- Fraunhofer Institute for Environmental Safety, and Energy Technologies UMSICHT, Oberhausen, Germany
Due to several benefits regarding human health, the flavonoid rutin gains interest in nutrition and pharmaceutical industry. In order to satisfy high quality standards during cultivation and the final product, plants are grown increasingly in controlled environments with LED-technology as artificial light source. In this study the effect of various light settings on rutin content and biomass of Levisticum officinale was investigated. For continuous tracking of the biomass during cultivation, RGB-Images were taken. The actual biomass after harvest showed a strong positive correlation with the number of leaf-pixels detected via image processing (R2 = 0.937). Concerning the effect of UV-B radiation on rutin synthesis, time of synthesis was investigated. Two days after UV-B treatment a significant increase in rutin was observed. A short exposure time in combination with a high irradiance of 1 W m2 also showed positive effects on the rutin content in lovage. No significant effect of UV-B light on fresh weight was shown, but the combination of supplementary green light and high total photosynthetic photon flux density (PPFD) resulted in an increase of biomass.
1 Introduction
Indoor farming and controlled environment agriculture systems (CEA) show enormous potential regarding sustainable plant cultivation close to the consumer. In CEA systems it is possible, to control abiotic stress scenarios, which are associated with targeted influences on plant quality (Espinosa-Leal et al., 2022). However, there is still a need for development and research, especially with respect to energy use and cultivation parameters. Depending on plant species, different lighting is required regarding yield and plant quality. Numerous studies focus on the effect of lighting on biomass or secondary metabolites, but the results are mostly plant-specific and cannot be transferred to other plant species without restrictions. This is particularly the case in terms of plant quality and the biosynthesis of phenolic compounds.
In our review from 2020, we point out partially contrary results of the effect of various light treatments on flavonoid content, even for plants belonging to the same family or variety (Thoma et al., 2020). Based on our results from 2022 regarding rutin content in lovage (Levisticum officinale W.D.J KOCH), no clear significant effect of specific wavelength components could be shown (Wack et al., 2022). No significant difference between two relatively high proportions of green light (20 and 40% of the total photosynthetic photon flux density (PPFD)) where shown. The presented study focuses on the effect of light on fresh weight (FW) and on rutin content in lovage. Following on from our previous studies, we answer the question, if relatively low green light proportions show significant effects on FW and rutin content. Additionally, the effect of UV-B light is investigated.
Lovage belongs to the Apiaceae family, and its leaves are often used as herbs for example in European cuisine. The roots are known to show high abundance of various essential oils containing monoterpenes and phthalides (Spréa et al., 2020). Beside essential oils, lovage plants contain valuable secondary metabolites. In comparison to dill (Anethum graveolens), parsley (Petroselinum crispum) and celery leaves (Apium graveolens L.), all belonging to the Apiaceae family, lovage shows the highest content of phenolic compounds and flavonoids, as well as the strongest antioxidant activity (Nour et al., 2017). Rutin is one of the dominant flavonoids in Levisticum officinale (Tvrda et al., 2019). Due to its anti-inflammatory and antioxidant properties, rutin has become increasingly important for the pharmaceutical industry (Meinhart et al., 2020). Promising results treating Alzheimer’s disease in rats with rutin were shown by the group of Moghbelinejad et al. (2014). Also, the treatment of renovascular hypertension in rats with rutin showed positive effects (Kaur and Muthuraman, 2016). Mascaraque et al. investigated the positive, anti-inflammatory effect of rutin treating colitis in mice (Mascaraque et al., 2014). These free radical scavenging properties are not only helpful for animals, but also for the plant itself. Additionally, it protects the plant against ultraviolet radiation (UV), since it is located in the upper epidermis of leaves and shows distinct absorbance in the UV-domain. For this reason, the triggering of the biosynthesis of rutin via exposure with UV light and other wavelength is conclusive.
In their review, Neugart and Schreiner summarize positive effects of UV-radiation in general on secondary metabolism in agricultural crops (Neugart and Schreiner, 2018). Several research groups investigated the effect of light on rutin synthesis using the example of buckwheat (Fagopyrum esculentum) (Suzuki et al., 2005; Tsurunaga et al., 2013; Seo et al., 2015; Nam et al., 2018). A combination of blue (B), red (R) and white (W) LEDs showed optimal results regarding rutin content in buckwheat (Seo et al., 2015). Huang et al. describe a significant increase of rutin and quercetin content in buckwheat plants under UV-B exposure (Huang et al., 2016). Kreft et al. showed that UV-B radiation stimulate rutin accumulation in buckwheat plants; especially in the leaves (up to 97%) and to a certain degree as well in the flowers (up to 17%). When increasing the UV-B radiation the rutin accumulation is decreasing (Kreft et al., 2002). The work of Gumerova et al. showed that a small B:R ratio or a relatively big amount of red light, resulted in a significantly higher rutin content (Gumerova et al., 2021). Most studies about lovage investigate its essential oils, their composition or germination response (Raal et al., 2008; Khodashenas et al., 2015; Spréa et al., 2020). Wack et al. found one of the significant parameters regarding rutin concentration in lovage was light intensity and developed a new extraction method for rutin (Wack et al., 2022). Furthermore, Lima et al. aimed in their study to evaluate the influence of red, blue and white and as well monochromatic light associated or not with UV-B on photosynthetic performance and phenolic compound production in microtomato plants cultivated via vertical agriculture. They identified that plants grown under blue light plus UV radiation had high photosynthetic rates and the fruits at all maturation stages from plants grown under blue light and blue light plus UV radiation had high rutin contents (Lima et al., 2023).
In this study, we investigate the effect of supplementary green light and total PPFD, of supplementary UV-B light, its exposure time, and the effect of the time of synthesis on fresh weight and rutin content in Levisticum officinale.
2 Materials and methods
2.1 Plant material and growth conditions
Three different experiments were conducted in a closed indoor cultivation room. For each experiment Levisticum officinale (seeds from SPERLI GmbH) was cultivated for 50 days under artificial LED light at a PPFD of 245 ± 12.5 μmol m−2 s−1, a photoperiod of 16/8 h of light/dark. The temperature was kept at 18/23 ± 1°C (night/day), the relative humidity at 68/78 ± 3% and CO2 content at 460 ± 60 ppm. The plants were cultivated in soil (seedling substrate, pH = 5.5, low level of nutrients) in cultivation trays (6 × 10 pots) using an automated ebb & flood irrigation system. Trays were watered every 2nd day for 5 min.
2.2 Light treatments
For all experiments, LED fixtures (GND Solutions GmbH) with four channels having peaks at 445, 660, 730 nm and white (5,000 K) where used. For pre-cultivation and for control groups, a blue to red ratio (B:R) of 1:1.7 was applied including 10% green light (500–600 nm) using white LEDs (Figure 1, black line). The total PPFD of 245 ± 12.5 μmol m−2 s−1 was measured using an integrating sphere with a spectrometer (CAS 140 CT array spectrometer, Instrument Systems) at the level of the canopy. The variance of ±12.5 μmol m−2 s−1 resulted from heterogenic light emission due to positioning of plants at the center or the edge of the cultivation area.
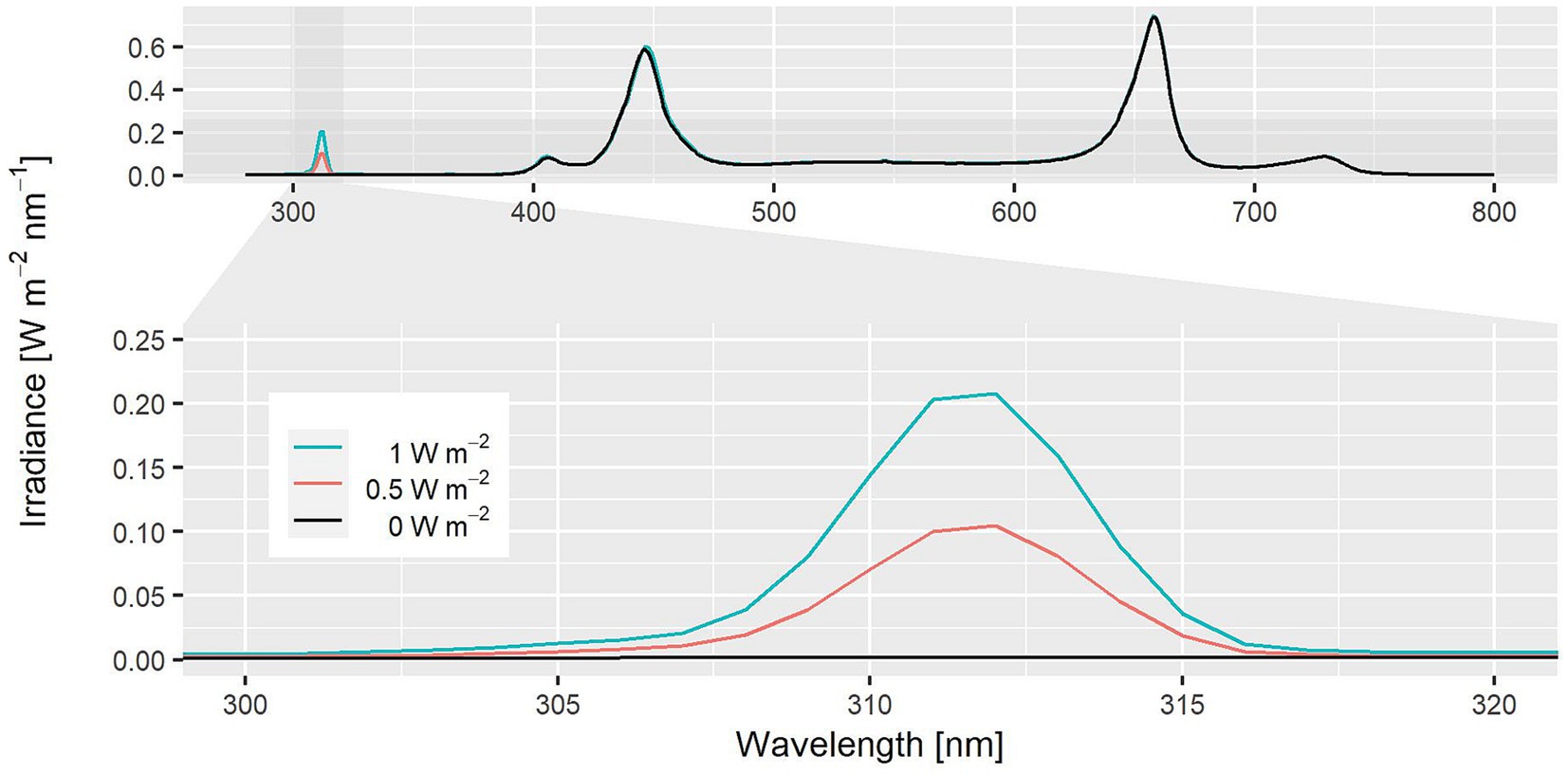
Figure 1. Light spectrum used for study III. Spectrum without UV-B was used for pre-cultivation (black).
2.2.1 Study I
In a first experiment, the effect of the total PPFD and green light on FW and rutin content was investigated employing a two-factorial design. Both factors were varied on two levels. The total PPFD was set to 135 and 223 μmol m−2 s−1, the relative amount of green light using white LEDs was set to 10 and 20% of the total PPFD (Figures 2A,B). The B:R ratio was kept constant at 1:1.7 for all four groups.
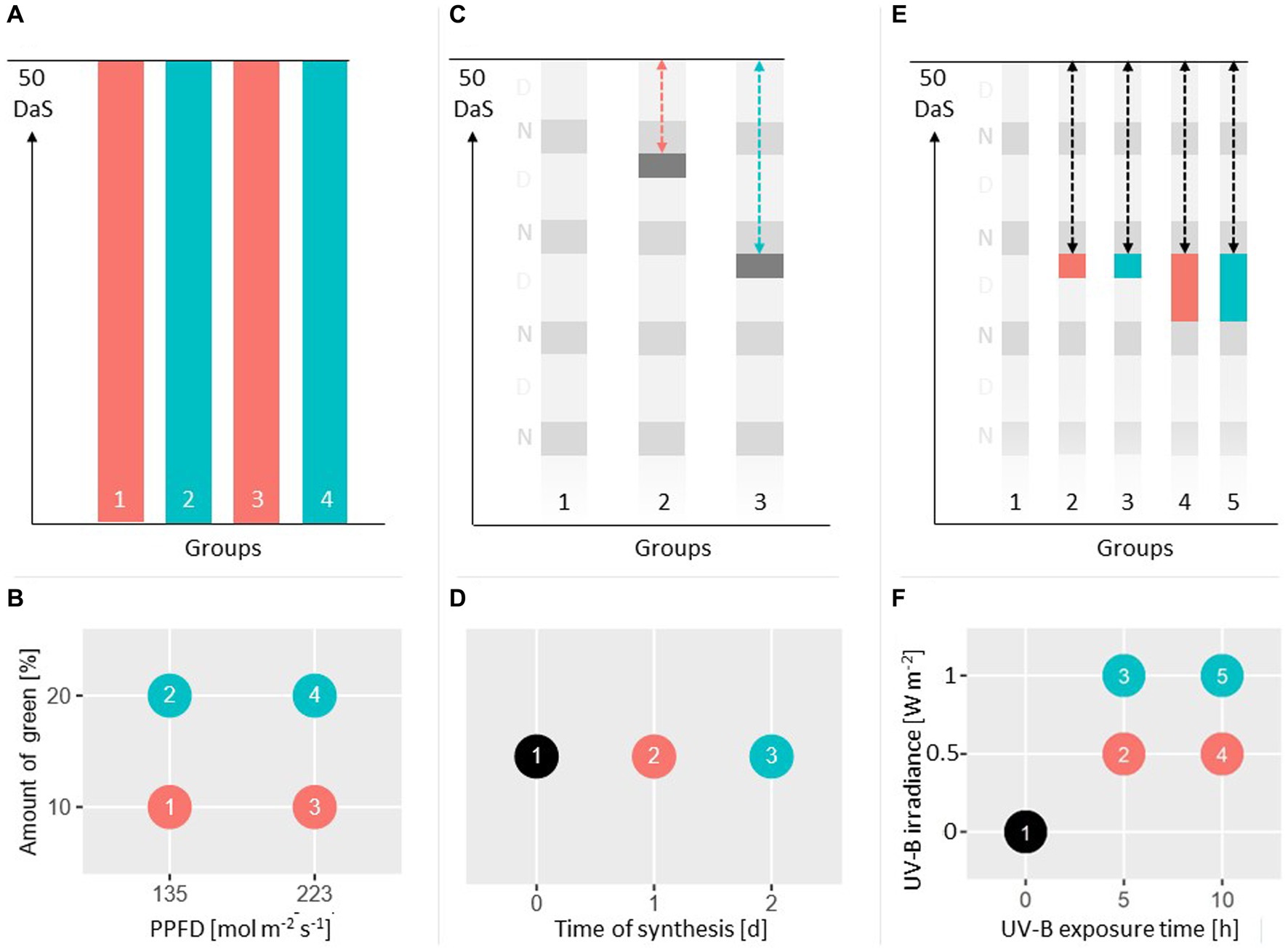
Figure 2. (A,C,E) Show the duration of lighting treatments of three studies. (B,D,F) Show the experimental designs with their corresponding factors and levels. In study I (A,B), four groups with varying amount of green light and varying PPFD were investigated. In study II (C,D), three groups with varying time of synthesis including a control group were investigated. In study III (E,F), five groups with varying UV-B irradiance and varying UV-B exposure time including a control group were investigated.
2.2.2 Study II
In order to investigate the effect of the time of synthesis tsynth on FW and rutin content, plants were exposed to supplementary UV-B light at EUV-B = 1 W m−2 for 5 h (Figures 2C,D). The factor tsynth was varied on 3 levels, 1 d, 2 d and a control group without supplementary UV-B radiation.
2.2.3 Study III
Employing a balanced two-factorial design including a control group, the effect of UV-B irradiance and exposure time tExp on fresh weight and rutin content was examined (Figures 2E,F). The UV-B irradiance was varied on two levels with EUV-B = 0.5 and 1 W m−2 (Figure 1) with a time of synthesis of 2 d. The two levels of the exposure time were set to tExp = 5 and 10 h.
2.3 Analysis of rutin content and fresh weight
For the determination of fresh weight (FW) and rutin content, leaves and stem were weighed and analyzed. The biomass was frozen using liquid nitrogen (−196°C) in order to reduce the potential effect of a volatile behavior of metabolites. Afterwards, the water content was reduced using a vacuum freeze dryer. For determining the exact amount of the total water content, a part of each sample was dried completely at 105°C for 24 h and the weight difference was measured. The remaining part of the sample was mechanically dispersed and stirred in an extraction solution (49.5% H2O, 49.5% Ethanol and 1% HCl) for 2 h at 22°C in darkness. To remove solid compounds from the extract, the viscous biomass was filtered using filter paper. Rutin content of the extract was determined via high performance liquid chromatography (HPLC, HPLC 1200 Quat Pump, Agilent Technologies) and related to dry weight (DW).
The FW was measured gravimetrically. Parallel, RGB cameras (red, green, blue color model) and image recognition techniques were used and the correlation between real FW and number of leaf pixels was investigated. Of each group, images were taken hourly during light period. Image processing and the calculation of the number of leaf pixels in each image was done in python. This was achieved by converting the images from RGB to hue, saturation, value domain (HSV color model) and setting pairs of threshold values: Hue (0–179): 0–70, Saturation (0–255): 25–170 and Value (0–255): 60–255.
In order to remove false detected pixels e. g. areas of soil, which show a smaller area of connected pixels, a small-bloob filter was applied. Selected regions having a contour less than 110 pixels were identified and subtracted from the number of leaf pixels. For each image at the end of cultivation, the number of leaf pixels and the corresponding FW was analyzed in terms of correlation. For the linear regression model, 144 samples were considered.
2.4 Statistical analysis
The data were analyzed in R Software (4.2.1) using the package ggplt2 for plotting (R Core Team, 2022). For the experiments, either one – or two-factorial designs were used. Each group consisted of n = 8 samples each containing 18 plants. To identify significant differences between groups, general linear models (GLM) were used, and analysis of variance (ANOVA) was performed. Assumptions of ANOVA were checked before, using Levene’s test (homogeneity of variances) and Shapiro–Wilk test (normality). Pairwise comparisons were done using Tukey post hoc test. If the normality assumption was violated, the Kruskal-Wallis test was applied. The results were considered as statistically significant at p ≤ 0.05.
3 Results
Lovage plants were monitored via RGB cameras, and the number of leaf pixels was detected via image processing algorithms. Figure 3 shows the linear regression between the number of leaf pixels and the fresh weight per plant determined gravimetrically. Considering 144 pairs of data, the coefficient of determination R2 was 0.94 with p < 2.2e-16. With increasing fresh weights, the data points disperse more due to leaf overlapping.
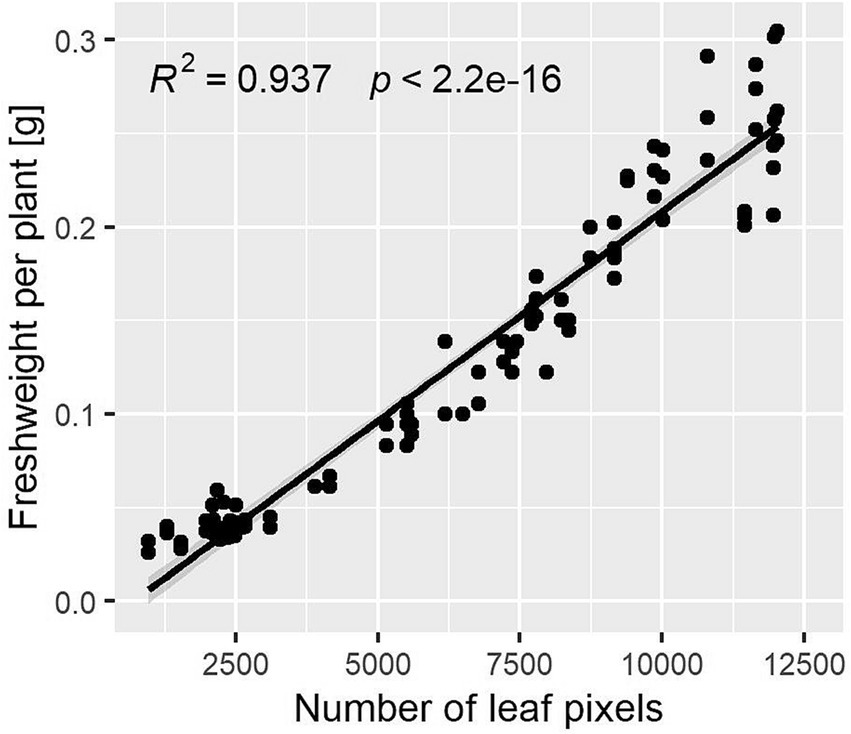
Figure 3. Simple linear regression of the relationship between the number of leaf pixels and fresh weight per plant determined gravimetrically (n = 144).
3.1 Study I
The results indicate that the FW is not significantly affected by the total PPFD (p = 0.43), but in terms of relative amount of green light, a significant effect could be shown (p = 0.008). Additionally, the interaction of both factors exhibits a significant effect with p = 1.9e-7. For both combinations, either at a low PPFD and a low amount of green light as well as at a high PPFD and a high amount of green light, a relatively high FW was measured. Figure 4A shows the pairwise p-values resulting from Tukey test with their corresponding significance codes 0 “***” 0.001 “**” 0.01 “*” 0.05. Highest amount of FW was measured at PPFD = 135 μmol m−2 s−1 and 10% of green light. A high amount of green light is not beneficial at a low PPFD. The Tukey test also shows, that at a low amount of green light (10%) a low PPFD (135 μmol m−2 s−1) leads to a higher FW than a high PPFD (223 μmol m−2 s−1). The increased fresh weight at a low PPFD is due to an increased water content, since the dry weight for this group was relatively low compared to the group at a high PPFD (Figure 4B). Baligar et al. investigated water use efficiency parameters (e.g., shoot weight per transpired water over the entire cultivation period) on various legume cover crops and found increasing values with an increasing PPFD (Baligar et al., 2020). These water use efficiency parameters provide important insights into the ability of plants to use water for producing high dry weights.
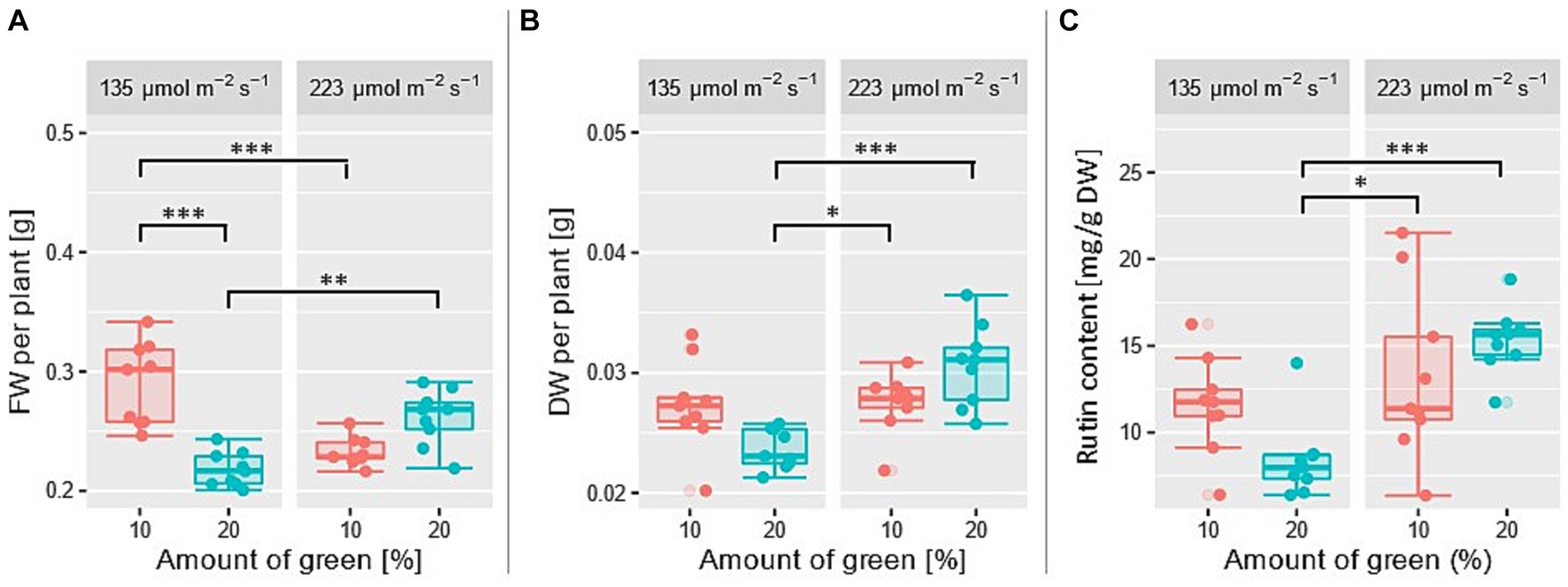
Figure 4. (A) Fresh weight per plant, (B) dry weight per plant, and (C) rutin content under four different treatments with n = 8. Amount of green light (10 and 20%) and PPFD (135 and 223 μmol m−2 s−1) was varied. Each data point is the average value of 18 plants. TukeyHSD test (p < 0.05), significance codes: 0 “***” 0.001 “**” 0.01 “*” 0.05.
Considering the data points for the group at a low PPFD analyzed by image processing, the regression model predicts a lower FW than the measured one. The reason is an increased elongation of the plants, where the leaf position is directed towards the light source. Thus, the angle between leaf and camera is not perpendicular anymore and a smaller area of pixels is detected by the algorithm.
Regarding rutin content, a significant effect of total PPFD (p = 0.0003) and the interaction of total PPFD and relative amount of green light (p = 0.021) could be demonstrated. Similar to FW, an increased rutin content could be observed for simultaneously low and simultaneously high factor settings (Figure 4C). Especially at a high PPFD and a high amount of green light, rutin content increases. No significant effect for the factor relative amount of green light could be shown (p = 0.59).
3.2 Study II
According to the Shapiro Wilk test, the data concerning FW were not distributed normal but showed a right skewed distribution (p = 0.0027) and thus cannot be analyzed via ANOVA. A potential explanation for the data distribution due to positioning of plants and heterogenic light distribution was investigated via ANOVA. No significant effects were identified. The data were analyzed with the Kruskal-Wallis test and no significant effect of tsynth on FW was found (Figure 5A).
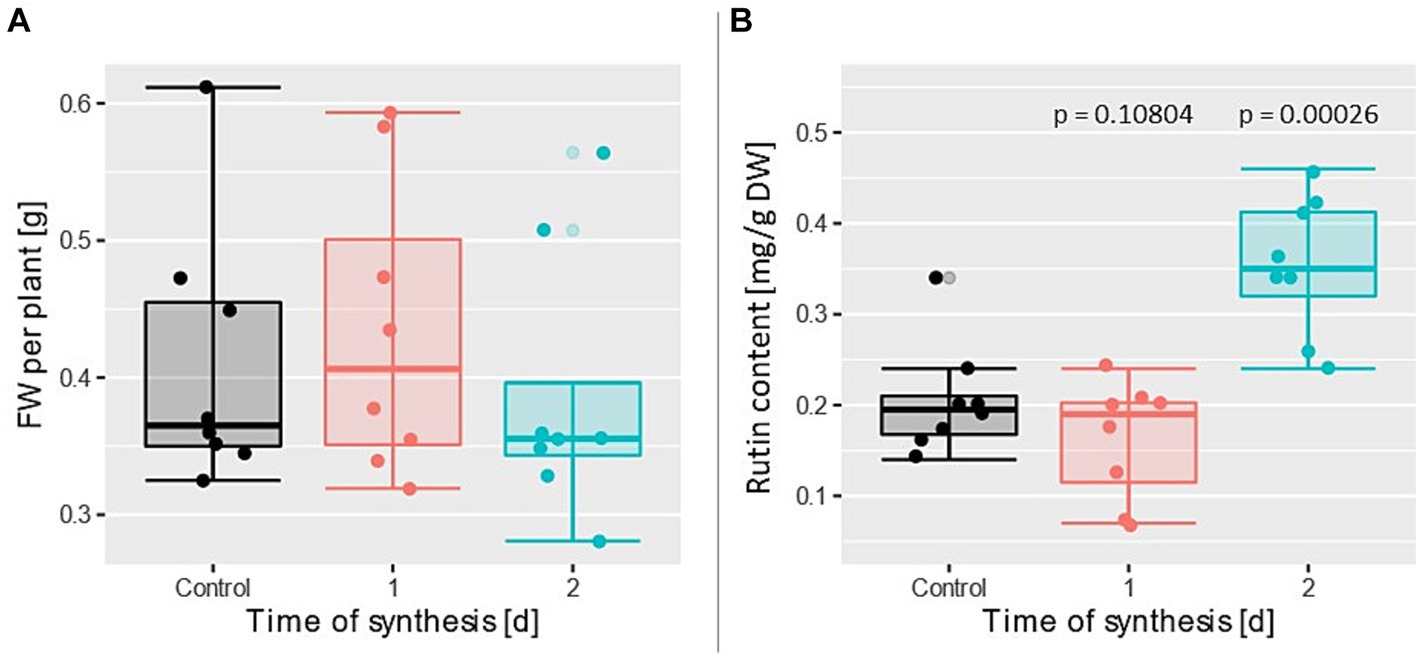
Figure 5. (A) Fresh weight per plant and (B) rutin content under three treatments with n = 8. Time of synthesis was varied. Each data point is the average value of 18 plants.
In contrast to that, a significant effect of tsynth on rutin content was observed with a p-value of 0.00026 between control group and the group with tsynth = 2 d (Figure 5B). The measured rutin content at tsynth = 2 d was almost twice as high as the values within the control group. At tsynth = 1 d no significant effect was observed (p = 0.108).
3.3 Study III
Due to right skewed data concerning FW (Shapiro Wilk test: p = 0.00027), Kruskal-Wallis test was used for variance analysis. As shown in Figure 6A, no significant effect on FW was found considering UV-B irradiance as well as exposure time (p = 0.061 and p = 0.32, respectively).
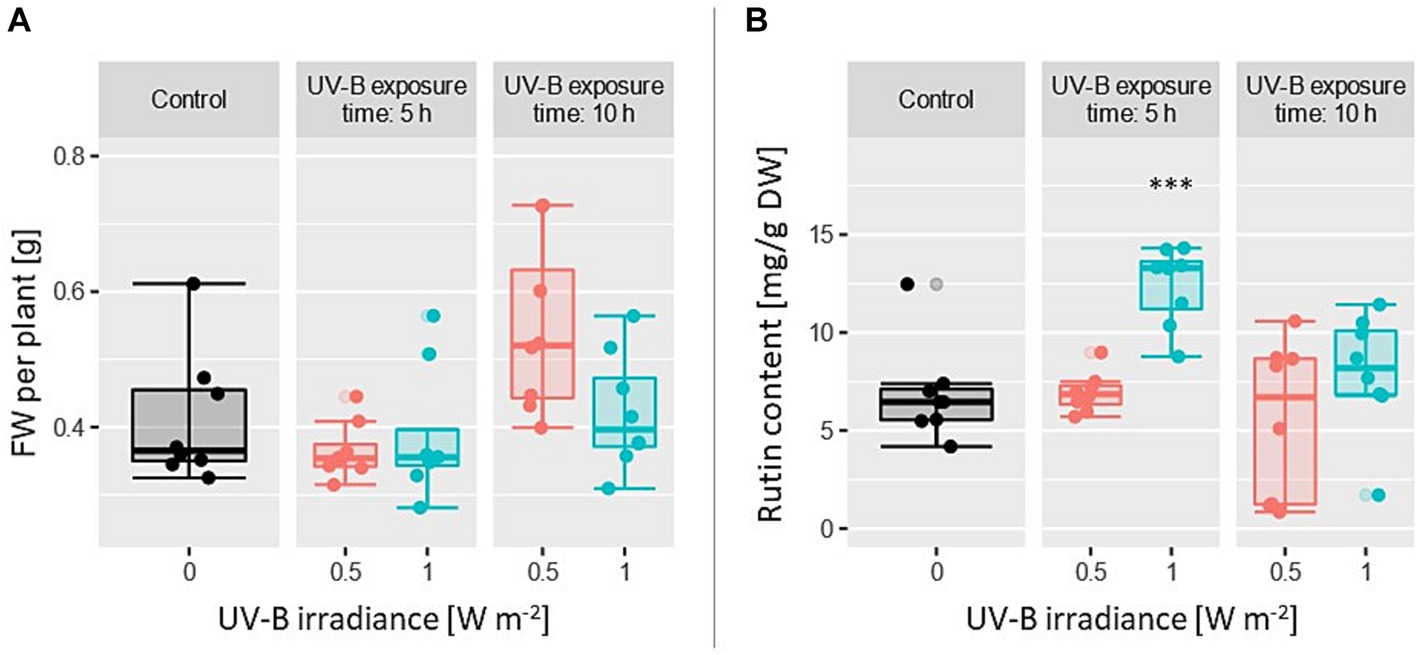
Figure 6. (A) Fresh weight per plant and (B) rutin content under five treatments with n = 8. UV-B irradiance (0, 0.5, and 1 W m−2) and UV-B exposure time (0, 5, and 10 h) including a control group were varied. Each data point is the average value of 18 plants.
In terms of rutin content, the data showed a slight heterogenic variance, which was confirmed by Levene’s test (p = 0.044). Since the p-value of Levene’s test is close to the significance level of α = 0.05, still an ANOVA was performed, but decreasing its significance level to α = 0.01. A significant effect of UV-B irradiance (p = 0.0044) and exposure time (p = 0.00023) was observed (Figure 6B). In comparison to the control group, the rutin content increased by 80% under supplementary UV-B at EUV-B = 1 W m−2 for 5 h. Exposing the plants for 10 h, rutin content is similar to the values within the control group.
4 Discussion
The fresh weight and the rutin content of Levisticum officinale was investigated under various light treatments.
4.1 Fresh weight
Regarding fresh weight, a certain amount of green light (20% of the total PPFD) shows beneficial effects in case the total PPFD is not too low. For being able to convert supplementary green light into chemical energy, it is necessary to exceed a certain threshold regarding the number of photons in the red and blue domain. For lovage, the threshold lies between 135 and 223 μmol m−2 s−1. This also means, that exposing lovage plants to a low PPFD, mainly blue and red light are responsibly for driving photosynthetic processes. Furthermore, it was shown that at a relative amount of green light of 10%, the FW of plants under 223 μmol m−2 s−1 is significantly lower than the FW of plants under 135 μmol m−2 s−1. The reason is a higher water content in plants exposed to a low PPFD. At supplemental proportions of green light at 20%, a significant increase of DW with increasing PPFD could be observed. For future experiments the additional investigation of water use efficiency parameters can provide valuable information regarding the production of DW and the effect of PPD (Baligar et al., 2020). Comparing the FW and DW results to the image processing data, an elongated growth can be observed for the group at a low PPPFD. Low light triggers phototropism processes in Levisticum officinale accompanied by an increased water content in order to enhance the amount of absorbed light.
Supplementary UV-B light does not show significant effects on the fresh weight of Levisticum officinale. The light harvesting complexes (LHC) being responsible for light absorption in terms of photosynthesis do not exhibit considerable absorption in the UV-B domain for lovage. Compared to lettuce (variety “Levistro”), supplementary UV-B light leads to a significantly decreased FW, but a slight increased DW. No effects could be observed for the red leaf variety “Carmoli” (Flores et al., 2023). These findings support the hypothesis, that the effect of light on biomass is specific for plant species.
4.2 Rutin content
With respect to our study from 2022, where no clear significance could be shown between the effect of two green light proportions (20 and 40% of the total PPFD) on rutin content, the presented work investigated the potential effect of lower green light proportions (Wack et al., 2022). The results indicate that green light as a sole factor does not have a significant effect, even at low levels. However, considering the interaction with a second factor, namely the PPFD, a trend is visible regarding the rutin content in lovage: exposing plants to a high PPFD of 223 μmol m−2 s−1 with a green light proportion of 20%, a small increase in rutin content is detectable (Figure 4C). It should be emphasized that this interaction is no significant effect, only a tendency.
The effect of different delay times (time of synthesis) between UV-B stress period and the measurement of rutin content were investigated. In Levisticum officinale a time of synthesis of 2 d showed a significant increase of rutin content. The cascade of light absorption via the photoreceptor UVR8, the consecutive change in gene expression and the final synthesis of rutin is in the order of 2 days. Additionally, a relatively short UV-B stress period of 5 h with an irradiance of 1 W m−2 is sufficient to significantly enhance the rutin content. Saad et al. for example found that the maximum response to salinity stress in Daucus carota cell culture took 9 days considering the total flavonoid content (Saad et al., 2021). The group of Altangerel compared the response of anthocyanin content in coleus plants after different stress treatments over time. Compared to saline stress, light stressed plants showed a significant increase in anthocyanins already after 36 h, whereas a significant response to saline stress was after 48 h (Altangerel et al., 2017). The response to light stress seems to be quicker than, e.g., to salinity stress, assuming a comparability between these two plants species.
Exposing lovage plants to a higher UV-B irradiance, the rutin content is not significantly affected with respect to the control group being not exposed to UV-B radiation. Due to the short exposure time, bleaching effects of already synthesized rutin do not play a major role. It seems, that high UV-B irradiances at 1 W m−2 disturb signal pathways of UVR8 and also the biosynthesis of rutin.
In order to enhance the rutin content of Levisticum officinale while having an increased fresh weight, we suggest a total PPFD at around 220 μmol m−2 s−1. Regarding the light spectrum, a B:R ratio of 1:1.7, supplementary green light (20% of the total PPFD) shows good results. Two days before harvest or intake through nutrition of lovage, supplementary UV-B light at 1 W m−2 for 5 h increase rutin content and thus the quality of the cultivar. For providing plants with high and constant quality, precise light recipes are essential, since already small variations can have significant effects not only in yield, but also in secondary metabolite content. Further research with respect to the increasing need of CEA systems is required, especially in terms of energy consumption.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
FT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. DS: Supervision, Writing – review & editing. AS: Supervision, Writing – review & editing. VK: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was partially supported by the Federal Ministry of Education and Research (BMBF) through the program Agricultural Systems of the Future in the framework of the “National Research Strategy BioEconomy 2030” under grant No. 031B0728A “SUSKULT – Development of a Sustainable Cultivation System of Resilient Metropolitan Regions” and through the program “Model Region Bioeconomy in the Rhenish mining area” under grant No. 031B1137BX “Model region, Phase 1, BioRevierPLUS: InnoLA, TP2-circular PhytoREVIER.”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Altangerel, N., Ariunbold, G. O., Gorman, C., Alkahtani, M. H., Borrego, E. J., Bohlmeyer, D., et al. (2017). In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 114, 3393–3396. doi: 10.1073/pnas.1701328114
Baligar, V. C., Elson, M. K., He, Z., Li, Y., Paiva, A. D. Q., Almeida, A. A. F., et al. (2020). Light intensity effects on the growth, physiological and nutritional parameters of tropical perennial legume cover crops. Agronomy 10:1515. doi: 10.3390/agronomy10101515
Espinosa-Leal, C. A., Mora-Vásquez, S., Puente-Garza, C. A., Alvarez-Sosa, D. S., and García-Lara, S. (2022). Recent advances on the use of abiotic stress (water, UV radiation, atmospheric gases, and temperature stress) for the enhanced production of secondary metabolites on in vitro plant tissue culture. Plant Growth Regul. 97, 1–20. doi: 10.1007/s10725-022-00810-3
Flores, M., Amorós, A., and Escalona, V. H. (2023). Changes in agronomic, antioxidant compounds, and morphology parameters of green and red lettuces (Lactuca sativa L.) by successive harvests and UV-B supplementation. Horticulturae 9:677. doi: 10.3390/horticulturae9060677
Gumerova, E. A., Akulov, A. N., and Rumyantseva, N. I. (2021) ‘Red and blue light have different effects on flavonols and proanthocyanidins accumulation in the cell culture of tartary buckwheat’, in AIP conference proceedings. Vol. 2388. AIP Publishing.
Huang, X., Yao, J., Zhao, Y., Xie, D., Jiang, X., and Xu, Z. (2016). Efficient Rutin and quercetin biosynthesis through flavonoids-related gene expression in Fagopyrum tataricum Gaertn. Hairy Root Cultures with UV-B Irradiation. Front. Plant Sci. 7:63. doi: 10.3389/fpls.2016.00063
Kaur, S., and Muthuraman, A. (2016). Therapeutic evaluation of rutin in two-kidney one-clip model of renovascular hypertension in rat. Life Sci. 150, 89–94. doi: 10.1016/j.lfs.2016.02.080
Khodashenas, M., Keramat, B., and Emamipoo, Y. (2015, 2015). Germination response of endangered medicinal plant, Levisticum officinale, to stratification and some plant growth regulators, biological and environmental. Science 7, 228–235.
Kreft, S., Strukelj, B., Gaberscik, A., and Kreft, I. (eds) (2002) Rutin in buckwheat herbs grown at different UV-B radiation levels: comparison of two UV spectrophotometric and an HPLC method.
Lima, I. H. A., Rodrigues, A. A., Resende, E. C., Da Silva, F. B., dos Santos Farnese, F., Silva, L. D. J., et al. (2023). Light means power: harnessing light spectrum and UV-B to enhance photosynthesis and rutin levels in microtomato plants. Front. Plant Sci. 14:1261174. doi: 10.3389/fpls.2023.1261174
Mascaraque, C., Aranda, C., Ocón, B., Monte, M. J., Suárez, M. D., Zarzuelo, A., et al. (2014). Rutin has intestinal antiinflammatory effects in the CD4+ CD62L+ T cell transfer model of colitis. Pharmacol. Res. 90, 48–57. doi: 10.1016/j.phrs.2014.09.005
Meinhart, A. D., Damin, F. M., Caldeirão, L., Teixeira-Filho, J., and Godoy, H. T. (2020). Rutin in herbs and infusions: screening of new sources and consumption estimation. Food Sci. Technol. 40, 113–120. doi: 10.1590/fst.01219
Moghbelinejad, S., Nassiri-Asl, M., Farivar, T. N., Abbasi, E., Sheikhi, M., Taghiloo, M., et al. (2014). Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol. Lett. 224, 108–113. doi: 10.1016/j.toxlet.2013.10.010
Nam, T. G., Kim, D.-O., and Eom, S. H. (2018). Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 27, 169–176. doi: 10.1007/s10068-017-0204-1
Neugart, S., and Schreiner, M. (2018). UVB and UVA as eustressors in horticultural and agricultural crops. Sci. Hortic. 234, 370–381. doi: 10.1016/j.scienta.2018.02.021
Nour, V., Trandafir, I., and Cosmulescu, S. (2017). Bioactive compounds, antioxidant activity and nutritional quality of different culinary aromatic herbs. Not. Bot. Horti Agrobot. Cluj Napoca 45, 179–184. doi: 10.15835/nbha45110678
R Core Team. (2022) R: a language and environment for statistical computing [computer program]. Available at:https://www.r-project.org/.
Raal, A., Arak, E., Orav, A., Kailas, T., and Müürisepp, M. (2008). Composition of the essential oil of Levisticum officinale W.D.J. Koch from some European countries. J. Essent. Oil Res. 20, 318–322. doi: 10.1080/10412905.2008.9700022
Saad, K. R., Kumar, G., Mudliar, S. N., Giridhar, P., and Shetty, N. P. (2021). Salt stress-induced anthocyanin biosynthesis genes and MATE transporter involved in anthocyanin accumulation in Daucus carota cell culture. ACS Omega 6, 24502–24514. doi: 10.1021/acsomega.1c02941
Seo, J.-M., Arasu, M. V., Kim, Y.-B., Park, S. U., and Kim, S.-J. (2015). Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chem. 177, 204–213. doi: 10.1016/j.foodchem.2014.12.094
Spréa, R. M., Fernandes, Â., Calhelha, R. C., Pereira, C., Pires, T. C. S. P., Alves, M. J., et al. (2020). Chemical and bioactive characterization of the aromatic plant Levisticum officinale W.D.J. Koch: a comprehensive study. Food Funct. 11, 1292–1303. doi: 10.1039/C9FO02841B
Suzuki, T., Honda, Y., and Mukasa, Y. (2005). Effects of UV-B radiation, cold and desiccation stress on rutin concentration and rutin glucosidase activity in tartary buckwheat (Fagopyrum tataricum) leaves. Plant Sci. 168, 1303–1307. doi: 10.1016/j.plantsci.2005.01.007
Thoma, F., Somborn-Schulz, A., Schlehuber, D., Keuter, V., and Deerberg, G. (2020). Effects of light on secondary metabolites in selected leafy greens: a review. Front. Plant Sci. 11:497. doi: 10.3389/fpls.2020.00497
Tsurunaga, Y., Takahashi, T., Katsube, T., Kudo, A., Kuramitsu, O., Ishiwata, M., et al. (2013). Effects of UV-B irradiation on the levels of anthocyanin, rutin and radical scavenging activity of buckwheat sprouts. Food Chem. 141, 552–556. doi: 10.1016/j.foodchem.2013.03.032
Tvrda, E., Varga, A., Slavik, M., and Arvay, J. (2019). Levistikum officinale nad its effects on bovine spermatozoa activity. J. Microbiol. Biotechnol. Food Sci. 8, 1212–1216. doi: 10.15414/jmbfs.2019.8.5.1212-1216
Wack, H., Somborn, A., Schlehuber, D., Deckert, S., and Keuter, V. (2022). Cultivation of lovage under exposure of light-emitting diode illumination and analysis of rutin produced by high performance liquid chromatographiy (HPLC), and ultraviolet-visible spectroscopy (UV-vis). Int. J. Food Sci. 2022, 1–8. doi: 10.1155/2022/6357893
Keywords: UV-B, rutin, antioxidants, secondary metabolites, flavonoids, lovage, Levisticum officinale, LED
Citation: Thoma F, Schlehuber D, Somborn A and Keuter V (2024) How does supplementary green light and UV-radiation affect biomass and rutin content in Levisticum officinale? Front. Sustain. Food Syst. 7:1322443. doi: 10.3389/fsufs.2023.1322443
Edited by:
José Pinela, Instituto Politécnico de Bragança, PortugalReviewed by:
Himanshi Jangir, University of Central Florida, United StatesLena Gálvez Ranilla, Catholic University of Santa María, Peru
Copyright © 2024 Thoma, Schlehuber, Somborn and Keuter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Felix Thoma, ZmVsaXguYWoudGhvbWFAZ21haWwuY29t; ZmVsaXgudGhvbWFAdW1zaWNodC5mcmF1bmhvZmVyLmRl
 Felix Thoma
Felix Thoma Dennis Schlehuber
Dennis Schlehuber Annette Somborn
Annette Somborn Volkmar Keuter
Volkmar Keuter