- 1Crop Protection Section, ICAR- Directorate of Onion and Garlic Research, Rajgurunagar, Pune, Maharashtra, India
- 2Department of Entomology, Post Graduate Institute, Mahatma Phule Krishi Vidyapeeth, Rahuri, Maharashtra, India
- 3Department of Entomology, Lovely Professional University, Jalandhar, Punjab, India
- 4Division of Physiology, Biochemistry and Post Harvest, ICAR-Central Plantation Crops Research Institute, Kasaragod, Kerala, India
Stingless bee honey, also known as pot honey, is a unique product that differs from other honey (Apis) in terms of flavor, chemical composition, biological characteristics, and sourness. Raw and by-products made from this honey have substantial use for its diverse health benefits and human dietary requirements. The physiochemical properties of honey from stingless bee mainly rely on nectar sources, geographic locations, climate, bee species, and handling and storage conditions. The honey contains reducing sugars, water, minerals, and ash content, and its characteristic features include color, acidity, pH, electrical conductivity, and viscosity. Further, it contains several biological and therapeutic constituents such as flavonoids, antioxidants, antibacterial, wound-healing, antidiabetic, and anticancer properties, and the Maillard reaction product hydroxymethylfurfural. This review summarizes the literature on the physical and biological properties and therapeutic constituents' use of stingless bee honey. It also highlights the quality standards available worldwide and required modifications in establishing universal standards for promoting this honey.
1 Introduction
Stingless bees are small to medium-sized bees found across Asia, Africa, America and Australia (Grüter, 2020) (Figure 1A). They visit many tropical flowering plants and have the potential to be commercial pollinators (Souza et al., 2021; Karuppaiah et al., 2023). Colonies of stingless bees are perennial and usually consist of hundreds or thousands of workers who exhibit highly eusocial behavior (Wille, 1983). About 500 stingless bee species are known around the world, belonging to the Meliponinae subfamily of the Apidae family in the Hymenoptera order (Shamsudin et al., 2019; Karuppaiah et al., 2022).
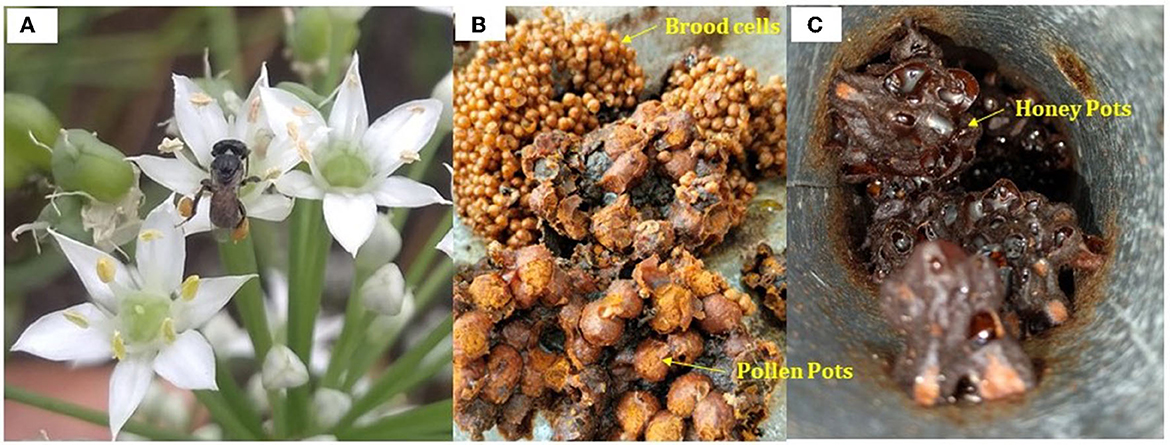
Figure 1. (A) Stingless bee, Tetragonula sp. foraging on wild onion; (B) Brood cells and pollen pots in the nest; (C) Honey pots.
Meliponiculture is the scientific method of stingless beekeeping, which has long been practiced in tropical nations. Stingless bee honey (SBH) is highly valued and is regarded to be more effective as an all-natural remedy for treating common ailments (Zulkhairi Amin et al., 2018). SBH is also referred to as “pot-honey” as they store honey in honey pots (Figures 1B, C) rather than honeycombs (Vit et al., 2004) and is widely regarded as having, nutritive, therapeutic, and medicinal properties conferring various health benefits (Nordin et al., 2018; Souza et al., 2021). Stingless bees dehydrate honey to a specific level and after that, the bacteria and yeasts, primarily Bacillus species, consume some of the sugar and ferment it anaerobically to produce alcohol, which is then fermented aerobically to produce acetic acid (Souza et al., 2006; Menezes et al., 2013). Other non-alcoholic fermentation processes also convert sugar into different kinds of acids and other byproducts that introduce bioactive components like antibiotics and antioxidant molecules to the honey (Almasaudi, 2021). The species-specific microbiomes and processing dynamics, that modify the properties of the honey are quite distinctive. Besides, it also adds enzymes and other compounds to honey, which may aid in preserving and digesting nutrients (Menezes et al., 2013).
Furthermore, the term “stingless bee honey” extracted from the Scopus database up to 2023 was used for understanding the connections between scientific output and stingless bee honey (Figure 2). According to Figure 2A, the largest circles represent the most common author-selected terms: “honey”, “stingless bee” “physicochemical properties”, “antioxidant” and “flavonoid”. Based on the bibliometric mapping performed using widely referred VOSviewer version 1.6.19 (Kwon, 2023; Liaqat et al., 2023), we could categorize the terms into four distinct sets. The red grouping emphasizes terms that are specific to honey and its physicochemical properties. The keywords grouped in green cluster are linked to the stingless bee honey's biological and therapeutic constituents, and the keywords assembled in blue cluster are connected to the physicochemical analysis of SBH. The terms in pink cluster focus on the microbial sensitivity tests of SBH. Figures 2A, B exemplifies that the physicochemical parameters of stingless bee honey hold great promise as a source of nutrition and therapy demonstrating a value in the human diet (Esa et al., 2022).
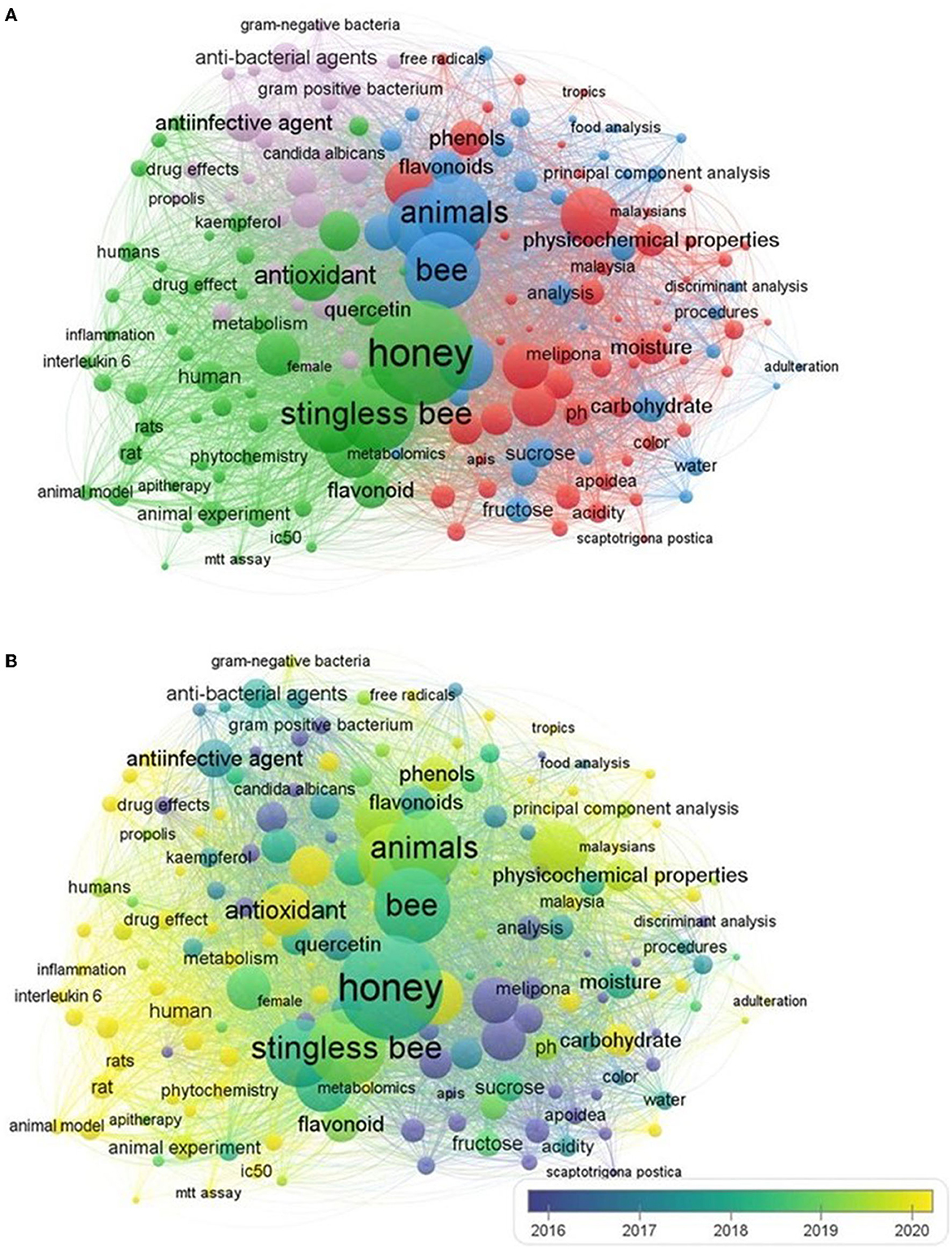
Figure 2. A bibliometric network map of scientific research on stingless bee honey. The network visualization based on four colored clusters (A) indicates the relatedness of the scientific journals and an overlay visualization (B) specifies a period of the occurrence of the keyword from 2016 (blue) to 2020 (yellow). This figure is created from VOSviewer version 1.6.19, and collected data were obtained from Scopus database utilizing keyword “stingless bee honey”.
This review is aimed to shed light on the physicochemical properties, constituents, therapeutic benefits and quality standard requirements of SBH. The informational summary in this review will be useful in better understanding SBH, its potential use and its scope in various economic benefits (Potts et al., 2016; Souza et al., 2021) graphically depicted in Figure 3.
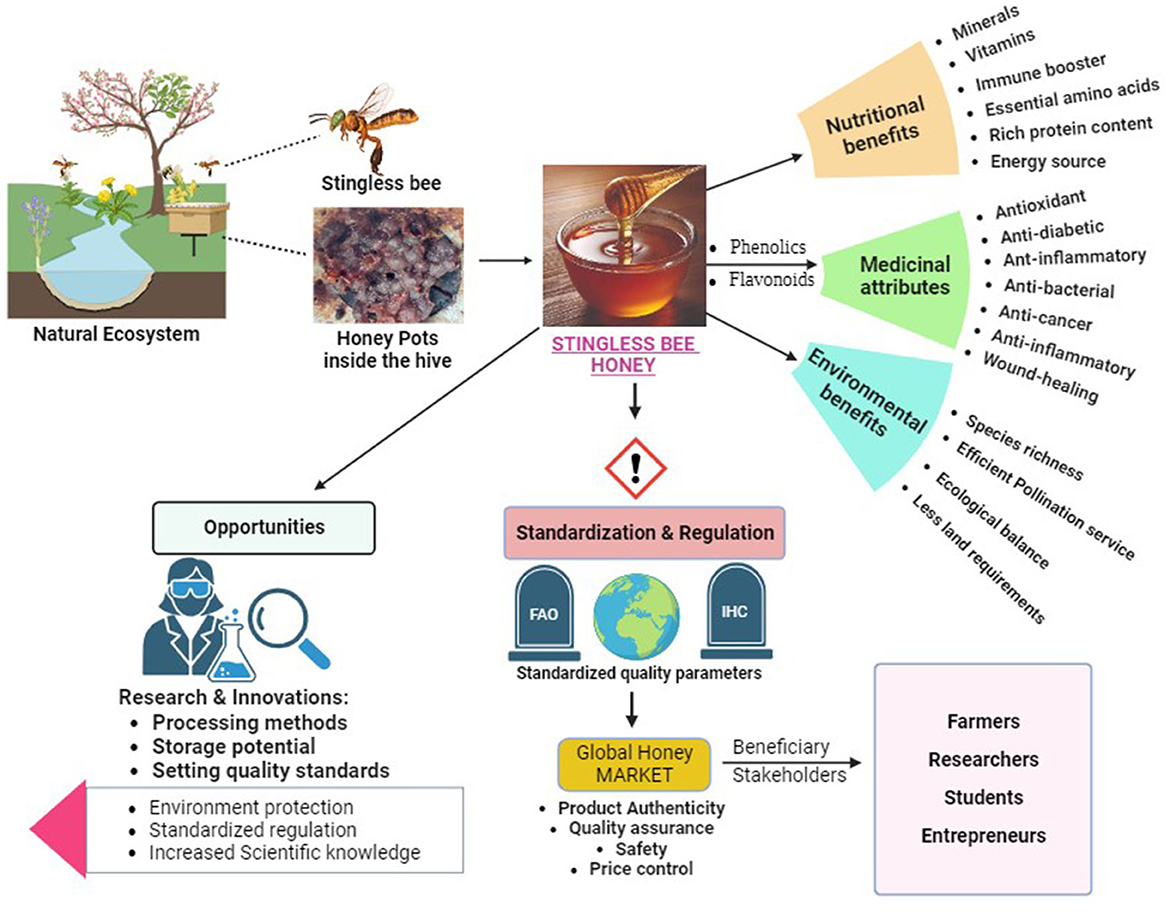
Figure 3. Graphical abstract of stingless bee honey as a nutritional food with therapeutic properties.
2 Biochemical features and constituents of SBH and Apis honey
The physicochemical parameters of pot honey have been extensively examined and compared to Apis mellifera produced floral honey (Lage et al., 2012; da Silva et al., 2013; Biluca et al., 2016; Chuttong et al., 2016; Nordin et al., 2018; Popova et al., 2021; Souza et al., 2021; Villacrés-Granda et al., 2021). Pot honey has distinct organoleptic and physicochemical properties when compared to A. mellifera produced floral honey (Biluca et al., 2016; Rosli et al., 2020; Popova et al., 2021). Water content, reducing sugar, acidity and pH, electrical conductivity, ash, color, viscosity, hydroxymethylfurfural (HMF), as well as various phenolic and flavonoid concentrations, are all included in the physiochemical profile of pot honey (Figure 4) (Bafo, 2019; Santisteban et al., 2019; Lavinas et al., 2023). These attributes are liable to change based on variables such as bee species, botanical sources, geographic origins, climate, collection period, processing, and storage procedures. Supplementary Table S1 summarizes the physicochemical characteristics of stingless bees and their regional variations.
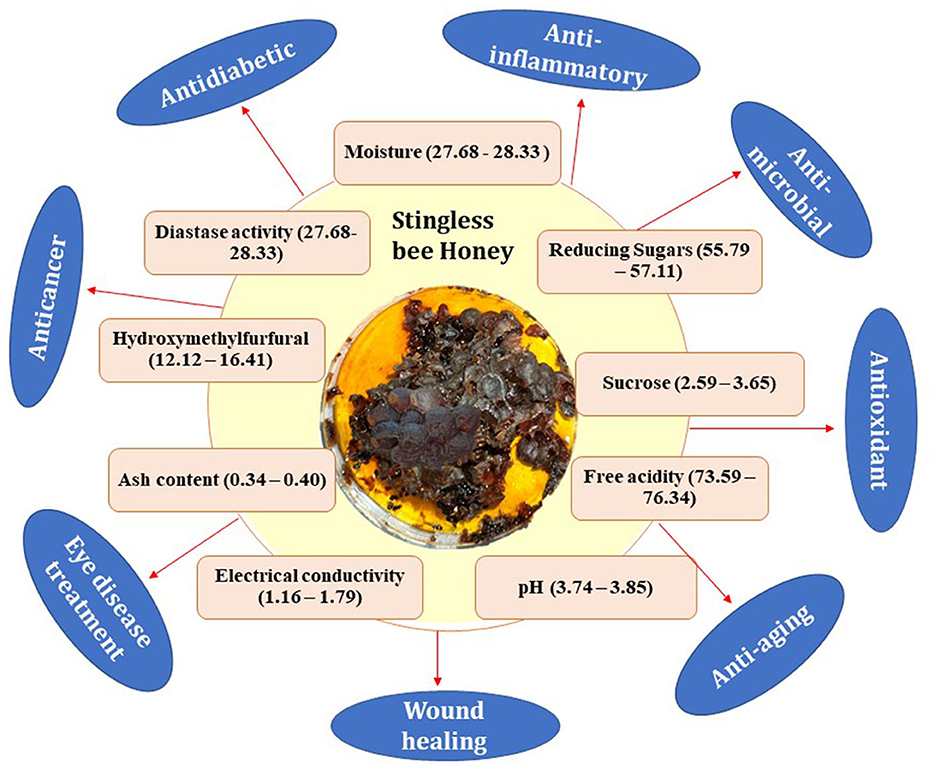
Figure 4. Schematic diagram of physicochemical attributes and medicinal properties of stingless bee honey.
2.1 Water content
SBH has a greater water content than Apis honey (Avila et al., 2019a), which exceeds 20% and can approach 40% (Biluca et al., 2016). In some situations, the water content of SBH can exceed 42% (v/v), especially when collected from humid areas (Marinus, 2006; Ramli et al., 2017). Chuttong et al. (2016) reported that the average moisture content of stingless honey was 31%. Melipona favosa and Melipona trinitatis had an average moisture content of 23.5% (Bijlsma et al., 2006; Nweze et al., 2017). The moisture content of Heterotrigona itama and Geniotrigona thoracica honey is 16.1-32.1% (Julika et al., 2020; Zawawi et al., 2022). Honey's water content is critical in inhibiting or limiting microbial growth. It is an essential factor in affecting honey's stability and influencing microbial response and the type of microorganisms found in honey (Ikhsan et al., 2022). As a result, pot honey deteriorates more quickly than apis honey (Nordin et al., 2018). The increase in water content provides an ideal environment for the growth of numerous bacteria associated with Meliponini bees. Bacilli of the genera Bacillus and Streptomyces were discovered in pot-honey samples (Ngalimat et al., 2019). These bacteria are thought to help prevent honey spoilage by suppressing the growth of pathogenic germs. The intricate interplay between honey's water content and microbial activity underscores the significance of maintaining optimal moisture levels for honey preservation, as deviations can either enhance or compromise its shelf life and quality.
2.2 Reducing sugars
Despite having a sweet taste, stingless bee honey often has less reducing sugar than Apis honey (Avila et al., 2018). Each 100 g of SBH contains fructose ranging from 15.0–48.4 g, glucose ranging 12.2–40.0 g, sucrose <0.01–7.3 g, fructose + glucose ranging 15.0–80.2 g, fructose/glucose 0.78–1.6 g, and glucose/water (G/W) (0.47–1.89) (Julika et al., 2020). The fructose concentration is mainly responsible for the variation in sweetness of SBH (González-Miret et al., 2005). The variation in sugar concentration in honey is influenced by a number of variables, such as the honey's botanical origin, the climate, and its geographic location (Pascual-Mate et al., 2018). In comparison to honey obtained in the dry season, honey extracted during the rainy months usually has a lesser amount of reducing sugars. Sugar levels decrease as a result of increased water content brought on by high humidity during the rainy season (Singh and Singh, 2018). The time of storage is yet another important component that may have an impact on the honey's diminishing sugar content. Higher quantities of reducing sugars and a reduction in acidity have been linked to longer storage times (Karnia et al., 2020). It's interesting to observe how various factors, from environmental conditions to storage duration, play a crucial role in shaping up the composition of stingless bee honey, affecting both its taste and nutritional characteristics.
2.3 Acidity and pH
Natural SBH has an acidic pH ranging from 3.2–4.5, giving it a distinct sour flavor and aroma (Sant'ana et al., 2020; Schvezov et al., 2020; Umaña et al., 2021; Andrade-Velásquez et al., 2023; Grandoa et al., 2023; López-Garay et al., 2023; Shaik et al., 2023). SBH is more acidic than Apis honey (Almeida-Muradian et al., 2014), which is due to the larger amount of organic acids, esters, and inorganic ions, including phosphate, chloride, sulfate, and nitrate. SBH acidity is also regulated by the source of nectar, bee species, and their enzymes or activity by bacterial communities (Finola et al., 2007; Lee et al., 2008; de Sousa et al., 2016; Machado De-Melo et al., 2018). Sugar fermentation into organic acids can also increase acidity (Evahelda et al., 2021). Fermentation takes place naturally in storage due to honey's inherently increased moisture content. Honey contains organic components that significantly lower sugar concentrations, such as fructose, glucose, maltose, and sucrose, making it ideal for the growth of osmophilic yeasts. Honey's higher moisture content (>17.1%) and storage temperature ranging from 23 to 27°C could encourage yeast development and fermentation (de Almeida-Muradian et al., 2014). These prevailing conditions favor the capacity of yeast' to convert the sugars glucose and fructose into ethyl alcohol and carbon dioxide. As a result of the alcohol gets converted into acetic acid and water in the presence of oxygen, offering a sour flavor (Ismail et al., 2021). The acidic pH of honey contributes to its antibacterial qualities by averting the existence and proliferation of harmful microbes (Lee et al., 2008; da Silva et al., 2013). It is also the secret to honey's shelf-life due to its stability against microbial spoilage (Ismail et al., 2021), and it allows honey to be compatible with many food products in terms of pH and acidity.
2.4 Electrical conductivity
The mineral concentration, salts, free organic acids, ash content, moisture content, proteins, and source of nectar all have a direct impact on the electrical conductivity (EC) of SBH (Prakash et al., 2015; Solayman et al., 2016; González-Montemayor et al., 2019). Specifically, the higher concentration of these constituents in the honey leads to high conductivity (da Silva et al., 2016; Baloš et al., 2018; Ismail et al., 2021; Mulugeta and Belay, 2022; Shaik et al., 2023). Although there are no significant differences in the electrical conductivity between SBH and Apis species honey, it was previously revealed to have a greater EC ranging from 0.72–0.16 mS cm−1 compared to Apis honey (Alvarez-Suarez et al., 2018), which falls below the limit of the EC value defined by the International Honey Commission (IHC) (should not exceed 0.8 ms/cm) (Shaik et al., 2023). This variance could possibly be explained by a greater amount of organic acids (as shown by higher acidity values), resulting in higher conductivity. The role of organic acids in this context is crucial, as they contribute to the ionization of compounds in honey, leading to increased electrical conductivity. Additionally, the presence of certain minerals and salts, which are known conductive agents, may further contribute to the observed differences in EC values between SBH and Apis honey varieties. Further research is needed to elucidate the specific compounds responsible for the conductivity variations and their potential implications for the quality and authenticity of honey products.
2.5 Minerals and ash
The mineral composition of honey is determined by its ash content. Honey contains essential minerals such as potassium, sodium„ calcium, and magnesium. In addition, honey may contain other minerals such as nickel, chlorine, phosphorus, sulfur, silicates, iron, copper, manganese, and more (Ameliya et al., 2023). SBH typically contains an appreciable amount of potassium, ranging from 10.55 mg to 448 mg 100g−1, accounting for one half of the total mineral constituents (Biluca et al., 2016; Sabir et al., 2021). Potassium is an essential mineral that is vital for several physiological tasks, such as heart and muscle function. SBH contains small amounts of calcium varying from 1.12–35.2 mg 100 g−1 (Biluca et al., 2016; Sabir et al., 2021), which is important for bone health and various physiological processes. Magnesium is essential for muscle and nerve function, as well as bone health (Ramli et al., 2020). Sodium is essential for maintaining proper fluid balance in the body. The average content of both magnesium and sodium was found to vary in SBH, ranging from 0.410 to 17.3 mg 100 g−1 for Mg and from 0.730–30.4 mg 100 g−1 for Na (Biluca et al., 2016; Sabir et al., 2021). Phosphorus is present in trace amounts in honey (Biluca et al., 2016), which is important for bone and tooth health and various cellular functions. Honey contains trace amounts of sulfur, which contributes to its characteristic flavor and aroma (Tafere, 2021). SBH also contain trace amounts of chlorine, an electrolyte that the body requires (Ajibola et al., 2012).
The mineral content of SBH is influenced by various factors, including the kind of flowers visited by bees for nectar, the botanical origin of the nectar, geographical location, bee species, sugar content, intensity of honey color, soil type of the plants where grown, climatic conditions, and honey processing as well as handling (Nanda et al., 2003; Lacerda et al., 2010; de Almeida-Muradian et al., 2013; Pereira et al., 2020; Gela et al., 2021). However, honey's ash level, which is correlated with its reducing sugar content and black color, suggests the presence of minerals (Venturini et al., 2007; Lacerda et al., 2010). The amount of ash content in a properly dehydrated SBH can be between 0.02 to 0.19% and 0.85% (Esa et al., 2022). The variation in mineral content underscores the intricate interplay between environmental factors and honey composition, making honey a dynamic and diverse natural product with potential health benefits derived from its rich mineral profile.
2.6 Color and viscosity
Honey's physical characteristics is initially recognized by consumers through its color (Figure 5). Scientifically, color is determined by the Pfund scale, which reveals that SBH can range from a light white hue, as shown in Scaptotrigona pectoralis honey (14.5 mm Pfund units), to different shades of light and dark amber, as seen in Plebeia molesta honey (Nordin et al., 2018; Avila et al., 2019a). Trigona honey is assessed to have a yellow-red hue value of 53.50 and a brightness level of 6.61±0.48 (Moniruzzaman et al., 2014). Pollen sources, sugar constituents, antioxidants like carotenoids, xanthophylls, and cyan pigments, minerals, amino acids, and phenolic substances (primarily flavonoids), and 5-HMF which is produced by honey heating or prolonged storage via the Maillard reaction, all contribute to substantial changes in honey color (Shamsudin et al., 2019; Tkáč et al., 2022). The color of the honey, however, does not vary with the bee species (Nordin et al., 2018; Avila et al., 2019a). Darker honey has more minerals, polyphenolic chemicals, dextrins, and acidity than lighter honey (Machado De-Melo et al., 2018).
Viscosity is important in honey's handling, storage, processing, and parameters of sensory quality, which ultimately influence consumer preference. SBH's viscosity is lower than that of Apis honey (do Vale et al., 2018). According to Ameliya et al. (2023), Trigona honey has a viscosity of 596.33 Cp, while Apis cerana honey has a viscosity of 956.33 Cp. Many factors influence it, including plant species sources, water content, temperature, quantity of fructose or glucose, granulation, and the chemical composition of honey. SBH has a decreased viscosity due to its higher moisture content, low polysaccharide content, and lower sugar content (Machado De-Melo et al., 2018). Understanding these physical characteristics, including color and viscosity, provides valuable insights into the diverse nature of honey and its appeal to consumers.
2.7 Hydroxymethylfurfural (HMF)
HMF, a heterocyclic organic molecule with six carbon atoms and functional groups for aldehyde and alcohol, is used as a measure to assess the freshness and aging of honey (Crane, 1983). It is an important intermediate product that results from two reactions; the Maillard reaction and the breakdown of 3-deoxyglucosone (Salis et al., 2021). HMF is normally absent in fresh honey, but throughout processing and aging, its content rises (Ismail et al., 2021). Low levels of HMF are anticipated in SBH due to its characteristics like high moisture and acidity content, as well as the dominance of fructose. These features limit the synthesis of HMF in honey. The concentration of HMF in SHB can range from 12.64 to 15.0 mg kg−1 (Guerrini et al., 2009; Biluca et al., 2014). Unfavorable storage circumstances, higher temperatures, lengthier heating processes, pH levels, and floral sources can all alter the concentration of HMF in honey (Ismail et al., 2021). According to the Codex Alimentarius Commission (Codex Alimentarius, 2019), honey from nations or regions experiencing tropical ambient temperatures, as well as blends that include such honey, shall not exceed the HMF value of 80 mg/kg honey. Monitoring and controlling HMF levels in honey are crucial not only for quality assurance but also for ensuring compliance with international standards and regulations. The specified limit serves as a benchmark to prevent excessive HMF formation, which could indicate improper processing, storage, or unfavorable environmental conditions. Adhering to these standards contributes to the overall quality and safety of honey products in the global market, providing consumers with confidence in the freshness and authenticity of the honey they consume.
2.8 Phenolics
Phenolic compounds are secondary metabolites found in plants and some animal products, and they are known for their potential health benefits due to their antioxidant properties, antimicrobial activity, and anti-inflammatory effects (Lin et al., 2016). SBH has a wide variety of phenolic compounds (more than 80), similar to traditional honey, such as flavonoids, phenolic acids (caffeic acid and gallic acid), and tannins (Dos Santos et al., 2021). These compounds are primarily derived from the nectar and pollen collected by the bees from various plant sources (Biluca et al., 2020). Certainly, fermentation and hydrolysis are methods known to efficiently transform glycosides into aglycones, enhancing the bioavailability of various compounds. Consequently, it is plausible that SBH may contain a higher concentration of phenolic compound aglycones as opposed to glycosides, potentially augmenting its health-promoting properties (Biluca et al., 2017; Dos Santos et al., 2021; Mokaya et al., 2022). Flavonoids have been studied for their possible health advantages, which include anti-inflammatory and antioxidant properties (Han et al., 2012). The composition of phenolic compounds in SBH can vary based on geographical location, botanical origin of the floral source, and bee species involved in honey production. The range of total phenolic content in SBH is 0.0543 ± 0.003 to 0.1760 ± 0.002 mg GAE/g (Mahani et al., 2022) to 7 to 66 mg GAE/g (da Silva et al., 2013). This biochemical variability highlights the importance of considering these factors when evaluating the potential health benefits of SBH. Overall, the rich and diverse phenolic profile of SBH underscores its significance as a natural product with promising health-promoting properties. Further research into the specific health impacts of individual phenolic compounds in SBH is warranted to fully understand its therapeutic potential.
3 Biological and therapeutic aspects of SBH
SBH contains a variety of biological, pharmacological, and physiological properties that can potentially be therapeutic for people and animals (Figure 4). The therapeutic value of these properties depends on the various categories of flavonoids and polyphenols present in stingless bee honey that have been correlated to these anti-inflammatory (Biluca et al., 2020), antidiabetic (Ali et al., 2020), antifungal (Hau-Yama et al., 2020), antimicrobial (Boorn et al., 2010; Nishio et al., 2016), antioxidant (Krishnasree and Ukkuru, 2015; Tuksitha et al., 2018), anticancer properties (Borsato et al., 2014), and ameliorating (Mohammad et al., 2020) constituents. Apinae honey has also been found to have significant medicinal uses, particularly in the treatment of neurological disorders (Al-Himyari, 2009; Al-Rahbi et al., 2014; Saxena et al., 2014), gastrointestinal tract diseases (Haffejee and Moosa, 1985; Ali and Al-Swayeh, 1997), neurological illnesses (Al-Himyari, 2009; Al-Rahbi et al., 2014; Saxena et al., 2014), as well as improving the hormones associated with fertility (Mohamed et al., 2013; Haron et al., 2014; Mosavat et al., 2014; Rajabzadeh et al., 2015a,b). New antimicrobial agents from natural honey are of great interest, and honey from many different species and botanical origins worldwide has drawn much research (Supplementary Table S2). Honey has been used for millennia to treat various illnesses, making it a hot topic for research (Costa-Neto and Oliveira, 2000).
3.1 Anti-cancer activity, cardiovascular therapy, antioxidant and anti-inflammatory activity
Flavonoids, particularly flavanones, flavones, and flavonols, which are present in honey, have extraordinary medicinal uses, including immunosuppressive and anticancer properties (Kustiawan et al., 2014; Karabagias et al., 2016). In particular, the caffeic acid ester from Trigona spp. demonstrates specific chemo-preventive effects. It significantly lowers the number of abnormal crypts and crypt foci, making it a viable colon cancer therapeutic alternative (Yazan et al., 2016).
Caffeic acid extracted from Melipona subnitida honey has been shown to be effective in the treatment of dyslipidemia, which is linked to cardiovascular disease (Bezerra et al., 2018). 4-hydroxyphenyl acid from Heterotrigona itama honey (Ramli et al., 2019) and gamma-Mangostin from Thailand stingless bee honey (Ishizu et al., 2019) have also shown promise in cardiovascular therapy.
Total phenolics and flavonoid content of SBH have been linked to its potential antioxidant properties (Praptiwi et al., 2023). SBH was used to formulate honey with antioxidant properties that help lessen oxidative reactions or free radicals in food systems and human health (Figure 6) (Tuksitha et al., 2018). In vitro, assays using Brazilian SBH have revealed 45% higher antioxidant and biological activity than those using A. mellifera honey (Avila et al., 2019b). According to Praptiwi et al. (2023), T. laeviceps honey contains 5,7-dihydroxy chromone, cnidimon C, puerarin, and irisflorentin, while Tetragonula carbonaria honey has been illustrated to contain gallic acid, pimaric acid, and pimaric acid isomers (Massaro et al., 2014). To the anti-inflammatory properties of SBH of various species, naringenin (Copmans et al., 2018; Badrulhisham et al., 2020; Mokarrami et al., 2022), myricetin (Sun et al., 2019), and phenylalanine (Mustafa et al., 2019) add equal benefits. Kaempferol from Trigona spp. (Ranneh et al., 2018); Apigenin (Zhang et al., 2019b; Singh et al., 2021; Yadav et al., 2022); Chrysin (Shooshtari et al., 2020; Al-Haleem et al., 2021; Zhang et al., 2021); Catechin (Ahmed et al., 2021); Caffeic acid (Arshad et al., 2020; Kadar et al., 2022); ferulic acid (Zhang et al., 2019a,b; Park et al., 2022); and 4-hydroxybenzoic acid (Ranneh et al., 2019) from various SHB have been documented for antioxidant and anti-inflammatory activities (Vattuone et al., 2007; Suntiparapop et al., 2012; Duarte et al., 2018; Seng and Tang, 2020; Wongsa et al., 2023).
3.2 Antimicrobial and anti-diabetic activity
The antimicrobial properties of SBH are well documented by several researchers based on its botanical origin (Hbibi et al., 2020; Zapata-Vahos et al., 2023). SBH is shown to have antibacterial action against Gram-positive bacteria including Bacillus subtilis, Micrococcus luteus, B. megaterium, and B. brevis, and Gram-negative bacteria, such as Escherichia coli and Pseudomonas syringae (Chanchao, 2009; Al-Hatamleh et al., 2020a,b; Cantero et al., 2021). Nishio et al. (2016) illustrated the in-vitro antibacterial activity of honey from Scaptotrigona bipunctata and Scaptotrigona postica against Gram-positive and Gram-negative bacteria, including multidrug-resistant strains. Miorin et al. (2003), Sgariglia et al. (2010) found that honey from Trigona angustula had antibacterial activity against both Gram-positive (Staphylococcus aureus and Enterococcus faecalis) and Gram-negative (Pseudomonas aeruginosa and E. coli). Brown et al. (2020) and Khongkwanmueang et al. (2020) reported that the honey of M. favosa (Tobago) and T. laeviceps (Thailand) contained a high amount of phenolics and flavonoids, which led to potent antimicrobial activity against yeast and pathogenic microbes (S. aureus, E. coli, S. pyogenes, and H. influenza). Lepidotrigona arciferal honey can also treat colds and coughs (Biswa et al., 2017). Unsaturated pyrrolizidine alkaloids (PA) of SBH have antifungal and antiviral qualities (Moreira et al., 2018; Tasca et al., 2018; Schramm et al., 2019; Mädge et al., 2020). The antibacterial properties of SBH are further correlated with the amount of hydrogen peroxide and other non-peroxide components, including lysozyme, phenolic acid, and flavonoid (Kwakman and Zaat, 2012). These chemical compositions show that non-phenolic chemical substances were responsible for the antibacterial effect (Brodkiewicz et al., 2018). Furthermore, it may be associated with the acidic property (low pH) (Avila et al., 2018), polyphenol concentration (Daglia, 2012), and protein content (Ramon-Sierra et al., 2020).
Aziz et al. (2017) demonstrated the antihyperglycemic effects of Apis honey on chemically induced diabetes. Stingless bee honey showed promise as an anti-diabetic drug by lowering histopathological alterations, oxidative stress expression levels, inflammation, and apoptotic indicators in pancreatic islets. In addition, stingless bee honey raised the insulin expression level (Aziz et al., 2017; Sahlan et al., 2020). According to Krishnasree and Ukkuru (2017), Trigona iridipennis honey has more enzyme inhibition against amylase and glucosidase. Caffeic acid (Jung et al., 2006; Estevinho et al., 2008; Khalil and Sulaiman, 2010; Rocha et al., 2012; Spilioti et al., 2014) and catechin (Koh et al., 2004; Afroz et al., 2016) are some bioactive compounds responsible for the antihyperglycemic effects of SBH.
3.3 Wound healing, anti-allergic activity and anti-aging property
SBH has shown promising results in clinical studies on wound healing (Al-Achi, 2008; Reni-Yusli et al., 2016; Ng et al., 2017), which is useful in the treatment of pathogens, wound debridement and inflammation suppression, scarring reduction, stimulation of angiogenesis, tissue granulation, and epithelium growth (Molan and Betts, 2004). Infections in the body, such as ulcers, skin infections, and rashes, are also treated (Kwapong et al., 2010). Protocatechuic acid, an important free phenolic acid found in SBH, is a powerful antioxidant that boosts cell proliferation and aids in healing wounds (Abd Jalil et al., 2017). Based on molecular docking modeling, polyphenol-rich SBHs having 3,5-di-O-caffeoylquinic acid, quercetin glucoside molecules, and caffeoyl-D-glucose have strong anti-allergic actions (Yong et al., 2023).
Oxygen serves as an antioxidant, safeguarding the human body from internal damage and inhibiting oxidative reactions in various types of food (Han et al., 2012). Natural honey has been shown to have anti-aging effects. SBH curtails reactive oxygen species (ROS) and free radicals produced during various metabolic activities (Habryka et al., 2021). Honey from H. itama increases the expression of collagen type I and metalloproteinase (MMP)-1 in human dermal fibroblast cells, acting as an anti-aging agent (Malik et al., 2020).
3.4 Other therapeutic values
In addition to its many therapeutic applications, SBH has been used to treat neurological disorders, infertility, and ocular diseases. In the course of cataract treatment, honey from Trigona spp. slows down the progression of the disease (Patricia, 2002), while honey from Meliponula spp. has been shown to shorten the duration of infection for eye disorders brought on by Staphylococcus aureus and Pseudomonas aeruginosa (Ilechie et al., 2012). Follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone levels can all be boosted by eating honey. Furthermore, it has been demonstrated that giving children continual honey supplementation will shorten their bouts of diarrhea (Haffejee and Moosa, 1985) and be used to treat gastrointestinal problems brought on by ethanol as well as increased vascular permeability (Yazan et al., 2018). It prevents dementia and other cognitive diseases (Al-Himyari, 2009) and enhances memory by increasing neuron proliferation in hippocampal regions (Al-Rahbi et al., 2014).
4 Quality standards requirement for stingless bee honey
Honey, being a natural product, possesses a varied composition that is influenced by numerous factors. These factors include the botanical and geographical source of honey, the intensity of nectar availability, prevailing climatic conditions, manipulations by beekeepers, the methods of handling and packaging, the duration, and the conditions of storage (Thrasyvoulou et al., 2018). Establishing specifications for the quality criteria of SBH is crucial when it comes to its direct consumption by humans (Lemos et al., 2018). While honey standards have been adjusted based on the botanical source, the species' origin has not been considered in these modifications (Owen, 2023). It is of utmost importance that these factors are given due consideration and that quality standards are established for other varieties of SBH as well (Vit et al., 2004).
The current definition of honey, according to Codex Alimentarius (2019), pertains exclusively to honey produced by honeybees and stored in a honeycomb made up of wax, thereby excluding honey stored by stingless bees in honey pots made of cerumen. Similarly, the directives of the International Honey Commission of the European Union (EU, 2014) limited the definition of honey to that obtained solely from the Apis species. Consequently, the commonly followed standards of these authorities do not encompass the definition of honey produced by stingless bees. To address this gap, the scientific community (Vit et al., 2004; Villas-Bôas and Malaspina, 2005; Carvalho et al., 2013; De Camargo et al., 2017; Malaysian Standards, 2017) has proposed regulatory standards (Table 1) for stingless bee honey, aiming to establish standardized criteria for its market acceptance and direct consumption by humans.
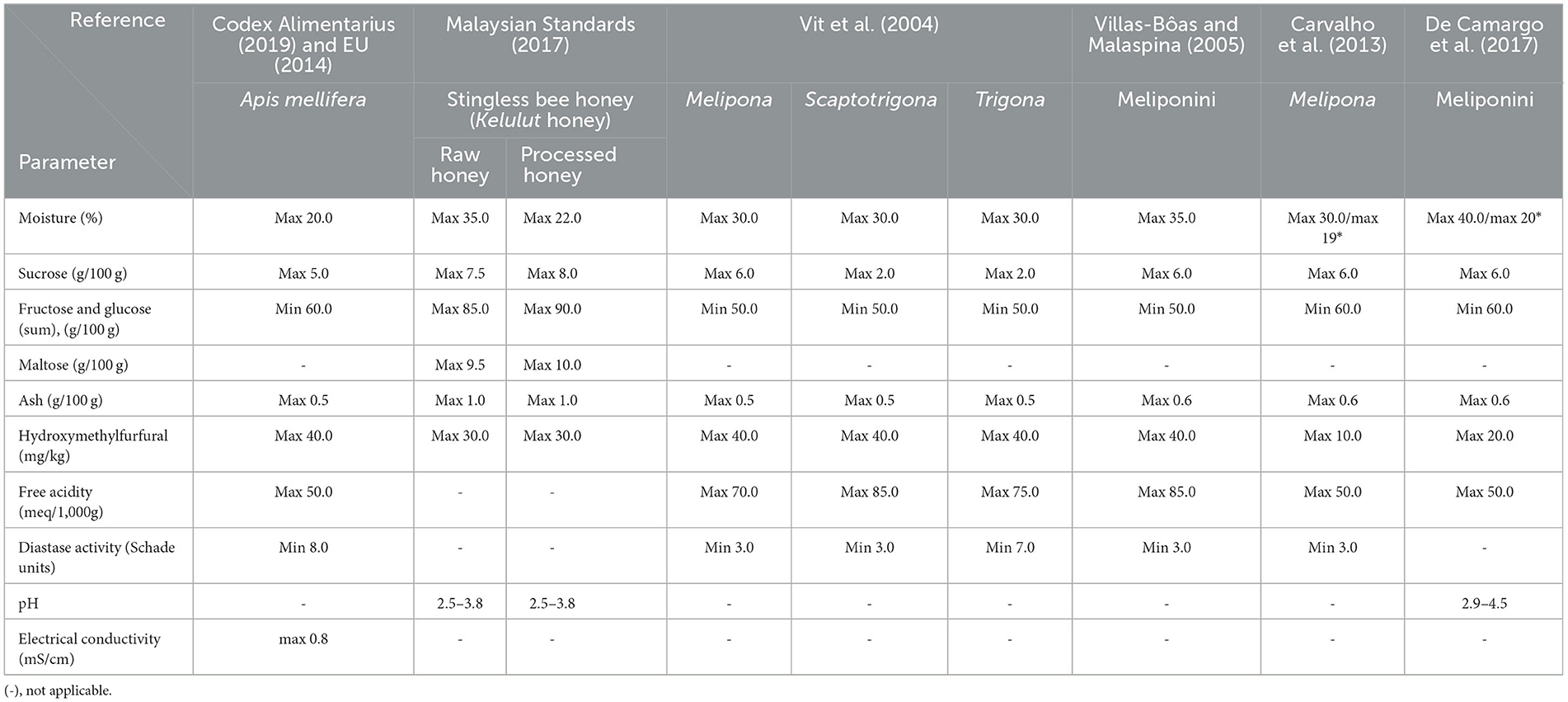
Table 1. Quality standard limits of the current legislation for Meliponini bee floral honey and Apis mellifera floral honey.
Stingless bees produce unique honey with physicochemical characteristics that differ from the honey produced by A. mellifera bees (the only once regulated). Stingless bee honey has more moisture and acidity, as well as less sugar, diastase activity, and 5-hydroxymethylfurfural (5-HMF) (Avila et al., 2018; Nordin et al., 2018). These parameters do not correspond to current regulatory restrictions (Codex Alimentarius, 2019). Moreover, the product is mostly sold informally and directly by producers without any certification of quality or authenticity, making it vulnerable to fraud. Hence, it is necessary to set general quality standards for SBH. It is crucial to revise these standards in order to accurately check the authenticity of honey products and prevent adulterated, fraudulent marketing. Failing to address this issue poses significant challenges in the market for the product.
A quality standard for SBH proposed by the Malaysian standard indicates a permissible moisture content of 35 g, sucrose of 7.5 g, and ash content of 1.0 g for each 100 g of honey. Furthermore, unlike the IHC norm, it considers a maximum reducing sugar content of 85 g per 100 g rather than a minimum content. Free acidity and diastase activity (DA) are not considered quality parameters, but the quantity of HMF content was kept close to the IHC standard. Phenolic compounds and pH values should be 2.5–3.8 in SBH (Malaysian Standards, 2017). At the same time, Vit et al. (2004) suggested increased moisture content (30 g/100 g), and sucrose (6 g/100 g) and a minimum reducing sugar content (50 mg/100 g). The ash contents were kept similar to IHC standards, while HMF should be <30 mg/kg. In recent times, certain Brazilian states, like Bahia (Brazil, 2014) and Sáo Paulo (Brazil, 2017), have introduced defined criteria for honey produced by stingless bees. The objective behind this is to ensure quality control and establish formal guidelines for the sale of this particular product. Consequently, establishments engaged in honey processing, accredited by the Federal Inspection Service, have managed to navigate past existing bureaucratic obstacles. They are now able to officially register and market stingless bee honey in the formal marketplace (Vit et al., 2004; Villas-Bôas and Malaspina, 2005; Carvalho et al., 2013; De Camargo et al., 2017).
Despite the growing number of studies into the physicochemical properties of these honeys, there is a great difficulty in uncovering a single standard that helps in determining their authenticity and quality (Braghini et al., 2021). Moreover, effective regulation is difficult owing to the world's large diversity of stingless bees (Braghini et al., 2021). Furthermore, when compared to A. mellifera bees (20 kg per hive/year) stingless bees produce less honey (1–5 kg per hive/year), (Chuttong et al., 2016). This makes it a rare product with a higher market value (US 100 USD/kg for stingless bee honey and US 20–40 USD/kg for A. mellifera honey) (Se et al., 2018; Shadan et al., 2018). In addition to honey, these bees manufacture other products in the hive, such as propolis and wax, which have been widely studied and deserve attention.
The physicochemical properties of Malaysian honey were influenced by many factors, including bee species, floral sources, seasonal factors, processing, geographical distribution, etc., (Ismail et al., 2021). The Malaysian Apis and Trigona honey differed remarkably in physicochemical parameters (Ismail et al., 2021). Given that stingless bee honey has very varied physicochemical characteristics, relying solely on physicochemical parameters to assess the quality and determine authenticity is insufficient (Braghini et al., 2021). To address this issue, it is of utmost importance to conduct further research focused on identifying potential chemical markers of stingless bee honey. Such markers will play a crucial role in distinguishing the entomological, geographical, and floral origin of this product (Braghini et al., 2021).
5 Conclusion
This literature review has provided an overview of the physiochemical characteristics of SBH and its prospective applications for an array of health benefits. The excellent health benefits have been outlined, including the potential to reduce inflammation, fight infections, heal wounds, treat diabetes, and fight cancer (as a chemopreventive agent). The therapeutic benefits of SBH are due to its physicochemical components, particularly their phenolic and flavonoid content. The data elucidated in this review highlighs the significant variations in stingless bee honey. This further emphasizes the need for criteria and guidelines to be developed to evaluate the honey quality of stingless bees. A further difficulty for quality control is identifying species. Since there are many different types of stingless bee honey, it is difficult for the regulator to create standards. Considering the fact that many species that still need to be investigated, the increasing demand for products from stingless bees and their usage in various medicinal applications requires additional validation studies and more comprehensive methods. In-depth studies on chemical characterization still need to be included and are imperative. In particular, if research is done to identify and enhance geographical elements, The scientific understanding and awareness of the distinctive qualities of each stingless bee species' honey will be essential to increasing the value of its products. Regulations are needed to safeguard this priceless and underutilized natural product's quality, safety, and authenticity. It is vital to address the uncertainty in global legislation by establishing specific minimum standards that must be met by all nations that produce, import, or export honey.
Author contributions
AG: Conceptualization, Software, Writing—original draft, Writing—review & editing. DS: Conceptualization, Writing—original draft, Writing—review & editing. PS: Supervision, Writing—review & editing. CP: Writing—review & editing. MP: Conceptualization, Writing—original draft, Writing—review & editing. TP: Software, Writing—review & editing. RD: Funding acquisition, Resources, Supervision, Writing—review & editing. SK: Supervision, Writing—review & editing. SR: Writing—review & editing, Formal analysis. VM: Funding acquisition, Resources, Writing—review & editing. VK: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Visualization, Writing—review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The stingless honey bee project undertaken by our team was supported by Department of Science and Technology (DST) - Science and Engineering Research Board (SERB), New Delhi (CRG/2021/004267).
Acknowledgments
We thank the financial grant (CRG/2021/004267) given by the Department of Science and Technology (DST) - Science and Engineering Research Board (SERB), New Delhi, India, as well as the facilities provided by the Indian Council of Agricultural Research and Directorate of Onion and Garlic Research (ICAR-DOGR), Pune, Maharashtra, India.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1324385/full#supplementary-material
References
Abd Jalil, M. A., Kasmuri, A. R., and Hadi, H. (2017). Stingless bee honey, the natural wound healer: a review. Skin Pharmacol. Physiol. 30, 66–75. doi: 10.1159/000458416
Afroz, R., Tanvir, E. M., Zheng, W., and Little, P. J. (2016). Honey-derived flavonoids: natural products for the prevention of atherosclerosis and cardiovascular diseases. Clin. Exp. Pharmacol. 6, 1000209. doi: 10.4172/2161-1459.1000209
Ahmed, H., Khan, M. A., Ali- Zaidi, S. A., and Muhammad, S. (2021). In silico and in vivo: evaluating the therapeutic potential of kaempferol, quercetin, and catechin to treat chronic epilepsy in a rat model. Front. Bioeng. Biotechnol. 9, 754952. doi: 10.3389/fbioe.2021.754952
Ajibola, A., Chamunorwa, J. P., and Erlwanger, K. H. (2012). Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. 9, 1–12. doi: 10.1186/1743-7075-9-61
Al-Achi, A. (2008). An Introduction to Botanical Medicines: History, Science, Uses, and Dangers. Westport, CT: Praeger Publishers.
Al-Haleem, A. E. N., Ahmed, H. I., and El-Naga, R. N. (2021). Lycopene and Chrysin through mitigation of neuroinflammation and oxidative stress exerted antidepressant effects in clonidine-induced depression-like behavior in rats. J. Diet. Suppl. 11, 1–20. doi: 10.1080/19390211.2021.1988797
Al-Hatamleh, M. A. I., Boer, J. C., Wilson, K. L., Plebanski, M., Mohamud, R., and Mustafa, M. Z. (2020a). Antioxidant-based medicinal properties of stingless bee products: recent progress and future directions. Biomol. 10, 1–28. doi: 10.3390/biom10060923
Al-Hatamleh, M. A. I., Hatmal, M. M., Sattar, K., Ahmad, S., Mustafa, M. Z., Bittencourt, M. D. C., et al. (2020b). Antiviral and immunomodulatory effects of phytochemicals from honey against COVID-19: Potential mechanisms of action and future directions. Molecules. 25, 5017. doi: 10.3390/molecules25215017
Al-Himyari, F. A. (2009). The use of honey as a natural preventive therapy of cognitive decline and dementia in the middle east. Alzheimer's & Dement. 5, P247. doi: 10.1016/j.jalz.2009.04.248
Ali, A. M., and Al-Swayeh, O. A. (1997). Natural honey prevents ethanol-induced increased vascular permeability changes in the rat stomach. J. Ethnopharmacol. 55, 231–238. doi: 10.1016/S0378-8741(96)01504-8
Ali, H., Abu Bakar, M. F., Majid, M., Muhammad, N., and Lim, S. Y. (2020). In vitro anti-diabetic activity of stingless bee honey from different botanical origins. Food Res. 4, 1421–1426. doi: 10.26656/fr.2017.4(5).411
Almasaudi, S. (2021). The antibacterial activities of honey. Saudi J. Biol. Sci. 28, 2188–2196. doi: 10.1016/j.sjbs.2020.10.017
Almeida-Muradian, L. B., Stramm, K. M., and Wstevinho, L. M. (2014). Efficiency of the FT-IR ATR spectrometry for the prediction of the physicochemical characteristics of Melipona subnitida honey and study of the temperature's effect on those properties. Int. J. Food Sci. and Technol. 49, 188–195. doi: 10.1111/ijfs.12297
Al-Rahbi, B., Zakaria, R., Othman, Z., Hassan, A., Ismail, Z. I. M., and Muthuraju, S. (2014). Tualang honey supplement improves memory performance and hippocampal morphology in stressed ovariectomized rats. Acta Histochem. 116, 79–88. doi: 10.1016/j.acthis.2013.05.004
Alvarez-Suarez, J. M., Giampieri, F., Brenciani, A., Mazzoni, L., Gasparrini, M., González-Paramás, A. M., et al. (2018). Apis mellifera vs Melipona beecheii Cuban polifloral honeys: a comparison based on their physicochemical parameters, chemical composition and biological properties. LWT. 87, 272–279. doi: 10.1016/j.lwt.2017.08.079
Ameliya, R., Martati, E., and Wulan, S. N. (2023). The physicochemical properties of local Indonesia honey Trigona and A. cerana produced in North Lombok, West Nusa Tenggara. Adv. Food Sci. Sustain. Agric. Agroind. Eng. 2023, 65–72.
Andrade-Velásquez, A., Hernández Sánchez, H., Dorantes-Álvarez, L., Palmeros-Sánchez, B., Torres-Moreno, R., Hernández-Rodríguez, D., et al. (2023). Honey characterization and identification of fructophilic lactic acid bacteria of fresh samples from Melipona beecheii, Scaptotrigona pectoralis, Plebeia llorentei, and Plebeia jatiformis hives. Front. Sustain. Food Syst. 7, 1113920. doi: 10.3389/fsufs.2023.1113920
Arshad, N. A., Lin, T. S., and Yahaya, M. F. (2020). Stingless bee honey reduces anxiety and improves memory of the metabolic disease-induced rats. CNS & Neurol. Disorders-Drug Targets. 19, 115–126. doi: 10.2174/1871527319666200117105133
Avila, S., Beux, M. R., Ribani, R. H., and Zambiazi, R. C. (2018). Stingless bee honey: Quality parameters, bioactive compounds, health-promotion properties and modification detection strategies. Trends in Food Sci. Technol. 81, 37–50. doi: 10.1016/j.tifs.2018.09.002
Avila, S., Hornung, P. S., Teixeira, G. L., Malunga, L. N., Apea-Bah, F. B., Beux, M. R., et al. (2019b). Bioactive compounds and biological properties of Brazilian stingless bee honey have a strong relationship with the pollen floral origin. Int. Food Res. J. 123, 1–10. doi: 10.1016/j.foodres.2019.01.068
Avila, S., Lazzarotto, M., Hornung, P. S., Teixeira, G. L., Ito, V. C., Bellettini, M. B., et al. (2019a). Influence of stingless bee genus (Scaptotrigona and Melipona) on the mineral content, physicochemical and microbiological properties of honey. J. Food Sci. Tech. 56, 4742–4748. doi: 10.1007/s13197-019-03939-8
Aziz, M. S. A., Giribabu, N., Rao, P. V., and Salleh, N. (2017). Pancreatoprotective effects of Geniotrigona thoracica stingless bee honey in streptozotocin-nicotinamide-induced male diabetic rats. Biomed. Pharmacother. 89, 135–145. doi: 10.1016/j.biopha.2017.02.026
Badrulhisham, N. S. R., Ab Hamid, S. N. P., Ismail, M. A. H., Yong, Y. K., Zakuan, N. M., Harith, H. H., et al. (2020). Harvested locations influence the total phenolic content, antioxidant levels, cytotoxic, and anti-inflammatory activities of stingless bee honey. J. Asia Pac. Entomol. 23, 950–956. doi: 10.1016/j.aspen.2020.07.015
Bafo, W. (2019). Meliponiculture and physicochemical properties of honey produced by the African stingless bee Plebeina hildebrandti Friese in Kalakamati village, Botswana. Botsw. J. Agric. Appl. Sci. 13, 33–42. doi: 10.37106/bojaas.2019.13
Baloš, M. Ž., Popov, N., Vidaković, S., Pelić, D. L., Pelić, M., Mihaljev, Ž., et al. (2018). Electrical conductivity and acidity of honey. Archives Veter. Med. 11, 91–101. doi: 10.46784/e-avm.v11i1.20
Bezerra, M. L. R., de Souza, E. L., de Sousa, J. M. B., Lima, M. D. S., Alves, A. F., Almeida, M. G., et al. (2018). Effects of honey from Mimosa quadrivalvis L. (malicia) produced by the Melipona subnitida D. (jandaira) stingless bee on dyslipidaemic rats. Food Funct. 9, 4480–4492. doi: 10.1039/C8FO01044G
Bijlsma, L., de Bruijn, L. L., Martens, E. P., and Sommeijer, M. J. (2006). Water content of stingless bee honeys (Apidae, Meliponini): interspecific variation and comparison with honey of Apis mellifera. Apidologie. 37, 480–486. doi: 10.1051/apido:2006034
Biluca, F. C., Braghini, F., Gonzaga, L. V., Costa, A. C. O., and Fett, R. (2016). Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae). J. Food Comp. Anal. 50, 61–69. doi: 10.1016/j.jfca.2016.05.007
Biluca, F. C., da Silva, B., Caon, T., Mohr, E. T. B., Vieira, G. N., Gonzaga, L. V., et al. (2020). Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 129, 108756. doi: 10.1016/j.foodres.2019.108756
Biluca, F. C., de Gois, J. S., Schulz, M., Braghini, F., Gonzaga, L. V., Maltez, H. F., et al. (2017). Phenolic compounds, antioxidant capacity and bio-accessibility of minerals of stingless bee honey (Meliponinae). J. Food Compos. Anal. 63, 89–97. doi: 10.1016/j.jfca.2017.07.039
Biluca, F. C., Della Betta, F., De Oliveira, G. P., Pereira, L. M., Gonzaga, L. V., Costa, A. C. O., et al. (2014). 5-HMF and carbohydrates content in stingless bee honey by CE before and after thermal treatment. Food Chem. 159, 244–249. doi: 10.1016/j.foodchem.2014.03.016
Biswa, R., Sarkar, A., and Khewa, S. (2017). Ethnomedicinal uses of honey of stingless bee by Nepali community of Darjeeling foothills of West Bengal. India. Ind. J. Trad. Knowl. 16, 648–653. Available online at: http://nopr.niscpr.res.in/handle/123456789/42659
Boorn, K. L., Khor, Y. Y., Sweetman, E., Tan, F., Heard, T. A., and Hammer, K. A. (2010). Antimicrobial activity of honey from the stingless bee Trigona carbonaria determined by agar diffusion, agar dilution, broth microdilution and time-kill methodology. J. Appl. Microbiol. 108, 1534–1543. doi: 10.1111/j.1365-2672.2009.04552.x
Borsato, D. M., Prudente, A. S., Doll-Boscardin, P. M., Borsato, A. V., Luz, C. F., Maia, B. H., et al. (2014). Topical antiinflammatory activity of a monofloral honey of Mimosa scabrella provided by Melipona marginata during winter in Southern Brazil. J. Med. Food. 17, 817–825. doi: 10.1089/jmf.2013.0024
Braghini, F., Biluca, F. C., Schulz, M., Gonzaga, L. V., Costa, A. C., and Fett, R. (2021). Stingless bee honey: A precious but unregulated product-reality and expectations. Food Rev. Int. 38, 683–712. doi: 10.1080/87559129.2021.1884875
Brazil (2014). “Agência Estadual de Defesa Agropecuária da Bahia – ADAB,” in Regulamento Técnico de Identidade e Qualidade do Mel de Abelha social sem ferrão, gênero Melipona (Salvador).
Brazil (2017). Secretaria de Agricultura e abastecimento. Resolução SAA - 52, de 3-10-2017. regulamento técnico de identidade, o padrão de qualidade e os requisitos do processo de beneficiamento do mel, destinado ao consumo humano elaborado pelas abelhas da subfamíia Meliponinae (Hymenoptera, Apidae), conhecidas como abelhas sem ferrão. São Paulo: Diário Oficial, 127.
Brodkiewicz, Y., Marcinkevicius, K., Reynoso, M., Salomon, V., Maldonado, L., and Vera, N. (2018). Studies of the biological and therapeutic effects of argentine stingless bee propolis. J. Drug Del. Therap. 8, 382–392. doi: 10.22270/jddt.v8i5.1889
Brown, E., O'Brien, M., Georges, K., and Suepaul, S. (2020). Physical characteristics and antimicrobial properties of Apis mellifera, Frieseomelitta nigra and Melipona favosa bee honeys from apiaries in Trinidad and Tobago. BMC Compl. Med. Ther. 20, 85. doi: 10.1186/s12906-020-2829-5
Cantero, T. M., da Silva Junior, P. I., Negri, G., Nascimento, R. M., and Mendonça, R. Z. (2021). Antimicrobial activity of flavonoids glycosides and pyrrolizidine alkaloids from propolis of Scaptotrigona aff. postica. BioRxiv. 42, 300–315. doi: 10.1101/2021.07.01.450350
Carvalho, C. A., Alves, L. D., Souza, R. M. D. O., Veras, B. D. A., Alves, S. D. O., Sodre, E. M., et al. (2013). “Proposta de regulamento técnico de qualidade fisico-química do mel floral processado produzido por abelhas do gênero Melipona,” in Stingless Bees Process Honey and Pollen in Cerumen Pots. Mérida, Venezuela: Facultad de Farmacia y Bioanálisis, Universidad de Los Andes, 1–9.
Chanchao, C. (2009). Antimicrobial activity by Trigona laeviceps (stingless bee) honey from Thailand. Pak. J. Med. Sci. 25, 364–369.
Chuttong, B., Chanbang, Y., Sringarm, K., and Burgett, M. (2016). Effects of long-term storage on stingless bee (Hymenoptera: Apidae: Meliponini) honey. J. Apic. Res. 8839, 1–11. doi: 10.1080/00218839.2016.1186404
Codex Alimentarius (2019). “Revised codex standard for honey,” in CODEX STAN 12-1981. Rome Italy: FAO/OMS, Codex Alimentarius Commission.
Copmans, D., Orellana-Paucar, A. M., Steurs, G., Zhang, Y., Ny, A., Foubert, K., et al. (2018). Methylated flavonoids as anti-seizure agents: Naringenin 4′, 7-dimethyl ether attenuates epileptic seizures in zebrafish and mouse models. Neurochem. Int. 112, 124–133. doi: 10.1016/j.neuint.2017.11.011
Costa-Neto, E. M., and Oliveira, M. V. M. (2000). Cockroach is good for asthma: zootherapeutic practices in Northeastern Brazil. Hum. Ecol. Rev. 7, 41–51. Available online at: https://www.jstor.org/stable/24706947
da Silva, I. A. A., da Silva, T. M. S., Camara, C. A., Queiroz, N., Magnani, M., de Novais, J. S., et al. (2013). Phenolic profile, antioxidant activity and palynological analysis of stingless bee honey from Amazonas, Northern Brazil. Food Chem. 141, 3552–3558. doi: 10.1016/j.foodchem.2013.06.072
da Silva, P. M., Gauche, C., Gonzaga, L. V., Costa, A. C. O., and Fett, R. (2016). Honey: Chemical composition, stability and authenticity. Food Chem. 196, 309–323. doi: 10.1016/j.foodchem.2015.09.051
Daglia, M. (2012). Polyphenols as antimicrobial agents. Curr. Opi. Biotech. 23, 174–181. doi: 10.1016/j.copbio.2011.08.007
de Almeida-Muradian, L. B., Stramm, K. M., Horita, A., Barth, O. M., da Silva de Freitas, A., and Estevinho, L. M. (2013). Comparative study of the physicochemical and palynological characteristics of honey from Melipona subnitida and Apis mellifera. Int. J. Food Sci. & Tech. 48, 1698–1706. doi: 10.1111/ijfs.12140
De Camargo, R. C. R., De Oliveira, K. L., and Berto, M. I. (2017). Stingless bee honey: technical regulation proposal. Braz. J. Food Technol. 20, e2016157. doi: 10.1590/1981-6723.15716
de Sousa, J. M. B., de Souza, E. L., Marques, G., de Toledo Benassi, M., Gullón, B., Pintado, M. M., et al. (2016). Sugar profile, physicochemical and sensory aspects of monofloral honeys produced by different stingless bee species in Brazilian semi-arid region. LWT-Food Sci. Tech. 65, 645–651. doi: 10.1016/j.lwt.2015.08.058
do Vale, M. A. D., Gomes, F. A., Cunha dos Santos, B. R., and Batista Ferreira, J. (2018). Honey quality of Melipona sp. bees in Acre, Brazil. Acta Agron. 67, 201–207. doi: 10.15446/acag.v67n2.60836
Dos Santos, A. C., Biluca, F. C., Braghini, F., Gonzaga, L. V., Costa, A. C. O., and Fett, R. (2021). Phenolic composition and biological activities of stingless bee honey: an overview based on its aglycone and glycoside compounds. Food Res. Int., 147, 110553. doi: 10.1016/j.foodres.2021.110553
Duarte, A. W. F., Vasconcelos, M. R. D. S., Oda-Souza, M., Oliveira, F. F. D., and And López, A. M. Q. (2018). Honey and bee pollen produced by Meliponini (Apidae) in Alagoas, Brazil: multivariate analysis of physicochemical and antioxidant profiles. Food Sci.Technol. 38, 493–503 doi: 10.1590/fst.09317
Esa, N. E. F., Ansari, M. N. M., Razak, S. I. A., Ismail, N. I., Jusoh, N., Zawawi, N. A., et al. (2022). A Review on recent progress of stingless bee honey and its hydrogel-based compound for wound care management. Mol. 27, 3080. doi: 10.3390/molecules27103080
Estevinho, L., Pereira, A. P., Moreira, L., Dias, L. G., and Pereira, E. (2008). Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 46, 3774–3779. doi: 10.1016/j.fct.2008.09.062
EU (2014). Directive 2014/63/ EU of the European parliament and of the council of 15 May 2014 amending council directive 2001/110/EC relating to honey. Off.J. Eur. Un. L 164, 1–5.
Evahelda, S., Aini, S. N., and Afriani, Z. L. (2021). Chemical characteristics of kelulut honey (Trigona Sp.) in Bangka Tengah District, Indonesia. Int. E-Conf. Sust. Agric. Farm. Sys. 694, 12072. doi: 10.1088/1755-1315/694/1/012072
Finola, M. S., Lasagno, M. C., and Marioli, J. M. (2007). Microbiological and chemical characterization of honeys from central Argentina. Food Chem. 100, 1649–1653. doi: 10.1016/j.foodchem.2005.12.046
Gela, A., Hora, Z. A., Kebebe, D., and Gebresilassie, A. (2021). Physico-chemical characteristics of honey produced by stingless bees (Meliponula beccarii) from West Showa zone of Oromia Region, Ethiopia. Heliyon, 7, e05875. doi: 10.1016/j.heliyon.2020.e05875
González-Miret, M. L., Terrab, A., Hernanz, D., Fernández-Recamales, M. Á., and Heredia, F. J. (2005). Multivariate correlation between color and mineral composition of honeys and by their botanical origin. J. Agric. Food Chem. 53, 2574–2580. doi: 10.1021/jf048207p
González-Montemayor, Á. M., Flores-Gallegos, A. C., Serrato-Villegas, L. E., López-Pérez, M. G., Montañez-Sáenz, J. C., and Rodríguez-Herrera, R. (2019). “Honey and Syrups: Healthy and natural sweeteners with functional properties,” in Natural Beverages, eds. Grumezescu, A. M., and Holban, A. M. Cambridge, MA: Academic Press, 143–177.
Grandoa, R. C., Weisa, C. M. S. C., Bertana, L. C., Tormena, L., Bonattob, C., Mossib, A. J., et al. (2023). Physicochemical characterization and acceptance of honey from stingless bees. Food Hum. 1, 71–77. doi: 10.1016/j.foohum.2023.04.005
Guerrini, A., Bruni, R., Maietti, S., Poli, F., Rossi, D., Paganetto, G., et al. (2009). Ecuadorian stingless bee (Meliponinae) honey: a chemical and functional profile of an ancient health product. Food Chem. 114, 1413–1420. doi: 10.1016/j.foodchem.2008.11.023
Habryka, C., Socha, R., and Juszczak, L. (2021). Effect of bee pollen addition on the polyphenol content, antioxidant activity, and quality parameters of honey. Antiox. 10, 810. doi: 10.3390/antiox10050810
Haffejee, I., and Moosa, A. (1985). Honey in the treatment of infantile gastroenteritis. BMJ 290, 1866–1867. doi: 10.1136/bmj.290.6485.1866
Han, J. Y., Ahn, S. Y., Kim, C. S., Yoo, S. K., Kim, S. K., Kim, H. C., et al. (2012). Protection of apigenin against kainate-induced excitotoxicity by anti-oxidative effects. Biol. Pharmac. Bull. 35, pp.1440–1446. doi: 10.1248/bpb.b110686
Haron, M. N., Rahman, W. F. W. A., Sulaiman, S. A., and Mohamed, M. (2014). Tualang honey ameliorates restraint stress-induced impaired pregnancy outcomes in rats. Eur. J. Integr. Med. 6, 657–663. doi: 10.1016/j.eujim.2014.07.001
Hau-Yama, N. E., Magaña-Ortiz, D., Oliva, A. I., and Ortiz-Vázquez, E. (2020). Antifungal activity of honey from stingless bee Melipona beecheii against Candida albicans. J. Apicul. Res. 59, 12–18. doi: 10.1080/00218839.2019.1665247
Hbibi, A., Sikkou, K., Khedid, K., El Hamzaoui, S., Bouziane, A., and Benazza, D. (2020). Antimicrobial activity of honey in periodontal disease: a systematic review. J. Antimicr. Chemother. 75, 807–826. doi: 10.1093/jac/dkz527
Ikhsan, L. N., Chin, K., and Ahmad, F. (2022). Methods of the dehydration process and its effect on the physicochemical properties of stingless bee honey: a review. Mol. 27, 7243. doi: 10.3390/molecules27217243
Ilechie, A. A., Kwapong, P. K., Mate-Kole, E., Kyei, S., and Darko-Takyi, C. (2012). The efficacy of stingless bee honey for the treatment of bacteria-induced conjunctivitis in guinea pigs. J. Exp. Pharm. 63–68. doi: 10.2147/JEP.S28415
Ishizu, E., Honda, S., Ohta, T., Vongsak, B., and Kumazawa, S. (2019). Component analysis and antiangiogenic activity of Thailand stingless bee propolis. Makara J. Tech. 23, 77–83. doi: 10.7454/mst.v23i2.3703
Ismail, N. I., Kadir, M. R. A., Zulkifli, R. M., and Mohamed, M. (2021). Comparison of physicochemical, total protein and antioxidant profiles between Malaysian Apis and Trigona honeys. Mal. J. Analys. Sci. 25, 243–256.
Julika, W. N., Ajit, A., Ismail, N., Aqilah, N., Naila, A., and Sulaiman, A. Z. (2020). Sugar profile and enzymatic analysis of stingless bee honey collected from local market in Malaysia. IOP Conf. Series: Mat. Sci. Eng. 736, 062001. doi: 10.1088/1757-899X/736/6/062001
Jung, U. J., Lee, M. K., Park, Y. B., Jeon, S. M., and Choi, M. S. (2006). Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J. Pharmacy. Exp. Therap. 318, 476–483. doi: 10.1124/jpet.106.105163
Kadar, M. A. N. N., Ahmad, F., Teoh, S. L., and Yahaya, M. F. (2022). Comparable benefits of stingless bee honey and caffeic acid in mitigating the negative effects of metabolic syndrome on the brain. Antiox. 11:2154. doi: 10.3390/antiox11112154
Karabagias, I. K., Dimitriou, E., Kontakos, S., and Kontominas, M. G. (2016). Phenolic profile, colour intensity, and radical scavenging activity of Greek unifloral honeys. Eur. Food Res. Tech. 242(8), 1201–1210. doi: 10.1007/s00217-015-2624-6
Karnia, I., Hamidah, S., and Thamrin, G. A. R. (2020). Pengaruh masa simpan madu kelulut (Trigona sp) terhadap kadar gula pereduksi dan keasaman. Jurnal Sylva Scienteae. 2, 1093–1099.
Karuppaiah, V., Gadge, A. S., Shirsat, D. V., Soumia, P. S., Mainkar, P., Kumar, S., et al. (2023). The complete mitochondrial genome of the Indian dammer bee, Tetragonula iridipennis, and the phylogenomics of Meliponini. Front. Ecol. Evol. 11, 1171242. doi: 10.3389/fevo.2023.1171242
Karuppaiah, V., Gadge, A. S., Soumia, P. S., Vitthal, D., Shirsat, S. S. S., and Mahajan, V. (2022). Survey and documentation of native stingless bees from western Maharashtra and their role in onion pollination. Sust. Agric. Innov. for Res. Agri-Food Sys. 321, 55362. doi: 10.55362/IJE/IESIC/2022/PROC
Khalil, M. L., and Sulaiman, S. A. (2010). The potential role of honey and its polyphenols in preventing heart disease: a review. Afr. J. Trad.Compl. Alt. Med. 7, 56693. doi: 10.4314/ajtcam.v7i4.56693
Khongkwanmueang, A., Nuyu, A., Straub, L., and Maitip, J. (2020). Physicochemical profiles, antioxidant and antibacterial capacity of honey from stingless bee Tetragonula laeviceps species complex. E3S Web of Conf. 141, 03007. doi: 10.1051/e3sconf/202014103007
Koh, S. H., Kwon, H., Kim, K. S., Kim, J., Kim, M. H., Yu, H. J., et al. (2004). Epigallocatechin gallate prevents oxidative-stress-induced death of mutant Cu/Zn-superoxide dismutase (G93A) motoneuron cells by alteration of cell survival and death signals. Toxicol. 202, 213–225. doi: 10.1016/j.tox.2004.05.008
Krishnasree, V., and Ukkuru, P. M. (2015). Phytochemical screening and antioxidant activity of different bee honeys. J. Med. Herbs and Ethnomed. 1, 38–44. doi: 10.5455/jmhe.2015-07-013
Krishnasree, V., and Ukkuru, P. M. (2017). In vitro antidiabetic activity and glycemic index of bee honeys. Ind. J. Trad. Knowl. 16, 134–140. Available online at: http://nopr.niscpr.res.in/handle/123456789/37026
Kustiawan, P. M., Puthong, S., Arung, E. T., and Chanchao, C. (2014). In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines. Asian Pacific J. Trop. Biomed. 4, 549–556. doi: 10.12980/APJTB.4.2014APJTB-2013-0039
Kwakman, P. H. S., and Zaat, S. A. J. (2012). Antibacterial components of honey. IUBMB Life. 64, 48–55. doi: 10.1002/iub.578
Kwapong, P., Aidoo, K., Combey, R., and Karikari, A. (2010). “Stingless bee: importance,” in Management and Utilisation: a Training Manual for Stingless Bee Keeping. Accra: Unimax Macmillan Ltd.
Kwon, C. Y. (2023). Research Trends of Pharmacopuncture: a bibliometric analysis using VOSviewer (2007-2023). J. Pharmacopuncture. 26, 227. doi: 10.3831/KPI.2023.26.3.227
Lacerda, J. J. D. J., Santos, J. S. D., Santos, S. A. D., Rodrigues, G. B., and Santos, M. L. P. D. (2010). Influence of physicochemical characteristics and elemental composition on the colors of honeys produced by Apis mellifera in southwestern Bahia using multivariate analysis. New Chem. 33, 1022–1026. doi: 10.1590/S0100-40422010000500003
Lage, L. G., Coelho, L. L., Resende, H. C., Tavares, M. G., Campos, L. A., and Fernandes-Salomão, T. M. (2012). Honey physicochemical properties of three species of the brazilian Melipona. Anais da Academia Brasileira de Ciências. 84, 605–608. doi: 10.1590/S0001-37652012005000051
Lavinas, F. C., Gomes, B. A., Silva, M. V. T., Nunes, R. M., Leitão, S. G., Moura, M. R. L., et al. (2023). Discriminant analysis of Brazilian stingless bee honey reveals an iron-based biogeographical origin. Foods. 12, 180. doi: 10.3390/foods12010180
Lee, H., Churey, J. J., and Worobo, R. W. (2008). Antimicrobial activity of bacterial isolates from different floral sources of honey. Int. J. Food Microbiol. 126, 240–244. doi: 10.1016/j.ijfoodmicro.2008.04.030
Lemos, M. S., Venturieri, G. C., Dantas Filho, H. A., and Dantas, K. G. (2018). Evaluation of the physicochemical parameters and inorganic constituents of honey from the Amazon region. J. Apic. Res. 57, 135–144. doi: 10.1080/00218839.2017.1338120
Liaqat, W., Altaf, M. T., Barutçular, C., Zayed, E. M., and Hussain, T. (2023). Drought and sorghum: a bibliometric analysis using VOS viewer. J. Biomol. Struct. Dyn. 2023, 1–13. doi: 10.1080/07391102.2023.2269279
Lin, D., Xiao, M., Zhao, J., Li, Z., Xing, B., Li, X., et al. (2016). An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Mol. 21, 1374. doi: 10.3390/molecules21101374
López-Garay, L. A., Trejo-Téllez, L. I., Gómez-Merino, F. C., Contreras-Oliva, A., Pérez-Sato, J. A., and Salinas-Ruiz, J. (2023). Propiedades fisicoquímicas de miel de Scaptotrigona mexicana de la Región Montañosa de Veracruz, México. Ecosistemas y Recursos Agropecuarios. 10, 1. doi: 10.19136/era.a10n1.3380
Machado De-Melo, A. A., Almeida-Muradian, L. B. D., Sancho, M. T., and Pascual-Maté, A. (2018). Composition and properties of Apis mellifera honey: a review. J. Apicul. Res. 57, 5–37. doi: 10.1080/00218839.2017.1338444
Mädge, I., Gehling, M., Schöne, C., Winterhalter, P., and These, A. (2020). Pyrrolizidine alkaloid profiling of four Boraginaceae species from Northern Germany and implications for the analytical scope proposed for monitoring of maximum levels. Food Add. Cont. - Part A. 37, 1339–1358. doi: 10.1080/19440049.2020.1757166
Mahani, M., Savitri, S. R., and Subroto, E. (2022). Hubungan kadar flavonoid dan aktivitas antioksidan madu dari berbagai provinsi di indonesia. J. Sains dan Teknologi Pangan 7, 5255–5268.
Malaysian Standards (2017). Kelulut (stingless bee) Honey-Specification: MS 2683: 2017. Cyberjaya: Department of Standard Malaysia.
Malik, A. N., Mohamed, M., Mustafa, M. Z., and Zainuddin, A. (2020). In vitro modulation of extracellular matrix genes by stingless bee honey in cellular aging of human dermal fibroblast cells. J. food Biochem. 44, e13098. doi: 10.1111/jfbc.13098
Marinus, J. S. (2006). Water content of stingless bee honeys (Apidae, Meliponini): interspecific variation and comparison with honey of Apis mellifera. Apidologie. 37, 480–486.
Massaro, C. F., Shelley, D., Heard, T. A., and Brooks, P. (2014). In vitro antibacterial phenolic extracts from “sugarbag” pot-honeys of Australian stingless bees (Tetragonula carbonaria). J. Agr. Food Chem. 62, 12209–12217. doi: 10.1021/jf5051848
Menezes, C., Vollet-Neto, A., Contrera, F. A. F. L., Venturieri, G. C., and Imperatriz-Fonseca, V. L. (2013). “The Role of useful microorganisms to stingless bees and stingless beekeeping,” in Pot-Honey, eds. P. Vit, S. Pedro, D. Roubik. New York: Springer. doi: 10.1007/978-1-4614-4960-7_10
Miorin, P., Levy Junior, N., Custodio, A., Bretz, W., and Marcucci, M. (2003). Antibacterial activity of honey and propolis from Apis mellifera and Tetragonisca angustula against Staphylococcus aureus. J. Appl. Microbiol. 95, 913–920. doi: 10.1046/j.1365-2672.2003.02050.x
Mohamed, M., Sulaiman, S. A., and Sirajudeen, K. N. S. (2013). Protective effect of honey against cigarette smoke induced-impaired sexual behavior and fertility of male rats. Toxicol. Ind. Health. 29, 264–271. doi: 10.1177/0748233711432568
Mohammad, S. M., Mahmud-Ab-Rashid, N. K., and Zawawi, N. (2020). Botanical origin and nutritional values of bee bread of stingless bee (Heterotrigona itama) from Malaysia. J. Food Qual. 2020, 1–12. doi: 10.1155/2020/2845757
Mokarrami, S., Jahanshahi, M., Elyasi, L., Badelisarkala, H., and Khalili, M. (2022). Naringin prevents the reduction of the number of neurons and the volume of CA1 in a scopolamine-induced animal model of Alzheimer's disease (AD): a stereological study. Int. J. Neurosci. 18, 1–8. doi: 10.1080/00207454.2022.2102981
Mokaya, H. O., Nkoba, K., Ndunda, R. M., and Vereecken, N. J. (2022). Characterization of honey produced by sympatric species of Afrotropical stingless bees (Hymenoptera, Meliponini). Food Chem. 366, 130597. doi: 10.1016/j.foodchem.2021.130597
Molan, P., and Betts, J. A. (2004). Clinical usage of honey as a wound dressing: an update. J. Wound Care. 13, 353–356. doi: 10.12968/jowc.2004.13.9.26708
Moniruzzaman, M., Chowdhury, M. A. Z., Rahman, M. A., Sulaiman, S. A., and Gan, S. H. (2014). Determination of mineral, trace element, and pesticide levels in honey samples originating from different regions of Malaysia compared to Manuka honey. BioMed. Res. Int. 1–10. doi: 10.1155/2014/359890
Moreira, R., Pereira, D. M., Valentão, P., and Andrade, P. B. (2018). Pyrrolizidine alkaloids: chemistry, pharmacology, toxicology and food safety. Int. J. Mol. Sci. 19, 1668. doi: 10.3390/ijms19061668
Mosavat, M., Ooi, F. K., and Mohamed, M. (2014). Effects of honey supplementation combined with different jumping exercise intensities on bone mass, serum bone metabolism markers and gonadotropins in female rats. BMC Complement. Altern. Med. 14, 126. doi: 10.1186/1472-6882-14-126
Mulugeta, M., and Belay, A. (2022). Comb honey and processed honey of Croton macrostachyus and Schefflera abyssinica honey differentiated by enzymes and antioxidant properties, and botanical origin. Heliyon. 8, 5. doi: 10.1016/j.heliyon.2022.e09512
Mustafa, M. Z., Zulkifli, F. N., Fernandez, I., Mariatulqabtiah, A. R., Sangu, M., Nor Azfa, J., et al. (2019). Stingless bee honey improves spatial memory in mice, probably associated with brain-derived neurotrophic factor (BDNF) and inositol 1, 4, 5-triphosphate receptor type 1 (Itpr1) genes. Evidence-Based Compl. Alt. Med. 1–11. doi: 10.1155/2019/8258307
Nanda, V., Sarkar, B. C., Sharma, H. K., and Bawa, A. S. (2003). Physico-chemical properties and estimation of mineral content in honey produced from different plants in Northern India. J. Food Comp. Anal. 16, 613–619. doi: 10.1016/S0889-1575(03)00062-0
Ng, W. J., Lye, P. Y., Chan, Y. J., Lau, Z. K., and Ee, K. Y. (2017). Synergistic effect of Trigona honey and ampicillin on Staphylococcus aureus isolated from infected wound, Inte. J. Pharma. 13, 403–407. doi: 10.3923/ijp.2017.403.407
Ngalimat, M. S., Rahman, R. N. Z. R., Yusof, M. T., Syahir, A., and Sabri, S. (2019). Characterisation of bacteria isolated from the stingless bee, Heterotrigona itama, honey, bee bread and propolis.Peer Journal. 7, Article e7478. doi: 10.7717/peerj.7478
Nishio, E. K., Ribeiro, J. M., Oliveira, A. G., Andrade, C. G. T. J., Proni, E. A, Kobayashi, R. K. T., et al. (2016). Antibacterial synergic effect of honey from two stingless bees: Scaptotrigona bipunctata Lepeletier, 1836, and S. postica Latreille, 1807. Sci. Rep. 6, 21641 doi: 10.1038/srep21641
Nordin, A., Sainik, N. Q. A. V., Chowdhury, S. R., Saim, A. B., and Idrus, R. B. H. (2018). Physicochemical properties of stingless bee honey from around the globe: a comprehensive review. J. Food Comp. Anal. 73, 91–102. doi: 10.1016/j.jfca.2018.06.002
Nweze, J. A., Okafor, J. I., Nweze, E. I., and Nweze, J. E. (2017). Evaluation of physicochemical and antioxidant properties of two stingless bee honey: a comparison with Apis mellifera honey from Nsukka, Nigeria. BMC Research Notes. 10, 4–9. doi: 10.1186/s13104-017-2884-2
Owen, E. R. (2023). “Geographical, entomological and botanical origins of honey,” in Honey – Composition and Properties, ed I. Muhammad (Intech Open). doi: 10.5772/intechopen.106414
Park, S., Moon, N. R., Kang, S., and Kim, D. S. (2022). Ferulic acid and vinpocetine intake improves memory function by enhancing insulin sensitivity and reducing neuroinflammation and oxidative stress in type 2 diabetic animals with induced Alzheimer's disease. J. funct. Foods 95, 105180. doi: 10.1016/j.jff.2022.105180
Pascual-Mate, A., Oses, S. M., Fernandez-Muino, M. A., and Sancho, M. T. (2018). Methods of analysis of honey. J. Apic. Res. 57, 38–74. doi: 10.1080/00218839.2017.1411178
Pereira, J. R., Campos, A. N. D. R., de Oliveira, F. C., Silva, V. R., David, G. F., Da Silva, J. G., et al. (2020). Physical- chemical characterization of commercial honeys from Minas Gerais, Brazil. Food Biosci. 36, 100644. doi: 10.1016/j.fbio.2020.100644
Popova, M., Gerginova, D., Trusheva, B., Simova, S., Tamfu, A. N., Ceylan, O., et al. (2021). A preliminary study of chemical profiles of honey, cerumen, and propolis of the African stingless bee Meliponula ferruginea. Foods 10, 997. doi: 10.3390/foods10050997
Potts, S. G., Imperatriz-Fonseca, V., Ngo, H. T., Aizen, M. A., Biesmeijer, J. C., Breeze, T. D., et al. (2016). Safeguarding pollinators and their values to human well-being. Nature 540, 220–229. doi: 10.1038/nature20588
Prakash, V., Martin-Belloso, O., Keener, L., Astley, S. B., Braun, S., McMahon, H., et al. (2015). Regulating Safety of Traditional and Ethnic Foods. Cambridge, MA: Academic Press.
Praptiwi, P., Fathoni, A., Efendy, O., Wulansari, D., and Agusta, A. (2023). Evaluation of antibacterial and antioxidant activities of stingless bee (Tetragonula laeviceps) hive from Purwodadi botanical garden and LC/MS Profiles of Dichloromethane fraction. AHSR. 56, 349–359. doi: 10.2991/978-94-6463-112-8_33
Rajabzadeh, A., Sagha, M., Gholami, M. R., and Hemmati, R. (2015a). Honey and vitamin E restore the plasma level of gonadal hormones and improve the fertilization capacity in noise-stressed rats. Crescent J. Med. Biol. Sci. 2, 064–068.
Rajabzadeh, A., Saki, G., Khodadadi, A., Sarkaki, A., Jafai, A., and Hemadi, M. (2015b). A survey of the relationship between noised pollution, honey and vitamin E and plasma level of blood sexual hormones in noise-exposed rats. Jentashapir J. Health Res. 6, 27331. doi: 10.5812/jjhr.27331
Ramli, A. S., Basrawi, F., Idris, D. M. N. D., bin Yusof, M. H., Ibrahim, T. K., Mustafa, Z., et al. (2017). A new dewatering technique for stingless bees honey. MATEC Web Conf. 131, 03014. doi: 10.1051/matecconf/201713103014
Ramli, E. S. M., Kamaruzzaman, M. A., Thanu, A. S., Mohamed, N., Fahami, N. A. M., and Yusuf, M. R. (2020). Potential benefical effects of stingless bee honey (kelulut honey) on bones exposed to long-term dexamethasone. Int. J. Food Res. 7, 23–33.
Ramli, N. Z., Chin, K. Y., Zarkasi, K. A., and Ahmad, F. (2019). The beneficial effects of stingless bee honey from Heterotrigona itama against metabolic changes in rats fed with high carbohydrate and high-fat diet. Int. J. Environ. Res. Public Health. 16, 4987. doi: 10.3390/ijerph16244987
Ramon-Sierra, J., Martinez-Guevara, J. L., Pool-Yam, L., MaganaOrtiz, D., Yam-Puc, A., and Ortiz-Vazquez, E. (2020). Effects of phenolic and protein extracts from Melipona beecheii honey on pathogenic strains of Escherichia coli and Staphylococcus aureus. Food Sci. Biotechnol. 29, 1013–1021. doi: 10.1007/s10068-020-00744-4
Ranneh, Y., Akim, A. M., Hamid, H. A., Khazaai, H., Fadel, A., and Mahmoud, A. M. (2019). Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr. Metabol. 16, 1–17. doi: 10.1186/s12986-019-0341-z
Ranneh, Y., Ali, F., Zarei, M., Akim, A. M., Abd Hamid, H., and Khazaai, H. (2018). Malaysian stingless bee and Tualang honeys: a comparative characterization of total antioxidant capacity and phenolic profile using liquid chromatography-mass spectrometry. LWT. 89, 1–9. doi: 10.1016/j.lwt.2017.10.020
Reni-Yusli, E.M, Bachtia, B., Fatma-Suni, D.B, Sutjiat, A., et al. (2016). Effect of Rambutan-honey and its flavonoid on TGF-b1 induce fibroplasia oral wound healing. Res. J. Med. Plants. 10, 435–442. doi: 10.3923/rjmp.2016.435.442
Rocha, L. D., Monteiro, M. C., and Teodoro, A. J. (2012). Anticancer properties of hydroxycinnamic acids-a review. Cancer Clin. Oncol. 1, 109–121. doi: 10.5539/cco.v1n2p109
Rosli, F. N., Hazemi, M. H. F., Akbar, M. A., Basir, S., Kassim, H., and Bunawan, H. (2020). Stingless bee honey: Evaluating its antibacterial activity and bacterial diversity. Insects. 11, 500. doi: 10.3390/insects11080500
Sabir, A., Agus, A., and Sahlan, M. (2021). The minerals content of honey from stingless bee Tetragonula laeviceps from different regions in Indonesia. Livest. Res. Rural. Dev. 33, 2.
Sahlan, M., Rahmawati, O., Pratami, D. K., Raffiudin, R., Mukti, R. R., and Hermasyah, H. (2020). The effects of stingless bee (Tetragonula biroi) honey on streptozotocin-induced diabetes mellitus in rats. Saudi J. Biol. Sci. 27, 2025–2030. doi: 10.1016/j.sjbs.2019.11.039
Salis, S., Spano, N., Ciulu, M., Floris, I., Pilo, M. I., and Sanna, G. (2021). Electrochemical determination of the ‘furanic index' in honey. Molecules 26, 4115. doi: 10.3390/molecules26144115
Sant'ana, R. D. S., de Carvalho, C. A. L., Oda-Souza, M., Souza, B. D. A., and Dias, F. D. S. (2020). Characterization of honey of stingless bees from the Brazilian semi-arid region. Food Chem. 327, 127041. doi: 10.1016/j.foodchem.2020.127041
Santisteban, R. M., Cabrera, S. P., Neto, J. F., Silva, E., Correia, R. C., Alves, R. F., et al. (2019). Análises melissopalinológicas, físico-químicas, atividade antirradicalar e perfil químico por UPLC-DAD-QTOF-MS/MS dos méis de Frieseomelitta doederleini (abelha branca): comparação com os fenólicos presentes nas flores de Mimosa tenuiflora (jurema preta). Química Nova. 42, 874–884 doi: 10.21577/0100-4042.20170407
Saxena, A. K., Phyu, H. P., Al-Ani, I. M., and Talib, N. A. (2014). Potential protective effect of honey against chronic cerebral hypoperfusion-induced neurodegeneration in rats. J. Anat. Soc. India. 63, 151–155. doi: 10.1016/j.jasi.2014.11.003
Schramm, S., Köhler, N., and Rozhon, W. (2019). Pyrrolizidine alkaloids: biosynthesis, biological activities and occurrence in crop plants. Mol. 24, 498. doi: 10.3390/molecules24030498
Schvezov, N., Pucciarelli, A. B., Valdes, B., and Dallagnol, A. M. (2020). Characterization of yateí (Tetragonisca fiebrigi) honey and preservation treatments: dehumidification, pasteurization and refrigeration. Food Cont. 111, 107080. doi: 10.1016/j.foodcont.2019.107080
Se, K. W., Ghoshal, S. K., Wahab, R. A., Ibrahim, R. K. R., and Lani, M. N. A. (2018). Simple approach for rapid detection and quantification of adulterants in stingless bees (Heterotrigona Itama) honey. Food Res. Int. 105, 453–460. doi: 10.1016/j.foodres.2017.11.012
Seng, E., and Tang, T. H. (2020). Anti-inflammatory properties of stingless bee honey may reduce the severity of pulmonary manifestations in COVID-19 infections? Malaysian J. Med. Sci. 27, 150–152. doi: 10.21315/mjms2020.27.3.16
Sgariglia, M. A., Vattuone, M. A., Vattuone, S. M. M., Soberon, J. R., and Sampietro, D. A. (2010). Properties of honey from Tetragonisca angustula fiebrigi and Plebeia wittmanni of Argentina. Apidol. 41, 667–675. doi: 10.1051/apido/2010028
Shadan, A. F., Mahat, N. A., Wan Ibrahim, W. A., Ariffin, Z., and Ismail, D. (2018). Provenance establishment of stingless bee honey using multi-element analysis in combination with chemometrics techniques. J. Forensic Sci. 63, 80–85. doi: 10.1111/1556-4029.13512
Shaik, M. I., Zulkifli, N. Z., Sayyed, J. A., Nannepaga, J. S., Gurusubramanian, G., and Abd Razak, S. B. (2023). Dehydration Treatment effect on the physicochemical properties and microbial population of stingless bee honey from three different species. Rec. Adv. Global Melipon. 2023, 121–140. doi: 10.4018/978-1-6684-6265-2.ch007
Shamsudin, S., Selamat, J., Sanny, M., Abd Razak, S. B., Jambari, N. N., Mian, Z., et al. (2019). Influence of origins and bee species on physicochemical, antioxidant properties and botanical discrimination of stingless bee honey. Int. J. Food Prop. 22, 239–264. doi: 10.1080/10942912.2019.1576730
Shooshtari, M. K., Sarkaki, A., Mansouri, S. M. T., Badavi, M., Khorsandi, L., and Dehcheshmeh, G. M. (2020). Protective effects of chrysin against memory impairment, cerebral hyperemia and oxidative stress after cerebral hypoperfusion and reperfusion in rats. Metab. Brain Dis. 35, 401–412. doi: 10.1007/s11011-019-00527-9
Singh, A., Upadhayay, S., and Mehan, S. (2021). Inhibition of c-JNK/p38MAPK signalling pathway by apigenin prevents neurobehavioral and neurochemical defects in ethidium bromide-induced experimental model of multiple sclerosis in rats: evidence from CSF, blood plasma and brain samples. Phytomed. Plus 1, 100139. doi: 10.1016/j.phyplu.2021.100139
Singh, I., and Singh, S. (2018). Honey moisture reduction and its quality. J. food Sci. Technol. 55, 3861–3871. doi: 10.1007/s13197-018-3341-5
Solayman, M., Islam, M. A., Paul, S., Ali, Y., Khalil, M. I., Alam, N., et al. (2016). Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: a comprehensive review. Compr. Rev. Food Sci. Food Saf. 15, 219–233. doi: 10.1111/1541-4337.12182
Souza, B., Roubik, D., Barth, O., Heard, T., Enríquez, E., Carvalho, C., et al. (2006). Composition of stingless bee honey: setting quality standards. Interciencia 31, 867–875.
Souza, E. C. A., Menezes, C., and Flach, A. (2021). Stingless bee honey (Hymenoptera, Apidae, Meliponini): a review of quality control, chemical profile, and biological potential. Apidologie 52, 113–132. doi: 10.1007/s13592-020-00802-0
Spilioti, E., Jaakkola, M., Tolonen, T., Lipponen, M., Virtanen, V., Chinou, I., et al. (2014). Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PloS ONE 9, e94860. doi: 10.1371/journal.pone.0094860
Sun, Z. Q., Meng, F. H., Tu, L. X., and Sun, L. (2019). Myricetin attenuates the severity of seizures and neuroapoptosis in pentylenetetrazole kindled mice by regulating the of BDNF-TrkB signaling pathway and modulating matrix metalloproteinase-9 and GABAA. Exp. Therap. Med. 17, 3083–3091. doi: 10.3892/etm.2019.7282
Suntiparapop, K., Prapaipong, P., and Chantawannakul, P. (2012). Chemical and biological properties of honey from Thai stingless bee (Tetragonula leaviceps). J. Apic. Res. 51, 45–52. doi: 10.3896/IBRA.1.51.1.06
Tafere, D. A. (2021). Chemical composition and uses of Honey: a review. J. Food Sci. Nutr. Res., 4, 194–201.
Tasca, J. A., Smith, C. R., Burzynski, E. A., Sundberg, B. N., Lagalante, A. F., Livshultz, T., et al. (2018). HPLC-MS detection of pyrrolizidine alkaloids and their N-oxides in herbarium specimens dating back to the 1850s. Appl. Plant Sci. 6, e1143. doi: 10.1002/aps3.1143
Thrasyvoulou, A., Tananaki, C., Goras, G., Karazafiris, E., Dimou, M., Liolios, V., et al. (2018). Legislation of honey criteria and standards. J. Apicultural Res. 57, 88–96. doi: 10.1080/00218839.2017.1411181
Tkáč, M., Vorlov,á, L., Borkovcov,á, I., and Golian, J. (2022). Physicochemical and bioactive characterization of beekeeper and market honeys. Emir. J. Food Agric. doi: 10.9755/ejfa.2022.v34.i4.2851
Tuksitha, L., Chen, Y. L. S., Chen, Y. L., Wong, K. Y., and Peng, C. C. (2018). Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak). J. Asia-Pacific Entomol. 21, 563–570. doi: 10.1016/j.aspen.2018.03.007
Umaña, E., Zamora, G., Aguilar, I., Arias, M. L., Pérez, R., Sánchez, L. A., et al. (2021). Physicochemical differentiation of stingless bee honey from Costa Rica. J. Apicultural Res. 19, 1–10. doi: 10.1080/00218839.2021.1903737
Vattuone, M. A., Quiroga, E. N., Sgariglia, M. A., Soberón, J. R., Jaime, G. S., Arriazu, M. E. M., et al. (2007). Compuestos fenólicos totales, flavonoides, prolina y capacidad captadora de radicales libres de mieles de Tetragonisca angustula Fiebrigi (Schwarz, 1938) y de Plebeia wittmanni. B. Latinoam. Caribe Pl. 6, 299–300.
Venturini, K. S., Sarcinelli, M. F., and Silva, D. (2007). Characteristics of Honey. Technical Bulletin of the Federal University of Espírito Santo-UFES.
Villacrés-Granda, I., Coello, D., Proaño, A., Ballesteros, I., Roubik, D. W., Jijón, G., et al. (2021). Honey quality parameters, chemical composition and antimicrobial activity in twelve Ecuadorian stingless bees (Apidae: Apinae: Meliponini) tested against multiresistant human pathogens. LWT 140, 110737. doi: 10.1016/j.lwt.2020.110737
Villas-Bôas, J. K., and Malaspina, O. (2005). Parâmetros físico-químicos propostos para o controle de qualidade do mel de abelhas indígenas sem ferrão no Brasil. Mensagem Doce 82, 6–16.
Vit, P., Medina, M., and Eunice Enríquez, M. (2004). Quality standards for medicinal uses of Meliponinae honey in Guatemala, Mexico and Venezuela. Bee World 85, 2–5. doi: 10.1080/0005772X.2004.11099603
Wille, A. (1983). Biology of the stingless bees. Annu. Rev. Entomol., 28, 41–64. doi: 10.1146/annurev.en.28.010183.000353
Wongsa, K., Meemongkolkiat, T., Duangphakdee, O., Prasongsuk, S., and Rattanawannee, A. (2023). Physicochemical properties, phenolic, flavonoid contents and antioxidant potential of stingless bee (Heterotrigona itama) honey from Thailand. Curr. Res. Nutr. Food Sci. 11, 1. doi: 10.12944/CRNFSJ.11.1.18
Yadav, R. K., Mehan, S., Sahu, R., Kumar, S., Khan, A., Makeen, H. A., et al. (2022). Protective effects of apigenin on methylmercury-induced behavioral/neurochemical abnormalities and neurotoxicity in rats. Human Exper. Toxicol. 41, 9603271221084276. doi: 10.1177/09603271221084276
Yazan, L. S., Zainal, N. A., Ali, R. M., Zali, M., Shyfiq, M. F., Sze, O. Y., et al. (2018). Antiulcer properties of kelulut honey against ethanol-induced gastric ulcer. Pertanika J. Sci. Technol. 26, 121–132.
Yazan, L. S., Zali, M. M. F. S., Ali, M. R., Zainal, N. A., Esa, N., Sapuan, S., et al. (2016). Chemopreventive properties and toxicity of kelulut honey in sprague dawley rats induced with Azoxymethane. BioMed Res. Int. 40, 36926. doi: 10.1155/2016/4036926
Yong, P. Y. A., Yip, A. J. W., Islam, F., Hong, H. J., Teh, Y. E., Tham, C. L., et al. (2023). The anti-allergic potential of stingless bee honey from different botanical sources via modulation of mast cell degranulation. Res. Square. doi: 10.1186/s12906-023-04129-y
Zapata-Vahos, I. C., Henao-Rojas, J. C., Yepes-Betancur, D. P., Marín-Henao, D., Giraldo Sánchez, C. E., Calvo-Cardona, S. J., et al. (2023). Physicochemical parameters, antioxidant capacity, and antimicrobial activity of honeys from tropical forests of Colombia: Apis mellifera and Melipona eburnea. foods. 12, 1001. doi: 10.3390/foods12051001
Zawawi, N., Zhang, J., Hungerford, N. L., Yates, H. S., Webber, D. C., Farrell, M., et al. (2022). Unique physicochemical properties and rare reducing sugar trehalose mandate new international regulation for stingless bee honey. Food Chem. 373, 131566 doi: 10.1016/j.foodchem.2021.131566
Zhang, S. H., Liu, D., Hu, Q., Zhu, J., Wang, S., and Zhou, S. (2019a). Ferulic acid ameliorates pentylenetetrazol-induced seizures by reducing neuron cell death. Epilepsy Res. 156, 106183. doi: 10.1016/j.eplepsyres.2019.106183
Zhang, X., Bu, H., Jiang, Y., Sun, G., Jiang, R., Huang, X., et al. (2019b). The antidepressant effects of apigenin are associated with the promotion of autophagy via the mTOR/AMPK/ULK1 pathway. Mol. Med. Rep. 20, 2867–2874. doi: 10.3892/mmr.2019.10491
Zhang, Y., Zhao, J., Afzal, O., Kazmi, I., Al-Abbasi, F. A., and Altamimi, A. S. A. (2021). Neuroprotective role of chrysin-loaded poly (lactic-co-glycolic acid) nanoparticle against kindling-induced epilepsy through Nrf2/ARE/HO-1 pathway. J. Biochem. Mol. Toxicol. 35:e22634. doi: 10.1002/jbt.22634
Keywords: antioxidant, honey, physicochemical, stingless bee honey, therapeutic
Citation: Gadge AS, Shirsat DV, Soumia PS, Pote CL, Pushpalatha M, Pandit TR, Dutta R, Kumar S, Ramesh SV, Mahajan V and Karuppaiah V (2024) Physiochemical, biological, and therapeutic uses of stingless bee honey. Front. Sustain. Food Syst. 7:1324385. doi: 10.3389/fsufs.2023.1324385
Received: 19 October 2023; Accepted: 05 December 2023;
Published: 04 January 2024.
Edited by:
Reza Rastmanesh, American Physical Society, United StatesReviewed by:
Otilia Bobis, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaRodica Margaoan, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Copyright © 2024 Gadge, Shirsat, Soumia, Pote, Pushpalatha, Pandit, Dutta, Kumar, Ramesh, Mahajan and Karuppaiah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vadivelu Karuppaiah, a2FydXBwYWlhaHYyMDA4QGdtYWlsLmNvbQ==
†Present address: Ankush S. Gadge, Research Extension Centre, Central Silk Board, Aurangabad, Maharashtra, India
 Ankush S. Gadge
Ankush S. Gadge Dhananjay V. Shirsat
Dhananjay V. Shirsat Parakkattu S. Soumia
Parakkattu S. Soumia Chandrashekhar L. Pote
Chandrashekhar L. Pote M. Pushpalatha
M. Pushpalatha Trupti Rajesh Pandit
Trupti Rajesh Pandit Ram Dutta
Ram Dutta Satish Kumar
Satish Kumar S. V. Ramesh
S. V. Ramesh Vijay Mahajan1
Vijay Mahajan1 Vadivelu Karuppaiah
Vadivelu Karuppaiah