- 1Food Security and Safety Focus Area, Faculty of Natural and Agricultural Sciences, North-West University, Mmabatho, South Africa
- 2Genetic Resources Center, International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria
Field experiments were conducted in two different agroecological locations of Ibadan and Ikenne in Nigeria from August through December during the 2019 and 2020 cropping seasons. The studies were set up to reduce reliance on inorganic nitrogen fertilizer and to embrace the use of nitrogen-fixing bacteria to improve legume production to increase farmers' output and profitability. Ten accessions of the Bambara groundnut (BGN) were used in the trials. Seeds of each BGN accession were coated with each of the following Bradyrhizobium japonicum strains (B. japonicum): FA3, RACA6, USDA110, and IRJ2180A before planting. Furthermore, Nitrogen (N) fertilizer (20 kg/ha, urea) was applied to seedlings without inoculation, and uninoculated seedlings (without inoculation and without fertilization) served as control. The experiment was, therefore, a factorial arrangement (10 BGN accessions, 4 B. japonicum strains, N fertilizer application, and an uninoculated control). The yield and yield components of the inoculated BGN accessions were significantly enhanced at both agroecological locations and seasons. Among the B. japonicum strains used for inoculation, RACA6 strains significantly enhanced the yield and yield component of TVSu-1698 than other inoculated BGN accessions with a mean value of 6,234 ± 87 kg ha−1 recorded in both locations and seasons, compared to the result obtained in the combination of TVSu-1698 with N fertilizer with a mean value of 3,264 ± 943 kg ha−1. By using TVSu-1698 with RACA6 strain, farmers can get 85% more yield than on average with other genotypes/strains combination, while an average yield of 60% could be obtained by farmers using N fertilizer application.
Introduction
Bambara groundnut (Vigna subterranea (L.) Verdc) (BGN) is a leguminous crop that is indigenous to Africa and is widely grown in the Sub-Saharan Africa region (Tan et al., 2020). It is an important nutritious food source that is affordable to rural dwellers (Mbosso et al., 2020) and is a drought-resistant crop (Olanrewaju et al., 2021b). It can perform well and produce good crop yield on marginal soil and soil facing water stress, compared to other leguminous crops (Ajilogba et al., 2022). BGN contains 64.4% carbohydrates, 23.6% protein, 6.5% fat, 5.5% fiber, and essential minerals (Halimi et al., 2019). It is relatively underutilized compared with other leguminous crops and has often been associated with small-scale subsistence farming with most of its cultivation and processing being done by women (Mubaiwa et al., 2018). BGN serves as food for both humans and animals and it helps in the correction of nutritional disorders in both humans and animals (Olanrewaju et al., 2021a). Its roots form a mutualistic association with root nodule bacteria. This helps to increase the nitrogen availability in the soil as the bacteria assimilate nitrogen present in the atmosphere, trap it, and make it available to the plant in the soil (Babalola et al., 2017). This process eventually helps increase soil fertility, which leads to improvement in crop yield (Bitire et al., 2022). The application of chemical fertilizers to improve the fertility of the soil and enhance crop yield poses a threat to ecosystems and human health. Hence, the use of biofertilizers to counter the harmful effect of chemical fertilizers on ecosystems and human health is being explored (Enagbonma and Babalola, 2019). Biofertilizers are affordable, non-toxic, and very easy to apply; they help to standardize the soil structure and biodiversity of agricultural land. Hence, they serve as a good substitute for chemical fertilizers (Thomas and Singh, 2019). Biofertilizers also include microbial inoculants which are organic products containing some specific microorganisms derived from the root zones of plants. They have been shown to support and increase the growth and yield of the plant by 10–40% (Kawalekar, 2013). These biofertilizers colonize the rhizosphere and the interior of the plant, increasing plant growth when inoculated to the seed, plant surface, or the soil directly (Raghuwanshi, 2012). Biofertilizers not only improve soil fertility and crop productivity by adding nutrients to the soil but also protect the plant from pests and diseases (Yadav and Sarkar, 2019). It has been established that the application of biofertilizers to crops helps improve the growth of the root system, extend its life, degrade harmful materials, increase the survival of seedlings, and reduce the time to flowering (Nosheen et al., 2021). After the continuous use of biofertilizers for 3 to 4 years in the field, there is no further need for their application as parental inocula are sufficient for growth and multiplication (Bumandalai and Tserennadmid, 2019). Furthermore, the rate of survival of most exotic rhizobia strains introduced into the soil often is too low to infect legume plants due to competition with native rhizobia strains already present in the soil. It is, therefore, recommended that proper inoculations may be required to obtain a better symbiosis (Thilakarathna and Raizada, 2017). Most importantly, applications of inorganic N fertilizers to agricultural crops have been continuously increasing for the last decades globally. The application of Nitrogen (N) fertilizer at a different rate was found to increase the vegetative traits, yield, nodulation, and amino acid content of BGN (Hasan et al., 2021). Moreover, the introduction of biofertilizers may be utilized in the agricultural economy to optimally ensure the sustainability of food production and incorporation into the crop-breeding programs (Uzoh and Babalola, 2018; Fasusi et al., 2021). Nevertheless, the use of biofertilizers in agriculture is usually considered safe and environmentally friendly compared to the chemical fertilizers (Glick, 2020; Fasusi et al., 2021). Some studies, therefore, suggest that indigenous rhizobia strains may be better adapted to local environmental stress conditions (low/high temperature) compared to introducing exotic rhizobia. Native rhizobia, therefore, may have the potential for local commercialization (Thilakarathna and Raizada, 2017). Research conducted involving the inoculation of Bradyrhizobium strains significantly increased (from 13 to 40%) pod yield in groundnut when compared with the uninoculated control (Asante et al., 2020). Farmers in the tropics usually have trouble procuring the inorganic N fertilizer to boost their legumes production due to the high cost, and those that can afford usually apply below the recommended quantities. Therefore, the objective of this study is to determine the variability in the yield and yield components of inoculated BGN accessions through biofertilizers (B. japonicum strains) in different geographical locations.
Materials and methods
Experimental sites
Experiments were carried out on the field at two different agroecological locations: Ibadan and Ikenne in Nigeria, from August-December 2019 and 2020 seasons. Ikenne can be characterized as having a tropical savanna, wet climate, located at latitude (Lat) 6° 52′ 56″ N and longitude (Long) 3° 42' 54″ E with temperatures between 22.5°C and 29.5°C. While Ibadan can be characterized as having a sub-humid climate, located at Lat 7° 21′ 30 N and Long 3° 45′ 54 E with temperatures between 21 and 30.5°C.
Field preparation
The fields used for the studies were prepared using a tractor-driven plow, harrowed to remove plant debris, and 2 m plots were made (row) with a spacing of 25 cm between plots and 1 m between each block. Wooden pegs were used to demarcate one block from another. The field was partitioned into three blocks in both locations and seasons with the aid of wooden pegs.
Experimental design
The experiment was arranged in a randomized complete block design (RCBD) in both locations and seasons, and plastic pegs listing the name of the BGN accession and treatment were fixed into the soil by the side of the plots.
Accessions
Ten BGN accessions: TVSu-378, TVSu-506, TVSu-787, TVSu-1606, TVSu-1698, TVSu-1739 TVSu-710, TVSu-365, TVSu-475, and TVSu-305 were randomly selected from early maturing accessions at the International Institute of Tropical Agriculture (IITA) Gene bank.
Treatments
Four strains of B. japonicum inoculum included in the study were: FA3, RACA6, USDA110, and IRJ2180A containing 2.8 × 107, 7.2 × 106, 4.3 × 107, and 1.4 × 107 cfu/ml respectively. N fertilizer application, urea (20 kg N/ha) was applied to seedlings of BGN accessions that were not coated with B. japonicum strains 2 weeks after planting. The control had uninoculated seedlings (without inoculation and fertilizer application).
Field layout
Each of the BGN accessions had six rows in a block (four rows for B. japonicum strains, one row for N fertilizer, and one row for uninoculated control) making a total of 60 rows in a block and was replicated three times (three blocks) making a total of 180 rows (120 rows for B. japonicum strains, 30 rows for N fertilizer, and 30 rows for uninoculated control) with no border effect.
Culturing bacteria
The B. japonicum strains coated to BGN accessions were grown on yeast extract mannitol agar (YEMA), (Bikrol et al., 2010). The samples were maintained in Congo red yeast extract mannitol agar (CRYMA) at 28°C for 3 days (Bikrol et al., 2010). The strains were selected from the soil microbiology laboratory (IITA), based on authenticity (a pot experiment in the growth room at 32°C, which involved the inoculation of six different strains FA3, RACA6, USDA110, IRJ2180A, IRc461, and IRc29 on Bambara groundnut seedlings). The soil used for authenticity was carefully sterilized using the autoclave at 120°C for 1 h and was allowed to cool before being filled into 2 kg pots in triplicates. Two seeds of BGN were sown into each pot and after emergence, 2 ml of the broth culture of the strains were carefully inoculated to the BGN and were carefully monitored throughout the vegetative stages. At 50% flowering, plant samples were uprooted for nodulation infectivity and the best nodulating strains were selected for further studies on the field in two locations.
Determination of indigenous rhizobia in soil
The amount of the indigenous rhizobia strain present in the soil in both locations and seasons was determined using the most probable number before planting was done, The experiments were set up separately by locations in the growth room (32°C) using sterile soil in 2 kg pots. Cowpea seeds were sown and replicated three times in the first season (2019), while BGN seeds were sown in the second season (2020) in the growth room. At 50% flowering, rhizobia were isolated from nodules on Congo red agar (Woomer, 1994) using the spread plate method. Two undamaged nodule samples were picked from each plant of BGN and were placed in sterile water for about 15 to 20 min to rehydrate them after which they were surface sterilized using 3% sodium hypochlorite for 3 mins. They were then rinsed with sterile water after which they were further sterilized with 95% ethanol and then rinsed with six changes of sterile water (Woomer, 1994). The nodules were then transferred into sterilized Petri dishes, crushed with a flamed glass rod, and mixed with a few drops of sterile water. A loop full of the crushed nodule was streaked on Congo red agar and then incubated at 28°C for 5–7 days, after which the isolates were purified and identified.
Treatments application
One kilogram of seeds from each BGN accession was coated with each of the selected B. japonicum strains (FA3, RACA6, USDA110, and IRJ2180A) using Arabic gum (20 g) dissolved in 200 ml of warm water to ensure that the B. japonicum strains stick firmly to the BGN accessions, and were carefully labeled to prevent mix up and allowed to dry with the seeds (Nodumax, IITA). Six seeds of each BGN accession were planted in each row in a block in both geographical locations and seasons. N fertilizer urea 20 kg ha−1 was applied to the seedlings of BGN accessions that were not inoculated with bacteria strains, and no treatments were applied to the uninoculated control plots.
Field maintenance
After emergence, seedlings were carefully monitored during the vegetative stage, and regular weeding was done manually with the aid of a hoe. Spraying of insecticides (termex) was done to control insects that might hinder flowering.
Pod characterization
After harvesting, pods were preserved in the drying room (17°C) and allowed to dry properly before threshing, after which characterization was done on harvested pods and seeds. Data were obtained on yield and yield components on harvested pods and seeds. Data collected on the pod were pod length and width, and seed length and width which were obtained using the vernier caliper. The weight of 100 seeds, pod weight per plot, and seed weight per plot were determined using the weighing balance, while the number of pods and seeds per plot was determined by counting.
Statistical analysis
Data collected on yield traits were subjected to a four-way analysis of variance (Accession* Treatments*Location*Season) using the statistical analysis system (SAS) package and means were separated using Ducan's multiple range test (DMRT) at P<0.05 (Zatybekov et al., 2017).
Field history
The fields used for this study (at Ibadan and Ikenne) do not have any previous record of inoculation of Bradyrhizobium or previous cultivation of BGN. The experiment was not sited in the same spot in the second season in both locations but was sited a few meters apart from the first season's experiment to avoid contamination from inoculation from the first season's experiment.
Data collections on yield
Data were collected from the harvested pod as described by the descriptor of BGN, International Plant Genetic Resources Institute (IPGRI) Rome (Italy), (IITA), and International Bambara groundnut Network (IPGRI I BAMNET, 2000).
Geographical origin and source of B. japonicum strains used in the study
The FA3 strain originated from Cameroon and was sourced from IRAT (IRAT- Institutes de Recherché Agronomiques Tropicales), (Brunel et al., 1988). The USDA110 strain originated in Florida, USA with its source from the USDA (United States Department of Agriculture), (Bai et al., 2003). IRJ2180A and RACA6 strains were from the IITA Headquarters, (Ibadan) Nigeria, (Okogun and Sanginga, 2003). All the strains have soybean and Bambara groundnut as major hosts.
Determination of macronutrient and exchangeable bases in soil
Method of soil collection
Soil samples were collected from both geographical locations and seasons with the aid of a soil auger in triplicates. After collection, soils were carefully labeled to prevent mix-up, sieved using a 2-mm sieve, and air dried at room temperature 32°C before subjecting it to further analysis (routine analysis, available nitrogen, phosphorus, organic carbon, etc.).
Method of soil analysis
The % of organic carbon (OC) was analyzed following the procedure outlined by Walkley and Black (1934), and the % of N was determined using the Kjedahl method (Mulvaney and Page, 1982). The available Phosphorus (P) analysis was carried out as described in Bray's method (Carter and Gregorich, 2007). The exchangeable potassium (K), calcium (Ca), and sodium (Na) were determined by ammonium acetate (Black et al., 1965).
Estimation of yield components
N =
P =
H =
S = (Leonard, 1980).
Where:
N = yield/plot
X = number of seeds planted in a plot
Y= number of shoots harvested in a plot
L = weight of harvested seed per plot (g)
P = Yield/plant
H = Yield/ha
W = Seed weight/plot (kg)
A = Area of the plot (m2)
S = Shelling %
T = Pod weight
G = seed weight
1ha=10,000 m2.
Results
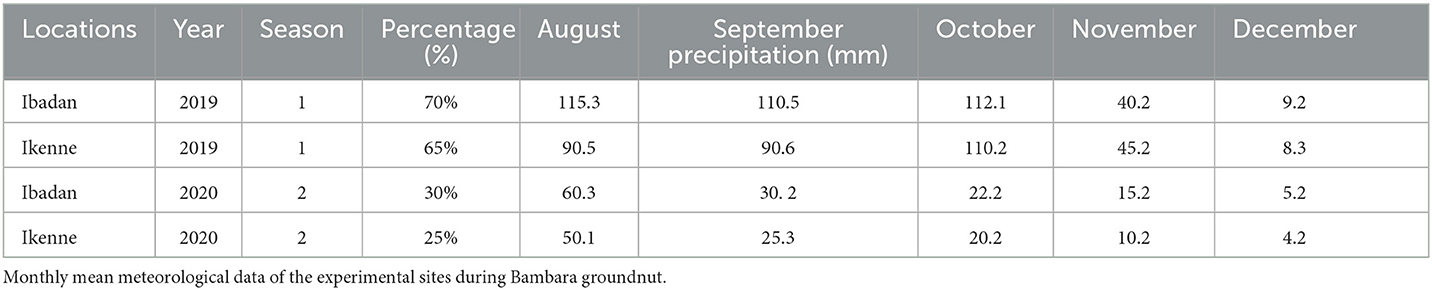
The rainfall distribution pattern recorded in Ibadan in the first season showed that higher rainfall patterns were recorded in August, September, and October, but a sudden decline in rainfall was recorded in November and December which did not pose any negative effect on the vegetative and the reproductive growth on the inoculated BGN accessions. This rainfall amount to about 70% rainfall distribution in the agroecological zones in the first season (Table 1). Similarly, in Ikenne, the rainfall pattern commenced with above-average rains in August and September, with a high increase in October, which eventually declined in November and December without a negative effect on the vegetative and reproductive stages of the legumes, and this amounted to 65% in those geographical locations in the first season (Table 1). Furthermore, in the second season, above-average rains were recorded in Ibadan and Ikenne in August with a decline from September through to December, which amounted to a 30% rainfall distribution in Ibadan and a 25% rainfall distribution in Ikenne. This adversely affected the soil's chemical characteristics and eventually affected both the vegetative and the reproductive stages of the inoculated BGN accessions in both locations due to low rainfall distribution (Table 1).
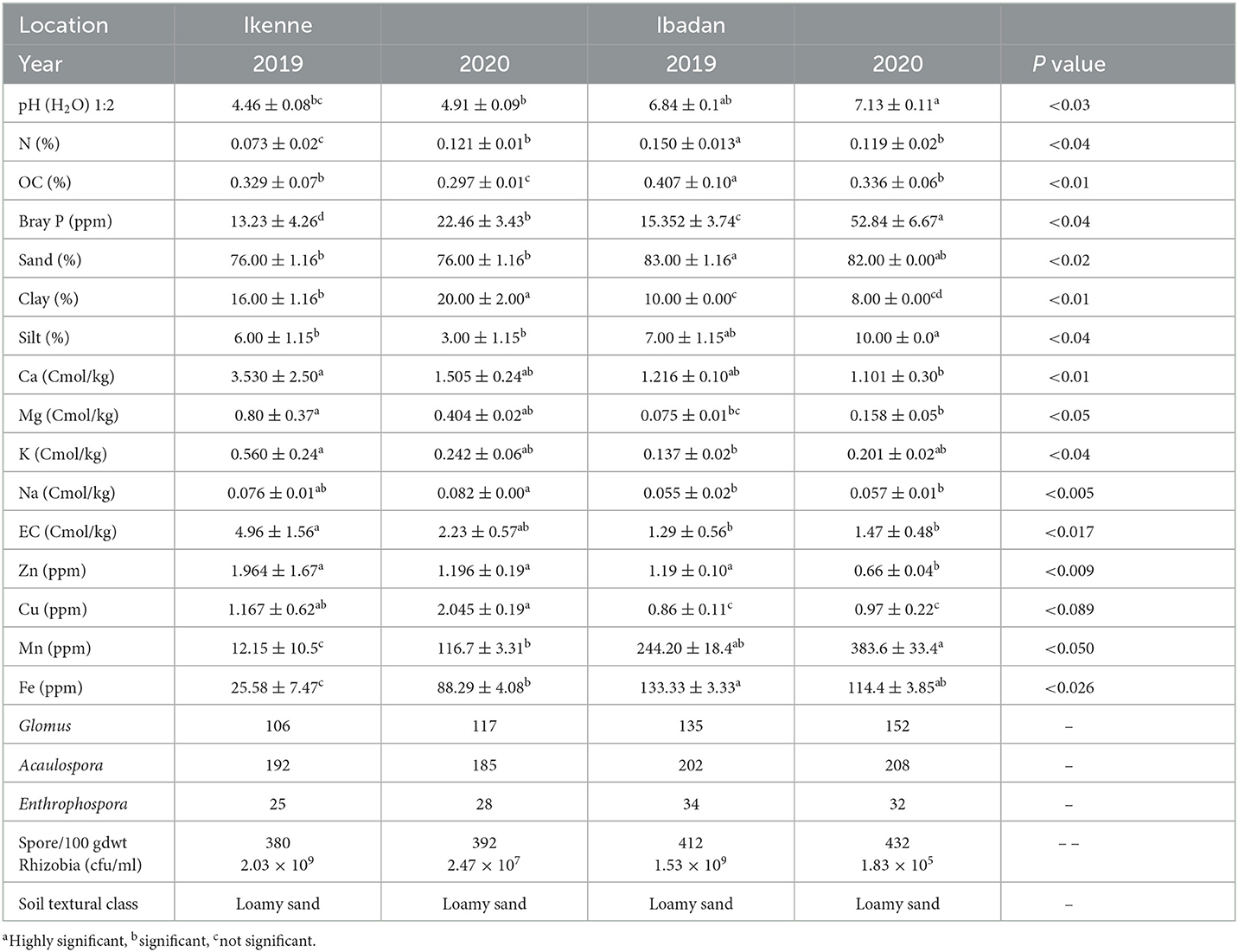
Characterization of the soil used in the studies
The soil in Ikenne was acidic in nature in both seasons, while the soil in Ibadan was neutral in both seasons. The % of organic carbon showed that the soil used was normal, while the % of nitrogen, phosphorus, and potassium showed that the nutrients were at sufficient levels in both locations and seasons. A higher % of phosphorus was recorded in the soil used in both locations and seasons (Table 2). The % of potassium present in the soil used showed that it was available in moderate quantities. The calcium from the soil in Ibadan 2019 showed that it was normal, while the calcium from the soil in Ikenne in the 2019 season was very high. Higher magnesium values obtained in Ikenne showed that it was sufficient in both locations and seasons, while lower quantities of Magnesium were obtained in the Ibadan soil. The particle size analysis was determined by the hydrometer method. The class of the soil, due to the proportion of each particle, showed that it was loamy sand because it ranged from 76.00 to 83.00% for % of sand, % of clay ranged from 8.00 to 19.00%, and the % of silt ranged from 3.00 to 10.00%. The recorded mycorrhizal spore count was low in Ibadan soil in 2019 and 2020, with a mean value of 382 and 392 respectively, compared to Ikenne with a mean value of 412 and 432, in both seasons (Table 2). The number of indigenous rhizobia present in the soil was determined using the most probable number (MPN). In Ikenne it was 2.03 × 109 cfu/ml and 2.47 × 107 cfu/ml in 2019 and 2020 respectively, while in Ibadan, the values obtained were 1.53 × 109 cfu/ml and 1.83 × 105 cfu/ml in 2019 and 2020 respectively. The available rhizobia count in the soil was moderately abundant (Vincent, 1970).
Rainfall pattern during BGN production in both locations
Adequate rainfall, ranging from 110 mm to 115 mm, was recorded in both locations (Ibadan and Ikenne) throughout the cultivation of the BGN accessions in the first season, but a slight break in rainfall was recorded in the second season in both locations for a few months, and the alternative means of water was introduced using irrigation in both locations but could not sustain the underutilized legume compared to the natural rain, which adversely affected the yield of inoculated accessions of BGN in the second season. The absence of rain caused a significant decrease in pod formation in both locations in the second season. This eventually resulted in low yield and yield components in both locations in the second season.
Response of BGN accessions to B. japonicum strains inoculation
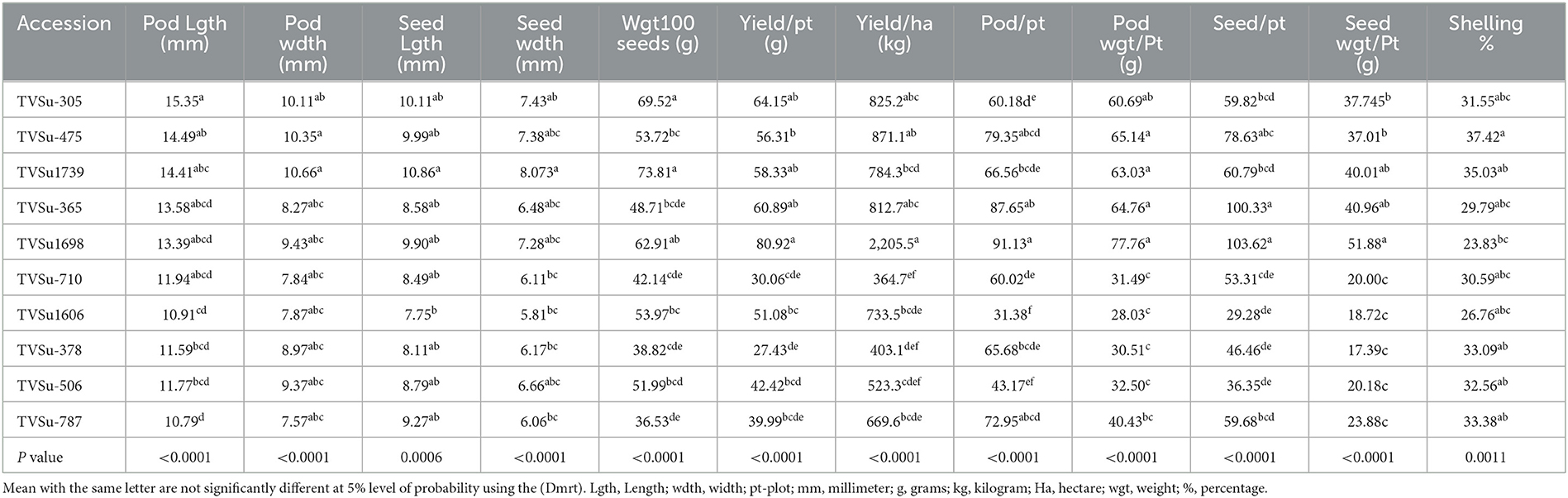
Analysis of variance showed that significant differences were recorded among BGN accessions in yield and yield components obtained in Ikenne and Ibadan in both seasons except for some common yield traits (Tables 3, 4). Furthermore, no significant differences were recorded among the B. japonicum strains inoculated to BGN accessions in the yield and yield components recorded in Ibadan and Ikenne separately in 2019 and 2020, except differences that were recorded in yield/plot and yield/ha in Ibadan, 2020 (Tables 3, 4). Most importantly, significant differences were not recorded in the interaction of BGN accessions with treatments in the yield and yield components in Ikenne and Ibadan (Tables 3, 4). The break in rainfall recorded in the second season resulted in a 60% decrease in yield compared to the first season. Moreso, the highest values were recorded in pod length (p < 0.0001), width (p < 0.0.001), the weight of 100 seeds (p < 0.0001), yield/plot (p < 0.0001), yield/ha (p < 0.0.0001), pod/plot (p < 0.0001), the weight of pod/plot (p < 0.0001), the weight of seed/plot (p < 0.0001), and shelling % (p < 0.012) in TVSu-305, TVSu-365, TVSu-1739, and TVSu-1698 compared to other inoculated BGN accessions (Table 5). Furthermore, TVSu-1698 showed the highest mean value on yield and yield components in both locations compared to other inoculated BGN accessions (Table 5). The result of the second season revealed that no significant differences were recorded among the inoculated BGN accessions in pod length, width, seed length, and width except in TVSu-378 and TVSu-787 in both locations (Table 6). A significant difference was recorded in the weight of 100 seeds (p < 0.0001), yield/plot (p < 0.0001), yield/ha (p < 0.0001), pod weight/plot (p < 0.0001), and seed weight/plot (p < 0.0001) in TVSu-1739 and TVSu-1698 and were not significantly different from other inoculated BGN accessions except TVSu-378 and TVSu-787 (Table 6). There was also no significant difference recorded among the inoculated BGN accessions in both locations in the pod length and width and seed length and width (Table 6). Significant differences were recorded in the inoculated BGN accession TVSu-1698 (p < 0.0001) which showed higher yield and yield components compared to other BGN accessions in both locations in the second season (Table 6). Reduced shelling percentages were recorded in both locations and seasons, which showed that the biofertilizer increased the grain size and yield of the BGN accessions and reduced the shelling percentages in both locations and seasons (Tables 6, 7). The result eventually revealed that in the first, second, and both seasons combined, TVSu-305 and TVSu-1698 responded to the inoculation with high yield and yield components in both locations and seasons than other inoculated BGN accessions which were not significantly different on yield and yield components (Table 7). But significant differences were recorded among the B. japonicum strains inoculation when both locations were combined in both seasons (Table 8).
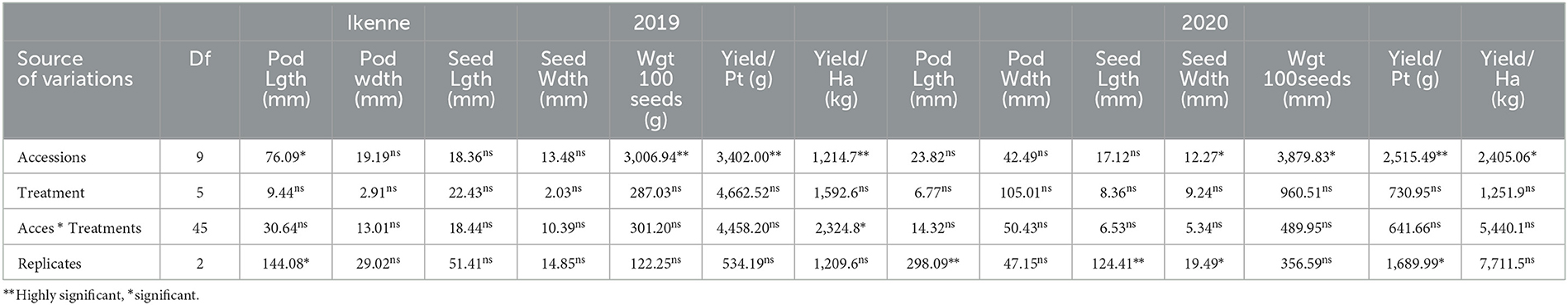
Table 3. Analysis of variance showing the effect of treatments on yield and yield components of BGN accessions in Ikenne, 2019 and 2020.
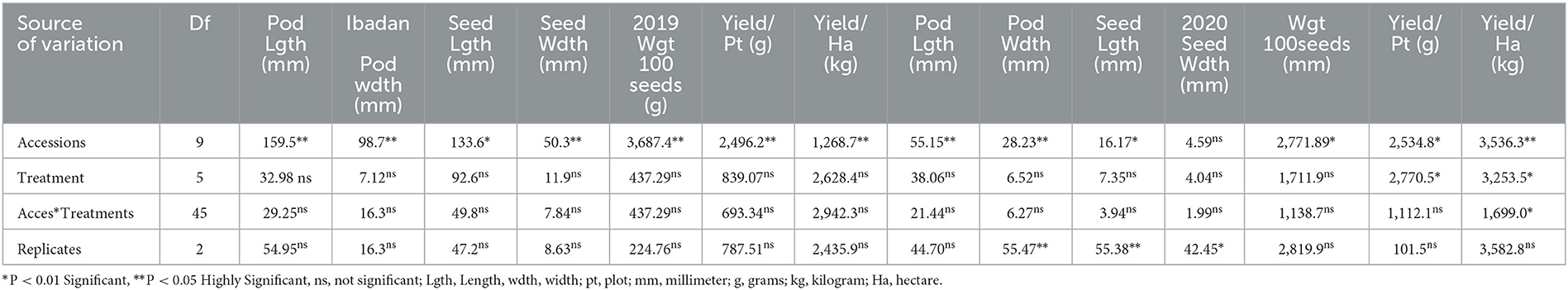
Table 4. Analysis of variance showing the effect of treatments on yield and yield components of BGN accessions in Ibadan, 2019 and 2020.
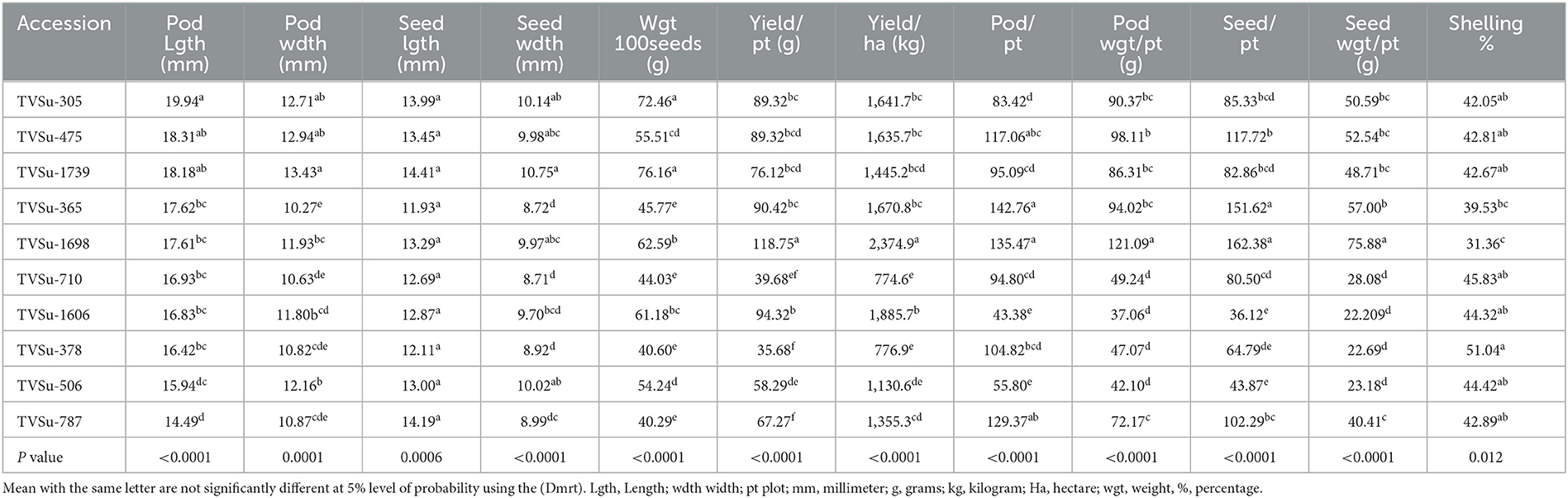
Table 5. Effects of treatments on yield and yield components of BGN accessions in Ibadan and Ikenne in the 2019 cropping season.
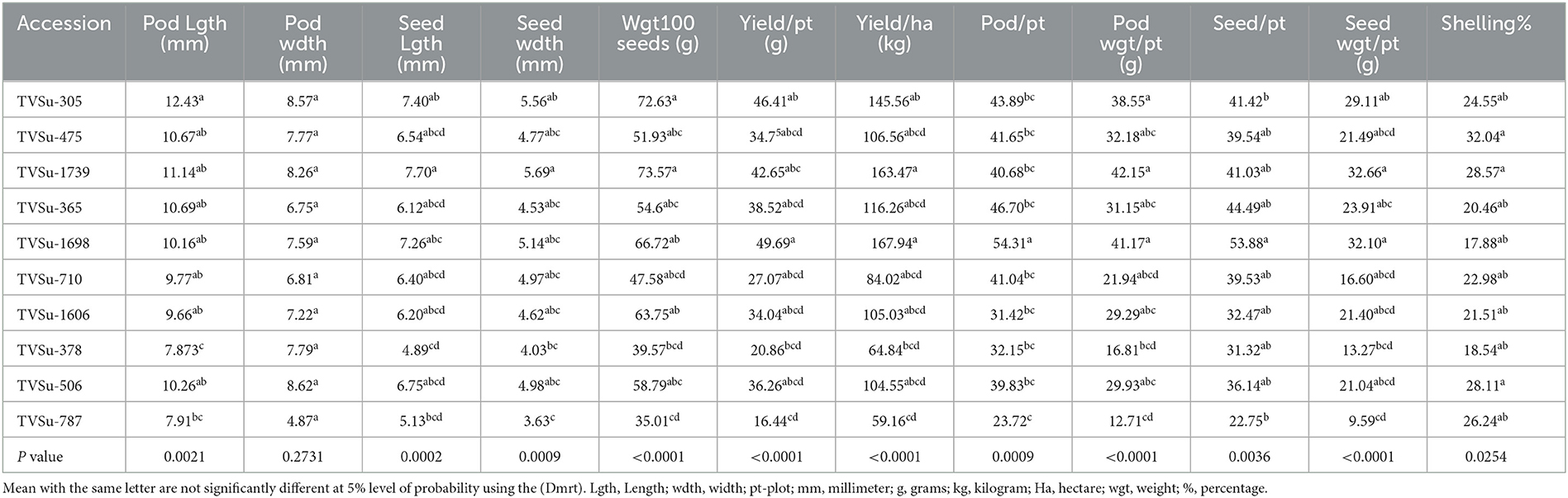
Table 6. Effects of treatments on yield and yield components of BGN accessions in Ibadan and Ikenne in 2020 cropping season.
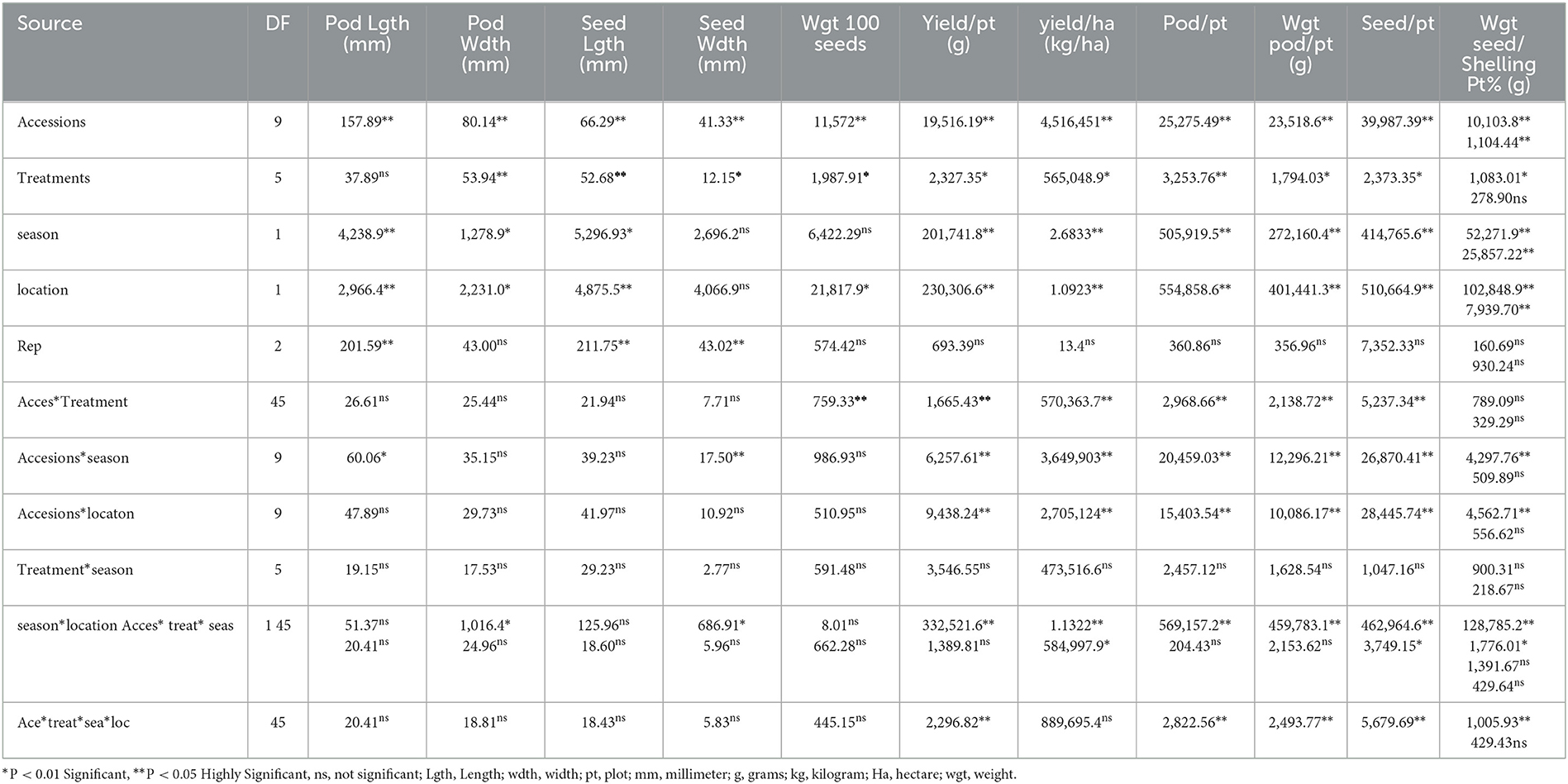
Table 8. Analysis of variance showing the effect of treatments on yield and yield components of BGN accessions in Ibadan and Ikenne in both seasons.
Interactions between accessions, strains, location, and season
The results obtained from the analysis of variance indicated that significant differences were recorded among inoculated BGN accessions and B. japonicum strains inoculation in the yield and yield components (Table 8). Furthermore, significant differences were recorded in both seasons, and locations, and replicated in some of the yield and yield components that were measured but no significant differences were recorded in seed width and weight of 100 seeds in both locations and seasons (Table 8). The results recorded in the interaction of accessions with strains, accessions with seasons, and accession with locations, reflected significant differences in some yield components such as pod/plot, yield/ha, yield/plot, the weight of pod/plot, seed/plot, and weight of seed/plot but no significant difference was recorded in seed size. Furthermore, there was no significant difference recorded in the interaction of treatments between the seasons, and the interaction of seasons with the location in all the yield components measured (Table 8). Moreover, in the interaction of seasons with locations, accessions with strains, and seasons with locations, significant differences were recorded in some of the yield and yield components (Table 8). The analysis of variance eventually showed variation in the yield and yield components of independent and interaction of variables which reflect the impact of the inoculation on BGN accessions. The analysis of variance indicated that significant differences were recorded among the strains inoculated to the BGN accessions in the yield and yield components (Table 8).
Interactions between BGN accessions and B. japonicum strain were recorded (Table 9). Significant differences were recorded in the interaction of BGN accessions with B. japonicum strains in the yield and yield components. Different variability was recorded in the yield and yield components of inoculated BGN accessions in both locations and cropping seasons (Table 9). The highest pod length and weight of 100 seeds was recorded in the combination of TVSu-305 with IRJ2018A with mean values of (26.7± 2.78 mm and 88.5± 6.67 g respectively) (Table 9). Moreso, the highest yield/plot was recorded in the combination of TVSu-365 with USDA110 with a mean value of (195.3± 39.7g) compared to other combinations (Table 9). Furthermore, the highest pod and seed width was recorded in the interaction of TVSu-305 with RACA6 with mean values of 15.1± 0.17 and 14.7± 2.19 mm respectively (Table 9). Finally, the highest weight in pod per plot and yield per hectare was recorded in the interaction of TVSu-1698 and RACA6 with a mean value of 327.2± 205.5 g and 6,234± 86.70 kg/ha respectively. The interaction of BGN accessions with B. japonicum strains revealed the relevance of inoculation on the legume genotype across locations, as different variability was recorded on yield and yield components of inoculated BGN accessions (Table 9). In some instances, higher values were recorded in the uninoculated control over the N fertilizer application. The highest yield recorded by TVSu-365 in the uninoculated control (4,236.3± 856.3 kg/ha) was a result of the genotype responding to the indigenous rhizobia in the uninoculated control better than other BGN accessions and the result obtained in TVSu-365 can also be attributed to the response of the indigenous rhizobia with genotype, in relation to the environments, and seasons (Table 9). TVSu-1606 and TVSu-1739 showed higher values in uninoculated control than N fertilizer application in the yield/plot and yield/ha recorded, which was also due to the presence of the indigenous rhizobia in the soils in both locations. The response recorded among the BGN accessions can also be because TVSu-365, TVSu-1606, and TVSu-1739 (genotype) responded to the indigenous rhizobia in the soil, compared to other BGN accessions.

Table 9. Mean and standard error of yield and yield components of BGN accessions in Ibadan and Ikenne in both cropping seasons.
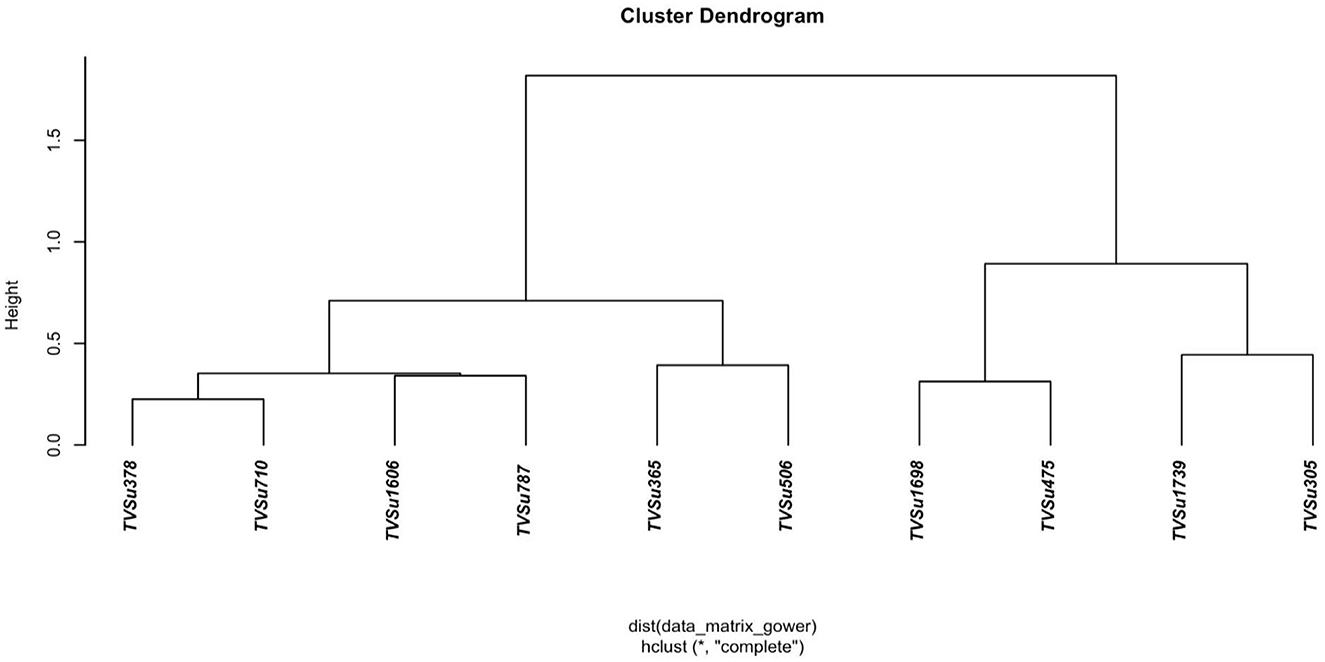
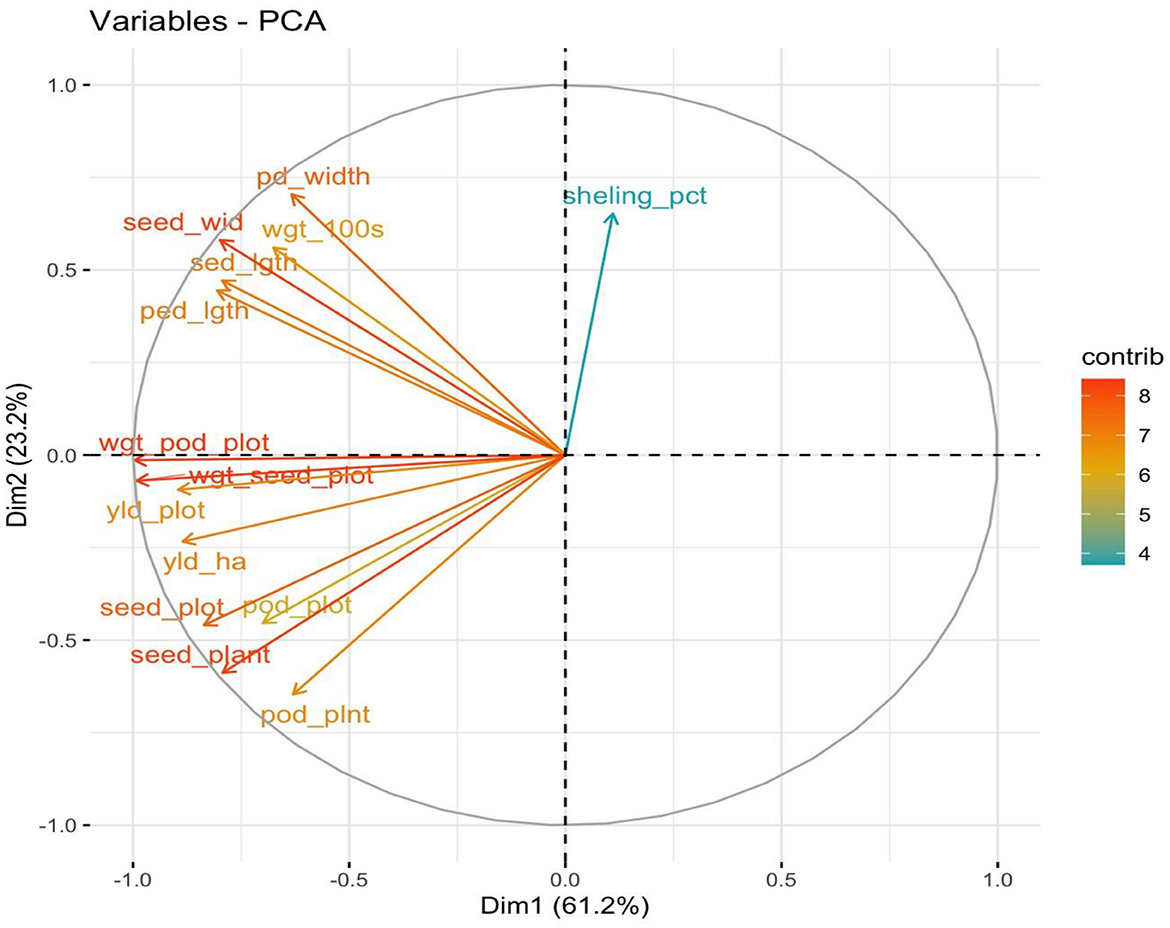
Figure 1 shows the result obtained by inoculating BGN accessions with B. japonicum strains. Different variability was recorded in the yield traits with the highest mean value seen in TVSu-365, TVSu-1698, TVSu-475, TVSu-305, and TVSu-1739 compared to other inoculated BGN genotypes. The results further revealed that no significant differences were recorded between TVSu-1698 and TVSu-365 in the yield/plot, yield/ha, pod weight, seed/plot, and seed per plot, but were significantly different from other inoculated BGN genotypes (Figure 1). While no significant differences were recorded among TVSu-475, TVSu-305, and TVSu-1739 in the pod length and width, seed length and width, and weight of 100 seeds, they were significantly different from other inoculated BGN accessions (Figure 1). A higher shelling % was recorded in TVSu-506 compared to other BGN genotypes (Figure 1). The cluster dendrogram shows the response of the BGN genotypes to the inoculation (Figure 2). It reveals that TVSu-1698, TVSu-1739, and TVSu-305 are not significantly different but are significantly different from TVSu-378, TVSu-710, TVSu-1606, TVSu-787, TVSu-365, and TVSu-506 which, among them, are not significantly different (Figure 2). Figure 3 illustrates the differences in the yield traits recorded among the BGN genotypes. It reflects equal variability in pod length and width, seed length, yield/plot, yield/ha, and seed/plot. Likewise, equal variability was recorded in the seed wgt/plot, pod weight/plot, and seed width (Figure 3).
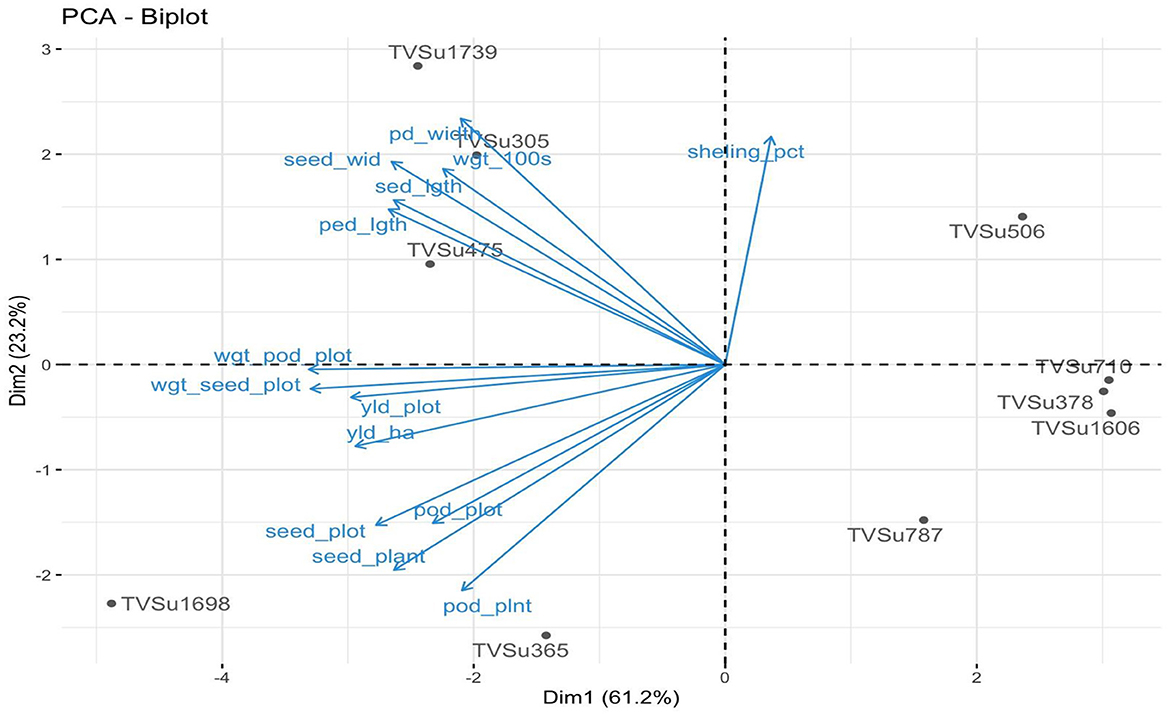
Figure 1. Principal components analysis (PCA) showing the interaction of the inoculated BGN genotypes and yield traits recorded.
Discussion
The differences recorded in the yield and yield components of the inoculated BGN accessions in both agroecological zones, in the second season compared to the first season, were a result of low rainfall and a break in rainfall recorded in the second season, which resulted in an average of 60% decrease in yield in the second season. Most importantly, the soil contributed partially to the improvement of the yield and the yield component recorded. The result of the soil sample showed that macronutrients, micronutrients, and biological nutrients needed for crop sustainability were available in adequate quantities in the soil, which buttressed the inoculation of the B. japonicum strains with enhanced optimum yield and yield components of BGN accessions. This is in accordance with an experiment conducted by Singh et al. (2022) which concluded that inoculation of beneficial microbes enhanced plant acquisition of macronutrients and micronutrients in legumes. Soil quality is a major factor that cannot be over-emphasized. The essential nutrients needed by the crop for optimum growth and development are readily available in the soil which contributed significantly to the yield response recorded in the inoculated BGN accessions in both locations and seasons. This is in agreement with the findings of Carter (2002) who reported on soil quality for sustainable land management and food production systems. Also, the pH of the soil in both locations is another major determinant of the success of the production of the legume as low yields were also recorded in acidic soil (Ikenne) compared to the soil with neutral conditions (Ibadan). This correlates with the findings of Hackney et al. (2019) who reported that soil acidity reduces nodulation and legume yield production. Inoculation of B. japonicum strains enhanced the yield and yield components of BGN accessions in relation to the environment where the study was conducted. However, it was noted that the N fertilizer application does not increase yield over the uninoculated control according to the analysis conducted on the available indigenous rhizobia present in the soil in both locations. The results showed that the indigenous rhizobia present in the soil were in moderate quantities and performed exceedingly better than the N fertilizer application in relation to yield and yield components recorded. This is in accordance with the study by Nyaga and Njeru (2020) who reported an increase in cowpea growth and production due to potential native rhizobia. Significant differences were recorded among BGN accessions, which showed that the underutilized legume responded to the inoculation in both seasons. The potential of the Bradyrhizobium strains inoculated to BGN accessions varies, thereby improving the yield and yield components in both locations and seasons. RACA6 strain improved substantially the yield of BGN accessions by 85% in both locations and seasons. Furthermore, USDA110 and IRJ2180A strains improved the yield of BGN accessions by 70%. FA3 strain improved the yield of BGN accessions by 80% in both locations and seasons. The response recorded in the N fertilizer application showed that N fertilizer contributed to yield improvements of BGN accessions by 60% compared to the uninoculated control with a yield improvement value of 50% which may be due to the presence of indigenous rhizobia in the soil used. The response recorded on yield and yield component of BGN accessions can be attributed to the genotype, in relation to the environments, and seasons. The results obtained are in line with the findings by Allito et al. (2021) who reported that inoculation of rhizobia increases the nodulation of faba bean compared to the uninoculated control. Superior performances were recorded in TVSu-1698 and TVSu-1739 among other inoculated BGN accessions, across locations in both cropping seasons. Though variability in yield was recorded among inoculated BGN accessions on yield and yield components, inoculation of B. japonicum strains to BGN accessions revealed significant differences among accessions in yield traits, except TVSu-1698 which showed a higher response to the inoculation with more seeds per area compared to other BGN accessions. The results demonstrated that inoculation of legumes with B. japonicum strains improves yield, which is in accordance with the study by Santos et al. (2019) where they concluded that inoculation with rhizobia and legumes raised great interest from researchers and companies in the 1970s. The result obtained from the interactions also revealed the relevance of the inoculation of B. japonicum strains to BGN accessions showing different variability in the yield and yield components measured. Furthermore, significant differences were recorded among the B. japonicum strains inoculated in the yield and yield components of the BGN accessions recorded. The success of the inoculation depends primarily on the type of the B. japonicum strain, the genotype of the legume, the environmental conditions, and the crop management (Abdullahi and Abubakar, 2022). Furthermore, significant differences were recorded in both seasons, locations, the interaction of accessions with strains, the interaction of accessions with seasons, and interaction of accessions with location on pod length and width, seed length, the weight of 100 seeds, yield/plot, yield/ha, the weight of pod/plot, and weight of seed/plot. This is in agreement with a study conducted using an Early Maturing Promiscuous Cultivar (TGX 1485) of inoculated soybean with one of the four B. japonicum strains (R25B, IRj2180A, IRc461, and IRc291) at Minna, Northern part of Nigeria. The four rhizobia inoculants increased all parameters including grain yield (Abdullahi et al., 2020). In addition, differences were recorded among the yield and yield components of the inoculated BGN accessions with TVSu-1698 showing a higher response to the inoculation in both locations in the yield and yield components measured than other BGN accessions. Precisely, significant differences were recorded in the fruits (pod), seed size, shape, and shelling %, in TVSu-1698 compared to other BGN accessions. Finally, it was obvious that the inoculation of the B. japonicum strains significantly enhanced the yield and yield components of BGN accessions than the N fertilizer-applied plants, and uninoculated control. Though in a few instances, N fertilizer application also showed a high impact on some BGN accessions which also reflects the importance of N fertilizer application, and indigenous rhizobia in the uninoculated control. The uninoculated plants and N-fertilized plants both produced nodules from indigenous rhizobia present in the soil (Getachew Gebrehana and Abeble Dagnaw, 2020). Most importantly, the BGN accessions responded to the B. japonicum inoculation across geographical locations and seasons which reflects the importance of the nitrogen-fixing bacteria (B. japonicum strains) over the N fertilizer application and the uninoculated control. The most yield components that were enhanced by the inoculation of the B. japonicum strains were yield/plots and yield/ha which were recorded in both geographical locations and cropping seasons (Asante et al., 2020). In summary, it was observed that the two native strains (RACA6 and IRJ2180A), and the non-native strains (FA3 and USDA110) improved the yield and yield components of some BGN accessions. RACA6 strain enhanced the yield of TVSu-1698 compared to the IRJ2180A strain and the two non-native strains. While the FA3 strain enhanced the yield of some BGN accessions (TVSu-710, TVSu-787, and TVSu-1739) compared to the USDA110 strain. The response recorded in the yield of TVSu-365, TVSu-1606, and TVSu-1739 without inoculation by strains means that farmers who cannot afford the commercial inoculant can cultivate TVSu-365, TVSu-1606, and TVSu-1739 and can take advantage of the indigenous rhizobia in the soil to improve the yield of the legume.
Conclusion
Using B. japonicum strains to inoculate legumes as a starter dose is an alternative option for farmers to improve the yield and yield components of legumes. In this study, the inoculation of B. japonicum strains significantly enhanced the yield and yield components of BGN accessions used in both locations and seasons. Precisely, FA3 and RACA6 strains enhanced the yield and yield components of BGN accessions compared to other B. japonicum strains and the inorganic N fertilizer in both locations and seasons. The use of B. japonicum strains, FA3 and RACA6 should be embraced by farmers in the tropics to improve productivity and to reduce reliance on inorganic N fertilizer application which most farmers in the tropics cannot afford, and those that can afford usually apply below recommended quantities. It is evident that the inoculation of bacteria strains performed exceedingly well and improved the yield and yield components of BGN accessions in both locations and seasons compared to inorganic N fertilizers applications and the uninoculated control. B. japonicum strains are easily affordable for farmers and pose no threat to the soil and human health after use compared to the N fertilizers application that usually contaminates the soil and causes adverse effects on human health after use. It may be necessary to conduct this study in the future using different rates of N fertilizer with B. japonicum strains on BGN accessions using multiple locations and possibly increase the number of plants tested per experimental condition. The findings from this study can help improve legume production output and combat hidden hunger in developing countries.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TB: conceptualization, methodology, and writing original draft preparation. OB and OO: supervision and editing. MA: project administration and fund acquisition. All authors discussed the results and contributed to the final manuscript.
Funding
Funding recieved from Crop trust through Genetic Resources Center, International Institute of Tropical Agriculture, Nigeria.
Acknowledgments
The authors are grateful to the Genetic Resources Center of the International Institute of Tropical Agriculture, Ibadan, Nigeria for germplasm, facilities, and financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullahi, A., and Abubakar, F. (2022). Sustainable way of improving grain legumes productivity against failure of introduced rhizobia inoculant. Bioscientist J. 10, 167–180. Available online at: http://www.bioscientistjournal.com
Abdullahi, A. A., Howieson, J., O'Hara, G., Terpolilli, J., Tiwari, R., and Yusuf, A. A. (2020). History of Rhizobia inoculants use for improving performance of grain legumes based on experience from Nigeria Just Enough Nitrogen. New York, NY: Springer, 101–113.
Ajilogba, C. F., Olanrewaju, O. S., and Babalola, O. O. (2022). Improving bambara groundnut production: insight into the role of omics and beneficial bacteria. Front. Plant Sci. 13, 133. doi: 10.3389/fpls.2022.836133
Allito, B. B., Ewusi-Mensah, N., Logah, V., and Hunegnaw, D. K. (2021). Legume-rhizobium specificity effect on nodulation, biomass production and partitioning of faba bean (Vicia faba L.). Sci. Rep. 11, 3678. doi: 10.1038/s41598-021-83235-8
Asante, M., Ahiabor, B. D. K., and Atakora, W. K. (2020). Growth, Nodulation, and Yield Responses of groundnut (Arachis hypogaea L.) as influenced by combined application of rhizobium inoculant and phosphorus in the Guinea Savanna zone of Ghana. Int. J. Agron. 2020, 1–7. doi: 10.1155/2020/8691757
Babalola, O. O., Olanrewaju, O. S., Dias, T., Ajilogba, C. F., Kutu, F. R., and Cruz, C. (2017). Biological nitrogen fixation: the role of underutilized leguminous plants Microorganisms for Green Revolution. New York NY: Springer, 431–443.
Bai, Y., Zhou, X., and Smith, D. L. J. C. (2003). Enhanced soybean plant growth resulting from coinoculation of Bacillus strains with Bradyrhizobium japonicum. J. Crop Sci. 43, 1774–1781. doi: 10.2135/cropsci2003.1774
Bikrol, A., Saxena, N., and Singh, K. (2010). Characterization of Bradyrhizobium strains isolated from different varieties of soybean with 16SrDNA RFLP from agricultural land of Madhya Pradesh, India. Indian J. Microbiol. 50, 404–411. doi: 10.1007/s12088-011-0077-6
Bitire, T. D., Abberton, M., Oyatomi, O., and Babalola, O. O. (2022). Effect of Bradyrhizobium japonicum strains and inorganic nitrogen fertilizer on the growth and yield of bambara groundnut (Vigna subterranea (L.) Verdc) accessions. Front. Sustain. Food Syst. 6, 139. doi: 10.3389/fsufs.2022.913239
Black, C. A., Evans, D., and White, J. (1965). Methods of soil analysis: chemical and microbiological properties. ASA.
Brunel, B., Cleyet-Marel, J.-C., Normand, P., and Bardin, R. (1988). Stability of Bradyrhizobium japonicum inoculants after introduction into soil. J. Appl. Environ. Microbiol. 54, 2636–2642. doi: 10.1128/aem.54.11.2636-2642.1988
Bumandalai, O., and Tserennadmid, R. (2019). Effect of Chlorella vulgaris as a biofertilizer on germination of tomato and cucumber seeds. Int. J. Aquatic Biol. 7, 95−99. doi: 10.22034/ijab.v7i2.582
Carter, M. R. (2002). Soil quality for sustainable land management: organic matter and aggregation interactions that maintain soil functions. Agron. J. 94, 38–47. doi: 10.2134/agronj2002.3800
Carter, M. R., and Gregorich, E. G. (2007). Soil Sampling and Methods of Analysis. London: CRC press.
Enagbonma, B. J., and Babalola, O. O. (2019). Potentials of termite mound soil bacteria in ecosystem engineering for sustainable agriculture. Annal. Microbiol. 69, 211–219. doi: 10.1007/s13213-019-1439-2
Fasusi, O. A., Cruz, C., and Babalola, O. O. (2021). Agricultural sustainability: microbial biofertilizers in rhizosphere management. Agriculture 11, 163. doi: 10.3390/agriculture11020163
Getachew Gebrehana, Z., and Abeble Dagnaw, L. (2020). Response of soybean to Rhizobial inoculation and starter N fertilizer on Nitisols of Assosa and Begi areas, Western Ethiopia. Environ. Syst. Res. 9, 1–11. doi: 10.1186/s40068-020-00174-5
Glick, B. R. (2020). Introduction to plant growth-promoting bacteria Beneficial plant-bacterial interactions. New York NY: Springer, 1–37.
Hackney, B., Jenkins, J., Powells, J., Edwards, C., De Meyer, S., Howieson, J., et al. (2019). Soil acidity and nutrient deficiency cause poor legume nodulation in the permanent pasture and mixed farming zones of south-eastern Australia. Crop Pasture Sci. 70, 1128–1140. doi: 10.1071/CP19039
Halimi, R. A., Barkla, B. J., Mayes, S., and King, G. J. (2019). The potential of the underutilized pulse bambara groundnut (Vigna subterranea (L.) Verdc.) for nutritional food security. J. Food Composit. Anal. 77, 47–59. doi: 10.1016/j.jfca.2018.12.008
Hasan, M., Uddin, M. K., Mohammed, M. T. M., Zuan, A. T. K., and Motmainna, M. (2021). Growth, yield, nodulation and amino acid content of bambara groundnut (Vigna subterranea) under inorganic and organic fertilizer application.
IPGRI I BAMNET (2000). Descriptors for bambara groundnut (Vigna subterranea), International Plant Genetic Resources Institute, Rome, Italy; International Institute of Tropical Agriculture,<city>Ibadan</city>, Nigeria. Journal of The International Bambara Groundnut Network, Germany.
Kawalekar, J. S. (2013). Role of biofertilizers and biopesticides for sustainable agriculture. J. Bio. Innov. 2, 73−78. doi: 10.4236/jbm.2018.66002
Leonard, D. K. (1980). Soils, Crops, and Fertilizer Use and Application (Field Manual for Crop Developments).
Mbosso, C., Boulay, B., Padulosi, S., Meldrum, G., Mohamadou, Y., Berthe Niang, A., et al. (2020). Fonio and bambara groundnut value chains in mali: issues, needs, and opportunities for their sustainable promotion. Sustainability 12, 4766. doi: 10.3390/su12114766
Mubaiwa, J., Fogliano, V., Chidewe, C., and Linnemann, A. R. (2018). Bambara groundnut (Vigna subterranea (L.) Verdc.) flour: a functional ingredient to favour the use of an unexploited sustainable protein source. PloS one 13, e0205776. doi: 10.1371/journal.pone.0205776
Nosheen, S., Ajmal, I., and Song, Y. (2021). Microbes as biofertilizers, a potential approach for sustainable crop production. Sustainability 13, 1868. doi: 10.3390/su13041868
Nyaga, J. W., and Njeru, E. M. (2020). Potential of native rhizobia to improve cowpea growth and production in semiarid regions of Kenya. Front. Agron. 2, 606293. doi: 10.3389/fagro.2020.606293
Okogun, J., and Sanginga, N. (2003). Can introduced and indigenous rhizobial strains compete for nodule formation by promiscuous soybean in the moist savanna agroecological zone of Nigeria? J. Biol. Fertil. Soils 38, 26–31. doi: 10.1007/s00374-003-0611-8
Olanrewaju, O. S., Oyatomi, O., Babalola, O. O., and Abberton, M. (2021a). Genetic diversity and environmental influence on growth and yield parameters of Bambara groundnut. Front. Plant Sci. 12, 352. doi: 10.3389/fpls.2021.796352
Olanrewaju, O. S., Oyatomi, O., Babalola, O. O., and Abberton, M. (2021b). GGE Biplot analysis of genotype × environment interaction and yield stability in bambara groundnut. Agronomy 11, 1839. doi: 10.3390/agronomy11091839
Raghuwanshi, R. (2012). Opportunities and challenges to sustainable agriculture in India. Nebio 3, 78−86. doi: 10.1007/978-981-15-3151-4_5
Santos, M. S., Nogueira, M. A., and Hungria, M. (2019). Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. Amb Exp. 9, 1–22. doi: 10.1186/s13568-019-0932-0
Singh, S. K., Wu, X., Shao, C., and Zhang, H. (2022). Microbial enhancement of plant nutrient acquisition. Stress Biol. 2, 1–14. doi: 10.1007/s44154-021-00027-w
Tan, X. L., Azam-Ali, S., Goh, E. V., Mustafa, M., Chai, H. H., Ho, W. K., et al. (2020). Bambara groundnut: an underutilized leguminous crop for global food security and nutrition. Front. Nutri. 7, 601496. doi: 10.3389/fnut.2020.601496
Thilakarathna, M. S., and Raizada, M. N. (2017). A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol. Biochemistr. 105, 177–196. doi: 10.1016/j.soilbio.2016.11.022
Thomas, L., and Singh, I. (2019). Microbial Biofertilizers: Types and Applications Biofertilizers for Sustainable Agriculture and Environment. New York, NY: Springer, 1–19.
Uzoh, I. M., and Babalola, O. O. (2018). Rhizosphere biodiversity as a premise for application in bio-economy. Agricult. Ecosyst. Environ. 265, 524–534. doi: 10.1016/j.agee.2018.07.003
Vincent, J. M. (1970). A manual for the practical study of the root-nodule bacteria. J. Basic Microbiol. 12, 440. doi: 10.1002/jobm.19720120524
Walkley, A., and Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Woomer, P. L. (1994). Most probable number counts. J. Meth. Soil Anal. Part 2 Microbiologic. Biochem. Prop. 5, 59–79. doi: 10.2136/sssabookser5.2.c5
Yadav, K. K., and Sarkar, S. (2019). Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environ. Ecol. 37, 89–93. Available online at: http://www.environmentandecology.com
Keywords: inoculation, bacteria strains, underutilized legume, fertilizer, yield
Citation: Bitire TD, Abberton M, Oyatomi O and Babalola OO (2023) Yield response of accessions of Bambara groundnut (Vigna subterranea (L) Verdc) inoculated with Bradyrhizobium japonicum strains. Front. Sustain. Food Syst. 7:1142123. doi: 10.3389/fsufs.2023.1142123
Received: 11 January 2023; Accepted: 27 February 2023;
Published: 27 March 2023.
Edited by:
Matteo Balderacchi, Independent Researcher, Piacenza, ItalyReviewed by:
Amritbir Riar, Research Institute of Organic Agriculture (FiBL), SwitzerlandMarika Pellegrini, University of L'Aquila, Italy
Copyright © 2023 Bitire, Abberton, Oyatomi and Babalola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olubukola Oluranti Babalola, T2x1YnVrb2xhLmJhYmFsb2xhQG53dS5hYy56YQ==
 Tope Daniel Bitire
Tope Daniel Bitire Michael Abberton
Michael Abberton Olaniyi Oyatomi
Olaniyi Oyatomi Olubukola Oluranti Babalola
Olubukola Oluranti Babalola