- 1Departamento Territorio, Ambiente y Paisaje, Centro Universitario Regional del Este, Universidad de la República (CURE-Udelar), Maldonado, Uruguay
- 2Departamento Sistemas Agrarios y Paisajes Culturales, Centro Universitario Regional del Este, Universidad de la República (CURE-Udelar), Rocha, Uruguay
- 3Laboratorio de Arqueología del Paisaje y Patrimonio, Facultad de Humanidades y Ciencias de la Educación (FHCE - Udelar), Montevideo, Uruguay
- 4Departamento de Biología Vegetal, Facultad de Agronomía, Universidad de la República (FAGRO - Udelar), Montevideo, Uruguay
Introduction: Agrobiodiversity and local knowledge are fundamental components in the domestication and structuring of rural landscapes. In a context of threats to agroecosystems resulting from changes in production systems and rural–urban migration processes, the conservation and valorization of agrobiodiversity is a pressing challenge. “Quebrada de los Cuervos and Sierras del Yerbal” is a protected landscape in Uruguay where a rural community of approximately 30 families with a long-standing tradition resides.
Methods: The research aimed at identifying current and abandoned (taperas) domestic contexts, and the plant genetic resources found in the area, categorizing their uses and management practices through interviews and participant observation.
Results and discussion: Ethnographic research revealed 185 species (121 exotic, 64 native) with diverse growth habits, 10 categories of uses, and 11 categories for management practices. The differences found between houses and taperas revealed that the abandonment of activities in rural areas is a relevant factor in the loss of agrobiodiversity. Among the 185 species, a notable group of plant genetic resources of high cultural significance is recognized due to their consensus of use, frequency of management practices, and number of uses. These include introduced fruit trees (peach, citrus, and fig) and native fruit trees (guayabo del país, pitanga, and arazá), vegetable landraces, native trees with multiple uses, yerba mate, and medicinal species such as Aristolochia fimbriata. For domestic contexts, a model of spatial distribution of agrobiodiversity is proposed, cultivated spaces where the plant genetic resources are located in home gardens and small plots, managed spaces where the resources are found in the surroundings of houses, and promoted and intervened wild spaces where the species are used from natural grasslands and wild environments. The obtained information reaffirms the need to conserve this biocultural landscape, placing agrobiodiversity and local knowledge as a focal point in the protected area. The management plan must be formulated with active participation from the rural community, aiming for valorization through integration into agroecological production chains, among other possibilities.
1. Introduction
Agrobiodiversity involves human intervention for its generation and evolution (Sthapit et al., 2016) and is defined as a dynamic network of relationships among people, living organisms, and the environment that responds to specific needs and circumstances (De Boef et al., 2013a). Agrobiodiversity encompasses biologically diverse species with relevant functional uses for humans. It is necessary for maintaining key functions within agroecosystems, and its importance lies in the fact that greater agrobiodiversity enhances agricultural systems’ resilience to changes (FAO, 1999; Newton et al., 2009). Within agrobiodiversity, plant species with real or potential value for humans are referred to as Plant Genetic Resources (UN Convention on Biological diversity, 1992). This definition explicitly links plant species with specific knowledge, which can be of scientific or traditional origin, leading to the so-called Local or Traditional Ecological Knowledge, or both [see discussion in Heckler (2009)]. Local ecological knowledge refers to an accumulated body of knowledge, practices, and beliefs that evolve through adaptive processes and are culturally transmitted from generation to generation. It encompasses the relationships between living organisms and their environment, takes a holistic approach, and recognizes the complexity of the ecological system (Berkes et al., 2000; Emperaire and Peroni, 2007). The loss of agrobiodiversity, or genetic erosion, is closely associated with the loss of local knowledge, which has multiple causes, including the simplification of agricultural habitats due to industrial agriculture, the abandonment of landraces, the rapid expansion of extensive monocultures, infrastructure growth, the mining industry, and rural depopulation, among others (Achkar, 2017; Baeza et al., 2022; Gallego et al., 2023).
Rural communities play a fundamental role in generating and maintaining agrobiodiversity, as they engage primarily in non-industrial forms of nature management and possess long-standing traditional knowledge (Toledo and Barrera-Bassols, 2008). Each socioculture interacts with its own landscape and biodiversity, resulting in a complex and wide range of interactions that give rise to specific biocultural patches. These local knowledge systems exist as “historical community consciousness” and represent the reservoir of human memory that allows the species to continuously adapt to a constantly changing complex world (Toledo and Barrera-Bassols, 2008). This can also be understood as a community of practice defined by a group of individuals who interact, learn together, establish relationships, and develop a sense of belonging around a specific domain of knowledge and associated practices (Wenger et al., 2002; Dabezies and Taks, 2021).
Several authors have linked the management of agrobiodiversity to the landscape (Wiersum, 1997; Clement, 1999; Clement and Cassino, 2018; Franco-Moraes et al., 2021). This approach considers cultural diversity as the main shaping agent in the domestication of species and landscapes, involving coevolutionary processes (Casas et al., 1997; Heckenberger et al., 2003; Clement et al., 2015; Reis et al., 2018; Franco-Moraes et al., 2023). Changes in plant populations result from changes in management practices, constituting a multidimensional, dynamic, and interactive process involving plants, the environment, and humans at different scales. It encompasses the management and domestication of individual species and entire agroecosystems, transforming a wild ecosystem into a managed and domesticated one. The process of landscape domestication occurs over time through interventions and manipulations of biotic and abiotic components, leading to ecological and demographic changes in plants and animals, increased occurrence of useful species, enhanced productivity of agroecosystems, and a more habitable landscape for humans. Clement and Cassino (2018) recognizes four categories of landscapes based on the degree of human intervention, including pristine, promoted, managed, and cultivated landscapes, although the existence of pristine landscapes is widely debated by the author. Within the cultivated landscape, in addition to large-scale crops, home gardens and small plots (“chacras”) can be included. These microenvironments within the agroecosystem serve as places for experimentation, species introduction, crop improvement, and refuges for unique genetic diversity (Watson and Eyzaguirre, 2001; Kumar and Nair, 2004).
Uruguay is located in the Pampa biome, the largest natural grasslands region in South America and one of the largest in the world. This region has undergone significant changes in land use/land cover in the past 20 years, primarily due to forest plantation and soybean cultivation (Baeza et al., 2022), resulting in a significant impact on biodiversity, agrobiodiversity and ecosystem services such as pollination, soil conservation, and water supply, among others, causing fragmentation and habitat loss. One of the national strategies to address these effects is the National System of Protected Areas (SNAP). In this context, the protected landscape “Quebrada de los Cuervos and Sierras del Yerbal” was established in 2008. This area is a part of the “Serranías del Este” ecoregion, characterized by its high degree of naturalness in ecosystems. It is home to a small rural community which consists of descendants of native populations, Creoles, and European colonizers. The predominant productive system is livestock farming on natural grasslands, carried out by traditional family farmers who engage in vegetable and fruit cultivation for self-consumption, while also raising poultry and pigs. They also maintain and utilize agrobiodiversity for various purposes. In this context, the protected landscape provides an exceptional opportunity to study agrobiodiversity and local knowledge.
The general objective of this study is to contribute to the understanding, valorization, and conservation of agrobiodiversity in the protected area “Quebrada de los Cuervos and Sierras del Yerbal” by delving into the study of plant genetic resources, the origin and transmission of local ecological knowledge, and their role in shaping landscape dynamics. Considering the hypothesis that there is a diverse set of species used in domestic contexts that are essential for survival, and that there is a resource management strategy by the region’s inhabitants, both present and past, we propose the following objectives: (1) to identify and characterize agrobiodiversity in domestic contexts within the Protected Landscape, (2) to conduct an ethno-agronomic approach (Flora, 2001) to study the uses and management of plant genetic resources in domestic contexts, (3) to propose guidelines that contribute to conserving and valorizing agrobiodiversity in the protected area.
2. Materials and methods
2.1. Study area and rural community
The study was conducted in the protected area “Quebrada de los Cuervos y Sierras del Yerbal” (32° 55’S, 54° 27¨W), Treinta y Tres Department, Uruguay (Figure 1). The area is located in the Pampa biome (Allen et al., 2011; Mengue et al., 2020), within the “Serranías del Este” ecoregion (Evia and Gudynas, 2000; Achkar et al., 2016), characterized by its undulating and rugged terrain, altitudes ranging from 50 to 350 meters above sea level, slopes between 5 and 30%, and a dense hydrographic network. The climate, according to the updated Köppen-Geiger classification, is of the Cfa type (Peel et al., 2007), humid subtropical. The area experiences an average annual rainfall of 1,300 mm, distributed throughout the year; however, there is considerable irregularity and variability between years. The average annual temperature is 17.8°C, with an average maximum of 23.3°C and an average minimum of 12.3°C.1 The predominant ecosystems are natural grasslands, hilly forests, riparian forests, and ravine forests. The protected area is part of the National System of Protected Areas, covering an area of 19,192 hectares dedicated to landscape and biodiversity conservation under the international IUCN category of “Protected Landscape” (Nudley, 2008; SNAP/DINAMA, 2010).
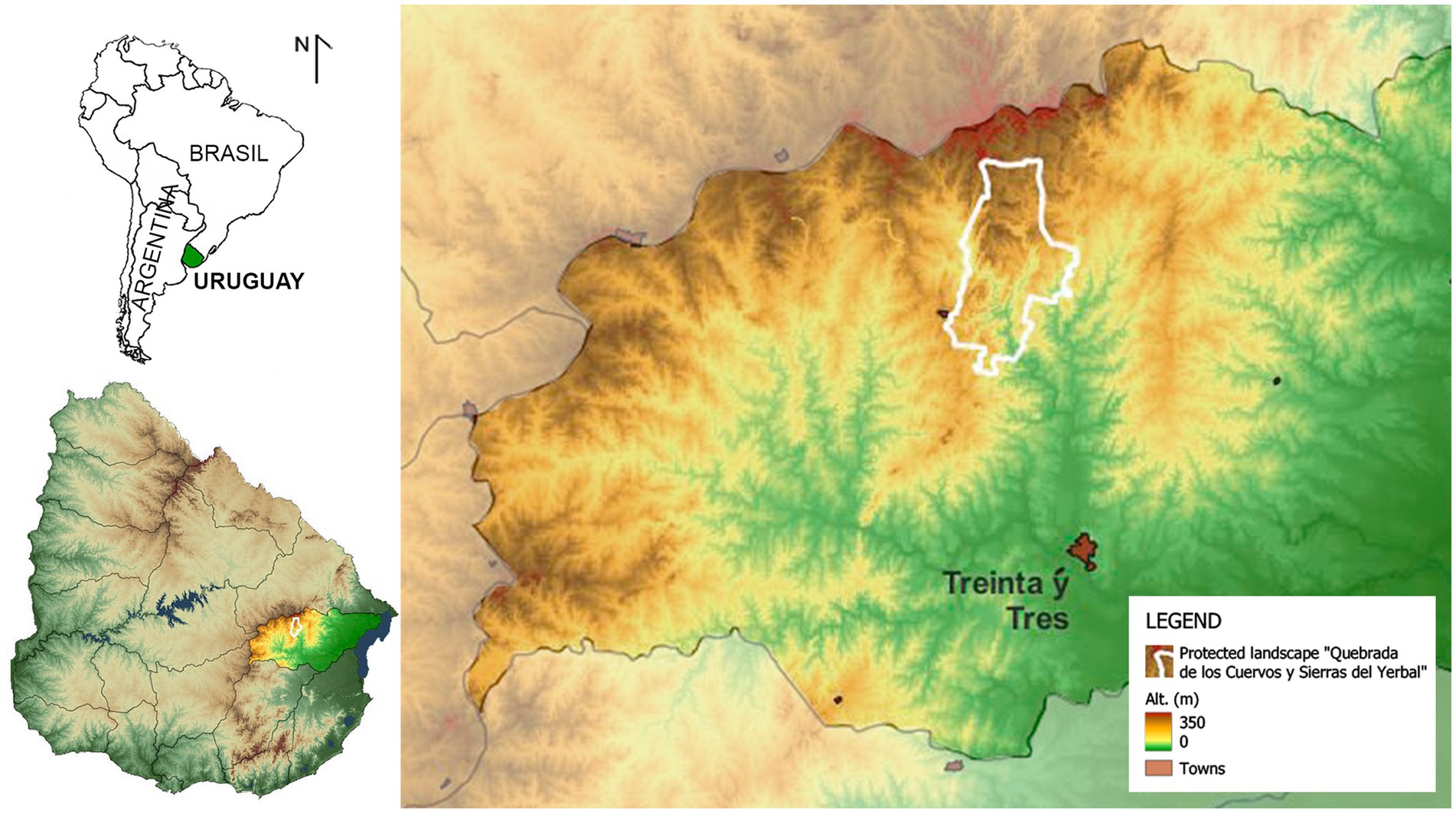
Figure 1. Geographic location and relief of the study area, “Quebrada de los Cuervos y Sierras del Yerbal” Protected Landscape, Treinta y Tres, Uruguay.
The protected area was inhabited by over 100 families, but currently, according to the information provided by the interviewees, only between 30 and 40 families reside in the area, indicating significant emigration forces at play (Achkar, 2017). The official rural population density is 0.34 inhabitants per square kilometer (INE, 2011). This population is primarily composed of descendants of european immigrants (Bica, 2019), with possible indigenous and/or African ancestry, resulting in a mixed population (Palermo, 2019; Clemente, 2021). The average size of properties is 350 hectares, with livestock farming as the main activity. However, the residents have recently engaged in eco and agrotourism activities.
2.2. Fieldwork
2.2.1. Field survey
Initially, a survey phase was conducted to extensively assess (Banning, 2002) the domestic contexts (DC) using satellite imagery from platforms such as Google Earth and Geoservicios IDEuy,2 1:50,000 cartography, field surveys, study of toponyms, and consultations with local informants. The term DC refers to inhabited locations typically comprised of one or more dispersed buildings and spaces utilized by the family for their daily activities. Abandoned locations were classified as “taperas” (traditional term used to denote abandoned houses), while inhabited ones were simply referred to as “houses.” For the documentation of each DC, a form was designed to record the place’s location, description, productive context, and ownership details if provided by informants. The data were organized in QGIS (v3.2) to generate a map illustrating the distribution of DCs. Subsequently, the obtained map guided a second survey phase in the field to locate and document each DC.
2.2.2. Primary assessment of agrobiodiversity
To gain an initial understanding of plant agrobiodiversity in the area, the species found in each visited DC were systematically identified, taking into consideration both cultivated spaces and their surrounding areas. The environments where the species were found were categorized as home gardens, small plots, vicinity of houses or taperas, and more distant areas encompassing grasslands, forests, and hilltops or rocky outcrops. The botanical identification was performed by the authors, who collected samples for subsequent verification at the Laboratory of Botany at the Regional University Center of the East Region (Universidad de la República). The nomenclature used was verified against the Plant List.3
2.2.3. Characterization of local knowledge
Based on the primary assessment and with the aim of obtaining detailed information regarding species, uses, and associated local knowledge, the DCs with the highest agrobiodiversity were selected, and connections were established with guardians and other key informants knowledgeable about these plant genetic resources. An ethnographic approach (Guber, 2014) was employed as a means of immersing in the context, exploring discourses, and gaining insight into the practices of the individuals (Restrepo, 2016). Techniques such as participant observation (Kawulich, 2006) and open and semi-structured interviews (Guber, 2001, 2014) were utilized, ensuring that the consent of each interviewee was obtained for the use of their provided data. A guideline was defined to cover topics such as family history and its connection to plant usage, the origin of knowledge, and the use and management of both wild and cultivated agrobiodiversity.
2.3. Data analysis
The data obtained from the surveys and interviews were systematically organized and analyzed both qualitatively and quantitatively. The following variables were recorded for each species: botanical family, origin (native or exotic, considering native species as those belonging to the Uruguayan flora), plant habit (annual herbaceous, perennial herbaceous, subshrub, shrub, tree, lichen), type(s) of DC (house or tapera) and environment where it is found (garden, small plots, adjacent environment, grassland, forest or rocky outcrops).
The recorded uses were classified into 11 categories: human consumption, animal feed, medicine, veterinary use, toxic and harmful use, fuel, construction, industry and crafts, environmental uses, ornamental, and social, symbolic, and ritual uses (Pardo de Santayana et al., 2014). The management practices were classified into 10 categories, based on an adapted proposal from various authors (Casas et al., 1996, 2014; Blancas et al., 2013; Furlan et al., 2017; Chamorro and Ladio, 2021): “tolerance” referring to species allowed to remain in environments where thinning, pruning, or weeding activities are carried out; “protection” implying actions taken to prevent damage caused by environmental factors to the species; “improvement” involving the favoring of individuals of the species or variety, for example, by eliminating competition, irrigation, seed dispersal, soil improvement (including soil cultivation and addition of fertilizers, among others); “propagation” referring to direct propagation of the species through seeds or vegetative methods; “transplantation” involving the moving individuals that have established naturally or were initially tolerated and then removed; “pruning” referring to the removal of parts of a plant with a specific goal; “gathering” involving direct harvesting of natural populations; “selection” referring to selecting certain phenotypes for reproduction; “community circulation” involving the exchange of plant materials among neighbors, family members, or other individuals; “care for inherited plants” involving the preservation of plants that were initially cultivated by others.
The following data were calculated: number of citations per species (NCs), understood as the number of interviews where the species was mentioned, number of uses per species (NUs), number of citations of use per species (NCUs), understood as the number of times the species was cited for a particular use, number of citations of management practices per species (NCMPs), understood as the number of times the species was cited for a specific management practice, number of management practices for each species (NMPs), and Consensus of Use index (CU%), calculated as NCs over the total number of interviewees.
To compare the agrobiodiversity of the two types of DCs, the Shannon-Wiener diversity index (H′) based on the frequency of species occurrence was estimated. Evenness was calculated as E = H′/lnS, where S represents the total species richness (Magurran, 1988; Magurran and McGill, 2011). Subsequently, a Detrended Correspondence Analysis (DCA; Sokal and Rohlf, 1995) was performed to ordinate the DCs based on the assemblages of plant genetic resources found in each one.
Qualitative information about local knowledge of the species, descriptions of uses and management practices, as well as data on the origin of knowledge, its generation, and propagation, was obtained from the analysis of the interviews.
3. Results
3.1. Domestic contexts and rural communities
A total of 54 domestic contexts were surveyed, consisting of 41 taperas and 13 houses (Figure 2). In most cases, these contexts comprise more than one building, with the main constructions generally made of stone, while secondary ones may be made of mud, brick, or stone. In the DCs, there are cultivated spaces (home gardens and small plots), mostly with clear boundaries, commonly fenced with stonewalls, wire fences, or metal sheets. These spaces are located in interior courtyards, around the house, with slate walkways and raised stone beds, or near the buildings with protective measures to prevent grazing. Taperas exhibit varying degrees of deterioration, ranging from abandoned houses to remnants of foundations that outline the shapes of past constructions. The protective features of previously cultivated areas no longer fulfill their function or only partially do so.
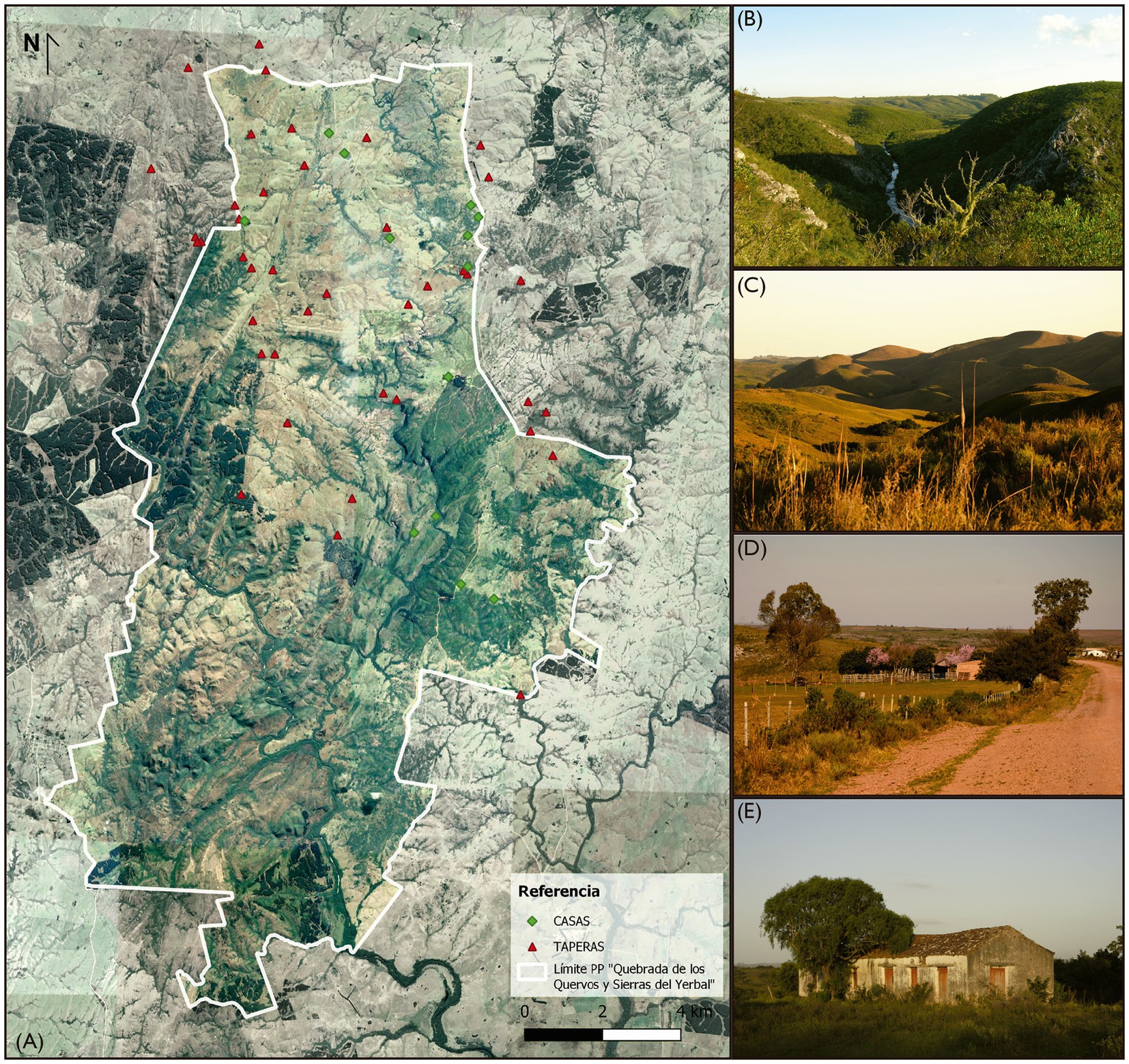
Figure 2. (A) Geographical distribution of the surveyed domestic contexts, including houses and taperas in the “Quebrada de los Cuervos and Sierras del Yerbal”, Treinta y Tres, Uruguay. (B) Quebrada de los Cuervos. (C) Sierras del Yerbal. (D) Surveyed domestic contexts in the Quebrada de los Cuervos and Sierras del Yerbal, house with a cultivated space fenced with wire, featuring fruit tree species such as Prunus persica and Citrus spp., with Eucalyptus in the nearby environment. (E) Well-preserved tapera with a Schinus molle tree in the front.
Twelve adult individuals were interviewed, of whom 67% were women and 33% were men, ranging in age from 20 to 70 years, although 75% of the interviewees were over 50 years old. The interviewees included 10 local residents (families with several generations in the area), one non-resident owner, and one representative from a local NGO. In most cases, multiple interviews were conducted with the same person, resulting in variable quality and depth of information, with an average of 37 species cited per informant and a range of 9 to 97.
3.2. Characterization and spatial distribution of agrobiodiversity
From the surveys and interviews, 185 species with associated uses were recorded, with 161 of them mentioned by the interviewees. These species belong to 66 botanical families, with 65 families of flowering plants (phanerogams) and one family represented by the lichen Usnea densirostra (Parmeliaceae). Seven of these families account for more than 40% of the species, namely Fabaceae (9%), Asteraceae (8%), Rosaceae (7%), Myrtaceae (5%), Rutaceae (5%), Solanaceae (5%), and Lamiaceae (4%). The Poaceae family, which along with Asteraceae has the highest number of species in Uruguay, is not well-represented in this study as it does not include forage species from natural grasslands. Figure 3A presents the distribution of families and species, including both native and exotic species, with 14 families shared between them. Figures 3C,D show the main families within each group. The distribution of growth habits among these species was as follows: 66 trees, 38 shrubs, 8 subshrubs, 44 perennial herbs, 28 annual herbs, and 1 lichen. These habits have different distributions between native and exotic species (Figure 3B).
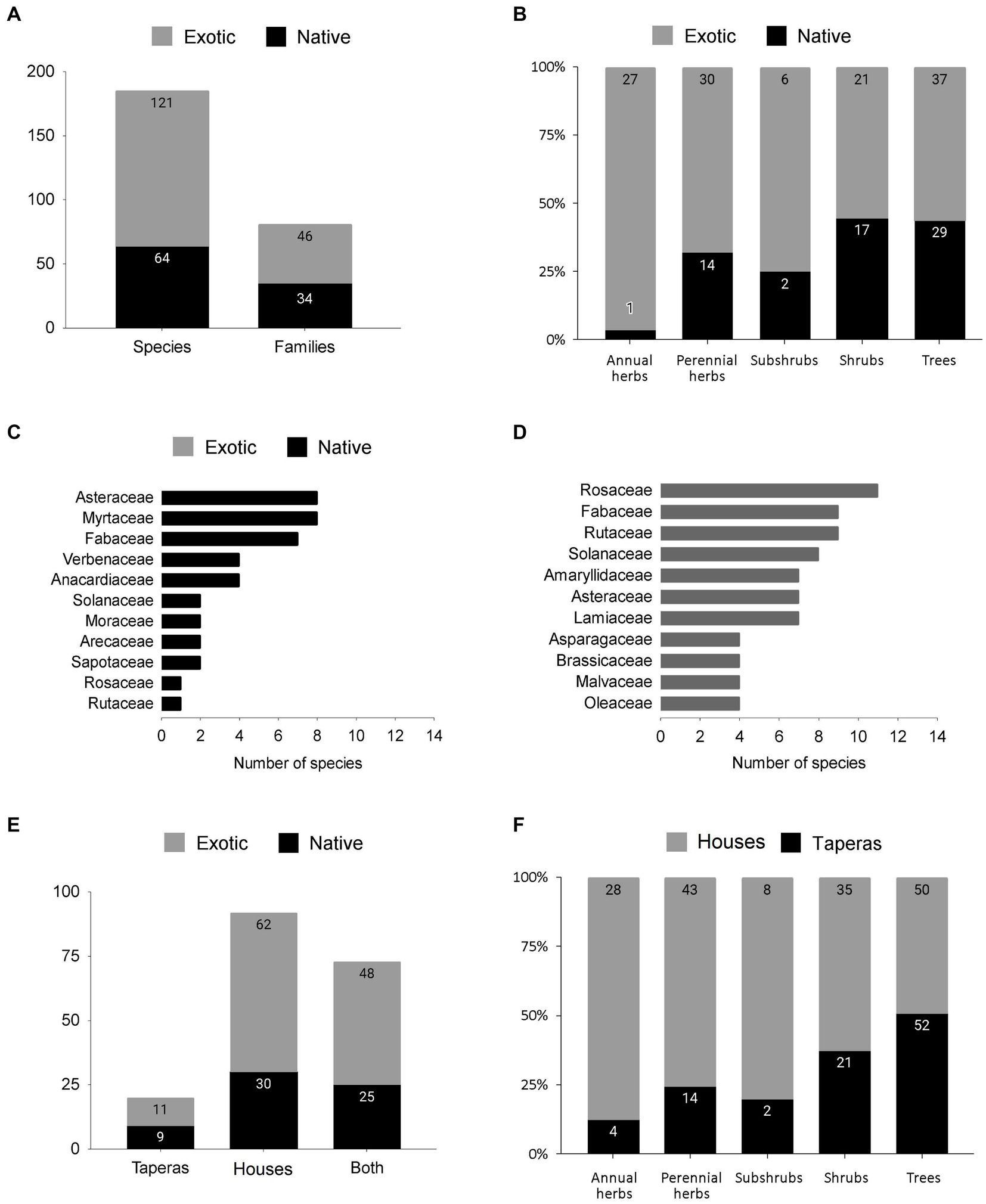
Figure 3. (A) Proportion of native to exotic plant genetic resources over a total of 185 species in 66 families of phanerogams and 1 lichen family. (B) Proportion of habits among exotic and native species. (C,D) The most important native (black) and exotic (gray) families. (E) Amount of species recorded exclusively in Taperas and Houses or in both, showing the exotic to native ratio (gray = exotic and black = native). (F) Proportion of habits in Houses (gray) and Taperas (black). Labels in bars mean the number of taxa in every category.
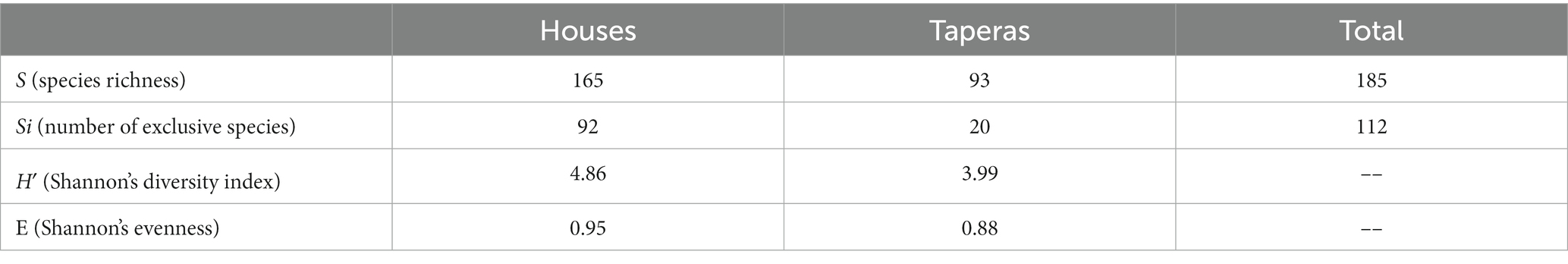
A total of 165 and 93 plant genetic resources were recorded in houses and taperas, respectively (Table 1). The number of species in houses ranged from 9 to 91, while in taperas it ranged from 0 to 25. The shared species between houses and taperas, as well as the exclusive species in each DC, can be seen in Figure 3E. There are differences in the composition of growth habits between houses and taperas (Figure 3F). Taking into account the relative frequencies in each DC, tree species are the most represented group in both DCs, accounting for 30% in houses and 56% in taperas. The distribution of habits in houses is more balanced, with 26% perennial herbs, 21% shrubs, 17% annual herbs, and 6% other habits, while in taperas, the rest of the habits consist of 23% shrubs, 15% perennial herbs, 4% annual herbs, and 2% other habits. More than 90% of vegetable crops and 75% of aromatic species are absent in taperas. The Shannon diversity index (H′) and evenness (E) values are shown in Table 1. Higher values are observed in houses compared to taperas, indicating higher richness and a greater number of species with comparable abundance in houses. Taperas, on the other hand, have fewer species with more extreme frequencies, resulting in lower levels of evenness.
The most frequent species and the exclusive ones in each DC are shown in Figure 4. Among the 30 most abundant species, approximately half are shared between both DCs, but their order of importance changes. Furthermore, houses and taperas are clearly separated into two groups in the DCA (Figure 5), with houses ordered toward the left and taperas toward the right of the graph. The first axis of ordination follows the reverse gradient of DC diversity. The separation into two groups was expected given the high proportion of exclusive species found in houses. Some of these species stand out in the ordination, along with other species that made a significant contribution. Species appearing in intermediate positions on the graph, such as Schinus lentiscifolius (Carobá) or Eucalyptus spp., are present in both houses and taperas.
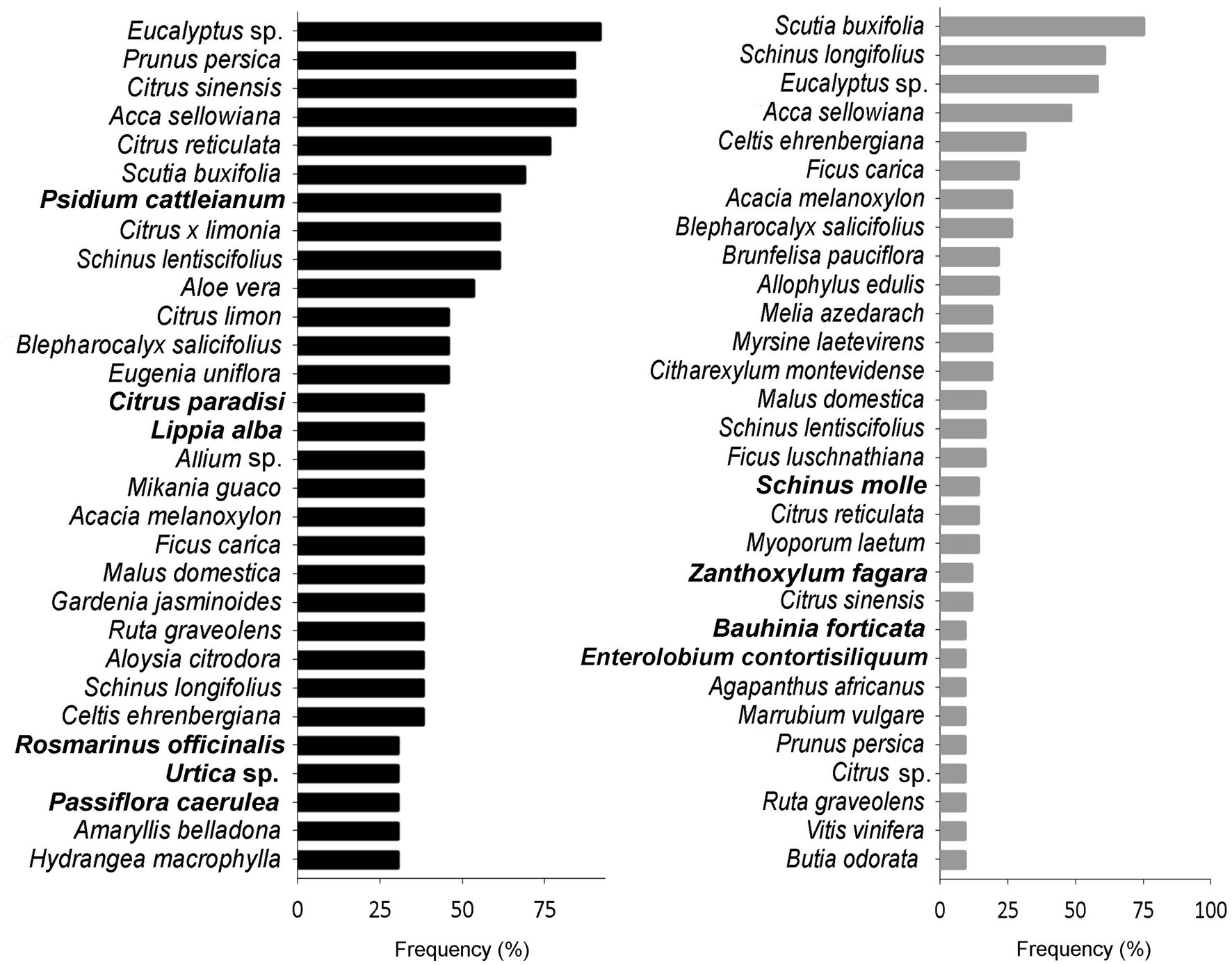
Figure 4. The top-30 most frequent species found in Houses (black) and Taperas (gray). In bold are shown the exclusive species in every domestic context.
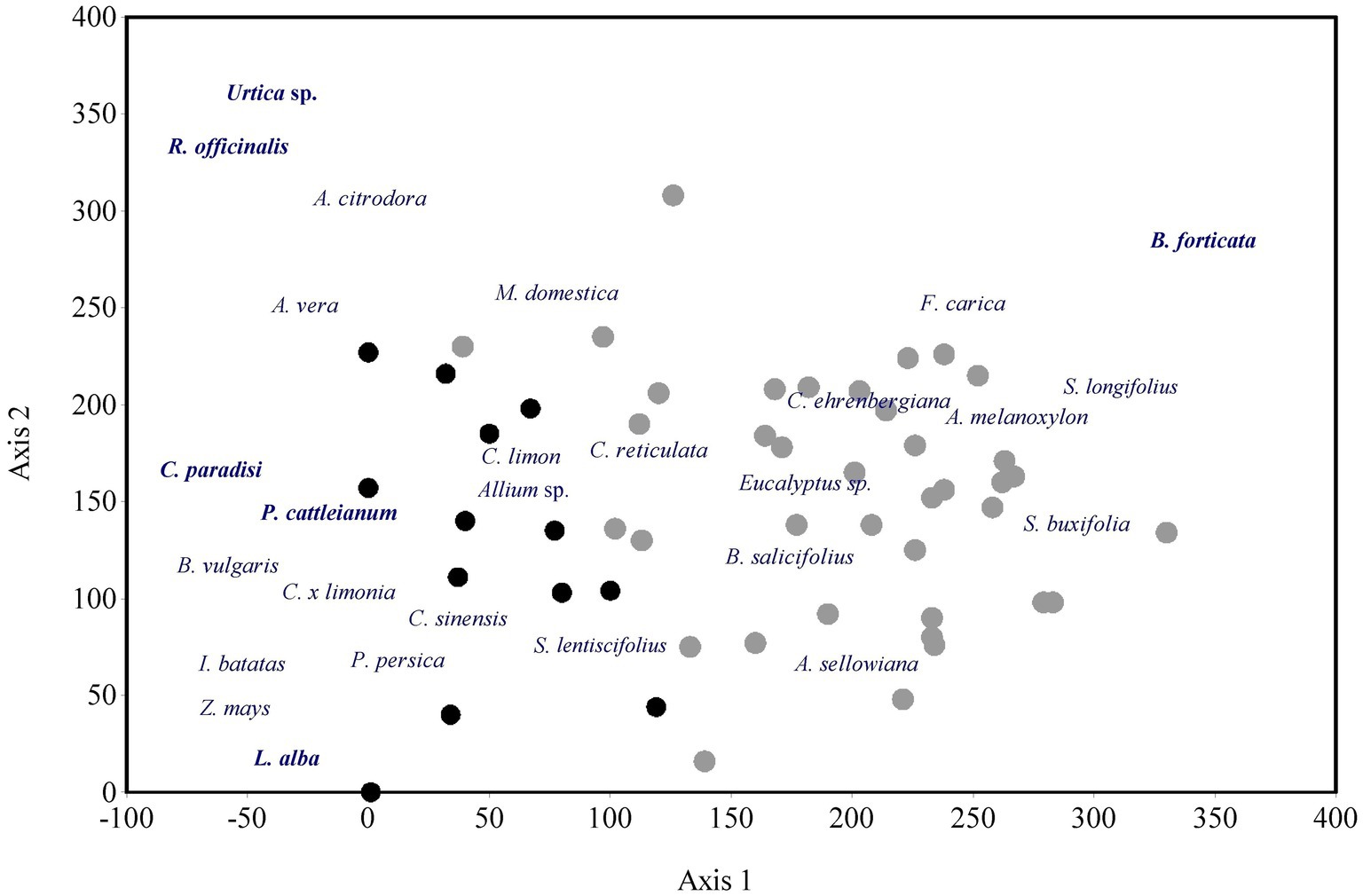
Figure 5. DCA ordination of Houses (black dots) and Taperas (gray dots) with respect to the frequency of species recorded. The first two eigenvalues were 0.467 and 0.316. The ordination of some of the most frequent plant genetic resources found in both domestic contexts are also shown.
Regarding the spatial distribution of plant genetic resources, the species were distributed as follows: 120 in home gardens, 17 on small plots, 82 in the surrounding area, 33 in natural grasslands, 44 in forests, and 21 on hilltops. There are 43 species present in the cultivated and non-cultivated environments, with the majority (35) being native species.
3.3. Uses of agrobiodiversity
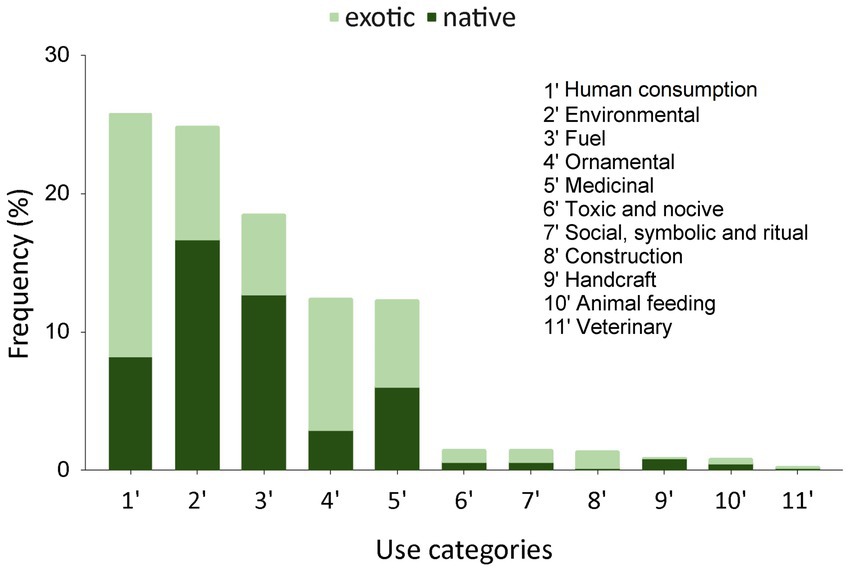
From the fieldwork, 1,199 records of plant uses emerged, including inferred uses from the survey (52%) and cited uses from interviews (48%). Uses were recorded for the 11 pre-established categories, and extensive local knowledge was found regarding the ways of using numerous native and exotic plant genetic resources. Figure 6 shows the frequencies of each use category, with the most frequent being: human consumption, environmental uses, fuel, ornamental, and medicinal. The figure also indicates that native species predominate in environmental and fuel uses, while medicinal uses show an equivalent use between exotic and native species, and the other two categories are predominantly exotic. When considering the number of species, the categories are ranked differently: human consumption, ornamental, medicinal, and environmental uses with 71, 62, 58, and 49 species, respectively. Fuel use was mentioned for 28 species, while toxic and harmful use, social, symbolic uses and ritual uses, animal feed, and industry and craftsmanship were cited for 7 to 12 species each. Construction and veterinary uses registered fewer species, 3 and 2, respectively.
The species with more than one use category (NU>1) constitute 45% of the species total, with native species having the highest number of NUs: Schinus lentiscifolius and Blepharocalyx salicifolius with 5 use categories, Scutia buxifolia, Acca sellowiana, Citharexylum montevidense and Daphnopsis racemosa with 4. It was observed that different use categories concentrate varying numbers of species with NU>1. Environmental, fuel, toxic and harmful uses practically encompass all species with more than 1 use, while medicinal uses have 64% of their species with more than 1 use, ornamental (50%), and human consumption (44%). Figure 7 provides an ordered list of species with the highest number of citations for their uses (NCUs) and the respective use categories for each species.
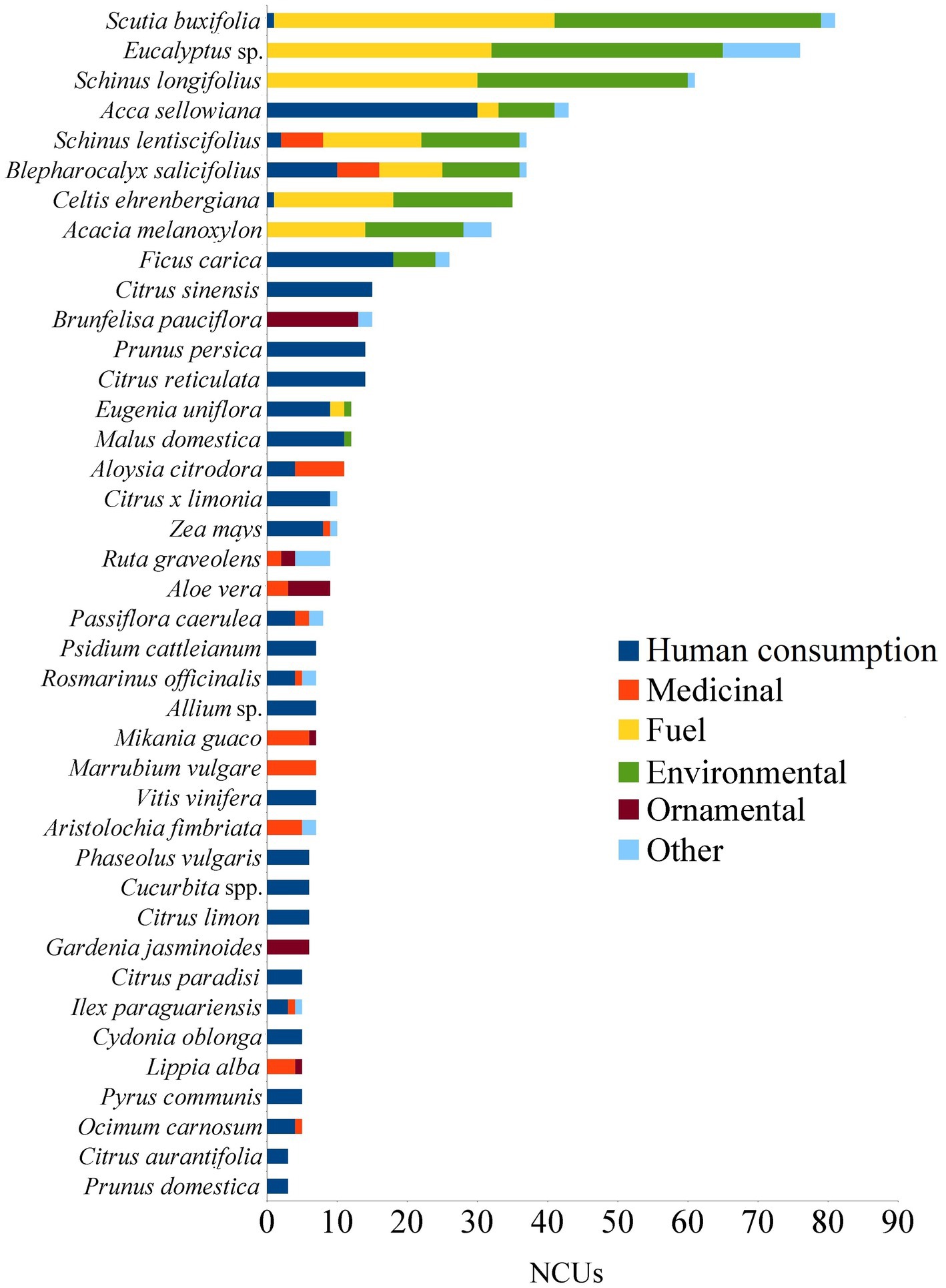
Figure 7. The top-40 most used species with respect to the number of cited uses (NCUs) for every use category. Minor uses are summarized as “other.
On the other hand, considering the Consensus of Use, the species with higher CU (>50%) are: Prunus persica, Citrus sinensis, Acca sellowiana, Eucalyptus spp., Schinus lentiscifolius, Scutia buxifolia, Zea mays, Citrus reticulata, Citrus x limonia, Eugenia uniflora, Psidium cattleianum, Cucurbita spp., Phaseolus vulgaris, Ficus carica, Urtica urens, and Blepharocalyx salicifolius. Table 2 presents the most cited species for the main use categories.
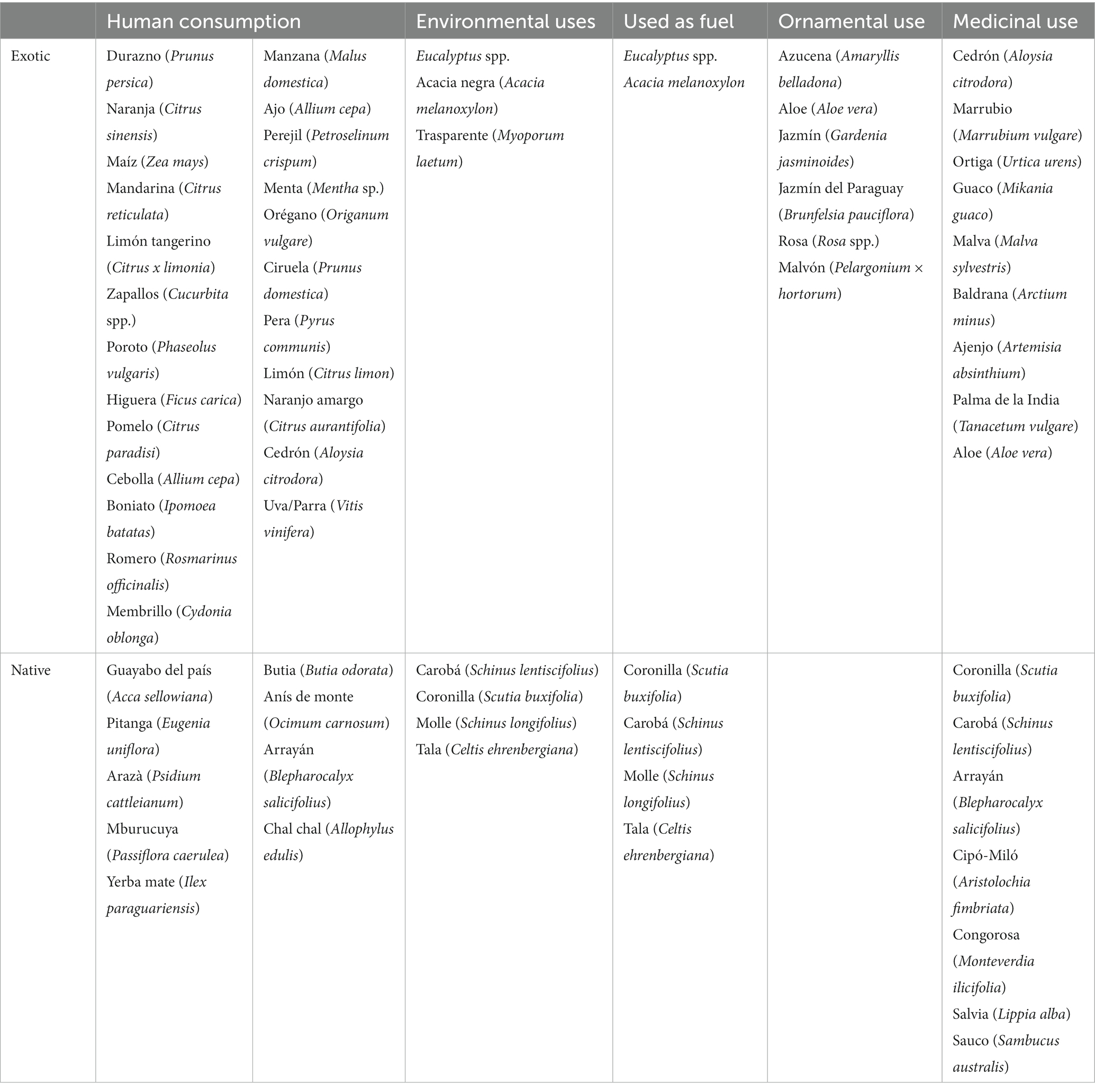
Regarding human consumption, various forms of food consumption were recorded, including fresh, cooked, or dried fruits and vegetables, alcoholic beverages (wine and liqueur), and non-alcoholic beverages (flavored water, juice, tea, and infusion), seasoning, sweets, and chewing products. Out of the 71 species cited for human consumption, 53 are exotic and are distributed among traditional productions: 27% fruit crops (19 species), 32% vegetable crops (23), and 11% aromatic plants (8). Images of some of the most relevant species for human consumption are presented in Figure 8. Among the 18 native food species, most are edible fruits that are usually consumed in situ when exploring forests, grasslands, or rocky outcrops. The most notable example is Blepharocalyx salicifolius. A.M. describes the taste and experience with the fruit: “Birds and humans feed on Arrayán, it leaves you with a refreshing sensation, like a mint candy, the aroma is very good.” Other species cited with this form of consumption are Schinus lentiscifolius, Celtis ehrenbergiana, Scutia buxifolia, Allophylus edulis, Citharexylum montevidense, Psidium salutare, Opuntia ficus-indica, Myrceugenia euosma, Passiflora caerulea, and it also happens with Acca sellowiana and Psidium cattleianum, although these last two are also found in cultivated environments. Lastly, the preparation of infusions from different parts of the plant was recorded for 3 native species: Ilex paraguariensis, Achyrocline satureioides and Ocimum carnosum.

Figure 8. (A) Fruit orchard: Peach (Prunus persica) and Citrus sp. (B) Fruit orchard: Tangerine lemon (Citrus x limonia) with fruit, surrounded by blooming peaches. (C,D) Guayabo del país (Acca sellowiana). (E) Fruit of the Tangerine lemon. (F,J) Ancient Fig tree (Ficus carica). (J) Detail of the Fig tree, showing a carving on the trunk, which is presumed to be the result of a healing practice. (G) Common bean (Phaseolus vulgaris). (H) Cidra (Cucurbita ficifolia). (I) Warted squash (Cucurbita spp.).
Environmental use was the second most cited use, being of equal importance as human consumption. The most common form of use was for the protection of humans and animals from extreme weather conditions, providing shade in summer and shelter from cold in winter, mainly protecting livestock from frost. Examples of some tree species in use can be observed in Figure 9. Most of the species in this use category are trees, and although the number of species is high, the use citations are concentrated in a few species (Table 2). Fuel use has similar characteristics, with fewer species since a selection is generally made from the previous category, emphasizing the quality of firewood for fuel.

Figure 9. (A) Coronilla (Scutia buxifolia). (B) Use of Coronilla in the construction category, as a post or wire rein. (C) Arrayán (Blepharocalyx salicifolius) in fruiting stage. (D) Carobá (Schinus lentiscifolius). (E) Carobá ancient tree managed with a single trunk.
Regarding the use of ornamental plants, although it was one of the uses with the highest number of species and a significant number of citations, these are well-distributed, and few species stand out. Traditionally ornamental genera such as Amaryllis, Rosa, Pelargonium, and Gardenia are notable. The native species mentioned as ornamentals were 9, each with only 1 or 2 citations: the palms Butia odorata and Syagrus romanzoffiana, Daphnopsis racemosa, Lippia alba, Prunus subcoriacea, Aspillia montevidensis, Cochliasanthus caracalla, and Phytolacca dioica. Regarding gardens and their beauty, M.S. recounts that in Amaro’s house, now in ruins, there was a “garden” framed between the buildings “that was beautiful, full of flowers, there was a huge orange tree in the middle surrounded by stones, and he cultivated plants in flowerbeds” (…) “On November 2nd, everyone would go to pick flowers for the dead.” These flowerbeds still exist today, with no flowers, and they are still delimited by standing stones.
For medicinal use, citations of species used for various diseases in the respiratory, digestive, circulatory, endocrine, immune and urinary systems were recorded. As well as for the skin, subcutaneous tissue, infectious and parasitic diseases; and against poisoning, and other medicinal uses. A variety of medicine preparation methods and application forms were also documented. Fifty-eight species were found with medicinal use, 30 of which are native, exhibiting various habits, with perennial herbs (22) being the most common, followed by shrubs (14), and finally, annual herbs, subshrubs, and trees (8, 8, and 6 respectively).
3.4. Agrobiodiversity management
Based on an ethnographic work, 1,338 records of management practices emerged, providing data for the 10 predefined categories of management, along with qualitative information on the application of each practice. Figure 10 shows the frequencies of management practices and their application to exotic and native species. The most frequent management practices are protection, propagation, and improvements, mainly applied to exotic species, followed by pruning, gathering, and tolerance, with the last two practices mostly applied to native species. Regarding the number of species receiving each practice, the order is as follows: protection (134), propagation (120), improvements (119), tolerance (54), gathering (45), pruning (36), community circulation (34), care for inherited plants (29), transplantation (14), and selection (11).
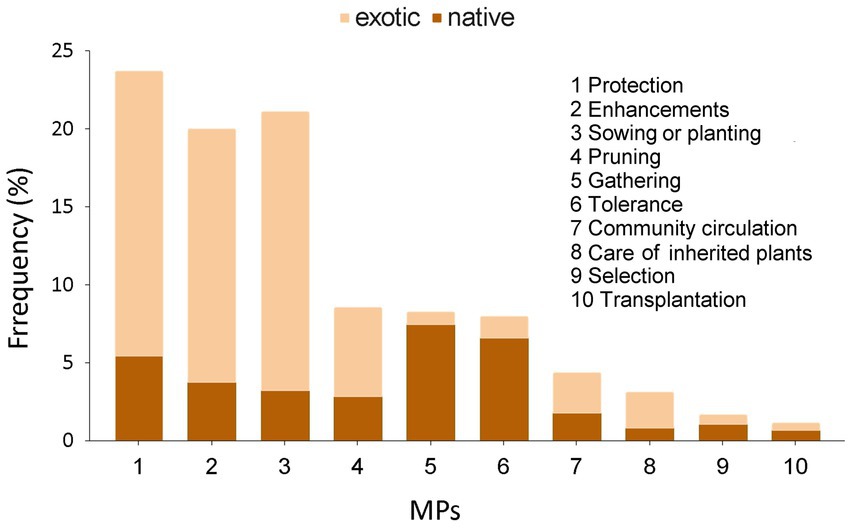
Figure 10. Frequency of citations of management practices (MPs) in native (dark) and exotic (light) species.
The recorded species with more than one management practice comprise 82% of the species total, with species ranging from 0 to 10 management practices. The species with the highest number of management practices are: Acca sellowiana (10), Prunus persica (9), Ilex paraguariensis (9), Prunus domestica (8), Schinus lentiscifolius (8), Ficus carica (7), Citrus x limonia (7), Ruta graveolens (7), Aristolochia fimbriata (7), Blepharocalyx salicifolius (7), and Psidium cattleianum (7). Figure 11 ranks the species according to the number of citations of management practices per species (NCMPs), highlighting those of greater cultural value.
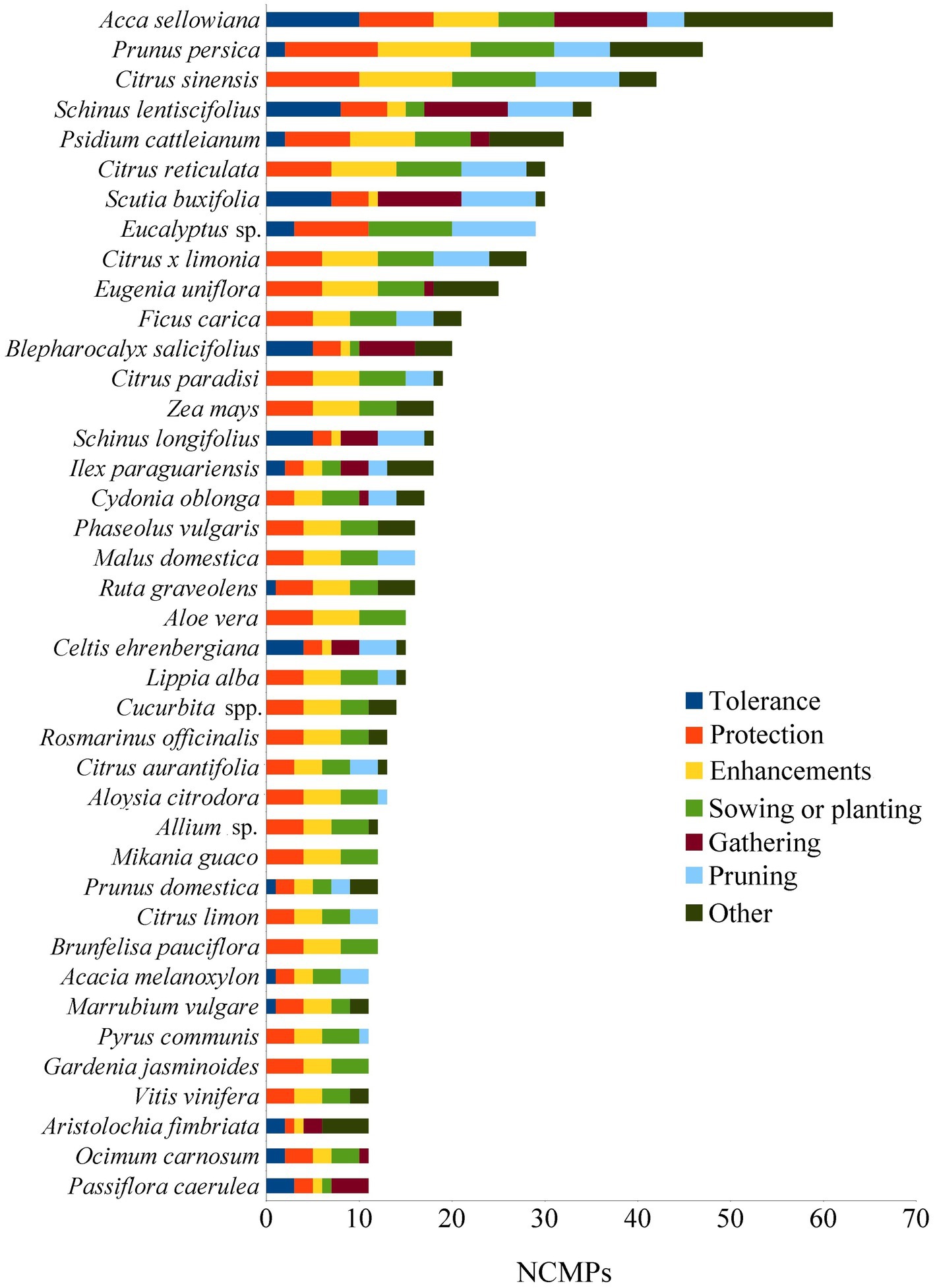
Figure 11. The top-40 most frequently managed species based on the NCMPs. Minor practices were summarized as “other” for better legibility.
Regarding protection, propagation, and improvement practices, they are mainly applied to species found in cultivated environments (gardens, small plots and holdings) and the surroundings of houses. Protection of these environments includes enclosures that prevent livestock from grazing, protect against wind and damage from other animals such as hares, parrots, and wild boars, as well as actions taken on plants to prevent insect attacks (e.g., ants). In this regard, P.R. indicates: “when there is a plague of parrots, you have to take turns scaring them away..” P.R. also mentions that after abandonment, when the previously maintained protections by the inhabitants deteriorate, livestock enter the farm or garden, breaking branches and browsing foliage, weakening and killing the specimens. As for propagation, it is carried out by sowing seeds obtained from collecting, self-production, exchange, purchased plants, or collected propagules. The recorded improvements include the addition of animal manure (chicken, horse, cow), soil preparation, sowing, irrigation, and removal of plants competing for space or light with the target plant.
Pruning was recorded in 36 species, including trees and some shrubs, mainly used for human consumption, environmental purposes, and fuel. Formation pruning is mainly performed on trees that provide shade and shelter for livestock, shaping a high-crowned tree that allows circulation underneath, as is the case with Scutia buxifolia, Schinus lentiscifolius, Schinus longifolius, or Celtis ehrenbergiana. On the other hand, pruning fruit trees aims at increasing fruit production and ensuring their health. Regarding sanitary pruning, P.R. provides an example indicating an important factor leading to the death of specimens after the abandonment of the DC, namely, the parasitism of “Yerba del pajarito” (Tripodanthus acutifolius), a native epiphyte hemiparasitic species that germinates and parasitizes trees, weakening the specimens. According to the account, the “Yerba del pajarito” is constantly controlled by residents in their homes, and a common management practice in fruit trees is to cut the branches that support early stages of its parasitism.
Gathering and tolerance practices are applied to 45 and 54 species, respectively, of which 89 and 74% are native, primarily recorded in medicinal, human consumption, fuel, and environmental uses. Some examples of native species where these practices are applied are: Acca sellowiana, Schinus lentiscifolius, Scutia buxifolia, Schinus longifolius, Celtis ehrenbergiana, Blepharocalyx salicifolius, Monteverdia ilicifolia, Ilex paraguariensis, Baccharis trimera, Baccharis articulata, and Passiflora caerulea. Some examples of exotic naturalized species are Cyclospermum leptophyllum, Arctium minus, and Urtica urens.
The care of inherited plants was mainly recorded in old specimens of Acca sellowiana, Prunus persica, Citrus x sinensis and Citrus x limonia, indicating that they were planted by previous generations. It also includes vegetable landraces, whose seeds have been conserved for several generations. A. states, “The squashes are from my father’s house. One type has a long neck, another one grows oval.”
Selection was recorded for five native species: Acca sellowiana, Ilex paraguariensis, Achyrocline satureioides, Psidium cattleianum, and Blepharocalyx salicifolius. In the case of Arrayán, A. indicates, “It’s the white Arrayán, the one with thin leaves and a white bark. I used it to treat uric acid. I collected seeds from these plants to share seedlings with this trait.” As for exotic species, selection was recorded in peach (Prunus persica), plum (Prunus domestica), fig (Ficus carica), as well as in landraces of maize (Zea mays), bean (Phaseolus vulgaris) and squash (Cucurbita spp.).
Community circulation occurs through various channels: among family members and/or neighbors, from wild plants to one or several neighbors’ homes, from taperas to houses, from institutional projects to neighbors and vice versa, and from houses to the wild. A.M. comments on peaches, “They have been in the area for many years” [...] “The peach trees were brought from the plants that were at Z.’s house. They have always planted them. They had an impressive peach orchard. Z. gave me two bags of peaches, and I did not have any, so I made jam. They made dried peaches, among many other things. I made seedlings with the seeds.” The same applies to native fruit trees, where seeds or seedlings are collected to be cultivated near the house, as is the case with Guayabo del país (Acca sellowiana) and Arazá (Psidium cattleianum). A.M. explains, “the ideal place for native fruit trees is to have them close to the house, so you can harvest them. Harvesting takes a long time, which I no longer have.” Another example is Marcela (Achyrocline satureioides). A.M. states, “I used to only collect it, but now I have learned to put it back into the soil. I use scissors to cut the flowers, then I let them dry on paper. I use the flower for tea and extract the seeds. I put the seeds back into the soil. I once made a flowerbed with those seeds in the backyard.” [...] “Marcela is a complicated plant to cultivate; you have to leave it alone. It prefers to live in the wild.” A.M. throws the plant near the house to have it there and in the hills to maintain the species and prevent its loss. She has observed that in some enclosed fields, a different, larger species of Marcela, called “Marcelones,” has grown. She is also collecting seeds from this species.
3.5. Prominent plant genetic resources
The cultural value of the species in this landscape can be observed in Figure 12 through the values of CU (Consensus of Use), NUs (Number of Uses), and NCPMs (Number of Citations per Mention). Qualitative information on the local knowledge gathered is presented for these species, including Yerba Mate (Ilex paraguariensis) and Cipo-miló (Aristolochia fimbriata), which are considered strategic resources by the community.
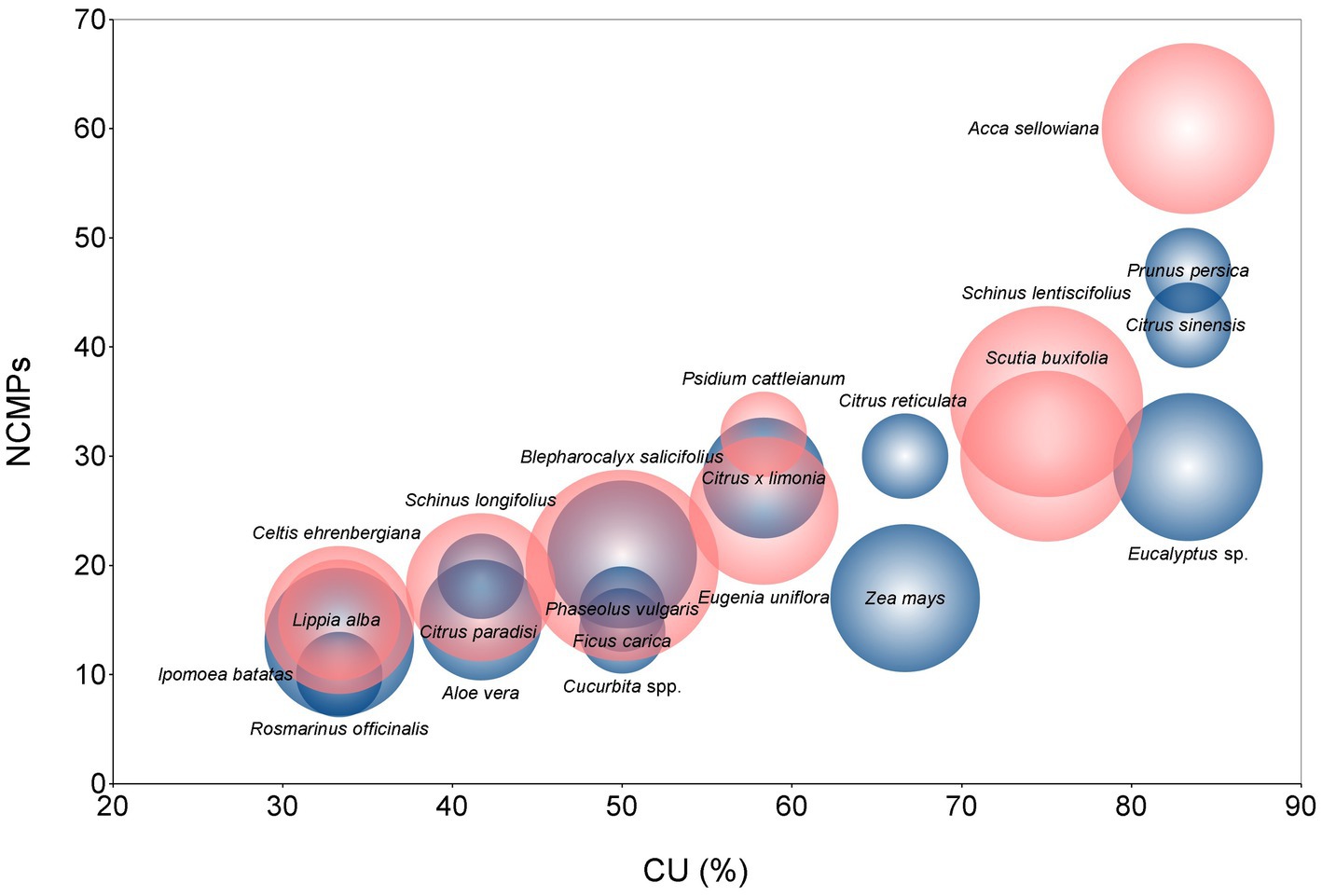
Figure 12. Selection of species with the highest consensus of use (CU) and NCMPs. Bubble sizes are proportional to the number of uses referred to in the interviews. Blue bubbles are exotic and pink ones are native species.
Peach (P. persica) is the most cited species by the interviewees and is highly present in households (Figures 8A,B), it is one of the species with the highest number of recorded management practices. These genetic materials have been in the area for several generations and exhibit significant variability in their fruit, skin color, pulp color, with the “white peach” being very common, along with clingstone and freestone varieties, and a wide harvest period ranging from November to February. There is local knowledge regarding its ecology and cultivation. A.M. states, “There are white-fleshed, yellow-fleshed, and red-fleshed peaches. The red one gives fruit in November, it’s the first one. The latest one is in February, and I always have peaches throughout the summer.” [...] “It’s not big but very tasty, very aromatic, it makes excellent liqueur, exquisite.” Varied ways of consumption were recorded, such as fresh fruits, dried (“orejones”), and the preparation of preserves and liqueurs. This species is found in gardens, where it receives fertilization, irrigation, training pruning, branch thinning, and sanitation pruning to eliminate the hemiparasitic plant “Yerba del pajarito” (Tripodanthus acutifolius). Peaches are propagated through seeds, which germinate spontaneously, and seedlings are allowed to continue their growth in situ or are transplanted to a definitive location. People also engage in sowing for subsequent transplantation. Seed and plant exchange and care for inherited plants was also recorded, indicating a long history of cultivation in the area. They are aware of their history: who brought the seeds, and where they came from.
The Citrus genus comprises seven fruit-bearing species in the area, and it was recorded in 92% of the surveyed households, mainly found in orchards, although there may be specimens in gardens and the surrounding area. The most used species within the genus are Orange, Mandarin, and Tangerine Lime. Some very old trees, according to accounts, could be 100 years old, and it is mentioned that there used to be orchards that sold oranges for the local industry. The “Tangerine Lime,” as it is called by the local inhabitants, is a citrus not commercially cultivated in Uruguay. According to our survey, its fruit is medium-sized, orange-colored, with orange and acidic pulp (Figure 8E). It produces abundantly throughout the year without presenting alternate bearing, as reported by the interviewees. Its uses include fresh consumption, the preparation of preserves, jams, and beverages such as juices and wine. The species propagates naturally through regeneration, where plants are allowed to sprout or are transplanted to a suitable location. There is community circulation and care for inherited plants.
Fig trees (Ficus carica) are found in gardens or orchards, in protected spaces, but they are also present in the less protected surroundings (Figure 8F). They are long-lived and resilient species, and very old specimens were observed in taperas. Knowledge about the qualities and variability of its fruit was recorded, with the presence of three types of plants: A.M. “I have two fig trees, one with large white figs and another called “honey fig.” Honey figs are white figs that, when ripe, release a sweet drop that resembles honey, very sweet.” Black-skinned fig trees were also found during the surveys. Although its primary use is human consumption, its environmental use for shade around the house was recorded, and symbolic or ritual uses were also mentioned, as A. recounts, “you can heal using the fig tree: you carve the sick person’s foot to cure hernia. When the tree wound is healed, the person gets cured.” It is worth noting that during the survey of taperas, a specimen with a carving resembling a small foot was recorded (Figure 8J). Furthermore, information about its propagation was collected, noting that root suckers emerge from the base of the tree, generate roots, and can be separated from the mother plant to generate a new identical plant.
Guayabo del país (Acca sellowiana) is a native fruit species whose fruits are consumed both fresh and processed into sweets (Figures 8C,D). In the area, there are wild specimens, specimens found in cultivated areas probably selected for their fruit, and specimens in small production plots installed by local organizations and academic groups. The interviewees shared general knowledge about the species and specific plants: A.M. said “there’s a new one, in a paddock, which is growing well because it does not have any predators. That tree bears very delicious fruit, a shiny, elongated fruit with a thin skin, it’s the type of Guayaba that is good to eat fresh.” A.M.: “Guayaba trees do not yield the same amount every year,” attributing it to climate change and noting that it can be observed in all fruit trees. P.R. comments, “Every house used to have old Guayaba trees. There was a time when Guayaba trees produced a lot, then there was a period when they stopped producing, and now the ones in the countryside are starting to produce again” [...] “When we were kids, in the afternoon, we would go out and look for Guayaba to eat.” Other uses were also recorded, such as animal feed, environmental uses, and fuel. The fruit harvest is done in wild plants (gathering), which are “monitored,” keeping track of their phenological status, particularly the fruit ripening stage. Local knowledge was recorded about the wild and domestic animals that eat the fruit, including sheep, wild boars, and rheas. The protection, improvement, and tolerance of plants in the immediate vicinity of the house were also noted. Improvements include measures such as removing plants of other species that compete with it, as M. comments: “I clear the area below it to make it clearer, I remove the surrounding plants.” Planting, cultivation, and transplantation of specimens from the wild to the garden or farm, or from one cultivated area to another, were also observed. Z.O. states, “I plant seeds everywhere, and then, when they sprout, I move the seedlings to another place” [...] “I planted this one, I took it from the root of another plant in the garden. It had a sprout, I took it out with a small shovel and planted it in a container, and then I planted it here. It was a little trunk, it had leaves...” The care of old plants inherited from previous inhabitants or family members was also confirmed, and there was knowledge on the history of these plants.
The Arazá (Psidium cattleianum) and Pitanga (Eugenia uniflora) are two native fruit species mainly mentioned for human consumption. Both species are highly present in both gardens and small plots, and are part of current development projects managed by local NGOs. J.P., a member of an NGO, defines these species as part of the most important plant genetic resources in the area. Local knowledge about both species was recorded. One of the interviewees, M., monitors wild arazá plants near her house, in the forest along a stream, so she can eat them: “Arazá need good moisture to produce large fruits. If you plant it in the field, it produces small fruits, but next to a stream, it produces nice large fruits. One branch fell to the ground, took root, and grew into a new plant.” A.M. planted Pitanga in her garden, a plant she brought from Treinta y Tres some 24 years ago. She has already harvested fruits, and made juice and wine.
The vegetable landrace varieties mentioned by the interviewees were maize, pumpkin and squash, beans, and sweet potato. They are usually grown in small plots and homegardens using agroecological multi-species systems. Information from the interviews reveals details about the landraces of Squash (Cucurbita spp.), their traits, and uses. Interviewee A mentions using all the landraces she has for making sweets, and some for stews: “Now I have a gray squash, white on the outside and orange on the inside. It belongs to my sister-in-law; they have had it for a long time. I like that strong color because of the color it gives the sweet.” She also has warted squashes (Figure 8I). She selects the seeds by choosing “the squash closest to the stem, the first one that does not grow as much on a trellis. I choose the seeds from the tastier ones: I save the seeds, taste the squash, and if it’s good, I plant it.” Another cucurbit mentioned is the Cidra (Cucurbita ficifolia) (Figure 8H). A.M. says, “I plant cidra every year, a significant amount can be harvested from half a hectare, with fruits weighing up to 30 kg. The plant has always been in the area; people used to grow it and it was passed on from one person to another. It was mainly used to feed animals, and they made sweets for the house. Cows and pigs were fed with it.” As for Maize, the interviews indicate its diverse uses over several generations. M.S.: “My family used to grow maize, and with the grains, they would grind them and make bread, mazamorra, and gofio.” Another interviewee (A.) explains how she selects the grains for planting in the next season: “With maize, I also choose good grains that are not diseased, with even rows. I remove the tassel and the back part, which always gets crossed. About the management, she says, “The ‘purple’ variety pigments the others. I plant them in the same field, separated by rows of squash.” Regarding the origin and circulation of the seeds, she says, “The seeds came from N’s aunt and I gave them to A.M..” The Beans included black beans (Figure 8G) and “frutilla” beans, which were the most commonly used. Interviewee A. recounted, “Black beans are delicious to eat and easy to cook. This year I harvested more than a bag of beans. I have had these seeds for 10 years; they were given to me by the husband of my daughter’s teacher, who was from Treinta y Tres. We eat those beans and share them with A.M.”
The species of Eucalyptus (Eucalyptus spp.) are present in most DCs, and they are among the species with the highest number of documented uses and management practices. Reports indicate that due to their rapid growth compared to native forest species, they are planted to fulfill various needs, such as livestock protection, providing shade and wind protection for homes, and serving as fuel for heating and cooking. Eucalyptus is also used in construction, particularly for posts, despite being known for its faster decay. It can be found near houses and planted as isolated stands within grasslands, forming sheltering groves. Protection is practiced in the early stages, and later they are managed through pruning. The branches and cut stems are used as fuel or for posts. In some cases, natural regeneration occurs, which is tolerated.
Carobá (Schinus lentiscifolius) (Figures 9D,E) and Coronilla (Scutia buxifolia) (Figures 9A,B) are iconic native species in this landscape, widely distributed in the “Quebrada de los Cuervos and Sierras del Yerbal.” They have high cultural value and serve multiple purposes. Both species are tolerated and managed in the vicinity of DCs. However, they are generally not permitted in cultivated areas due to their space requirements. Several interviewees mentioned that their management involves pruning lateral branches and shaping the crown in a way that allows the trunk to thicken and occupy less surface area in the field. This allows animals to seek shelter underneath the trees, providing firewood and protection for livestock (Figures 9A,B,D). The interviewees also agree that felling the trees is not a good option because it encourages basal regrowth, and the tree occupies even more space. This management approach is also applied to Tala (Celtis ehrenbergiana) and Molle (Schinus longifolius). Other reported uses of Carobá include medicinal applications for stomach ailments such as acidity or heartburn, consumption of its fruit as a seasoning or chewable, and animal feed. The other uses of Coronilla include the consumption of its chewable fruit and the utilization of its trunk to build fences or enclosures. In all cases, the uses are derived from wild plants.
Arrayán (Blepharocalyx salicifolius) is another common species in the native forest of the area (Figure 9C), highly valued among local inhabitants. Four categories of use were recorded for this species, with the most cited use being medicinal as a digestive aid for stomach ailments. Local knowledge was documented, including phenotypic selection for medicinal use based on differences in bark, leaves, and fruit. One interviewee, M., mentions, “I have an Arrayán plant that I grew from a seed collected in the forest to provide plants to a neighbor who wants to take it because she says it’s good for cholesterol, and the one she has there has a light yellow fruit, not red like the ones here.” This statement also highlights the community circulation of the species. Other uses of Arrayán include human consumption of its fruit as candy or chewable. One of the interviewees explored the creation of processed products such as jam or liqueur. A.M. states, “I’ve collected and made liqueurs with Arrayán using both the fruit and the leaves.” (...) “There are different plants with different fruits, more red or more orange, and they ripen at different times, so you can choose.” (...) “In general, I gather the fruits, separating them by color, and make one liqueur with the orange ones and another with the red ones. The fruit is very small, though, and you have to gather a large quantity. Each tree yields a lot, but the fruits do not ripen all at once, so you spend several days collecting a large amount.” It is a species that is not planted due to its abundance and is harvested from wild specimens. If Arrayán trees grow near DCs, they are tolerated.
The Yerba mate plant (Ilex paraguariensis) has three main uses: human consumption, medicinal purposes, and social, symbolic and ritual uses. It is one of the species with the highest number of management practices and the most extensive qualitative information recorded. According to P.R. ‘s accounts, “all these streams have Yerba mate.” The interviewee does not recall the local use of this particular population, although they did participate in the harvesting and processing of Yerba mate in other nearby areas. P.R. describes the process of Yerba mate production, stating, “It used to be harvested in June and transported to the house in carts. The branches would be placed inside the shed on wire racks, a fire was made at the door using good firewood, and embers were spread throughout the shed. The leaves were gradually roasted and prepared, then ground using manual grinders or pounded with a mortar and pestle. The final product was packaged in wooden barrels weighing 60 to 70 kg. We produced a large quantity.” (...) “The mate was left to age for a year. New batches were extremely bitter.”
Currently, a local NGO with a farmer is implementing a development project based on the wild population present in the area and the planting of specimens in an agroforestry system. According to the accounts of P.P. and A.D, the species is propagated through locally collected seeds as well as those introduced from other locations. Seedlings are generated in containers and, upon reaching a certain height, planted in the riparian and ravine forests. The ancient plants are cared for and harvested to produce yerba for personal consumption.
The Cipó-Miló (Aristolochia fimbriata) is a species of great local importance, as indicated in the accounts. It is a native species, but it is not commonly found in wild spaces in the Quebrada de los Cuervos and Sierras del Yerbal. Instead, it is found in ruderal spaces or in some of the old taperas. It is used in cases of venomous snake bites, which were once common in rural life in the sierras. The accounts suggest that in the past, it was used to save the life of a person bitten by a snake when reaching a healthcare center in time was impossible, or even before such facilities existed. Nowadays, it is used for bitten dogs and also to treat insect bites. The plant has a reserve rhizome, known as “batata,” and the remedy is prepared by chopping the rhizome and soaking it in white alcohol, sometimes with the addition of tobacco and aspirin. Locals apply this preparation to the bite or sting, and, in some cases, it is ingested while trying to reach a healthcare center. In terms of management practices for the species, if necessary, harvesting is done in the wild, and it is tolerated if found in a DC. Various cultivation practices are applied, such as transplantation, protection, and improvements. There is a sense of communal circulation, and it is one of the species where the care of inherited plants can be observed.
3.6. Origin, reproduction and transmission of local knowledge
The knowledge recorded in the studied rural community comes from multiple sources. While ancestral knowledge transmitted from generation to generation is present and continues to be passed down, there are other sources of information that interact and hybridize with the traditional knowledge. Among these sources are younger generations who bring knowledge acquired from agricultural schools or universities, books they acquire or receive from visitors, scholars, or government employees, who often also offer training courses or workshops. Civil society organizations promote different types of projects, and external groups bring new knowledge and share it with the community, as was the case with a Guaraní family that lived in the area for a year and shared construction techniques and knowledge about medicinal plants. Furthermore, experimentation and observation also generate knowledge on an ongoing basis, which is retained and transmitted. A.M., referring to a specific species, states, “The sheep eat it…We cleaned it up and conducted an experiment to see what would happen. We are learning from the plant; sometimes, it tells us a little about itself.”
Lastly, when asked about the exchange of information among neighbors, A.M. indicates that there has always been an exchange of information in rural schools, where people would gather and frequently engage in community tasks to support the institution. The interviewee also mentions that the presence of the protected area serves as a meeting place where neighbors start to go. “These projects that involve the neighbors are very important because there is a more fluid exchange of different knowledge among the neighbors. If there are no meetings, there is no discussion about these things.” [...] “Before, on a day off, you would go visit your neighbor. Now times have changed, and there is no time to visit neighbors. Many things are lost, like communication, and we do not work together on certain things anymore.” [...] “Plants used to move more because when you visited your neighbor, the first thing you would talk about was the garden, and there you would see the plants you did not have and take them with you. Same thing with seeds.”
4. Discussion
4.1. Agrobiodiversity and local knowledge
Our study confirms that the rural community of “Quebrada de los Cuervos and Sierras del Yerbal” utilizes and manages a wide agrobiodiversity that covers important daily life needs. Although the number of respondents is not high, it accounts for 40% of the households in the study area. Future studies may explore some age or gender limitations or biases, among other aspects. Various plant genetic resources and local knowledge intertwine in this territory to provide goods and services such as food, medicine, shaping the environment and constructions, fuel, as well as social and spiritual goods, allowing the habitability of the landscape. The hierarchy of uses for human consumption, ornamental, medicinal, environmental uses, and fuel coincides with other studies (Caballero-Serrano et al., 2016; Mariel et al., 2021; Rosero-Toro et al., 2022) highlighting the importance of provisioning, cultural, and regulatory ecosystem services provided by subsistence economies. Agrobiodiversity is part of a multiple-use strategy of resources and ecosystems (Toledo and Barrera-Bassols, 2008; Casas et al., 2014; Furlan et al., 2017) that ensures resilience, food security, and the maintenance of the needs of rural communities.
The wide documented diversity of 185 species, 121 exotic and 64 native, is a biocultural heritage of this community. Out of the 64 native species used, 51 are considered national plant genetic resources (Rivas, 2007; Vidal et al., 2018, 2021), and only four are considered priority species for conservation (Soutullo et al., 2009), including Ilex paraguariensis and Psidium cattleianum as local resources. With the indicators used, a group of 24 species with high levels of cultural significance is defined (Figure 12), including vegetable landraces, native tree species, native and exotic fruit trees, some medicinal species, in addition to Ilex paraguariensis and Aristolochia fimbriata. The most diverse environments are the home gardens and the surroundings of the house, highlighting the use of 51 native species from non-cultivated environments.
Among the 71 species recorded for human consumption, there is a high number of fruit trees, with about 33 species, predominantly from the Rosaceae, Rutaceae, and Myrtaceae families, in line with other studies (Furlan et al., 2017; Chamorro and Ladio, 2021; Mariel et al., 2021). There are important exotic fruit species at the local and national level, such as Citrus spp., peach, apple, plum, grape, and quince. It is likely that for some of these crops, there is secondary genetic variability generated in situ, adapted to the local management practices and environmental conditions. Among the native fruit species, the ones with the highest regional and international recognition are Acca sellowiana, Psidium cattleianum, Eugenia uniflora, and Butia odorata (Thorp and Bieleski, 2002; Vignale and Bisio, 2005; Vignale et al., 2016, 2018; Speroni et al., 2018). Additionally, other species were recorded that could be classified as small fruits (berries), such as Blepharocalyx salicifolius, Allophylus edulis, Citharexylum montevidense, Chrysophyllum gonocarpum, Psidium salutare, Passiflora caerulea, Myrceugenia euosma, and Celtis ehrenbergiana. Native fruits, particularly berries, have great nutritional and medicinal value and have been used by indigenous and traditional populations since ancient times (Furlan et al., 2017; Schmeda-Hirschmann et al., 2019; Rivas et al., 2020, 2023; Chamorro and Ladio, 2021).
The presence of landraces of common bean, maize, sweet potato, squash and pumpkin is traditional in family production systems (Burgueño et al., 2015; Mello et al., 2017; Pereira, 2017; Favaro and Piazza, 2019; Cuadro et al., 2024). Over time, adaptation and selection processes have resulted in a significant diversity of landraces in the Pampa biome (Almeida et al., 2020). However, these landraces are currently facing strong genetic erosion due to migration from rural to urban areas and the substitution of landraces with modern cultivars. This affects the adaptive capacity, evolutionary potential of the crops, resilience of agroecosystems, and the livelihoods of farmers and rural communities (Khoury et al., 2022). In this regard, characterizing landraces, providing ex situ support, and valuing them are crucial actions within a conservation and management plan for agrobiodiversity in the protected landscape.
Tree species play a fundamental role in rural communities, not only by providing non-timber forest products (NTFPs), but also for environmental and fuel uses, leading to the incorporation of multiple species in their domestic and productive systems, as observed in numerous communities (Dawson et al., 2014). Preferred species for these uses include native species such as Scutia buxifolia, Schinus lentiscifolius, Schinus longifolius, and Celtis ehrenbergiana. Additionally, the general use of native forests is cited to meet further needs. These species are generally multipurpose, consistent with other studies (Dawson et al., 2014; Caballero-Serrano et al., 2016; Morales et al., 2017). In addition, carbon sequestration, nutrient cycling, and water purification should be added to direct benefits.
Medicinal species play a fundamental role in the health and daily life of rural communities in Uruguay (Prieto and Bustamante, 1996; Castiñeira et al., 2018; Tabakian, 2019). Our study revealed a wide diversity of species with various habits and uses that people maintain in their gardens or directly collect from nature, with a 50% component of native species. Comparing our findings with comprehensive studies on medicinal species in the northern region of the country (Castiñeira et al., 2018; Tabakian, 2019) there is significant overlap in introduced and numerous native species. However, some different species are notable, such as Schinus lentiscifolius, Aristolochia fimbriata, Anemia tomentosa, Ocimum carnosum, and Psidium salutare. The first two species hold high cultural significance for our study area. This demonstrates that while there are widely used species, there are also territorial specificities in plant genetic resources and local knowledge.
In the set of species used, the native component is high (35%), which increases to 45% when considering species of high cultural significance or specific uses such as medicinal plants (52%), environmental uses (59%), and fuel (57%). Several authors (Caballero-Serrano et al., 2016; Tabakian, 2019) emphasize cultural factors as determinants of diversity in plant use, in addition to physical and socioeconomic factors. Chamorro and Ladio (2021) report 39% of native species in use in Patagonia, where the respondents were mestizos and criollos with some Mapuche influence. Caballero-Serrano et al. (2016) found 64% of native species in use in the Ecuadorian Amazon. Tabakian (2019) documented 70% of native medicinal plants in use in northern Uruguay, interviewing descendants of indigenous peoples. Our work confirms the use and manipulation of native species to obtain goods and services, increasing the availability of useful plants through diverse management practices; this likely triggered incipient domestication processes (Casas et al., 1997, 2014). One example is Acca sellowiana, which has a wild population with extensive diversity (Rivas et al., 2007; Baccino, 2011; Calvete, 2013; Puppo et al., 2014), accompanied by selected individuals managed in cultivated environments, transplanted from the wild, tolerated, or obtained from other locations. Many of the surveyed native and landraces are listed internationally as Neglected and Underutilized Species (NUS) with agri-food value. Some of the native species include Acca sellowiana, Eugenia uniflora, Psidium cattleianum, and Ilex paraguariensis, while introduced species include Cydonia oblonga, Citrus reticulata, Citrus limon, Phaseolus spp., and various species and landraces of cucurbits, among others (Hernández Bermejo et al., 2019). NUS crops, due to their limited use or cultivation abandonment, are subject to genetic erosion (Padulosi et al., 2011; Barbieri et al., 2014).
The substantial wealth of local knowledge regarding native and exotic plant genetic resources is the result of production, hybridization, and transgenerational transmission of knowledge. This legacy is a product of a cultural syncretism, incorporating knowledge from indigenous, colonial-missionary, and criollo populations that have converged in the area for the past 300 years (Bica, 2019; Palermo, 2019; Torres, 2019), as other authors have noted for nearby regions (Castiñeira et al., 2018; Tabakian, 2019; Vidal et al., 2021). Throughout this long process, knowledge related to specific practices flows through individuals and in relation to the environment. It is transmitted, acquired, and discarded based on trial and error, giving rise to new knowledge about introduced and local species. Currently, this entire legacy interacts with other sources of knowledge that have entered the area through academia and new ruralities (Pochettino and Lema, 2008; Toledo and Barrera-Bassols, 2008).
4.2. Agrobiodiversity loss and local knowledge
The high number of taperas allows us to infer that numerous families who worked the land using agrobiodiverse systems once lived in the area. Currently, only 30 to 40 families reside there, according to the provided data, highlighting the significant impact of rural population migration to urban centers, a trend that has been occurring in Uruguay for decades (Achkar, 2017; Cortés-Capano et al., 2020; Vidal et al., 2021). This migration is part of a global trend resulting from the establishment of the agro-industrial model, which jeopardizes the conservation of agrobiodiversity and biocultural heritage (Toledo and Barrera-Bassols, 2008). With the abandonment of the area, knowledge and seeds are lost as people leave, and the lack of generational turnover further endangers the conservation of cultural and biological diversity.
The difference in the number of species found in houses and taperas, the values of the Shannon index, and the ordination analysis, combined with the fact that out of 93 species recorded in the taperas only 33 are repeated in more than 10% of them, reflect the rapid loss of species and the fragility of most resources in the abandoned cultivation gardens and plots. On the other hand, several resources that are highly present in houses significantly decrease in frequency in taperas, particularly some traditional fruit crops. However, there are accounts stating that all houses had specimens of these species. The diversity of species maintained in houses is sustained by the care and management practices of the inhabitants, clearly demonstrating that the main factor contributing to the loss of diversity is the cessation of these management practices. The time it takes for species to disappear after abandonment varies (Clement, 1999), and losses are associated with the botanical habits of the species. There is a significant reduction in the number of herbaceous species from houses to taperas, with more than 90% of vegetable crops, 75% of aromatic plants, and 57% of medicinal plants lost, while species used for environmental and fuel purposes, mainly trees and shrubs, increase.
The loss of local knowledge, either due to changes in customs or the departure of knowledgeable individuals from the area, may explain the presence of 20 exclusive species in taperas. One such case is Bauhinia forficata, which is only found in taperas and is not mentioned in the interviews. There are national and international records of the medicinal use of this species for urinary system diseases and diabetes, among other illnesses (Prieto and Bustamante, 1996; Caffaro et al., 2015; Tabakian, 2019). Another example of knowledge loss over time is that of Ilex paraguariensis. Although it is not present in the taperas, it can be found in the forests and has given its name to four watercourses in the area: “Yerbal Chico,” “Yerbal Grande,” “Yerbalito,” and “Cañada de la Yerba.” Documented stories exist about the yerba mate plantations in these hills that supplied the Eastern and Río Grande Jesuitic missions (Bonetti, 2010; López Mazz et al., 2020). In our study, knowledge about this species emerged in a few interviews, and although they provided detailed descriptions of cultivation practices and the technique of harvesting and processing yerba mate, it could be inferred that there was likely an ancient knowledge that is practically extinct in the area.
4.3. Rural communities, knowledge and plants: interactions that transform and shape landscapes
Rural communities manage agrobiodiversity in different ways and in multiple environments, both in cultivated and wild areas, as described by Casas et al. (1997), Clement (1999), and Wiersum (1997). The qualitative and quantitative analysis of the data allows us to propose a model of organization and management of space and resources carried out by the local inhabitants. It is a complex, multi-use strategy in which plant genetic resources are found in diverse environments, at different scales, and in a variety of interactions between humans and the environment. By interpreting how different plant genetic resources are grouped in space according to their category of use, the combination of management practices and their frequency, the distance in relation to the DC, and the habits and origin of the species, we can distinguish spaces with different characteristics. Based on the classification proposed by Clement (1999) for landscapes or environments, we identify four spaces of use, with DCs and the life of the local inhabitants as the center (Table 3):
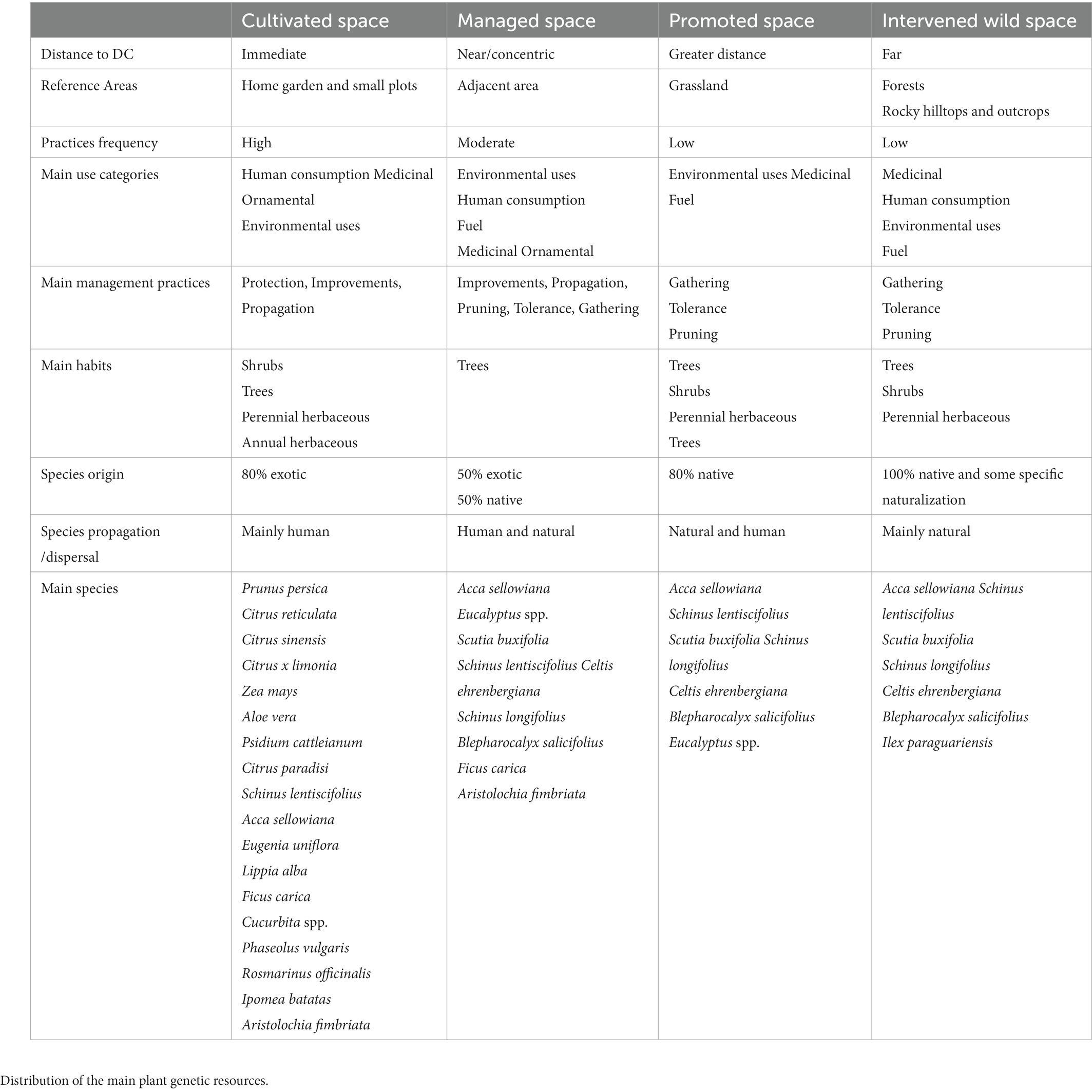
Table 3. Characteristics of cultivated, managed, promoted, and intervened wild spaces in domestic contexts.
Cultivated spaces: These are delimited and protected areas, closely integrated with or near the house, where daily plant care takes place. Home gardens and small plots play a crucial role in species domestication, serving as repositories of germplasm and experimental sites. The resources in these spaces are intensively and consistently managed. Within the study area, the majority of exotic agrobiodiversity is cultivated, primarily for human and animal consumption, medicinal purposes, and ornamental use. High-intensity management practices, such as protection, improvement, and propagation, are performed with greater frequency; while pruning, tolerance, and gathering practices are present with medium-frequency. Lastly, community circulation, care of inherited plants, selection, and transplantation, although less frequent, occur twice as often compared to other spaces.
Managed space: It is a concentric area around the house, without defined boundaries or livestock protection, but with daily care and interventions. It contains a concentration of tree species, forming a small-scale agroforestry system with a 50% native component. The main uses include environmental purposes, human consumption, fuel, with some medicinal and ornamental species present. In general, trees are pruned to provide shade during summer and protection against cold in winter, or sometimes arranged to form windbreaks. The intensity of management in this space is moderate, with the most frequent management practices being protection, propagation, pruning, improvement, tolerance, and gathering. Other practices occur less frequently, including the care of inherited plants.
Promoted spaces: These spaces consist of the property’s grasslands where livestock production takes place. Grazing with different animal loads and the burning of “maciegas” (non palatable grasses) are common practices in this pastoral system to control less efficient species for livestock, which modifies species populations and undoubtedly the landscape (Rivas and Condon, 2015). Aside from forage species, this space mainly comprises native tree species and some shrubs, primarily used for environmental purposes, medicine, and fuel. The intensity of management is lower than in the previous spaces, and the main practices are gathering and tolerance. Pruning may occur for trees that provide shelter for the livestock beneath their canopy.
Intervened wild spaces are areas of natural vegetation such as forests and rocky outcrops. They can be located within or outside the family farmer’s property, in proximity to the house or along daily routes (school path, pasture edges, roadside, etc.). These natural formations undergo some degree of modification due to human and livestock traffic, occasional vegetation thinning for livestock shelter, and the presence of escaped or naturalized species from cultivation. Interventions may also include the cultivation of Ilex paraguariensis in agroforestry systems for subsequent harvesting. The species in these spaces are mostly native, with some exclusive to these environments. They are primarily used for medicinal purposes, human consumption, environmental uses, and fuel. This is also where the majority of species used for industry and craftsmanship are found, as well as a high proportion of toxic and harmful species. The intensity of management for the studied species is similar to the promoted space. The most frequent management practice is gathering, while other practices such as pruning, transplanting seedlings, selection, community circulation, and care of inherited plants occurs at a lower frequency.
The location of certain plant genetic resources and their corresponding practices is not fixed; there are movements of species from wild spaces to cultivated spaces and vice versa. Some native species are transplanted or propagated for cultivation, while a few examples of certain crops appear in wild environments, whether as a result of human activity or natural dispersal. In the same vein, the exchange of plants and seeds between neighbors and from taperas to cultivated spaces is part of this dynamic.
The natural dispersal of fruits and seeds is also a part of this dynamic, influencing the distribution of plant genetic resources in various spaces (Table 3). Specifically, 56 local native species (87.5% of the total native species), primarily utilized in managed, promoted, and intervened wild spaces, depend on natural dispersal, though not exclusively. Some of these species also emerge in cultivated spaces, being tolerated and protected. Most of these species are trees, predominantly exhibiting zoochory syndromes (Ramírez and Säumel, 2022). On the other hand, the herbaceous plants, mainly from the Asteraceae family, exhibit anemochory syndromes, while only a few species show autochory syndromes.
Although there is no research on frugivorous fauna in the protected area, some interviews conducted in this study mention birds, including the Rhea americana, as dispersal agents. The vertebrate fauna of Quebrada de los Cuervos and Sierras del Yerbal comprises 138 bird species, 29 species of mammals, amphibians, and reptiles (SNAP/DINAMA, 2010), to which cattle (as a potential dispersal agent) must be added. While there is no evidence to suggest that the dispersing fauna is at risk of conservation in the protected area, the crucial role these species play in landscape conservation is recognized (Green and Dennis, 2007; Wright, 2007), along with the need for future ethnographic and ecological research.
This spatial differentiation allows us to propose that landscape management processes are taking place in the Sierras del Yerbal. The differential human-nature interaction in different spaces is a way of extending domestic units (Stampella, 2015) and ultimately shapes what we have referred to in our work as the domestic context. The inhabitants use the territory for their daily needs, just as they use their gardens and small plots for plants that are not present in natural environments, while naturally abundant resources are directly harvested. Recognizing these assemblages of species, uses, and differential management of the territory, applied persistently, allows us to visualize the human imprint on the historical processes of landscape modification and domestication (Franco-Moraes et al., 2021). The transformation of the environment based on cultural criteria leads to the creation of specific biocultural landscapes (Peroni et al., 2013; Hong et al., 2014). The current challenge of conserving the protected landscape largely relies on recognizing these aspects and integrating them into the area’s planning and management.
4.4. Local community, agrobiodiversity, and conservation in protected areas
Agrobiodiversity, a significant component of biodiversity, depends on human intervention for its generation, maintenance, and future evolution (Sthapit et al., 2016). It delivers valuable ecosystem services, including provisioning, cultural, and regulatory services, not only to local inhabitants but also to the global population (Wood et al., 2015; Caballero-Serrano et al., 2016). However, agrobiodiversity is often overlooked in conservation objectives and management plans of protected areas, where it is only tangentially considered through the conservation plans of “natural” ecosystems. Integrating agrobiodiversity as a focal point in in-situ conservation strategies for the “protected landscape” category of the IUCN would serve the purpose of conserving the human-environment interaction that shapes the observed landscapes.
Our research reveals a sustained interaction process between rural communities, plant genetic resources, and environmental conditions. The role of local communities is internationally recognized and needs to be studied locally to design appropriate guidelines for agrobiodiversity conservation and management (De Boef et al., 2013b). The power of local knowledge relies not only on keen observation but also on experiential learning (Morris, 2006; Eden, 2012). Many practical knowledge systems employed by local communities regulate species diversity, create habitat heterogeneity at the landscape scale, and adjust the intensity of use, thus increasing the diversity of available biological resources (Berkes et al., 2000; Assis et al., 2013; Reis et al., 2018; Araujo et al., 2021). The resource management practices of communities reflect a knowledge system based on cultural practices aligned with their objectives and the need for future conservation (Jackson et al., 2007), forming authentic “communities of practice” (Dabezies and Taks, 2021) that safeguard biocultural landscapes (Rivas et al., 2023).
In this regard, conservation objectives of the area cannot be pursued independently of social and rural development goals (Cortés-Capano et al., 2020). It is necessary to revise the perception of farmers as degraders of natural systems and recognize them as custodians and creators of agrobiodiversity and the landscape, as they play a key part in the solution (Cortés-Capano et al., 2020; Dawson et al., 2021). The sustainability of agroecosystems must consider environmental, social, and economic aspects. Therefore, production carried out by farmers within protected areas, integrating their local knowledge and agrobiodiversity, is crucial for landscape conservation. In-situ conservation, a dynamic approach that integrates biophysical, socioeconomic, and cultural components, allows for ongoing evolutionary processes in agroecosystems (Maxted et al., 1997; Rivas et al., 2010). It encompasses the concepts of conservation through use (Halffter, 2002) and community-based biodiversity management (MCB), which promotes local governance and community empowerment (Jarvis et al., 2011; De Boef et al., 2013b).
Furthermore, landscape conservation should not solely rely on farmers; it requires policymakers to generate and implement incentives that facilitate and promote in-situ conservation of agroecosystems while improving the quality of life for inhabitants (Rivas et al., 2010; Lacerda et al., 2020). In the current national context, protected landscape areas could play a leading role in the https://www.gub.uy/ministerio-ganaderia-agricultura-pesca/comunicacion/publicaciones/plan-nacional-para-fomento-produccion-bases-agroecologicas/plan-nacional across its four strategic pillars: (1) promoting and facilitating the adoption of agroecological practices, increasing the number of farmers practicing this system within the area; (2) facilitating access to products, distribution, and generating consumers by emphasizing the value of agrobiodiversity, fostering local farmers’ markets, establishing production networks, and agroecological certification to access national, regional, and international markets; (3) contributing to ecosystem conservation through the rescue, production, and use of native and local genetic resources while recognizing the rights of farmers, and (4) promoting training, research, and extension processes in the area.
5. Conclusion
The research revealed a high number of plant species used and managed by the rural community in the protected landscape of “Quebrada de los Cuervos and Sierras del Yerbal,” which cover various needs of the daily life of its inhabitants. This agrobiodiversity and the local knowledge about it constitute a landscape where biological and cultural diversity intertwine. A group of native and introduced plant genetic resources of high cultural significance stands out due to their agreed-upon use, diversity of uses, and management practices.
The comparison between the agrobiodiversity of houses and old rural buildings clearly indicates that the abandonment of domestic contexts is a primary cause of agrobiodiversity loss. The in-situ conservation of agrobiodiversity and local knowledge is intrinsically associated with the conservation of the biocultural landscape and, therefore, the permanence of family production systems in their domestic contexts.
The proposal regarding the differential use of spaces in domestic contexts reflects the historical and ongoing management of the landscape, reaffirming the close link between agrobiodiversity and the domestication of landscapes. The challenge of current conservation in the protected landscape largely rests on recognizing these aspects and integrating them into the planning and management of the area.
The threat faced by these rural landscapes worldwide is no different from that occurring in the Pampa biome. In the protected landscape of “Quebrada de los Cuervos and Sierras del Yerbal,” it is a priority to include agrobiodiversity as a relevant focal object of conservation and to generate a participatory management plan that involves the local community from the outset. The conservation and valorization strategy of plant genetic resources requires public policies that support production, commercialization, and agroecological certification as alternatives to encourage the permanence of farmers in rural areas and promote generational turnover. Academia has a relevant role to play through the deployment of transdisciplinary strategies where the generated information is taken into account by decision-makers.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MP: study conception and design, data collection, analysis and interpretation of results, draft manuscript preparation and final edition. CG and MR: study conception and design, analysis and interpretation of results, draft manuscript preparation, and final edition. AC: data collection. AL: analysis and interpretation of results and draft manuscript preparation. All authors contributed to the article and approved the submitted version.
Funding
This research has been conducted in the frame of partial supports from la Comisión Sectorial de Investigación Científica (CSIC), Universidad de la República, Uruguay and the Agencia Nacional de Investigación e Innovación (ANII), Uruguay.
Acknowledgments
The authors would like to express their sincere gratitude to the postgraduate program in Agricultural Sciences at the Faculty of Agronomy, Universidad de la República, Uruguay. They extend their heartfelt appreciation to the individuals from “Quebrada de los Cuervos y Sierras del Yerbal” for their hospitality, support, and contribution to the research endeavors. Their willingness to share their knowledge has greatly enriched this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Achkar, M. (2017). “El bioma pampa: un territorio en disputa” in Olhares sobre o Pampa: um território em disputa. eds. C. R. Flores and E. Wizniewsky (Porto Alegre: Evangraf), 125–139.
Achkar, M., Díaz, I., Dominguez, A., and Pesce, F. (2016). Uruguay. Una visión desde la geografía. 1ra edición Montevideo: Banda Oriental.
Allen, V. G., Batello, C., Berretta, E. J., Hodgson, J., Kothmann, M., Li, X., et al. (2011). An international terminology for grazing lands and grazing animals. Grass Forage Sci. 66, 2–28. doi: 10.1111/j.1365-2494.2010.00780.x
Almeida, N. C., Vidal, R., Bernardi Ogliari, J., Costich, E., and Chen, J. (2020). Relationships among American popcorn and their links with landraces conserved in a microcenter of diversity. Genet. Resour. Crop. Evol. 67, 1733–1753. doi: 10.1007/s10722-020-00935-2
Araujo, J. J., Rojas, J. L., Keller, H. A., and Hilgert, N. I. (2021). Landscape management among the Guarani of the Atlantic Forest of Misiones, Argentina: the case of the Syagrus Romanzoffiana (Cham.) glassman (Arecaceae) palm tree. Ethnobiol. Conserv. 10, 1–19. doi: 10.15451/EC2021-04-10.22-1-19
Assis, A. L., Zank, S., Peroni, N., and Hanazaki, N. (2013). “Traditional people and the conservation of biodiversity in Brazil,” in Community biodiversity management. Promoting resilience and the conservation of plant genetic resources, eds. W. BoefDe, A. Subedi, N. Peroni, M. Thijssen, and E. O’Keeffe (Wageningen, Netherlands: Routledge), 133–140.
Baccino, E. (2011). Estructura genética de cuatro poblaciones silvestres de Acca sellowiana (Berg) Burret situadas en el noreste de Uruguay. [thesis]. [Montevideo, (Uruguay)]: Universidad de la República.
Baeza, S., Vélez-Martin, E., De Abelleyra, D., Banchero, S., Gallego, F., Schirmbeck, J., et al. (2022). Two decades of land cover mapping in the Río de la Plata grassland region: the MapBiomas Pampa initiative. Remote Sens. Appl. 28:100834. doi: 10.1016/j.rsase.2022.100834
Banning, E. B. (2002). Archaeological survey. Manuals in archaeological method, theory and technique. 2nd Berlin: Springer Science & Business Media.
Barbieri, R. L., Costa Gomes, J., Alersia, A., and Padulosi, S. (2014). Agricultural biodiversity in southern Brazil: integrating efforts for conservation and use of neglected and underutilized species. Sustainability 6, 741–757. doi: 10.3390/su6020741
Berkes, F., Colding, J., and Folke, C. (2000). Rediscovery of traditional ecological knowledge as adaptive management. Ecol. Appl. 10, 1251–1262. doi: 10.1890/1051-0761(2000)010[1251,ROTEKA]2.0.CO;2
Bica, C. (2019). Paisajes rurales del Este de Uruguay. Investigación arqueológica sobre la producción artesanal de cal en la cuenca del A.o Yerbal Grande. [thesis]. [Montevideo, (Uruguay)]: Universidad de la República.
Blancas, J., Casas, A., Pérez-Salicrup, D., Caballero, J., and Vega, E. (2013). Ecological and socio-cultural factors influencing plant management in Náhuatl communities of the Tehuacán Valley, Mexico. J. Ethnobiol. Ethnomed. 9, 1–22. doi: 10.1186/1746-4269-9-39
Burgueño, B., Carbone, J. P., Fontaine, F., and Nansen, K. (2015). Importancia de la conservación de semillas criollas y su relación con los sistemas de producción familiar en una zona del departamento de Tacuarembó. [thesis]. [Canelones (Uruguay)]: Consejo de Educación Técnico Profesional, Universidad del Trabajo del Uruguay.
Caballero-Serrano, V., Onaindia, M., Alday, J. G., Caballero, D., Carrasco, J. C., McLaren, B., et al. (2016). Plant diversity and ecosystem services in Amazonian homegardens of Ecuador. Agric. Ecosyst. Environ. 225, 116–125. doi: 10.1016/j.agee.2016.04.005
Caffaro, K. M., Araújo Junior, J. X., Santos, J. M., Santos, R. M., Capesatto, E. A., and Bastos, M. L. A. (2015). Integrative review article on medical use and pharmacological activities Bauhinia genus plant. J. Nurs. 9, 9399–9405. doi: 10.5205/reuol.6812-75590-1-ED.0908sup201509
Calvete, A. (2013). Contribución al mejoramiento participativo del Guayabo del país (Acca sellowiana Berg. Burret) en el paisaje protegido Quebrada de los Cuervos. [thesis]. [Montevideo, (Uruguay)]: Universidad de la República.
Casas, A., Blancas, J., Otero-Arnaiz, A., Cruse-Sanders, J., Moreno, A., Camou, A., et al. (2014). Manejo y domesticación de plantas en Mesoamérica, in Botânica na América Latina: Conhecimento, interaҫão e difusão, eds. T. R. Dos Santos, C. W. NacimentoDo, L. C. Lima, and F. A. Ribeiro (Brazil: Sociedade Botânica do Brasil).
Casas, A., Caballero, J., Mapes, C., and Zárate, S. (1997). Manejo de la vegetación, domesticación de plantas y origen de la agricultura en Mesoamérica. Bol. Soc. Bot. Méx. 61, 31–47. doi: 10.17129/botsci.1537
Casas, A., Vázquez, M. D. C., Viveros, J. L., and Caballero, J. (1996). Plant management among the Nahua and the Mixtec in the Balsas River basin, Mexico: an ethnobotanical approach to the study of plant domestication. Hum. Ecol. 24, 455–478. doi: 10.1007/BF02168862
Castiñeira, E., Canavero, A., and Pochettino, M. L. (2018). Comparison of medicinal plant knowledge between rural and urban people living in the biosphere reserve “bioma Pampa-Quebradas del Norte”, Uruguay: an opportunity for biocultural conservation. Ethnobiol. Conserv. 7, 1–34. doi: 10.15451/ec2018-03-07.04-1-34
Chamorro, M. F., and Ladio, A. (2021). Management of native and exotic plant species with edible fruits in a protected area of NW Patagonia. Ethnobiol. Conserv. 10, 1–24. doi: 10.15451/ec2021-02-10.14-1-24
Clement, C. R. (1999). 1492 and the loss of amazonian crop genetic resources. I. the relation between domestication and human population decline. Econ. Bot. 53, 188–202. doi: 10.1007/BF02866498
Clement, C. R., and Cassino, M. F. (2018). “Landscape domestication and archaeology” in Encyclopedia of global archaeology. ed. C. Smith (New York: Springer International Publishing), 1–8.
Clement, C. R., Denevan, W. M., Heckenberger, M. J., Junqueira, A. B., Neves, E. G., Teixeira, W. G., et al. (2015). The domestication of Amazonia before European conquest. Proc. R. Soc. B Biol. Sci. 282:20150813. doi: 10.1098/rspb.2015.0813
Clemente, I. (2021). “La Frontera sureste de Uruguay: territorio y sociedad, in Fronteras en construcción” in Practices sociales, políticas públicas y representaciones espaciales desde Sudamérica. eds. T. Porcaro and E. S. Sandes (Ciudad Autónoma de Buenos Aires: Alejandro Gabriel Benedetti)
Cortés-Capano, G., Toivonen, T., Soutullo, A., Fernández, A., Dimitriadis, C., Garibotto-Carton, G., et al. (2020). Exploring landowners’ perceptions, motivations and needs for voluntary conservation in a cultural landscape. People Nature 2, 840–855. doi: 10.1002/pan3.10122
Cuadro, M. G., Vidal, R., and Bianco, M. B. (2024). Procesos Culturales y Conocimientos tradicionales asociados a variedades criollas en tres localidades uruguayas. Rev. Econ. Sociol. Rural. 62:e269359. doi: 10.1590/1806-9479.2022.269359
Dabezies, J. M., and Taks, J. (2021). Environmental knowledge and the definition of a community of practice. Improvisation and identity of the Butiaceros of southern Uruguay. Geoforum 118, 30–37. doi: 10.1016/j.geoforum.2020.10.008
Dawson, N., Carvalho, W. D., Bezerra, J. S., Todeschini, F., Tabarelli, M., and Mustin, K. (2021). Protected areas and the neglected contribution of indigenous peoples and local communities: struggles for environmental justice in the Caatinga dry forest. People Nature. 1–17. doi: 10.1002/pan3.10288
Dawson, I. K., Leakey, R., Clement, C. R., Weber, J. C., Cornelius, J. P., Roshetko, J. M., et al. (2014). The management of tree genetic resources and the livelihoods of rural communities in the tropics: non-timber forest products, smallholder agroforestry practices and tree commodity crops. For. Ecol. Manag. 333, 9–21. doi: 10.1016/j.foreco.2014.01.021
De Boef, W., Peroni, N., and Hanazaki, N. (2013a). “People, biodiversity and landscapes,” in Community biodiversity management. Promoting resilience and the conservation of plant genetic resources, eds. W. BoefDe, A. Subedi, N. Peroni, M. Thijssen, and E. O’Keeffe (Routledge: Wageningen, Netherlands), 125–132.
De Boef, W., Thijssen, M., and Sopov, M. (2013b). Agrobiodiversity, livelihoods and markets, in community biodiversity management. Promoting resilience and the conservation of plant genetic resources, eds. W. BoefDe, A. Subedi, N. Peroni, and M. Thijssen (Wageningen, Netherlands: Routledge), 177–187.
Eden, S. (2012). Counting fish: performative data, anglers’ knowledge-practices and environmental measurement. Geoforum 43, 1014–1023. doi: 10.1016/j.geoforum.2012.05.004
Emperaire, L., and Peroni, N. (2007). Traditional management of agrobiodiversity in Brazil: a case study of manioc. Hum. Ecol. 35, 761–768. doi: 10.1007/s10745-007-9121-x
Evia, G., and Gudynas, E. (2000). Ecología del Paisaje en Uruguay. Aportes para la conservación de la Diversidad Biológica. Montevideo, Uruguay: AECI/Junta de Andalucía/MVOTMA. BOOK.
FAO (1999). Agricultural biodiversity, multifunctional character of agriculture and land conference, background paper 1.
Favaro, M., and Piazza, S. (2019) Caracterización de variedades criollas hortícolas en sistemas productivos del noreste de Canelones, zonas Pantanoso del Sauce y Tapia. [BSc thesis]. [Montevideo (Uruguay)]: Universidad de La República.
Flora, C. B. (2001). Interactions between agroecosystems and rural communities. Boca Ratón: CRC Press.
Franco-Moraes, J., Clement, C. R., Cabral de Oliveira, J., and Oliveira, A. A. d. (2021). A framework for identifying and integrating sociocultural and environmental elements of indigenous peoples’ and local communities’ landscape transformations. Perspect. Ecol. Conserv. 19, 143–152. doi: 10.1016/j.pecon.2021.02.008
Franco-Moraes, J., Viana Braga, L., and Clement, C. R. (2023). The Zoʻé perspective on what scientists call “forest management” and its implications for floristic diversity and biocultural conservation. Ecol. Soc. 28:37. doi: 10.5751/ES-13711-280137
Furlan, V., Pochettino, M. L., and Hilgert, N. I. (2017). Management of fruit species in urban home gardens of Argentina Atlantic Forest as an influence for landscape domestication. Front. Plant Sci. 8:e01690. doi: 10.3389/fpls.2017.01690
Gallego, F., López-Mársico, L., Tommasino, A., Altesor, A., Casás, M., and Rodríguez, C. (2023). Legacy effects after seven years of afforestation with Pinus taeda in a natural grassland. Restor. Ecol. 31:e13865. doi: 10.1111/rec.13865
Green, R. J., and Dennis, A. J. (2007). “Management implications and conservation” in Seed dispersal. Theory and its application in a changing world. eds. A. J. Dennis, E. W. Schupp, R. J. Green, and D. A. Westcott (Wallingford: CABI), 519–521.
Guber, R. (2001). La etnografía: método, campo y reflexividad. Enciclopedia latinoamericana de sociocultura y comunicación 146
Guber, R. (2014). Prácticas Etnográficas. Ejercicios de reflexividad de antropólogas de campo Buenos Aires: Instituto de Desarrollo Económico y Social (IDES). 224.
Heckenberger, M. J., Kuikuro, A., Kuikuro, U. T., Russell, J. C., Schmidt, M., Fausto, C., et al. (2003). Amazonia 1492: Pristine Forest or cultural parkland? Science 301, 1710–1714. doi: 10.1126/science.1086112
Heckler, Serena . (2009). Introduction, in landscape, Process and power re-evaluating traditional environmental knowledge. Oxford, New York: Berghahn Books.
Hernández Bermejo, J. E., Pochettino, M. L., Herrera, F., Labarca, Y., and Tarifa, F. (2019). Cultiva. NUS newsletter. Córdoba, España: Red Iberoamericana de Cultivos Infrautilizados y Marginados con Valor Agroalimentario. CYTED.
Hong, S. K., Bogaert, J., and Min, Q. W. (2014). Biocultural landscapes: Diversity, functions and values. New York: Springer Dordrecht Heidelberg.
INE (2011). Resultados del Censo de Población 2011: población, crecimiento y estructura por sexo y edad. Montevideo, Uruguay.
Jackson, L. E., Pascual, U., and Hodgkin, T. (2007). Utilizing and conserving agrobiodiversity in agricultural landscapes. Agric. Ecosyst. Environ. 121, 196–210. doi: 10.1016/j.agee.2006.12.017
Jarvis, D. I., Hodgkin, T., Sthapit, B. R., Fadda, C., and López-Noriega, I. (2011). An heuristic framework for identifying multiple ways of supporting the conservation and use of traditional crop varieties within the agricultural production system. Crit. Rev. Plant Sci. 30, 125–176. doi: 10.1080/07352689.2011.554358
Kawulich, B. B. (2006). La observación participante como método de recolección de datos. For. Qual. Soc. Res. 6:23.
Khoury, C. K., Brush, S., Costich, D. E., Curry, H. A., de Haan, S., Engels, J. M. M., et al. (2022). Crop genetic erosion: understanding and responding to loss of crop diversity. New Phytol. 233, 84–118. doi: 10.1111/nph.17733
Kumar, B. M., and Nair, P. K. R. (2004). The enigma of tropical homegardens. Agrofor. Syst. 61, 135–152. doi: 10.1007/978-94-017-2424-1_10
Lacerda, A. E. B., Hanischff, A. L., and Nimmo, E. R. (2020). Leveraging traditional agroforestry practices to support sustainable and agrobiodiverse landscapes in southern Brazil. Land 9, 1–19. doi: 10.3390/LAND9060176
López Mazz, J. M., Marin Suárez, C., Dabezies Damboriarena, J. M., and Tejerizo García, C. (2020). Arqueología de la esclavitud africana en la frontera uruguayo-brasileña: el caso de la Estancia de los Correa (Rocha, Uruguay). Arqueología 26, 181–201. doi: 10.34096/arqueologia.t26.n2.5942
Magurran, A. E. (1988). Ecological diversity and its. Measurement. Princeton, N.J.: Princeton University Press. doi: 10.1007/978-94-015-7358-0
Magurran, A. E., and McGill, B. J. (2011). Biological diversity: Frontiers in measurement and assessment. Oxford: Oxford University Press.
Mariel, J., Carrière, S. M., Penot, E., Danthu, P., Rafidison, V., and Labeyrie, V. (2021). Exploring farmers’ agrobiodiversity management practices and knowledge in clove agroforests of Madagascar. People Nature 3, 914–928. doi: 10.1002/pan3.10238
Maxted, N., Hawkes, J. G., Ford-Lloyd, B. V., and Williams, J. T. (1997). A practical model for in situ genetic conservation. Plant Genet. Conserv. The in situ approach, eds. N. Maxted, B.V. Ford-Lloyd, and J. G. Hawkes (London: Chapman & Hall). 339–367. doi: 10.1007/978-94-009-1437-7_22
Mello, K., Gonzalez, L., Childe, R., Protti, J. L., and Aguirre, S. (2017). “Estudio exploratorio sobre la situación de semillas criollas en Rivera” in Anais do 9o Saläo internacional de ensino, pesquisa e extensäo (SIEPE) (Santana do Livramento: Universidad Federal do Pampa), 21–23.
Mengue, V. P., Dias de Freitas, M. W., Silva da Silva, T., Fontana, D. C., and Scottá, F. C. (2020). LAND-USE and land-cover change processes in Pampa biome and relation with environmental and socioeconomic data. Appl. Geogr. 125:102342. doi: 10.1016/j.apgeog.2020.102342
Morales, D., Molares, S., and Ladio, A. (2017). A biocultural approach to firewood scarcity in rural communities inhabiting arid environments in Patagonia (Argentina). Ethnobiol. Conserv. 6, 1–17. doi: 10.15451/ec2017-08-6.12-1-17
Morris, C. (2006). Negotiating the boundary between state-led and farmer approaches toknowing nature: an analysis of UK agri-environment schemes. Geoforum 37, 113–127. doi: 10.1016/j.geoforum.2005.01.003
Newton, A. C., Begg, G. S., and Swanston, J. S. (2009). Deployment of diversity for enhanced crop function. Ann. Appl. Biol. 154, 309–322. doi: 10.1111/j.1744-7348.2008.00303.x
Nudley, N. (2008). Directrices para la aplicación de las categorías de gestión de áreas protegidas. Gland, Suiza: UICN.
Padulosi, S., Bergamini, N., and Lawrence, T. (2011). On-farm conservation of neglected and underutilized species: Status, trends and novel approaches to cope with climate change. in Proceedings of the International Conference, Frankfurt, 307.
Palermo, E. (2019). La región histórica del norte uruguayo en la segunda mitad del siglo XIX, 1850–1900. Porto Alegre: FCM.
Pardo de Santayana, M., Morales, R., Aceituno, L., and Molina, M. (2014). Inventario español de los conocimientos tradicionales relativos a la biodiversidad: primera fase, introducción, metodología y fichas. Madrid: Ministerio de Agricultura, Alimentación y Medio Ambiente, Secretaría General Técnica.
Peel, M. C., Finlayson, B. L., and Mcmahon, T. A. (2007). Updated world map of the Koppen-Geiger climate classification. Hydrol. Earth Syst. Sci. Discuss. Euro. Geosci. Union 11, 1633–1644. doi: 10.5194/hess-11-1633-2007
Pereira, S. (2017). Prospección de variedades criollas hortícolas y sus conocimientos tradicionales asociados en el Palmar de Castillos, departamento de Rocha. [thesis]. [Montevideo (Uruguay)]: Facultad de Agronomía, Universidad de La República.
Peroni, N., Albuquerque, U., Assis, A. L., and Freintas Lins, E. (2013). “The domestication of landscapes and cultural keystone species in a context of community biodiversity management in Brazil,” in Community biodiversity management. Promoting resilience and the conservation of plant genetic resources, eds. W. BoefDe, A. Subedi, N. Peroni, M. Thijssen, and E. O’Keeffe (Wageningen, Netherlands: Routledge), 146–150.
Pochettino, M. L., and Lema, V. S. (2008). La variable tiempo en la caracterización del conocimiento botánico tradicional. Darwin 46, 227–239.
Puppo, M., Rivas, M., Franco, J., and Barbieri, R. L. (2014). Propuesta de descriptores para Acca sellowiana (Berg.) Burret. Rev. Bras. Frutic. 36, 957–970. doi: 10.1590/0100-2945-393/13
Ramírez, L. R., and Säumel, I. (2022). Native forest metacommunity structures in Uruguay shaped by novel land-use types in their surroundings. Ecol. Evol. 12:e8700. doi: 10.1002/ece3.8700
Reis, M. S., Montagna, T., Mattos, A. G., Filippon, S., Ladio, A. H., da Cunha Marques, A., et al. (2018). Domesticated landscapes in araucaria forests, southern Brazil: a multispecies local conservation-by-use system. Front. Ecol. Evol. 6:e00011. doi: 10.3389/fevo.2018.00011
Rivas, M. (2007). Recursos fitogenéticos nativos de Uruguay. Montevideo, Uruguay: Asesoría al Sistema Nacional de Áreas Protegidas.
Rivas, M., Barbieri, R. L., Marchi, M., Sosinski, E., and Amorim, F. (2020). “La Red Palmar/Rota dos Butiazais - Una red internacional para la conservación de los Palmares de Butiá mediante su uso sostenible” in Palmeras NUS al sur de la América austral. eds. N. Hilbert, M. L. Pochettino, and E. Hernández (Argentina/España: CYTED), 195–221.
Rivas, M., Clausen, A., and León, P. (2010). “Conservación in situ de recursos fitogenéticos de importancia para la agricultura y la alimentación” in Estrategia en los Recursos Fitogenéticos para los países del Cono Sur Montevideo. ed. A. Berretta (PROCISUR/IICA: Uruguay), 61–74.
Rivas, M., and Condon, F. (2015). “Plant domestication and utilization: the case of the Pampa biome” in Advances in plant breeding strategies: Breeding, biotechnology and molecular tools. eds. J. Al-Khayri, S. M. Jain, and D. Johnson (Cham: Springer), 3–24.
Rivas, M., Dabezies, J. M., and del Puerto, L. (2023). Historical evolution and multidimensional characterisation of the Butia palm landscape: a comprehensive conservation approach. Land 12:648. doi: 10.3390/land12030648
Rivas, M., Vignale, B., Camussi, G., Puppo, M., and Pritsch, C. (2007). Los recursos genéticos de Acca sellowiana (Berg.) Burret en Uruguay, in Avances de Investigación en Recursos Genéticos en el Cono Sur II (Uruguay: PROCISUR, IICA).
Rosero-Toro, J. H., Gómez, H. D. C. D., Ruan-Soto, F., and Santos-Fita, D. (2022). Can cultural significance in plants be explained by domestication and usage spaces? A study case from a coffee producing community in Huila, Colombia. Ethnobiol. Conserv. 10, 1–17. doi: 10.15451/ec2021-06-10.28-1-24
Schmeda-Hirschmann, G., Jiménez-Aspee, F., Theoduloz, C., and Ladio, A. (2019). Patagonian berries as native food and medicine. J. Ethnopharmacol. 241:111979. doi: 10.1016/j.jep.2019.111979
Sokal, R.R., and Rohlf, F. J. (1995). Biometry: the principles and practice of statistics in biological research. 3rd, New York: W.H. Freeman and Co.
Soutullo, A., Alonso, E., Arrieta, D., Beyhaut, R., Carreira, S., Clavijo, C., et al. (2009). Especies prioritarias para la conservación en Uruguay. Montevideo, Uruguay: SNAP-DINAMA/GEF/PNUD.
Speroni, G., Vignale, B., and Cabrera, D. (2018). “Psidium cattleyanum Sabine” in Arazá, araçá-da-praia, araçazeiro, guayabo fresa, strawberry guava. eds. R. Leggiadro, M. Galván, and E. Grille (San José: Instituto Interamericano de Cooperación para la Agricultura (IICA)).
Stampella, P. C. (2015). Historia local de Naranja amarga (Citrus x aurantium L., Rutaceae) del viejo mundo asilvestrada en el corredor de las antiguas Misiones Jesuíticas de la provincia de Misiones (Argentina). Caracterización desde una perspectiva interdisciplinaria. [Doctoral thesis]. [La Plata (Argentina)]: Universidad Nacional de la Plata.
Sthapit, B., Lamers, H. A. H., and Rao, V. R. (2016). Tropical fruit tree diversity: good practices for in situ and on-farm conservation. London and New York: Routledge. doi: 10.4324/9781315758459
Tabakian, G. (2019). Estudio comparativo de plantas medicinales vinculadas a tradiciones indígenas y europeas en Uruguay. Bonplandia 28:135. doi: 10.30972/bon.2823855
Thorp, G., and Bieleski, R. (2002). Feijoas: Origins, cultivation and uses. E. 1ra. Auckland, New Zeland: D. Bateman, Ltd.
Toledo, V., and Barrera-Bassols, N. (2008). La Memoria Biocultural. La importancia ecológica de las sabidurías tradicionales. 1ra. Barcelona, España: Junta de Andalucia.
Torres, M. G. (2019). Movilidad, fugas y mestizaje en la Banda Oriental del Uruguay: una primera aproximación, Buenos Aires. Revista TEFROS 17, 114–142.
UN Convention on Biological diversity (1992). Handbook on the convention on biological diversity. Available at: https://www.cbd.int/doc/handbook/cbd-hb-01-en.pdf (accessed June 13, 2023).
Vidal, R., Rivas, M., Chiappe, M., Quintero, D., Castro, X., Calvete, A., et al. (2021). Conocimientos Tradicionales asociados a los usos de los Recursos Genéticos en Uruguay. Montevideo, Uruguay: PNUD Uruguay.
Vidal, R., Rivas, M., Chiappe, M., Quintero, D., Piazza, S., Calvete, A., et al. (2018). Relevamiento de los Recursos Fitogenéticos con conocimientos tradicionales asociados - Proyecto “Fortalecimiento de los Recursos Humanos, los marcos legales y las capacidades institucionales para aplicar el protocolo de Nagoya.” Montevideo, Uruguay: Facultad de Agronomía/PNUD.
Vignale, B., Cabrera, D., Rodríguez, P., and Machado, G. (2016). Selección de frutales nativos en Uruguay. Horticultura Argentina 35, 19–29.
Vignale, B., Jolochin, G., and Cabrera, D. (2018). “Eugenia uniflora L.” in Pitanga, Ñangapiré, Pitangueira, Pitanguero, Surinam cherry, Cayenne cherry. eds. R. Leggiadro, M. Galván, and E. Grille. 1ra ed (Montevideo: Instituto Interamericano de Cooperación para la Agricultura IICA)
Watson, J. W., and Eyzaguirre, P. B. (2001). Home gardens and in situ conservation of plant genetic resources. In Proceedings of the Second International Home Gardens Workshop (Witzenhausen, Federal Republic of Germany: international plant genetic resources institute, Rome), 192.
Wenger, E., McDermott, R., and Snyder, W. M. (2002). Cultivating communities of practice: A guide to managing knowledge - seven principles for cultivating communities of practice. Boston, USA: Harvard Business School Press.
Wiersum, K. F. (1997). From natural forest to tree crops, co-domestication of forests and tree species, an overview. Neth. J. Agric. Sci. 45, 425–438. doi: 10.18174/njas.v45i4.503
Wood, K. A., Stillman, R. A., and Goss-Custard, J. D. (2015). Co-creation of individual-based models by practitioners and modelers to inform environmental decision-making. J. Appl. Ecol. 52, 810–815. doi: 10.1111/1365-2664.12419
Keywords: plant genetic resources, local knowledge, in situ conservation, protected areas, domesticated landscape, agrobiodiversity, Pampa biome, genetic cultural erosion
Citation: Puppo M, Gianotti C, Calvete A, Leal A and Rivas M (2023) Landscape, agrobiodiversity, and local knowledge in the protected area “Quebrada de los Cuervos y Sierras del Yerbal,” Uruguay. Front. Sustain. Food Syst. 7:1240991. doi: 10.3389/fsufs.2023.1240991
Edited by:
León-Sicard Tomás Enrique, Universidad Nacional de Colombia, ColombiaReviewed by:
Vinicio J. Sosa, Instituto de Ecología (INECOL), MexicoSyam Viswanath, Kerala Forest Research Institute, India
Copyright © 2023 Puppo, Gianotti, Calvete, Leal and Rivas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: María Puppo, bWFyJiN4MDAwRUQ7YS5wdXBwb0BjdXJlLmVkdS51eQ==; Mercedes Rivas, bXJpdmFzQGZhZ3JvLmVkdS51eQ==
 María Puppo
María Puppo Camila Gianotti
Camila Gianotti Alejandra Calvete1
Alejandra Calvete1 Mercedes Rivas
Mercedes Rivas