of antioxidants and other compounds of nutritional value (Vervoort et al., 2011) and affect juice color (Rattanathanalerk et al., 2005).
Non-thermal processing methods such as high-pressure processing (HPP), pulsed electric fields, radiation, and ultrasound have recently received attention for use in juice processing. These methods may retain the sensory characteristics and nutrient content of the juice, to levels similar to those found in raw or fresh fruits, in addition to removing microbial risk from the juice. Irradiation with UV-C light (200–280 nm) has the potential to reduce microbial loads in food and on packaging surfaces while having little effect on sensory and product quality (Abdul Karim Shah et al., 2016). Furthermore, UV-C treatment can improve the quality of fruits and vegetables by increasing the synthesis of secondary metabolites and antioxidant enzymes (González-Aguilar et al., 2007). For instance, the increased total phenolic content and antioxidant activity were obtained in UV-C treated banana at the dose of 0.01–0.3 kJ/m2 (Mohamed et al., 2017), tomato at 3.7 kJ/m2 (Charles et al., 2008), and blueberries at 4.3 kJ/m2 (Perkins-Veazie et al., 2008). Romanazzi et al. (2006) found that grapes irradiated with UV-C at 0.36 kJ/m2 induced catechin and trans-resveratrol, which later became a treatment to increase antioxidants in grapes for making a functional wine with the health-enhancing properties of resveratrol. The UV-C exposure at 13.2, 26.4, and 39.6 kJ/m2 also increased vitamin C levels in the “Phulae” pineapple pulp (Sari et al., 2016). However, the use of UV-C can shorten the shelf life of juice products due to microbial spoilage, in particular yeasts and molds. It is probably attributable to the complex cell structure of the fungi, which makes them more resistant to UV-C treatment (Torkamani and Niakousari, 2011).
As a result, combining UV-C and other non-thermal processing treatments may have a synergistic benefit and increase the shelf life of fruit juice, including “Nanglae” pineapple juice. HPP is an emerging technology that originated in Japan and has been used for a decade in food packaging and beverage production (Knorr, 1993). HPP can inactivate microorganisms such as bacteria, yeasts, and molds, as well as deactivate enzymes and chemical reactions, resulting in fewer quality changes and the preservation of aroma, taste, and nutritional value (Balasubramaniam et al., 2008). For example, HPP has been shown to retain more antioxidants and ascorbic acid in strawberry and blackberry purees than thermal processing (Abera, 2019). HPP treatment has also significantly improved the levels of total carotenoids in foods compared to untreated ones (Gamlath and Wakeling, 2011). When pressures ranging from 400 to 600 MPa were applied to tomato puree and orange juice (Sanchez-Moreno et al., 2005, 2006), a significant increase in all types of carotenoids was observed. Our previous study revealed that HPP at 400 and 600 MPa for 5 min reduced microbial load in “Nanglae” pineapple juice (Chuensombat et al., 2019). Because the average pH of “Nanglae” pineapple is around 4.6 (Kongsuwan et al., 2009), HPP at 600 MPa is recommended for application with “Nanglae” pineapple juice according to the Food Drug Administration Thailand. (2019). However, there is little information on the use of HPP in the production of “Nanglae” pineapple juice. Moreover, HPP is insufficient on its own to inactivate some enzymes that can cause deterioration and should be used in conjunction with other methods (Chandra et al., 2021). To the best of our knowledge, no research has been published on the use of UV-C in conjunction with HPP to preserve the quality of fruit juice. Therefore, the purpose of this study was to determine the combined effect of UV-C and HPP treatments on quality of “Nanglae” pineapple juice during cold storage compared to conventional heat treatment.
A fully ripened and defect-free “Nanglae” pineapples were purchased from an orchard in Chiang Rai, Thailand. The pineapples were washed with tap water, peeled, cored, and cut into small pieces using a sharp stainless-steel knife. The pineapple juice was extracted using a hydraulic press (Owner Foods Machinery Co., Ltd.), collected, and immediately used for further experimentation.
The UV-C radiation chamber (Figure 1) was built with slight modifications from Sari et al. (2016). The UV-C lamps (Sylvania Ultraviolet G8W, 253.7 nm) were placed 30 cm above and below the rack, with four lamps each. The UV-C intensity in this experiment was 1.14 mW/cm2 measured using a digital UV-C meter (LUTRON UVC-254SD, Taiwan). The pineapple juice was poured into a 22 × 25 cm glass container and filled to a height of 1 cm (approximately 550 mL) before being exposed to UV-C radiation for 4.38 min to receive a dose of 3 kJ/m2. After treatment, 130 mL of pineapple juice was poured into a sterile polyethylene terephthalate (PET) bottle and prepared for high-pressure processing.
UV-C-treated pineapple juice was subjected under high-pressure processing at 600 MPa for 5 min using a high-pressure generator (Bao Tou KeFa High Pressure Technology Co., Ltd., China) with a capacity of 3.0 L at room temperature (25°C). Reverse osmosis water was used as the pressure transmitting fluid. The treatment time in this study excluded the time of increasing and releasing the pressure. After treatment, all samples were stored at 5 ± 1°C. Quality changes of pineapple juice were determined every 7 days for 91 days. Untreated pineapple juice was used as a control.
Pineapple juice was mixed thoroughly and heated at 80 ± 5°C for 10 min according to Chia et al. (2012). After heat treatment, 130 mL of pineapple juice was immediately filled into a sterile polyethylene terephthalate (PET) bottle, lidded and cooled down before storing at 5 ± 1°C.
Total aerobic microbial counts and yeast and mold counts were determined using 3M Petrifilm™ Aerobic Count Plates and 3M Petrifilm™ Yeast and Mold Count Plates, respectively (AOAC, 2000). Aliquots (1.0 mL) of pineapple juice were taken and decimally diluted in 9.0 mL of 0.1% peptone water. Aliquots were then pipetted onto the film, spread, and incubated at 35 ± 1°C for 48 ± 3 h. The numbers of colony forming units (CFU)/mL were expressed as decimal logarithms. The Aerobic Count Plate was considered below the quantification limit of 1.48 log CFU/mL when <30 colonies were counted; the Yeast and Mold Count Plate was considered below the quantification limit of 1.18 log CFU/mL when <15 colonies were counted.
The pH of pineapple juice was measured using a pH meter (Binder, Scientific Promotion Co., Ltd.). The total soluble solids content was measured using a digital hand refractometer (ATAGO, Japan), and the values were expressed as °Brix. Titratable acidity was determined according to the method of the Association of Official Agricultural Chemists (AOAC, 2000). Pineapple juice (5 mL) with 1% phenolphthalein solution as an indicator was titrated with 0.1 N of sodium hydroxide. The values were expressed as a percentage of citric acid. The color of pineapple juice was measured using a CR-10 color reader (Konica Minolta, Inc., Japan) and expressed as L* (lightness) and b* (brightness) values.
Ascorbic acid was analyzed titrimetrically according to the method of the Association of Official Agricultural Chemists (AOAC, 2000). Aliquots (2 mL) of pineapple juice were added to 5 mL of metaphophoric acid-acetic acid solution and titrated with 2,6-dichloroindophenol. The values were expressed as mg per 100 mL juice (mg/100 mL).
Total carotenoid contents were determined according to Desobry et al. (1997) with some modifications. Juice samples (5 mL) were mixed with 95% hexane. Then, the sample was homogenized for 1 min and centrifuged at 2,000 × g, 4°C for 5 min. The yellow layer was collected to measure the absorbance at 454 nm using a spectrophotometer (GENESYS 10S UV-Vis, ThermoFisher Scientific). The results were expressed as mg per 100 mL juice (mg/100 mL).
Total phenolic compounds were determined according to International Organization for Standardization 14502-1. (2005) with some modifications. Pineapple juice (250 μL) was mixed with 1,250 μL of 10% (v/v) Folin-Cioculteu phenol's reagent and 1,000 μL of 7.5% (w/v) of sodium carbonate. The mixture was incubated at room temperature (25°C) for 60 min. Then, the absorbance was measured at 765 nm using a microplate reader (Multiskan Go, ThermoFisher Scientific). Gallic acid was used as a standard, and the results were expressed as mg of gallic acid equivalents (GAE) per 100 mL juice (mg of GAE/100 mL).
Total protein contents were measured according to Bollag et al. (1996). Aliquots (300 μL) of pineapple juice were mixed with 3 mL of Bradford reagent (95% ethanol, 86% phosphoric acid and Coomassie Brilliant Blue G 250). The absorbance was measured at 595 nm using a spectrophotometer (GENESYS 10S UV-Vis, ThermoFisher Scientific). Bovine serum albumin (BSA) was used as a standard. The results were expressed as mg per 100 mL juice (mg/100 mL).
DPPH radical scavenging activity was determined according to Khalaf et al. (2008) with some modifications. Pineapple juice (50 μL) was mixed with 1,950 μL of 60 μM DPPH solution prepared by dissolving DPPH in 95% methanol. The mixture was incubated in the dark for 30 min then the absorbance measured at 517 nm using a microplate reader (Multiskan Go, ThermoFisher Scientific). Trolox was used as a standard, and the results expressed as μmol of Trolox per 100 mL juice (μmol Trolox/100 mL).
Ferric reducing antioxidant power (FRAP) was determined according to Benzie and Strain (1996). Pineapple juice (400 μL) was added to 2.6 mL of FRAP solution prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-S-triazine (TPTZ) in 40 mM hydrochloric acid (HCl) and 20 mM iron (III) chloride (FeCl3) in ratio of 10:1:1 (v/v/v). The mixture was incubated at 37°C for 30 min and measured the absorbance at 595 nm using a microplate reader (Multiskan Go, ThermoFisher Scientific). Ferrous sulfate (FeSO4) was used as a standard. Results were expressed as μmol of FeSO4 per 100 mL juice (μmol FeSO4/100 mL).
All data were analyzed using two-way analysis of variance (ANOVA) (IBM SPSS Statistics 22.0). A two-way ANOVA analysis was performed to estimate the effect of treatment and storage period on juice quality. The data were expressed as means ± standard deviations (SD) of three replications. Significance testing was performed using Duncan's multiple range tests; differences were taken as statistically significant when P < 0.05.
The effect of treatment, storage period, and their interaction on quality of “Nanglae” pineapple juice are shown in Table 1. Treatments and treatment × storage period interaction had a significant effect on all quality attributes, whereas storage period was only statistically significant on pH, titratable acidity, L*, b*, ascorbic acid, total phenolic compounds, total protein content and DPPH.
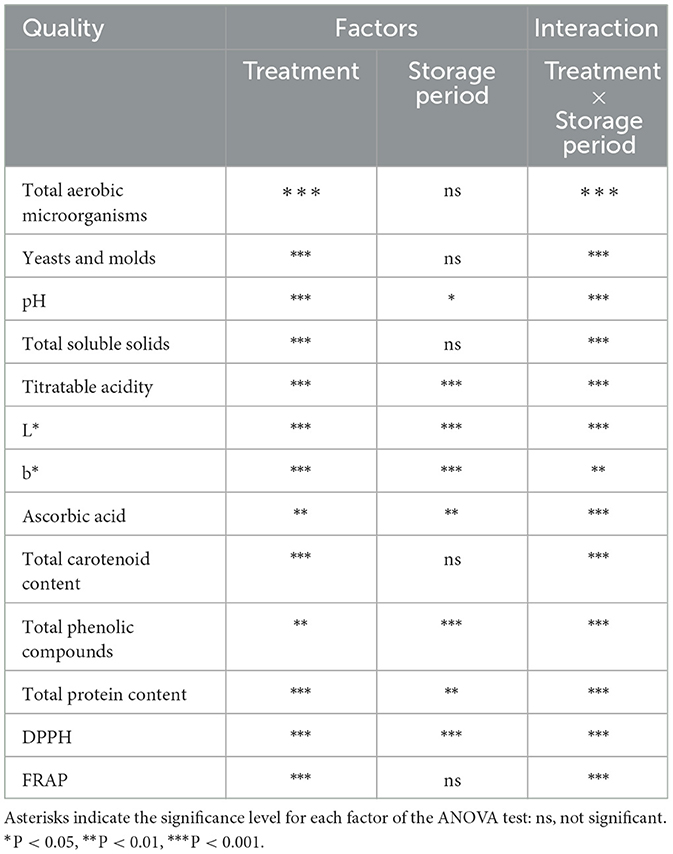
Table 1. Two-way ANOVA analyses of effect of treatments, storage period and their interaction on pineapple juice quality.
Table 2 shows the initial microbial counts, as well as those after CHT and UV-C + HPP treatments during storage. Untreated pineapple juice initially contained 7.22 log CFU/mL of aerobic microorganisms and 5.24 log CFU/mL for yeasts and molds. Both CHT and UV-C + HPP treatments reduced numbers of aerobic microorganisms to <1.48 log CFU/mL after treatment and for the entire 91-day storage period. Throughout the storage period, the yeast and mold counts in juice treated with UV-C + HPP were <1.18 log CFU/mL. Juice treated with CHT, on the other hand, contained yeasts and molds with viable cells exceeding 1.18 log CFU/mL after 63 days of storage. The increase in yeast and mold counts could be explained by heat resistance of fungi and yeasts as suggested by Shearer et al. (2002). Another possibility is that fruits with low pH and high simple carbohydrate components are ideal substrates for fungal growth, making the juice more susceptible to fungal rather than bacterial contamination (Moss, 2008). Molds, such as Byssochlamys fulva and Neosartorya fischeri, can withstand high pasteurization temperatures, low pH concentrations of about 3–4.5, and low oxygen tension in order to produce pectinolytic enzymes that affect juice stability during storage (Tournas et al., 2006). This study revealed that UV-C combined with HPP was comparable to CHT for preserving pineapple juice by inactivating aerobic microorganisms for 91 days of storage. For yeast and mold, however, the combined treatments outperformed the CHT by keeping yeast and mold counts below the quantification limit of 1.18 log CFU/mL for 91 days, whereas the CHT only did so for 63 days. Both technologies are well known for their ability to retard the growth of yeasts and molds. UV-C irradiation kills microorganisms by altering the chemical structure of DNA chains, causing cellular damage (Chang et al., 1985). As a result, cyclobutane pyrimidine dimers are produced, causing DNA molecule distortion, which may result in cell replication malfunctions and cell death (Dai et al., 2012). Several studies have revealed that fungal cell membrane damage has been observed following HPP treatment, implying that HPP is an important trigger of cell death (Hocking et al., 2006; Varela-Santos et al., 2012; Buerman et al., 2020).
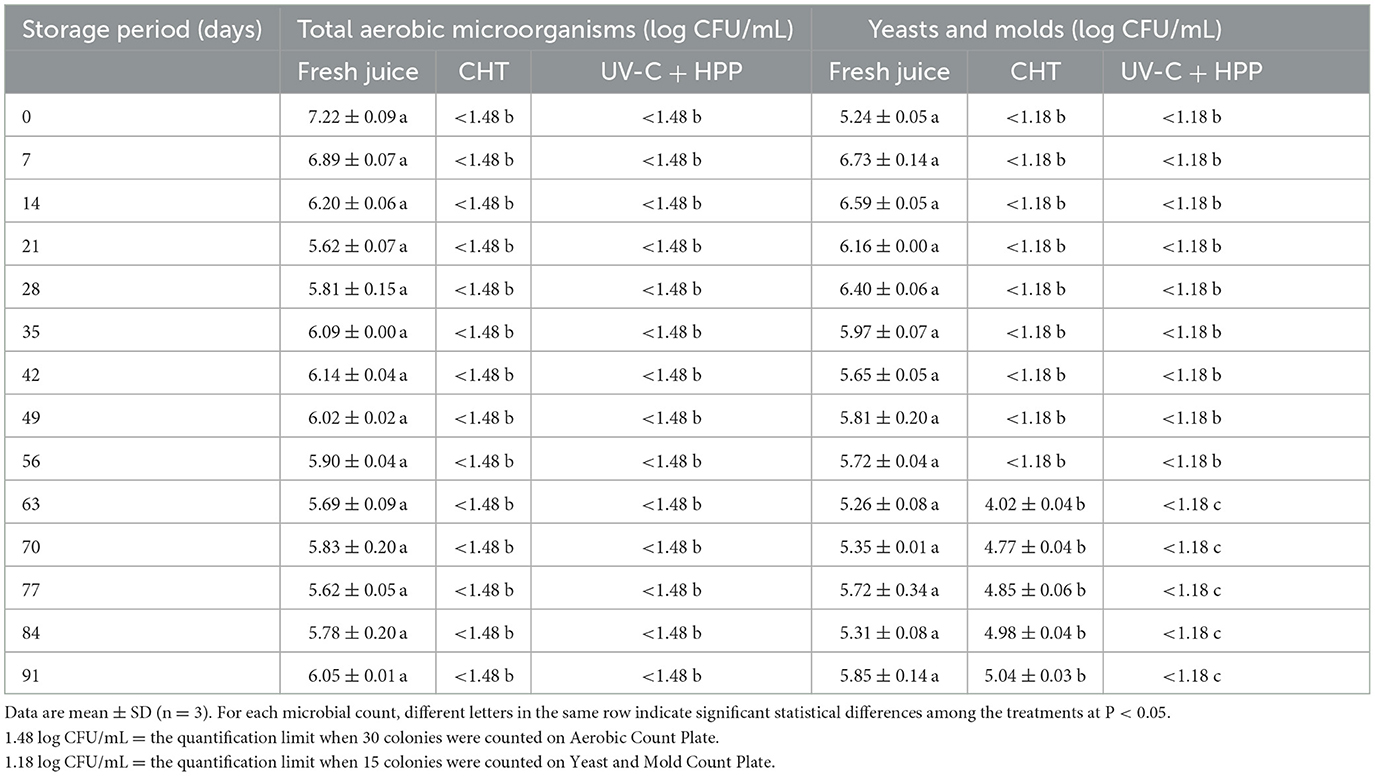
Table 2. Total aerobic microorganisms and total combined yeasts and molds in fresh pineapple juice, CHT-treated juice, and UV-C + HPP treated juice during storage at 5 ± 1°C for 91 days.
The pH of a solution indicates its acidity or alkalinity. Pineapple juice's pH value is determined by its organic acid content, classifying it as acidic food (Cárnara et al., 1995). In this study, the initial pH of fresh juice (untreated control), after CHT and after UV-C + HPP treatments were 4.98, 4.95, and 4.94, respectively (Figure 2). During the first 28 days of storage, the pH of pineapple juice did not differ between treatments; however, it significantly increased (P < 0.05) in untreated pineapple juice on day 35 of storage and remained significantly higher than those in the CHT and UV-C + HPP treatments until the end of storage. The increased pH in untreated pineapple juice was most likely caused by spoilage microorganisms, such as yeasts, and lactic and propionic acid bacteria, which thrive in acidic juice and adjust the juice pH to suit their growth (Cortés et al., 2008; Lima Tribst et al., 2009). The findings in this study agree with this statement; aerobic bacteria, yeasts, and molds were found in fresh juice, which has a slightly higher pH than CHT and UV-C + HPP treated juice.
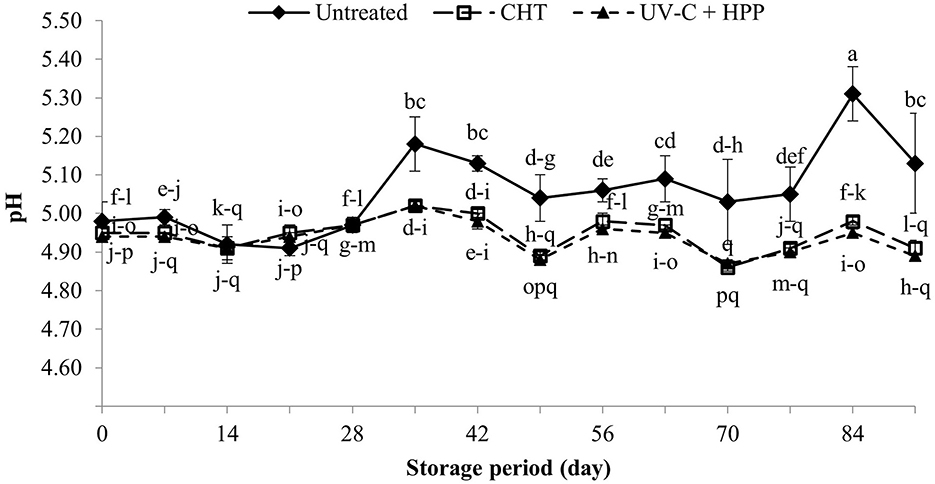
Figure 2. The pH of fresh pineapple juice (untreated), CHT treated juice, and UV-C + HPP treated juice during storage at 5 ± 1°C for 91 days. Data shown are means ± SD of three replications. The different letters are significantly different at P < 0.05.
The main soluble solid in fruit juice is sugar; other soluble solids include organic acid, amino acids, and soluble proteins (Kinhal, 2019). The TSS of pineapple juice after CHT and UV-C + HPP treatments was significantly higher (P < 0.05) than that of fresh pineapple juice (Figure 3). The CHT treatment yielded the highest TSS, which could be due to water evaporation during thermal treatment, resulting in concentrated juice (Tandon et al., 2003). Several studies have revealed that UV-C processing has no effect on the TSS of fruit juices such as apple juice (Caminiti et al., 2012), pineapple juice (Shamsudin et al., 2014), and orange juice (Bazaraa et al., 2022). In this study, however, the increased TSS in the UV-C + HPP treatment could be attributed to high pressure causing increased solubility of insoluble components in the juice because most natural compounds become more soluble under compression (Kaushik et al., 2014). During the storage period, the TSS in CHT and UV-C + HPP treatments did not differ between the initial and the last 91 days of storage, whereas the TSS in the untreated fresh juice was slightly lower at the end of storage compared to that at the initial day. The reduction in TSS during storage might be due to microorganisms using soluble solids for their activities (Wu et al., 2021). The microbial counts revealed in our study were consistent with this statement, in which decreased TSS with increased microbial populations during storage was observed in fresh juice.
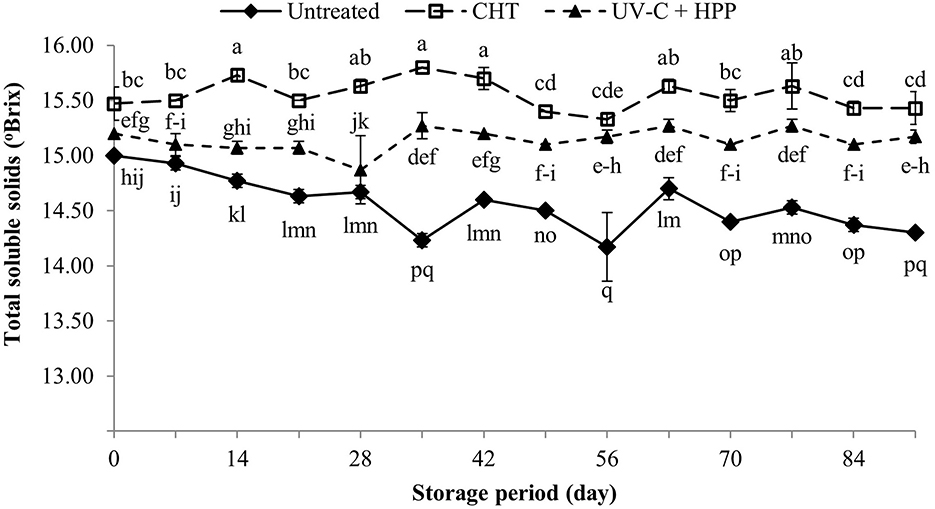
Figure 3. Total soluble solids of fresh pineapple juice (untreated), CHT treated juice, and UV-C + HPP treated juice during storage at 5 ± 1°C for 91 days. Data shown are means ± SD of three replications. The different letters are significantly different at P < 0.05.
The titratable acidity of juice is determined by organic acids, and the major organic acid in pineapple juice is citric acid. On the initial day, there was no significant difference in the TA of pineapple juice among the treatments (Figure 4). However, when compared with CHT and UV-C + HPP treated pineapple juice on day 21, the TA of untreated pineapple juice was significantly lower (P < 0.05), and it remained significantly lower until the end of storage. The decreasing TA in untreated pineapple juice could be due to microorganisms reducing acidity through the fermentation of organic acids resulting in juice spoilage (Sodeko et al., 1987). The TA of pineapple juice decreased during storage in all treatments. Similarly, a decrease in acidity throughout storage period was also observed in mandarin juice (Pareek et al., 2011), apple juice (Hameed et al., 2019), and Kinnow mandarin juice (Singh et al., 2009).
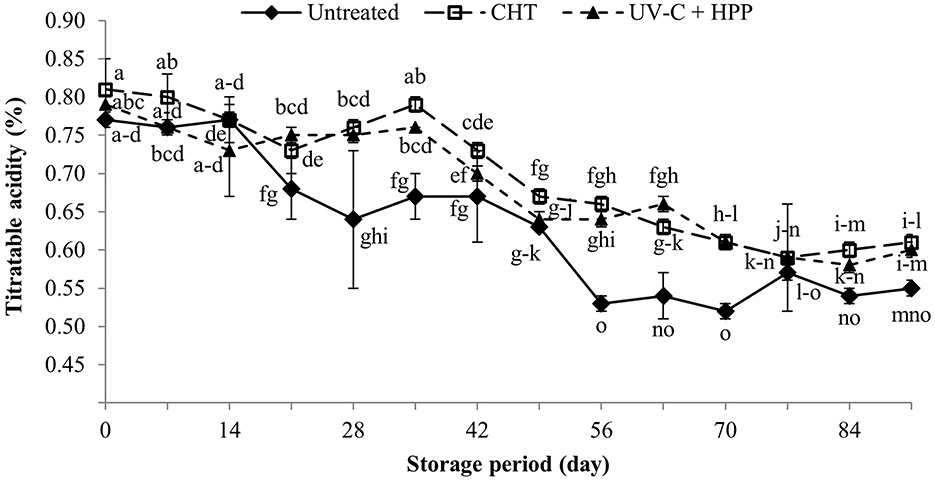
Figure 4. Titratable acidity of fresh pineapple juice (untreated), CHT treated juice, and UV-C + HPP treated juice during storage at 5 ± 1°C for 91 days. Data shown are means ± SD of three replications. The different letters are significantly different at P < 0.05.
Figure 5 depicts the color changes in “Nanglae” pineapple juice during storage at 5 ± 1°C for 91 days. On the initial day, there was no difference between the treatments; however, only the CHT treatment developed separation between clear juice and sediment on day 7 of storage. After 60 days of storage, the color of pineapple juice in all treatments was darker than those of the initial day. The presence of bubbles in untreated pineapple juice indicates that the juice has fermented. The color of pineapple juice was measured using the L* (lightness) (Figure 6A) and b* (yellow-blue) (Figure 6B) values. The L* value of CHT pineapple juice increased significantly (P < 0.05) after treatment compared to UV-C + HPP treated juice. The increase in the L* value after heat treatment indicates transparent juice as a result of the degradation of enzymes, proteins, colorants, and pigments (Umair et al., 2022). For instance, the loss of carotenoid pigment following heat treatment results in lighter juice (Aghajanzadeh et al., 2021), which is supported by the findings of this study. The L* value in all samples decreased slightly during storage. Similarly, a decrease in L* value was found in orange juice (Wibowo et al., 2015). The decreasing L* value indicates that the color of pineapple juice darkens with storage, most likely due to browning of the juice (Roig et al., 1999). There was no difference in b* between juice given the CHT and UV-C + HPP treatments, but there was a significant difference (P < 0.05) between treated and untreated pineapple juice. The untreated juice may have a higher b* value because the pigment is affected by heat and UV-C, as well as the precipitation of insoluble particles (Zhao, 2007).

Figure 5. Appearance of fresh pineapple juice (untreated), CHT treated juice, and UV-C + HPP treated juice during storage at 5 ± 1°C for 91 days: (A) on the initial day, (B) after 7 days, (C) after 35 days, and (D) after 91 days.
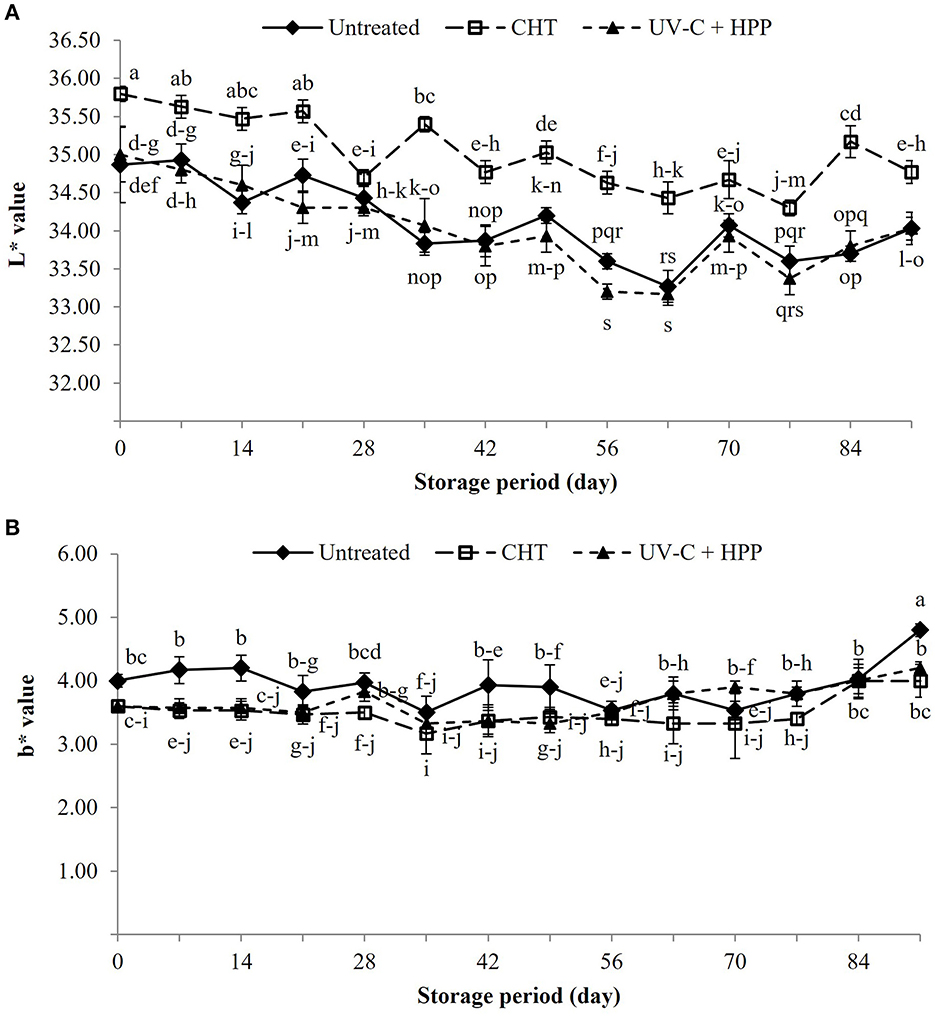
Figure 6. Color as measured by (A) L* and (B) b* values of fresh pineapple juice (untreated), CHT treated juice, and UV-C + HPP treated juice during storage at 5 ± 1°C for 91 days. Data shown are means ± SD of three replications. The different letters are significantly different at P < 0.05.
Ascorbic acid, also known as vitamin C, is a nutrient that is beneficial to humans. It is a water-soluble antioxidant that helps to protect compounds from free radicals in the extracellular and intracellular spaces of biological systems (Kaur and Kapoor, 2001). Changes in ascorbic acid content in food processing can indicate the quality of food after processing and during storage (Tewari et al., 2017). Ascorbic acid is a heat-sensitive compound that degrades after being heated due to heat-induced oxidation (Hounhouigan et al., 2014). In this study, the ascorbic acid content of juice was reduced by 64% after CHT (1.77 mg/100 mL) and by the UV-C + HPP treatment (1.77 mg/100 mL) compared to untreated fresh juice (4.91 mg/100 mL) (Figure 7). There was no difference between CHT and UV-C + HPP treatment in ascorbic content in the juice from the initial day until the end of the 91-day storage period. In contrast, the initial high ascorbic acid content in the untreated control rapidly decreased and remained at the same level of ascorbic content in the CHT and UV-C + HPP treatments on day 28 until the end of storage. Because ascorbic acid is a strong absorber of UV-C light, it appears normal that fruit juice with a high ascorbic content loses a significant amount of its original content after being exposed to UV-C light (Abdul Karim Shah et al., 2016), as observed in several fruit juices, including grape juice (Chia et al., 2012; Shamsudin et al., 2014), “King David” apple juice (Falguera et al., 2011), and pineapple juice (Goh et al., 2012). In addition, the degradation of ascorbic acid following UV-C treatment is dose dependent, with much greater degradation occurring with higher UV-C doses applied (Koutchma et al., 2016). Following HPP treatment, the soluble oxygen concentration in the juice increases, resulting in rapid ascorbic acid degradation (Kimura et al., 1994; Oey et al., 2006). However, ascorbic acid in fruit juice after high-pressure treatment barely degrades. In most cases, the HPP treatment preserved ascorbic acid content, such as in “Navel” orange juice (De Ancos et al., 2020), aloe vera-litchi mixed beverage (Swami Hulle and Rao, 2015), and apple juice (Kim et al., 2012). In a previous study, we found that HPP-treated pineapple juice retained more ascorbic acid than heat-treated juice (Chuensombat et al., 2019). In this study, the decrease in ascorbic acid after UV-C + HPP treatment could be presumably due to the UV-C light sensitivity of ascorbic acid.
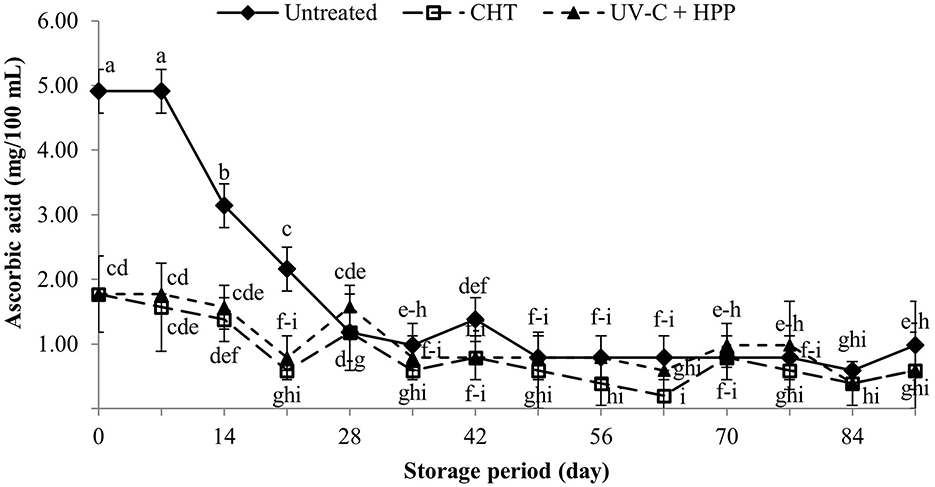
Figure 7. Ascorbic acid of fresh pineapple juice (untreated), CHT treated juice, and UV-C + HPP treated juice during storage at 5 ± 1°C for 91 days. Data shown are means ± SD of three replications. The different letters are significantly different at P < 0.05.
The carotenoid content of pineapple flesh is higher than that of pineapple juice. The primary carotenoids in pineapple juice are violaxanthin (50%), leuteoxanthin (8%), β-carotene (9%), and neoxanthin (8%) (Morgan, 1966). In this study, the initial carotenoid content in UV-C + HPP treated pineapple juice (0.51 mg/100 mL) was significantly higher (P < 0.05) than in CHT (0.34 mg/100 mL) and remained higher until the end of the 91-day storage period (Figure 8). Furthermore, CHT significantly decreased (P < 0.05) carotenoids in juice compared to the untreated control. During thermal processing, heat causes a significant loss of carotenoids. The chain of conjugated double bonds in carotenoids is responsible for their susceptibility to degradation under conditions such as high temperature, light, and oxygen (Khalil et al., 2015). The increased carotenoid contents in the UV-C + HPP treatment could be attributed to the UV-C treatment. The carotenoid content of an orange, carrot and celery juice blend also increased following UV-C treatment (Nornisa et al., 2018). UV-C acts as abiotic stress and may induce the production of reactive oxygen species (ROS) (Kovács and Keresztes, 2002). As a result, non-enzymatic antioxidants such as glutathione (GSH), phenolics, and carotenoids are activated to overcome the excess ROS (Nyathi and Baker, 2006).
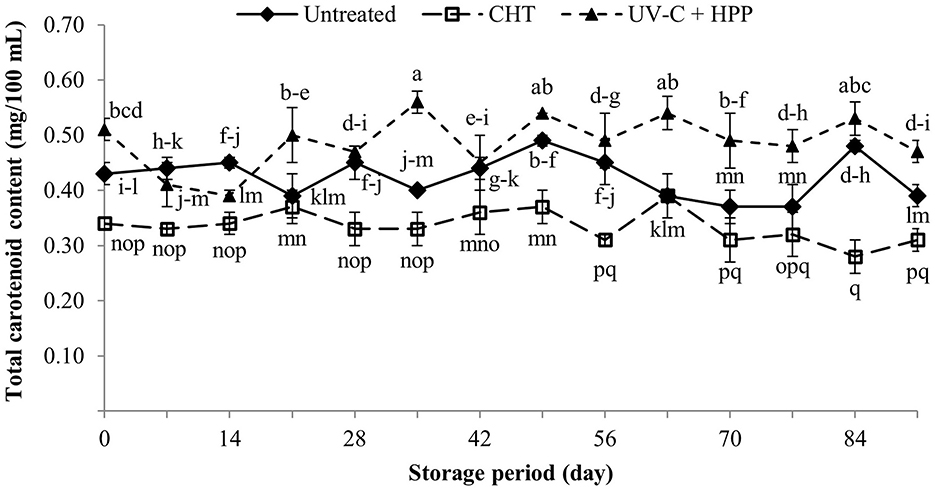
Figure 8. Total carotenoid content of fresh pineapple juice (untreated), CHT treated juice, and UV-C + HPP treated juice during storage at 5 ± 1°C for 91 days. Data shown are means ± SD of three replications. The different letters are significantly different at P < 0.05.
Phenolic compounds are plant secondary metabolites that play an important role in the color and flavor development of fruit juice and wine (Aguilar-Rosas et al., 2007). Furthermore, phenolic compounds are natural antioxidants that aid in the neutralization of free radicals; however, they decline over time. Figure 9 depicts the changes in total phenolic compounds of CHT and UV-C + HPP treated pineapple juice during storage at 5 ± 1°C for 91 days. TPC levels in the UV-C + HPP treatment were not different from those in the untreated control, though they were higher than those in the CHT on the initial day following treatment. During the entire storage period, however, pineapple juice treated with UV-C + HPP contained significantly less (P < 0.05) TPC than CHT and control. The higher TPC in CHT-treated pineapple juice could be attributed to the inactivation of the enzyme that degrades phenolic compounds (Odriozola-Serrano et al., 2008). However, UV-C treatment combined with other processing techniques has improved the retention of phenolic compounds in fruit juice. According to Sew et al. (2014), combining UV-C and mild heat treatments preserved the total phenol content in pineapple juice. While combined treatments of UV-C and sonication retained phenolic compounds in mango juice (Santhirasegaram et al., 2015). More research is needed to determine whether the combined treatments work synergistically or antagonistically.
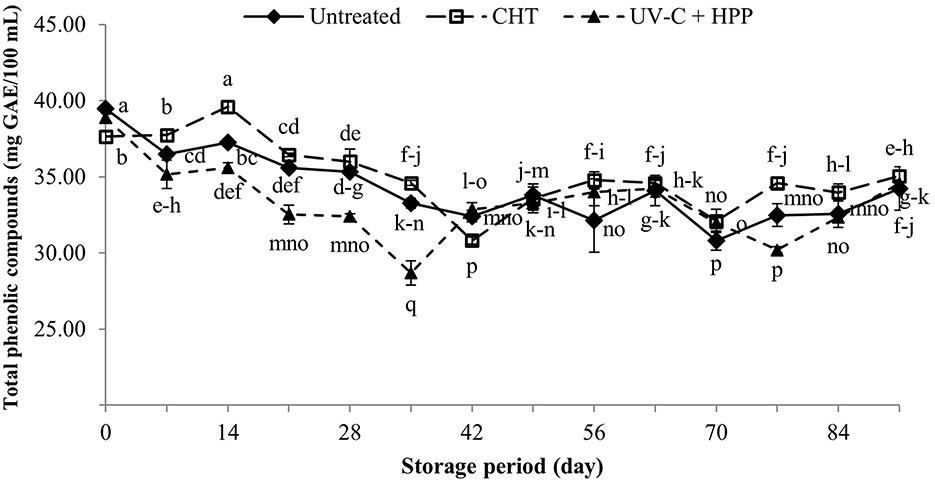
Figure 9. Total phenolic compounds of fresh pineapple juice (untreated), CHT treated juice, and UV-C + HPP treated juice during storage at 5 ± 1°C for 91 days. Data shown are means ± SD of three replications. The different letters are significantly different at P < 0.05.
Pineapple contains proteolytic enzymes called bromelains that account for nearly half of the protein in pineapple (Smith, 2003). Over the storage period, pineapple juice treated with UV-C + HPP had significantly higher protein contents (P < 0.05) than CHT-treated pineapple juice (Figure 10). However, the protein content of untreated pineapple juice was the highest (15.00 mg/100 mL), followed by UV-C + HPP treated pineapple juice (13.56 mg/100 mL) and CHT-treated juice (5.73 mg/100 mL). This indicates that there was a significant loss of protein in pineapple juice after heat treatment. The protein losses in CHT pineapple juice can be described as high-temperature hydrogen bond breakdown and protein structure unfolding with loss of activity (Koizumi et al., 2007). Protein denaturation in UV-C + HPP could be attributed to the pressure sensitivity of high molecular weight components such as protein (Chandra et al., 2021), causing the destabilization of noncovalent bonds in the tertiary structure, particularly hydrophobic and ionic bonds (Chapleau et al., 2004). Despite the fact that protein loss occurred in the pineapple juice immediately following the UV-C + HPP treatment, its level remained close to that of fresh juice throughout the storage period.
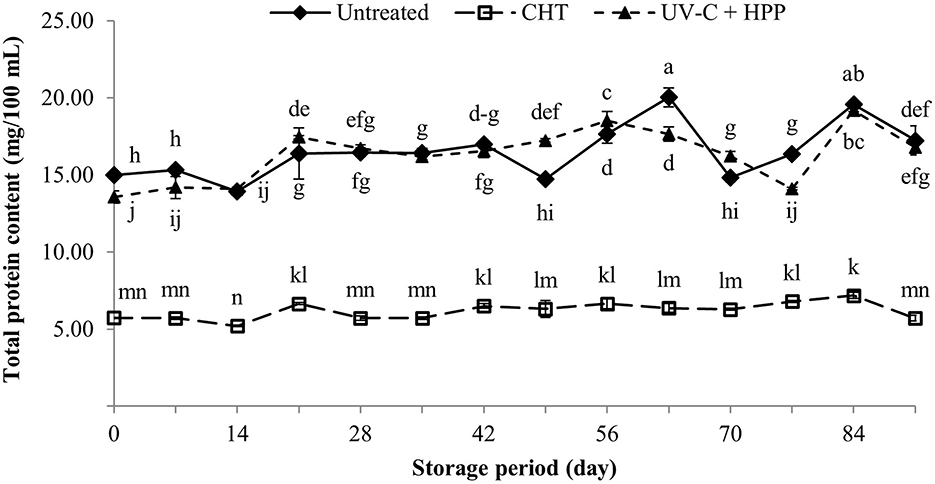
Figure 10. Total protein content of fresh pineapple juice (untreated), CHT treated juice, and UV-C + HPP treated juice during storage at 5 ± 1°C for 91 days. Data shown are means ± SD of three replications. The different letters are significantly different at P < 0.05.
The antioxidant activity is related to antioxidant phytochemicals, with phenolic compounds serving as the primary antioxidant and was determined by the reduction of DPPH and FRAP (Figure 11). The DPPH activity of all treatments remained stable after treatment until day 42 of storage before rapidly decreasing on day 49. The DPPH value of pineapple juice did not differ between the CHT and UV-C + HPP treatments, but it was significantly lower (P < 0.05) than the untreated juice. After 91 days of storage, DPPH was decreased by 8.0% in UV-C + HPP treated juice (125.5 μmol Trolox/100 mL) and 6.3% in CHT treated juice (127.8 μmol Trolox/100 mL) when compared to fresh juice (136.4 μmol Trolox/100 mL). Similarly, juice treated with UV-C + HPP had the lowest FRAP value compared to CHT and fresh juice throughout the storage period, except on day 91 where FRAP value was reduced by 10.1% in the UV-C + HPP treatment (617.5 μmol FeSO4/100 mL) and 22.8% in the CHT (530.0 μmol FeSO4/100 mL) as compared to fresh juice (686.7 μmol FeSO4/100 mL). These findings were consistent with lower levels of TPC and ascorbic acid in the pineapple juice in both the CHT and UV-C + HPP treatments compared to untreated fresh juice.
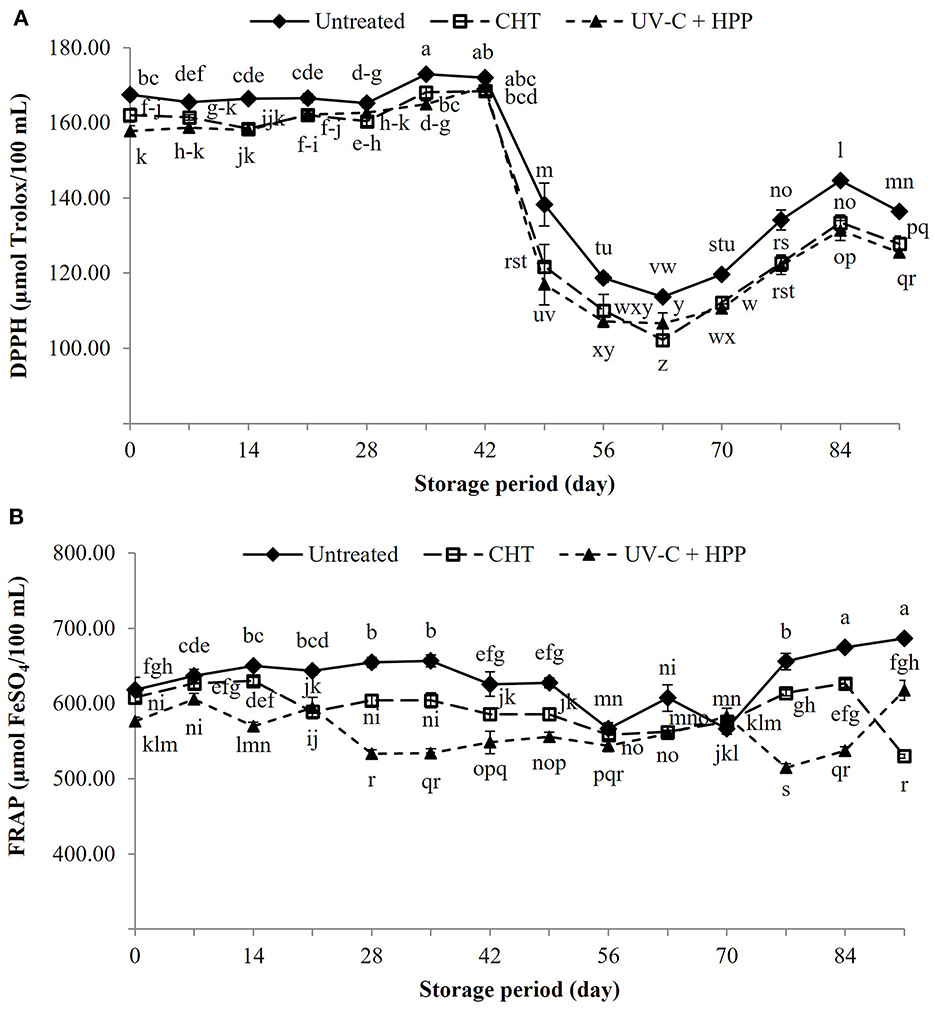
Figure 11. Antioxidant activity as measured by (A) DPPH and (B) FRAP of fresh pineapple juice (untreated), CHT treated juice, and UV-C + HPP treated juice during storage at 5 ± 1°C for 91 days. Data shown are means ± SD of three replications. The different letters are significantly different at P < 0.05.
Although, the use of UV-C in combination with HPP treatment has not been reported in any fruit juice, the effect of UV-C irradiation or HPP treatment on antioxidant activity in fruit juices has been controversial. Fruit juice treated with HPP had either an increase or a decrease in antioxidant capacity. For instance, HPP treatment reduced DPPH and FRAP values in Korla pear juice (500 MPa, 10 min), red grapefruit juice (550 MPa, 10 min), and orange juice (550 MPa, 70 sec) (Gao et al., 2015; Zhao et al., 2016; Vieira et al., 2018); while apple juice (300–600 MPa, 5 min), apple juice with Sabah Snake Grass leaves (300–500 MPa, 5 min) and strawberry-apple-lemon juice (500 MPa, 15 min) showed an increase in these values (Feng et al., 2020; Hasni et al., 2020; Szczepanska et al., 2021). In some cases, such as mulberry juice (Zou et al., 2016) and pitaya juice (Quiroz-González et al., 2020), HPP had no effect on antioxidant activity. Similarly, UV-C treatment did not affect antioxidant capacity in some fruit juices, including an apple-cranberry blend, grape juice, and carrot juice (Caminiti et al., 2011; Pala and Toklucu, 2013a; Hernández-Carranza et al., 2016). Mango and starfruit juice, on the other hand, showed an increase in antioxidant capacity after UV-C treatment (Bhat et al., 2011; Santhirasegaram et al., 2015), whereas apple and orange juice showed a decrease (Caminiti et al., 2012; Pala and Toklucu, 2013b). Thus, the optimal condition of the combined UV-C and HPP treatment should be studied further in order to boost the nutritional level of the juice.
The combination of UV-C (3 kJ/m2) and high-pressure processing (600 MPa for 5 min) treatments demonstrated its potential to preserve the quality of “Nanglae” pineapple juice. The combined treatments effectively reduced the microbial loads in pineapple juice similarly to conventional heat treatment and retained carotenoids and protein contents close to those in the fresh juice throughout the 91-day storage period. The juice quality in terms of pH, total soluble solids, titratable acidity, ascorbic acid, total phenolic compounds, and antioxidant capacity did not differ between conventional heat treatment and the combined UV-C and HPP treatments. This study suggests that UV-C and HPP may have a synergistic effect on microbial inactivation while having less impact on quality than conventional heat treatment. However, more research into the optimal conditions for microorganism inactivation while retaining nutritional and sensory quality is recommended. Consequently, the combination of these two technologies may eventually replace traditional heat treatment.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
PS, NC, SS, and MN designed and conducted the experiments and analyzed the data. PS, NC, and MN prepared the first draft of the manuscript. SS and MN critically reviewed and corrected the English. All authors have read and approved the manuscript before submission.
The authors would like to thank Mae Fah Luang University, Chiang Rai, Thailand, for providing research facilities, and Prof. Dr. Paul Holford from Western Sydney University, Australia, for proofreading and editing this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdul Karim Shah, N. N., Shamsudin, R., Abdul Rahman, R., and Adzahan, N. M. (2016). Fruit juice production using ultraviolet pasteurization: a review. Beverages. 2, 22. doi: 10.3390/beverages2030022
Abera, G. (2019). Review on high-pressure processing of foods. Cogent Food Agri. 5, 1568725. doi: 10.1080/23311932.2019.1568725
Aghajanzadeh, S., Ziaiifar, A. M., and Verkerk, R. (2021). Effect of thermal and non-thermal treatments on the color of citrus juice: a review. Food Rev. Int. 1–23. doi: 10.1080/87559129.2021.2012799
Aguilar-Rosas, S., Ballinas-Casarrubias, M., Nevarez-Moorillon, G., Martin-Belloso, O., and Ortega-Rivas, E. (2007). Thermal and pulsed electric fields pasteurization of apple juice: Effects on physicochemical properties and flavour compounds. J. Food Eng., 83, 41–46. doi: 10.1016/j.jfoodeng.2006.12.011
AOAC (2000). Official Methods of Analysis. (17th Eds.). Arlington, VA., USA: Association of Official Analytical Chemistry.
AOAC (2000). Official Methods of Analysis of Association of Official Analytical Chemists (17th ed.). Gaithersburg, MD, USA: AOACInternational.
Balasubramaniam, V. M., Farkas, D., and Turek, E. J. (2008). Preserving foods through high-pressure processing. Available online at: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2008/november/features/preserving-foods-through-high-pressure-processing (accessed 3 April, 2022).
Bazaraa, W. A., Eissa, H. A., Helmy, S. A., Ramadan, M. T., and Aboelhaggag, R. M. (2022). Effect of ultra violet (UV-C) and cold storage on orange juice quality. Food Sci. Technol. Int. 0, 10820132221117750. doi: 10.1177/10820132221117750
Benzie, I. F., and Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239, 70–76. doi: 10.1006/abio.1996.0292
Bhat, R., Ameran, S. A., Ching Voon, H., Karim, A. A., and Min Tze, L. (2011). Quality attributes of starfruit (Averrhoa carambola L.) juice treated with ultraviolet radiation. Food Chem., 127, 641–644. doi: 10.1016/j.foodchem.2011.01.042
Bollag, D. M., Rozycki, M. D., and Edelstein, S. J. (1996). Protein Concentration Determination Protein Methods, (2nd ed.). p. 107–154. New York: Wiley- Liss Inc.
Buerman, E. C., Worobo, R. W., and Padilla-Zakour, O. I. (2020). High pressure processing of spoilage fungi as affected by water activity in a diluted apple juice concentrate. Food Control. 107, 106779. doi: 10.1016/j.foodcont.2019.106779
Caminiti, I. M., Noci, F., Munoz, A., Whyte, P., Morgan, D. J., Cronin, D. A., et al. (2011). Impact of selected combinations of non-thermal processing technologies on the quality of an apple and cranberry juice blend. Food Chem. 124, 1387–1392. doi: 10.1016/j.foodchem.2010.07.096
Caminiti, I. M., Palgan, I., Muñoz, A., Noci, F., Whyte, P., Morgan, D. J., et al. (2012). The effect of ultraviolet light on microbial inactivation and quality attributes of apple juice. Food Bioprocess Technol., 5, 680–686. doi: 10.1007/s11947-010-0365-x
Cárnara, M., Díez, C., and Torija, E. (1995). Chemical characterization of pineapple juices and nectars. Principal components analysis. Food Chem. 54, 93–100. doi: 10.1016/0308-8146(95)92668-A
Chandra, R. D., Prihastyanti, M. N. U., and Lukitasari, D. M. (2021). Effects of pH, high pressure processing, and ultraviolet light on carotenoids, chlorophylls, and anthocyanins of fresh fruit and vegetable juices. eFood. 2, 113–124. doi: 10.2991/efood.k.210630.001
Chang, J. C., Ossoff, S. F., Lobe, D. C., Dorfman, M. H., Dumais, C. M., Qualls, R. G., et al. (1985). UV inactivation of pathogenic and indicator microorganisms. Appl. Environ. Microbiol. 49, 1361–1365. doi: 10.1128/aem.49.6.1361-1365.1985
Chapleau, N., Mangavel, C., Compoint, J. P., and de Lamballerie-Anton, M. (2004). Effect of high-pressure processing on myofibrillar protein structure. J. Sci. Food Agric. 84, 66–74. doi: 10.1002/jsfa.1613
Charles, M. T., Goullet, A., and Arul, J. (2008). Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit IV. Biochemical modification of structural barriers. Postharvest Biol. Technol., 47, 41–53. doi: 10.1016/j.postharvbio.2007.05.019
Chia, S. L., Rosnah, S., Noranizan, M. A., and Wan Ramli, W. D. (2012). The effect of storage on the quality attributes of ultraviolet-irradiated and thermally pasteurised pineapple juices. Int. Food Res. J. 19, 1001–1010.
Chuensombat, N., Rungraeng, N., Setha, S., and Suthiluk, S. (2019). A preliminary study of high pressure processing effect on quality changes in “Nanglae” pineapple juice during cold storage. Journal of Food Science and Agricultural Technology. Spcl Iss. 5, 13–18.
Cortés, C., Esteve, M. J., and Frígola, A. (2008). Color of orange juice treated by high intensity pulsed electric fields during refrigerated storage and comparison with pasteurized juice. Food Control. 19, 151–158. doi: 10.1016/j.foodcont.2007.03.001
Dai, T., Vrahas, M. S., Murray, C. K., and Hamblin, M. R. (2012). Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev Anti Infect Ther. 10, 185–195. doi: 10.1586/eri.11.166
De Ancos, B., Rodrigo, M. J., Sánchez-Moreno, C., Pilar Cano, M., and Zacarías, L. (2020). Effect of high-pressure processing applied as pretreatment on carotenoids, flavonoids and vitamin C in juice of the sweet oranges ‘Navel' and the red-fleshed ‘Cara Cara'. Food Res Int. 132, 109105. doi: 10.1016/j.foodres.2020.109105
Deak, T. (2014). “Thermal treatment,” in Food Safety Management. Cambridge, MA: Academic Press. p. 423–442. doi: 10.1016/B978-0-12-381504-0.00017-2
Desobry, S. A., Netto, F. M., and Labuza, T. P. (1997). Comparison of spray-drying, drum-drying and freeze-drying for β-carotene encapsulation and preservation. J. Food Sci. 62, 1158–1162. doi: 10.1111/j.1365-2621.1997.tb12235.x
Falguera, V., Pagán, J., and Ibarz, A. (2011). Effect of UV-C irradiation on enzymatic activities and physicochemical properties of apple juices from different varieties. LWT Food Sci. Tech. 44, 115–119. doi: 10.1016/j.lwt.2010.05.028
Feng, X., Zhou, Z., Wang, X., Bi, X., Ma, Y., and Xing, Y. (2020). Comparison of high hydrostatic pressure, ultrasound, and heat treatments on the quality of strawberry–apple–lemon juice blend. Foods. 9, 218. doi: 10.3390/foods9020218
Ferreira, E. A., Siqueira, H. E., Boas, E. V. V., Hermes, V. S., and Rios, A. D. O. (2016). Bioactive compounds and antioxidant activity of pineapple fruit of different cultivars. Rev. Bras. Frutic. 38. doi: 10.1590/0100-29452016146
Food and Drug Administration Thailand. (2019). General criteria for high-pressure pasteurization [High-Pressure Processing (HPP)]. Available online at: https://www.fda.moph.go.th/sites/food/Permission/4.9_HPP.pdf (accessed 12 August, 2022).
Gamlath, S., and Wakeling, L. (2011). “Trends in high pressure processing of foods: food quality and bioactive components,” in New Topics in Food Engineering, Comeau, M. A. (ed). Hauppauge, N.Y.: Nova Science Publishers. p.121–149.
Gao, G., Zhao, L., Ma, Y., Wang, Y., Sun, Z., and Liao, X. (2015). Microorganisms and some quality of red grapefruit juice affected by high pressure processing and high temperature short time. Food Bioprocess Technol. 8, 2096–2108. doi: 10.1007/s11947-015-1556-2
Goh, S. G., Noranizan, M., Leong, C. M., Sew, C. C., and Sobhi, B. (2012). Effect of thermal and ultraviolet treatments on the stability of antioxidant compounds in single strength pineapple juice throughout refrigerated storage. Int. Food Res. J. 19, 1131–1136.
González-Aguilar, G., Zavaleta-Gatica, R., and Tiznado-Hernández, M. (2007). Improving postharvest quality of mango ‘Haden'by UV-C treatment. Postharvest Biol. Technol. 45, 108–116. doi: 10.1016/j.postharvbio.2007.01.012
Hameed, F., Kumar, A., and Hamid, N. (2019). Effect of thermal treatment and storage on the quality of apple juice. J. Pharmacogn Phytochem. 8, 1976–1979.
Hasni, H. N., Koh, P. C., Noranizan, M. A., Tahir, P. N. F. M. M., Mohamad, A., Limpot, N., et al. (2020). High-pressure processing treatment for ready-to-drink Sabah Snake Grass juice. J. Food Process. Preserv. 44, e14508. doi: 10.1111/jfpp.14508
Hernández-Carranza, P., Ruiz-López, I. I., Pacheco-Aguirre, F. M., Guerrero-Beltrán, J. Á., Ávila-Sosa, R., and Ochoa-Velasco, C. E. (2016). Ultraviolet-C light effect on physicochemical, bioactive, microbiological, and sensorial characteristics of carrot (Daucus carota) beverages. Food Sci. Technol. Int. 22, 536–546. doi: 10.1177/1082013216631646
Hocking, A. D., Begum, M., and Stewart, C. M. (2006). “Inactivation of fruit spoilage yeasts and moulds using high pressure processing.,” in Advances in Food Mycology, Hocking, A. D., Samson, R. A., Thrane, U. (Eds.). New York, NY: Springer. p. 239–246. doi: 10.1007/0-387-28391-9_16
Hounhouigan, M. H., Linnemann, A. R., Soumanou, M. M., and Van Boekel, M. A. J. S. (2014). Effect of processing on the quality of pineapple juice. Food Rev. Int. 30, 112–133. doi: 10.1080/87559129.2014.883632
International Organization for Standardization 14502-1. (2005). Determination of Substances Characteristic of Green and Black Tea—Part 1: Content of Total Polyphenols in Tea—Colorimetric Method Using Folin-Ciocalteu Reagent. International Organization for Standardization, Geneva.
Kaur, C., and Kapoor, H. C. (2001). Antioxidants in fruits and vegetables–the millennium's health. Int. J. Food Sci. 36, 703–725. doi: 10.1046/j.1365-2621.2001.00513.x
Kaushik, N., Kaur, B. P., and Rao, P. S. (2014). Application of high pressure processing for shelf life extension of litchi fruits (Litchi chinensis cv. Bombai) during refrigerated storage. Food Sci. Technol. Int. 20, 527–541. doi: 10.1177/1082013213496093
Khalaf, N. A., Shakya, A. K., Al-Othman, A., El-Agbar, Z., and Farah, H. (2008). Antioxidant activity of some common plants. Turk J Biol. 32, 51–55.
Khalil, A. W., Ali, J., Paracha, G. M., Iman, S., and Hassan, S. (2015). Effect of heat treatments on some quality parameters of carrot (Dascus carota L.) juice. World J. Dairy Food Sci. 10, 55–59.
Kim, H., Leem, K. H., Lee, S., Kim, B. Y., Hahm, Y., Cho, H. Y., et al. (2012). Effect of high hydrostatic pressure on immunomodulatory activity of cloudy apple juice. Food Sci Biotechnol. 21, 175–181. doi: 10.1007/s10068-012-0022-4
Kimura, K., Ida, M., Yosida, Y., Ohki, K., Fukumoto, T., and Sakui, N. (1994). Comparison of keeping quality between pressure-processed jam and heat-processed jam: changes in flavor components, hue, and nutrients during storage. Biosci. Biotechnol. Biochem. 58, 1386–1391. doi: 10.1271/bbb.58.1386
Kinhal, V. (2019). Brix as a Metric of Fruit Maturity. Available online at: https://felixinstruments.com/blog/brix-as-a-metric-of-fruit-maturity/ (accessed 9 March, 2022).
Knorr, D. (1993). Effect of high-hydrostatic-pressure processes on food safety and quality. Food Technol. 47, 156–161.
Koizumi, M., Hirai, H., Onai, T., Inoue, K., and Hirai, M. (2007). Collapse of the hydration shell of a protein prior to thermal unfolding. J. Appl. Crystallogr. 40, 175–178. doi: 10.1107/S0021889807003354
Kongsuwan, A., Suthiluk, P., Theppakorn, T., Srilaong, V., and Setha, S. (2009). Bioactive compounds and antioxidant capacities of Phulae and Nanglae pineapple. As. J. Food Ag-Ind. S44–S50.
Koutchma, T., Popović, V., Ros-Polski, V., and Popielarz, A. (2016). Effects of ultraviolet light and high-pressure processing on quality and health-related constituents of fresh juice products. Compr. Rev. Food Sci. Food Saf. 15, 844–867. doi: 10.1111/1541-4337.12214
Kovács, E., and Keresztes, Á. (2002). Effect of gamma and UV-B/C radiation on plant cells. Micron. 33, 199–210. doi: 10.1016/S0968-4328(01)00012-9
Lima Tribst, A. A., de Souza Sant'Ana, A., and de Massaguer, P. R. (2009). Microbiological quality and safety of fruit juices—past, present and future perspectives. Crit. Rev. Microbiol. 35, 310–339. doi: 10.3109/10408410903241428
Mohamed, N. T. S., Ding, P., Kadir, J., and Ghazali, H. M. (2017). Potential of UVC germicidal irradiation in suppressing crown rot disease, retaining postharvest quality and antioxidant capacity of Musa AAA “Berangan” during fruit ripening. Food Sci. Nutr. 5, 967–980. doi: 10.1002/fsn3.482
Morgan, R. (1966). Chemical studies on concentrated pineapple juice 1. Carotenoid composition of fresh pineapples. J. Food Sci. 31, 213–217. doi: 10.1111/j.1365-2621.1966.tb00482.x
Moss, M. O. (2008). Fungi, quality and safety issues in fresh fruits and vegetables. J. Appl. Microbiol. 104, 1239–1243. doi: 10.1111/j.1365-2672.2007.03705.x
Nornisa, N., Somasundram, C., and Razali, Z. (2018). The effects of UV-C treatment on the quality of orange, carrot and celery juice blend. J Food Sci Nutr. 1, 1–7. doi: 10.35841/food-science.1.3.1-7
Nyathi, Y., and Baker, A. (2006). Plant peroxisomes as a source of signalling molecules. Biochim. Biophys. Acta - Mol. Cell Res. 1763, 1478–1495. doi: 10.1016/j.bbamcr.2006.08.031
Odriozola-Serrano, I., Soliva-Fortuny, R., and Martín-Belloso, O. (2008). Changes of health-related compounds throughout cold storage of tomato juice stabilized by thermal or high intensity pulsed electric field treatments. Innov. Food Sci. Emerg. Technol. 9, 272–279. doi: 10.1016/j.ifset.2007.07.009
Oey, I., Verlinde, P., Hendrickx, M., and Loey, A. (2006). Temperature and pressure stability of L-ascorbic acid and/or [6s] 5-methyltetrahydrofolic acid: a kinetic study. Eur. Food Res. Technol. 223, 71–77. doi: 10.1007/s00217-005-0123-x
Pala, C., and Toklucu, A. K. (2013a). Effect of UV-C light processing on some quality characteristics of grape juices. Food Bioprocess Technol. 6, 719–725. doi: 10.1007/s11947-012-0808-7
Pala C. Toklucu A. K. (2013b), Physicochemical and ensory properties of UV-C processed orange juice its microbial stability during refrigerated storage. LWT-Food Sci. Technol. 50, 426–341. 10.1016/j.lwt.2012.09.001
Pareek, S., Paliwal, R., and Subrata, M. (2011). Effect of juice extraction methods and processing temperature-time on juice quality of Nagpur mandarin (Citrus reticulata Blanco) during storage. J. Food Sci. Technol. 48, 197–203. doi: 10.1007/s13197-010-0154-6
Perkins-Veazie, P., Collins, J. K., and Howard, L. (2008). Blueberry fruit response to postharvest application of ultraviolet radiation. Postharvest Biol. Technol. 47, 280–285. doi: 10.1016/j.postharvbio.2007.08.002
Quiroz-González, B., Rodríguez-Martínez, V., Welti-Chanes, J., del García-Mateos, M. R., Corrales-García, J., Ybarra-Moncada, M. C., et al. (2020). Refrigerated storage of high hydrostatic pressure (HHP) treated pitaya (Stenocereus pruinosus) juice. Rev. Mex. Ing. Quim. 19, 387–399. doi: 10.24275/rmiq/Alim588
Rattanathanalerk, M., Chiewchan, N., and Srichumpoung, W. (2005). Effect of thermal processing on the quality loss of pineapple juice. J. Food Eng. 66, 259–265. doi: 10.1016/j.jfoodeng.2004.03.016
Roig, M., Bello, J., Rivera, Z., and Kennedy, J. (1999). Studies on the occurrence of non-enzymatic browning during storage of citrus juice. Int. Food Res. J. 32, 609–619. doi: 10.1016/S0963-9969(99)00128-3
Romanazzi, G., Mlikota Gabler, F., and Smilanick, J. L. (2006). Preharvest chitosan and postharvest UV irradiation treatments suppress gray mold of table grapes. Plant Dis. 90, 445–450. doi: 10.1094/PD-90-0445
Sanchez-Moreno, C., Plaza, L., De Ancos, B., and Cano, M. P. (2006). Impact of high pressure and traditional thermal processing of tomato puree on carotenoids, vitamin C and antioxidant activity. J.Sci. Food Agric. 86, 171–179. doi: 10.1002/jsfa.2321
Sanchez-Moreno, C., Plaza, L., Martinez, P., De Ancos, B., Martin-Belloso, O., and Cano, M. P. (2005). Impact of high pressure and pulsed electric fields on bioactive compounds and antioxidant activity of orange juice in comparison with traditional thermal processing. J. Agric. Food Chem. 53, 4403–4409. doi: 10.1021/jf048839b
Santhirasegaram, V., Razali, Z., George, D. S., and Chandran, S. (2015). Effect of thermal and non-thermal processing on phenolic compounds, antioxidant activity and sensory attributes of Chokanan mango (Mangifera indica L.) juice. Food Bioprocess Technol. 8, 2256–2267. doi: 10.1007/s11947-015-1576-y
Sari, L. K., Setha, S., and Naradisorn, M. (2016). Effect of UV-C irradiation on postharvest quality of ‘Phulae' pineapple. Sci. Hortic. 213, 314–320. doi: 10.1016/j.scienta.2016.09.049
Sew, C. C., Ghazali, H. M., Martin-Belloso, O., and Mohd Adzahan, N. (2014). Effects of combining ultraviolet and mild heat treatments on enzymatic activities and total phenolic contents in pineapple juice. Innov. Food Sci. Emerg. Technol. 26, 511–516. doi: 10.1016/j.ifset.2014.05.008
Shamsudin, R., Noranizan, M. A., Yap, P. Y., and Mansor, A. (2014). Effect of repetitive ultraviolet irradiation on the physico-chemical properties and microbial stability of pineapple juice. Innov. Food Sci. Emerg. Technol. 10, 166–171. doi: 10.1016/j.ifset.2014.02.005
Shearer, A. E., Mazzotta, A. S., Chuyate, R., and Gombas, D. E. (2002). Heat resistance of juice spoilage microorganisms. J. Food Prot. 65, 1271–1275. doi: 10.4315/0362-028X-65.8.1271
Singh, S. V., Jain, R. K., and Gupta, A. K. (2009). Changes in quality of debittered kinnow juice during storage. J. Food Sci. Technol. 46, 598–600.
Smith, L. G. (2003). “Pineapples,” in Encyclopedia of Food Sciences and Nutrition (2nd ed). Caballero, B. (ed.). p. 4567–4575. Oxford: Academic Press. doi: 10.1016/B0-12-227055-X/00928-7
Sodeko, O. O., Izuagbe, Y. S., and Ukhun, M. E. (1987). Effect of different preservative treatments on the microbial population of Nigerian orange juice. Microbios. 51, 133–143.
Swami Hulle, N., and Rao, P. (2015). Effect of high pressure and thermal processing on quality changes of aloe vera-litchi mixed beverage (ALMB) during storage. J. Food Sci. Technol., 53, 359–369. doi: 10.1007/s13197-015-2056-0
Szczepanska, J., Pinto, C. A., Ska , pska, S., Saraiva, J. A., and Marszałek, K. (2021). Effect of static and multi-pulsed high pressure processing on the rheological properties, microbial and physicochemical quality, and antioxidant potential of apple juice during refrigerated storage. LWT Food Sci. Technol. 150, 112038. doi: 10.1016/j.lwt.2021.112038
Tandon, K., Worobo, R. W., Churey, J. J., and Padilla-Zakour, O. I. (2003). Storage quality of pasteurized and UV treated apple cider. J. Food Process. Preserv. 27, 21–35. doi: 10.1111/j.1745-4549.2003.tb00498.x
Tewari, S., Sehrawat, R., Nema, P. K., and Kaur, B. P. (2017). Preservation effect of high pressure processing on ascorbic acid of fruits and vegetables: a review. J. Food Biochem. 41, e12319. doi: 10.1111/jfbc.12319
Torkamani, A., and Niakousari, M. (2011). Impact of UV-C light on orange juice quality and shelf life. Int. Food Res. J. 18, 1265–1268.
Tournas, V. H., Heeres, J., and Burgess, L. (2006). Moulds and yeasts in fruit salads and fruit juices. Food Microbiol. 23, 684–688. doi: 10.1016/j.fm.2006.01.003
Umair, M., Jabeen, S., Ke, Z., Jabbar, S., Javed, F., Abid, M., et al. (2022). Thermal treatment alternatives for enzymes inactivation in fruit juices: recent breakthroughs and advancements. Ultrason Sonochem. 86, 105999. doi: 10.1016/j.ultsonch.2022.105999
Varela-Santos, E., Ochoa-Martinez, A., Tabilo-Munizaga, G., Reyes, J. E., Pérez-Won, M., Briones-Labarca, V., et al. (2012). Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innov. Food Sci. Emerg. Technol. 13, 13–22. doi: 10.1016/j.ifset.2011.10.009
Vervoort, L., Van der Plancken, I., Grauwet, T., Timmermans, R. A., Mastwijk, H. C., Matser, A. M., et al. (2011). Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice: Part II: Impact on specific chemical and biochemical quality parameters. Innov. Food Sci. Emerg. Technol. 12, 466–477. doi: 10.1016/j.ifset.2011.06.003
Vieira, F. N., Lourenço, S., Fidalgo, L. G., Santos, S. A. O., Silvestre, A., et al. (2018). Long-term effect on bioactive components and antioxidant activity of thermal and high-pressure pasteurization of orange juice. Molecules. 23, 2706. doi: 10.3390/molecules23102706
Wibowo, S., Vervoort, L., Tomic, J., Santiago, J. S., Lemmens, L., Panozzo, A., et al. (2015). Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 171, 330–340. doi: 10.1016/j.foodchem.2014.09.007
Wu, W., Xiao, G., Yu, Y., Xu, Y., Wu, J., Peng, J., et al. (2021). Effects of high pressure and thermal processing on quality properties and volatile compounds of pineapple fruit juice. Food Control. 130, 108293. doi: 10.1016/j.foodcont.2021.108293
Zhao, L., Wang, Y., Hu, X., Sun, Z., and Liao, X. (2016). Korla pear juice treated by ultrafiltration followed by high pressure processing or high temperature short time. LWT-Food Sci. Technol. 65, 283–289. doi: 10.1016/j.lwt.2015.08.011
Zhao, Y. (2007). Berry Fruit: Value-Added Products for Health Promotion. Food Science and Technology (1st ed.). New York, USA: CRC press. doi: 10.1201/9781420006148
Keywords: UV-C, high-pressure processing, pineapple juice, pasteurization, bioactive compounds
Citation: Suthiluk P, Chuensombat N, Setha S and Naradisorn M (2023) Synergistic effect of UV-C irradiation and high-pressure processing in reducing microbial load in “Nanglae” pineapple juice compared to conventional heat treatment. Front. Sustain. Food Syst. 7:979943. doi: 10.3389/fsufs.2023.979943
Received: 28 June 2022; Accepted: 17 March 2023;
Published: 30 March 2023.
Edited by:
Mahendran Radhakrishnan, Indian Institute of Food Processing Technology, IndiaReviewed by:
Preetinder Kaur, Punjab Agricultural University, IndiaCopyright © 2023 Suthiluk, Chuensombat, Setha and Naradisorn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matchima Naradisorn, bWF0Y2hpbWFAbWZ1LmFjLnRo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.