- 1Campus Rio Verde, Goiano Federal Institute, Rodovia Sul Goiana, Rio Verde, Goiás, Brazil
- 2Agronomy Department, Agronomy School, Federal University of Goiás (UFG), Goiânia, Brazil
- 3Department of Biochemical Engineering, School of Chemistry, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil
The demand for dyes from natural sources to substitute synthetic dyes for application in the food industry has been continuously increasing due to some synthetic dyes being associated with several problems, including hypersensitivity, carcinogenesis, and negative environmental impacts. Furthermore, dyes from natural sources (like pigments) are generally regarded by the consumer as safer or with fewer side effects—a fact that requires in-depth investigation—, which increases the commercial interest in such products. In this sense, great focus has been given to the biotechnological potential of Monascus sp. to produce red, orange, and yellow pigments using different types of the fermentation process (submerged or in solid-state fermentation), substrates, and process parameters (temperature, pH, agitation, aeration, etc.), aiming at optimizing and reducing costs in pigment production. In general, Monascus pigment has shown stability at neutral and basic pH, at elevated temperatures for a few hours, and to some metallic ions while not showing stability at acidic pH, elevated temperatures for many hours, and in the presence of light. Applications of Monascus pigment with colorant function in foods (candies, bread, yogurt, cheese, beer, and meat products) reported improvement in the color aspect by sensory analysis. The application of Monascus pigment still seems promising and incipient, demonstrating that it needs to be further studied, mainly concerning the stability of the pigment in vivo systems (inside the food) where adverse conditions are combined. Regulatory issues are heterogeneous around the world, which creates difficulties to expand production and commercialization but also demonstrates the need for studies to confirm its safety. In this sense, this mini-review presents the potential, strategies, and challenges of Monascus pigment for food application.
1. Introduction
The global demand for natural pigments is constantly growing, a fact that place pressure on the sector to offer more products to meet this demand. In this sense, was estimated that the global pigment market—considering the demand from textiles, food and beverages, paints and coatings, and cosmetic industries— was USD 2.0 billion in 2021, reaching USD 2.8 billion by 2028. And this growth is also in line with the demand from the consumer for sustainable raw materials (MarketWatch, 2022).
The use of pigments is part of human culture. This pigment application aims to (i) make the products where they are applied more attractive for consumption and (ii) afford, standardize, or intensify the color, providing identity to certain products, among others (Manan et al., 2017; Muthusamy et al., 2020). In the food manufacturing process and during storage, the original color can often be lost or degraded. Faced with this obstacle, the food industry resorts to the use of synthetic (synthesized/produced in a laboratory or industrial setting) or natural dyes (substances derived from natural sources) to correct or provide original color, improving the attractiveness of products for consumption and maintaining their visual identity (Dikshit and Tallapragada, 2018).
In this context, pigments from natural sources such as plants (flowers, fruits, and leaves), animals (insects), and microorganisms (fungi, bacteria, yeasts, and algae) stand out because they act as dyes (Sudhakar et al., 2016; Muthusamy et al., 2020; Aman Mohammadi et al., 2022). The increase in demand for pigments can be associated with the consumer market's perception that this type of product is safer and more environmentally friendly (Kobylewski and Jacobson, 2012). It is commonly reported that pigments are considered safe, non-toxic, non-carcinogenic, biodegradable, and of low risk to the environment (Wrolstad and Culver, 2012; Dikshit and Tallapragada, 2018) as they do not interfere with the aquatic biota, not showing an undesirable/harmful tendency toward allergic reactions, intolerances, in addition to other effects such as mutagenicity and potential carcinogenic effect (Dikshit and Tallapragada, 2018; Aman Mohammadi et al., 2022). However, like any other product available for use, natural pigments need to be extensively studied for toxicity and safety (Kobylewski and Jacobson, 2012), as several other widely used components have been banned due to proven adverse effects, including synthetic dyes such as ponceau, tartrazine, and sunset yellow, for example (Kobylewski and Jacobson, 2012; Poorniammal et al., 2021). Additionally, is important to emphasize that legal aspects related to doses and approval of natural pigments use are important for feasibility and safety of use (Commission Regulation, 2014).
Thus, due to its high demand, the evaluation of toxicology, economical, and technologically viable new sources of natural pigments has increased. Depending on the type of pigment and the color that is necessary to assign to the food, the extraction of pigments of plant origin in high quantities may not be feasible, in addition to their limited application due to low thermal stability or at different pHs. Pigments of plant origin may not be available throughout the year and their products depend on environmental conditions, demand a large area of cultivation, and compete with vegetables that would be destined for human consumption (Sudhakar et al., 2016; Fernández-López et al., 2020; Poorniammal et al., 2021). In addition, the pigment extraction process often generates a large amount of waste material for the extraction of a small amount of pigments (Sharma et al., 2021).
The pigments produced by microorganisms have shown low production costs compared with plant pigments –especially when agro-industrial waste and by-products are used as a substrate for their production (Lemes et al., 2021, 2022). It is important to highlight that the question of costs for the use of by-products is still a challenge –but dependent on the type of by-product, seasonality, and quantity generated, as well as the process used, geographic location, among other factors– and need to be overcome for complete viability of the process (Carvalho et al., 2023).
On the other hand, microbial pigments present higher yields, as well as simpler and cleaner extraction and purification processes compared with plant pigments. Pigments from microorganisms show no seasonal variation (as with pigments extracted from plant sources, for example), and demonstrate the possibility of improvements in the process to increase production yield and ease of scalability (Galaffu et al., 2015; Panesar et al., 2015; Aman Mohammadi et al., 2022; Bakhshi et al., 2022).
In addition to benefiting the coloring of products—and overcoming toxicological and regulatory issues—pigments of microorganisms can provide health benefits when consumed, as their composition contains bioactive compounds from the secondary metabolism of the producing organism itself, including alkaloids, phenols, flavonoids, polysaccharides, terpenes, and several other widely studied compounds. These bioactivities enable a reduction of the incidence of many diseases of cardiovascular origin, in addition to chronic degenerative diseases and cancer, in addition to acting as antioxidant and antimicrobial components (Lavecchia et al., 2013).
In this sense, among several microorganisms producing pigments, the natural pigment produced by Monascus sp. –which is used in products and processes of several countries specially in Asia– has gained huge prominence for food application (the advantages are presented in item 2), demonstrating the potential for the production of yellow, orange, and red pigments by use of different types of the fermentation (submerged or in solid-state fermentation), substrates (synthetic, natural and also by-products), and process parameters (temperature, pH, agitation, aeration, etc.,). Furthermore, Monascus pigment has been associated with antioxidant, antiosteoporosis, anti-inflammatory, and antimicrobial, properties, as well as anti-diabetic, antidepressant, anticancer, antiobesity, neurocytoprotective, antihypertensive, and hepatoprotective effects (Feng et al., 2016; Agboyibor et al., 2018; Chaudhary et al., 2022). In this way, this mini-review presents the trend of production and application Monascus pigment in foods, as well as its strategies and challenges.
2. Monascus sp. and its pigment production potential
Monascus is a homothallic fungus related to the Ascomycetes class and Monascaceae family and are the famous producers of several important secondary metabolites, for example, pigments (Mahmoud et al., 2021). The genus Monascus is described as a filamentous, aerobic, saprophytic, prototrophic, mesophilic (optimum temperature of 30–35°C), xerophilic fungus, with respiration-fermentative metabolism (Feng et al., 2016; Agboyibor et al., 2018), and reproduction both sexually (asci) and asexual (conidia). Over glucose in the culture medium, microorganisms of this genus can form ethanol under aerobic conditions, and therefore can be classified as Crabtree negative with limited respiration (Crabtree, 1929).
Monascus fungus has 7 species belonging to the genus, but the most relevant species in the food industry are Monascus purpureus, Monascus ruber, and Monascus pilosus (Carvalho et al., 2005; Agboyibor et al., 2018). The advantage of this genus over other microorganisms is due to the easy production of the pigment mainly on non-expensive substrates and this pigment presents characteristics such as bioactive properties as antioxidant and antimicrobial activities, good solubility in water and ethanol that facilitates the extraction step, and also its safety for consumption (Vendruscolo et al., 2016).
Monascus are filamentous fungi and can grow on simple substrates and produce different types of metabolites, including alcohols, vitamins, enzymes, polysaccharides, fatty acids, flavor compounds, flocculants, ketones, organic acids, and antioxidant, antibiotic, and antihypertensive agents (Vendruscolo et al., 2016). The growth and secondary metabolism of these microorganisms are directly influenced by several cofactors, such as the sources of carbon and nitrogen and conditions used to obtain the compound including pH, and temperature, among others (Chen and Johns, 1994; Huawei et al., 2019).
The secondary metabolites, like occur with natural pigments, produced by M. purpureus and M. ruber are extremely important and have an economic impact (Agboyibor et al., 2018; Chaudhary et al., 2022). Among the pigments produced by Monascus sp. are ankaflain and monascin (yellow), monascorubrin and rubropunctatin (orange), and monascorubramine and rubropunctamine (purple-red) (Figure 1) (Kim and Ku, 2018). The pigments produced by Monascus sp. have a stereotype of azaphilones, which are a family of natural cyclic compounds that have at least one chiral center. These azaphilones have a great interest in demonstrating biological activities, which include adipogenesis and lipolysis (antiobesity effect), anticancer, and anti-inflammatory activities, as well as antidepressant, anti-osteoporosis, and anti-diabetic effects, and others (Gao et al., 2013; Agboyibor et al., 2018).
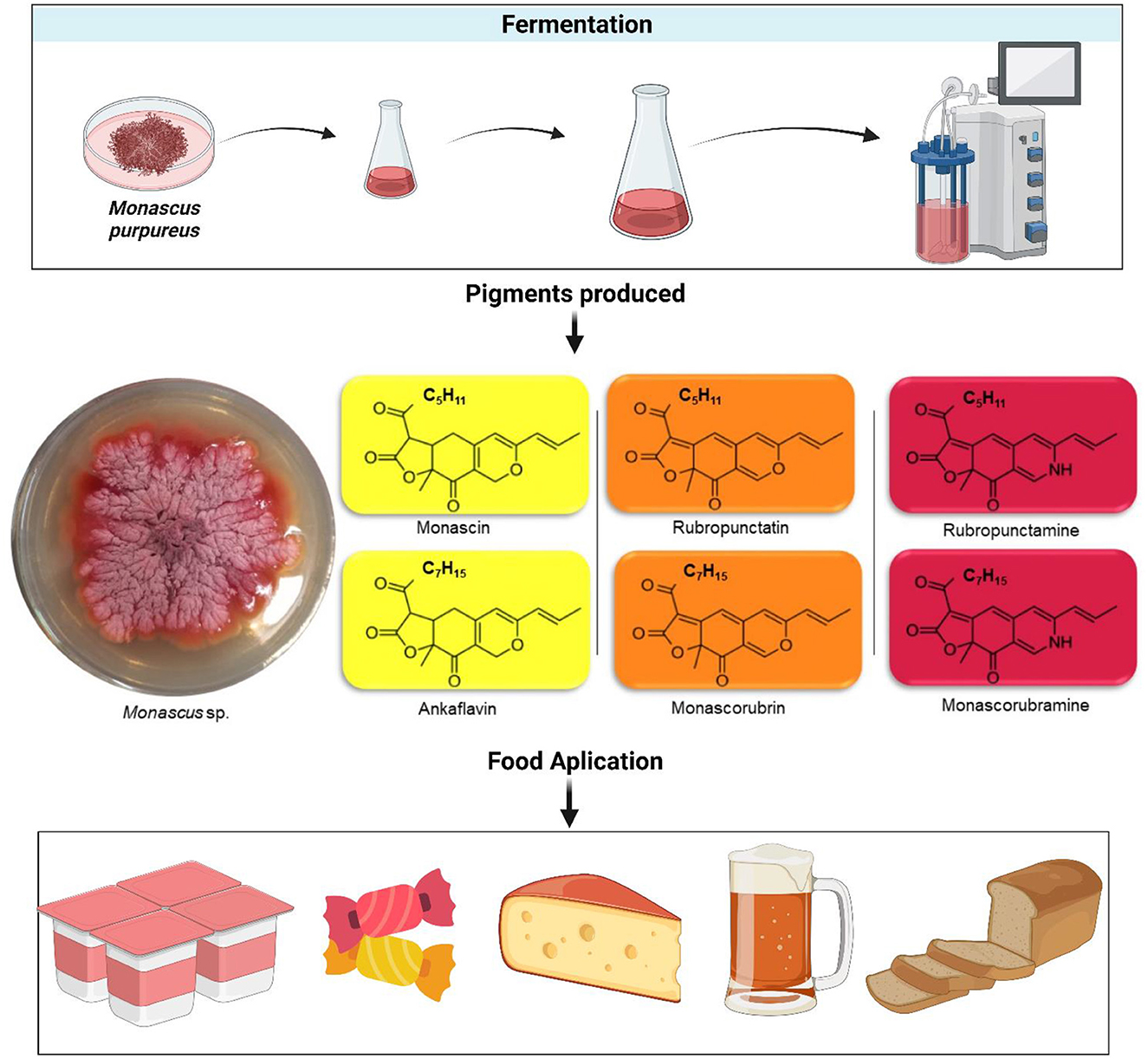
Figure 1. Fermentation scheme, chemical structure, and food application of pigments produced by Monascus species.
Obtaining pigments from fermentation using Monascus can be carried out by (i) solid-state fermentation and (ii) submerged fermentations. The (i) solid-state fermentation consists of cultivation with a low amount of water (De Carvalho et al., 2006; Zhang et al., 2013; Almeida et al., 2019) using a wide variety of agricultural substrates such as fruit pulp, grains, straw, and bagasse (Lemes et al., 2021). In addition, solid-state fermentation has low water content in the medium reducing contamination problems, and offers high volumetric productivity, concentrated target compounds, tolerance of high substrate concentration, and less waste and water generation (Lemes et al., 2021). On the other hand, solid-state fermentation presents difficulty in scale-up and control of process parameters such as pH, heat, moisture, and nutrient homogenization, among other, problems with heat build-up and higher impurity product, increasing the complexity of the recovery protocol due to the heterogeneity of the medium and complexity of the matrix used, impacting recovery and purification costs (Couto and Sanromán, 2006).
The (ii) submerged fermentation that are the microorganisms growing in a liquid medium with nutrients, which demonstrates the ease of homogenization and process control such as oxygenation, pH, and temperature (Agboyibor et al., 2019; Chai et al., 2020; Abdollahi et al., 2021; Abuthahir et al., 2021; Chen et al., 2021; de Almeida et al., 2021). In addition, the pigments are secreted into the fermentation medium and then recovered in a separation step, such as centrifugation or using simple solvent extraction (Lemes et al., 2021). Furthermore, submerged fermentation allows the proper mixing of nutrients due to the high amount of free water and is a method of easy handling and scaling up. Nevertheless, the target products tend to be diluted at the end of fermentation (Lemes et al., 2021), but they can be easily solved using membrane concentration processes or a suitable technique for pigment processing (Vandanjon et al., 1999).
Regardless of the method chosen for fermentation, the availability of nutrients in the chosen medium and the fermentation conditions directly impact the synthesis of pigments by the species of fungus used, making it necessary to investigate and provide a broad interpretation of primary and secondary metabolism (Carvalho et al., 2005; Agboyibor et al., 2018).
Pigments obtained from the secondary metabolism of Monascus sp. are originates from medium-chain fatty acids, such as octanoic acid, which in turn are synthesized by the fatty acid metabolic pathway and bind to the chromophore structure through a transesterification reaction, resulting in the orange pigment (monascorubrin—C23H26O5 or rubropunctatin—C21H22O5 in trans-esterification with octanoic acid). The reduction of the orange pigment, monascorubramine, gives rise to the yellow pigment (ankaflavin—C23H30O5 or monascin—C21H26O5). The red pigments (monascorubramine—C23H27NO4 and rubropunctamine—C21H23NO4) are produced by the reaction of the orange pigment with compounds that contain NH3 and NH2 in the molecule (Feng et al., 2016; Chen et al., 2017).
Once produced, pigments need to be properly obtained, separated, and identified, since their value and application are directly related to purity degree (Mukherjee and Singh, 2011) and stability, which can be affected by factors such as light, oxygen, and metal ions presence, pH levels, as well as high temperatures (Vendruscolo et al., 2016; Abdollahi et al., 2021). Separation methods vary according to the location of the pigment (intracellular or extracellular) production and the need for use for each application. The ratio of intracellular and extracellular pigments produced by Monascus is dependent on fungus culture conditions, such as carbon, nitrogen, and pH sources. Therefore, conditions can be used to convert intracellular into extracellular pigments such as cultivation at pH 8.5 which reduces from 75 to 17% intracellular pigments (Orozco and Kilikian, 2008). As the aim of the industry is not normally to add steps in the production process, pigment extraction has been directed toward extracellular produced pigments. Monascus extracellular pigments can be obtained using appropriate organic solvents (Carvalho et al., 2005) and simple, low-cost, and easy physical methods considered environmentally friendly such as separation by centrifugation or filtration (Almeida et al., 2019; de Almeida et al., 2021). On the other hand, intracellular pigments—less frequently used due to their difficulty of extraction and low yield—need to be “released” using some method that promotes cell lysis (rupture of the cell wall) for subsequent separation, which can involve multiple steps as the use of specific organic solvents (Carvalho et al., 2005), ultrasound-assisted extraction (Kraboun et al., 2017), extractive fermentation with surfactant (Chen et al., 2018), ethanol/ammonium sulfate aqueous two-phase system (Dong et al., 2020), among several other procedures. Each of the operations used can significantly affect the yield, purity, and functional properties of the target compound (Gautério et al., 2022).
The methods of pigments identification and/or purification that may or may not be used for Monascus pigment are including ultraviolet (UV) spectrometer, luminescence, spectrophotodensitometer, nuclear magnetic resonance spectroscopy, enzyme immunoassay (ELISA), immunochromatographic tests, capillary zone electrophoresis, thin layer chromatography (TLC), mass spectrometry, capillary chromatography micellar electrokinetics, mass spectrometry, high-performance liquid chromatography (HPLC) with UV light, fluorescence (FLD), liquid chromatography-mass spectrometry (LC-MS), LC-MS/MS, LC-FLD, ultra-performance liquid chromatography-quadrupole/time-of- flight mass spectrometry (UHPLC-DADQToF-MS), and gas chromatography-mass spectrometry (GC-MS). Among these, HPLC is the most used technique for the determination of 80% of organic compounds worldwide due to its precision and is considered the most effective in the detection of natural pigments (Liu and Chen, 2019; Singh and Mehta, 2020).
In some cases, a sequence or combination of the mentioned techniques can be used for separation and better identification of the pigments, allowing the complete characterization of the compound, as well as the verification of its purity and even its stability. These determinations are important to ensure standardization, comply with regulatory aspects in different countries, avoid adulterations, and even check for the presence of undesirable components, such as toxic components, among others, allowing for proper and safe application in products. However, the application of Monascus pigment has also been carried out directly, that is, the fermented extract itself where the pigment was produced is added to the food product without the need for isolation and/or purification (as will be discussed in subitem 3).
3. Monascus pigment for food applications: Trends and challenges
The addition of natural pigments to food has several advantages, with the main purpose of stimulating and improving sensory perception, in addition to reducing the use of synthetic components, due to health and safety concerns (Egea and Marcionilio, 2022). In this sense, due to its production capacity, the Monascus pigment is often evaluated for its application in food and beverages, including dairy products, candies, bakery products, cereals, meat products, and beverages, among others (Table 1) (Mal'a et al., 2010; Gomah et al., 2017; Darwesh et al., 2020; Monteiro et al., 2021; Abdel-Raheam et al., 2022; Srianta et al., 2022).
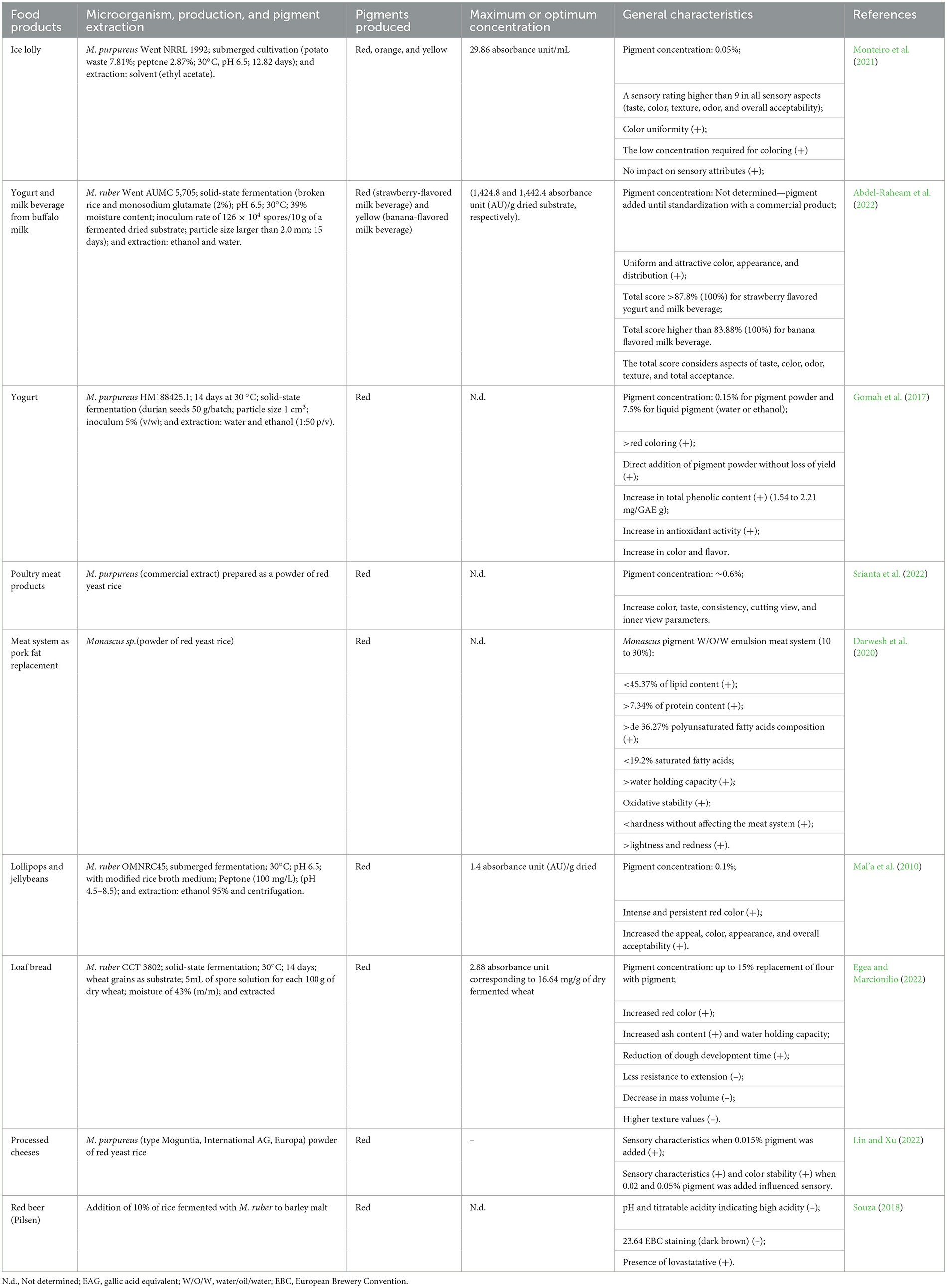
Table 1. Applications of Monascus purpureus and Monascus ruber pigments in food products (2000–2022 years) (+) positive and (–) negative aspects.
With the advent of the plant-based market, dyes for application in meat analogs, especially red dyes, have been studied more to meet the needs of the industry. To our surprise, only two of the studies that we found tested Monascus pigment in meat products (Mal'a et al., 2010; Zheng et al., 2023). In meat analogs currently, the main colorings used are annatto extracts (E 160b), lycopene, beet juice extract, or leghemoglobin to imitate meat red color, and titanium dioxide to imitate chicken color. However, when using these dyes, ingredients to promote thermal stability must be added (Boukid, 2021; Ishaq et al., 2022).
As with any component applied to food, pigments are subject to changes triggered by the physical-chemical characteristics of the matrices, as well as by operating conditions (pH, acidity, temperature, salts, among others) during processing and shelf life, and may impact on pigment degradation, structural alteration, and even the loss of bioactive, technological, and sensorial capacity, mainly about its colorific capacity (Egea and Marcionilio, 2022; Oliveira Filho et al., 2023).
In this sense, the Monascus pigment has already had its stability evaluated under different conditions (Abdollahi et al., 2021; de Almeida et al., 2021), to allow its application and/or improvement of its stability to avoid changes in its properties (Vendruscolo et al., 2013; Xu et al., 2020a). Among the main limitations, related to its degradation, factors such as light, oxygen, metal ions, pH levels, and high temperatures are always highlighted (Agboyibor et al., 2018; Abdollahi et al., 2021), which vary according to the producing microorganism, way of obtaining, and method of extraction, among others.
Regarding pigment stability, it has also been reported that the stability is affected by different types of solvents during the extraction process, with an influence on yield, stability, and pigmentation capacity (Srianta et al., 2022). Furthermore, Monascus pigment does not completely lose pigmentation at temperatures up to 97.8°C (Abdollahi et al., 2021; da Silva et al., 2022), pH in the range of 3 to 8 (da Silva et al., 2022), presence of several components, including salts (2, 5%) (Abdollahi et al., 2021) and solvents (Srianta et al., 2022), which corroborates and justifies its efficient application in different food matrices.
As reported in Table 1, the different Monascus pigments have already been produced in different presentations (liquid and powder) and applied in different products such as cheese, yogurt, lollipops, jellybeans, meat products, bread, and beers demonstrating an impact positive in color, attractiveness, uniformity of color, pigment stability, in addition to high sensory scores for all aspects evaluated (taste, color, odor, texture, and total acceptance). In addition, it was verified that the addition of pigment confers the presence of bioactive substances, mainly phenolic compounds, with special antioxidant and antimicrobial activities. In all the cases cited, the added pigment seemed to withstand the process conditions, not being degraded to the point of making its application unfeasible, much less influencing the attractiveness and acceptance by the consumers (Mal'a et al., 2010; Gomah et al., 2017; Darwesh et al., 2020; Monteiro et al., 2021; Abdel-Raheam et al., 2022; Srianta et al., 2022).
Several strategies can be used to guarantee the stability of the pigment, such as pigment encapsulation using precipitation, spray or freeze drying, or liposome membranes methods with a different encapsulating agent (dextrin, maltodextrin, sodium caseinate, gum Arabic, among others) and carrier agents (propylene glycol, mannitol, among others), which can result in products with different structures and stability profiles (Priatni, 2015; Jian et al., 2017; Xu et al., 2020b; Ali et al., 2022; Long et al., 2023).
In the case of the protection offered by the sodium caseinate microcapsule, for example, the thermal degradation (100°C) of the encapsulated Monascus pigment demonstrated to follow the degradation of the pigment without encapsulation when the heating time is increased at pH 3.0 (Ali et al., 2022). On the other hand, the microcapsule can contribute by increasing the solubility and decreasing the precipitation of the Monascus pigment in an acid medium (Jian et al., 2017; Ali et al., 2022).
Spray or freeze/dried encapsulation methods require efforts to verify encapsulating agents and carriers that demonstrate better performance in preserving the Monascus pigment color after the process has been carried out. For example, Xu et al. (2018) verified that the combination of maltodextrin as an encapsulating agent and mannitol as a carrier agent increases the hydrophilicity and solubility of the encapsulated Monascus pigment, resulting in a greater red color.
Another way to increase the stability of Monascus pigment using the spray drying/freeze drying encapsulation process is to associate this process with combined treatment, for example, the emulsification/internal gelation method. Zhang et al. (2023) demonstrated that thermal degradation (50–90°C) was lower when spray-dried followed by freeze-dried combined with emulsification/internal gelation were applied compared to Monascus pigment extract without application of emulsification technique /internal gelation. Furthermore, even encapsulated, Monascus pigment showed low stability to illumination treatment (500 lx), pH below 5 and above 9, and the presence of Ca2+, Cu2+ and Fe2+. This occurs because the emulsification/internal gelation technique combined with drying methods results in a controlled release of Monascus pigment under specific conditions, improving the stability profile of the pigment.
Encapsulation of Monascus pigment using liposome membranes using a thin-film ultrasonic method demonstrated stability at pH (2, 3, 4, 5, 6, 7, 8, 9, and 10), thermal (water bath at 60, 70, 80, and 90°C/5 h), light (500 lx), storage in refrigeration (4°C for 30 days in the dark), and in vitro simulated gastrointestinal digestion stability and stronger inhibitory effect on MKN-28 cells (gastric cancer cell line) by damaging the integrity of cells (Long et al., 2023). In addition, liposomes containing rubropunctatin (a Monascus pigment) demonstrated greater water solubility, stability to light (tungsten lamp (500 W, wavelength of 597–622 nm), also increasing anticancer activity and stimulating apoptosis-promoting mechanism (Xu et al., 2020b).
Although the encapsulation processes seem efficient in increasing the stability of Monascus pigment, the literature still lacks the application of these encapsulates, regardless of the method, directly in the food product where all adverse conditions are combined (acidic pH, high processing temperatures, exposure light during storage, among others).
However, for products that present adequate stability, the most common is to add them before the heat treatment to ensure the microbiological safety of the food, having already verified the maintenance of its properties in time/temperature binomials of 68°C/30 min, 90°C/5 min, 70°C/10 min, among others (Abdel-Raheam et al., 2022). In cases where the pigment has reduced stability, measures such as the addition after heat treatment can be considered, requiring additional care to avoid contamination that seems to have minor effects on the degradation of pigments such as sterilization procedures with filtering membranes and also the use of high pressure, ultrasound, for example (Sant'Ana, 2014; Singh et al., 2019). In addition, auxiliary stability-improving techniques, such as nanotechnology, can be successfully employed in this regard (Bhandari et al., 2022).
In addition to the concern with the stability of the pigment for application in food products, another concern is related to the regulatory and safety aspects of the Monascus pigment. Safety aspects for food products are still not very homogeneous across the world, since the use of Monascus pigment is restricted for application in products in the United States and the European Union countries, but allowed in Asian countries, where it is traditionally consumed in typical products (Manan et al., 2017; Liu et al., 2018). In countries where fermented products containing Monascus pigment are allowed, there is a position that the amount of pigment must be controlled, so for these countries, the safety of using Monascus pigment is related to the amount ingested (Commission Regulation, 2014). In the case of countries where the use of Monascus pigment is still not allowed, it is argued that the aspects of health hazards—the same ones related to synthetic pigments— are not yet fully elucidated and should be better analyzed before releasing for use. In addition, some countries raise the possibility of production and high doses of citrinin—a mycotoxin displaying hepatotoxic and nephrotoxic effects on humans—as one of the reasons for banning it, but which can be circumvented with the genetic manipulation of strains that do not produce the component, or with the control of the conditions of the fermentation process (Lin and Xu, 2022). In some cases, limits have been established for the presence of citrinin in food products containing Monascus pigment, such as in Japan, European countries, and the United States (Commission Regulation, 2014).
Thus, it is clear that the Monascus pigment is an important ingredient for the development of food products and can be used properly to improve the attractiveness, as well as the nutritional and bioactive aspects of food, provided that the limiting operational conditions are respected.
4. Conclusion and future directions
Several advantages related to the production and use of the Monascus pigment are presented when compared to synthetic and vegetable pigments, but it is still necessary to advance in points such as its economic viability, toxicity, mycotoxin production depending on the strain, safe consumption doses, among others, to make its application homogeneous, unrestricted and safe.
Regulatory aspects are still quite divergent around the world—as with any other product still in the implementation phase—and are mainly related to the citrinin content and the lack of studies on the effects of Monascus pigments and their doses on human health, which must be investigated and answered appropriately.
The main advantages associated with Monascus sp. for pigment production are related to its high pigment production capacity using non-expensive substrates in its cultivation and production of pigments with bioactive properties, good solubility (water and ethanol), and sensorial properties. The Monascus pigment can be efficiently applied in food with a color impact, conferring attractiveness, pleasant appearance, and color uniformity as well as promoting sensory aspects such as taste, color, odor, texture, and total acceptance.
Another aspect to be considered is the need to apply auxiliary stabilization techniques depending on the operational processing conditions, which may include the use of encapsulation in different systems, for example. Furthermore, economical and environmentally viable strategies must be studied, to offer a stable and low-cost pigment.
Additionally, due to its bioactive properties, with health effects, including anti-obesity activity as well as obesity-related-diseases, such as hyperlipidemia, steatohepatitis, and hyperglycemia the pigment stands out as an emerging compound in the pharmaceutical industry, which further contributes to the need for in-depth studies.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES—Finance Code 001), FAPEG, Conselho Nacional de Desenvolvimento Científico (CNPq), and Instituto Federal Goiano (IF Goiano/PROPPI - Process nos. 23216.000940.2022-92 and 23218.000604.2023-19).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Raheam, H. E., Alrumman, S. A., Gadow, S. I., El-Sayed, M. H., Hikal, D. M., Hesham, A. E-. L., et al. (2022). Optimization of monascus purpureus for natural food pigments production on potato wastes and their application in ice lolly. Front. Microbiol. 13, 862080. doi: 10.3389/fmicb.2022.862080
Abdollahi, F., Jahadi, M., and Ghavami, M. (2021). Thermal stability of natural pigments produced by monascus purpureus in submerged fermentation. Food Sci. Nutr. 9, 4855–62. doi: 10.1002/fsn3.2425
Abuthahir, S. S. F., Venil, C., Malathi, M., and Devi, P. R. (2021). Optimization of submerged fermentation for enhanced production of canthaxanthin by dietzia maris Aurccbt01. Mater. Today Proceed. 47, 2132–7. doi: 10.1016/j.matpr.2021.05.150
Agboyibor, C., Kong, W-. B., Chen, D., Zhang, A-. M., and Niu, S-. Q. (2018). Monascus pigments production, composition, bioactivity and its application: a review. Biocatal. Agric. Biotechnol. 16, 433–47. doi: 10.1016/j.bcab.2018.09.012
Agboyibor, C., Kong, W-. B., Zhang, A-. M., and Niu, S-. Q. (2019). Nutrition regulation for the production of monascus red and yellow pigment with submerged fermentation by monascus purpureus. Biocatal. Agric. Biotechnol. 21, 101276. doi: 10.1016/j.bcab.2019.101276
Ali, I., Al-Dalali, S., Hao, J., Ikram, A., Zhang, J., Xu, D., et al. (2022). The stabilization of monascus pigment by formation of monascus pigment-sodium caseinate complex. Food Chem. 384, 132480. doi: 10.1016/j.foodchem.2022.132480
Almeida, A., Lima, T., Santos, N., Santana, R., Santos, S., and Egea, M. (2019). An alternative for corn bran byproduct: fermentation using monascus purpureus. Nutr. Food Sci. 50, 515–527. doi: 10.1108/NFS-06-2019-0177
Aman Mohammadi, M., Ahangari, H., Mousazadeh, S., Hosseini, S. M., and Dufossé, L. (2022). Microbial pigments as an alternative to synthetic dyes and food additives: a brief review of recent studies. Bioprocess Biosyst. Eng. 45, 1–12. doi: 10.1007/s00449-021-02621-8
Bakhshi, F., Jahadi, M., Ghasemisepro, N., and Jahanfar, S. (2022). Modeling red monascus pigment production on date waste substrate using submerged cultivation. J. Food Biosci. Technol. 12, 15–26.
Bhandari, M., Sharma, R., Sharma, S., Bobade, H., and Singh, B. (2022). Recent advances in nanoencapsulation of natural pigments: emerging technologies, stability, therapeutic properties and potential food applications. Pigment Resin Technol. doi: 10.1108/PRT-04-2022-0050
Boukid, F. (2021). Plant-based meat analogues: from niche to mainstream. Europ. Food Res. Technol. 247, 297–308. doi: 10.1007/s00217-020-03630-9
Carvalho, A. S. S., Sales, J. C. S., Nascimento, F. V., Ribeiro, B. D., Souza, C. E. C., Lemes, A. C., et al. (2023). Lipase production by yarrowia lipolytica in solid-state fermentation using amazon fruit by-products and soybean meal as substrate. Catalysts 13, 289. doi: 10.3390/catal13020289
Carvalho, J. C., Oishi, B. O., Pandey, A., and Soccol, C. R. (2005). Biopigments from monascus: strains selection, citrinin production and color stability. Brazil. Arch. Biol. Technol. 48, 885–94. doi: 10.1590/S1516-89132005000800004
Chai, X., Ai, Z., Liu, J., Guo, T., Wu, J., Bai, J., et al. (2020). Effects of pigment and citrinin biosynthesis on the metabolism and morphology of monascus purpureus in submerged fermentation. Food Sci. Biotechnol. 29, 927–37. doi: 10.1007/s10068-020-00745-3
Chaudhary, V., Katyal, P., Poonia, A. K., Kaur, J., Puniya, A. K., Panwar, H., et al. (2022). Natural pigment from monascus: the production and therapeutic significance. J. Appl. Microbiol. 133, 18–38. doi: 10.1111/jam.15308
Chen, G., Wang, M., Tian, X., and Wu, Z. (2018). Analyses of monascus pigment secretion and cellular morphology in non-ionic Surfactant Micelle Aqueous solution. Microb. Biotechnol. 11, 409–19. doi: 10.1111/1751-7915.13038
Chen, M-. H., and Johns, M. R. (1994). Effect of carbon source on ethanol and pigment production by monascus purpureus. Enzyme Microb. Technol. 16, 584–90. doi: 10.1016/0141-0229(94)90123-6
Chen, W., Chen, R., Liu, Q., He, Y., He, K., Ding, X., et al. (2017). Orange, red, yellow: biosynthesis of azaphilone pigments in monascus fungi. Chem. Sci. 8, 4917–25. doi: 10.1039/C7SC00475C
Chen, X., Gui, R., Li, N., Wu, Y., Chen, J., Wu, X., et al. (2021). Production of soluble dietary fibers and red pigments from potato pomace in submerged fermentation by monascus purpureus. Process Biochem. 111, 159–66. doi: 10.1016/j.procbio.2021.09.011
Commission Regulation (2014). No 212/2014 of 6 March 2014 Amending Regulation (Ec) No. 1881/2006 as Regards Maximum Levels of the Contaminant Citrinin in Food Supplements Based on Rice Fermented with Red Yeast Monascus Purpureus.
Couto, S. R., and Sanromán, M. A. (2006). Application of solid-state fermentation to food industry—a review. J. Food Eng. 76, 291–302. doi: 10.1016/j.jfoodeng.2005.05.022
Crabtree, H. G. (1929). Observations on the carbohydrate metabolism of tumours. Biochem. J. 23, 536. doi: 10.1042/bj0230536
da Silva, J. R., da Silva, T. T., de França Queiroz, A. E. S., Moreira, K. A., and Ribeiro, D. S. (2022). Avaliação do processo de liofilização na estabilidade dos pigmentos produzidos por monascus purpureus Cct 3802. Res. Soc. Develop. 11, e97111738807. doi: 10.33448/rsd-v11i17.38807
Darwesh, O. M., Matter, I. A., Almoallim, H. S., Alharbi, S. A., and Oh, Y-. K. (2020). Isolation and optimization of monascus ruber Omnrc45 for red pigment production and evaluation of the pigment as a food colorant. Appl. Sci. 10, 8867. doi: 10.3390/app10248867
de Almeida, A. B., Santos, N. H., de Lima, T. M., Santana, R. V., de Oliveira Filho, J. G., et al. (2021). Pigment bioproduction by monascus purpureus using corn bran, a byproduct of the corn industry. Biocatalysis Agric Biotechnol. 32, 101931. doi: 10.1016/j.bcab.2021.101931
De Carvalho, J. C., Pandey, A., Oishi, B. O., Brand, D., Rodriguez-Léon, J. A., Soccol, C. R., et al. (2006). Relation between growth, respirometric analysis and biopigments production from monascus by solid-state fermentation. Biochem. Eng. J. 29, 262–9. doi: 10.1016/j.bej.2006.01.008
Dikshit, R., and Tallapragada, P. (2018). “Chapter 3—Comparative Study of Natural and Artificial Flavoring Agents and Dyes,” in Grumezescu AM, Holban AM, Natural and Artificial Flavoring Agents and Food Dyes (Cambridge, MA: Academic Press), p. 83–111. doi: 10.1016/B978-0-12-811518-3.00003-X
Dong, C., Baomin, G., Weibao, K., Shiquan, N., Shuling, Y., Yang, Y., et al. (2020). Extraction and stability of monascus pigments from fermentation broth of monascus purpureus Yy1-3 using ethanol/ammonium sulfate aqueous two-phase system. Food Sci. 41, 91–9. doi: 10.7506/spkx1002-6630-20190408-095
Egea, M. B., and Marcionilio, S. M. (2022). A Biodiversidade Como Fonte De Compostos Bioativos: Moléculas E Aplicações. Rio Verde: IF Goiano. doi: 10.54879/978-65-87469-20-1.2022.01.013
Feng, Y., Shao, Y., Zhou, Y., Chen, W., and Chen, F. (2016). “Monascus pigments,” in Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants, eds E. J. Vandamme and J. R. Revuelta (Weinheim: Wiley-VCH), 497–535. doi: 10.1002/9783527681754.ch18
Fernández-López, J. A., Fernández-Lledó, V., and Angosto, J. M. (2020). New insights into red plant pigments: more than just natural colorants. RSC Adv. 10, 24669–82. doi: 10.1039/D0RA03514A
Galaffu, N., Bortlik, K., and Michel, M. (2015). An Industry Perspective on Natural Food Colour Stability. Colour Additives for Foods and Beverages. Cambridge, MA: Elsevier, p. 91–130. doi: 10.1016/B978-1-78242-011-8.00005-2
Gao, J-. M., Yang, S-. X., and Qin, J-. C. (2013). Azaphilones: chemistry and Biology. Chem. Rev. 113, 4755–811. doi: 10.1021/cr300402y
Gautério, G. V., Silvério, C., Egea, M. B., and Lemes, A. C. (2022). β-Glucan from Brewer's spent yeast as a techno-functional food ingredient. Front. Food Sci. Technol. doi: 10.3389/frfst.2022.1074505
Gomah, N. H., Abdel-Raheam, H., and Mohamed, T. (2017). Production of natural pigments from monascus ruber by solid state fermentation of broken rice and its application as colorants of some dairy products. J. Food Dairy Sci. 8, 37–43. doi: 10.21608/jfds.2017.37112
Huawei, Z., Chengtao, W., Jie, Q., Bingjing, Z., Bing, Z., Chuangyun, D., et al. (2019). Determining a Suitable Carbon Source for the Production of Intracellular Pigments from Monascus Purpureus Hbsd 08. Bingley: Pigment and Resin Technology. doi: 10.1108/PRT-05-2019-0042
Ishaq, A., Irfan, S., Sameen, A., and Khalid, N. (2022). Plant-based meat analogs: a review with reference to formulation and gastrointestinal fate. Current Res. Food Sci. 7, 973–983. doi: 10.1016/j.crfs.2022.06.001
Jian, W., Sun, Y., and Wu, J. Y. (2017). Improving the water solubility of monascus pigments under acidic conditions with gum arabic. J. Sci. Food Agric. 97, 2926–33. doi: 10.1002/jsfa.8130
Kim, D., and Ku, S. (2018). Beneficial effects of Monascus Sp. Kccm 10093 pigments and derivatives: a mini review. Molecules 23, 98. doi: 10.3390/molecules23010098
Kobylewski, S., and Jacobson, M. F. (2012). Toxicology of food dyes. Int. J. Occup. Environ. Health 18, 220–46. doi: 10.1179/1077352512Z.00000000034
Kraboun, K., Tochampa, W., Jittrepotch, N., Rojsuntornkitti, K., Chatdamrong, W., Kongbangkerd, T., et al. (2017). Optimization of ultrasonic-assisted extraction for monacolin K, antioxidant activity, pigment and citrinin of monascal waxy corn by response surface methodology. Food Appl. Biosci. J. 5, 115–31.
Lavecchia, T., Rea, G., Antonacci, A., and Giardi, M. T. (2013). Healthy and adverse effects of plant-derived functional metabolites: the need of revealing their content and bioactivity in a complex food matrix. Crit. Rev. Food Sci. Nutr. 53, 198–213. doi: 10.1080/10408398.2010.520829
Lemes, A., Coelho, M., Gautério, G., de Paula, L., de Oliveira Filho, J., Egea, M., et al. (2022). Industrial wastes and by-products: a source of functional foods, nutraceuticals, and biopolymers. Biopolymers in nutraceuticals and functional foods. Royal Soc. Chem. 329–60. doi: 10.1039/9781839168048-00329
Lemes, A. C., Egea, M. B., de Oliveira Filho, J. G., Gautério, G. V., Ribeiro, B. D., Coelho, M. A. Z., et al. (2021). Biological approaches for extraction of bioactive compounds from agro-industrial by-products: a review. Front. Bioeng. Biotechnol. 9, 802543. doi: 10.3389/fbioe.2021.802543
Lin, L., and Xu, J. (2022). Production of fungal pigments: molecular processes and their applications. J. Fungi. 9, 44. doi: 10.3390/jof9010044
Liu, L., and Chen, J. (2019). Systems and Synthetic Biotechnology for Production of Nutraceuticals. Singapore: Springer doi: 10.1007/978-981-15-0446-4
Liu, L., Zhao, J., Huang, Y., Xin, Q., and Wang, Z. (2018). Diversifying of chemical structure of native monascus pigments. Front. Microbiol. 9, 3143. doi: 10.3389/fmicb.2018.03143
Long, P., Zhu, L., Lai, H., Xu, S., Dong, X., Shao, Y., et al. (2023). Monascus red pigment liposomes: microstructural characteristics, stability, and anticancer activity. Foods 12, 447. doi: 10.3390/foods12030447
Mahmoud, G. A-. E., Soltan, H. A., Abdel-Aleem, W. M., and Osman, S. A. (2021). Safe natural bio-pigment production by monascus purpureus using mixed carbon sources with cytotoxicity evaluation on root tips of Allium Cepa L. J. Food Sci. Technol. 58, 2516–27. doi: 10.1007/s13197-020-04758-y
Mal'a, P., Baranová, M., Marcinčáková, D., and Nagy, J. (2010). Organoleptic evaluation of poultry meat products with wheat protein–seitan, coloured by microbial natural pigment. Assam Univ. J. Sci. Technol. 5, 1–5.
Manan, M., Mohamad, R., and Ariff, A. (2017). Monascus Spp.: a source of natural microbial color through fungal biofermentation. J. Microbiol. Exp. 5, 1–19. doi: 10.15406/jmen.2017.05.00148
MarketWatch (2022). Global Natural Pigment Market Share, Size and Forecast Till 2028. Available online at: https://www.marketwatch.com/press-release/global-natural-pigment-market-share-size-and-forecast-till-2028-2022-12-17 (accessed December 17, 2022).
Monteiro, A. B. P., Prados, C. R. M. G., Silva, M. L. R., Silva, E. P., Damiani, C., and Vendruscolo, F. (2021). Production of monascus pigments by solid-state cultivation of wheat grains and application in bread formulations. Int. J. Gastronomy Food Sci. 24, 100313. doi: 10.1016/j.ijgfs.2021.100313
Mukherjee, G., and Singh, S. K. (2011). Purification and characterization of a new red pigment from monascus purpureus in submerged fermentation. Process Biochem. 46, 188–92. doi: 10.1016/j.procbio.2010.08.006
Muthusamy, S., Udhayabaskar, S., Udayakumar, G. P., Kirthikaa, G., and Sivarajasekar, N. (2020). “Properties and applications of natural pigments produced from different biological sources-a concise review,” in Sustainable Development in Energy and Environment. Springer Proceedings in Energy, eds V. Sivasubramaniam, A. Pugazhendi, and I. Moorthy (Singapore: Springer), 105–119. doi: 10.1007/978-981-15-4638-9_9
Oliveira Filho, J. G., Sousa, T. L., Sousa, M. F., Peres, D. S., Danielli, L. Z., Lemes, A. C., et al. (2023). “Bioavailability and Delivery Mechanisms of Nutraceuticals in Nanoparticles Derived from Biopolymers,” in Sreerag Gopi PB, Matej Brai, editor. Biopolymers in Nutraceuticals and Functional Foods (Croydon, UK: The Royal Society of Chemistry), p. 101–21. doi: 10.1039/9781839168048-00101
Orozco, S. F. B., and Kilikian, B. V. (2008). Effect of Ph on citrinin and red pigments production by monascus purpureus Cct3802. World J. Microbiol. Biotechnol. 24, 263–8. doi: 10.1007/s11274-007-9465-9
Panesar, R., Kaur, S., and Panesar, P. S. (2015). Production of microbial pigments utilizing agro-industrial waste: a review. Current Opin. Food Sci. 1, 70–6. doi: 10.1016/j.cofs.2014.12.002
Poorniammal, R., Prabhu, S., Dufossé, L., and Kannan, J. (2021). Safety evaluation of fungal pigments for food applications. J. Fungi. 7, 692. doi: 10.3390/jof7090692
Priatni, S. (2015). Encapsulation and stability study of monascus fermented rice extract. Proc. Chem. 17, 189–93. doi: 10.1016/j.proche.2015.12.118
Sant'Ana, A. S. (2014). “Physical Removal of Microfloras|Filtration,” in Encyclopedia of Food Microbiology (Second Edition), eds Batt, C. A., Tortorello, M. L. (Oxford: Academic Press), p. 36–41. doi: 10.1016/B978-0-12-384730-0.00252-4
Sharma, M., Usmani, Z., Gupta, V. K., and Bhat, R. (2021). Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 41, 535–63. doi: 10.1080/07388551.2021.1873240
Singh, J., and Mehta, A. (2020). Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: a review. Food Sci. Nutr. 8, 2183–204. doi: 10.1002/fsn3.1474
Singh, P., Singh, A., and Saini, P. (2019). “Effect of high pressure processing on quality of fruits and vegetable products,” in Trends and Prospects in Processing of Horticultural Crops, ed Prasad, I. (New Delphi: Today and Tomorrow's Printers and Publishers)
Souza, T. F. C. (2018). Cerveja Com Adição De Arroz Fermentado Em Estado Sólidos Pelo Fungo Monascus Ruber E Identificação De Metabólitos Secundários [Beer with Addition of Fermented Rice in Solid Condition by Fungus Monascus Ruber and Identification of Secondary Metabolites]. Goiânia: Universidade Federal de Goiás.
Srianta, I., Kuswardani, I., Ristiarini, S., Kusumawati, N., Godelive, L., Nugerahani, I., et al. (2022). Utilization of durian seed for monascus fermentation and its application as a functional ingredient in yogurt. Bioresources Bioprocess. 9, 1–14. doi: 10.1186/s40643-022-00619-y
Sudhakar, P., Latha, P., and Reddy, P. V. (2016). “Chapter 15—Plant Pigments,” In: Sudhakar P, Latha P, Reddy PV, Phenotyping Crop Plants for Physiological and Biochemical Traits (Cambridge, MA: Academic Press), p. 121–7. doi: 10.1016/B978-0-12-804073-7.00015-6
Vandanjon, L., Jaouen, P., Rossignol, N., Quéméneur, F., and Robert, J-. M. (1999). “Concentration and desalting by membrane processes of a natural pigment produced by the marine diatom haslea ostrearia simonsen,” in Progress in Industrial Microbiology (Cambridge, MA: Elsevier), p. 393–402. doi: 10.1016/S0079-6352(99)80132-5
Vendruscolo, F., Bühler, R., de Carvalho, J., de Oliveira, D., Moritz, D., Schmidell, W., et al. (2016). Monascus: a reality on the production and application of microbial pigments. Appl. Biochem. Biotechnol. 178, 211–23. doi: 10.1007/s12010-015-1880-z
Vendruscolo, F., Müller, B., Moritz, D., de Oliveira, D., Schmidell, W., Ninow, J., et al. (2013). Thermal stability of natural pigments produced by monascus ruber in submerged fermentation. Biocatal. Agric. Biotechnol. 2, 278–84. doi: 10.1016/j.bcab.2013.03.008
Wrolstad, R. E., and Culver, C. A. (2012). Alternatives to those artificial FdandC food colorants. Annu. Rev. Food Sci. Technol. 3, 59–77. doi: 10.1146/annurev-food-022811-101118
Xu, D., Xie, J., Feng, X., Zhang, X., Ren, Z., Zheng, Y., et al. (2020a). Preparation and evaluation of a rubropunctatin-loaded liposome anticancer drug carrier. RSC Adv. 10, 10352–60. doi: 10.1039/C9RA10390B
Xu, D., Xu, Y., Liu, G., Hou, Z., Yuan, Y., Wang, S., et al. (2018). Effect of carrier agents on the physical properties and morphology of spray-dried monascus pigment powder. LWT 98, 299–305. doi: 10.1016/j.lwt.2018.08.056
Xu, D., Zheng, B., Che, Y., Liu, G., Yuan, Y., Wang, S., et al. (2020b). The stability, microstructure, and microrheological properties of monascus pigment double emulsions stabilized by polyglycerol polyricinoleate and soybean protein isolate. Front. Nutr. 7, 543421. doi: 10.3389/fnut.2020.543421
Zhang, H., Zhu, L., Shao, Y., Wang, L., He, J., He, Y., et al. (2023). Microencapsulation of monascus red pigments by emulsification/internal gelation with freeze/spray-drying: process optimization, morphological characteristics, and stability. LWT 173, 114227. doi: 10.1016/j.lwt.2022.114227
Zhang, L., Li, Z., Dai, B., Zhang, W., and Yuan, Y. (2013). Effect of submerged and solid-state fermentation on pigment and citrinin production by monascus purpureus. Acta Biol. Hung. 64, 385–94. doi: 10.1556/ABiol.64.2013.3.11
Keywords: bioprocesses, monascorubramine, natural pigment, color, biological properties
Citation: Egea MB, Dantas LA, Sousa TLd, Lima AG and Lemes AC (2023) The potential, strategies, and challenges of Monascus pigment for food application. Front. Sustain. Food Syst. 7:1141644. doi: 10.3389/fsufs.2023.1141644
Received: 10 January 2023; Accepted: 06 March 2023;
Published: 23 March 2023.
Edited by:
Laurent Dufossé, Université de la Réunion, FranceReviewed by:
Juliano Lemos Bicas, State University of Campinas, BrazilJulio Montañez, Autonomous University of Coahuila, Mexico
Copyright © 2023 Egea, Dantas, Sousa, Lima and Lemes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariana Buranelo Egea, bWFyaWFuYS5lZ2VhQGlmZ29pYW5vLmVkdS5icg==
 Mariana Buranelo Egea
Mariana Buranelo Egea Luciana Arantes Dantas
Luciana Arantes Dantas Tainara Leal de Sousa2
Tainara Leal de Sousa2 Alan Gomes Lima
Alan Gomes Lima Ailton Cesar Lemes
Ailton Cesar Lemes