- 1ICAR Research Complex for North Eastern Hill Region, Umiam, Meghalaya, India
- 2Assam Agricultural University, Jorhat, Assam, India
Introduction: Under a changing climate, the fragile ecosystems of the Eastern Himalayas (EH) are persistently challenged by prolonged dry spells and erratic rainfall. Identification of suitable high-yielding crops with higher moisture stress tolerance and adaptability is paramount for the region. Although the region received a good amount of rainfall in the rainy season, the winter months, viz., November to March, rarely received any rain. Even within the rainy season, there are several intermittent drought spells that hinder crop productivity.
Methods: The present study has used field and microcosm experiments to assess the year-round cultivation potential and extent of moisture stress tolerance in the lesser-known buckwheat crop of the region.
Results and discussion: Sowing of buckwheat from mid-September to mid-December produced better grain yield, the highest being when sowing in October (9.83 q ha−1) and the crop was found suitable to grow all through the year for higher green biomass (12.6–38.4 q ha−1). The moisture stress tolerance of buckwheat was significantly enhanced by increased total root length and root surface area by 12.4 and 34.7%, respectively. Increased photo-protective carotenoids, chlorophyll b, and favorable stomatal attributes with substantial epicuticular wax have significantly improved the moisture stress tolerance of Buckwheat. In addition, leaf proline was found 25.4% higher and total soluble protein, reducing sugar, and cell membrane stability were found 29.2, 38.1, and 36.5% lower compared to the control, respectively. A significantly lower rate of water loss (25.6%) with its stomatal and non-stomatal adaptations and versatile pollen structural traits under moisture stress over control, make the buckwheat crop potentially more stress tolerant and economical crop for EH of India.
1. Introduction
Agriculture functioning under fragile agro-ecologies of the Eastern Himalayan Region (EHR) is severely constrained by rapid degradation of land resources, undulating hill topography, the prevalence of shifting cultivation, and frequent occurrence of environmental and edaphic stresses, leading to low crop productivity (Hetherington and Woodward, 2003; Das et al., 2017; Krishnappa et al., 2017). High incidence of rainfall during the pre-kharif (pre-monsoon) and kharif (monsoon) seasons coupled with conventional and traditional crop cultivation practices result in unprecedented soil erosion with drastic nutrient losses, which hampers the agricultural productivity in the region (Das et al., 2019,2020). As most of the hill farmers in the EHR practice organic farming, which requires adaptation to environmental stressors as well as nutrient-efficient crops (Layek et al., 2021), lesser-known indigenous crops with greater economic and culinary potential would be more beneficial (Seitinthang, 2014; Krishnappa et al., 2017; Das et al., 2019,2020). Although the region receives a high amount of annual rainfall (≈2,200–2,400 mm per annum), the occurrence of prolonged and intermittent dry spells is becoming a common phenomenon during the post-monsoon season under a changing climate (Krishnappa et al., 2019; Hajong et al., 2022). However, the occurrence of most rainfall from April to October and very little rainfall (≈6–8% of total rainfall) during the period from November to March results in moisture deficiency for crops grown after the rainy season. Since 80% of the crop area consists of rainfed agriculture in the region, apparent climate change and vulnerability potentially affect agriculture production by inimically reducing cropping intensity (131%) in regions with acute soil moisture deficits across hill slopes (Krishnappa et al., 2017; Hajong et al., 2022; Layek et al., 2022). The impacts of climate change are causes for concern in every province of the Eastern Himalayas with a greater degree of meteorological drying or lower rainfall experienced between 1991 and 2007 (Saikia et al., 2013), leading to multiple socio-economic consequences (Ravindranath et al., 2011). Moreover, acid soil stress is also one of the major crop production limitations (>80% geographical area) as it results in reduced phosphorus availability and substantial inadequacies in micronutrient supply with pronounced elemental toxicities (Manoj, 2011; Manoj et al., 2011). Hence, ideal crop diversification and intensification approaches require exploration and development of crops with multi-stress tolerant abilities.
Buckwheat (Fagopyrum esculentum L.) is one of the lesser known, underutilized, and gluten-free pseudocereal, serving multiple health and culinary benefits, with immense cultivation potential in the region (Skerritt, 1986). Buckwheat is used for both greens and grain purposes. Tender shoots are used as a leafy vegetable, and flowers and leaves are used for extraction of rutinoids (Krishnappa et al., 2023). As buckwheat exhibits fast growing ability with denser and fibrous roots, it helps in soil binding and overcoming erosion in hill slopes and competes with and checks weed growth (Krishnappa et al., 2019, 2023). Buckwheat is commonly cultivated under marginal and degraded lands of EHR with poor nutrients, lesser moisture regimes and low input agriculture (Hajong et al., 2019, 2022). Since moderate to severe moisture stress prevails during the post-kharif (post-monsoon) season in the region, buckwheat cultivation is subjected to varied levels of moisture stress and invariable soil acidity at the root and shoot level (Yang et al., 2013; Krishnappa et al., 2019). Since crop growth largely depends on the ability of roots to acquire essential water and nutrients from the rhizosphere, the impediments at the root system level by toxic elements (Al and Fe) hampers the ability of the roots to support a highly metabolizing shoot system, especially under low soil moisture stress conditions (Yang et al., 2013). Novel research efforts are, therefore, urgently required to understand the nature of moisture stress tolerance for developing potential high yielding crops for multiple stress environments (Atkinson and Urwin, 2016). Even though buckwheat, which has various food, fodder, and medicinal values, bear unique growth characteristics, viz., early maturing with short duration and ability to grow in nutrient-poor degraded soils, understanding the stress adaptation response under invariable moisture stress conditions of the region is of greater significant importance (Michiyama and Hayashi, 1998; Krishnappa et al., 2019). Rooting depth, distribution, and root traits are often considered as key parameters for measuring plant tolerance under moisture stress (Lynch and Brown, 2012). The impact of moisture stress in many crop plants has been explored for the unique roles of root morphology, root architecture, and shoot growth alterations as a part of robust plant stress responses for improved water and nutrient uptake (Schroeder et al., 2001; Seki et al., 2007; Abenavoli et al., 2016; Das et al., 2021). Under short-term water stress, plants increase their water use efficiency (WUE) by reducing stomatal aperture and thereby reducing their transpiration rate. However, under conditions of prolonged water deficit, plants frequently produce leaves with reduced stomatal conductance resulting from altered stomatal density (SD) and size (Doheny-Adams et al., 2012; Franks et al., 2015). Under continued moisture stress, it is important to understand how crop adaptability manifest as the extent of change in root growth, stomatal structure, and cuticle synthesis as major means of producing stomatal and non-stomatal barriers for reduced water loss through plant leaves (Jones, 1998; Seki et al., 2007).
Moisture stress at the reproductive stages cause drying of anthers and decreases pollen viability and activity in rice (Saragih et al., 2013) and pea (Gusmao et al., 2012). This indicates that ultimate grain yield is a product of robust pollen viability by a tolerant crop under stress conditions (Gusmao et al., 2012; Sakhi et al., 2014). The close linkage between the genotypes that set seeds effectively and maintain adequate pollen viability for attaining higher yield under moisture stress was owed to increased gametophyte tolerance (Sakhi et al., 2014). The advent of new elemental imaging techniques like energy-dispersive X-ray microanalysis (EDX) has hastened the provision of an understanding of elemental distributions and quantification in cells and tissues (Jiang et al., 2015). As EDX is commonly used for both qualitative and quantitative analysis, it enables researchers to identify both types of elements as well as the percentage of each element’s concentration within the sample. Since these techniques encompass little or no sample preparation and are mostly non-destructive, they are very beneficial for proper energy and resource management (McCully et al., 2010). Since soil moisture is a major limiting factor for crop growth and a primary hurdle when it comes to reproductive growth in winter crops like buckwheat in the EHR, it is pertinent to understand the extent to which moisture stress tolerance is reflected in terms of root and shoot growth changes with an apparent increase in resource use efficiency and gametophyte-related traits in buckwheat under hill slopes. In cognizance of the above, this study primarily aimed to examine the potential of buckwheat for year-round cultivation in Meghalaya, India, by conducting a field experiment and understanding moisture stress adaptive mechanisms through a microcosm experiment.
2. Materials and methods
2.1. Field experiment
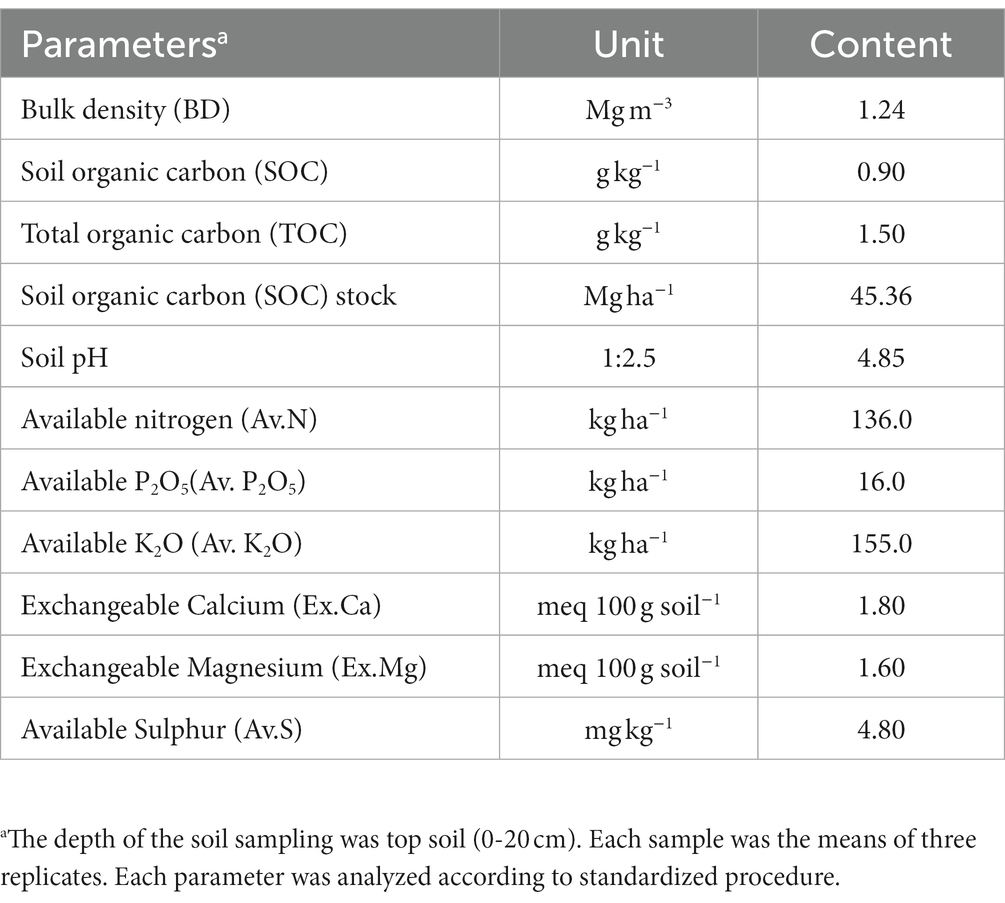
The field experiment was conducted in the upper hills of the water management field of the ICAR Research Complex for the North Eastern Hill Region, Umiam, Meghalaya, India. The experimental site was located at a latitude of 25.4° N and longitude of 91.54° E and has an altitude of 950 m above the mean sea level. The soil of the experimental site was clay loam in texture with a substantially low amount of available nitrogen (231.5 kg N ha−1) and phosphorus (P) (16.2 kg P2O5 ha−1) and a high amount of potassium (K) (255.0 kg K2O kg ha−1). The pH and organic carbon (SOC) content of the soil was 4.85 and 1.35%, respectively. The initial soil samples had a bulk density of 1.24 Mg m−3 and a maximum water holding capacity of 43–46% (Table 1). The field experiment was conducted for 2 consecutive years (2014 and 2015). The crop was sown month wise (during the first week of every month) with a seed rate of 30 kg ha−1 with 30 cm line spacing. Monthly averages of meteorological data during the period of experimentation are presented in Figure 1. The long-term (30 years) average annual rainfall of the study site was ≈2,450 mm and the average annual rainfall over the 2 years of the study was 2356.5 mm, and this was distributed mainly during the April to October period of the year (Figure 1). In respect to weather parameters, maximum temperatures varied between 29.0°C and 30.6°C over the years, the minimum temperature being recorded within the range of 5.5–5.6°C. The year 2015 (2565.6 mm rainfall) received 19.5% higher rainfall compared to 2014 (2147.4 mm). However, there was not any significant difference in maximum temperature, minimum temperature, and maximum RH across the 2 years of experimentation (Figure 1).
2.2. Experimental details and layout
The buckwheat cultivar used for study was Fagopyrum esculentum Moench (var.gossi local) locally adapted to mid altitudes of Meghalaya (Krishnappa et al., 2023). The experiment was initiated during 2014 by uniform ploughing of the field using a power tiller followed by surface leveling. Sowing of the crop was done on Days 1 or 2 of the first week of every month. Spacing of 30*10 cm and recommended nutrient package and inter cultivation practices (60:60:40 kg N, P2O5 and K2O/ha) were followed as per the recommendation for the region (Krishnappa et al., 2023). Well-decomposed Farmyard manure (FYM) (0.5% N & 0.21% P2O5) was applied on the basis of an equivalent N dose to meet the requirements of 60:60:40 kg N, P2O5 and K2O ha−1. The P requirement was supplemented through rock phosphate as the soil in this region is severe to moderately acidic and P availability was rated as very low. The experiment was laid out in randomized block design (RBD) and replicated thrice. The gross plot size was 5.0 m × 4.0 m.
2.3. Microcosm experiment for moisture stress study
The microcosm experiment was carried out during 2015. The size of the pot selected for the microcosm experiment was 5 kg soil holding capacity (≈5litre volume) with the following dimensions: 26.0 cm diameter at the top with 13.9 cm diameter at base and height 22.0 cm. The pots were filled with topsoil (0–20 cm) which is freshly collected from buckwheat field with similar soil properties (Table 1).
2.4. Induction of moisture stress
Two moisture stress treatments, viz., one by withdrawing and providing reduced water and another by providing sufficient water at regular interval, were used for studying moisture stress tolerance. Soil used in the experiment had a low pH (5.20), was medium in nitrogen (238.2 kg N ha−1) and phosphorus (15.1 kg P2O5 ha−1), was medium in available potassium (243.3 kg K2O ha−1), and falls into the category of silty clay loam. Withdrawal of water was initiated after 35 days of crop sowing when plants attained the 4–5 leaf stage. Under moisture stress, survival watering (with amount of water required to bring the soil till 60% field capacity-FC) was provided as and when plants showed moisture stress symptoms like mild wilting. Moderate to severe moisture stress (to reach soil water till 60% FC with 10–13% soil moisture) was induced while coinciding with the active growth stage (40–45 DAS). Initially, the amount of water required for bringing the soil (5 kg pot−1) to 100% field capacity was arrived at by weighing the pots with dry soil and weighing the pots after overnight water saturation. After knowing the actual amount of water required to bring the 5 kg soil to sufficient moisture conditions (100% FC), only 60% of that amount of water was added when the plants showed mild wilting symptoms so that plants were maintained under continuous water stress conditions (Krishnappa et al., 2019; Hajong et al., 2022).
2.5. Recording of growth and yield attributes
The plant height and total biomass of buckwheat were also documented during harvest. The crop duration was calculated from the date of sowing till the attainment of physiological maturity. At the end of the first field experiment, the yield of grains after physiological maturity was used to compute the HI (Eq. 1). Nutritional quality of grain was quantified by following the standard procedure for crude fiber (Marnyard, 1970), crude protein (Kjeldahl, 1883), soluble carbohydrates (Appleman et al., 1927), fat (soxlet extraction), starch (Anthrone method), and ash content (Nielsen, 2003). Economic yield (Eq. 2) and stover yield (Eq. 3) were estimated after oven drying of plants obtained from centre of each plot and estimates were brought to 13% tissue moisture content. The HI determined by using Eq. 1 was expressed as a %.
2.6. Physiological observations
2.6.1. Determination of leaf relative water content (LRWC) and rate of leaf water loss
To measure LRWC, matured and fully expanded leaves were harvested and immediately weighed for fresh weight, then immersed and incubated overnight in petriplate containing water to record the maximum turgid leaf weight and dry weight taken after 48 h of oven drying at 72°C. Later, required RWC was calculated by using the standard formula expressed as a percentage (Layek et al., 2022).
For assessing the rate of water loss (ROWL), fresh leaves were harvested from the field and quickly carried to laboratory to be spread in a ventilated place, and periodical fresh weights were recorded at timely intervals (at 1 h intervals) till zero fresh weight difference was attained (Max.4 h). ROWL is expressed as the amount of water lost per unit leaf area per unit time (ml cm−2 h−1).
2.6.2. Leaf pigment analysis
The concentrations of chlorophyll and carotenoid in fresh and matured leaves after stress periods were estimated by an acetone extraction method. The known weight of leaf tissue (0.25 g) was fully homogenized in 15 mL of 80% acetone: 20% water solution using a pre-cooled pestle and mortar at 4°C. A pinch of CaCO3 was added to the extraction solution during grinding of the samples to neutralize any plant acids that might be liberated during grinding. After thorough grinding, the extracted solution was filtered through filter paper (Whatman No.42) into a volumetric flask and the volume of the extract amounted to 25 mL using the same 80% acetone: 20% water solution ratio. Each sample was placed in a cuvette, and absorbance was recorded at 645, 663, and 480 nm through a UV visible spectrophotometer (UV-2100). The absorbance values were substituted into standard formulae to calculate the chl a, chl b, and total chlorophyll (chla+b), which are expressed in mg/g whereas the carotenoid pigment content was expressed as μg/g on a fresh weight basis (Misyura et al., 2012; Das et al., 2021; Layek et al., 2022).
2.6.3. Measurement of shoot traits
The fully expanded and matured buckwheat leaf, the fourth leaf from the top, was used for measurement of leaf thickness (LT). LT was measured using the absolute digital vernier caliper (Mitutoyo corp. Japan) at the broadest part of the leaf excluding major veins with an accuracy of ±0.01 mm and expressed in μm (Hazarika et al., 2020). LT was determined through a direct reading with gentle pressing of the caliper to avoid overestimation and any injury to the intact leaf (Vile et al., 2005). For recording the shoot and root dry weight, the fresh and air-dried root and shoot samples were oven dried at 72°C for 48 h or till reaching a constant weight and expressed in terms of g/plant. The root-to-shoot ratio was calculated by dividing root dry weight (RDW) by shoot dry weight (SDW). Total dry matter (TDM) was derived by adding SDW and RDW and is expressed on a single plant basis (Hajong et al., 2022).
2.6.4. Determination cell membrane stability (CMS)
The CMS was determined as a percent leakage of cell contents in the fresh leaves of buckwheat leaves (Sullivan and Ross, 1979; Hazarika et al., 2020). Finely cut leaf pieces weighing up to 0.5 g were taken from the top third leaf and immersed in 50 mL of deionized water and incubated under laboratory conditions for 3 h. At the end of the 3 h, initial electrical conductivity (C1) was measured using a conductivity meter (ELICO Ltd., India.). Then the beaker containing deionized water with leaf pieces was boiled over a hot water bath for 30 min and cooled to record the final electrical conductivity (C2).
Cell membrane integrity was computed and expressed % by Eq. 4:
2.6.5. Estimation of leaf proline
A known quantity of fresh leaves (100 mg) from moisture stress treatments was collected and homogenized into fine powder with liquid nitrogen initially and added 10 ml of 3% sulfosalicylic acid after which centrifugation at 13000 rpm for 10 min was undertaken. The supernatant of 2 mL from each sample and 2 mL of acidified ninhydrin solution (1.25 g ninhydrin dissolved in 30 mL acetic acid at boiling temperature to which 20 mL of orthophosphoric acid were mixed) and 2 mL of 100% acetic acid were mixed and allowed to boiling for 1 h. Samples were quickly transferred onto ice-water to stop further reaction, and they were left to cool down for at least 20 to 30 min. Finally, 4 mL of toluene was added to each sample at room temperature; this was mixed thoroughly, and the absorbance was recorded at 520 nm (Bates et al., 1973). To assay the proline content of the sample, a standard curve was prepared in the range of 0.25 to 160 μM proline.
2.6.6. Root architecture measurements
The changes in root size and distribution of buckwheat as affected by moisture stress were assessed by smooth uprooting of the plants from pots by careful loosening of the soil surrounding root system and washing to remove adhering rhizospheric soil with a gentle flush of water (Bohm, 1979; Das et al., 2021). The turgid and fresh roots were scanned after air drying by a root scanner by using winrhizo R software (Regent instruments Inc., Quebec, Canada) to measure the finer details of root architecture as a response to moisture stress. The intact turgid root system was evenly spread on a transparent fiber tray (30 cm x 20 cm) without overlapping and imaging was done at a resolution of 200 dpi (dots per inch) with Epson v 700 perfection scanner (Arsenault et al., 1995; Abenavoli et al., 2016; Hazarika et al., 2020; Hajong et al., 2022; Layek et al., 2023).
2.6.7. Determination of stomatal attributes
Stomatal attributes like stomatal frequency (no. of stomata/unit area), stomatal index (no. of stomata per epidermal cells), and stomatal size were assessed under 40X using a compound microscope (BX- 50F, Olympus, Japan). Since buckwheat leaves have hypo-stomatic properties, which bear more stomata beneath leaf surface, stomatal counting was made in both the surface by applying translucent nail polish and shade drying for 20–30 min. The leaf surfaces have been cut into 2.0–2.5 cm2 dimensions and the layer of nail polish impression was gently removed with the help of forceps to place the same on microscopic slides with a few drops of water before a glass cover slip was placed on top to cover. Stomatal numbers on every sample surface were counted at three different microscopic fields under 40X magnification of compound microscope along with related observations on stomatal length, breadth, and number of guard cells in the particular microscopic field (Hazarika et al., 2020; Layek et al., 2023).
2.6.8. SEM (scanning electron microscope) analysis
Fully matured leaves from different moisture stress treatments have been collected and allowed to air dry at room temperature without any initial pre-treatment, and the same leaves were subsequently carefully incubated between two sheets of pleated paper with little pressure for 48–76 h without any vacuum (Hajong et al., 2022). All care has been taken to remove moisture from the leaves with minimal distortion to ensure the leaf cells remain intact and there is maximal preservation of the original cell forms and structures. Shrinkage of the epidermal cells and stomata and distraction to the micro-morphology of leaf cuticle was cautiously observed to minimize the damage during processing. Intact dried leaf samples were cut with a scalpel (1 × 1 cm) and mounted with carbon tape on a stub (Kosma et al., 2009; Lahlali et al., 2014). Both lower and upper surfaces were viewed with a scanning electronic microscope (SEM) adjusted at a high voltage range in between 200 V and 30 kV and a pressure range between 10 Pa and 130 Pa (Low vacuum) (JSM 6360, JEOL Ltd., Japan). Sections were ideally taken from the middle of the leaves to avoid differential thickness along the leaf. All the parameters in the SEM were measured with a XT Docu Soft Imaging System GmbH. The magnification range variable used was in between 30X and 100,000X. All parameters mentioned were adjusted depending on the type of leaf surface of the plant. Energy-dispersive X-ray analysis data were also recorded with an EDX detector to generate more information about a sample chemical composition, including what elements are present as well as their distribution and concentration (Krishnappa et al., 2019; Hajong et al., 2022).
2.6.9. Pollen surface morphology and pollen wall structure
Slightly open flowers were harvested into a petridish during cool morning hours (around 8.00 AM) on the 25th day after application of moisture stress. The anthers were carefully cut open to dehisce the pollen sacs. Mature pollen grains were gold coated (Edwards S150B Sputter Coater, Wilmington, MA, United States) before examination using SEM (JEOL JSM-35) and imaged using a charge-coupled device (CCD) navigation camera (Adams and Morton, 1972; Salem et al., 2007). It involved acetolysis and then dehydration via an ethanol series of 25, 50, 90, and 100% (twice). In vitro pollen germination and tube growth were observed using a pollen germination medium. Fresh pollen grains were gathered from anthers of slightly opened flowers that were collected during morning cool hours (around 8.00 AM). Pollen grains were placed onto the germination medium on microscopic slides that were then placed individually above moistened filter paper in 90 mm Petri dishes. Petri dishes were sealed with parafilm to maintain high humidity, and they were placed in an incubator. After 12 h of incubation, germination of pollen grains was examined using direct microscopic observation with a compound microscope at 40X (BX - 50F, Olympus, Japan). Pollen grains were considered germinated when the pollen tube length was greater than the grain diameter.
2.7. Statistical analysis
The required statistical analysis of experimental data recorded in the current study which was based on RBD was performed by using a standard method of analysis of variance (ANOVA) to study the effect of sowing month and its interaction with grain yield. The significance was tested using the standard error of mean (S.Em ±) for each treatment, and the critical difference (CD) was at a 5% level of probability (p < 0.05 in all cases) that has been worked out for each of the treatment means (Gomez and Gomez, 1984).
3. Results
3.1. Cultivation potential of buckwheat
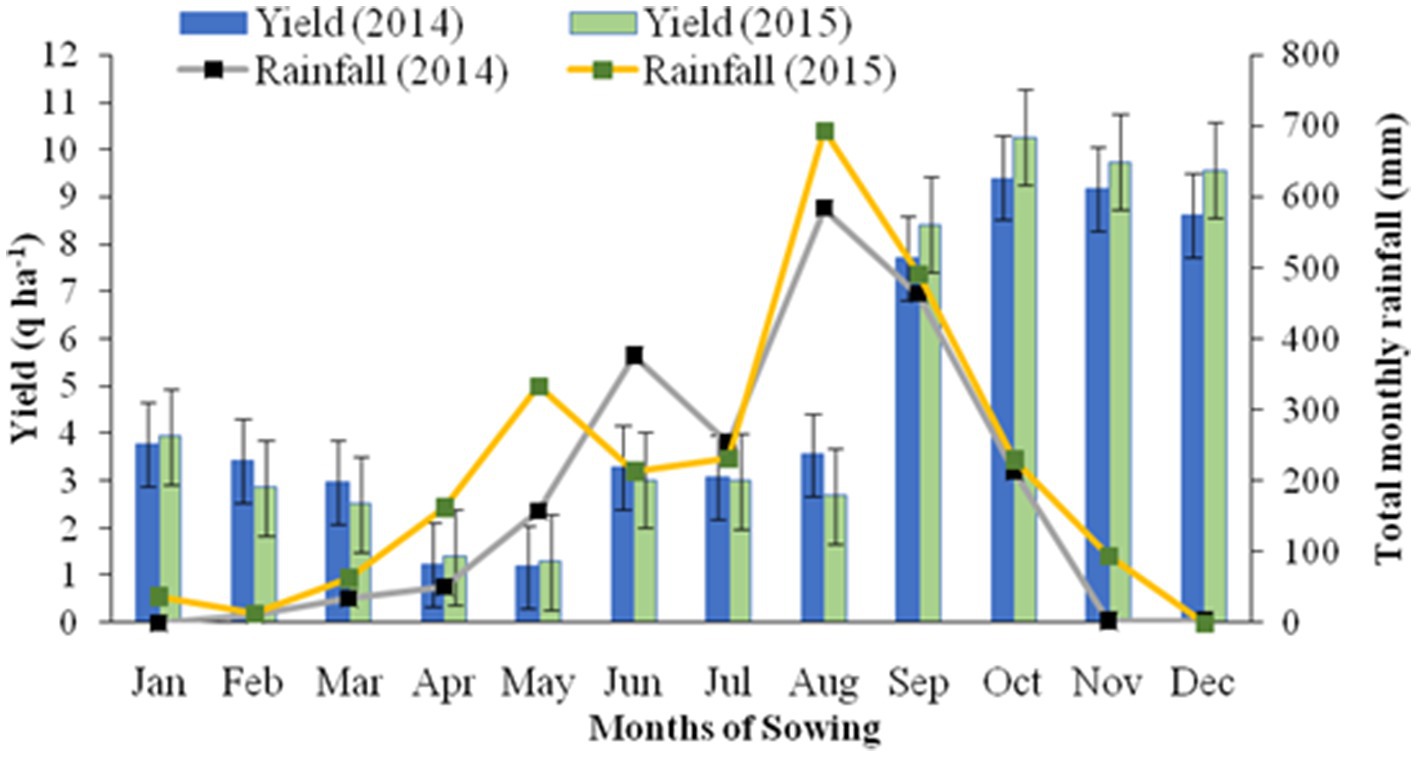
The crop duration of buckwheat and plant height at harvest did not vary significantly across the years of experimentation (Table 2). However, the crop duration and plant height at harvest varied significantly across the sowing treatments. While the crops matured after 133 days if sown in the months of January and 124 days if sown in February, buckwheat matured very early, in only 101 and 102 days if sown in the month of September and July, respectively. The grain yield of buckwheat also did not change significantly over the years. The highest mean yield of buckwheat (pooled of year 2014 and 2015) was recorded for the month of sowing in October (9.83 q ha−1) followed by sowing in November (9.45 q ha−1) and December (9.09 q ha−1). The lowest yield was recorded for sowing in the month of May (1.23 q ha−1) and April (1.32 q ha−1). As buckwheat needs cold and dry weather during maturity, sowing in October to December results in high yield as harvesting coincides with dry weather. High-intensity rain, especially during the rainy season that is very prevalent in North Eastern India, resulted in significantly lower yield for buckwheat compared to the yield of the crops sown during the winter months. The year 2014 recorded significantly higher straw yield (20.16 q ha−1) as against year 2015 (18.84 q ha−1). The harvest index was recorded to be higher in 2014 as compared to 2015. Among the months of sowing, the highest HI was recorded in the month of November (33.99%) followed by December (33.51%) and October (32.62%). Except for HI, all the other parameters were not significantly influenced by the interaction effect of years of experimentation and month of sowing (Tables 2, 3). The highest HI was recorded for the buckwheat grown in the month of November in 2015 (34.46%) followed by month of December for 2015 (33.94%). The lowest HI was recorded for the month of May and year 2015 (6.04%) followed by May and year 2014 (6.31%). Although buckwheat can grow well in the months of the rainy season and can produce good vegetative growth, if maturity coincides with rain, the yield decreases drastically which is clearly reflected with substantial reduction in HI (Table 3; Figure 1). Meanwhile the soil moisture measured was found to be highest (44.0%) during July and lowest during months of January (14.5%) and February (15.1%) (Supplementary Figure S1).
Table 2. Crop morphology and yield attributes of buckwheat as influenced by different sowing months.
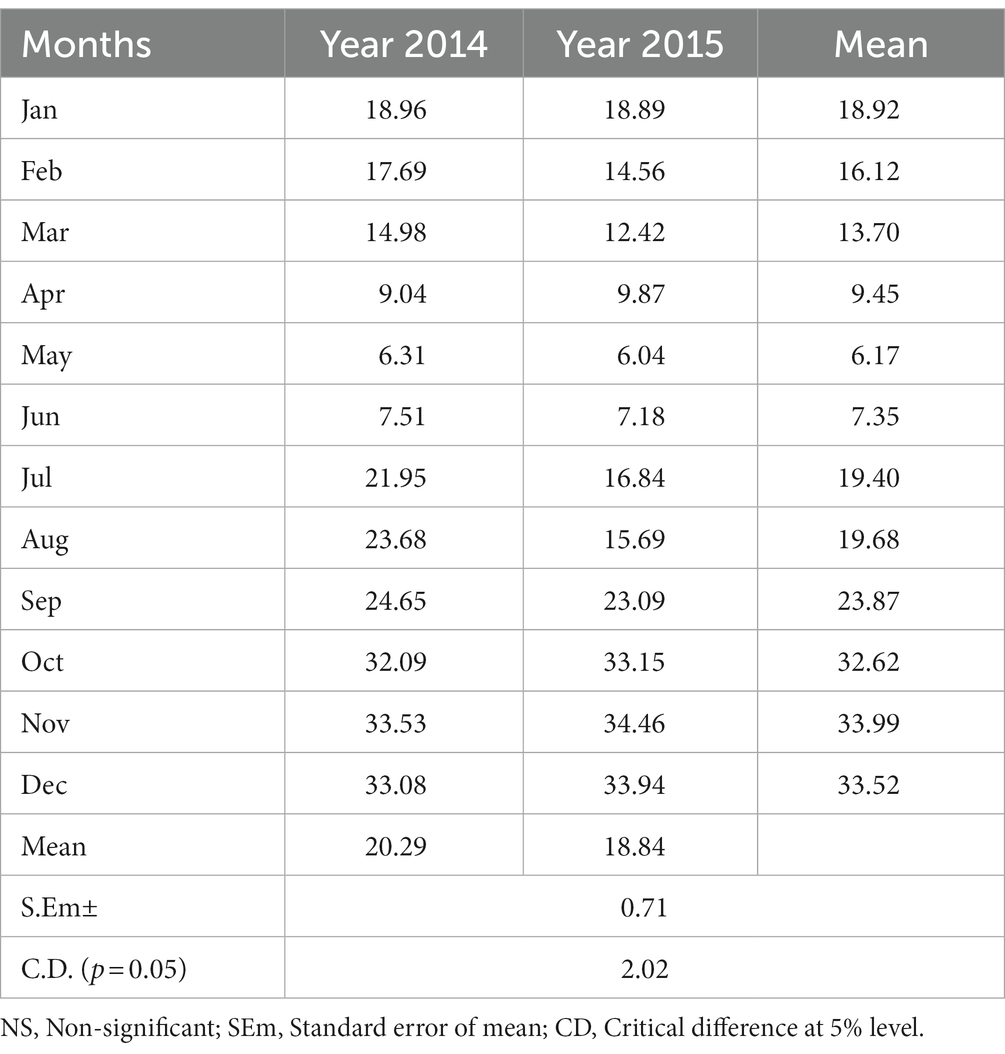
Table 3. Interaction effects of years of experiment and month of sowing on harvest index (%) of buck wheat.
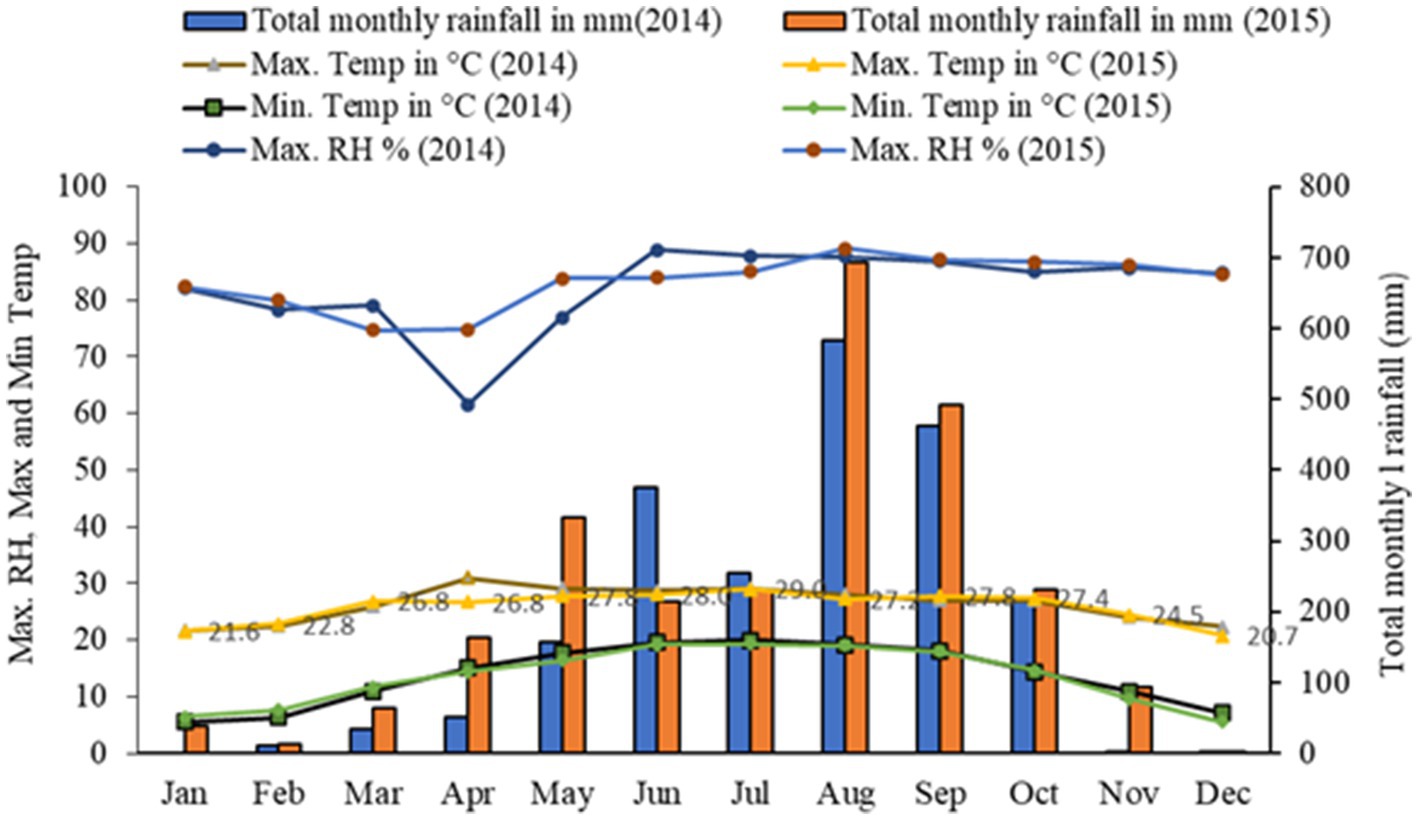
It is clear from Figure 2 that the buckwheat yield recorded in 2015 was relatively higher than in 2014, especially for sowing in the months of September to December. Higher rainfall in 2015 followed by enhancement in soil moisture and subsequent use by buckwheat especially for the crop sown in the winter months resulted in significantly higher yield compared to 2014. Higher rainfall in 2015 compared to 2014 helped to supply soil moisture for buckwheat in the months of November to March as there was very little or no rain in this period and the crop is mostly grown without irrigation, whereas the reverse (low yield in 2015) was recorded for sowing in the months of February, March, June, July, and August since the crops sown in these months coincided with heavy rain at flowering or maturity especially in year 2015 (Figure 2).
In addition, the nutrient composition of buckwheat seeds collected from the crops sown during the October month yielded the highest productivity at 11.43% crude protein, 21.34% crude fiber, 2.88% fat, 62.20% soluble carbohydrate, and 2.15% ash, starch (70–91%), iron (60–100 ppm), and zinc (20–30 ppm) (Supplementary Table S1). Moreover, cultivation of buckwheat crop under the fragile hill ecosystems of the Himalayas was found to be remunerative, generating an annual income of approx. Rs. 45,000–60,000 ha−1 with a shorter crop duration of 96–116 days under low-input conditions and multipurpose utility under resource constrained conditions.
3.2. Moisture stress response of buckwheat
The performance of buckwheat under moisture stress conditions was observed to be lowered as reflected in terms of reduced shoot growth, decreased leaf size and turgidity, and increased root growth (Figure 3; Table 4 and Supplementary Figure S2). The number of leaves per plant, shoot dry weight, total dry matter (TDM), and plant height were decreased significantly to the tune of 23.5, 45.1, 34.7, and 33.5%, respectively, whereas the root dry weight and R/S ratio increased to the extent of 55.4 and 181%, respectively, which is indicative of the significant impact of moisture stress on above- and below-ground biomass (Table 4). The plant height significantly reduced under moisture stress to the extent of 33.7% compared to control. In addition, total root length, root surface area, and root volume are key root architecture components that varied significantly under moisture stress conditions compared to the control (Table 4 and Supplementary Figure S2), which accounted for an increase of 12.4, 34.7, and 25.4% compared to the control (non-moisture stress) condition, respectively. Leaf RWC was reduced under moisture stress conditions due to inadequate water availability for plant uptake, confirming the presence of water stress during experimentation with the buckwheat plant (Table 4). Moreover, total soluble protein, reducing sugar, and CMS were reduced to the tune of 29.2, 38.1, and 36.5%, respectively, whereas leaf proline content was increased to the extent of 25.4% under moisture stress conditions as compared to control (Table 5).

Figure 3. Effect of moisture stress on growth and development of buckwheat. Leaf size changes under control (A) and moisture stress (B), whole plant level changes under control (C) and moisture stress (D) and growth changes in field condition under control (E) and moisture stress (F) at ICAR Research Complex for North Eastern Hill Region, Umiam, Meghalaya (Scale bars for panel E,F is 1 to 10). The above images were taken during field evaluation from the best sowing month of December representing higher grain productivity. Control is well watered condition with 100%FC and moisture stress is reduced water availability condition to the extent of 60% FC.
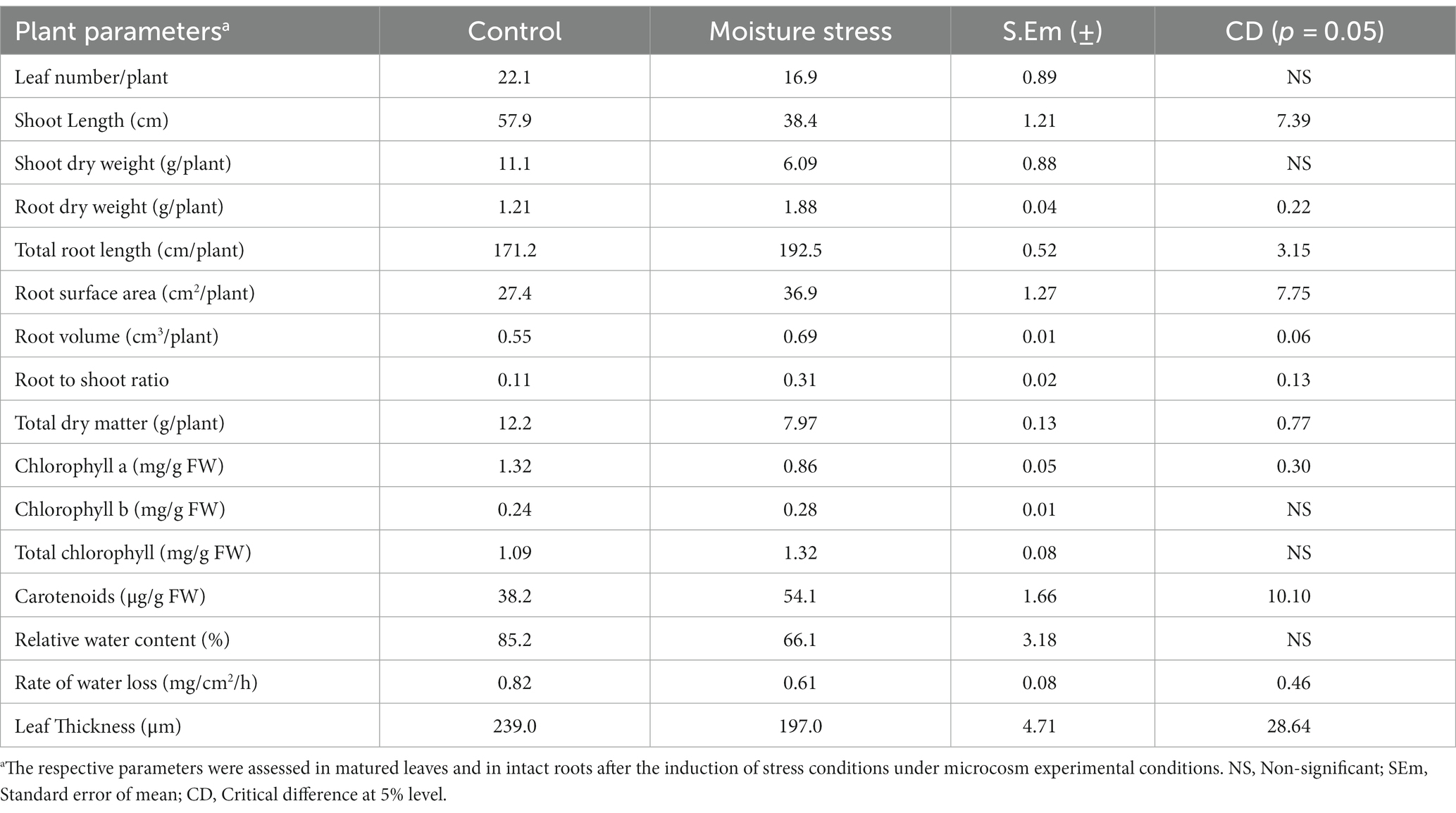
Table 4. Morpho-physiological traits of buckwheat under moisture stress and control conditions of microcosm experiment.
Under moisture stress conditions, the stomatal length was increased while the width was reduced drastically in both of the surfaces of the leaves (Supplementary Figure S3) whereas the stomatal frequency in the abaxial and adaxial surfaces was reduced to the 42.8 and 66.6%, respectively, under stress over control conditions (Figure 4). Apart from the significant differences in stomatal size the differences in, the stomatal frequency and stomatal index were noticed in both the surfaces under stress and non-stress environments. The stomatal index was reduced significantly both in the abaxial and adaxial surfaces to the extent of 72.6 and 55.1%, respectively, under moisture stress conditions compared to control. Between the two leaf surfaces of buckwheat, the abaxial surface has more stomata than the adaxial surface (Figure 4). The high-resolution SEM images showed that under moisture stress the stomatal size significantly decreased or closed partially in abaxial surface whereas under controlled conditions stomatal aperture remained open to a greater extent (Figure 5). Moreover, both layers of buckwheat leaves showed thick deposition of the amorphous cuticle layer without any crystal particulate structure and without any pores for the loss of water droplets (Figure 5). In addition, tiny micro-foldings were observed on the surface of the cuticular layer in both leaf surfaces of buckwheat. But these micro-foldings are loosely arranged and glossy in appearance under control conditions, exposing more of the cuticular surface area to water loss whereas the same layer appeared tight, coarse, non glossy, and dry under moisture stress conditions (Figure 5).
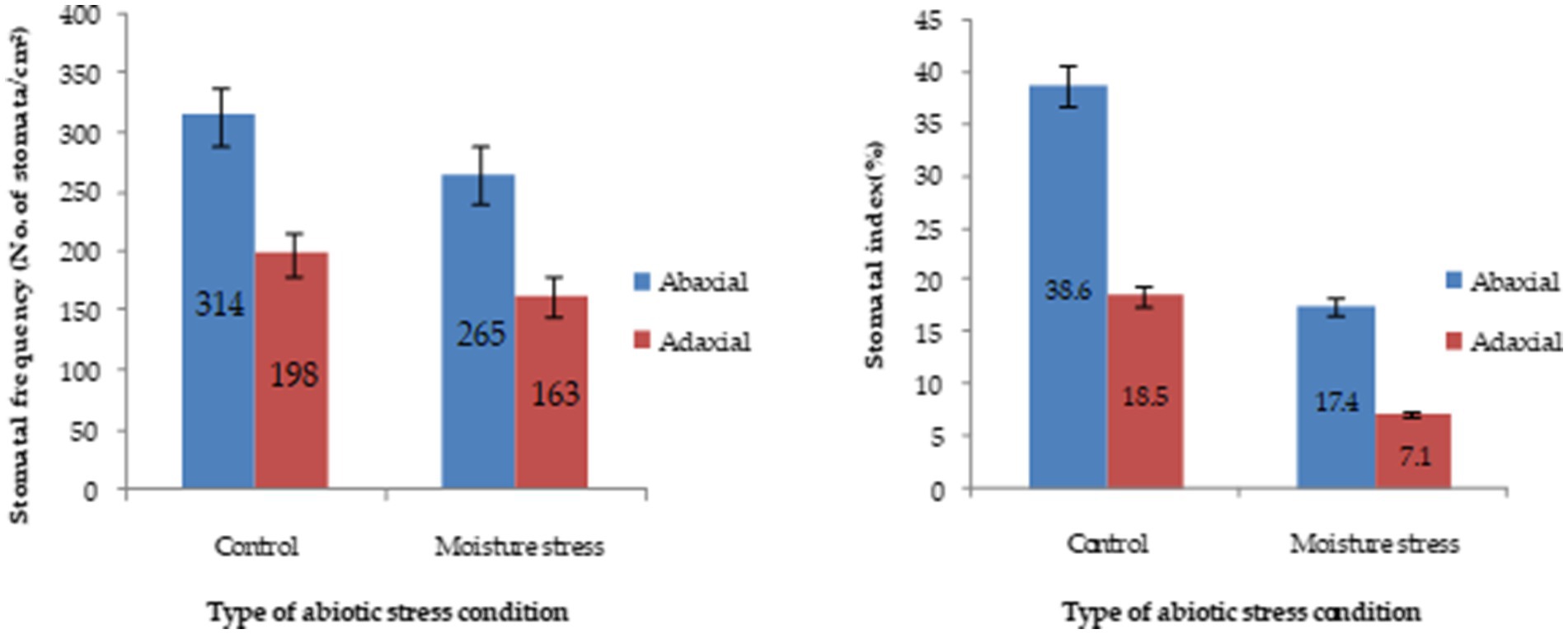
Figure 4. Stomatal characteristics of buckwheat grows under moisture stress and control conditions. Stomatal and epidermal cell counting was done from three microscopic fields after taking impression of the leaves. Stomatal frequency and stomatal index were calculated and averaged from three fields.
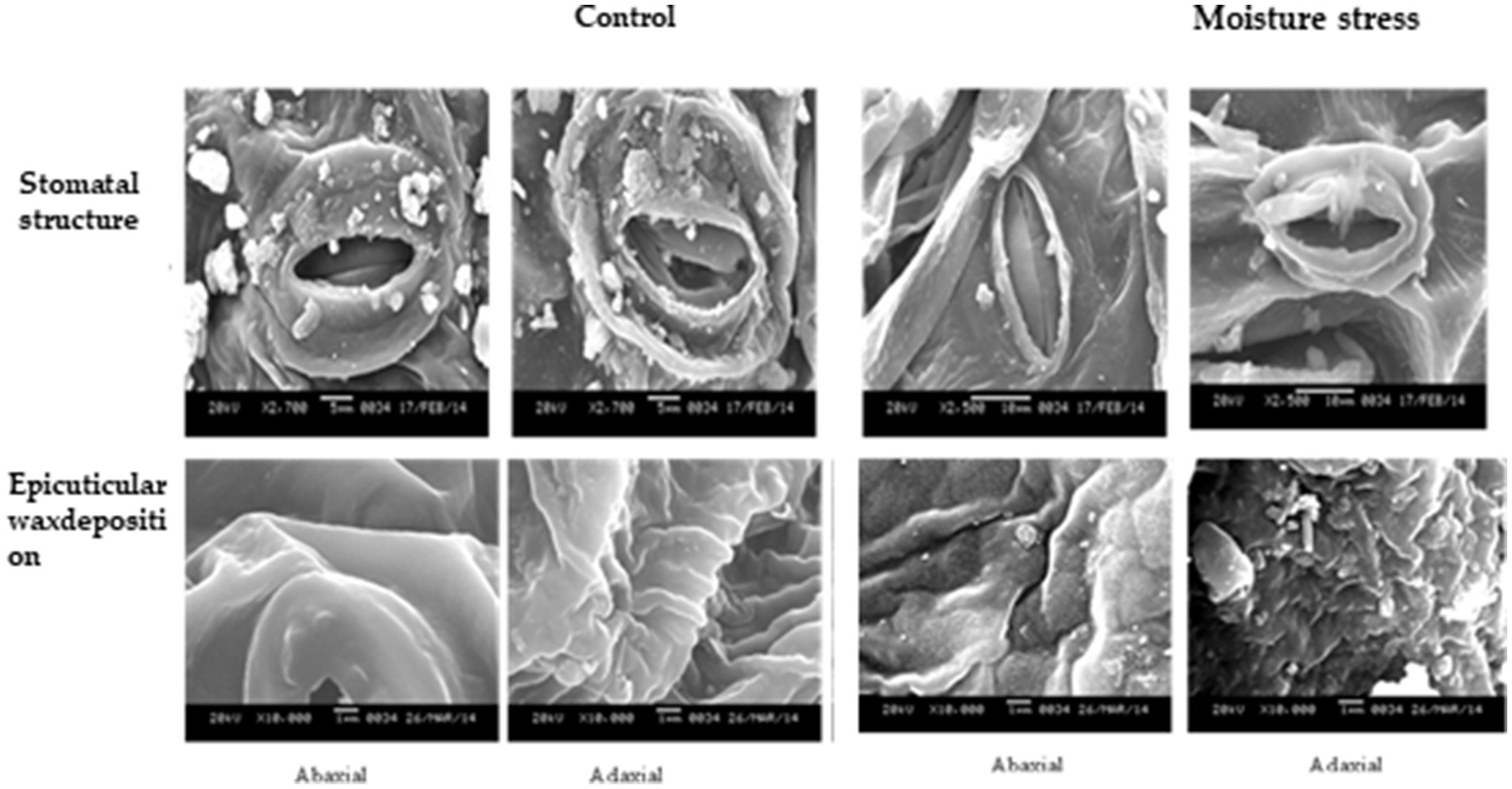
Figure 5. SEM micrographs of ahasial and adasial leaf surface of buckwheat grow under moisture stress and contral conditions. Fully and leaves were collected at the end of moisture stress and shade dried for 5–6 he after which leaves were incubated between two sheets of pleated paper for sing mostan After complete drying the leaves taken for processing and viewing under SEM.
Energy-dispersive X-ray microanalysis (EDX) is a suitable technique for analyzing elements at the microscopic level simultaneously equipped with SEM harboring an energy-dispersive system for quantitative electron probe X-ray microanalysis; this indicated that under control, more dense elemental concentration and accumulation were noticed owing to less hindrance to nutrient uptake whereas the extent of element deposition is reduced under moisture stress (Supplementary Figure S4). EDX data were recorded as a graphical representation and comparison of elemental contents as a percentage of relative weight values from both surface of leaves reflect relative abundance of Ca, K, Mg, and Cl under sufficient moisture whereas minimal quantities of K, Mg, N, Mn, and Cl were recorded under moisture stress conditions (Supplementary Figure S4). Significant pollen morphological differences were observed in both the images captured with two orientations between the whole pollen grains studied from two moisture stress conditions (Figure 6).

Figure 6. SEM images of pollen grains of Buckwheat grown under control and moisture stress conditions. Pollen images captured at two orientations at the same resolution for assessing the extent of structural variation as affected by moisture stress. Pollen grains were carefully collected from anthers of slightly opened flowers.
The pollen wall and surface features with reticulate sculpturing were observed to be almost similar under moisture stress and control without much change. Each pollen grain contained a series of tiny holes in line, each capable of acting as a potential germinating pore. When a pollen grain lands on the buckwheat stigma, a pollen tube germinates from one of these pores and grows through the style to the ovary. Similarly, when cultured in vitro, each pollen grain germinates from the tiny pore present per grain (Supplementary Figure S2). Our results reveal that pollen size is reduced significantly under moisture stress (602*732 μM) compared to the control (644*795 μM) whereas pollen germination was observed more under control than moisture stress (Supplementary Figure S5).
4. Discussion
4.1. Versatile growth habit and short crop duration potentially augments year-round cultivation of buckwheat
Buckwheat production is influenced by various biotic and abiotic stress factors under the fragile hilly ecosystems of the Eastern Himalayas. In our study, higher grain production of the crop was achieved when the crop was sown during the months of September to December. This may be attributed to increased biomass production, a favorable temperate climate, and relatively shorter days during the rabi season (winter months during the post-monsoon period) at mid to high altitudes and also due to higher partitioning efficiency of the crop, even at a soil moisture status of 14.5 to 15.1%, which is common phenomena during winter in the region (Figure 2). During the kharif season, higher rainfall and increased temperature followed by oversaturation of soil resulted in lower grain yield as compared to buckwheat sown in the winter months. This might be due to the absence of a favorable climate like a cooler temperature and low-humidity environment. Relatively higher rainfall in 2015 compared to 2014 was helpful in supplying more soil moisture to buckwheat in the months of November to March as there was very little or no rain in this period (Figure 2).
The crop duration of buckwheat and plant height at harvest are not significantly influenced by years of experimentation and varied with sowing treatments, indicating the sensitivity of crop growth to climatic factors like rainfall, temperature, and humidity. Changes in the crop maturity during various months of sowing reflect its specificity in day length requirement and cumulative days. Highest grain yield was recorded for crops sown in October (9.83 q ha−1) followed by sowing in November (9.45 q ha−1) and December (9.09 q ha−1), indicating the feasibility of buckwheat cultivation during rain-free months of the Eastern Himalayas. The lowest yield was recorded for sowing in the months of May (1.23 q ha−1) and April (1.32 q ha−1) because of the high temperature and high soil moisture. As buckwheat needs cold and dry weather during maturity, sowing in October to December results in high yield as harvesting coincides with dry weather (Krishnappa et al., 2019; Hajong et al., 2022). The HI varied significantly across sowing months, the highest HI being recorded in the month of November (33.99%) followed by December (33.51%) and October (32.62%). This might be due to higher biomass partitioning and other ecological factors that are influenced by low moisture and lower humidity in the environment (Hajong et al., 2022). Although the buckwheat can grow well during months of the rainy season and can produce good vegetative growth, biomass partitioning was affected drastically during rainy days owing to an unfavorable climate, especially high temperatures. Moreover, grain maturity coincides with rain and the yield decreases due to lower grain size and quality deterioration, which is clearly reflected with substantial reduction in harvest index. Considering its abilities of higher biomass production and quicker growth, the crop could be the right choice for reducing soil erosion and also offering a better source of fodder for livestock than other similar crops like Barley and Oats (Krishnappa et al., 2017; Hajong et al., 2022). Moreover, incorporation of green biomass into soil is also more likely to improve soil health in the degraded lands of the Eastern Himalayas (Das et al., 2017). Day length plays a pivotal role in photo-periodism and significantly influenced main stem growth, flowering behavior, and seed-set in buckwheat like in other cereals like wheat and barley, which have been shown to be mostly influenced by the sowing date by earlier researchers (Michiyama et al., 2004). Pfeiffer (1993) also suggested that under moisture stress and problematic soil conditions, the moisture-stress-tolerant crop triticale was assessed for its distinct yield, growth superiority, and adaptive advantage over other moisture stress susceptible crops like wheat. Fayaz and Arzani (2011) showed the better performance of the triticale crop over wheat in terms of improved grain yield potential under moisture stress conditions. Significant genotypic variability and physio-morphological efficiency influence moisture stress tolerance of buckwheat under hill environments (Hajong et al., 2019, 2022). Moreover, buckwheat is reported to be rich in rutin (quercetin-3-rutinosid) and contain lower fat and higher mineral nutrients and other polyphenols with significant utility in the pharmaceutical industry and nutritional security (Skerritt, 1986; Luther, 1992; Seitinthang, 2014). Moreover, it has a very low content of prolamins, which makes it a valuable source of dietary protein for gluten-sensitive individuals; additionally, buckwheat is rich in nutritional factors and antioxidants (Shiratori and Nagata, 1986; Skerritt, 1986; Bonafaccia et al., 2003; Seitinthang, 2014). Higher income from buckwheat cultivation can make the crop more remunerative and make it suitable for preventing soil erosion across hill slopes, restoration of soil fertility, as well as a better alternative source of green fodder under the stressful environments of the hill ecosystem. Appropriate technology evolution with necessary policy interventions for adequate value addition and marketing might help in popularizing this crop in the conditions of the Eastern Himalayas. Buckwheat could be a robust non-cereal crop because it is more productive than other cereals with better nutrient content in the grain and green leaves under abiotic stress conditions of marginal hill environments (Shiratori and Nagata, 1986; Oettler, 2005).
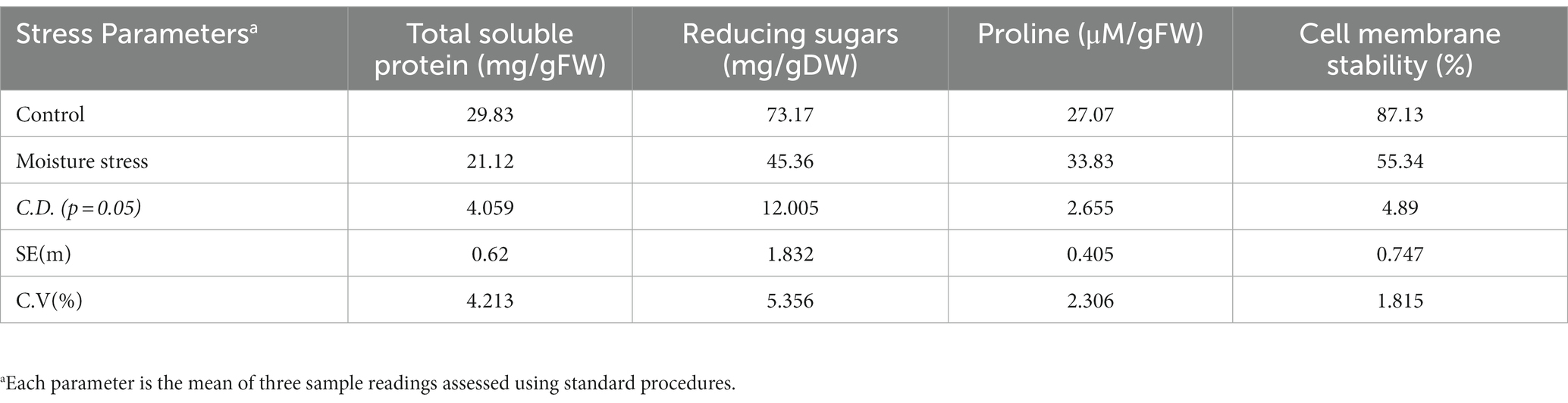
Table 5. Physio-biochemical parameters of buckwheat leaves as influenced by moisture stress conditions.
4.2. Altered root morphology and leaf pigmentation incrementally favored moisture stress tolerance in buckwheat
With the field evaluation, moisture stress response and innate crop resilience studies were thought to be necessary for a unique and lesser known crop like buckwheat. In this connection, stress response studies to unravel apparent physio-morphological and biochemical traits or mechanisms of buckwheat from the perspective of erratic rainfall under current changing climate in the Eastern Himalayas were undertaken. Possible changes in root shoot growth alterations, stomatal characteristics, and leaf surface features would be remarkably varied under moisture stress levels of 10–15%. Since the root system is slowly explored as a key source for moisture stress tolerance and adaptation with an array of optimum root traits or phenes, the plant’s ability to alter its root system would increase the stress tolerance substantially. Improved root parameters might act as pivotal moisture stress protective mechanisms, enabling the plant to explore increased quantities of water and essential nutrients from a deeper layer of the soil, exposing the adequate surface area of the root system (Bañoc et al., 2000). Buckwheat has the unique advantage of modifying roots in accordance with prevailing soil water status under soil moisture constraints, as was evident in our study, indicating the versatility of crop adaptability in terms of altering root plasticity. This buckwheat crop seems to enhance the root growth under moisture stress environments, but equally it sacrifices the shoot growth via a large reduction in stomata density. It was interesting to witness the retention of higher leaf water balance for increased metabolic activity by buckwheat, which is only possible through regulation of water absorption through roots and water loss through transpiration. Similar studies assessing the impact of root growth phenotyping on stress tolerance have been conducted for many crop plants (Abenavoli et al., 2016; Hazarika et al., 2020; Das et al., 2021; Hajong et al., 2022; Layek et al., 2023), including shoot growth alterations were also considered as robust for moisture stress response studies for better water and nutrient uptake and adequate plant growth and development (Schroeder et al., 2001; Seki et al., 2007).
Optimum quantities of leaf pigments are needed for sufficient harvesting of light and conversion it into chemical energy. Buckwheat accumulated higher leaf chlorophyll pigments (chlorophyll b) and carotenoids under moisture stress conditions compared to the control (Table 4). This differential and incremental increase in leaf pigments, especially protective pigments with antioxidant capacities like chlorophyll b and carotenoids, are found to be a suitable rescue system for the crop to thrive and impart stress adaptability under moisture stress conditions for balanced light absorption and reduced photo-inhibition (Ashraf and Mehmood, 2000; Tikkanen et al., 2012; Krishnappa et al., 2017). The chlorophyll a to b ratio was decreased under moisture stress (3.07) compared to under control conditions (5.5), substantiating the higher accumulation of secondary pigment chlorophyll b by buckwheat. Increased chlorophyll b and carotenoid content by buckwheat could be useful in two major photo-protection systems: i) as an antioxidant that can scavenge free radicals generated by excess solar radiation energy and ii) through NPQ enhancement to emit excess solar radiation energy. Regulation of photosynthetic apparatus by inducing necessary changes in leaf pigments under fluctuating light conditions under hilly locations of the Eastern Himalayas is important for onset of an appropriate physiological mechanism and adaptation in stress resilient crops like buckwheat (Seki et al., 2007). This unique ability would be beneficial for maintaining higher leaf photosynthesis and metabolic activity under stress conditions (Hajong et al., 2022). Moreover, rutin, quercetin, quercitrin, and other flavonoids synthesized in the leaves of higher plants could be at greater advantage for protecting the plants against UV radiation, diseases, and predators. In addition, retention of moderate leaf thickness in buckwheat compared to other rabi crops under moisture stress conditions is very beneficial and signifies a potential edge in engaging more layers of mesophyll cells for higher photosynthetic activity and higher sugar synthesis, which are required for sustaining biomass and yield under low moisture conditions of hill slopes (Vile et al., 2005; Hazarika et al., 2020). Apart from pivotal root architectural response, buckwheat is capable of synthesizing and accumulating higher quantities of compatible solutes like proline under moisture stress with significant reduction in soluble leaf protein, cell membrane stability, and reducing sugar of the leaves (Irigoyen and Einerich, 1992; Arteaga et al., 2020). A major part of the soluble protein of leaves is the Rubisco enzyme (30–50%); more reduction in the soluble protein under moisture stress directly impact photosynthetic activity of leaves. Water deficits generally reduce protein synthesis and accelerate their degradation, causing reduced photosynthetic activity in the plant (Irigoyen and Einerich, 1992). Overall photosynthesis of the plant was significantly reduced under a water deficit, decreasing sugar accumulation in the leaves. On the other hand, few studies have shown drought to increase the accumulation of soluble sugars (Irigoyen and Einerich, 1992; Du et al., 2020). Buckwheat is a unique hill slope crop with fast growing ability and possibly decreased reducing sugar owing to a significant reduction in leaf pigments, leaf area, and total soluble protein (Irigoyen and Einerich, 1992). Rajbhandari (2004) showed the positive and significant relationship between LAI and PAR interception in buckwheat genotypes, which are closely linked to kernel yields, locations of experimentation, and crop season. However, the ROWL of buckwheat leaves was recorded to be lower even after 4 h compared to other rabi crops like lentil or pea, adequately proving the existence of robust stomatal and non-stomatal regulation of water loss from the leaves. This leverage of reduced water loss from leaves might have enabled moisture-stress-affected crops to retain more water for basic metabolic activity under moisture constraints (Table 4). Retention of higher relative water content (RWC) and minimized rate of water loss (ROWL) in leaves form important part of moisture stress tolerance in buckwheat like other moisture-stress-tolerant crops (Soltys-Kalina et al., 2016; Czyczyło-Mysza et al., 2018).
4.3. Stomatal attributes and a robust pollen structure substantially confer moisture stress tolerance in buckwheat
Stomatal opening or closing is generally a major means of controlling water loss from plants while allowing for photosynthesis. Gas exchange is regulated by controlling the stomatal aperture and density on the epidermis (Nawrath, 2006; Fayaz and Arzani, 2011). Stomatal size and stomatal density are considerably influenced by plant species and abiotic environmental perturbations such as changes in atmospheric CO2 concentration, light intensity, temperature, soil water, and nutritional status (Ishimaru et al., 2001; Soltys-Kalina et al., 2016). This mechanism of reducing the lower number of stomata per unit leaf area and per unit number of epidermal cells under stress conditions by buckwheat might have helped the plant retain basic quantities of water for higher survivability and sustaining minimal metabolic activity under stress conditions (Joshi and Paroda, 1991; Jones, 1998; Xu and Zhou, 2008; Sirichandra et al., 2009). Besides stomatal control, increased cuticle synthesis and accumulation as a major means of non-stomatal regulation or barrier for free water loss through plant leaves is a prime defense mechanism that can prevent water loss under moisture stress conditions (Jones, 1998; Schroeder et al., 2001; Jetter et al., 2006). Cuticular wax is typically a complex mixture of dozens of hydrocarbon compounds (Jetter et al., 2006); they make up the primary structure of the cuticle and play an important role in limiting non-stomatal water loss under stress conditions (Riederer and Schreiber, 2001). Therefore, it was interesting to study the presence of leaf barriers for transpirational water loss in turgid buckwheat leaf. A non-porous waxy layer with micro-foldings acted as a barrier to water loss, which is very much beneficial to the plant to combat water limited conditions. Under moisture stress, the folding of cuticular wax was reduced compared to in the control to form a non-porous and tight waxy barrier. It saves the plant from possible wilting under water stress conditions more so than that of the crystallized cuticle layer predominantly found in moisture stress susceptible crops (Zhang et al., 2007). The results are in accordance with the findings of Christa and Soral-Smietana (2008), indicating the sensitivity of stomatal and non-stomatal mechanisms induced under moisture stress and providing the adequate basis for retention of higher water content and a lower rate of water loss even under moisture stress conditions (Krishnappa et al., 2019). Interestingly, EDX data recorded in the current study indicated higher accumulation of essential elements under control than moisture stress conditions. This might be because buckwheat is potentially a moisture-stress-tolerant crop that maintains robust root growth plasticity that could possibly come to the rescue of the plant to take up minimal quantities of essential elements (McCully et al., 2010; Krishnappa et al., 2019).
Pollen morphology studies under stress conditions indicate that buckwheat is a stress-tolerant crop able to maintain an optimum pollen size with an appropriate wall structure with tiny pores. The extent of pollen germination and tube growth in the study even under moisture stress conditions shows the adequate versatility of the buckwheat crop to maintain its optimum intact pollen structure and pollen viability (Figure 6 and Supplementary Figure S5). But, even under moisture stress conditions, buckwheat could maintain an intact pollen size and viability for proper pollen germination and subsequent seed set. Buckwheat’s resilience, by allowing a minimal effect of moisture stress on reproductive growth under stress conditions of the Himalayan hill slopes, is very useful for the crop’s ecological fitness (Hajong et al., 2019; Krishnappa et al., 2019). Similar studies of pollen morphology have been conducted to analyze the crop performance and productivity under moisture stress and heat stress conditions (Sakhi et al., 2014; Jiang et al., 2015).
5. Conclusion
This study implies that the buckwheat is suitable for cultivation for higher grain productivity during the winter months (mid-September to October), but it was also found to be better than other crops for producing higher green biomass during all through the year. Root morphological traits, photo-protective pigments, and leaf proline were substantially enhanced and emanated as efficient stress rescue mechanisms in buckwheat to overcome inimical moisture stress conditions of hill slopes of Eastern Himalaya under a changing climate. In view of its increased resilience and sustained productivity under stressful environments within marginal hill environments, it was found to be remunerative and a potential stress-tolerant crop for increased cropping intensity and food security for prevailing low-input agriculture in the region. Further, physio-biochemical stress-adaptive mechanisms for improved nutrient and water use efficiency traits with stress-induced cellular and metabolic adjustments and a hormone-mediated signaling network that regulates a multitude of stress-responsive metabolic processes need to be studied.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
KR and DR conceived and designed the experiments, investigated, and wrote the article. DR, BK, and VM performed the funding acquisition, project administration, supervision, editing, and reviewing. AD, JL, and NR wrote the article and performed data curation, formal analysis, and editing. US, AS, and PM performed the investigation, methodology, validation, and formal analysis. KM, PM, and ND contributed resources, reagents, materials, methodology, and conceptualization. All authors contributed to the article and approved the submitted version.
Acknowledgments
Authors thankfully acknowledge the active support and guidance provided by Director, ICAR-Research complex for North Eastern Hill Region, Umiam, Meghalaya and the All India Coordinated Research Project (AICRP) in Water management section of ICAR Research complex for North Eastern Hill region, Umiam, Meghalaya. The authors thankfully regard the technical support and supervision rendered by the honorable Director, ICAR Research Complex for North Eastern Hill region, Umiam, Meghalaya for the smooth conduct of present study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1190807/full#supplementary-material
References
Abenavoli, M. R., Leone, M., Sunseri, F., Bacchi, M., and Sorgona, A. (2016). Root phenotyping for drought tolerance in bean landraces from Calabria (Italy). J. Agron. Crop Sci. 202, 1–12. doi: 10.1111/jac.12124
Adams, R. J., and Morton, J. K. (1972). An improved technique for examining pollen under the scanning electron microscope. Pollen Spores 14, 203–212. Available at: https://digitalcommons.usu.edu/bee_lab_a/29
Appleman, C. O., Loomis, W. E., Phillips, T. G., Tottingham, W. E., and Willaman, J. J. (1927). The determination of soluble carbohydrates. Plant Phy. 2, 195–204. doi: 10.1104/pp.2.2.195
Arsenault, J. L., Pouleur, S., Messier, C., and Guay, R. (1995). WinRHIZO, a root-measuring system with a unique overlap correction method. Hort. Sci. 30, 906D–9906D. doi: 10.21273/HORTSCI.30.4.906D
Arteaga, S., Yabor, L., Díez, M. J., Prohens, J., Boscaiu, M., and Vicente, O. (2020). The use of proline in screening for tolerance to drought and salinity in common bean (Phaseolus vulgaris L.) genotypes. Agronomy 10:817. doi: 10.3390/agronomy10060817
Ashraf, M., and Mehmood, S. (2000). Response of four brassica species to drought stress. Environ. Exp. Bot. 30, 93–100. doi: 10.1016/0098-8472(90)90013-T
Atkinson, N. J., and Urwin, P. E. (2016). The interaction of plant biotic and abiotic stresses: from genes to the field. J. Exp. Bot. 63, 3523–3544. doi: 10.1093/jxb/ers100
Bañoc, D. M., Yamauchi, A., Kamoshita, A., Wade, L. J., and Pardales, J. R. (2000). Dry matter production and root system development of rice cultivars under fluctuating soil moisture. Plant Prod. Sci. 3, 197–207. doi: 10.1626/pps.3.197
Bates, L. S., Waldren, R. P., and Teare, I. D. (1973). Rapid determination of free proline for water stress studies. Plant Soil 39, 205–207. doi: 10.1007/BF00018060
Bohm, W. (1979). Methods of studying root systems. Ecol stud., 33, Springer-Verlag, Berlin, pp. 151.
Bonafaccia, G., Gambelli, L., Fabjan, N., and Kreft, I. (2003). Trace elements in flour and bran from common and tartary buckwheat. Food Chem. 83, 1–5. doi: 10.1016/S0308-8146(03)00228-0
Christa, K., and Soral-Smietana, M. (2008). Buckwheat grains and buckwheat products-nutritional and prophylactic value of their compoenents-a review. Czech J.Food Sci. 26, 153–162. doi: 10.17221/1602-CJFS
Czyczyło-Mysza, I. M., Marcińska, I., Skrzypek, E., Bocianowski, J., Dziurka, K., Rančić, D., et al. (2018). Genetic analysis of water loss of excised leaves associated with drought tolerance in wheat. Peer J 6:e5063. doi: 10.7717/peerj.5063
Das, A., Ghosh, P. K., Lal, R., Saha, R., and Ngachan, S. V. (2017). Soil quality effect of conservation practices in maize–rapeseed cropping system in eastern Himalaya. Land Degrad. Dev. 28, 1862–1874. doi: 10.1002/ldr.2325
Das, A., Layek, J., Idapuganti, R. G., Basavaraj, S., Lal, R., Rangappa, K., et al. (2020). Conservation tillage and residue management improves soil properties under upland rice–rapeseed system in the subtropical eastern Himalayas. Land Degrad. Dev. 31, 1775–1791. doi: 10.1002/ldr.3568
Das, A., Layek, J., Ramkrushna, G. I., Rangappa, K., Lal, R., Ghosh, P. K., et al. (2019). Effects of tillage and rice residue management practices on lentil root archiecture, productivity and soil properties in India’s lower Himalayas. Soil Tillage Res. 194:104313. Available at: https://www.sciencedirect.com/science
Das, A., Rangappa, K., Basavaraj, S., Dey, U., Haloi, M., Layek, J., et al. (2021). Conservation tillage and nutrient management practices in summer rice (Oryza sativa L.) favoured root growth and phenotypic plasticity of succeeding winter pea (PisumsativumL.) under eastern Himalayas, India. Heliyon 7:e07078. doi: 10.1016/j.heliyon.2021.e07078
Doheny-Adams, T., Hunt, L., Franks, P. J., Beerling, D. J., and Gray, J. E. (2012). Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth CO2 gradient. Philos. Trans. R Soc. B Biol. Sci. 367, 547–555. doi: 10.1098/rstb.2011.0272
Du, Y., Zhao, Q., Chen, L., Yao, X., Zhang, W., Zhang, B., et al. (2020). Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 146, 1–12. doi: 10.1016/j.plaphy.2019.11.003
Fayaz, N., and Arzani, A. (2011). Moisture stress tolerance in reproductive growth stages in triticale (X Triticosecale Wittmack) cultivars under field conditions. Crop Breeding J. 1, 1–12.
Franks, P. J. W., Doheny-Adams, T., Britton-Harper, Z. J., and Gray, J. E. (2015). Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 207, 188–195. doi: 10.1111/nph.13347
Germ, M. (2004). Environmental factors stimulate synthesis of protective substances in buckwheat. Proceedings of the 9th international symposium on buckwheat, Prague. 55–59.
Gomez, K. A., and Gomez, A. A. (1984). Statistical procedure for agricultural research. International Rice research institute, 2nd John Wiley and Sons, New York, Singapore
Gusmao, M., Siddique, K. H. M., Flower, K., Nesbitt, H., and Veneklaas, E. J. (2012). Water deficit during the reproductive period of grass pea (Lathyrussativus L.) reduced grain yield but maintained seed size. J. Agron. Crop Sci. 198, 430–441. doi: 10.1111/j.1439-037X.2012.00513.x
Hajong, S., Rangappa, K., Dasaiah, H. G., Moirangthem, P., Saikia, U. S., and Ahlawat, S. P. (2019). Morpho-physiological efficiency of buckwheat (Fagopyrumsp) for enhanced stress tolerance at mid altitudes of Meghalaya presented and published in the proceedings of 14th international symposium on buckwheat 03–06 sept 2019, Shillong, India.
Hajong, S., Rangappa, K., Dasaiah, H. G., Moirangthem, P., Saikia, U. S., Bhattacharjee, B., et al. (2022). Genotypic variability and physio-morphological efficiency of buckwheat (Fagopyrumspp.) under moisture stress at mid-altitudes of Meghalaya (India). Crop Pasture Sci. 74, 204–218. doi: 10.1071/CP22062
Hazarika, S., Nabam, A., Thakuria, D., Kataki, S., and Krishnappa, R. (2020). Lime equivalence of organic manures and scope of their utilization as acid soil amendments. Arch. Agron. Soil Sci. doi: 10.1080/03650340.2020.1749266
Hetherington, A. M., and Woodward, F. I. (2003). The role of stomata in sensing and driving environmental change. Nature 424, 901–908. doi: 10.1038/nature01843
Irigoyen, D. J. J. W., and Einerich, M. S.-D. (1992). Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa). Physiol. Plant. 84, 55–60. doi: 10.1111/j.1399-3054.1992.tb08764.x
Ishimaru, K., Shirota, K., Higa, M., and Kawamitsu, Y. (2001). Identification of quantitative trait loci for adaxial and abaxial stomatal frequencies in Oryza sativa. Plant PhysiolBiochem. 39, 173–177. doi: 10.1007/s10681-004-2559-7
Jetter, R., Kunst, L., and Samuels, A. L. (2006) in Composition of plant cuticular waxes. In biology of the plant cuticle, annual plant reviews. eds. M. Riederer and C. Müller, vol. 23 (Oxford, UK: Blackwell), 145–181.
Jiang, Y., Lahlali, R., Karunakaran, C., Kumar, S., Davis, A. R., and Bueckert, R. A. (2015). Seed set, pollen morphology and pollen surface composition response to heat stress in field pea. Plant Cell Environ. 38, 2387–2397. doi: 10.1111/pce.12589
Jones, H. G. (1998). Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 49, 387–398. doi: 10.1093/jxb/49.Special_Issue.387
Joshi, B. D., and Paroda, R. S. (1991). Buckwheat in India. NBPGR, Shimla Sci. Monogr. No. 2, 1–117.
Kjeldahl, J. (1883). NeueMethodezurBestimmung des Stickstoffs in organischenKörpern. Fresen. J. Anal. Chem. 22, 366–382. doi: 10.1007/BF01338151
Kosma, D. K., Bourdenx, B., Bernard, A., Parsons, E. P., Lu, S., Joube’s, J., et al. (2009). The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 151, 1918–1929. doi: 10.1104/pp.109.141911
Krishnappa, R., Dipjyoti, R., Meghna, H., Anjan, K. S., Uday, S. S., Kaberi, M., et al. (2017). Physiological efficiency and drought tolerance ability of buckwheat (Fagopyrumesculentum L.) under hill slopes of north eastern Himalayan region. Proceedings of inter drought-V, Hyderabad, 21–25 February, 2017.
Krishnappa, R., Kumar, A., Choudhury, B. U., Moirangthem, P., Layek, J., Rajkhowa, D., et al. (2023) Buckwheat- potential stress tolerant crop for mid hills of Himalaya under changing climate. In Pseudocereals - recent advances and new perspectives, Intech Open, London
Krishnappa, R., Rajkhowa, D., Saikia, U. S., Moirangthem, P., Sarma, A. K., Deshmukh, N. A., et al. (2019). Physiological responses of buckwheat (Fagopyrum esculentum L.) for stressful environments under fragile hill ecosystems of eastern Himalaya. Presented and published in the proceedings of 14th international symposium on buckwheat 03–06 sept 2019, Shillong, India
Lahlali, R., Jiang, Y., Kumar, S., Karunakaran, C., Liu, X., Borondics, F., et al. (2014). ATR-FTIR spectroscopy reveals involvement of lipids and proteins of intact pea pollen grains to heat stress tolerance. Front. Plant Sci. 5:747. doi: 10.3389/fpls.2014.00747
Layek, J., Das, A., Ghosh, P. K., Rangappa, K., Lal, R., Idapuganti, R. G., et al. (2022). Double no-till and rice straw retention in terraced sloping lands improves water content, soil health and productivity of lentil in Himalayan foothills. Soil Till Res. 221:105381. doi: 10.1016/j.still.2022.105381
Layek, J., Das, A., Ramkrushna, G. I., Krishnappa, R., Ghosh, P. K., Lal, R., et al. (2021). Managing rice fallow lands of the eastern Indian Himalayas: impacts of residue management and varietal interventions on soil properties, carbon stocks, and productivity. Land Degrad. Dev. 32, 4871–4888. doi: 10.1002/ldr.4067
Layek, J., Rangappa, K., Das, A., Ansari, M. A., Choudhary, S., Rajbonshi, N., et al. (2023). Evaluation of millets for physio-chemical and root morphological traits suitable for resilient farming and nutritional security in eastern Himalayas. Front. Nutr. 10:1198023. doi: 10.3389/fnut.2023.1198023
Luther, Z. (1992). Polyphenol classification and tannin content of buckwheat seeds (Fagopyrum esculentummoench). Fagopyrum 12, 36–42.
Lynch, J. P., and Brown, K. (2012). New roots for agriculture-exploiting the root phenome. Phil. Trans. R. Soc. 367, 1598–1604. doi: 10.1098/rstb.2011.0243
Manoj, K. (2011). North eastern region: soil and water management imperatives for food security in a changing climate. Curr. Sci., 101–1119.
Manoj, K., Patra, A. K., and Swarup, A. (2011). Impact of climate change on fertilizer demand in agriculture: concerns and imperatives for food security in India. Indian J. Fert. 7, 48–62.
McCully, M. E., Canny, M. J., Huang, C. X., Miller, C., and Brink, F. (2010). Cryo-scanning electron microscopy (CSEM) in the advancement of functional plant biology: energy dispersive X-ray microanalysis (CEDX) applications. Funct. Plant Biol. 37, 1011–1040. doi: 10.1071/FP08304
Michiyama, H., and Hayashi, H. (1998). Differences of growth and development between summer and autumn-type cultivars in common buckwheat (Fagopyrum esculentum Moench). Jpn. J. Crop Sci. 67, 323–330. doi: 10.1626/jcs.67.323
Michiyama, H., Tsuchimoto, K., Tani, K. I., Hirano, T., Hayasashi, H., and Campbell, C. (2004). Influence of day length on the growth of stem, flowering, the morphology of Flower clusters, and seed-set in buckwheat (FagopyrumesculentumMoench) proceedings of the 9th international symposium on buckwheat, Prague, 35–40.
Misyura, M., Colasanti, J., and Rothstein, S. J. (2012). Physiological and genetic analysis of Arabidopsis thaliana anthocyanin biosynthesis mutants under chronic adverse environmental conditions. J. Exp. Bot. 2012, 695–709. doi: 10.1093/jxb/ers328
Nawrath, C. (2006). Unraveling the complex network of cuticular structure and function. CurrOpin Plant Biol. 9, 281–287. doi: 10.1016/j.pbi.2006.03.001
Oettler, G. (2005). Centenary review. The fortune of a botanical curiosity-triticale: past, Present and future. J. Agric. Sci. 143, 329–346. doi: 10.1017/S0021859605005290
Pfeiffer, W. H. (1993).Triticale improvement strategies at CIMMYT: Exploiting adaptive patterns and end-use orientation. Triticale Topics, 11,:18-27
Rajbhandari, B. P. (2004).Eco-physiological aspects of common buckwheat. Proceedings of the 9th international symposium on buckwheat, Prague
Ravindranath, N. H., Rao, S., Sharma, N., Nair, M., Gopalakrishnan, R., Rao, A. S., et al. (2011). Climate change vulnerability profiles for North East India. Curr. Sci. 2011, 384–394.
Riederer, M., and Schreiber, L. (2001). Protecting against water loss: analysis of the barrier properties of plant cuticles. J. Exp. Bot. 52, 2023–2032. doi: 10.1093/jexbot/52.363.2023
Saikia, U. S., Goswami, B., Rajkhowa, D. J., Venkatesh, A., Ramachandran, K., Rao, V. U. M., et al. (2013). Shift in monsoon rainfall pattern in the north eastern region of India post 1991. J. Agromet. 15, 162–164. doi: 10.54386/jam.v15i2.1467
Sakhi, S., Okuno, K., Shahzad, A., and Jamil, M. (2014). Evaluation of sorghum (Sorghum bicolor) Core collection for drought tolerance: pollen fertility and mean performance of yield traits and its components at reproductive stage. Int. J. Agric. Biol. 16, 251–260.
Salem, M. A., Kakani, V. G., Koti, S., and Reddy, K. R. (2007). Pollen-based screening of soybean genotypes for high temperatures. Crop Sci. 47, 219–231. doi: 10.2135/cropsci2006.07.0443
Saragih, A. A., Puteh, A. B., Ismail, M. R., and Mondal, M. (2013). Pollen quality traits of cultivated (Oryza sativa L. Ssp. Indica) and weedy (Oryzasativa’var. Nivara) rice to water stress at reproductive stage. Aust. J. Crop. Sci. 8, 1106–1112.
Schroeder, J. I., Kwak, J. M., and Allen, G. J. (2001). Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410, 327–330. doi: 10.1038/35066500
Seitinthang, L. H. (2014). Cropping pattern of north East India: an appraisal. American Res. Thoughts. 1, 488–498.
Seki, M., Umezawa, T., Urano, K., and Shinozaki, K. (2007). Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 10, 296–302. doi: 10.1016/j.pbi.2007.04.014
Sirichandra, C., Wasilewska, A., Vlad, F., Valon, C., and Leung, J. (2009). The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action. J. Exp. Bot. 60, 1439–1463. doi: 10.1093/jxb/ern340
Skerritt, J. H. (1986). Molecular comparison of alcohol-soluble wheat and buckwheat proteins. Cereal Chem. 63, 365–369.
Soltys-Kalina, D., Plich, J., Strzelczyk-Żyta, D., Śliwka, J., and Marczewski, W. (2016). The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breeding sci. 66, 328–331. doi: 10.1270/jsbbs.66
Sullivan, C. Y., and Ross, M. W. (1979). “Selecting for drought and heat resistance in grain sorghum” in Stress Phy. In crop plants. eds. H. Mussell and R. C. Staples (New York: John Wiley and Sons), 263–281.
Tikkanen, M., Grieco, M., Nurmi, M., Rantala, M., Suorsa, M., and Aro, E. M. (2012). Regulation of the photosynthetic apparatus under fluctuating growth light Phil. Trans. R. Soc. B: Bio Sci. 367, 3486–3493. doi: 10.1098/rstb.2012.0067
Vile, D., Garnier, E., Shipley, B., Laurent, G., Navas, M. L., Roumet, C., et al. (2005). Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann. Bot. 96, 1129–1136. doi: 10.1093/aob/mci264
Xu, Z., and Zhou, G. (2008). Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 59, 3317–3325. doi: 10.1093/jxb/ern185
Yang, Z. B., Rao, I. M., and Hrost, W. J. (2013). Interaction of aluminium and drought stress on root growth and crop yield on acid soils. Plant Soil 372, 3–25. doi: 10.1007/s11104-012-1580-1
Zhang, J. Y., Broeckling, C. D., Sumner, L. W., and Wang, Z. Y. (2007). Heterologous expression of two Medicago truncatula putative ERF transcription factor genes, WXP1 and WXP2, in Arabidopsis led to increased leaf wax accumulation and improved drought tolerance, but differential response in freezing tolerance. Plant Mol. Biol. 64, 265–278. doi: 10.1007/s11103-007-9150-22
Keywords: buckwheat, epicuticular wax, hill ecosystem, moisture stress, root morphology, stomatal characters, winter season
Citation: Rangappa K, Rajkhowa D, Layek J, Das A, Saikia US, Mahanta K, Sarma AK, Moirangthem P, Mishra VK, Deshmukh NA, Rajbonshi N and Kandpal BK (2023) Year-round growth potential and moisture stress tolerance of buckwheat (Fagopyrum esculentum L.) under fragile hill ecosystems of the Eastern Himalayas (India). Front. Sustain. Food Syst. 7:1190807. doi: 10.3389/fsufs.2023.1190807
Edited by:
Pankaj Kumar Arora, Babasaheb Bhimrao Ambedkar University, IndiaReviewed by:
Ajaz Ahmad Lone, Sher-e-Kashmir University of Agricultural Sciences and Technology, IndiaArtur Pinski, University of Silesia in Katowice, Poland
†Present address:
Anjan Kumar Sarma, Faculty of Science, Assam Down Town University, Guwahati, Assam, IndiaNishant Anandrao Deshmukh, National Research Centre for Grapes, Pune, Maharashtra, India
Copyright © 2023 Rangappa, Rajkhowa, Layek, Das, Saikia, Mahanta, Sarma, Moirangthem, Mishra, Deshmukh, Rajbonshi and Kandpal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krishnappa Rangappa, a3Jpc2hwaHlzaW9sb2d5QGdtYWlsLmNvbQ==; Jayanta Layek, amF5YW50YS5pY2FyQGdtYWlsLmNvbQ==
‡These authors share first authorship
 Krishnappa Rangappa
Krishnappa Rangappa Dipjyoti Rajkhowa
Dipjyoti Rajkhowa Jayanta Layek
Jayanta Layek Anup Das
Anup Das Uday Sankar Saikia1
Uday Sankar Saikia1 Vinay Kumar Mishra
Vinay Kumar Mishra